The lived experiences of people with psychiatric disorders vary significantly in terms of symptoms, frequency, and impact on their lives, challenging both clinicians and researchers to match effective treatments with patients who can benefit most, said David Gray, vice president of chemistry at Cerevel Therapeutics. Targeting the glutamatergic mechanisms (see Chapters 3 and 4) has made a substantial difference for some individuals, he said, but other mechanisms and treatment options are also emerging, supported by preclinical and clinical data. Moreover, even for the mechanisms discussed earlier, many unknowns exist with respect to the subtle modes of action, different modes of target modulation, and different downstream effects that may be keys to efficacy, said Gray.
The search for novel antidepressants appears to be converging on a final common pathway—the glutamate receptor (NMDA/AMPA) to mTOR pathway—which modulates cellular activity in the prefrontal cortex, said Robert Davis, senior vice president and chief scientific officer at Intracellular Therapeutics. Multiple entry points into this pathway provide potential new targets for antidepressants, he said. Moreover, it is a very tightly controlled system with many checks and balances, he added. Several novel approaches to evaluating the role of this pathway in psychiatric illnesses are discussed in this chapter although this is not a comprehensive review.
mTOR SIGNALING—A TARGET FOR DEPRESSION AND PSYCHOSIS
Rapamycin, a naturally occurring antifungal and immunosuppressive compound product discovered in 1975 (Vézina et al., 1975) opens the door
to the mechanistic target of rapamycin (mTOR) pathway. The mTOR pathway regulates cellular growth and the cellular response to nutrients through the activity of two protein complexes called mTORC1 and mTORC2, said Eddine Saiah, chief scientific officer at Navitor Pharmaceuticals. mTORC1 plays an especially important role in brain and mood disorders by regulating synaptogenesis via protein synthesis, axon elongation, and dendritic branching, said Saiah. For example, several studies have shown that the mTOR pathway is suppressed in patients with major depressive disorder and in animals that are chronically stressed, said Saiah. In addition, the efficacy of ketamine requires the activation of the mTORC1 signaling pathway and is blocked by rapamycin (Duman et al., 2016), he said (see Figure 5-1). These discoveries suggested the potential to identify direct mTORC1 activators that might have antidepressant effects similar to ketamine, but without the psychotomimetic effects and abuse potential, said Saiah.
Research by several groups, including David Sabatini and colleagues at the Whitehead Institute and MIT, also led to the discovery of amino acid sensors that are required for the activation of mTORC1 and the development of small molecule modulators of these sensors that can also activate mTORC1. At Navitor Pharmaceuticals, Saiah and colleagues discovered a compound called NV-5138 that activates mTORC1 by disrupting the interaction between the amino acid sensor Sestrin2 and a protein called Gator2 (Sengupta et al., 2019). In animal models, they demonstrated that orally administered NV-5138 has favorable pharmacokinetics, activates key synaptic proteins in brain regions implicated in depression, shows efficacy comparable to ketamine, and is dependent on BDNF signaling (Kato et al., 2019). Saiah said that subsequent Phase 1 studies with NV-5138 have shown similar pharmacokinetics and efficacy even after a single dose.
These studies established NV-5138 as a first-in-class direct mTORC1 activator, said Saiah. It gets into the brain, is rapidly absorbed, shows rapid and persistent molecular target engagement, provides rapid relief of core symptoms of depression, and has a favorable safety and tolerability profile. Saiah added that preclinical models demonstrate the efficacy of NV-5138 for up to 7 days, even though the compound has a half-life of only 2–4 hours. One possible explanation, he said, is that once the compound triggers mTORC1 to initiate protein synthesis, the drug can be cleared from the system in 2–4 hours while still mediating its effects over the course of 1 week.
SEROTONIN 5-HT2 RECEPTOR AGONISTS FOR MENTAL DISORDERS
In Chapter 2, Steven Paul described how drugs that target serotonin receptors have been used for decades to treat depression and psychosis.
