1
Introduction: The Imperative for Global Investment in Influenza Vaccines
The year 2020 will be remembered as “the year of COVID-19” (Lurie et al., 2021). As of July 2021, the number of reported COVID-19 cases surpassed 184 million, resulting in more than 4 million reported deaths worldwide (CRS, 2021). The pandemic has created an economic crisis, which some scholars estimate may cost the global economy more than $10 trillion (Glassman and Smitham, 2021). Unemployment levels are currently comparable to the Great Depression of the 1930s, 95 million people worldwide are estimated to have entered extreme poverty in 2020, and an estimated 80 million more are undernourished relative to pre-pandemic levels. The global economic downturn has produced estimates of a 5.3 percent drop in global trade for 2020, imposing a particularly heavy toll on trade-dependent developing and emerging economies (CRS, 2021).
While scientists and public health policy makers may not have been able to predict that it would be specifically SARS-CoV-2, the cause of COVID-19, that would cause the next major pandemic, they did underscore the vital importance of preparing for a major respiratory pandemic due to an as-yet-unknown pathogen, or “Disease X” (Simpson et al., 2020). In its 2019 Strategy document, the World Health Organization (WHO) stated, “Although it is impossible to predict when the next pandemic might occur, its occurrence is considered inevitable, and it could well occur during the time frame of this strategy. Given increased economic globalization, urbanization and mobility, the next pandemic will spread further and faster, and could lead to significant disruptions” (WHO, 2019). That same year, the Global Preparedness Monitoring Board (GPMB) discussed “preparing
for the worst: a rapidly spreading, lethal respiratory pathogen pandemic … such as an especially deadly strain of influenza” (GPMB, 2019).
Despite the devastation wrought by COVID-19, from an epidemiological perspective, it does not represent a “worst-case” scenario. Epidemiologists use the R0 (“reproductive number”) metric to indicate how contagious an infectious disease is;1 the 1918–1919 pandemic influenza had an estimated R0 of 2.0–3.0, which is close to the estimated initial R0 for SARS-CoV-2 (2.5, or a range of 1.8–3.6) (Petersen et al., 2020). With a similar case fatality ratio (CFR, a measure of how lethal a disease is) so far for COVID-19, the 1918–1919 pandemic,2 and new variants of highly pathogenic influenza viruses circling the globe annually, one could argue that pandemic influenza reflects an even more dire risk to global and economic health than SARS-CoV-2. Indeed, according to the U.S. Centers for Disease Control and Prevention (CDC) guidance on “categories of viral severity,” the transmissibility and lethality of COVID-19 place it as a category three and two, respectively, with category five being the most severe (CDC, 2007). Experts worry that the risk for pandemic influenza may be even higher during the COVID-19 era due to an increased risk of overtaxed surveillance and testing systems and predict that another major flu pandemic is likely in the next 10–30 years (U.S. Senate, 2017).
The year 2020 also saw “unprecedented” innovation and scientific collaboration that produced new technologies, such messenger RNA (mRNA) vaccines, resulting in 3–4 times more COVID-19 vaccine doses projected to be available by the end of 2021 than what was possible for all vaccines in 2019 (IFPMA, 2021).3 These vaccines were able to be developed in record speed at least in part because of fortunate viral attributes, decades-long investments in mRNA technology research, and pre-pandemic research on the spike protein, the major target for COVID-19 vaccines (Ball, 2020). However, the next pandemic pathogen could have an R0 of not 1, 3, or 4, but 12–18, similar to measles, and a CFR not between 1 and 10 but in the range of 40–80 or higher, like the viruses that cause untreated Nipah (WHO, 2018), Marburg (WHO, 2021a), or Ebola (WHO, 2021b) disease. Vaccine production could be more complicated than for COVID-19 if this
___________________
1 R0 is generally reported as a numeric value or range whereby an outbreak is expected to continue if the R0 value is >1 and is expected to end if the R0 is <1 (Delamater et al., 2019).
2 These numbers need to be interpreted with care; for instance, we may wish to substitute CFR with infection fatality rate (IFR), as well as ultimately measuring mortality by way of overall pandemic excess mortality; see, for example, Ritchie et al. (2021) and He et al. (2020).
3 The announced cumulative supply target of COVID-19 vaccines is up to 14 billion doses by the end of 2021. This represents 3–4 times the pre-COVID-19 annual global demand for all vaccines (3.5–5.5 billion) (WHO, 2019).
hypothetical pathogen—like influenza viruses—has a high rate of mutation or mechanisms for evading human immune responses.
Although promising new technologies were advanced for COVID-19 vaccines, scholars have argued that collaboration across the research and development (R&D) ecosystem has largely “fumbled” during the pandemic (Lurie et al., 2021). For instance, the absence of global entities with a clear mandate, responsibility, or resources to initiate product development in a pandemic was exposed. No global organizations support a viral pathogen repository, host their sequence information, grow and share virus isolates for essential research, organize and collect biological reference materials from patients to support developing and validating new diagnostic tests, and incentivize manufacturing by buying raw materials. Some organizations, such as the Coalition for Epidemic Preparedness Innovations (CEPI), which was launched in 2017, have used their own resources to mobilize vaccine developers to pivot ongoing R&D to COVID-19. But they lack broad mandates to coordinate vaccine preparedness; CEPI, for example, could only fund early R&D and not late-stage trials. This has caused delays in vaccines reaching those who need them most and amplified distribution inequities. New multilateral frameworks, such as that of the COVAX Facility, have been launched to fill governance gaps, such as procuring vaccines for global allocation. These single-disease frameworks were largely reactive, lack permanent legal charters, and have faced issues with securing adequate financing from high-income countries.
