3
Results
LITERATURE SEARCH RESULTS
The objective was to assess the status of evidence on riboflavin as a candidate nutrient for a future Dietary Reference Intake (DRI) review. The results are intended to support decision making about the sufficiency of evidence on new nutrient outcome relationships or other related criteria to support a comprehensive systematic review to update DRI nutrients in the United States and Canada. Key steps were to (1) develop an analytic framework on the basis of appropriate markers of the adequate intake (the absence of a deficiency state) of riboflavin, (2) consult subject-matter experts on riboflavin, (3) use the analytic framework to develop and carry out a literature scan with relevant text words and controlled vocabulary, (4) review abstracts and full-text publications for relevance relative to prespecified criteria, and (5) reach consensus on the relevance of the evidence to the DRI process.
Figure 3-1 presents the results. The searches produced 3,479 records. After removing 753 duplicates, 2,726 unique records remained. During title and abstract screening, 2,635 records were excluded, leaving 91 articles for full-text screening; the committee excluded 57 articles then (see Chapter 4 for a discussion of exclusion reasons). An article was identified outside of the original literature search and was screened and determined to be eligible for inclusion, resulting in 35 included articles.
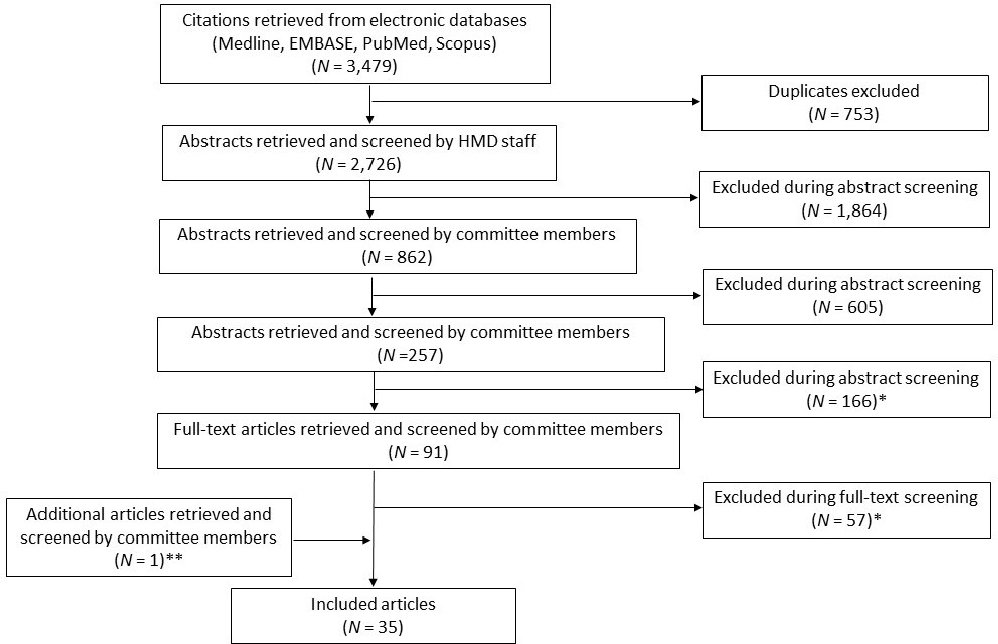
* See Table 3-1 for description of exclusion reasons.
** This article was identified outside of the original literature search.
Excluded Studies
During screening, the committee removed articles for any reason identified in the prespecified exclusion criteria; however, it notes that many of these had more than one disqualifying reason. Table 3-1 shows the number of excluded articles by reason. Appendix E presents a list of articles that were excluded throughout the screening process.
TABLE 3-1 Number of Articles Excluded as Not Relevant to the Prespecified Criteria (number of records/articles excluded during abstract and full-text screening and the reason for exclusion; prespecified criteria are listed in Chapter 2, Table 2-1)
| Exclusion Reason | Number of Articles | |
|---|---|---|
| Excluded During Abstract Screening | Excluded During Full-Text Screening | |
| Lack of independent effect of riboflavin | 99 | 10 |
| No validated biomarker of interest | 37 | 4 |
| Insufficient data to assess dietary intake response | 15 | |
| ≥20% of individuals had existing chronic disease | 13 | 3 |
| No outcome of interest | 9 | 14 |
| Mechanistic study | 1 | 0 |
| Methods not considered adequate or relevant | 1 | 3 |
| No intervention/exposure of interest | 1 | 3 |
| No population subgroup of interest | 1 | 0 |
| Not living in a high- or middle-income country | 0 | 3 |
| Case report or case series | 0 | 1 |
| No full text available | 0 | 1 |
| Duplicate | 4 | 0 |
| Total | 166 | 57 |
Included Studies
Using Covidence,1 the committee evaluated the full text for each of the 91 articles identified for the first level of full-text screening. Tables 3-2 and 3-3 provide descriptions of studies included for adequacy and chronic disease outcomes, respectively, including a notation of the outcome category identified in the analytic framework to which each relates (see Chapter 2, Figure 2-1): adequacy (N = 24), chronic disease (N = 11), or toxicity (N = 0).
