2
Basic and Translational Science
This chapter addresses the first of the four dimensions of vaccine research, development, and manufacturing examined by the committee. Key findings about vaccine research and development (R&D) from COVID-19 in relation to each of these dimensions is presented in Box 2-1. It begins by describing the development of COVID-19 vaccines and then surveys the current landscape of influenza vaccines, expanding on the overview in Chapter 1. The third section considers how lessons learned from the devel-
opment of COVID-19 vaccines can be applied to both seasonal and pandemic influenza vaccines in the dimension of basic and translational science. The final section presents recommendations drawn from those findings.
As discussed in Chapter 1, current seasonal and pandemic influenza vaccines are based on traditional vaccine platforms and concepts that induce strain-specific immunity. The seasonal vaccines require annual reformulation and readministration, or inclusion of a novel matched virus in case of a pandemic (Krammer and Palese, 2015). This process takes months, during which the population is vulnerable to infection. Broadly protective or universal influenza vaccines have the theoretical potential to mitigate the need for seasonal reformulation and annual vaccination and may also significantly enhance pandemic preparedness. However, major developments in this area are necessary to fulfil these purposes, and these types of vaccines are therefore not expected to sustain pandemic preparedness in the short term. In addition, implementation of seasonal influenza virus vaccination programs is not considered feasible in many low- and middle-income countries (LMICs). A broadly protective, universal, low-cost influenza virus vaccine that could be given as an initial prime–boost regimen with potential booster doses given at longer intervals would make influenza vaccines affordable to LMICs and would enhance vaccine coverage in high-income countries (HICs). Some evidence to date indicates that broadly protective vaccines may not achieve the high efficacy needed to control pandemic influenza and may therefore not be the solution to pandemic preparedness. Such vaccine candidates may need to be combined with vaccines targeting strain-specific neutralizing antibody epitopes (Wei et al., 2020; Zeigler et al., 2021). This chapter addresses priorities for basic and translational science that can help achieve these goals through sustained funding.
THE DEVELOPMENT OF COVID-19 VACCINES
Basic research fueled the development of a diverse vaccine portfolio for COVID-19, which began right after the first online publication of the viral sequence. Based on previous basic research on severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), targets for the vaccines were quickly identified and industry, government laboratories, and academia started development of vaccines.
Platforms Used to Develop COVID-19 Vaccines
Next to traditional vaccine platforms, novel platforms are being used successfully for COVID-19. Several vaccines were developed, each with a different efficacy, ranging from 51 to 95 percent, results that may depend on
the platform, the location, and the time the clinical trials were conducted, as well as the included populations. The first authorized vaccines in many countries were the vaccines produced by Pfizer-BioNTech and Moderna, that make use of a modified mRNA platform (Gavi, 2021). The vaccines consist of modified mRNA encoding the prefusion-stabilized, full-length spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles for delivery (Baden et al., 2020; Polack et al., 2020). Both vaccines were found to be safe in clinical trials; however, acute allergic reactions were reported after vaccine rollout to the population, with a reported 2.5–11.1 cases of anaphylaxis per million doses given (Blumenthal et al., 2021). With the spread of SARS-CoV-2 over the course of the pandemic, several variant viruses have emerged—some are designated as variants of concern and others as variants of interest (Subbarao, 2021). The levels of neutralizing antibody in the sera of vaccinated individuals to some of these variants, such as the beta and delta variants, was lower than against the vaccine strain (Stowe et al., 2021) though the vaccines are still highly effective in preventing severe illness and death, as they were designed to provide cross-protection against variants. The mRNA vaccine platform offers the flexibility to design booster vaccine candidates against such variants to improve efficacy. Both Pfizer-BioNTech and Moderna started phase I trials studying such variants of their vaccines in early 2021.
Another vaccine platform used by several manufacturers is the replication-deficient viral vector platform. AstraZeneca developed a vaccine in collaboration with the University of Oxford that uses a recombinant replication-deficient chimpanzee adenovirus (ChAdOx1) vector encoding the spike protein of SARS-CoV-2 (Voysey et al., 2021). The single-dose vaccine developed by Janssen (Johnson & Johnson) uses a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding the full-length, stabilized spike protein (Oliver et al., 2021). The Gamaleya National Research Centre for Epidemiology and Microbiology in Russia developed a heterologous recombinant adenovirus vaccine based on recombinant adenovirus type 26 and type 5 encoding the full-length spike protein of SARS-CoV-2 (Logunov et al., 2021). Finally, the vaccine developed by CanSino Biologics in China is a one-dose regimen like the Janssen vaccine, using adenovirus type 5 as the viral vector to encode the spike protein (Peshimam and Farooq, 2021).
Two candidates using a protein subunit vaccine platform are those developed by Novavax and the Vector Institute. The Novavax vaccine uses a stable, prefusion recombinant spike protein nanoparticle that is adjuvanted with Matrix-M. On June 14, 2021, Novavax released the results of its phase III trial (Novavax, 2021). Sanofi Pasteur also has a recombinant protein-based vaccine adjuvanted with GlaxoSmithKline’s (GSK’s) AS03 adjuvant in a phase III study that was initiated in May 2021 (Sanofi, 2021).
Finally, several vaccines based on whole inactivated virus have been developed. A vaccine produced by the Chinese company Sinopharm received authorization in China and from the World Health Organization (WHO) (Al Kaabi et al., 2021; Zimmer et al., 2021). A vaccine produced by another Chinese company, Sinovac, was authorized for use in China and by WHO (2021b). A vaccine by Bharat BioTech is authorized for use in India (Pokharel, 2021).
Initiatives to Fund COVID-19 Vaccine Development
To stimulate the development of COVID-19 vaccines, countries—individually and together in consortia—funded the development of multiple candidates. The United States launched Operation Warp Speed (OWS) in May 2020 as an initiative by the U.S. Department of Health and Human Services (HHS). This public–private partnership had the objective to deliver 300 million doses of a safe and effective vaccine for COVID-19 by January 2021 (GAO, 2021). The partnership included federal bodies and biopharmaceutical companies working on vaccines. OWS provided funding or contracts to support the development of the vaccines by Moderna (mRNA platform), AstraZeneca and the University of Oxford (replication-defective vector platform), Johnson & Johnson (Janssen, replication-defective vector), Novavax (protein adjuvanted), and GSK and Sanofi (protein adjuvanted) (Slaoui and Hepburn, 2020). Pfizer and BioNTech (mRNA platform) did not accept federal funding but contracted with OWS to secure 100 million doses for the United States (Zimmer and Thomas, 2020).
The Coalition for Epidemic Preparedness Innovations (CEPI), a global partnership including public, private, philanthropic, and civil society organizations, was launched in 2017 to develop vaccines for future pandemics. CEPI also aims to enable equitable access to the vaccines developed through the COVAX initiative, allowing early access of novel vaccines to LMICs. It has pledged investments of up to $1.2 billion in a diverse portfolio of vaccine candidates based on multiple platforms. Figure 2-1 depicts COVID-19 vaccines supported by CEPI. Furthermore, it has laid out a series of protocols to facilitate rapid clinical studies (CEPI, 2020). The European Union committed to investing €1 billion from the Horizon 2020 program for research and innovation to the Coronavirus Global Response Initiative, with €350 million available for vaccine development (European Commission, 2021). These and other generous funding initiatives and collaborations among many players facilitated the rapid development of COVID-19 vaccines.
The first vaccine, produced by Pfizer-BioNTech, was authorized 8 months after clinical trials began (Ball, 2021). COVID-19 vaccine development was rapid but not enough to prevent hundreds of millions of infections (WHO, 2021a) and thousands of deaths. Thus, it is crucial that the vaccination strategy for pandemic influenza involves both preparedness,
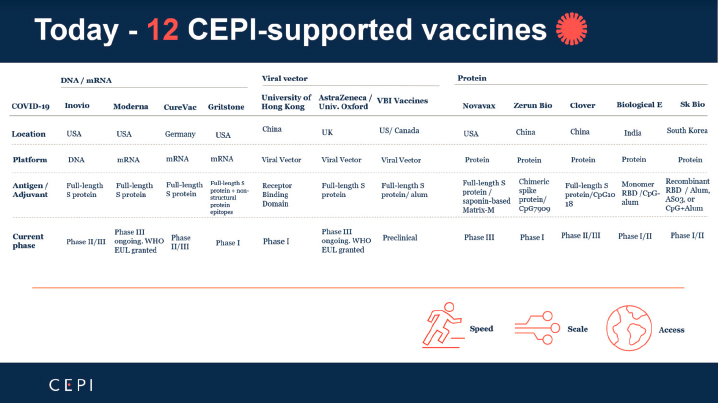
SOURCE: Saville, 2021.
including off-the-shelf vaccines that can be strategically deployed to slow down the global spread of infection, and response, activating the rapid development and manufacture of highly effective vaccines.
