1
Introduction
Since the mass production of penicillin began in the 1940s, antimicrobials have drastically improved human health, preventing death from bacterial infection and lowering the risk associated with surgery and other lifesaving medical procedures (Ventola, 2015). These medicines are often credited with driving a sharp rise in life expectancy in the latter half of the twentieth century (Hutchings et al., 2019; Sullivan, 2018). But almost as quickly as the first family of antibacterials was introduced, its usefulness declined. Within 6 years of the introduction of penicillin, roughly a quarter of staphylococcal infections in hospitals (where the drug was often used) were no longer susceptible to it (Chambers, 2001). Penicillin resistance continued to spread, by the 1970s being as common in community-acquired infections as in hospitals (Chambers, 2001).
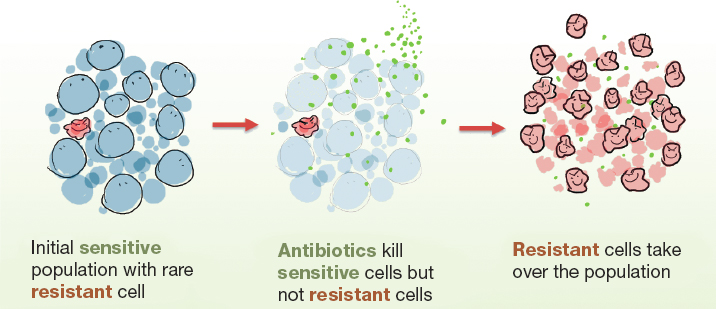
The genes that cause microbes (bacteria, viruses, fungi, and parasites) to survive against the organisms that try to kill them or stop their growth (antimicrobials) are not new. Genomic analysis of permafrost soil samples has found resistance to most antimicrobials in use today existed 30,000 years ago (D’Costa et al., 2011; Perry et al., 2016). While selective pressure from modern antimicrobial medicines has undoubtedly encouraged the survival of resistant organisms (simplified in Figure 1-1), they are an ancient and persistent part of the ecosystem. As Gerry Wright, director of McMaster University (2011) Institute for Infectious Disease Research, explained, “[antimicrobials] are part of our natural world and therefore we need to be incredibly careful in how we use them. Microorganisms have figured out a way of how to get around them well before we even figured out how to use them.”
SOURCE: Yunxin Joy Jiao, reprinted with permission.
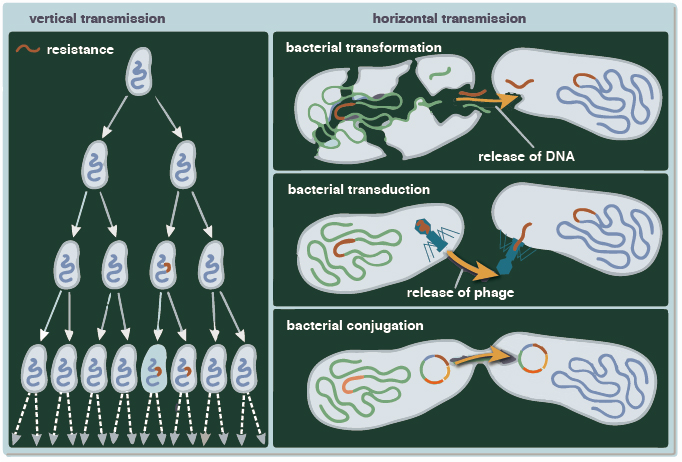
Part of the challenge lies in the many ways microorganisms have for responding to selective pressures. One way is through vertical gene transmission, the classic, Darwinian evolution wherein beneficial gene mutations are passed from one generation to another. But microorganisms, especially bacteria, can pass genes to unrelated organisms, even to other species, in processes described as horizontal or lateral gene transfer (Abe et al., 2020; Keeling and Palmer, 2008). Described as “the movement of genetic information between organisms … except for those from parent to offspring,” horizontal gene transmission is thought to be the dominant means of evolution in microbes (Abe et al., 2020; Burmeister, 2015). Horizontal transmission processes, shown in Figure 1-2, can rapidly spread beneficial genes across microbial communities, ultimately accounting for much of the baseline genetic variability then acted upon by selection pressure (Hall et al., 2020).
