The fifth session of the workshop focused on addressing regenerative medicine manufacturing and supply chain challenges with systems-level approaches. The session was moderated by Krishnendu Roy, the Robert A. Milton Chair Professor, the director of the National Science Foundation’s (NSF’s) Engineering Research Center for Cell Manufacturing Technologies (CMaT), and the director of the Marcus Center for Therapeutic Cell Characterization and Manufacturing at the Wallace H. Coulter Department of Biomedical Engineering at the Georgia Institute of Technology and Emory University. The session featured presentations on using artificial intelligence (AI) in cell and gene therapies, in modeling manufacturing processes in regenerative medicine, and in modeling the supply chain and other processes involved in cell therapy manufacturing and distribution. The session’s objective was to highlight opportunities where systems thinking approaches could address inefficiencies with manufacturing and the supply chain related to regenerative medicine.
ARTIFICIAL INTELLIGENCE IN CELL AND GENE THERAPIES
Iya Khalil, the global head of the AI Innovation Center, and Seshu Tyagarajan, the global head of Late Stage CMC Strategy, Cell and Gene Therapies, Novartis Technical Operations, both at Novartis, provided an overview of current work with AI for manufacturing cell and gene therapies, outlining how they are approaching and solving problems and the types of data they are using in this process.
New Opportunities to Apply Artificial Intelligence
The possibilities of AI are being realized in many fields, Khalil said, whether in building a disease model from omics data or understanding a particular medicine’s manufacturing or supply chain process or some other application. The reason for the use of AI in so many fields, she added, is the unprecedented ability today to collect all kinds of data, including measures on the patient level, on the granular level of a genomic, molecular, or phenotypic screen readout, or actual health outcomes at a single point of time or over time. It also includes knowledge about how to generate an effective cell therapy or immune drug. The ability to collect all of these data, coupled with advances in AI, creates the potential for new learning (Topol, 2019). Deep learning algorithms are a class of AI algorithms that can be applied to discern complex patterns underlying the data, Khalil said. As more data are fed into those algorithms, the potential for accurate algorithms increases, exceeding what was possible with machine learning 5 to 10 years ago, Khalil said. Combined with the capabilities of computational power, these
advances in AI contribute to strengthening the overall ability to learn from data, make predictions from data, and reason based on those data.
Using Deep Learning Algorithms to Detect Diabetic Retinopathy
Khalil provided examples of how this process is being applied to diabetic macular degeneration as it relates to follow-on diseases such as diabetic retinopathy. Starting with retinal images, high-performance supercomputing and AI can be used to achieve promising outcomes and develop new knowledge from the data that previously could not be accessed. For example, results from an algorithm for detecting diabetic retinopathy and macular edema were comparable to the capabilities of ophthalmologists (Gulshan et al., 2016). Beyond matching or potentially exceeding ophthalmologists’ diagnostic abilities, algorithms are also finding new associations beyond imaging data. They are, for example, combining imaging data with biomarker data in an effort to learn how macular degeneration might impact outcomes, such as adverse cardiac events (Poplin et al., 2018). In 2018 an AI-based diabetic retinopathy diagnostic system was approved by the Food and Drug Administration (FDA), so this algorithm can now be applied at the point of care (Abràmoff et al., 2018). These developments demonstrate what can potentially be achieved with AI and data, Khalil said.
Harnessing Core Artificial Intelligence Capabilities
Khalil said that her group is working on harnessing core AI capabilities that involve deep learning and that extend to approaches involving causal learning. Data are harnessed from a variety of sources, including text and files analyzed using natural language processing (NLP) and image analytics, such as readouts of flow data from manufacturing processes. AI is applied to an automated analysis—so that it is no longer a tedious job for humans—and combined with advances in visual analytics.
Applying Artificial Intelligence to Drive Exploration and Empowerment
Together, these combined advances in capabilities allow for more than just an in-depth look at one AI field, Khalil said. Her group is applying these capabilities in two different ways: (1) AI exploration for breakthrough discoveries and (2) AI empowerment of citizen scientists. Exploration involves gaining a solid understanding of the fundamental mechanisms and processes that drive what is observed and the outcomes that are being modeled. Empowerment involves creating capabilities not only for the deep-data AI scientists who know how to use these tools, but also for the data scientists—and even non-data scientists—who want to improve
their abilities to interact and reason with data. Harnessing the power and potential of AI is important, Khalil said, because it can drive fundamental discoveries and empower scientists and clinicians throughout an organization. She briefly shared examples of applications of AI, including applying visual analytics methods to flow cytometry data (Bachthaler and Weiskopf, 2008; Ray and Pyne, 2012). Beyond analyzing images, Khalil’s team is also gaining insights from unstructured data. They achieve these insights by using NLP capabilities to analyze massive amounts of corpus text data and automating the ability to learn and extract adverse FDA events from text data and apply this to drug labels (Ly et al., 2018). Insights can also be extracted from electronic health record (EHR) data, a task that formerly required the deployment of many people to pull data from NLP records and EHRs. However, various AI and NLP methods can scale this quickly, making it much easier and more straightforward to use the data from EHRs to predict treatment outcomes (Liu et al., 2019).
