1
Introduction
Throughout 2020 and 2021, the global response to COVID-19 demonstrated the importance of vigilance and preparedness for infectious diseases. In this context, there was a marked demand placed on vaccine research and development (R&D), with researchers, federal agencies, public health institutions, manufacturers, and regulators being rapidly mobilized to develop an effective vaccine as quickly as possible. Throughout this process, many opportunities and hurdles were identified that can inform the development of a conceptual framework for limiting the spread of future viral outbreaks, including pandemic influenza. The U.S. National Influenza Vaccine Modernization Strategy 2020–2030 (HHS, 2020) and the World Health Organization (WHO) Global Influenza Strategy 2019–2030 (WHO, 2019), have both called for improving influenza vaccines with respect to both effectiveness and scalable manufacturing technologies. As demonstrated throughout the response to COVID-19, the process of R&D of novel vaccines can be significantly optimized when stakeholders are provided with the resources and technologies needed to support research and when data are shared in real time (Kinsella et al., 2020). This report will identify the most important drivers of success in this model and provide recommendations for using those findings to support vaccine R&D for prevention and control of both pandemic and seasonal influenza outbreaks.
This report is the product of one of four studies conducted under an initiative carried out by the National Academy of Medicine (NAM) within the National Academies of Sciences, Engineering, and Medicine at the request of the U.S. Department of Health and Human Services’ Office of Global Affairs. The purpose of this initiative was to use lessons learned from the
response to the COVID-19 pandemic to advance vaccine preparedness for pandemic and seasonal influenza. The NAM convened an international committee of domestic and global experts who met to discuss the key issues that arose during the COVID-19 response and to identify considerations for the development of a framework for preparedness for the next pandemic, both domestically and globally. The discussions of this international committee served as the basis for the statements of task for the ad hoc committees charged with conducting the four concurrent National Academies consensus studies.
The present report was produced by the Committee on Vaccine Research and Development Recommendations for Advancing Pandemic and Seasonal Influenza Preparedness and Response, convened to identify opportunities for optimizing vaccine R&D processes in the event of an influenza pandemic, and to determine whether those opportunities could be used to inform the development of seasonal and universal influenza vaccines. The full charge to the committee responsible for this report is provided in Box 1-1. The committee included 10 members with academic and professional expertise in immunology, virology, vaccine manufacturing, and vaccine surveillance, as well as other related focus areas. The committee biographies are provided in Appendix A. Regrettably, one committee member passed away during the study.
STUDY APPROACH AND SCOPE
This study is intended to address the need to optimize influenza vaccine technology based on current capabilities, a need identified in the 2019–2030 WHO Global Influenza Strategy (WHO, 2019), the 2020–2030 U.S. National Influenza Vaccine Modernization Strategy (HHS, 2020), and other documents. This report presents the findings of the committee, based on discussions during a series of 10 meetings that occurred over a span of 4 months, March through August 2021. During its first meeting, the committee decided that its charge would best be met by focusing on the following four dimensions of vaccine R&D with a vision of supporting the rapid development of between 4.15 and 8.31 billion doses of influenza vaccine globally in a timely and equitable manner: (1) basic and translational science, (2) clinical science, (3) manufacturing science, and (4) regulatory science. The committee would like to emphasize the importance of building adequate capacity in these four dimensions to ensure global preparedness for the next impending influenza outbreak.
The first seven committee meetings included open sessions during which speakers presented information relevant to the committee’s charge. Meeting themes were developed based on one of the above four dimensions. All public meeting agendas can be found in Appendix D. The open sessions were followed by closed sessions during which the committee reflected on key findings, conclusions, and recommendations relating to the speakers’
presentations, as well as the meeting topic at hand. The 8th, 9th, and 10th meetings focused on arriving at committee consensus on outlines developed following the prior meetings, which were used by the committee and study staff to write this report.
This report is intended to provide an overview of a myriad of complex issues defined in the committee’s Statement of Task (see Box 1-1); it makes no claim to providing comprehensive or systematic examination of all the available evidence on these issues. Figure 1-1 shows how each major element of the Statement of Task maps to the chapters of the report.
The content of this report was informed by the extensive expertise of the committee members, which was applied in formulating recommendations for improving influenza vaccine R&D. The findings, recommendations, and conclusions in this report also reflect evidence and accounts related to the COVID-19 pandemic that were published prior to August 2021, such as peer-reviewed journal articles, case studies, and news media articles. It should be noted that this study was initiated and carried out while the COVID-19 response was actively under way; thus, the literature available on vaccine R&D at that time may not reflect the full breadth of the growing evidence base. To circumvent such limitations, the committee relied heavily on expert accounts provided by the many speakers at its open sessions who provided key insights about vaccine R&D efforts led by various organizations in response to COVID-19, which helped to ensure that the committee had a good understanding of developments in R&D capacity as they occurred.
BACKGROUND
Previous pandemics were caused by influenza A viruses. The influenza pandemic in 1918–1919, caused by an H1N1 virus with genes of avian origin, is the largest pandemic recorded and is estimated to have caused 50 million deaths (Johnson and Mueller, 2002; Taubenberger and Morens, 2006). In 1957, the world experienced an H2N2 pandemic that was estimated to have caused 1.1 million excess deaths (Viboud et al., 2016). In 1968 a pandemic was caused by an H3N2 virus strain. This virus contained two genes from an avian virus and six genes from the 1957 H2N2 virus (Kawaoka et al., 1989). This pandemic resulted in an estimated 1 million deaths (CDC, 2019b).
The 2009 influenza pandemic was caused by a novel influenza A H1N1 virus that originated in pigs. The first cases were identified in February 2009 in Mexico (Hsieh et al., 2011). Two months later, the first U.S. cases were identified when two pediatric cases in southern California were reported to the U.S. Centers for Disease Control and Prevention (CDC, 2009). On April 25, WHO declared a public health emergency of
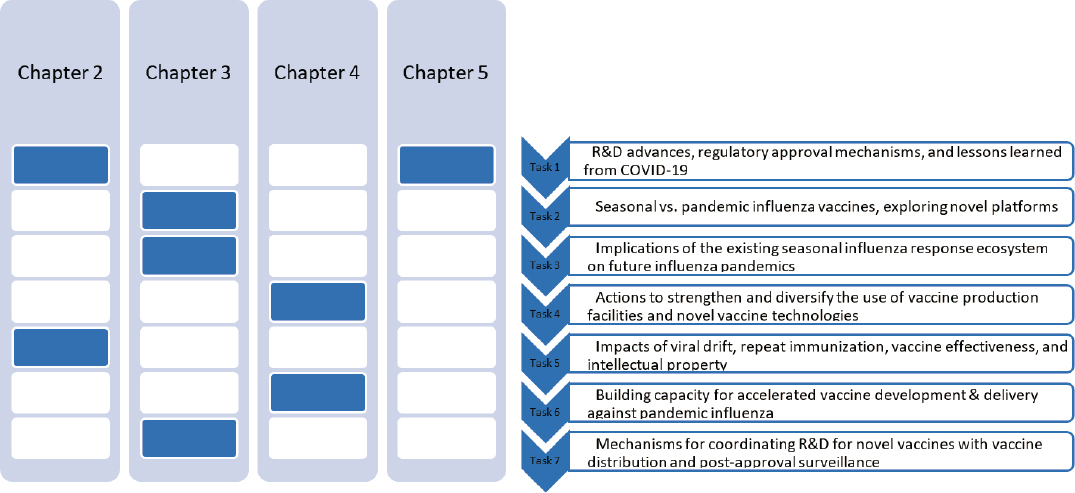
NOTES: The committee will address seven major themes, outlined above in their corresponding chapters. Chapter 2 will address tasks 1 and 5; Chapter 3 will address tasks 2, 3, and 7; Chapter 4 will address tasks 4 and 6; and Chapter 5 will also address task 1.
international concern and activated the International Health Regulations (IHR) that were developed after the severe acute respiratory syndrome coronavirus (SARS) outbreak in 2003. On June 11, 2009, WHO declared a pandemic. The first vaccine virus candidates were developed a month after the public health emergency was declared, and vaccine seed strains were made available within weeks (CDC, 2019c). However, it took months for the vaccine to be manufactured and distributed, and by then, the peak was over, and the effects of the vaccine were limited. While the response was much more rapid in terms of vaccine development than what was seen during COVID-19, this pandemic showed that despite an early decision to develop vaccines, their manufacturing and distribution were too slow to limit the spread of the first wave of the virus (WHO, 2011). As of August 10, 2021, the recorded COVID-19 death toll was 4.3 million (LePan, 2021) but is estimated to be as high as 16 million. Figure 1-2 shows a history of global pandemics throughout history.