CURRENT INFLUENZA VACCINES
Influenza vaccines have been used for more than 50 years and provide moderate protection with a vaccine effectiveness rate varying between 10 and 60 percent (Paules and Fauci, 2019). An important difference between vaccine development for COVID-19 and influenza is that trials compare the efficacy in very different populations. For COVID-19, the trials compared people not having previously been exposed to the virus or a vaccine with those that were vaccinated, while for influenza previous encounters with influenza viruses may affect the response to any tested vaccine. Therefore, given that the COVID-19 vaccines were tested in a naïve population, these vaccines could show a large effect size when comparing the vaccinated group with the non-vaccinated controls. Novel influenza vaccines, on the other hand, would be tested in populations that are likely not naïve to previous influenza exposure or even influenza vaccines. Therefore, the expected effect size for such studies would be less dramatic than those shown by COVID-19 studies. Basic research includes substantial efforts to find targets for universal or broadly effective influenza vaccines and novel correlates of protection (Corder et al., 2020). Developments in the fields of
virology, vaccinology, structural biology, and immunology have contributed to improvements in pandemic preparedness and seasonal influenza vaccines. While prior influenza pandemics and worrying outbreaks of avian influenza have fueled work on influenza pandemic preparedness, the COVID-19 pandemic has resulted in a major paradigm shift in vaccine technology (Billington et al., 2020). Prior to COVID-19, mRNA platforms had been studied and defined, but there was no model for widespread adoption and distribution. In particular, the cold-chain requirements of these vaccines, especially the Pfizer vaccine requiring initial cold storage conditions of about –75°C posed a challenge for global distribution (PATH, 2020). Similarly, the sole vector-based vaccine that had been licensed for human use was an Ebola vaccine, which had limited distribution, compared to vector-based vaccines for COVID-19 (FDA, 2019). Consequently, there is much to be learned from the development, evaluation, licensing, and mass vaccination of COVID-19 vaccines for future pandemic preparedness.
Production of Seasonal Influenza Vaccines in 2021
As discussed in Chapter 1, the vast majority of the available vaccines against seasonal influenza viruses in 2021 are inactivated egg-grown vaccines, with 15.5 percent produced using cell culture platforms (Sparrow et al., 2021). For trivalent inactivated vaccines, one egg is usually needed for production of a single vaccine dose. Live attenuated influenza vaccine production has usually a higher yield as every egg can produce approximately 10 quadrivalent vaccine doses (Mohn et al., 2018). The production of pandemic vaccines in eggs takes an estimated 20–28 weeks, which poses a major time challenge. The 2009 H1N1 pandemic resulted in increased hen flocks for production of eggs for better preparedness all year, but egg production still has a long lead time. For cell culture vaccines, this timeframe can potentially be reduced to 12 weeks (Wright, 2008). However, there are proprietary obstacles associated with this technique as few cell lines have been approved and licensed (Barr et al., 2018).
Limitations of Current Seasonal Influenza Vaccines
The vaccine effectiveness (VE) of the seasonal influenza vaccine ranges from 10 to 60 percent (CDC, 2020; Paules and Fauci, 2019) (see Chapter 1). This effectiveness may be decreased because of egg-adaptive mutations that occur during production. Decreases in effectiveness may also be caused by the ongoing evolution of the circulating viruses; this can lead to a mismatch between the circulating strain and the vaccine because of the time delay between strain selection and the administration of vaccines, in particular for A(H3N2) viruses (Divino et al., 2020; The Scripps Research
Institute, 2017; Zost et al., 2017). Availability of cell-based vaccines is limited because of historical factors, with the egg-based platform having been used for decades, as well as initial higher production costs (Cox et al., 2015; Rockman et al., 2020). Few countries have adopted cell-based vaccines in large quantities despite some evidence that cell-based vaccines more closely match circulating virus strains than egg-based vaccines and may be more effective (Boikos et al., 2020; DeMarcus et al., 2019; Gouma et al., 2020; Izurieta et al., 2018; Klein et al., 2020; Krishnarajah et al., 2021; Rajaram et al., 2020a; Wang et al., 2020). As annual seasonal influenza vaccination is recommended by many countries and widespread adoption is subsidized by governments, priority is often given to the most cost-efficient vaccine if sufficiently supported by evidence, which currently relies on eggs (Friede et al., 2011). Despite this, Ruiz-Aragon et al. (2020) recently showed how using cell-based quadrivalent influenza vaccines as an alternative to egg-based vaccines is actually cost-effective and lowers burden of disease, as the higher vaccine effectiveness in cell-based vaccines put less of a strain on the health care system. In a pandemic context, these barriers may be overcome by access to reserve funding. The committee recognizes that the current manufacturing capacity, availability, and use of egg-based vaccines has held back innovation of novel platforms for influenza vaccines, particularly when contrasted with COVID-19 vaccine development.
In addition to production time, the availability of eggs is a limiting factor hindering pandemic-level vaccine production (Milián and Kamen, 2015; Ulmer et al., 2006). When a novel A(H1N1) strain emerged in 2009, seed virus production started in April, a pandemic was declared in June, and vaccines were approved in September and deployed in October (Rappuoli and Dormitzer, 2012). Despite the advantage of an established process, vaccine supply during the following 2 months was insufficient to cover target groups recommended for vaccination, as also seen for COVID-19 (IOM, 2010). Recognizing insufficient supply and vaccine nationalism, in which governments of high-income countries made deals with manufacturers to ensure distribution to their population thus inhibiting the global supply of vaccines, occurred during the 2009 influenza pandemic and COVID-19 pandemic, the committee urges the building of infrastructure for surge capacity to ensure preparedness during the next impending influenza pandemic. After further research has been conducted, WHO can evaluate on the basis of the acquired data whether widespread investment into novel vaccine platforms is warranted and advise on vaccines to be stockpiled.
Egg-based vaccine production is an engrained part of the public health response to influenza. For new vaccines to be commercially viable they have to be at least as effective as the standard of care—egg-based vaccines (Newland et al., 2021). These requirements by regulatory agencies based on confirming the presence of HI antibodies has been an impediment to devel-
opment of new vaccines. There is a need to create and agree upon correlates of protection for vaccines that do not induce HI antibodies. Defining such correlates needs to be determined in the context of exposure using studies into vaccine efficacy during an outbreak. Vaccines produced using novel platforms that target the traditional antigenic targets for influenza, such as HA, can use the HI assay as a correlate of protection. However, innovations to these assays are needed to ensure they can be performed faster, cheaper, and with more consistent results.
The COVID-19 pandemic was pivotal for the evolution of mRNA vaccines, which had not been tested in large populations previously. However, preclinical research resulting from the SARS-CoV and MERS-CoV outbreaks provided the foundation for the current COVID-19 vaccines. mRNA vaccines came to the forefront during COVID-19 because they could be rapidly manufactured and deployed, and they were more immunogenic than recombinant protein vaccines (Lederer et al., 2020). Importantly, the COVID-19 pandemic provided the first opportunity to demonstrate that mRNA vaccines can be safe and highly effective, leading to licensure (Baden et al., 2020; Polack et al., 2020). A number of factors will influence whether mRNA vaccine platforms are adopted for influenza vaccine development, including relative efficacy, manufacturing capacity, and costs of manufacture and distribution.
