6
Clinical Trials in the Era of COVID-19 and Beyond
The goal of drug development is to assure quality, safety, and efficacy of products that are delivered to patients, said Sven Stegemann, professor for patient-centric drug development and manufacturing at the Graz University of Technology. This is achieved through a defined program that involves discovery, development, clinical trials, and presenting evidence for regulatory approval.1 In the past few decades, this process has
___________________
1 For more information, see https://www.fda.gov/drugs/drug-information-consumers/ fdas-drug-review-process-ensuring-drugs-are-safe-and-effective (accessed November 11, 2020).
been adjusted to provide fast access to innovative treatments through approaches such as fast track, breakthrough, and orphan drug development.2 Since the outbreak of COVID-19, Stegemann said, the existing procedures are showing their limitations for developing treatments and vaccines for the populations most affected, and in a timeframe that is necessary. Researchers are taking on a “joint ethical responsibility” to address these new challenges quickly without compromising on quality or safety. Acting thoughtfully and in sync with other stakeholders, he said, will bring innovative medicines to patients faster; these innovations “might be considered a quantum leap in health care a few years from now.”
In this session, speakers considered how clinical trials, research, and health care have changed in response to the COVID-19 pandemic, and explored how some of these changes might be beneficial for the future.
A REGULATORY PERSPECTIVE
FDA responded rapidly to the COVID-19 pandemic, said Harpreet Singh, acting division director of the FDA Division of Oncology 2. Multiple guidances on a variety of topics were released, including one on the conduct of clinical trials during COVID-19.3 This expedited guidance was released without public comment on March 19, 2020, said Singh, and has been updated several times based on questions asked and clarifications needed. The document provides general guidance on common issues, and asks sponsors with specific questions to contact the appropriate FDA review division, she said.
The guiding principle of the clinical trial guidance is patient safety, said Singh. The guidance states that “ensuring the safety of trial participants is paramount,” and that any necessary modifications to trials should ensure safety. The document encourages investigators and sponsors to engage with their Institutional Review Boards (IRBs) on issues of continued accrual, drug administration, and trial participation. Sponsors may consider alternatives to trial protocols, including alternative offsite methods for safety assessments, the use of technology to facilitate remote data collection and monitoring, and alternative secure delivery methods for investigational products such as oral therapies and IV therapies. In addition, the document clarifies the requirements for investigators and sponsors to interact with and report to FDA and IRBs. COVID-19 screen-
___________________
2 For more information, see https://www.fda.gov/patients/learn-about-drug-and-deviceapprovals/fast-track-breakthrough-therapy-accelerated-approval-priority-review (accessed November 11, 2020).
3 For more information, see https://www.fda.gov/regulatory-information/search-fdaguidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency (accessed November 11, 2020).
ing as a part of health care does not need to be reported as a protocol amendment unless the data are part of the research objectives, and protocol modifications to protect life and well-being may be implemented before IRB or FDA approval. Most importantly, said Singh, it is critical that all of these changes and contingency plans be well documented to track the challenges and opportunities moving forward.
Singh noted that many of these considerations are relevant to trials with older adults. This “grand experiment” during COVID-19 has forced the rapid adaptation of new models and approaches, and will have lessons learned for moving forward. For example, said Singh, a decentralized or hybrid trial approach—in which little to no travel is required and digital health technology facilitates the trial—might be used during COVID-19, and may also serve to address some of the barriers to clinical trial participation by older adults. These barriers, said Singh, include the geographic location of most clinical trials, difficulty with transportation to the site, requirements for frequent visits to the clinical site, and a preference for treatment by one’s own physician. Decentralized trials can address these barriers by using local labs and local imaging facilities for safety assessments; using telemedicine visits or wearable technologies to communicate with patients and collect data; and using alternative methods of product delivery, such as local administration of IV drugs or shipping of oral medications.
