5
Radioactive Sources and Alternative Technologies in Sterilization
This chapter describes the principles (Section 5.1) and use of radioactive sources and alternative technologies in sterilization. The applications discussed include sterilization of medical devices and health care products to eliminate microorganisms (Section 5.2), irradiation of food and agricultural products to eliminate harmful bacteria and a variety of microorganisms and insects or to extend their shelf life (Section 5.3), and sterilization of pests to manage their reproduction (Section 5.4). References to specific technologies and in some cases to specific commercial products and manufacturers do not necessarily constitute or imply their endorsement by the committee.
5.1 STERILIZATION PRINCIPLES
Radiation for sterilization applications is typically derived from one of three sources: gamma rays (cobalt-60), electron beam (e-beam) accelerators, and accelerators that produce x-rays for irradiation. The materials to be sterilized are typically packaged on a conveyor that transports them at a controlled speed from a loading area to a treatment area where they are irradiated and then returned to an unloading area. Sterilization by gamma-ray, e-beam, and x-ray modalities is broadly similar in terms of the transfer of energy and interaction with matter (see Appendix F). The main differences in the different modalities are related to dose rate, exposure time, penetration depth, and product compatibility.
Gamma rays from a cobalt-60 source are emitted omnidirectionally, whereas e-beam and high-energy x-ray photons are directed toward the product to be irradiated. Consequently, assuming same flux of electrons and gamma rays, e-beam dose rates are much higher than gamma-ray dose rates, resulting in significantly reduced exposure times (seconds versus minutes or hours) and allowing for higher throughput. The penetration of high-energy x-rays used for sterilization is comparable to that of gamma rays. Consequently, treatments have comparable uniformity, usually expressed by the ratio between maximum and minimum dose (dose uniformity ratio or DUR) deposited in the irradiation configuration. This makes it easier to meet both the dose required for sterilization and remain below the maximum dose tolerated by the product.
The penetrating ability of electrons is less than that of gamma rays. However, this does not imply that e-beam sterilization is limited in application to low-density or smaller products. Many products can be satisfactorily sterilized by e-beam with acceptable DUR through repackaging of the product to individual boxes to reduce the need for large penetration depth. This is in contrast to x-ray treatments where products can be treated en masse directly on pallets, and gamma-ray treatments where products are typically processed in totes or carriers. Additional strat-
egies such as exposure of a product from two sides using dual e-beams may achieve required sterilization doses throughout the product with acceptable maximum dose.
Irradiation facilities used for sterilization can operate on a contract basis or be incorporated in a company’s production line (in-house facilities). Most irradiation facilities are contract facilities that are designed around specific products but provide flexibility and adapt to the customers’ needs. That is, contract irradiation facilities can be used for sterilization that serves multiple purposes, and the dosages are adjusted depending on the products that require treatment (see Table 5.1 for typical dose requirements in sterilization). A smaller portion of irradiation facilities are in-house; that is, they are owned and operated by the company that is also the provider of the irradiated product. The global contract irradiation market is dominated by two companies, Steris and Sterigenics, which, combined, control approximately 85 percent of the sterilization market. The parent company for Sterigenics is Sotera Health, which also owns Nordion, the largest global supplier of cobalt-60. The sterilization market, particularly for medical devices, is operating at or near capacity1 but the industries that these facilities serve are growing.
Traditionally, cobalt-60 has been the most commonly used radioisotope in industrial sterilization. High-activity (1–5 MCi [37–185 PBq]) industrial irradiators are typically used for medical device sterilization because they can deliver high doses and can achieve large product throughput. High-activity irradiators can also be used for other applications including decontamination of packaging, cosmetics, pharmaceuticals, pet toys, and more rarely, phytosanitary applications and the sterile insect technique (SIT). A special configuration of high-activity industrial irradiators is a panoramic irradiator. Panoramic irradiators have the highest total activity of all radioactive sources. Approximately 98 percent of civilian source activity in the United States is in the form of cobalt-60, mostly due to these high-activity irradiators (NRC, 2008). Panoramic irradiators are used primarily to sterilize single-use medical products and devices (about 70 percent of their use), but they are also used to sterilize other products.
Inside a panoramic irradiator, the cobalt-60 sources are placed in a planar array of a few square meters. Individual pellets of cobalt are placed in steel rods called “pencils” that are arranged in a rack that holds them in a plane. The product to be irradiated remains in its original packaging and either remains on pallets or is placed on cartons that are transferred into totes. To utilize the gamma photons as efficiently as possible and to make the dose distribution as uniform as possible, the conveyor system surrounds the source on both sides and products travel on multiple levels in multiple passes. When retracted, the source is shielded either by water (wet storage) or by a shield such as lead or other appropriate material (dry storage). Because the photons are emitted in all directions, on average only about 30 percent of the emitted energy is deposited in the product (IAEA, n.d.). Irradiation of products occurs within an isolated bunker, typically an enclosure with thick concrete shielding, to protect workers from radiation.
Most low-activity (~1 MCi [37 PBq]) industrial irradiators are used for food irradiation, because this application typically requires a lower dose and throughputs. Low-activity irradiators can also deliver doses suitable for other sterilization applications, but at slower speed.
TABLE 5.1 Typical Irradiation and Dose Range
| Product | Purpose of Irradiation | Typical Dose Range (kGy) |
|---|---|---|
| Health care products | Sterilization | 15–30 |
| Sterile insect technique | Reproductive sterilization for pest management | 0.1–0.5 |
| Meat, poultry, fish | Delaying spoilage, killing certain pathogenic bacteria (e.g., salmonella) | 1–7 |
| Phytosanitation | Inactivating insects | 0.1–1 |
| Spices and other seasonings | Killing a variety of microorganisms and insects | 1–30 |
SOURCE: Adopted and revised from IAEA, 2006.
___________________
1 Nordion noted in a recent white paper (Nordion, 2021) that there is some room for growth in processing capacity, about 50 percent, in the gamma facilities that already exist.
The sections below provide some information on specific technologies and trends for the different sterilization applications.
5.2 MEDICAL DEVICE STERILIZATION
The U.S. medical device market is valued at $156 billion and is growing 5–7 percent annually2 because of increasing demand for existing medical devices and because of the availability of new products. Many single-use devices must be sterilized after manufacture. The annual growth rate of the U.S. market for medical device sterilization is about the same as for the medical device market. Global trends in medical device sterilization are similar to those in the United States. Demand for sterilization of single-use medical equipment was accelerated in 2020 owing to the COVID-19 pandemic and need for single-use disposable components for testing kits and vaccines.
Although sterilization represents a small percentage of the medical device’s production cost, it is considered a vital processing step within the manufacturing of medical devices and has direct impacts on the ability to commercialize a product. Many medical devices and other products are developed and manufactured with a selected sterilization modality. Any change in the sterilization process will require adjustments in the design of the product and revalidation of the product and its packaging as well as of the sterilization process itself. Large medical product manufacturers (about 20 percent of the overall market) operate their own sterilization facilities as well as contract with third-party sterilization service providers. Smaller manufacturers contract their sterilization.
The Food and Drug Administration (FDA) requires sterilization of all invasive medical and dental devices including syringes, surgical gloves, artificial joints, and implantable devices such as orthopedics and heart valves. Additionally, many single-use materials (e.g., tubing, bags, and filters) used in the production of pharmaceuticals and vaccines must be sterilized by an approved method and process. FDA generally requires a sterility assurance level (SAL) of 10–6 for invasive medical products.3 The SAL for noninvasive medical devices is lower, 10–3 or 1 in 1,000 probability of finding one nonsterile device. All three irradiation modalities (gamma, e-beam, and x-ray) are recognized in the applicable International Organization for Standardization (ISO) 11137 standard, and therefore the transference of the sterilization dose between the three modalities is broadly recognized. However, FDA does not recognize the equivalence of the three modalities in terms of biocompatibility.
