6
A Blueprint for Action
Given the many years of research and the well-oiled machine for vaccine production, the world is better prepared for the next impending influenza outbreak than it was for the COVID-19 pandemic. Nonetheless, lessons learned from the response to COVID-19 can help ensure preparedness for a future influenza pandemic. Vaccine research and development (R&D) took major steps forward in 2020 and 2021, with novel vaccine platforms obtaining Emergency Use Authorization that enabled the rapid immunization of millions of people to curb the COVID-19 pandemic. Yet, while vaccines were available for COVID-19 within 1 year of its onset, there remains significant room for improvements that would speed up vaccine research, development, and manufacturing. Having vaccines earlier in a future influenza pandemic could significantly reduce both the burden of disease and the social and economic consequences. Valuable resources have been developed, such as the Influenza Vaccines Roadmap, developed by the Center for Infectious Disease Research and Policy, to develop a framework for expedient R&D of influenza vaccines (CIDRAP, 2021). This report builds on existing work and investigates what lessons can be learned from the development of COVID-19 vaccines given the current state of influenza vaccine research.
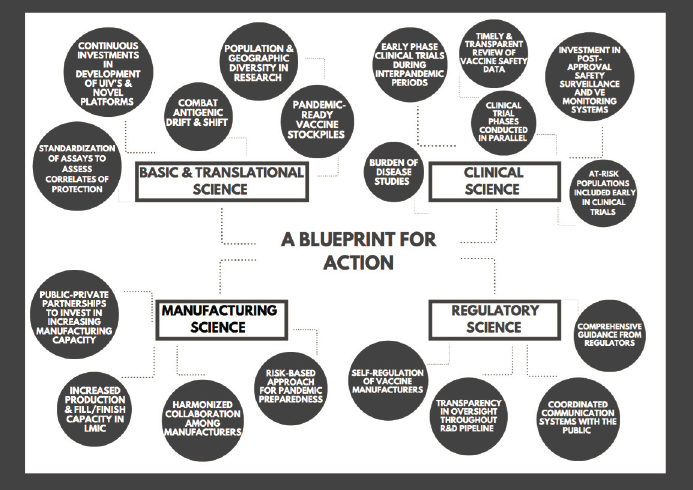
During its deliberations, the committee explored a range of topics that it decided to categorize in the dimensions of basic and translational science, clinical science, manufacturing science, and regulatory science. The recommendations presented in this chapter can serve collectively as a blueprint for action to accelerate the vaccine development process, increase access to vaccines, and limit global vaccine inequity. These recommendations are
designed to respond to the priority needs for seasonal and pandemic influenza identified below.
PRIORITY NEEDS FOR ADVANCING THE DEVELOPMENT OF VACCINES FOR SEASONAL AND PANDEMIC INFLUENZA
Investments to Support Influenza Vaccine Research and Development
Continuous funding streams are essential for vaccine R&D. During pandemics, funding is generally sufficient, but it often decreases considerably once a pandemic has ended, resulting in halted or limited research. Seasonal influenza vaccines have low to moderate effectiveness and require renewal every year because of antigenic drift of the virus and the short-lived immunity induced by vaccines (Gerdil, 2003; Rajaram et al., 2020). Today, influenza vaccines are based largely on inactivated virus grown in embryonated chicken eggs (Bender, 2019; Gerdil, 2003). Even though this is a successful way of producing vaccines, more effective vaccines are urgently needed, as are novel vaccine platforms for quick access during a pandemic.
A priority need, then, is for the allocation of a continuous and permanent stream of funding to support the development of broadly protective influenza vaccines, as well as novel vaccine platform technologies. For pandemic preparedness, the additional development of vaccines targeting conserved epitopes, or other types of broadly protective vaccine constructs, such as supraseasonal, pan-subtype, or pan-group, is vital to developing a pandemic-ready stockpile. Given the great potential of the mRNA vaccine platform, as illustrated by its use to produce two successful vaccines for COVID-19 within 12 months, further study of this platform for production of influenza vaccines is clearly warranted. Additionally, recombinant protein vaccines are of interest for influenza, and so it would be valuable to invest in improving the responsiveness of this platform. Adjuvants are another valuable area of investment because of their effects on antigen sparing for egg-based, cell-based, and recombinant vaccines. Efficacy data from animal models and early clinical trials are required to establish the most suitable platforms for investments.
