3
Critical Components for Vaccine Manufacturing
Producing large amounts of a vaccine in a short period of time entails more than developing the proper biological construct—such as a candidate vaccine virus, recombinant antigen, or mRNA—as quickly as possible. After a new vaccine is designed and shown to be safe and effective, the main challenge that manufacturers face is scaling production. A manufacturing plant may require about “9,000 different materials sourced from some 300 suppliers across approximately 30 different countries” to produce vaccines (WTO, 2020, p. 16). Manufacturers must also procure adequate supplies of more than 100 different critical components, including glass vials, filters of various types, tubing, stabilizing agents, and disposable bags (Hatchett et al., 2021).
If the supply of one of these components cannot meet the demand, then the entire production of a vaccine can grind to a halt. That is, a resilient and robust supply chain is essential, both to operate at one scale during “normal” years of seasonal influenza and to dramatically scale up during a pandemic. In addition, vaccine manufacturing at scale requires a highly trained workforce that understands the exact procedures needed for a product that requires quality standards and regulatory approval for use in humans. During a pandemic with the need to scale production rapidly, a shortage of trained workers can restrict the quantity of vaccine that is produced (Hatchett et al., 2021; Tarbet et al., 2013).
In this chapter the committee discusses the challenges of ensuring ample supplies of the critical components for vaccine manufacturing and the capacity of the global supply chain for critical vaccine components. The first section below delineates the critical components that are needed broadly
across vaccine manufacturing platforms. The next five sections consider five aspects of them: manufacturing preparedness and response, forecasting demand, production, stockpiling, and management and allocation. The final two sections consider workforce issues and distributed manufacturing networks.
Table 3-1 summarizes the recommendations in this chapter, delineated by the U.S. and global or regional actors for their implementation.
TABLE 3-1 Summary of Recommendations on Critical Components for Vaccine Manufacturing
| Global or Regional Actor | Recommendation | Domestic Actor(s) |
|---|---|---|
|
Recommendation 3-1: Global pandemic manufacturing and supply chain task force |
|
|
||
|
||
|
||
|
||
|
||
|
||
|
Recommendation 3-2: Preparedness and response capability framework |
|
|
Recommendation 3-3: Critical analytics |
|
| Global or Regional Actor | Recommendation | Domestic Actor(s) |
|---|---|---|
| Recommendation 3-4: Vaccine manufacturing workforce development and capacity |
|
|
|
Recommendation 3-5: Global network of sustainable on-demand vaccine manufacturing capacity |
|
THE CRITICAL COMPONENTS
The committee adopted the following definitions for components, inputs, analytics, equipment, and personnel for its analysis of critical components:
- Component (in the vaccine production process): Any highly specialized input (e.g., raw material or consumable), analytic (e.g., quality control test reagent or standard), equipment (e.g., bioreactor or pump), infrastructure (e.g., quality management system or facility), or workforce personnel (e.g., highly specialized personnel) required for the successful production of a releasable vaccine.
- Critical input: Any highly specialized raw material or consumable (e.g., chemical, reagent, buffer, cell culture media, adjuvant, vial, bag, tubing, or filter) whose shortage has the potential to cause a significant delay in the manufacture or release of an unadulterated filled and finished vaccine.
- Critical analytic: Any quality control material (e.g., reagent or standard) whose shortage has the potential to cause a significant delay in the manufacture or release of an unadulterated filled and finished vaccine.
- Critical equipment: Any highly specialized equipment (e.g., a single use bioreactor, nucleic acid synthesizer, protein synthesizer, separation or purification apparatus, or fill-finish equipment) whose shortage has the potential to cause a significant delay in the manufacture or release of an unadulterated filled and finished vaccine.
- Critical workforce personnel: Any highly specialized or highly trained person (e.g., process engineer, lipid chemist, quality con
trol analyst, facility engineer, or quality assurance director) whose shortage has the potential to cause a significant delay in the manufacture or release of an unadulterated filled and finished vaccine.
During the COVID-19 pandemic, many of the critical components were in short supply (Hatchett et al., 2021). For example, mRNA vaccines that were authorized for use under emergency approval required relatively large amounts of ionizable cationic lipids to create the lipid nanoparticles in which the mRNA constructs were encased for delivery to the appropriate sites in the body (Crommelin et al., 2021; Schoenmaker et al., 2021). Synthesizing these lipids entails a complex, multistep process, and only a few facilities worldwide were set up to carry out that synthesis (McCoy, 2021). Even then, the process of turning the lipids into lipid nanoparticles that encase mRNA molecules requires specialized facilities capable of Good Manufacturing Practices (promulgated by the U.S. Food and Drug Administration [FDA]) and machinery that are also limited in number (Hatchett et al., 2021).
Though manufacturers had a 10-month lead time between developing and producing a vaccine at scale, securing sufficient quantities of ionizable cationic lipids and the associated production capabilities to make billions of doses of vaccine proved to be a heroic undertaking. The Biden administration’s National Strategy for the COVID-19 Response and Pandemic Preparedness recognized this challenge specifically in its call to use the Defense Production Act (DPA) to accelerate the manufacture, delivery, and administration of the COVID-19 vaccine (White House, 2021): see Box 3-1. Given that efforts are now under way to produce mRNA vaccines for influenza (Laguipo, 2021; Pfizer, 2021), ionizable cationic lipids may likely be on the list of critical components for seasonal and pandemic influenza vaccines.
MANUFACTURING PREPAREDNESS AND RESPONSE
Supply constraints and shortages of critical components occur frequently, particularly during surge demand for vaccines. Globalization has produced an increasingly complex supply chain for biopharmaceutical manufacturers, as well as medical products generally, creating an imperative to identify the critical components needed for influenza vaccine manufacturing and track their availability. Consequently, it is important to determine where bottlenecks and challenges may occur to ensure an uninterrupted supply of vaccines.
In many ways, the COVID-19 pandemic serves as a relevant test case to highlight similar challenges for influenza (Hatchett et al., 2021). Bottlenecks
in the availability of vaccine components need to be identified and appropriately tracked to enable an uninterrupted supply of vaccines. Some challenges include problems obtaining single-use assemblies (e.g., disposable bioreactor bags and tubing, and purification and sterilization filters, cell culture media, lipids, vials), certain specialty chemicals and reagents (e.g., adjuvant components), and final fill and finish components and containers (e.g., multi-dose glass vials) to name a few. A Chatham House report highlighting the supply chain challenges for COVID-19 vaccines underscores where these bottlenecks can appear: see Figure 3-1 (Hatchett et al., 2021).
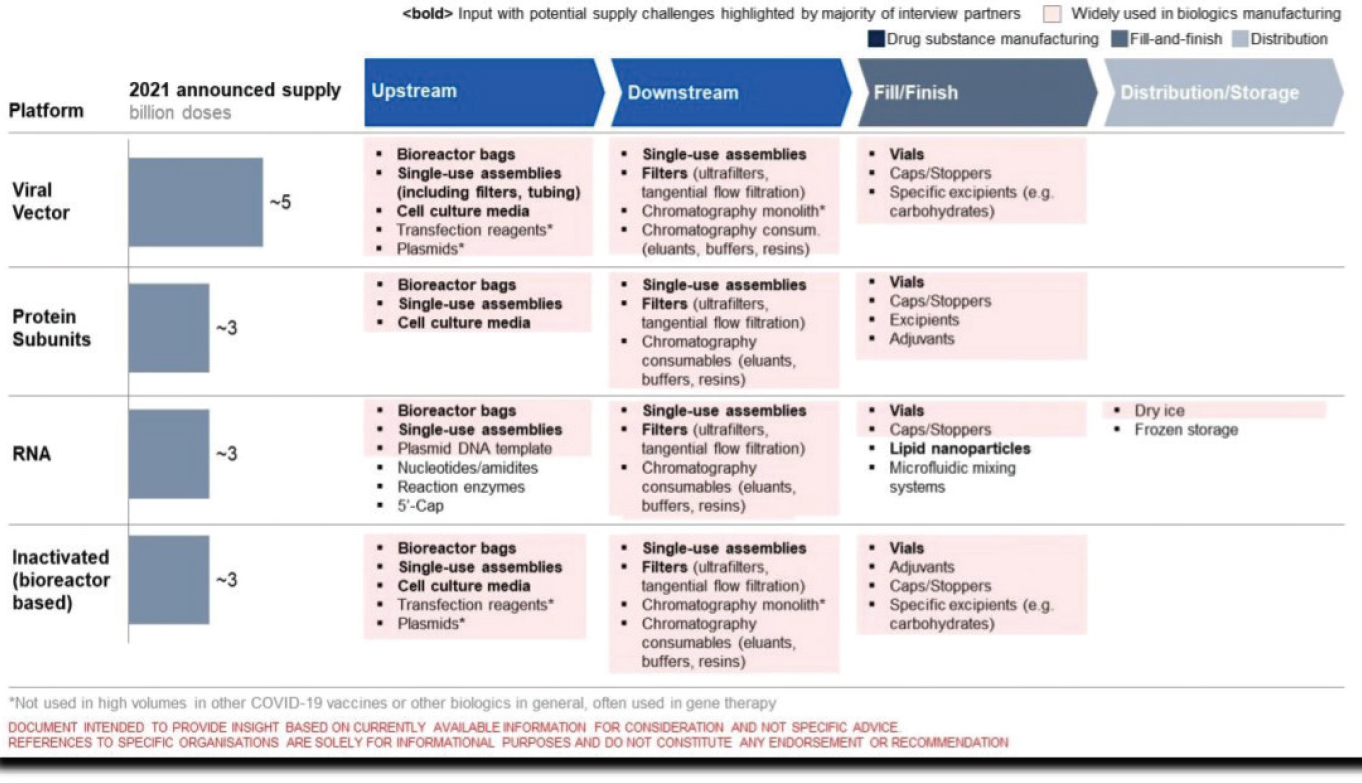
NOTES: Upstream and downstream refer to the stages in vaccine manufacturing and not the global supply chain. Document intended to provide insight based on currently available information for consideration and not specific advice. References to specific organizations are solely for informational purposes and do not constitute any endorsement or recommendation. * Not used in high volumes in other COVID-19 vaccines or other biologics in general, often used in gene therapy.
SOURCE: Hatchett et al., 2021, p. 14.