The inclusive dates of the search were also set to capture studies that may not have been included in the first DRI review (IOM, 1998). The search’s beginning dates varied by database but included at least 1969 on. Only six of the included studies were cited in the 1998 DRI report (denoted by an asterisk in Tables 3-2 and 3-3). Ten were published before that report but not captured in its evidence review; 19 of the 35 included articles were published after that report.
___________________
1 See https://www.covidence.org (accessed July 15, 2021).
TABLE 3-2 Assessment of Relevance of Reviewed Full-Text Articles Relevant to Riboflavin DRIs on the Basis of Prespecified Criteria Identified to Indicate the Relation of Nutrient Intake to Adequacy
| Author (Year) | Study Design | Country and Population Description | Intervention | Diet Assessment Method | Biomarker | Comparator | Additional Comments |
|---|---|---|---|---|---|---|---|
| Alexander et al. (1984)* | Cross-sectional observation al study | United States 24 women aged ≥70 years living in a care facility; excluded individuals who were not in generally good health, not ambulatory, or taking drugs known to interfere with calcium or riboflavin metabolism |
No intervention; participants free to consume a single multivitamin supplement containing 1.7 mg riboflavin per day | 3-day food intake (examination of all food served compared to uneaten; self-reported intake not monitored in dining room and monitored intake of supplement) | Urinary riboflavin (single 24-hour collection) EGRAC |
No supplement (N = 15/22) | Two intake levels with biomarkers |
| Belko et al. (1983)* | Controlled feeding trial | United States 12 women aged 19–27 years; excluded individuals with history of recurrent or chronic disease, hematological or physical signs of health impairment, abnormal EKG, history of knee, |
Exercise for 6 weeks (3 weeks short and 3 weeks long exercise) | Controlled diet adjusted for riboflavin per calorie intake ratio (0.6 mg/1000 kcal) and EGRAC <1.25 in acclimation and first sedentary periods with | EGRAC | No exercise for 6 weeks (2 weeks acclimatizati on and 2 × 2 weeks sedentary) | Data reported individually for riboflavin intake and EGRAC |
| joint, or back injury, or intake of drugs, including oral contraceptives; participants instructed to abstain from vitamin supplements for 2 months prior to start of study | semisynthetic ice cream | ||||||
| Belko et al. (1984)* | 2 period crossover designed controlled feeding trial | United States 12 moderately overweight women aged 22–39 years; excluded individuals with weight for height outside of specified range; hematological or physical signs of health impairment; abnormal EKG; history of knee, joint or back injury; intake of drugs; history of fad or crash dieting; participants instructed to abstain from vitamin supplements for 6 |
Exercise (150 minutes per week) for 5 weeks | Controlled diet (0.8 mg/1,000 kcal consumed) | Urinary riboflavin (3 days of 24-hour collections) EGRAC |
No exercise for 5 weeks | Individual data reported for the two biomarkers; mean riboflavin intake can be calculated based on mean energy intake |
| weeks prior to start of study. | |||||||
| Boisvert et al. (1993a) | Study 1: cross-sectional Study 2: nonrandom ized experiment al study |
Guatemala Study 1: Adults older than 50 years. Mainly free-living residents in rural settings; others were institutionalized. Some participants had very low socioeconomic status Study 2: A subset of riboflavin-deficient participants (N = 9) were also enrolled in a riboflavin supplementation study |
Study 2: 10 mg/d riboflavin for 3 days. EGRAC measured on day 4 and weeks 3, 5, 7, and 11; no placebo | Milk intake estimated by participants or observed by authors over a period of several days | EGRAC | Before and after supplementat ion only in the subset with low EGRAC | Results provide data on EGRAC due to short-term supplementation of riboflavin in participants with low EGRAC values; no dose–response data |
| Boisvert et al. (1993b)* | Controlled feeding study | Guatemala Phase 1: 14 adults; mean age 70.9 years Phase 2: 15 adults (5 from phase 1); mean age 71.1 years |
Incremental increase in riboflavin intake after basal period from 0.9 mg/day for 2–5 weeks to 1.1 mg/day for 3 weeks | Controlled diets: Phase 1: moderate carbohydrate, higher fat (typical Western diet); Phase 2: higher carbohydrate, lower fat | EGRAC (weekly) Urinary riboflavin excretion (weekly, 24-hour collection) |