In short, while experts knew in 2019 that a severe, novel respiratory pandemic could be on the horizon, pandemic preparedness governance writ large has been largely a “conductor-less orchestra.” In Lurie’s words, the COVID-19 pandemic has laid bare the “fragility of our global system of preparedness and response to pandemics, and the fragmentation of our research and development ecosystem” (Lurie et al., 2021). Economies across the world have been crippled by COVID-19, and yet it is far from the “worst-case” scenario for a future pandemic of “Disease X.”
WHAT IF 2020 HAD BEEN THE “YEAR OF INFLUENZA” INSTEAD?
New strains of influenza emerge each year, but it is far from a new threat. This—and the fact that the influenza virus has a large animal reservoir and high mutation rate, making it difficult to predict which strains will circulate worldwide and decreasing average vaccine efficacy4—has
___________________
4 Efficacy is the performance of an intervention under ideal and controlled circumstances (Singal et al., 2014).
often led to public complacency and intermittent buy-in for influenza pandemic preparedness efforts. Despite advances in vaccines and therapeutics, an estimated 1 billion cases of seasonal influenza annually occur worldwide (WHO, 2019). This amounts to a global incidence in almost an eighth of the world’s population every year (Palache et al., 2020). Three to 5 million of these are severe and, on average, 290,000–650,000 lead to respiratory deaths (GBD 2017 Influenza Collaborators, 2019; Ruscio et al., 2020). Furthermore, these are likely underestimates because major gaps remain in data available to estimate the burden of influenza, particularly in low- and middle-income countries (LMICs) (Bresee et al., 2018; WHO, 2021c).
Pandemic strains of influenza are capable of high lethality; the 1918–1919 pandemic had an estimated death toll of up to 50 million (WHO, 2019). In the United States alone, if COVID-19 caused deaths at the same rate, the total death toll would approach 2 million (Ewing, 2021). Influenza pandemics occurred in 1957–1958, 1968–1969, and 2009–2010, resulting in 1–4 million, 1–4 million, and 100,000–400,000 deaths, respectively. Collectively, influenza pandemics can also cause consistently high economic burdens (see Table 1-1). The 2009 H1N1 pandemic, for instance, is estimated to have cost $45–$55 billion despite its comparatively low lethality. The World Bank has estimated that a global influenza pandemic with the scale and virulence of 1918 influenza would cost the modern world economy US$3 trillion, or up to 4.8 percent of the global gross domestic product (GDP); even a moderately virulent pandemic would amount to a 2.2 percent loss of global GDP; and losses in sub-Saharan Africa would be equivalent to 1 year’s worth of economic growth (GPMB, 2019).
In such a context, investing in pandemic preparedness—and particularly vaccination—is among the “best buys” in global health and the most cost-effective investment possible (Gouglas et al., 2018; The Rockefeller Foundation, 2021).5 Yet, worldwide influenza vaccine6 distribution before COVID-19 remained highly variable geographically (see Figure 1-1). This is both a demand and supply issue. Many countries, particularly LMICs, do not know the health and economic burden of seasonal influenza, have
___________________
5 Considering the vast sums spent so far on domestic stimulus and pandemic suppression in developed countries—$1.9 trillion in the most recent U.S. stimulus package alone—the sum needed for more comprehensive immunization is minimal, especially when weighed against the cost of another infection surge.
6 Looking at seasonal vaccine access is an important proxy for pandemic influenza vaccines because it appears that COVID-19 may have increased acceptance of influenza vaccination in previously unvaccinated individuals and may have motivated uptake in newly eligible candidates. See Bachtinger et al. (2021).
TABLE 1-1 Snapshot of Estimated Economic Costs of Pandemics
| Pandemic/Epidemic | Financial Losses (US$) | Type of Calculation | Study | Citation |
|---|---|---|---|---|
| H1N1 (1918) | GDP losses: 3 percent (Australia), 15 percent (Canada), 17 percent (United Kingdom), 11 percent (United States) | productivity loss | McKibbin and Sidorenko (2006) | Yamey et al. (2017) |
| SARS (2003) | $52.2 billion* | productivity loss | Lee and McKibbin (2004) | Yamey et al. (2017) |
| H1N1 (2009) | $45,000,000–$55,000,000 | total cost | Resolve to Save Lives (2019) | GPMB (2019) |
| Ebola (2013) | $2.8 billion (Guinea, Liberia, and Sierra Leone) | economic and social impact | World Bank 2014–2015 West Africa Ebola crisis: impact update | Yamey et al. (2017) |
| Ebola (2014–2016) | $53 billion | economic and social impact | Huber et al. (2018) | GPMB (2019) |
| Ebola (2014–2016) | $4.3 billion | lost productivity and trade | CDC (2019) | NASEM (2019) |
| COVID-19 (2020–2021) | $10.3 trillion (estimate) | forgone output | Glassman and Smitham (2021) | Glassman and Smitham (2021) |
| COVID-19 (2020–2021) | $11 trillion | forgone output | Georgieva (2020) | The Rockefeller Foundation (2021) |
| Expected cost: influenza pandemic akin to the scale and virulence of the 1918 pandemic | $3 trillion | not given | Frangoul (2014) | GPMB (2019) |
| Expected cost: influenza pandemic akin to the scale and virulence of the 1918 pandemic | $500 billion | intrinsic losses | Fan et al. (2018) | NASEM (2019) |
* The calculations suggest that the cost in 2003 of SARS for the world economy as a whole is close to $US40 billion.
national influenza vaccination plans and programs, especially for adults, or prioritized seasonal influenza vaccination. For example, in 2009, the estimate of worldwide capacity for producing monovalent7 pandemic influenza vaccines was 2.7 billion doses compared to the total worldwide population of approximately 6.85 billion, enough doses for approximately one-third of that population. By 2019, this capacity had risen to approximately 6.4 billion doses, or enough to inoculate about three-quarters of the global population, in large part due to the efforts of WHO’s Global Action Plan for Influenza Vaccines (GAP, see Chapter 2) and improved vaccine technologies, such as increasing the use of adjuvants (Rockman et al., 2020).
