5
Stewardship and Infection Prevention
As Chapter 2 discussed, there are many factors driving the misuse and overuse of antimicrobials and the emergence of resistance. Limited local laboratory capacity, for example, can force the extensive use or prolonged courses of empiric antimicrobial treatment. In this regard, the overuse of these medicines is in many ways a proxy indicator of other gaps in the health system, such as problems with infection control and uneven access to medicines, preventative services, or primary care (Denyer Willis and Chandler, 2019). Efforts to promote rational antimicrobial use will be futile without attention to these underlying problems.
The Centers for Disease Control and Prevention (CDC) defines antimicrobial stewardship as “the effort to measure and improve how antibiotics are prescribed by clinicians and used by patients” (CDC, 2021e). Stewardship can also be thought of as an effort to match antimicrobial use to need, with an emphasis on the right medicine, in the right dose, for the right length of time. Drug selection, dose, and duration influence potential adverse effects to patients and contribute to the development of resistance (Gerding, 2001). More recent frameworks emphasize duration of treatment and correct de-escalation (described in Chapter 2) as other important dimensions of stewardship (Goebel et al., 2021).
In its Global Action Plan on AMR, the World Health Organization (WHO) cites the optimal use of antimicrobial medicines in human and animal health as one of its main objectives (Mendelson and Matsoso, 2015). In the United States, both the 2015 and 2020 National Action Plans for Combating Antibiotic-Resistant Bacteria emphasized supporting
stewardship programs and infection prevention in humans and animals (FTF CARB, 2020; Mendelson and Matsoso, 2015).
Successful antimicrobial stewardship will protect the drugs we have, thereby prolonging their useful life in recognition of the fact that the pace of drug development has not and cannot keep pace with the emergence of resistance (Doron and Davidson, 2011). Good stewardship strikes the optimal balance between prescribing effective treatment and avoiding unnecessary risks, be they the short-term risk to the patient or long-term risks to society by encouraging resistance.
This chapter presents the committee’s analysis of key bottleneck problems related to stewardship and infection prevention, in both humans and animals. This is not an exhaustive analysis of every possible tool for stewardship or infection prevention. Education of providers, for example, is one necessary precursor for better stewardship. In training and in professional development, health professionals are taught the essentials of antimicrobial treatment, including, most obviously, correct diagnosis, but also drug choice and dose, duration of treatment, and de-escalation (Goebel et al., 2021). The committee commends the greater attention to antimicrobial stewardship in preclinical and continuing education emerging across health professions (Augie et al., 2021; Espinosa-Gongora et al., 2021; Gotterson et al., 2021; Holz et al., 2021; Nasr et al., 2021; Van Katwyk et al., 2018).
At the same time, knowledge of correct stewardship practices is rarely enough to alter providers’ behavior. Qualitative research across six low- and middle-income countries found awareness of antimicrobial resistance and knowledge of the role of providers to combat it consistently very high (Goebel et al., 2021). This does not necessarily translate into changes in prescribing patterns, however, as such decisions are influenced by larger social and economic factors (Goebel et al., 2021). In the face of environmental conditions that encourage infection, the cost, time, and tools required for diagnosis, and managing the expectations of patients, their families, or, in veterinary medicine, animal owners, it can be difficult for providers to change behavior, or to argue that, in some cases, such change would be advisable (Goebel et al., 2021). In short, the relationship between providers’ knowledge and their practice is not direct or linear (Denyer Willis and Chandler, 2019). For this reason, attention to providers’ behavior and their awareness of good stewardship practices has been described as “the tip of the iceberg” (Chandler et al., 2016).
This chapter presents the committee’s judgment regarding key points where policy intervention could improve antimicrobial stewardship in the United States. It also discusses tools that could help mitigate the problem in low- and middle-income countries where the burden of resistance is greatest. Though not an exhaustive list, the steps recommended in this
chapter have potential to encourage more judicious use of antimicrobials as well as promising preventive measures.
STEWARDSHIP IN HUMAN MEDICINE IN THE UNITED STATES
In its definition of antimicrobial stewardship, the CDC emphasizes both the prescription and use of antimicrobials, a distinction that can be difficult to track (CDC, 2021e).
Stewardship in hospitals was the focus of the agency’s 2014 report Core Elements of Hospital Antibiotic Stewardship, the first in a series of guidance documents (Sanchez, 2016). This immediate emphasis on hospital stewardship was well founded. By CDC estimates, 30 to 50 percent of antimicrobial use in hospitals is unnecessary (e.g., to treat a viral infection) or inappropriate (e.g., use of the wrong drug for a particular bacteria) (CDC, 2021b). Because of the lag time on microbiological diagnosis, hospital prescribing relies heavily on the broad-spectrum drugs that are often used inappropriately (Doron and Davidson, 2011).
The frequency of misuse in hospitals is a concern as infections can spread quickly and because hospitals are, by definition, places for infirm and immunocompromised people for whom infections pose serious risks. Box 5-1 describes how, even when hospital staff have heightened attention to infection control, drug-resistant pathogens can spread quickly. Hospitals are also, compared to other practice settings, structured environments with multiple checks on medicine use and patient compliance as well as in-house laboratory and pharmacy systems. For these reasons, hospitals are an obvious starting point for efforts to promote antimicrobial stewardship.
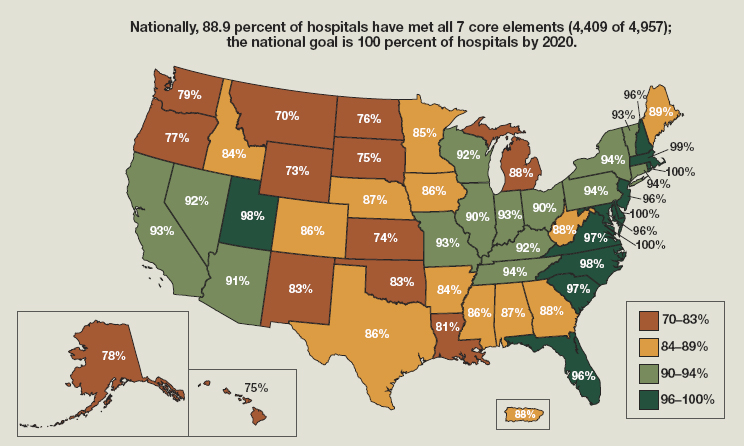
National attention to stewardship in hospitals has elicited considerable progress over a relatively short time. Starting in 2017, the Joint Commission, the organization that accredits hospitals, has assessed hospital stewardship programs as part of their review (Joint Commission, 2016). Between 2014 and 2017 the number of U.S. hospitals with stewardship programs conforming to CDC guidelines almost doubled (CDC, 2019b). A Centers for Medicare & Medicaid Services (CMS) rule that went into effect in 2019 requires hospitals to have antibiotic stewardship as part of their infection control efforts (ASM, 2019). Attention from CMS and the Joint Commission command the attention of hospital administrators, making it easier to ask for financial support for stewardship activities (Joint Commission, 2016). In 2014 less than 40 percent of U.S. hospitals had a stewardship program (Pollack et al., 2016). By 2019, almost 89 percent did (see Figure 5-1).
The success in improving hospital stewardship over the last 5 years is heartening, but the institutions left without functional stewardship
programs are some of the most challenging ones to reach. CDC surveys indicate that hospitals with 25 or fewer beds, many of them designated Critical Access Hospitals that support rural or remote areas, account for most of the remaining hospitals without complete stewardship programs (CDC, 2020g). These hospitals have fewer staff, a reflection of their smaller patient load, and cannot often support the expertise in infectious disease and specialty pharmacy outlined in CDC guidance. Collaborations with other hospitals are one effective way to overcome this barrier (CDC, 2018; StratisHealth, 2020). Using telemedicine to connect to academic medical centers is one particularly promising strategy, as discussed in Box 5-2.
It is difficult to overstate the importance of federal leadership in bringing attention to antimicrobial stewardship in hospitals. Joint Commission standards and a CMS rule command the attention of hospital leadership and make it easier for stewardship staff to get protected time
NOTE: Reference to specific commercial products, manufacturers, companies, or trademarks does not constitute its endorsement or recommendation by the U.S. Government, Department of Health and Human Services, or Centers for Disease Control and Prevention.
SOURCE: Adapted from CDC, 2020d.
and salary support for their work (StratisHealth, 2020). In the absence of such a rule, it can be difficult to persuade hospital administrators of the value of the antimicrobial stewardship activities (Kapadia et al., 2018; StratisHealth, 2020). This is partly because the relationship between stewardship activities and changes in burden of resistance are not clear or direct; even the best stewardship program will not necessarily improve indicators of resistance in the hospital (Doron and Davidson, 2011).
The rapid improvement in hospital stewardship programs in the United States is a success; tele-health programs and outreach to smaller community hospitals are promising tools to reach remaining hospitals (Shively et al., 2020). By 2020, 88.9 percent of hospitals had implemented all seven of the CDC’s core elements of antimicrobial stewardship, falling short of the agency’s goal of 100 percent of hospitals having quality stewardship programs in place by 2020 (CDC, 2020b).