The current breakdown of sterilization modalities used by the medical industry in terms of prevalence is 50 percent ethylene oxide (EtO) gas fumigation, 40 percent cobalt-60 gamma radiation, 10 percent e-beam radiation, and less than 1 percent other (including x-ray radiation). Gamma-ray irradiation therefore continues to dominate the radiation sterilization market (about 80 percent of all irradiation modalities), followed by e-beam (about 20 percent), and x-ray at only a very small share. Some general advantages and disadvantages of these different modalities are shown in Table 5.2.
5.2.1 Radioisotope Technologies
Cobalt-60 has been used for sterilization of medical devices since the 1960s. There is substantial knowledge of its effects on both the reduction of microorganisms to prevent disease and changes to material properties, as well as considerable experience in its use. The equipment used in cobalt-60-based sterilization is easy to use and maintain and is generally reliable. Importantly, medical device manufacturers have experience with the requirements to validate this modality of sterilization for new products.
There are approximately 200 large gamma irradiation facilities worldwide in more than 50 countries that are predominately used for medical device sterilization. A schematic representation of a gamma sterilization facility is shown in Figure 5.1. In these facilities, there are an estimated 400 megacurie (MCi) of cobalt-60 installed,4 but, according to a recent white paper by Nordion, the licensed capacity exceeds 600 MCi, which implies that there is
___________________
2 Kathleen Hoffman, Sotera Health Services, LLC, presentation to the committee on October 13, 2020.
3 This means a 1 in 1 million probability of finding a nonsterile unit.
4 The United States has slightly greater than 50 percent of the total installed cobalt-60 sterilization capacity contained in 50 commercial irradiation facilities.
TABLE 5.2 Comparison of Most Common Modalities in Sterilization
| Ethylene Oxide Fumigation | Gamma Irradiation | E-Beam Irradiation | X-ray Irradiation | |
|---|---|---|---|---|
| Typical products | Radiation-sensitive products including surgical kits, tubing sets, tracheostomy equipment, catheters | Syringes, surgical drape and gowns, gloves, staplers, wound dressings, implants including stents, pacemakers, orthopedic devices, food products | Medical devices that require limited penetration, labware, clean-room supplies, tissues, food products | Potentially similar to gamma but limited current acceptance |
| Market breakdown | ~50% | ~40% | ~10% | < 1% |
| Pros | Ability to penetrate pallets of finished products; good option for radiation-sensitive products | Quick processing times; good penetration of finished products | Quickest processing times | Potentially quick processing times; good penetration of finished products |
| Cons | Longer processing times; ethylene oxide residuals; use of a hazardous gas | Cannot treat radiation-sensitive products; use of cobalt-60, a radioactive material | Cannot treat radiation-sensitive products; limited product penetration | Cannot treat radiation-sensitive products; current availability; limited acceptance; energy inefficiencies |
SOURCE: Modified from presentation by Kathleen Hoffman, Sotera Health Services, LLC, October 13, 2020.
some room for growth in existing irradiators (Nordion, 2021). The same white paper recognizes that current demand for cobalt-60 exceeds supply by approximately 5 percent (Nordion, 2021). Nordion is investing in expansion of its cobalt-60 production capacity in existing and potentially new reactors to meet current and projected demand.
Expansion of production capacity for a facility that has radioactive sources such as a cobalt-60 gamma sterilization facility can be achieved simply by adding source elements to those already in place in the facility. This is an advantage over the expansion process for e-beam and x-ray facilities, which are typically designed to run at a
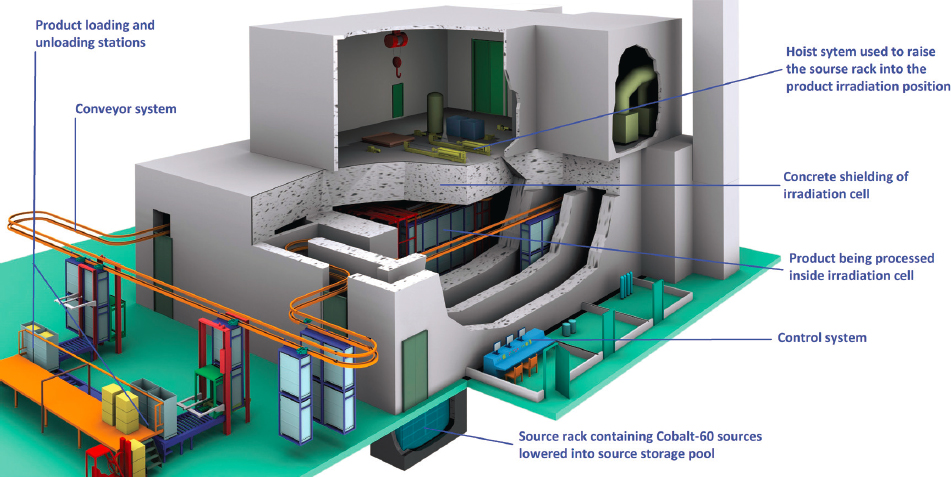
SOURCE: Image courtesy of SQHL Radiation Engineering Technology Co., Ltd.
specific capacity based on their installed technology. Increased capacity may require adding production lines and expanding the facility or adding new facilities to accommodate another installed e-beam or x-ray device.
Several factors are prompting industry and users to look for alternatives to gamma irradiation in sterilization. These include reducing dependence on a single modality, scarcity of cobalt-60 supply, increased regulations governing cobalt-60 transportation and commercial use, and increasing price of cobalt-60.
5.2.2 Alternative Technologies
As noted earlier, e-beam irradiation, x-ray irradiation, and EtO fumigation are modalities currently used for medical device sterilization. These methods are not necessarily a direct replacement for one another. Pros and cons of these different modalities are summarized in Table 5.2 and discussed in the following sections.
E-Beam Irradiation
Today there are about 75 high-energy e-beam facilities globally located in 12 countries and about 15–20 e-beam facilities dedicated to medical device sterilization (IIA, 2017) in the United States. A schematic representation of an e-beam irradiation facility is illustrated in Figure 5.2. About 10 percent of single-use medical device sterilization is performed using e-beams. This underutilization is likely because of the significant capital investment needed to transition to this technique and the demanding equivalency studies that need to be performed so that FDA and regulators in other countries recognize the equivalence to gamma sterilization in terms of biocompatibility.
Use of e-beam in sterilization has grown over the past decade at a rapid pace (Sugden, 2019). According to one source, from 2005 to 2015, on average 4 e-beam systems per year were installed, and from 2016 to 2019, the number increased to about 12 systems per year. Based on different projection scenarios, an additional 200 to 400 e-beam systems could be installed over the next 10 years.5 Use of e-beam in sterilization is expected to continue to grow due to pressure to replace cobalt-60 irradiation or EtO but also due to improvements in accelerator technology.
