4
Technology and Manufacturing Partnerships
PLATFORM TECHNOLOGIES IN THE COVID-19 ERA
Industry partnerships for platform technologies (technologies used as a base on which other applications, processes, or technologies are developed) forged during the COVID-19 pandemic are poised to offer a disruptive moment,1 including for those used for influenza vaccines. COVID-19 has accelerated the shift toward messenger RNA (mRNA) and recombinant vaccines. In its May 4, 2021 earnings call, Pfizer reported 2021 sales projections that estimated that its COVID-19 vaccine—“Comirnaty”—would generate 1-year sales that far exceeded those of any pharmaceutical product in history (LaMattina, 2021). Unsurprisingly, Pfizer is looking to harness the mRNA platform technology advancements to develop a more effective influenza vaccine. The company’s chief scientific officer and president of research and development (R&D), Michael Dolsten, informed investors that Pfizer will start clinical trials for its influenza mRNA vaccine during the third quarter of 2021 (LaMattina, 2021).
The COVID-19 pandemic has also helped to attract and drive new players into the vaccine market. Only 14 percent of organizations involved in pandemic-related product development had commercialized vaccines, although many had experience in developing vaccines against Zika virus, Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV-1, H1N1 influenza, and various other diseases (Chagar et al.,
___________________
1 Techopedia. https://www.techopedia.com/definition/3411/platform (accessed January 14, 2022).
2021). More than 20 COVID-19 vaccines are being produced around the world. Figure 4-1 depicts the production locations. Table 4-1 lists the vaccine manufacturers by platform.
About two-thirds of the current COVID-19 vaccines in the pipeline rely on established technologies such as protein subunit vaccines, nonreplicating viral vectors, and inactivated viruses. The remaining use newer technologies—including mRNA and adenovirus vectors—that could serve as “plug-and-play” platforms for vaccines against other pathogens. To confer protection against infectious diseases, mRNA vaccines introduce a piece of mRNA that corresponds to a protein or a component thereof in the virus, inducing cells in the body to produce that protein, triggering an immune response that produces antibodies against the protein that persist and can prevent infection and serious illness after exposure to the target virus. COVID-19 vaccines based on the adenovirus vector technology trigger an immune response using a nonreplicating viral vector that delivers the genetic code for the spike protein on the surface of the SARS-CoV-2 virus. Both mRNA and adenovirus approaches to vaccine development have been studied for other emerging viruses, including Zika and MERS. Zabdeno, a vaccine against Zaire ebolavirus developed using adenovirus vector technology, was licensed by the European Medicines Agency in 2020.
Funding from a range of agencies and organizations—including the U.S. Department of Defense, the U.S. Biomedical Advanced Research and Development Authority (BARDA), the U.S. National Institutes of Health (NIH), the United Kingdom, and the Coalition for Epidemic Preparedness Innovations (CEPI)—has been instrumental in developing the technologies
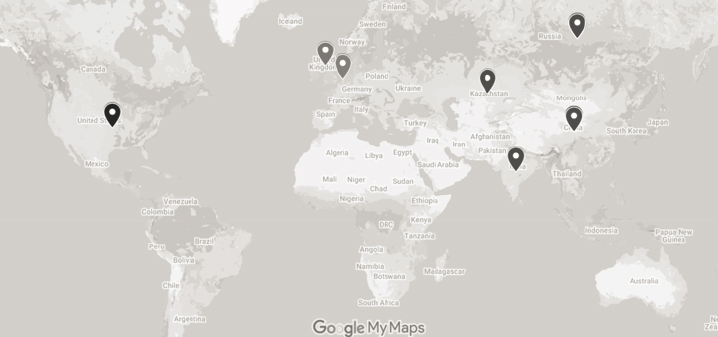
SOURCE: Based on data from the COVID-19 Vaccine Manufacturing Potential map, with data through March 2021. http://vaxmap.org (accessed March 31, 2021).
TABLE 4-1 Approved Vaccines, Manufacturers, and Platforms
| Vaccine Type | Name | Developer | Country |
|---|---|---|---|
| Nonreplicating viral vector | COVID-19 Vaccine Oxford/AstraZeneca (AZD1222) | BARDA, OWS | United Kingdom |
| Inactivated vaccine | BBIBP-CorV | Beijing Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | China |
| Inactivated vaccine | Covaxin (BBV152) | Bharat Biotech, Indian Council of Medical Research; Ocugen, National Institute of Virology | India |
| Inactivated vaccine | WIBP-CorV | Wuhan Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | China |
| Inactivated vaccine | CoviVac | Chumakov Federal Scientific Center for Research and Development of Immune and Biological Products | Russia |
| Inactivated vaccine | QazVac (QazCovid-in) | Research Institute for Biological Safety Problems | Kazakhstan |
| Inactivated vaccine | KCONVAC | Minhai Biotechnology Co.; Kangtai Biological Products Co. Ltd. | China |
| Inactivated vaccine (formalin with alum adjuvant) | CoronaVac | Sinovac, PT Bio Farma, Instituto Butantan | China |
| mRNA-based vaccine | Comirnaty (BNT162b2) | Pfizer, BioNTech; Fosun Pharma | United States |
| mRNA-based vaccine | Moderna COVID19 Vaccine (mRNA-1273) | Moderna, BARDA, NIAID | United States |
| Nonreplicating viral vector | COVID-19 Vaccine Janssen (JNJ-78436735; Ad26. COV2.S) | Janssen Vaccines (Johnson & Johnson) | The Netherlands, United States |
| Peptide vaccine | EpiVacCorona | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology, Rospotrebnadzor | Russia |
| Recombinant adenovirus vaccine (rAd26 and rAd5)/ nonreplicating viral vector | Sputnik V | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Russia |
| Vaccine Type | Name | Developer | Country |
|---|---|---|---|
| Recombinant adenovirus vaccine (rAd26)/nonreplicating viral vector | Sputnik Light | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Russia |
| Recombinant vaccine | ZF2001 | Anhui Zhifei Longcom Biopharmaceutical, Institute of Microbiology of the Chinese Academy of Sciences | China, Uzbekistan |
| Recombinant vaccine (adenovirus type 5 vector)/ nonreplicating viral vector | Convidicea (PakVac, Ad5-nCoV) | CanSino Biologics | China |
| Protein subunit | CIGB-66 | Center for Genetic Engineering and Biotechnology | Thailand |
| Protein subunit | MVC-COV1901 | Medigen Vaccine Biologics Corp. | Taiwan |
| Nonreplicating viral vector | Covishield | Serum Institute of India (Oxford/AstraZeneca formulation) | India |
| Inactivated vaccine | COVID-19 inactivated | Shifa Pharmed Industrial | Iran |
| mRNA | TAK-919 | Takeda (Moderna formulation) | Japan |
| DNA | ZyCoV-D | Zydus Cadila | India |
NOTE: BARDA = Biomedical Advanced Research and Development Authority; NIAID = National Institute of Allergy and Infectious Diseases; OWS = Operation Warp Speed.
SOURCE: Airfinity or the COVID19 Vaccine Tracker, https://covid19.trackvaccines.org/vaccines/approved (accessed September 30, 2021). Data through September 2021.
used in new vaccines (Sabin-Aspen Vaccine Science and Policy Group, 2021). Manufacturers of vaccines using mRNA and protein subunits will likely be incentivized to shift to new antigenic targets, including for influenza, to continue capitalizing on the new technology developed for COVID-19. This represents a potentially transformative inflection point in the influenza vaccine manufacturing landscape. Certain technologies supported by BARDA for pandemic influenza—mRNA (Moderna), adenovirus 26 (Janssen), recombinant protein (Sanofi), and nanoparticle (Novavax)—were adapted for SARS-COV-2 with support from the U.S. government and ultimately granted emergency use authorization by the
U.S. Food and Drug Administration. Although it would require substantial R&D followed by clinical trials, this underscores the potential to transfer platform technologies for pandemic influenza vaccines and therapeutics to other emerging infectious diseases and vice versa (Newland et al., 2021).
NEW INDUSTRY PARTNERSHIPS DURING COVID-19
The launch of these new platform technologies during the COVID-19 pandemic has led to a plethora of new industry partnerships, many forged among companies that have traditionally been in direct competition. In the words of the Sabin-Aspen Vaccine Science and Policy Group (2019, p. 19), “Nothing in recent times has showcased the payoff of basic science investment as much as COVID-19 vaccines—a decades-long overnight success story that drew on years of earlier research to confront a devastating new virus.” As of September 2020, partnerships were developing around half of all COVID-19 vaccine candidates, including those in clinical trials. Almost half of those partnerships involved two research entities, and about 40 percent included a research entity and a player in the development, manufacturing, and/or commercialization spaces (Chagar et al., 2021). Inevitably, some of these partnerships will ebb post-pandemic, but others will likely facilitate more rapid access to vaccines. Moreover, effective partnerships should—in theory—become easier to form. As new products are developed, this could also contribute to expediting access to vaccines. For example, Indonesia serves as the regional hub for the production and distribution of Chinese vaccines across Southeast Asia (Nugroho, 2020).
In some cases, the crucible of the COVID-19 pandemic catalyzed an “all-hands-on-deck” attitude among traditional competitors in the pharmaceutical industry. For instance, Sanofi has partnered or plans to partner with the manufacturers of three major COVID-19 vaccines to expand production capacity. In January 2021, it announced plans to share access to its manufacturing infrastructure and expertise with Germany’s BioNTech to produce 125 million vaccine doses, followed the next month by declaring a similar partnership with Johnson & Johnson to produce 12 million doses of its vaccine per month. Beginning in September 2021, Sanofi will partner with Moderna by using its manufacturing capacity and infrastructure to support the production of 200 million vaccine doses (Keown, 2021).