Considerations in determining the best platforms for further development include issues associated with expansion of manufacturing capacity and feasibility of scale-up in low- and middle-income countries (LMICs), as well as effectiveness, speed, and cost. For each new platform, standardized assays, reagents, and animal models will be needed. Research on surrogate markers of protection for each platform would facilitate the establishment of the regulatory requirements for such vaccines.
Beyond the development of novel platforms and next-generation vaccines, significant investment in research aimed at identifying methods for
overcoming viral drift effects and optimizing adjuvant manufacturing could yield knowledge that would aid in the rational design of vaccines and, more specifically, the development of vaccines for particular subpopulations.
Finally, building a framework for collaboration or using an existing organization to distribute know-how domestically and internationally would provide vital support for global vaccine development.
Clinical Research into Novel Influenza Vaccines to Increase Pandemic Preparedness
The main gap in the development of next-generation influenza vaccines is the clinical development. Given the costly nature of such studies and the current approval process for seasonal influenza vaccines, there is little incentive for the industry to conduct such trials. Seasonal egg-based vaccines need to be complemented by candidate vaccine and vaccine adjuvant combinations that have undergone multicenter international trials to establish safety and immunogenicity. The conduct of clinical trials throughout the world, from the early stages of development, would fill the clinical funding gap and ensure global preparedness.
To this end, public-sector investments are essential to improve the data-sharing infrastructure so vaccine manufacturing centers could mobilize quickly and efficiently (such as using the highly effective nongovernmental GISAID data-sharing structure), which in turn would help shorten the time from sequence discovery and publication to global distribution of vaccines. Financial aid could stimulate vaccine manufacturers to take a risk-based approach to pandemic influenza preparedness, understanding that the investments made would be a form of insurance that would limit the costs of the next impending influenza outbreak. To qualify for these funding initiatives, manufacturers would be required to participate in R&D, data sharing, technology adaptation, and training activities with international partners. In addition, it would be important to stimulate technology transfer for both scale-up and scale-out. Scientific evidence supporting design strategies such as WHO prequalification or licensing could be used to reduce risk.
Many countries, in particular LMICs, do not have seasonal influenza vaccine programs as there is a low perceived urgency of the threat of influenza relative to the cost (Ortiz and Neuzil, 2019; Williams et al., 2021). Figures 4-2 and 4-3 show the prevalence of adult immunization programs in the world and reflect that many countries have influenza programs in place, particularly in Europe and the Americas. However, there appears to be a significant disparity in the existence of these programs between high-income countries (HICs) and LMICs. However, the costs and impact of seasonal influenza are high, and the expansion of seasonal programs would improve pandemic preparedness. Therefore, burden-of-disease stud-
ies, including cost–benefit analyses and analyses of economic productivity losses, are needed to create a case for local governments to adopt a seasonal vaccine program.
For clinical studies of novel seasonal influenza vaccines, accurate determination of postapproval vaccine effectiveness will be essential. Furthermore, postapproval safety monitoring will need to be performed on a global scale to detect any safety signals. Important priorities include sustaining local networks of clinical trials and research capacities, increasing the involvement of LMICs in clinical trials, and helping LMICs develop distribution capacities. A global coordinated pharmacovigilance system is needed to complement this work, with an increase in surveillance and pharmacovigilance systems in LMICs, providing the ability to calculate background rates, conduct rapid case-control studies, and assess subpopulations.
To enable clinical trials in LMICs, a wide variety of stakeholders will have to collaborate in a global network of funding and coordination to ensure that challenges are overcome and duplication of effort is minimized. Given the potential for competing research priorities among stakeholders, regional and international research groups, nongovernmental organizations, humanitarian organizations, international agencies such as WHO, and funding agencies, coordination will be key in moving the field forward.
Expanding Influenza Vaccine Manufacturing Capacity
The great majority of the world’s vaccine manufacturing capacity is based in HICs (Sparrow et al., 2021). Even though there are some big players in LMICs, it is essential to expand manufacturing capacity in these regions. The COVID-19 pandemic has shown that countries and regions with manufacturing capacity have an advantage in early access to pandemic vaccines, and during the COVID-19 pandemic, vaccine nationalism delayed the equitable global distribution of vaccines (Hyder et al., 2021). International action is required to build on the momentum of and attention to COVID-19 vaccine development to strengthen the manufacturing landscape for influenza vaccines.