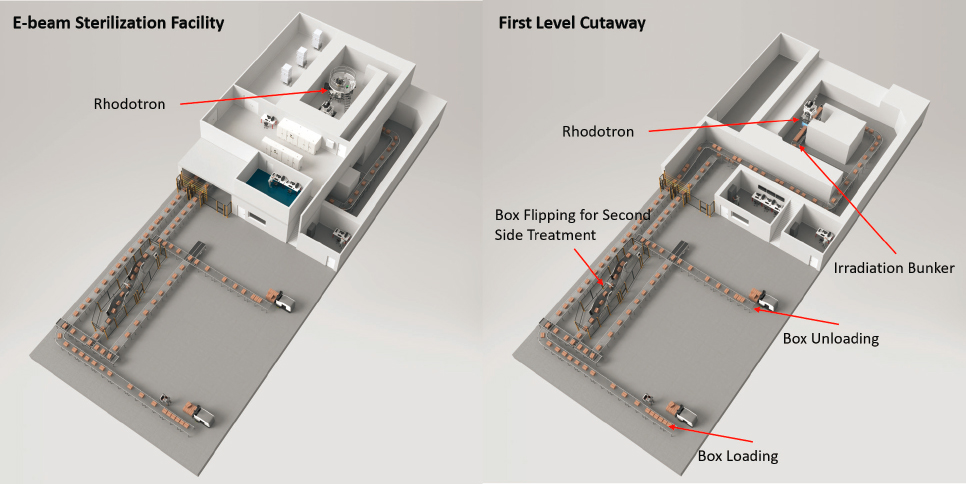
SOURCE: IBA Industrial.
___________________
5 Email communication between Christophe Malice, IBA, and Ourania Kosti, National Academies, on February 1, 2021.
Conventional linear accelerators (linacs) used in industrial sterilization operate at normal ambient temperatures. The radiofrequency power they use induces electrical currents in the surface of the accelerating cavities, which generates heat and dissipates some of the power provided to the cavity. This heat generation forces the accelerators to operate in a pulsed mode with high instantaneous power at pulse frequencies of 100–500 Hz but low average power. The energy dissipation means that overall energy efficiency is generally less than 50 percent.
Superconducting linacs are used in discovery science at places such as national laboratories. These accelerators dissipate very little of the energy input as heat. As a result, overall efficiencies can be 80 percent or more. Additionally, the output beam can be continuous. This enables beams with higher average power than conventional linacs and can overcome the inefficiency of the bremsstrahlung process to produce useful x-ray beams. Superconducting linacs are presently at Technology Readiness Level 4 but are being developed for commercial applications.
Today the two main suppliers of accelerators for sterilization are Mevex and IBA Industrial. Mevex produces conventional linacs. The linac can produce an e-beam or can be used with an x-ray converter system placed at the end of the scanning system to convert the electrons to x-rays. IBA has developed an accelerator, Rhodotron®, which recirculates the beam multiple times across the diameter of a toroidal accelerating cavity. After each crossing, a magnet bends the beam through approximately 190 degrees for another crossing, creating a flower petal–like trajectory. When the beam has reached either 7.5 or 10 megaelectron volt (MeV) the beam exits the accelerator and goes on to irradiate the product. Each of the reversing magnet locations is an opportunity for a beam exit port. IBA also developed the Rhodotron® Duo system that benefits from the regulatory limits for x-rays (7.5 MeV) and electrons (10 MeV). Rhodotron® Duo has an exit port at each of these energies through separate beam lines and therefore allows a single accelerator to provide both x-rays and e-beams simultaneously.
X-ray Technology
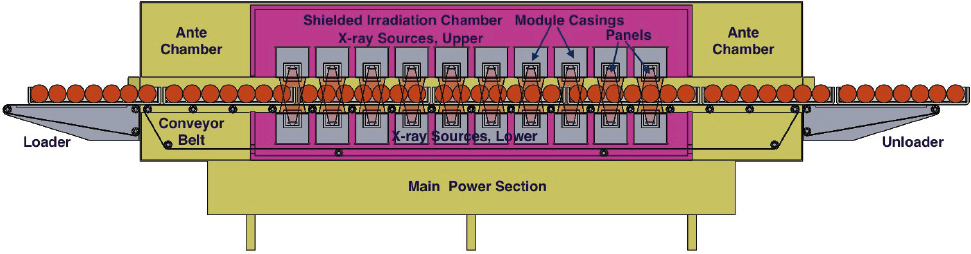
Accelerators used as a high-energy photon sources (1–10 MeV) could be the most straightforward replacement of gamma irradiation because the radiation penetration in the two modalities is similar. However, as discussed in Appendix F, sterilization using x-rays is inefficient. To generate 15 kilowatt (kW) of x-ray power, at least 120 kW of e-beam power is required. The requirement for robust, dependable, high-power e-beams has been a primary factor in the delay of adoption of x-rays for sterilization.
X-ray sterilization systems are commercially available, but they are in limited use. A schematic representation of an x-ray sterilization facility is shown in Figure 5.3. X-ray technology currently accounts for less than 1
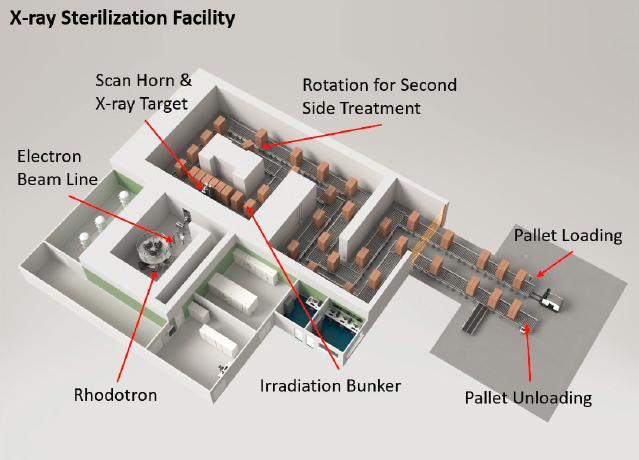
SOURCE: IBA Industrial.
percent of medical device sterilization volume. A 2016 white paper published by IBA, one of the major suppliers of accelerators, analyzed the differences between x-ray and gamma irradiation for sterilization (Dethier, 2016). IBA’s assessment was that “a chicken-and-egg” dilemma has held back the adoption of x-ray irradiation, because medical device manufacturers have resisted committing to this technology due to lack of operational x-ray facilities while the sterilization service providers have not invested in x-ray facilities due to lack of a commitment from the medical device manufacturers, resulting in market stasis. IBA’s analysis indicated that x-ray irradiation can be cost-competitive with gamma irradiation, especially for activity levels greater than 1.4 MCi (51.8 PBq) (Dethier, 2016). A more recent budgetary investment and operational costs analysis for a gamma irradiation and x-ray irradiation facility dedicated to medical device sterilization showed that the costs (initial investment and first-year operating costs) for an x-ray facility were about $17 million, which is 2.5 times lower than those of a gamma facility.6 The committee did not verify the accuracy of either of these analyses. If accurate, the present cost of cobalt-60 has moved the cost-competitive point down to 1 MCi. An additional potential operational benefit to e-beam facilities is the potential to deploy e-beam in-line in product production prior to boxing of products. This would offer a significant potential advantage for use of e-beam that is not possible with gamma irradiation.
An x-ray sterilization facility has operated in Däniken, Switzerland, since 2012. At that facility, a 700-kW Rhodotron® e-beam has a fixed water-cooled tantalum target to convert accelerated electrons into x-rays. However, the existence of only one x-ray facility posed problems with accruing customers due to business continuity concerns in the event of process interruption. More recently, sterilization service providers have announced their intent to invest in x-ray facilities. In 2019 and 2020, Steris AST, which owns and operates the Däniken facility, announced its intention to open additional x-ray facilities in Germany, Malaysia, the Netherlands, and Thailand, and three in the United States. In addition, Steri-Tek added x-ray to its existing e-beam services in Fremont, California, in 2019. The company also announced a new facility including x-ray in Dallas, Texas. Whether x-ray technology will capture significant market share from gamma or e-beam technologies for medical device sterilization will depend on a number of factors, including market growth.
