8
A Role for the United States in Coordinated Global Action
The COVID-19 pandemic has reinforced how microbial pathogens can spread rapidly and without respect for national borders. The spread of resistant pathogens and resistance genes can be equally rapid but more insidious. The consequences of the spread of SARS-CoV-2 were relatively clear and direct, while resistant pathogens may move around the world undetected until they are established in a population.
The global dissemination pathways for antibiotic-resistant bacteria are well documented and include international travelers carrying resistant bacteria on their skin, in their gut, or in their upper respiratory system (Arcilla et al., 2017; Nurjadi et al., 2015; Reuland et al., 2016; Walker et al., 2018; Worby et al., 2020). Returning travelers may also contract a resistant infection as a patient in a country where these infections are more prevalent and infection control measures inadequate (CDC, 2021; Khawaja et al., 2017). Importation of contaminated food, fish, and animals is another important dissemination pathway, as is the migration of wildlife (Arnold et al., 2016; Jung and Rubin, 2020). Once within the community and especially within a health system, resistant bacteria are difficult to control. As Chapter 3 discussed, the health and economic effects of antimicrobial resistance may extend far beyond the initial infection and its effects.
For all these reasons, a national strategy to combat antimicrobial resistance will depend on global investment and sustained international engagement integrated across human, animal, and environmental health. Part of the challenge of responding to antimicrobial resistance is
that, while the U.S. strategy and action plan, like most countries’ strategies and action plans, evoke a One Health grounding, putting it into practice is difficult (GCOA and IDSA, 2021). Ultimately, every implementing agency involved in the response to antimicrobial resistance has its own mandate and mission, and none of these is explicitly a One Health mission. As the previous chapter explained, the implementing agencies are to be commended on their progress in meeting the goals set in the National Action Plan for Combating Antimicrobial Resistance, 2015–2020. At the same time, meaningful and measurable progress against antimicrobial resistance will hinge on cross-sectoral policies and the balancing of human, animal, and environmental health priorities. A recent assessment of 11 countries’ progress against their national action plans found the environmental component of most national strategies needs more attention and that integrating private-sector involvement into the national strategy is also challenging (GCOA and IDSA, 2021). In short, the holistic management of a One Health agenda is challenging for most countries.
This challenge of coordinating a One Health response is compounded when working internationally, making global coordination essential. In surveillance, for example, an investment in improving our national system will return more if comparable effort is directed to integrating surveillance data internationally. A surveillance system that only detects and responds to resistant bacteria in the United States will be at a constant disadvantage, missing early signals of emerging resistance. An international link would provide U.S. authorities with prompt information about emerging threats, allowing them to communicate these risks to health officials throughout the United States. Early warning about such threats could help providers at hospitals, nursing homes, and other health care facilities to detect, prepare for, and control the widespread transmission of the resistant pathogens within their networks.
The integration of surveillance data from human, animal, and environmental sources will be a critical component of a global strategy against antimicrobial resistance. The largest increases in antimicrobial consumption over the past 2 decades, for both humans and livestock, have occurred in low- and middle-income countries (Klein et al., 2021; Tiseo et al., 2020). Between 2000 and 2015 these countries saw a 90 percent increase in use of antimicrobials on the World Health Organization (WHO) Watch List, including most of the highest-priority agents among the critically important antimicrobials for human medicine (Klein et al., 2021). As the Global Burden of Disease modeling shows, low- and middle-income countries bear the highest burden of resistant infections; deaths rates from resistant infections were highest in sub-Saharan Africa (AMR Collaborators, in review).
Low- and middle-income countries frequently have a high burden of human infectious diseases and a growing demand for animal-source foods (IHME, 2021; Our World in Data, 2021; Tarawali, 2018). These needs can contribute to inappropriate antimicrobial use, especially when linked with poor health systems, inadequate infection prevention programs, weakly regulated access to antimicrobials, and minimal laboratory diagnostic capacity. For the rest of this century, human population growth will be concentrated in low-income countries, mostly in tropical or subtropical latitudes—sub-Saharan Africa’s population is projected to double in the next 30 years and reach over 3 billion by 2100 (Vollset et al., 2020). This population trend will likely drive increased antimicrobial use in humans, animals, and crops.
The need for coordinated action against antimicrobial resistance is urgent and growing. An international investment in this problem is both morally compelling and in the best interest of the United States. As the COVID-19 pandemic has illustrated, infectious pathogens have no regard for national borders. COVID-19 has drawn attention to the ways in which the United States can use its technical depth in science and medicine for global public benefit. The National Institutes of Health (NIH) Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership, discussed in Chapter 6, for example, brought together multiple government agencies, the European Medicines Agency, and various representatives from academia, industry, and philanthropy to hasten the development of novel medical products (NIH, 2021).
A national response to antimicrobial resistance proportionate to the size and scope of the threat would work across government agencies and in collaborative, bilateral and multilateral relationships internationally. A program modeled on the President’s Emergency Plan for AIDS Relief (PEPFAR) may be best suited to this problem.
Since its founding in 2003 PEPFAR has invested over $85 billion in the fight against the HIV epidemic, saving over 20 million lives in 50 countries and contributing to a 39 percent reduction in AIDS deaths since 2010 (Department of State, 2021; UNAIDS, 2020). Key to this success was the coordinated work of multiple government agencies, including those that are also essential to combat the threat of antimicrobial resistance.
An antimicrobial-resistance program modeled off PEPFAR would expand the global engagement of U.S. agencies. Key to this engagement would be the strengthening of international surveillance for resistant pathogens through increased support for both national and multilateral surveillance systems, such as the WHO’s Global Antimicrobial Resistance and Use Surveillance System (GLASS), described in Chapter 4, and the coordinated efforts of the tripartite program on antimicrobial resistance of the WHO, the Food and Agriculture Organization of the United Nations (FAO), and the World Organisation for Animal Health (known by the his-
torical acronym OIE). Such support would allow for a better understanding of the true burden of resistant infections, allowing for better targeted response strategies, and provide critical, early warning information about emerging resistant threats. A PEPFAR-like program would also allow U.S. agencies to mitigate the need for antibiotics by improving infection prevention and antimicrobial stewardship in both human and animal health in low- and middle-income countries. Lastly, it would provide sustained leadership and accountability in the form of a Global Coordinator for Antimicrobial Resistance modeled on the Global AIDS Coordinator. The global coordinator would help ensure accountability. This person could also require rigorous program evaluations, setting up a cycle of increasingly more effective and better targeted interventions.
