During the second session of the workshop, presenters and attendees explored how systems thinking approaches may be applied to challenges associated with identifying the critical quality attributes in the discovery, regulation, and manufacturing of regenerative medicine. The session featured a fireside chat with Amy Abernethy, the principal deputy commissioner at the Food and Drug Administration (FDA), on the regulation of regenerative medicine products in the context of systems thinking approaches which was moderated by Anne Plant, a National Institute of Standards and Technology (NIST) Fellow and the former chief of the Biosystems and Biomaterials Division at NIST. Jane Lebkowski, the president of Regenerative Patch Technologies, moderated the subsequent panel discussion on the costs associated with not implementing systems thinking approaches. The panelists, who gave brief presentations as part of the panel included Adrian Bot, the vice president of translational medicine at Kite Pharma, Inc.; Bala Manian, the chief executive officer (CEO) of Mojave Bio, Inc.; Douglas Olson, the president and the CEO of Buhlmann Diagnostics Corp and a scientific advisory board member at Cell Manufacturing Technologies (CMaT); and Sally Temple, the scientific director, the principal investigator, and the co-founder of the Neural Stem Cell Institute. This session’s objective was to understand the challenges associated with identifying critical quality attributes in the discovery, regulation, and manufacturing of regenerative medicine products and how systems thinking approaches may be applied.
SYSTEMS THINKING AND THE REGULATION OF REGENERATIVE MEDICINE PRODUCTS
Plant opened the session by noting that progress in the collection of large quantities of data, which is often omics-based, has made large datasets available for both biological and clinical sciences. As the development of advanced therapies becomes increasingly reliant on large datasets, it brings advantages as well as technical challenges in data collection, analysis, and the development of appropriate models to provide context to those data in order to provide predictive knowledge. Abernethy described opportunities and challenges associated with access to big data and systems thinking in the context of the regulatory landscape for regenerative medicine.
Systems Thinking and Large-Scale Data in the Regulatory Landscape
The conceptual approach of using large-scale datasets across the medical product development process—from early development to late-stage post-marketing evaluation—is of great importance to FDA, Abernethy said. An advantage of systems thinking is the opportunity to link various steps of the development process through datasets. This is helpful in characterizing products, understanding pharmacology and toxicology information, and discerning clinical effects. Using large-scale datasets to consider issues of efficacy, safety, and approval of drugs in the regenerative medicine space will help improve the quality of medical products, she said; however, despite great interest in using large-scale datasets to accelerate drug development, this is not yet a widespread practice.
Interrelating large-scale datasets of deep omics (deep learning applied to omics data) on the discovery side together with clinical data can support clinical evaluation. For example, Abernethy said, next-generation genomic sequencing data have been combined with electronic health record data to create appended datasets that marry those two types of information. In turn, those datasets could then be married with information about the manufacturing process of various regenerative therapies. Likewise, the datasets could be combined with information related to health care delivery. Combining these datasets can elucidate interrelated features across clinical development, quality manufacturing, and quality implementation on the health care delivery side. Furthermore, Abernethy said, this process can distinguish the issues of manufacturing and health care delivery that are more important to focus on from those that are of lesser consequence. However, the ability to gather seemingly disparate information by considering an entire set of interrelationships requires subject-matter expertise—not only in mathematics, but also in all of the different areas involved—to be able to understand the data in context. For FDA, this means developing the
technical cross-checking capabilities needed to confirm findings. Thus, she said, there is much work to be done across the entire process.
Efforts to Combine Large-Scale Data Across the Wider Ecosystem of Components
Are there examples, Plant asked, of efforts that combine data over a wide ecosystem of components and then bring those data into the discovery, manufacturing, or delivery processes? Abernethy answered that these processes are not yet well-developed, but that some efforts are showing promise. For example, FDA is conducting a Model-Informed Drug Development pilot program1 in which clinical pharmacologists, statisticians, and other FDA professionals are working with industry and drug developers to use preclinical and early clinical data to improve the planning of clinical trials, identify dosing more carefully, and predict the most consequential trial outcomes more accurately. Using information gathered from sources that historically were excluded from the trial design process, this process is increasing the knowledge used in the clinical trials context. Furthermore, essential preclinical safety models have been created to inform researchers as to what safety events might be seen in the clinical trial space. The pilot program, Abernethy said, is an important step in developing communication between regulators and the industry, and she suggested that a critical step in moving forward will be to create a common language to enable stakeholders to determine who is responsible for each step and how best to document datasets, methods, and outputs.
