Proceedings of a Workshop
| IN BRIEF | |
|
April 2021 |
LEARNING FROM RAPID RESPONSE, INNOVATION, AND ADAPTATION TO THE COVID-19 CRISIS
Proceedings of a Workshop—in Brief
The world continues to grapple with the profound and unprecedented impacts of the COVID-19 pandemic, which by January 2021 had infected more than 90 million people worldwide and taken over 1.9 million lives.1 The crisis quickly mobilized action by universities, industry, and federal, state, and local governments to organize resources and networks; instigate new partnerships; adapt to changing and uncertain circumstances; and innovate solutions to mounting public health and economic challenges. The crisis has also catalyzed broader conversations about the pace of science, the agility of scientific collaboration and partnership, the importance of international scientific coordination, and the significance of the public value of science.
The Government-University Industry Research Roundtable (GUIRR) hosted a virtual workshop on October 14–16, 2020, to consider lessons learned to date about rapid response, innovation, and adaption related to COVID-19, more than 6 months into the global pandemic. The panels, discussions, and breakout sessions connected stakeholders to share how institutions have overcome barriers to accelerating research within and beyond the science community; fostered unprecedented collaboration; and tested the responsiveness and resilience of the research enterprise. The workshop built on a series of workshops held between May and August to consider the emerging challenges of the pandemic.2 While the long-term impacts of the pandemic on the agility and productivity of the research enterprise are not certain at this juncture, early and continuous reflection on these broad questions by a diverse array of stakeholders is important to future investigative efforts.
GUIRR co-chairs Laurie Leshin (Worcester Polytechnic Institute) and Al Grasso (former president, MITRE Corporation) moderated panels around four themes: (1) Addressing inequities of the crisis; (2) Informing future emergency preparedness efforts; (3) Crisis-driven collaborations across government, universities, and industry; and (4) Adaptations to federal funding and research regulation. A keynote address by Christopher Austin (National Institutes of Health [NIH]) reinforced the importance of cross-sector partnerships that GUIRR exemplifies to accelerate translational research during the pandemic. Breakout sessions on the final day of the workshop allowed for more informal discussion.
This proceedings in–brief, prepared by a rapporteur, summarizes the workshop. It is not intended to present consensus recommendations or conclusions by GUIRR or the participants.
KEYNOTE: CATALYZING TRANSLATIONAL INNOVATION IN THE AGE OF COVID-19
According to Christopher Austin, the National Center for Advancing Translational Sciences (NCATS) views its response to COVID-19 through a similar lens as GUIRR: that is, taking advantage of the “triple helix” of government, universities, and industry. According to Austin, the crisis has not only brought out lesions in the system—it has also shown that the system can adapt and perform beyond what was thought possible. “The bottom line is this experience makes me more optimistic that the triple helix can make the medical translation process work better than it does,” he said. “Although
__________________
1 World Health Organization COVID-19 Dashboard: https://covid19.who.int/.
2 National Academies of Sciences, Engineering, and Medicine. 2020. Resilience of the Research Enterprise During the COVID-19 Crisis: Proceedings of a Workshop Series–in Brief. Washington, DC: The National Academies Press. https://doi.org/10.17226/26014.
![]()
COVID has been an enormous tragedy, there is important learning to take to heart.”
Using slides prepared by his NIH colleague Anthony Fauci, Director of the National Institute of Allergy and Infectious Disease, Austin first presented a scientific overview of SARS-CoV-2, noting it is one of many coronaviruses that are mostly common and mild. Others were severe but died out before they became global pandemics. In contrast, SARS-CoV-2 has infected millions of people and has many clinical presentations, levels of severity, and methods of transmission. Unlike other viruses, it infects respiratory, vascular, and gastro-intestinal cells. Long-term effects may occur after the infection is gone. Age and underlying medical conditions put people at increased risk.
As underscored throughout the workshop, Austin noted the dramatic disparities in incidence and death rates across racial and ethnic populations. Possible reasons include that Black and Latinx populations are more likely to be employed as frontline workers who are exposed. Other reasons are related to social and structural determinants of health. Racial and ethnic minority populations are more likely to live in densely crowded (by neighborhood and household) conditions, and these populations have a disproportionate burden of underlying health conditions, including cardiovascular disease, morbid obesity, and kidney and liver disease.3
He highlighted the status of investigational therapies and vaccines. Focusing on NCATS, he said the Center works to move, or translate, every step of the process between lab findings and medical practice “better, faster, and cheaper.” Many NCATS platforms were concentrating on opioids as part of NIH’s Helping to End Addiction Long-Term (HEAL) initiative. Although opioid addiction remains a problem (indeed, worsened by the pandemic, he noted), many of these labs had to close and NCATS made a major pivot to COVID-19.4
In highlighting NIH efforts, he stressed the principles behind them related to urgency and collaboration. “I would argue they are the same principles we ought to apply to every single disease,” he said. “There is a scientific, medical, and moral obligation to do the same for cancer patients, or people with diabetes, schizophrenia, rare diseases, or anything else…. This is a moment in time to ask ourselves, ‘Are we going to do things differently or not, now that we have shown it can be done?’”
NCATS set up an Open Data Portal to overcome the problem that data are difficult to share quickly.5 Researchers are sharing negative and positive data–all the “secret sauce.” Clinicians are often reluctant to share experience in real time, but NCATS and the Food and Drug Administration (FDA) created a mobile app called CURE ID to allow health care workers to contribute knowledge and learn from others. It was launched on December 5, 2019, for rare tropical diseases. Less than a month later, the first COVID-19 case was diagnosed and added to the app. It has provided critical information, especially in the early days of the disease when there were no systematic data, and it will remain useful for other diseases. Another related effort that pivoted to COVID-19 is the Critical Path Institute (C-Path). Through this collaboration, housed at the University of Arizona, clinicians identify existing drugs to treat infectious diseases.6
A serosurvey conducted by NCATS and three other NIH Institutes is evaluating healthy people exposed to the infection, which will assist with decisions about opening the economy and with investigations of immunity and correlates of prevention. He stressed that no one institute could have done the survey alone.
NIH’s single biggest program is the Clinical and Translational Science Awards (CTSA) program, involving 60 large academic health centers and 100 affiliates. Two CTSA-funded trials, at New York University and Vanderbilt, are using plasma from recovered patients (i.e., convalescent plasma) to investigate whether antibodies protect from and treat the illness. One challenge in designing the trials is what he called a “clinical whack-a-mole” because the virus is not geographically stable. It illustrates the need for standing national capacity to do studies quickly, he said.
COVID-19 has allowed for making clinical data quicker and easier to mine. The Center for Data to Health (CD2H) is now linking patient data in a secure way. “That was a dream back in April. It is now a reality,” he said. As of mid-October, it contained data from almost 1 million patients (120,000 of whom had COVID-19); 600 million lab results; and 1 billion rows of data. Additional sites are offering their data, which will grow the database even further. “This will be a research translational engine of unmatched scale,” he said. “It could be done because we had exemptions to data-sharing under the health emergency pandemic declaration by HHS.” He expressed hope that it will exhibit so much value, policies limiting shared data for cancer and other diseases will be overcome.
