To open the workshop, three speakers shared their personal perspectives on key focus areas of the series: person-centeredness and inclusivity in clinical trials; the role of digital technology in conducting clinical trials; and resilience, sustainability, and transparency of the clinical trials enterprise. Terris King, former director of the Office of Minority Health at the Centers for Medicare & Medicaid Services (CMS), shared his vision for building a more person-centered and inclusive clinical trials enterprise by 2030. Robert Califf, head of Clinical Strategy and Policy at Verily Life Sciences and Google Health, reflected on the use of existing and emerging technologies for achieving the aspirations for a transformed 2030 clinical trials enterprise. Elliott Levy, senior vice president for R&D Strategy and Operation at Amgen Inc., shared his vision for a future of clinical research that could better meet the needs of patients and society. Each talk was followed by a facilitated breakout group discussion.
ENVISIONING A MORE PERSON-CENTERED AND INCLUSIVE CLINICAL TRIALS ENTERPRISE
A Perspective on Person-Centeredness and Inclusivity: Moving Forward Together
King’s community has experienced high rates of COVID-19 cases during the pandemic. He described his work participating in focus groups for Johns Hopkins University and the Robert Wood Johnson Foundation, and arranging for conversations between several pharmaceutical companies and his congregation to discuss vaccination and to address issues with misinformation. Through these activities, King heard that distrust of the biomedical research and health care systems persists. He told workshop participants that “many African Americans … would rather die than take a vaccine that many of you would offer.” He shared the perspective that many from his community believe that others only care about the health of Black communities now given the COVID-19 pandemic because they realize everyone’s health is interconnected. The sense of many Black communities around the country, King said, is that “if entities talking to us would talk about more than vaccine hesitancy, if health care institutions would also talk about the health issues and concerns that we had before COVID ever came, we might trust that they’re actually meaning us good for when this is over, and we might actually listen to them in terms of taking a vaccine.”
Long before the pandemic, the promise of precision medicine was that treatment of disease could be tailored to individuals based on genetic and other factors. At the time, King said, the need for greater participation by African Americans and other underrepresented minorities in clinical trials
was acknowledged. Yet, minority populations are still underrepresented in clinical trials, hindering the development and application of precision medicine treatments that could benefit them. “We [Black communities and clinical trialists] need each other in terms of trials,” King said. “We need each other in terms of participation. We need each other to move forward.” African Americans suffer from high rates of diabetes, hypertension, and obesity (NCHS, 2021), and are more likely to die from COVID-19 than white Americans (CDC, 2021). Many researchers, clinicians, and policy experts have suggested that the disparity in COVID-19 mortality rates might be associated with social determinants of health and/or underlying comorbidities, such as diabetes, but the true reason is still unknown. Understanding and addressing the health concerns of African Americans has not been a priority for research, King noted.
Building trust with Black communities involves making the health of African Americans a priority and being transparent about the process and the products of medical research. King said the clinical trials enterprise is using “the wrong message and the wrong messengers” when trying to engage minority populations in clinical trials. What is needed, he continued, is a person-centered model of community-based participatory research that reaches African Americans in the spaces where they gather to share their faith and their fears and where trusted relationships are built. These spaces include not just churches, but also community sanctuaries such as beauty salons and barbershops. Trusted community members and pastors in these spaces can work with researchers and the pharmaceutical industry to build programs that convey, with complete transparency, the benefit of the pharmaceutical products for the community.
King emphasized the need to invest in the community, and provide stipends to the trusted community members to enable them to educate and engage others in the research process. “What we discovered from COVID is we’re connected. Let’s use this process to connect and build processes that work for both parties,” he said, adding that both parties must humble themselves so they can learn from each other. He noted that this approach to engaging the community is not new and has been successful in other health settings (e.g., Project Dulce for improving diabetes care in underrepresented populations).1 In conclusion, King said, “Let’s work together and build a vision for 2030 to save the least, the lost, and those who lack support.”
___________________
1 For more information, see https://www.scripps.org/services/metabolic-conditions/diabetes/diabetes-professional-training (accessed August 3, 2021).