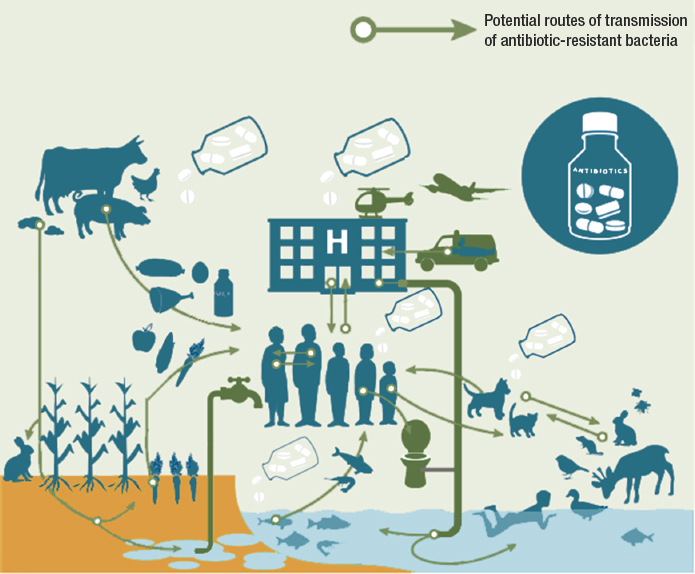
Furthermore, microbes move easily across habitats, living in water and soil as well as in animals and humans. Because of the interconnectedness of these habitats, shown in Figure 1-3, pressures in one setting can easily affect others, creating a reservoir of resistance genes. “All the genes [in a microbial community] that directly or indirectly contribute to resistance” make up the resistome (Wright, 2010). Monitoring changes in the resistome and the concentration of different antimicrobials and resistance genes can give insight into emerging resistance patterns, a topic discussed more in Chapter 4.
Soil is an especially diverse and important reservoir of both antimicrobials and resistance genes (Bello-Lopez et al., 2019; Nesme and Simonet, 2015). Most of the antibiotics developed in the so-called golden age of drug discovery, and even until the 1990s, were developed from soil microorganisms (Nesme and Simonet, 2015). The soil resistome has changed markedly with the use of antimicrobial medicines. Research in archived soil
samples from the 1940s to the early 2000s has found a dramatic increase in the genes causing resistance to all classes of antibiotics tested, with tetracycline-resistance genes alone increasing 15-fold between the 1970s and 2000s (Knapp et al., 2010).
Clinical isolates tell a similar story. Samples from the British antimicrobial reference laboratory have shown an exponential rise in resistance to carbapenems, a group of broad-spectrum antibiotics often used as the drug of last resort for resistant infections (Papp-Wallace et al., 2011; Shallcross et al., 2015) (see Figure 1-4). A 2016 report from the Organisation for Economic Co-operation and Development (OECD) found the prevalence
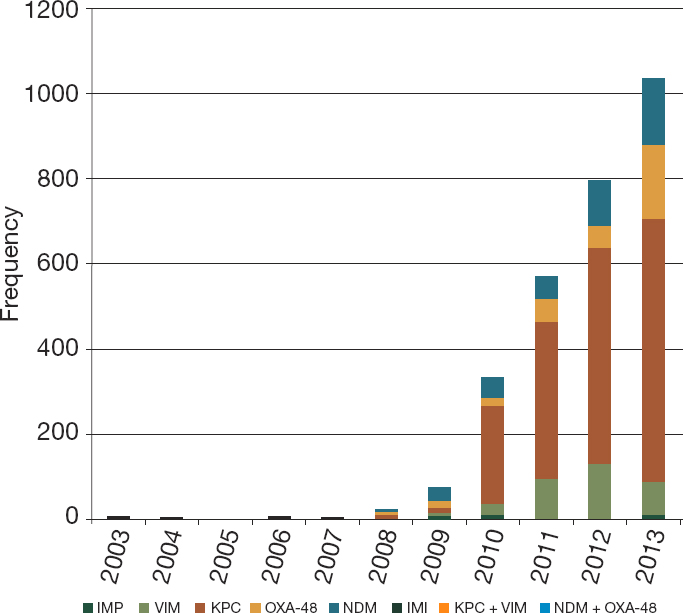
NOTES: Resistance and Healthcare Associated Infections unit, from UK laboratories. IMI = imipenem-hydrolyzing beta-lactamases; IMP = imipenemase metallobeta-lactamase; KPC = Klebsiella pneumoniae carbapenemases; NDM = New Delhi metallo-beta-lactamase; OXA-48 = oxacillinase-48; VIM = Verona integron-encoded metallo-beta-lactamases.