What would the global community have done to scale up influenza vaccine production if we were experiencing a pandemic influenza strain with relatively high lethality (1–2 percent) this year? Chapter 3 includes more detail about where influenza vaccine manufacturing partnerships are taking place and how they have evolved over the past 15 years. As of 2019, approximately 79 percent of pandemic influenza vaccine production capacity (and 80–95 percent of seasonal influenza vaccine production) uses egg-based systems, which means that pandemic influenza vaccine production takes time; the large caveat for the above estimate that 6.4 billion
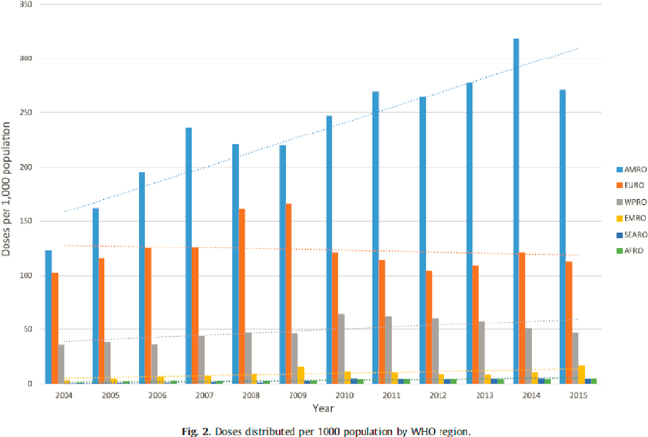
SOURCE: Palache et al., 2021 (CC BY-NC-ND 4.0).
___________________
7 Monovalent vaccines contain a single strain of a single antigen; polyvalent vaccines contain two or more strains/serotypes of the same antigen (WHO, 2021d).
influenza vaccines8 could be produced during a pandemic is that it is based on a full year’s manufacturing output. Realistically, it would take about 3–6 months for full-scale manufacturing to begin, because it requires preparing and testing a suitable viral strain, and another 6–9 months to scale up to full capacity. Modern vaccine platform technologies, such as recombinant proteins and mRNA, can produce more quickly than egg-based systems and may improve yields because they do not require the full pandemic virus or candidate vaccine virus and are not limited by the supply of embryonated eggs available to grow viral strains. Since 2009, the number of manufacturers has also increased (as of 2020, at least 32 had licensed influenza vaccines), but a full two-thirds of the total was still produced by only seven manufacturers, most of them in wealthy countries (Rockman et al., 2020; Sparrow et al., 2021).
If it had been a new pandemic influenza virus that circulated this year, the potential vaccine production capacity could, therefore, have been available in a high enough volume to cover most vulnerable populations worldwide. However, this is largely theoretical; it assumes that surveillance systems would quickly identify the spillover virus, the country in which the virus was identified would be willing to share viral samples or sequence information, the pandemic strain would grow as well in eggs or cell cultures as seasonal influenza strains, and a clear—and early—signal would appear for manufacturers to switch from producing seasonal to pandemic influenza vaccines. (That ability to switch could also be affected by how far seasonal vaccine production has progressed; it diminishes as seasonal production advances.) It also assumes that the pandemic virus would supplant circulating seasonal influenza viruses (so no need for seasonal influenza vaccines), that manufacturers would be willing to switch production, with the understanding that the market for seasonal influenza vaccines already in production would cease but a market for the pandemic vaccines would exist and that the vaccines would be affordable for LMICs. As of 2018, the WHO Pandemic Influenza Preparedness (PIP) Framework (see Chapter 2) had secured commitments from industrial partners to provide 400 million doses of vaccine for LMICs. This means that only about 400 million of the
___________________
8 The most recent WHO analysis of pandemic influenza vaccine production capacity estimates it in terms of a “best-case scenario,” 8.31 billion doses over 12 months, and a “moderate-case scenario,” 4.15 billion doses on the same time line. The best case assumes manufacturers would operate at full scale with no limitations on supplies/reagents, pandemic strains would grow equally well in eggs and cell-based systems as seasonal strains do, and a moderate amount of antigen would be required to elicit an adequate immune response. The moderate scenario similarly assumes that manufacturers can all operate at full scale with no supply or reagent limitations and that pandemic strains would grow equally well; it departs from the best-case scenario by estimating that more (twice) the amount of antigen would be required than seasonal strains for an adequate immune response (Sparrow et al., 2021).
estimated 6.4 billion dose production capacity for the pandemic vaccines would have been explicitly allocated for low-cost distribution to LMIC member states through WHO, which is a drop in the ocean in terms of capacity needed (and these doses might only be delivered after the national needs of vaccine-producing countries have been met). Finally, it assumes that countries would have national influenza vaccination plans to direct distribution. As the most recent data show, these plans are highly variable across regions; in 2018, 85 percent of LMICs did not have national seasonal influenza vaccination policies or programs, which form the cornerstone for pandemic vaccination (Morales et al., 2021).
