As with the stress-related disorders discussed in Chapter 2, the sex bias for neurodevelopmental and neurodegenerative diseases is striking, said Li Gan, Burton P. and Judith B. Resnick Distinguished Professor of Neurodegenerative Diseases at Weill Cornell Medicine. Advances in transcriptomic and circuit approaches have expanded the understanding of sex biases in these disorders, although much remains to be learned. Nonetheless, recent findings in the study of autism, schizophrenia, Alzheimer’s disease (AD), and other tauopathies have provided new opportunities to dissect disease mechanisms, identify new targets, develop biomarkers, and design better preclinical and clinical studies, said Gan.
AUTISM
The pervasive neurodevelopmental disorders known collectively as autism spectrum disorders (ASDs) have about a four-fold higher prevalence in males than in females, although the phenotypic presentation and overall severity are largely similar across both sexes, said Donna Werling, assistant professor of genetics at the University of Wisconsin–Madison (Baio et al., 2018). About 1.7 percent of children in the United States receive an ASD diagnosis on the basis of symptoms in two key domains: social communication deficits and restricted or repetitive behaviors or interests, she said. Many genetic variants have also been associated with increased ASD risk.
One approach to understanding the underlying mechanisms that give rise to these sex differences would be to analyze differences in the molecular
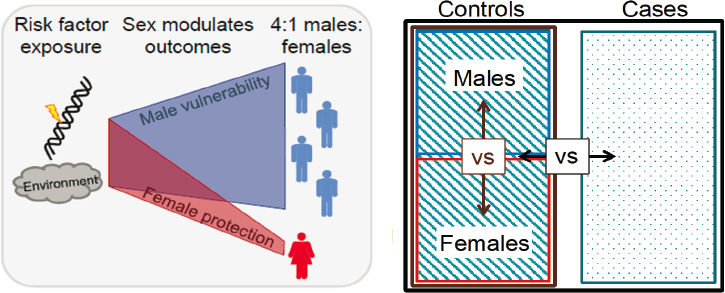
status of brain tissue between females and males with autism, said Werling. However, given the fact that phenotypes are similar, a more compelling case could be made for investigating the biology involved in sex-differentiated risk mechanisms. She hypothesized that males may be biologically more vulnerable to certain risk factors and/or that something in the biology of females attenuates the impact of those risk factors such that fewer females present with ASD symptoms (see Figure 3-1). From a transcriptomic perspective, the goal is to find genes and their associated functions that drive male vulnerability and/or female protection.
Werling cited four landmark studies of transcriptomic analysis in brain tissue from people with autism (Gupta et al., 2014; Parikshak et al., 2016; Velmeshev et al., 2019; Voineagu et al., 2011). These studies used nonindependent sample sets and, as expected given the difference in prevalence, included more males than females. Thus, said Werling, most of what is known about transcriptomic patterns in the brain comes from only 69 individuals with autism, and only 14 of these are female. These studies also focused on a limited set of brain regions, primarily the frontal and prefrontal cortex and the cerebellum.
The first three of these studies analyzed co-expressed gene modules and showed that in the ASD frontal and temporal cortex there was decreased transcription of modules associated with neuronal and synaptic function and increased expression of modules associated with immune, astrocyte, and
SOURCE: Presented by Donna Werling, September 23, 2020.
microglial function (Gupta et al., 2014; Parikshak et al., 2016; Voineagu et al., 2011). These patterns were not male specific.
The fourth study sought to understand whether these patterns indicate alterations in cell type composition or function, said Werling. Investigators in Arnold Kriegstein’s lab conducted single-cell-expression analysis from the prefrontal cortex and anterior cingulate cortex in 15 donors with ASD (Velmeshev et al., 2019). They identified 17 cell clusters that correspond to specific cortical cell types. Of these, only one cell type was proportionately more abundant in ASD samples than controls—protoplasmic astrocytes. They also conducted differential expression analyses within specific cell types, which showed that many of the most strongly down-regulated genes in cells from patients diagnosed with ASD were primarily in layer two/three excitatory neurons and a class of interneurons called vasoactive intestinal polypeptide interneurons, while the genes that were most strongly up-regulated were observed in protoplasmic astrocytes and microglia.