Austin concluded with comments about the public-private partnership launched in April through the Foundation for the National Institutes of Health (FNIH) to develop a coordinated, fast-track research response.7 Called the
__________________
3 See https://jamanetwork.com/journals/jama/fullarticle/2766098.
4 See https://ncats.nih.gov/covid19-translational-approach. For more discussion about the HEAL initiative and the compounding impacts of the pandemic on the opioid crisis, see discussion with Nora Volkow, director of the National Institute on Drug Abuse: https://heal.nih.gov/news/news/2020-rx-summit.
5 See https://ncats.nih.gov/expertise/covid19-open-data-portal.
6 See https://c-path.org/c-path-launches-cure-drug-repurposing-collaboratory-to-accelerate-identification-of-new-uses-of-existing-drugs-to-treat-infectious-diseases-including-covid-19/.
7 See https://www.nih.gov/research-training/medical-research-initiatives/activ.
Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV), it has been able to prioritize drugs under a master protocol, initiate clinical trials, and ensure alignment across efforts.
Looking across NIH and its partners, Austin extracted lessons he has learned. First, he said, it is possible to go from fundamental discovery to therapeutics and vaccines much more quickly than has historically occurred. Corners have not been cut, he stressed. “What’s different is that inefficiency, duplication, time lags, and lack of sharing was wrung out of the system,” he said. While he recognized that the extent and pace is not maintainable for all diseases, important elements include the sense of urgency by all involved; a recalculation of benefits and risks based on the urgency; willingness to share based on that recalculation; and unprecedented proactive collaboration.
Second, Austin noted the higher return on investment for all, even with the sharing of credit and profits. But he warned that many conditions, regulatory and policy exemptions, and additional funding will likely not continue without proactive steps by all participants. “What levers do we pull to make this the new normal?” he asked. “That’s what patients deserve. That’s what the science will allow.”
In discussion after Austin’s remarks, Grasso concurred with Austin about the need to sustain partnerships and collaboration, and asked participants how to maintain the “risk appetite.” A participant asked how NIH will study the long-term impact of COVID-19. Austin said he expects NIH leadership will turn to this issue, but NCATS in particular is codifying what it has done to date. The pandemic also showed the challenges of relying on large academic health centers for clinical trial recruitment, given that much of the successful recruitment into COVID-19 trials came from community hospitals. The issues of health disparities have been magnified as well, Austin added, noting that he sees an NIH-wide commitment to utilizing the COVID-19 learnings as a catalyst for change. Finally, he expressed hope that this experience will enable others to appreciate the value of translational science.
ADDRESSING INEQUITIES OF THE COVID CRISIS
The first workshop panel addressed how the public health community is working to advance solutions and build trust to confront the disproportionate impact of COVID-19 on racial and ethnic communities hardest hit by the pandemic.
Leana Wen (George Washington University) emphasized that COVID-19 revealed underlying, long-standing disparities through disproportionate death rates and hospitalization rates by race and ethnicity among adults and children. Reasons include underlying medical conditions and living conditions. “Social distancing is a privilege that many people do not have,” she said. Access to healthy food is inequitable: Wen noted that in Baltimore, where she served as health commissioner, one in three Black people live in a “food desert,” versus one in 12 white people. She explained that poor diet can lead to diabetes, heart issues, and other medical concerns, which in turn can make COVID-19 more serious. While Wen said that systemic racism must be addressed over the long-term, she suggested 8 solutions now:
- Get data, because otherwise “we are flying blind.” A statewide average may miss important information in specific communities.
- Target testing to different populations. For example, drive-in testing is not accessible to many people. Mobile or walk-in testing, or partnerships with churches, may be better options.
- Hire contact tracers from within communities. They can also become part of the public health workforce.
- Set up isolation and quarantine facilities for people who cannot quarantine at home. This underscores that housing and food access are health issues.
- Institute worker protections. There needs to be enough personal protective equipment (PPE) for clerks, drivers, and others. Protections for social distancing are also needed.
- Ensure health insurance coverage, including for long-term consequences of COVID-19.
- Fund schools. While re-opening cannot be rushed, one consequence of school closures has been widening educational disparities.
- Support local public health systems that are on the front lines.
In the discussion that followed, Wen was asked how disparities might manifest when a vaccine is available, in terms of distribution and acceptance. She distinguished between three groups who seem resistant to vaccines: those already opposed to vaccinations, those newly skeptical based on political pressures, and, to the point of the question, communities of color, where distrust of medical research and institutions is rooted in centuries of systemic racism and disparities in care. “We need public education using trusted members of the community,” she suggested. “We also need a framework for equitable distribution.” She referred to the National Academies’ framework proposed for equitable allocation of a vaccine as a good starting point.8 She commended the presidents of two Historically Black Colleges
__________________
and Universities (HBCUs) who had offered support from their institutions in the search for a vaccine. “They put their own credibility and capital on the line,” she said.
Wen agreed that the public’s trust in scientific information about COVID-19 seems to have shifted, as the scientific understanding of the virus—and particularly the environments that enable its spread—has changed since the beginning of the pandemic. She urged the need to enlist credible messengers to stress that re-evaluation and change is a good public health response. Many people want to do the right thing but do not know how, she said, and they need simple tools and better messaging.
Equitable solutions were the thread of several comments. One participant asked about how the United States can help countries with fewer resources. Wen noted that an infection somewhere is an infection everywhere, but also argued that the United States is not in a position to help other countries because it does not have the disease under control. Finally, a participant noted the disproportionate impact of remote work on female scientists. Wen agreed with the pressure on women, and Laurie Leshin pointed out that institutions must build support structures and remove barriers.
George Mensah (National Heart, Lung, and Blood Institute, NIH) focused his presentation on NIH’s response to the mounting evidence of disparities in the burden of COVID-19, and implications for future pandemics and public health. He shared an infographic created by the Centers for Disease Control and Prevention (CDC) that serves not only to explain potential sources of infection and ways to reduce those risks, but also the number of cases, hospitalizations, and deaths by racial and ethnic group (see Figure 1).