As David Fidler put it at the Readiness for Microbial Threats 2030: Exploring Lessons Learned Since the 1918 Influenza Pandemic workshop (NASEM, 2018), the global governance structures for influenza “may turn out to be gossamer strands across the mouth of a cannon.” The world can produce more influenza vaccines today than in 2009 but has not yet solved the serious issues of vaccine access and equity and managing the “switch” from seasonal to pandemic vaccine production and the poor market-based incentives for producing influenza vaccines.
A DISRUPTIVE MOMENT TO RECONSIDER INFLUENZA IN THE WIDER PANDEMIC PREPAREDNESS LANDSCAPE
In many ways, the global health community entered the twenty-first century with optimism and a sense of shared will and values. As Farrar (2019) recounts, “There was clear political commitment to multilateral agencies, the United Nations, the World Health Organization and others as the essential international architecture that brought all the countries of the world together to share challenges and ensure, as far as possible, equitable solutions.” But this era of biomedical achievements did not, in Farrar’s words, serve “as the springboard to enhanced health for everyone everywhere, as science advanced with clear public and political support for ensuring the benefits of these advances in improving lives for all.” The situation at the beginning of the beginning of the century’s third decade is quite different. According to Farrar, “increasing nationalism and a retreat from a sense of common public good … challenge the optimism of the first decade of the twenty-first century.”
Some see the global health community as naively approaching pandemic preparedness with fuzzy notions about how acting for the public good alone will produce equitable access to vaccines and technologies. The reality is that good will and altruism are readily defeated by politics and money. Pandemic financing writ large has a poor track record in terms of sustained financing, which is often episodic and typically flows during a crisis and ebbs afterward (GPMB, 2019). During the 2014–2015 Ebola outbreak, for
example, epidemic and pandemic preparedness increased to 16 percent of global health aid in 2015, then fell to 7 percent by 2017. Despite numerous new organizations, systems, and processes, particularly since the 2009 H1N1 pandemic, preparedness for an influenza pandemic and pandemics in general remains underfunded both nationally and through international funding mechanisms. In 2019, the Institute for Health Metrics and Evaluation estimated that it made up slightly less than 0.9 percent of development assistance for health (global health aid) (Glassman and Smitham, 2021).
The Review on Antimicrobial Resistance (AMR), established in 2014 by the UK Prime Minister and chaired by economist Lord Jim O’Neill, is perhaps the most poignant example of the challenges of attracting sustained financial investments for global public goods—even when they are considered high risk by epidemiologists and “best buys” by economists. The first AMR Review paper estimated that drug-resistant infections could cause the deaths of 10 million by 2050, at a total cost to the global economy of up to $100 trillion, and the final report called for urgent incentives for investment in antibiotics. Yet, a follow-up report in 2019 called progress “remarkably disappointing” and stated that “what is missing, despite endless words, is a firm commitment of monies from governments or pharmaceutical companies.” As Chatham House summarized, the concern is that “governments are waiting for the crisis to escalate to justify large-scale spending on AMR, while pharmaceutical companies are waiting for governments to panic and start throwing more money at the problem” (Dall, 2019).
Could political will invigorated by the COVID-19 pandemic be harnessed to change this pattern and promote investments in influenza pandemic preparedness as a global public good? COVID-19 has produced a recognition of a need for strong incentive frameworks to balance the fact that sovereign leaders have the responsibility to protect their own country’s populations first. Glaring shortfalls now on display during the COVID-19 pandemic appear to be triggering much-needed global action. For example, the U.S. Secretary of State, Antony Blinken, in remarks to the UN Security Council on February 17, 2021 (U.S. Department of State, 2021) announced an intent to “advance the creation of a long-overdue sustainable financing mechanism for health security, so we can leave the world more prepared for future outbreaks than it was for this pandemic.” In remarks to the G7 in February 2021 (White House, 2021), U.S. President Biden announced actions to improve the health and safety of the U.S. population by protecting vulnerable populations worldwide. He called on the G7 partners to create a sustainable health security financing mechanism aimed at building the capacity to end the COVID-19 pandemic and prevent future ones. In addition, in January 2021, the G20 launched a High-Level Independent Panel (HLIP) on financing for global pandemic preparedness and response (PPR) (Italian Ministry of Economy and Finance, 2021). The charge to the G20 panel was to identify gaps in
the existing financing systems for global pandemic prevention, surveillance, preparedness, and response and propose actionable solutions to address these gaps, leveraging resources from the public, private, and philanthropic sectors and international finance institutions. Although these measures are promising, their outcomes remain to be seen, particularly after the acute phase of the COVID-19 pandemic ends.
The enormous innovations in vaccine production that have emerged during the COVID-19 pandemic, including new technology platforms using mRNA and recombinant proteins, may hold the key to new vaccines for influenza—and for new markets to support sustainability for the many new vaccine manufacturing facilities created over the last decade. Yet, they may also lead to misguided optimism. Influenza viruses are biologically quite different from SARS-CoV-2, having much higher rates of mutation among other properties. Much R&D is required to establish the generalizability of the new approaches to vaccine manufacturing and to continue to develop the workforce necessary to sustain it. This approach will probably not be adequate in a world in which new pandemic threats can arise at any time with a potentially much higher R0. On the other hand, care needs to be taken not to throw the baby out with the bathwater or consider the past too deeply. For all its defects and failures, the global influenza system is among the most well established and best functioning among the existing global health governance structures for infectious diseases (Carroll et al., 2021). Many elements of the system, which are reviewed in Chapter 2, are working as they were meant to and should be highlighted for this and possibly expanded to include other pathogens with pandemic potential.