There have long been calls to make clinical trials more patient centered, said Singh; the acceleration of approaches such as decentralized trials may be a “silver lining” to the pandemic. Former FDA Commissioner Gottlieb expressed support for such approaches before the pandemic, stating in 2019 that “pragmatic and hybrid clinical trials, including decentralized trials that are conducted at the point of care—and that incorporate real-world evidence—can help clinical trials become more agile and efficient by reducing administrative burdens on sponsors and those conducting trials, and can allow patients to receive treatments from community providers without compromising the quality of the trial or the integrity of the data being collected.”4
In closing, Singh asked workshop participants to consider three issues:
- Can researchers leverage clinical trials conducted during the COVID-19 pandemic to understand the implications of remote assessments and decentralized trial procedures for older adults?
___________________
4 For more information, see https://www.fda.gov/news-events/speeches-fda-officials/ breaking-down-barriers-between-clinical-trials-and-clinical-care-incorporating-real-world-evidence (accessed November 12, 2020).
- What are the challenges and opportunities for use of telemedicine to provide care for older adults? For example, what challenges are faced by older adults with visual, hearing, or cognitive impairments?
- What are the practical lessons that clinicians can take from increased digitization and use of technology in clinical trials as they relate to older adults? For example, could the Centers for Medicare & Medicaid Services (CMS) support coverage of devices for older adults with certain impairments?
A CLINICIAN PERSPECTIVE
Many common acute infections disproportionately affect older adults, said John Powers, professor of clinical medicine at The George Washington University School of Medicine. This is due to differences in immune function and response, as well as the frequency of comorbidities and co-medications among older adults. In addition, the effect of interventions—drugs and vaccines—differ between younger and older adults. COVID-19 is “another in a list” of infectious diseases that disproportionately affect older persons, he said. According to the Centers for Disease Control and Prevention, 8 out of 10 people who have died from coronavirus have been age 65 or older. The risk of hospitalization “goes up almost logarithmically” by age (see Figure 6-1).
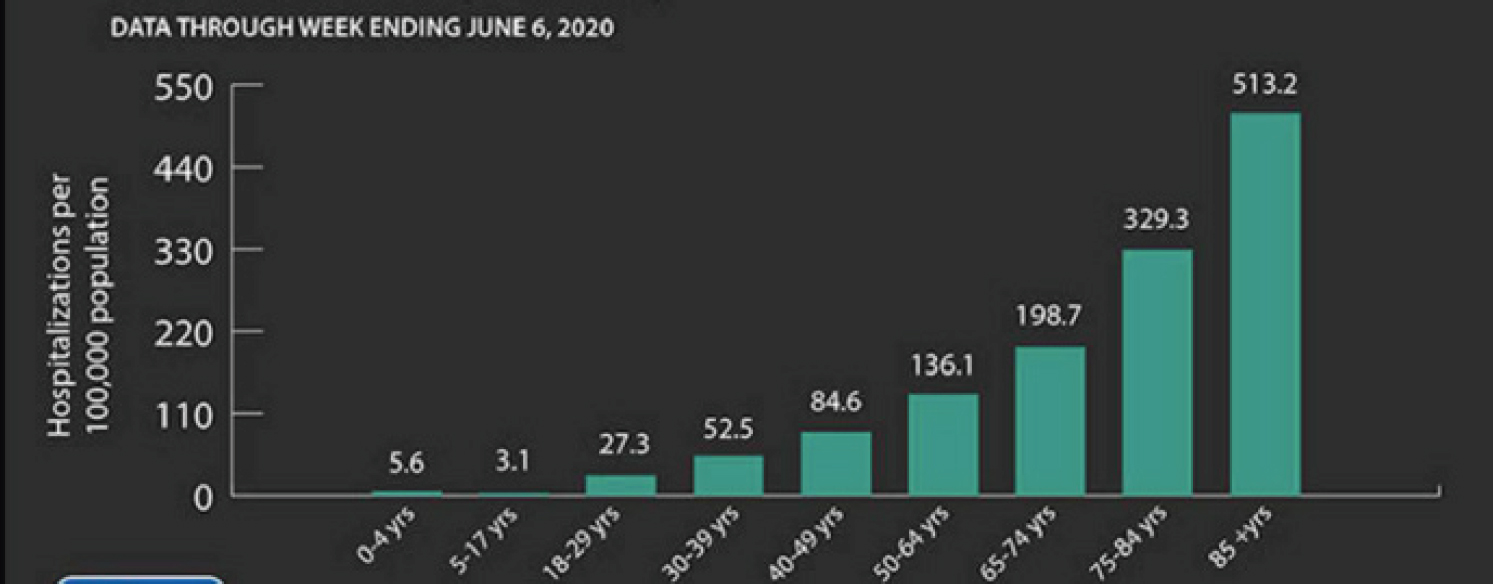
NOTES: As age rises, so does the chance of hospitalization for COVID-19, with the oldest old (85+ years) at 513.2/100,000 people compared with different age groupings. Data shown reflect hospitalizations from COVID-19 through June 6, 2020.