Recommendation 8-1: Congress should expand the United States global engagement on antimicrobial resistance by (1) strengthening surveillance of resistant pathogens both by supporting existing, multilateral surveillance systems and by expanding U.S. agencies’ international surveillance programs; (2) reducing need for antimicrobials by broadening agencies’ work on infection prevention and antimicrobial stewardship in humans and animals; and (3) ensuring sustained leadership and critical evaluation by creating a Global Coordinator for Antimicrobial Resistance similar to the Global AIDS Coordinator.
The urgent threat of antimicrobial resistance requires a sustained global response from the United States. The committee does not envision this program would replace U.S. support for various international efforts such as the Tripartite Program. Rather, the proposed program would work in collaboration with existing networks and build off existing bilateral relationships in order to better coordinate global and national efforts. For example, the Centers for Disease Control and Prevention (CDC) already works in over 60 countries in bilateral partnerships with ministries of health and other host country organizations (CDC, 2017). USAID works in over 100 countries; the agency’s strategic plan emphasizes the prevention of outbreaks and infectious diseases (USAID, 2021c; USAID and Department of State, 2018).
The main novel contributions of the proposed program are the emphasis on supporting countries’ surveillance capabilities and the global surveillance effort for antimicrobial resistance; the efforts made to reduce the need for antimicrobials in low- and middle-income countries; and the creation of the global coordinator. By implementing these programs the United States could expand its leadership in combating antimicrobial resistance around the world.
STRENGTHENING SURVEILLANCE
Since its start in 2015, the WHO GLASS program has enrolled 109 countries (WHO, 2020). GLASS provides a global system for collecting national data on antimicrobial use and resistance and a snapshot of each country’s microbial surveillance systems. The Emerging Antimicrobial Resistance Reporting tool is an important component of GLASS, as it provides WHO with early notification about new resistance patterns (WHO, 2021c). The system provides a critical service in low- and middle-income countries, where the burden of infectious disease and antimicrobial use can create hot spots for the emergence and spread of resistant pathogens.
GLASS also coordinates with regional networks, including the Central Asian and European Surveillance of Antimicrobial Resistance, the European Antimicrobial Resistance Surveillance Network, the Latin American Network for Antimicrobial Resistance Surveillance, and with the UK Fleming Fund, which helps low- and middle- income countries fight antimicrobial resistance by improving surveillance (WHO, 2017). These data sources are skewed, however, toward higher-income countries (WHO, 2020). In 2019, less than 15 percent of low- and middle-income countries reported any antimicrobial resistance information to these networks, and most of what was reported was from selected hospital and clinical laboratories (WHO, 2020). These samples are not representative of national burdens of resistant pathogens, as data may be based on bacterial isolates from only a few surveillance sites (Schnall et al., 2019; WHO, 2020). Since a surveillance system is only as good as the information that is entered into it, the lack of nationally representative, high-quality data on antimicrobial use and resistance limits the WHO’s and member countries’ ability to conduct risk assessments or to use these data to monitor resistant threats.
Since 2015 the WHO has expanded GLASS’ scope to include information on antimicrobial use, health care–associated infections, and resistant pathogens in food (WHO, 2021c). It has also developed a capacity-building component that includes technical support and laboratory strengthening (WHO, 2021c). The sustained effort from the WHO has also drawn attention to the need for antimicrobial surveillance and helped ministries in low- and middle-income countries build better integrated surveillance systems from the start (Charitonos et al., 2019; The Fleming Fund, 2016). GLASS also provides implementation guidance specifically tailored to meet the challenges encountered in low- and middle-income countries, such as lack of a national action plan on antimicrobial resistance, the need for an accredited, coordination laboratory at a sentinel surveillance site, and the need for training clinical, laboratory, information technology, and public health personnel about antimicrobial resistance (Seale et al., 2017).
The GLASS program has also made efforts to measure antimicrobial consumption in certain countries (WHO, 2021d). Surveys can be useful to measure antimicrobial consumption, especially in structured settings such as hospitals (Versporten et al., 2018). Measuring consumption in animals, and even in outpatient human medicine, can be more complicated. Sales data, still widely used as a proxy for use in animals, are almost impossible to interpret (FDA, 2020). Proposed measures of use include veterinary antimicrobial sales, as well as total consumption expressed per 1,000 people per day, and consumption ratios of broad- to narrow-spectrum antimicrobials (ECDC et al., 2017). All these measures have biases, and it is not clear which are best suited to facilitate comparisons between settings and over time.
One Health Surveillance
In 2018 GLASS piloted a program for surveillance of extended-spectrum beta-lactamase Escherichia coli in human samples, poultry, water (specifically sewage), market runoff, and urban rivers in nine countries (WHO, 2021b). Such efforts are indicative of a long-term One Health vision for the program. But for the most part, GLASS’s target population for routine surveillance is patients on whom clinical samples were drawn at health facilities (WHO, 2021a). This choice is understandable, and the narrow focus may help country surveillance experts and the WHO support staff keep the scope manageable and quality good. One useful role the United States could play would be in building off the GLASS framework to give more attention to animal and environmental sentinel surveillance.
This One Health attention would not necessarily need to be built from scratch. The FAO also supports countries in developing surveillance for resistant pathogens in food and agriculture. It provides guidelines on data management and susceptibility testing tools for aquaculture, and supports reference centers in eight countries to build tools and knowledge about antimicrobial surveillance in food and food-producing animals (FAO, 2016, 2019, 2021a,b,c; Smith, 2019). The WHO and the FAO Codex Alimentarius Commission also supports the monitoring of resistant pathogens in food and agriculture (FAO, 2016). The U.S. effort would do well to work with these and other networks to integrate data from a range of sources. In veterinary surveillance in particular the efforts being made in low- and middle-income countries are often still in early stages. Deliberate attention from the United States could be a catalytic investment in moving animal and environmental surveillance forward.
To start, U.S. efforts could aim to integrate more and different types of surveillance information, as described in Chapter 4, to give a better pic-
ture of the burden of resistance across human, animal, and environmental health. Coordinating for data standardization and automated capture, for example, would reduce the reporting burden on hospitals, clinics, and public health laboratories, something that would be disproportionately valuable in parts of the world with shortages of health workers and infrastructure. Similarly, an effort to automate surveillance would mean faster turnaround on information, something valuable in low- and middle-income countries, where the opportunity costs of wasting time or resources are high and good data to guide antimicrobial stewardship activities are scarce.