Between March 2020 and May 2021, numerous efforts were mounted to understand the lessons of COVID-19 at the global, regional, national, and local levels. Many reports have been or will soon be issued to document these lessons and make recommendations for increased preparedness and effectiveness for dealing with future threats, including influenza.9 These efforts are well grounded in science and robust analytics but tend to be focused on the past, including the COVID-19 pandemic over the past 18 months. The
___________________
9 Such efforts include those by the U.S. National Academies of Sciences, Engineering, and Medicine (https://nam.edu/programs/advancing-pandemic-and-seasonal-influenza-vaccine-preparedness-and-response-a-global-initiative); the Lancet COVID-19 Commission (https://covid19commission.org); the IPPR Report by the University of California, San Francisco’s Institute for Global Health Sciences: The United States’ Response to COVID-19: A Case Study of the First Year, April 15, 2021 (https://globalhealthsciences.ucsf.edu/sites/globalhealthsciences.ucsf.edu/files/covid-us-case-study.pdf); the Pan European Pandemic Commission’s Report Rethinking Policy Priorities in the Light of Pandemics, March 2021 (https://www.euro.who.int/__data/assets/pdf_file/0010/495856/Pan-European-Commission-Call-to-action-eng.pdf); and the G20 High Level Independent Panel on Financing the Global Commons for Pandemic Preparedness and Response (https://pandemic-financing.org/about-us). All websites accessed December 17, 2021.
current need is for recommendations that aim at countering future pandemics in ways that ensure significantly more effective measures than the world has been able to mount. It is easy to be reactive, but we need to be strategic.
Is this, therefore, a disruptive moment and an opportunity to truly rethink the whole system for managing respiratory viral diseases with pandemic potential? We may never have a better chance to undertake the required reforms. As the health, social, and economic consequences of a severe pandemic are now clear to all, the current push to increase and stabilize pandemic financing, partly inspired by COVID-19, may be either the latest rendition of the usual reactive pattern or the beginning of a genuine effort to achieve more effective governance and financing structures for sustained pandemic preparedness. The key may be to harness the current political will and momentum evident in the efforts of the G7, the G20, and many other groups to establish effective mechanisms for the longer term. However, this leaves open major questions. How should the speed of sharing viral samples versus equity in benefits (such as vaccine access) be handled? This is particularly salient due to recent policy proposals, such as the TRIPS intellectual property (IP) waiver and global pandemic treaty (Velásquez and Syam, 2021). What partnership models can help to keep platform-based technologies scaled up during COVID-19 “warm” (i.e., actively producing vaccines or other products) for influenza, and how can they be best coordinated globally? What scale of investment is required for pandemic influenza vaccination, and where should it be channeled? To what extent do the governance structures for influenza—often considered a “known” and non-dangerous disease—require reconceptualization if they are to attract such investments?
CHARGE TO THE COMMITTEE ON GLOBAL COORDINATION, PARTNERSHIPS, AND FINANCING
At the request of the U.S. Department of Health and Human Services, the National Academies of Sciences, Engineering, and Medicine (the National Academies) will spearhead an effort to assess the global impact that capabilities, technologies, processes, and policies developed for COVID-19 could have on pandemic and seasonal influenza global preparedness and response, especially regarding vaccine development. The content will be based on diverse evidence to ensure that the report can be contextualized, adapted, and implemented across various circumstances internationally. The effort includes four work streams that collectively will culminate in a comprehensive series of reports with recommendations to improve research and development, production, manufacturing, planning, scale-up, and timely distribution of influenza vaccines and countermeasures rapidly around the globe while protecting incentives for innovation.
To contribute to this effort, an ad hoc committee under the auspices of the National Academies will describe the current global governance landscape for influenza vaccines and vaccination; analyze the effectiveness and replicability of global coordination and financing models formed in response to recent viral pandemics and epidemics; and provide recommendations for governance frameworks, partnerships, and financing mechanisms that may promote sustainable influenza vaccine preparedness and response. Specifically, the committee will
- Review existing global governance frameworks, partnerships, and intergovernmental treaties (e.g., the PIP Framework and International Health Regulations [IHR]) for seasonal and pandemic influenza vaccine development, manufacturing, and distribution, particularly those developed in response to H1N1, and identify any major barriers to the sustainable implementation of these frameworks and partnerships.
- Review multilateral responses and public–private partnerships developed and implemented during COVID-19 (e.g., the ACT Accelerator) and other viral outbreak events (e.g., the Ebola epidemic), and highlight any approaches (e.g., R&D, liability frameworks, distribution, and innovative business models) that may confer significant advantages for national preparedness and regional and global coordination of seasonal and pandemic influenza vaccines and vaccination.
- Drawing on the above reviews and the benefits and challenges presented by relevant global frameworks (e.g., the Nagoya Protocol and the PIP Framework), propose practical and feasible recommendations for sustainable governance frameworks and coordination mechanisms to increase global and regional preparedness for influenza; improve international influenza vaccine research, manufacturing, and equitable distribution and access; and address global challenges such as vaccine confidence.
- Review the theoretical basis of incentivizing vaccine research, manufacturing, and distribution in the pandemic preparedness context, and identify any relevant examples of the successful and sustainable design and use of incentives, particularly for low-resource settings.
- Provide recommendations for the contexts in which specific financing strategies and mechanisms (e.g., incentives, risk pooling, and trust funds) can be sustainably adapted. These mechanisms will ideally encourage national investment in pandemic influenza preparedness and response, while optimizing the effectiveness of development assistance for health.
