A common theme throughout the workshop discussions was the importance of prioritizing the patient—patients’ needs and preferences should be at the forefront throughout the drug R&D process. Christin Veasley, director of the Chronic Pain Research Alliance, emphasized the value of including the perspective of people who have lived experience with these conditions. Karen Winkfield, executive director of the Meharry-Vanderbilt Alliance, a strategic partnership between the Meharry Medical College and the Vanderbilt Medical Center, discussed ways to incorporate patient expertise throughout the design and implementation of clinical trials. Erica Woodahl from the University of Montana, who studies genetic variation in indigenous populations, provided insights on the barriers and opportunities for broader access to biomedical research for underserved and unstudied populations. Jason Mellad, chief executive officer (CEO) and founder of Start Codon—a UK-based accelerator, which offers funding to rapidly develop life science innovations—shared his thoughts on the promise of more equitable research.
CHRONIC PAIN: A PATIENT’S JOURNEY
According to a 2011 National Academies report, chronic pain afflicts 100 million Americans and is associated with more than $500 billion in direct medical treatment costs and lost productivity each year (IOM, 2011). Chronic pain conditions, which may result from various underlying diseases or conditions, share many attributes with other chronic conditions, said Veasley. Little information is available about the underlying biological mechanisms and causes of chronic pain, and animal models, translation, and research investment are insufficient, she explained. Few new drugs or other non-pharmacologics have been approved for the treatment of chronic pain. Although numerous therapies are on the market, data are insufficient to determine which treatments will be effective for which populations and which individuals. “And of course, our health care system is failing people with these conditions because we do not have team-based interdisciplinary care,” concluded Veasley.
On a personal level, she said, capturing any one individual’s experience is difficult. Veasley described the physical, emotional, spiritual, environmental, and vocational toll that one such disease—chronic pain—can take on individuals. She described living with a chronic disease as feeling like one is tethered to a ball and chain that grows heavier over time: In the beginning, a patient diagnosed with a chronic disease may feel upset, but motivated to find the right doctor, the right care, and a cure. Over time, the patient may see multiple doctors and try various treatments, while dealing with the long-term physical, psychological, and emotional burden
of the disease. As the burden of chronic disease grows heavier and heavier, it becomes harder and harder to move forward.
Veasley said the clinical trials enterprise is not designed to address the complexity of chronic conditions, particularly when it comes to issues related to comorbidity and multimorbidity. She pointed out that basic research tools (e.g., animal models) are not built to address questions about multimorbidity, and that clinical trials are often not adequately powered to assess disease heterogeneity and often exclude people with multiple chronic conditions. She added that disease classification is often based on signs and symptoms rather than the underlying mechanisms of disease. Lastly, payers frequently do not incentivize the type of interdisciplinary care needed to effectively treat people with chronic disease.
Veasley said the goal should shift from finding a cure to determining how to live well with a chronic condition. She concluded that this shift would require changing the entire system, not just addressing the issue disease by disease. This type of systems-level change would involve collaboration across different entities working as patient and advocacy organizations to examine each level of the drug R&D process to identify what needs to happen, determine who should take action, and how to incentivize that action.
THE IMPORTANCE OF THE PATIENT VOICE
Winkfield argued that a patient-centered approach to clinical trials is vital and offered suggestions for how to incorporate patients’ input and expertise throughout the drug R&D process. She spoke about some lessons learned from her work in oncology and health equity that could be broadly applicable for other types of chronic illnesses. She expressed hope that researchers, regulators, clinicians, and patients can begin to think more critically about a patient-centered approach to the design and implementation of clinical trials.
When considering how to incorporate patient input before a concept for a trial has begun, Winkfield encouraged stakeholders to be mindful and creative. She acknowledged that this is not necessarily easy, but there are examples of success (see Box 2-1). The Wake Forest Comprehensive Cancer Center’s Advocates for Research in Medicine (ARM) program was established as a mechanism for people with personal experience with cancer—survivors, caregivers, and individuals at high risk for cancer—to review research proposals and advise on clinical trials.
Winkfield stated that engaging patients means engaging patients from different communities—another area in need of improvement. A snapshot from the American Association for Cancer Research Cancer Disparities Progress Report shows that while differences in overall cancer death rates
between Black and White Americans are less pronounced today compared to 30 years ago, Black men and women continue to have the highest risk of cancer death (AACR, 2020). She pointed out that these health disparities apply across the board, including cardiovascular disease, diabetes, and HIV/AIDS, and are due in large part to disparities in access to care and the quality of care provided (NASEM, 2017).