SOURCE: Shallcross et al., 2015, from Public Health England.
of resistance in clinical testing to have increased in 23 of 26 OECD countries in the years between 2009 and 2014,1 rising about 5 percentage points on average, despite largely stable rates of antibiotic use (OECD, 2016). In low- and middle-income countries, where certain infections are more common, as are the crowding, poor sanitation, and limited clinical infection control that make transmission more likely, the situation is worse (Alvarez-Uria et al., 2016). By some estimates, resistant infections are 66 percentage points more common in lower-middle income countries than in rich ones (Alvarez-Uria et al., 2016).
Later sections of this report discuss the global nature of antimicrobial resistance and the challenges of responding to a health crisis with multiple root causes that manifests itself differently in different parts of the same country, or even within the same state or county. It is challenging to marshal a national response to such a varied and disparate threat. A coordinated, strategic response is essential and something the U.S. government has set out in its National Strategy for Combating Antibiotic Resistant Bacteria (PCAST, 2015, 2020; The White House, 2014).
CHARGE TO THE COMMITTEE
The first national action plan for antimicrobial resistance was released in 2015, the result of President Obama’s Executive Order Combating Antibiotic-Resistant Bacteria, which created both an interagency task force to implement the National Strategy for Combating Antibiotic-Resistant Bacteria (released at the same time) and an independent presidential advisory council to make recommendations to the secretary of health on the government’s implementation of the national strategy (CDC, 2020; HHS, 2021; Obama, 2014). The strategy and the national action plan that guides its implementation, drive the U.S. government’s response to the problem of antimicrobial resistance (PCAST, 2015). Its five goals are shown in Box 1-1. Figure 1-5 shows the recent timeline of relevant U.S. government publications.
When the first national strategy and action plans were in their last year, but before the release of the 2020 to 2025 documents, Congress directed the National Institute of Allergy and Infectious Diseases (NIAID) to convene a consensus committee under the auspices of the National Academies of Sciences, Engineering, and Medicine (hereafter, the National Academies) to examine national progress against the goals shown in Box 1-1.2 NIAID is an important implementer of the national action plan, but it is only one of many agencies involved. With this in mind, the study
___________________
1 As indicated through an aggregate combination of six common pathogen-drug combinations.
2 HR 1865 Further Consolidated Appropriations Act, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1865/text (accessed April 8, 2021).
sponsors requested input from their counterparts across the task force to develop the statement of task for this study, shown in Box 1-2. More information about the committee members answering this charge can be found in Appendix A.
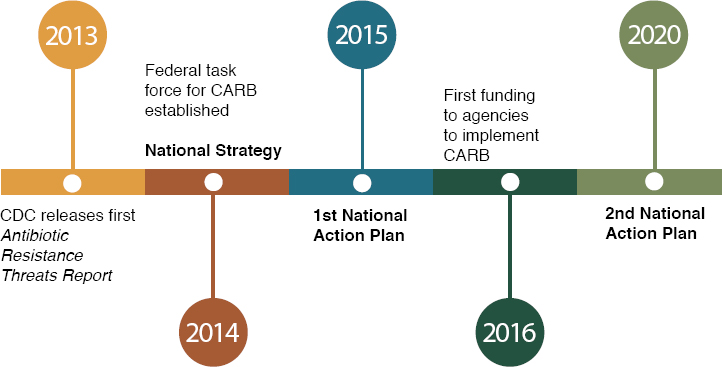
SOURCE: CDC, 2020.