Work related to the coronavirus disease 2019 (COVID-19) is another area where researchers are intermarrying large datasets, Abernethy said. Computational modeling of repurposed drugs that have potential as therapeutics for COVID-19 is being overlaid with data from the use of those drugs in real clinical environments as well as with data about the risk of developing COVID-19. These data are being used to help identify potential drugs that can be repurposed as COVID-19 therapeutics and to determine priorities for clinical trials (Gordon et al., 2020). In the regenerative medicine space, long-term data are needed to better understand the long-term safety consequences of therapeutics. FDA issued guidance for cell and gene therapies in January 2020 that highlighted that the long-term safety consequences of gene therapies are not yet understood.2 Preclinical models appended to
___________________
1 More information about the Model-Informed Drug Development pilot program is available at https://www.fda.gov/drugs/development-resources/model-informed-drug-development-pilot-program (accessed December 8, 2020).
2 FDA’s cellular and gene therapy guidances are available at https://www.fda.gov/vaccinesblood-biologics/biologics-guidances/cellular-gene-therapy-guidances (accessed December 8, 2020).
clinical trials can inform what safety issues to monitor for, while follow-up information from insurance claims and electronic health records data can aid in better understanding longer-term clinical impacts, Abernethy added.
Shifting Dynamics in the Manufacturing Process
There is a push–pull dynamic in the manufacturing space, Plant said, between having a manufacturing process and then wanting to change, improve, or streamline that process, which may involve a change in starting materials. The philosophy of “product is the process” seems to have worked for some biological therapies, she said, but that philosophy may not apply as aptly to cell therapies, in which a philosophy of “product is the product” may be more appropriate. Will it shift the dynamic to have more data and a better understanding of what is important to measure in order to predict product success? she asked. Abernethy predicted that there will be continued movement in “product-is-the-process” thinking, especially in the regenerative medicine space with respect to n-of-1 or n-of-a-few therapies, which will require continued novel thinking and development.
Abernethy remarked that when she joined FDA in 2019, she discovered that the elements of the tightly controlled, professionalized manufacturing process were not specifically appended to an understanding of how patients perform. At that time, cross-linking manufacturing details and optimization details with electronic health records data went against common practice, she said. Currently, in late 2020, manufacturing plants are being built to include a series of intricate sensors. In the shift toward n-of-1 or n-of-a-few therapies, work is under way to interrelate details along the manufacturing pathway to clinical outcomes. This could help researchers explore the interrelationships among changes made to the manufacturing process, any resulting changes in the product, and the possible effects of those changes in people. Systems thinking, she added, provides an opportunity to think broadly about how different pieces can interrelate, given the tether of data and computation that is being created, and which can be used to understand the ripple effects.
Regulatory Challenges in Shifting Toward Systems Thinking
In shifting toward a systems thinking approach, Abernethy said, there are many areas FDA may need to address thoughtfully. For example, regulatory certainty is important for medical product development because it enables decisions about where to invest and it informs expectations along the pathway. Thus, FDA is cautious in making changes, as any shifts in FDA’s approach have the potential to cause downstream effects on safety and effectiveness and to shift regulatory expectations in an environment in
which people want certainty about what process to expect. Therefore, in exploring an area such as systems thinking and the interrelationship of multiple datasets, FDA would benefit from becoming more comfortable with the possible changes in the development frame. Efforts to link up various components are already ongoing in preclinical toxicity and post-marketing studies, she said, and she suggested that industry should also engage in this type of work.