0.65–0.70 mg/day riboflavin for 2–5 weeks (basal period) | EGRAC and urinary riboflavin values reported graphically relative to mean riboflavin intake for each phase of the controlled diets |
| All riboflavin deficient but healthy. | 1.3 mg/day for 3 weeks 1.5 mg/day for 2–3 weeks |
||||||
| Breskin et al. (1985) | Case-control study | United States 30 children aged 10 to 108 months; recruited at a well-child clinic |
N/A | Two 24-hour recalls Contents of supplements collected from labels |
EGRAC | No reported use of riboflavin supplements Supplement use based on intake data, not an intervention. |
Some dose–response data (riboflavin intake–EGRAC) and a correlation coefficient |
| Choi et al. (2015) | Cross-sectional study | Korea 412 adults, aged 20–64 years; excluded adults who were not in good health, had known illnesses, or took medications |
N/A | 3 consecutive 24-hour recalls; measured dietary and total (dietary plus supplemental ) riboflavin intake | Urinary riboflavin excretion (spot urine; measured in 149 participants) | Non-supplement users Supplement use was based on intake data not an intervention |
Some dose–response data (riboflavin intake–EGRAC) and a correlation coefficient |
| Harrill et al. (1982) | Supplemen tal feeding trial | United States 18 women living in a nursing home; median age 89 years; participants not receiving |
Daily high-calorie nutritionally balanced liquid supplement providing an | 2 × 2-day weighed food record preceding and following supplemental | Urinary riboflavin (casual sample collected before 8 am before and | Compared to baseline usual food intake | Intake response based on mean urinary riboflavin values reported before and after supplementation with known amount of riboflavin |
| vitamin supplements and with inadequate food intake | additional 0.65 mg riboflavin for 30 days, along with a large panel of other nutrients | feeding period | after supplementa l feeding) | ||||
| Hiraoka (2001) | Cross-sectional study | Japan 150 female students, aged 21–22 years, not taking vitamin supplements |
N/A | 3-day weighed food record | Whole blood total riboflavin (determined by HPLC) | Quartiles of riboflavin intake, estimated from intake data | Some dose–response data (riboflavin intake–EGRAC) and a correlation coefficient |
| Jiang (2006) | Randomized controlled trial | China 101 school children aged 9–11 years |
0.625 mg/day riboflavin for 37 days | None | Urinary riboflavin (collected on days 1, 20, and 37); EGRAC (method not reported) | Placebo B-vitamin compound supplement | Objective: determine if fortifying a food with riboflavin will improve biomarkers of riboflavin status |
| Jungert et al. (2020) | Cross-sectional study | United Kingdom 407 healthy adults aged 18–92 years; excluded individuals taking vitamin B supplements or medicines that interfere with |
N/A | 4-day food diary | EGRAC | None | Reports mean intake and biomarker for middle-aged and older groups |
| vitamin B metabolism | |||||||
| Lewis and King (1980) | Controlled feeding trial | United States 13 women aged 19–25 years |
Self-selected oral contraceptive use 3 mg/day for 12 days |
Semi-purified liquid diet (no riboflavin) plus a daily 3 mg riboflavin supplement for 12 days | EGRAC | Self-selected nonuse of oral contraceptives | Basal dietary intake of riboflavin based on 3-day food record prior to controlled feeding; pre-study riboflavin intake reported |
| Lo (1984) | Cross-sectional study | China 11,200 children aged 12–19 years from 31 schools Clinical observations in 1,313 children |
N/A | 3-day intake of food for each school measured by weighing the total amount before and after cooking and subtracting leftovers; intake from supplements not assessed | None | None | Compares intake and clinical deficiency signs for adequacy in children |
| Madigan et al. (1998) | Study 1: cross-sectional Study 2: nested double-blind, |
United Kingdom Study 1: 92 free-living adults aged 65–91 years; excluded individuals who scored low on a |
Study 1: dietary intake assessment Study 2: 1.6 or 25 mg/day |
Diet history (conducted by one trained nutritionist through interview in | EGRAC | Study 2: placebo | Study 1 (cross-sectional) reports some intake and EGRAC response data Study 2 (double-blind randomized controlled trial) showed that 25 |