There are other clinical settings where there is room for improvement in the rational use of antimicrobials. In its 2019 report, Antibiotic Use in the United States, the CDC identified problems with outpatient prescribing practices, including unnecessary use of fluoroquinolones for urinary tract
and respiratory tract infections, overly long antibiotic treatment for sinus infections and community-acquired pneumonia, and the misuse of azithromycin in children (CDC, 2019b). The agency’s Core Elements of Outpatient Antibiotic Stewardship emphasized that a responsibility for stewardship was distributed across the health system, including primary care providers, and also urgent and emergent care, pharmacies, dental practices, and many outpatient specialty providers and clinics (Sanchez, 2016). Rapid, reliable diagnostic information could do much to improve these troubling practices, a matter discussed in more detail later in this chapter.
Nursing Homes, Long-Term Acute Care Hospitals, and Dialysis Centers
There are several clinical practice settings similar to hospitals in their misuse of antimicrobials, vulnerable patient populations, and an administrative structure conducive to implementing change. Recent government response to the COVID-19 pandemic recognizes the unique importance of these practice settings, with the CDC creating special outbreak control teams to deploy to nursing homes, dialysis clinics, and other skilled nursing settings to prevent and control the spread of SARS-CoV-2 and other infectious diseases (CDC, 2021c). Nursing homes, long-term acute care hospitals, and dialysis centers all have a financial relationship with CMS. These settings are an obvious choice as the next step in the push for improved antimicrobial stewardship.
Nursing Homes
Nursing homes, the live-in health facilities that provide 24-hour supervision and skilled nursing support, are home to an estimated 1.3 million Americans (Harris-Kojetin et al., 2016; NIA, 2017b). Some nursing home residents are admitted for short stays, for physical or occupational therapy after an injury or surgery, for example, but the vast majority are there permanently because their conditions require constant skilled nursing and supervision (Harris-Kojetin et al., 2016; NIA, 2017b). About 80 percent of nursing home residents are over 65 years of age (Harris-Kojetin et al., 2016). Their care is often complicated by comorbidities such as dementia (36 percent prevalent), diabetes (37 percent prevalent), heart disease (36 percent prevalent), and hypertension (77 percent prevalent) (Harris-Kojetin et al., 2016). Limiting infections through stewardship is especially important in nursing homes, as infection control measures such as isolation and donning gowns and gloves are not always practical or suitable in the setting (Cohen et al., 2015). Unlike in hospitals, where the attending physician or other in-house provider is often responsible for prescriptions, nursing home residents are free to choose their provider
(CMS, 2021g; LaBore, 2014). This person is not generally affiliated with the nursing home and would not necessarily have the same perspective on the institution’s stewardship goals as the in-house staff.
The CDC released its Core Elements of Antibiotic Stewardship for Nursing Homes in 2015, setting out steps for nursing homes to improve their antibiotic prescribing and reduce inappropriate use (CDC, 2015b). Yet a recent survey found that only a third of nursing homes had comprehensive antimicrobial stewardship programs (Fu et al., 2020). The most recent compendium of data on CMS-certified nursing homes reported that problems with infection control were the most common citation for nursing homes in the years 2010 to 2014; citations for improper use of medicines have also become more common (CMS, 2015).
An estimated 70 percent of nursing home residents receive antimicrobials in a year (CDC, 2020a). Point prevalence surveys indicate about 8 percent of nursing home residents are using antimicrobial medicines at any given time, with about a third of these being broad-spectrum antibiotics (Thompson et al., 2021b). Data from nursing homes in 10 states indicate that for every hundred nursing home residents, 2.7 are being treated with antibiotics for urinary tract infections (Thompson et al., 2020).
Such trends are concerning, as nursing home residents are often frail and have immune systems compromised by advanced age and comorbidities. Clostridioides difficile infection, an infection often stemming from inappropriate or excessive use of antimicrobials, is endemic in nursing homes and can be deadly for residents (Mayo Clinic, 2020; Yu et al., 2016). About 10 percent of patients who acquire C. difficile infection in nursing homes die within 30 days (Yu et al., 2016).
Long-Term Acute Care Hospitals
Long-term acute care hospitals (also called long-term care hospitals) are sometimes confused with long-term care (i.e., nursing home), but they are different (NIA, 2017a,b). Long-term acute care is a specialized hospital for patients who are too infirm to be discharged to a nursing home, but not dynamic enough to warrant care in a regular, acute care hospital (ASHA, 2021). Many are discharged directly from intensive care units, bringing with them the associated risks of gram-negative, drug-resistant infections (ASHA, 2021; Kadri, 2020; Strich and Kadri, 2019). At admission, more than 60 percent of these patients are either infected or colonized with methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, or both (Gould et al., 2006).
An estimated 120,000 Medicare beneficiaries are treated in long-term care hospitals every year (Makam et al., 2019). Medicare national data indicate that only about 19 percent of patients successfully return home
after time in long-term acute care (CMS, 2021f). Fewer than half survive 12 months after admission; median survival is about 8 months (Makam et al., 2019). Patients in long-term care hospitals stay, on average, for 25 days or longer, often for conditions that involve prolonged use of ventilators and central lines, wound or burn care, and dialysis (ASHA, 2021; CMS, 2019b; Jacob et al., 2019). Infections associated with central lines, catheters, and ventilators are common. National surveys of long-term acute care have found 84 percent of S. aureus bloodstream infections acquired from central lines are resistant to methicillin; 44 percent of Enterococcus faecalis urinary tract infections acquired from catheters are resistant to vancomycin (Chitnis et al., 2012; Gould et al., 2006). A regional study found that the highly resistant Klebsiella pneumoniae that produce an enzyme (carbapenemase) that renders them non-susceptible to the carbapenem class antibiotics, are 10 to 54 percent prevalent in long-term acute care (Lin et al., 2013). Colonization with resistant bacteria (meaning the presence of a pathogen without its damaging tissue or causing illness) can easily become chronic among these patients (O’Fallon et al., 2009). Resistant K. pneumoniae can be especially persistent; 83 percent of colonized patients retain K. pneumoniae for the duration of their stay in long-term acute care (Haverkate et al., 2016).
Survey data indicate a mismatch between perception and actual risk of antimicrobial-resistant infections in long-term acute care. A study in Detroit found that while almost two-thirds of staff consider antimicrobial resistance to be a serious national problem, only 38 percent saw it as a problem in their hospital (Mushtaq et al., 2017). The same respondents showed low awareness of some stewardship principles, missing 77 percent of opportunities to de-escalate antimicrobial treatment (Mushtaq et al., 2017).
Dialysis Centers
The vast majority (98 percent) of the estimated 520,000 hemodialysis patients in the United States receive maintenance dialysis at outpatient centers (Apata et al., 2021). These patients are immunocompromised almost by definition, and dialysis involves repeated bloodstream access, often with central venous catheters (Apata et al., 2021; CDC, 2020e). Bloodstream infections are a serious risk for dialysis patients, and mortality after sepsis is 100 to 300 times higher for them than for the general population (Sarnak and Jaber, 2000).
The balancing of risk and benefit that all prescribers confront in the use of antimicrobials is heightened in people with kidney disease. The relationship between drug concentration and time that underlies decisions about dosing is altered in dialysis patients because they cannot filter medicines effectively between sessions (Eyler and Shvets, 2019). As
a group, these patients also have some of the highest rates of colonization with drug-resistant bacteria in the world, making effective dosing clinically important but difficult in practice (Wang et al., 2019). Colonization with vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus are both about 6 percent prevalent in dialysis patients (Zacharioudakis et al., 2014, 2015).
There are serious problems with antimicrobial stewardship in nursing homes, long-term acute care, and dialysis centers. These practice settings also all have a financial relationship with CMS that could be used to encourage implementation of good stewardship practices.
Recommendation 5-1: The Centers for Medicare & Medicaid Services should require nursing homes, long-term acute care hospitals, and dialysis centers to have antimicrobial stewardship programs and include that information on the Care Compare website. These programs should, at a minimum, designate key staff, a system for preauthorization of restricted antimicrobials, and a process for regular review of all antimicrobial prescriptions.
This recommendation is consistent with recent action at CMS. In a 2016 rule, the agency required nursing homes to have antimicrobial stewardship programs in place by late 2017 that would set out a system for monitoring use and recording lapses in infection control (CMS, 2016; Cooper, 2020). Similarly, the CMS rule requiring antimicrobial stewardship in hospitals would apply to long-term acute care hospitals as well, though there is no implementing guidance specific to this setting (CMS, 2019a). Plans to expand stewardship requirement for dialysis centers and other practice settings that participate in CMS are pending (Cooper, 2020).