THE CURRENT STATE OF INFLUENZA VACCINE MANUFACTURING AND THE LIMITATIONS OF EGG-BASED VACCINES
If the COVID-19 pandemic had been caused by influenza, it is unclear whether vaccine access would have been broader. Three types of seasonal
influenza vaccines are available: (1) inactivated influenza vaccines (IIVs), (2) live attenuated influenza vaccines (LAIVs), and (3) recombinant vaccines. Both IIVs and LAIVs can be manufactured using embryonated chicken eggs as the substrate; IIVs can also rely on an animal cell–based substrate that does not require chicken eggs. In contrast, recombinant vaccines are manufactured synthetically without requiring a candidate vaccine virus (CVV) sample. The substrate for production of these vaccines is either egg based (i.e., using embryonated chicken eggs) or cell based (using cell culture).
In 2019, the 12-month global production capacity for seasonal and pandemic influenza was estimated to be 1.48 billion doses for the former if manufacturers were operating at full-scale capacity (Sparrow et al., 2021) and for the latter, 4.15 billion doses assuming “moderate case” production capacity and 8.31 billion doses for “best-case” capacity. The global production of all influenza vaccines remains highly reliant on egg-based processes: in 2019, egg- and cell-based vaccines represented 84.5 percent and 15.5 percent, respectively, of the global production capacity for seasonal vaccines. Of the overall pandemic influenza vaccine production capacity, 79 percent was egg based and 21 percent cell based.2 The vast majority of global production capacity is used for IIVs (seasonal: 89.6 percent; pandemic: 88.9 percent), followed by recombinant vaccines (seasonal: 5.4 percent; pandemic: 7.7 percent) and LAIVs (seasonal: 5.0 percent; pandemic: 3.4 percent).
Influenza vaccines are manufactured using a distributed model, although the majority of the production capacity—particularly for pandemic influenza—is located in high-income countries (HICs). According to the analysis of global production capacity of seasonal and pandemic influenza vaccines in 2019 (Sparrow et al., 2021), the distribution of the 40 active production facilities by World Health Organization (WHO) region is skewed toward the Western Pacific Region (20 facilities), although active facilities were located in all regions except for the African Region (European Region: 9; Region of the Americas: 7; South-East Asia Region: 3; Eastern Mediterranean Region: 1) (Sparrow et al., 2021). None was located in low-income countries, while 20 facilities were in HICs, representing 69 percent of global seasonal vaccine production capacity and 80 percent of pandemic vaccine production capacity. Of the remaining 20 facilities, 15 were located in upper-middle-income countries and five in low- and middle-income countries (LMICs).
___________________
2 These proportions are different from those for seasonal vaccine production because two manufacturers who produce seasonal vaccines in eggs have approval to produce cell-based vaccines for pandemics, some of the manufacturers with cell-based productions produce quadrivalent vaccines, and one manufacturer has an approved facility for cell-based IIV for a pandemic but does not produce seasonal flu vaccines.
Existing production facilities for seasonal influenza could be switched over to produce pandemic influenza vaccines. However, even in the best-case scenario, it would take 4–6 months after the declaration of a pandemic for vaccines to be available for deployment and months longer to maximize production capacity (Sparrow et al., 2021). For an entirely new IIV, it would take at least 23–24 weeks after selecting the virus and uploading its genetic sequence to produce the first doses.3 LAIVs have a slightly shorter time from sequencing to deployment, about 21 weeks. Recombinant vaccines can be produced on a much more rapid time line than either, largely because they are not dependent on a CVV, but they represent only a small fraction of the current global vaccine production capacity.
The technologies that dominate the influenza vaccine manufacturing system are, therefore, not optimal. While egg-based technologies have a long history and an excellent safety profile, they have limited efficacy (ranging from 60 percent in 2010–2011 to only 10 percent in 2004–2005) (NASEM, 2019), longer production times, and susceptibility to bottlenecks in the production process. Manufacturing capacity could be expanded using cell culture (i.e., producing antigens), but this has even lower yields than eggs (although some new technologies, such as strain modification, may allow cell culture to produce higher yields). Recombinant technologies have shown high productivity for influenza but would also lead to shortages of adjuvants.
The mRNA and platform technologies could potentially manufacture vaccines more rapidly than the egg-based method, which could also contribute to containing and controlling the spread of influenza. However, the egg-dominated market might have deterred some manufacturers from taking the risk on mRNA and other new technologies if the world had been hit by an influenza pandemic instead. On the other hand, an influenza pandemic could also have given mRNA and other recombinant technologies an earlier proving ground than COVID-19, and some companies might have attempted to apply mRNA or other new platforms to influenza. Successes with new platforms could relatively quickly render egg-based technology obsolete.
NEW TECHNOLOGIES FOR INFLUENZA ON THE HORIZON
Such new technologies are on the horizon for influenza but still far from ready for widespread rollout. In recent years, the field of influenza vaccine platform technology has expanded significantly, with 106 vaccine
___________________
3 Timing includes the time needed for (1) the CVV to be prepared, characterized, safety tested by the reassorting laboratories, and shipped to manufacturers; (2) the manufacturers to assess the CVVs for yield and growth characteristics, prepare clinical lots, and conduct clinical trials and for vaccine production, formulation, packaging, and distribution; (3) the Essential Regulatory Laboratories to prepare reagents necessary to measure potency and for lot release; and (4) national regulatory authorities to assess the vaccine for licensure and release it.
candidates in various clinical trial phases. As of July 2021, 26 new technologies had reached clinical trials, and 90 were in the late preclinical trial stage, according to the Universal Influenza Vaccine Technology Landscape (CIDRAP, 2021). Figure 4-2 provides a visual depiction of the global vaccine landscape at the preclinical and early clinical trial phases, with six different types of platforms: recombinant proteins, non-virus-like particles (nanoparticles), virus-like particles (VLPs), virus-vectored, nucleic acid, and recombinant influenza virus. Table 4-2 provides a breakdown of the vaccine platforms by clinical trial phase, with a significant number (85 out of 106) in the preclinical phase.
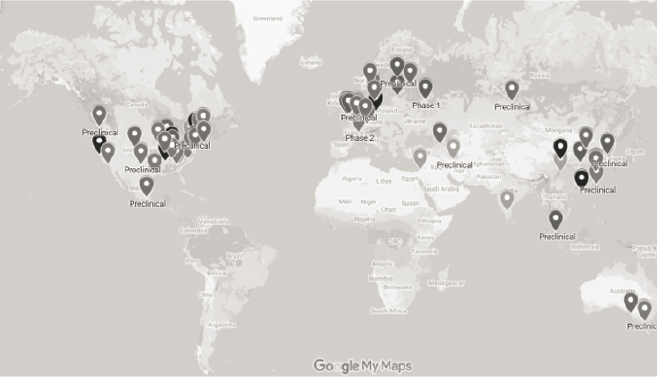
SOURCE: Based on data from CIDRAP (2021), as of June 1, 2021. https://ivr.cidrap.umn.edu (accessed December 19, 2021).
TABLE 4-2 Influenza Vaccine Platforms by Clinical Trial Phase
| Vaccine Platform | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Trial Phase | Recombinant Proteins | Non-Virus-Like Nanoparticles | Virus-Like Particles | Virus Vectored | Nucleic Acid Based | Recombinant Influenza Virus Based | Total | |
| Preclinical | 19 | 17 | 16 | 13 | 13 | 7 | 85 | |
| Phase I | 3 | 2 | 1 | 1 | 2 | 2 | 11 | |
| Phase II | 1 | 1 | 0 | 3 | 0 | 2 | 7 | |
| Phase III | 1 | 1 | 1 | 0 | 0 | 0 | 3 | |
| Total | 24 | 21 | 18 | 17 | 15 | 11 | 106 | |
SOURCE: Data from CIDRAP (2021), as of June 1, 2021. https://ivr.cidrap.umn.edu (accessed December 19, 2021).
Recombinant Technology Vaccines
Recombinant technology vaccines in development for influenza include quadrivalent vaccines, VLPs, and nanoparticles. Quadrivalent technology (FluBlok, Sanofi Pasteur, Lyon) involves using genetically modified baculoviruses to insert tailored RNA into insect cells, where vaccine proteins are then grown. Initial research suggests that the FluBlock vaccine may be at least 30 percent more efficacious than the standard vaccines in adults aged >50 years (Venkatesen, 2017). VLPs (Medicago, Quebec) are used to produce recombinant DNA VLP vaccines in tobacco leaf cells (Ward et al., 2021) and could allow for vaccine production to begin just 5–6 weeks after the declaration of a pandemic if the virus strain is known. As of 2019, this technology was in Phase III trials. Nanoparticle technology being developed by Novavax showed a favorable safety profile, which was confirmed in Phase III trials.4
mRNA Vaccines
As of May 2019, Phase I clinical trials had tested two first-generation mRNA vaccines (Moderna, Norwood MA) against two highly pathogenic influenza strains (Bender, 2019). In 2018, Pfizer and BioNTech announced a partnership to develop mRNA influenza vaccine, with BioNTech to receive $120 million up front from Pfizer plus up to $305 million more, depending on development achievement; tiered royalties on future sales were expected to be in the double-digit percentage range (Reuters, 2018). In January 2021, Moderna announced three new vaccine programs targeting seasonal influenza, HIV, and Nipah virus. The company also expects to extend mRNA work to 24 programs in five therapeutic areas. The influenza vaccines—mRNA1010, mRNA-1020, and mRNA-1030—will target seasonal types A and B (Laguipo, 2021). In April 2021, Moderna’s chief executive officer, Stephane Bancel, announced that it is planning to develop an influenza vaccine in tandem with its COVID vaccine as a single booster shot (Bever, 2021).
ACCELERATING THE DEVELOPMENT OF PLATFORM TECHNOLOGIES FOR INFLUENZA
Accelerating the development of platform technologies for influenza vaccines over the next 3–5 years will enhance the speed of production and potential efficacy. While developing pre-pandemic strain-specific vaccines is viewed as an insurance policy, this approach is costly and may be futile if vaccines for a putative pandemic strain are never needed because the pandemic does not materialize, as happened with the 2005 H5N1 influenza
___________________
4 See https://www.novavax.com/our-pipeline#nanoflu (accessed December 19, 2021).
outbreak. Viruses also have time to mutate over the required 6 months or more needed for egg-based technologies, which compromises the efficacy of the final product, as occurred with the H7N9 vaccine in 2017. Post hoc development of vaccines has also often been an ineffective response to a pandemic given the slowness of egg-based technologies.