The U.S. Perspective
The U.S. government has authorized mechanisms to address supply constraints and stockouts of critical components during public health emergencies in the past. In 2006, President George W. Bush signed the Pandemic and All-Hazards Preparedness Act (P.L. 109-417), which authorizes appropriations to improve preparedness and response activities to events like pandemic influenza and bioterrorism (Lister and Gottron, 2007). As part of preparedness initiatives, the act grants the secretary of the U.S. Department of Health and Human Services (HHS) the authority to hold meetings and execute specific agreements with various potential countermeasure developers that would otherwise violate antitrust laws. DPA enables cooperation among private industries with competing interests to plan and coordinate measures to support national defense efforts through other agencies, such as the Federal Emergency Management Agency and the U.S. Department of Defense (Cecire and Peters, 2020).
During COVID-19, the United States has enacted a series of export restrictions through the DPA that require manufacturers to prioritize work with the federal government to provide materials that are necessary for national defense (White House, 2021), which included export restrictions on critical components needed to produce COVID-19 vaccines. While the United States did not restrict exports of COVID-19 vaccines, it did impose restrictions on the export of key raw materials needed to manufacture COVID-19 vaccines. There are concerns that this kind of restriction could limit the supply of these components to global manufacturers (Jain and Rocha, 2021). While the direct effects of these actions are still unknown, it is clear that export restrictions have the potential to limit global access to critical components, potentially hindering vaccine manufacturing capacity worldwide (Casey and Cimino-Isaacs, 2021).
There is an opportunity to help address some of the challenges through the antitrust authority. Ideally, the authority would be ready and available when a national or global public health emergency is declared by the HHS secretary or a global public health emergency of international concern is declared by the director general of the World Health Organization (WHO). However, the committee found a lack of institutional memory in the agencies about when and how to use this authority; this needs to be remedied. The agencies with the power to invoke antitrust authority do not present the only challenge. Some manufacturers have trepidation in participating in community discussions with competitors under voluntary agreements afforded by mechanisms such as the DPA (Title VII) and decline to participate in those discussions. Additionally, the DPA has gone through many iterations (Lawson and Rhee, 2020), which could create difficulties in understanding its authorities and implications.
According to a U.S. Government Accountability Office (GAO) report on Operation Warp Speed (GAO, 2021), vaccine supply chains were strained for numerous reasons. The global demand for critical components, workforce disruptions caused by the COVID-19 pandemic, and export restrictions implemented by some countries where critical components are produced contributed to an unprecedented strain on the supply chain. The report noted that one manufacturing facility had to wait 4 to 12 weeks to obtain supplies normally available within 1 week. GAO also found that manufacturers faced challenges in hiring and training personnel with the specialized skills needed to operate vaccine manufacturing processes (GAO, 2021).
A Global Perspective
Supply of most critical components for manufacturing influenza vaccines currently relies on an uncoordinated, decentralized global supply chain. During the COVID-19 pandemic, the Coalition for Epidemic Preparedness Innovations (CEPI) established an aggregated list of critical supplies. The list included such items as bioreactor bags, filters, tubing, and clips (see Appendix A). The CEPI list offers an opportunity to gain a broad understanding of the components that have been affected, lead times, and the landscape of critical use components and to track potential gaps in the supply chain for these components. Following the Global COVID-19 Vaccine Supply Chain & Manufacturing Summit in March 2021, COVAX1 chartered a Supply Chain and Manufacturing Task Force to address urgent shortages and expedite cross-border transit of critical components for manufacturing COVID-19 vaccines (COVAX Manufacturing Task Force, 2021). Within the task force, work stream 0 aims to “create an aligned supply baseline” and “conduct supply and manufacturing ecosystem mapping,” and work stream 1 aspires to “create partnerships to enhance visibility of [vaccine] input supplies” and “accelerate export permits [and] custom clearance for critical SKUs (stock keeping units)” (COVAX, 2021b, p. 3).
The task force has worked to identify the core critical consumables, determine where constraints exist, and assess how to mitigate bottlenecks to enable uninterrupted vaccine production across platforms. This mechanism will also allow COVAX participants to request any materials for vaccine production from the COVAX Marketplace. Initially, COVAX will focus on providing materials from six critical supply areas: bioreactor bags, single-use assemblies, cell culture media, lipids, vials, and stoppers (CEPI, 2021;
___________________
1 COVAX is the acronym for COVID-19 Vaccines Global Access; see fn. 9 and the text discussion in Chapter 2.
Kuchler, 2021). In the future, the marketplace could potentially expand to provide other critical medical supplies, including vaccines, in the event of a global shortage. Currently there is no global monitoring or oversight for these materials, such that in a flux situation when there is a surge demand for vaccines, these consumables are effectively and efficiently allocated to the key vaccine manufacturing sites in response to pandemic demands (COVAX Manufacturing Task Force, 2021).
COVAX’s Manufacturing Task Force and CEPI’s list of critical components could serve as models for similar efforts to identify potential bottlenecks in influenza vaccine supply chains for both domestic and global vaccine manufacturing capacity. COVAX has also initiated the COVAX Marketplace to facilitate access to critical supplies and alleviate some of these bottlenecks: see Box 3-2. Other organizations and governments have also created entities to address similar challenges. On September 22, 2021, the United States and the European Union announced the launch of the joint COVID-19 Manufacturing and Supply Chain Taskforce. The announcement acknowledges that global coordination for vaccine production and distribution is key to addressing the COVID-19 pandemic. It also states that the vaccine component supply chains of the United States and the European Union are interconnected, which directly affects global availability of vaccines. This task force aims to address bottlenecks in supply
chains, assess global supply and demand of vaccines and components, and collaborate to increase global production of vaccines and supplies (European Union, 2021). Both COVAX and the new U.S.-EU task force represent significant steps toward increased collaboration for vaccine manufacturing, highlighting the importance of component availability.
The few models that assess supply chain resilience generally fail to consider a “comprehensive strategy necessary for scaling up vaccine production and distribution,” instead focusing on single links in the supply chain (Golan et al., 2021, p. 2). Improving supply chain resilience depends on “mapping the layers of suppliers, manufacturing plants, distributors, and other elements of the logistics network” and identifying vulnerable points and bottlenecks (Simchi-Levi and Simchi-Levi, 2020, para. 2). Indeed, as the COVID-19 pandemic has illustrated, global pandemics can disrupt multiple links in the supply chain, again pointing to the need for a comprehensive view of the supply chain involved in vaccine manufacturing. One result of the multiple disruptions to these supply chains is that over half of the companies involved in the supply chain intend to move away from single sourcing critical components to manufacture vaccines (Remko, 2020). While national mechanisms exist to define, identify, and appropriately track the availability of critical components necessary to manufacture seasonal or pandemic influenza vaccines (albeit subject to the usual political and fiscal “tides” of funding), such a functional, ready-made mechanism does not currently exist at the global level.
COVAX developed a critical supply tracking mechanism specific to COVID-19 vaccine production needs that might serve as a model for influenza vaccines during a public health emergency. Several global entities, such as the Global Preparedness Monitoring Board, the World Trade Organization (WTO), and WHO, have either the authority or convening power to create globally coordinated mechanisms to define, identify, and appropriately track the availability of critical components necessary to manufacture influenza vaccines during a public health emergency. However, without national and global identification and tracking mechanisms for critical components—both during non-surge demand periods (to test and evaluate operations) and surge demand periods, such as regional or global influenza outbreaks—the supply chain is at high risk for crippling supply constraints or outright stockouts.
Trade barriers, such as export restrictions, are also a potential concern internationally. Both the European Union and India moved to restrict vaccine exports, which resulted in slower vaccine delivery to other regions of the world (Peters and Prabhakar, 2021). However, these restrictions were initiated after COVID-19 cases increased in these regions and domestic public health responses were needed (Kar et al., 2021). In addition to critical components, several countries initiated export restrictions for various pharmaceutical and medical supplies in response to COVID-19. The
global community has responded to the potential complications of export restrictions, with the G20 stating that these measures must be “targeted, proportionate, transparent, and temporary,” and must not “create unnecessary barriers to trade or disruption to global supply chains” (Casey and Cimino-Isaacs, 2021, p. 2).
The committee notes that there is precedence for the establishment of international entities to facilitate visibility and increase access to critical components for vaccine manufacturing. The COVAX Manufacturing Task Force and the new U.S.–EU joint task force have made strides toward streamlining procurement of critical components. While both of these examples are limited in scope, the committee believes that a global entity could be established, building on this foundation, to further increase visibility and access to critical vaccine components for broader pandemic preparedness.
RECOMMENDATION 3-1: The G20 should constitute a Global Pandemic Manufacturing and Supply Chain Task Force as a permanent structure, governed by a globally inclusive body, with technical responsibilities to ensure global pandemic influenza manufacturing and supply chain preparedness and response. The task force governance should be designed to bring together relevant U.S. and international governmental agencies; industry associations, such as the International Federation of Pharmaceutical Manufacturers & Associations and the Developing Countries Vaccine Manufacturers Network; private philanthropic organizations; and international nongovernmental organizations.
RECOMMENDATION 3-1(a): The G20 should commission an independent panel of manufacturing and supply chain experts to conduct a review of the technical capabilities and governance structure of the COVAX Manufacturing Task Force to extract lessons learned, assess its suitability for pandemic influenza, and inform the design of the structure, management, and governance of the committee’s recommended task force.
RECOMMENDATION 3-1(b): U.S. government entities, including the U.S. Department of Health and Human Services and its agencies (such as the U.S. Food and Drug Administration, the Biomedical Advanced Research and Development Authority, and the Centers for Disease Control and Prevention), the U.S. Trade Representative, the U.S. Department of Commerce, the U.S. Agency for International Development, and others should work collaboratively with the committee’s recommended task force in specific areas and, as identified in this report, take a global leadership role in activities under the task force.
FORECASTING DEMAND
Without robust forecasting, a stockout of even a single critical component can disrupt not just the unit of operations that uses that component, but also processes both upstream and downstream, which can ultimately disrupt all vaccine production in a facility. Most manufacturers have their own forecasting process to procure and stockpile critical inputs, equipment, and analytics. Therefore, manufacturers know their seasonal vaccine market and can plan accordingly, oftentimes months to years in advance. While workable for routine annual immunization needs, such a forecasting approach is not a fit-for-purpose mechanism in a regional or global influenza outbreak.
Demand for Critical Components
The demand for critical components is known as derived demand, since it is developed from the demand forecast for specific vaccines. In the past, when all influenza vaccines were made using egg-based processes, forecasting the demand for critical components was fairly straightforward and largely unchanged from year to year. Today, however, with recombinant technology used to make one approved influenza vaccine and other technologies primed to contribute to or completely change influenza vaccine production, forecasting derived demand has become challenging because production technologies require several common and several unique critical components. In fact, a key question to answer even before forecasting derived demand is how much demand there will be for each vaccine produced by a different process. Part of the answer might depend on differing attributes or target product profiles for each vaccine type. It is unclear today who would make that decision or how it would be made.