This section provides brief background information on the following:
- influenza virus and its effects on humans,
- viral antigenic drift and shift and the implications for influenza vaccine development,
- the landscape of influenza vaccine platforms,
- the effectiveness of seasonal influenza vaccines,
- the potential for developing a universal vaccine, and
- vaccine development in the context of the COVID-19 pandemic.
Chapter 2 delves into several of these topics in greater detail.
The Influenza Virus and Its Effects on Humans
The influenza virus is a segmented, negative-sense RNA virus that can be divided into four types: A, B, C, and D. Influenza types A and B cause seasonal epidemics and influenza A viruses cause sporadic pandemics. Influenza A viruses are further classified into subtypes, based on the antigenicity of the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA). To date, 18 HA and 11 NA subtypes have been discovered (CDC, 2019a), of which viruses containing 3 HA (H1, H2, and H3) and 2 NA (N1 and N2) subtypes have caused epidemics in humans (Taubenberger and Morens, 2010). The virion’s envelope also contains proteins that serve as ion channels (M2). The matrix protein (M1) provides structure to the virion, and within the virion, the segmented viral RNA is localized, bound to nucleoprotein and polymerase proteins (Gómez-Puertas et al., 2000).
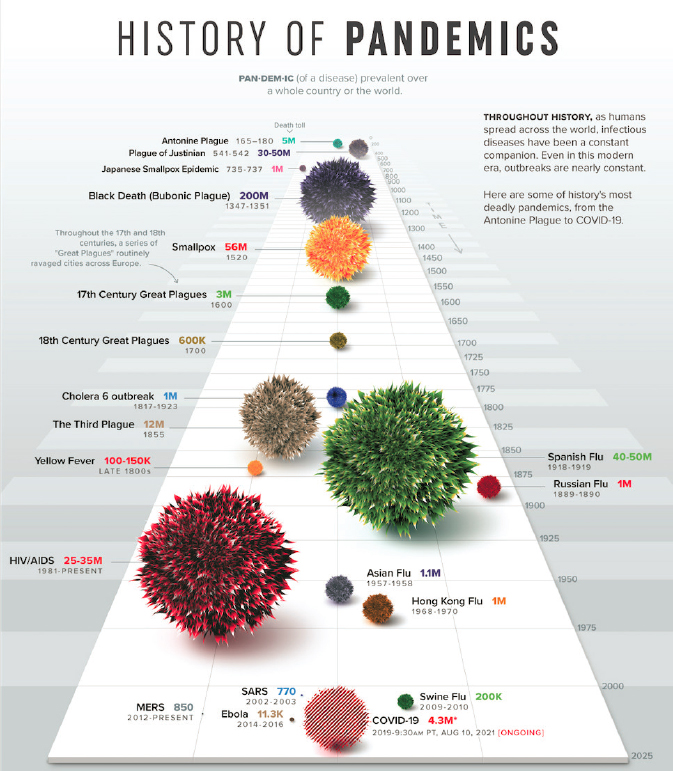
SOURCE: LePan, 2021.
Disease Caused by Influenza Viruses
The influenza virus HA attaches to the terminal α-sialic acid on the surface of the host cell and this strong binding initiates the endocytosis process (Luo, 2012). A reduction in the pH in the endosome allows the viral membrane to fuse with the host membrane; these processes are mediated by the M2 ion channel and HA. The fusion results in the release of the eight RNA viral segments into the nucleus. The RNA segments are translocated to the nucleus where they are transcribed and replicated (Luo, 2012). mRNA is transported back into the cytoplasm for translation and virion RNA seg-
ments exit the nucleus and are transported to the cell surface where new progeny virions assemble and bud.
After infection with an influenza virus, respiratory disease generally occurs within 1 to 4 days, and is characterized by a sudden onset of fever, (dry) cough, headache, muscle and joint pain, severe malaise, sore throat, and other respiratory symptoms. Symptoms peak on day 2 or 3 after infection and resolve in most cases within 9 days. Viral shedding peaks at day 2 and lasts an average of 4.8 days (Carrat et al., 2008).
In general, influenza is a self-limited illness (Ghebrehewet et al., 2016; Paules and Subbarao, 2017). Symptoms can, in severe cases, lead to pneumonia and death. Severe disease occurs worldwide in an estimated 3–5 million cases every year, resulting in 290,000–650,000 deaths (Iuliano et al., 2018; Jha et al., 2020; WHO, 2021c). Most of the severe cases and deaths occur in groups at high risk of complications of influenza, including children under the age of 5, during pregnancy, people older than 65 years, people with chronic diseases, and patients with immunosuppressive conditions or taking immunosuppressive drugs (Ortiz et al., 2018; WHO, 2021b). These high case numbers result in associated health care costs also being high at an estimated $2.0–$5.8 billion annually in the United States alone (Yan et al., 2017).
Immune Response to Natural Influenza Infection
Given that the incubation period of influenza is short, acute effects as induced by the innate immune response are essential to curb infection. Viral RNA can be recognized by pattern-recognition receptors (PRRs), such as toll-like receptors (Zhu et al., 2021). The activation of PRRs results in the production of proinflammatory cytokines and type I interferons, resulting in the inhibition of viral replication in host cells. Alveolar macrophages play an important role in the innate immune response and limit the spread of the virus by phagocytosis of infected cells. Dendritic cells present antigen to CD4+ and CD8+ T cells to stimulate adaptive cellular responses and can exert cytolysis (interferon killer dendritic cells).
The adaptive immune response forms the second line of defense against viral infection, consisting of humoral and cellular responses. The humoral response includes the production of IgA, IgM, and IgG antibodies that are mainly directed against HA and NA. A proportion of the antibodies directed against HA can neutralize virus infectivity by inhibiting the attachment to the receptor and viral entry, while the antibodies against NA inhibit its enzymatic activity resulting in reduced viral spread. Additionally, natural killer (NK) cells can recognize and lyse infected cells bound to antibodies, a process called antibody-dependent cell cytotoxicity. Antibodies directed against M2 protein are produced to a limited extent, and antibod-
ies directed against nucleoproteins may result in complement-mediated cytotoxicity (Sedova et al., 2019).
Cellular immune responses consist of both CD4+ and CD8+ T cells (Jameson et al., 1999). CD4+ T cells mainly function as helper cells, producing cytokines that stimulate B cell responses (humoral, Th2 response) and promote cellular responses (Th1 response). The main activity of CD8+ T cells is as cytolytic T lymphocytes, which are activated in lymphoid tissues by antigen-presenting cells, recruiting these cells to the infection site. Cytolytic T lymphocytes kill virus-infected cells by producing and releasing perforins and granzymes, or by inducing apoptosis through the Fas/FasL pathway. Additionally, these cells also produce a series of cytokines that promote antigen presentation by stimulation of major histocompatibility complex molecule expression (Janway et al., 2001).
Viral Antigenic Drift and Shift and Implications for Vaccine Development
Influenza viruses are characterized by their ability to rapidly undergo antigenic changes caused by mutations in the antibody-binding sites of the HA and NA viral surface proteins, a process called antigenic drift. The virus acquires mutations because the viral RNA-dependent RNA polymerase does not have proofreading activity, resulting in a high error rate causing mutations in the genes and proteins (Drake, 1993). The HA is also under positive selection from immune pressure, resulting in mutations in key antigenic sites that allow the virus to escape neutralization by antibodies induced by prior infection or vaccination. Additionally, antigenic shift may occur when two viruses coinfect a cell. Coinfection results in reassortment of gene segments, giving rise to new gene constellations and introduction of novel gene segments, resulting in a novel virus that has the potential to cause a pandemic (Lopez and Legge, 2020).