| placebo-controlled trial | mental state questionnaire, used vitamin supplements or medication known to affect riboflavin or vitamin B6 metabolism, or not in general good health Study 2: 45 participants from Study 1 |
riboflavin or placebo for 12 weeks | participants’ own homes) | mg/day riboflavin supplementation lowered EGRAC when compared to “before treatment” and the 1.6 mg/day supplementation group | |||
| McKinley et al. (2002) | Randomized controlled trial | United Kingdom 52 healthy adults aged ≥60 years with sub-optimal riboflavin status (EGRAC ≥1.20); excluded individuals with B-vitamin supplementation; gastrointestinal disease; hematological disorders; drugs known to affect vitamin B6 or riboflavin metabolism; vascular, hepatic, or renal disease; |
1.6 mg/day riboflavin supplement versus placebo for 12 weeks | None | EGRAC | Placebo | Supplementation and biomarker data |
| impaired cognitive function; serum creatinine over 130 µmol/l; or serum vitamin B12 concentration less than 111 pmol/l | |||||||
| Mobarhan et al. (1982) | Cross-sectional study | Italy 107 boys aged 7–10 years with no clinical evidence of acute or chronic disease 7 individuals with EGRAC >1.2 (but no signs of clinical riboflavin deficiency) treated with 10 mg/day riboflavin for 2 weeks |
N/A | 24-hour recall (repeated six times per individual on alternate days) Intake from supplements not assessed |
EGRAC | None | Response to supplementation (lowering of EGRAC) in all individuals supplemented |
| Powers et al. (2011) | Randomized controlled trial | United Kingdom 117 healthy women aged 19–25 years with moderately low riboflavin status (EGRAC value >1.40); excluded individuals who had pre-existing hematologic |
2 or 4 mg riboflavin or placebo for 8 weeks | 4-day food diary | EGRAC | Placebo | Both EGRAC and hemoglobin increased in the intervention arm, suggesting a role of riboflavin with iron metabolism |
| disorders; given blood in the previous 3 months; been taking nutritional supplements; any diagnosed gastrointestinal problem; or had been pregnant or breastfeeding | |||||||
| Rutishauser et al. (1979) | Cohort study | United Kingdom 23 relatively healthy adults aged 72–86 years; followed for 60 weeks |
N/A | A daily qualitative record kept of all food and drink consumed for 1 year and 1 day of food weighing every 6–8 weeks; a record of vitamin supplements containing riboflavin kept, and the contribution they made to the mean daily intake added to the dietary intake | EGRAC | None | Assessment of between-subject change in riboflavin intake and EGRAC over time; not a strong correlation between EGRAC and intake |
| Shaw (1993) | Cohort study | China 61 healthy pregnant women, average age 29±4 years; recruited at 26±2 gestational weeks; excluded individuals who were anemic, diagnosed with any clinical signs of deficiency, or receiving prescribed supplements; included individuals who self-chose to take prenatal supplements; accounted for supplement use and riboflavin content in dietary intake data |
N/A | 24-hour recall Dietary intake assessment accounting for supplement use (specific supplement providing 2 mg/tablet) |
EGRAC measured at 26 and 36 gestational weeks and 6 weeks postpartum | None | At 26 weeks gestation, prevalence of deficiency reduced in supplement users |
| Suprapto et al. (2002) | Randomize d controlled trial | Indonesia 84 pregnant women, aged <35 years, 13–28 weeks gestation; single pregnancy; in good health; excluded women with preeclampsia, congestive heart disease, tuberculosis, and acute infections |
4 treatment groups: (1) IF iron-folate + 5 mg glucose (placebo), (2) IFR iron-folate + 5 mg riboflavin, (3) IFA iron-folate + 2.75 mg retinyl palmitate, (4) IFRA iron-folate + 5 mg riboflavin + 2.75 mg retinyl palmitate; given daily for 60 days | 24-hour recall Intake from supplements not assessed |
EGRAC | Placebo (iron-folate + 5 mg glucose) | Iron-folate +5 mg riboflavin supplementation yielded greater hemoglobin than iron-folate; comparison of IF versus IFR groups allowed independent assessment of riboflavin effect |
| Szczuko et al. (2011) | Cross-sectional study | Poland 120 adults aged 22–25 years; excluded individuals with hemolytic anemia, lower activity of glucose-6-phosphate |