FDA may also need to be able to clarify the characteristics of datasets, determine their fit for purpose, identify the appropriate analyses to enact for various tasks, and develop communication about those tasks, Abernethy said. Furthermore, different tasks are needed for various spaces. For instance, the specific tasks required in modeling preclinical toxicity and clinical trials differ from those involved in modeling to replace some part of the pathway. Familiarity with these different tasks can then enable the development of a set of prescribed events, such as guidance documents. For this multistep pathway Abernethy envisioned a series of pilots, such as the Model-Informed Drug Development pilot, along with those guidances for important components (e.g., conducting 15 years of follow-up research). Finally, she suggested that FDA should participate—either directly or via public-private partnerships—in projects to build regulatory familiarity, enabling regulators to develop the expertise required for this new approach.
Facilitating Systems Thinking Among FDA Sponsors
Is FDA planning, Plant asked, on taking any actions to facilitate systems thinking on the part of FDA sponsors? The Model-Informed Drug Development pilot is a good example of encouraging this shift, Abernethy replied. In the context of the current pandemic, FDA is participating in the COVID-19 Evidence Accelerator.3 This set of activities is being conducted via a public–private partnership organized by the Reagan-Udall Foundation, FDA’s congressionally mandated foundation. The Foundation has brought together stakeholders who have data—predominantly clinical data and real-world data relevant to COVID-19—and those willing to work on analyzing data in a transparent way to quickly address critical questions. Abernethy described this effort as an example of how FDA is increasing its capacity to use these datasets and to work toward better understanding about (1) data that are fit-for-purpose, (2) problems with data characterization and quality, (3) appropriate and accurate analyses, and (4) the integration of new capabilities such as artificial intelligence. Transparency
___________________
3 More information about FDA’s action to harness real-world data to inform COVID-19 response efforts is available at https://evidenceaccelerator.org (accessed December 17, 2020).
in this process allows everyone to observe and comment on the work being done, she added.
Data Sharing in Regenerative Medicine
Do the low numbers of clinical trials, patients, and products inherent in regenerative medicine make shared databases necessary?, asked Krishnendu Roy. Would FDA, he continued, participate in a registry containing de-identified patient measurements and outcomes that allows for sharing datasets large enough to include characterization measurements and manufacturing parameters? Abernethy noted that other areas of medicine, such as rare diseases, also have low numbers and a similar need to devise sharing mechanisms to allow data aggregation in order to achieve adequate cohort size and detail. Several different sharing mechanisms exist, including data pooling, which is similar to the data warehousing model, and the federated model. Other models use a series of linkage techniques to aggregate data, while an intentional model develops single, core registries or parallel registries that are joined. Different models have value for different types of tasks, so Abernethy suggested that when one selects a model, it is important to consider the tasks that will be initiated and it may be helpful to think of datasets as central resources that can be curated in such a way that one can make comparisons among them. Examples of reference datasets can be found in the laboratory and microbiology space, she said. Abernethy did not comment on the specific direction FDA plans to take in this area, but she suggested that the organization’s Orphan Products Grants Program may pertain to this topic.4
Does FDA, Plant asked, offer incentives to stimulate data sharing among companies that may have proprietary information concerns? Companies may have different motivations depending on the circumstances, Abernethy said; the specific circumstances involved may lend themselves to one of the four models of data sharing previously mentioned. For instance, with rare diseases the patient community may provide the motivation to create a sharing mechanism. Currently, the COVID-19 pandemic combined with available health technologies have created a business opportunity to establish aggregated pools of data and perform high-level curation in order to sell those data to downstream purchasers. FDA does provide direct stimulation in certain areas, Abernethy said, with the Orphan Products Grants Program being one example.
___________________
4 More information about the Orphan Products Grants Program is available at https://www.fda.gov/industry/developing-products-rare-diseases-conditions/orphan-products-grantsprogram (accessed December 8, 2020).
Technical and Human Capabilities Required for Systems Thinking
Which areas related to systems level thinking warrant attention? Plant asked. Data sharing, data linkage, and having individuals with the abilities required to work with these datasets are critical areas, Abernethy replied. To become adequately equipped to receive this type of work, FDA is currently developing the ability to work with similar datasets and to cross-verify results from different teams, which requires technical capabilities and appropriate expertise.