Enhancing Outcomes in a More Person-Centered and Inclusive Clinical Trials Enterprise: Breakout Discussion Highlights
A summary of the points made by individual breakout group participants was provided in plenary session by Natalie Rotelli of Eli Lilly and Company, Mark Unruh of The University of New Mexico, Jonathan Watanabe of the University of California, Irvine, and Jeanne Regnante of the LUNGevity Foundation on behalf of four breakout groups. The following topics were highlighted by breakout group participants as being of interest for further discussion in the subsequent workshop meetings (see Chapter 3). This section is the rapporteurs’ summary of the breakout group reports by Rotelli, Unruh, Watanabe, and Regnante, and should not be construed as reflecting agreement among any group. All suggestions and proposals are reported for discussion purposes only.
Improving Representation and Relevance
The results of a clinical trial should be relevant to trial participants and to the broader patient population. Breakout discussants observed that only a small subset of the general population participates in clinical trials, which compounds the challenges of enrolling a diverse and representative trial population. Participants discussed how elements of study design could create barriers to participation for minorities (e.g., inclusion and exclusion criteria, convenience of site locations, or appointment hours) and how new approaches to participation that leverage digital health technologies might increase access to clinical trial participation. The need for metrics to demonstrate progress toward the goal of improving representation and relevance was raised by discussants, and it was noted that measures of success should be driven by what is meaningful to communities.
Engaging and Preparing a More Diverse Clinical Research Workforce
Representation applies not only to trial participants, but also to those designing and conducting the trials. Breakout discussants suggested that the clinical trials workforce should reflect the community it serves. Engaging investigators beyond those affiliated with traditional academic research institutions was discussed as one way to broaden diverse representation among both investigators and participants, and to potentially enhance the speed of participant accrual. Later in the workshop, individual workshop speakers and participants discussed in more depth approaches to improve workforce diversity (see Chapter 2), and build trust and sustain long-term relationships with communities and community providers (see Chapter 5).
Improving Community Engagement and Fostering Trust
The need for sustained investments in building communities and maintaining trust was a theme across the breakout discussions. Breakout discussants observed that the clinical trials enterprise seems “piecework” in that generally each trial is set up independently, making it difficult to have a sustainable impact in a given community. The importance of leveraging established partnerships to engage target communities was highlighted in the breakout discussions. The role of community-based participatory research was highlighted, including the need to consider community researchers as part of the clinical research team. Lessons may be learned from the response to the COVID-19 pandemic, which involved leveraging existing skills, resources, and infrastructure within communities (e.g., community-based pharmacies).
ENVISIONING AN OPTIMIZED CLINICAL TRIALS ENTERPRISE THROUGH THE USE OF TECHNOLOGIES
A Perspective on the Use of Digital Technologies: Taking Action for Impact
Califf described the development of COVID-19 vaccines as “a real triumph,” but added that the clinical trials enterprise in general did not deliver. “[T]he clinical trials industry … is at the point now where digital transformation is going to have an impact,” Califf said. “And the way people handle it will determine the winners and the losers as things shake out.” He provided examples of digital disruption in other industries, such as the transformation of photography from film and paper to digital and the movement from video rental to digital streaming, in which the digital disruption was driven by external organizations while the original businesses resisted change (see Steinhubl et al., 2019). He suggested that the clinical trials enterprise embrace the coming digital disruption and adapt technologies to improve clinical research and care.
Califf referred participants to the vision statement by the Clinical Trials Transformation Initiative (CTTI) on transforming clinical trials for 2030, which he said was in line with the focus of this National Academies workshop.2 He focused his remarks on seven technology-related actions that he said have the potential to transform the field.
Califf suggested that one approach could be to replace human labor through automation, while not replacing human jobs. He observed that some manual processes in the conduct of clinical trials could be auto-
___________________
2 See https://ctti-clinicaltrials.org/who_we_are/transforming-trials-2030 (accessed April 13, 2022).
mated, but concerns about regulatory oversight have stalled progress. “We have to move into a regulatory regime that supports and does not inhibit automation,” he said. Automation could enhance the efficiency of virtual visits, virtual monitoring, auditing, and statistical process control, for example. Califf asserted that automation could reduce the time spent doing mundane, repetitive tasks and allow trial staff to spend more time on higher value activities, including interacting with clinical trial participants.