Ethylene Oxide Gas Fumigation and Other Methods
In North America, about 50 percent of sterilization of single-use medical devices is performed using EtO gas fumigation. EtO gas fumigation involves exposure of the packaged product on pallets within an air-tight and humidified chamber that varies in size but can be up to 70 m3. Ethylene oxide gas is appropriate for sterilization of medical devices and other products that cannot be treated with ionizing radiation or other modalities due to their size, shape, complexity, or material composition. These products include catheters, IV tubing, and endotracheal and angiographic balloons. However, there are pressures to reduce the emission levels and the residual gas remaining on sterilized products generated by EtO fumigation processes. The Environmental Protection Agency is expected to issue a proposal on emission standards for EtO commercial sterilization operations in 2021. This proposal could drive more products that are currently sterilized with EtO to be sterilized with other modalities including irradiation.
There are other options for sterilization of medical devices. For example, steam and dry heat have been used to sterilize products for decades, but the high temperatures used in these modalities preclude treatment of many heat-sensitive materials. Vaporized hydrogen peroxide and nitrogen dioxide are emerging as alternatives to EtO, and some commercial companies are starting to offer these methods as part of their sterilization services.
5.2.3 Alternative Technology Adoption Considerations
Increasing concerns about cobalt-60 source availability and EtO emissions and residuum levels, together with other factors, have put pressure on medical device suppliers to look for alternative technologies to meet demand for device sterilization. Several industry representatives who briefed the committee, as well as other experts, project that utilization of e-beam and x-ray irradiation sterilization will increase to cover market demand that will probably not be covered by gamma irradiation. The committee agrees with the general conclusion of a previous report
___________________
6 Communication between Cherenkov Consulting S.C. and Ourania Kosti, National Academies, on March 10, 2021.
that e-beam irradiator installations will continue and, in addition, several x-ray irradiators will also be installed in the near future (IAEA, 2019a). The committee does not expect a full transition to alternative technologies for sterilization in the next decade. Instead, all major medical device sterilization modalities—gamma, e-beam, x-ray, and EtO—are expected to contribute to the reliability of the market.
Transitioning from gamma irradiation to e-beam and x-ray irradiation for sterilization is theoretically straightforward because all are approved sterilization methods that are recognized by an established standard (ISO 11137). However, sterilization using gamma rays from cobalt-60 has, over decades, resulted in extensive experience and data on the performance of materials. That information is sparse for the performance of the same materials following sterilization with e-beams and x-rays. Manufacturers who consider adopting an alternative technology will need to determine material compatibility and suitability of alternative technologies and undergo revalidation studies to ensure sterilization equivalency and absence of material alteration. This may require submissions of new 510(k) premarket applications or premarket application supplements, depending on the regulatory classification of the product and the product design or other changes required. Also, because many products are sold globally, multiple approvals are required. There is no uniformity in international regulation or, necessarily, in the knowledge and familiarity of the various regulatory bodies with alternative modalities for sterilization. Revalidation is a long process that can take years and can be a significant business risk. Health care markets are generally risk averse given the possible direct consequences to human health of changes in established processes.
To help address the information gap issue described above, Pacific Northwest National Laboratories, with support from the National Nuclear Security Administration (NNSA), coordinated a collaborative project with medical device sterilization industry participation aiming to collect data on the performance of gamma-ray, e-beam and x-ray irradiation for a wide range of medical devices (Fifield et al., 2019). The team, known as Team Nablo,7 used two prototypical commercial devices currently sterilized using cobalt-60 gamma radiation and irradiated them at four doses relevant to sterilization. The devices selected for the analysis included components comprising six distinct polymer materials commonly used in the medical device industry and were tested for function and color change. The team concluded that the e-beam and x-ray modalities are viable alternatives to cobalt-60 gamma radiation (Fifield et al., 2021). This successful collaboration and other ongoing efforts by the team will provide peer-reviewed data that are required for regulatory submissions and are a model for future comparison studies.
Transitioning from gamma irradiation to alternative technologies also requires substantial facility modifications with transition costs that are expected to be large. These costs vary depending on whether an existing cobalt-60 facility is transitioning to an alternative technology, constructing an operating de novo cobalt-60 or an alternative technology irradiation facility, or developing parallel operation by adding an alternative technology at an existing cobalt-60 irradiation facility. NNSA tasked Sandia National Laboratories (Sandia) to examine the costs, benefits, and challenges associated with operating a gamma industrial panoramic irradiator facility compared to a nonradioisotopic irradiator replacement. The study is being conducted in phases and is expected to conclude in summer 2021. At the conclusion of the study, Sandia will provide a report to NNSA with a decision strategy to allow a facility to analyze whether alternative technology may be a viable option. The report will also include lessons learned for future engagement on alternative technology adoption.8
5.3 FOOD IRRADIATION
Food can be irradiated for either safety or phytosanitary purposes. These two distinct processes and goals have some common elements, and for that reason they are discussed together in this section.
Food safety treatments aim to reduce the transmission of foodborne illnesses, extend the shelf life of products, prevent sprouting, and slow the ripening process. Phytosanitation aims to protect domestic crops from invasive species carried across borders by imported fruits, vegetables, and other food products. Fresh fruits and vegetables can harbor a broad range of pests, which unless properly controlled could spread widely, resulting in economic loss. The most common insects of concern in the transport of fresh produce are fruit flies, butterflies and moths,
___________________
7 In memory of Samuel V. Nablo, known by many in the radiation processing industry.
8 Jodi Lieberman, Sandia National Laboratories, presentation to the committee on February 25, 2021.
and mealybugs. The goal of phytosanitation is not to kill but to stop development or reproduction of any pests that may be carried by the products. Because mortality is not always the goal of the treatment, live target pests may be found. Therefore, it is essential that the treatment ensures that they are unable to reproduce. The treatment of products for phytosanitary purposes can be applied in the originating country, at the port of entry, or in transit.
The most common modality for food treatment for safety purposes is heat (pasteurization). Other modalities include high-pressure processing and emerging technologies including pulsed field and ultraviolet treatment. The use of irradiation for food safety has been approved in 40 countries, but it remains extremely limited with significant variation among countries regarding its acceptability. China is the largest consumer of irradiated food with more than 1 million tons of food per year currently processed by irradiation, an increase from 600,000 tons in 2015 (MeiXu, 2021). However, even in China where food irradiation is accepted, it is still only used in few niche applications, for example, to treat pickled chicken products, spices, and dehydrated vegetables.
FDA has approved a variety of foods for irradiation in the United States including beef, pork, poultry, crustaceans, fresh fruit and vegetables, shell eggs, and spices and seasonings. However, very little food is currently irradiated in the United States for food safety primarily because of lack of public acceptance of irradiated food (see discussion in Section 5.3.3). The same applies to Europe where food irradiation has declined in the past two decades. The largest category of food irradiated in the United States is spices. Irradiated foods are also provided to hospitalized patients who are immunocompromised due to disease or therapy and to astronauts to avoid getting foodborne illness in space.
The most common modality for phytosanitation in the United States is fumigation with chemicals, for example, methyl bromide (about 95 percent). Other modalities such as heat (44–48°C), cold (0–2°C), and irradiation collectively account for the remaining 5 percent. Heat treatment, irradiation, and fumigation are applied before shipment of food products or upon arrival and are usually done in a matter of hours or less. Cold treatments are typically applied in transit, as they take 12 to 22 days to be effective. The appropriate phytosanitation method and the protocol for that method are dependent on the pest and food product. Irradiation stops pest development; the other methods kill pests (Hallman, 2007).