Given that this work involves both hard systems (e.g., modeling, mathematics) and soft systems, such as human interaction, Plant asked Abernethy what she views as the bigger challenge: getting humans to understand the changes and to work together or getting the mathematical and technical details right. Abernethy reflected on her experiences of earning a Ph.D. in informatics, serving as the founding director of the Center for Learning Health Care at Duke University, and building datasets at a health technology company before coming to FDA. Throughout those experiences, she said, the most challenging aspect of bringing data together and applying them across disparate fields was the human component. This underscores the need to develop a common language to ensure an equal voice for all stakeholders, from mathematicians to clinicians to drug developers.
Impact of Systems Thinking on Cost Reduction
Could systems thinking, a workshop participant asked, contribute to reducing the costs of cell and gene therapy, and, if so, does FDA have a role in achieving that goal? Abernethy noted that cost is not specifically considered in FDA’s medical product approval process for safety and effectiveness. However, efficiency does directly influence the overall cost of product development, and systems thinking will likely make trials more efficient, which should in turn affect issues related to cost. Additionally, she said, she expects that systems thinking will likely cause a shift toward platform thinking as it relates to cell and gene therapies, which could create scalable opportunities and thus reduce costs. Systems thinking in health care delivery, especially for therapies such as chimeric antigen receptor (CAR) T cell therapy, can increase optimization across the service chain and have resulting value. Although these types of datasets can increase efficiency, they are expensive to generate and can be costly to manage. She cautioned that a focus on generating and using these datasets as a cost-saving measure can potentially undermine the ability to perform this work with the quality and credibility that is expected.
USING SYSTEMS THINKING APPROACHES FOR CELL THERAPY PRODUCT DEVELOPMENT
Role of Patients in Systems-Based Thinking
Olson described his experience as patient number 2 on the initial University of Pennsylvania clinical trial of CART19.5 At the time of the clinical trial, the only recipients of the treatment had been mice, so there was little knowledge regarding what to expect in humans. In serving as a patient advocate with CMaT, Olson has followed CAR T therapeutic research and the tremendous variability in patient response and toxicities. He emphasized that patients have an important role to play in providing feedback about their experiences with CAR T therapy in clinical trials. Given that it is an individualized therapy and each patient is different, he said, it will be crucial to be able to predict what patients should expect from the therapy in terms of both experience and outcome. In talking with patients who are considering or beginning CAR T cell therapy, he has found that the most common question he is asked is what they should expect. He gives the same answer as physicians do: that every patient is an individual, and responses can vary. However, Olson said he also tries to include a message of hope. As a patient, he said, he is excited to see systems-based approaches being used to gain a better understanding of an individual patient’s biological make-up and of how patients are likely to respond to a therapy because that will be of great value in supporting these patients.
Iterative Process of Defining Critical Quality Attributes
Temple discussed the value of systems thinking to therapeutic cell product development. Cell products are a relatively new and growing area of therapy development. Unlike small molecules, cells are highly complex and dynamic, and cell products are often mixtures of multiple cell types. Because the complexity involved in these products far exceeds that of typical drugs, she suggested distilling down that complexity to the critical quality attributes of each cell product that should be monitored during good manufacturing practices (GMPs).
Temple described three steps in the iterative process of defining critical quality attributes. The first step is to gather key information about the cell product. Cutting-edge techniques, such as high-content image analysis, single-cell gene expression, and protein expression, can be used to acquire a wealth of information about cells. New techniques are also continually
___________________
5 More information about clinical trial NCT01029366 is available at https://clinicaltrials.gov/ct2/show/NCT01029366 (accessed December 8, 2020).
being created for cell study. At this step of the process, specific data, methodologies, and time points should be selected strategically to generate in-depth and wide information, Temple said. The next step is to collect information about the patient and his or her response to treatment, including data on genetics, blood analytics, imaging, and functional outcome measures. Collecting these data, which are complex, can be costly to both the patient and the study, so both the types of data and the time-frame for collection should be carefully considered. The third step is to integrate the information and correlate product characteristics with patient outcomes in order to identify critical quality attributes, which are then incorporated into the GMP process. Temple emphasized that this is an iterative process that requires systems-level thinking. Although the challenges involved are considerable, she said, this process of defining critical quality attributes is essential to ensure reliable manufacturing of products that are safe and effective for patients.