SOURCES: As presented by John Powers, August 6, 2020. See the Centers for Disease Control and Prevention webpage at https://www.cdc.gov/coronavirus/2019ncov/need-extra-precautions/older-adults.html (accessed December 17, 2020).
The disproportionate impact of infectious diseases on older adults has implications for the conduct of clinical trials as well as for value-based clinical care, said Powers. There are implications for inclusion and exclusion criteria, interventions studied, outcomes measured, and the value of medical interventions. He said that value in health care “means improving patient outcomes, not just improving processes.” There is a growing trend toward patient-focused drug development and the collection of real-world evidence about how interventions are used in practice, he said, but clinical trials often still do not focus on the outcomes or populations that are most relevant.
There is also a need for clinical trials to measure outcomes that matter to patients, particularly for older adults, Powers said. A patient-reported outcome (PRO) is information gathered from patients without interpretation from anyone else. Using PROs is useful because many of the effects of a disease can only be known by the person experiencing the disease (e.g., pain). Additionally, patients may have a different perspective on the outcomes that matter (Powers et al., 2016). For example, more than 99 percent of people survive COVID-19, but survivors may have symptoms that bother them and impair their day-to-day function. Many COVID-19 studies use indirect outcome measures such as the doctor’s decision to prescribe oxygen, or the doctor’s decision to put a patient in the hospital or the intensive care unit. By contrast, PROs can directly measure the patient’s health status.
PROs can be used as endpoints in infectious disease studies, said Powers. To develop meaningful PROs, patients should be interviewed in order to understand what symptoms are important and to be able to phrase questions in a way that patients understand. For example, some have suggested that COVID-19 patients should be asked about “feverishness,” but Powers evaluated the use of this word and found that patients do not understand it. Powers and his colleagues have developed a PRO instrument to measure influenza-like illness (Powers et al., 2018). It asks patients to use a daily diary to assess their symptoms across six body systems: nose, throat, eyes, chest/respiratory, GI, and body/systemic. The tool was developed and tested in adults with influenza, but could also be used for assessing patients with COVID-19.
There are some challenges to using these types of patient-reported measures in older adults, said Powers; however, some of these may be based on assumptions rather than evidence. For example, there are assumptions that older adults are not able to answer questions regarding their own health because they are too sick or suffer from dementia. While this may be true in some cases, said Powers, it is not true for all older adults. There are also assumptions that older adults cannot use electronic devices for reporting outcomes. If this is the case, said Powers, inves-
tigators can provide training on the devices or conduct data collection by telephone. He shared a study in which PROs, along with laboratory confirmation, were used as efficacy endpoints in a trial of a respiratory syncytial virus vaccine in older adults (Yu et al., 2019).
In conclusion, Powers said, there is a need to include older persons in clinical studies to obtain better real-world evidence, particularly given the potential differences in effectiveness and adverse effects. PROs can be used to directly measure the impact of a disease on a patient’s life, and these measures can and should be used in infectious disease and in older patients. Better measurement of patient outcomes, said Powers, will lead to better value in health care for those most affected.