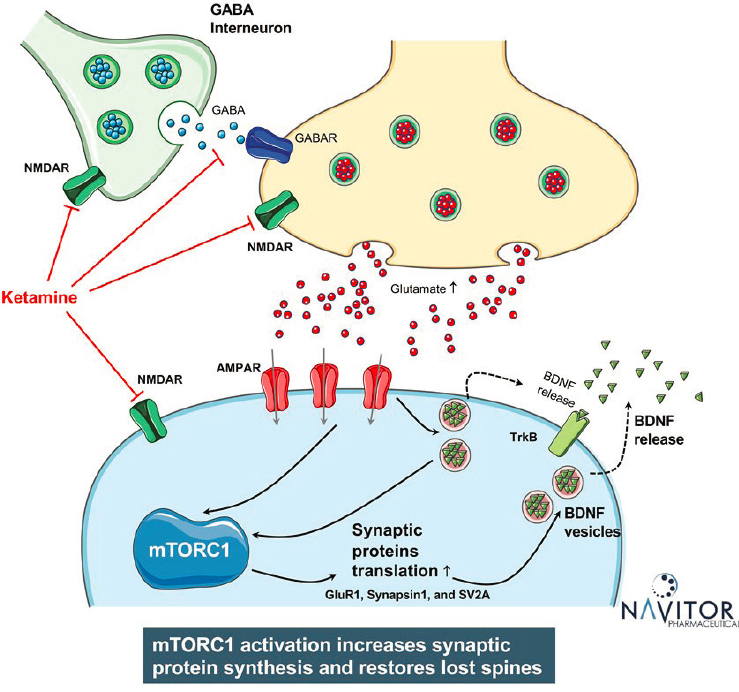
SOURCE: Presented by Eddine Saiah, March 9, 2021.
5-HT2A agonists also include a class of drugs called psychedelics or classic serotonergic hallucinogens, such as LSD, psilocybin, and mescaline, said Gabriella Gobbi, professor of psychiatry at McGill University. Although LSD was synthesized in the 1930s and shown to have dramatic psychological effects, abuse of the drug in the 1960s stifled clinical research for decades (Passie et al., 2008). Interest in using LSD therapeutically reemerged in the 1990s (Carhart-Harris and Goodwin, 2017). Earlier, Paul described how drugs that target serotonin receptors have been used for decades to treat depression and psychosis. 5-HT2A agonists also include
a class of drugs called psychedelics or classic serotonergic hallucinogens, such as LSD, psilocybin, and mescaline, said Gobbi. Although LSD was synthesized in the 1930s and shown to have dramatic psychological effects, abuse of the drug in the 1960s stifled clinical research for decades (Passie et al., 2008). Interest in using LSD therapeutically reemerged in the 1990s (Carhart-Harris and Goodwin, 2017).
In 2016, Gobbi and colleagues demonstrated the electrophysiological effects of increasing doses of LSD on serotonin and dopamine neurons in different brain regions (De Gregorio et al., 2016). What they found was that low doses of LSD decreased serotonin firing activity, but with continued treatment over 7 days, activity increased. This observation led them to investigate long-term treatment with microdoses of LSD, said Gobbi. Using several different behavioral tests in mice, they showed no effect on depression or anhedonia, but increases in social interaction (De Gregorio et al., 2021).
To determine the neurological basis of these behavioral changes, Gobbi and colleagues used electrophysiological methods to show that LSD increased activity at 5-HT2A and AMPA receptors in the medial prefrontal cortex (mPFC), but not 5-HT1A or NMDA receptors, she said. Using optogenetics in mouse models, they went on to show that inhibiting excitatory neurons in the mPFC nullified the prosocial effects of LSD. Gobbi said they also showed that LSD exerts its effects through the mTORC pathway. Still puzzled by the observation that LSD had prosocial effects but did not reduce depressive behaviors, Gobbi said they used a stress model in which animals were stressed for 15 days, and showed that low doses of LSD for 7 days reversed the symptoms of anxiety. The next question, said Gobbi, is whether low doses of LSD can be effective clinically for patients with depression, social anxiety, and/or autism spectrum.
TAAR1/5-HT1AR AGONISTS IN SCHIZOPHRENIA TREATMENT
Kenneth Koblan, chief scientific officer at Sunovion Pharmaceuticals, described a recently developed antipsychotic drug that, unlike many conventional medications for psychosis, does not antagonize monoamine receptors. This agent, SEP-856, was granted breakthrough therapy designation by FDA, said Koblan. SEP-856 is an agonist at the TAAR1 receptor (trace amine receptor 1) as well as at the 5-HT1A receptor, he said.
SEP-856 was discovered with an in vivo screening approach using the SmartCube® system developed by PsychoGenics, said Koblan. SmartCube® uses reference compounds and pattern recognition software to build databases that link compounds to the types of behavioral phenotypes those compounds cause in rodents (Alexandrov et al., 2015). SmartCube® then compares this database against novel compounds to predict the potential
antipsychotic signatures of those compounds. Using this approach to optimize for antipsychotic compounds that do not interact at the D2 receptor or at 5-HT2A receptors, Koblan’s team discovered SEP-856, a small compound that has an antipsychotic-like profile in a number of different animal assays. They found that SEP-856 at low doses has antidepressant effects, while at higher doses it has antipsychotic effects (Dedic et al., 2019) (see Figure 5-2). In vitro screening confirmed that the agent does not interact at the D2 or 5-HT2A receptor. “We believe that it modulates presynaptic dopamine synthesis capacity,” Koblan said regarding its hypothesized mechanism of action.