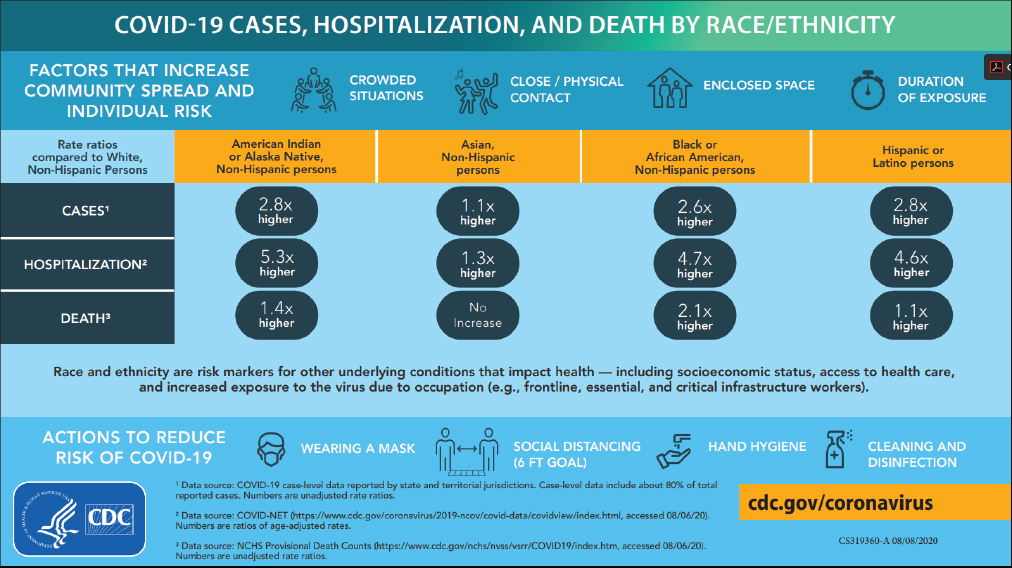
Source: George Mensah, NIH. Presentation at the workshop of the Government-University-Industry Research Roundtable on “Learning from Rapid Response, Innovation, and Adaptation to the COVID Crisis,” held October 14, 2020.
One of NIH’s five strategic priorities related to COVID-19 research is to “prevent and redress poor COVID-19 outcomes in health disparity and vulnerable populations.”9 Mensah summarized three programs: Rapid Acceleration of Diagnostics (RADx-UP), to accelerate diagnostics in underserved populations; Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV), to accelerate COVID-19 drug and vaccine development with safety, speed, and diverse and inclusive participation; and the Community Engagement Alliance (CEAL) Against COVID-19, which supports urgent community engagement and outreach to address misinformation and distrust in communities that have been hit hardest by the pandemic. He noted that all three, as well as other NIH efforts, are characterized by rapid and adaptive response, and inclusive participation.
__________________
According to Mensah, the response to COVID-19 has broader implications for clinical and public health practice and research. He suggested the following areas of impact:
- Attention to social determinants of health is crucial for reducing disparities in COVID-19 and other diseases and conditions.
- Active community engagement is needed to build and sustain trusting relationships with the hardest-hit, underserved communities.
- In all actions to address disparities, it is necessary to “move at the speed of trust.”
- Trusted voices and messengers at the national and local levels are important resources.
- Innovative and strategic public-private partnerships are critical to accelerate the development of drugs, vaccines, and other effective interventions.
Mensah was asked about how to balance a rapid response with the need to “move at the speed of trust.” He suggested regular, iterative communication across government agencies, voluntary health organizations, research institutions, and community-based organizations to eliminate duplication and improve coordination. He also commented that while it is necessary to use established resources now for expediency, it is necessary in the long-term to build and tap into new networks. A linkage between the National Virtual Biotech Lab run by the Department of Energy and NIH was suggested—Mensah agreed that such a linkage would support NIH’s strategic plan for COVID-19 research, specifically its priority to support related to basic science and improve fundamental knowledge about the disease.
Chris Lyttle (Robert Woods Johnson Foundation [RWJF]) explained RWJF’s Systems for Action (S4A) program, housed at the Colorado School for Public Health. It developed to identify novel mechanisms for aligning delivery and financing systems in medical care, public health, and social and community services, with the recognition that the connection between public health and personal medical care is often fragmented by inefficiencies and inequitable outcomes because of a lack of social services and support. As Lyttle noted, the pandemic has exacerbated the need for critical social and medical needs, while also creating upstream barriers to healthy living, and downstream health consequences.
The S4A program is testing novel mechanisms for aligned systems and services across sectors to learn from and move beyond the disconnections. Mechanisms being tested include new alliances and partnerships; intergovernmental and public-private ventures; new financing and payment arrangements; incentives for individuals, organizations, and communities; cross-sector governance and decision-making structures; information exchange and decision support; new technology; community engagement, public values, and preferences; and new workforce and staffing models.
Projects supported by the program rely on networks of partners and collaborators from across sectors and disciplines—throughout research, health, wellness, education, social services, and insurance communities, and involving academia and state and municipal governments. Lyttle shared two projects, including a study in Indiana that integrated electronic health records with other data to improve population and clinical health; and a comprehensive care program in Chicago that proactively monitors seniors. Lyttle noted that additional funded studies will be announced in the coming weeks, with a specific aim to measure health equity outcomes.
INFORMING FUTURE EMERGENCY PREPAREDNESS EFFORTS
The second panel addressed existing and newly formed networks to address the pandemic and future emergencies. Three presenters discussed what they have learned about guiding agile emergency response networks at the national, state, and local levels, and what gaps remain for future challenges.
To provide context for the state of research and practice on public health emergency response before the pandemic, David Abramson (New York University) presented the work of the National Academies’ Committee on Evidence-Based Practices for Public Health Emergency Preparedness and Response (PHEPR).10 He clarified that he would present the committee’s findings and add his own comments, but that they should not be considered as representing the views of the committee. He focused on one of the charges that CDC, the study sponsor, requested the committee to address: to provide recommendations for future research to address critical gaps and improve evidence, as well as processes needed to improve the overall quality of evidence within the field.
The committee stressed the need for a systems perspective to achieve optimal public health outcomes related
__________________
to preparedness and mitigation, as well as to response and recovery after a disaster. Abramson noted that an amalgam of public and private entities must be coordinated during a disaster, and ideally the connections and relationships are built beforehand to create an infrastructure for response.
In reviewing the PHEPR research base, it became clear to the committee that there is little research in many critical areas, especially at a systems level. Most data come from simulations and training, and not actual disasters. He recognized the challenges in assembling data during an emergency, when so many other things are going on. The government has supported a few attempts to stimulate and coordinate PHEPR research, but has not sustained them. Among the current coordinated efforts is Disaster Research Response (DR2), funded by the National Institute of Environmental Health Science and the National Library of Medicine.11
Among the challenges identified by the committee: (1) Identifying and prioritizing the research needs to avoid duplication and gaps; (2) Research process challenges, particularly the need for rapid funding and access to impacted areas; (3) Infrastructure and logistics challenges; (4) Coordinating and managing relationships—especially during chaotic period of response and recovery (see Figure 2).