Taken together, said Werling, these studies show a consistent pattern across tissues and different data modalities of reduced expression of neuronal and synaptic genes and elevated expression of immune and glial genes.
To link these patterns to sex differential risk mechanisms, Werling showed data from an earlier study she conducted as a graduate student in Daniel Geschwind’s lab (Werling et al., 2016). Using RNA sequencing data from the BrainSpan consortium,1 she and her colleagues showed that in the neurotypical cortex, there was female-biased expression of the genes associated with synaptic and neuronal function that had shown reduced expression in brain tissue from people with ASD, and male-biased expression of genes associated with immune and glial cell function that had been shown to be up-regulated in autism. An analysis across the entire range of cortical brain regions and age samples available in the BrainSpan dataset showed that genes that are up-regulated in the ASD brain and are associated with the functions of astrocytes and microglia show male-biased expression, particularly during mid-fetal development and later in adulthood. Neuron-associated genes down-regulated in ASD showed a less striking female bias (Li et al., 2018; Parikshak et al., 2016). These data suggest that the sex-differential function of glia and/or neurons may contribute to ASD pathobiology and/or protective mechanisms, respectively, said Werling.
Although more data are needed to develop a detailed understanding of these sex-related pathobiological and protective mechanisms, transcriptomic studies have provided directions for more focused follow-up, she said. However, these studies have been limited by the relative paucity of brain tissue from autistic females. In addition, she said, to understand the brain circuitry
___________________
1 For more information, see https://www.brainspan.org (accessed October 24, 2020).
involved, transcriptomic analysis of more brain regions is needed, as well as data from multiple time points across the life span.
SCHIZOPHRENIA
Schizophrenia is a serious and heterogeneous psychiatric illness with well-established sex differences in incidence, age of onset, symptomatology across the life span, and outcomes, according to Panagiotis Roussos, professor of genetics and genomic sciences and psychiatry at the Icahn School of Medicine at Mount Sinai. For example, onset in men typically occurs in the early 20s, while the incidence in women peaks in the mid- to late 20s and then again in middle age (Abel et al., 2010). Women are more likely to experience depression, while men more often present with negative symptoms such as lack of motivation, anhedonia, and flat affect. Roussos noted that there is conflicting evidence related to sex differences in the expression of positive symptoms such as hallucinations and delusions. As the disease progresses, women are more likely than men to show reduced psychotic symptoms and better cognitive and global functioning, said Roussos. These sex differences suggest that different underlying mechanisms may occur in males and females; furthermore, there is strong evidence for contributions from both genetic and environmental factors, he said.
To examine sex-related transcriptomic changes associated with schizophrenia, Roussos and colleagues turned to the multi-cohort CommonMind Consortium (CMC) dataset,2 using tissue from four brain banks (Hoffman et al., 2019). This resource enabled them to obtain RNA sequence data on tissue from the dorsolateral prefrontal cortex, Brodmann areas 9 and 46 in 778 individuals (497 males, 281 females). The control to case ratio of 1.3 was consistent between males and females (see Figure 3-2).
Their analysis demonstrated robust and highly reproducible differential gene expression between cases and controls across cohorts, similar to what has been observed in previous studies, said Roussos. For example, the KCNK1 gene, which codes for a potassium channel subunit, was expressed at lower levels in schizophrenia samples across both the CMC and Human Brain Collection Core (HBCC) cohorts.
Differential expression analysis between males and females also demonstrated concordant sex signatures in the two cohorts. Combined results from the two cohorts identified 686 differentially expressed genes. Not surprisingly, the genes with the biggest effect sizes were located on the X and Y chromosomes, but there were also highly significant sex differences for many more genes residing on autosomes, said Roussos. Pathway analysis on these gene signatures identified molecular pathways related to