Attention to surveillance would complement U.S. government agencies’ existing programming. For example, the CDC is a national lead agency on the Global Health Security Agenda, an effort to strengthen the prevention, detection, and response to infectious threats (CDC, 2021). The CDC also leads the national Antibiotic Resistance Solutions Initiative, a program that improves capacity for surveillance, response, and containment of resistant pathogens in the United States (CDC, 2020f; PCAST, 2020). As part of the Global Health Security Agenda, USAID has worked with OIE and FAO on surveillance of zoonotic disease and on strengthening of veterinary laboratories, albeit on a relatively small scale (USAID, 2019a). The Department of Defense (DOD), through the Defense Threat Reduction Agency, has supported increased laboratory capacity for detecting specific zoonotic pathogens that pose a clear risk to human health (DARPA, 2019). The committee commends these efforts and encourages agencies to build off the knowledge gained from these programs to improve information flow between these systems and human health surveillance networks and to work toward transferable strategies for use in low- and middle-income countries.
One useful strategy to build surveillance capacity is the network of networks approach (Novossiolova et al., 2020). In both agriculture and human medicine, there are so many diverse systems for collecting animal health information, many of them managed by industry, that working across networks is always desirable (Ashley et al., 2018; Lees and Prince, 2017). The network of networks approach has an advantage of redundancy; a signal missed in local- or provincial-level surveillance may be picked up at the national level (Ashley et al., 2018; Lees and Prince, 2017). It can also integrate data from different types of surveillance systems. For example, the CDC works through its Antibiotic Resistance Laboratory Network and other networks such as PulseNet, which monitors foodborne outbreaks, to detect and respond to resistant pathogens (CDC, 2019; PCAST, 2020). This approach would be invaluable in low- and middle-income countries where there are often surveillance networks not part of the WHO GLASS system (Africa CDC, 2018; Gandra et al., 2020).
In working against a complex health threat, most countries and organizations will understandably want to channel their efforts into direct health programs, likely programs for human health. This instinct is understandable; politicians and health experts already have ideas about what their communities need and how to use their resources. At the same time, any programming or policy intended to combat antimicrobial resistance depends on a foundation of information that surveillance networks supply. Without this information it will be almost impossible to know if programs are having their intended effect or if resources are being allocated wisely. Yet a recent analysis of 11 countries’ ability to meet their commitments to combating antimicrobial resistance found that so far the problem has failed to inspire political action, especially evident in government allocations for surveillance, stewardship, and environmental management (GCOA and IDSA, 2021). The report described the environmental programming as “vastly underfunded, preventing the full integration of a One Health approach across sectors” (GCOA and IDSA, 2021). This may be the foundational problem complicating the global response to antimicrobial resistance. Leadership from the U.S. government on developing surveillance would have meaningful ramifications across the world.
REDUCING NEED
The emergence and spread of antimicrobial-resistant bacteria is the inevitable consequence of antimicrobial use. Mitigating this problem requires attention to antimicrobial use, especially in global hotspots. A higher burden of infectious disease and problems with infection control in health care contribute to the greater need for antibiotics in low- and middle-income countries. There is also a problem of inappropriate antimicrobial use, often a consequence of limited access to medicines and to quality health services (Das and Horton, 2016).
Controlling Infection
One path to reduce the need for antimicrobials is to attack the root problems such as crowding, contaminated water and food, lack of sanitation, and inadequate infection prevention measures (Holmes et al., 2016). Improving wastewater management and sanitation in low- and middle-income countries may be the most important step to controlling antimicrobial resistance globally (GCOA and IDSA, 2021). This is an area where the USAID has considerable experience and relatively consistent involvement across administrations. The agency’s water and sanitation programs operated in 51 countries, providing safe drinking water for over 53 million people between 2008 and 2019 (USAID, 2020, 2021b). One role the
suggested program could take on might be expanding on USAID water and sanitation programs, as well as those implemented by foundations and nongovernmental organizations, to measure the effects of improving the water and sanitation infrastructure on the burden of infectious disease and antimicrobial use. Structured appropriately, analysis should be able to identify what components of the program drove the decrease, leading to better understanding of the relationship between hygiene, antimicrobial use, and the development of resistance.
The CDC (2020b,d,e) also has a global water, sanitation, and hygiene portfolio, including a program that aims to improve sanitation in hospitals and clinics in eight partner countries. As Chapters 2 and 5 discussed, hospital-acquired infections are dangerous; they can spread rapidly among a vulnerable, often immunocompromised, population. Figure 8-1 shows estimates of the sanitation conditions in health facilities (including hospitals, clinics, and dispensaries) across 78 low- and middle-income countries. The serious problems with piped water, infrastructure for handwashing and toilets, and unsafe waste disposal are all risk factors for the development and spread of antimicrobial-resistant infections. The CDC’s programs emphasize observation and analysis of the conditions in the health center, evaluations, and in-depth interviews with administrators and staff (CDC, 2020a). Sometimes the programs introduce tools for hand hygiene or new latrines (CDC, 2020c). These efforts, much like the agency’s global program in health care infection prevention, have the potential to control the emergence and spread of resistant pathogens. Such work would be an excellent target for expansion and scaling up in partner countries.
In low- and middle-income countries, the need for infection prevention is paramount but often difficult to put into practice. Problems with a reliable supply chain for disinfectants, personal protective equipment, and other consumables are common problems (Vilar-Compte et al., 2017). There is also evidence that lack of staff training on infection control and

SOURCES: CDC, 2020e; Cronk and Bartram, 2018.
a shortage of infection control experts is at the root of the problem, as are general crowding and problems disposing of biomedical waste (Manchanda et al., 2018). Both the CDC and USAID have considerable experience in these areas. Expanding programming would serve the dual goals of protecting local communities and mitigating emergence and global spread of resistant pathogens.
The problem of unstable supply chains applies to medicines as well. Frequent stock outs and poor demand forecasting, combined with a background problem of substandard medicine quality, all contribute to the emergence of resistance (Pisa and McCurdy, 2019). The supply of veterinary antimicrobials may be even less reliable, with some evidence indicating that informal, unregulated channels account for the vast majority of veterinary antimicrobials in some countries (Poupaud et al., 2021). This is an area where USAID has some experience that could be expanded on in implementing this recommendation (USAID, 2019b).