Because of the pandemic and seasonal risks, it is essential that circulating influenza viruses are closely monitored. The WHO Global Influenza Surveillance and Response System (GISRS) is a worldwide surveillance network that monitors influenza viruses (WHO, 2021a). A WHO advisory group of experts reviews the influenza surveillance data generated by GISRS at biannual meetings to decide whether the seasonal influenza vaccine composition requires updating. Annual seasonal influenza vaccines are based on these surveillance data, and given the limited time available to manufacture vaccines for worldwide distribution, timely development is crucial (Hay and McCauley, 2018).
The WHO GISRS surveillance system ensures that virus strains that are prevalent during the previous season and likely to cause disease in the following season are known. Virus samples are collected and analyzed year-round by the WHO Collaborating Centers (located in Atlanta, Bei-
jing, London, Melbourne, and Tokyo) (CDC, 2019e; WHO, 2013). Based on the antigenic and genetic characterization, antigenic cartography and predictive modeling, WHO recommends candidate viruses for the composition of the seasonal vaccines. This is done twice per year to account for seasonal influenza in the Northern and Southern Hemispheres (Ziegler et al., 2018).
Seed virus strains of the candidate viruses are produced by genetic reassortment of H1N1 and H3N2 viruses. For this genetic reassortment, the genes encoding the HA and NA proteins of the recommended influenza A viruses are reassorted with the internal protein genes of influenza A/Puerto Rico/8/34 (PR8 strain) to generate high-growth reassortant viruses. The PR8 strain is adapted to growth in chicken eggs to ensure high yield and fast production of vaccines and is safe for humans (Vemula et al., 2017). For influenza B viruses, reassortant viruses or wildtype viruses are used (Ping et al., 2016). The high growth reassortant viruses are distributed to vaccine manufacturers to start the production of vaccines.
The Landscape of Influenza Vaccine Platforms
Egg-Based Vaccine Platform
About 95 percent of the licensed seasonal influenza vaccines are manufactured in embryonated chicken eggs. The trivalent inactivated vaccine (TIV) comprises three circulating influenza strains, of which two are influenza A (H3N2 and H1N1) and one influenza B. The quadrivalent inactivated vaccine (QIV) was approved by the U.S. Food and Drug Administration (FDA) in 2012 and includes an additional B strain. Both the TIV and QIV can be manufactured in three formulations: inactivated whole virus, detergent-split virus, or subunit virus. Whole virus vaccines are immunogenic but are associated with a high level of reactogenicity. The virus is inoculated in embryonated chicken eggs and allantoic fluid is harvested. The virus is then chemically inactivated using formalin or β-propiolactone, concentrated, and purified to remove any nonviral protein contamination, such as chicken ovalbumin (Gerdil, 2003). Thiomersal or formaldehyde is used as a preservative to prevent bacterial or fungal growth. Because of their reactogenicity, whole virus vaccines have been largely replaced by split and subunit vaccines, in which additional treatment with detergents or solvents has disrupted the viral lipid envelope. The resulting incomplete viral particles contain HA, NA, and matrix proteins. These vaccines have lower reactivity than the whole virus vaccines.
Vaccines developed to mimic natural mucosal infection can theoretically induce both cellular and humoral immunity (Zhou et al., 2016). Live attenuated influenza vaccines are engineered to grow below 33°C, to
limit the replication in the upper respiratory tract after inhalation (White et al., 2021). An example of this type of vaccine is FluMist/Fluenz (Zhou et al., 2016). These vaccines were first developed by serial passage of the virus in eggs under suboptimal conditions to create temperature-sensitive phenotypes. These strains then formed the “master donor” strains. These donor strains are used to contribute the internal genes, except HA and NA genes, to generate new vaccine strains with the HA and NA of the circulating strains by classical reassortment in eggs or reverse genetics (see below). Additional molecular changes have been made to internal genes to attenuate the virus and confer temperature sensitivity. The idea behind this concept is that these strains can replicate in the nasal passage but not in the respiratory tract and, in that way, elicit mucosal immune responses while not causing disease (Zhou et al., 2016).
The egg-based vaccine platform has been used since the 1940s and is well established. Because of these characteristics, it is a relatively cheap method to produce vaccines. However, it does come with some limitations that affect efficacy and limit use in case there is a high demand for vaccines, such as during a pandemic (Milián and Kamen, 2015). The egg-based platform, as described, is a time-consuming process. It takes approximately 6 months to go from the selection of strains to manufacturing vaccines to worldwide distribution (Gerdil, 2003). Given the extensive timeline required to manufacture egg-based vaccines, there is only limited time for errors. Limited availability of embryonated chicken eggs is a risk factor for this process and may delay production.
Another limitation of this platform is that the egg-based system is prone to mutations in the virus strain as it adjusts to growth in eggs (Subbarao and Barr, 2019; Wu et al., 2019). This may result in a discrepancy between the strain of the vaccine and the circulating strain, limiting the effectiveness of the vaccine, which is specifically a problem for seasonal vaccines (Pushko and Tretyakova, 2020). Additionally, there is a risk of allergic reactions to any residual egg proteins in the vaccine for people with allergies to egg antigens. However, egg allergy is not a contraindication to receiving influenza vaccine in many countries (Pushko and Tretyakova, 2020).
Cell-Based Vaccine Platform
An alternative to the egg-based approach is the use of qualified cell lines for vaccine production. This technique can use reassortant viruses or can make use of reverse genetics, a procedure that uses molecular techniques to generate a specific virus phenotype (Hegde, 2015; Wong and Webby, 2013). Using plasmid-based systems, viruses can be generated from cloned cDNA (Fodor et al., 1999; Neumann et al., 1999). After amplifying specific viral RNA fragments, the RNA is cloned into plasmids containing an
RNA polymerase I promoter sequence to drive generation of viral RNA in an antisense direction. A second RNA polymerase II promoter on the same plasmid, but in sense direction, generates mRNA for transcription of viral proteins. Alternatively, viral proteins can be expressed from a second set of plasmids. These plasmids are subsequently transfected into cells and provide the cells with all the information needed to make influenza viruses. After culture of the transfected cells, viable virus is recovered from the culture supernatant. Cell lines used to produce influenza vaccines include the Vero African monkey kidney cell, Madin-Darby canine kidney (MDCK), and the human-derived cell lines (Hegde, 2015) adapted to grow in suspension. Vaccine production using cell lines has several advantages. This technique is more easily scalable than the egg-based platform, as cell lines are widely available and can be expanded quickly. An example of an MDCK-developed vaccine that has demonstrated the potential efficiencies of this approach in productivity improvements is Flucelvax (CDC, 2021). Cells can be cryopreserved and scaled up in times of high need, which is a clear advantage in urgent situations such as pandemics if the proper facilities are available (Wong and Webby, 2013). Furthermore, this platform offers ease of manipulations in manufacturing and reduces risk of allergic reactions as the vaccine does not contain any egg components (Wong and Webby, 2013). Finally, there is a lower risk of viral mutations as seen in the egg-based platform, resulting in a vaccine with higher similarity to circulating virus strains (Harding and Heaton, 2018).
There is a risk of an adventitious virus in the cells being unintentionally introduced into the manufacturing and affecting vaccine quality. Additionally, there may be intellectual property (IP) issues around the application of reverse genetics for seasonal influenza. Therefore, this method requires extensive screening, and the process might have to be intensified to obtain the required yield.
Recombinant Influenza Vaccines
Another seasonal influenza vaccine platform that does not use chicken eggs is the recombinant protein influenza vaccine system. This is a relatively novel method that makes use of a baculovirus expression vector system. Baculoviruses are DNA viruses that can infect insects and are engineered using recombinant DNA technology to replace the gene for polyhedrin with an influenza gene of interest, such as a gene encoding a specific HA (Fabre et al., 2020). Baculovirus specifically infects Spodoptera frugiperda ovarian cells (Felberbaum, 2015). Because of the powerful promoter gene, a high level of HA protein production can be obtained. The SF+ cells are cultured in a fermenter and infected with the baculovirus that is engineered to express selected influenza genes. The culture is maintained for 2–4 days,
the supernatants are collected, and viruses are purified by centrifugation. The protein (for the vaccine) is then extracted from the virus with detergent (Cox and Hashimoto, 2011; Invitrogen Life Technologies, n.d.). This method allows rapid scaling up, speeds up the vaccine production process, and is suitable for highly pathogenic viruses, which could be essential when a pandemic hits. A limitation of this platform is that the insect cells may not modify the expressed protein posttranscriptionally the same way as mammalian cells do, which may slightly affect the proteins antigenicity (Wong and Webby, 2013). FluBlok is an FDA-approved example of a recombinant HA trivalent vaccine derived using SF+ cells (Fabre et al., 2020; Felberbaum, 2015).