Process of Cell Therapy Development as a System
Manian discussed the process of cell therapy development as a system (see Figure 3-1) and explored how systems thinking approaches from the field of engineering can affect manufacturing efficiencies. In any manufacturing process, he said, raw materials—cells, in this case—have a substantial impact on the output of the process. However, in the development of cell therapies, cell procurement often receives inadequate consideration with respect to procurement timing, phenotypic distribution, cell health, and the impact of apheresis on cell quality. The next step in the process after procurement is cell selection and enrichment, which involves considerations of yield and the health of the cells that will later be engineered. Cell activation and stimulation make up the next step, and here the focus is on achieving consistent, repeatable, and well-controlled activation. Adopting a philosophy that the “process is the product”—which often frames the process as a series of steps performed repeatedly in an identical fashion—can lead one to overlook the tremendous degree of interaction among components of the process, Manian said. On the other hand, taking the appropriate measurements during this phase can facilitate adjustments as needed between the components of the process. During the next step—genetic modification (i.e., genetic engineering)—receptor density and epitope conservation are often overlooked, despite the technological capability and ability to observe those variables on the production floor. The next step is cell expansion, in which considerations of quantity and cell health are important. The concentration and purity of the drug product need to be monitored as expanded cells are harvested, he said. During the formulation and release of the drug product, the focus is on function and pyrogenicity as well as on the impact
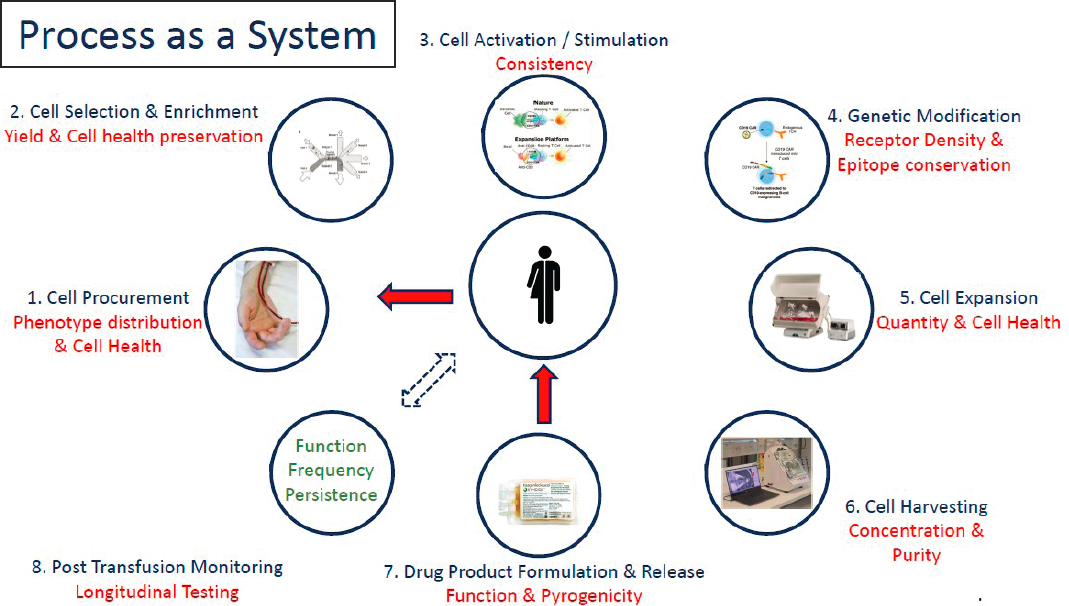
SOURCE: Bala Manian workshop presentation, October 22, 2020.
of pyrogenicity after infusion because cell and gene product raw materials are not sterile. The final step is post-transfusion patient monitoring, which is conducted longitudinally over a long period of time. The data generated throughout all components of this manufacturing system are then collected across patients and used to improve the ability to produce consistent and reliable products. This system can be characterized at the component level by examining the interactions among components. Furthermore, Manian said, this process can be framed as a closed-loop system, which can act as a guidance in the adjustment of parameters to produce a reliable product that is consistent over time.