Regardless of food product or application, FDA regulates the use of radiation on foods, as a food additive (as opposed to a physical process) with oversight of its use shared with the U.S. Department of Agriculture (USDA). FDA has determined that gamma (using cobalt-60 or cesium-137), x-ray, and e-beam are equally safe and effective for approved food irradiation treatments, including both pathogen reduction and phytosanitary applications (USDA, 2016). FDA also determines the labeling requirements for irradiated food with the international Radura symbol for irradiation, along with the appropriate statement on the food label. Bulk foods such as meats, eggs, fruits, and vegetables are required to be labeled with the Radura symbol. However, FDA does not require that individual ingredients in multi-ingredient foods such as spices be so labeled.
Phytosanitary requirements for U.S. imports and regional shipments are determined by USDA and enforced by USDA’s Animal and Plant Health Inspection Service (APHIS) in conjunction with its state partners. There are more than 150 domestic phytosanitary treatment facilities certified by USDA in the United States and overseas. Of those, only three import facilities (two e-beam and one cobalt-60) use radiation for phytosanitary purposes. There is also one cobalt-60 and one x-ray facility for treatment of regional shipments. Internationally, USDA has eight cobalt-60 facilities and one e-beam facility. Countries other than the United States that have imported fresh commodities disinfected via irradiation are Australia, Indonesia, Malaysia, Mexico, New Zealand, and Vietnam.
Phytosanitary requirements for U.S. exports are typically determined through bilateral agreements between the United States and importing countries or multilateral trade agreements. Each country has different effective approved dosages, although most follow guidelines issued by the International Standards for Phytosanitary Measures (ISPM 18 and 28) (FAO and IPPC, 2019).
5.3.1 Radioisotope Technologies
For food irradiation, the minimum absorbed dose needs to be sufficient to achieve the purpose of the application (food safety or phytosanitary) and the maximum absorbed dose must not compromise wholesomeness, structural integrity, smell, or taste. ISO 14470 (2011) specifies requirements for the development, validation, and routine
control of food treatments using ionizing radiation. The doses used for food safety irradiation are greater than those used for phytosanitary purposes. A dose lower than 2 kilogray (kGy) is used to delay sprouting of vegetables and aging of fruits. Doses between 1 and 10 kGy are used to reduce the levels of pathogenic organisms, similar to pasteurization. Doses greater than 10 kGy are used to achieve sterility (analogous to canning) or to decontaminate certain food ingredients such as spices.
In phytosanitation, the most commonly used generic dose is 400 Gy, but indications suggest that this is higher than necessary to serve the purpose (Hallman and Blackburn, 2016). This generic dose is specific to an insect category (e.g., fruit flies) but nonspecific to the food product, which simplifies the applied protocol when compared with other phytosanitary treatments.
Most food and agricultural products treated by irradiation are processed in facilities using gamma radiation from cobalt-60.9 These facilities typically are not dedicated facilities but instead multipurpose facilities primarily used for medical device sterilization. At a recent symposium,10 it was stated that the use of gamma irradiation for food poses security, economic, and availability challenges; that multipurpose facilities are typically optimized for medical device sterilization; and that the technology is not well suited for countries where food security remains an issue. As a result, fewer cobalt-60 facilities are being built for food irradiation. For example, in China, there were about 130 cobalt-60 facilities in 2019 responsible for 70 to 80 percent of irradiated food (MeiXu, 2021). No new facilities have been built in the past 5 years. Instead, as noted in Section 5.3.2, China is investing in e-beam facilities, and 5 to 10 new facilities have been built each year for the past 5 years. However, new gamma facilities were recently completed in Vietnam11 and India.12
5.3.2 Alternative Technologies
Heat treatments and chemical additives are often used for food safety and phytosanitary purposes, and steam and EtO are used for spices and certain food products. For example, canned foods are rendered commercially sterile using pressurized, saturated steaming of products packed into a steam chamber. Microbial death occurs based on numerous factors including the time and temperature of treatment and thermal resistance characteristics of the target organism.
Methyl bromide fumigation is by far the most common phytosanitary treatment method in the United States. Despite increased regulatory costs over the past several decades, the method remains highly cost-effective and can be performed in simple facilities. The primary disadvantage of methyl bromide fumigation is that the chemical has long been recognized as a significant ozone-depleting substance, and its use for noncritical applications has been phased out as the result of international agreements. Although post-harvest phytosanitary uses have been indefinitely exempted from these restrictions, there remains domestic and international pressure to reduce its use for health, environmental, or occupational safety reasons. As a result, USDA actively encourages the use of alternatives including ionizing radiation, for phytosanitary treatments when feasible (Pillai et al., 2014). The commercial availability of recapture systems and the development of processes to contain, destroy, or reuse methyl bromide after use in order to reduce the negative impacts have not altered USDA’s position on the treatment.
The main disadvantage of cold treatments for phytosanitary purposes is the long required treatments that are typically applied after packing during lengthy transport. The relatively long treatment periods may also constitute a business risk in the case of power outages or equipment failures. For some products, a treatment interruption that leads to an increase in temperature as little as 1°C, even for a short period of time, could require the process to be restarted. The speed of heated-air phytosanitary treatments varies depending on a wide range of factors, including the product and product packaging, the facility size and design, and the humidity present in the air at the facility location. Heated-air treatment is one of the most challenging phytosanitary treatments to manage because many variables can affect its efficacy. For example, faster, forced-air treatments might damage treated commodities,
___________________
9 See http://www-naweb.iaea.org/nafa/fep/crp/fep-xray-application-food-irradiation.html.
10 International Food Irradiation Symposium, March 9–11, 2021.
11 See https://iiaglobal.com/news/offer-irradiation-services-expanding-vietnam.
12 See https://iiaglobal.com/news/more-gamma-irradiators-in-india.
while slower applications could fail if pests are able to acclimate to the increasing temperature through “heat-shock proteins.”
E-beam and x-ray modalities can be effectively used for both food safety and phytosanitary purposes. In the United States, the upper limit for e-beam processing used on foods is 10 MeV and for x-ray processing is 7.5 MeV. Outside the United States, the limit for e-beam processing is also 10 MeV, but for most countries the maximum allowable energy for x-ray processing is 5 MeV. A few other countries, for example, Canada, India, Indonesia, and the Republic of Korea, also allow the use of 7.5 MeV x-rays for irradiation of food products. The increase from 5 to 7.5 MeV makes use of x-ray technology more economical and also enables increased throughput.
Food processing applications of e-beam technology can be broadly divided into three applications: low-energy (< 1 MeV), medium-energy (1–8 MeV), and high-energy (8–10 MeV) applications. Current low-energy applications include the in-line sterilization of packaging materials and the in-line disinfestation and sterilization of seed surfaces. Medium-energy applications include phytosanitary treatment of packaged fruits and vegetables. High-energy applications include irradiation for food safety purposes of spices, packaged meats, seafood, and other foods.
In 2014, the Food and Agriculture Organization of the United Nations (FAO) and the International Atomic Energy Agency (IAEA) initiated a Coordinated Research Project (CRP) with the objective to accelerate development and facilitate implementation of practical techniques for the irradiation of food and agricultural products using e-beam and x-ray. Project results are expected in June 2021.13 In addition, the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture in collaboration with the IAEA Division of Physical and Chemical Sciences launched a new 5-year (2020–2025) CRP, titled Innovating Radiation Processing of Food with Low Energy Beams from Machine Sources. In September 2020, under an Asia-Pacific Regional Cooperative Agreement comprising 22 government parties, the IAEA also launched a project titled Promoting Food Irradiation by Electron Beam and X Ray Technology to Enhance Food Safety, Security and Trade. The purpose of this project is to address reliance of food irradiation on cobalt-60 gamma facilities and promote alternative irradiation technologies.