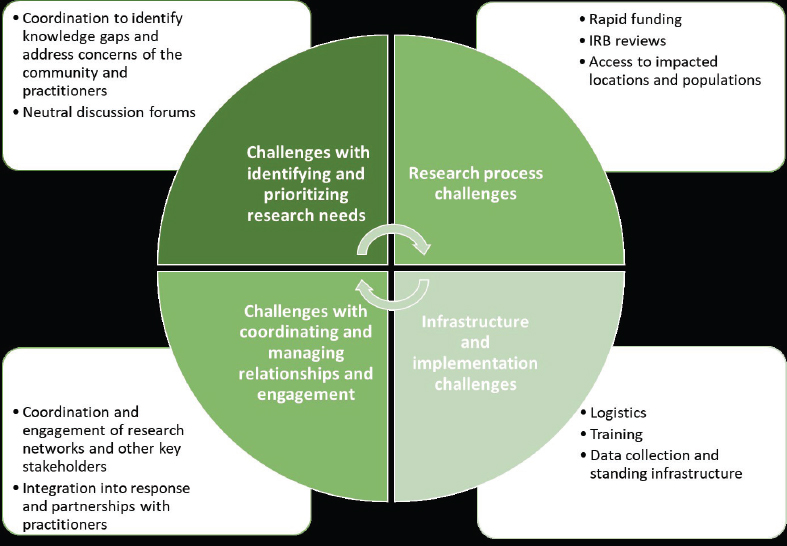
Source: David Abramson, New York University. Presentation at the workshop of the Government-University-Industry Research Roundtable on “Learning from Rapid Response, Innovation, and Adaptation to the COVID Crisis,” held October 14, 2020. See National Academies of Sciences, Engineering, and Medicine. 2020. Evidence-Based Practice for Public Health Emergency Preparedness and Response. Washington, DC: The National Academies Press. https://doi.org/10.17226/25650.
Overall, he said, the committee concluded that the evidence base underlying the nation’s response to public health emergencies is seriously deficient, which hampers effective responses. The committee offered recommendations for the development of a federally-supported public health disaster science framework, which could be used to produce generalizable findings to help inform the PHEPR field. Such coordination could include the development of a common research agenda across federal agencies and a more robust and accelerated funding mechanism for collecting and implementing “lessons learned” from disaster responses. The Extreme Events Research networks funded by the National Science Foundation (NSF) in the social sciences and engineering serve as a potential model.
The committee began its work before COVID-19, Abramson noted, but it became clear as they finished that the pandemic reinforced the need for a better public health emergency evidence base. Stable funding, robust design and conduct of research studies, development of the research workforce, and collaboration between public health practitioners and researchers are all vital, as COVID-19 has shown.
With speed so essential after a disaster, a participant asked how to put a response system in place. Abramson noted a 2014 conference jointly sponsored by NIH and the Institute of Medicine that had looked at standing up a Rapid
__________________
Response Research infrastructure.12 Rapid response teams could develop pre-approved Institutional Review Board (IRB) agreements, for example. In his own work, he and colleagues try to get into the field as soon as possible and hope get funding down the road. He suggested the National Science Foundation’s rapid response grants as a template.13
A participant asked whether lessons from the response to COVID-19 will help prepare for the next emergency. Abramson opined that although the federal response has been handicapped, many state and local responses have been optimized. From a research point of view, he said, much will be learned from the variation of their responses. Abramson was also asked how the committee thought about educating the next generation of public health professionals to meet this need. He said some schools of public health have developed competencies around emergency preparedness, which CDC funded. Without stable funding, however, scholarship and educational programming are hard to sustain. He noted that NYU and several other institutions offer disaster certificates, but there is not yet a robust network.
Carolyn Kirk (Massachusetts Technology Collaborative, or MassTech) described the response to meet PPE needs in Massachusetts. MassTech is a quasi-public economic development agency for the Commonwealth of Massachusetts, which works collaboratively with government, industry, and academic stakeholders. The agency has an innovation institute, a cyber center, an e-Health institute, a broadband institute, and—most relevant for Kirk’s presentation to GUIRR—a center for advanced manufacturing. The agency also leads the state’s advanced manufacturing collaborative, an effort to spur engagement between the public and private sector efforts around manufacturing. Thus, the infrastructure to support a response to the state’s PPE challenge was in place when COVID-19 struck.
Kirk recalled the early challenges of the pandemic – supply chains for PPE were drying up. Through the work of MassTech and its partners, Massachusetts turned to its own manufacturers to pivot to PPE production. The Manufacturing Emergency Response Team (MERT) was created to identify manufacturers that could produce the needed materials, a group made up of government leaders, R&D Centers, academic experts, and manufacturers. The governor declared a state of emergency on March 10. Within 19 days, MERT had set up a portal for manufacturers to “declare their intent” to produce PPE. Within 30 days, many manufacturers were ready with orders to fill. Within 80 days, a PPE testing infrastructure was set up. And on Day 90, Massachusetts Governor Charlie Baker visited a factory as the one millionth face mask rolled off the production line.
The MERT effort was aligned with the governor’s command center, which identified which PPE was in short supply, providing critical real-time intelligence to the MERT. Almost 1,000 manufacturers came through the portal. A triage process took place to identify those that could pivot at scale quickly. Manufacturers were put through what the MERT called “the gauntlet” to figure out which product they could produce quickly based on their existing capabilities; for example, apparel manufacturers were making medical products for the first time. “The right experts showed up on Day 1,” Ms. Kirk added, which was critical to success, such as experts in testing or FDA approvals. As of Kirk’s presentation in October 2020, about 50 companies had made and placed in the market more than 12 million pieces of PPE since March.
The state set up a $16-million grant program for much-needed capital investment. Also important was the PPE testing infrastructure in collaboration with several local labs, because the usual labs for FDA certification were backed up. The testing also revealed that many products from other countries, such as K95 masks, did not perform as labeled.
An article in the New England Journal of Medicine, submitted by one of MERT’s medical advisors Dr. Mark Zeidel of Beth Israel Lahey, described how the state opened up these supply chains.14 In addition, MassTech and several partners received an NSF grant to codify the experience for future emergency responses.15
When asked about surprises along the way, Kirk said the first was the lack of a federal response. “We felt we were completely on our own. We didn’t lament it, we just networked and did what we had to do,” she said. “But looking back, if we didn’t have the university partners, the manufacturing base, the innovation culture, we would not have been able to do what we did.” How to do this on a larger scale was discussed. Kirk has fielded questions from other states—she said she now pushes Massachusetts manufacturers out to these other states to expand their customer base. This may address one of the challenges of sustainability since by pivoting to PPE, the companies have forsaken their usual customers and business lines.
In the final presentation of this panel, Seth Marder (Georgia Tech) presented a proposed framework for what he is calling a Network of Agile Science and Engineering Centers (NASEC). He noted the idea stems from his experience putting together interdisciplinary teams and taking a systems-level view of challenges, and was inspired by his observations of institutions’ emergency responses to the COVID crisis. He said many points raised by Abramson and Kirk point
__________________
12 Institute of Medicine. 2015. Enabling Rapid and Sustainable Public Health Research During Disasters: Summary of a Joint Workshop by the Institute of Medicine and the U.S. Department of Health and Human Services. Washington, DC: The National Academies Press. https://doi.org/10.17226/18967.
13 See https://www.nsf.gov/pubs/2009/nsf09034/nsf09034.jsp.
14 See https://www.nejm.org/doi/full/10.1056/NEJMc2009432.
15 See https://www.eurekalert.org/pub_releases/2020-10/uoml-ma100220.php.
to the value of an entity like NASEC, which could be led by academic institutions and involve other entities, and could prevent disjointed and inefficient responses to future disasters.