Medical Tourism
As with investment in surveillance, an investment in infection control would benefit the United States. Not only do health care–acquired infections contribute to the global burden of antimicrobial resistance, they have a more direct effect on the United States when its residents travel overseas for health care. It is difficult to know the volume of the market for medical tourism. Estimates of the number of patients traveling for health care range from 4 to 16 million, with roughly 1 million of them originating in the United States (Chen and Wilson, 2013; Dalen and Alpert, 2019). Of the top 10 destinations for medical tourism, 6 are middle-income countries (Costa Rica, India, Malaysia, Mexico, Thailand, and Turkey) (Dalen and Alpert, 2019). The care sought there can be weight loss, fertility, or cardiac treatments; surgeries are also common, including transplantation, cosmetic surgery, and dentistry (Dalen and Alpert, 2019). Before COVID-19, this practice was predicted to be increasing, and it may return as the pandemic fades (Chen and Wilson, 2013; Dalen and Alpert, 2019).
Medical tourists are at high risk of acquiring a resistant infection (Chen and Wilson, 2013). Once home, these patients can be the source of drug-resistant outbreaks in their home countries (Bokhary et al., 2021). In 2018 the CDC identified an outbreak of carbapenem-resistant Pseudomonas aeruginosa among patients who had traveled to Mexico for bariatric surgery (Baum, 2019). The previous year, the CDC reported on an outbreak of nontuberculous mycobacteria surgical site infections among 52 patients in nine states, all of whom had undergone cosmetic surgery in the Dominican Republic (Gaines et al., 2018). These pathogens can then spread in U.S. hospitals and clinics, increasing the risk to other patients in the system. And while medi-
cal tourists are a special category of high-risk traveler, ordinary travelers frequently become colonized with drug-resistant pathogens, commonly resistant enteric bacteria, and may carry them a month or longer (Arcilla et al., 2017; Bokhary et al., 2021). Travelers may carry home bacteria that mirror the microbiological milieu of the place visited. A recent well-publicized case of a young volunteer in India who needed an emergency amputation and contracted multiple drug-resistant bacteria (including, but not limited to, Pseudomonas aeruginosa, Klebsiella pneumoniae, Morganella morganii, and Enterococcus) are reminders of the vulnerability of the health system to imported resistant pathogens (IDSA, 2021; PBS, 2013).
Stewardship and Research
Antimicrobial stewardship also faces unique challenges in low- and middle-income countries (Galindo-Fraga et al., 2018). In an effort to support countries in their health security programs, the WHO (2021e) provides a voluntary external evaluation of countries’ capabilities to comply with the International Health Regulations and respond to a variety of health threats, including antimicrobial resistance. A recent analysis of these evaluations in sub-Saharan Africa found that countries’ antimicrobial stewardship was the weakest link in overall response plans for antimicrobial resistance (Elton et al., 2020). It follows that attention to national stewardship guidelines, which most countries in the region still need to create and implement, would produce major benefits in terms of controlling resistance (Elton et al., 2020). Antimicrobial stewardship is an area U.S. government agencies, especially the CDC and the NIH, have expertise, broadly across humans and animals and narrowly in hospitals.
Stewardship is also an area where government agencies could build on existing efforts. The WHO (2019), for example, has a toolkit to guide the implementation of antimicrobial stewardship programs in low- and middle-income countries. Some research indicates that a lack of funding and staff can be serious barriers to implementing this toolkit, however (Maki et al., 2020). This is an area where U.S. government assistance modeled on the PEPFAR program could be helpful. Attention to the health workforce in partner countries was central to the PEPFAR strategy (USAID, 2021a). PEPFAR gives considerable attention to monitoring health workers, how they are deployed, and their capacity, central to routine monitoring and program management (USAID, 2021a). This kind of information would be useful in considering how to use staff most effectively in antimicrobial stewardship programs. More distal influences on antimicrobial stewardship, including management of the medicines supply chain, the capacity of microbiology laboratories, and the education of the health workforce, would also be key work areas for the proposed program.
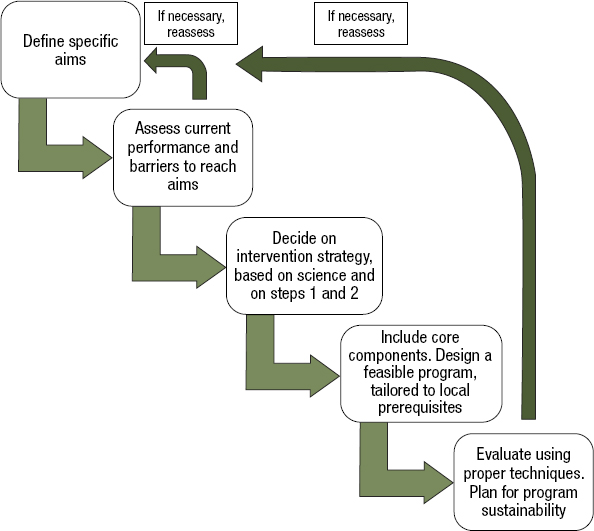
Other barriers to implementing antimicrobial stewardship in low- and middle-income countries include a lack of microbiology laboratory capacity, patient expectations, and the need to provide “just in case” treatment for patients in rural or remote areas (Maki et al., 2020; Mathew et al., 2020). The best steps to address these barriers will vary by setting, though an emphasis on building laboratory capacity and tools to develop better, more reliable antibiograms may be common across many settings (Mathew et al., 2020; Nicholson et al., 2018). By working in partnership with host country governments, U.S. experts could be part of an iterative process to tailor stewardship programs to the local context (see Figure 8-2). Such international partnerships may also be good tools to drive more political will for designing stewardship programs suitable to the challenges and context in low- and middle-income countries (Mathew et al., 2020).
Respiratory and gastrointestinal infections lead to considerable antibacterial use even when the causative pathogen is viral. As Chapters 5 and 6 discussed, widespread vaccination could reduce the need for antimicrobials in both human and animal health, but the evidence for such an effect is limited and based largely on data from Europe and North America. The strategy described in Chapter 5 to improve our under-
SOURCE: Resman, 2020.
standing of vaccination’s effect on mitigating antimicrobial resistance in humans could be equally valuable in studying animal vaccination. Chapter 6 describes a strategy to help bring more animal health products to market, and attention to the ability of such products to reduce antimicrobial use as indicators of resistance would be important to measure in the rollout of these products. Respiratory and gastrointestinal disease drive both prophylactic and therapeutic antimicrobial use in terrestrial animals. Additional research on the downstream value and cost-effectiveness of vaccines and preventive products for use in animals could help drive a cycle of investment, development, and use of these products.