The Committee’s Approach to Its Charge
The committee met six times, each meeting via videoconferencing and spread over several days. The agendas for the public meetings are shown in Appendix C. In closed session, the committee debriefed on the material presented at public meetings and on the literature presented in this report. Committee members also had regular videoconferences to develop their conclusions and recommendations. Members of the public submitted articles and other information for the committee’s review, available upon request from the National Academies’ Public Records Office.
To better understand the progress various agencies involved in the National Action Plan on Combating Antibiotic-Resistant Bacteria made from 2015 to 2020, the committee commissioned an analysis from the Center for Infectious Disease Research and Policy at the University of Minnesota. The researchers drew on published sources and key informant interviews to evaluate agencies’ work. Their analysis is presented as a supplementary web appendix available at https://www.nap.edu/resource/26350/Background_Analysis.pdf.
At the committee’s first meeting, representatives from NIAID and other task force agencies gave an overview of their work and an orientation to the task. One area where they gave the committee some leeway was in its interpretation of the term antimicrobial resistance. This term can refer to resistance in many kinds of microbes, including viruses and protozoa, and to multidrug-resistant strains of mycobacteria that cause tuberculosis. The committee chose to narrow the scope of this study to include antibacterials, excepting tuberculosis medicines and antifungals. Even though many of the points in this report could be broadly applicable, resistance to antimalarials, bioterrorism agents, and antivirals, such as those used to treat hepatitis and HIV, are outside of the scope. This is consistent with the national strategy and the priority pathogens listed in the Centers for Disease Control and Prevention’s (CDC’s) Antibiotic Resistant Threats in the United States, 2019 (CDC, 2019). This strategy is also consistent with the National Strategy for Combating Antimicrobial Resistance. The 2020 to 2025 strategy does not discuss malaria or HIV programming, and mentions tuberculosis only tangentially in relation to global surveillance for resistant infections (PCAST, 2020).
In defining its scope, the committee recognizes that the emergence of resistance is a common biological process largely similar for HIV, tuberculosis, and malaria as for other resistant infections. The scope of public health programming and funding for HIV, tuberculosis, and malaria vastly outweighs the national and international resources directed to other resistant infections, however. Though the underlying mechanisms causing resistance are the same, the response to these diseases is not comparable to that for bacterial and fungal pathogens more broadly. Many of the recommendations presented in this report to counter resistance to antibiotics or antifungals are transferable to other types of resistance. The committee members determined that attention to gaps in the response to bacterial and fungal resistance broadly would allow them to make more meaningful recommendations than had they concentrated on HIV, tuberculosis, and malaria, recognizing nonetheless that the underlying mechanism of resistance to medicines is similar across infectious diseases.
The study sponsors at NIAID also gave the committee considerable leeway in interpreting the charge, “to examine and quantify” the impact
of antimicrobial resistance in the United States. While this charge could be interpreted as a call for original data collection and analysis, the sponsor supported the committee’s strategy to address this point with review of the recent health and economic literature on the topic, especially the major national and international publications that have driven some of the recent public dialogue. This is not a systematic review in the style of a Cochrane review or an exhaustive analysis of every publication on the topic, however.
Part of the challenge of responding to antimicrobial resistance lies in the varied and dynamic nature of the threat. The burden of resistant pathogens differs widely from one place to another, even within different hospitals in the same county, and from one year to the next. It is difficult to predict which pathogens will emerge as serious threats and which will subside, partly because human response influences the future disease burden from any pathogen. For this reason, the committee avoided a reactive emphasis on the pathogens driving the burden of resistant infections today in favor of a broader, more adaptive strategy applicable to a range of bacteria and fungi. This is not to say that this report avoids drawing upon and citing the CDC Urgent Threats and the World Health Organization Priority Pathogens lists (CDC, 2019; WHO, 2017). Rather, in tailoring its recommendations the committee refrained from making recommendations regarding any particular pathogen in favor of those with broader applicability. In short, the committee chose a more fundamental approach to the problem, in line with the One Health view of antimicrobial resistance in humans, animals, and the environment discussed more in the next chapter. For this reason, individual infections (methicillin-resistant Staphylococcus aureus, for example) are not the subject of different report chapters, but the interconnectedness of resistance with other topics in health and disease are discussed throughout.