While Winkfield recognized that part of the challenge in addressing these health disparities is that minorities tend to be underrepresented in clinical trials, she emphasized the need to hear from minority communities to better understand the underlying issues that lead to disparities in engagement in clinical trials. For example, a recent study published in JAMA Oncology showed that while 20 percent of multiple myeloma patients are Black, only 5 percent of people engaged in the clinical trials that were used to grant U.S. Food and Drug Administration (FDA) drug approval were Black (Loree et al., 2019). Winkfield emphasized the importance of access to and diversity in clinical trials in ensuring that drug developments benefit all communities. When designing trials, she asked stakeholders to consider whether a trial meets the needs of the intended patients affected by the dis-
ease, and whether the makeup of trial participants reflects the diversity of real-world populations. She reiterated the importance of engaging patients and their community early on in the research process and development of a trial.
In addition to considering racial and ethnic health disparities, Winkfield pointed out the need to consider the impact of geography on health disparities and access to clinical trials. For example, rural populations may have less access to clinical research sites and are often underrepresented in clinical trials. Additionally, individuals with fewer financial resources may be less likely to take part in clinical trials. She referenced the American Society of Clinical Oncology policy statement on addressing financial barriers to patient participation in clinical trials, which includes a number of recommendations concerning that issue.1
In closing, Winkfield highlighted a paper she and colleagues published in JCO Oncology Practice that offers an actionable framework to address cancer care disparities (Winkfield et al., 2021). Although the framework was developed for the oncology space, she suggested that many of the actionable items could be broadly applicable to other chronic diseases. She pointed to the importance of community engagement in the framework, saying, “You have to hear from your stakeholders in order to make a difference, in order to make sure that your clinical trials are meeting the needs of the populations you are trying to serve.”
INCLUDING UNDERSERVED POPULATIONS
Woodahl provided insight on how to ensure broader access to biomedical research for underserved and unstudied populations. Her work is focused on indigenous populations, she said, but added that her observations should be generalizable to other underrepresented populations.
Woodahl’s research is in the area of precision medicine and pharmacogenomics. As she explained, the goal of precision medicine is to identify variability among individuals in genes, environment, and lifestyle that can be useful in preventing and treating disease. In general, populations are heterogeneous, but medications are mainly delivered in a one-size-fits-all fashion, with a standard starting dose or regimen. However, not all patients respond to a medication in the same way: Some may not respond at all to the standard therapy, while others may experience adverse events when given a standard dose. One of the goals of pharmacogenetics is to better identify non-responders and individuals who are more likely to experience adverse events prior to initiating therapy.
___________________
1 For more information, see https://ascopubs.org/doi/full/10.1200/JCO.18.01132 (accessed July 15, 2021).
Predicting the response of individuals to particular medications requires large-scale genetics studies, such as genome-wide association studies (GWAS). Despite dramatic growth in GWAS over the past decade and a half, Woodahl said, the majority of studies have been limited to populations of European descent (see Figure 2-1). The next two populations with the greatest inclusion in GWAS are individuals of East Asian descent and of South Asian descent. Far fewer individuals of African ancestry are included in GWAS, and indigenous people worldwide make up less than 0.02 percent of participation.
The lack of inclusivity in GWAS is a problem, Woodahl said, because it is unclear whether medical innovations coming out of these studies will be as useful for populations for which few data are available. Additionally, the availability of medical innovations is often limited to large academic medical centers, which are generally located in large urban areas. Populations living in rural areas may have comparatively less access to recent advances in precision medicine.
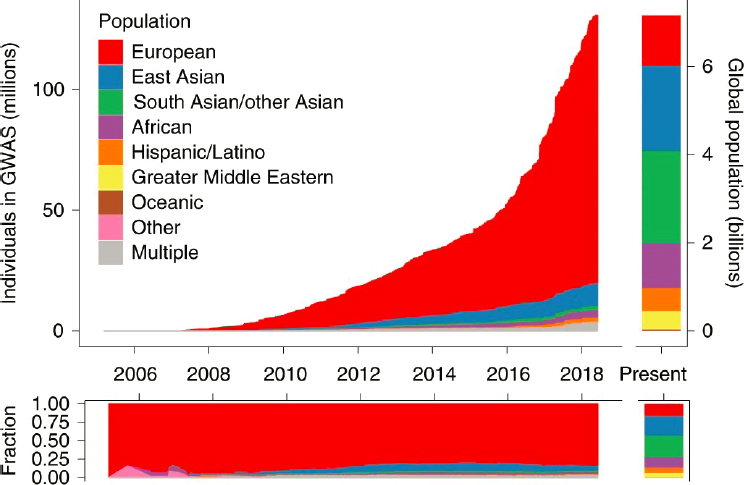
NOTE: GWAS = genome-wide association study.