As context, Marder commented that natural disasters, including earthquakes, tsunamis, storms, and fires, and human-made disasters, including chemical, biological, and cyber terrorist attacks, require a concerted and intelligent response to mitigate, and perhaps offset, the negative impacts on society. A significant number and wide variety of natural disasters (e.g., floods, fires) occur every year, scattered across the country. Localized, often ad hoc and spontaneous networks respond. While this response has mitigated the severity of the current crisis, COVID-19 also points out the vulnerabilities related to supply chain, robust national-level testing, communications, and other needs. He noted that the premise of the NASEC concept recognized that each crisis is different, but all share underlying needs for effective policy, clear communication, technical expertise, organized coordination, and rapid mobilization of resources.
Under his concept, NASEC would have teams trained and in place, he explained, with a workforce that is multidisciplinary, agile, and with a strong sense of service to the nation. Each NASEC center would have a core expertise, such as earthquakes or cybersecurity. In addition to core scientific and engineering capabilities, each center would have expertise in policy, project management, and other skills. Working groups would plan, develop best practices, create databases, and identify new opportunities. The network would have created the ties among them before disasters arise and could take up the slack for each other if a disaster impacts one location.
Although he has developed preliminary ideas about implementation, timelines, and budgets, Marder said what is important at this point is to put out the idea for discussion. A participant noted the existence of other connecting groups, such as Centers of Excellence. Marder said NASEC centers could work across domains with a larger-scale view. Training for domain experts in regulatory constraints from the start is also essential, he added.
Kirk noted that what resonated with her about Marder’s concept was in her interactions with the Federal Emergency Management Agency (FEMA), she was told they did not have confidence around a disease crisis. A layer of confidence and expertise to deal with different types of crises is important, she said. Abramson commented about what he sees as a failure of crisis management at the federal level. Better awareness and training are needed, he said. A participant asked about changes needed in funding mechanisms, peer review, and academic culture to accomplish intersectional aims. Abramson said currently, few institutes at NIH support intersectional work. He suggested more systems building and rapid funding mechanisms.
PARTNERSHIPS AND COLLABORATIONS IN RESPONSE TO THE COVID-19 CRISIS
The first part of this session consisted of a review of several programmatic partnerships enabled through the COVID-19 Healthcare Coalition. It was followed by three presentations about other partnerships and collaborations formulated in response to the pandemic: between private companies across sectors; between universities and industry; and between the private sector; university alliances, and state governments.
COVID-19 Healthcare Coalition
Jay Schnitzer (MITRE) described the coalition, which he co-leads with John Halamka (Mayo Clinic Platform).16 As the first COVID-19 cases were reported, the private sector realized the need to lessen the clinical impact of the virus and enable health care delivery systems to function. The COVID-19 Healthcare Coalition was formed with five guiding principles: (1) everyone participates for the benefit of the country rather than individual company; (2) open sharing of plans; (3) no money exchanged; (4) verbal agreements and minimal bureaucracy; and (5) agreement to these terms allow for entry. More than 1,000 companies, large and small, have joined. MITRE operates the coalition on behalf of the private sector. Liv Blackmon (MITRE) moderated a series of short presentations from coalition members about the three focus areas: clinical care outcomes, critical supplies, and public guidance and policy.
John Halamka highlighted how quickly the coalition formed and dealt with clinical issues. For example, as convalescent plasma was identified as a potential treatment, it became important to involve blood banks, community organizations, cloud-based platforms, and researchers. “The Fight Is in Us” became the umbrella campaign under which this took place. Brian Anderson (MITRE) continued by explaining how “The Fight Is in Us” campaign engaged with the government, particularly Operation Warp Speed (OWS), to target people who had recovered from COVID-19 and might donate blood. Celebrities assisted as trusted spokespeople. Organizing principles for this public-private partnership were engagement, evidence, and equity, which, he noted, apply to other areas of the coalition’s work. Katy Warren (MITRE) explained the outreach effort. A website provides information and resources, with the goal to encour-
__________________
16 For more information, see https://c19hcc.org.
age people to donate.17 The site leverages the Microsoft Azure Health bot to prequalify potential donors and inform them of nearby donation centers.18 No personal data are collected. Working with OWS, they targeted 14 high-interest communities. These now have location-specific landing pages.
Moving to other coalition work, Kunal Rambhia (MITRE) discussed wastewater-based epidemiology (WBE), or surveillance, as a cost-effective, early-warning indicator for COVID-19, and one that can be repurposed to detect other diseases or substances. WBE is part of a broader response, he stressed. Researchers already working on WBE focused on SARS-CoV-2, and it has been used successfully in group and congregate settings. The field is quickly maturing to advance methods from sample collection and processing to data analysis and decision making. Knowledge-sharing and partnerships are essential to incorporate WBE into routine disease surveillance. One collaborator, Rolf Halden (Arizona State University), highlighted the field’s U.S. milestones since 2004, leading up to work on SARS-CoV-2. As an example, data revealed through WBE pointed out Guadalupe, a community of 6,000 mostly Latinx and Native Americans within Tempe, Arizona, was a COVID-19 hotspot. This allowed for a focused response on Guadalupe that helped suppress infections. He stressed the importance of high-quality sample collection, and of continuous rather than grab sampling. NSF is funding a SARS-CoV-2 wastewater surveillance network that should provide useful insights to mature this approach to disease surveillance.
Taylor Wilkerson (MITRE) and Neelima Ramaraju (Llamasoft) focused on the coalition’s PPE supply chain work. Wilkerson said the coalition has created tools and models for managers, guidance documents, and novel methods to minimize demand (e.g., technology to re-use N95s). The coalition brings together academic researchers with expertise in analytics, public health, and practical experience. For example, its COVID-19 Demand Model offers new ways to forecast demand, including surge scenarios. Ramaraju collaborated on a white paper to preparing the health care supply chain for the pandemic’s next phase.19 It offers seven recommendations: (1) create a command center; (2) take a systems approach to supply chain; (3) improve extended supply chain visibility; (4) create a scenario plan to mitigate risks; (5) rethink demand planning processes; (6) be agile; and (7) focus on key relationships.20 It is available to organizations as a guiding document, she said. While focused on health care, the guidance could be applied to other sectors.
Paul Jarris (MITRE) next described Sara Alert™, a standards-based, open source tool for secure monitoring and reporting, which was developed with epidemiologists and public health officials to automate the monitoring of exposed and infected individuals. The free cloud-based system automates the active monitoring of ill individuals in isolation and case contacts in quarantine via text, email, or phone. De-identified, aggregated data are also available for analysis. To date, 650,000 individuals in 639 jurisdictions have been enrolled. The open source code is also available on GitHub. Academic institutions are also interested in implementing the system, and they have partnered with Oak Ridge Associated Universities (ORAU). Kenneth Tobin (OARU) then described how Sara Alert™ is being customized for universities, starting with an early adopter program. Based on this feedback, they are refining the system for campus use. A low-cost subscription service will be available for the spring semester. Institutions can use Sara Alert™ through their states or OARU. Its API connects with other systems.