Prior Strain Immunity and the Role of Antigenic Drift
Influenza vaccines that are currently on the market induce neutralizing antibodies against the head of the influenza virus HA protein (Krammer, 2019). Due to antigenic drift among circulating virus strains (see Chapter 1), as well as antibody decay, the protection afforded by vaccine-induced antibodies is short lived. The strains used to formulate influenza vaccines are reviewed biannually, and vaccines are readministered to keep up with changes in the viruses. Despite extensive efforts to update vaccine strains to keep pace with antigenic drift, the poor vaccine effectiveness against A(H3N2) viruses persists (Belongia et al., 2017; Rajaram et al., 2020b). As discussed above, this subtype has undergone more rapid antigenic change resulting in greater mismatch between vaccine and circulating strains (Bedford et al., 2015; Paules et al., 2017). Mutations and antigenic change can also arise upon adaptation of viruses to growth in eggs, adding to the risk that the circulating strains and the vaccine strain are different, again limiting vaccine efficacy (Hampson et al., 2017; Paules et al., 2017; Zost et al., 2017). Repeated vaccination may also contribute to poor vaccine immunogenicity (Huang et al., 2017; Leung et al., 2021; Mosterín Höpping et al., 2016; Thompson et al., 2016) and effectiveness against A(H3N2) viruses
(Belongia et al., 2017; Saito et al., 2017; Morimoto and Takeishi, 2018). Effects of influenza virus exposure history on influenza vaccine responses are complex and not well understood. It is well established that infection or vaccination with a prevailing strain will boost antibody titers against prior strain variants. This phenomenon was termed original antigenic sin because it was thought that the continued production of antibodies against the earliest encountered strain could be detrimental to production of antibodies against the prevailing strain (Francis, 1960). More recent studies demonstrate that titer rise is greatest against the prevailing strain and decreases with increasing antigenic distance from the prevailing strain (Fonville et al., 2014). This back-boosting of antibody titers against strains encountered earlier in life is thought to be mediated by recall of memory B cells that recognize epitopes that are conserved between prior and prevailing strains (Fonville et al., 2014; Kelvin and Zambon, 2019; Nguyen-Contant et al., 2021). Continued back-boosting of antibodies against past strains may better account for the tendency for antibody titers to be highest against early life strains than the original antigenic sin hypothesis. Similarly, memory recall is likely to account for protection associated with prior infections (Fonville et al., 2014), including infection occurring early in life. Imprinting is a phenomenon whereby protection against novel A(H5N1) and A(H7N9) viruses has been associated with birth cohort and in turn with early life infection with viruses that share protective stem epitopes (Gostic et al., 2016). Nevertheless, the substantial evidence from repeated vaccination studies also indicates that existing immunity can attenuate antibody responses to prevailing vaccine strains. The antibody focusing hypothesis has been proposed to account for differences in effects of existing immunity and in effects of repeat vaccination between seasons. This hypothesis suggests that previously induced B cells dominate and focus the response on epitopes that are conserved between prior and prevailing vaccine strains, limiting protection when the epitope changes (Cobey and Hensley, 2017; Krammer, 2019). Greater understanding of the mechanisms underlying the effects of existing immunity is vital for vaccine development.
Conclusion 2-1: A priority focus for future research on influenza vaccines is how to overcome issues associated with viral antigenic drift and annual vaccination, and the potential of novel vaccine platforms to circumvent such effects. Improved characterization of B cell responses may help to validate current theories that vaccines recall existing memory B cells but do not efficiently induce de novo B cell responses against variant epitopes in new vaccine strains. This may further guide the rational design of vaccines and adjuvants to provide broader and more durable immune responses.
The Need for Universal Influenza Vaccines
Vaccines targeting conserved epitopes of the influenza virus show promising immunogenicity. Universal influenza vaccines (UIV) theoretically have the potential to provide protection against both seasonal and pandemic strains (Arinaminpathy et al., 2020) (see Chapter 1). As influenza viruses are diverse, there are many possible ways to define “universal” coverage of a vaccine. Criteria for UIV range from the degree and duration of protection to the strains it protects against (Gottlieb and Ben-Yedidia, 2014). WHO has called for the development of a vaccine that “prevents severe disease from all forms of influenza A by 2027, which would prevent pandemics” (Eisenstein, 2019). Other institutions, such as the National Institute of Allergy and Infectious Diseases (NIAID), have developed strategic plans for developing UIVs that hold different criteria (Erbelding et al., 2018). NIAID criteria state that a universal vaccine should: (1) “be at least 75 percent effective,” (2) “protect against group I and II influenza A viruses,” (3) “have durable protection that lasts at least 1 year,” and (4) “be suitable for all age groups” (NIAID, 2021). New technologies have become available and accessible now more than ever and could help move us to a new era of influenza vaccines. These could aid in developing vaccines that not only induce antibody responses but also CD8+ cytotoxic T cell responses that may provide more long-lasting effectiveness.
There are multiple barriers to the development of UIV candidates. The main gap in the development of UIVs is the necessary clinical research (see Chapter 3). Basic research has provided a number of candidates for UIVs as well as vaccine platforms, but challenges associated with clinical development, such as regulatory and economic barriers, contribute to a bottleneck in development (Cox et al., 2015; Nachbagauer and Krammer, 2017). Many of the UIV approaches are studied in silos; being tested as individual products that may or may not lead to an effective vaccine that can protect against future drift variants. An approach would be to combine different strategies in a multivalent vaccine (e.g., head and stem HA in combination, hemagglutinin (HA) and neuraminidase (NA) UIV, internal influenza proteins plus outer glycoproteins) or heterologous prime-boost strategies. Little testing is being performed with these strategies, which have been used in other vaccine development programs, such as AIDS vaccines. To implement such plans, it would be valuable for institutions to explore these approaches via funding support and working with issues related to intellectual property to create a culture of shared goals.
Early clinical trials (phase I and II) are critical for the generation of safety and immunogenicity data required to identify promising antigens and platform constructs and to select candidates for phase III efficacy trials. These types of vaccines are unlikely to replace seasonal vaccines soon, but
they could be rolled out as an early pandemic control measure that does not require prior knowledge of the pandemic strain. Additionally, ongoing research is developing ways to improve the effectiveness of seasonal vaccines, such as improved adjuvants, novel platforms, and inclusion of NA antigens. These investigations may result in influenza vaccine candidates with a broader protection (e.g. “supraseasonal,” or pan-H1 or pan-H3 vaccines) than the strain-specific seasonal vaccines currently in use.
It has been suggested that the COVID-19 pandemic could have been significantly offset if there had been an off-the-shelf prepared vaccine. It is predicted that even if such an off-the-shelf vaccine had a much lower efficacy, it could have slowed the progression of the pandemic while more specific and efficacious vaccines were being developed (Graham, 2021). To respond to a pandemic, the breath of the immune response may be more critical than the durability of the response. Therefore, improvement of seasonal influenza vaccines may offer the most relevant options in the near future for pandemic response preparedness.
Conclusion 2-2: The continued development and clinical assessment of vaccines targeting conserved epitopes and improved seasonal influenza vaccines with the goal of establishing a pandemic-ready stockpile is a priority. However, the usefulness of broadly protective influenza virus vaccines/UIV is not limited to pandemic preparedness. Highly effective UIV candidates would also abolish the need for matched seasonal vaccines and thereby increase vaccine uptake globally, leading to a reduced burden of seasonal influenza on global health and decreased susceptibility of the global population against zoonotic influenza virus outbreaks or emerging pandemic influenza viruses.
BASIC AND TRANSLATIONAL SCIENCE: APPLYING LESSONS FROM THE DEVELOPMENT OF COVID-19 VACCINES TO VACCINES FOR INFLUENZA
Funding Basic Research
The committee recognizes that the long-term investments in basic research have resulted in infrastructure to support the development of seasonal, broad, and universal influenza vaccines, with novel immune correlates of protection (Lim et al., 2020; Newland et al., 2021). Previous investments in basic research were essential for rapid vaccine development; and academic and industry researchers with established off-the-shelf protocols and know-how were able to move quickly to conduct needed research on novel vaccines for COVID-19. These tools will also aid in improving influenza pandemic preparedness. Similarly, sustained and appropriate invest-
ments in basic and translational science on influenza (Erbelding et al., 2018) and earlier novel coronavirus outbreaks (Begum et al., 2020) provided an evidence base for rapid development of stabilized spike-based COVID-19 vaccines (Begum et al., 2020; Wu et al., 2021), experience which can be capitalized on for future influenza vaccine work (Erbelding et al., 2018).
During interpandemic periods, funding has generally been insufficient to build and maintain capacity for manufacture and deployment of pandemic vaccines. There were many opportunities to prepare for the emergence of more SARS-CoV-like viruses between 2002 and 2020 (Saif, 2020). However, as the SARS-CoV epidemic ended and MERS-CoV outbreaks declined, and as a consequence of the decrease in perceived likelihood of need and profitability of these vaccines, funding to maintain R&D of these vaccines was reduced (Begum et al., 2020). During an outbreak, it is typical for funding to be available for the R&D of strategies leading to a phase I study. However, as soon as the outbreak resolves, funding is decreased because funding agencies are quick to move on (GPMB, 2019). For novel influenza pandemic strains, fluctuations in funding combined with the need to wait until there is active transmission to test vaccine efficacy significantly slows vaccine development, as seen with the Ebola vaccine. To ensure vaccine preparedness for the next pandemic it is essential to interrupt this pattern of discontinuation of funding for vaccine development once case numbers decline and the likelihood for potential profit margins decreases. There is a large discrepancy between the cost of a pandemic and what it would cost to consistently fund the research required for preparedness. In a non-pandemic context, funding has been insufficient for investment in capacity building and improving the public health infrastructure, thus limiting sustainable efforts to prepare for an outbreak (NAM, 2016). A continuous source of funding is necessary to prevent patterns of spikes and deserts in the funding landscape, as the current strategy is highly ineffective. While funding vaccine research is costly, ad hoc clinical trials introduce far more financial and operational inefficiencies. For example, cost analyses show that the price of developing a new vaccine from the preclinical testing phase through phase IIA trials is up to $350 million (Gouglas et al., 2018). For perspective, total global spending on COVID-19 vaccines is projected to be as high as $157 billion through 2025 (Mishra, 2021). A World Bank report estimates that a severe pandemic such as the influenza H1N1 pandemic of 1918 could cost 5 percent of the global gross domestic product. It also estimates that the annual cost of a moderate to severe pandemic would cost $570 billion annually or 0.7 percent of global income, while funding pandemic preparedness would cost just $4.5 billion, less than $1 per person (IWG, 2017). Economic impact studies like this show how costly it is to the global economy when the world is underprepared for a pandemic.
