2
Methodological Approach to Evidence Scanning
The Committee on Scanning for New Evidence on Riboflavin to Support a Dietary Reference Intake Review held meetings and discussions to develop a systematic approach to identifying and summarizing relevant publications on potential relationships between riboflavin intake from food and supplements and health outcomes, including relevant indicators and biomarkers, which could serve as a basis for revising intake recommendations through the Dietary Reference Intake (DRI) process.
APPROACH
The committee first developed an analytic framework for riboflavin based on appropriate clinical and biochemical markers of adequate (not deficient) and excessive intakes (see Figure 2-1). The goal of an analytic framework is to identify DRI-relevant clinical outcomes and dose–response data and provide clarity for assessment of relevant articles retrieved during the literature search (Brannon et al., 2016). Using its analytic framework, the committee designed a literature search strategy with the objective of identifying articles on riboflavin intake (from both food and supplements) and health outcomes, including chronic disease risk, that could inform a future DRI review. The committee began by developing key questions, to serve as an organizational tool for identifying search terms, and prespecified inclusion and exclusion criteria, to inform eligibility of the search results for subsequent data extraction and tabulation. The committee focused on the DRI framework structure for a systematic review, which is based on the relationship between nutrient intake (exposure) and health outcomes (Russell et al., 2009).
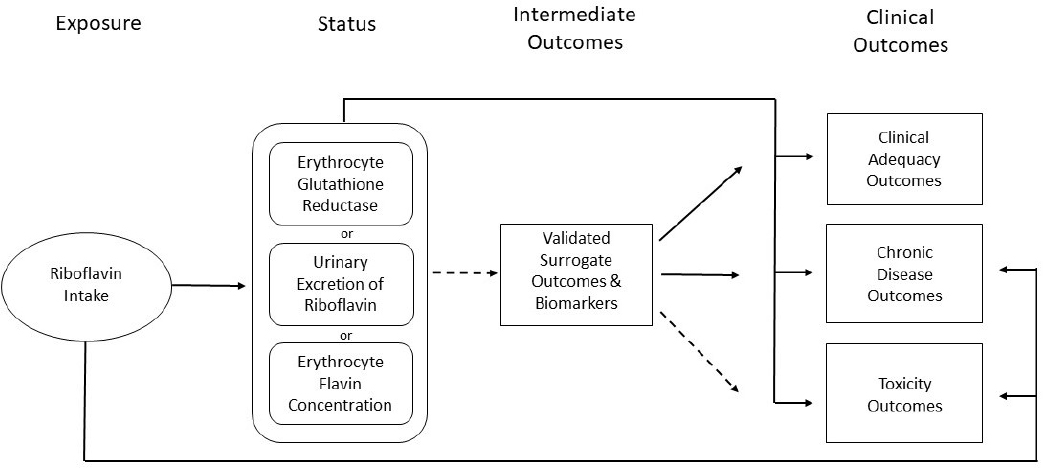
NOTES: The solid lines represent established associations among factors. The dotted lines represent associations with validated surrogate outcomes and biomarkers. The arrows represent the direction of an association.
SOURCE: Adapted from Russell et al., 2009.
Developing the Eligibility Criteria
Subject-Matter Expert Input
To ensure the evidence scan was comprehensive, the committee identified subject-matter experts to provide information related to developing its analytic framework. The committee convened a 1-day meeting with these experts (see Appendix B for the agenda). Relevant expertise represented included metabolically related B vitamins, nutrition in the life cycle, relationships of riboflavin to chronic health conditions, and nutritional toxicology. The subject-matter experts were asked (ahead of time) to develop their presentations (see Chapter 1, Box 1-1).
Eligibility Criteria
Informed by input from the subject-matter experts, the committee developed a draft set of prespecified criteria for assessing the relevance of identified evidence, which was refined following an internal check to determine if the search retrieved anticipated articles (see detailed description). The criteria were organized using the population, interventions (exposures), comparators, outcomes, and study designs (PI[E]COD) framework; Table 2-1 shows the final criteria.