A universal influenza vaccine would represent a real game changer and eliminate or greatly reduce all of these problems at once. Progress is under way on multiple fronts—from R&D to financing—to develop this holy grail. Ideally, it would confer 3–5 years of protection from morbidity and mortality caused by all influenza A subtype viruses and influenza B lineage viruses, both circulating and emerging (“drifted and shifted”). The 2018 Strategic Plan developed by NIH’s National Institute of Allergy and Infectious Diseases (NIAID) highlights NIAID’s commitment to support the research needed to advance the development of a universal influenza vaccine that provides long-lasting protection against multiple strains for both seasonal and potentially pandemic influenza (Erbelding et al., 2018). The European Union’s Framework Programme for Research and Technological Development, part of Horizon 2020, also supports major initiatives to develop novel influenza vaccines through (1) improving understanding of immunity against the virus and immune response to vaccines; (2) identifying genetic biomarkers that characterize highly pathogenic strains; and (3) using new immunization technologies, formulations, and vectors and delivery systems, and vaccination methods (Navarro-Torné et al., 2019). The European Union expects that its Joint Influenza Vaccine Effectiveness Studies program will positively influence societal acceptance of influenza vaccines in Europe, result in improved vaccine coverage, and ultimately reduce the burden of disease. If it proves a successful model, it could be expanded to other vaccines in EU national and subnational immunization programs.
Development of a universal influenza vaccine is garnering financial support from various philanthropic donors and initiatives. In 2018, the Bill & Melinda Gates Foundation (BMGF) partnered with FluLab to launch a $12 million Universal Influenza Vaccine Development Grand Challenge seeking bold, innovative, and transformative ideas to be realized through interdisciplinary collaboration outside of the scope of traditional efforts. In the same year, the Wellcome Trust announced its support for the Center for Infectious Disease Research and Policy (CIDRAP) to develop a roadmap for R&D for influenza vaccines to accelerate progress toward universal or broadly protective influenza vaccines; it was published in September 2021.5
A 2020 report by the Sabin-Aspen Vaccine Science and Policy Group surveyed the current state of progress toward a universal influenza vaccine,
___________________
5 See https://www.cidrap.umn.edu/ongoing-programs/influenza-vaccines-roadmap (accessed December 19, 2021).
noting that the rate of research, development, and innovation has generally decelerated in recent years, despite substantial investments in basic research. It will likely remain stagnant if the vaccine market remains focused and reliant on outdated processes for tracking and selecting likely seasonal virus strains and using egg-based manufacturing processes, both of which deter investment and innovation toward a universal vaccine. The group argued that accelerating development will require overcoming the fragmentation and lack of goal-oriented coordination that pervades the system by (1) creating an entity to lead and coordinate this effort, (2) advancing an R&D agenda focused on transformational change that includes a broader range of scientific perspectives, and (3) communicating the true impact of influenza and the urgent need for a universal vaccine.
A number of entities could play such a role in a universal vaccine effort if their mandates and missions were expanded. For example, CEPI is a global partnership among public, private, philanthropic, and civil society organizations; its mission6 is to “accelerate the development of vaccines against emerging infectious diseases and enable equitable access to these vaccines for people during outbreaks.”7 CEPI has identified a number of gaps in the landscape that it was designed to fill. First, CEPI works to counter known threats through “proof-of-concept safety testing” and will establish vaccine stockpiles as a preparedness measure. Second, CEPI funds new and innovative platform technologies with a potential to “accelerate the development and manufacturing of vaccines against unknown pathogens,” with an intent to meet a time line of 16 weeks from antigen identification to product release for clinical trials. Third, CEPI supports and coordinates activities to improve the collective response to epidemics, strengthen capacity in at-risk countries, and advance the regulatory science that governs vaccine development. CEPI has no specific role in the influenza ecosystem, but an expanded mandate could allow it to lead a global effort for developing a universal influenza vaccine if supported by sufficient funding.
Another entity that could play a leading or coordinating role in the search for a universal influenza vaccine is BARDA.8 It prepares for the next influenza pandemic by supporting development, licensure, and manufacturing of improved products to detect, treat, and prevent seasonal and pandemic influenza, which can be rapidly manufactured. BARDA has a strong track record of bringing medical countermeasures for influenza across the finish line, supporting a broad portfolio of pandemic countermeasures licensed or approved by the U.S. Food and Drug Administration. By
___________________
6 See https://cepi.net/about/whyweexist (accessed December 19, 2021).
7 See https://cepi.net (accessed December 19, 2021).
8 See https://www.phe.gov/about/barda/Pages/PI.aspx (accessed December 19, 2021).
partnering with industry product developers and manufacturers, BARDA supports developing cutting-edge influenza vaccines, diagnostics, and therapeutics and maintains stockpiles of influenza vaccine antigens and adjuvants. However, it is a U.S. government agency and not charged with global international coordination as its major focus.
In February 2021, the European Commission (EC) announced a proposal for its Health Emergency Preparedness and Response Authority (HERA) Incubator, premised on the understanding that expanding vaccine production capacity in Europe would require more integrated and strategic public–private partnerships with the pharmaceutical industry. The EC also established the Task Force for Industrial Scale-Up of COVID-19 Vaccines to help ramp up production capacity and alleviate supply chain bottlenecks (Hoen et al., 2021). HERA is currently primarily focused on variants of SARS-CoV-2 that are causing great concern because of their increased transmissibility. The European Union is setting up a European biodefense preparedness plan “HERA Incubator” to bring together researchers, biotech companies, manufacturers, regulators, and public authorities to monitor variants, exchange data, and cooperate on adapting vaccines. The plan will focus on detecting, analyzing, and adapting to virus variants; speeding up regulatory approval of vaccines; providing guidance on data requirements and facilitating the certification of new or repurposed manufacturing infrastructures; and supporting the speedy mass production of adapted or novel COVID-19 vaccines. The EU investment in state-of-the-art vaccine and drug R&D and manufacturing capacities will be one of the core blocks for future pandemic response but will also contribute to EU strategic autonomy in the area of health and the positioning of the European health care industry. It is, however, just being organized at the time of this report.
Other entities might be able to play a role in coordinating and leading a push for a universal influenza vaccine. The Global Funders Consortium for Universal Influenza Vaccine Development, established in 2017 to convene organizations and governments that fund R&D for universal influenza vaccines in particular, has been and can continue to be involved in identifying funding gaps and promoting dialogue between key stakeholders.
It is the committee’s consensus that CEPI merits serious consideration for wider global coordination. Its current mission and mandate, including its investment case emphasis on developing a universal vaccine against coronaviruses, would allow it to assume this role. This would not require an extensive mission expansion but would benefit from expanding CEPI’s support of “platform-agnostic” technologies. It will also be important to improve coordination for future influenza vaccine research at CEPI and/or HERA and BARDA with institutional R&D capabilities that go beyond the United States, particularly in Germany (e.g., Max Planck Institutes), Japan, and South Korea, and engage regional entities for R&D mobilization.
While the remarkable effectiveness of mRNA vaccines against SAR-CoV-2 makes the technology attractive for many vaccines, a universal influenza vaccine is still a science problem more than a manufacturing problem. Toggling between seasonal and pandemic influenza vaccine production has long been considered the best strategy to maintain a “warm” base for a pandemic vaccine when it is needed, and platform technologies offer an opportunity to move beyond this dynamic because they could be used for seasonal influenza vaccines and other pathogens.
GEOGRAPHICALLY DISTRIBUTED AND REGIONAL MANUFACTURING MODELS (“HUBS”)
Momentum is gathering toward a shift to models such as distributed manufacturing and regional development hubs that bring vaccine production closer to the end users. Greater scale in global manufacturing capacity could reduce the extent of rationing in a pandemic; greater geographic distribution of global manufacturing capacity could reduce the inequalities of access that arise in rationing. However, a trade-off exists between these two approaches.
For influenza and other types of vaccines using new platform technologies, this shift could help drive much broader and more equitable access worldwide. This momentum is reflected in the G20 Summit’s 2021 Rome Declaration, which calls for voluntary licensing and technology transfer to increase global production of COVID-19 vaccines and has spurred billions of dollars in pledges from G20 members and vaccine manufacturers (Talor, 2021). At the same summit, the EC announced that it will provide €1 billion in support for a Team Europe initiative on manufacturing and access to vaccines, medicines, and health technologies in Africa (European Commission, 2021).
In a pandemic, LMICs that do not have local vaccine production capacity or contractual supply agreements with producers of medical countermeasures must rely on internationally coordinated pandemic influenza vaccine deployment. Sustainable local manufacturing capacity is one way to address the risk of inequitable and delayed vaccine availability (WHO, 2013). In June 2021, The Rockefeller Foundation urged G7 leaders to adopt its Updated Action Plan for Global COVID-19 Vaccination, which calls for regional manufacturing hubs in the Global South to narrow the gap between vaccine manufacturing capacity between the developed and developing worlds (The Rockefeller Foundation, 2021).
The aim of decentralized manufacturing is to reduce delays and gaps in access to vaccines through regional production. The basic premise of “decentralized” or “distributed” manufacturing is that it disperses production across various locations (Harrison et al., 2018). Adopting this model alters manufacturers’ organizational structure in a range of
advantageous ways, such as democratizing supply, creating additional jobs in more locations, and allowing more flexibility in response to changes in demand. Distributed supply chain hubs in parallel to manufacturing facilities also present a market opportunity for countries to supply the bags, filters, and other items required for vaccine supply chains. This model differs from the “hub-and-spoke model,” whereby vaccines can be stored in ultra-cold freezers in centralized production “hubs” and then transported to regional “spokes” for short-term storage and administration, because decentralized facilities are not reliant on a central manufacturing hub.