Conclusion 2-3: Sufficient funding for continuous research in the basic science of influenza vaccines is paramount to ensuring preparedness for a pandemic outbreak. Current investments are inadequate during interpandemic periods.
Using Novel Platforms for Influenza Vaccines
The COVID-19 pandemic has been a driving force for vaccine innovation, and has shown us the value of the mRNA vaccine technology (Chu et al., 2021; Vasireddy et al., 2021). At present, mRNA vaccines are expensive, capacity to manufacture them is limited, reactogenicity is relatively high—which may limit use in young children, and it is difficult to distribute them to LMICs given the cold chain required for these vaccines (Knezevic et al., 2021; Rosa et al., 2021). However, COVID-19 mRNA vaccines were developed rapidly out of need, without time to test ways in which to improve stability. With further developments and improvements of this platform, and uptake by more manufacturers, these challenges can be overcome. For example, during the COVID-19 pandemic response, for the first time, there was an essentially seamless transition between trial phases with a single protocol (NCIRS, 2020). Accordingly, further R&D on novel platforms is suggested, including increasing the number of manufacturers in different geographic areas to overcome these challenges. Expanding the number of manufacturers globally can also aid in situations where there are shortages caused by other factors unrelated to a pandemic, such as infrastructure damage caused by natural disasters.
Recombinant protein vaccines may be a cost-effective and efficient way of producing influenza vaccines compared to the mRNA platform as they have already been shown to be safe and effective and the infrastructure for production is already in place (Dunkle and Izikson, 2016; Pollet et al., 2021). Recombinant protein vaccines, such as FluBlok, have been developed and licensed for influenza but require a dose of HA that is three times higher than that used in egg-based vaccines (Wong and Webby, 2013). Development of protein vaccines for pandemic influenza may be less challenging than for SARS-CoV-2, as different HA proteins are expressed at comparable and relatively high levels using recombinant baculovirus insect cell expression systems (Jazayeri and Poh, 2019; Krammer, 2020). While recombinant protein vaccines are relatively easy to develop and manufacture, a rate-limiting step in the development for such vaccines for pandemic influenza may be the need for adjuvants to increase immunogenicity and efficacy (Coffman et al., 2010; Pollet et al., 2021). Generally, each vaccine antigen-adjuvant combination that is developed must be licensed, rather than generic licenses for adjuvants, which poses a challenge for develop-
ment of new combinations. However, research into adjuvant combinations should not be forgotten in a vaccine R&D strategy because of its relative value to optimizing vaccine efficacy and dose sparing. Preclinical toxicity studies and clinical trials are therefore required for each new combination. Continuous research is needed to understand the mechanism of action and safety of adjuvants and enhanced efforts to find more efficacious adjuvants than currently available. Additionally, there is a need for licensing and building capacity to manufacture adjuvants. This will require funding to increase preclinical and clinical trial facilities.
The differences in cost of vaccines between various older and more novel vaccine platforms (e.g., egg-based versus cell culture, mRNA, or recombinant protein based) are defined by a plethora of factors, including the materials used for the production and intellectual property. Given that some vaccine platforms (reverse genetics, mRNA, recombinant protein) are relatively new, proprietary issues may result in higher costs when aiming to expand capacity. Next to the direct costs of vaccine manufacturing, the clinical work required to determine safety and efficacy to allow the vaccines to be considered for authorization and approval is an extremely costly process that represents a high risk for industry (Plotkin et al., 2017).
Conclusion 2-4: With respect to vaccine development platforms, a priority is the development of strategies for the rational choice of platforms that balances vaccine effectiveness, speed of development, and cost as the pandemic progresses. For instance, a moderately effective vaccine might be acceptable if readily available, and it can be replaced by more effective or lower cost vaccines later.
Standardizing Assays, Reagents, and Animal Models
To strengthen and adapt the vaccine development pipeline to accommodate new vaccine platforms, standardization of assays, reagents, and animal models is essential. Allowing sufficient flexibility in the details of assay design, especially for later-stage development, is essential to allow for the refinement of assays that is needed to achieve the automation, throughput, and performance standards needed to support the large-scale clinical trials that lead to vaccine authorization or licensure. After basic research characterization of potential targets for vaccines, the first phase of vaccine development involves preclinical research, including both in vitro work and animal studies to test safety and immunogenicity. Animal studies usually involve rodents and, in later stages, ferrets and sometimes non-human primates for immunological studies (FDA, 2015). Development of new reagents, particularly for ferrets, guinea pigs, and hamsters, is necessary if vaccine assessment will be performed in these preclinical animal
models. Following those studies, vaccine candidates are assessed in regulatory applications, such as the investigational new drug application to the U.S. Food and Drug Administration (FDA), before receiving approval for in-human testing. Human studies involve three main phases, followed by a postmarketing surveillance phase (FDA, 2015; Slaoui and Hepburn, 2020). Clinical trials start with phase I studies in a small group of adults to assess safety; the type and extent of the immune response may be monitored. In the absence of safety concerns, the vaccine will move to phase II studies, which are conducted in a larger group of several hundred adults and may include people from risk groups. The safety, immunogenicity, proposed dosing and dosing schedule will be determined in these studies. Finally, the vaccine candidate moves to phase III studies, which include thousands to tens of thousands of individuals, often in a double-blind, randomized, placebo-controlled study to assess safety and efficacy. Efficacy studies may determine whether the vaccine prevents infection with the virus; prevents (severe) disease, hospitalization, or death; and induces a protective immune response by assessing antibody production and other immune markers (FDA, 2015).
To achieve clinical endpoints, the prevalence of the virus in a population (attack rate) is a determinant of how many people need to be studied and for how long. Given the relatively low incidence of clinically apparent seasonal influenza, clinical trials are often large, making them costly and time consuming. This discourages pharmaceutical companies from engaging in next-generation influenza virus vaccine development. Having immune correlates of protection to assess in clinical trials would streamline the vaccine development pipeline. New vaccine platforms require their own immune correlates, and these should be assessed using standardized assays to allow for the comparison of immunogenicity and efficacy between different vaccine candidates and for decision making. For COVID-19, this was not predetermined and comparing vaccine trial results was therefore challenging. Correlates of protection that are being assessed for COVID-19 are not yet well defined, though neutralizing antibody responses to the spike protein are often used as a proxy and can be assessed by multiple assays reported as values or titers (Khoury et al., 2021; Klasse et al., 2021). Convalescent plasma was often included as a comparator; however, this was not standardized across studies. International standards were not readily available and clinical trials were developed and conducted before standards were available. This example indicates the need for research to define correlates of protection and assay standardization before a pandemic, given the short response time in pandemic conditions.
After the development of novel vaccines, there is a need to develop new endpoints to provide guidance to regulatory agencies, like FDA, for assessment of novel vaccines that do not induce hemagglutinin inhibition
(HI) antibodies (Lim et al., 2020; Weir and Gruber, 2016). New seasonal influenza virus strains are selected on the basis of specific hemagglutination-inhibiting (HI) antibody responses, and seasonal influenza vaccines do not require field efficacy trials each time they are updated (Weir and Gruber, 2016). When updating a vaccine containing new antigens for the seasonal influenza vaccine, manufacturers submit a supplement for their existing vaccine license. Supplements for live attenuated, but not inactivated, vaccines require a study in approximately 300 adults to demonstrate sufficient attenuation (Weir and Gruber, 2016). The current system promotes the types of vaccines already available as it uses HI antibodies as the correlate of protection for influenza. Age also affects immunity, which is not currently considered in the correlate of protection, but it should be addressed (Reber et al., 2012). Another effect of the focus on the HI assay is the lack of attention to NA in assessing VE. Traditional vaccines contain some NA, but the amount of NA in vaccines is not standardized, and NA antibodies are not measured in a standardized assay (Gomez Lorenzo and Fenton, 2013). Therefore, standardization and sole focus on one type of assay and correlates of protection might stand in the way of innovation. During the influenza season, countries such as the United States, Canada, Russia, Australia, and multiple European countries routinely use the test-negative design to estimate VE, and conduct ad hoc assessments of vaccine immunogenicity (Sullivan et al., 2014). The committee proposes investments that support research into new clinical endpoints on an ongoing basis, and that assays to assess correlates of protection are standardized for each vaccine platform.