TELEHEALTH APPLICATIONS
Although telehealth has been reimbursable since 1997, said Erika Ramsdale, assistant professor at the University of Rochester Medical Center, limitations and restrictions on reimbursement have meant that implementation of telehealth has lagged behind the technology.5 Many of these restrictions were lifted due to the COVID-19 pandemic,6 and there has been rapid uptake of the use of telehealth. For example, the number of Medicare beneficiaries receiving telehealth services each week went from nearly zero to approximately 1.7 million over the course of 1 month (see Figure 6-2). At the Wilmot Cancer Institute, where Ramsdale practices, phone and video visits ramped up to replace in-person visits after a drop-off in March. Ramsdale noted that the use of telehealth was fairly similar for those under age 65 and those 65 and older.
There is evidence that older adults are less likely to have access to home broadband or devices than younger people (Anderson and Perrin, 2017). However, said Ramsdale, age is not the only factor behind this “digital divide.” Numerous intersecting and interacting factors modify access to the Internet, she said. Older adults with higher levels of education and higher incomes are much more likely to have access to Internet and home broadband (see Figure 6-3). A recent study found that about 38 percent of older adults are not ready for telemedicine—that is, they do not have access to technology, they are not comfortable with technology, or they have age-related issues that prevent them from using technology (Lam et al., 2020). However, said Ramsdale, the “readiness” of
___________________
5 For more information, see S 385, Comprehensive Telehealth Act of 1997. See https://www.congress.gov/bill/105th-congress/senate-bill/385 (accessed November 12, 2020).
6 For more information, see HR 748, Coronavirus Aid, Relief, and Economic Security Act. See https://www.congress.gov/bill/116th-congress/house-bill/748 (accessed November 12, 2020).
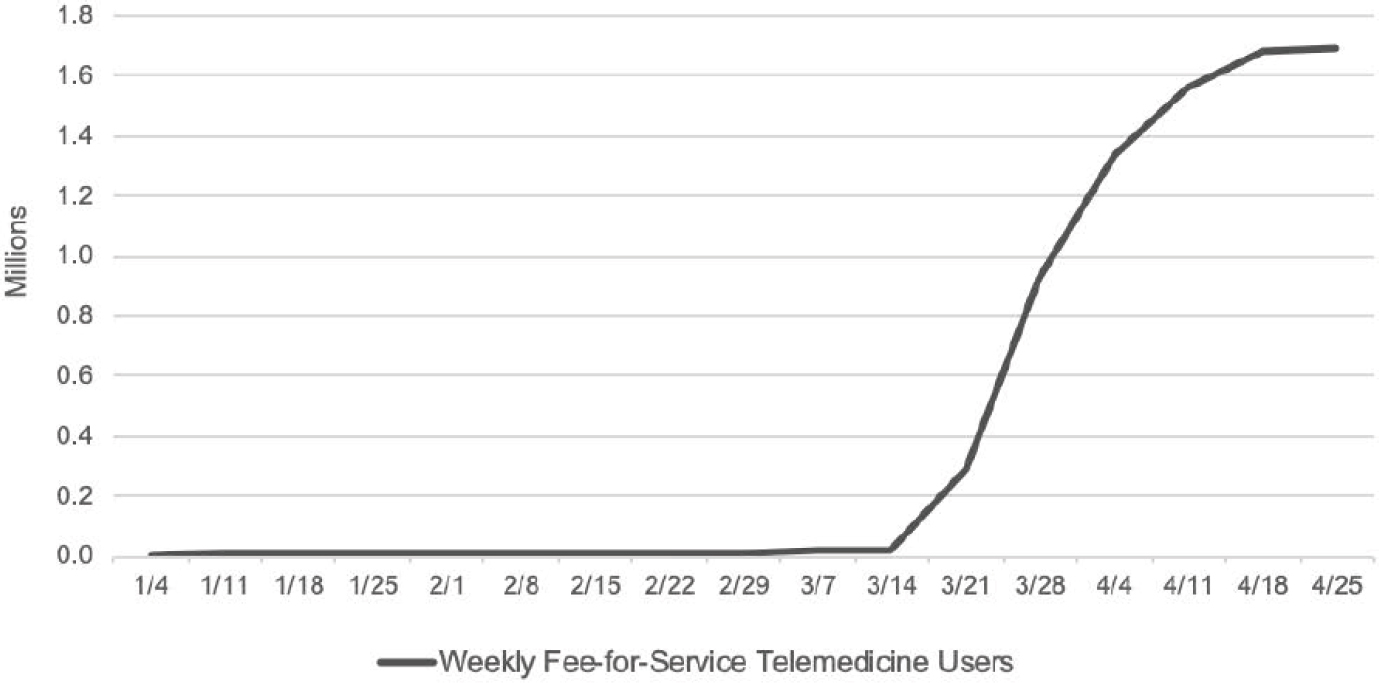
NOTE: There is an increase in use of telehealth services in March, which coincides with public health steps to mitigate the spread of COVID-19 in the United States.