ENSURING SUSTAINED LEADERSHIP
The ambitious, global program envisioned in this recommendation represents a significant investment from the U.S. taxpayer. Given the scope of this investment and the need for coordination with an increasingly large groups of stakeholders both in the United States and abroad, there is a need for a designated national leader on the antimicrobial-resistance effort. This role would be modeled off the Global AIDS Coordinator, with the same responsibility for monitoring and oversight of international response (FBS, 2018).
PEPFAR’s Global AIDS Coordinator oversees all the U.S. government’s international work on combating HIV and AIDS, helping ensure efficiency and avoiding any duplication of effort. PEPFAR’s success is in some ways attributable to this level of oversight, and the committee sees value in replicating this feature of PEPFAR to the efforts on antimicrobial resistance, creating a global antimicrobial-resistance coordinator. This person’s responsibilities could include monitoring progress in reducing antimicrobial use and emerging resistance in the United States, as well as overseeing the government’s activities in low- and middle-income countries. For example, the global coordinator envisioned in this recommendation would remain abreast of the work of the public–private partnership described in Recommendation 6-4. At the same time, the Department of Health and Human Services’ (HHS’s) Office of Global Affairs might provide continuous technical input to support the coordinator’s efforts.
As is evident from this report, addressing the global threat of antimicrobial resistance and its direct effects on the U.S. health care system is complex, engaging multiple U.S. government agencies, various international organizations in human and animal health, national health systems, pharmaceutical and food production industries, and philanthropic partners (e.g., Wellcome Trust). Combating and controlling resistant pathogens requires ongoing action in a range of sectors, including hospital, laboratories, infrastructure (e.g., WASH), and animal, fisheries,
and environmental management. These actions range from providing incentives for antibiotic discovery to changing health care practices or regulatory frameworks.
Coordinating the response to the threat of antimicrobial resistance is challenging, both within the U.S. government and with international partners such as WHO, OIE, and FAO, and in international economic forums such as the G20, the G7, and the Organisation for Economic Co-operation and Development. HHS’s Office of Global Affairs has the main coordinating responsibility for these tasks. This role extends beyond technical coordination and includes multinational and intergovernmental commitments. The National Action Plan for Combating Antibiotic-Resistant Bacteria, 2020–2025 also calls on other U.S. government departments, including the Department of State and DOD to engage with multinational organizations to provide financial and technical expertise on antimicrobial resistance (FTF CARB, 2020). However, the committee notes that although many departments are involved in global antimicrobial-resistance efforts, there is no mandate for coordination across all of the involved organizations (although there is some coordination and collaboration called for among a few agencies).
A recent review of 11 countries’ response to antimicrobial resistance found that antimicrobial resistance is a low priority across most of the countries analyzed (GCOA and IDSA, 2021). By designating a Global Antimicrobial Resistance Coordinator similar to the Global AIDS Coordinator, the U.S. government could demonstrate national commitment to the problem and help build it in partner counties. The global coordinator could also work to mobilize interest of other key funding organizations, as the Global AIDS Coordinator has done in the past (Das, 2007). The interdisciplinary One Health nature of response to antimicrobial resistance makes accountability for results more important. Given the number and diversity of stakeholders in this field, there is considerable potential for duplication of effort or failure to build off of parallel and complementary work.
The committee recognizes that antimicrobial resistance is in some ways a more complicated problem than HIV as it is not the result of any one pathogen or type of exposure. But some of the lessons learned from preventing and treating HIV are applicable to other global health crises, including antimicrobial resistance. Because of PEPFAR, there are processes in place for working with multiple government partners, intergovernmental agencies, the pharmaceutical industry, and various private philanthropic organizations. One common criticism of PEPFAR and other disease-specific programs is that they create distortions in health delivery and in the workforce where services of good quality are provided only to some, HIV-positive people for example, because of the vast donor infra-
structure and spending to support the program for one disease (Biesma et al., 2009; Tangcharoensathien and Patcharanarumol, 2010). A global program for antimicrobial resistance might be able to build off the lessons learned from other global health initiatives and avoid this pitfall (Atun et al., 2008; Biesma et al., 2009).
Through its very nature, response to antimicrobial resistance involves action across different parts of the human, animal, and environmental health systems. Channeling resources wisely into such a varied response is, the committee recognizes, daunting. But by supporting a truly systemic, One Health response, this program may be able to drive meaningful progress on a range of health indicators. An investment in microbiology laboratory capacity, for example, could reverberate across the health system. Attention to animal health could improve livelihoods and food security. Across these sectors, the global coordinator would work as a champion, building support for antimicrobial resistance with international organizations and with counterpart programs abroad.
REFERENCES
Africa CDC (Centres for Disease Control and Prevention). 2018. Africa CDC framework for antimicrobial resistance, 2018-2023. https://africacdc.org/download/africa-cdc-framework-for-antimicrobial-resistance (accessed July 23, 2021).
AMR Collaborators (unpublished). Global burden of bacterial antimicrobial resistance in 2019. Lancet. In review.
Arcilla, M. S., J. M. van Hattem, M. R. Haverkate, M. C. J. Bootsma, P. J. J. van Genderen, A. Goorhuis, M. P. Grobusch, A. M. O. Lashof, N. Molhoek, C. Schultsz, E. E. Stobberingh, H. A. Verbrugh, M. D. de Jong, D. C. Melles, and J. Penders. 2017. Import and spread of extended-spectrum beta-lactamase-producing enterobacteriaceae by international travellers (combat study): A prospective, multicentre cohort study. Lancet Infectious Diseases 17(1):78-85.
Arnold, K. E., N. J. Williams, and M. Bennett. 2016. “Disperse abroad in the land”: The role of wildlife in the dissemination of antimicrobial resistance. Biology Letters 12(8):20160137.
Ashley, E. A., J. Recht, A. Chua, D. Dance, M. Dhorda, N. V. Thomas, N. Ranganathan, P. Turner, P. J. Guerin, N. J. White, and N. P. Day. 2018. An inventory of supranational antimicrobial resistance surveillance networks involving low- and middle-income countries since 2000. Journal of Antimicrobial Chemotherapy 73(7):1737-1749.
Atun, R. A., S. Bennett, A. Duran. 2008. When do vertical (stand-alone) programmes have a place in health systems? Copenhagen: World Health Organization. Regional Office for Europe, Health Evidence Network, European Observatory on Health Systems and Policies. https://apps.who.int/iris/handle/10665/107977 (accessed September 22, 2021).
Baum, S. G. 2019. Carbapenem-resistant p. Aeruginosa from medical tourism. NEJM Journal Watch. https://www.jwatch.org/na49219/2019/06/06/carbapenem-resistant-paeruginosa-medical-tourism (accessed July 19, 2021).