N/A | 7-day food report Intake from supplements not assessed |
EGRAC | Comparison of intakes and corresponding EGRAC values |
| dehydrogenase, or hypothyreosis | |||||||
| Tavares et al. (2009) | Randomized controlled trial | Portugal 42 adults aged 60–94 years with low riboflavin status (EGRAC 1.2); excluded individuals with hematological disorders; gastrointestinal, cardiovascular, hepatic or renal disease; impaired cognitive function; or taking B-vitamin supplements or drugs that affect riboflavin metabolism |
10 mg/day riboflavin or placebo for 28 days | None | EGRAC | Placebo | Supplementation improved homocysteine and EGRAC but not ferritin, uric acid, or C-reactive protein |
| Tremblay et al. (1984)* | Randomized controlled trial | Canada 14 elite adult swimmers; mean age 19 years; no vitamin supplement usage in the prior 6 months |
60 mg/day riboflavin | Dietary questionnaire | EGRAC | Placebo | No dietary data or dietary intake data provided, only a statement that EGRAC and intakes indicated adequacy; no effect of supplement on performance, EGRAC, or max VO2 |
| Vir and Love (1977) | Cross-sectional study | United Kingdom 196 adults, aged ≥65 years; some institutionalized, some free living; excluded individuals with acute illness and on a modified diet; those taking a multivitamin grouped separately |
N/A | Not reported | EGRAC | None | Biochemical deficiency detected in 7.1% of individuals; multiple levels of riboflavin in supplements administered |
* Study cited in 1998 DRI report (IOM, 1998).
NOTE: EGRAC = Erythrocyte glutathione reductase activity/activation coefficient; EKG = electrocardiogram; HPLC = high-performance liquid chromatography; kcal = kilocalorie; max VO2 = maximal oxygen consumption; mg = milligram; N/A = not applicable; pmol = picomole; µmol/l = micromole/liter.
TABLE 3-3 Assessment of Relevance of Reviewed Full-Text Articles Relevant to Riboflavin DRIs on the Basis of Prespecified Criteria Identified to Indicate the Relation of Nutrient Intake to an Identified Health Outcome Related to Risk of Chronic Disease
| Author (Year) | Study Design | Country and Population Description | Intervention | Diet Assessment Method | Biomarker | Comparator | Additional Comments |
|---|---|---|---|---|---|---|---|
| He et al. (2009) | Cluster randomized controlled trial with 21 townships | China 2,250 adults, aged 40–69 years |
Riboflavin-fortified salt (100–150 mg/kg) for 6 years (N = 9 townships, N = 950 for outcome) | Not determined | EGRAC (N = 155 intervention and 120 comparator) | Common (nonfortified) salt (N = 12 townships, N = 1,300 for outcome) | Risk of esophageal atypical dysplasia and cancer |
| Leske et al. (1995)* | Case-control study | United States 1,380 adults aged 40–79 years; 945 cases with lens opacities (posterior subscapular, nuclear, or cortical) or mixed cataract; 435 controls |
N/A | FFQ Intake from supplements was not assessed |
EGRAC | Control group | Lens opacity (a biomarker for cataract risk) associated with low riboflavin status (as well as other nutrients, including protective association with higher vitamin E and glycine status) |
| Liu et al. (2020) | Cohort study | China 12,245 adults aged ≥18 years (mean 41.2 years); excluded pregnant women and individuals with hypertension at baseline; median followup was 7.8 years; included individuals who reported that they smoked |
N/A | 3 consecutive 24-hour dietary recalls combined with household food inventory | None | Follow-up compared to baseline and quartiles of intake | Dose–response characterization of riboflavin intake to systolic and diastolic BP as well as hypertension onset (intake–outcome association); but no biomarkers of riboflavin status |
| Intake from supplements not assessed | |||||||
| Moore et al. (2019) | Cross-sectional cohort | Northern Ireland and the Republic of Ireland 5,186 community-dwelling adults aged ≥60 years; excluded individuals with diagnosis of dementia or receiving vitamin B12 injections |
N/A | FFQ validated for collection of B vitamins; included intake from supplements and fortified foods | EGRAC | Examined nonfortified foods, fortified foods, and supplement use on EGRAC and depression and anxiety | |
| Rooney et al. (2020) | Randomized controlled trial | United Kingdom 47 adults with the MTHFR genotype; excluded supplement users |