Innovation and Cross-Sector Partnership
The next set of presenters explored other innovative partnerships to address COVID-19, beginning with Raymond Chiu (3M Health Care). 3M, he noted, sells globally, and manufactures as close to each market as possible, including domestic production. At the start of the pandemic, the company prioritized protecting its employees while increasing production levels and expanding partnerships. The company tripled its capacity to manufacture N95 equipment through industry partnerships with other companies, the U.S. Department of Defense, and local governments. Early on, in addition to ramping up production, governance and communication systems had to be put in place to make decisions. Chiu noted that maintaining quality and fighting against fraud and price-gouging have been important factors throughout the pandemic response.
Understanding COVID-19 as an airborne challenge required a spectrum of equipment, which drove 3M to collaborate with other producers. The company collaborated with Cummins and with Ford in PPE production including N95 respirators and powered air purifying respirators—within days, the companies exchanged design drawings and prototypes for critical equipment. Chiu emphasized that mutual trust and goals dictated the rapid pace. 3M continues
__________________
17 See http://www.fightisinus.org.
18 See https://azure.microsoft.com/en-us/services/bot-services/health-bot/.
19 Preparing Supply Chain Operations for the Next Phase of the COVID-19 Response: https://c19hcc.org/static/catalog-resources/c19_supply_chain_operations.pdf.
20 “Preparing Supply Chain Operations for the Next Phase of the COVID-19 Response,” COVID-19 Healthcare Coalition Supply Chain Working Group, May 27, 2020, https://c19hcc.org/static/catalog-resources/c19_supply_chain_operations.pdf.
to partner with these other entities, including Hewlett Packard, NISSHA Medical Technologies, MIT, and Purdue University on pandemic response-related research and production.
At the end of the day, he said, to achieve science, speed, and scale, it is important to have innovation in fundamental science and capabilities; engineering and scale manufacturing expertise and infrastructure; and external collaborations.
Drew Bennett (University of Michigan) said the University of Michigan brought together its health care system, medical school, and engineering school to respond to the early demands of the pandemic. The Michigan Medicine/Engineering Task Force and a Procurement Support Team were set up to build and acquire necessary equipment and supplies. He noted his own background as a respiratory therapist provided him a unique perspective.
Early key priorities identified were PPE acquisition and alternative sourcing; PPE sterilization, reuse, and validation; aerosolization containment; and ventilator sharing capabilities. The task force prioritized its activities to make decisions about “build versus buy,” with a health center command center determining what was essential, what constituted short term needs (face shields, masks, and gowns), mid-term needs (state incentives and the development of new manufacturing capacity), and long-term needs (near-sourcing so that products are made close to where they are needed).
Sharing several examples of innovation responses from the University of Michigan community, Bennett noted that the local maker community quickly mobilized to design and create face shields and other short-term PPE needs, while corporate and community partners stepped up to address the identified short- and mid-term priorities, including immediate development, manufacture, and supply of various PPE options and other materials from Ford, GM, Toyota, and other first tier automotive suppliers. Within a matter of weeks, innovations to control and contain aerosolization by tenting patients and to build ventilator capacity were prototyped and received FDA emergency use authorization.
Bennett stressed the importance of communications, coordination, resource identification, leadership support, and team work. When asked whether the University’s Mcubed program, which ran through June 2020, had an impact on the University’s pandemic response, Bennett noted that the culture of cross-disciplinary research and collaboration at the institution was robust, which likely strengthened its ability to respond.
Paula Johnson (Wellesley College) described how Massachusetts colleges and universities developed a testing infrastructure partnership among themselves and with the Broad Institute and the state government that allowed the schools to repopulate their campuses during the fall semester. “The speed in which we acted was remarkable and at staggering cost,” she said. “It was equally remarkable to witness the meaningful ways in which colleges and universities stepped up to support the state’s emergency response.” In the spring of 2020, this effort included alumni, faculty, and students donating and making PPE, as well as opening dorms and other facilities to frontline workers, homeless individuals, and others.
Johnson noted that GUIRR co-chair Laurie Leshin chairs the Association of Independent Colleges and Universities in Massachusetts (AICUM) and served on the governor’s reopening advisory board. A Higher Education Working Group was formed and was charged with developing a comprehensive, phased framework to repopulate campuses. Guiding principles to safely repopulate include to (1) protect the health and safety of students, faculty, staff, and surrounding communities; (2) enable students to make meaningful progress toward their educational goals; (3) contribute to research and innovation; and (4) minimize adverse economic impact, including to the state economy.
Although there is no one-size-fits-all solution to repopulating campuses, it became clear that asymptomatic or surveillance testing would be crucial, Johnson said. However, there was no national or state guidance nor infrastructure for testing on the massive scale needed. A testing working group, which Johnson chairs, thus formed part of the effort. Critical to its success, she said, was that high-level leaders committed their time and AICUM provided essential support and guidance.
Testing, she noted, is one part of a response that includes contact tracing, quarantine and isolation protocols, and prevention strategies. The testing group tapped into the emerging science about how often to test, what type of test to use, and other decisions. They partnered with the Broad Institute, which normally conducts biomedical and genomics research with MIT and Harvard. The Broad offered testing services to institutions across the state, with speed, accuracy, and scale.
Procuring the test kits is far from the final step, she stressed. The process is complicated, to encompass scheduling students, checking them in, collecting the tests, transporting the specimens to the Broad, and distributing and acting on the results, as well as tracking students who missed their tests. Information technology interfaces were developed for each step, including scheduling to avoid bottlenecks at the Broad. The partnership with the Broad made it possible to bring students back to campus, she stated.
In terms of lessons learned, Leshin noted some papers have already been published about the process to reopen campuses. Johnson reflected that large universities who can build their own facilities are in a relatively strong posi-
tion, but schools without the capacity that Massachusetts has are more vulnerable. She called for a way to incent this kind of movement. “We as a country have not invested in public health infrastructure,” she said. “We should do better.”
ADAPTIONS TO FEDERAL FUNDING AND RESEARCH REGULATION
The final set of presentations focused on examples of flexibility and collaboration by federal funding and regulatory agencies during the pandemic to accelerate scientific discovery and translation. The first two speakers commented on aspects of Operation Warp Speed, the effort led by the federal government to develop and deliver 300 million doses of COVID-19 vaccine by January 2021. The final speaker commented on the pandemic’s impact on NIH’s extramural research community.21
Gary Disbrow (Biomedical Advanced Research and Development Authority [BARDA]) explained BARDA’s mission to make available medical countermeasures through public-private partnerships to drive innovation off the bench to the patient. A decade of investments in platform technologies under flexible arrangements facilitated the agency’s rapid pivot to COVID-19 vaccine and monoclonal antibody candidates. “We’ve been preparing for a pandemic since 2009,” he said. The goal is to defeat, or flatten, a pandemic curve (Figure 3).