Applying Artificial Intelligence to Learn Cause and Effect from Data
Another fundamental capability harnessed specifically in cell and gene therapy is the ability to learn cause and effect from data, Khalil said. One data approach is to try to learn these complex patterns from the space between inputs and outputs, which Khalil described as a “bit of a black box.” Another approach is to strive for transparency and a better understanding of the underlying processes in the system that gave rise to the data. Through causal learning and graphical causal learning methods, it is now possible to analyze datasets that include sets of inputs that modulate responses in the system and then learn how these factors form a causal network that can be mapped out and whose behavior can be predicted (Schadt et al., 2005). This causal network demonstrates influences on outcomes (e.g., whether the nodes being measured are causal for the outcome or reactive to the outcome). Developing the capability to perform a “sorting of causality” is helpful in addressing confounding factors, which are major underlying issues with these types of datasets. For instance, if only large-scale patterns of associations in datasets are evaluated, it can lead to the incorrect inference that insulin and amputation are merely associated, when in fact there is an underlying cause: the confounding factor that having diabetes will influence changes in insulin levels, which can influence the possibility of amputation. Therefore, it is actually diabetes, not insulin, that is directly associated with amputation. Underlying statistical and mathematical probabilities allow cause and effect to be sorted out mathematically, she added. Technology has been developed for learning cause-and-effect associations not only at a small graphical level, but also at scale.
Other AI capabilities are being used in cell and gene therapy across different data modalities and types that allow work with imaging data, NLP data, and sorting out cause and effect. There is a huge potential, Khalil said, to learn to identify the various key steps in the complex manufacturing process for patient-specific cell therapy, starting with the patient, then applying treatment and cell therapy into the patient, and finally monitoring the patient long-term, collecting a variety of data modalities throughout the process. A goal is to learn how AI and machine learning models can be applied to understand the manufacturing process, then determine how to intervene to optimize those processes.
Cell and Gene Therapy: Supporting the End-to-End Patient Journey
The long-term vision and mission of cell and gene therapy development at Novartis is to understand the end-to-end patient journey as fully as possible, Tyagarajan said. The goal is to improve every step along that journey (e.g., understanding and improving the incoming material can aid in better patient selection, which in turn can lead to improvements in robust manufacturing). Consequently, these advances could reduce vein-to-vein time, improve predictability, and ultimately enable the product to reach a greater patient population. She acknowledged that cell and gene therapy is a nascent field with many challenges, such as process variability, donor-to-donor variability, availability of raw materials, a shortage of analytical assays, a need for analytical development, limited choice of vendors, logistics and supply chain issues, a shortage of trained personnel, and rapid timelines. Thus, she said, she and her colleagues are always looking for ways to learn more about the patient journey so that processes can be expedited and barriers removed.
Developing Autologous Cell Therapies
The process of developing autologous chimeric antigen receptor (CAR) T cell therapy begins with cell collection from the patient, Tyagarajan explained. The patient’s T cells are selected, modified, expanded, formulated, frozen, and sent back to the patient for treatment via infusion. A systems approach is used to identify the factors that influence each data-collection point in the process: patient characteristics, apheresis, manufacturing, final product, cellular kinetics, and outcome (see Table 6-1). Because each step affects the next step or steps, there are dependencies involved that are not well understood. Multiple datasets are collected at each stage, including various types of patient data, apheresis information, extensive manufacturing information, product measurements, cellular kinetics data,
TABLE 6-1 Factors Influencing Each Stage in the Development of Autologous Cell Therapies
| Data-Collection Stage | Considerations |
|---|---|
| Patient |
|
| Apheresis |
|
| Manufacturing |
|
| Final product |
|
| Cellular kinetics |
|
| Outcome |
|
SOURCE: Seshu Tyagarajan workshop presentation, October 23, 2020.
and pharmacokinetics and pharmacodynamics information. AI is used to derive answers from these datasets, she added.
Advancing the Field of Cell and Gene Therapy
There are three research questions Novartis is pursuing to implement AI in the burgeoning field of cell and gene therapy, Tyagarajan said. These include (1) how to achieve a better understanding of the cell and gene therapy manufacturing process by identifying the factors that are most important, (2) whether those factors and their associated data can be used
to predict clinical outcomes, and (3) how to rapidly translate lessons from these initial explorations into the next generation of clinical trials. She outlined some of the strategies Novartis is using to address these questions.
To achieve a better understanding of the factors that are important for manufacturing, Tyagarajan and her colleagues are analyzing available data by combining them into multiple datasets. One set of data is coalesced into the patient group, another set into the apheresis group, and others into the manufacturing dataset and the final product dataset. The goal of coalescing these datasets is to predict the out-of-sample value of a node in the graph in order to identify the individual factors important for manufacturing a better product. To determine whether these factors can be used to predict patient outcomes, the number of influencing factors is increased to identify other factors that may be confounding. Dose and final product characteristics are added to the first group of datasets with the goal of identifying how these factors influence response and how the nodes in the graph interact with each other. The aims of this process are to (1) identify confounders of the problem, (2) create a model to predict response with dose and confounders, and (3) develop a way to marginalize out the confounders. Confounders between dose and response can include patient characteristics (e.g., body mass index, age, tumor size) and product characteristics.