From a technical point of view, the establishment of correlates of protection and their broad use across different vaccines, platforms, developers, and regulatory agencies would be facilitated by the use of standardized assays and reagents. For influenza, there have already been efforts to standardize protocols and establish quality assurance systems. The Standardization and Development of Assays for Assessment of Influenza Vaccine Correlates of Protection (FLUCOP) initiative was developed with the goal of standardizing assays for correlates of protection and is focused on providing protocols and training videos to make these assays available globally. Funding for FLUCOP has come from the European Union and will end in 2022 (FLUCOP, 2020). Additionally, the Consortium for the Standardization of Influenza Seroepidemiology (CONSISE) started in 2011 as a partnership of various global organizations without specific funding. CONSISE aims to standardize influenza serology worldwide, and has developed standardized research protocols for epidemiology, clinical data collection, and biological sample collection, processing, and research (CONSISE, 2021).
Conclusion 2-5: Advances in influenza vaccine development will require standardized assays, reagents, and animal models for the development of novel correlates of protection. This work should be facilitated by WHO’s development of international standards for these research tools.
Global Expansion of Clinical Research
Global collaboration and distribution of know-how and reagents are essential for the development of vaccines, correlates of protection, and global clinical trial capacity (human capital). Clinical research presents a fundamental gap in the R&D of vaccines. There are several candidates for universal influenza vaccines and several new vaccine platforms, but clinical testing is a bottleneck that should be prioritized. Clinical development should be carried out on a global scale, requiring global coordination. However, as of 2021, most of the universal influenza vaccine trials are performed in the United States. At the time of print, the National Institutes of Health (NIH) reports as many as 25 clinical trials being conducted globally. Of these, 11 are in the United States, 6 in Europe, 4 in the Middle East, and 2 in North Asia (NIH, 2021). It is essential that vaccines are tested in diverse populations and, therefore, expansion of the conduction of clinical trials globally is vital.
Impediments to the necessary collaboration include resource limitations and the complexities of multi-national trials, including regulatory hurdles and incompatibility. Few countries have the capacity and expertise to perform such trials. Note that WHO has previously encouraged LMIC manufacturers to move into the influenza vaccine space, and these efforts were supported in the past by funding from the Biomedical Advanced Research Development Authority (BARDA) in the United States (Berlanda Scorza, 2017). However, there is a need for a local market to sustain influenza vaccine development. There will be a need to build appropriate facilities and train local experts, which will require political support and a local market for influenza vaccine to sustain production.
RECOMMENDATIONS
Based on the findings and conclusions presented in this chapter, the committee makes the following recommendations regarding the research, development, and manufacturing of seasonal and pandemic influenza vaccines in the dimension of basic and translational science.
Recommendation 2-1: The U.S. Department of Health and Human Services, through the National Institute of Allergy and Infectious Diseases, the Biomedical Advanced Research and Development Authority,
and the U.S. Department of Defense, as well as other corresponding governmental and funding agencies domestically and abroad, should invest, proportionate to the enormous costs of pandemics, in basic and translational research to reveal a diverse array of influenza vaccines, using different platforms, viral targets, adjuvants, and delivery systems. This will allow selection of the candidates most fit for purpose to be brought to authorization and sufficient production and distribution to optimize the control of influenza across diverse settings and phases of pandemics and epidemics.
Recommendation 2-2: The World Health Organization should advocate and coordinate with multi-lateral stakeholders (e.g., the Coalition for Epidemic Preparedness Innovations), governments, funding agencies, the vaccine industry, and philanthropic organizations to build global capacity for robust and internationally comparable preclinical, clinical, and immunological assessments of influenza vaccine candidates, including novel candidates that use innovative structures, targets, and delivery systems to potentially broaden or improve protection.
Recommendation 2-3: International research networks (e.g., the National Institutes of Health/U.S. Centers for Disease Control and Prevention funding networks) supported by governments and funding agencies, the World Health Organization, and the vaccine industry should support, carefully plan, and conduct multi-center international clinical trials and field studies to compare emerging vaccines with standard vaccines in, among others, geographically, demographically, and immunologically diverse populations to inform rational and situation-based use and manufacture of an extended array of vaccines.
Recommendation 2-4: National regulators should engage with the vaccine industry and academic researchers in the development, standardization, and implementation of innovative assays to evaluate vaccines that induce immunity through mechanisms other than strain-specific neutralizing hemagglutination-inhibiting antibodies in order to reach consensus on the validation of these assays that will allow approval or licensure of influenza vaccines based on a broader range of assays that reflect induction of immunity.
REFERENCES
Al Kaabi, N., Y. Zhang, S. Xia, Y. Yang, M. M. Al Qahtani, N. Abdulrazzaq, M. Al Nusair, M. Hassany, J. S. Jawad, J. Abdalla, S. E. Hussein, S. K. Al Mazrouei, M. Al Karam, X. Li, X. Yang, W. Wang, B. Lai, W. Chen, S. Huang, Q. Wang, T. Yang, Y. Liu, R. Ma, Z. M. Hussain, T. Khan, M. Saifuddin Fasihuddin, W. You, Z. Xie, Y. Zhao, Z. Jiang, G. Zhao, Y. Zhang, S. Mahmoud, I. ElTantawy, P. Xiao, A. Koshy, W. A. Zaher, H. Wang, K. Duan, A. Pan, and X. Yang. 2021. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: A randomized clinical trial. JAMA 326(1):35–45.
Arinaminpathy, N., S. Riley, W. S. Barclay, C. Saad-Roy, and B. Grenfell. 2020. Population implications of the deployment of novel universal vaccines against epidemic and pandemic influenza. Journal of the Royal Society Interface 17(164):20190879.
Baden, L. R., H. M. El Sahly, B. Essink, K. Kotloff, S. Frey, R. Novak, D. Diemert, S. A. Spector, N. Rouphael, C. B. Creech, J. McGettigan, S. Khetan, N. Segall, J. Solis, A. Brosz, C. Fierro, H. Schwartz, K. Neuzil, L. Corey, P. Gilbert, H. Janes, D. Follmann, M. Marovich, J. Mascola, L. Polakowski, J. Ledgerwood, B. S. Graham, H. Bennett, R. Pajon, C. Knightly, B. Leav, W. Deng, H. Zhou, S. Han, M. Ivarsson, J. Miller, and T. Zaks. 2020. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England Journal of Medicine 384(5):403–416.
Ball, P. 2021. The lightning-fast quest for COVID vaccines—and what it means for other diseases. Nature 589(7840):16–18.
Barr, I. G., R. O. Donis, J. M. Katz, J. W. McCauley, T. Odagiri, H. Trusheim, T. F. Tsai, and D. E. Wentworth. 2018. Cell culture-derived influenza vaccines in the severe 2017-2018 epidemic season: A step towards improved influenza vaccine effectiveness. NPJ Vaccines 3:44.
Bedford, T., S. Riley, I. G. Barr, S. Broor, M. Chadha, N. J. Cox, R. S. Daniels, C. P. Gunasekaran, A. C. Hurt, A. Kelso, A. Klimov, N. S. Lewis, X. Li, J. W. McCauley, T. Odagiri, V. Potdar, A. Rambaut, Y. Shu, E. Skepner, D. J. Smith, M. A. Suchard, M. Tashiro, D. Wang, X. Xu, P. Lemey, and C. A. Russell. 2015. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature 523(7559):217–220.
Begum, J., M. Nasir Akbar, K. Dev, B. Buyamayum, W. Mohd Yaqoob, and M. Raza. 2020. Challenges and prospects of COVID 19 vaccine development based on the progress made in SARS and MERS vaccine development. Transboundary and Emerging Diseases. August 20.
Belongia, E. A., D. M. Skowronski, H. Q. McLean, C. Chambers, M. E. Sundaram, and G. De Serres. 2017. Repeated annual influenza vaccination and vaccine effectiveness: Review of evidence. Expert Review of Vaccines 16(7):1–14.
Berlanda Scorza, F. 2017. Advancing new vaccines against pandemic influenza in low-resource countries. Vaccine 35(40):5397–5402.
Billington, J., I. Deschamps, S. C. Erck, J. L. Gerberding, E. Hanon, S. Ivol, J. W. Shiver, J. A. Spencer, and J. Van Hoof. 2020. Developing vaccines for SARS-CoV-2 and future epidemics and pandemics: Applying lessons from past outbreaks. Health Security 18(3):241–249.
Blumenthal, K. G., L. B. Robinson, C. A. Camargo Jr., E. S. Shenoy, A. Banerji, A. B. Land-man, and P. Wickner. 2021. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA 325(15):1562–1565.