Effectiveness of Seasonal Influenza Vaccines
Vaccine effectiveness (VE) ranges from 10 to 60 percent for each seasonal vaccine component but has been relatively low for A(H3N2) viruses (Paules and Fauci, 2019). In the 2017–2018 season in the United States, the overall adjusted VE was 36 percent, with only 25 percent VE for the influenza A(H3N2) virus and 42 percent for influenza B viruses (Flannery et al., 2018). Even though effectiveness is low, the U.S. CDC still recommends vaccination as it prevents some infections, hospitalizations, and deaths. In a 9-year study of seasonal influenza from 2005 to 2014, Foppa et al. (2015) estimated that more than 40,000 lives were saved by vaccination. More recently, data from the 2018–2019 influenza season indicates vaccination prevented an estimated 4.4 million illnesses and 3,500 deaths in the United States alone (Chung et al., 2020).
The prediction of which virus strains will be circulating is an imperfect process. Therefore, vaccine strains do not always match the circulating strains, limiting VE. The egg-based vaccine platform has the additional limitation that it may result in changes in the viral genome owing to adaptation of the virus to grow in eggs (Ping et al., 2015). Additionally, there are VE challenges in specific high-risk populations, such as the elderly, owing to immunosenescence (Smetana et al., 2018), and in immunocompromised persons (Beck et al., 2012).
Repeat Immunization Effects: The Effect of Immune History or Imprinting
Another reason for the limited responses to seasonal influenza vaccination is immune history. Antibodies elicited by vaccination tend to decay within a year, in particular in older adults (Young et al., 2017). Because of antigenic drift, repeated annual administration of vaccines are required to keep up with changes in the virus. Despite extensive efforts to update
vaccine strains to correct for antigenic drift, VE has been poor, particularly against H3N2 viruses (Allen and Ross, 2021).
Prior immunization can modify the VE of influenza vaccines. As part of the “antigenic sin” hypothesis, two observations are of importance: (1) antibody titers are highest to strains encountered in early childhood, and (2) vaccine antibody responses are dominated by major antigens of viruses encountered in primary childhood infections (Francis, 1960). It was originally hypothesized that people produce antibodies directed against strains encountered during early childhood at the expense of the response toward the current strain (Davenport et al., 1953). However, this hypothesis has been modified, and it is now thought that an encounter of a new influenza virus strain back-boosts titers against previously encountered strains; thus, back-boosting diminishes with antigenic distance from the new strain (Fonville et al., 2014; Lessler et al., 2012). This indicates that memory B cells induced by prior infections are activated by vaccination (Palm and Henry, 2019). Annual vaccination may accelerate this antibody refocusing on previously encountered epitopes, resulting in memory responses that do not neutralize the current strain. However, prior infection does boost the immune response and infection results in a broader antibody range than vaccination (Dugan et al., 2020). Therefore, the antibody-focusing hypothesis was developed suggesting that previously induced B cells dominate and focus the response to highly conserved epitopes between prior and prevailing vaccine strains, limiting protection when the epitope changes (Cobey and Hensley, 2017).
Correlates of Protection
The primary correlate of protection in use for seasonal influenza vaccines are hemagglutination inhibition (HI) antibody titers. HI antibodies are directed against the HA that binds sialic acid on human cells, including red blood cells, resulting in agglutination of red blood cells in solution. In the HI assay, this mechanism is used to determine whether antibodies block this cross-linking and inhibit hemagglutination (CDC, 2019d). HI antibodies are a surrogate for virus neutralization, because antibodies that block sialic acid binding inhibit the ability of the virus to infect cells (Kaufmann et al., 2017). HI antibody titers are widely used and are accepted by regulators as a correlate of protection for inactivated influenza vaccines, given that this surrogate is reasonably likely to provide clinical benefit.
However, the HI assay does not measure antibodies directed against the HA stem or other viral proteins, nor can it detect T cell responses. Therefore, this assay is not suitable for any of the universal or broadly protective vaccine candidates. In addition, the HI assay is an indirect measure, and
the assay is a less sensitive measure that can be inconsistent. Therefore, innovation in measuring antibodies to HA is needed.
Universal Vaccine Development
Given the antigenic drift of influenza viruses and limitations with regard to long-lasting antibody response, there is a need to provide seasonal vaccines every year. Targeting the more conserved regions of the virus and improving vaccine-induced immunity could potentially mitigate these issues. Additionally, such strategies could be used to develop pandemic vaccines, decreasing the time it takes to produce vaccines in the traditional way. There are multiple targets of interest for the development of such universal or broadly effective vaccines.
The influenza virus requires the HA glycoprotein to attach to sialic acid-containing proteins on human cells and for the fusion between viral and endosome membranes to release viral RNA into the cell’s cytoplasm (Krammer et al., 2018a). Given the importance of HA for the virulence of the virus, the HA protein is the main target for vaccines. There are two main groups of HA: group 1 and group 2, and antibodies mostly cross-react within groups (group 1: H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17, H18; and group 2: H3, H4, H7, H10, H14, H15). Seasonal vaccines target the head domain of the HA protein that easily mutates. The HA stem and certain head regions are less prone to mutations, and antibodies toward them can potentially provide broader protection as they could neutralize various subtypes. However, these regions elicit limited immune responses (Estrada and Schultz-Cherry, 2019). Therefore, new vaccination strategies are required to target these regions. Vaccination using chimeric HA is one such option. In this strategy, the immunodominant head domain of seasonal vaccines is replaced with an avian head domain, to which humans are naïve (Nachbagauer et al., 2021). Multiple vaccinations with different heads and the same stem can elicit an immune response to targets that remain consistent, in this case, stem epitopes. An alternative to this approach replaces not the whole head, but only certain antigenic sites in the head domain to stimulate immune responses toward the conserved regions of the head. This strategy is called the mosaic HA vaccine. The Computational Optimized Broadly Reactive Antigens (COBRA) approach uses multiple HA sequences of a specific subtype to elicit broad cross-reactive responses toward the HA head and stem (Giles and Ross, 2011). The mini HA antigen vaccination approach uses epitopes only expressed on the HA stem that are structurally engineered to produce a stable construct. Repeated vaccination with these antigens can boost humoral responses toward the HA stem. Another option is the use of nanoparticle immunogens containing multiple HA glycoprotein
trimers designed computationally and constructed in a controlled way so that the four HAs of licensed quadrivalent influenza vaccines are included. These “mosaic” nanoparticle immunogens have recently been shown to elicit antibody responses in preclinical models against heterologous viruses by targeting the conserved HA stem (Boyoglu-Barnum et al., 2021).
NA is a glycoprotein expressed on the surface of the virus and infected cells. The virus uses this enzyme to release the virion from infected cells. Human anti-NA antibodies can be highly and broadly protective as these inhibit the enzymatic activity of NA. NA drifts slower than HA and is well conserved within each subtype (Westgeest et al., 2012). NA content is currently not standardized in commercial vaccines, but NA inhibition has been shown to be a correlate of protection in both animal studies (Chen et al., 2018; Krammer et al., 2018b) and human studies (Maier et al., 2020; Memoli et al., 2016; Monto et al., 2015). Recombinant NA vaccines can be produced in a similar fashion as recombinant HA vaccines, with the potential to supplement current seasonal vaccines by targeting NA specifically, although there might be challenges regarding NA stability.
Internal proteins of the influenza virus are also of interest to target with vaccination strategies. The nucleoprotein and polymerase subunits are highly conserved; however, these proteins are not easily accessible for antibodies. Infected cells produce high levels of these proteins and present the antigens on major histocompatibility complex molecules, which may elicit cellular immune responses. Therefore, these proteins are investigated as potential targets for T cell-based vaccines (Carragher et al., 2008). This could be achieved using vector-based, nucleic acid, and recombinant protein vaccines.
The M2e ectodomain is a viral ion channel with limited expression on the virion but is also expressed on the cell surface of infected cells at relatively high density. The M2 protein is primarily expressed in the trans-membrane and intracellular regions. However, the M2e ectodomain consists of 23 amino acids on the N terminus and is highly conserved, making it a target of interest (Saelens, 2019). Vaccines targeting M2e can induce infection-permissive (not sterilizing) immunity. In small animal models it generally protects well against infection but may permit some virus replication and morbidity.