The committee approached its charge and recommendations with an effort to identify key problems and barriers to their solutions. This should not be understood as the committee’s judgement on the relative merit of strategies that are not the topic of recommendations. Similarly, in making its recommendations the committee tried to strike a balance between innovation and practicality, directing the recommendations to organizations for which the suggested action would be challenging but feasible.
The Organization of This Report
In their deliberation, committee members worked to find common threads and common gaps in response to antimicrobial resistance across sectors. Some of their recommendations are relevant only to animal health, others only to human medicine, some cut across multiple sectors. Rather than being organized by topic (agriculture, medicine, economics)
the report handles these topics together and presents the committee’s analysis by theme. This chapter introduces the topic and the background for this study. The next chapter discusses the scope of the problem and provides context on global action against antimicrobial resistance. Chapter 3 reviews the literature on the health and economic burden of resistance. Chapter 4 discusses surveillance and tools for strengthening it, and Chapter 5 discusses ways to prevent infection and improve stewardship. Chapter 6 turns to the market for new medical products and steps that could make this market work better for public health. Chapter 7 looks at the national action plan and the U.S. government’s response from 2015 to 2020, and the last chapter suggests a role for the United States in the global response to antimicrobial resistance. These chapters contain the reasoning supporting the committee’s recommendations, based on literature review and the result of its deliberations.
This report presents actions that, in the committee’s judgment, have strong promise to control the problem of antimicrobial resistance. The recommendations highlight important gaps and solvable problems but do not constitute an exhaustive list of potential policy actions against antimicrobial resistance. Many of the actions recommended are within the purview of the U.S. government, a strategy the committee sees as suitable to its charge and the congressional mandate for this study. At the same time, action against as complex and global a threat as antimicrobial resistance cannot be limited to any one country or to government action. With this in mind, the committee gave considerable attention to ways to involve international organizations, foundations, and the private sector in this work, work which, to be effective, must look internationally at least as much as domestically.
REFERENCES
Abe, K., N. Nomura, and S. Suzuki. 2020. Biofilms: Hot spots of horizontal gene transfer (hgt) in aquatic environments, with a focus on a new hgt mechanism. FEMS Microbiology Ecology 96(5):fiaa031.
Alvarez-Uria, G., S. Gandra, and R. Laxminarayan. 2016. Poverty and prevalence of antimicrobial resistance in invasive isolates. International Journal of Infectious Diseases 52:59-61.
Bello-Lopez, J. M., O. A. Cabrero-Martinez, G. Ibanez-Cervantes, C. Hernandez-Cortez, L. I. Pelcastre-Rodriguez, L. U. Gonzalez-Avila, and G. Castro-Escarpulli. 2019. Horizontal gene transfer and its association with antibiotic resistance in the genus aeromonas spp. Microorganisms 7(9):363.
Biomerieux. 2020. A global approach to human and animal health. https://amr.biomerieux.com/en/challenges/from-farm-to-food-to-people-one-health (accessed July 13, 2021).
Burmeister, A. R. 2015. Horizontal gene transfer. Evolution, Medicine, and Public Health 2015(1):193-194.
CARB (National Action Plan for Combating Antibiotic-Resistant Bacteria). 2020. National action plan for combating antibiotic resistant bacteria. Federal Task Force on Combating Antibiotic-Resistant Bacteria. https://www.hhs.gov/sites/default/files/carb-national-action-plan-2020-2025.pdf (accessed April 8, 2021).
CDC (Centers for Disease Control and Prevention). 2019. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: Centers for Disease Control and Prevention, Department of Health and Human Services. www.cdc.gov/DrugResistance/Biggest-Threats.html (accessed April 8, 2021).
CDC. 2020. U.S. national action plan for combating antibioticresistant bacteria (national action plan). Atlanta, GA: Centers for Disease Control and Prevention, Department of Health and Human Services.
Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerging Infectious Diseases 7(2):178-182.