However, decentralization also raises challenges related to ensuring oversight, unified decision making, stressing the supply chain, maintaining product quality, and ensuring a downstream market (demand for the products). None of these are specific to influenza vaccine production. No global institutional architecture exists to handle them, and development finance institutions often struggle to establish business cases for developing vaccines for a pandemic event with uncertain timing. During COVID-19, CEPI performed early manufacturing scale-up, and BARDA-supported scale-up through Operation Warp Speed (OWS), but this support was provided after the pandemic began. In addition, any expansion in production capacity must be accompanied by expanding suppliers of raw materials and consumables, which presents another set of challenges.
The argument for the regional hub model is particularly strong in Africa, which had received less than 2 percent of COVID-19 vaccines worldwide as of April 2021 (WHO, 2021). In April 2021, WHO issued a global call for “expression of interest” in establishing COVID-19 mRNA vaccine technology transfer hubs, which could scale up production and access. Through a partnership with COVAX, the Africa Centres for Disease Control and Prevention (CDC), a network of universities, and an industry consortium (Biovac, Afrigen Biologics and Vaccines), the first such hub is planned for South Africa. This is a crucial step in building geographically distributed manufacturing hub capacity, but additional investments will be necessary on a much larger scale. Between April and June 2021, the Partnership for Africa Vaccine Manufacturing was also launched by the Africa CDC and the African Union. The goal of the partnership is that by 2040, African countries should produce at least 60 percent of the vaccines they use, as opposed to the current 1 percent (Africa CDC and African Union, 2021). In April 2021, Rwandan president Paul Kagame also remarked that Rwanda has also discussed creating an mRNA vaccine plant.
One potential manufacturer, GreenLight BioSciences, has proposed small, modular mRNA plants that can be built in Massachusetts and shipped worldwide. Each could take advantage of its own locally sourced raw materials and potentially provide up to 17 million doses per month at an estimated cost of about $200 million per mini-vaccine plant (Greenlight, 2021). Similar momentum is building in the Latin America and Caribbean
regions. As of May 2021, only 3 percent of the population in Latin American countries were fully vaccinated for COVID-19, leading Pan American Health Organization (PAHO) Director Christine Etienne to call for regional hub development: “We must ramp up production for the entire vaccine value chain—from the ingredients that go into vaccines to the vials and syringes that help us deliver them—without compromising quality” (PAHO, 2021).
Geographically distributed manufacturing is a largely unregulated field. Regional manufacturers have struggled to know what others are working on and where they may obtain relevant technical advice and training, which must be kept up to date for quality, safety, and efficiency. CEPI recently (May 2021) launched a survey9 to map the landscape of vaccine manufacturing capacity and capability in Africa, Southeast Asia, the Middle East, and Latin America. These issues will also need to be addressed to facilitate establishing effective and successful regional hubs. Political and economic stability, and other factors related to regulatory, infrastructural, and supply chain capacity, must be part of the equation for planning future geographically distributed manufacturing hubs, if they are to be effective and sustainable.
CAPABILITIES REQUIRED FOR THE TRANSITION TO PLATFORM TECHNOLOGIES
An inevitable consequence of the shift from egg-based vaccines to platform technologies will be a transitional period of several years for seasonal vaccine manufacturing characterized by a degree of instability and uncertainty. To support the transition and increase influenza vaccine production capacity, six capabilities are required, all of which rely on strong global partnerships:
- Robust early-phase R&D—particularly Phase III clinical trials—focused on platform technologies for influenza and, ideally, a platform-based universal influenza vaccine;
- Sufficient worldwide capacity for manufacturing vaccines to facilitate the success of distributed or regional hubs;
- Effective business models to drive demand and use facilities for products other than pandemic vaccines;
- Systematic workforce training for sustainable technology transfer and a nimble response to subsequent shifts in technologies;
- Expansion of regionally integrated regulatory capacity for vaccines; and
- Appropriate consideration of end users’ needs and supply chain bottlenecks, particularly for a geographically distributed hub model.
___________________
9 See https://cepi.net/news_cepi/survey-launched-by-cepi-to-track-multinational-vaccine-manufacturing-capacity-for-use-in-future-epidemics-and-pandemics (accessed December 19, 2021).
BARRIERS AND PATHWAYS TO SUCCESS FOR EFFECTIVE GLOBAL PARTNERSHIPS TO SUPPORT NEXT-GENERATION INFLUENZA VACCINES
This section surveys current barriers and pathways to success for effective global partnerships for influenza vaccine development and manufacturing based on each of these essential capabilities for influenza vaccine production.
R&D: Barriers
The unprecedented pace of vaccine R&D propelled by the COVID-19 pandemic demonstrated the value that can be achieved through cooperation and resource pooling. However, it also exposed a range of “orphan problems” in the development landscape, which no entity is accountable for addressing in a pandemic context (Sabin-Aspen Vaccine Science and Policy Group, 2021), including the lack of mechanisms for (1) sharing newly discovered viral strains and subsequent mutations, (2) safeguarding intellectual property rights, (3) ensuring liability protection, and (4) harmonizing regulatory processes. It also highlighted the need for advance planning to forge partnerships to rapidly develop and test new vaccines in LMICs.
The gaps identified in CIDRAP’s Influenza Vaccines Research and Development Roadmap mirror many of these wider orphan problems and barriers in the domains of policy, governance, financing, and regulation (Moore et al., 2021). For instance, substantial financing risks and inadequate incentives are significant barriers to bringing broadly protective vaccines to market (WHO, 2016). The “valley of death” refers to the phases spanning early discovery, to Phase III clinical trials, to regulatory approval and early commercialization, when manufacturers bear a disproportionate and asymmetrical burden of the risk. For example, they incur substantial R&D and licensure costs, despite uncertain or risky outcomes and no revenue yet generated or guaranteed. This can be a substantial disincentive to enter the vaccine market (Douglas and Samant, 2018). Barriers also inhibit the later phases—Phase III trials, licensure, and monitoring—that require the greatest financial inputs but lack global coordination and sustainable, collaborative models with appropriate market incentives (Rappuoli et al., 2021). Gaps in R&D governance include no coordinated commitment to funding R&D for universal influenza vaccines, which warrants new public–private partnerships similar to those established for COVID-19 vaccine development (Krammer et al., 2018).
The predominant commercial model for global vaccine production is based on annual reformulation and egg-based technology, which is a barrier to investing in new vaccines with a potentially broader effect. This underscores the need to consider innovative strategies for financing the development of new types of influenza vaccines (Douglas and Samant, 2018). Improving vaccine effectiveness, coupled with more attractive “branding” through coordinated
communications strategies, could help to stimulate investment in innovative R&D strategies. Developing innovative approaches will require substantial additional investments in infrastructure across R&D and production—particularly to overcome challenges during the valley of death. Coordinated partnerships involving national governments, the pharmaceutical industry, philanthropic organizations, and academia will be critical in advancing new vaccines through clinical trials and licensure (Bresee et al., 2019). As Nicole Lurie has recommended, “For future outbreaks and potential pandemics, it is crucial to identify key areas of research as quickly as possible, and have established mechanisms to rapidly release funds from a pre-positioned pool to jumpstart the research and development response” (Lurie et al., 2021, p. 1234).
Further barriers are posed by a lack of transparent sharing of technology information that would allow scientists worldwide to collaborate on innovations (Kavanagh et al., 2021). For instance, the platform designed by the Global Research Collaboration for Infectious Disease Preparedness (GloPID-R) supports scientists and research funders in identifying optimal research solutions and channeling the necessary funds into those solutions rapidly, which also conserves resources and avoids duplication. Glopid-R has facilitated collaboration between India and the European Union focused on next-generation influenza vaccines (Chaudhury, 2020). However, its role is limited to coordination and information sharing among research funders. Moreover, although it includes 29 major research funders, it lacks sufficient financial backing. Although GloPID-R is a valuable first step, such platforms must be supported by sufficient mandates and resources to be significantly impactful (Lurie et al., 2021).
The Ebola vaccine is a cautionary example of failure to adequately coordinate R&D across public–private partnerships; poor coordination delayed deployment of an existing vaccine until 1 year into the epidemic. In addition to the absence of shared protocols, global authorities did not clearly communicate how many doses would be needed. The vaccine candidate that ultimately proved effective (VSV-ZEBOV; now licensed as Ervebo) had been developed a decade earlier and could have been advanced and ready to deploy much sooner given appropriate pathways and incentives (Sabin-Aspen Vaccine Science and Policy Group, 2021).
R&D: Partnership Pathways for Success
Elements from OWS offer instructive examples of how partnerships can help forge pathways for success in vaccine R&D. For instance, OWS facilitated the selection of multiple platforms and companies, then provided coordinated assistance to companies at multiple steps along the development chain. Specifically, the partnership between the U.S. Department of Defense (DoD) and the U.S. Department of Health and Human Services (HHS) spurred the quickest vaccine development in history by providing at-risk funding for
manufacturing and testing candidates from five companies—AstraZeneca, Janssen Pharmaceuticals (a unit of Johnson & Johnson), Moderna Inc., Novavax Inc., and Sanofi. (Pfizer Inc. opted to use its own resources for its COVID-19 vaccine.) Although OWS was successful in accelerating the development of safe and effective vaccines, it was less successful in expanding manufacturing capacity and facilitating the administration of those vaccines, with around 2–3-fold fewer vaccine doses administered than planned.
A major boon to OWS was the use of clear criteria for company selection based on robust preclinical and early-stage clinical trial data that suggested the potential to enter Phase III field efficacy trials between July and November 2020, with outcomes by the first half of 2021. Additional criteria included platform technologies permitting rapid, effective manufacturing and companies that demonstrated industrial process scalability, yields, and consistency such that they would be capable of producing more than 100 million doses by mid-2021. Moreover, OWS selected different platforms to mitigate the risk that any single platform or specific vaccine candidate would fail due to any number of problems related to safety, efficacy, industrial manufacturability, and scheduling. OWS included two platforms that had not been used in a licensed vaccine but theoretically could be quickly adapted and scaled up (the mRNA and replication-defective live-vector platforms) and one that had been proven—the recombinant-subunit-adjuvanted protein platform.