Increasing Immunogenicity and Duration of Immune Response
To improve the current seasonal vaccines, manufacturers have aimed to improve the immunogenicity of vaccines by producing high-dose formulations of the vaccines. These higher dose formulations have been shown to improve the immunogenicity (high-dose Fluzone and Flublok) (Cox et al., 2015). Additionally, adjuvants can elicit more robust immune responses, as has been shown in the vaccine Fluad, which uses an oil-in-water adjuvant
(Tregoning et al., 2018). The research into increasing the immunogenicity has largely focused on the older population who experience immunosenescence, and current research is investigating the benefit of these dose and adjuvants changes in children with an immature immune system. Novel vaccine platforms such as DNA, RNA, and vector virus vaccines may be able to deliver high doses of antigen or provide adjuvant properties to vaccines, potentially improving immunogenicity. Additionally, vaccines developed with these platforms have the potential to induce not only antibody responses, but also CD8+ T cell responses, potentially improving VE. However, whether these platforms indeed improve the immunogenicity for influenza vaccines remains to be investigated.
Vaccine Development in the Context of the COVID-19 Pandemic
After the WHO Country Office in China was notified of cases of pneumonia with unknown cause in Wuhan, China, on December 31, 2019 (WHO, 2020a), it took less than 2 weeks before the first genome sequence of the new coronavirus was made public on January 10, 2020 (Paden et al., 2020). The virus was later named SARS-CoV-2, and infection causes coronavirus disease 2019 (COVID-19). Two months after the sequence was first published, WHO declared COVID-19 a pandemic (WHO, 2020b). Based on previous work on SARS and MERS, the spike protein of the virus was detected as a target for vaccines, and development started after the publishing of the sequence. Even though previous research played a vital role in allowing the quick development of vaccines, the lack of funding in between the SARS and the COVID-19 pandemics slowed down the speed of development of vaccine candidates.
Vaccines were developed based on novel vaccine platforms, a development that rested on many years of basic science research (see Figure 1-3). The first phase I/II trials for vaccines used the mRNA vaccine platform and were conducted by Moderna and Pfizer-BioNTech, starting on March 16, 2020 (Moderna, 2020a) and April 23, 2020, respectively (Pfizer, 2020b). The first phase III clinical trial results were announced in November (Pfizer, 2020a) and December 2020 (Moderna, 2020b), after which Emergency Use Authorization (EUA) was provided for multiple vaccines (FDA, 2021b,c). On February 27, 2021, FDA issued EUA for a third vaccine, a one-shot regimen produced by Johnson & Johnson (Janssen) using a replication-deficient viral vector platform (FDA, 2021a). Other COVID-19 vaccines are in development and in use, and the development of various platforms has sped up vaccine development and created a greater global capacity to manufacture the many doses needed in a pandemic. Vaccine rollout over the world is currently ongoing, with more EUA of vaccine candidates to be expected in the upcoming months.
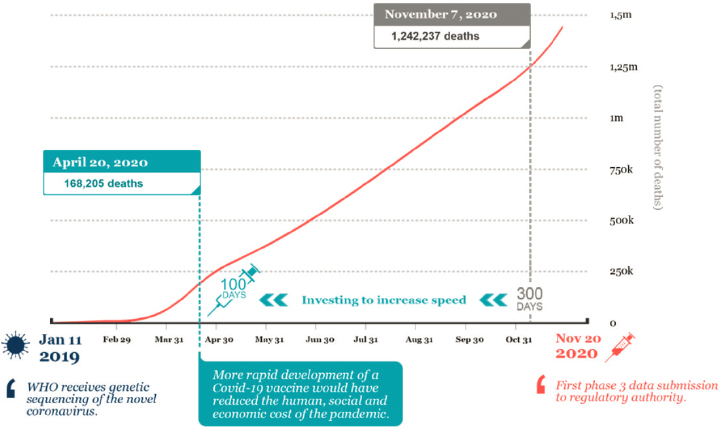
NOTE: It took about 300 days from virus characterization to submission of phase III data.
SOURCE: Saville, 2021.
ORGANIZATION OF THE REPORT
The remaining chapters of this report present the committee’s findings regarding the four dimensions of vaccine R&D outlined above: basic and translational science (Chapter 2), clinical science (Chapter 3), manufacturing science (Chapter 4), and regulatory science (Chapter 5). Each of these chapters ends with the committee’s recommendations, informed by those findings, for applying lessons learned from the development of vaccines for COVID-19 to advance research, development, and manufacturing of vaccines for both pandemic and seasonal influenza within the respective dimension.
REFERENCES
Allen, J. D., and T. M. Ross. 2021. Next generation methodology for updating HA vaccines against emerging human seasonal influenza A(H3N2) viruses. Scientific Reports 11(1):4554. https://doi.org/10.1038/s41598-020-79590-7.
Beck, C. R., B. C. McKenzie, A. B. Hashim, R. C. Harris, University of Nottingham Influenza and the ImmunoCompromised Study Group, and Jonathan S. Nguyen-Van-Tam. 2012. Influenza vaccination for immunocompromised patients: Systematic review and meta-analysis by etiology. Journal of Infectious Diseases 206(8):1250–1259. https://doi.org/10.1093/infdis/jis487.
Boyoglu-Barnum, S., D. Ellis, R. A. Gillespie, G. B. Hutchinson, Y. J. Park, S. M. Moin, O. J. Acton, R. Ravichandran, M. Murphy, D. Pettie, N. Matheson, L. Carter, A. Creanga, M. J. Watson, S. Kephart, S. Ataca, J. R. Vaile, G. Ueda, M. C. Crank, L. Stewart, K. K. Lee, M. Guttman, D. Baker, J. R. Mascola, D. Veesler, B. S. Graham, N. P. King, and M. Kanekiyo. 2021. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 592(7855):623–628. https://doi.org/10.1038/s41586-021-03365-x.
Carragher, D. M., D. A. Kaminski, A. Moquin, L. Hartson, and T. D. Randall. 2008. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. Journal of Immunology (Baltimore, MD: 1950) 181(6):4168–4176. https://doi.org/10.4049/jimmunol.181.6.4168.
Carrat, F., E. Vergu, N. M. Ferguson, M. Lemaitre, S. Cauchemez, S. Leach, and A. J. Valleron. 2008. Time lines of infection and disease in human influenza: A review of volunteer challenge studies. American Journal of Epidemiology 167(7):775–785. https://doi.org/10.1093/aje/kwm375. https://www.ncbi.nlm.nih.gov/pubmed/18230677.
CDC (U.S. Centers for Disease Control and Prevention). 2009. Swine influenza A (H1N1) infection in two children—Southern California, March–April 2009. Morbidity and Mortality Weekly Report 58(15):400–402.
CDC. 2019a. Types of influenza viruses. https://www.cdc.gov/flu/about/viruses/types.htm (accessed October 20, 2021).
CDC. 2019b. 1968 pandemic (H3N2 virus). https://www.cdc.gov/flu/pandemic-resources/1968-pandemic.html (accessed October 20, 2021).
CDC. 2019c. 2009 H1N1 pandemic timeline. https://www.cdc.gov/flu/pandemic-resources/2009-pandemic-timeline.html (accessed October 20, 2021).
CDC. 2019d. Antigenic characterization. https://www.cdc.gov/flu/about/professionals/antigenic.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fflu%2Fprofessionals%2Flaboratory%2Fantigenic.htm (accessed October 20, 2021).
CDC. 2019e. CDC’s World Health Organization (WHO) Collaborating Center for Surveillance, Epidemiology and Control of Influenza. https://www.cdc.gov/flu/weekly/who-collaboration.htm (accessed October 20, 2021).
CDC. 2021. Cell-based flu vaccines. https://www.cdc.gov/flu/prevent/cell-based.htm (accessed October 20, 2021).
Chen, Y. -Q., T. J. Wohlbold, N. -Y. Zheng, M. Huang, Y. Huang, K. E. Neu, J. Lee, H. Wan, K. Thatcher Rojas, E. Kirkpatrick, C. Henry, A.-K. E. Palm, C. T. Stamper, L. Yu-Ling Lan, D. J. Topham, J. Treanor, J. Wrammert, R. Ahmed, M. C. Eichelberger, G. Georgiou, F. Krammer, and P. C. Wilson. 2018. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 173(2):417–429. https://www.sciencedirect.com/science/article/pii/S0092867418303106 (accessed October 20, 2021).