A meeting convened by Chatham House, COVAX, the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), the Biotechnology Innovation Organization (BIO), and the Developing Countries Vaccine Manufacturers Network (DCVMN) in March 2021 highlighted the challenges of forecasting the supply of critical single-use and raw materials needed for vaccine manufacturing (Hatchett et al., 2021). The resulting report, as well as testimony the committee received during several open sessions, noted the limited data available to forecast supply and manufacturing needs for manufacturing vaccines that themselves have uncertain demand. Private firms, such as Airfinity, have created proprietary forecasting models (Airfinity, 2021). Given that forecasting critical components is key to efficient and effective use of vaccine manufacturing capacity during a pandemic situation, further work by the public sector seems warranted. Without national and global forecasting mechanisms for critical
components during surge demand periods (i.e., regional or global influenza outbreaks, epidemics, or pandemics), the supply chain is left vulnerable.
The committee notes that unlike the COVID-19 pandemic, which involved creating a vaccine against an entirely new pathogen (SARS-CoV-2) using new technology (lipid nanoparticle-encased mRNA), each manufacturer of an influenza vaccine, whether for seasonal or pandemic influenza, would know in advance the supplies needed and, in most cases, have contracts in place to secure those supplies. Presumably, the initial contracted supplies and critical components would enable a vaccine manufacturer to start production while its suppliers of those components increased their production—assuming they have the capacity and workforce to do so—to meet ongoing demand of pandemic influenza. During the COVID-19 pandemic, suppliers of critical components (e.g., bioreactor bags and single-use assemblies) faced severe capacity challenges, and in response, they increased their production capacity by 50 percent in 2020 and planned another 50 percent expansion in 2021 (Kuchler and Miller, 2021).
Accurate forecasting of potentially supply-constrained critical components for vaccine manufacturing is required to ensure that the supply chain is predictable and that sufficient critical components are available where and when they are needed to safeguard the uninterrupted vaccine production supply that is forecast. Critical components necessary for vaccine production, including a skilled workforce and necessary trainers, need to be mobilized nearly instantly to address pandemic influenza in a compressed timeframe for vaccines to have a meaningful public health impact. As such, national and global mechanisms are needed to actively forecast critical component bottlenecks, inventories, and supply requirements for forecasted pandemic influenza vaccine demand, both nationally and globally. Additionally, these mechanisms need to consider the potential for hoarding and how to disincentivize it.
PRODUCTION
Given the narrow window of opportunity for influenza vaccines to alter the course of a pandemic, the timeline for scaling up and scaling out the supply of critical components for influenza vaccine production is projected to be even shorter than during the current COVID-19 pandemic (Sparrow et al., 2021). This window can be widened with the use of nonpharmaceutical interventions, such as masks and increased testing capabilities, as evidenced by decreased transmission of seasonal influenza during the worldwide COVID-19 restrictions. This compressed timeline for vaccine production will likely result in the urgent need for expanding manufacturing capacity and concomitant expanded supply needs for critical components. In addition to increasing production of supplies in
existing and expanded critical component manufacturing facilities, other facilities could be co-opted to rapidly convert to manufacturing critical supply components and vaccine. For example, these could include use of veterinary vaccine manufacturing facilities, lyophilization capacity in the food industry, and glass manufacturing for consumer products. Identification and testing of these capacities in advance of a pandemic would help to ensure their feasibility to contribute to the critical component supply chain.
The lead times and capacities to ramp up production of critical components to supply vaccine manufacturing demands vary widely. Some critical components can be manufactured fairly quickly, but for others, manufacturing timelines can be months or more (Hatchett et al., 2021). Likewise, rapidly scaling up production of some critical components can be achieved readily, whereas scaling up production for others is quite challenging absent significant at-risk capital investment. Having insight into potential supply bottlenecks and the length of time to produce and scale up production of critical components is essential for accurate supply forecasting and vaccine production planning.
Scaling up manufacturing capacity to meet surge critical inputs, analytics, and equipment supply demands, such as during an influenza pandemic, may have long lead times and require significant at-risk capital investment and on-going maintenance and readiness costs (Mirasol, 2020). Development of a novel vaccine candidate can require upwards of $1 billion and take more than a decade (Røttingen, 2016). Developing a new influenza vaccine from existing technologies is also anticipated to take approximately 10 years, although the cost may be lower (an estimated $376.72 million) (Chit et al., 2014). No current global mechanism exists to coordinate and prioritize production of potentially supply-constrained critical components, including their distribution, and the processes to facilitate their global passage. At-risk capital and on-going maintenance cost investments in critical component production prior to a surge in the supply demand, along with national and global coordination and prioritization mechanisms for critical component production during surge demand periods (i.e., regional or global influenza outbreaks, epidemics, or pandemics) are needed to enhance supply chain stability.
STOCKPILING
The committee notes that a real-time list of critical component supplies would yield an understanding of which components are most affected and the lead times needed for additional production to begin. While one approach to addressing future bottlenecks in critical components would be for manufacturers to stockpile them, there is little incentive for them to bear
the cost in doing so. One proposed approach is to augment or replace conventional strategic stockpiles by a “commons-based strategy” composed of networks of repositories, extant inventories, and participants (Handfield et al., 2020, p. 1068). Essential to the success of a “global commons”2 supply chain during a public health emergency is an effective centralized governance body with oversight of a comprehensive, distributed, cross-functional public–private sector network rather than a singular stockpile or repository. It is assumed that this strategy requires more funding on a longer-term basis. However, it has been called to question whether this system “might be any less subject to the usual political and fiscal ‘tide’ than the one it seeks to replace” (Handfield et al., 2020, p. 1086). Funding stability is a critical consideration. Currently, U.S. and global public health emergency responses are treated “as a light switch that can be flipped on and off” (Handfield et al., 2020, p. 1067), usually resulting in delayed and inadequate responses, in part due to underresourced preparedness initiatives including strategic stockpiling (Finkenstadt et al., 2020; Gerstein, 2020). However, “an entirely new model is needed to create incentives and invest in capabilities that provide a more battle-ready plan for future biological invasions’’ (Handfield et al., 2020, p. 1068), one that balances preparedness (“just in case”) with managing costs of ongoing inventory (“just in time”) and sustainability.
Because the specific composition of a well-matched influenza vaccine for use in a pandemic remains uncertain until the specific influenza strain causes an outbreak (Paules and Fauci, 2019), stockpiling pre-pandemic influenza vaccines carries a high cost and a risk of stockpiling poorly matched vaccines (Yen et al., 2015). The concept of modernizing stockpiles for vaccines can be a costly, yet an expected endeavor. Innovation and continual improvement of post-approval vaccine composition and manufacturing processes is common, and entities such as WHO have provided guidance on regulatory practices to ensure product quality (WHO, 2021c). As such, stockpiling vaccines manufactured by previous processes may raise questions about authorization for their use and how such stockpiled vaccines could be brought back into the regulatory quality system of the manufacturer during an emergency.
Currently, vaccine manufacturers, rather than the public sector, physically store public-sector vaccine stockpiles (BARDA, 2019). However, vaccine nationalism, which occurs when countries make contracts with manufacturers in advance to secure vaccines for their own populations apart from global collaboration, can threaten global vaccine access during a pandemic (Bloom et al., 2020). Public-sector vaccine stockpiles are not
___________________
2 “Global commons” refers to critical products that, during a global event such as COVID-19, need to be (1) abundant, (2) credible/usable, and (3) accessible in ways that meet the public’s needs.
useful globally if they cannot leave the country. Stockpiling certain critical components for influenza vaccine manufacturing to enable immediate surge supply, at the onset of an influenza outbreak, may provide an attractive alternative to stockpiling influenza vaccines. However, no current national or global mechanism exists to strategically stockpile critical components for influenza vaccine manufacturing. A new strategic stockpiling model is needed to create incentives and investments in capabilities that provide a more agile, fit-for-purpose, and sustainable influenza outbreak-ready plan for meeting an immediate surge demand for critical components for vaccine manufacturing. While the value of strategic stockpiles remains unquestioned in public health emergency responses, such as for pandemic influenza, establishing and sustaining fit-for-purpose strategic stockpiles of critical components for influenza vaccine manufacturing has many challenges.
MANAGEMENT AND ALLOCATION
Uncoordinated and unpredictable management and allocation of critical components, particularly during an influenza pandemic, can lead to bottlenecks and stockouts (Sparrow et al., 2021) and to hoarding and inefficient distribution of inventory (Khamsi, 2020). In the event that a limited supply of critical components exists, allocation of those materials will be crucial for manufacturing vaccines in a timely manner and thus for saving lives. A potential issue is that suppliers may need to balance domestic and global demands, especially if the countries in which those suppliers are based impose export restrictions. In addition, the allocation processes will vary for pre-competitive (e.g., commoditized) versus competitive (e.g., proprietary or patent-protected) critical components. A report by Chatham House noted,
Any specific initiative that would involve sharing of information or collaboration between industry players would require careful antitrust review before potentially being implemented. That analysis would be specific to the situation and would need to be considered on a case-by-case basis. (Hatchett et al., 2021, p. 22)
Critical components determined as pre- or non-competitive could be allocated through a predetermined governance mechanism that includes lawful private-sector collaborations, whereas “expeditious guidance” or more highly regulated governance mechanisms would be needed for critical components subject to compliance with antitrust laws.
Bilateral contractual obligations for critical components have elements that affect global allocation and coordination. These obligations may include timing elements that indicate the shipping and receipt of products,
which drives the strategy in how shipments are provided. Some manufacturers are challenged in meeting these obligations, making it difficult to prioritize domestic and global needs. Specifically, despite the fact that India is the home of the Serum Institute, one of the largest vaccine producers in the world, the country was notably behind in its ability to produce sufficient COVID-19 vaccine for other countries, through COVAX, as a key supplier. Due to a surge in COVID-19 cases in India in the spring of 2021 and shortages of critical inputs, the Serum Institute could not meet its initial contractual obligations with COVAX to supply vaccine for other countries (Cohen, 2021; Menon, 2021). This situation is not limited to one manufacturer or one country: it potentially threatens production for many vaccine suppliers. Scarce supplies of critical inputs and a surge in cases of pandemic influenza have the potential to cause delays in vaccine production. The global system for supply of inputs, along with regulatory issues, political concerns, and vaccine nationalism create the potential for more bottlenecks in the production and delivery of vaccines (Clift, 2021).