1.6 mg/day riboflavin or placebo for 16 weeks | None | EGRAC | Placebo | 1.6 mg/day riboflavin supplementation lowered EGRAC, systolic BP, and diastolic BP (compared to placebo group) in MTHFR TT individuals |
| Shi et al. (2014) | Cohort study | China 1,227 adults aged ≥20 years; excluded individuals with extreme weight change; diagnosed diabetes, stroke, or cancer at baseline |
N/A | FFQ (to estimate dietary intake patterns during previous year) 3-day weighed food diary (at baseline) |
None | N/A | Dose–response characterization of riboflavin intake to systolic and diastolic BP and hypertension onset (intake–outcome association); but no biomarkers of status; also accounts for effects of overweight/obesity and anti-hypertensives on the dose–response relationship |
| Shin and Kim (2019) | Cross-sectional | Korea | N/A | 24-hour dietary recall; included | None | N/A | Greater risk of metabolic syndrome in women consuming less than 1.2 |
| 6,092 adults, aged ≥19 years; nonsmokers (Data from Korea National Health and Nutrition Examination Survey) |
intake of dietary supplements | mg riboflavin/day, especially in postmenopausal women | |||||
| Theofylaktop oulou et al. (2014) | Cross-sectional study | Norway 3,727 adults aged 46–47 years 3,324 adults aged 70–72 years |
N/A | FFQ included intake from supplements | Plasma riboflavin | Role in modulating kynurenine pathways, which may be related to risk of chronic disease and immune function Measured dietary intake data and chronic disease outcomes; however, plasma riboflavin not included in the PI(E)COD criteria or analytic framework |
|
| Ward et al. (2020) | Cross-sectional cohort study | United Kingdom and Republic of Ireland 6,076 adults aged 18–102 years recruited from two national cohorts |
N/A | Dietary information (including vitamin supplement use) collected for cohort participants, but methods not reported | EGRAC | N/A | Reported a significant effect of riboflavin status on blood pressure in individuals with the MTHFR C677 TT genotype; however, no dietary intake data for riboflavin included |
| Wilson et al. (2012) | Cohort study and nested crossover | United Kingdom | 1.6 mg/day riboflavin for 16 weeks | Not determined | EGRAC | Placebo | Cohort of CVD patients; MTHFR 677 C→T genotype a major determinant of BP; |
| intervention trial | 83 adult CVD patients in cohort; 29 in intervention trial | systolic and diastolic BP highest in TT genotype relative to other genotypes; intervention with 1.6 mg riboflavin/day (versus placebo) lowered BP significantly and to a greater extent than BP-lowering drugs in this cohort, specific for the MTHFR TT genotype; little exposure to riboflavin-enriched or - fortified foods in this Irish population | |||||
| Wilson et al. (2013) | Randomized controlled trial | United Kingdom 91 hypertensive adults with the MTHFR 677TT genotype; mean age of 69 years Excluded individuals with a history of gastrointestinal, hepatic, renal, or hematological disorders or taking B-vitamin supplements, anticonvulsant therapy, or any other drugs known to interfere with folate or B-vitamin metabolism |
1.6 mg/day riboflavin or placebo for 16 weeks | None | EGRAC | Placebo | Supplementation improved systolic (~5 mmHg) but not diastolic blood pressure |
NOTE: BP = blood pressure; CVD = cardiovascular disease; EGRAC = erythrocyte glutathione reductase activity/activation coefficient; FFQ = food frequency questionnaire; Hg = mercury; kg = kilogram; mg = milligram; mm = millimeter; MTHFR = methylenetetrahydrofolate reductase; N/A = not applicable; PI(E)COD = population, interventions (exposures), comparators, outcomes, and study designs.
REFERENCES
Alexander, M., G. Emanuel, T. Golin, J. T. Pinto, and R. S. Rivlin. 1984. Relation of riboflavin nutriture in healthy elderly to intake of calcium and vitamin supplements: Evidence against riboflavin supplementation. American Journal of Clinical Nutrition 39(4):540–546.
Belko, A. Z., E. Obarzanek, H. J. Kalkwarf, M. A. Rotter, S. Bogusz, D. Miller, J. D. Haas, and D. A. Roe. 1983. Effects of exercise on riboflavin requirements of young women. American Journal of Clinical Nutrition 37(4):509–517.
Belko, A. Z., P. E. Obarzanek, and R. Roach. 1984. Effects of aerobic exercise and weight loss on riboflavin requirements of moderately obese, marginally deficient young women. American Journal of Clinical Nutrition 40(3):553–561.