Chung, J. R., M. A. Rolfes, B. Flannery, P. Prasad, A. O’Halloran, S. Garg, A. M. Fry, J. A. Singleton, M. Patel, C. Reed, U.S. Influenza Vaccine Effectiveness Network, Influenza Hospitalization Surveillance Network, and the Assessment Branch, Immunizations Services Division, Centers for Disease Control and Prevention. 2020. Effects of influenza vaccination in the United States during the 2018–2019 influenza season. Clinical Infectious Diseases 71(8):e368–e376.
Cobey, S., and S. E. Hensley. 2017. Immune history and influenza virus susceptibility. Current Opinion in Virology 22:105–111. https://doi.org/10.1016/j.coviro.2016.12.004.
Cox, M. M., and Y. Hashimoto. 2011. A fast track influenza virus vaccine produced in insect cells. Journal of Invertebrate Pathology 107(Suppl):S31–S41. https://doi.org/10.1016/j.jip.2011.05.003.
Cox, M. M. J., R. Izikson, P. Post, and L. Dunkle. 2015. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Therapeutic Advances in Vaccines 3(4):97–108.
Davenport, F. M., A. V. Hennessy, and T. Francis, Jr. 1953. Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. Journal of Experimental Medicine 98(6):641–656. https://doi.org/10.1084/jem.98.6.641.
Drake, J. W. 1993. Rates of spontaneous mutation among RNA viruses. Proceedings of the National Academy of Sciences 90(9):4171–4175. https://doi.org/10.1073/pnas.90.9.4171.
Dugan, H. L., J. J. Guthmiller, P. Arevalo, M. Huang, Y.-Q. Chen, K. E. Neu, C. Henry, N.-Y. Zheng, L. Y.-L. Lan, M. E. Tepora, O. Stovicek, D. Bitar, A.-K. E. Palm, C. T. Stamper, S. Changrob, H. A. Utset, L. Coughlan, F. Krammer, S. Cobey, and P. C. Wilson. 2020. Preexisting immunity shapes distinct antibody landscapes after influenza virus infection and vaccination in humans. Science and Translational Medicine 12(573):eabd3601. https://doi.org/10.1126/scitranslmed.abd3601.
Estrada, L. D., and S. Schultz-Cherry. 2019. Development of a universal influenza vaccine. Journal of Immunology 202(2):392–398. https://doi.org/10.4049/jimmunol.1801054.
Fabre, M. L., P. N. Arrías, T. Masson, M. L. Pidre, and V. Romanowski. 2020. Baculovirus-derived vectors for immunization and therapeutic applications. Emerging and Reemerging Viral Pathogens 197–224. https://doi.org/10.1016/B978-0-12-814966-9.00011-1.
FDA (U.S. Food and Drug Administration). 2021a. Janssen COVID-19 vaccine. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine.
FDA. 2021b. Moderna COVID-19 vaccine. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine#:~:text=The%20emergency%20use%20authorization%20allows,of%20age%20and%20older (accessed October 20, 2021).
FDA. 2021c. Pfizer-BioNTech COVID-19 vaccine. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine (accessed October 20, 2021).
Felberbaum, R. S. 2015. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnology Journal 10(5):702–714. https://doi.org/10.1002/biot.201400438.
Flannery, B., J. R. Chung, E. A. Belongia, H. Q. McLean, M. Gaglani, K. Murthy, R. K. Zimmerman, M. P. Nowalk, M. L. Jackson, L. A. Jackson, A. S. Monto, E. T. Martin, A. Foust, W. Sessions, L. Berman, J. R. Barnes, S. Spencer, and A. M. Fry. 2018. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness - United States, February 2018. Morbidity and Mortality Weekly Report 67(6):180–185. https://doi.org/10.15585/mmwr.mm6706a2.
Fodor, E., L. Devenish, O. G. Engelhardt, P. Palese, G. G. Brownlee, and A. García-Sastre. 1999. Rescue of influenza A virus from recombinant DNA. Journal of Virology 73(11):9679–9682. https://doi.org/10.1128/JVI.73.11.9679-9682.1999.
Fonville, J. M., S. H. Wilks, S. L. James, A. Fox, M. Ventresca, M. Aban, L. Xue, T. C. Jones, N. M. H. Le, Q. T. Pham, N. D. Tran, Y. Wong, A. Mosterin, L. C. Katzelnick, D. Labonte, T. T. Le, G. van der Net, E. Skepner, C. A. Russell, T. D. Kaplan, G. F. Rimmelzwaan, N. Masurel, J. C. de Jong, A. Palache, W. E. P. Beyer, Q. M. Le, T. H. Nguyen, H. F. L. Wertheim, A. C. Hurt, A. D. M. E. Osterhaus, I. G. Barr, R. A. M. Fouchier, P. W. Horby, and D. J. Smith. 2014. Antibody landscapes after influenza virus infection or vaccination. Science (New York, N.Y.) 346(6212):996–1000. https://doi.org/10.1126/science.1256427.
Foppa, I. M., P. Y. Cheng, S. B. Reynolds, D. K. Shay, C. Carias, J. S. Bresee, I. K. Kim, M. Gambhir, and A. M. Fry. 2015. Deaths averted by influenza vaccination in the U.S. during the seasons 2005/06 through 2013/14. Vaccine 33(26):3003–3009.
Francis, T. 1960. On the doctrine of original antigenic sin. Proceedings of the American Philosophical Society 104(6):572–578. http://www.jstor.org/stable/985534 (accessed October 20, 2021).
Gerdil, C. 2003. The annual production cycle for influenza vaccine. Vaccine 21(16):1776–1779. https://doi.org/10.1016/s0264-410x(03)00071-9.
Ghebrehewet, S., P. MacPherson, and A. Ho. 2016. Influenza. BMJ 355:i6258. https://doi.org/10.1136/bmj.i6258.
Giles, B. M., and T. M. Ross. 2011. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 29(16):3043–3054. https://doi.org/10.1016/j.vaccine.2011.01.100.
Gómez-Puertas, P., C. Albo, E. Pérez-Pastrana, A. Vivo, and A. Portela. 2000. Influenza virus matrix protein is the major driving force in virus budding. Journal of Virology 74(24):11538–11547. https://doi.org/10.1128/jvi.74.24.11538-11547.2000.
Harding, A. T., and N. S. Heaton. 2018. Efforts to improve the seasonal influenza vaccine. Vaccines 6(2):19. https://doi.org/10.3390/vaccines6020019.
Hay, A. J., and J. W. McCauley. 2018. The WHO global influenza surveillance and response system (GISRS)—A future perspective. Influenza and Other Respiratory Viruses 12(5):551–557. https://doi.org/10.1111/irv.12565.
Hegde, N. R. 2015. Cell culture-based influenza vaccines: A necessary and indispensable investment for the future. Human Vaccines & Immunotherapeutics 11(5):1223–1234. https://doi.org/10.1080/21645515.2015.1016666.
HHS (U.S. Department of Health and Human Services). 2020. National Influenza Vaccine Modernization Strategy 2020-2030. https://www.phe.gov/Preparedness/planning/nivms/Documents/nivms-2020-2030.pdf (accessed October 20, 2021).
Hsieh, Y.-H., S. Ma, J. X. V. Hernandez, V. J. Lee, and W. Y. Lim. 2011. Early outbreak of 2009 influenza A (H1N1) in Mexico prior to identification of pH1N1 virus. PLOS ONE 6(8):e23853. https://doi.org/10.1371/journal.pone.0023853.
Invitrogen Life Technologies. n.d. Guide to baculovirus expression vector systems (BEVS) and insect cell culture techniques. https://tools.thermofisher.com/content/sfs/manuals/bevtest.pdf (accessed October 20, 2021).