Global public–private mechanisms to manage the ownership, financing, prioritization, and allocation of critical components will be required during an influenza pandemic to avoid bottlenecks and prevent stockouts of critical components that would disrupt influenza vaccine manufacturing. Pooled procurement, or coordinated buying through the public sector, could also help to secure supplies of critical components. Assuming uncertainty partially affects capacity for critical components, this approach could mitigate risks and increase incentives for the producers of critical components to manufacture with more certainty while avoiding unnecessary and disruptive competition and allowing more strategic redirection of critical components to meet global and local public health needs. IFPMA notes that the efficiency and availability of existing capacity is related to supply sustainability and that demand is dependent on such factors as vaccine “roll-out and absorptive capacity for vaccination programs,” acknowledging the connection between low capacity and uncertainty (Hatchett et al., 2021, p. 9). Commitment from global leaders to stewardship of global commons, combined with consistent, long-term public funding insulated from political influences, are key components in effective management of the supply of critical inputs. No current global mechanism exists to own or finance or allocate, including prioritization and distribution, the critical components for influenza vaccine manufacturing. However, the Global Fund’s model of pooled procurement for infectious diseases, including malaria and HIV (The Global Fund, 2021), could serve as an example of a framework that could be applied to critical components of influenza vaccines (Huff-Rousselle, 2012).
Some global commons, classified as in the pre-competitive space, could be managed through public–private partnerships without a declared public
health emergency. Other global commons that depend on a declared public health emergency, classified in the competitive space, could be managed through public–private partnerships if mechanisms exist for antitrust safe havens. National or global mechanisms for active management (ownership, financing, and allocation) and critical component supply during surge demand periods (i.e., regional or global influenza outbreaks, epidemics, or pandemics) are important to maintaining a robust supply chain.
RECOMMENDATION 3-2: The Office of the Secretary of the U.S. Department of Health and Human Services (HHS) and its technical agencies (including the Office of Global Affairs, the Assistant Secretary for Preparedness and Response, and the Biomedical Advanced Research and Development Authority), in collaboration with appropriate global technical counterparts, should provide technical and resourcing support to the committee’s recommended task force (see Recommendation 3-1) to develop a comprehensive pandemic preparedness and response capability framework that comprises three elements:
- End-to-end visibility of critical inputs: in collaboration with the World Trade Organization, the Coalition for Epidemic Preparedness Innovations, the Developing Countries Vaccine Manufacturers Network, and the International Federation of Pharmaceutical Manufacturers & Associations, evaluate a means to define, identify, and track (e.g., through barcodes and block-chain technologies) the global real-time availability of potentially supply-constrained critical inputs necessary to manufacture vaccines for pandemic influenza, known as the essential global commons list for pandemic influenza vaccine manufacturing.
- Resiliency assessment and analysis: in collaboration with other U.S. agencies (including the Office of Science and Technology Policy, the U.S. Trade Representative, and the U.S. Agency for International Development) provide technical and resourcing support for the committee’s recommended task force to forecast supply and demand of critical inputs, including workforce personnel and training needs for pandemic influenza vaccine manufacturing, and perform a resiliency assessment of the current end-to-end network to identify vulnerabilities in physical inputs, as well workforce gaps, that may impede pandemic influenza vaccine manufacturing.
- Preparedness, response, and global coordination: working with the U.S. Department of State, coordinate efforts both within HHS and across other U.S. government entities to provide technical and resourcing support to the committee’s recommended
task force to develop technical capabilities to ensure sourcing, production, distribution, risk management, and coordination of critical components necessary for manufacturing seasonal and pandemic influenza vaccines, including capabilities to ensure globally effective preparedness and response.
Critical Assays and Analytics
Vaccine potency is a critical test to ensuring that once manufactured, the appropriate dose is administered. Manufacturing and assessing influenza vaccines are often challenged by the constant mutation of viruses, making it difficult to assess a vaccine’s efficacy and to produce well-validated and reliable potency assays (Minor, 2015). In the event of a pandemic, it is crucial to share these assays to quickly begin the scale up and production of vaccines. As outlined in Chapter 2, the WHO’s Global Influenza Surveillance and Response System (GISRS) plays an integral role in virus surveillance and serves as a model platform for global collaboration and timely sharing of viruses, reagents, and other information to anticipate and respond to threats such as pandemic influenza (Hay and McCauley, 2018).
Analytics are important to assessing the impact of vaccines on public health (WHO, 2021a). This has been especially relevant during the COVID-19 pandemic to assess the effectiveness of vaccines against the circulating variants. A global network does exist to produce some critical analytics; however, it has inadequate resources to meet the timely and large supply needs for standards and other critical analytics during surge demand for vaccines (Sorescu et al., 2021). The Agility Project is an example of an existing mechanism. The project, although not a fully integrated network, was initiated by CEPI and consists of a partnership with Public Health England, the National Institute for Biological Standards and Control in the United Kingdom, and the Global Initiative on Sharing All Influenza Data Initiative. The project’s goal is to assess emerging variants of COVID-19 in order to inform vaccine strain changes and maintain vaccine effectiveness (Kumar et al., 2021). Similarly, the WHO Global Influenza Program, which houses the GISRS network, produces reference standards and critical analytic reagents annually for seasonal influenza (Hay and McCauley, 2018; WHO, 2017). However, GISRS may have inadequate resources to meet the timely and large supply needs for reference standards and other critical analytical reagents during surge demands to address influenza pandemics.
As part of Regulatory Preparedness for Human Pandemic Influenza Vaccines, published in 2007, WHO noted the inherent variability in assay systems and cautioned against comparing results from different studies. To that point, one study analyzed serological Zika-positive samples from different laboratories around the globe and found a more than 100-fold
difference in neutralization titers for some blood samples (Mattiuzzo et al., 2019). WHO (2007) further warned that in the absence of internationally validated and harmonized assays, inconsistent data should be cautiously interpreted. The ramifications of inadequate harmonization of critical assays are particularly pronounced during global infectious disease outbreaks. During outbreaks of emerging viruses, noted Mattiuzzo and colleagues (2019), “speed and accuracy in detection of infection are critical factors to control the spread of the disease” (p. 1). The COVID-19 pandemic is a recent example where WHO (2021e) suggested starting with international standards, which would allow comparability of results from different assays that could facilitate and harmonize evaluation of diagnostics, vaccines, therapeutics, and other products. Box 3-3 provides a description of the public health value of regulatory harmonization and convergence.
RECOMMENDATION 3-3: The U.S. Food and Drug Administration and the regulatory arm of the World Health Organization (WHO) should evaluate the development of fast turnaround batch release (including potency and stability-indicating) assays for seasonal and pandemic influenza vaccine manufacturing and ready global access to international reference standards and benchmark comparators (e.g., immunological reagents) for use in product analytics and clinical trials. The WHO Collaborating Centres for Influenza should facilitate the
development of internationally harmonized and prioritized assays acceptable to regulatory bodies. A long-term goal should be set to achieve global regulatory harmonization and convergence of the analytical standards and assays, in partnership with the International Coalition of Medicines Regulatory Authorities.
TRAINING AND READINESS OF CRITICAL WORKFORCE PERSONNEL
Forecasting the needs for the existing workforce, including its size, composition, readiness, and availability, as well as the need for additional trained, highly specialized, experienced workers, presents an additional bottleneck in meeting the surge needs in manufacturing capacity and vaccine demand during an outbreak. While some of the existing or reserve workforce may be redeployed to fill gaps in staffing needs at expanded manufacturing facilities, an accurate forecast of workforce demand and supply is needed to make prudent investments in training to help ensure that a sufficient surge workforce is available in real-time during an outbreak. Training programs may be more effective during an outbreak if informed by an accurate workforce composition forecast (WHO, 2012). Coordination between training programs to comprehensively meet a surge demand is key.
A highly skilled workforce—fit-for-purpose, fully trained, scalable, and ready for surge demand—is a critical component for influenza vaccine manufacturing, particularly in response to a pandemic, yet no global plans or mechanisms exist to ensure availability or readiness of on-demand critical workforce personnel. Access to an available fully trained, highly skilled workforce reserve supplemented by on-demand workforce training is critical for responding to a pandemic. Currently, the state of training for the manufacturing workforce varies. There is no ready, on-demand, “reserve corps” of personnel for deployment during a surge demand for influenza vaccine manufacturing nationally or globally. The COVAX Manufacturing Task Force advocates for the need for free movement of critical components for vaccine manufacturing, which includes a skilled workforce (COVAX Manufacturing Task Force, 2021).
In addition, to prepare for the readiness and availability of highly skilled, experienced, critical workforce personnel and effective trainers requires long lead times. Therefore, a skilled workforce can be considered a critical component for vaccine manufacturing. Forecasting the needs for critical workforce personnel and training is essential for both seasonal and pandemic influenza vaccine manufacturing. Both require significant investments of time and money. There are training packages that are available for manufacturers and their workforce. Some include off-the-shelf Good
Manufacturing Practices (as promulgated by FDA) training and microbiology containment training (ECA, 2021). Others, such as the Novartis vaccinology course, provide in-person training and hands-on experience within functioning manufacturing facilities (Podda, 2010).
Training is not the only challenge, however. Moving a trained workforce quickly to where they are needed can also prove to be difficult, especially during a pandemic or public health emergency. As such, WTO and IFPMA acknowledge the need to facilitate movement of skilled manufacturing workforce personnel, in addition to the supplies needed for manufacturing (Bigger, 2021). A reserve workforce would allow specific people to “parachute in” to support manufacturing on the floor of a facility to bolster vaccine production. For example, those trained in chemical engineering, downstream processing, or quality control and assurance could travel without visa controls relatively quickly.
The lack of a highly trained workforce knowledgeable in all complex manufacturing processes of vaccine manufacturing, as well as enabling functions, such as quality control and assurance for mRNA vaccines developed for COVID-19, has been one of the biggest challenges in decentralizing manufacturing across the globe. For example, lipid and microfluidics experts are critical for efficacious mRNA vaccines and are needed to assist with the manufacturing process (Hatchett et al., 2021). Biologics manufacturing is an art as well as a science. Absent the time to develop comprehensive and highly robust critical quality attributes, critical process parameters, and well-defined process space, technology transfer is challenging and risky. It is extremely difficult to transfer processes without expertise at the recipient facility. Training staff who have the requisite know-how and experience are needed as “boots in factory,” so that the process can be observed, absorbed, and performed repetitively by those who must learn the art of production. Consistent with the training process for many skilled and technical occupations, it is essential for new trainees to work alongside experienced staff for some period to allow for observation, learning in real-life situations, and the ability to share the valuable knowledge that is gained only through experience.