Systems Biology in Support of Cell Therapy
Bot described how systems biology thinking is used in the context of CAR T cell therapy. He provided an overview of CAR T cell technology and the modern approaches based on machine learning that are used to understand the separate components of the system and bring them together in an integrative model. The current version of CAR T cell therapy involves genetically engineering lymphocytes harvested through apheresis from each individual patient. The critical step in this multi-step manufacturing process is the use of viral vectors to introduce a synthetic gene; this gene expresses a synthetic molecule that enables immune T cells to lock onto target cells, recognize them, and eliminate them from the body. To date, the three products that have been approved in this area are all directed at B-cell lymphomas and leukemias and express an antigen called cluster of differentiation 19, or CD19.
There are data available from both clinical and post-marketing studies on thousands of patients who have been treated with CAR T cells, Bot said. This provides an opportunity to integrate data from manufacturing, delivery, and patient outcomes into larger models, potentially contributing to an unprecedented understanding of how immune cells interact with various organs of the body and how they mediate response or toxicities. To move these efforts forward, Bot said, three questions should be considered:
- How can we better characterize products by identifying the quality product attributes that matter most?
- How can we better define and understand the mechanisms of treatment resistance—either primary treatment resistance or secondary treatment resistance (relapse)—in order to optimize such processes as manufacturing, clinical protocols, and the development of next-generation interventions?
- How can we understand the novel toxicities associated with CAR T cells, which are potent in terms of immunological activity and can lead to toxicities (e.g., cytokine release syndrome)?
All of the information collected can be fed back into biostatistics databases and used to generate models through collaboration among those with expertise in multiple disciplines, Bot said. The components of these models (e.g., product attributes, tumor characteristics, clinical outcomes, pharmacokinetics, pharmacodynamics) can then be interrogated and combined. Next, machine learning is used to define and prioritize the components that have the most influence on the outcome and which components can be used as leads for process optimization, next-generation interventions, and product characterization.
This process and its component parts constitute a systems biology approach, Bot said. He presented a model of CAR T cell therapy that depicts how CAR T cells work, why they sometimes fail, and how they are identified. The critical product attributes of CAR T cells include intrinsic T cell fitness or expansion capability, T cell polyfunctionality, CAR functional avidity, and receptor density. These attributes influence the outcome of therapy. Additionally, the model incorporates tumor-associated factors and other patient characteristics that interact with product attributes, producing information on pharmacokinetics, pharmacodynamics profiles, clinical efficacy, and toxicity. Tumor biology research is rapidly expanding the knowledge base about the role of the tumor immune microenvironment and of how the oncogenic landscape interacts with product attributes to influence clinical response or treatment resistance. This knowledge helps optimize the manufacturing process and maximize the number of specialized T cells that mediate clinical efficacy without increasing the toxicity profile. In parallel, systems biology thinking enables the creation of networks of cells and molecules that jointly determine the outcome of T cell interventions, including inflammatory toxicities. Bot said that this type of thinking helps narrow down molecules and actionable targets involved in managing, mitigating, and preventing toxicities associated with CAR T cell interventions.
DISCUSSION
Implementing Systems-Based Thinking in Cell Characterization
Throughout the session, Lebkowski said, the importance of identifying cell types, cell function, cell phenotype, and cell properties was demonstrated. How, she asked, could systems-based thinking be implemented in, for example, the process of cell characterization? The type of data gathering for a cell product that Temple described in her presentation is
not particularly compatible with a GMP process, she said. Rather, it is used to understand the product in depth in a research environment. For example, this type of high-level data analysis of a product is used to look at gene expression at the single-cell level and to identify the emergent properties that pertain to pathways and cell states. Multimodal integration may involve sets of omics data (e.g., single-cell transcriptomics), live-cell imaging, and computer-based imaging. Bringing together different modalities to make sense of this information is challenging, but these large-scale analyses can be used to identify emergent properties and predict how to develop products that are safe and useful. Then the product needs to be investigated thoroughly in a research setting to determine how to integrate the different forms of data in order to select which product should be put through a GMP process, she said.