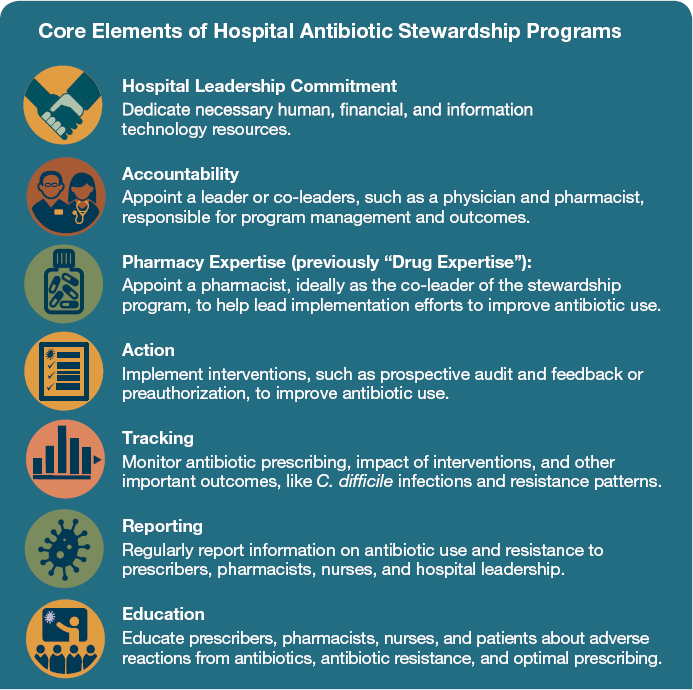
The CDC 2015 guidance, Core Elements of Antibiotic Stewardship in Nursing Homes, will be invaluable in implementing this recommendation. Although there are no parallel, tailored antimicrobial stewardship guidelines for dialysis or for long-term acute care, the core elements outlined in other CDC stewardship documents (leadership, accountability, pharmacy expertise, action, tracking, reporting, and education) are broadly applicable to a range of these settings (see Figure 5-2). The CDC cites the same core elements in its 2015 guidance on antimicrobial stewardship in nursing homes (CDC, 2015b).
There are also similarities among the three types of practice settings. All rely heavily on nurses and pharmacists (Apata et al., 2021; Katz et al., 2017; Sloane et al., 2016). Physicians are not necessarily, or even commonly, on site; they base their prescribing decisions heavily on nurses’ reports. When physicians are on site, it tends to be on a rotating basis,
making it difficult to find one sufficiently integrated into day-to-day activities to have a sense of ownership of a stewardship program. As in critical access hospitals, infectious disease specialists are not generally on staff, and telehealth may be the best option when specialist consultations are needed (Apata et al., 2021; Petrak, 2014).
There are steps that could make stewardship a higher priority for the in-person staff in these settings. The Agency for Healthcare Research and Quality (AHRQ) provides simple toolkits to help nursing homes implement their stewardship programs. These toolkits emphasize the appointing of stewardship champions on staff, and the clear assigning of responsibility for different pieces of the program (AHRQ, 2016a,b). The AHRQ guidance encourages involving external pharmacy consultants and prescribing physicians in the implementation of stewardship programs (AHRQ, 2016a). Hospital research suggests that pharmacists are
SOURCE: CDC, 2019c.
often willing to take responsibility for stewardship, acting as champions of the stewardship program (Livorsi et al., 2021). In addition to reviewing culture data, pharmacists can serve as a check on appropriate ordering, dosing, duration of treatment, and de-escalation. The structure of the stewardship program will vary based on the size and resources of the setting, but coordination with prescribers will be important across settings.
Regardless of who leads the stewardship program, regular review of all antimicrobial use will be an essential first step to ensuring rational use. This review is difficult when recordkeeping is inadequate, as is common in dialysis clinics (D’Agata et al., 2018). Record keeping in nursing homes can also be uneven; a recent national survey found only about half used electronic medical records (Bjarnadottir et al., 2017). While the electronic system is not absolutely necessary for reviewing antimicrobial use, it greatly eases the process, making strategies like remote audit and feedback on prescribing possible. This strategy significantly decreased antimicrobial use and C. difficile infection in long-term acute care (Beaulac et al., 2016).
Expanding stewardship may be an opportunity to modernize documentation processes, especially in nursing homes and dialysis centers. At a minimum, records should clearly cite the indication for every antimicrobial prescribed; the dose and duration of treatment; as well recording antibiotic “time-outs” or breaks in treatment to determine if the drug is working. The review would give the stewardship team a chance to encourage de-escalation and to avoid parenteral therapy when oral treatment is possible.
Medical records are also useful in developing a pre-authorization process for restricted antimicrobials. Pre-authorization is a key part of hospital stewardship; it refers to the standing approval of an infectious disease specialist (physician or pharmacist) for empiric treatment with antimicrobials (Eljaaly et al., 2018). In dialysis, preauthorization could emphasize the rational use of vancomycin, properly a drug of last resort and not one that should be used out of habit. In nursing homes, preauthorization might give more attention to the treatment of a positive urine culture, discouraging the use of antimicrobials to treat asymptomatic presence of microbes in urine. Whenever possible the pre-authorized treatment would be integrated into the electronic medical record system. Automatic prompts in electronic medical records have been shown to improve antimicrobial prescribing practices in outpatient medicine and could be used in these settings as well (Meeker et al., 2016).
Payment and Cost Savings
The committee recognizes that implementing stewardship programs adds work for managers and staff at these facilities. But historical evidence
from hospitals suggests these costs can be more than made up in savings on medicines, both from defaulting to cheaper antibiotics and using shorter treatment courses (CDC, 2015a). Given the common overuse and misuse of antibiotics in the settings targeted by this recommendation, the benefits of more rational use, both to the individual facility and to society, are likely to be even greater. Modeling indicates that implementing stewardship programs in hemodialysis clinics would save between $100 and $229 million, prevent between 2,000 and 4,645 C. difficile and multidrug-resistant infections, and avoid between 600 and 1,340 deaths every year (D’Agata et al., 2018). Research in nursing homes has not found evidence that stewardship programs reduce infection, hospitalization, or mortality rates among residents, but do tend to reduce unnecessary antibiotic use and improve adherence to stewardship guidelines (Feldstein et al., 2018). Less can be said about long-term acute care, though a pilot study in Michigan found that reductions in spending on antibiotics alone saved $55,000 in the first 3 months after implementing a stewardship program (Mushtaq et al., 2017).
There are also similarities in business models among these practice settings. In the United States, almost 70 percent of nursing home care and 79 percent of long-term acute care is for-profit (CDC, 2021f; MedPAC, 2020). Dialysis clinics are even less diverse; two for-profit chains alone control 72 percent of the U.S. dialysis market (Childers et al., 2019; Levin et al., 2020). In this situation, it may help to frame antimicrobial stewardship as a step to lowering future costs, especially if coupled with wider use of electronic records, as the cost savings might accrue to a different department than the one making the investment in stewardship.
Care Compare and Implementation
What is more, implementation does not have to be an overnight, disruptive change. The CDC guidance to nursing homes encourages gradual implementation, starting with one or two changes and adding more pieces to the strategy over time (CDC, 2021e). To start, CMS could work with providers in these settings to define the barriers to good stewardship and strategies to change their practices (Resman, 2020). When inappropriate use is driven more by the expectations of patients or their families, then strategies to improve communication and education might be an early starting point. Some research indicates ways of describing patients or residents may encourage behavior at odds with good stewardship or patient care (Chambers et al., 2019). The pattern of describing a resident as having frequent urinary tract infections, for example, tends to lead to the over treating of asymptomatic infection (Chambers et al., 2019). These patterns can change, especially when the stewardship program has an educational emphasis rather than a punitive one.
CMS could also help draw attention to stewardship by including it in the quality measures that inform its star rating system for nursing homes and dialysis and its Care Compare database (CMS, 2021c). CMS created the star rating system for nursing homes in 2008; it draws on inspection reports, staffing review, and quality measures such as vaccination coverage and percentage of residents with urinary tract infections, pressure sores, and in physical restraints (CMS, 2020; Horton et al., 2020). The rating system for dialysis is a more recent development, first implemented in 2015 and revised in 2019 (University of Michigan Kidney Epidemiology and Cost Center, 2018). The dialysis rating system relies on quality measures relating to mortality, hospitalization, transfusions; bloodstream infection is also included (Horton et al., 2020).
The rating systems for nursing homes and dialysis is primarily a tool to help consumers and their families navigate their options (CMS, 2021c; University of Michigan Kidney Epidemiology and Cost Center, 2018). Over time, insurance companies and state regulators have used the rating system for incentive payments, referrals, and loans (AHCA/NCAL, 2020). Today the Medicare Care Compare database acts as a clearinghouse for independent quality assessments, as well as patient surveys when available (CMS, 2021b). For long-term acute care, the website also allows for benchmarking against national averages on the hospital’s rates of C. difficile infection and catheter- and central line–associated infections (CMS, 2021c).
The Care Compare database is meant to be easy to use and to consolidate relevant information into one website (CMS News and Media Group, 2020). By giving more emphasis to antimicrobial stewardship in the public indicators on Care Compare, Medicare could help draw attention to the importance of stewardship programs among providers and Medicare beneficiaries.