A global, 4-week Phase 2 clinical study in acutely psychotic patients showed that across all geographies and subgroups, participants randomized to SEP-856 rather than placebo improved significantly on the Positive and Negative Syndrome Scale (PANSS), a scale used to measure the severity of symptoms in patients with schizophrenia. Participants were then able to roll over into an open-label safety study for 6 months, where they showed continued improvement. These robust effects were seen not only in PANSS
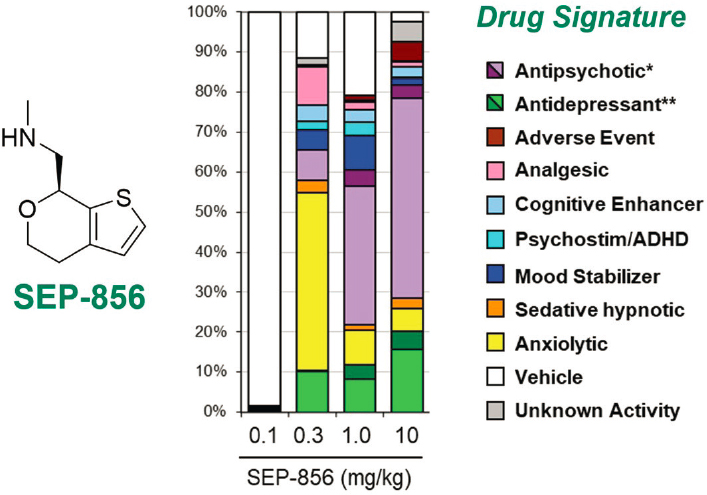
NOTE: * antipsychotic (purple) and high-dose antipsychotic (dark purple); ** antidepressant (green) and high-dose antidepressant (dark green).
SOURCES: Presented by Kenneth Koblan, March 9, 2012; adapted from Dedic et al., 2019.
scores, but in all other key secondary endpoints, including the Clinical Global Impressions Severity (CGI-S) scale and the Brief Negative Symptom Scale (BNSS); there was no evidence of treatment-emergent adverse effects, said Koblan (Koblan et al., 2020).
M1/M4 ACETYLCHOLINE MUSCARINIC RECEPTOR TARGETING FOR PSYCHOSIS
Multiple lines of evidence suggest that muscarinic acetylcholine receptors (mAChRs) are involved in the pathogenesis of schizophrenia, said Alan Breier, the Indiana University Mental Health Research and Education Senior Professor of Psychiatry.
Therefore, they represent excellent therapeutic targets, he explained. Five types of mAChRs are expressed in the brain; two of them, M1 and M4, have been implicated in neuropsychiatric disorders, said Breier. M4 serves as a gatekeeper for acetylcholine release. One hypothesis is that activation of M4 receptors may exert antipsychotic effects by decreasing acetylcholine release, which leads to decreased firing of dopamine neurons, he said. Several agents that target M4 and M1 receptors are in development for schizophrenia.
Breier focused his attention on xanomeline, a muscarinic agonist that engages all five mAChRs, but preferentially engages M1 and M4. A Phase 2 placebo-controlled clinical trial of xanomeline in patients with Alzheimer’s disease unexpectedly showed dose-dependent significant improvement in psychotic symptoms, said Breier (Bodick et al., 1997). This was followed by a small exploratory study in patients with refractory schizophrenia, which demonstrated significant improvements in psychotic symptoms according to multiple clinical measures, including PANSS (Shekhar et al., 2008).
Although the results of both xanomeline studies looked promising, Breier said there were high rates of side effects that commonly result from activating the peripheral cholinergic system, including nausea, vomiting, diarrhea, and excessive sweating. As a result, he said, the drug was licensed by Eli Lilly and Company to Karuna Therapeutics. Scientists at Karuna Therapeutics hypothesized that combining xanomeline with an older generic drug called trospium chloride, which reduces peripheral cholinergic side effects, might mitigate those unwanted outcomes while leaving the antipsychotic effects intact. A recently published Phase 2 study demonstrated that this combined therapy improved the patients’ PANSS total score, with similar improvements observed in clinical subscales that assessed the drug’s impact on positive and negative psychosis symptoms (Brannan et al., 2021).