Organizations and governments can help meet this need by supporting and encouraging vaccine manufacturers to prioritize and harmonize influenza vaccine development and manufacturing capacity. The approach taken by the Coalition for Epidemic Preparedness Innovations (CEPI) to address the COVID-19 pandemic provides a valuable model for effectively mobilizing and optimizing global assets to respond to a pandemic viral outbreak, and this model could inform the pathway for influenza preparedness. The global network of influenza centers of excellence could advance progress on this pathway by increasing collaboration and expanding to ensure capacity building in LMICs. The sustainable financial vehicles discussed above
would encourage and support LMICs in financing vaccine manufacturers to carry out R&D for seasonal and pandemic influenza vaccines.
Global Changes to Regulatory Systems to Improve Preparedness
During the COVID-19 pandemic, regulatory systems laid out clear requirements and worked together with industry partners in sharing data. In so doing, they facilitated overlapping preclinical and clinical phase studies, which resulted in the rapid development of novel vaccines directed at SARS-CoV-2 (Excler et al., 2021). However, pandemic influenza vaccines would ideally be developed even more rapidly, particularly for a potentially deadlier strain. To aid this process, clear guidelines need to be developed for preclinical and clinical studies to be performed in the interpandemic period.
Based on the results of clinical studies conducted for next-generation vaccines for seasonal influenza, then, regulators need to develop pathways for the rapid review of pandemic strain vaccines. These pathways could involve some interpandemic studies, such as phase I and II studies. Results of seasonal influenza studies suggest the possibility that a pandemic response may require only a strain change and demonstration of correlates of protection, followed by postauthorization confirmation of effectiveness.
Likewise, regulators need to develop clear pathways for the development of new vaccine platforms for seasonal influenza that are designed to generate data on multiple correlates of protection, efficacy, and safety. Given the crucial importance of data sharing between industry and regulators, regulators also need to develop protocols for these processes, as well as for chemistry, manufacturing, and control process inspections. Prepopulated regulatory assessment protocols with standardized endpoints and selection of sites at risk would support these efforts. Preparing such protocols before a pandemic occurs would allow for rapid development of high-quality vaccines. Early discussions with regulators and WHO are critical for the development of such protocols. During COVID-19, CEPI created an advisory group to communicate challenges from the regulators to work through regulatory challenges (CEPI, 2020). CEPI has also urged vaccine developers to use international standards for testing of efficacy, which is essential to establish correlates of protection. Additionally, CEPI engaged with academic journals to insist on the use of international standards for publishing. Together, these measures can streamline vaccine development with high standards.
Even though some effort was made to include more diverse populations in clinical trials for COVID-19 vaccines, diversity remained limited. For instance, pregnant individuals and children younger than 16 years were not included in initial clinical trials (Hwang et al., 2020; Subbaraman, 2021; Van Spall, 2021), while minority populations and the elderly were underrepresented (Flores et al., 2021). Therefore, regulators need to develop guid-
ance for pandemic vaccine development for subpopulations at risk, including children, pregnant individuals, elderly people, and those with comorbidities.
Regulatory capacity also needs to be increased globally to support the rapid development of vaccines. WHO could act as a coordinator for the sharing of data among regulators, including data on efficacy and safety, postmarketing surveillance data, and product issues. With increased regulatory capacity and collaboration, core global regulatory standards of Emergency Use Authorization can support the development of a strong, stable global vaccine supply.
The COVID-19 pandemic showed the importance of clear and transparent public communications for vaccine acceptance in populations. Reduced trust and credibility among the public were the result of multiple factors, including a lack of transparency in the dissemination of data regarding the safety and risk of various vaccine candidates and results of risk–benefit analyses, along with the politicization of vaccine development. To address these issues, regulators and the industry could adopt a set of practices throughout the life cycle of vaccine development to ensure the transparency of data, including the release of trial data. It is essential for all communications to be based on science and as independent as possible of commercial and political interests. Improvement of diversity and inclusion in studies can aid in the communication to the population. In coordination with trusted scientific leaders, national regulators could advance clear and transparent public communications by collaborating on a global level to establish a coordinated communication capacity aimed at helping the public understand differences in recommendations in regions for the same vaccine across countries and regions. Community advisors could gather information and work together with regulators to provide guidance and real-time feedback on communication.