Another approach that Califf proposed was to provide digital support that makes the work easier and more fun. Califf offered suggestions for how digital support might enhance the conduct of clinical trials. For example, trial participation can be made more engaging for patients through the use of gamification—the application of game design elements to non-game situations. Decision support tools for clinical trials and clinical practice could help providers delegate some routine health care activities to other staff, which would share the workload across the health care team and enable providers to focus on other priority tasks. The use of passive measurement technologies (with informed consent) can enhance virtual visits and reduce the burden of data collection. Digital support can also enable more home health visits, and “digital phenotypes” can help ensure that the technology used is appropriate for the individual (e.g., some patients might need more personal interaction or might be less technology literate).
A third approach Califf suggested was to scale research in a way that is representative, reliable, and powerful. He said the dependence on manual processes limits the ability to reach populations of potential trial participants. For common chronic diseases, many of those who are eligible for a trial can face barriers to participation, such as not living near a trial site. Rare disease trials can be challenging to enroll in small areas and can require coordination across health systems and geographies. Digital health technologies can enable the conduct of research at the scale needed for studies to be representative, reliable, and adequately powered to produce meaningful data, he said.
A fourth approach Califf suggested was to involve patients and participants directly in research. Digital health technologies can enable direct interaction with patients and potential trial participants to gain input on their priorities, preferences, and concerns (e.g., features of trial design and outcomes of importance to patients). Technology can also enable self-reporting by participants, which Califf said can add depth and context to clinical and functional outcomes measures.
Califf proposed creating communities of learning and research as another approach. Concerns about patient and participant privacy and data integrity have resulted in a system that does not facilitate interac-
tion among the broad range of stakeholders in clinical research, Califf observed. He said it is time to develop communities of learning in the clinical research enterprise.
Califf emphasized the need to integrate research and practice. He noted that the quality of electronic health records (EHR) data and claims data is improving, and standards, common data models, and automated curation methods that are being developed and deployed can help support the advancement of a learning health system.
Lastly, Califf pointed to the use of cloud computing to federate data, information, and knowledge. Technology can be used to optimize the collection, storage, curation, and global sharing of data for regulatory and technology assessment purposes, Califf said. Technology has enabled the ability to “bring the questions to the data,” rather than just bring data to the questions, he continued. “[W]e are going to be much better off if we create global datasets that are available, with the proper protections, to a variety of people to try to understand what the data mean and to participate in the research in a direct way,” Califf said.
Fundamental Non-Technical Issues to Be Addressed
Several non-technical issues need to be addressed if technologies are to be used to their fullest extent in clinical trials, Califf said. These include the interrelated issues of how to govern the privacy and confidentiality of health-related data; prioritization of clinical studies (and who determines priorities); and how to balance the risks versus the benefits of clinical trial participation.
Ultimately, he said, “Digital technology can either be a rising tide that raises all boats if we make it equitable … or it can be used much like it is now in most of our health systems … to segment populations to optimize the situation for some people, particularly those who are already digitally enabled.”
Using Technology to Optimize the Clinical Trials Enterprise: Breakout Discussion Highlights
A summary of the points made by individual breakout group participants was provided in plenary session by Celia Witten of the Center for Biologics Evaluation and Research (CBER) at FDA, Sam Roosz of Crescendo Health, Ed Seguine of Clinical Ink, and Jeanne Regnante of the LUNGevity Foundation on behalf of breakout groups. The following topics were highlighted as being of interest for further discussion in subsequent workshop meetings (see Chapter 4). This section is the rapporteurs’ summary of the breakout groups reports by Witten, Roosz, Seguine,
and Regnante, and should not be construed as reflecting any group. All suggestions and proposals are reported for discussion purposes only.