SOURCES: As presented by Erika Ramsdale, August 6, 2020. Originally from Verma, 2020.
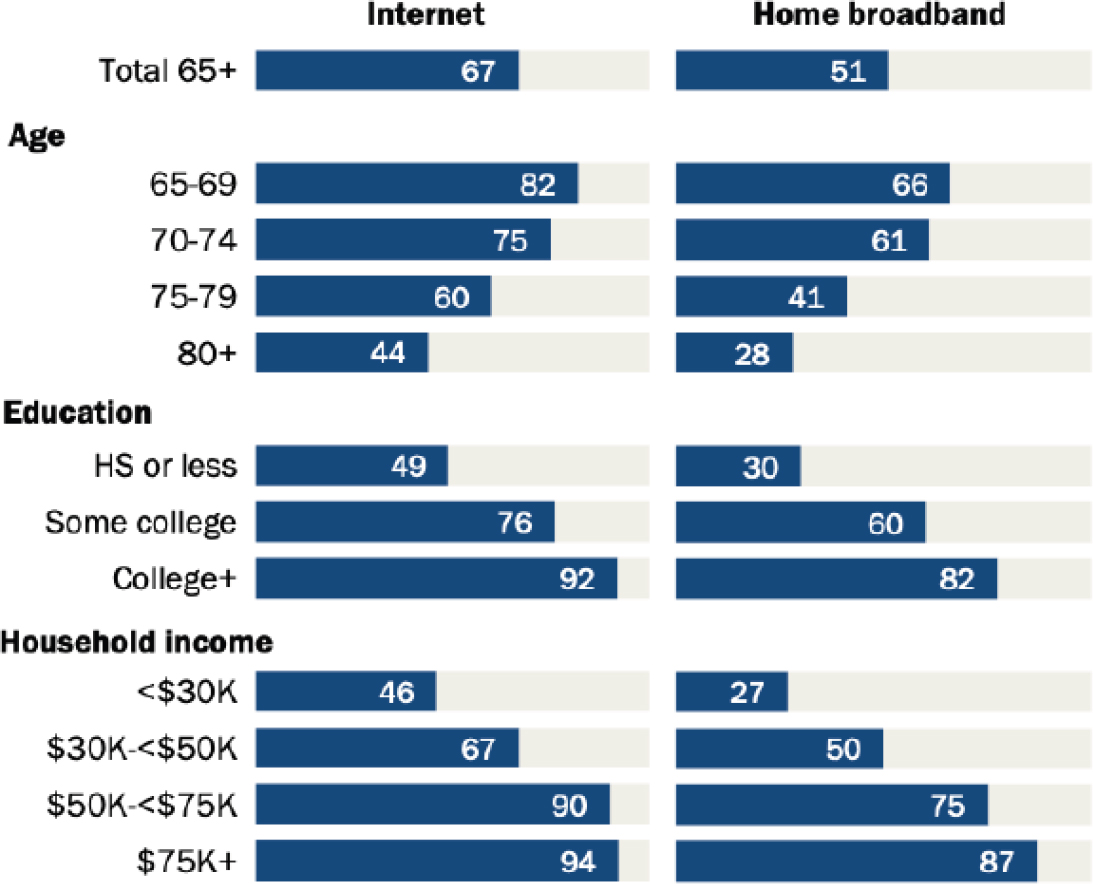
NOTES: Adults over age 80 are less likely than younger older adults to have broadband or Internet access, but age is not the only factor. Higher income and education indicate that adults are more likely to have broadband and Internet access, regardless of age.