Global Coordination and Cooperation
CEPI and funding from the Wellcome Trust and the Bill & Melinda Gates Foundation were essential to the development of vaccines for COVID-19 using new platforms. CEPI was established to develop rapid-response platforms for new diseases with the potential to cause pandemics. The inclusion of MERS-CoV on its priority list helped prepare for the COVID-19 pandemic. CEPI began supporting vaccine development in January 2020 and had a diversified portfolio with established and novel technologies (CEPI, 2020). It additionally supported the R&D efforts by developing robust assays for determining a vaccine’s immunogenicity. CEPI and other groups have also been tracking virus variants to determine their potential effect on vaccine effectiveness. The COVAX response facilitated unprecedented access to funds, but may benefit from measures to improve sustainability
to account for future influenza outbreaks. CEPI’s approach to addressing the COVID-19 pandemic provides a valuable model for how to effectively mobilize and optimize global assets to address a pandemic virus outbreak. This model could be used to address future pandemic influenza outbreaks (see Figure 6-1). However, one significant disadvantage of this model is that CEPI does not provide access to its assay protocols for early phase trials, and consequently limits reproducibility of assays. This disadvantage should be addressed in developing a sustainable model for addressing future outbreaks. An optimal harmonized framework for clinical development of vaccines for influenza would require coordinated funding for clinical trials that include diverse populations and are distributed globally. Manufacturing and fill and finish facilities would need to be expanded globally to allow early equitable access to vaccines. Global standards, based on regulatory standards, would support coordinated vaccine effectiveness and safety surveillance, providing a comprehensive and robust picture of these vital dimensions of all vaccines.
RECOMMENDATIONS
With the above priority needs in mind, the committee formulated recommendations in the dimensions of basic and translational science, clinical science, manufacturing science, and regulatory science (see Figure 6-2).
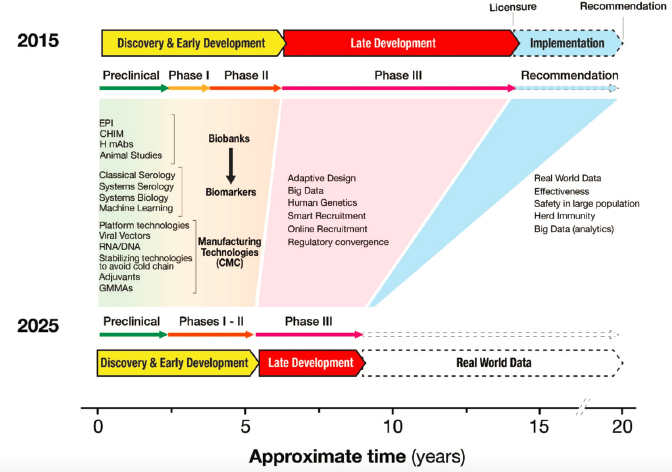
SOURCE: Black et al., 2020.
NOTE: LMIC = low- and middle-income country; R&D = research and development; UIV = universal influenza vaccines.
Basic and Translational Science
Recommendation 2-1: The U.S. Department of Health and Human Services, through the National Institute of Allergy and Infectious Diseases, the Biomedical Advanced Research and Development Authority, and the U.S. Department of Defense, as well as other corresponding governmental and funding agencies domestically and abroad, should invest, proportionate to the enormous costs of pandemics, in basic and translational research to reveal a diverse array of influenza vaccines, using different platforms, viral targets, adjuvants, and delivery systems. This will allow selection of the candidates most fit for purpose to be brought to authorization and sufficient production and distribution to optimize the control of influenza across diverse settings and phases of pandemics and epidemics.