Boikos, C., G. C. Sylvester, J. S. Sampalis, and J. A. Mansi. 2020. Relative effectiveness of the cell-cultured quadrivalent influenza vaccine compared to standard, egg-derived quadrivalent influenza vaccines in preventing influenza-like illness in 2017-2018. Clinical Infectious Diseases 71(10):e665–e671.
CDC (U.S. Centers for Disease Control and Prevention). 2020. Influenza (flu) vaccine effectiveness studies. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm (accessed October 19, 2021).
CEPI (Coalition for Epidemic Preparedness Innovations). 2020. 2020 annual progress report: Covering the period from 1 January–31 December. https://cepi.net/wp-content/uploads/2021/03/CEPI-2020-Annual-Report.pdf (accessed October 19, 2021).
Chu, L., R. McPhee, W. Huang, H. Bennett, R. Pajon, B. Nestorova, B. Leav, and mRNA Study Group. 2021. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 39(20):2791–2799.
Cobey, S., and S. E. Hensley. 2017. Immune history and influenza virus susceptibility. Current Opinion in Virology 22:105–111.
Coffman, R. L., A. Sher, and R. A. Seder. 2010. Vaccine adjuvants: Putting innate immunity to work. Immunity 33(4):492–503.
CONSISE (Consortium for the Standardization of Influenza Seroepidemiology). 2021. About us. https://consise.tghn.org/about (accessed October 19, 2021).
Corder, B. N., B. L. Bullard, G. A. Poland, and E. A. Weaver. 2020. A decade in review: A systematic review of universal influenza vaccines in clinical trials during the 2010 decade. Viruses 12(10):1186.
Cox, N. J., J. Hickling, R. Jones, G. F. Rimmelzwaan, L. C. Lambert, J. Boslego, L. Rudenko, L. Yeolekar, J. S. Robertson, J. Hombach, and J. R. Ortiz. 2015. Report on the second WHO integrated meeting on development and clinical trials of influenza vaccines that induce broadly protective and long-lasting immune responses. Vaccine 33(48):6503–6510.
DeMarcus, L., L. Shoubaki, and S. Federinko. 2019. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine 37(30):4015–4021.
Divino, V., G. Krishnarajah, S. I. Pelton, J. Mould-Quevedo, V. R. Anupindi, M. DeKoven, and M. J. Postma. 2020. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season. Vaccine 38(40):6334–6343.
Dunkle, L. M., and R. Izikson. 2016. Recombinant hemagglutinin influenza vaccine provides broader spectrum protection. Expert Review of Vaccines 15(8):957–966.
Eisenstein, M. 2019. Towards a universal flu vaccine. Nature 573(7774):S50–S52.
Erbelding, E. J., D. J. Post, E. J. Stemmy, P. C. Roberts, A. D. Augustine, S. Ferguson, C. I. Paules, B. S. Graham, and A. S. Fauci. 2018. A universal influenza vaccine: The strategic plan for the National Institute of Allergy and Infectious Diseases. Journal of Infectious Diseases 218(3):347–354.
European Commission. 2021. EU research and innovation supporting vaccine development for COVID-19. https://ec.europa.eu/info/research-and-innovation/research-area/health-research-and-innovation/coronavirus-research-and-innovation/vaccines_en (accessed October 19, 2021).
FDA (U.S. Food and Drug Administration). 2015. Drug development and review definitions. https://www.fda.gov/drugs/investigational-new-drug-ind-application/drug-development-and-review-definitions (accessed October 19, 2021).
FDA. 2019. First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response. https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virusdisease-marking-critical-milestone-public-health (accessed October 19, 2021).
FLUCOP Standardization and Development of Assays for Assessment of Influenza Vaccine Correlates of Protection. 2020. Standardisation and development of assays for assessment of influenza vaccine correlates of protection. https://flucop.eu (accessed October 19, 2021).
Fonville, J. M., S. H. Wilks, S. L. James, A. Fox, M. Ventresca, M. Aban, L. Xue, T. C. Jones, N. M. H. Le, Q. T. Pham, N. D. Tran, Y. Wong, A. Mosterin, L. C. Katzelnick, D. Labonte, T. T. Le, G. van der Net, E. Skepner, C. A. Russell, T. D. Kaplan, G. F. Rimmelzwaan, N. Masurel, J. C. de Jong, A. Palache, W. E. P. Beyer, Q. M. Le, T. H. Nguyen, H. F. L. Wertheim, A. C. Hurt, A. D. M. E. Osterhaus, I. G. Barr, R. A. M. Fouchier, P. W. Horby, and D. J. Smith. 2014. Antibody landscapes after influenza virus infection or vaccination. Science 346(6212):996–1000.
Francis, T. 1960. On the doctrine of original antigenic sin. Proceedings of the American Philosophical Society 104(6):572–578.
Friede, M., L. Palkonyay, C. Alfonso, Y. Pervikov, G. Torelli, D. Wood, and M. P. Kieny. 2011. WHO initiative to increase global and equitable access to influenza vaccine in the event of a pandemic: Supporting developing country production capacity through technology transfer. Vaccine 29:A2–A7.
GAO (U.S. Government Accountability Office). 2021. Operation Warp Speed: Accelerated COVID-19 vaccine development status and efforts to address manufacturing challenges. https://www.gao.gov/products/gao-21-319 (accessed October 19, 2021).
Gavi (The Vaccine Alliance). 2021. The COVID-19 vaccine race—weekly update. https://www.gavi.org/vaccineswork/covid-19-vaccine-race (accessed October 19, 2021).
Gomez Lorenzo, M. M., and M. J. Fenton. 2013. Immunobiology of influenza vaccines. Chest 143(2):502–510.
Gostic, K. M., M. Ambrose, M. Worobey, and J. O. Lloyd-Smith. 2016. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 354(6313):722–726.
Gottlieb, T., and T. Ben-Yedidia. 2014. Epitope-based approaches to a universal influenza vaccine. Journal of Autoimmunity 54:15–20.
Gouglas, D., T. Thanh Le, K. Henderson, A. Kaloudis, T. Danielsen, N. C. Hammersland, J. M. Robinson, P. M. Heaton, and J.-A. Røttingen. 2018. Estimating the cost of vaccine development against epidemic infectious diseases: A cost minimisation study. Lancet Global Health 6(12):e1386–e1396.
Gouma, S., S. J. Zost, K. Parkhouse, A. Branche, D. J. Topham, S. Cobey, and S. E. Hensley. 2020. Comparison of human H3N2 antibody responses elicited by egg-based, cell-based, and recombinant protein-based influenza vaccines during the 2017-2018 season. Clinical Infectious Diseases 71(6):1447–1453.
GPMB (Global Preparedness Monitoring Board). 2019. Pandemic preparedness financing status update. https://www.gpmb.org/#tab=tab_1 (accessed October 19, 2021).
Graham, B. S. 2021. Lessons learned from COVID-19 for pandemic preparedness: Vaccine R&D for advancing pandemic and seasonal influenza preparedness, learning from the COVID-19 vaccine experience.
Hampson, A., I. Barr, N. Cox, R. O. Donis, S. Siddhivinayak, D. Jernigan, J. Katz, J. McCauley, F. Motta, T. Odagiri, J. S. Tam, A. Waddell, R. Webby, T. Ziegler, and W. Zhang. 2017. Improving the selection and development of influenza vaccine viruses—report of a WHO informal consultation on improving influenza vaccine virus selection, Hong Kong SAR, China, 18–20 November 2015. Vaccine 35(8):1104–1109.
Huang, K.-Y. A., S.-C. Chang, Y.-C. Huang, C.-H. Chiu, and T.-Y. Lin. 2017. Antibody responses to trivalent inactivated influenza vaccine in health care personnel previously vaccinated and vaccinated for the first time. Scientific Reports 7:40027.
IOM (Institute of Medicine). 2010. The 2009 H1N1 influenza vaccination campaign: Summary of a workshop series. Washington, DC: The National Academies Press.
IWG (International Working Group on Financing Preparedness). 2017. From panic and neglect to investing in health security: Financing pandemic preparedness at a national level. Washington, DC: World Bank Group.
Izurieta, H. S., Y. Chillarige, J. Kelman, Y. Wei, Y. Lu, W. Xu, M. Lu, D. Pratt, S. Chu, M. Wernecke, T. MaCurdy, and R. Forshee. 2018. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. Journal of Infectious Diseases 220(8):1255–1264.
Jazayeri, S. D., and C. L. Poh. 2019. Development of universal influenza vaccines targeting conserved viral proteins. Vaccines 7(4):169.
Kelvin, A. A., and M. Zambon. 2019. Influenza imprinting in childhood and the influence on vaccine response later in life. Euro Surveillance 24(48):1900720.