STEWARDSHIP IN ANIMAL MEDICINE IN THE UNITED STATES
While the basic concepts of antimicrobial stewardship are the same in human and animal medicine, the practices differ considerably. CDC guidance on antimicrobial stewardship in hospitals, outpatient medicine, nursing homes, and critical access facilities all emphasize the role of executive leadership and accountability for stewardship throughout the health system; they all also stress the role of pharmacists (CDC, 2019c, 2020g, 2021a). These intervention points have no direct parallel in veterinary medicine. Despite recent trends toward consolidation, about half of veterinarians work in practices that employ fewer than 100 people (Ouedraogo et al., 2018). Most veterinarians dispense medicines from their practice without a pharmacy intermediary (Morley et al., 2005). For these reasons, the American Veterinary Medical Association (AVMA) emphasizes the role
of the veterinarian “individually and as a profession” in antimicrobial stewardship (AVMA, 2021a).
One core element of antimicrobial stewardship that does apply across a range of human and animal medicine practices is tracking. It is impossible to measure progress against any goal, especially a complex, multifaceted endpoint like antimicrobial stewardship, without understanding patterns of use or being able to measure the effect of interventions. Better information about antimicrobial use in animals is a serious barrier to better stewardship. Of particular concern is the veterinary use of antimicrobials thought to promote pathogens’ cross-resistance to human antimicrobials (Singh and Bhunia, 2019).
Tracking Antimicrobial Use in Animals
The United States does not have a strong system to track antimicrobial use in animals. While the Food and Drug Administration (FDA, 2020e) requires companies that make animal medicines to report their annual antimicrobial sales, the actual use of the drugs is harder to measure. Veterinarians may buy medicines that they do not use or do not use immediately (FDA, 2020e). It is also difficult to make inferences about use without information about the number and species of animals treated. Large differences in size and metabolism among species make it impossible to draw meaningful conclusions about trends in consumption from sales information (FDA, 2020e). Even sales data are only available for food-producing animals. Much less can be said about antimicrobial use in pets, as Box 5-3 explains.
More accurate information about antimicrobial use on farms comes from the U.S. Department of Agriculture (USDA) and FDA research. The USDA’s National Animal Health Monitoring System (NAHMS) conducts regular (every 5 to 10 years) surveys of antimicrobial use and resistance in different animals (Bright-Ponte, 2020). Recent surveys in cattle and swine feedlots provided a baseline for comparisons of how FDA guidance on judicious antimicrobial use may change practices (Bright-Ponte, 2020; USDA, 2017, 2019). These surveys include questions about the farmer’s relationship with a veterinarian and include analysis of biological samples from the animals and the farms’ records (USDA, 2017, 2019). Collecting these data more frequently or widely would be complicated logistically. Cattle agriculture in particular is characterized by considerable market fragmentation (Bright-Ponte, 2020). There are also wide differences in record keeping on farms (Bright-Ponte, 2020). Despite agreement that the indication for using an antimicrobial and the dose, duration, and route administered (e.g., injected, orally, in feed) are key data to capture, it remains challenging to do so (Bright-Ponte, 2020).
At the writing of this report, the FDA Center for Veterinary Medicine had pilot projects under way to get additional information on antimicrobial use in animals. In 2016, it funded two 5-year cooperative agreements, one characterizing antimicrobial use in U.S. beef feedlots and dairies, the
other collecting data on antibiotic use in U.S. poultry and swine production (USDA, 2018). The agency announced another cooperative agreement examining antimicrobial use in dogs and cats in 2020 (FDA, 2020d). The committee commends FDA on these efforts that will give valuable insight into the relationship between antimicrobial use in animals and the emergence of the resistance and will inform long-term strategies on how best to monitor antimicrobial use. The CDC (2021d) also has projects in place to strengthen tracking and data collection on farms.
Capturing Prescription Data
At the same time, considerable information on drug choice, indication, dose, route of administration, and species is lost at the farm level. Prescriptions are one way to measure consumption of, and indication for, antimicrobials. Since 2017, FDA rules have required a veterinarian’s written authorization for the use of certain drugs in animal feeds (Clark, 2017). The same rules disallow the use of medically important antimicrobials without veterinary oversight (Clark, 2017). Since veterinary medicines do not necessarily go through a pharmacy, however, not all states require veterinarians to provide prescriptions, though AVMA (2021b,c) recommends they always be made available upon request. To this end, some states encourage veterinarians to use electronic prescribing systems, and the electronic prescribing software is already in use (AVMA, 2021d).
The FDA Center for Veterinary Medicine has the mandate to monitor animal medicines and to conduct research that advances this work (FDA, 2020b). The center could promote better antimicrobial stewardship by investing in strategies to advance the use of electronic prescriptions and to encourage the sharing of prescription information in proprietary hands. In the near term, the agency can continue to research ways to better estimate antimicrobial use in animals.
Recommendation 5-2: The Food and Drug Administration’s Center for Veterinary Medicine should establish a process and clear metrics to facilitate better tracking of antimicrobial consumption in animals. This information would support the design and implementation of stewardship programs.
Prescription data would help make more sense of raw antimicrobial use information as it would clarify what species is being treated; it would also allow insight into where stewardship programs are working and what practices help promote them (Pinto Ferreira, 2017). Better prescription data would also afford better understanding of antimicrobial use
in companion animals, something not currently tracked. Unfortunately, there are no user-friendly technologies to collect prescription information (Pinto Ferreira, 2017). Therefore, mandatory electronic prescriptions are a valuable long-term goal in veterinary medicine. FDA should encourage veterinarians and farmers to work toward this goal, communicating how better tracking of antimicrobial use in animals would do much to improve our understanding of effective stewardship. With better data, it would be possible to reward producers for good antimicrobial stewardship through tax breaks or other incentive programs (Pinto Ferreira, 2017).
The agency could also emphasize the accompanying benefits of electronic prescribing. For example, it can help veterinarians, particularly those who work with a diverse range of species, automatically calculate correct dosages. It would also allow insight into the extra-label (called off-label) prescribing of human medicines in animals, a practice not uncommon in companion animals (Goggs et al., 2021; Papich, 2020). Although FDA prohibits the off-label use of certain antimicrobials, notably fluoroquinolones and cephalosporins, in food-producing animals, there is considerable ambiguity regarding other drugs, and better insights into the need for—and real-world use of—medicines in veterinary practice would be useful (FDA, 2021a).
Electronic prescriptions also provide an entry point for steps to control the prescription of critically important human antimicrobials. A recent randomized, controlled trial in the United Kingdom found that by monitoring electronic prescriptions it was possible to alert veterinarians when their prescribing of the highest priority human medicines was above the median (Singleton et al., 2021). When combined with regular meetings and education about stewardship and benchmarking of the practice prescribing patterns, also facilitated through review of electronic records, prompts about prescription practice reduced the use of critically important antimicrobials by almost 40 percent in cats and over 23 percent in dogs (Singleton et al., 2021).
In human medicine, research on electronic records is difficult as the data is usually proprietary (Adibuzzaman et al., 2017; Gliklich et al., 2014). There is time to avoid or control this problem in animal medicine by encouraging data accessibility in the early stages of the shift to electronic prescribing.
The goal of this recommendation is to make accessible the information about dose, duration, and indication for how antimicrobials are used and in what species. Advancing this goal may mean better outreach to private industry. The largest veterinary provider in the United States, for example, is Mars, Incorporated, which owns one of the largest veterinary laboratory chains in the country (Kelloway, 2018; Veterinary Practice News Editors, 2017). As FDA has relationships with Mars and other
animal health companies, it could involve them in the discussion about accessibility and monitoring of antimicrobial consumption data.
In its communication, the agency should emphasize the value of aggregate information about antimicrobial use and the need to identify patterns of judicious use as well as misuse. This is consistent with international trends. In Denmark, for example, the VetStat central database, a national repository of electronic prescribing and other reporting requirements for farmers and pharmacies, has been in use since 2001 (AACTING, 2021). VetStat data have been used to estimate daily doses of active ingredients per 100 animals, a much higher level of precision than is now possible in the United States (AACTING, 2021). By tracking VetStat data, Danish authorities identified a rise in antimicrobial consumption between 2001 and 2009, mostly driven by use in pigs (FAO and Denmark Ministry of Environment and Food, 2019). The national regulatory authority used this information to establish antibiotic use thresholds and a warning system for farms exceeding this threshold, reducing antimicrobial consumption by 90 percent (relative to 2009 levels) in less than 5 years (DVFA, 2017).
Similar monitoring systems are taking hold across Europe. In 2019, the European Medicines Agency issued regulations on monitoring the use of antimicrobials in animals, encouraging the monitoring of veterinary prescriptions as a means to understand use (EMA, 2020). In response, European countries are developing national databases similar to Denmark’s VetStat to collect and store electronic prescriptions (Chirollo et al., 2021; Government of Ireland, 2021; Koper et al., 2020).