SOURCES: As presented by Erika Ramsdale, August 6, 2020. Originally from Anderson and Perrin, 2017.
older adults varied considerably by characteristics such as race, location, income, education, and self-rated health. Unfortunately, she said, “some of the people that most need to access high levels of care are the least able to do it in the COVID era.”
Telehealth has many benefits for older adults, said Ramsdale. It limits their reliance on mobility and access to transportation. Patients may be more comfortable in their own homes, and it is easier for families or caregivers to join an appointment or conversation (Joinson, 2001). Since the pandemic began, Ramsdale said that she has been able to conduct some tasks more easily via telehealth. For example, reviewing a patient’s medications is difficult to do in the clinic because they forget to bring all of their medications with them, she said. With a telehealth appointment, Ramsdale can easily be shown their medications, their routine for taking them (e.g., using daily pillboxes), and any potential issues (e.g., pills are disorganized). Another benefit, she said, is being able to evaluate the home environment and identify any problems, such as fall risks.
Ramsdale shared several examples of recent or current studies that have examined the use of telehealth among older adults. The first was a study that looked at the use of virtual house calls for patients with Parkinson’s (Beck et al., 2017). The study found that there was no difference in clinical outcomes compared with in-person visits, and not only were patients satisfied with virtual visits, but they preferred them (55 percent to 18 percent). However, said Ramsdale, the caveat is that the trial participants were not representative of the general population—96 percent were white, 73 percent were college educated, and nearly all had Internet access at home. Another in-progress trial is looking at palliative care and the potential benefits of virtual access to supports, including caregiver support groups, chaplains, social workers, and psychotherapists (Kluger et al., 2020). The investigator on this study, Benzi Kluger, told Ramsdale, “Older adults more frequently find televideo burdensome and are more likely to choose phone or mail options if offered. However, the vast majority do fine and often involving younger relatives, friends, or neighbors in visits can get them over the hump.” Ramsdale is currently in the preimplementation phase of a trial looking at older adults with cancer and polypharmacy. The intervention arm will receive a virtual pharmacist assessment and a deprescribing intervention. Referring to Rothschild’s presentation, Ramsdale said she is in the “shut up and listen” phase, which involves focus groups with stakeholders and a prepilot iterative adaptation of the intervention. The trial is designed as a hybrid effectiveness–implementation trial (see Tobin’s presentation), which aims to measure efficacy outcomes as well as the implementation of the intervention in the real world.
Remote geriatric assessments have been conducted for more than a decade, said Ramsdale, beginning with “very low tech” thick paper pack-
ets mailed to patients and now moving into using electronic methods. Most measures for geriatric assessment are reported by patients, with reasonable concordance with objective measures. However, said Ramsdale, measuring cognition remotely has been challenging. Although the Montreal Cognitive Assessment has been validated for virtual administration (Chapman et al., 2021; DeYoung and Shenal, 2019), people with dementia or cognitive impairment may have difficulty accessing the necessary technology or understanding questions over the phone. During the pandemic, Ramsdale and her colleagues have performed four or five remote cognitive evaluations per week, but there have been high rates of difficulty hearing questions and suspicions about reliability due to patients “cheating” or difficulty monitoring some aspects of the tests. There is a software platform called CAMPFIRE that can be used for cognitive assessments; it collects PROs and objective measures, and reports the data to the provider. The platform attempts to mimic the in-person experience (e.g., an iPad and a stylus to mimic paper administration), and uses technology that can track attention and eye movement (Elkin-Frankston, 2017; Jacobs et al., 2019).
“Access to home digital technology in the COVID era and beyond is access to health care,” said Ramsdale. Given the promise and the ubiquity of telehealth, the Internet, enabled devices, and tech support should be considered medical needs. Of course, said Ramsdale, there will be some age-related barriers such as hearing, vision, and cognition. However, there are ways to overcome these barriers, including innovative technologies, support, and software tailored to different needs. Ultimately, there is a need for more implementation research to study how to best connect older adults with the technology they need to participate in clinical research or receive health care. Clinicians can leverage the “unfortunate opportunity” of the pandemic to rapidly iterate ideas, gather pilot data, and further the promise of telehealth for older adults.