Khoury, D. S., D. Cromer, A. Reynaldi, T. E. Schlub, A. K. Wheatley, J. A. Juno, K. Subbarao, S. J. Kent, J. A. Triccas, and M. P. Davenport. 2021. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nature Medicine 27(7):1205–1211.
Klasse, P. J., D. F. Nixon, and J. P. Moore. 2021. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Science Advances 7(12):eabe8065.
Klein, N. P., B. Fireman, K. Goddard, O. Zerbo, J. Asher, J. Zhou, J. King, and N. Lewis. 2020. Vaccine effectiveness of cell-culture relative to egg-based inactivated influenza vaccine during the 2017–18 influenza season. PLOS ONE 15(2):e0229279.
Knezevic, I., M. A. Liu, K. Peden, T. Zhou, and H. N. Kang. 2021. Development of mRNA vaccines: Scientific and regulatory issues. Vaccines 9(2):1–11.
Krammer, F. 2019. The human antibody response to influenza a virus infection and vaccination. Nature Reviews Immunology 19(6):383–397.
Krammer, F. 2020. SARS-CoV-2 vaccines in development. Nature 586(7830):516–527.
Krammer, F., and P. Palese. 2015. Advances in the development of influenza virus vaccines. Nature Reviews Drug Discovery 14(3):167–182.
Krishnarajah, G., V. Divino, M. J. Postma, S. I. Pelton, V. R. Anupindi, M. DeKoven, and J. Mould-Quevedo. 2021. Clinical and economic outcomes associated with cell-based quadrivalent influenza vaccine vs. standard-dose egg-based quadrivalent influenza vaccines during the 2018–19 influenza season in the United States. Vaccines 9(2):80.
Lederer, K., D. Castaño, D. Gómez Atria, T. H. Oguin 3rd, S. Wang, T. B. Manzoni, H. Muramatsu, M. J. Hogan, F. Amanat, P. Cherubin, K. A. Lundgreen, Y. K. Tam, S. H. Y. Fan, L. C. Eisenlohr, I. Maillard, D. Weissman, P. Bates, F. Krammer, G. D. Sempowski, N. Pardi, and M. Locci. 2020. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity 53(6):1281–1295.
Leung, V. K. Y., A. Fox, L. A. Carolan, M. Aban, K. L. Laurie, J. Druce, Y.-M. Deng, M. A. Slavin, C. Marshall, and S. G. Sullivan. 2021. Impact of prior vaccination on antibody response and influenza-like illness among Australian healthcare workers after influenza vaccination in 2016. Vaccine 39(24):3270–3278.
Lim, W. W., N. H. L. Leung, S. G. Sullivan, E. J. Tchetgen, and B. J. Cowling. 2020. Distinguishing causation from correlation in the use of correlates of protection to evaluate and develop influenza vaccines. American Journal of Epidemiology 189(3):185–192.
Logunov, D. Y., I. V. Dolzhikova, D. V. Shcheblyakov, A. I. Tukhvatulin, O. V. Zubkova, A. S. Dzharullaeva, A. V. Kovyrshina, N. L. Lubenets, D. M. Grousova, A. S. Erokhova, A. G. Botikov, F. M. Izhaeva, O. Popova, T. A. Ozharovskaya, I. B. Esmagambetov, I. A. Favorskaya, D. I. Zrelkin, D. V. Voronina, D. N. Shcherbinin, A. S. Semikhin, Y. V. Simakova, E. A. Tokarskaya, D. A. Egorova, M. M. Shmarov, N. A. Nikitenko, V. A. Gushchin, E. A. Smolyarchuk, S. K. Zyryanov, S. V. Borisevich, B. S. Naroditsky, and A. L. Gintsburg. 2021. Safety and efficacy of an RAD26 and RAD5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397(10275):671–681.
Milián, E., and A. A. Kamen. 2015. Current and emerging cell culture manufacturing technologies for influenza vaccines. BioMedical Research International 2015.
Mishra, M. 2021. World to spend $157 billion on COVID-19 vaccines through 2025 report. https://www.reuters.com/business/healthcare-pharmaceuticals/world-spend-157-billion-covid-19-vaccines-through-2025-report-2021-04-29 (accessed October 19, 2021).
Mohn, K. G. I., I. Smith, H. Sjursen, and R. J. Cox. 2018. Immune responses after live attenuated influenza vaccination. Human Vaccines & Immunotherapeutics 14(3):571–578.
Morimoto, N., and K. Takeishi. 2018. Change in the efficacy of influenza vaccination after repeated inoculation under antigenic mismatch: A systematic review and meta-analysis. Vaccine 36(7):949–957.
Mosterín Höpping, A., J. McElhaney, J. M. Fonville, D. C. Powers, W. E. P. Beyer, and D. J. Smith. 2016. The confounded effects of age and exposure history in response to influenza vaccination. Vaccine 34(4):540–546.
Nachbagauer, R., and F. Krammer. 2017. Universal influenza virus vaccines and therapeutic antibodies. Clinical Microbiology and Infection 23(4):222–228.
NAM (National Academy of Medicine). 2016. The neglected dimension of global security: A framework to counter infectious disease crises. Washington, DC: The National Academies Press. Pp. 17–22.
NCIRS (National Centre for Immunisation Research and Surveillance). 2020. Phases of clinical trials https://www.ncirs.org.au/phases-clinical-trials (accessed October 19, 2021).
Newland, M., D. Durham, J. Asher, J. J. Treanor, J. Seals, R. O. Donis, and R. A. Johnson. 2021. Improving pandemic preparedness through better, faster influenza vaccines. Expert Review of Vaccines.
Nguyen-Contant, P., M. Y. Sangster, and D. J. Topham. 2021. Squalene-based influenza vaccine adjuvants and their impact on the hemagglutinin-specific B cell response. Pathogens 10(3):355.
NIAID (National Institute of Allergy and Infectious Diseases). 2021. NIAID strategic plan for a universal influenza vaccine. https://www.niaidcivics.org/news/2019/10/niaid-strategic-plan-for-a-universal-influenza-vaccine (accessed October 19, 2021).
NIH (National Institutes of Health). 2021. https://clinicaltrials.gov/ct2/results/map?term=universal+influenza+vaccine&map= (accessed October 19, 2021).
Novavax. 2021. Novavax COVID-19 vaccine demonstrates 90% overall efficacy and 100% protection against moderate and severe disease in prevent-19 phase 3 trial. Gaithersburg, MD: Novavax, Inc.
Oliver, S. E., J. W. Gargano, H. Scobie, M. Wallace, S. C. Hadler, J. Leung, A. E. Blain, N. McClung, D. Campos-Outcalt, R. L. Morgan, S. Mbaeyi, J. MacNeil, J. R. Romero, H. K. Talbot, G. M. Lee, B. P. Bell, and K. Dooling. 2021. The advisory committee on immunization practices’ interim recommendation for use of Janssen COVID-19 vaccine—United States, February 2021. Morbidity and Mortality Weekly Report 70(9):329–332.
PATH. 2020. Vaccine cold chain Q&A. https://www.path.org/articles/vaccine-cold-chain-q (accessed October 19, 2021).
Paules, C. I., and A. S. Fauci. 2019. Influenza vaccines: Good, but we can do better. Journal of Infectious Diseases 219(Suppl 1):S1–S4.
Paules, C. I., S. G. Sullivan, K. Subbarao, and A. S. Fauci. 2017. Chasing seasonal influenza—the need for a universal influenza vaccine. New England Journal of Medicine 378(1):7–9.
Peshimam, G. N., and U. Farooq. 2021. CanSinoBio’s COVID-19 vaccine 65.7% effective in global trials, Pakistan official says. Reuters. https://www.reuters.com/article/us-healthcoronavirus-vaccine-pakistan/cansinobios-covid-19-vaccine-65-7-effective-in-globaltrials-pakistan-official-says-idUSKBN2A81N0 (accessed October 19, 2021).
Plotkin, S., J. M. Robinson, G. Cunningham, R. Iqbal, and S. Larsen. 2017. The complexity and cost of vaccine manufacturing - An overview. Vaccine 35(33):4064–4071.
Pokharel, K. 2021. India’s Bharat Biotech says vaccine is effective against delta variant. https://www.wsj.com/articles/indias-bharat-biotech-says-vaccine-is-effective-against-delta-variant-11625325879 (accessed October 19, 2021).