Boisvert, W. A., C. Castaneda, I. Mendoza, G. Langeloh, N. W. Solomons, S. N. Gershoff, and R. M. Russell. 1993a. Prevalence of riboflavin deficiency among Guatemalan elderly people and its relationship to milk intake. American Journal of Clinical Nutrition 58(1):85–90.
Boisvert, W. A., I. Mendoza, C. Castaneda, L. De Portocarrero, N. W. Solomons, S. N. Gershoff, and R. M. Russell. 1993b. Riboflavin requirement of healthy elderly humans and its relationship to macronutrient composition of the diet. Journal of Nutrition 123(5):915–925.
Breskin, M. W., C. M. Trahms, and B. Worthington-Roberts. 1985. Supplement use: Vitamin intakes and biochemical indexes in 40- to 108-month-old children. Journal of the American Dietetic Association 85(1):49–56.
Choi, J. Y., Y. N. Kim, and Y. O. Cho. 2015. Evaluation of riboflavin intakes and status of 20-64-year-old adults in South Korea. Nutrients 7(1):253–264.
Harrill, I., M. Kunz, and A. Kylen. 1982. Dietary supplementation and nutritional status in elderly women. Journal of Nutrition for the Elderly 1(3–4):3–14.
He, Y., L. Ye, B. Shan, G. Song, F. Meng, and S. Wang. 2009. Effect of riboflavin-fortified salt nutrition intervention on esophageal squamous cell carcinoma in a high incidence area, China. Asian Pacific Journal of Cancer Prevention 10(4):619–622.
Hiraoka, M. 2001. Nutritional status of vitamin A, E, C, B1, B2, B6, nicotinic acid, B12, folate, and beta-carotene in young women. Journal of Nutritional Science and Vitaminology 47(1):20–27.
IOM (Institute of Medicine). 1998. DRIs for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press. https://doi.org/10.17226/6015.
Jiang, Y. Y. 2006. Effect of B vitamins–fortified foods on primary school children in Beijing. Asia Pacific Journal of Public Health 18(2):21–25.
Jungert, A., H. McNulty, L. Hoey, M. Ward, J. J. Strain, C. F. Hughes, L. McAnena, M. Neuhauser-Berthold, and K. Pentieva. 2020. Riboflavin is an important determinant of vitamin B-6 status in healthy adults. Journal of Nutrition 150(10):2699–2706.
Leske, M. C., S. Y. Wu, L. Hyman, R. Sperduto, B. Underwood, L. T. Chylack, R. C. Milton, S. Srivastava, and N. Ansari. 1995. Biochemical factors in the Lens Opacities case-control study. Archives of Ophthalmology 113(9):1113–1119.
Lewis, C. M., and J. C. King. 1980. Effect of oral contraceptive agents on thiamin, riboflavin, and pantothenic acid status in young women. American Journal of Clinical Nutrition 33(4):832–838.
Liu, M., C. Zhou, Z. Zhang, Q. Li, P. He, Y. Zhang, H. Li, C. Liu, and X. Qin. 2020. Inverse association between riboflavin intake and new-onset hypertension: A nationwide cohort study in China. Hypertension 1709–1716.
Lo, C. S. 1984. Riboflavin status of adolescents in southern China. Average intake of riboflavin and clinical findings. The Medical Journal of Australia 141(10):635–637.
Madigan, S. M., F. Tracey, H. McNulty, J. Eaton-Evans, J. Coulter, H. McCartney, and J. J. Strain. 1998. Riboflavin and vitamin B-6 intakes and status and biochemical response to riboflavin supplementation in free-living elderly people. American Journal of Clinical Nutrition 68(2):389–-
395.
McKinley, M. C., H. McNulty, J. McPartlin, J. J. Strain, and J. M. Scott. 2002. Effect of riboflavin supplementation on plasma homocysteine in elderly people with low riboflavin status. European Journal of Clinical Nutrition 56(9):850–856.
Mobarhan, S., G. Maiani, and E. Zanacchi. 1982. Riboflavin status among rural children in southern Italy. Human Nutrition: Clinical Nutrition 36(1):71–79.
Moore, K., C. F. Hughes, L. Hoey, M. Ward, C. Cunningham, A. M. Molloy, J. J. Strain, K. McCarroll, M. C. Casey, F. Tracey, E. Laird, M. O’Kane, and H. McNulty. 2019. B-vitamins in relation to depression in older adults over 60 years of age: The Trinity Ulster Department of Agriculture (TUDA) cohort study. Journal of the American Medical Directors Association 20(5):551.