DIGITIZATION OF MEDICINE
Eric Topol, founder and director of the Scripps Research Translational Institute, told workshop participants about a study that launched in March 2020 called DETECT (Digital Engagement & Tracking for Early Control & Treatment).7 During the COVID-19 pandemic, access to testing has been an issue, said Topol. Even if there was expanded access, testing cannot be done on all 330 million Americans on a regular basis, and there is the risk of false negatives. Digital surveillance is a way to get around
___________________
7 For more information, see NCT04336020 at https://clinicaltrials.gov/ct2/show/ NCT04336020 (accessed November 12, 2020).
this issue, said Topol. More than 100 million Americans have a smart watch or fitness wristband that collects heart rate data. Topol and his colleagues have found that certain signals in the heart rate data—including elevated resting heart rate, less physical activity, and more sleep—can be used as surrogates for fatigue. Tracking fatigue on a population level by geographic area can help detect an outbreak of flu-like illness, including COVID-19. Topol expressed his hope that more people would join the DETECT study, stating that this digital surveillance tool could be helpful in “getting a much better grip” on the COVID-19 pandemic in the United States.
A PATIENT PERSPECTIVE
“The concept of patient centeredness in health care has come a long way since I was diagnosed with breast cancer 20 years ago,” said Beverly Canin, member of the Cancer and Aging Research Group. There has been a major movement toward centering patients, including PCORI’s engagement with patients,8 FDA and NIH routinely including patients in their decision making,9 and patients serving as members of peer review panels.
Unfortunately, COVID-19 has spotlighted what has long been known: There are large disparities in health care outcomes among racial, ethnic, socioeconomic, and age groups.10 COVID-19 has exposed the systemic weaknesses in health care systems, and despite programs to overcome these disparities, we have made only incremental progress, she said (Zimmerman and Anderson, 2019). The question is whether COVID-19 will be a catalyst for more incremental progress or major change that addresses systemic issues and entrenched disparities. This workshop so far, said Canin, is “encouraging” in its focus on including and engaging older adults in clinical research, and the open acknowledgment of the barriers to doing so. However, “we have been vividly reminded by COVID-19 that the more we know, the more we do not know.” There is a need to keep the focus on what matters most for patients and the providers who care for them.
Canin told workshop participants about a recent survey that was conducted to measure “cancer care providers’ attitudes toward the barriers and facilitators related to the care for these patients during the pandemic” (BrintzenhofeSzoc et al., 2021). Canin said that the findings in Brintzen-
___________________
8 For more information, see https://www.pcori.org/about-us/our-programs/engagement/ public-and-patient-engagement (accessed November 12, 2020).
9 For one example, see https://allofus.nih.gov/about/all-us-research-program-overview (accessed November 12, 2020).
10 For more information, see https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html (accessed November 12, 2020).
hofeSzoc and colleagues (2021), as well as a manuscript under review at the time of the workshop, underscored some of the discussions in this workshop. Communication among patients, caregivers, and health care providers is recognized as vital to optimizing outcomes for patients, but it is not always practiced. Canin shared her personal experience from 20 years ago when an oncologist refused to discuss whether the literature supported her need for chemotherapy. Other findings included a need for more training in cultural sensitivity and culturally appropriate communication strategies, both in the clinic and in public health messaging; more innovative outreach strategies; and leveraging the relationships that formal and informal community-based organizations have with distinct patient populations.
Canin closed by emphasizing the need to delve deeper into the systemic causes of exclusion and disparities, as well as systemic racism. She challenged workshop participants to question their complicity in perpetuating the status quo, and to examine what can be done to address these major issues.
DISCUSSION
Stegemann led panelists and workshop participants in a wide-ranging discussion on a variety of topics including remote consent and applications for technology in research and clinical care.