___________________
2 For more information, see http://commonmind.org (accessed October 26, 2020).
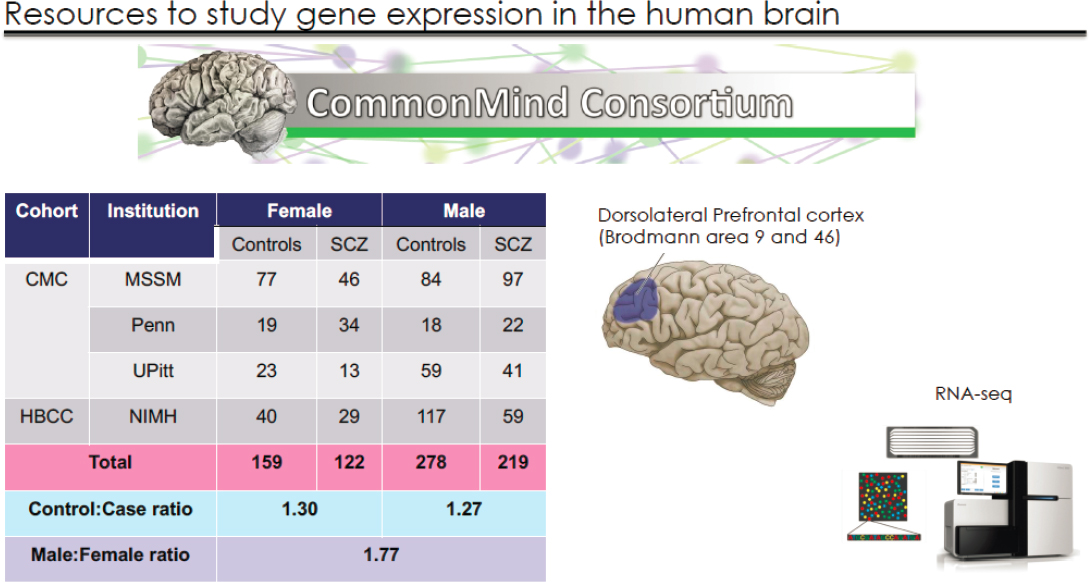
NOTES: The CommonMind Consortium (CMC) dataset includes the original CMC cohort comprising brains from Mount Sinai, the University of Pennsylvania Brain Bank, and the University of Pittsburgh (the MSSM-Penn-Pitt cohort), as well as the National Institute of Mental Health’s Human Brain Collection Core (NIMH-HBCC) cohort, for a total of 778 brains. RNA sequencing studies were conducted on tissue from Brodmann areas 9 and 46.
SOURCE: Presented by Panagiotis Roussos, September 23, 2020.
epigenome regulation, chromosome X inactivation, hormonal regulation, and synaptic transmission.
Next, they performed sex interaction analysis to detect sex-by-diagnosis differences between males and females. Although some genes showed concordance across cohorts, none were significant after multiple test corrections, said Roussos. He suggested that increasing the size of the cohorts would increase the power and could enable identification of significant interactions.
Roussos noted that sex differences in schizophrenia could result either from differences in directionality of gene expression (e.g., genes that are up-regulated in females with schizophrenia, but down-regulated in males with schizophrenia) or from different effect sizes with the same directionality. To examine this possibility, he and his colleagues performed case-control analysis separately for males and females. This analysis indicated that the effect size in females is only 71 percent of that in males, which could explain the more severe clinical phenotype observed in males in other studies.
Roussos and colleagues also used multiscale embedded gene co-expression network analysis (MEGENA) to analyze sex-by-diagnosis differences in co-expression modules rather than individual genes. Many modules yielded robust signals, he said. In a study available in preprint format, pathway analysis indicated that these modules represent molecular functions such as metabolism, hormone synthesis, signaling pathways, and regulation of neurotransmission (Hoffman et al., 2020).
ALZHEIMER’S DISEASE
More than 5 million Americans currently have a diagnosis of AD, and about two-thirds of these are women, said Timothy Hohman, associate professor of neurology at the Vanderbilt University Medical Center (Alzheimer’s Association, 2019). Moreover, he said, there is substantial evidence of sex differences in the drivers of AD pathology and the downstream response to pathology, suggesting that sex should be integrated into precision medicine models of the disease.