Biesma, R. G., R. Brugha, A. Harmer, A. Walsh, N. Spicer, and G. Walt. 2009. The effects of global health initiatives on country health systems: A review of the evidence from HIV/AIDS control. Health Policy and Planning 24(4):239-252.
Bokhary, H., K. N. A. Pangesti, H. Rashid, M. Abd El Ghany, and G. A. Hill-Cawthorne. 2021. Travel-related antimicrobial resistance: A systematic review. Tropical Medicine and Infectious Disease 6(1):11.
CDC (Centers for Disease Control and Prevention). 2017. CDC works worldwide 24/7 to protect the U.S. From disease threats. https://www.cdc.gov/globalhealth/what/default.htm (accessed July 16, 2021).
CDC. 2019. About Pulsenet. https://www.cdc.gov/pulsenet/about/index.html (accessed July 22, 2021).
CDC. 2020a. Global water, sanitation, & hygeine (WASH): Data collection tools and communication. https://www.cdc.gov/healthywater/global/healthcare-facilities/tools.html (accessed July 19, 2021).
CDC. 2020b. Global water, sanitation, & hygiene (WASH). https://www.cdc.gov/healthywater/global/index.html (accessed July 19, 2021).
CDC. 2020c. Global water, sanitation, & hygiene (WASH). https://www.cdc.gov/healthywater/global/healthcare-facilities/innovations.html (accessed July 19, 2021).
CDC. 2020d. Global water, sanitation, & hygiene (WASH): Where we work. https://www.cdc.gov/healthywater/global/healthcare-facilities/locations.html (accessed July 19, 2021).
CDC. 2020e. WASH in healthcare facilities. https://www.cdc.gov/healthywater/global/healthcare-facilities/overview.html (accessed July 16, 2021).
CDC. 2020f. What CDC is doing: AR solutions initiative. https://www.cdc.gov/drugresistance/solutions-initiative (accessed June 16, 2021).
CDC. 2021. CDC and the global health security agenda. https://www.cdc.gov/globalhealth/security/index.htm (accessed July 16, 2021).
Charitonos, K., A. Littlejohn, H. Kaatrakoski, A. Fox, V. Chaudhari, T. Seal, and N. Tegama. 2019. Technology-supported capacity building on AMR surveillance: Findings from the pilot phase. Milton Keynes, England: The Open University.
Chen, L. H., and M. E. Wilson. 2013. The globalization of healthcare: Implications of medical tourism for the infectious disease clinician. Clinical Infectious Diseases 57(12):1752-1759.
Cronk, R., and J. Bartram. 2018. Environmental conditions in health care facilities in low- and middle-income countries: Coverage and inequalities. International Journal of Hygiene and Environmental Health 221(3):409-422.
Dalen, J. E., and J. S. Alpert. 2019. Medical tourists: Incoming and outgoing. American Journal of Medicine 132(1):9-10.
DARPA (Defense Advanced Research Projects Agency). 2019. A new layer of medical preparedness to combat emerging infectious disease. https://www.darpa.mil/news-events/2019-02-19 (accessed July 16, 2021).
Das, P. 2007. Mark Dybul: US global aids coordinator in charge of PEPFAR. Lancet 369(9568):1161.
Das, P., and R. Horton. 2016. Antibiotics: Achieving the balance between access and excess. The Lancet 387(10014):102-104.
Department of State. 2021. The United States President’s Emergency Plan for AIDS Relief. https://www.state.gov/pepfar (accessed September 22, 2021).
ECDC (European Centre for Disease Prevention and Control), EFSA BIOHAZPanel (European Food Safety Authority Panel on Biological Hazards), and CVMP (EMA Committee for Medicinal Products for Veterinary Use). 2017. ECDC, EFSA and EMA Joint Scientific Opinion on a list of outcome indicators as regards surveillance of antimicrobial resistance and antimicrobial consumption inhumans and food-producing animals. EFSA Journal 15(10):5017.
Elton, L., M. J. Thomason, J. Tembo, T. P. Velavan, S. R. Pallerla, L. B. Arruda, F. Vairo, C. Montaldo, F. Ntoumi, M. M. Abdel Hamid, N. Haider, R. Kock, G. Ippolito, A. Zumla, T. D. McHugh, and the PANDORA-ID-NET Consortium. 2020. Antimicrobial resistance preparedness in sub-Saharan African countries. Antimicrobial Resistance & Infection Control 9(1):145.
FAO (Food and Agriculture Organization of the United Nations). 2016. FAO and WHO activities to support monitoring and surveillance of antimicrobial resistance in the food and agriculture sectors. http://www.fao.org/fao-who-codexalimentarius/news-and-events/news-details/en/c/1381093 (accessed July 16, 2021).
FAO. 2019. Monitoring and surveillance of antimicrobial resistance in bacteria from healthy food animals intended for consumption. In Regional Antimicrobial Resistance Monitoring and Surveillance Guidelines. Bangkok, Thailand: Food and Agriculture Organization of the United Nations.
FAO. 2021a. Antimicrobial resistance: FAO reference centres for antimicrobial resistance. http://www.fao.org/antimicrobial-resistance/resources/reference-centres/en (accessed July 16, 2021).
FAO. 2021b. Antimicrobial resistance: Surveillance and monitoring. http://www.fao.org/antimicrobial-resistance/key-sectors/surveillance-and-monitoring/en (accessed July 16, 2021).
FAO. 2021c. FAOreferencecentres forantimicrobialresistance. https://www.fao.org/antimicrobialresistance/resources/reference-centres/en (accessed October 11, 2021).
FBS (Functional Bureau Strategy). 2018. Office of the U.S. Global aids coordinator and health diplomacy (S/GAC). https://www.state.gov/wp-content/uploads/2019/01/FBS-SGAC_UNCLASS_508.pdf (accessed July 19, 2021).
FDA (Food and Drug Administration). 2020. FDA releases annual summary report on antimicrobials sold or distributed in 2019 for use in food-producing animals. https://www.fda.gov/animal-veterinary/cvm-updates/fda-releases-annual-summary-report-antimicrobials-sold-or-distributed-2019-use-food-producing (accessed May 4, 2021).
FTF CARB (Federal Task Force for Combating Antibiotic-Resistant Bacteria). 2020. National action plan for combating antibiotic-resistant bacteria 2020-2025. Washington, DC: Office of the Assistant Secretary for Planning and Evaluation, Department of Health and Human Services.