Polack, F. P., S. J. Thomas, N. Kitchin, J. Absalon, A. Gurtman, S. Lockhart, J. L. Prez, G. Pérez Marc, E. D. Moreira, C. Zerbini, R. Bailey, K. A. Swanson, S. Roychoudhury, K. Koury, P. Li, W. V. Kalina, D. Cooper, R. W. Frenck, L. L. Hammitt, Ö. Türeci, H. Nell, A. Schaefer, S. Ünal, D. B. Tresnan, S. Mather, P. R. Dormitzer, U. Şahin, K. U. Jansen, and W. C. Gruber. 2020. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. New England Journal of Medicine 383(27):2603–2615.
Pollet, J., W.-H. Chen, and U. Strych. 2021. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Advanced Drug Delivery Reviews 170:71–82.
Rajaram, S., C. Boikos, D. K. Gelone, and A. Gandhi. 2020a. Influenza vaccines: The potential benefits of cell-culture isolation and manufacturing. Therapeutic Advances in Vaccines and Immunotherapy 8:2515135520908121.
Rajaram, S., R. Wojcik, C. Moore, R. Ortiz de Lejarazu, S. de Lusignan, E. Montomoli, A. Rossi, A. Pérez-Rubio, A. Trilla, V. Baldo, R. Jandhyala, and G. Kassianos. 2020b. The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: Literature review and expert consensus. Vaccine 38(38):6047–6056.
Rappuoli, R., and P. R. Dormitzer. 2012. Influenza: Options to improve pandemic preparation. Science 336(6088):1531–1533.
Reber, A. J., T. Chirkova, J. H. Kim, W. Cao, R. Biber, D. K. Shay, and S. Sambhara. 2012. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging and Disease 3(1):68–90.
Rockman, S., K. L. Laurie, S. Parkes, A. Wheatley, and I. G. Barr. 2020. New technologies for influenza vaccines. Microorganisms 8(11):1745.
Rosa, S. S., D. M. F. Prazeres, A. M. Azevedo, and M. P. C. Marques. 2021. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 39(16):2190–2200.
Ruiz-Aragón, J., R. Gani, S. Márquez, and P. Alvarez. 2020. Estimated cost-effectiveness and burden of disease associated with quadrivalent cell-based and egg-based influenza vaccines in spain. Human Vaccines & Immunotherapeutics 16(9):2238-2244.
Saif, L. J. 2020. Vaccines for COVID-19: Perspectives, prospects, and challenges based on candidate SARS, MERS, and animal coronavirus vaccines. European Medical Journal. March 24.
Saito, N., K. Komori, M. Suzuki, K. Morimoto, T. Kishikawa, T. Yasaka, and K. Ariyoshi. 2017. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: A test-negative case-control study in japan. Vaccine 35(4):687–693.
Sanofi. 2021. Sanofi and GSK initiate global phase 3 clinical efficacy study of COVID-19 vaccine candidate. May 27, 2021. https://www.sanofi.com/en/media-room/press-releases/2021/2021-05-27-07-30-00-2236989#:~:text=Home-,Sanofi%20and%20GSK%20initiate%20global%20Phase%203%20clinical,of%20COVID%2D19%20vaccine%20candidate&text=PARIS%20and%20LONDON%20%E2%80%93%20May%2027,protein%20COVID%2D19%20vaccine%20candidate (accessed October 19, 2021).
Saville, M. 2021. Presentation. Updated figure provided by Coalition for Epidemic Preparedness.
Scripps Research Institute. 2017. How flu shot manufacturing forces influenza to mutate: Egg-based production causes virus to target bird cells, making vaccine less effective. https://www.sciencedaily.com/releases/2017/10/171030134625.htm (accessed October 19, 2021).
Slaoui, M., and M. Hepburn. 2020. Developing safe and effective COVID vaccines—Operation Warp Speed’s strategy and approach. New England Journal of Medicine 383(18):1701–1703.
Sparrow, E., J. G. Wood, C. Chadwick, A. T. Newall, S. Torvaldsen, A. Moen, and G. Torelli. 2021. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine 39(3):512–520.
Stowe, J., N. Andrews, C. Gower, E. Gallagher, L. Utsi, R. Simmons, S. Thelwall, E. Tessier, N. Groves, G. Dabrera, R. Myers, C. Campbell, G. Amirthalingham, M. Edmuds, M. Zambon, K. Brown, S. Hopkins, M. Chand, M. Ramsay, and J. Lopez Bernal. 2021. Effectiveness of COVID-19 vaccines against hospital admission with the delta (b.1.617.2) variant. Public Health England.
Subbarao, K. 2021. The success of SARS-CoV-2 vaccines and challenges ahead. Cell Host Microbe 29(7):1111–1123.
Sullivan, S. G., S. Feng, and B. J. Cowling. 2014. Potential of the test-negative design for measuring influenza vaccine effectiveness: A systematic review. Expert Review of Vaccines 13(12):1571–1591.
Thompson, M. G., A. Naleway, A. M. Fry, S. Ball, S. M. Spencer, S. Reynolds, S. Bozeman, M. Levine, J. M. Katz, and M. Gaglani. 2016. Effects of repeated annual inactivated influenza vaccination among healthcare personnel on serum hemagglutinin inhibition antibody response to A/Perth/16/2009 (H3N2)-like virus during 2010–11. Vaccine 34(7):981–988.
Ulmer, J. B., U. Valley, and R. Rappuoli. 2006. Vaccine manufacturing: Challenges and solutions. Nature Biotechnology 24(11):1377–1383.
Vasireddy, D., P. Atluri, S. V. Malayala, R. Vanaparthy, and G. Mohan. 2021. Review of COVID-19 vaccines approved in the United States of America for emergency use. Journal of Clinical Medicine Research 13(4):204–213.
Voysey, M., S. A. C. Clemens, S. A. Madhi, L. Y. Weckx, P. M. Folegatti, P. K. Aley, B. Angus, V. L. Baillie, S. L. Barnabas, Q. E. Bhorat, S. Bibi, C. Briner, P. Cicconi, A. M. Collins, R. Colin-Jones, C. L. Cutland, T. C. Darton, K. Dheda, C. J. A. Duncan, et al. 2021. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397(10269):99–111.
Wang, W., E. Alvarado-Facundo, R. Vassell, L. Collins, R. E. Colombo, A. Ganesan, C. Geaney, D. Hrncir, T. Lalani, A. E. Markelz, R. C. Maves, B. McClenathan, K. Mende, S. A. Richard, C. Schofield, S. Seshadri, C. Spooner, G. C. Utz, T. E. Warkentien, M. Levine, C. L. Coles, T. H. Burgess, M. Eichelberger, and C. D. Weiss. 2020. Comparison of A(H3N2) neutralizing antibody responses elicited by 2018–2019 season quadrivalent influenza vaccines derived from eggs, cells, and recombinant hemagglutinin. Clinical Infectious Diseases. September 8.
Wei, C.-J., M. C. Crank, J. Shiver, B. S. Graham, J. R. Mascola, and G. J. Nabel. 2020. Next-generation influenza vaccines: Opportunities and challenges. Nature Reviews Drug Discovery 19(4):239–252.
Weir, J. P., and M. F. Gruber. 2016. An overview of the regulation of influenza vaccines in the United States. Influenza and Other Respiratory Viruses 10(5):354–360.
WHO (World Health Organization). 2021a. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int (accessed October 19, 2021).
WHO. 2021b. WHO validates Sinovac COVID-19 vaccine for emergency use and issues interim policy recommendations. https://www.who.int/news/item/01-06-2021-whovalidates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (accessed October 19, 2021).
Wong, S.-S., and R. J. Webby. 2013. Traditional and new influenza vaccines. Clinical Microbiology Reviews 26(3):476–492.
Wright, P. F. 2008. Vaccine preparedness—are we ready for the next influenza pandemic? New England Journal of Medicine 358(24):2540–2543.
Wu, K., A. P. Werner, J. I. Moliva, M. Koch, A. Choi, G. B. E. Stewart-Jones, H. Bennett, S. Boyoglu-Barnum, W. Shi, B. S. Graham, A. Carfi, K. S. Corbett, R. A. Seder, and D. K. Edwards. 2021. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. January 25.
Zeigler, D. F., E. Gage, and C. H. Clegg. 2021. Epitope-targeting platform for broadly protective influenza vaccines. PLOS ONE 16(5):e0252170.
Zimmer, C., and K. Thomas. 2020. Was the Pfizer vaccine part of the government’s Operation Warp Speed? The New York Times. November 10.
Zimmer, C., J. Corum, and S.-L. Wee. 2021. Coronavirus vaccine tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html (accessed October 19, 2021).
Zost, S. J., K. Parkhouse, M. E. Gumina, K. Kim, S. Diaz Perez, P. C. Wilson, J. J. Treanor, A. J. Sant, S. Cobey, and S. E. Hensley. 2017. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proceedings of the National Academy of Sciences 114(47):12578–12583.


