AD is marked by two primary neuropathologies in the brain—amyloid beta (Aβ) plaques and neurofibrillary tangles composed of tau protein—which drive neurodegeneration and cognitive impairment, said Hohman. Over the past 20 years, therapy development had been driven by the amyloid cascade hypothesis, he said, yet more than 50 clinical trials targeting amyloidosis have failed to result in an effective approved therapy. Hohman presented results from his lab and others integrating sex-specific genomic, transcriptomic, and biomarker data into disease models that aim to identify molecular drivers of AD risk and resilience, which can be translated into novel therapeutic targets.
In both human autopsy studies and mouse models of AD, females have been shown to have higher levels of both Aβ plaques and neurofibrillary tangles, suggesting that the neuropathology is fundamentally different between males and females, said Hohman (Carroll et al., 2010; Oveisgharan et al., 2018; Yue et al., 2011). Moreover, for a given level of neuropathology demonstrated at autopsy, females showed a more rapid decline in cognition compared with males (Barnes et al., 2005). Females also showed more rapid hippocampal atrophy, which is a marker of neurodegeneration, and more rapid cognitive decline in response to AD neuropathology (Koran et al., 2017).
Sex differences have also been demonstrated for genetic risk factors of AD, said Hohman. A large meta-analysis showed that female carriers of the APOE4 allele, the strongest genetic risk factor for AD, are at higher risk of developing AD than their male counterparts (Neu et al., 2017). Beyond APOE, however, Hohman said there are additional sex-specific genomic drivers of AD neuropathology that emerge downstream of amyloidosis, suggesting that there may be a sex-specific genetic architecture of AD. Indeed, there are striking differences in cell-specific gene expression patterns that change in the male and female brain, even in mid-life. For example, with increased age, the male brain shows changes in genes expressed in neurons and genes involved in synaptic transmission and dendritic growth, while the aging female brain shows gene expression changes in many of the support cells of the brain—microglia, endothelial cells, astrocytes, and oligodendrocytes (Sanfilippo et al., 2019). Moreover, this pattern is recapitulated in the AD brain, said Hohman.
Single-cell transcriptomics studies have also demonstrated sex differences in cell types impacted by AD neuropathology, added Hohman. Specifically, neuronal genes are down-regulated in females; while in males, oligodendrocyte genes are up-regulated (Mathys et al., 2019) (see Figure 3-3).
In recent years, sex-specific transcriptomic data have provided many more examples of notable sex differences in transcriptomic networks related to AD, said Hohman. Yet, as with other disease areas, distinguishing cause from consequence remains a major challenge. One approach he and others have used to try to disentangle cause and consequence has been to go back to genome-wide association study (GWAS) data and ask whether genomic predictors act in a sex-specific manner. The original study was a GWAS of cerebrospinal fluid levels of Aβ42, a biomarker of brain amyloid levels. This analysis showed strong associations with several genetic loci—APOE on chromosome 19, as well as loci on chromosomes 1 and 6 (Deming et al., 2017). When Hohman and colleagues performed a reanalysis that integrated sex into the model, they showed that the chromosome 6 association was driven entirely by females, and they identified a single-nucleotide
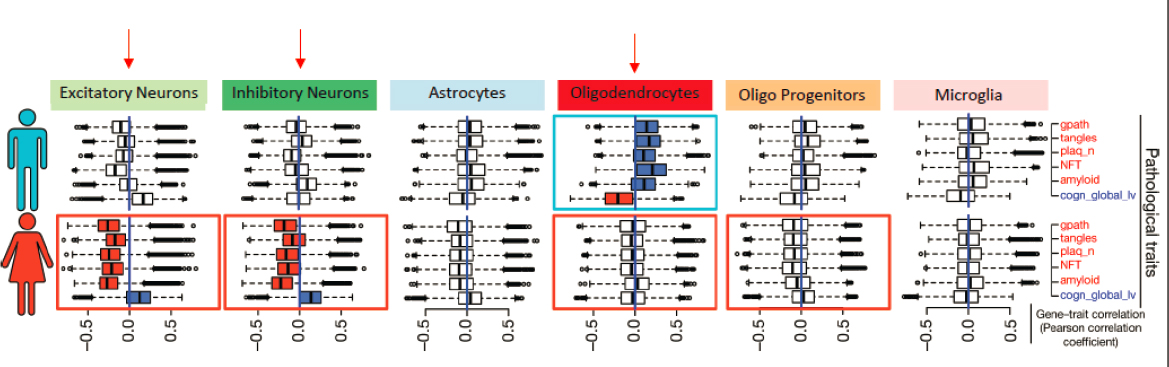
SOURCES: Presented by Timothy Hohman, September 23, 2020; Mathys et al., 2019.