5.3.3 Alternative Technology Adoption Considerations
Irradiation does not yet have a significant place among food processes. Consumer acceptance is still perceived as the main challenge with adopting this method in food safety. Consequently, the potential of the technology to reduce foodborne diseases and post-harvest losses remains mostly untapped. The use of irradiation to prevent the spread of invasive insect pest is the only recent development toward expanding food irradiation.
Overall, the global trade of food products treated with irradiation for phytosanitary purposes has increased from approximately 5,000 tons of foods in 2007 to more than 45,000 in 2019 (Hénon, 2021). The majority of the increase in recent years is in countries that either need irradiated products for their domestic market or see an opportunity to develop markets overseas. Examples include Australia, India, Thailand, and Vietnam. It is likely that irradiation for phytosanitary purposes in these and other countries will continue to increase in coming years.
Although the incidence of foodborne disease disproportionally affects populations in low- and middle-income countries (LMICs), it is unlikely that food irradiation will be implemented in many of these. For example, in Africa, except in Egypt and South Africa, the lack of resources and basic infrastructure cannot support food irradiation technologies at the scale necessary to be effective. Other actions are more urgent than irradiation to enhance food safety in African countries, including improving handling, transportation, and food storage conditions.
Food irradiation has demonstrated practical benefits when used for food safety and phytosanitary purposes. In phytosanitation, the method employed by producers for a given product is subject to several constraints: it must effectively eliminate or neutralize the targeted pest while having a negligible negative impact on the product itself, must be cost-effective, must be environmentally acceptable, and must meet the specific requirements for the product at the consumer location (Hallman, 2007). Irradiation has several advantages over other phytosanitary treatments. Whereas development of heat, cold, and fumigation protocols involve studying each fruit pest combination, generic irradiation treatments can be developed for a pest species irrespective of commodity (Hallman, 2011). Disadvan-
___________________
tages include logistical bottlenecks due to current limited availability of the technology and lack of an independent verification of treatment efficacy because pests may be found alive after treatment during commodity inspection.
With increasing restrictions placed on the use of chemical fumigants by importing countries, the use of phytosanitary irradiation is increasing around the world.14 In the United States, it is recognized that alternatives to methyl bromide for use as phytosanitary measures are needed, particularly because there may be future restrictions on the use of methyl bromide. Tentative plans exist for commissioning four irradiation facilities in the United States for phytosanitary purposes: two in Texas (one e-beam, one x-ray), one in South Florida (x-ray), and one in New Jersey (x-ray or possibly cobalt-60). Some of these facilities might be operational in the next 3 years.15 In Australia, Steritech recently opened a purpose-built hybrid e-beam/x-ray facility approved for multiple fresh produce commodities and export markets.
The biggest investor in the world by far on food irradiation is China. The country is investing in e-beam for food irradiation for safety purposes, with 5 to 10 new machines being installed each year during the past 5 years. In 2019 there were about 78 e-beam accelerators in the country. Adoption of food irradiation for safety purposes is stagnant in many parts of the world including the United States and Japan, and is declining in Europe. Several reasons have been provided to explain these trends.
First, a major factor influencing the adoption of irradiated food is public understanding and acceptance of the process. Although experts mostly agree that irradiation is an effective way to provide safer products to the consumer (WHO et al., 1981), food processors and distributors are hesitant to provide a product for which there is general consumer wariness and negative perceptions. These perceptions include concerns that irradiated food makes food radioactive and therefore can cause cancer and that irradiation changes the chemical composition of the food or that it diminishes the nutritional value of the food product (Castell-Perez and Moreira, 2021). FDA has issued statements noting that these negative perceptions are false.16 There is some evidence that the more consumers understand the use of radiation as an effective means to prevent foodborne diseases, the higher the probability that the irradiated food will be accepted and purchased (Castell-Perez and Moreira, 2021).
Second, there is lack of coordination and harmonization of regulations in international trade. The Codex Alimentarius (or food code) Commission (Codex 1984) has recommended harmonized standards for irradiated foods and an international code of practice for the operation of radiation facilities used for the treatment of foods. These standards state that irradiated foods should be accompanied by shipping documents identifying the irradiator, date of treatment, lot identification, dose, and other details of treatment. The International Consultative Group on Food Irradiation (ICGFI), established under the aegis of FAO, the World Health Organization, and the IAEA, worked from 1982 to 2004 to harmonize food irradiation standards around the world. Among its activities, the ICGFI made collections of national regulations, issued codes of practice for irradiation of different foods, and proposed authorization of irradiation by class of food, a generic approval that simplifies trade.
Third, currently food irradiation is primarily outsourced, with multipurpose radiation processing centers in locations that can serve a broad number of potential customers and offering their services on a contract basis to a broad range of businesses. This model for food irradiation service is generally considered to be unfavorable to expansion of this application because of the associated costs with transfer of food products to the facility and the long turnaround (about 4 days), which may have adverse effects on some products. It has been suggested that private investments in e-beam and x-ray machines that integrate them in the manufacturing or packaging line might change the industry’s views on food irradiation (Pillai, 2021).
Fourth, labeling of irradiated food is believed to have a negative impact on acceptance of irradiated food. Food irradiation in Europe was active, especially in Belgium, France, and the Netherlands but decreased rapidly after the enforcement of a European Union (EU) regulation in 1999 requiring the strict labeling of irradiated foods.17 Specifically, commercial food irradiation in France reached 20,000 tons in 1998 but dropped to 3,000 tons in
___________________
14 Laura Jeffers, USDA, presentation to the committee on January 6, 2021.
15 Email communication between Laura Jeffers, USDA, and Ourania Kosti, National Academies, on January 26, 2021.
16 See https://www.fda.gov/food/buy-store-serve-safe-food/food-irradiation-what-you-need-know.
17 Framework Directive 1999/2/EC covers the process, labeling, and conditions for authorizing food irradiation and Implementing Directive 1999/3/EC lists the foods and ingredients allowed to be treated with ionizing radiation.
2005 (Kume et al., 2009). In 2018, the EU, under the Better Regulation framework, initiated an assessment as to whether the 1999 Directive remains relevant and effective.
5.4 INSECT STERILIZATION
SIT aims to control pests that can damage crops and other plants by sterilizing males of the pests. It applies radiation doses sufficient to render them sterile, but without weakening them so that they are still able to compete with wild males for mating. If released in sufficient numbers, the irradiated male pests reduce the population by mating with females that produce no offspring (see Figure 5.4). Sterile insects from a facility can be shipped to other countries for the purpose of pest control management.
The late pupal stage is generally preferred for irradiation because it is more practical to handle and ship pupae and easier to achieve an acceptable balance between competitiveness and sterility. Upon arrival at final destination, the irradiated pupae or adults need to be cleared by the national phytosanitary and customs authorities. Sterile insects need to conform to international accepted quality control standards and operation procedures (FAO et al., 2019). Prior to their release, sterile insects emerge from the pupal stage, are fed and mature, and then loaded into delivery vehicles for aerial or ground release. Regimens of chilling and oxygen deprivation are being used as means of prolonging the storage time of irradiated pupae and adults during transportation without reducing longevity and the ability of males to perform in the field.