The committee recognizes that additional data accessibility requirements put a burden on veterinarians and may be met with resistance from producers. The shift to electronic prescribing and the central monitoring systems similar to what can be found in Europe is, to be clear, a long-term goal. Production systems in the United States are different from those in Europe, so it is unlikely that duplicating the VetStat system would be a suitable goal for this country. There are other ways to monitor antimicrobial use to inform stewardship programs in each state. In any case, the monitoring system used is less important than the measures of use derived.
There is no standardized system to measure antimicrobial use in animals (Kasabova et al., 2019). Units of measurement for antimicrobial use include expressions of the mass of the active substance administered, the dose (how many milliliters of medicine used multiplied by the mg/ml concentration of active ingredient), or a count of days treated or courses of medicine administered (Sanders et al., 2020). Mass and dose measures then need to be divided by some indicator of the target animal population: average number of animals treated, mass of the meat produced, or standardized weight of the animals treated, for example (Sanders et al.,
2020). Different estimates of the population treated and animal weights affect the estimates of use (Kasabova et al., 2019). Count-based measures such as the number of days treated per year have some advantage in being, essentially, indicators of treatment incidence, something relatively direct to calculate and meaningful to both farmer and veterinarian (Sanders et al., 2020). For this reason, count measures may be more amenable to benchmarking and comparisons among farms (Sanders et al., 2020).
Measuring antimicrobial use and prescribing practices in animals is related to concerns about veterinary drug labeling. Not all veterinary antimicrobials have up-to-date labels that reflect current standards of judicious use (The Pew Charitable Trusts, 2016). For example, 28 percent of medically important antimicrobials used in animal feed have no defined duration of use, introducing guesswork for the veterinarian and possibly exposing the animal to an unnecessarily prolonged treatment (FDA, 2021b). The FDA Center for Veterinary Medicine’s recent work to support better antimicrobial stewardship calls for updating the approved use and conditions of antimicrobials and to the labels that inform their use (FDA Center for Veterinary Medicine, 2018). To this end, the agency has mobilized funding for research to establish duration limits for antimicrobials in the feed of food-producing animals (FDA, 2020c). The committee commends these steps, and sees that attention to monitoring prescribing patterns could be a complement to FDA’s work to revise and update antimicrobial labels. Ultimately, action in both areas is needed to promote judicious use of antimicrobials in veterinary medicine.
In any effort to measure antimicrobial use or to promote stewardship in animal agriculture, FDA should work with and strengthen collaboration with USDA. The ongoing and proposed additional surveillance studies conducted through USDA’s NAHMS program are valuable tools to this end (USDA, 2014). USDA also has a valuable agricultural extension network that can be used for education and outreach. Research has shown agricultural extension staff to be a trusted source of information on antimicrobial stewardship for farmers (Ekakoro et al., 2019; Wemette et al., 2020). Extension programs can also do much to improve information management on the farm and promote the best practices in biosecurity, both of which control antimicrobial use (Baudoin et al., 2021; Clark et al., 2012; Henriksson et al., 2018). For these reasons, agricultural extension is already highlighted in USDA and CDC’s antimicrobial resistance programming (NIMSS, 2017).
Implementation of this recommendation would pave the way for better information on how antimicrobials are used in animals. This is an important and necessary step for better antimicrobial stewardship.
Generating these data is not, in itself, enough to inform policy, however. In setting up a system for tracking antimicrobial use, FDA would need to consider steps to ensure the information was properly analyzed and interpreted. This could come from within the agency, though designating an independent third party for analysis might be a better way to overcome industry reluctance to share sensitive information.
The Need for Animal-Specific Breakpoints
In addition to better understanding how veterinarians use antimicrobials, the cause of good stewardship (using the right drug, in the right dose, for right duration) in veterinary medicine is held back by challenges in availability and use of veterinary diagnostic tests. Some of the factors that encourage reliance on empiric treatment in human medicine apply to veterinary medicine as well (e.g., slow turnaround time for diagnostic test results). These problems are amplified, however, by several factors unique to animals. First is the logistical challenge of collecting diagnostic samples on a farm. If the sample can be drawn in a minimally disruptive way, during milking for example, the logistical burden is lower than if testing disrupts the animal’s routine (Lubbers, 2021). The process of bringing the animal into a chute to draw a sample is stressful for the animal and sometimes dangerous for its handlers (Lubbers, 2021). Especially when large animals are involved, the safety concerns alone are enough to encourage empiric treatment (Lubbers, 2021). There are also financial barriers. In veterinary medicine the animal owner generally pays out of pocket not only for medicines, but for diagnostic testing used to inform treatment. The veterinarian and his or her client must weigh this additional expense, around $20 to $110 per sample, against the likelihood of the result yielding novel information that would alter clinical treatment (ISU, 2021). Finally, even after the samples are drawn and submitted for testing, the veterinarian may not be able to act on the information returned because there are no established susceptibility breakpoints for that microbe–drug combination in the species tested.
Establishing susceptibility breakpoints requires balancing information on the mechanism by which an organism is resistant to a drug, the range and distribution of observed minimum inhibitory concentrations, the pharmacokinetic and pharmacodynamic properties that influence drug concentration in tissue, and data on clinical outcomes from similar cases (Humphries et al., 2019). As a recent review paper explained, “[B]reakpoint decisions are rarely clear-cut” and are therefore often the work of expert committees convened by international organizations (Weinstein and Lewis, 2020). The best known of these are the Clinical Laboratory Standards Institute (CLSI), run in partnership with the International Stan-
dards Organization, and the European Committee in Antimicrobial Susceptibility Testing (EUCAST) (Kahlmeter et al., 2019). CLSI breakpoints and interpretative criteria are widely used in the United States and internationally (Weinstein and Lewis, 2020). CLSI is also approved by FDA as a “standards development organization,” meaning that FDA (2020a) accepts most CLSI interpretative criteria for susceptibility tests. EUCAST, founded in 1997 by the European Society for Microbiology and Infectious Disease, serves a similar role in Europe; its breakpoints are also used internationally (EUCAST, 2021).
Most antimicrobial susceptibility test guidelines were developed for human pathogens, but work on veterinary breakpoints has followed. Since the late 1980s, CLSI has convened the Sub-Committee on Veterinary Antimicrobial Susceptibility Testing to develop interpretive breakpoints for bacterial pathogens in animals (Lubbers, 2021). EUCAST convened its Veterinary Committee on Antimicrobial Susceptibility Testing (VetCAST) in 2015 (EUCAST, 2021). These two volunteer groups develop interpretative standards and guidelines for their respective organizations and the regulatory agencies that reference them. Both groups rely heavily on independent research and on clinical trial data submitted by the drug companies.
Breakpoints in veterinary medicine are specified not just by microbe–drug combination, but also by species and disease process (Toutain et al., 2017; Watts et al., 2018). Even when the drug and pathogen are constant, the drug may be administered differently in different animals. Differences in physiology and metabolism among species further influence the way the drug moves (pharmacokinetics) and its ultimate efficacy. Therefore, the ability to develop new susceptibility test breakpoints depends on collecting and creating pharmacokinetic-pharmacodynamic data for different drugs in different species and on convening experts to review and interpret this data. Both the data and the expertise to review it are somewhat scarce (Damborg, 2021; Toutain et al., 2017). Despite agreement that more animal-specific breakpoints are needed, it is difficult to keep up momentum for the process (FAO, 2019; Toutain et al., 2017). The time and expense of building the evidence base to inform breakpoint analysis is a complicated precursor to any interpretation of test criteria.
There are, therefore, too few interpretive breakpoints for antimicrobial susceptibility tests in animals, especially in food-producing animals (Toutain et al., 2017; Watts et al., 2018). Such breakpoints are vital to antimicrobial stewardship in veterinary medicine; they are also a cornerstone of surveillance and monitoring resistance patterns. Despite decades of effort from standard-setting organizations, development of needed breakpoints has not kept pace with the demand for them, especially in light of increasing emphasis on antimicrobial stewardship in veterinary medicine.
Deliberate effort at the level of the federal government would encourage the research needed to develop these breakpoints for key drug, pathogen, and species combinations.
Recommendation 5-3: The Food and Drug Administration’s Center for Veterinary Medicine should convene an advisory committee to coordinate development of antimicrobial susceptibility test breakpoints in animals and identify priority animal, drug, and pathogen combinations. When necessary, the Center for Veterinary Medicine would fund the research needed to develop the priority breakpoints.
There are many combinations of pathogen, drug, and animal species of interest in veterinary medicine. Choosing priorities for breakpoint development from among these many combinations should be done in a more deliberate way, with more open communication among clinicians who use the test results and the diagnostics laboratories that generate them, as well as the standards organizations that set the breakpoints, and the scientists who do the pharmacokinetic and pharmacodynamic research. The FDA (2020f) advisory committee system is designed to bring such varied stakeholder groups together and to get advice from niche subject-matter experts outside of government.
This committee would work with the CLSI Veterinary Antimicrobial Susceptibility Testing subcommittee and with clinical stakeholders to assess the various microbe–drug–species combinations and identify the most urgent needs for animal health and public health. The committee need not start from scratch. AVMA recently published an assessment of species-specific antimicrobial-resistant pathogens that affect animal health (AVMA, 2020). Pathogens identified in this document could serve as the starting point for the proposed advisory committee. This list could be immediately narrowed to pathogens treated with antibiotics that are important to human medicine (e.g., cephalosporins, fluoroquinolones, and macrolides) and to zoonotic pathogens that affect both animals and humans (e.g., Salmonella and Campylobacter).