Search Strategy
The committee developed a search strategy that included a search syntax containing specific text words (i.e., natural language terms) and controlled vocabulary terms (medical subject headings [MeSH]) to search Medline for peer-reviewed, published literature. The
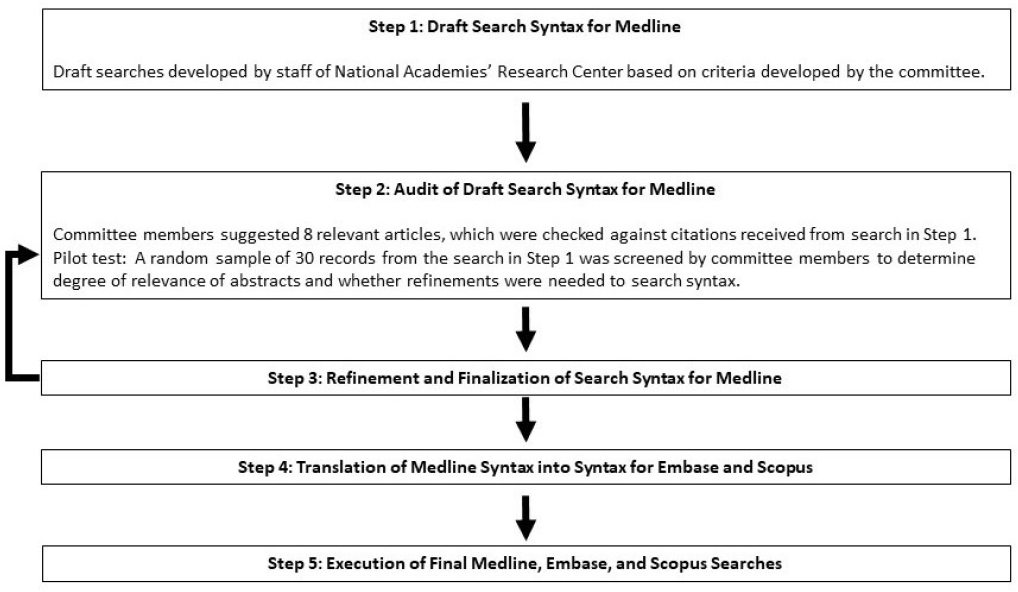
committee used the National Academies’ Research Center as a resource for the search. The staff created a draft list of potentially relevant text words and MeSH terms (see Figure 2-2, Step 1).
The committee developed an internal check to assess whether the search strategy was either too broad or too narrow (see Figure 2-2, Step 2). Committee members suggested eight key known articles that they expected to find in the results. Staff checked whether the preliminary search identified these articles. Staff also conducted an abridged check of a random 300 records to determine whether it would be necessary to refine the search syntax. Committee members reviewed a subset of a random 30 records. The search syntax was refined several times before the Medline search strategy was finalized (see Figure 2-2, Step 3). The committee next translated the search syntax for use in Embase (using Ovid) and Scopus (see Figure 2-2, Steps 4 and 5).
TABLE 2-1 Prespecified Criteria for Assessing the Relevance of Identified Evidence
| Component | Criteria |
|---|---|
| Populations |
Healthy individuals of any age living in a high- or upper-middle-income country at the time of the studya
Exclude: |
| Interventions/Exposures |
Riboflavin intake—amount,e frequency, duration Indicators/biomarkers, such as
|
Exclude:
|
|
| Comparators | Riboflavin of different doses, frequencies, and/or durations No consumption of foods that are major sources of riboflavin (e.g., dairy) No riboflavin enrichment/fortification/restoration No riboflavin supplementation |
| Outcomes |
Clinical adequacy outcomesh
Toxicity outcomes
Chronic disease risk outcomes
|
| Study Designs |
Exclude:
|
|
|
a Countries are classified according to definitions from the World Bank: https://data.worldbank.org/income-level/high-income (accessed January 28, 2021) and https://data.worldbank.org/income-level/upper-middle-income (accessed January 28, 2021).