Recommendation 2-2: The World Health Organization should advocate and coordinate with multilateral stakeholders (e.g., the Coalition for Epidemic Preparedness Innovations), governments, funding agencies, the vaccine industry, and philanthropic organizations to build global capacity for robust and internationally comparable preclinical,
clinical, and immunological assessments of influenza vaccine candidates, including novel candidates that use innovative structures, targets, and delivery systems to potentially broaden or improve protection.
Recommendation 2-3: International research networks (e.g., the National Institutes of Health/U.S. Centers for Disease Control and Prevention funding networks) supported by governments and funding agencies, the World Health Organization, and the vaccine industry should support, carefully plan, and conduct multi-center international clinical trials and field studies to compare emerging vaccines with standard vaccines in, among others, geographically, demographically, and immunologically diverse populations to inform rational and situation-based use and manufacture of an extended array of vaccines.
Recommendation 2-4: National regulators should engage with the vaccine industry and academic researchers in the development, standardization, and implementation of innovative assays to evaluate vaccines that induce immunity through mechanisms other than strain-specific neutralizing hemagglutination-inhibiting antibodies in order to reach consensus on the validation of these assays that will allow approval or licensure of influenza vaccines based on a broader range of assays that reflect induction of immunity.
Clinical Science
Recommendation 3-1: The World Health Organization, in collaboration with national public health agencies (e.g., the U.S. Centers for Disease Control and Prevention, the European Centre for Disease Prevention and Control, the China Center for Disease Control and Prevention, and the Africa Centres for Disease Control and Prevention) should conduct burden-of-disease studies in low- and middle-income countries to understand factors such as the health and economic burden of influenza illness and barriers to immunization in adult, pregnant, and pediatric populations to ensure development of infrastructure and capacity needed for pandemic vaccine development and implementation. Cost–benefit analyses should include additional economic productivity losses caused by delayed access to a vaccine in a pandemic.
Recommendation 3-2: The International Coalition of Medicines Regulatory Authorities and the World Health Organization, in partnership with national regulatory (e.g., the U.S. Food and Drug Administration and the European Medicines Agency) and public health agencies (e.g., the U.S. Centers for Disease Control and Prevention, the European Cen-
tre for Disease Prevention and Control, the China Center for Disease Control and Prevention, and the Africa Centres for Disease Control and Prevention) should invest, on a global level, in data infrastructure and capacity building to conduct real-time sentinel site surveillance of vaccine safety and effectiveness of different vaccine products deployed for use in epidemics and pandemics in diverse populations (e.g., age group, gender, race/ethnicity, geographic, presence of comorbidities, pregnancy, and socioeconomics) including a plan to ensure coordination, collaboration, and data sharing across these sentinel surveillance sites.
Recommendation 3-3: The International Coalition of Medicines Regulatory Authorities and the World Health Organization (Global Advisory Committee on Vaccine Safety) should ensure international coordination and collaboration on the timely and transparent review of vaccine safety data during epidemics and pandemics to support real-time decision making about the use of vaccines. Safety data should be made available to support country-level benefit–risk assessments, particularly for low- and middle-income countries relying on regional data from sentinel sites conducting safety surveillance.
Manufacturing Science
Recommendation 4-1: The U.S. Department of Health and Human Services and the World Health Organization should develop a plan for a sufficient and self-sustainable global supply of influenza vaccines for pandemics. This includes
- Convening, supporting, and encouraging multi-national, public, and private vaccine manufacturers to benchmark, prioritize, and harmonize influenza vaccine manufacturing; and
- Enhancing and expanding support of the global influenza vaccine manufacturing network, creating manufacturing hubs for greater collaboration, and building capacity to address challenges in manufacturing in low- and middle-income countries.
Recommendation 4-2: Vaccine manufacturers should take a risk-based approach to pandemic influenza preparedness. This approach would be most effective if incentivized, and could include
- Participating during research and development, data sharing, technology adoption, and training activities with international partners;
- Expanding internal capacity to assess the production needs and their risks;
- Using scientific evidence to design strategies to reduce risks (e.g., World Health Organization prequalification, licensing, and marketing); and
- Formalizing technology transfer (scale-up and scale-out) activities taking into consideration timelines and the outcomes for equitable costs, access, and distribution.