The next step in the process involves grouping data, applying machine learning models, and learning from random forest analysis to build a system of intelligence. The system of intelligence is composed of a user interface, a data ingestion pipeline of clinical and other datasets, and a knowledge ingestion pipeline of medical and biological knowledge. Statistics, machine learning, and cloud computing are applied to all of the system’s components to yield insights that must then be validated. Creating this system of intelligence for cell and gene therapy is in its early stages, having commenced only a year ago, but Tyagarajan said that much work is taking place during this promising stage of development.
MODELING THE MANUFACTURING PROCESS IN REGENERATIVE MEDICINE
Theresa Kotanchek, the chief executive officer of Evolved Analytics, LLC, highlighted notable challenges in cell manufacturing, various modeling processes to address those challenges, and recent projects and achievements.
Grand Challenges in Cell Manufacturing
Kotanchek opened by briefly listing eight grand challenges in the field of cell manufacturing (see Box 6-1). NSF’s CMaT was formed to address
such challenges with the vision of transforming the manufacture of cell-based therapeutics into a “large-scale, lower-cost, reproducible, and high-quality engineered process for broad industry and clinical use,” Kotanchek said. The group is seeking to become a visionary and strategic international resource and an exemplar for developing new knowledge, innovative technologies, a diverse workforce, and enabling standards for the cell production and characterization processes.1 The organization convenes critical stakeholders—including practitioners, industry professionals, regulators, and patient advocates—to address the necessary requirements for developing a lingua franca to facilitate communication among those working in biologics, informatics and analytics, and manufacturing and industrial engineering. CMaT is strategically positioned to address challenges in the emerging cell manufacturing industry, particularly those related to quality, cost, speed, and agility, she added. She focused on CMaT’s work with multivariate critical quality attributes (CQAs), multivariate critical process parameters (CPPs), and how these influence the progress toward real-time monitoring.
___________________
1 More information about CMaT is available at http://cellmanufacturingusa.org/vision (accessed January 8, 2021).
Dynamic Sampling Platform
Kotanchek described CMaT’s dynamic sampling platform, which is designed to engineer reproducible, predictive measurement and assay technologies that enable batch and continuous monitoring of cell state and product. CMaT is working to develop nondestructive, in-line, closed system analysis using real-time sampling, reporters/sensors, and imaging tools as process analytic technologies. It is also developing three-dimensional disease and organoid models, also known as “potency-on-a-chip.” The goal is to use this dynamic sampling platform in a robust way in both discovery and process development.
T Cell Characterization Project
The power of CMaT’s approach, Kotanchek explained, lies in the recognition that key deliverables are needed in the formation of new scientific knowledge and in new tools and technologies that can be used to build integrated systems. Furthermore, CMaT recognizes that these building blocks can in turn be applied to different testbeds. She focused on an example specific to the T cell testbed. CMaT’s work in this area links into its integrated, crosscutting engineering system, which is intended for use both for understanding the system and for predictive purposes, she added.
Kotanchek highlighted a project focused on variability in the assessment and omics characterization of CAR T cells through an integrative computational pipeline. This work explored how degradable microscaffold microcarrier cultures can expand more central memory and lymph-node-homing T cells (Dwarshuis et al., 2019). The goals of the project were to control these T cell expansion processes and to develop a workflow to enable multi-omics characterization and unbiased modeling of the end product for early, predictive signatures of quality during manufacturing. The project aims first to understand the variance and then to establish CQAs and CPPs that are predictive of potency, safety, and consistency.
The first phase of the project focused on examining the experimental process parameters by optimizing the microscaffold’s input process parameters and looking at the responses of viability and CD4/CD8 naïve-memory attributes, Kotanchek said. The group employed DataModeler software, which uses evolutionary computing, to perform predictive optimization and identify regions for further improvement on extrapolation. Next, they executed a sequential, adaptive experimental design for further testing and validation, which enabled them to move into an optimized region that had not been part of the original study. Big data is often a focus of systems thinking, Kotanchek said, but much of their early program work actually
involved smaller datasets and the use of the evolutionary toolset to gain a deeper understanding.
The second phase of the project—a multi-omics integrative approach for early predictive signatures—looked at the impact on microcarriers of process parameters, secretome profiles, and metabolites, Kotanchek said. This involved merging process parameters, secretomes, and nuclear magnetic resonance (NMR) data and measuring the total live CD4 naïve-memory cells, total live CD8 naïve-memory cells, and the ratios of CD4 to CD8. The objective was to examine if CD4 and CD8 cells could be controlled simultaneously, Kotanchek said, and this study also included a comparison of different machine learning analyses, along with linear modeling, to identify the best ways forward.