Gaines, J., J. Poy, K. A. Musser, I. Benowitz, V. Leung, B. Carothers, J. Kauerauf, N. Mollon, M. Duwell, K. Henschel, A. De Jesus, S. K. Head, K. Lee, N. Arboleda, and D. H. Esposito. 2018. Notes from the field: Nontuberculous mycobacteria infections in U.S. medical tourists associated with plastic surgery—Dominican Republic, 2017. Morbidity and Mortality Weekly Report 67(12):369-370.
Galindo-Fraga, A., M. Villanueva-Reza, and E. Ochoa-Hein. 2018. Current challenges in antibiotic stewardship in low- and middle-income countries. Current Treatment Options in Infectious Diseases 10(3):421-429.
Gandra, S., G. Alvarez-Uria, P. Turner, J. Joshi, D. Limmathurotsakul, and H. R. van Doorn. 2020. Antimicrobial resistance surveillance in low- and middle-income countries: Progress and challenges in eight South Asian and Southeast Asian countries. Clinical Microbiology Reviews 33(3):e00048-00019.
GCOA and IDSA (Global Coalition on Aging and Infectious Diseases Society of America). 2021. 2021 AMR Preparedness Index. https://globalcoalitiononaging.com/wp-content/uploads/2021/06/GCOA-AMR-Preparedness-Index_FINAL.pdf (accessed July 12, 2021).
Holmes, A. H., L. S. Moore, A. Sundsfjord, M. Steinbakk, S. Regmi, A. Karkey, P. J. Guerin, and L. J. Piddock. 2016. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 387(10014):176-187.
IDSA (Infectious Diseases Society of America). 2021. Patient stories: David Ricci. https://www.idsociety.org/public-health/patient-stories/david-ricci (accessed July 19, 2021).
IHME (Institute for Health Metrics and Evaluation). 2021. Communicable, maternal, neonatal, and nutritional diseases—level 1 cause. http://www.healthdata.org/results/gbd_summaries/2019/communicable-maternal-neonatal-and-nutritional-diseases-level-1-cause (accessed July 20, 2021).
Jung, D., and J. E. Rubin. 2020. Identification of antimicrobial resistant bacteria from plant-based food products imported into Canada. International Journal of Food Microbiology 319:108509.
Khawaja, T., J. Kirveskari, S. Johansson, J. Väisänen, A. Djupsjöbacka, A. Nevalainen, and A. Kantele. 2017. Patients hospitalized abroad as importers of multiresistant bacteria—a cross-sectional study. Clinical Microbiology and Infection 23(9):673.e671-673.e678.
Klein, E. Y., M. Milkowska-Shibata, K. K. Tseng, M. Sharland, S. Gandra, C. Pulcini, and R. Laxminarayan. 2021. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000-15: An analysis of pharmaceutical sales data. Lancet Infectious Diseases 21(1):107-115.
Lees, V. W., and C. Prince. 2017. Lessons learned from the evolution of terrestrial animal health surveillance in Canada and options for creating a new collaborative national structure. Canadian Veterinary Journal 58(5):459-465.
Maki, G., I. Smith, S. Paulin, L. Kaljee, W. Kasambara, J. Mlotha, P. Chuki, P. Rupali, D. R. Singh, D. C. Bajracharya, L. Barrow, E. Johnson, T. Prentiss, and M. Zervos. 2020. Feasibility study of the World Health Organization health care facility-based antimicrobial stewardship toolkit for low- and middle-income countries. Antibiotics (Basel) 9(9):556.
Manchanda, V., U. Suman, and N. Singh. 2018. Implementing infection prevention and control programs when resources are limited. Current Treatment Options in Infectious Diseases 10(1):28-39.
Mathew, P., J. Ranjalkar, and S. J. Chandy. 2020. Challenges in implementing antimicrobial stewardship programmes at secondary level hospitals in india: An exploratory study. Front Public Health 8:493904.
Nicholson, A., I. Tennant, L. White, C. A. Thoms-Rodriguez, L. Cook, S. Johnson, T. Thompson, J. Barnett, and L. Richards. 2018. Correction to: The knowledge, attitudes and practices of doctors regarding antibiotic resistance at a tertiary care institution in the caribbean. Antimicrobial Resistance & Infection Control 7(1):77.
NIH (National Institutes of Health). 2021. Medical research initiatives. https://www.nih.gov/research-training/medical-research-initiatives (accessed September 30, 2021).
Novossiolova, T., L. Bakanidze, and D. Perkins. 2020. Effective and comprehensive governance of biological risks: A network of networks approach for sustainable capacity building. In Synthetic biology 2020: Frontiers in risk analysis and governance. Cham, Switzerland: Springer. Pp. 313-349.
Nurjadi, D., B. Friedrich-Janicke, J. Schafer, P. J. Van Genderen, A. Goorhuis, A. Perignon, A. Neumayr, A. Mueller, A. Kantele, M. Schunk, J. Gascon, A. Stich, C. Hatz, E. Caumes, M. P. Grobusch, R. Fleck, F. P. Mockenhaupt, and P. Zanger. 2015. Skin and soft tissue infections in intercontinental travellers and the import of multi-resistant staphylococcus aureus to Europe. Clinical Microbiology and Infection 21(6):567.e1-567.e10.
Our World in Data. 2021. Infectious disease death rates, 2016. https://ourworldindata.org/grapher/infectious-disease-death-rates (accessed July 20, 2021).
PBS. 2013. Transcript: Hunting the nightmare bacteria. https://www.pbs.org/wgbh/frontline/film/hunting-the-nightmare-bacteria/transcript (accessed July 19, 2021).
PCAST (President’s Council of Advisors on Science and Technology). 2020. National action plan for combating antibiotic-resistant bacteria, 2020-2025. Washington, DC: The White House. Federal Task Force on Combating Antibiotic-Resistant Bacteria.
Pisa, M., and D. McCurdy. 2019. Improving global health supply chains through traceability. CGD Policy Paper. Washington, DC: Center for Global Development.
Poupaud, M., V. Putthana, A. Patriarchi, D. Caro, A. Agunos, N. Tansakul, and F. L. Goutard. 2021. Understanding the veterinary antibiotics supply chain to address antimicrobial resistance in LAO PDR: Roles and interactions of involved stakeholders. Acta Tropica 220:105943.
Resman, F. 2020. Antimicrobial stewardship programs; a two-part narrative review of stepwise design and issues of controversy part i: Step-wise design of an antimicrobial stewardship program. Therapeutic Advances in Infectious Disease 7:2049936120933187.