The committee would still face a problem of insufficient data about veterinary pathogens. In general, information about veterinary antimicrobials are scarce, and often the proprietary data of pharmaceutical companies. FDA has the authority to ask drug sponsors to collect more data during the approval process and to encourage them to work with CLSI’s VAST (Veterinary Antimicrobial Susceptibility Testing) subcommittee to generate the information needed to develop susceptibility test breakpoints. The advisory committee could provide guidance on what data are needed for establishing breakpoints and what methods should be used to
generate the data. These may include epidemiological studies, pharmacokinetic and pharmacodynamic data, and clinical trials.
Currently, CLSI’s Veterinary Antimicrobial Susceptibility Testing subcommittee develops breakpoints with volunteer effort, based on data availability and willingness of an individual committee member to champion an effort (Watts et al., 2018). This process is not efficient or sustainable. The proposed advisory committee would evaluate the current process and identify ways to improve it. Particularly, the committee could consider funding research to generate data that are critically needed for developing breakpoints.
In the longer term, the committee could consider ways to increase the pool of qualified experts to participate in veterinary breakpoint development. This may include training strategies in the United States and enhanced collaboration and coordination between CLSI-VAST and VetCAST to take advantage of expertise available in different countries. Increasing international collaboration may have the added benefit of paving the way for more harmonized methods internationally.
The advisory committee could also work with veterinary organizations such as the USDA National Animal Health Laboratory Network, the FDA Veterinary Laboratory Investigation and Response Network, and the American Association of Veterinary Laboratory Diagnosticians to educate their members about the relationship between antimicrobial susceptibility test data and antimicrobial stewardship. Both epidemiological and clinical studies are needed to assess the effectiveness of national and regional stewardship programs. Better education and member outreach, something the associations have experience with, could help strengthen efforts to increase diagnostic testing.
There is also a need for new quality control and testing methods that these organizations could help develop. Although progress has been made in standardizing susceptibility test methods, there are still considerable needs remaining. For example, some pathogens grow slowly or require special culture conditions. There is a special need for testing methods for the so-called fastidious pathogens, organisms that will only grow in the presence of specific nutrients or atmosphere, such as mycoplasma, mycobacteria, and anaerobes (Watts et al., 2018). Since these pathogens grow slowly or require special culture conditions, they are not amenable to standard laboratory methods but are important for animal health. Attention to speeding the development of tests for them would be a meaningful use of the advisory committee’s effort.
The advisory committee could also identify a standardized system for veterinary diagnostic labs to report susceptibility test data to veterinarians. Currently, most veterinary diagnostic labs in the United States use disc diffusion and broth microdilution (Dargatz et al., 2017). Yet there is
considerable variability in how the results are reported (e.g., a numeric or categorical measures of susceptibility) and the forms used for reporting. This variability arises in part from the uncertainty in breakpoints this recommendation aims to reduce. It also causes confusion among the users of the data and inconsistency in their ability to act on the results (Dargatz et al., 2017). The standardized reporting system would be developed with input from commercial test developers, veterinarians, and other end users and would provide not only interpretation of the results (e.g., pathogen is susceptible, intermediate, or resistant) but also quantitative data (e.g., minimum inhibitory concentrations).
Attention from FDA could help make veterinary susceptibility testing less ad hoc, but after setting out the priority pathogen, drug, and species combinations there will still be a need for pharmacokinetic and pharmacodynamic data to establish the needed breakpoints, especially for generic drugs. By designating funding for this research, the agency could remove another major barrier to better antimicrobial stewardship in animals.
DIAGNOSTIC STEWARDSHIP IN THE UNITED STATES
Across human and animal medicine, accurate, fast diagnostic tests are needed to promote antimicrobial stewardship. By making test results available to clinicians before they start empiric treatment, diagnostic testing can avoid much unnecessary empiric treatment. In a 2018 commentary, Jim O’Neill, the lead commissioner of the O’Neill report, described rapid diagnostics as “the single biggest potential game changer in the fight against antimicrobial resistance” (Collier and O’Neill, 2018). In low- and middle-income countries, diagnostics have the potential to save millions of lives; an estimated 405,000 child deaths from bacterial pneumonia could be avoided with diagnostic tests (Moeller et al., 2007). In the United States, their value would be more on the side of avoided unnecessary or poorly targeted treatments.
As this report has explained, much of the error in treating infectious disease stems from uncertainty, an abundance of caution weighted in favor of the patient, even if the patient’s interests are not aligned with the larger interests of society. This human calculus encourages treatment, and treatment with broad-spectrum antibiotics, on the possibility that the patient would benefit. Research in British primary care practices, where antibiotic prescribing is generally much more restrained than in the United States, still indicates that between 8 and 23 percent of antibiotic prescriptions are inappropriate and could be avoided with better diagnostics (Smieszek et al., 2018).
The well-founded fear of failing to treat a serious infection is reflected in formal treatment guidelines. For example, surveillance of gonococcal
isolates in the United States since 2009 has shown an alarming trend in resistance to azithromycin, with elevated minimum inhibitory concentrations of azithromycin seen in almost 5 percent of isolates by 2018 (St Cyr et al., 2020). These data prompted the CDC to revise first-line treatment guidelines for gonorrhea to ceftriaxone, a WHO Watch Group medicine, in 2020 (St Cyr et al., 2020; WHO, 2021). This is a prudent revision and one needed in response to rising levels of azithromycin resistance. If there were a fast, reliable way to distinguish azithromycin-susceptible cases from the azithromycin-resistant ones, then more targeted use of the second-tier treatment would be possible. Molecular assays to rule out fluoroquinolone-resistant gonococci by detecting the gyrA gene would allow for prediction of ciprofloxacin susceptibility (Hemarajata et al., 2016). Such tests would, in turn, slow the spread of resistance and preserve the useful life of antimicrobial medicines.
Despite wide agreement that rapid diagnostic tests could reduce unnecessary reliance on antimicrobials, their uptake has been slow and uneven (PCAST, 2020; Review on Antimicrobial Resistance, 2015). Some of the barriers relate to the product development pipeline (e.g., regulatory hurdles, clinical trials, and data validation) and will be discussed in the next chapter. There are also useful diagnostic tests already on the market that are not used widely enough to drive better stewardship.
Rapid, point-of-care diagnostic tests, when used appropriately, could have considerable benefit for antimicrobial use and patient outcomes. At the same time, these tests can also lead to an overuse of testing that may have the opposite effect on antimicrobial use than was intended. Diagnostic stewardship helps ensure that the right test and the most clinically relevant results are being reported on the right patient, avoiding unnecessary therapeutics and inappropriate management (Messacar et al., 2017). Testing for bacterial pharyngitis caused by Streptococcus pyogenes is often rapid and performed at the point of care, but in the absence of defined bacterial pharyngitis symptoms, a positive test (due to colonization rather than infection) may lead to a misdiagnosis of bacterial pharyngitis, in turn leading to overuse of antibiotics (Thompson et al., 2021a). Another example are urine cultures, which are notoriously overused and overinterpreted, leading to the overdiagnosis of urinary tract infections in patients with no symptoms, who happen to have bacteria in the urine (asymptomatic bacteriuria) a syndrome that does not warrant treatment (Chan-Tack et al., 2020). In using or developing rapid diagnostics for urinary tract infections, speeding the time to results is important, but it is also important to consider the target patient population for the test. Understanding the test performance and clinical interpretation in specific populations, such as patients in long-term care facilities, pregnant women, and children, will be critical in optimizing the use of these novel diagnostics for urinary tract infections (Patel et al., 2021).
Clinical microbiologists are important stewards of these diagnostic tests, particularly as molecular developments yield more complex tests that put more interpretative demands on laboratory staff. Communication between clinical microbiologists and prescribers helps ensure that rapid diagnostic tests are used at the right time on the right patient for optimal patient care.
One rapid diagnostic test that would optimize patient care is a point-of-care test to distinguish viral from bacterial infection. A blood test that can make this distinction in 12 hours, rapid only in comparison to traditional culture and disk susceptibility testing methods, is projected to hasten de-escalation in hospitals, with the potential to reduce antimicrobial use by 14 percent (Yui et al., 2020). In a trial at a large teaching hospital, multiplex PCR on positive blood cultures, along with antimicrobial stewardship, reduced use of broad-spectrum antimicrobials (Banerjee et al., 2015). The same technology can be used at point of care to assist in identifying viral infections (i.e., respiratory virus infections) in outpatient medicine and have performed better than antigen tests in terms of targeting treatment and improving workflow in the clinic (Beal et al., 2020). Nevertheless, at a cost of more than $100 a test for consumables alone, the diagnostic is considerably more expensive than an antibacterial medicine (Genome Web, 2012). Recent CMS reimbursement guidelines clarify that such tests will not be covered unless certain additional patient criteria are met, such as the patient’s serious or critical illness and underlying conditions (e.g., cystic fibrosis, chronic obstructive pulmonary disease) (CMS, 2021e).