The approaches adopted by OWS also enabled customized partnerships with each pharmaceutical company, thus allowing for tailored support of all major aspects of the vaccine development chain. For instance, Pfizer was provided with a financial incentive to bear the R&D risk. The other five companies were required to adhere to published “tenets” as a condition of receiving support. For those companies, OWS took on 80–100 percent of the financial risk by funding both R&D and manufacturing. If Phase III trials had failed, all funding would have been provided by the U.S. government. Furthermore NIH network–associated clinical trial sites—representing 20–40 percent of sites—were engaged to operationalize the Phase I–III trials. Although Pfizer manufactured its own vaccine, OWS was involved in all operational aspects of manufacturing for Moderna’s vaccine, including accessing and ordering raw materials, obtaining special equipment and machinery, installation, and other support. The other four companies used sites for drug substance manufacturing that were part of a BARDA collaborative network. Alongside technical teams from those companies, OWS supported the equipping of sites, hiring of talent, and validating of work to support scale-up of manufacturing (GAO, 2021).
Markets and Business Models: Barriers for Pandemic Flu Vaccines
The Sabin-Aspen Vaccine Science and Policy Group identified two major barriers to effective vaccine markets that are not specific, but are appli-
cable, to influenza vaccines. The first is the misalignment between financial incentives and public health needs (the “profit-based model”); the second is the disproportionate concentration of markets in HICs.
About 80 percent of the US$ value of the global vaccine market is generated in HICs, despite those countries accounting for roughly only 20 percent of the annual volume of vaccines consumed (Shulman et al. 2021). LMICs account for about 18 percent of the dollar value of the global vaccine market but approximately 80 percent of the annual volume (WHO, 2019a). Manufacturers deeply entrenched in the influenza vaccine market have a residual commitment to arguing that a separate capacity needs to be retained for seasonal influenza. The complexity of the manufacturing process generally requires building new plants for each vaccine—which can take up to 5 years at a cost of at least $350 million in the United States or $150 million in India (Sabin-Aspen Vaccine Science and Policy Group, 2021). Concerns also abound regarding the fate of egg-based vaccine production facilities worldwide when companies with next-generation mRNA technologies begin to apply them to influenza vaccines.
A delicate balance must be struck between having facilities keep capacity warm between pandemics without relying on them absolutely for routine production. Although this issue will persist with the advent of new technologies, diversification could contribute to greater efficiencies (Sell et al., 2021). Either manufacturing capacity will need to be rapidly rolled out but not always needed or billions of dollars will need to be spent building capacities that are unnecessary in steady-state situations and cannot transition to necessary products. It is clear that the latter option is probably infeasible. Additional concerns proliferate around how to maintain trained staff and current regulatory licenses to enable rapid activation in a pandemic. From the manufacturers’ perspective, however, it is impracticable to operate at 10 percent capacity to prepare for ramping up to 100 percent in an emergency. Mechanisms for initiating the switch after WHO declares a Public Health Emergency of International Concern (PHEIC) are still not agreed upon and often remain at the discretion of individual countries (Rockman et al., 2020). From the manufacturers’ standpoint, switching means taking on major risk and potentially losing the return on a huge investment.
The WHO Global Action Plan (GAP) manufacturing program (2006–2019) was designed to bolster the market for influenza vaccines and sustainably increase manufacturing capacity. GAP aimed to increase the supply of a pandemic vaccine and thereby reduce the gap between the potential vaccine demand and supply anticipated during an influenza pandemic (WHO, n.d.).10 The GAP experience offers three valuable lessons (see Box 4-1): (1) the development and manufacture of influenza vaccines is highly complex,
___________________
10 See https://www.who.int/influenza_vaccines_plan/resources/gap_faq.pdf?ua=1 (accessed December 19, 2021).
for both egg-based vaccines and forthcoming novel-platform technologies; (2) the parallel time lines for manufacturing and developing demand for vaccines are interdependent and both equivalently years long; and (3) it is critical to think deeply about creating demand, especially if the technology has narrow applications. GAP leadership indicates that, if launching a similar initiative now, they would likely look beyond both the egg-based and “seasonal influenza vaccine market” models.
Markets and Business Models: Pathway to Success
Few blueprints exist for how to keep facilities warm, although mRNA technologies could transform this landscape (WHO, 2013). However, to address these challenges, a wealth of resources and expertise can be provided by regional and global industrial organizations, such as the African Vaccine Manufacturing Initiative, Developing Country Vaccine Manufacturing Network (DCVMN), Developing Country Vaccine Regulatory Network, Association of South-East Asian Nations, and International Federation of Pharmaceutical Manufacturers & Associations (WHO, 2013). Organizations
such as the DCVMN, which are supported through public–private partnerships or foundations, have successfully expanded capacity to meet demand in certain regions—especially in parts of Latin America (Sabin-Aspen Vaccine Science and Policy Group, 2021). Gavi and BMGF investments have helped to counter incentives that drive production of high-margin vaccines, contributing to an increase in the number of companies producing basic vaccines for low-income countries (from 5 in 2000 to 18 in 2020) (Sabin-Aspen Vaccine Science and Policy Group, 2021). It will be critical to encourage greater involvement of coordination structures, such as the DCVMN. Unlike multinationals, participating countries rely heavily on grants, loans, and seed funding from public and philanthropic sources. For instance, at least half the non-influenza-specific vaccine doses procured by Gavi and UNICEF in recent years have come through the DCVMN (Hayman and Pagliusi, 2020).
Manufacturing Capacity: Barriers
In the push for distributed manufacturing, it is important not to underestimate the difficulties intrinsic to actually accomplishing one-time
technology transfers. Simplistic distributed manufacturing initiatives may not be appropriate or successful (WHO, 2011). An analysis by the MIT Center for Biomedical Innovation of distributed versus centralized biologics manufacturing identified areas of cost efficiencies (MIT Center for Biomedical Innovation, 2017). Figure 4-3 summarizes the scenario: a centralized model with a single high-volume plant is replaced by six smaller, distributed plants with the same total production capacity. The cost centers in this model were qualitatively ranked as being better (strength/green in Figure 4-3), approximately the same (similar/yellow), or worse (weakness/red); while the model indicates that distributed production can provide efficiencies (such as the time to product access), it is also predicts critical increases in operational and regulatory costs.
Attempts to promote distributed manufacturing of antiretrovirals have not been widely successful. A critical factor for success (or failure) of these efforts is the commitment from LMIC governments. Major barriers encountered are the need for steady and consistent government policy, adequate infrastructure, qualified technical and managerial human resources, robust production-distribution logistics systems, strong national regulatory agencies, and an international market (Pinheiro Edos et al., 2014).
GAP (2006–2019) and BARDA’s International Influenza Vaccine Manufacturing Capacity Building Program employed a partnership model based on distributed, egg-based vaccine manufacturing capacity (see Box 4-1). GAP was supported by an independent technical advisory group (Francis and
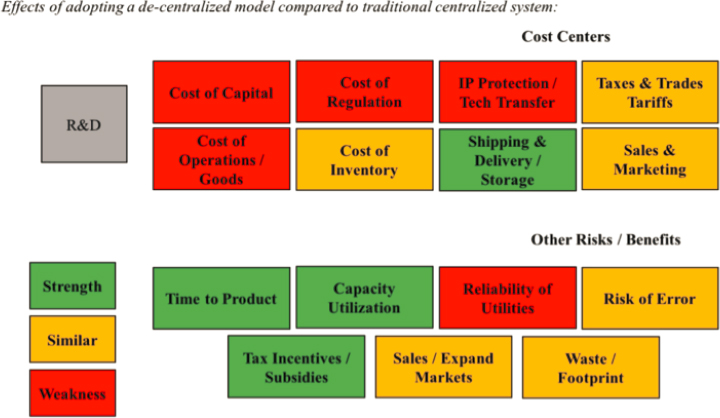
SOURCE: MIT Center for Biomedical Innovation, 2017.
Grohmann, 2018) to assist WHO in selecting proposals for funding and providing programmatic support for successful grantees. Participation in this voluntary program was based on manufacturers’ submitting letters of intent to WHO declaring their interest in developing influenza pandemic vaccine capacity. Across the program’s lifetime, 14 manufacturers were provided with financial support over four review processes (2007, 2009, 2011, 2013); WHO allocated approximately $50 million through 2015. According to BARDA, every $1 of funding leveraged $17 in local investment. Despite the program’s successes, it was merely a drop in the bucket in terms of expanding global vaccine manufacturing capacity. It was estimated that successful grantees with a licensed vaccine and functional facility could collectively produce a maximum of 675.2 million doses of pandemic vaccine in 12 months, which represented only 8.13 percent of the total global capacity in 2019.
Manufacturing Capacity: Partnership Pathways to Success
As illustrated by the GAP program, distributed vaccine production has many pathways to failure and a narrow road to success (McLean et al., 2016). It is critical to consider both the distributed manufacturing location and capacity. For instance, small-scale country manufacturing could be sufficient to meet a country’s own needs, whereas larger capacity operations in countries with small populations could allow for rapid production to support regional needs. As demonstrated by the cooperation among PATH, BARDA, and GAP, multi-agency involvement can leverage agencies’ respective strengths—for example, BARDA financed PATH’s involvement, particularly in the arena of workforce development.
Lessons learned from GAP suggest the following key predictors of success for a program aiming to expand manufacturing capacity through partnerships (see Box 4-1):
- Political will and stability, which can be bolstered by requiring all manufacturers applying to a program to send a letter of support from their ministerial agency;
- Large-scale investment from local governments;
- A skilled workforce, even if it is only experienced with basic vaccine quality assurance, quality control, and fill-finishing;
- A strong regulatory agency, with involvement of WHO/U.S. Food and Drug Administration (FDA) regulatory systems and training opportunities; and
- Available expertise for in-facility training, such as subject-matter experts, technical advisory groups (including ex-industry and regulatory expertise), or a pool of consultants.