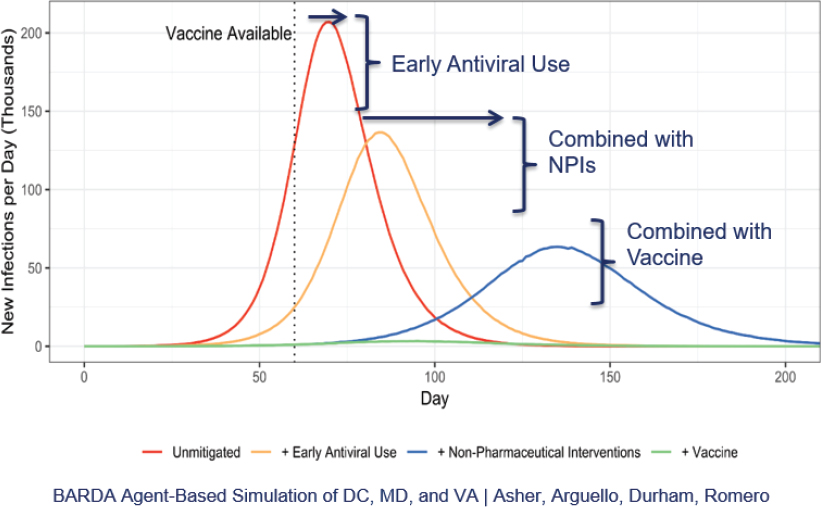
Source: Gary Disbrow, Biomedical Advanced Research and Development Authority. Presentation at the workshop of the Government-University-Industry Research Roundtable on “Learning from Rapid Response, Innovation, and Adaptation to the COVID Crisis,” held October 15, 2020.
Through a COVID-19 market research portal, more than 3,800 submissions have been received to date for therapeutics, diagnostics, vaccines, and other types. Extensive conversations have taken place with developers of more than 500 of them. BARDA also developed a medical countermeasure development strategy to accelerate development, mitigate risk, and scale domestic manufacturing. Task forces were set up to review market submissions and to avoid duplication.
In terms of flexibility, Disbrow pointed to a new, streamlined funding vehicle, called an Easy Broad Agency Agreement (EZ BAA) that can go from posting a solicitation to award within 30 days. With a ceiling under $750,000, it has been particularly useful in developing diagnostics. GUIRR co-chair Al Grasso praised the EZ BAA, but asked about the dollar threshold. Disbrow said a higher amount would require more data and a longer proposal process. He said BARDA reached out to DoD’s extensive acquisition workforce for assistance and this arrangement will carry forward.
Disbrow then turned to describing BARDA’s role in Operation Warp Speed (OWS). OWS is co-led by the Department of Defense and the Department of Health and Human Services, and according to Disbrow, it is an unprecedented effort to develop countermeasures against the pandemic that encompass vaccines, therapeutics, capacity ex-
__________________
21 The workshop did not include a presentation on the National Science Foundation’s Rapid Response Research (RAPID) mechanism, which was used to a significant extent by researchers to support proposals conducting non-medical, non-clinical-care research exploring various aspects of virus transmission and prevention, This program existed at NSF before the pandemic. For more information on RAPID, see: https://beta.nsf.gov/science-matters/rapid-responders-how-nsf-support-enabling-fight-against-covid-19-real-time and https://www.nsf.gov/pubs/2020/nsf20052/nsf20052.jsp.
pansion, and kitting and distribution. In vaccine development, the federal government is assuming the financial burden of setting up parallel activities to expedite development. Under normal circumstances, companies would not scale up manufacturing for a vaccine candidate, for example, until after clinical trial results. OWS assumes the financial burden of the scale up activities to enable immediate forward movement on production once safety and efficacy of vaccines (and other COVID responses) are achieved and proven. BARDA supported efforts to establish domestic manufacturing capacity and response capabilities for multiple therapeutics candidates; rapid deployable capabilities including wearable virus detection, diagnostic technologies, and remote monitoring technologies; vaccine candidates; and COVID diagnostics tests. Much of this support was expressed through partnerships with NIH, FDA, and the private sector.22
When asked to reflect on some lessons learned from the handling of this pandemic, Disbrow noted that BARDA covers preparedness and response. Preparedness receives less funding, but he hoped the crisis would show that additional R&D funding to look at threat-agnostic medical countermeasures is worth the investment. The agency receives $300 million to prepare for endemic influenza. In contrast, billions of dollars are being spent on COVID-19, and trillions more to support the economy. Incremental increases for preparedness would allow for development of threat-agnostic technologies.
Janet Woodcock (OWS) further explained Operation Warp Speed, which initially focused on vaccines. It became clear that therapeutics and other aspects will be critical before and even after a vaccine was developed. She noted the hundreds of applications for funding submitted, mostly for repurposed drugs. The goal of OWS was to have products—supported by evidence—available in commercial volume by the end of calendar year 2020.
As mentioned by Disbrow, in this nontraditional method of federal support, OWS has been deeply involved in supporting manufacturing and distribution products that may well not be successful. Most clinical trials are platform trials, which seem most efficient, but they are taking place in large health centers that do not reach most patients where they are. OWS is trying to get treatments in the hands of doctors as quickly as possible. The vetting process evaluates possibilities. For anything that looks promising, “we swoop in to see how can support,” she said.
The issue about how to make the clinical trial system more inclusive was discussed. She replied that companies stand up trials for single use, and other clinical trial networks are not supported adequately. In addition, communities are not involved. She contrasted the U.S. system with the United Kingdom. She noted that the RECOVERY trial has enrolled tens of thousands of UK participants in a randomized control trial that has yielded tremendous information.23 She urged master protocols to develop longitudinal studies to understand diseases and test interventions to continuously improve outcomes, rather than trials that are set up to focus on a specific therapy or vaccine and then close down.
An audience member asked both Disbrow and Woodcock about sustaining the R&D and manufacturing capacity enabled by OWS. Disbrow agreed with the concern to sustain the capacity after the immediate need because the federal government as the only purchaser is not a viable solution. Woodcock said redundancy of supply is necessary and a cost-effective solution is advanced manufacturing. The federal government would be well-served to invest in the growth of U.S. advanced manufacturing, she suggested, and Disbrow agreed. When asked what the government could do to accelerate advanced manufacturing, Woodcock said that there were not regulatory barriers to advanced manufacturing developments, as the FDA has been a cheerleader of in this area. Incentives could be offered, she suggested, including tax incentives or credits. She also noted that the pandemic has underscored the need for the United States to have an “evidence generation machine” in place through a sustainable clinical trial system.