Point-of-care tests for infections account for some of the highest volume of diagnostic tests performed (Bonislawski, 2019). These tests also have very low profit margins for their manufacturers; there is no advantage to a high test volume when every test is individually run (Bonislawski, 2019). Recent reductions in the CMS reimbursement for diagnostics could discourage use of point-of-care tests (Sears, 2018).
Furthermore, the problem of diagnostic stewardship is not just a lack of tests. Sometimes tests are available and not used (Pulcini et al., 2012). The clinical decision to prescribe an antimicrobial is influenced by the test performance and indication, reimbursement for it, and provider attitudes. The rapid antigen test for streptococcal infection, for example, is a cheap test that is widely used to direct antimicrobial treatment for pharyngitis (Barakat et al., 2019; NLM, 2020). At least with adult patients, a negative antigen test provides a reason to deny antimicrobials to a patient who may be asking for them. (In children, the strep antigen test performance is not sufficiently reliable and confirmatory culture is necessary [Barakat et al., 2019; Cohen et al., 2016]). Nevertheless, these tests are thought to decrease antimicrobial treatment relatively little (Cohen et al., 2016). At
the same time, novel point-of-care diagnostic tests may be improving this picture. Nucleic acid amplification tests for group A streptococcal pharyngitis have gained use in recent years and show diagnostic accuracy comparable to that of gold-standard culture methods (Luo et al., 2019).
Tools to diagnose a viral infection in outpatient medicine could, if used widely, avert even more unnecessary treatment as empirical treatment is usually the default in these settings (Cooke et al., 2020). But most rapid tests are expensive, and they carry cost implications in terms of diverted staff time (Okeke et al., 2011). Coupled with a pressure on clinical laboratories to save money, additional spending on testing is hard to justify without solid evidence of its value (Caliendo, 2015).
The limited use of rapid diagnostics has downstream negative consequences for antimicrobial resistance (Roope et al., 2019). It is unlikely that any diagnostic test can undercut first- or even second-line antimicrobial treatments on direct cost alone. Yet society has an urgent need for wider use of these tests to allow for antimicrobial stewardship.
Reimbursing the full value of diagnostic tests would be a meaningful step toward better stewardship, but determining this value is not straightforward. Diagnostic testing is one early step on a path of treatment decisions, wherein later decisions are partially predetermined by earlier ones (Ferrante di Ruffano et al., 2012). Rapid, accurate results are valuable only if they change treatment decisions early on this path, as there is plausible reason to assume that rapid molecular drug susceptibility test results would do. Prescribers have no incentive to use a broad-spectrum antibiotic against clear indication of the narrow-spectrum drug indicated. At the same time, the value of these tests, especially in terms of changes in patient outcomes such as morbidity and length of hospital stay, or financial outcomes such as cost of treatment or repeated office visits, are not usually readily apparent to doctors or administrators.
Furthermore, the switch to wider reliance on microbiological diagnostics depends on provider behavior, something that is influenced by practice guidelines that emphasize diagnostic use. For example, concerns about multidrug-resistant tuberculosis prompted the CDC (2009) to call for more research on molecular testing for drug resistance. This research informed the 2017 revision to practice guidelines, including a recommendation to use rapid, molecular drug susceptibility tests on certain patients (Lewinsohn et al., 2017). When formal treatment guidelines reference diagnostic use, providers have clear reason to use them, although lag time to change practice can be lengthy (Morris et al., 2011).
There is wide agreement that antimicrobial stewardship should include patient and provider education as well as technological tools such as better diagnostics (O’Neill, 2016; PCAST, 2020). A lack of compelling evidence on the value of diagnostic testing, however, prevents its inclusion in practice
guidelines. The Department of Health and Human Services (HHS) could help remove that barrier by supporting the outcomes research on diagnostic testing that the CDC, the Infectious Diseases Society of America (IDSA), and other societies use to inform their practice guidelines.
Recommendation 5-4: The Department of Health and Human Services agencies, including the Centers for Disease Control and Prevention, the Food and Drug Administration, and the Centers for Medicare & Medicaid Services, and the Patient-Centered Outcomes Research Institute should support outcomes research in diagnostic testing to drive an iterative process of guidelines development and to influence reimbursement for diagnostic testing.
The problem of widespread empiric therapy is at the center of antimicrobial resistance. Generic antibiotics will almost always be cheaper than even inexpensive diagnostic tests, discouraging providers from curtailing inappropriate antimicrobial use. Reliance on diagnostic testing has the potential to alter this pattern, but the use of these tests is limited (Trevas et al., 2021). The failure of diagnostic stewardship is a thorny and circular problem, driven by cost and human behavior as much as evidence. When confronted by a problem with multiple competing causes it can be difficult to identify the root cause, a dilemma that can lead to inaction. The committee recognizes that generating evidence on the value of diagnostic testing will not in itself alter clinicians’ behavior or bring down the cost of test kits. But without explicit attention to this evidence base it is difficult to encourage clinicians to use the tests or to justify subsidizing their cost. The first step in compensating tests based on their value is establishing that value with evidence.
This recommendation echoes IDSA’s recent call for “improved study designs to better capture the clinical and economic benefits of diagnostics” (Trevas et al., 2021). Large multicenter studies evaluating the value of diagnostics tests are done mostly for regulatory approval, and are therefore focused on the tests accuracy, not on its economic or clinical value, outcomes delineated in Box 5-4 (Trevas et al., 2021). As this box shows, the cost savings associated with diagnostic testing are often accrued downstream, not in the departments closest to testing. For example, a rapid test for methicillin-resistant Staphylococcus aureus eventually saved $1.5 million in less than 2 years on the avoided costs of contact precautions (extra protective gowns and gloves, isolating the patients in private rooms, etc.) (Shenoy et al., 2013).
The evidence needed will be challenging to generate, as it must include both clinical trials and clinical laboratories in its design. Health
records and claims data, sometimes called real-world data, can also be important sources of data for outcomes research (FDA, 2021c). The participation of multiple clinical sites is also essential as the inferences made from aggregate data are more generalizable and better able to detect small but meaningful treatment differences (Kahn et al., 2012).
A lack of statistical power to detect differences can also be a serious problem in diagnostics research, something that can be avoided with multisite studies. Previous research at a large teaching hospital found that rapid diagnostics cause doctors to use antimicrobials more judiciously, but was not powered to detect difference on other outcomes (Banerjee et al., 2015). Multisite studies are also more expensive to run (Lovegreen et al., 2018). There is also a need for industry participation across sites that sometimes requires the involvement of a coordinating center (Smith et al., 2019).
The Antibiotic Resistance Leadership Group has a research framework in place that would lend itself to the type of outcome research envisioned in this recommendation. The National Institute of Allergy and Infectious Diseases funds the group to design and execute clinical research related to antibiotic-resistant bacteria, including research related to improving diagnosis (ARLG, 2021b). The group’s scientific agenda emphasizes “practice-changing guidelines” and has identified diagnostics as a broad research priority (ARLG, 2021a,b). This diagnostic research portfolio is weighted toward assessment of new diagnostic tools and biomarkers but does mention strategies to make best use of diagnostic tests (ARLG, 2021a). Although this group is not funded to do diagnostic outcome studies, its existing research and laboratory network could be a starting point for pursuing these questions.
Cost is still a major barrier to conducting outcomes research on diagnostic tests, however. HHS agencies could reduce this barrier by making such studies an explicit priority and mobilizing funding for them. Though not a major research funder, CMS does sponsor research relating to new payment policies and the effect of the agency’s policies on its customers and beneficiaries (CMS, 2012). The CDC also funds research that feeds into the iterative process of guidelines development. As the national leader in developing public health guidelines, the CDC (2012) has an interest in supporting the evidence base that informs them and directing attention to serious gaps. The Patient-Centered Outcomes Research Institute (PCORI), a large public research funder, is also in a good position to investigate the relationship between antimicrobial diagnostic test use and health outcomes. PCORI’s (2014) mandate is to improve the quality of evidence informing clinical and health policy decisions.
Even with sufficient evidence to inform treatment guidelines, rapid diagnostic tests still face an uphill battle, with many clinicians choosing to wait for traditional culture and susceptibility testing before de-escalating or changing treatment. For example, genotypic assays that screen for the mecA gene can accurately determine resistance or susceptibility to methicillin in staphylococci, including Staphylococcus aureus (Bakthavatchalam et al., 2017). Use of these rapid tests are referenced in multiple treatment guidelines (Hanson et al., 2020; Uyeki et al., 2019). Yet there was a lag time of
several years before the tests gained wide acceptance (Banerjee et al., 2015; Ehren et al., 2020). Though aware of these barriers, the committee encourages more attention to the evidence linking diagnostic testing with patient outcomes. Without this evidence in hand, it will be that much harder to start the process of changing clinical behavior or test reimbursement.