Remote Consent
Stegemann noted that older adults sometimes have cognitive impairments such as dementia, and he asked panelists to address the issue of obtaining remote consent for older adults to participate in research. Singh started by saying that FDA has guidance that specifically addresses how to obtain remote or virtual consent from a legally authorized representative of an impaired patient.11 However, she noted that FDA guidance is not legally binding, and that many states, institutions, and health care systems do not permit remote consent. She wondered if there is a false perception that FDA does not allow remote consent, and suggested that there is a need to engage with those who are resistant to determining the barriers. Ramsdale said that there is a lot of institutional variability on this matter, and Powers noted that institutions tend to be resistant to change. He told a story about a newspaper article from the 1800s about how a new device in medicine “will never take hold.” The device was
___________________
11 For more information, see https://www.fda.gov/regulatory-information/search-fdaguidance-documents/informed-consent (accessed November 12, 2020).
the stethoscope. One way to encourage change, he said, is to disseminate evidence; one example is showing people that remote consent for older adults is already happening and that it works. Topol added an example from his own work; the trial enrolled 6,000 people with an average age of more than 70, and all consented remotely.
On a related subject, Canin said that consent forms often use language that is not user friendly. She said that the language is often complex medical language rather than “something that was easy for a layperson to understand.” She noted that this is a barrier to trial participation that does “not need to be there.” With the health inequities and disparities in this country, she said, researchers should be facilitating access to research participation rather than setting up barriers.
Applications for Technology in Research and Clinical Care
Stegemann asked panelists whether the pandemic will have an impact on how technology is used in research and clinical care. Canin responded that while technology has great promise, a parallel strategy is needed to reach people who do not have access to technology. Powers said that technology is often thought about in terms of “bells and whistles,” but that technologies that have been around for 100 years—such as the mail and the telephone—can be used to reach populations who have reduced access to modern technologies. Ramsdale added that reaching rural populations can be accomplished by “bringing the care to people where they are,” whether that is with high-tech bells and whistles or simply having a research coordinator onsite at a community clinic. Stegemann concluded that there is no single answer, but that more tools are needed to reach all people.
With the increased use of telemedicine during the pandemic, Stegemann wondered if video visits are changing the nature of the patient–provider relationship. Ramsdale responded that the research on this point is mixed; while some research shows that patients are more likely to divulge information to a computer, there is also research that suggested that patients can be self-conscious and anxious during video visits.12 She speculated that the use of technology would likely change the patient–provider relationship, but it is unclear exactly what the effect would be. Topol emphasized the importance of in-person care, relationships, and human touch, and stated his wish that the “intimate, precious relationship that was the epitome of empathy and communication” not be eroded further by the use of telemedicine.
___________________
12 For a sample article summarizing these phenomena, see https://www.theatlantic.com/health/archive/2020/08/telemedicine-has-resurrected-house-call/614992 (accessed November 12, 2020).
Powers noted that this does not have to be an “either/or” situation; despite the prevalence and benefits of technology, it will never completely replace in-person visits. For example, noted Stegemann, technology could be used for brief interactions between in-person visits to increase communication and potentially improve adherence. Canin noted that some people may prefer video visits when the alternative is in-person visits with both parties masked and distanced.
COVID-19 as an Impetus for Change
“We need to insist that a lot of these changes continue” past the COVID-19 pandemic, said Powers. Many changes that have occurred during the pandemic—such as remote trials and increased use of telemedicine—“should have happened already and COVID was just the thing that goosed us along to move a little faster toward implementing them.” Singh agreed and said FDA is actively engaging with industry to learn what is happening during the pandemic and what potential challenges may arise. For example, she said, is the integrity of the data impacted when a trial is conducted remotely? How can practices such as televisits and shipping medications be used to improve future trials? She noted that creativity during the pandemic has opened doors to new ways of doing things, and this “should persist past COVID.” Ramsdale, recalling Canin’s presentation, said the pandemic has “forced us to think about realigning with some of our priorities.” The rifts in society—such as health inequities and lack of access to care—are now “glaringly obvious,” said Ramsdale, and there is an opportunity to address them. Ramsdale added that making changes is a matter of “where we are putting our attention [and] where we are putting our money” and policy solutions are needed to overcome institutional inertia. However, said Canin, these changes will only happen if “we as individuals take it on as a commitment to make it happen.”
This page intentionally left blank.