Lebkowski asked whether Temple envisions a focus on critical quality attributes—perhaps tweaking or revising them as part of the product development process—to better understand the function and the interaction of particular cells in a human. To do so, Temple said, patient data will need to be gathered and compared with what is being discovered about the cell product. This is challenging because (1) it is demanding from the patients’ perspective, (2) it is expensive to study in a clinical trial, and (3) it can be difficult to determine what to measure and for how long to measure it. However, critical quality attributes cannot be identified until patient data, including data about response, are incorporated into these analyses, Temple said.
Implementing Systems-Based Approaches in CAR T Cell Therapy
A major challenge in implementing systems-based approaches in CAR T cell therapy, Bot said, is the need to scale up and scale out the datasets in the post-commercial setting. He and his colleagues are trying to harmonize biomarker approaches and protocols by partnering with registries such as the international or regional bone marrow transplant registries and are working with physicians and patients to obtain appropriate consent for additional data collection. It is, Bot said, “absolutely critical” to identify, train, and validate predictive algorithms for CAR T cell intervention toxicities and efficacy. There has been significant progress over the past 5 years in terms of knowledge about the key determinants of response to CAR T cell intervention. However, he said, more work is needed to create strong predictive algorithms based on systems biology thinking, which can only be accomplished in partnership with appropriate stakeholders in the commercial post-marketing setting. To forge such relationships, he has partnered with academic collaborators and other major comprehensive cancer centers that are collecting data. Through collaboration, he said, data can be deposited and analyzed in an organized fashion.
Integrating Systems-Based Approaches into Manufacturing
What, Lebowski asked, are some of the opportunities and challenges in integrating systems-based approaches into the manufacturing system? Manian pointed to the intersection between design thinking and systems thinking. Design thinking is often involved in development in considering how a product will be manufactured. Although the measurement technologies used to understand and establish mechanisms of cell therapy are complex, generational biomarkers or surrogate measurements can provide insights into these mechanisms. Manian suggested that measurements of system components should not be used to understand a process, but instead should be implemented on the manufacturing floor. Because manufacturing is a continuous process that cannot be stopped for lengthy periods of time, measurements in a manufacturing space must be conducted within 15 minutes or so and must provide information in a way that is actionable. Viewed in that context, there are many tools that are currently available to take measurements at each component of the process, Manian said. Based on the information gathered, adjustments can be made in the subsequent process in response to the indications of the data. This need not be an open loop system; it can also be a closed-loop system. He suggested that information on the incoming material is the point at which interaction currently needs to occur because little characterization is currently being done in this area. Moving the process to the point of cell procurement can have a huge impact on the overall control of the processes, he added. Furthermore, each component of a system can be framed in terms of making measurements. For example, viral vectors are currently examined only in terms of control-arm viral vector delivery. However, many other components can be explored, such as generated receptor densities, uniformity, variation across patients, impact on cell health, and the survivability of engineered cells. Manian suggested that, over time, the processes of characterizations will lead to a better understanding of the overall manufacturing process. “Just as we understand interaction between cells, we need to understand the interaction between components of the manufacturing system,” he said.
Improving Patient Data Collection
Given the variability driven by different starting materials and diverse patient characteristics, Lebkowski asked, are clinical trials collecting sufficient data about patients and products to integrate aspects of manufacturing or patient variability into systems-based thinking? If it is not sufficient, she continued, which types of data should be collected and how can data collection, particularly from patients, be improved? Bot replied that CAR T cells are based on the patient’s own immune system, which is a collection
of heterogeneous T cells, B cells, and other immune cells, and in this case the T cells are of particular interest. Although patient heterogeneity in T cells is overwhelming, collecting data about that heterogeneity will be critical for identifying the most important attributes in patients’ pre-treatment immune systems in terms of influencing product characteristics and subsequent clinical outcomes. In the face of this heterogeneity, fully leveraging this opportunity will require single-cell omics analysis of characteristics such as T cell fitness, expansion capability, resistance to apoptosis (i.e., cell death), and the ability to engage multiple immune programs simultaneously, which seems to be linked to clinical performance. All of this information can be used to build better predictive algorithms that are more actionable in delivery, Bot said, because they rely on data from pre-manufacturing characteristics of the immune system. Furthermore, this will help pave the way to a new generation of cell interventions based on designer, off-the-shelf cells that are sourced either from stem cells differentiated ex vivo or from well-defined donors. This would result in a tremendous reduction in inter-patient variability in the next-generation products, he said.