Powers, H. J., M. H. Hill, S. Mushtaq, J. R. Dainty, G. Majsak-Newman, and E. A. Williams. 2011. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). American Journal of Clinical Nutrition 93(6):1274–1284.
Rooney, M., T. Bottiglieri, B. Wasek-Patterson, A. McMahon, C. F. Hughes, A. McCann, G. Horigan, J. J. Strain, H. McNulty, and M. Ward. 2020. Impact of the MTHFR C677T polymorphism on one-carbon metabolites: Evidence from a randomised trial of riboflavin supplementation. Biochimie 173:91–99.
Rutishauser, I. H., C. J. Bates, A. A. Paul, A. E. Black, A. R. Mandal, and B. K. Patnaik. 1979. Long-term vitamin status and dietary intake of healthy elderly subjects. 1. Riboflavin. The British Journal of Nutrition 42(1):33–42.
Shaw, N. S. 1993. Riboflavin status in late pregnancy, postpartum and cord blood. Nutrition Research 13(2):147–155.
Shi, Z., B. Yuan, A. W. Taylor, S. Zhen, H. Zuo, Y. Dai, and G. A. Wittert. 2014. Riboflavin intake and 5-year blood pressure change in Chinese adults: Interaction with hypertensive medication. Food and Nutrition Bulletin 35(1):33–42.
Shin, W.-Y., and J.-H. Kim. 2019. Low riboflavin intake is associated with cardiometabolic risks in Korean women. Asia Pacific Journal of Clinical Nutrition 28(2):285–299.
Suprapto, B., Widardo, and Suhanantyo. 2002. Effect of low-dosage vitamin A and riboflavin on iron-folate supplementation in anaemic pregnant women. Asia Pacific Journal of Clinical Nutrition 11(4):263–267.
Szczuko, M., T. Seidler, M. Mierzwa, E. Stachowska, and D. Chlubek. 2011. Effect of riboflavin supply on student body’s provision in north-western Poland with riboflavin measured by activity of glutathione reductase considering daily intake of other nutrients. International Journal of Food Sciences and Nutrition 62(4):431–438.
Tavares, N. R., P. A. Moreira, and T. F. Amaral. 2009. Riboflavin supplementation and biomarkers of cardiovascular disease in the elderly. The Journal of Nutrition, Health & Aging 13(5):441–446.
Theofylaktopoulou, D., A. Ulvik, O. Midttun, P. M. Ueland, S. E. Vollset, O. Nygard, S. Hustad, G. S. Tell, and S. J. P. M. Eussen. 2014. Vitamins B2 and B6 as determinants of kynurenines and related markers of interferon-gamma-mediated immune activation in the community-based Hordaland Health Study. British Journal of Nutrition 112(7):1065–1072.
Tremblay, A., F. Boilard, and M. F. Breton. 1984. The effects of a riboflavin supplementation on the nutritional status and performance of elite swimmers. Nutrition Research 4(2):201–208.
Vir, S. C., and A. H. Love. 1977. Riboflavin status of institutionalised and non-institutionalised aged. International Journal for Vitamin and Nutrition Research 47(4):336–344.
Ward, M., C. F. Hughes, J. J. Strain, R. Reilly, C. Cunningham, A. M. Molloy, G. Horigan, M. Casey, K. McCarroll, M. O’Kane, M. J. Gibney, A. Flynn, J. Walton, B. A. McNulty, A. McCann, L. Kirwan, J. M. Scott, and H. McNulty. 2020. Impact of the common MTHFR 677C→T polymorphism on blood pressure in adulthood and role of riboflavin in modifying the genetic risk of hypertension: Evidence from the JINGO project. BMC Medicine 18(1):318.
Wilson, C. P., M. Ward, H. McNulty, J. J. Strain, T. G. Trouton, G. Horigan, J. Purvis, and J. M. Scott. 2012. Riboflavin offers a targeted strategy for managing hypertension in patients with the
MTHFR 677TT genotype: A 4-y follow-up. The American Journal of Clinical Nutrition 95(3):766–772.
Wilson, C. P., H. McNulty, M. Ward, J. J. Strain, T. G. Trouton, B. A. Hoeft, P. Weber, F. F. Roos, G. Horigan, L. McAnena, and J. M. Scott. 2013. Blood pressure in treated hypertensive individuals with the MTHFR 677TT genotype is responsive to intervention with riboflavin: Findings of a targeted randomized trial. Hypertension 61(6):1302–1308.
