SIT has been used against relatively few insect species including the New World screwworm, tsetse flies, a variety of fruit flies, and some moths. Notably, SIT is not used broadly for control of mosquitoes to help eradicate mosquito-borne diseases such as dengue and malaria because of the difficulty of irradiating males without reducing their mating competitiveness and survival (Lees et al., 2015). In addition, the pupal stage of mosquitos is short and therefore they cannot be shipped long distances. Instead, mosquitos need to be treated and released locally. Global cooperation on the development of SIT to control mosquitoes intensified following the Zika epidemic in 2015–2016. A new technique, supported by the IAEA in cooperation with FAO, involves a combination of SIT and the incompatible insect technique18 to suppress mosquito populations. Successful findings have been published elsewhere (Zheng et al., 2019).
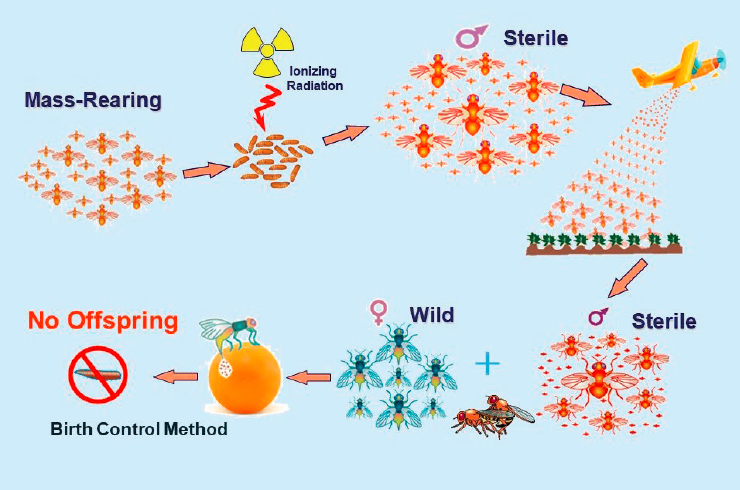
SOURCE: Rui Cardoso Pereira, IAEA, presentation to the committee on January 28, 2021.
___________________
18 In this method, released males are infected with the maternally inherited endosymbiotic bacteria Wolbachia, resulting in sterile matings with field females that are not infected with the same Wolbachia strain, a phenomenon known as cytoplasmic incompatibility.
For SIT, agametic (no production of gametes) sterility is not intended or used because it is important that spermatozoa are in fact produced. If sterile males were unable to produce sperm, competition in remating females would likely lead to fertile sperm winning over (nonexistent) sperm from sterile males. This would lead to most or all of the eggs from females that mate more than once being fertilized by unmodified sperm and therefore being viable, unless all of their mates are sterile (Alphey et al., 2006).
There are two main challenges with SIT: (a) radiation-induced performance decrease and (b) sex separation. For (a), radiation damage occurs to both somatic and germline cells, so the radiation dose required to sterilize also reduces the performance of the sterilized male insects, rendering them less potent (Parker and Mehta, 2007). To compensate for this loss of effectiveness, more sterilized insects need to be reared and released, which is more expensive (Alphey, 2016). For (b), for most pest insects, no practical means for large-scale sex separation is available, yet release of a male-only population is desirable for two reasons. First, females may damage fruit, even if they have been sterilized, thus directly causing some of the damage that the control program is intended to reduce. Second, if males and females are released together, the males may court the sterile females, and consequently not seek out the wild females as effectively as if they had been released without sterile females (Alphey et al., 2006). Sterile male-only releases of the Mediterranean fruit fly increased the effectiveness of population suppression by three- to five-fold versus male and female release during large-scale field trials (Rendón et al., 2004).
Gamma rays, x-rays, and e-beams function in a broadly similar way to achieve insect sterilization: ionizing radiation disrupts normal cellular function in pests by breaking chemical bonds within DNA and other biomolecules (Barkai-Golan and Follett, 2017; Follett, 2014; Hallman and Blackburn, 2016). This damage can be direct, as electrons are removed from biological molecules, such as DNA, RNA, or proteins, or indirect, from the free radicals formed during the ionization of water molecules within the biological systems.
As of February 2021, the IAEA’s Directory of SIT programs (DIR-SIT)19 listed more than 50 SIT programs in 26 countries, and an update was expected later in the year. Most SIT programs use cobalt-60 gamma rays. Gamma-based SIT can take place in sterilization facilities using panoramic irradiators or using smaller self-contained dry-storage irradiators; most of these facilities are dedicated facilities used for SIT purposes only.20 The largest program that uses gamma irradiation is in Guatemala and can produce 3 billion sterile males per week primarily for use in the United States (California and Florida), Guatemala, and Mexico. Most gamma facilities are much smaller and irradiate fewer than 200 million insects per week. One facility, in Spain, uses e-beam, and it can irradiate 500 million insects per week. There are about 10 x-ray irradiators in use, most of them for newly established mosquito programs. This is an increase from four x-ray irradiators 4 years ago. Analysis of the activity (cobalt-60) or power (e-beam and x-ray) of treatment facilities listed in the IAEA DIR-SIT database and the quantity of insects these facilities process (i.e., size of the program), demonstrates that alternative technologies could be used by any SIT program, irrespective of size.
5.4.1 Radioisotope Technologies
The standard technique for SIT uses gamma rays, sourced most commonly from cobalt-60. A few programs also use cesium-137. In conventional self-shielded irradiators, the sample chamber is surrounded by several rods or “pencils” of the radioisotope. The dose rate of the cell is determined by the activity of the source and the absorbed dose delivered to the insects is controlled by adjusting the exposure time. An IAEA representative who briefed the committee noted that use of cobalt-60 for SIT is a reliable method, but it faces two challenges.21 First, in 2008, Nordion discontinued production of its Gammacell 220, the source most commonly used for irradiating insects for sterilization purposes, raising concerns about future availability of small-scale irradiators that support a number of SIT projects. Today, at least two companies, Foss Therapy Services and the Institute of Isotopes Company, produce gamma-irradiation devices suitable for SIT. Foss Therapy Services also developed the procedures and tooling necessary to perform field reloads of Nordion’s Gammacell 220. Second, the growing logistical complexi-
___________________
19 See https://nucleus.iaea.org/sites/naipc/dirsit/SitePages/World-Wide%20Directory%20of%20SIT%20Facilities%20(DIR-SIT).aspx.
20 To the committee’s knowledge, the only program currently using a contract irradiation facility is the Spanish medfly program.
21 Rui Cardoso Pereira, IAEA, presentation to the committee on January 28, 2021.
ties of the shipment of radioisotopes across international borders are making the reloading of existing sources and purchasing of new ones increasingly difficult.
5.4.2 Alternative Technologies
X-rays (energy range around 150 kV to 250 kV) can be suitable replacements for radioactive sources for smaller SIT programs that irradiate fewer than 100 million insects per week. Testing of x-ray machines around 2008, at the Insect Pest Control Laboratory of the FAO/IAEA Agriculture and Biotechnology Laboratories in Seibersdorf, Austria, revealed issues with the reliability of the x-ray tube (IAEA, 2012a). The newer, second-generation x-ray tube (see discussion in Section 4.1.2) has not been tested by the laboratory. According to one expert, it is likely that the improvements seen in blood irradiation using the second-generation x-ray tube would also be reflected in SIT, because the principle of irradiation for the two applications is similar.22 There is little field experience available to conclude about the reliability of the recently deployed x-ray machines. However, it is generally thought that reliability remains an issue, and x-ray machines require frequent maintenance and service. As noted in Chapter 4, Stellarray’s Flat Panel X-ray Source is also designed for SIT applications (see Figure 5.5). This new technology could increase throughput and improve dose distribution, making x-ray technology an appropriate alternative to gamma-based SIT.