SOURCES: Presented by Erica Woodahl on February 22, 2021, at the Innovation in Drug Research and Development for Prevalent Chronic Diseases workshop; Martin et al., 2019.
Woodahl laid out a few of the barriers to increasing diversity in research:
- There is the perception that past research has provided little benefit to underrepresented groups. Woodahl referenced the term “helicopter research” to describe projects in which researchers go into a community to obtain samples and data, then leave and publish their papers. The community receives little direct benefit. Going forward, Woodahl asked stakeholders to consider how to address this issue and ensure that proposed projects are of interest and have provided value to the people in the community participating in the research.
- Among minority populations, including American Indian and Alaska Native communities, there is a lack of trust in the research enterprise. As an example, Woodahl pointed to a case in which researchers at Arizona State University had shared genetic samples collected for diabetes research with other researchers without the consent of the Havasupai Tribe.2 The case led to backlash by indigenous communities against research. In 2002, the Navajo Nation put a moratorium on genetic research.3
- Concerns about data sharing and ownership can impede research efforts. The National Institutes of Health (NIH) requires data-sharing agreements when it issues genetic research grants, but Woodahl has worked with communities that were hesitant to relinquish control of their own data. She suggested that more flexibility is needed to accommodate diverse populations.
- A number of the communities that Woodahl works with are geographically distant from academic institutions, which requires investigators to travel large distances to reach community participants and build trusting relationships.
- Given issues with geographical remoteness, there may be a lack of laboratory infrastructure at community-based sites (e.g., it may be difficult to maintain the integrity of biological samples that must be transported from a clinical site back to an academic lab). Additionally, there may be a shortage of expert personnel at the community level.
___________________
2Havasupai Tribe of the Havasupai Reservation v. Arizona Board of Regents and Therese Ann Markow, 1 CA-CV 07-0454 and 1 CA-CV 07-0801 (Arizona Court of Appeals, 2009).
3 Approving a Moratorium on Genetic Research Studies Conducted Within the Jurisdiction of the Navajo Nation Until Such Time That a Navajo Nation Human Research Code Has Been Amended by the Navajo Nation Council, HSSCAP-20-02 (2002).
Given these and many other considerations, communities may not be comfortable agreeing to participate in a research project without developing a clear understanding of the issues and having trust in the investigators. Woodahl relies on community engagement as a way to include diverse communities in her research. Her approach is to understand what communities considering participation in research want to engage in and how that research can be mutually beneficial to the researchers and the community as equal stakeholders. This approach requires that she build in sufficient time to develop relationships with community participants and sustain that partnership over time.
Woodahl is part of a community–academia partnership between the University of Montana and Native Americans belonging to the Confederated Salish and Kootenai Tribes. The partnership has carried out studies in cancer and cardiovascular pharmacogenomics, and currently has projects on the genetic and seasonal contributions to vitamin D sufficiency. A community advisory board meets with the researchers every month to talk about research progress, recruiting, new grant opportunities, and other relevant matters. The researchers have held genetic education workshops to provide opportunities for community advisory board members to learn about genetics and gain some hands-on training. Community advisory board members have been invited to visit Woodahl’s lab at the University of Montana to see how the samples are stored and processed. She stressed the importance of this community engagement as core to her team’s community-based participatory research model.
Woodahl shared some of the approaches she and her team have taken to recruit study participants. She described the importance of attending community events, such as pow wows and health fairs, as a way to recruit research participants. She noted that these approaches may be different from clinical research done in academic medical centers. For example, when she and her team drive out to the reservation they bring all of the supplies and equipment—portable freezers, centrifuges, blood collection supplies—that they need to recruit participants.
Woodahl recognized that the work involved is substantial, but said it is worth the effort to help ensure that medical innovations benefit all populations. More population-specific biorepositories are needed to advance research on chronic diseases. To build these resources, Woodahl suggested thinking outside the box about ways to include underserved populations. “Community engagement is an essential piece in ensuring that we have better access to research for underserved populations and [we] hope that this will help address health care disparities,” she said.