Determining the Modeling Options
When considering different algorithmic approaches, underlying assumptions are often overlooked, Kotanchek said. These underlying assumptions are generally tied to “what we believe we know and what we do not know.” Specifically, they pertain to what is believed to be known about driving variables and about the model structure itself. For example, if the driving variables are known and the model form is linear, then linear regression may suffice. In cases where the driving variables are known and the model structure is nonlinear, then a nonlinear regression parameter may be appropriate. If the driving variables are known but the model structure is not, then a variety of powerful machine learning tools can be applied (e.g., neural networks, support vector machine model, random forests, and symbolic regression).
Symbolic regression can also be used in situations where there is uncertainty about the driving variables and of the model structure, Kotanchek said. This technique offers a powerful way to identify variables via the evolutionary process, providing explicit transparent models that can be tested. Evolution automatically handles the symbolic regression model development, generating novelty from the data that can then be exploited. Solutions are found by simulating natural evolutionary selection, with the end result being mathematical expressions instead of biological species. Novelty is generated by competition in the fitness of those expressions—in terms of accuracy versus complexity—to generate explicit algebraic, transparent, interpretable models. In this way, symbolic regression can be used as an augmented intelligence tool for hypothesis generation, enabling a domain expert to examine the most accurate solutions that emerge.
Learning and Extrapolating from Collections and Ensembles of Models
Not all models are created equal, Kotanchek noted, so the focus is on exploiting the collection of models that offer the greatest accuracy and the lowest degree of complexity. Focusing on the collection of models—rather than on specific models—can help to answer questions about: (1) modeling potential, (2) the complexity-versus-accuracy trade-off, (3) the number of variables required to get accurate models, (4) variable presence, (5) variable combinations, (6) variable and combination distributions, and (7) emergent metavariables that can provide further insight. The discussion of big data versus big insight is an important one in this type of work, Kotanchek said. The size of datasets can vary considerably—from 2 to 10,000 variables and from 10 to 1 million records—but the diversity of the dataset can be as important as its size; a dataset of 9 million records in which all but 10 are equivalent will not provide much insight into the early development process, for example, because it does not offer a diversity of data. It is critical to look at all data, she emphasized, including data that do not yield the anticipated result. The data themselves may help to determine the appropriate trade-off of complexity versus accuracy. Automated hypothesis generation and refinement serve to develop explicit algebraic models, reward simplicity and accuracy, and focus on the “good” and simple models. Many models are contenders, and all contenders can be exploited for insight and guidance. Iteration toward a final model set involves a focus on the variables that have the most impact on the outcome and on implementing a trustable model from the set of good and simple models.
A collection of models can be used to build “ensembles” of accurate-fit, trustable models. Ensembles are created by starting with interesting models that have reasonable accuracy and complexity, as well as desirable variables, variable combinations, and dimensionality. The models are automatically chosen to maximize the diversity of error residuals. Models within an ensemble will agree where constrained by data and diverge when exposed to novel parameter conditions. They can be used to guide decision making and to enable active learning and active design of experiments, Kotanchek added.
The diversity of the accurate-fit models in an ensemble is used to generate trust metrics, Kotanchek explained. To illustrate how ensembles are useful in extrapolation, she drew an analogy with the trajectory of a single bounce of a ball. If the simple trajectory is known and genetic programming were applied only to the first half of the bouncing ball dataset, it could generate hundreds of unique models to identify the prediction for the continued trajectory within seconds. A collection of simple models with high accuracy could then be selected to extract a diverse model set and generate an ensemble. This ensemble’s trimmed mean provides a projection;
however, the space has not been fully exploited, so further information needs to be collected. If one took the same type of data and looked at other machine learning comparisons, they would not necessarily provide accurate projections.
The power of ensembles is in the active design of experiments through active learning, Kotanchek said. Using modeling, an ensemble can be generated that provides a projection for the trajectory. Even though the predicted trajectory would be inside the nominal data range, more data points are needed to proceed. By adding samples at the point of maximum divergence (i.e., along the top of the arc of the ball’s bounce), it is possible to lock down model behavior, arriving at predicted trajectories that have little divergence from the actual trajectory. Thus, an active experiment design process can drive uncertainty out of the model. If experiments are expensive and time limited, Kotanchek added, ensembles can indicate areas where data collection will be most beneficial and informative. This concept of trustable models is the foundation of active experiment design, she emphasized.
Using Active Design of Experiments
CMaT has some key achievements that can be attributed to the active design of experiments, Kotanchek noted. These include optimizing conditions for maximizing memory T cells, designing the aforementioned CD4/CD8 multi-omics integration outputs, and designing the parameters for microcarriers and their interactive effects. CMaT has been able to merge the multi-omics dataset to run comparisons of accuracy and prediction across various machine learning methods (e.g., conditional inference forest, random forest, gradient boosted trees, and symbolic regression). These comparisons include all omics data, including data on process parameters over different time periods, process parameters with NMR, and process parameters with cytokine, Kotanchek said. The machine learning arm was performed to correlate and identify CPPs; it was effective in identifying cytokine dependency, examining the cytokines’ interactive effect, and predicting total live memory, CD4, and CD8. In addition, CMaT used multi-omics prediction profiles based on NMR media analysis of ethanol and lactate and microcarrier characteristics to look at optimization. These processes can be performed simultaneously or independently if the objective is to optimize one versus the other, Kotanchek said.