Regulatory Science
Recommendation 5-1: The U.S. Food and Drug Administration and other national regulators (e.g., European Medicines Agency) working with the scientific community and pharmaceutical industry should enhance comprehensive guidance for the development of influenza vaccines on novel platforms through Emergency Use Authorization to full licensure. This guidance should provide pathways for seasonal and pandemic influenza.
Recommendation 5-2: The U.S. Food and Drug Administration and other national regulators (e.g., European Medicines Agency) should commit to transparency in the oversight of clinical trials, review of data, authorization, and approval of pandemic influenza vaccines, including the release of facility inspection findings, clinical trial protocols, and clinical data that are the basis of decision making. Regulators should convene independent advisory committees to systematically review data, make recommendations, and build public understanding and confidence prior to the authorization or approval of novel vaccines.
Recommendation 5-3: The World Health Organization and the International Coalition of Medicines Regulatory Authorities should encourage and support the coordination between regulatory and public health agencies (e.g., the U.S. Centers for Disease Control and Prevention, the European Centre for Disease Prevention and Control, the China Center for Disease Control and Prevention, and the Africa Centres for Disease Control and Prevention) when announcing different decisions on the same or similar vaccines, to explain the different underlying circumstances and judgments.
Recommendation 5-4: Vaccine manufacturers should adopt a code of conduct for press releases and other communications regarding vaccine trial results and other matters that emphasizes the critical role of regulatory review.
REFERENCES
Bender, E. 2019. Accelerating flu protection. Nature 573(7774):S60–S61.
Black, S., D. E. Bloom, D. C. Kaslow, S. Pecetta, and R. Rappuoli. 2020. Transforming vaccine development. Seminars in Immunology 50:101413.
CEPI (Coalition for Epidemic Preparedness Innovations). 2020. 2020 annual progress report: Covering the period from 1 January - 31 December. Oslo, Norway: CEPI.
CIDRAP (Center for Infectious Disease Research and Policy). 2021. Influenza vaccines roadmap. https://www.cidrap.umn.edu/ongoing-programs/influenza-vaccines-roadmap (accessed October 19, 2021).
Excler, J.-L., M. Saville, S. Berkley, and J. H. Kim. 2021. Vaccine development for emerging infectious diseases. Nature Medicine 27(4):591–600.
Flores, L. E., W. R. Frontera, M. P. Andrasik, C. del Rio, A. Mondríguez-González, S. A. Price, E. M. Krantz, S. A. Pergam, and J. K. Silver. 2021. Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials. JAMA Network Open 4(2):e2037640.
Gerdil, C. 2003. The annual production cycle for influenza vaccine. Vaccine 21(16):1776–1779.
Hwang, T. J., A. G. Randolph, and F. T. Bourgeois. 2020. Inclusion of children in clinical trials of treatments for coronavirus disease 2019 (COVID-19). JAMA Pediatrics 174(9):825–826.
Hyder, A. A., M. A. Hyder, K. Nasir, and P. Ndebele. 2021. Inequitable COVID-19 vaccine distribution and its effects. Bulletin of the World Health Organization 99(6):406.
Ortiz, J. R., and K. M. Neuzil. 2019. Influenza immunization in low- and middle-income countries: Preparing for next-generation influenza vaccines. Journal of Infectious Diseases 219(Suppl 1):S97–S106.
Rajaram, S., C. Boikos, D. K. Gelone, and A. Gandhi. 2020. Influenza vaccines: The potential benefits of cell-culture isolation and manufacturing. Therapeutic Advances in Vaccines and Immunotherapy 8:2515135520908121.
Sparrow, E., J. G. Wood, C. Chadwick, A. T. Newall, S. Torvaldsen, A. Moen, and G. Torelli. 2021. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine 39(3):512–520.
Subbaraman, N. 2021. Pregnancy and COVID: What the data say. Nature 591(7849):193–195.
Van Spall, H. G. C. 2021. Exclusion of pregnant and lactating women from COVID-19 vaccine trials: A missed opportunity. European Heart Journal ehab103.
Williams, S. R., A. J. Driscoll, H. M. LeBuhn, W. H. Chen, K. M. Neuzil, and J. R. Ortiz. 2021. National routine adult immunisation programmes among World Health Organization member states: An assessment of health systems to deploy COVID-19 vaccines. Eurosurveillance 26(17):2001195.