Could the collection of induced pluripotent stem (iPS) cells from individual patients help, Lebkowski asked, to tease out some degree of heterogeneity from patient to patient? That is an interesting idea, Temple said, because iPS cell technology is enhancing the ability to produce many different types of tissue. This tissue can be produced in three dimensions to build organoids that are representative of gut, brain, skin, and inner ear. iPS cells could be used to study individuals, although CAR T technology may not yet have advanced far enough to create the relevant cells. Temple said that a challenge with iPS cells is their tendency to be in an immature state, but work is under way to learn how to mature those cells.
“We look for a lost key under the light because that is where the light is,” Manian said. Typically, cell therapy is approached in terms of making measurements with existing instrumentation; instead, he said, the process should start with defining the parameters critical to quality in order to determine the type of measurements needed. New methods developed to generate information about phenomena—such as cytokine profiling and phenotyping—across multiple patients and multiple types of drugs for the same disease state will likely further reveal how systems biology can affect manufacturing.
Are there opportunities, Lebkowski asked, to improve patient communication, for example, by explaining why organizations and developers of regenerative medicine therapies are trying to collect extensive panels of data from subjects who might be entering a trial? Olson said he has found from both his own personal experience and from his advocacy work engaging with patients contemplating this therapy that a major challenge is to distill the large amount of data that already exist and to find ways to apply those
data to each individual patient and predict what patients can expect based on their individual characteristics. Information already available should be framed more effectively for patients considering or beginning CAR T cell therapy so as to help them understand the therapy. The huge growth in the volume of CAR T data over the past decade is astounding, he added, but continuing to collect data on each individual’s experience and matching it to biological parameters is the work that still lies ahead.
Approaching Systems Thinking via Collaboration
Given the substantial resources required for systems thinking approaches, Lebkowski asked if a single sponsor would be adequate or if a consortium would be required. Kite Pharma, Inc., began this work, Bot said, by launching a consortium with large academic organizations that participate in clinical trials and sponsor research at the post-marketing stage for both axicabtagene ciloleucel (brand name Yescarta®) and brexucabtagene autoleucel (brand name Tecartus®), the two Kite products approved to date. Current work involves generating big data, using a harmonized biomarker protocol aligned with a schedule of assessments, integrating data via methodologies and analytics aligned with a single command structure, and analyzing data with pre-specified protocols and machine learning. However, advancing the field by accelerating real-world data generation will require a consortium across different sponsors and organizations in the industry to share data and collaborate with regulators. Bot suggested that this would contribute to optimizing patient management by learning how to increase the rate of durable responses and predicting or mitigating toxicities that may require intensive management in a hospital setting.
Costs of Not Implementing Systems Thinking
What are the costs to the field of regenerative medicine, Lebkowski asked, if systems thinking approaches are not implemented? Temple described a real-world example of a company that was generating a cell therapy for spinal cord injury and discovered that some element of the manufacturing process led to failure of that product. Although the product may have passed the criteria for release, it was clear that important aspects of the product were not being captured in the data collected. The clinical process for GMP is expensive because of the complexity of cells, she added. In her experience, she said, more comprehensive testing before release would be ideal, but it becomes cost prohibitive. However, an inadequate understanding of all of components in a system also comes at a cost (i.e., the cost of failure of the enterprise and, most importantly, of failure to deliver a beneficial product to patients). Temple underscored the need
to characterize therapeutic products more carefully and to integrate that information with patient data to predict responses more accurately and identify subpopulations of patients that are most likely to benefit from those products.
Manian highlighted the need to standardize processes to establish a framework with competencies that can be implemented. If systems thinking is not applied, then individualized patient-by-patient processes will remain unaffordable for a large segment of the population. Bot added that the costs of not implementing systems thinking are the missed opportunities to expand the footprint of potentially curative interventions in early disease stages and to benefit as many patients as possible.


















