G
Aluminum Fuel
In 2013, a Massachusetts Institute of Technology (MIT) student accidentally discovered that aluminum BBs heated on a hot plate with a small amount of activation metals (2–4 percent by weight of gallium and indium) would react vigorously with water. Subsequent investigation has revealed that common forms of aluminum (e.g., beverage cans and aluminum electrical wire) can be activated simply by heating them in an oven together with the activation metals, to eliminate the aluminum oxide not just on the surface but throughout the entire volume of the aluminum. Experiments show that the resulting activated metal is highly reactive with water. Once activated, aluminum can also be ground up and mixed with common oils, such as canola oil or mineral oil, to create a paste or liquid version of the fuel that reacts equally well. The collected off-gas (nominally, hydrogen) can be used in commercial fuel cells or internal combustion engines, and the liquid effluent is mildly basic. Experimenters have disposed of residual liquids as non-hazardous waste.
Using the activated aluminum reaction, proof-of-concept prototypes have been built to power: a one-man-portable battery charging system; a BMW sedan; a two-stroke combustion engine; a 100 W fuel cell driving a watercraft motor; and to inflate a stratospheric balloon with hydrogen lifting gas. Reaction heat has also been used to produce the pressure needed for reverse osmosis water purification.
TECHNOLOGY READINESS
Both the production of activated aluminum and its reaction with water have been demonstrated reliably using known feedstock, but operation under the full range of field conditions with various aluminum and water contaminants requires additional investigation. For example, researchers previously thought that the activated aluminum could not be reacted with saline water, but commonly available additives since have been observed to allow the reaction to proceed fully. Copper contamination in the aluminum or water also inhibits the reaction, but other additives may mitigate this effect. Reactant water impurities carried over into the hydrogen and steam off-gas stream could foul fuel cell membranes—a problem already constraining use of reformed fossil fuel sources. Liquid effluent characteristics also would be strongly influenced by ingredients and reaction conditions. Reaction heat management also poses an engineering challenge, as the reaction proceeds more rapidly at elevated temperatures. Work remains to optimize the reaction rate, reactor cooling, reaction controls, and other system considerations.
MILITARY APPLICATION
Investigators have postulated concepts that would leverage this reaction for military applications. Figure G.1 illustrates prospective flow paths from material source to tactical use. Aluminum feedstock—either pure
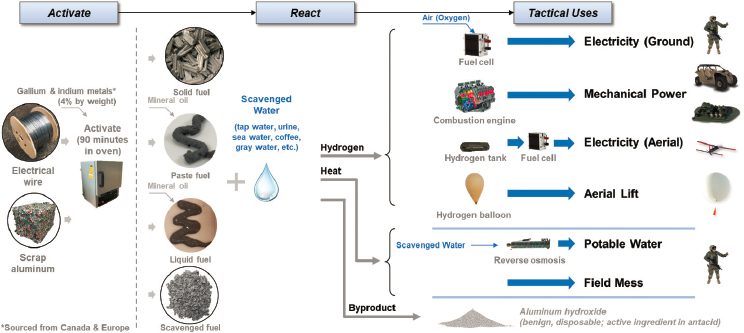
“primary” aluminum or “secondary” scrap aluminum—might be activated in a location where a modest amount of energy is available to heat it together with the activation metals. Alternately, aluminum could be activated commercially and transported in sealed containers to the point of use. The activation metals currently are sourced from Canada and Europe. Sourcing reactant water from local resource sources or waste streams could provide a logistic and security benefit. The aluminum-water reaction produces hydrogen, heat, aluminum hydroxide, and residual water with impurities. Practically speaking, hydrogen is a useful fuel and heat may be captured or rejected; liquid effluent might be considered as waste. In this context, figures of merit would include energy density and volume for the aluminum alone (assuming water is locally available), or for the combination of aluminum and water. Safety implications would be more nuanced, considering fire, chemical, and other hazards.
The aluminum-water reaction technology is still under investigation but may become useful for military application along with commercially available hydrogen technologies. Hydrogen-fueled vehicles exist for ground, marine, and aerial applications, but logistics impose a particular constraint. Activated aluminum could enable the production of hydrogen close to the tactical edge, potentially using local energy and water sources. For this reason, the Marine Corps reconnaissance community is currently considering the adoption of hydrogen-powered vehicles, rather than just battery-powered electric vehicles. The study team is investigating benefits and challenges for military use cases, for example:
- Mounted maneuver. Activated aluminum and locally available water could produce hydrogen in forward areas to fuel cells on electric vehicles. Reactors and fueling stations could be located in forward bases; hydrogen fuel cells would have reduced thermal, noise, and visible exhaust signatures compared to current combustion engine technologies. Watercraft might operate onboard aluminum-water reactors for on-demand supply. However, this study has highlighted the continued advantage of hydrocarbon fuel over hydrogen or other alternatives for heavy vehicles, especially in maneuver operations.
- Dismounted maneuver. Soldiers could carry pouches of activated aluminum for multiple uses. Reactant water could come from sources of opportunity (surface water, seawater, rainwater, or urine) and could be added to produce hydrogen (for fuel cell battery chargers) and heat (useful for comfort, or food/water heating). Soldiers currently carry various energy sources such as (diverse) batteries, ration heaters (combustible trioxane and water-activated magnesium-iron heat sources), and PV panels.
- Perhaps sealed pouches of aluminum pellets could serve as a relatively safe, compact, universal energy source with long shelf life.
- Reconnaissance and communications. Aerostats have been used for over a century and a half for military observation purposes. Field Manual FM 4-193 outlined procedures to produce hydrogen in the field using an aluminum-caustic reactor in forward locations. Each of the military services has contemporary programs to deploy stratospheric balloons for surveillance and communications; the Navy has demonstrated rapid inflation of balloons with hydrogen lift gas. Similarly, Group 1 and Group 2 unmanned aerial vehicles could be fueled with gaseous hydrogen with significant range improvements over similarly sized battery-powered variants.
- Base camps and stability operations. Soldiers in forward bases face competing challenges of efficiency (outcome vs. “boots on the ground”) and vulnerability (security and logistics dependencies). Activated aluminum technology could represent a relatively compact, safe, and flexible energy storage mechanism to support base camp functions such as power (electronic systems and lighting) and heat (food, water, and space). The technology offers potential resilience attributes given its simplicity: Aluminum might be recycled from local waste or packaging materials (e.g., pallets). Activation heat could be scavenged from the sun or local biomass source. Current renewable energy base camp solutions, intended to reduce logistic effort, depend on international shipment of electronic PV or wind systems. Activated aluminum technology might be implemented as a locally produced technology for both field forces (base camps) and indigenous communities (stability operations).
- Logistics. By using aluminum as a fuel, a “distributed logistics” approach may be possible, in contrast to more linear and long-distance logistics lines of communication necessitated by petroleum resupply operations. Scrap aluminum could be sourced from neighboring regions and transported in shipping containers or on trash barges, potentially less vulnerable to hostile attack compared to fuel tankers.
Activated aluminum may not be a promising energy storage mechanism to replace hydrocarbon fuels for energy-intensive combat vehicles, but its inherent simplicity and flexibility may provide value in various remote situations and/or in longer-term, small-scale operations.