polymorphism responsible for the association that is linked with expression of three different SERPIN genes (Deming et al., 2018). SERPINs, according to Hohman, are candidate markers of amyloidosis. They are protease inhibitors that have been shown to inhibit Aβ toxicity, likely by regulating neutrophil infiltration. Interestingly, there are sex differences in the neutrophil infiltration pattern, which appears to be modulated by estradiol. Although the gene is not expressed in the brain, staining for the SERPINB1 protein in the AD brain reveals its presence all around plaques, said Hohman.
Hohman and colleagues have used the same reanalysis of GWAS data approach to identify sex-specific genomic predictors by looking for associations with positron emission tomography imaging of amyloid, autopsy measures of neurofibrillary tangles, cognitive decline, and resilience to AD. These studies are providing new insight into sex-specific patterns and biological pathways that could be important for understanding how sex interacts with risk of and resilience to AD, he said.
TAUOPATHIES
AD is one of a group of disorders called tauopathies, so named because a pathological hallmark is the deposition of tau protein in the brain as neurofibrillary tangles. AD is not only more common in women, as mentioned earlier, but women also have more tau tangles than men when controlled for APOE4 carriage and age (Oveisgharan et al., 2018). AD is considered a secondary tauopathy because neurofibrillary tangles coexist with Aβ plaques as well as aberrantly activated microglia surrounding the plaques, added Gan. Long considered a consequence of pathology, she said that microglia are now believed to play a central role, in large part because of recent GWAS data, which have so far identified 29 AD risk loci and potentially 215 genes, many of them enriched in or in some cases exclusively found in microglia and macrophages (Jansen et al., 2019) (see Figure 3-4). Moreover, Gan noted that myeloid cells, such as macrophages and microglia, exhibit the strongest sex differences among immune cells in mice, with greater transcriptomic activation of immune pathways in females (Gal-Oz et al., 2019).
Microglia normally play a protective role as a first responder to injury, clearing aggregates and cell debris and initiating tissue repair through a neurotrophic effect, said Gan. In neurodegenerative disease, microglia respond inappropriately, causing excessive synaptic pruning and release of neurotoxic cytokines and chemokines. To understand sex differences in how microglia respond to tau, Gan and colleagues used RNA sequencing to profile messenger RNA (mRNA) and micro RNA (miRNA) in the brains of P301S transgenic mice, a mouse model of tauopathy (Kodama et al., 2020).
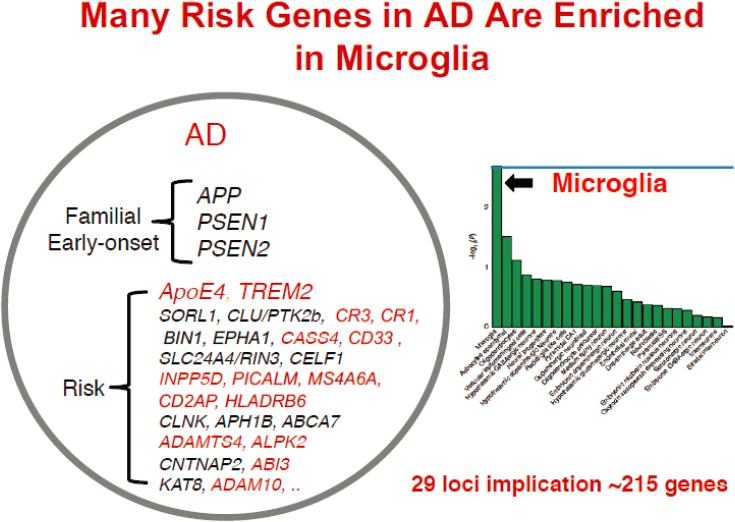
SOURCES: Presented by Li Gan, September 23, 2020; Jansen, 2019.
Although the pathological changes are similar in males and females, there was a substantial difference between male and female mice in terms of differential expression of mRNA and miRNA.