Reuland, E. A., G. J. Sonder, I. Stolte, N. Al Naiemi, A. Koek, G. B. Linde, T. J. van de Laar, C. M. Vandenbroucke-Grauls, and A. P. van Dam. 2016. Travel to Asia and traveller’s diarrhoea with antibiotic treatment are independent risk factors for acquiring ciprofloxacin-resistant and extended spectrum beta-lactamase-producing enterobacteriaceae—a prospective cohort study. Clinical Microbiology and Infection 22(8):731.e1-731.e7.
Schnall, J., A. Rajkhowa, K. Ikuta, P. Rao, and C. E. Moore. 2019. Surveillance and monitoring of antimicrobial resistance: Limitations and lessons from the gram project. BMC Medicine 17(1):176.
Seale, A. C., N. C. Gordon, J. Islam, S. J. Peacock, and J. A. G. Scott. 2017. AMR surveillance in low and middle-income settings—a roadmap for participation in the global antimicrobial surveillance system (GLASS). Wellcome Open Research 2:92.
Smith, P. 2019. The performance of antimicrobial susceptibility testing programmes relevant to aquaculture and aquaculture products. Rome, Italy: FAO Fisheries and Aquaculture Circular.
Tangcharoensathien, V., and W. Patcharanarumol. 2010. Commentary: Global health initiatives: Opportunities or challenges? Health Policy and Planning 25(2):101-103.
Tarawali, S. 2018. Food of animal origin: Demand and diversity. Expert panel: Food of Animal Origin 2030: Solutions to Consumption Driven Challenges, Berlin, Germany. https://cgspace.cgiar.org/rest/rest/bitstreams/65116fb8-0066-4366-a6c8-3cb03c0cf791/retrieve (accessed July 16, 2021).
The Fleming Fund. 2016. Antimicrobial resistance in low and middle income countries. https://www.flemingfund.org/wp-content/uploads/172ffba73bab28bc2bd75659114785c7.pdf (accessed September 22, 2021).
Tiseo, K., L. Huber, M. Gilbert, T. P. Robinson, and T. P. Van Boeckel. 2020. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 9(12):918.
UNAIDS. 2020. Seizing the moment: Tackling entrenched inequalities to end epidemics. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS.
USAID (U.S. Agency for International Development). 2019a. Global health security agenda. https://www.usaid.gov/sites/default/files/documents/1860/Health_Project_Fact_Sheet_-_Global_Health_Security_Agenda.pdf (accessed July 16, 2021).
USAID. 2019b. Supply chain. https://www.usaid.gov/global-health/health-systemsinnovation/health-systems/supply-chain (accessed July 19, 2021).
USAID. 2020. Global water and development. In Report of Water and Sanitation Activities FY 2018/2019. Washington, DC: U.S. Agency for International Development.
USAID. 2021a. Human resources for health: Optimizing the health workforce for hiv service delivery. https://www.usaid.gov/global-health/health-areas/hiv-and-aids/technical-areas/human-resources-health (accessed July 19, 2021).
USAID. 2021b. Water security and sanitation. https://www.usaid.gov/sites/default/files/documents/2020_OnePager_final_revised_1.4.21.pdf (accessed July 16, 2021).
USAID. 2021c. Who we are. https://www.usaid.gov/who-we-are (accessed July 16, 2021).
USAID and Department of State. 2018. Joint strategic plan FY 2018-2022. https://www.usaid.gov/sites/default/files/documents/1870/JSP_FY_2018_-_2022_FINAL.pdf (accessed July 16, 2021).
Versporten, A., P. Zarb, I. Caniaux, M. F. Gros, N. Drapier, M. Miller, V. Jarlier, D. Nathwani, H. Goossens, and Global-PPS Network. 2018. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. The Lancet Global Health 6(6):e619-e629.
Vilar-Compte, D., A. Camacho-Ortiz, and S. Ponce-de-Leon. 2017. Infection control in limited resources countries: Challenges and priorities. Current Infectious Disease Reports 19(5):20.
Vollset, S. E., E. Goren, C. W. Yuan, J. Cao, A. E. Smith, T. Hsiao, C. Bisignano, G. S. Azhar, E. Castro, J. Chalek, A. J. Dolgert, T. Frank, K. Fukutaki, S. I. Hay, R. Lozano, A. H. Mokdad, V. Nandakumar, M. Pierce, M. Pletcher, T. Robalik, K. M. Steuben, H. Y. Wunrow, B. S. Zlavog, and C. J. L. Murray. 2020. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the global burden of disease study. Lancet 396(10258):1285-1306.
Walker, T. M., M. Merker, A. M. Knoblauch, P. Helbling, O. D. Schoch, M. J. Van Der Werf, K. Kranzer, L. Fiebig, S. Kröger, and W. Haas. 2018. A cluster of multidrug-resistant mycobacterium tuberculosis among patients arriving in Europe from the horn of Africa: A molecular epidemiological study. The Lancet Infectious Diseases 18(4):431-440.
WHO (World Health Organization). 2017. Global antimicrobial resistance surveillance system (GLASS) report—early implementation. Geneva, Switzerland: World Health Organization.
WHO. 2019. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: A WHO practical toolkit. Geneva, Switzerland: World Health Organization.
WHO. 2020. Global antimicrobial resistance and use surveillance system (GLASS) report: Early implementation 2020 is the third GLASS report. Geneva, Switzerland: World Health Organization.
WHO. 2021a. GLASS-AMR module. https://www.who.int/initiatives/glass/glass-routine-data-surveillance (accessed July 16, 2021).
WHO. 2021b. GLASS-One Health module. https://www.who.int/initiatives/glass/glass-modules-7 (accessed July 16, 2021).
WHO. 2021c. Global antimicrobial resistance and use surveillance system (GLASS). https://www.who.int/initiatives/glass (accessed June 14, 2021).
WHO. 2021d. Global antimicrobial resistance and use surveillance system (GLASS) report: 2021. Geneva, Swirzerland: World Health Organization.
WHO. 2021e. Joint external evaluation (JEE). https://www.euro.who.int/en/health-topics/health-emergencies/international-health-regulations/monitoring-and-evaluation/joint-external-evaluation-jee (accessed July 13, 2021).
Worby, C. J., A. M. Earl, S. E. Turbett, M. Becker, S. R. Rao, E. Oliver, A. Taylor Walker, M. Walters, P. Kelly, and D. T. Leung. 2020. Acquisition and long-term carriage of multidrug-resistant organisms in us international travelers. Open Forum Infectious Diseases 7(12):ofaa543.




