OWS underscored the importance of providing coordination and assistance to companies about how to form partnerships to address limited manufacturing capacity. For example, BARDA assisted one company in identifying an additional manufacturing partner to increase production, and the U.S. Army Corps of Engineers oversaw construction projects to expand capacity at vaccine facilities. The Partnership for Influenza Vaccine Introduction (PIVI) further highlighted the value and necessity of long-term sustained investment in generating demand.
Various models can be implemented to apply these predictors of success to future programs, ranging from distributed models, to picking some “winners” for continued capacity (i.e., geographically distributed hubs), to manufacturer trade-offs in wealthy countries. With a fully distributed model, it is not possible to achieve the economies of scale needed to attain low-cost manufacturing. According to an analysis of scale of production and its influence on costs for influenza vaccines at WHO, manufacturing costs were much greater at a small than a large scale. Although local production might seem like the best option for achieving a sustainable supply, governments may not have considered how this strategy may increase costs. Decisions about engaging in country-based distributed manufacturing should be underpinned by national buy-in and a full understanding that vaccines may be more expensive.
Workforce Development and Technology Transfer: Barriers
In terms of driving manufacturing to capacity, human capital is often more important than monetary capital. The worldwide dearth of experts who have experience with critical technology and quality control processes presents a substantial challenge for distributed manufacturing. No global facilities or consortia exist to train personnel for full-scale vaccine R&D and manufacturing or connect stakeholders across all elements of the time line (Cawein et al., 2017). Additionally, a larger trained workforce is necessary to assist funders, such as the World Bank, in assessing and monitoring projects. Barriers to workforce development include the requirements for both sustained investment and high-level country buy-in over the long term, which is challenging in the face of changing political and organizational and administrations.
Workforce Development and Technology Transfer: Pathways to Success
GAP had a dedicated focus on workforce development, which was a key element of its success. Both Utah State University and North Carolina State University provided hands-on training to the program’s workforce, which included support in clinical development and the design of clinical
trial protocols. At BARDA-supported academic institutions, more than 250 technical staff from developing countries attended vaccine manufacturing training programs coupled with onsite technical support. BARDA also partnered with PATH to provide targeted technical support to manufacturers of influenza vaccines nearing eligibility for licensure.
The GAP experience illustrates that training over a few years is critical: manufacturers were encouraged to send 2–3 staff members to these facilities each year, which was reinforced with onsite training. Over several years, this contributed to building a “back bench.” Another valuable workforce development strategy is an annual meeting onsite as a forum for providing updates and sharing experiences. For example, SII engaged in knowledge exchange with China while building its LAIV manufacturing capacity. Similarly, assistance in addressing workforce gaps was a critical component of OWS’s success in increasing yields; DoD collaborated to expedite visa approval for key technical personnel, including technicians and engineers needed to assist with installing, testing, and certifying critical equipment from overseas. DoD personnel were also requested to serve as quality control staff at two manufacturing sites.
Regulatory Capacity: Barriers
Obtaining regulatory approval for vaccines requires national regulators to be experienced in assessing and monitoring sites for compliance with core Good Manufacturing Practice principles, especially for new sites. However, some LMICs lack sufficient capacity to provide rapid expert reviews of novel vaccines and thus rely on evaluations by agencies in other countries. This contributes to delays in licensing (and therefore access), lack of harmonized standards for ensuring product safety, efficacy, and quality, and problems with labeling and packaging. It also often leads to inconsistent local, and additional clinical trial, requirements (Sabin-Aspen Vaccine Science Policy Group, 2021).
In many LMICs, this limited or absent regulatory capacity is linked to the lack of national seasonal influenza vaccine policies. An evaluation of such policies in 194 WHO member states from 2014 to 2018 was conducted through the WHO/UNICEF Joint Reporting Form on Immunization to chart the evolution of influenza pandemic preparedness and identify challenges in sustaining equitable vaccine access (Morales et al., 2021). In 2018, only 79 percent (154 countries) reported influenza data via the form; 103 consistently had vaccination policies, and 65 consistently provided no evidence of a policy. Policies were most frequent in the WHO Regions of the Americas (89 percent of countries) and Europe (89 percent); they were less frequent in the Western Pacific (62 percent), Eastern Mediterranean (57 percent), Southeast Asia (27 percent), and Africa (11 percent). The type of
vaccine technology used was not widely known, especially in low-income countries (WHO, 2019b).
Regulatory Capacity: Partnership Pathways to Success
It can be difficult for vaccine manufacturers to navigate the regulatory pathway of establishing the superiority of their technology through appropriate efficacy markers. This is a particular challenge for geographically distributed hub models. Partnerships forged during the COVID-19 pandemic have demonstrated WHO’s crucial role. It coordinates vaccine approvals internationally through its Prequalification Program, established in 2001, and also employs its Emergency Use Listing procedure for vaccines. WHO can help enable swift authorization of products for use in countries with limited ability to conduct their own evaluations (Sabin-Aspen Vaccine Science and Policy Group, 2021).
The Africa CDC Consortium for COVID-19 Vaccine Clinical Trials, launched July 2020 by the African Union Commission, may provide an instructive example of how to regionally support testing, regulatory approval, and access to COVID-19 vaccines in alignment with geographically distributed hubs (Africa CDC, 2020). Developing the Ebola vaccine also illustrates the benefits of providing prequalification and early regulatory guidance for vaccines in LMICs. Designating the Ebola vaccine as a breakthrough therapy with priority review status triggered a fast-track mechanism that allowed FDA to provide intensive guidance at the earliest phases of clinical trials. As part of the review process, FDA coordinated with international regulatory agencies and based its approval, in part, on research conducted outside of the United States (Fritz, 2020; Sabin-Aspen Vaccine Science Policy Group, 2021).
Supply Chain Partnerships: Barriers
A substantial barrier in the vaccine supply chain is lack of coordination with end users about their health systems’ capacity to absorb vaccines and ancillary supplies during a pandemic. During the COVID-19 pandemic, input supply challenges were observed across all steps in the vaccine manufacturing process, including bioreactor bags, single-use systems, cell-culture media, filters, upstream and downstream gamma sterilization, and fill-and-finish supplies, such as vials. Such individual challenges are amplified via compounded risk, whereby the absence of any single input can disturb the entire process. This is linked to the limited data available for forecasting supplies and manufacturing needs, which requires collaboration between public and private sectors followed by clear communication of the potential
forecasted needs (IFPMA, 2021). Multinational organizations are often reluctant to rapidly adopt single-use systems due to concerns that specific supplies may not be available. Thus, strategies to approach and strengthen the connectivity of single-use systems warrant careful consideration.
Supply Chain Partnerships: Pathways to Success
An effective supply chain requires three components: (1) interchangeability (developing a “USB style”), (2) diversification to manage redundancy, and (3) speed and flexibility from regulators. The transition to mRNA vaccines and platform technologies requires highly specialized equipment and personnel. In addition to the need for sufficient available capacity (e.g., trained workforce to perform quality control), the input supply chain is critically important. This includes raw materials, consumables, and equipment across the value chain (i.e., upstream, downstream, and through to fill-and-finish). Some of these inputs are common to all types of vaccines and even to other biologic therapeutics; however, other inputs are specific to each technology platform. Certain materials, such as glass vials, are associated with significant risks in the upstream supply chain (IFPMA, 2021).
Lessons learned during the COVID-19 pandemic reveal many opportunities to reinforce supply chains. It highlighted the critical importance of developing multidisciplinary, government, and expert-led partnerships with the ability to build and commandeer lists of critical supplies, in order to avoid supply chain breakdowns, coordinate their delivery, and prioritize contracts. OWS provided federal assistance to address manufacturing supply chain challenges. This contribution effectively facilitated manufacturing COVID-19 vaccines when—due to global demand—companies were waiting 4–12 weeks for items that would have been available for shipment in about 1 week pre-pandemic. Through the program, DoD and HHS provided federal assistance to (1) expedite procurement and delivery of critical manufacturing equipment, (2) develop lists of critical supplies common across the six OWS vaccine candidates, (3) expedite delivery of necessary equipment and goods to the United States, and (4) place prioritized ratings on 18 supply contracts for vaccine companies under the Defense Production Act.
The UK Vaccine Task Force, launched in May 2020, included experts from the military and pharmaceutical industry and civil servants with expertise in preclinical and clinical development, regulatory issues, manufacturing, and project management. Close support by the UK government in building supply chains for pharmaceutical firms, and “effectively commandeering” a manufacturing facility while securing exclusive access to another, have been cited as major factors in the success of this effort (Hoen et al., 2021).
KEY FINDINGS AND CONCLUSIONS
Fostering Influenza Platform Innovation
- The current vaccine manufacturing capacity for influenza vaccines would be insufficient to vaccinate the world, even over 12 months.
- The goal in the next 3–5 years should be to progressively pursue development and assessment of new platform technologies to improve vaccine effectiveness and expand the technology options to optimize vaccine production. A universal influenza vaccine could be a game changer that could take the threat of influenza—both seasonal and pandemic—off the table. Platform innovation will require a combination of early R&D incentives, including support of Phase I–III clinical trials for platform and recombinant-based technologies.
- Encouraging and incentivizing voluntary industry partnerships will assist with developing platform technologies—and initiating their technology transfer—and should also be designed to support the partnerships needed for a universal influenza and/or next-generation vaccines. Because of the competitive nature of the vaccine enterprise, this sharing should take place under the auspices of government partnerships, with appropriate intellectual property protections.
- Several existing organizations may be able to lead large-scale R&D and clinical trials for influenza platform technologies, including large-scale global action in LMICs, if they are given expanded mandates that are matched with significant infusions of funding. These could include CEPI, BARDA, and the HERA Incubator.
- As a public–private partnership with a multilateral approach, CEPI has an existing platform with scientific expertise and networks that span the first three steps in the vaccine development process: preclinical development, clinical development, and scale-up (Yamey et al., 2020). Adding funding for the advanced development could provide transaction cost efficiency compared to launching a new mechanism, and large investments could allow CEPI to extend its expertise to Phase III trials and potentially technology transfer.