Questions Driving the Systems Thinking Approach
When using a dynamic sampling platform, the issues to address include the identification of key factors, system design, and the potential for better solutions, Kotanchek said. A workflow approach can enable information to
be extracted to inform the appropriate targets and designs for the dynamic platform. Active design of experiments is powerful, and system integration is paramount for the active learning process, she added. The active learning workflow integrates data sources, statistics and visualization, and data consolidation; this is followed by looking at model space descriptors, doing the modeling, deploying it through subject-matter experts, and then completing validation analysis. This information feeds back into the active design of the next set of experiments, and the cycle begins anew. She highlighted the importance of identifying the objective of the analysis at each phase of discovery, development, design, validation, optimization, process control, supply chain, commercialization, or strategy. This involves considering questions about variable selection and relationships, prediction, optimization and deployment, risk management, and insight and understanding (see Box 6-2). Kotanchek closed by encouraging researchers to exploit the data that are available, particularly experimental and manufacturing knowledge that relates to efforts that are not generating the target response. This type
of data can be powerful in understanding the difference between a patient group that is responding and a patient group that is not.
NOVEL SUPPLY CHAIN AND COST MODELING FOR CELL THERAPIES
Ben Wang, a professor, the executive director, and the Gwaltney Chair in Manufacturing Systems at the Georgia Tech Manufacturing Institute, discussed the application of systems thinking in manufacturing engineering, with a focus on novel supply chain and process modeling for regenerative medicine and cell therapy manufacturing and distribution. He presented several case studies of projects that have adopted a multidisciplinary approach to addressing the systems issue by building a team across different universities and disciplines. He also outlined two systems-based modeling projects and reviewed simulation case studies related to capacity planning, supply chain disruptions, demand surges and priority queue, and the cost of goods and automation.
Systems Thinking Lenses
Wang introduced three lenses through which to view systems thinking in manufacturing. The first is the technology readiness level/manufacturing readiness level spectrum, which moves through the stages of basic research, representative production environment, pilot product, low rate, full rate, and then finally the market. The second lens involves the value chain, whose stages include raw materials, fabrication, inspection, packaging, logistics, distribution, therapy delivery to patients, and long-term patient monitoring. The third lens is the stakeholders’ view, in which systems are conceptualized as an ecosystem where all stakeholders work together in the effort to optimize a set of objectives. Not all objectives are aligned across various stakeholders, however, and objective optimizations can conflict in some cases, leading to potential dilemmas in decision making, Wang said.
Supply Chain Challenges in the Regenerative Medicine Ecosystem
To frame the use cases he presented, Wang provided an overview of how process modeling can be used to address supply chain challenges. Regenerative medicine supply ecosystems are complex, Wang said, and he highlighted three challenges in managing regenerative medicine supply chains (see Figure 6-1). First, the large variety of products and supply chain issues creates a system that involves both “push” and “pull” dynamics; interactions between the push and pull factors creates challenges. Next, in order to make regenerative medicine truly personalized, the health
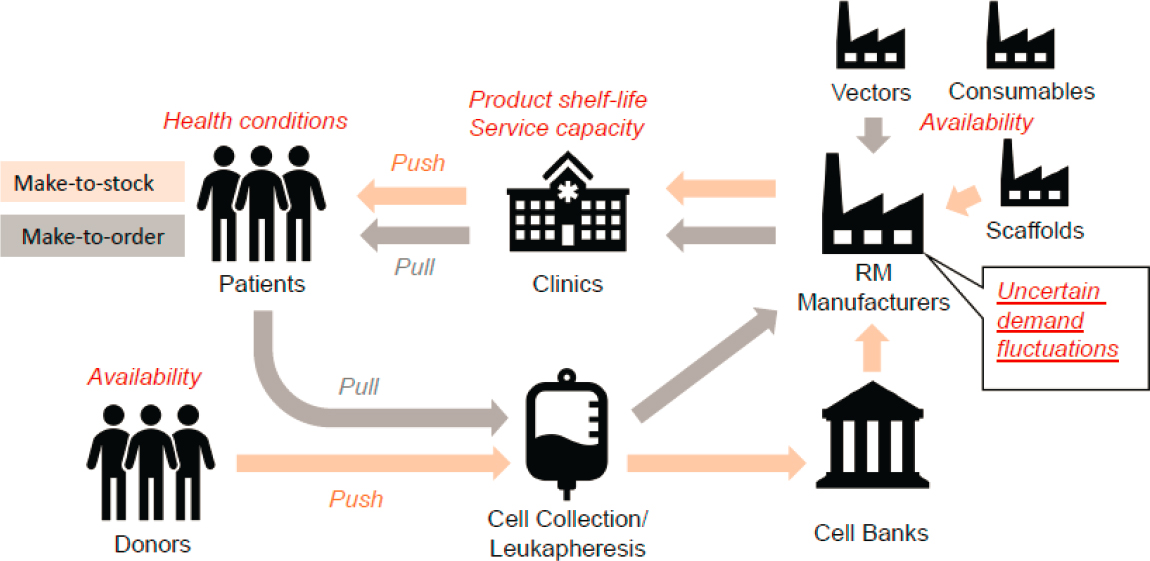
NOTE: RM = regenerative medicine.