Breier noted that while there were no significant improvements in cognition relative to placebo, a post-hoc subgroup analysis suggested that participants with high baseline impairment did improve significantly.
Importantly, he said, the combination of xanomeline and trospium demonstrated an improved safety profile with much lower rates of adverse events than were seen in studies of xanomeline alone. Meanwhile, he continued, two Phase 3 studies are under way.
NMDA MODULATION TO IMPROVE NEGATIVE AND COGNITIVE SYMPTOMS IN SCHIZOPHRENIA
Venkatesha Murthy, global head of psychiatry in the Takeda Neuroscience Therapeutic Area Unit, commented on the role of NMDA receptor modulation in the treatment of negative symptoms and cognitive impairment associated with schizophrenia. Clinical trials with bitopertin, a GlyT-1 inhibitor that modulates NMDA receptors failed to demonstrate the successful treatment of schizophrenia’s negative symptoms (Bugarski-Kirola, 2017). Similarly, a recent Phase 2 study of luvadaxistat, a D-amino acid oxidase inhibitor, did not meet its primary endpoint for reducing the severity of negative symptoms in stabilized schizophrenic patients (Neurocrine Biosciences, 2021). With neither of these two different and well-characterized proof-of-concept clinical trials achieving their target endpoints for alleviating negative symptoms, Murthy suggested that, “we should exercise extreme caution in pursuing NMDA modulators for these effects, and also potentially consider a different regimen.”
Although luvadaxistat was not found to improve negative symptoms, the drug developer for this compound reported that signals of improved cognitive performance were observed in some schizophrenia patients (Neurocrine Biosciences, 2021). Similarly, a recent Phase 2 study of the glycine transporter-1 inhibitor, BI 425809, also demonstrated improved cognition in schizophrenia patients. (Fleischhacker et al., 2021). Given these indicators, the effects of NMDA modulators on cognitive performance need to be deeply characterized and replicated, said Murthy.
THE ADDED COMPLEXITY OF POLYPHARMACY
Only rarely does a drug interact with just one target or one pathway, said Davis. New targets and opportunities, as well as new challenges, may be identified by focusing attention on drug actions beyond receptors. Looking beyond receptors will add complexity to studies as well as the potential generation of large amounts of data that must be deconvoluted. Moreover, he noted, new tools are needed to facilitate the translation of novel compounds from nonclinical to clinical settings.
Among the drugs discussed at this workshop, several also have pronounced polypharmacologic activity, said Bryan Roth, the Michael Hooker Distinguished Professor of Pharmacology at the University of North
Carolina at Chapel Hill School of Medicine. For example, LSD could be called either one of the “dirtiest” drugs or the one with the most robust pharmacology, hitting probably 50 targets in the brain with very high potency. Which target is responsible for its effects remains unclear. Roth continued by noting that it is similarly unclear whether the effects of xanomeline result from actions on the M1 receptor, the M4 receptor, both, or lower affinity action at other receptors. Paul suggested that both M1 and M4 receptor targeting may be important—M1 more for treating the precognitive effects and negative symptoms and M4 more for treating psychosis. In Roth’s lab and others, researchers are leveraging computational approaches to construct roadmaps for developing drugs that interact with multiple molecular targets.
Brier emphasized that hitting more than one target may be required for efficacy in complex diseases such as schizophrenia and depression. The extraordinary heterogeneity of these disorders adds further complexity, added Roth. Hundreds or maybe even thousands of loci may contribute to the genetic risk of these disorders, so it is not surprising that more than one molecular target would need to be engaged to affect the broad patient population. Whether targeted therapies for individual patients may ultimately prove beneficial remains an unanswered question, said Roth.
Roth continued by remarking that unbiased proteomic and single-cell transcriptomic technologies are interrogating “a whole universe of intracellular pathways” and are studying those pathways longitudinally. Thanks to the National Institutes of Health’s Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative and other initiatives, he said, it is now possible to delve into the detailed chemical and molecular biology of drug actions for existing drugs and use that knowledge to identify even safer and more effective medications. He added that tools are also now available to examine whether these various drugs work similarly on a network level by altering excitatory and inhibitory neurotransmission pre- or postsynaptically.
Other clinical endpoints could be useful in evaluating novel compounds, including those with polypharmacological properties, for their antipsychotic effects. For example, Koblan cited technological innovation around the use of electroencephalography, while Gobbi added that the measures of sleep duration and sleep quality have also been recently used as outcome measures in the development of drugs for neurological disorders.
This page intentionally left blank.