A library of training materials and on-demand trainers are needed to enable manufacturers of vaccines and vaccine-critical components to select specific and relevant training for their workforce for scaling up and scaling out influenza vaccine manufacturing and its critical components. A standing “reserve corps” of highly trained and experienced technology transfer chemistry, manufacturing, controls, and quality control and assurance experts that can be deployed immediately are also needed to enable vaccine manufacturers to scale up and scale out influenza vaccine manufacturing during a response to pandemic influenza. Such an on-demand reserve corps could serve as an immediate workforce when and where needed.
Workforce Preparedness Drills
Tabletop exercises and live-action “war games” with associated before-action and after-reviews have proven to be effective preparedness drills for emergency responses, including public health emergencies (Ghiga et al., 2021; Pegg, 2020; UNSIC, 2008). The committee found that no current national or global mechanism exists to train or have ready the critical workforce personnel that would be needed to effectively meet the needs for vaccine manufacturing in response to an influenza pandemic.
The committee believes that establishing a public-sector funded pandemic preparedness drill could create a ready manufacturing workforce. The drill would begin when annual influenza strains are announced. The drill could be initiated with a tabletop exercise and a before-action review of the live action “war game” exercise. The goals of the live action drill would be: completion of Phase 1 or 2 clinical trials within 100 days by the originator manufacturer; and, in parallel, initiation of a technology transfer to one or more low- and middle-income countries (LMICs) partners by the originator manufacturer, with the first lot manufactured by these partner(s) in 100 days. An after-action review could be conducted as the final step in the drill. The pandemic preparedness drill would be repeated annually and include activation of a global reserve corps to assist in both goals, which includes training during technology transfer. This workforce could be a reserve corps from the Biomedical Advanced Research and Development Authority (BARDA); they would be required to participate in training every year and serve as boots on the ground with an LMIC manufacturer. To ensure manufacturing readiness for an influenza pandemic response, regular, recurrent preparedness drills (e.g., annual live-action exercises using off-the-shelf available resources and protocols, and tabletop exercises) would need to be conducted to refresh and assess scale-up and scale-out of influenza vaccine manufacturing.
Vaccine Manufacturing and Development Technology Hubs
There is currently no long-term training program ensuring that countries are ready to receive the technology needed to create sustainable vaccine manufacturing capacity at the country or regional levels. A technology hub is a collaborative space where ideas and product development come together to build concepts into realities (Youtie and Shapira, 2008). These innovative environments have multiple purposes and are used in a variety of industry sectors. The state of Michigan, through the Michigan Economic Development Corporation, established 21 technology hubs where technology-based firms, entrepreneurs, and researchers worked together to accelerate product development and form unique partnerships to promote
innovation, new ideas, and research and development across various industries (Ann Arbor Spark, 2021). Public–private partnerships have been anchored in institutions of higher education and formed the catalysts for the technology hubs, which not only attracted but also retained talented professionals.
In 2007, WHO applied the hub concept to boost technology knowledge transfer on vaccine manufacturing in LMICs (Friede et al., 2011). The Netherlands Vaccine Institute (NVI), a governmental vaccine manufacturer, was the facilitator of the 5-year initiative, which expanded production capacity of influenza vaccines in 11 countries while also organizing training (the hub) for the country’s scientists and regulators to understand the new technologies. This initiative, a component of the WHO Global Action Plan (GAP) program, has seen successes, with grantees stating that the program was integral to project implementation, and was projected to continue producing vaccines. As a result of this initiative, approved influenza vaccines were produced in four LMICs, and five other LMICs developed clinical lots. With only $50 million in investment by WHO overall, this initiative resulted in significant and sustained increases in manufacturing capacity (Grohmann et al., 2016). WHO is seeking to replicate the success of its 2007 capacity-building technology transfer hubs to provide training to manufacturers in LMICs, prioritizing mRNA-vaccine technology. Learning from the 2007 initiative, a follow-on program would confront intellectual property barriers by enabling access to patents, technical know-how, materials, and data to maximize the effectiveness of the technology transfer hubs (Hoen et al., 2021). Table 3-2 lists additional educational structures that have elements of a technology hub structure for innovation in vaccine manufacturing.
Drawing inspiration from WHO and others, the committee explored the value of establishing regional technology hubs for experiential learning through a total immersion certification program lasting 1–2 years with a train-the-trainer option. The technology hubs would not function as research entities. Instead, they would build capacity for manufacturing, clinical trials (if indicated), quality assurance, and regulatory aspects of vaccine manufacturing. As envisioned, the educational experience would bring trainees through the entire manufacturing supply chain to learn while making a vaccine, similar to the International Influenza Vaccine Manufacturing Capacity Building Program set up by BARDA in conjunction with PATH and WHO’s GAP (BARDA, 2019). In that program, operational from 2006 to 2019, BARDA used a multisite training model to build manufacturing capacity for more than 250 technical staff from 13 developing countries. Training took place in the United States and at local country facilities, as well as through a center of excellence at the NVI (Grohmann et al., 2016). A majority of these countries are still producing WHO-prequalified vaccines (WHO, 2021b).
TABLE 3-2 Examples of Vaccine Manufacturing Educational Structures
| Program | Description | Partnership |
|---|---|---|
| Texas A&M University | University-industry collaboration for workforce training to mass-produce COVID-19 vaccine candidates (Reilly, 2021) | Texas A&M University system collaborates with FUJIFILM Diosynth Biotechnologies Texas |
| Illinois Supply Chain Management Training Program | A certificate program on essential supply chain management skills to mee local workforce needs (Cision, 2021) | Illinois Department of t Commerce and Economic Opportunity partners with the Association for Supply Chain Management |
| East Africa Vaccine Supply Chain Center of Excellence | A Rwanda-based program that trains workforce personnel in managing and developing innovations for vaccine supply chains (Gavi, 2021) | Gavi, The Vaccine Alliance; United Parcel Service; the International Federation of Pharmaceutical Wholesalers; and LOGIVAC Center partner with the University of Rwanda |
| Developing Countries Vaccine Manufacturers Network (DCVMN) | An alliance of vaccine manufacturers from developing countries that engage in vaccine research, development, manufacturing, and supply of accessibl and quality vaccines (DCVMN, 2021) | As of 2020, DCVMN partnered with 41 developing country manufacturers from e 14 countries and territories |
| Novartis Vaccines Institute for Global Health | A 2-year program for a master’s degree in vaccinology that trains students on various topics, including vaccine development, immunology, infectious diseases, clinical research methodology, epidemiology, biostatistics, clinical data management, regulatory issues, and manufacturing (Podda, 2010) | The program is a partnership between the University of Siena Medical School and Novartis Pharmaceuticals |
| International Influenza Vaccine Manufacturing Capacity Building Program | The program is a partnership that enhances sustainable influenza vaccine production capacity in under-resourced countries by expanding global vaccine manufacturing capacity, ensuring a skilled workforce that knows how to make current Good Manufacturing Practice quality vaccine, and providing in-country technical implementation assistance (Schafer, 2014) | A partnership among the Biomedical Advanced Research and Development Authority, the Global Action Plan of the World Health Organization, and PATH (BARDA, 2019) |
Creating such an entity would require funding, which the committee believes could come from public–private partnerships. Industry support could be financial or in kind. For example, innovative biotechnology companies could donate equipment for training, which would benefit the donor by having a trained workforce to use the equipment. Other
advantages to regional, long-term training include building relationships with other trainees and instructors; ensuring a well-trained, regional workforce ready to address the needed surge capacity during a pandemic; and providing a career progression for retaining staff. Retaining staff can be a challenge, but employer investment in employee’s career development and providing opportunities for learning can be a strong incentive to stay with an organization (Hoss, 2021; Lefkowitz, 2018). For vaccine manufacturing, experience with a cross-sector technology hub could prove to be such an incentive. While considering benefits for employees, it is also important to consider the effect of “brain drain” when workers from LMICs move to high-income countries, resulting in a lack of trained professionals in LMICs and weakened health systems. This is a complex issue with multifaceted causes, and mechanisms would need to be considered to address this effect (Cometto et al., 2013). While the provision of a framework for how the regional hubs would provide structure for training, the program would not be overly prescriptive to allow for regional adaptations of targeted training appropriate for a given region. There is a need for long-term experiential education and training programs ensuring countries are ready to receive technology transfer in advance of a public health crisis.
RECOMMENDATION 3-4: Improving vaccine manufacturing workforce development and capacity should be prioritized by relevant global stakeholders:
- Government agencies, commercial entities, nongovernmental organizations, and academic institutions with the requisite knowledge and skill sets should partner with advanced and developing vaccine manufacturers to develop vaccine manufacturing and development technology hubs.
- The U.S. Department of Health and Human Services and its technical agencies, including the Office of Global Affairs, with nongovernmental partners, such as PATH, should develop and implement a medical countermeasure “university” for training a vaccine manufacturing and delivery critical workforce.
DISTRIBUTED MANUFACTURING NETWORKS
Ensuring equitable access to influenza vaccines for all, especially during a pandemic, requires a well-coordinated globally distributed sustainable vaccine manufacturing network. The design of such a network requires balancing speed, efficiency, flexibility, and responsiveness to regional needs to ensure equitable access. Current influenza production facilities are largely concentrated in a few high-income regions (see Table 2-4 in Chapter 2).
The capability of LMICs to produce influenza vaccines within their regions is critical to reduce the global threat of pandemic influenza, provide international stability and security, and increase vaccine access (Bresee, 2019; WHO, 2016). However, as discussed in Chapter 2, the current distribution of influenza vaccine manufacturing facilities is skewed toward particular countries and regions. Of the 40 facilities globally, half are in the Western Pacific, less than one-quarter are in the European region, and none is in the African region (Sparrow et al., 2021).
COVID-19 has demonstrated the importance of expanded worldwide capacity for vaccine production to meet demand. The pandemic created the need to rapidly develop one or more candidate vaccines that could be produced and distributed under a wide variety of conditions worldwide. Vaccinating billions of people against COVID-19 has been a driving force behind programs to bring the pandemic under control. This has been a challenge for the existing global vaccine manufacturing capacity and has presented a far greater challenge for areas of the world lacking such capabilities.