SOURCE: Ben Wang workshop presentation, October 23, 2020.
conditions of the patient must be considered throughout the entire process. This involves assessing the real-time impact of patient health conditions on production and supply chain planning and execution. The third challenge is that uncertainties abound, including uncertainties related to demand fluctuations, inevitable machine breakdowns, quality control and process failures, and supply chain disruptions.
Process Modeling to Address Supply Chain Challenges
Digital modeling has become an efficient and cost-effective approach to exploring the dynamics of the supply chain and the process models, Wang said. Many existing digital models focus on a narrow band of activities in the entire value chain, thus missing critical elements and tools (Lam et al., 2018). Wang described a supply chain and process modeling project designed to address this issue through four main objectives. The first is to build a decision-support toolset to incorporate all stakeholders’ perspectives into supply chain system design, planning, and control. This involves attempting to optimize a set of stakeholder objectives that are not necessarily aligned and that may conflict. Second, the project aims to develop and validate digital models of manufacture and quality assurance for regenerative medicine and cells to support reliable, scalable manufacturing of quality, affordable therapeutics. These include models for a single production facility and for a network of production facilities. The third objective is to inform standards and the regulatory community in the process of standardizing this nascent industry. Finally, the project seeks to support and participate in education and workforce development, the importance of which cannot be overemphasized, Wang said.
The project adopted a two-pronged approach. The first prong is to develop a detailed view of a single production facility, such as a cell manufacturing facility or a regenerative medicine, tissue, or organ factory. Each component of the process is examined, from specimen collection to the point at which a therapy is packaged, inspected, and ready to be shipped. This involves assessing processing stations, quality control stations, human operators, and moving vehicles within the production facility. The second prong explores a network of facilities and generates various production model configurations. For example, a centralized production model would apply to a large factory being built in the United States to distribute products to clinics and hospitals throughout North America. A point-of-care production model would apply to a miniature production facility located at each hospital or clinic in the system. Configurations on the spectrum between centralized and point-of-care models uses the regional production hub model, which would apply to cases in which several factories
are located throughout the country, each serving the hospitals and clinics within a corresponding region. These hubs may or may not interact with one another, reflecting the dynamic nature of this modeling, he added. Using digital modeling, the project has created models of both autologous CAR T cell manufacturing and allogeneic induced pluripotent stem (iPS) cell manufacturing, Wang said. It has developed simulation tools that provide decision makers with large amounts of information pertaining to production situations, use of resources, labor, lead times, quality, inventories, ordering status, and failure rates.
Use Case: Production Capacity Planning
Wang described a use case for production capacity planning. Capacity planning is of prime importance in a nascent industry such as regenerative medicine and cell manufacturing, he emphasized, where players do not have the decades of experience that players do in other industries, such as the aerospace and automotive industries. Capacity planning is critical for informing decisions such as the number of bioreactors to establish, the labor requirements needed, and the level of automation to consider over the next 5–15 years. Analytical and simulation tools can help decision makers and plant managers design the best configuration, he said.
Use Case: Supply Disruption Simulations
Wang presented another use case involving simulations on supply chain disruptions (e.g., a disruption in which a natural or humanmade disaster causes a major shortage in the production system of a key reagent). A simulation can be used to compare two production configurations. The first configuration is a simplified system comprised of one factory and one reagent supplier. The simulation predicted that a 60-day complete supply disruption would lead this lean production configuration to a substantial, lasting backlog, preventing any return to steady-state queue times. The second configuration contains one factory and two reagent suppliers. In this simulation, only one of the two suppliers is affected, while the other continues to operate. In this case, the facility would face a 50 percent supply disruption rather than a complete disruption. The result is shorter backlogs than in the first scenario and an eventual return to the original production steady state. Another simulation compared the configuration of one factory and one supplier with a configuration that included a second factory with its own supplier. In this example, a supply disruption affected the first factory’s production, but the second factory and second supplier had no disruption. As in the preceding simulation, if the first factory halted
production until the 60-day supply disruption ceased, the result would be a permanent backlog, with no return to steady state. In contrast, if the first factory were to transfer 50 percent of orders to the second factory, the resulting backlog would be time-limited, and eventually a return to steady state would be achieved. Thus, order transfer is one feasible risk-mitigation strategy, Wang said.
Use Case: Demand Surge Simulations
A more complicated simulation that Wang described involved responses to a significant demand surge. By comparing the impact of three risk-mitigation policies, it is possible to model demand surges of various intensities (e.g., 2- to 5-fold increases in demand of 20- to 60-day durations). The first risk-mitigation policy is to accelerate manufacturing, which might involve temporarily eliminating some of the final inspections in order to ship therapies more quickly to the sickest patients. The simulation revealed that this policy is useful for smaller demand surges and that the impact is independent of the duration of the surge. The second policy is a priority queue, in which the sickest patients are prioritized in cases where a surge causes wait time for therapy. This policy was found to be effective when the surge level is high, he said. The third policy is to combine accelerated manufacturing and priority queuing to employ mitigation strategies effective for both lower and higher surge levels, resulting in improvement across surge intensities and durations.