The Use of Digital Health Technologies in Trials
Breakout participants discussed the need to intentionally consider whether the use of digital health technologies in clinical trials would be deployed as a tool to more effectively mine data from communities (i.e., with limited return of information or benefit to the community) versus being used as a tool to work more collaboratively with patients and communities (e.g., to reduce the burden of trial participation and return information back to individuals and communities, and to build value and transparency in the research enterprise). The acceptability of technologies and innovative methodologies in regulatory submissions may not be clearly established, so breakout participants suggested that guidance for industry from regulators might be needed so that sponsors can more confidently deploy these technologies in trials. Participants also discussed the need for training of clinical operations staff to ensure they are confident in the use of current and new technologies for clinical trials. The lack of clear and consistent terminology across industry regarding the use of technologies in trials was also raised. Breakout participants highlighted the need to disseminate information about initiatives and best practices for the use of technologies in clinical trials. The importance of applying lessons from the response to the COVID-19 pandemic to the use of technology in clinical research was also a recurring theme of discussion (see Chapter 4).
Technology and Trial Participants
The role of technology in trial recruitment was discussed, including the use of advanced analytics to identify potential participants from underrepresented groups, and engaging and establishing relationships with communities through the use of social media. The need to better leverage the power of communication was highlighted, including communication campaigns to educate the public about the benefits of trials and trial technologies. It was observed that access to technology tools varies across communities. Breakout participants discussed the value of investing in access to technology resources within communities, with a focus on technology that would fit into trial participants’ daily lives.
Data Collection and Sharing
Breakout participants discussed the need for harmonization of data collection and tools that can facilitate data sharing and translation across
data systems, so that data collection efforts do not hinder existing workflows and practices. The need to engage patients in identifying the outcomes of interest to them first, before designing the study, was also raised. Participants discussed the importance of responsibly and transparently sharing data from clinical trials with communities to build public trust in clinical research and add value back to the community.
ENVISIONING A MORE RESILIENT, SUSTAINABLE, AND TRANSPARENT CLINICAL TRIALS ENTERPRISE
A Perspective on the State of Clinical Trials in 2021
The clinical research enterprise primarily serves the needs of three key stakeholder groups: sponsors, patients, and societies, Levy said.3 Clinical research is conducted by the industry sponsors, who seek to improve the speed, efficiency, and success rates of their trials. At the same time, it is important to remember that clinical research is ultimately conducted for the benefit of the patients and for communities impacted by the costs and burdens of disease. Each stakeholder group has its own distinct interests which, he observed, can be in conflict to some extent (i.e., the interest of one might be only satisfied at the expense of another). However, Levy said, “what the pandemic taught us … is that a greater focus on the needs of patients and societies is, in fact, consistent with the industry’s needs for greater efficiency and productivity and therefore we can transform the clinical research enterprise in a way that benefits all parties, including industry.” Levy considered the current and future states of research from the perspective of each stakeholder group.
Sponsor Perspective: Enhance Efficiencies
Clinical research is a high-risk, costly, complex enterprise with poor success rates and low return on investment, Levy said. He explained that, in the absence of price increases, such an industry can only survive in a capital-rich environment. From a sponsor perspective, increasing operational efficiencies is essential. Levy outlined four areas for opportunity:
- Continuous process improvement could yield improvements in efficiency which, while incremental, could compound over time. Improved process efficiency also benefits patients by, for example,
___________________
3 Levy noted that the opinions expressed in his presentation are solely his own and do not necessarily represent those of his employer or any other party.
- reducing site workload, thereby allowing sites to focus more on patients than process.
- Platform trials, adaptive trial designs, and the use of historical clinical trial comparator data could increase trial efficiencies. Patients can benefit as well, Levy said. For example, use of a historical comparator means that participants are less likely to receive an ineffective clinical comparator.
- Trial simplification could result in cost savings (e.g., large outcomes trials following on the initial registration trial).
- Substitution of real-world evidence for evidence gathered in the course of traditional clinical trials could increase trial efficiency. Levy said he expects increased attention and use of real-world evidence in the coming years.
Levy pointed out that improving industry efficiency may also help reduce the burden of trial participation for patients and increase the volume of reliable and relevant data available to support evidence-based decision making on the part of stakeholders across the clinical trials enterprise.