The concentration of vaccine manufacturing capacity in a few regions and countries presents barriers to equitable access that extend beyond logistics concerns. Vaccine nationalism, in which one country or group of countries prioritizes its own interests, has limited vaccine access for many countries without such capacity. It has also caused many countries to enter bilateral agreements that often work against the longer-term interests of the country or region. As noted above, manufacturing vaccines is a complex, multistep process from the production of individual components to the fill-and-finish process prior to distribution. It is common for a vaccine to have a multinational pedigree when factoring in the components and the locations in which each step in the process takes place. Examples can readily be found where a vaccine packaged in Europe contains elements from multiple European countries, as well as from Asia and the Americas (Bown and Bollyky, 2021). Building manufacturing capacity in regions with low capacity relative to their population does not require establishing full capacity to manufacture vaccines from start to finish. A country with the capacity to participate at scale in creating components or carrying out some critical step in the vaccine-making process allows for full participation with other countries as a partner and stakeholder in vaccine production. This partnership may, in turn, benefit other countries with which each country shares regional, political, or economic ties; countries with at least one critical component would have some degree of market power and allow for mutually beneficial partnerships.
Global and even regional networking and sharing of resources for vaccine manufacturing present significant political and logistical challenges, especially in a pandemic. The ability to switch as quickly as possible from seasonal to pandemic influenza vaccine manufacturing is critical at the on-
set of an influenza pandemic (Rockman et al., 2020; Sparrow et al., 2021), assuming that the manufacturing platform will be the same. Before that switch can be implemented, multiple entities need to be engaged in identifying and getting a seed influenza virus to local manufacturers. Even this initial step can be fraught with political and logistical challenges. As noted below, global manufacturing networks that rely on the complex global supply chain are even more challenging to manage during a public health emergency of international concern—a less than ideal situation during an influenza pandemic.
COVAX launched the Manufacturing and Supply Chain Task Force to urgently address shortages and expedite cross-border transit of critical components for manufacturing COVID-19 vaccines and to facilitate global and regional vaccine manufacturing networks (COVAX, 2021b). In South Korea, SK Bioscience rapidly pivoted and used seasonal influenza vaccine facilities for COVID-19 vaccine manufacturing (Kim, 2021), while other manufacturing efforts that relied on a global network struggled. This demonstrates how flexibility within manufacturing facilities can be leveraged to respond to immediate threats. Global and regional shipping was logistically challenging and caused vaccine manufacturing delays. The rate at which COVID-19 vaccine manufacturing escalated made a very complex supply chain even more challenging (Bown and Bollyky, 2021).
Leveraging ongoing lessons learned from the COVID-19 vaccine response, COVAX’s Manufacturing and Supply Chain Task Force explores how LMICs can become a part of a regional manufacturing network or hub. Specifically, COVAX’s work stream 3 aims to improve vaccine manufacturing in LMICs by also providing support for various technology transfer and training centers. These centers provide innovation assessment, as well as the initial startup drug substance or drug product for formulation and filling (COVAX, 2021a). The centers also support regional manufacturing and distribution centers.
Countries and regions could also take up different stages of the vaccine manufacturing process. One such example can be seen with Johnson & Johnson’s COVID-19 vaccine. The company sent drug substance and product to South Africa for formulation and filling at Aspen Pharmacare Holdings Ltd., South Africa’s biggest drug maker. The vaccines will then be distributed throughout Africa (Cele, 2021; Jerving, 2021). This is one example of expanding and strengthening regional capacity for processing and supplying vaccines. This effort helps build regional capacity, particularly in parts of the world that are underserved or with a low number of facilities. There are certain countries that have a key aspiration to be able to establish vaccine manufacturing, particularly in the context of COVID-19.
Creating an enabling political environment and a supportive business ecosystem for local or regional vaccine production is an essential role for
LMIC governments (Ncube, 2021). Governments should be educated on the importance of supporting regional vaccine manufacturing and view those efforts as a public health and national security issue rather than a favorable business proposition. Political will is needed not only to create, but also to sustain regional vaccine manufacturing: that is, creating an understanding that paying more for regional vaccine is a national security and public health investment and that regional production should not be seen as a cost-saving measure. Similarly, global and regional pooled procurement organizations need to use their purchasing power to develop and support such globally distributed influenza vaccine manufacturing networks. Multinational and nongovernmental organization support is also a critical element to create and sustain frameworks for pooled procurement and a robust globally distributed vaccine manufacturing capacity. There are currently no coordinated global or regional mechanisms to establish and sustain an effective global and/or regional network for manufacturing influenza vaccines that ensures equitable vaccine access globally or regionally during an influenza pandemic. As discussed above, countries with robust seasonal influenza vaccine programs are better prepared to respond to a pandemic with their established capacity and greater familiarity with vaccines. Without global and regional vaccine manufacturing networks or robust local and regional vaccine production established and sustained to reduce the global threat of pandemic influenza, global supply and equitable access to pandemic influenza vaccines will remain uncertain and at highest risk. Vaccine production comprises drug substance, formulation, and fill- and-finish operations. There are many different platform technologies (see Chapter 2) with varying economies of scale following widespread adoption of the vaccine into national programs: see Figure 3-2.
There are various options for filling operations, including sterile bags, single- or multi-dose vials of varying sizes, and blow/fill/seal technology, each with a different cost structure and implications for storage, distribution, administration, and wastage. Production facilities could be integrated across all three stages or not integrated across different manufacturers for each stage and across geographies. Such choices have implications for overall coordination and management of the manufacturing supply network, resiliency, and total costs. While a larger number of vaccine production facilities are in higher-income countries, developing-country vaccine manufacturers supply over half of the vaccines used in developing countries (Munira et al., 2019). Firms take numerous factors into account when determining choices about integrated versus non-integrated manufacturing and location of facilities across the globe. These factors include existing process capabilities in vaccine manufacturing, including access to talent and other inputs including technology transfer, policy barriers, government incentives, size of local demand, and the need for resiliency.
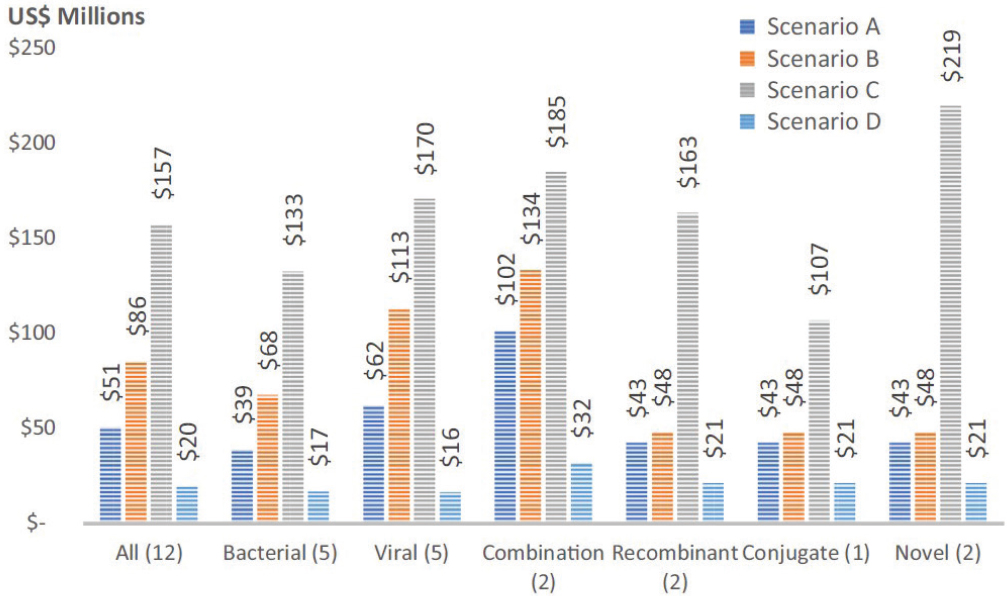
NOTE: Scenario A: 20 million annual doses—1 vaccine; Scenario B: 20 million annual doses—5 vaccines; Scenario C: 100 million annual doses—5 vaccines; Scenario D: 100 million annual doses—1 vaccine.
SOURCE: Munira et al., 2019, p. 1247.
RECOMMENDATION 3-5: The Office of Global Affairs, in coordination with other U.S. interagency stakeholders and working closely with global agencies, such as the World Health Organization, should provide technical and resourcing support to the committee’s recommended task force to evaluate the feasibility, structure, and sustainability of a globally distributed network of regional and local vaccine manufacturing capacity.
REFERENCES
Airfinity. 2021. Predictions of Phase 3 trial readouts.https://www.airfinity.com/predictions (accessed August 3, 2021).
Ann Arbor Spark. 2021. Michigan smartzones.https://annarborusa.org/entrepreneurialservices/michigan-smartzones (accessed August 17, 2021).
BARDA (Biomedical Advanced Research and Development Authority). 2019. International influenza vaccine manufacturing capacity building program. Washington, DC: U.S. Department of Health and Human Services.
Bigger, L. 2021. Session 2: Mapping vaccine manufacturing and trade. Paper read at COVID-19 Vaccine Supply Chain and Regulatory Transparency Technical Symposium, June 29. Webcast. World Trade Organization. https://www.wto.org/english/tratop_e/trips_e/technical_symposium_2906_e.htm (accessed October 12, 2021).
Bloom, D. E., D. Cadarette, and D. L. Tortorice. 2020. An ounce of prevention.https://www.imf.org/external/pubs/ft/fandd/2020/09/vaccine-finance-epidemics-and-preventionbloom.htm (accessed August 17, 2021).
Bollyky, T. J., and C. P. Bown. 2020. The tragedy of vaccine nationalism: Only cooperation can end the pandemic. Foreign Affairs 99:96.
Bown, C. P., and T. J. Bollyky. 2021. How COVID-19 vaccine supply chains emerged in the midst of a pandemic. Peterson Institute for International Economics. https://www.piie.com/publications/working-papers/how-covid-19-vaccine-supply-chains-emerged-midstpandemic (accessed October 12, 2021).
Bresee, J. S., K. E. Lafond, M. McCarron, E. Azziz-Baumgartner, S. Y. Chu, M. Ebama, A. R. Hinman, A. Xeuatvongsa, S. Bino, D. Richardson, R. M. Porter, A. Moen, M. McKinlay, G. Sahakyan, S. Wangchuk, P. Ruowen, Z. Yongchao, C. Linlin, C. Daouda, O. Tarkhan-Mouravi, P. Gould, P. Muthoka, G. O. Emukule, S. S. Chaves, M. A. Widdowson, D. Otorbaeva, V. Khanthamaly, K. Stavridis, V. Mikic, N. Furtuna, D. Capmari, B. Alexander, E. Dueger, M. Kamolzoda, J. Mott, A. Bin Salah, M. Mazur, A. Maria Ropero Alvarez, S. J. Olsen, S. Mirza, C. Sofia Arriola, J. Seward, S. Kluglein, A. F. Bolster, N. Minh Hang, J. W. McFarland, N. Ha Thu, T. Thi Minh Nguyen, and the PIVI Partners Group. 2019. The partnership for influenza vaccine introduction (PIVI): Supporting influenza vaccine program development in low and middle-income countries through public-private partnerships. Vaccine 37(35):5089–5095.