The result of this study is a report on the current barriers to effective global coordination and sustainable financing for seasonal and pandemic influenza vaccines and vaccination and the ways in which innovations and lessons learned from the COVID-19, Ebola, and 2009 H1N1 responses may address these barriers. The Committee on International Coordination, Partnerships, and Financing will coordinate with other related initiatives, including the three other consensus study committees in this initiative, to ensure the widespread dissemination and monitoring of its recommendations.
To approach this broad—and time-sensitive—task, the committee held a series of meetings from March through July 2021. Four meetings focused on the existing major global governance frameworks and regulations related to influenza vaccination, gaps in and barriers to these frameworks and regulations, areas in which the committee could make recommendations to fill these gaps, and ways in which influenza governance and financing structures could be integrated with wider respiratory pathogen and pandemic governance infrastructures. A series of smaller meetings of working groups focused on pathogen sharing, global partnerships for technology and manufacturing, and vaccine financing and access. Each of the three working groups developed findings and conclusions in foundational areas to address the Statement of Task. Working group and full committee meeting sessions gathered information from 21 expert speakers, many with experience with responses to COVID-19, Ebola, and H1N1 influenza (see Appendix I for meeting agendas). The final committee meeting focused on revising crosscutting recommendations and delineating areas of paramount importance for future research that could not reasonably be addressed on the study’s current short time line, such as vaccine confidence.
STRUCTURE OF THE REPORT
This report’s recommendations are based on a single premise: starting with the first human detection of a novel influenza virus with pandemic potential, how can the global health community move as quickly as possible to develop vaccines and equitably immunize as many people as possible worldwide? This is not discounting the importance of the human–animal nexus; it was determined that analyzing such surveillance systems went beyond the reasonable scope and time allocation for this study. Chapter 2 provides a review of existing global structures, partnerships, and frameworks for influenza vaccination, including those that may detect viral spillovers from animals to humans. Chapter 3 also considers financing mechanisms that may improve surveillance in hot spot areas for spillover.
Four main steps were required to tackle this premise and make specific recommendations for improving influenza global coordination, partnerships, and financing mechanisms (see Figure 1-2). These steps correspond
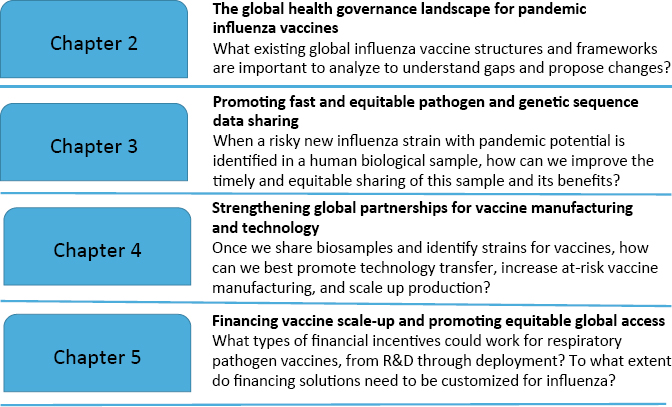
to each of the upcoming chapters. Chapter 3 focuses on how, once a viral spillover from animals to humans is detected, the global community can ensure that samples are shared quickly but also as equitably as possible. Chapter 4 turns to global partnerships for vaccine technology and manufacturing; it considers, after pharmaceutical companies obtain relevant biosamples, what types of partnerships can best scale up manufacturing and create a sustained demand for vaccines. Chapter 5 focuses on how countries can ensure that the most people in the most places around the world receive vaccines as quickly as possible. It addresses the scale of financing and incentives required to improve access to influenza and other vaccines during a future pandemic. The concluding chapter presents the committee’s recommendations and proposes ways to reframe preparedness for influenza pandemics to incentivize effective vaccine production and distribution in the post-COVID era and areas of the Statement of Task that should be prioritized by policy makers or further studied.
Across all chapters, the committee considers the areas in which the solution for pandemic influenza PPR should focus on influenza specifically and where influenza vaccination is best approached as a subset of solutions for broader respiratory pandemics. Ultimately, the committee argues that influenza presents a major pandemic threat and that addressing it will require enhanced global coordination, partnerships, and financing for respiratory pathogen PPR, particularly vaccines. Countering influenza is a global imperative for not only public health and economic reasons but also equity reasons.
REFERENCES
Bachtiger, P., A. Adamson, J. J. Chow, R. Sisodia, J. K. Quint, and N. S. Peters. 2021. The impact of the COVID-19 pandemic on the uptake of influenza vaccine: UK-wide observational study. JMIR Public Health Surveillance 7(4):e26734.
Ball, P. 2020. The lightning-fast quest for COVID vaccines—and what it means for other diseases. Nature 589:16–18.
Bresee, J., J. Fitzner, H. Campbell, C. Cohen, V. Cozza, J. Jara, A. Krishnan, and V. Lee. 2018. Progress and remaining gaps in estimating the global disease burden of influenza. Emerging Infectious Diseases 24(7):1173–1177.
Carroll, D., S. Morzaria, S. Briand, C. K. Johnson, D. Morens, K. Sumption, O. Tomori, and S. Wacharphaueasadee. 2021. Preventing the next pandemic: The power of a global viral surveillance network. BMJ 372:n485.