b Includes enrichment or replacement in addition to fortification at the time of the study.
cAs identified by the study authors.
d This does not apply to case-control studies that included a healthy control group.
e Identify dose, if from a dietary supplement.
f Identify if spot or 24-hour urine collection.
g See Figure 2-1 for biomarkers of riboflavin status.
h Several indicators have been used to estimate the adequacy of riboflavin status, including erythrocyte glutathione reductase, erythrocyte flavin concentration, and urinary excretion.
i Includes methods for measuring dietary intake and physiologic or other nutrient status measures.
The committee considered the potential of duplicating studies identified in the previous DRI report (IOM, 1998). Because that report did not involve a systematic review, the committee searched the literature from January 1969 to January 2021. The committee’s decision to extend its date range to include articles that were expected to be captured in the first DRI review is based on recognizing the difference between the narrative review process used in the first set of DRI reviews and the comprehensive systematic review process that underpins all current DRI reviews. The methodological structure of the two approaches defines how studies are identified and selected and data are reported. In the comprehensive systematic review process, selecting and assessing reported data are prespecified and informed by the analytic framework. In contrast, narrative reviews do not have a defined structure for identifying and selecting relevant studies, which may limit data availability and potentially influence data interpretation. Therefore, given the methodological differences, this evidence scan included the years covered in the 1998 report.
Only English-language reports were searched. Figure 2-2 summarizes the full search process, which was finalized and executed (Step 5) on February 25 and 26, 2021. Appendix C provides details of the literature searches.
Screening
All search results were uploaded to a systematic review management program (Covidence1) for screening. Following the internal assessment, trained staff performed an initial title and abstract screening of each record retrieved using the prespecified criteria in Table 2-1. Next, pairs of committee members independently screened each title and abstract that staff deemed potentially eligible. Conflicts were resolved by discussion.
Before proceeding to the full-text review, the committee discussed and refined the eligibility criteria, primarily to ensure shared understanding and consistent application of these (see Table 2-1). Using the refined criteria, single committee members performed a second round of title and abstract screening of the remaining articles. Next, pairs of committee members screened the full text of each article to determine eligibility for data extraction based on the refined criteria. Except during the second round of title/abstract screening, all records were double-screened independently, and conflicts were resolved by discussion or a third screener.
Data Extraction
The data extraction was carried out using Covidence. Relevant data for each study were extracted by two committee members, one as a primary extractor and the other who verified the data; conflicts were resolved by discussion between the two. If they agreed that a study was
___________________
1 See https://www.covidence.org (accessed July 15, 2021).
eligible, data on study characteristics, population characteristics, intake assessment methods, riboflavin status, adequacy outcomes, and chronic disease outcomes were extracted and verified.
REFERENCES
Brannon, P. M., C. M. Weaver, C. A. Anderson, S. M. Donovan, S. P. Murphy, and A. L. Yaktine. 2016. Scanning for new evidence to prioritize updates to the Dietary Reference Intakes: Case studies for thiamin and phosphorus. American Journal of Clinical Nutrition 104(5):1366–1377.
IOM (Institute of Medicine). 1998. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press. https://doi.org/10.17226/6015.
Russell, R., M. Chung, E. M. Balk, S. Atkinson, E. L. Giovannucci, S. Ip, A. H. Lichtenstein, S. T. Mayne, G. Raman, A. C. Ross, T. A. Trikalinos, K. P. West, Jr., and J. Lau. 2009. Opportunities and challenges in conducting systematic reviews to support the development of nutrient reference values: Vitamin A as an example. American Journal of Clinical Nutrition 89(3):728–733.
This page intentionally left blank.