Casey, C., and C. Cimino-Isaacs. 2021. Export restrictions in response to the COVID-19 pandemic. Washington, DC: Congressional Research Service.
Cecire, M. H., and H. M. Peters. 2020. The Defense Production Act of 1950: History, authorities, and considerations for Congress. Washington, DC: Congressional Research Service.
Cele, S. 2021. South Africa sees J&J vaccines cleared for delivery “soon.” Bloomberg, June 2. https://www.bloomberg.com/news/articles/2021-06-02/south-africa-sees-j-j-vaccines-cleared-for-delivery-soon (accessed August 13, 2021).
CEPI (Coalition for Epidemic Preparedness Innovations). 2021. CEPI launches COVAX marketplace to match buyers and sellers of critical manufacturing supplies and speed up global access to COVID-19 vaccines through COVAX. https://cepi.net/news_cepi/cepi-launches-covax-marketplace-to-match-buyers-and-sellers-of-critical-manufacturingsupplies-and-speed-up-global-access-to-covid-19-vaccines-through-covax (accessed August 13, 2021).
Chit, A., J. Parker, S. A. Halperin, M. Papadimitropoulos, M. Krahn, and P. Grootendorst. 2014. Toward more specific and transparent research and development costs: The case of seasonal influenza vaccines. Vaccine 32(26):3336–3340.
Cision. 2021. State of Illinois and ASCM launch first-of-its-kind training program to prepare residents for jobs fueled by growing e-commerce industry. PR Newswire. https://www.prnewswire.com/news-releases/state-of-illinois-and-ascm-launch-first-of-its-kindtraining-program-to-prepare-residents-for-jobs-fueled-by-growing-e-commerce-industry-301221680.html (accessed August 17, 2021).
Clift, C. 2021. Scaling up COVID-19 vaccine production: What are the problems and implications? The BMJ Opinion, March 17. Blog. https://blogs.bmj.com/bmj/2021/03/17/scaling-up-covid-19-vaccine-production-what-are-the-problems-and-implications (accessed October 12, 2021).
Cohen, J. 2021. The pandemic surge at home is threatening an Indian vaccinemaker’s bid to protect the world. Science, May 17. https://pulitzercenter.org/stories/pandemic-surge-home-threatening-indian-vaccinemakers-bid-protect-world (accessed October 12, 2021).
Cometto, G., K. Tulenko, A. S. Muula, and R. Krech. 2013. Health workforce brain drain: From denouncing the challenge to solving the problem. PLoS Medicine 10(9):e1001514.
COVAX (COVID-19 Vaccines Global Access). 2021a. COVAX manufacturing taskforce—Workstream 3. https://www.who.int/publications/m/item/covax-manufacturing-taskforce (accessed October 12, 2021).
COVAX. 2021b. Supply chain & manufacturing taskforce. https://cepi.net/wp-content/uploads/2021/05/20210512_COVAX-Supply-Chain-Manufacturing-Taskforce-ACT-AIntroduction.pdf (accessed October 12, 2021).
COVAX Manufacturing Task Force. 2021. COVAX manufacturing task force to tackle vaccine supply challenges. https://www.gavi.org/vaccineswork/covax-manufacturing-task-force-tackle-vaccine-supply-challenges (accessed May 21, 2021).
Crommelin, D. J. A., T. J. Anchordoquy, D. B. Volkin, W. Jiskoot, and E. Mastrobattista. 2021. Addressing the cold reality of mRNA vaccine stability. Journal of Pharmaceutical Sciences 110(3):997–1001.
DCVMN (Developing Countries Vaccine Manufacturers Network). 2021. About DCVMN. https://www.dcvmn.org/-About- (accessed August 13, 2021).
ECA. 2021. Live online training: GMP for vaccine manufacturers. https://www.gmpcompliance.org/training/gmp-course-conference/gmp-for-vaccine-manufacturers (accessed September 21, 2021).
EU (European Union). 2021. United States–European Commission joint statement: Launch of the joint COVID-19 manufacturing and supply chain taskforce.https://ec.europa.eu/commission/presscorner/detail/en/statement_21_4847 (accessed September 28, 2021).
Finkenstadt, D. J., R. Handfield, and P. Guinto. 2020. Why the U.S. still has a severe shortage of medical supplies. Harvard Business Review, September. https://hbr.org/2020/09/why-the-u-s-still-has-a-severe-shortage-of-medical-supplies (accessed August 17, 2021).
Friede, M., L. Palkonyay, C. Alfonso, Y. Pervikov, G. Torelli, D. Wood, and M. P. Kieny. 2011. WHO initiative to increase global and equitable access to influenza vaccine in the event of a pandemic: Supporting developing country production capacity through technology transfer. Vaccine 29(Suppl 1):A2–A7.
GAO (U.S. Government Accountability Office). 2021. Operation Warp Speed: Accelerated COVID-19 vaccine development status and efforts to address manufacturing challenges. Washington, DC.
Gavi (Gavi, The Vaccine Alliance). 2021. Setting new standards: East Africa’s centre of excellence for health supply chains. https://www.gavi.org/news/media-room/setting-new-standards-east-africas-centre-excellence-health-supply-chains (accessed August 8, 2021).
Gerstein, D. M. 2020. The strategic national stockpile and COVID-19: Rethinking the stockpile. RAND Corporation. https://www.rand.org/pubs/testimonies/CTA530-1.html (accessed October 12, 2021).
Ghiga, I., S. Richardson, A. M. R. Álvarez, M. Kato, D. Naidoo, S. Otsu, P. T. Nguyen, P. N. Nguyen, and T. Nguyen. 2021. PIPdeploy: Development and implementation of a gamified table top simulation exercise to strengthen national pandemic vaccine preparedness and readiness. Vaccine 39(2):364–371.
Golan, M. S., B. D. Trump, J. C. Cegan, and I. Linkov. 2021. The vaccine supply chain: A call for resilience analytics to support COVID-19 vaccine production and distribution. In COVID 19: Systemic risk and resilience, edited by B. Trump, J. Keenan, and I. Linkov. London: Springer. Pp. 389–437.
Grohmann, G., D. P. Francis, J. Sokhey, and J. Robertson. 2016. Challenges and successes for the grantees and the technical advisory group of WHO’S influenza vaccine technology transfer initiative. Vaccine 34(45):5420–5424.
Handfield, R., D. J. Finkenstadt, E. S. Schneller, A. B. Godfrey, and P. Guinto. 2020. A commons for a supply chain in the post-COVID-19 era: The case for a reformed strategic national stockpile. The Milbank Quarterly 98(4):1058–1090.
Hatchett, R., M. Saville, M. Downham, T. Cueni, L. Bigger, P. Arthur, R. Suri, S. Prasad, and R. Bech Hansen. 2021. Towards vaccinating the world: Landscape of current COVID-19 supply chain and manufacturing capacity, potential challenges, initial responses, and possible “solution space”—A discussion document. Coalition of Epidemic Preparedness Innovations. https://www.ifpma.org/wp-content/uploads/2021/03/Summit_Landscape_Discussion_Document.pdf (accessed October 1, 2021).
Hay, A. J., and J. W. McCauley. 2018. The WHO Global Influenza Surveillance and Response System (GISRS)—A future perspective. Influenza and Other Respiratory Viruses 12(5):551–557.
Hoen, E., C. Garrison, P. Boulet, K. Mara, and K. Perehudoff. 2021. Scaling-up vaccine production capacity: Legal challenges and recommendations. The Independent Panel for Pandemic Preparedness and Response. https://theindependentpanel.org/wp-content/uploads/2021/05/Background-paper-6-Scaling-up-vaccinationlegal-aspects.pdf (accessed October 12, 2021).
Hoss, S. 2021. Fueling workforce development through cross-sector collaboration. Forbes, June 25. https://www.forbes.com/sites/forbesnonprofitcouncil/2021/06/25/fuelingworkforce-development-through-cross-sector-collaboration/?sh=23927c592fc6 (accessed August 17, 2021).
Huff-Rousselle, M. 2012. The logical underpinnings and benefits of pooled pharmaceutical procurement: A pragmatic role for our public institutions? Social Science & Medicine 75(9):1572–1580.
Jain, R., and E. Rocha. 2021. U.S. curbs on raw material exports could dent new Quad alliance’s vaccine push. Reuters, March 11. https://www.reuters.com/article/us-healthcoronavirus-vaccines-quad/u-s-curbs-on-raw-material-exports-could-dent-new-quadalliances-vaccine-push-idUSKBN2B31M1 (accessed October 12, 2021).
Jerving, S. 2021. Deal to send COVID-19 vaccines from South Africa to Europe dismantled. Devex, September 2. https://www.devex.com/news/deal-to-send-covid-19-vaccines-from-south-africa-to-europe-dismantled-101532 (accessed October 15, 2021).
Kar, S. K., R. Ransing, S. M. Y. Arafat, and V. Menon. 2021. Second wave of COVID-19 pandemic in India: Barriers to effective governmental response. EClinicalMedicine 36:100915.
Khamsi, R. 2020. If a coronavirus vaccine arrives, can the world make enough. Nature 580(7805):578–580.
Kim, W.-S. 2021. SK Bioscience halts flu vaccine output to focus on COVID vaccines. The Korea Economic Daily, March 30.
Kuchler, H. 2021. COVAX launches marketplace for COVID vaccine inputs to boost production. Financial Times, July 15.https://www.ft.com/content/81c94b7d-e30c-43ff-b9e0-4191be8d64cb (accessed October 12, 2021).
Kuchler, H., and J. Miller. 2021. Shortage of giant plastic bags threatens global vaccines rollout. Financial Times, February 17. https://www.ft.com/content/b2f4f9cf-af80-428f-a198-2698ceb4c701 (accessed October 14, 2021).
Kumar, A., W. E. Dowling, R. G. Román, A. Chaudhari, C. Gurry, T. T. Le, S. Tollefson, C. E. Clark, V. Bernasconi, and P. A. Kristiansen. 2021. Status report on COVID-19 vaccines development. Current Infectious Disease Reports 23(6):9.