Iuliano, A. D., K. M. Roguski, H. H. Chang, D. J. Muscatello, R. Palekar, S. Tempia, C. Cohen, J. M. Gran, D. Schanzer, B. J. Cowling, P. Wu, J. Kyncl, L. W. Ang, M. Park, M. Redlberger-Fritz, H. Yu, L. Espenhaim, A. Krishnan, G. Emukule, L. van Asten, S. P. da Silva, S. Aungkulanon, U. Buchholz, M.-A. Widdowson, and J. S. Bresee for the Global Seasonal Influenza-Associated Mortality Collaborator Network. 2018. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 391(10127):1285–1300. https://doi.org/10.1016/S0140-6736(17)33293-2.
Jameson, J., J. Cruz, M. Terajima, and F. A. Ennis. 1999. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. Journal of Immunology 162(12):7578–7583. https://www.jimmunol.org/content/jimmunol/162/12/7578.full.pdf (accessed October 20, 2021).
Janway, C. A., P. Travers, Jr., M. Walport, and M. J. Shlomchik. 2001. Immunobiology: The Immune System in Health and Disease. 5th ed. New York: Garland Science.
Jha, B. K., R. Pandit, R. Jha, and K. D. Manandhar. 2020. Overview of seasonal influenza and recommended vaccine during the 2016/2017 season in Nepal. Heliyon 6(1):e03304. https://doi.org/10.1016/j.heliyon.2020.e03304.
Johnson, N. P. A. S., and J. Mueller. 2002. Updating the accounts: Global mortality of the 1918–1920 Spanish influenza pandemic. Bulletin of the History of Medicine 76(1):105–115. http://www.jstor.org/stable/44446153 (accessed October 20, 2021).
Kaufmann, L., M. Syedbasha, D. Vogt, Y. Hollenstein, J. Hartmann, J. E. Linnik, and A. Egli. 2017. An optimized hemagglutination inhibition (HI) assay to quantify influenza-specific antibody titers. Journal of Visualized Experiments (130):55833. https://doi.org/10.3791/55833.
Kawaoka, Y., S. Krauss, and R. G. Webster. 1989. Avian-to-human transmission of the pb1 gene of influenza A viruses in the 1957 and 1968 pandemics. Journal of Virology 63(11):4603–4608.
Kinsella, C. M., P. D. Santos, I. Postigo-Hidalgo, A. Folgueiras-González, T. C. Passchier, K. P Szillat, J. O. Akello, B. Álvarez-Rodríguez, and J. Martí-Carreras. 2020. Preparedness needs research: How fundamental science and international collaboration accelerated the response to COVID-19. PLOS Pathogens 16(10):e1008902. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7546461/pdf/ppat.1008902.pdf (accessed October 20, 2021).
Krammer, F., G. J. D. Smith, R. A. M. Fouchier, M. Peiris, K. Kedzierska, P. C. Doherty, P. Palese, M. L. Shaw, J. Treanor, R. G. Webster, and A. García-Sastre. 2018a. Influenza. Nature Reviews Disease Primers 4(1):3. https://doi.org/10.1038/s41572-018-0002-y.
Krammer, F., R. A. M. Fouchier, M. C. Eichelberger, R. J. Webby, K. Shaw-Saliba, H. Wan, P. C. Wilson, R. W. Compans, I. Skountzou, and A. S. Monto. 2018b. NAction! How can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio 9(2):e02332-17. https://doi.org/10.1128/mBio.02332-17.
LePan, N. 2021. Visualizing the history of pandemics. https://www.visualcapitalist.com/history-of-pandemics-deadliest (accessed October 20, 2021).
Lessler, J., S. Riley, J. M. Read, S. Wang, H. Zhu, G. J. D. Smith, Y. Guan, C. Q. Jiang, and D. A. T. Cummings. 2012. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLOS Pathogens 8(7):e1002802. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1002802 (accessed October 20, 2021).
Lopez, C. E., and K. L. Legge. 2020. Influenza A virus vaccination: Immunity, protection, and recent advances toward a universal vaccine. Vaccines 8(3):1–23. https://doi.org/10.3390/vaccines8030434.
Luo, M. 2012. Influenza virus entry. Advances in Experimental Medicine and Biology 726:201–221. https://doi.org/10.1007/978-1-4614-0980-9_9.
Maier, H. E., R. Nachbagauer, G. Kuan, S. Ng, R. Lopez, N. Sanchez, D. Stadlbauer, L. Gresh, A. Schiller, A. Rajabhathor, S. Ojeda, A. F. Guglia, F. Amanat, A. Balmaseda, F. Krammer, and A. Gordon. 2020. Pre-existing antineuraminidase antibodies are associated with shortened duration of influenza A(H1N1)pdm virus shedding and illness in naturally infected adults. Clinical Infectious Disease 70(11):2290–2297. https://doi.org/10.1093/cid/ciz639.
Memoli, M. J., P. A. Shaw, A. Han, L. Czajkowski, S. Reed, R. Athota, T. Bristol, S. Fargis, K. Risos, J. H. Powers, R. T. Davey, J. K. Taubenberger, A. Moscona, J. Crowe, and D. Hoft. 2016. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 7(2):e00417-16. https://doi.org/doi:10.1128/mBio.00417-16.
Milián, E., and A. A. Kamen. 2015. Current and emerging cell culture manufacturing technologies for influenza vaccines. BioMedical Research International. https://doi.org/10.1155/2015/504831.
Moderna. 2020a. Moderna announces first participant dosed in NIH-led phase 1 study of mRNA vaccine (mRNA-1273) against novel coronavirus. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-first-participant-dosed-nihled-phase-1-study (accessed October 20, 2021).
Moderna. 2020b. Moderna announces publication of results from the pivotal phase 3 trial of the Moderna COVID-19 vaccine in the New England Journal of Medicine. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-publication-results-pivotal-phase-3-trial (accessed October 20, 2021).
Monto, A. S., J. G. Petrie, R. T. Cross, E. Johnson, M. Liu, W. Zhong, M. Levine, J. M. Katz, and S. E. Ohmit. 2015. Antibody to influenza virus neuraminidase: An independent correlate of protection. Journal of Infectious Diseases 212(8):1191–1199. https://doi.org/10.1093/infdis/jiv195.
Nachbagauer, R., J. Feser, A. Naficy, D. I. Bernstein, J. Guptill, E. B. Walter, F. Berlanda-Scorza, D. Stadlbauer, P. C. Wilson, T. Aydillo, M. A. Behzadi, D. Bhavsar, C. Bliss, C. Capuano, J. M. Carreño, V. Chromikova, C. Claeys, L. Coughlan, A. W. Freyn, C. Gast, A. Javier, K. Jiang, C. Mariottini, M. McMahon, M. McNeal, A. Solórzano, S. Strohmeier, W. Sun, M. Van der Wielen, B. L. Innis, A. García-Sastre, P. Palese, and F. Krammer. 2021. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nature Medicine 27(1):106–114. https://doi.org/10.1038/s41591-020-1118-7.
Neumann, G., T. Watanabe, H. Ito, S. Watanabe, H. Goto, P. Gao, M. Hughes, D. R. Perez, R. Donis, E. Hoffmann, G. Hobom, and Y. Kawaoka. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proceedings of the National Academy of Sciences 96(16):9345–9350. https://doi.org/10.1073/pnas.96.16.9345.
Ortiz, J. R., J. Hickling, R. Jones, A. Donabedian, O. G. Engelhardt, J. M. Katz, S. A. Madhi, K. M. Neuzil, G. F. Rimmelzwaan, J. Southern, D. J. Spiro, and J. Hombach. 2018. Report on eighth WHO meeting on development of influenza vaccines that induce broadly protective and long-lasting immune responses: Chicago, USA, 23–24 August 2016. Vaccine 36(7):932–938. https://doi.org/10.1016/j.vaccine.2017.11.061.
Paden, C. R., Y. Tao, K. Queen, J. Zhang, Y. Li, A. Uehara, and S. Tong. 2020. Rapid, sensitive, full-genome sequencing of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases 26(10):2401–2405. https://doi.org/10.3201/eid2610.201800.
Palm, A.-K. E., and C. Henry. 2019. Remembrance of things past: Long-term B cell memory after infection and vaccination. Frontiers in Immunology 10:1787. https://doi.org/10.3389/fimmu.2019.01787.
Paules, C. I., and A. S. Fauci. 2019. Influenza vaccines: Good, but we can do better. The Journal of Infectious Diseases 219(Suppl 1):S1–S4.