Looking beyond operational efficiencies, Levy suggested that the expanding use of data and technology in trial design and execution will significantly improve the speed, efficiency, and success of clinical trials in the coming decade. Real-world data collected in health care settings can help provide a more complete picture of local patient characteristics and standards of care, which can be used to refine eligibility criteria and site selection, making trial enrollment more efficient and predictable. The increasing availability of patient-level genomic and proteomic data will enable identification of patients who would be most likely to benefit from the investigational intervention. This would enable smaller, faster studies, Levy said, and increase value for patients and society. Improved analytics, artificial intelligence, and machine learning can be applied to generate faster, more rigorous systematic reviews to inform the development of research questions and study designs, and to screening incoming clinical trial data for safety and other signals. There is also potential for new data collection approaches, such as passive data collection by wearable devices, to expand in scope over the coming decade and contribute to improved trial design and execution.
Patient Perspective
Levy outlined some patient-centered elements that he believes will be parts of future clinical trials:
- “Patients will routinely participate in the design of clinical trials,” Levy said.
- Trials will be more accessible through increased selection of sites in community settings, and through the increased use of remote trial conduct methods that were more broadly deployed and validated during the COVID-19 pandemic (see Figure 2-1).
- Adaptive trial designs can reduce the amount of non-informative testing to which participants are subjected, and platform trials can increase the likelihood of participants receiving an effective therapy.
- Advances in genomics and proteomics will allow for tailoring of treatments to individual patient needs and increase the probability of patient benefit.
“All these changes, which are made in the interest of patients, will benefit sponsors by improving recruitment, retention, and data quality,”
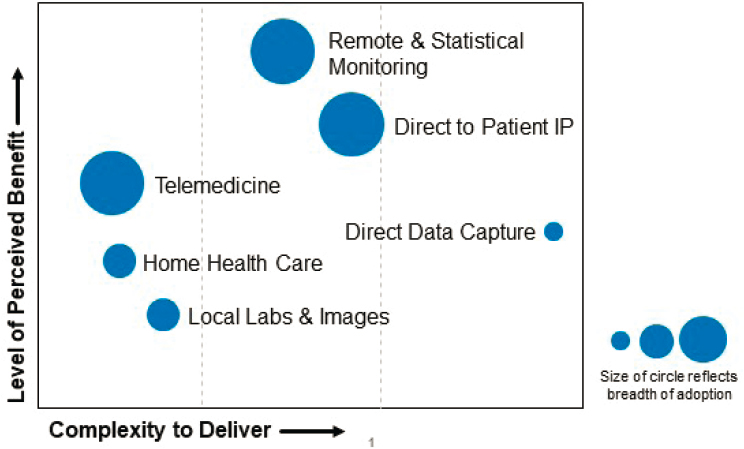
NOTES: Approaches adopted (to varying degrees as indicated by circle size) included telemedicine, remote and statistical monitoring, shipment of investigational product (IP) directly to patients, home health care, local collection of laboratory and imaging data, and direct data capture. Levy noted that the effort expended to deploy these approaches was generally acceptable to the sponsor and enabled the sponsor to safely continue and complete the study during the COVID-19 pandemic.
SOURCE: Levy presentation, January 26, 2021.
Levy added. As an example, he described Amgen’s approach to end-to-end patient engagement in the drug development process. Prior to the start of Phase 1, Amgen gathers patient input on their unmet treatment needs. When a target had been identified, patients provide input on their desired attributes for the products, and specify elements of the study design that would enable or encourage them to participate (e.g., dosing regimens, outcomes measures). The patient voice is also increasingly included in sponsor interactions with regulatory authorities and payers.
Societal Perspective
Levy observed that not enough clinical trial data are available on minority populations because of the underrepresentation of minority participants in clinical trials. He noted that participating in clinical trials “is a problem with deep historical roots” for many African Americans, and added that limited access to clinical trials in general compounds the barriers to participation for many underrepresented populations. The competitive model, by default, can limit the sharing of clinical trial data, and drives biopharmaceutical investment toward areas where incentives are greatest. This can lead to investments that are not aligned with societal need, leading to a lack of trust in the clinical research enterprise, Levy said.
There is opportunity for improvement in the value that the clinical trials enterprise delivers to societies, Levy said. He listed a few steps toward a future state that better promotes health equity and public trust:
- The balance between collaboration and competition should be reset to maintain incentives for innovation while expanding the scope of precompetitive collaboration and data sharing.
- A modified incentive system could help drive investments toward clinical research that is in better alignment with public health priorities, which in turn can help build public trust in the enterprise.