Use Case: Cost Modeling and Automation
Wang described a final use case that modeled automation for allogeneic iPS cell production and cost. Four different equipment configurations were compared: (1) a manual operation biosafety cabinet, (2) an automatic operation biosafety cabinet, (3) a manual operation isolator system, and (4) an automatic operation isolator system. Modeling revealed several takeaways. First, manual operations require much more labor, resulting in a lower throughput compared with automated systems. Also, isolators are more expensive and more labor intensive than biosafety cabinets. Finally, automation with biosafety cabinets has the lowest unit cost, whereas automation with isolators has the highest output. All of the configurations are assumed to be operating under the same cleanroom conditions, Wang said, but the factors of variability of cell quality and contamination risks have yet to be well studied.
DISCUSSION
Cell Quality in Manufacturing
How, Roy asked, could cell quality or CQAs influence the supply chain and automation system in the manufacturing pipeline? Cell quality and characterization or analytics were not considered in the model of automation cost and efficiency in Wang’s final simulation example, he noted. Wang replied that from a manufacturing standpoint, standardization and simplification stages should take place before automation. First, he said, it is important to understand the process variables and CQAs as well as their relationship and interplays, which can simplify the process as much as possible. The second step is standardizing the process. It is a risky proposition to automate before the systems are standardized and most of the uncertainty has been removed, he cautioned.
Data Protection in Storage, Processing, and Sharing
What are some of the challenges associated with data storage, processing, and sharing, a workshop participant asked, particularly in translational settings when working with patient samples? This is a daily consideration in her work with Novartis, Tyagarajan said. Multiples sets of regulations are in place, including the Health Insurance Portability and Accountability Act, European Union regulations, and regional regulations. Furthermore, Novartis has internal regulations that must be met before looking at clinical trial data. After considering regulatory requirements, the company determines whether data can be stored in an environment that belongs to Novartis but is external to the location of the clinical trial data. When data are stored in this way, they are anonymized to remove as many patient identifiers as possible. In certain instances, Tyagarajan said, it is not possible to completely remove all patient-identifying data because linking between different databases can require preserving certain patient-identifying elements. In those cases, access to that particular column of data is controlled and limited to specific individuals. In such circumstances, calling for what are known as pseudonymized variables requires documentation that explains why these data cannot be completely anonymous. The company also conducts internal compliance checks to ensure that patient data are protected to the greatest extent possible. A sizable challenge in employing sophisticated machine learning approaches, Khalil added, is creating a method of aggregating and learning from data and feedback loops. This is particularly challenging for the manufacturing process in the context of the compliance required, she said.
Is there data sharing outside your company, Roy asked Tyagarajan, and do registries have a role to play with anonymized manufacturing data? Post-market chimera data are deposited in the Center for International Blood and Marrow Transplant Research database, he noted, but they do not include manufacturing and characterization data. Tyagarajan discussed several differences between registry data and clinical trial data which indicate that the latter are a much more complete dataset than registry data. Furthermore, as registries are not mandated in the United States, they are dependent on physicians’ voluntary efforts to input information. Finally, patients may be less likely to report back to their physicians if they are doing well, contributing to incomplete data. An additional consideration relates to patient data acquired at different time intervals. For certain patients, data points may be collected at 6 months, 9 months, and 12 months. For others, it may be at 6 months and 12 months, and for still others it may be at 12 months and 24 months. Novartis has developed the pseudonymization process to help overcome this data gap. Partnerships and collaborations are needed, and this will require contracts and other formal steps, Tyagarajan said.
Registries have an important role to play, Khalil emphasized. While compliance can limit the data that can be shared, the AI Innovation Center advances patient care as much as possible within compliance. Registries can serve as a link between the manufacturing process and data on patient response over time. This type of registry can serve as a common platform where data modalities from those two pieces can be brought together, she said. With ongoing patient monitoring and the linkage of manufacturing with long-term patient outcomes, algorithms can be generated to provide the information that is most important for patients and doctors. However, she added, this will require collaboration among pharmaceutical companies, clinicians, and patient groups. The Multiple Myeloma Research Foundation has a registry on multiple myeloma patients followed longitudinally over time and, at this point, may even include data on novel cell therapies.2 That registry has enabled advances in predicting disease progression and identifying patients’ risk levels. These types of algorithms have benefits across the entire ecosystem, she concluded.
Discussions about accessing appropriate clinical data for these areas of research are ongoing, Kotanchek added. The next steps will involve (1) looking at long-term treatment effects, (2) integrating that knowledge with information from the manufacturing side in a way that allows the appropriate data to be shared, and (3) using the resulting deepened understanding to take additional action. She said that CMaT’s efforts are potentially powerful but that access to clinical data in relation to those programs is
___________________
2 More information on the Multiple Myeloma Research Foundation registry is available at https://mmrfcurecloud.org (accessed January 8, 2021).
essential. Thus, partnerships are paramount to realizing the full potential of what is possible.