To understand the impact on the disease process of differential miRNA expression, they showed that in the absence of Dicer, an enzyme that regulates the maturation and function of miRNA, many more transcripts were modulated in male microglia than in female microglia. When they crossed the Dicer knockouts with tau transgenic mice, they identified a cluster of transcripts in male mice that were nearly absent in female mice. They also showed that ablation of mature miRNAs exacerbated tau pathology in male but not female mice. Taken together, these findings indicate that microglial miRNAs influence homeostasis and tau pathogenesis in a sex-specific manner in tauopathy mice, said Gan.
TREM2 is perhaps the strongest AD risk gene after APOE and is highly expressed in microglia, so Gan and colleagues investigated its effects on AD pathology in a sex-specific manner. To do this, they collaborated with Dennis Dickson of the Mayo Clinic, who provided access to a set of
brain samples from individuals with a TREM mutation called R47H that increases the risk of AD and frontotemporal degeneration (FTD, a primary tauopathy) by 2- to 4.5-fold. By sequencing nearly 85,000 nuclei with either the common variant or R47H, they showed differential expression of microglial genes in males and females with very little overlap. Gan noted that a subcluster of genes that are highly expressed in females but not males were highly enriched for interferon response.
The R47H mutation also exerts strong sex-specific effects on microglial transcriptomes in female but not male mice, said Gan. Female but not male mice with the mutation showed declines in spatial learning and memory.
In addition to their function as mediators of the brain’s immune response, microglia also have physiological roles, said Beth Stevens, associate professor of neurology at Harvard Medical School and faculty at the Broad Institute of the Massachusetts Institute of Technology and Harvard. She added that although microglia have been studied in AD and other neurodegenerative diseases, there appears to be a convergence with other brain diseases such as schizophrenia at the level of the biology, genetics, proteomics, and other physiological pathways involved. Single-cell omics and epigenetic studies are “cracking open the field” in exciting ways, and it has become increasingly clear that there are sex differences in various models, especially in the context of environmental perturbations, she said.
For example, as noted earlier, synaptic pruning is an important function of microglia that can lead to pathological synapse loss in AD and other disorders. Several years ago, Steven McCarroll and colleagues identified a structural variant in the complement protein C4 that is the largest common risk factor for schizophrenia (Sekar et al., 2016). More recently, they showed that the same variant also reduces risk for the autoimmune diseases systemic lupus erythematosus and Sjögren’s syndrome and that they act more strongly in men than in women (Kamitaki et al., 2020). This observation may provide mechanistic insight into therapeutics and biomarkers for these diseases and offers a molecular foothold with which to dissect mechanisms using induced pluripotent stem cells (IPSCs) as well as nonhuman primate and other animal models, said Stevens.
Because microglia also appear to play a role in autism, Gan suggested that it might be useful to interrogate the pathways involved to determine whether this convergence helps explain sex differences. Werling noted that the two disorders have opposing sex skews in prevalence and that microglia are involved at different stages of development. So there could be completely different processes that just happen to involve the same cell types, or there could be a thread of convergence, she said. Moreover, according to Stevens, making sense of the transcriptomic states of microglia and their relationship to function will require new model systems because their functions are so diverse. She said induced microglial stem cells may help
to answer some of these questions by exposing them to different challenges and then assessing single-cell transcriptomics and epigenetics. Other groups are grafting these cells into mouse models with different genetic backgrounds or reporters to see what happens when they get to the brain. Stevens added that access to fresh human brain tissue could also advance understanding of the sex differences in disease-associated microglia (DAM) omics and function. While most of the work on establishing DAM signatures has been done in mouse models, Roussos said his lab has shown that the DAM signature in human brain is very different from that in mouse brain.
Nilüfer Ertekin-Taner, professor of neurology and neuroscience at Mayo Clinic Florida, added that while single-cell analysis can be very informative, analytic approaches that enable deconvoluting existing large-scale, bulk brain RNA-sequencing data could also be useful. She noted that shared datasets from thousands of individuals with a variety of different diseases are available for this type of analysis.
This page intentionally left blank.