- A systematic effort is needed to increase diversity among trial participants. Levy suggested, “Clinical trial diversity can be increased. We already know how. What is most needed is simply the will and the discipline to systematically apply existing methods.”
The COVID-19 pandemic has been a driver of change in clinical research and health care and has led to increased sharing of data and other proprietary information (AstraZeneca, 2020; COVID R&D Alliance, 2021; FDA, 2020a; Janssen Vaccines and Prevention, 2020; Moderna TX, 2020; Pfizer, 2020; TransCelerate, 2020). For example, the major COVID-19 vaccine trial protocols were publicly posted, which Levy said would previously have been “unthinkable.” The public disclosure of COVID-19 vac-
cine trial data drew attention to the issue of diversity in trial populations and has fostered discussions of minority underrepresentation in these trials. One notable example of this type of collaboration, Levy said, is the COVID R&D Alliance of major biopharmaceutical companies, which is focused on accelerating development of therapies for COVID-19 though repurposing, trial acceleration, data sharing, and pandemic preparedness. Another example is TransCelerate BioPharma, Inc., a nonprofit collaborative established in 2012 by the major biopharmaceutical companies to advance clinical research. In response to COVID-19, TransCelerate developed and launched a platform for sharing patient-level data from COVID-19 trials among researchers to inform future trial design and conduct (e.g., refining eligibility criteria, optimizing endpoints for assay sensitivity).
The interests of the sponsors, patients, and societies are not necessarily in conflict, Levy concluded. A more patient- and society-focused clinical research enterprise can also be more efficient and productive for industry trial sponsors.
Building a More Resilient, Sustainable, and Transparent Clinical Trials Enterprise: Breakout Discussion Highlights
A summary of the points made by individual breakout group participants was provided in plenary session by Clay Johnston of the Dell Medical School, Peyton Howell of Parexel, Jeanne Regnante of the LUNGevity Foundation, and Celia Witten of CBER at FDA on behalf of each of the four breakout groups. The following topics were highlighted as being of interest for further discussion in subsequent workshop meetings (see Chapter 5). This section is the rapporteurs’ summary of the breakout group reports by Johnston, Howell, Regnante, and Witten, and should not be construed as reflecting any group. All suggestions and proposals are reported for discussion purposes only.
Moving Toward Community-Based Trials
Participants discussed ways to better integrate clinical research and routine health care. In the wake of the COVID-19 pandemic and the need for the clinical trials enterprise to be better prepared for the next pandemic, there may be motivation for investments to improve the clinical trials enterprise. Participants discussed the creation of a clinical trials network that is community based, which could quickly transition from routine trials for chronic conditions to trials needed to respond to the next public health emergency. It was observed, however, that many communities lack the infrastructure needed for efficient participation in current clinical trials.
Approaches to address systemic racism and how to bring clinical trials to communities were discussed. Breakout discussants shared ideas for engaging trusted community members as brokers, involving the community in the development of trial networks, and fostering a clinical trials workforce that reflects the patients in the communities they serve.
Workforce and Workflow
Participants discussed the need to develop career paths and incentives for primary care and community-based physicians to act as clinical trial investigators in multicenter trials. Similarly, incentives for academic investigators to participate in large platform trials versus initiating their own smaller trials were discussed. Breakout discussants emphasized the need to fund the conduct and expansion of community-based participatory research and training and to provide incentives for community-based researchers. Workflow issues were also discussed, such as the pressures on clinical investigators to meet the competing demands of clinical trials and health care delivery.
Evidence Generation and Regulatory Review
The generation of quality data to support regulatory review was a key topic of interest. Participants discussed the role of institutional review boards (IRBs) in preventing uninformative trials from moving forward, and how enhanced coordination between regulators and industry sponsors might help ensure that data generated through novel methods will be acceptable for regulatory review and approval. The use of real-world data in clinical trials was highlighted as a means to bridge clinical research and health care delivery, and the need for standardized definitions of data elements in EHRs was noted. Breakout discussants suggested there may be lessons learned based on the UK RECOVERY Trial (see Chapter 5) and other ongoing efforts that have successfully coordinated clinical trials and enabled the sharing of standardized trial data.