D’Costa, V. M., C. E. King, L. Kalan, M. Morar, W. W. Sung, C. Schwarz, D. Froese, G. Zazula, F. Calmels, R. Debruyne, G. B. Golding, H. N. Poinar, and G. D. Wright. 2011. Antibiotic resistance is ancient. Nature 477(7365):457-461.
Hall, R. J., F. J. Whelan, J. O. McInerney, Y. Ou, and M. R. Domingo-Sananes. 2020. Horizontal gene transfer as a source of conflict and cooperation in prokaryotes. Frontiers in Microbiology 11:1569.
HHS (Department of Health and Human Services). 2021. Presidential advisory council on combating antibiotic-resistant bacteria (PACCARB). https://www.hhs.gov/ash/advisory-committees/paccarb/index.html (accessed September 23, 2021).
Hutchings, M. I., A. W. Truman, and B. Wilkinson. 2019. Antibiotics: Past, present and future. Current Opinion in Microbiology 51:72-80.
Keeling, P. J., and J. D. Palmer. 2008. Horizontal gene transfer in eukaryotic evolution. Nature Reviews Genetics 9(8):605-618.
Knapp, C. W., J. Dolfing, P. A. Ehlert, and D. W. Graham. 2010. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environmental Science & Technology 44(2):580-587.
McMaster University. 2011. Resistance to antibiotics is ancient. www.sciencedaily.com/releases/2011/08/110831155334.htm (accessed April 8, 2021).
Nesme, J., and P. Simonet. 2015. The soil resistome: A critical review on antibiotic resistance origins, ecology and dissemination potential in telluric bacteria. Environmental Microbiology 17(4):913-930.
Obama, B. 2014. Executive order 13676 of September 18, 2014: Combating antibiotic-resistant bacteria. Federal Register 184(79):56931-56935. https://obamawhitehouse.archives.gov/the-press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria (accessed December 15, 2021).
OECD (Organisation for Economic Co-operation and Development). 2016. Antimicrobial resistance, policy insights: Organisation for Economic Co-operation and Development. https://www.oecd.org/health/health-systems/AMR-Policy-Insights-November2016.pdf (accessed April 8, 2021).
Papp-Wallace, K. M., A. Endimiani, M. A. Taracila, and R. A. Bonomo. 2011. Carbapenems: Past, present, and future. Antimicrobial Agents and Chemotherapy 55(11):4943-4960.
PCAST (President’s Council of Advisors on Science and Technology). 2015. National action plan for combating antibiotic-resistant bacteria. Washington, DC: Federal Task Force on Combating Antibiotic-Resistant Bacteria, White House.
PCAST. 2020. National action plan for combating antibiotic-resistant bacteria, 2020-2025. Washington, DC: Federal Task Force on Combating Antibiotic-Resistant Bacteria, White House.
Perry, J., N. Waglechner, and G. Wright. 2016. The prehistory of antibiotic resistance. Cold Spring Harbor Perspectives in Medicine 6(6):a025197.
Shallcross, L. J., S. J. Howard, T. Fowler, and S. C. Davies. 2015. Tackling the threat of antimicrobial resistance: From policy to sustainable action. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 370(1670):20140082.
Sommer, M., and G. Dantas. 2014. How to fight back against antibiotic resistance. American Scientist 102(1):42–51. https://static1.squarespace.com/static/5402b1a0e4b02a7794494453/t/55147a26e4b01b71130b9ed5/1427405350832/Dantas+Sommer+2014+AR.pdf (accessed September 22, 2021).
Sullivan, T. 2018. Antibiotics are often used at the end of life, but at what cost? Health Affairs Blog. https://www.healthaffairs.org/do/10.1377/hblog20180308.826415/full (accessed April 8, 2021).
The White House. 2014. National strategy for combating antibiotic resistant bacteria. Washington, DC: The White House.
Ventola, C. L. 2015. The antibiotic resistance crisis: Part 1: Causes and threats. P&T 40(4):277-283.
WHO (World Health Organization). 2017. WHO publishes list of bacteria for which new antibiotics are urgently needed. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed July 13, 2021).
Wright, G. D. 2010. The antibiotic resistome. Expert Opinion on Drug Discovery 5(8):779-788.