Even though high-energy (5 to 10 MeV) electrons can be used to sterilize insects, they are not a suitable alternative for most SIT programs, which tend to be small, because of the high cost and large size of an e-beam facility. RadiaBeam Systems, a small business funded by NNSA through the Small Business Innovation Research program, is building an inexpensive, compact 3-MeV linac as the radiation source for a self-contained irradiator for SIT and other applications. The company’s goal is to produce a more compact, lower-cost linac that can function in environments with unstable electrical power. The company is currently building a prototype that plans to test at the IAEA Insect Pest Control Laboratory. At least two other companies, Mevex and Nuctech (China), also aim to produce similar compact accelerator systems for SIT.
Genetic Methods
Modern genetic methods are an alternative modality for SIT. These methods are typically divided into two distinct categories: (a) population suppression, containment, or eradication; and (b) population transformation or replacement. The first category has the same goals as SIT and is the only one discussed in this section. The second category has the goal to reduce or block the insect’s ability to transmit a disease, avoiding the emergence of an empty ecological niche. Because the goal of this genetic method is different from that of SIT, it is not discussed here.
Population suppression, containment, or eradication aims to reduce or even eliminate specific insect species by developing genes that are (conditionally) lethal or that make the insect unable to reproduce. A variety of systems
SOURCE: Courtesy of Stellarray, Inc.
___________________
22 Communication between Andrew Parker, IAEA (retired), and Ourania Kosti, National Academies, on February 19, 2021.
and possible genes are being researched for these purposes. One system, referred to as Release of Insects carrying a Dominant Lethal (RIDL), works by transmitting a transgene combination causing dominant embryo-specific lethality in the offspring (Alphey et al., 2006). Potent sterile insects are created, with the ability of the sterile males to produce and transfer competitive sperm. This dominant lethality is suppressible by addition of tetracycline in the larval diet, thereby enabling rearing of such strains. This allows the generation of competitive sterile insects that can transfer competitive sperm carrying the transgene to wild females. The embryos produced by the females carry the dominant transgene and, in the absence of the tetracycline additive, the embryos die (Schetelig et al., 2007). The RIDL biotechnology was developed by Oxitec (a spinoff from Oxford University) and adopted to tackle dengue fever disease. Oxitec scientists genetically modified the Aedes aegypti, a vector for dengue fever disease, creating the patented RIDL product of the strain A. aegypti OX513A. Despite controversy regarding the approach (Gene Watch UK, 2012), field trials have taken place in the Cayman Islands, Malaysia, and Brazil (Servick, 2016). A pilot program will release 750 million genetically modified mosquitoes into the Florida Keys in 2021 (Wilcox, 2021).
Sex selection (genetic sexing) has been achieved through a similar dominant, tetracycline-repressible genetic lethal system that works by killing individuals (females) that carry the lethal system, unless it is switched off by the tetracycline repressor (Thomas et al., 2000). Laboratory studies for sex selection using this technique, including one with New World screwworms, have been successful. In addition, coding for a marker gene containing fluorescent proteins has been added. This allows the transgenic insect to be readily visible from the wild type. The lead strain in this study carries a gene insertion that kills females very efficiently if present in two copies, and the strain is raised without the repressor. If the strain is raised without the repressor, only the males survive (Concha et al., 2016).
Employment of the repressible lethal systems also has the potential to effect genetic containment. Presently, mass rearing facilities for SIT raise large numbers of pest insects, which become beneficial only once sterilized. A large-scale release of these insects before sterilization, or of insects not irradiated to the proper level, could lead to large economic losses. This could be mitigated by use of the repressible lethal genetic system, because the insects are provided the repressor chemical only in the rearing facility (Alphey et al., 2006).
5.4.3 Alternative Technology Adoption Considerations
Migration from gamma irradiation to other modalities for SIT is needed because of the challenges with acquiring and transporting radioactive sources for insect sterilization, as described in Section 5.4.1. More than 10 years ago experts had projected that for these reasons the era of the small-scale gamma irradiators for SIT programs is getting close to an end and these irradiators would be replaced by x-ray machines (Mastrangelo et al., 2010). Although this has yet to happen, the committee concludes that it is technically feasible to use e-beam or x-ray for SIT. However, full adoption of these technologies in large SIT programs will require improvements in terms of reliability, cost, and dose uniformity.
It is likely that most panoramic irradiators will continue to operate and provide services for large SIT programs in the foreseeable future. However, the growing interest and demand for the development and application of SIT against mosquito vectors will likely increase demand for further developing x-ray sources that can be used by smaller local SIT programs.
The use of genetically modified insects for pest control is a complex topic that has sparked intense public debate. In the context of genetic engineering of mosquitoes for disease control, studies found that about 60 to 70 percent of adults in the United States favored releases of modified mosquitoes (Funk and Hefferon, 2018; Winneg et al., 2018). In 2016, the Florida Keys Mosquito Control District conducted a nonbinding referendum among Monroe County residents as part of a proposed trial release of a genetically engineered A. aegypti mosquito developed by Oxitec. Fifty-seven percent of the residents voted in favor of the trial, but 65 percent of residents of the suburb where the release was to occur were opposed (Servick, 2016). Several recurring comments on the topic included genetically modified mosquitos may carry new pathogens harmful to humans and animals, a new type of mosquito will be added to the environment, and there are limited test results documented. It is likely that in order to build public trust and acceptance of the use of genetically modified mosquitos, all of these concerns will need to be addressed by public authorities, regulatory agencies, and elected officials.
5.5 CHAPTER 5 FINDING
Finding 13: A progressive transition to alternative technologies is taking place in sterilization applications. Utilization of e-beam technologies in medical device sterilization has increased during the past 10–15 years, both domestically and internationally, and it is expected to continue to increase to meet growing demand for this application. Several companies have also announced plans to open new x-ray sterilization facilities. Alternative technologies for other sterilization applications, including food irradiation for safety and phytosanitary treatments and insect sterilization, are also increasingly accepted as viable replacements for radioactive sources in many countries.
The U.S. medical device sterilization market is growing about 5 to 7 percent annually. The current prevalence of sterilization modalities used by the medical industry is 50 percent EtO gas fumigation, 40 percent cobalt-60 radiation, 10 percent e-beam irradiation, and less than 1 percent other (including steam and x-ray irradiation). These methods are not necessarily a direct replacement for one another. Use of e-beam in sterilization has increased over the past decade at a rapid pace and is expected to continue to increase due to pressure to replace cobalt-60 irradiation or EtO gas fumigation but also due to improvements in accelerator technology. From 2005 to 2015, on average 4 e-beam systems per year were installed, and from 2016 to 2019, the number increased to about 12 systems per year. Based on different projection scenarios, an additional 200–400 e-beam systems could be installed over the next 10 years. X-ray sterilization systems are commercially available, but are in limited use. At least two companies have announced plans to open new x-ray sterilization facilities.
Despite the public perception and other challenges with food irradiation for food safety and phytosanitary purposes in the United States and Europe, alternative technologies are being increasingly adopted elsewhere. The biggest investor in the world by far on food irradiation is China. The country is investing in e-beam for food irradiation for safety purposes with 5 to 10 new machines being built each year during the past 5 years. Although the incidence of foodborne disease disproportionally affects populations in LMICs, it is unlikely that food irradiation will be implemented in many of these countries. For example, in Africa, except Egypt and South Africa, the lack of resources and basic infrastructure cannot support food irradiation technologies at the scale necessary to be effective.
X-ray technologies are increasingly accepted alternatives to gamma irradiation for SIT, with multiple projects around the world adopting the technology to control regional mosquito populations.
This page intentionally left blank.


