Paules, C., and K. Subbarao. 2017. Influenza. Lancet 390(10095):697–708. https://doi.org/10.1016/s0140-6736(17)30129-0.
Pfizer. 2020a. Pfizer and BioNTech conclude phase 3 study of COVID-19 vaccine candidate, meeting all primary efficacy endpoints. https://www.pfizer.com/news/press-release/pressrelease-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine (accessed October 20, 2021).
Pfizer. 2020b. BioNTech and Pfizer announce completion of dosing for first cohort of phase 1/2 trial of COVID-19 vaccine candidates in Germany. https://www.pfizer.com/news/press-release/press-release-detail/biontech-and-pfizer-announcecompletion-dosing-first-cohort (accessed October 20, 2021).
Ping, J., T. J. S. Lopes, C. A. Nidom, E. Ghedin, C. A. Macken, A. Fitch, M. Imai, E. A. Ma-her, G. Neumann, and Y. Kawaoka. 2015. Development of high-yield influenza A virus vaccine viruses. Nature Communications 6(1):8148.
Ping, J., T. J. Lopes, G. Neumann, and Y. Kawaoka. 2016. Development of high-yield influenza B virus vaccine viruses. Proceedings of the National Academy of Sciences 113(51):E8296–E8305. https://doi.org/10.1073/pnas.1616530113.
Pushko, P., and I. Tretyakova. 2020. Influenza virus like particles (VLPs): Opportunities for H7N9 vaccine development. Viruses 12(5):518. https://search.proquest.com/scholarly-journals/influenza-virus-like-particles-vlps-opportunities/docview/2401567847/se-2?accountid=152665 (accessed October 20, 2021).
Saelens, X. 2019. The role of matrix protein 2 ectodomain in the development of universal influenza vaccines. Journal of Infectious Diseases 219(Suppl 1):S68–S74. https://doi.org/10.1093/infdis/jiz003.
Saville, M. 2021. Lessons learned from COVID-19 vaccine development. PowerPoint presentation. Oslo, Norway: Coalition for Epidemic Preparedness Innovations.
Sedova, E. S., D. N. Scherbinin, A. A. Lysenko, S. V. Alekseeva, E. A. Artemova, and M. M. Shmarov. 2019. Non-neutralizing antibodies directed at conservative influenza antigens. Acta Naturae 11(4):22–32. https://doi.org/10.32607/20758251-2019-11-4-22-32.
Smetana, J., R. Chlibek, J. Shaw, M. Splino, and R. Prymula. 2018. Influenza vaccination in the elderly. Human Vaccines & Immunotherapeutics 14(3):540–549. https://doi.org/10.1080/21645515.2017.1343226.
Subbarao, K., and I. Barr. 2019. A tale of two mutations: Beginning to understand the problems with egg-based influenza vaccines? Cell Host & Microbe 25(6):773–775. https://doi.org/10.1016/j.chom.2019.05.012.
Taubenberger, J. K., and D. M. Morens. 2006. 1918 Influenza: The mother of all pandemics. Emerging Infectious Diseases 12(1):15–22. https://doi.org/10.3201/eid1201.050979.
Taubenberger, J. K., and D. M. Morens. 2010. Influenza: The once and future pandemic. Public Health Reports 125(Suppl 3):16–26. https://pubmed.ncbi.nlm.nih.gov/20568566.
Tregoning, J. S., R. F. Russell, and E. Kinnear. 2018. Adjuvanted influenza vaccines. Human Vaccines & Immunotherapeutics 14(3):550–564.
Vemula, S. V., E. E. Sayedahmed, S. Sambhara, and S. K. Mittal. 2017. Vaccine approaches conferring cross-protection against influenza viruses. Expert Review of Vaccines 16(11):1141–1154. https://doi.org/10.1080/14760584.2017.1379396.
Viboud, C., L. Simonsen, R. Fuentes, J. Flores, M. A. Miller, and G. Chowell. 2016. Global mortality impact of the 1957–1959 influenza pandemic. Journal of Infectious Disease 213(5):738–745. https://doi.org/10.1093/infdis/jiv534.
Westgeest, K. B., M. de Graaf, M. Fourment, T. M. Bestebroer, R. van Beek, M. I. J. Spronken, J. C. de Jong, G. F. Rimmelzwaan, C. A. Russell, A. D. M. E. Osterhaus, G. J. D. Smith, D. J. Smith, and R. A. M. Fouchier. 2012. Genetic evolution of the neuraminidase of influenza A (H3N2) viruses from 1968 to 2009 and its correspondence to haemagglutinin evolution. Journal of General Virology 93(Pt 9):1996–2007. https://doi.org/10.1099/vir.0.043059-0.
White, C. L., K. Chiem, D. R. Perez, J. Santos, S. Cardenas Garcia, A. Nogales, and L. Martínez-Sobrido. 2021. A new master donor virus for the development of live-attenuated influenza B virus vaccines. Viruses 13(7):1278. https://doi.org/10.3390/v13071278.
WHO (World Health Organization). 2011. Main operational lessons learnt from the WHO Pandemic Influenza A (H1N1) Vaccine Deployment Initiative. Geneva, Switzerland: World Health Organization. https://www.who.int/influenza_vaccines_plan/resources/h1n1_vaccine_deployment_initiaitve_moll.pdf (accessed October 20, 2021).
WHO. 2013. Global epidemiological surveillance standards for influenza. https://www.who.int/influenza/resources/documents/WHO_Epidemiological_Influenza_Surveillance_Standards_2014.pdf (accessed October 20, 2021).
WHO. 2019. Global influenza strategy 2019–2030. Geneva, Switzerland: World Health Organization.
WHO. 2020a. COVID-19 - China. https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229 (accessed October 20, 2021).
WHO. 2020b. WHODirector-General’s opening remarks at the media briefing on COVID-19- 11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed October 20, 2021).
WHO. 2021a. Global Influenza Surveillance and Response System (GISRS). https://www.who.int/initiatives/global-influenza-surveillance-and-response-system (accessed October 20, 2021).
WHO. 2021b. Vaccination. World Health Organization Regional Office for Europe. https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination (accessed October 20, 2021).
WHO. 2021c. Influenza-estimating burden of disease. https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/seasonal-influenza/burden-of-influenza (accessed October 20, 2021).
Wong, S.-S., and R. J. Webby. 2013. Traditional and new influenza vaccines. Clinical Microbiology Reviews 26(3):476–492. https://doi.org/10.1128/CMR.00097-12.
Wu, N. C., L. Huibin, A. J. Thompson, D. C. Wu, W. W. S. Ng, R. U. Kadam, C.-W. Lin, C. M. Nycholat, R. McBride, W. Liang, J. C. Paulson, C. K. P. Mok, and I. A. Wilson. 2019. Preventing an antigenically disruptive mutation in egg-based H3N2 seasonal influenza vaccines by mutational incompatibility. Cell Host & Microbe 25(6):836–844. https://doi.org/10.1016/j.chom.2019.04.013.
Yan, S., D. Weycker, and S. Sokolowski. 2017. US healthcare costs attributable to type A and type B influenza. Human Vaccines and Immunotherapy 13(9):2041–2047. https://doi.org/10.1080/21645515.2017.1345400.
Young, B., X. Zhao, A. R. Cook, C. M. Parry, A. Wilder-Smith, and M. Chen I-Cheng. 2017. Do antibody responses to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine 35(2):212–221.
Zhou, B., V. A. Meliopoulos, W. Wang, X. Lin, K. M. Stucker, R. A. Halpin, T. B. Stockwell, S. Schultz-Cherry, and D. E. Wentworth. 2016. Reversion of cold-adapted live attenuated influenza vaccine into a pathogenic virus. Journal of Virology 90(19):8454–8463. https://doi.org/10.1128/JVI.00163-16.
Zhu, W., C. Dong, L. Wei, and B. Z. Wang. 2021. Promising adjuvants and platforms for influenza vaccine development. Pharmaceutics 13(1):68. https://doi.org/10.3390/pharmaceutics13010068.
Ziegler, T., A. Mamahit, and N. J. Cox. 2018. 65 years of influenza surveillance by a World Health Organization-coordinated global network. Influenza and Other Respiratory Viruses 12(5):558–565. https://doi.org/10.1111/irv.12570.
This page intentionally left blank.


