THE VALUE OF RESEARCH ON UNDERSERVED POPULATIONS
Looking to the future, Mellad said he believes research that better serves underrepresented populations and improves equity will be “one of the most disruptive sets of innovations of the 21st century.” Minority populations are generally underrepresented in most clinical trials, he said. According to an FDA report, only 7 percent of patients in U.S. clinical drug trials from 2015 through 2019 were Black, compared with 13–14 percent in the general population (FDA, 2017). Ensuring that all populations are well represented needs to be a consideration beyond the clinical trial phase of drug R&D, he said. Including diverse samples must be a priority for preclinical work as well. “It is just not enough to find the individuals to be in your trial once you have a drug that is ready to go to market or diagnostic,” he said. “You need to also be thinking about that when it comes to the biomarker discovery or therapeutic discovery upstream in the preclinical phase.”
Mellad offered a brief description of a case study that illustrated a type of disparity that is often overlooked. Pulse oximeters are important devices for monitoring blood oxygen levels, which is particularly important for patients with diseases like chronic obstructive pulmonary disease (COPD) or COVID-19. It turns out, he said, that the usual pulse oximeters may not provide accurate readings for individuals with darker skin tones (Sjoding et al., 2020). Given that Blacks are three times more likely to suffer a poor outcome from COVID-19, inaccurate, at-home pulse oximeters exacerbate the issue. The lesson, he said, is that it is not simply equal access to a technology or a drug that is important; how the technology or drug was developed and whether it addresses the needs of the entirety of a diverse population is also crucial.
Getting access to populations that are underrepresented in clinical trials will be key to this work, Mellad said, and several companies trying to address this problem are getting interest from investors. The company Egality,4 he noted, is working on the recruitment of patients for clinical trials to improve the representation of minority participants. The company Hurdle5 is focused on managing patient care for underserved populations. By working with such companies, researchers will gain access to more patients from underrepresented populations, facilitating not only more representative populations for clinical trials but also access to data that will help identify novel pathways. “The two go hand in hand,” he said. “When I am looking for opportunities for investment, I am always thinking not only about the need that these companies are solving today but also how they
___________________
4 For more information, see https://www.egality.health (accessed June 29, 2021).
5 For more information, see https://www.startuphealth.com/hurdle (accessed June 29, 2021).
can pivot in the future when they build those informed consent databases of information, which will be valuable.”
REFERENCES
AACR (American Association for Cancer Research). 2020. AACR cancer disparities progress report 2020. http://www.CancerDisparitiesProgressReport.org (accessed July 16, 2021).
FDA (U.S. Food and Drug Administration). 2017. Global participation in clinical trials report, 2015–2016. https://www.fda.gov/files/drugs/published/2015---2016-Global-Clinical-TrialsReport.pdf (accessed June 29, 2021).
IOM (Institute of Medicine). 2011. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press.
Joosten, Y. A., T. L. Israel, N. A. Williams, L. R. Boone, D. G. Schlundt, C. P. Mouton, R. S. Dittus, G. R. Bernard, and C. H. Wilkins. 2015. Community engagement studios: A structured approach to obtaining meaningful input from stakeholders to inform research. Academic Medicine 90(12):1646–1650.
Loree, J. M., S. Anand, A. Dasari, J. M. Unger, A. Gothwal, L. M. Ellis, G. Varadhachary, S. Kopetz, M. J. Overman, and K. Raghav. 2019. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncology 5(10):e191870.
Martin, A. R., M. Kanai, Y. Kamatani, Y. Okada, B. M. Neale, and M. J. Daly. 2019. Clinical use of current polygenic risk scores may exacerbate health disparities. Nature Genetics 51:584–591.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Communities in action: Pathways to health equity. Washington, DC: The National Academies Press.
Sjoding, M. W., R. P. Dickson, T. J. Iwashyna, S. E. Gay, and T. S. Valley. 2020. Racial bias in pulse oximetry measurement. New England Journal of Medicine 383(25):2477–2478.
Wake Forest Baptist Health. n.d. Office of Cancer Health Equity. https://www.wakehealth.edu/Locations/Facilities/Comprehensive-Cancer-Center/Office-of-Cancer-Health-Equity (accessed July 15, 2021).
Winkfield, K. M., J. M. Regnante, E. Miller-Sonet, E. T. González, K. M. Freund, and P. M. Doykos on behalf of the Cancer Continuum of Care for Medically Underserved Populations Working Group. 2021. Development of an actionable framework to address cancer care disparities in medically underserved populations in the United States: Expert roundtable recommendations. JCO Oncology Practice 17(3):e278–e293.