- OWS is another example of what might work for influenza vaccine platform development, both for R&D and for manufacturing scalability to have surge capacity during a pandemic. The approach employed by OWS for COVID-19 vaccines was to support a variety of technologies, recognizing that the most effective platform for a particular antigenic target in a newly emerged pathogen will remain unknown until later phases of development. OWS provided
- federal assistance to address supply chain challenges, which effectively facilitated manufacturing by reducing waiting times during a period of high demand. Federal assistance was also provided to expedite both procuring and delivering critical manufacturing equipment and delivering necessary equipment and goods to the United States. Other program attributes include its strong central management, sufficient funding for its objectives, and high level of oversight. Giving BARDA a broader remit may allow it to overcome one limitation of OWS: it did not account for global need by building out how the United States works with industry.
- It will be important to improve coordination for future influenza vaccine research at CEPI, HERA, BARDA, and NIAID; build institutional R&D capabilities beyond the United States, such as in Germany (e.g., Max Planck Institute), Japan, and South Korea; and engage regional entities for R&D mobilization.
Supporting Geographically Distributed Regional Manufacturing Hubs
- Geographically distributed manufacturing hubs are a way to provide scaled-up vaccine manufacturing in LMICs. The difficulty is in how to scale up industry partnerships and apply them globally, in support of a geographically distributed manufacturing hub model.
- Sustainable and successful geographically distributed manufacturing hubs face several challenges in the context of influenza vaccination. They require (1) government commitment and strong industry involvement, (2) strong business models, (3) the capacity to develop a product that can be licensed and exported (e.g., workforce training for technology transfer and regulatory capacity), and (4) keeping national plans updated, to guide who will get vaccines, where, and how.
- The GAP program focused on egg-based technologies; any future global partnerships for diversified manufacturing and supply chain coordination should be designed to sustain newer technologies and provide long-term demand certainties. Increasing seasonal influenza vaccine demand as a principle for expanding global manufacturing capacity will not be sufficient to generate a pandemic vaccine market on the scale required.
- Moving to geographically distributed manufacturing hubs requires significant inputs to ensure that the surrounding “ecosystem” is in place for technology transfer and workforce development for platform technologies, regulatory capacity to produce vaccines, and a business model that creates a market for products developed
- using platform technologies (with the ability to rapidly switch to pandemic production mode) during times of no disease pandemics.
- A business model for pandemic influenza vaccines requires a dual-use paradigm for manufacturing facilities to keep them functioning between pandemics. This should encourage investment in platform technologies for vaccines for both seasonal influenza and other pathogens. It should pay at least as much attention to workforce development, regulation, and supply chain production as to manufacturing scale-up.
- This downstream market issue (demand for products developed) is different from upstream technology and R&D issues and not specific to influenza. No global institutional architecture exists to handle this issue, and development finance institutions often struggle to develop business cases for developing vaccines for a future pandemic event with uncertain timing.
- During COVID-19, supply chains broke down and have often been the reason that we could not produce more vaccines faster. Geographical distribution is not just about having factories and research facilities; it is also about ensuring that these factories have key inputs that they need to produce vaccines and the flexibility to diversify their production. Supply chain commodity production requires similar attributes.
- Developing geographically distributed supply chain hubs in parallel to manufacturing facilities presents a market opportunity for countries to invest as suppliers in the bags, filters, and other items required for vaccines. Supply chains are often treated as if they are only something you need for manufacturing, but being a supplier is also a business.
REFERENCES
Africa CDC (Centres for Disease Control and Prevention). 2020. Africa CDC Consortium for COVID-19 vaccine clinical trials. https://au.int/sites/default/files/documents/39350-doc-africa_cdc_consortium_for_COVID-19_clinical_trials_-_eng.pdf (accessed October 23, 2021).
Africa CDC and African Union. 2021. Partnerships for African Vaccine Manufacturing (PAVM) Task Force communiqué: Africa projects 60% vaccines production by 2040. https://www.thisdaylive.com/index.php/2021/06/29/africa-projects-60-vaccines-production-by-2040 (accessed December 30, 2021).
BARDA (Biomedical Advanced Research and Development Authority). n.d. International influenza vaccine manufacturing capacity building program. https://www.medicalcounter-measures.gov/barda/influenza-and-emerging-infectious-diseases/international-influenza-vaccine-manufacturing-capacity-building-program/ (accessed January 7, 2022).
Bender, E. 2019. Accelerating flu protection: Can the latest techniques speed up the dangerously slow production of flu vaccines? Nature 573:S60–S61. https://www.nature.com/articles/d41586-019-02756-5?error=cookies_not_supportedpercentcode=0d3fa8fb-ff26-49a4-8f74-2114493b6b6f (accessed July 22, 2021).
Bever, L. 2021. Coronavirus vaccine technology is paving the way for a whole new approach to flu shots. The Washington Post, April 11, 2021. Washington, DC.
Bresee, J. S., K. E. Lafond, M. McCarron, E. Azziz-Baumgartner, S. Y. Chu, M. Ebama, A. R. Hinman, A. Xeuatvongsa, S. Bino, D. Richardson, R. M. Porter, A. Moen, M. McKinlay, G. Sahakyan, S. Wangchuk, P. Ruowen, Z. Yongchao, C. Linlin, C. Daouda, O. Tarkhan-Mouravi, P. Gould, P. Muthoka, G. O. Emukule, S. S. Chaves, M.-A. Widdowson, D. Otorbaeva, V. Khanthamaly, K. Stavridis, V. Mikic, N. Furtuna, D. Capmari, B. Alexander, E. Dueger, M. Kamolzoda, J. Mott, A. Bin Salah, M. Mazur, A. Maria Ropero Alvarez, S. J. Olsen, S. Mirza, C. Sofia Arriola, J. Seward, S. Kluglein, A. F. Bolster, N. Minh Hang, J. W. McFarland, N. Ha Thu, and T. Thi Minh Nguyen. 2019. The partnership for influenza vaccine introduction (PIVI): Supporting influenza vaccine program development in low- and middle-income countries through public–private partnerships. Vaccine 37(35):5089–5095.
Cawein, A., E. Emini, M. Watson, J. Dailey, J. Donnelly, D. Tresnan, T. Evans, S. Plotkin, and W. Gruber. 2017. Human capital gaps in vaccine development: An issue for global vaccine development and global health. Annals of the New York Academy of Sciences 1395(1):3–11.
Chagar, A., M. Thomas, L. Zuo, and M. Watson. 2021. The R&D response to COVID-19: What can we learn for the vaccine ecosystem? Sabin-Aspen Vaccine Science and Policy Group. https://www.sabinaspengroup.org/the-rd-response-to-covid-19-what-can-we-learn-for-the-vaccine-ecosystem (accessed October 23, 2021).
Chaudhury, D. R. 2020. India, EU working to develop new generation of influenza vaccines: Envoy. The Economic Times. https://economictimes.indiatimes.com/industry/healthcare/biotech/healthcare/india-eu-working-to-develop-new-generation-of-influenza-vaccines-envoy/articleshow/75215403.cms?from=mdr (accessed December 19, 2021).
CIDRAP (Center for Infectious Disease Research and Policy). 2020. Universal influenza vaccine technology landscape. https://www.cidrap.umn.edu/universal-influenza-vaccine-technology-landscape (accessed July 20, 2021).
Douglas, R. G., and V. B. Samant. 2018. The vaccine industry. Plotkin’s Vaccines 41–50.
Erbelding, E. J., D. J. Post, E. J. Stemmy, P. C. Roberts, A. D. Augustine, S. Ferguson, C. I. Paules, B. S. Graham, and A. S. Fauci. 2018. A universal influenza vaccine: The strategic plan for the National Institute of Allergy and Infectious Diseases. The Journal of Infectious Diseases 218(3):347–354.
European Commission. 2021. 1 billion Team Europe Initiative on manufacturing and access to vaccines, medicines and health technologies in Africa. https://ec.europa.eu/commission/presscorner/detail/en/IP_21_2594 (accessed October 23, 2021).
Francis, D. P., and G. Grohmann. 2011. WHO Influenza Vaccine Technology Transfer Initiative: Role and activities of the Technical Advisory Group. Vaccine 29S:A45–A47.
Fritz, M. 2020. Drug approval during a public health crisis. Regulatory Review. https://www.theregreview.org/2020/02/11/fritz-drug-approval-during-public-health-crisis (accessed October 23, 2021).
GAO (U.S. Government Accountability Office). 2021. Operation Warp Speed: Accelerated COVID-19 vaccine development status and efforts to address manufacturing challenges. GAO-21-319. Washington, DC: U.S. Government Accountability Office.
Greenlight Biosciences. 2021. A blueprint to vaccinate the world. https://www.greenlightbiosciences.com/wp-content/uploads/2021/03/How-to-vaccinate-the-world-Green-Light-0326.pdf (accessed October 23, 2021).
Grohmann, G., D. P. Francis, J. Sokhey, and J. Robertson. 2016. Challenges and successes for the grantees and the Technical Advisory Group of WHO’s influenza vaccine technology transfer initiative. Vaccine 34(45):5420–5424.
Harrison, R. P., S. Ruck, Q. A. Rafiq, and N. Medcalf. 2018. Decentralised manufacturing of cell and gene therapy products: Learning from other health care sectors. Biotechnology Advances 36(2):345–357.
Hayman, B., and S. Pagliusi. 2020. Emerging vaccine manufacturers are innovating for the next decade. Vaccine X 5:100066.
Hoen, E., C. Garrison, P. Boulet, K. Mara, and K. Perehudoff. 2021. Scaling up vaccine production capacity: Legal challenges and recommendations. Background paper 6. Commissioned by The Independent Panel for Pandemic Preparedness and Response. https://theindependentpanel.org/wp-content/uploads/2021/05/Background-paper-6-Scaling-up-vaccinationlegal-aspects.pdf (accessed October 24, 2021).