CDC (U.S. Centers for Disease Control and Prevention). 2007. Interim pre-pandemic planning guidance: Community strategy for pandemic influenza migration in the United States—early, targeted, layered use of nonpharmaceutical interventions. https://www.cdc.gov/flu/pandemic-resources/pdf/community_mitigation-sm.pdf?fbclid=IwAR1sMmehOSZ8hHRKEPJEP2hUbkXbNMlS4sGRqQ7s5iLWOpyfQDErd4Wg_SE (accessed June 29, 2021).
CDC. 2019. 2014–2016 Ebola outbreak in West Africa. https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html (accessed October 23, 2021).
CRS (U.S. Congressional Research Service). 2021. Global economic effects of COVID-19. Report R46270. Updated July 9, 2021. https://crsreports.congress.gov/product/pdf/R/R46270 (accessed July 16, 2021).
Dall, C. 2019. UK report cites lack of progress on AMR review steps. https://www.cidrap.umn.edu/news-perspective/2019/10/uk-report-cites-lack-progress-amr-review-steps (accessed July 1, 2021).
Delamater, P. L., E. J. Street, T. F. Leslie, Y. T. Yang, and K. H. Jacobsen. 2019. Complexity of the basic reproduction number (R0). Emerging Infectious Diseases 25(1):1–4.
Ewing, E. T. 2021. Measuring mortality in the pandemics of 1918–19 and 2020–21. Health Affairs Blog. https://www.healthaffairs.org/do/10.1377/hblog20210329.51293/full (accessed July 16, 2021).
Fan, V. Y., D. T. Jamison, and H. Summers. 2018. Pandemic risk: How large are the expected losses? Bulletin of the World Health Organization 96(2):129–134. https://pubmed.ncbi.nlm.nih.gov/29403116 (accessed October 23, 2021).
Farrar, J. 2019. Science, innovation and society: What we need to prepare for the health challenges of the twenty-first century? International Health 11(5):317–320.
Frangoul, A. 2014. Counting the costs of a global epidemic. CNBC.com. https://www.cnbc.com/2014/02/05/counting-the-costs-of-a-global-epidemic.html (accessed October 23, 2021).
GBD (Global Burden of Disease) 2017 Influenza Collaborators. 2019. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. The Lancet Respiratory Medicine 7(1):69–89.
Georgieva, K. 2020. A New Bretton Woods Moment (speech at 2020 annual meeting, International Monetary Fund. https://www.imf.org/en/News/Articles/2020/10/15/sp101520-a-new-bretton-woods-moment (accessed October 23, 2021).
Glassman, A., and E. Smitham. 2021. Financing for global health security and pandemic preparedness: Taking stock and what’s next. https://www.cgdev.org/blog/financing-global-health-security-and-pandemic-preparedness-taking-stock-whats-next (accessed June 16, 2021).
Gouglas, D., T. Thanh Le, K. Henderson, A. Kaloudis, T. Danielsen, N. C. Hammersland, J. M. Robinson, P. M. Heaton, and J.-A. Røttingen. 2018. Estimating the cost of vaccine development against epidemic infectious diseases: A cost minimisation study. The Lancet Global Health 6(12):e1386–e1396.
GPMB (Global Preparedness Monitoring Board). 2019. A world at risk: Annual report on global preparedness for health emergencies. Geneva, Switzerland: World Health Organization.
He, D., S. Zhao, Y. Li, P. Cao, D. Gao, Y. Lou, and L. Yang. 2020. Comparing COVID-19 and the 1918–19 influenza pandemics in the United Kingdom. International Journal of Infectious Diseases 98:67–70.
Huber, C., L. Finelli, and W. Stevens. 2018. The economic and social burden of the 2014 Ebola outbreak in West Africa. Journal of Infectious Diseases 218(Suppl 5):S698–S704. https://doi.org/10.1093/infdis/jiy213 (accessed October 23, 2021).
IFPMA (International Federation of Pharmaceutical Manufacturers & Associations). 2021. Towards vaccinating the world: Landscape of current COVID-19 supply chain and manufacturing capacity, potential challenges, initial responses, and possible “solution space”—a Discussion Document. https://www.ifpma.org/wp-content/uploads/2021/03/Summit_Landscape_Discussion_Document.pdf (accessed October 21, 2021).
Italian Ministry of Economy and Finance. 2021. The G20 establishes a high-level independent panel on financing the global commons for pandemic preparedness and response. https://www.mef.gov.it/en/inevidenza/The-G20-establishes-a-High-Level-Independent-Panel-on-financing-the-Global-Pandemic-Preparedness-and-Response-00001 (accessed October 21, 2021).
Lee, J. W., and W. J. McKibbin. 2004. Estimating the global economic costs of SARS. In Learning from SARS: Preparing for the next disease outbreak: Workshop Summary. Washington, DC: The National Academies Press.
Lurie, N., G. T. Keusch, and V. J. Dzau. 2021. Urgent lessons from COVID 19: Why the world needs a standing, coordinated system and sustainable financing for global research and development. The Lancet 397(10280):1229–1236.
McKibbin, W. J., and A. A. Sidorenko. 2006. Global macroeconomic consequences of pandemic influenza. Centre for Applied Macroeconomic Analysis, Crawford School of Public Policy, Australian National University. https://cama.crawford.anu.edu.au/pdf/working-papers/2006/262006.pdf (accessed October 21, 2021).
Morales, K. F., D. W. Brown, L. Dumolard, C. Steulet, A. Vilajeliu, A. M. Ropero Alvarez, A. Moen, M. Friede, and P. Lambach. 2021. Seasonal influenza vaccination policies in the 194 WHO member states: The evolution of global influenza pandemic preparedness and the challenge of sustaining equitable vaccine access. Vaccine X 8:100097.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. Readiness for microbial threats 2030: Exploring lessons learned since the 1918 influenza pandemic: Proceedings of a workshop. Washington, DC: The National Academies Press.