Challenges Related to Lack of Patient Data
Are the types of patient data currently being collected (e.g., product characterization data, outcome data) sufficient, Roy asked, to predict treatment success? The lack of patient data in their clinical trials is a major challenge, Tyagarajan said. Her group uses the clinical trial dataset to train registry data, she said, but AI and machine learning require larger amounts of data. Because the field of cell and gene therapy is in its infancy, even the most advanced companies in the field have limited patient data. Thus, researchers often look to data collected by regional registries. These registries collect data from patients on a voluntary basis, and each has its own regulations. From a clinical outcome perspective, it is not possible to acquire the necessary amount of data from registries, but it is currently one of the only options available, Tyagarajan said.
In the chemical and biologics arena, situations with relatively low n and a high number of potential input variables are not uncommon, Kotanchek said. This has driven efforts to exploit the capability of symbolic regression to compress down to the highest probabilistic variables and variable combinations and then generate hypotheses. In contrast, more traditional machine learning tools are dependent on a very high n, which is used for feature identification. Symbolic regression capability allows for explicit inputs that can be checked by a domain expert to confirm that they make sense before they are used for building and testing. Therefore, the framework can be used to design the next set for validation, rather than waiting for the large n to arrive to drive those conclusions, she added.
The field of cell and gene therapy could learn from more mature industries that conduct process modeling for continuous improvement purposes, Roy said. Efforts are under way to structure informatics so that it can be reused across the enterprise to support various types of decision making, Kotanchek added. While confounding issues of patient data protection do present a substantial challenge, more investment is needed to support the reuse of data, she continued. Data assets are as critical as physical assets or human assets, yet they remain largely unexploited. New insights are valuable, Tyagarajan said, but they can create a time-consuming challenge. If a new insight pertains to a process that has already been validated, then it must be inputted into the regulatory filing. The philosophy of “process is product” may not apply to cell and gene therapy, Roy said. But if “the product is the product,” then the process can vary to achieve that product. He suggested that the regulatory side of cell and gene therapy may need to evolve toward that mindset as well.
Availability of Simulation Tools
Are the evolutionary computing simulations that were discussed earlier in the session open source or proprietary? Kotanchek said that the results she shared came from a data modeler desktop tool available via licensed software, and evolutionary algorithms are also available. Open-source tools are not always the most efficient and do not always have constraints concerning overfitting, she said; this should be taken into account when choosing a tool. Sufficient algorithms are available in the public domain and in publications, Khalil added, but one challenge is the limited availability of experts in machine learning approaches who are actively working with clinical experts and experts in the manufacturing process to understand the fundamentals of the problem at hand. This expertise is needed to determine the appropriate underlying mathematics for those fundamentals. The uncertainty in a given ensemble should also be considered so that algorithms can be tailored to address that uncertainty. At that point, experts can develop methods to test the signals that are coming from the data. All of the components for that process already exist, Khalil said.
Facility Relocation Considerations
There are two sets of variables that companies must consider in relocation planning when thinking about optimization and modeling tools to assist companies looking to relocate a facility, Wang said. The first set is tangible and consists of configurations that can be based on the pros and cons of various generated models. The second set is less tangible and involves labor, skilled workforce, regulations, investment, infrastructure, and transportation. Roy asked how the supply chain fits into these variables. The initial investment (i.e., the capital expenditure) is a variable, Wang said. Long-term considerations involve variables related to cost, including labor, as well as logistics of storage, distribution, and access to ports, airports, and highway systems. This represents a different level of systems thinking, Roy commented. Moreover, while a company can enter a new market using an existing facility, relocating a facility is a huge hurdle, Tyagarajan said. Trained operators are the hardest commodity to obtain, but relocation is difficult without them. Timelines should be clearly established when planning a relocation, Tyagarajan said. The relocation process typically takes years, during which time the original process may have changed—and new knowledge acquired—that would need to be integrated into the facility, requiring another filing. The field of cell and gene therapy is moving much faster than the mammalian cell culture field, making it difficult to keep pace with the new technologies being developed and the associated commercial
process that is filed. A process can become redundant by the time it is filed, Tyagarajan said, which creates another challenge.
Biological Information and Artificial Intelligence
Sui Huang, a professor at the Institute for Systems Biology, asked if biological knowledge—as mastered only by the human scientific mind—can be excluded from processes that rely on AI to extract actionable insights from big data or use automatic insights to determine causation. Our philosophy is to build biological measures into the process, Khalil said. This includes aspects such as cell viability, flow data from gene expression and omics, and process measures. She said that they take a probabilistic machine learning approach to the whole system. For example, the data may indicate that a key node, such as a certain genome, will drive cell count. Perhaps that genome was not measured in the first instantiation of the data. In this case, knowledge about how to account for hidden confounders or probabilistic models can be applied to capture the effect and then to inform collection of additional data. Khalil suggested that this is related to the issue of registries versus manufacturing because different types of data modalities can be combined to arrive at underlying biological mechanisms that are known to interact with process mechanisms.
This page intentionally left blank.
