The final speaker of the session, Michael Lauer (NIH) spoke about the status of medical research not directly related to COVID-19. Like many parts of the economy, institutions across the medical research enterprise locked down in March and operations remain far from normal. During Congressional testimony in May, NIH Director Francis Collins estimated $10 billion in lost productivity, but Lauer said the amount will probably be higher. It may also threaten a generation of researchers. In April, the Congressional Research Service published the report Effects on the Federal Research and Development Enterprise.24 It describes the disruptions accurately, according to Lauer, including shuttered labs, clinical trials stopped, and conferences cancelled. Required telework has disparate effects and anxiety is high, especially for early-career investigators. Data suggest disproportionate effects on scientists, looking at the effect on careers, especially for bench scientists and for women.25
NIH did not see a decline in the number of grant applications, or a decline in those with women as principal investigators, at its June 5 deadline. Data from the October 5 deadline will be examined. NIH has introduced some flexi-
__________________
22 For more information on OWS, see: https://www.nih.gov/about-nih/who-we-are/nih-director/testimony-operation-warp-speed-researching-manufacturing-distributing-safe-effective-coronavirus-vaccine, and for an update on OWS contracts for vaccines see: https://crsreports.congress.gov/product/pdf/IN/IN11560.
23 See https://www.recoverytrial.net/.
24 See https://crsreports.congress.gov/product/pdf/R/R46309.
bility in submitting applications, including deadlines and research guidance related to humans and animals. Peer review has been conducted virtually. NIH is conducting two surveys with institutional leaders and with scientists and scientific staff to identify the impacts of COVID-19 on extramural research.
Lauer then turned to the pace of publications and other outreach. The NIH Office of Portfolio Research maintains a website of all publications related to COVID-19; it has about 74,000 items, 50,000 of which were peer reviewed and the rest are preprints.26 Timeliness of dissemination of research results has been important and an example of how it should happen. While he agreed with Woodcock that the NIH clinical trial apparatus is far from ideal, he noted that National Institute of Allergy and Infectious Diseases clinical trial looking at the drug Remdesivir took place from February to April, and on May 22, a preliminary version of an article appeared in the New England Journal of Medicine.
He considered some of the lessons learned to date. He pointed to an article in Cell by Erin Gibson and others that stated COVID-19 “magnified the systemic issues plaguing academic research….” and urged use of the crisis as an opportunity to re-set.27 In Lauer’s view, the impact on research has been “bipolar,” with COVID-19-related research very active but with devastating shocks for other types of research. There have been losses of productivity, time, and opportunities, with extensive, disproportionate disruptions. Dramatic changes in results dissemination have occurred with the possibility of a reset in how publication happens in the future.
A participant commented on the challenge in recruiting and bringing in new employees in a remote environment. Research on which organizations, what kinds of work, and which people have thrived would be interesting, and Lauer agreed that could be an amazing natural experiment. Another commented on the future of multidisciplinary research collaborations, which often are built on trust that comes from in-person interactions. Lauer concurred, based on his own experience with different research groups. Personal discussions led to other projects and collaborations. The inability to have those kinds of events may have long-term effects.
Related to earlier discussion about the clinical trial system, Lauer pointed to an editorial by Anthony Fauci and Clifford Lane that pointed out much of the COVID literature is filled with uncontrolled observation studies or small trials, and this gap extends to other diseases.28 They pointed to the RECOVERY Collaborative Group in the United Kingdom mentioned by Woodcock, which had 15 percent of the entire COVID hospitalized population in a trial (versus 4 percent, at most, in the United States).
Lauer noted that the United Kingdom was conducting widespread clinical trials in the 1980s and 1990s in cardiovascular disease, because they had infrastructure to get them done. “They made the trials simple, that was crucial,” he said. “It is better to enroll 10,000 or 20,000 people in a trial and collect limited information about them, rather than collect massive amounts of information on just 100 people but get no good answers on interventions.” He said welcome changes in the United States include work by some Clinical and Translational Science Awards, the model of the Patient-Centered Outcomes Research Institute (PCORI), and NIH’s All of Us initiative. COVID-19 magnifies or highlights the need for clinical trials to answer necessary questions, he said, and said he thinks the momentum will carry.
NETWORKING GROUPS AND CONCLUDING THOUGHTS
On the third day of the workshop, participants broke into more informal networking groups by topic: Celebrating Victories and Learning from Failures; Engineering Responses to COVID-19; Future of International Research Collaborations; Viral Testing; Convergent Research; and Economic Impacts of COVID-19 on Research Institutions. Brief reports from the groups included the following:
- People are taking to the idea of remote work and are appreciative of their organization for supporting it. It works best for small or intact teams; bringing in new people is a challenge.
- COVID-19 has been a call to action to engineers who “see a problem and run to fix it.” This has been happening at different scales, all over the world. Seven or eight months into the pandemic, it is now a good time to see what has been done and what comes next.
- COVID-19 has raised a lot of issues about international collaboration in the new era. It would be useful to see the gains and losses from restricting international exchange.
- COVID-19 provides a great example of the need for convergent research to include the humanities, medi-
__________________
-
cine, and social science, in addition to biomedical sciences. Post-pandemic ideas to empower and stimulate change include making research challenge-driven and funding the teams, not just specific ideas, so they can be innovative.
- An issue of great concern is how the break in research will affect postdoctoral and graduate students. Special bridge programs from the agencies could be helpful to aid early-career researchers in particular.
- The pandemic necessitated regular meetings of diverse and interdisciplinary groups exchanging information and quickly learning from each other. The sharing of business efficiencies and other innovations might not have happened without the demanding conditions of the pandemic, and this has emphasized the importance of strong networks and continued cross-sectoral dialogue.
DISCLAIMER: This Proceedings of a Workshop—in Brief was prepared by Paula Whitacre as a factual summary of what occurred at the meeting. The statements made are those of the author or individual meeting participants and do not necessarily represent the views of all meeting participants; the planning committee; or the National Academies of Sciences, Engineering, and Medicine.
PLANNING COMMITTEE: Chaouki T. Abdallah, Georgia Institute of Technology; Olivia M. Blackmon, MITRE Corporation; and Cristina Thomas, 3M.
STAFF: Susan Sauer Sloan, Director, GUIRR; Megan Nicholson, Program Officer; Lillian Andrews, Senior Program Assistant; Clara Savage, Senior Finance Business Partner; Cyril Lee, Financial Assistant.
REVIEWERS: To ensure that it meets institutional standards for quality and objectivity, this Proceedings of a Workshop Series—in Brief was reviewed by Grace O’Sullivan, Arizona State University, and Yannis Yortsos, University of Southern California. Marilyn Baker, National Academies of Sciences, Engineering, and Medicine, served as the review coordinator.
SPONSORS: This workshop was supported by the Government-University-Industry Research Roundtable membership, National Institutes of Health, Office of Naval Research, Office of the Director of National Intelligence, and the United States Department of Agriculture.
For more information, visit http://www.nas.edu/guirr.
Suggested citation: National Academies of Sciences, Engineering, and Medicine. 2021. Learning from Rapid Response, Innovation, and Adaptation to the COVID-19 Crisis: Proceedings of a Workshop—in Brief. Washington, DC: The National Academies Press. https://doi.org/10.17226/26131.
Policy and Global Affairs

Copyright 2021 by the National Academy of Sciences. All rights reserved.