Laguipo, A. B. B. 2021. Moderna to develop three new mRNA-based vaccines against HIV, influenza, and the Nipah virus. https://www.news-medical.net/news/20210113/Moderna-starts-to-develop-three-new-mRNA-based-vaccines-against-HIV-influenza-and-the-Nipah-virus.aspx (accessed July 15, 2021).
Lawson, A., and J. Rhee. 2020. Usage of the Defense Production Act throughout history and to combat COVID-19. Yale School of Management.https://som.yale.edu/blog/usage-of-the-defense-production-act-throughout-history-and-to-combat-covid-19 (accessed October 12, 2021).
Lefkowitz, R. 2018. 2018 workplace learning report: The rise and responsibility of talent development in the new labor market. LinkedIn. https://learning.linkedin.com/resources/workplace-learning-report/download-report (accessed August 15, 2021).
Lister, S., and F. Gottron. 2007. The Pandemic and All-Hazards Preparedness Act (P.L. 109-417): Provisions and changes to preexisting law. Washington, DC: Congressional Research Service.
Mattiuzzo, G., Knezevic, I., Hassall, M., Ashall, J., Myhill, S., Faulkner, V., Hockley, J., Rigsby, P., Wilkinson, D. E., Page, M., and collaborative study participants. 2019. Harmonization of Zika neutralization assays by using the WHO International Standard for anti-Zika virus antibody. NPJ Vaccines 4:42. https://doi.org/10.1038/s41541-019-0135-3.
McCoy, M. 2021. Lipids, the unsung COVID-19 vaccine component, get investment.https://cen.acs.org/business/outsourcing/Lipids-unsung-COVID-19-vaccine/99/web/2021/02 (accessed August 10, 2021).
Menon, S. 2021. India vaccination: Does it have enough doses for all adults? BBC. https://www.bbc.com/news/world-asia-india-55571793 (accessed August 17, 2021).
Minor, P. D. 2015. Assaying the potency of influenza vaccines. Vaccines (Basel) 3(1):90–104.
Mirasol, F. 2020. Meeting the challenges for scaling up vaccine manufacturing systems. BioPharm International 33(12):46–50.
Munira, S. L., J. T. Hendriks, I. I. Atmosukarto, M. H. Friede, L. M. Carter, J. R. G. Butler, and A. C. A. Clements. 2019. A cost analysis of producing vaccines in developing countries. Vaccine 37(9):1245–1251.
Ncube, B. M., A. Dube, and K. Ward. 2021. Establishment of the African Medicines Agency: Progress, challenges and regulatory readiness. Journal of Pharmaceutical Policy and Practice 14(1):29.
PAHO (Pan American Health Organization). 2014. Strategic Development Plan 2014–2020 of the Pan American Network for Drug Regulatory Harmonization (PANDRH). PANDRH Series—Technical Document No. 14. Washington, DC. https://www.paho.org/hq/dmdocuments/2015/drug-harmonization-Strategic-Plan-PANDRH-04-20-2015.pdf (accessed October 12, 2021).
PAHO. 2019. Regulatory reliance principles: Concept note and recommendations. Ninth Conference of the Pan American Network for Drug Regulatory Harmonization (PANDRH). (San Salvador, 24 to 26 October, 2018). Washington, DC. https://iris.paho.org/bitstream/handle/10665.2/51549/PAHOHSS19003_eng.pdf (accessed October 12, 2021).
Paules, C. I., and A. S. Fauci. 2019. Influenza vaccines: Good, but we can do better. Journal of Infectious Diseases 219(Suppl 1):S1–S4.
Pegg, D. 2020. What was Exercise Cygnus and what did it find? The Guardian. https://www.theguardian.com/world/2020/may/07/what-was-exercise-cygnus-and-what-did-it-find (accessed August 13, 2021).
Peters, R., and D. Prabhakar. 2021. Export restrictions do not help fight COVID-19. https://unctad.org/news/export-restrictions-do-not-help-fight-covid-19 (accessed August 19, 2021).
Pfizer. 2021. New RNA technology could get the flu vaccine right, every year. https://www.pfizer.com/news/featured_stories/featured_stories_detail/new_rna_technology_could_get_the_flu_vaccine_right_every_year (accessed August 13, 2021).
Podda, A. 2010. Aims and role of Novartis Vaccines Institute for Global Health (NVGH). Procedia in Vaccinology 2(2):124–127.
Reggi, V. 2017. Medicines regulatory harmonization: International collaboration as a key to improve public health. Medicine Access @ Point of Care 1. https://doi.org/10.5301/maapoc.0000001.
Reilly, M. 2021. Research center trains COVID vaccine manufacturers. Texas A&M Today, April 22. https://today.tamu.edu/2021/04/22/research-center-trains-covid-vaccine-manufacturers (accessed August 13, 2021).
Remko, V. H. 2020. Research opportunities for a more resilient post-COVID-19 supply chain—Closing the gap between research findings and industry practice. International Journal of Operations & Production Management 40(4):341–355.
Rockman, S., K. Laurie, and I. Barr. 2020. Pandemic influenza vaccines: What did we learn from the 2009 pandemic and are we better prepared now? Vaccines 8(2):211.
Røttingen, J.-A. 2016 November 15-16. Alternative models for influenza vaccine R & D financing. Paper presented at Third WHO Consultation on Global Action Plan on Influenza Vaccines (GAPIII), Geneva, Switzerland.
Schafer, J. 2014. BARDA international influenza vaccine manufacturing capacity building program. https://www.who.int/phi/DAY1_04_Schafer_Panel1_BARDA_PM_Dubai2014.pdf (accessed August 17, 2021).
Schoenmaker, L., D. Witzigmann, J. A. Kulkarni, R. Verbeke, G. Kersten, W. Jiskoot, and D. J. A. Crommelin. 2021. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. International Journal of Pharmaceutics 601:120586.
Simchi-Levi, D., and E. Simchi-Levi. 2020. Building resilient supply chains won’t be easy. Harvard Business Review, June. https://hbr.org/2020/06/building-resilient-supply-chains-wont-be-easy (accessed September 28, 2021).
Sorescu, S., J. L. González, and A. Andrenelli. 2021. Using trade to fight COVID-19: Manufacturing and distributing vaccines. Organisation for Economic Co-operation and Development. https://www.oecd.org/coronavirus/policy-responses/using-trade-to-fight-covid-19-manufacturing-and-distributing-vaccines-dc0d37fc (accessed October 12, 2021).
Sparrow, E., J. G. Wood, C. Chadwick, A. T. Newall, S. Torvaldsen, A. Moen, and G. Torelli. 2021. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine 39(3):512–520.
Tarbet, E. B., J. T. Dorward, C. W. Day, and K. A. Rashid. 2013. Vaccine production training to develop the workforce of foreign institutions supported by the BARDA influenza vaccine capacity building program. Vaccine 31(12):1646–1649.
The Economist. 2021. American export controls threaten to hinder global vaccine production. https://www.economist.com/science-and-technology/2021/04/22/american-export-controls-threaten-to-hinder-global-vaccine-production (accessed August 25, 2021).
The Global Fund. 2021. Procurement tools. https://www.theglobalfund.org/en/sourcingmanagement/procurement-tools (accessed August 17, 2021).
UNSIC (United Nations System Influenza Coordination). 2008. Simulation exercises on influenza pandemic responses in the Asia-Pacific region. With Asian Disaster Preparedness Center; Kenan Institute Asia. http://towardsasaferworld.org/sites/default/files/unsic_pandemic_complete.pdf (accessed October 12, 2021).
White House. 2021. National strategy for the COVID-19 response and pandemic preparedness. National-Strategy-for-the-COVID-19-Response-and-Pandemic-Preparedness.pdf (accessed October 12, 2021).
WHO (World Health Organization). 2007. Regulatory preparedness for human pandemic influenza vaccine. Expert Committee on Biological Standardization. https://www.who.int/biologicals/publications/trs/areas/vaccines/influenza/Human_pandemic_Influenza_Vaccines_BS2074_01Feb08.pdf (accessed October 12, 2021).
WHO. 2012. WHO/DHHS joint workshop on enhancing the global workforce for vaccine manufacturing. Cape Town, South Africa, November 30–December 2, 2011. https://www.who.int/phi/WHO_IER_TTI_12.1_WEGWVM_Report_FINAL_May2012.pdf (accessed October 12, 2021).
WHO. 2016. Key elements of sustainability for influenza vaccine manufacturing in low and middle income countries. World Health Organization. https://apps.who.int/iris/handle/10665/251695 (accessed December 2, 2021).
WHO. 2017. Terms of reference for national influenza centers of the Global Influenza Surveillance and Response System.https://www.who.int/influenza/gisrs_laboratory/national_influenza_centres/tor_nic.pdf (accessed October 12, 2021).
WHO. 2021a. Immunization analysis and insights. https://www.who.int/teams/immunizationvaccines-and-biologicals/immunization-analysis-and-insights/surveillance (accessed September 17, 2021).
WHO. 2021b. List of vaccine producing countries with functional NRAs. https://www.who.int/initiatives/who-listed-authority-reg-authorities/list-of-vaccine-prod-countries (accessed September 28, 2021).
WHO. 2021c. Regulation of post approval changes to vaccines. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/regulation-of-post-approval-changes-to-vaccines (accessed September 16, 2021).
WHO. 2021d. Regulationa and prequalification.https://www.who.int/teams/regulationprequalification/regulation-and-safety/regulatory-convergence-networks/harmonization (accessed October 12, 2021).
WHO. 2021e. 38th WHO regulatory update on COVID-19.https://cdn.who.int/media/docs/default-source/medicines/regulatory-updates/covid-19/38th-who-regulatory-update-oncovid-19_12sep2021.pdf?sfvrsn=a17518a9_5&download=true (accessed October 12, 2021).
WTO (World Trade Organization). 2020. Developing and delivering COVID-19 vaccines around the world.https://www.wto.org/english/tratop_e/covid19_e/vaccine_report_e.pdf (accessed October 12, 2021).
Yen, C., T. B. Hyde, A. J. Costa, K. Fernandez, J. S. Tam, S. Hugonnet, A. M. Huvos, P. Duclos, V. J. Dietz, and B. T. Burkholder. 2015. The development of global vaccine stockpiles. Lancet Infectious Diseases 15(3):340–347.
Youtie, J., and P. Shapira. 2008. Building an innovation hub: A case study of the transformation of university roles in regional technological and economic development. Research Policy 37(8):1188–1204.




