IFPMA (International Federation of Pharmaceutical Manufacturers & Associations). 2021. Towards vaccinating the world: Landscape of current COVID-19 supply chain and manufacturing capacity, potential challenges, initial responses, and possible “solution space”—a discussion document. https://www.ifpma.org/wp-content/uploads/2021/03/Summit_Landscape_Discussion_Document.pdf (accessed October 24, 2021).
Kavanaugh, M. M., L. O. Gostin, and M. Sunder. 2021. Sharing technology and vaccine doses to address global vaccine inequity and end the COVID-19 pandemic. JAMA 326(3):219–220.
Keown, A. 2021. Sanofi partners with Moderna to answer global demand for COVID-19 vaccines. https://www.biospace.com/article/sanofi-to-manufacture-200-million-doses-of-the-moderna-vaccine-its-third (accessed July 19, 2021).
Krammer, F., A. Garcia-Sastre, and P. Palese. 2018. Is it possible to develop a “universal” influenza virus vaccine?: Potential target antigens and critical aspects for a universal influenza vaccine. Cold Spring Harbor Perspectives in Biology 10(7):a028845.
Laguipo, A. 2021. Moderna to develop three new mRNA-based vaccines against HIV, influenza, and the Nipah virus. https://www.news-medical.net/news/20210113/Moderna-starts-to-develop-three-new-mRNA-based-vaccines-against-HIV-influenza-and-the-Nipah-virus.aspx (accessed October 23, 2021).
LaMattina, J. 2021. Beyond its COVID-19 vaccine, Pfizer’s foray into flu vaccines can also prove lucrative. Forbes, May 6, 2021. https://www.forbes.com/sites/johnlamattina/2021/05/06/beyond-its-covid-19-vaccine-pfizers-foray-into-flu-vaccines-can-also-prove-lucrative/?sh=7794eac13306 (accessed July 19, 2021).
Lurie, N., G. T. Keusch, and V. J. Dzau. 2021. Urgent lessons from COVID-19: Why the world needs a standing, coordinated system and sustainable financing for global research and development. The Lancet 397(10280):1229–1236.
McLean, K. A., S. Goldin, C. Nannei, E. Sparrow, and G. Torelli. 2016. The 2015 global production capacity of seasonal and pandemic influenza vaccine. Vaccine 34(45):5410–5413.
MIT (Massachusetts Institute of Technology) Center for Biomedical Innovation. 2017. Comparing centralized vs. distributed models of biologics manufacturing and supply for global health: A discussion of challenges and opportunities. https://cbi.mit.edu/wp-content/uploads/2020/11/Scoping-Report-Global-Biologics-Supply.pdf (accessed July 21, 2021).
Moore, K., J. Ostrowsky, A. Kraigsley, A. Mehr, and M. Osterholm. 2021. Commentary: From hope to reality: The promise of the influenza vaccines R&D roadmap. https://www.cidrap.umn.edu/news-perspective/2021/01/commentary-hope-reality-promise-influenza-vaccines-rd-roadmap (accessed June 8, 2021).
Morales, K. F., D. W. Brown, L. Dumolard, C. Steulet, A. Vilajeliu, A. M. Ropero Alvarez, A. Moen, M. Friede, and P. Lambach. 2021. Seasonal influenza vaccination policies in the 194 WHO member states: The evolution of global influenza pandemic preparedness and the challenge of sustaining equitable vaccine access. Vaccine 8:100097.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. Exploring lessons learned from a century of outbreaks: Readiness for 2030: Proceedings of a workshop. Washington, DC: The National Academies Press.
Navarro-Torné, A., F. Hanrahan, B. Kerstiëns, P. Aguar, and L. Matthiessen. 2019. Public health–driven research and innovation for next-generation influenza vaccines, European Union. Emerging Infectious Diseases 25(2):e180359.
Newland, M., D. Durham, J. Asher, J. J. Treanor, J. Seals, R. O. Donis, and R. A. Johnson. 2021. Improving pandemic preparedness through better, faster influenza vaccines. Expert Review of Vaccines 20(3):235–242.
Nugroho, A. 2020. Indonesia is set to become the hub for Chinese vaccines in Southeast Asia. How does the country benefit? https://theconversation.com/indonesia-is-set-to-become-the-hub-for-chinese-vaccines-in-southeast-asia-how-does-the-country-benefit-148534 (accessed October 24, 2021).
PAHO (Pan American Health Organization). 2021. PAHO director calls for closing “glaring” vaccine gap by expanding vaccine production in Latin America and the Caribbean. https://www.paho.org/en/news/19-5-2021-paho-director-calls-closing-glaring-vaccine-gap-expanding-vaccine-production-latin (accessed July 20, 2021).
Pinheiro Edos, S., K. Brüning, M. F. Macedo, and A. C. Siani. 2014. Production of antiretroviral drugs in middle- and low-income countries. Antiviral Therapy 19(Suppl 3):49–55.
Rappuoli, R., E. De Gregorio, G. Del Giudice, S. Phogat, S. Pecetta, M. Pizza, and E. Hanon. 2021. Vaccinology in the post−COVID-19 era. Proceedings of the National Academy of Sciences 118(3):e2020368118.
Reuters. 2018. Pfizer bets on biotech flu vaccine in $425 million BioNTech alliance. https://www.reuters.com/article/us-pfizer-biontech-alliance/pfizer-bets-on-biotech-flu-vaccine-in-425-million-biontech-alliance-idUSKBN1L10F5 (accessed July 20, 2021).
The Rockefeller Foundation. 2021. One for all: An action plan for financing global vaccination and sustainable growth. New York: The Rockefeller Foundation.
Rockman, S., K. Laurie, and I. Barr. 2020. Pandemic influenza vaccines: What did we learn from the 2009 pandemic and are we better prepared now? Vaccines 8(2):211.
Sabin-Aspen Vaccine Science and Policy Group. 2019. Accelerating the development of a universal influenza vaccine. https://www.aspeninstitute.org/wp-content/uploads/2019/07/sabin-aspen-report-digital.pdf (accessed July 20, 2021).
Sabin-Aspen Vaccine Science and Policy Group. 2021. Powering vaccine R&D: Opportunities for transformation. https://www.sabinaspengroup.org/powering-vaccine-rd-opportunities-for-transformation (accessed July 20, 2021).
Schafer, J., and Sparrow, E. 2021. Lessons learned from the Global Action Plan for Influenza Vaccines (GAP)’s technology transfer partnerships and the Biomedical Advanced Research and Development Authority (BARDA)’s International Influenza Vaccine Technology Manufacturing Capacity Program. https://www.nationalacademies.org/event/05-11-2021/lessons-learned-from-global-influenza-technology-transfer-partnerships-meeting-3-supplementary-session (accessed August 24, 2021).
Sell, T. K., D. Gastfriend, M. Watson, C. Watson, L. Richardson, L., A. Cicero, T. Inglesby, and N. Connell. 2021. Building the global vaccine manufacturing capacity needed to respond to pandemics. Vaccine 39(12):1667–1669.
Shulman, J., R. Ahsan, and K. O’Malley. 2021. Understanding global vaccine economics and research and development. Sabin-Aspen Vaccine Science and Policy Group. https://www.sabinaspengroup.org/understanding-global-vaccine-economics-and-research-and-development (accessed July 21, 2021).
Sparrow, E., J. G. Wood, C. Chadwick, A. T. Newall, S. Torvaldsen, A. Moen, and G. Torelli. 2021. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine 39(3):512–520.
Talor, A. 2021. The G20 pledge drive. https://dukeghic.org/2021/05/25/the-g20-pledge-drive (accessed July 20, 2021).
Venkatesen, P. 2017. Recombinant influenza vaccine in adults aged 50 years or older. The Lancet Respiratory Medicine 5(8):613.
Ward, B. J., P. Gobeil, A. Séguin, J. Atkins, I. Boulay, P.-Y. Charbonneau, M. Couture, M-A. D’Aoust, J. Dhaliwall, C. Finkle, K. Hager, A. Mahmood, A. Makarkov, M. P. Cheng, S. Pillet, P. Schimke, S. St-Martin, S. Trépanier, and N. Landry. 2021. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Natural Medicine 27:1071–1078.
WHO (World Health Organization). 2011. Increasing access to vaccines through technology transfer and local production. Geneva, Switzerland: World Health Organization. https://www.who.int/phi/publications/Increasing_Access_to_Vaccines_Through_Technology_Transfer.pdf (accessed October 24, 2021).
WHO. 2013. Report of the workshop on business modelling for sustainable influenza vaccine manufacturing. Geneva, Switzerland: World Health Organization. https://www.who.int/influenza_vaccines_plan/resources/BusinessModeling_report_final.pdf (accessed December 19, 2021).
WHO. 2016. Key elements of sustainability for influenza vaccine manufacturing in low- and middle-income countries. Geneva, Switzerland: World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/251695/WHO-HIS-TTi-GAP-16.3-eng.pdf?sequence=1isAllowed=y (accessed October 24, 2021).
WHO. 2019a. Global vaccine market report. Geneva, Switzerland: World Health Organization.
WHO. 2019b. Partnership Contribution (PC) Preparedness High-Level Implementation Plan II 2018–2023, Revised Version. Geneva, Switzerland: World Health Organization.
WHO. 2021. WHO supporting South African consortium to establish first COVID mRNA vaccine technology transfer hub. News Release, June 12, 2021. Geneva, Switzerland: World Health Organization. https://www.who.int/news/item/21-06-2021-WHO-supporting-South-African-consortium-to-establish-first-COVID-mRNA-vaccine-technology-transfer-hub (accessed October 24, 2021).
WHO. n.d. Global Action Plan for Influenza Vaccines: Frequently asked questions. https://www.who.int/influenza_vaccines_plan/resources/gap_faq.pdf?ua=1 (accessed December 19, 2021).
Yamey, G., M. Schäferhoff, M. Pate, M. Chawla, K. Ranson, F. Zhao, R. Hatchett, and R. Wilder. 2020. Funding the development and manufacturing of COVID-19 vaccines. Duke Global Working Paper 20. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3575660 (accessed December 17, 2021).




