Palache, A, T. Tsai, Y. Vasiliev, A. Abelin, R. Hollingsworth, B. Taylor, and P. Barbosa. 2020. Global influenza vaccine distribution survey demonstrates urgency of implementation of objective 3 of WHO influenza strategy 2019–2030. Internal Medicine Review 6(2):1–27.
Palache, A., S. Rockman, B. Taylor, M. Akcay, J. K. Billington, P. Barbosa, IFPMA Influenza Vaccine Supply task force. 2021. Vaccine complacency and dose distribution inequities limit the benefits of seasonal influenza vaccination, despite a positive trend in use. Vaccine 39(41):6081–6087.
Petersen, E. M., U. Koopmans, D. H. Go, N. Hamer, F. Petrosillo, M. Castelli, M. Storgaard, S. Al Khalili, and L. Simonsen. 2020. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. The Lancet Infectious Diseases 20(9):e238–e244.
Resolve to Save Lives. 2019. A disease threat anywhere is a disease threat everywhere. https://resolvetosavelives.org/assets/Resources/RTSL_Fact_Sheet_3_22_19.pdf (accessed November 4, 2021).
Ritchie, H., E. Ortiz-Ospina, D. Beltekian, E. Mathieu, J. Hasell, B. Macdonald, C. Giattino, C. Appel, L. Rodés-Guirao, and M. Roser. 2021. Mortality risk of COVID-19. https://ourworldindata.org/mortality-risk-covid (accessed April 18, 2021).
The Rockefeller Foundation. 2021. One for all: An action plan for financing global vaccination and sustainable growth. New York: The Rockefeller Foundation.
Rockman, S., K. Laurie, and I. Barr. 2020. Pandemic influenza vaccines: What did we learn from the 2009 pandemic and are we better prepared now? Vaccines 8(2):211.
Ruscio, B., A. Bolster, J. Bresee, and London Shaping Meeting Participants. 2020. Shaping meeting to explore the value of a coordinated work plan for epidemic and pandemic influenza vaccine preparedness. Vaccine 38(16):3179–3183.
Simpson, S., M. C. Kaufmann, V. Glozman, and A. Chakrabarti. 2020. Disease X: Accelerating the development of medical countermeasures for the next pandemic. The Lancet Infectious Diseases 20(5):e108–e115.
Singal, A. G., P. D. R. Higgins, and A. K. Waljee. 2014. A primer on effectiveness and efficacy trials. Clinical and Translational Gastroenterology 5(1):e45.
Sparrow, E., J. G. Wood, C. Chadwick, A. T. Newall, S. Torvaldsen, A. Moen, and G. Torelli. 2021. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine 39(3):512–520.
U.S. Department of State. 2021. Secretary Antony J. Blinken remarks to the UN Security Council briefing on COVID-19 and vaccine access. https://www.state.gov/secretaryantony-j-blinken-remarks-to-the-un-security-council-briefing-on-covid-19-and-vaccine-access (accessed December 17, 2021).
U.S. Senate, Committee on Foreign Relations, Subcommittee on Multilateral International Development, Multilateral Institutions, and International Economic, Energy, and Environmental Policy. 2017. Testimony of Dr. Rebecca Katz: The World Health Organization and Pandemic Preparedness. June 20, 2017. Washington, DC.
Velásquez, G., and N. Syam. 2021. A new WHO international treaty on pandemic preparedness and response: Can it address the needs of the global south? Geneva, Switzerland: South Centre.
White House. 2021. Fact sheet: President Biden to take action on global health through support of COVAX and calling for health security financing. https://www.whitehouse.gov/briefing-room/statements-releases/2021/02/18/fact-sheet-president-biden-to-take-action-on-global-health-through-support-of-covax-and-calling-for-health-security-financing (accessed July 16, 2021).
WHO (World Health Organization). 2018. Nipah virus fact sheet. https://www.who.int/news-room/fact-sheets/detail/nipah-virus (accessed July 1, 2021).
WHO. 2019. Global influenza strategy 2019–2030. Geneva, Switzerland: World Health Organization.
WHO. 2021a. Fact sheet: Marburg virus disease. https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease (accessed July 1, 2021).
WHO. 2021b. Ebola virus disease fact sheet. https://www.who.int/en/news-room/fact-sheets/detail/ebola-virus-disease (accessed July 1, 2021).
WHO. 2021c. Global influenza surveillance and response system (GISRS). https://www.who.int/initiatives/global-influenza-surveillance-and-response-system (accessed July 1, 2021).
WHO. 2021d. Vaccine safety basics. https://vaccine-safety-training.org/types-of-vaccine.html (accessed December 17, 2021).
Yamey, G., M. Schaferhoff, O. K. Aars, B. Bloom, D. Carroll, M. Chawla, V. Dzau, R. Echalar, I. S. Gill, T. Godal, S. Gupta, D. Jamnison, P. Kelley, F. Kristenen, C. Mundaca- Shah, B. Oppenheim, J. Pavlin, R. Salvado, P. Sands, R. Schmunis, A. Soucat, L. Summers, A. El Turabi, R. Waldman, and E. Whiting. 2017. Financing of international collective action for epidemic and pandemic preparedness. The Lancet Global Health 5(8):E742–E744. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(17)30203-6/fulltext#articleInformation (accessed October 23, 2021).


