STRATEGIES TO PREVENT THE EMERGENCE OF RESISTANCE, ESPECIALLY IN LOW- AND MIDDLE-INCOME COUNTRIES
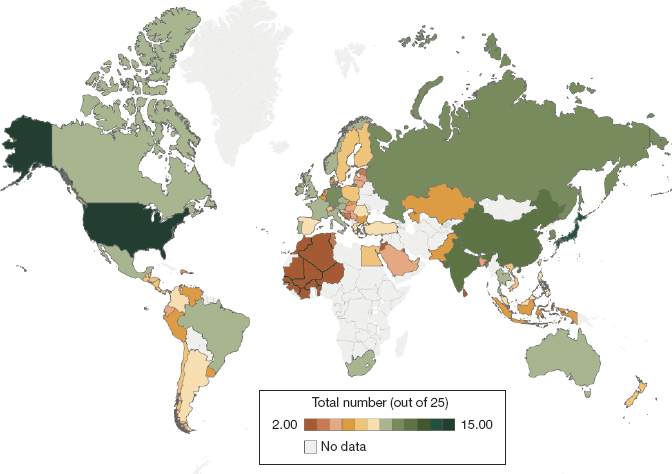
As Chapter 2 explained, the need for effective, good-quality antimicrobials is greater in low- and middle-income countries than in the United States, and access is a serious problem. The burden of infectious disease is higher in these parts of the world, requiring more justifiable courses of antimicrobials but also prompting more unjustified use. Governments have less to spend on health, and patients have less to spend on medicines, putting even relatively inexpensive generic antimicrobials out of reach for many (Craig, 2019). Rational selection of antimicrobials is also complicated when newer treatments are not available. Of the 21 new antibiotics to come to market between 1999 and 2014, 90 percent of countries registered 10 or fewer (Craig, 2019). See Figure 5-3.
Given the greater need for antimicrobials and problems with access to medicines, interventions to curb the unnecessary use of antimicrobials are harder to implement in low- and middle-income countries. The lack of diagnostic testing and microbiology laboratories is a serious barrier to stewardship (Okeke et al., 2011; Pierce et al., 2020). Without better, rapid diagnostics coming to the market, a point discussed more in the next chapter, it is difficult to encourage more judicious antimicrobial use in low- and middle-income countries. Sales restrictions would be unwise when access to medicines is a problem, nor are they likely to be effective. The sale of antimicrobials without a prescription may be banned in some low- and middle-income countries but is still common practice (Horumpende et al., 2018; Jacobs et al., 2019; Muri-Gama et al., 2018; Sulis et al., 2020). Despite formal requirements for a prescription, more than half of antimicrobials are dispensed without one in Vietnam, about 46 percent in Bangladesh, and 36 percent in Ghana (Do et al., 2021). Even if sales restrictions were enforceable, they are not likely to be effective when only a relatively small share of the population is able to see a licensed prescriber in the first place (Bebell and Muiru, 2014; Craig, 2019; Tattevin et al., 2020). Broad targets to reduce consumption are also not appropriate given the burden of disease (Tattevin et al., 2020). In many low- and middle-income countries good antimicrobial stewardship could mean more, appropriate use, not less.
NOTE: Countries in Central American and Francophone West Africa reported at regional levels.
SOURCE: Frost et al., 2019.
Much antibiotic use in low- and middle-income countries is for diarrheal disease and respiratory tract infections (Bielicki and Fink, 2020). Antibiotics are also often given to patients with fever against the chance that they have a life-threatening bacterial bloodstream infection like typhoid or bacteremia, but in fact more tropical fevers are caused by vector-borne diseases such as malaria and dengue (Adrizain et al., 2019; Batwala et al., 2011). Vector control, safe drinking water, and improved sanitation could all do much to reduce the need for antimicrobials, a topic discussed more in Chapter 8.
Especially in developing countries, antimicrobial stewardship plans need to take a broad view, with an eye on reducing the need for antimicrobials. The WHO has put considerable emphasis on infection prevention in its toolkits for antimicrobial stewardship programs in low- and middle-income countries, though these toolkits are intended for use in clinical medicine, where concepts like infection prevention are necessarily somewhat narrow in scope (Pierce et al., 2020; WHO, 2019a). Action against the more distal determinants of infection has the potential to elicit a more meaningful reduction in use.
Establishing the Value of Prevention Through Vaccination
Vaccines have the potential to reduce the need for antimicrobials and control the spread of resistance in the parts of the world where the problem is worst (Lipsitch and Siber, 2016). Though not a substitute for essential infrastructure or a functional health system, vaccines can prevent common respiratory and diarrheal diseases, something all the more valuable when improved sanitation and clean water are missing. Although many studies have assessed the efficacy of vaccines in reducing infections, few high-quality studies evaluate their effect on antibiotic use and antimicrobial resistance.
Figure 5-4 shows several possible pathways through which use of vaccines could reduce antimicrobial resistance. The most obvious is by reducing the selective pressure from antimicrobials used to prevent and treat bacterial infections. Table 5-1 reviews other pathways and examples of the relationship between vaccines and antimicrobial use.
Vaccines for Bacterial Infections
Especially in children, there is good evidence that pneumococcal and influenza vaccines predict less antimicrobial use and fewer courses initiated (Buckley et al., 2019). A recent study drawing on data from 18 low- or middle-income countries found that at current coverage levels, pneumococcal conjugate vaccine averted almost 24 million courses of antibacterials among children under 5 (Lewnard et al., 2020).
SOURCE: Lipsitch and Siber, 2016.
TABLE 5-1
Vaccines Can Work Through Many Pathways to Reduce Bacterial Infections
| Pathway Through Which Vaccines Can Reduce Antimicrobial Resistance | Examples | Evidence |
|---|---|---|
| Preventing common community-acquired bacterial infections | Hib, TCV, cholera, PCV, COVID-19, as well as Shigella, enterotoxigenic E. coli, N. gonorrhoeae, and group B strep vaccines in development | Some, particularly for Hib and PCV |
| Preferentially targeting antimicrobial-resistant lineages of infectious bacteria | PCV | Some, PCV |
| Preventing hospital-acquired infections | MRSA, CRE, and Acinetobacter vaccines in development | No population study data yet, vaccines still in pipeline |
| Protecting against diseases that make patients prone to secondary bacterial infection | Influenza, measles, COVID-19 | Little, and mostly concerning antimicrobial use |
| Preventing nonbacterial infections that produce syndromes that prompt antimicrobial use or misuse | Influenza, rotavirus, COVID-19, malaria, and dengue, as well as RSV vaccine in development |
NOTE: CRE = carbapenem-resistant Enterobacteriaceae; Hib = Haemophilus influenzae type b; MRSA = Methicillin-resistant Staphylococcus aureus; PCV = pneumococcal conjugate vaccine; RSV = respiratory syncytial virus; TCV = typhoid conjugate vaccine.
It follows that by reducing use of antibiotics, immunization would in turn slow the emergence of resistance. It is also plausible that vaccines act against resistance indirectly, by reducing the need for hospitalization and thereby reducing contact with amplifying reservoirs of resistant pathogens. For example, in the United States, before the Haemophilus influenzae type B vaccine (called Hib) was licensed for infants, there was increasing evidence of ampicillin resistance in meningitis, bacterial pneumonia, and epiglottitis, all diseases caused by invasive Hib (Jansen et al., 2018). The widespread use of the Hib vaccine virtually eliminated invasive Hib, including infections caused by resistant strains in the United States and in low- and middle-income countries (Agrawal and Murphy, 2011).
Pneumococcal conjugate vaccines have a similar effect,1 protecting against Streptococcus pneumoniae, a common bacterial pathogen that can cause meningitis, pneumonia, septicemia, and otitis media and is the worldwide leading cause of pneumonia among children under 5 (CDC,
___________________
1 So called because of their outer coating or capsule from the target bacterial serotypes conjugated to a carrier.
2020f; WHO, 2019b). There are many types of S. pneumoniae, and vaccines are designed to protect against the serotypes that cause the most disease, which are also the serotypes most associated with resistant infections (Klugman and Black, 2018). For this reason, serotypes used in the vaccine are occasionally changed in response to epidemiological surveillance.
Since their introduction, multiple studies, mostly in high-income countries, have shown an association between pneumococcal conjugate vaccines and reduced antibiotic use, reduced use of second-line antibiotics, and reduced incidence of resistant infections (Klugman and Black, 2018). In the United States, rates of invasive pneumococcal disease not susceptible to penicillin dropped 64 percent among children under 5 and 45 percent among adults older than 65 following the first introduction of pneumococcal conjugate vaccine in the United States (Hampton et al., 2012). The same pattern held after the vaccine’s expanded serotype coverage was introduced in 2010 (Tomczyk et al., 2016). Pneumococcal isolates collected from children with invasive infections have shown decreases in resistance to penicillin, cephalosporins, and trimethoprimsulfamethoxazole after the introduction of a 13-serotype pneumococcal conjugate vaccine, though this research is mainly from the United States and Europe (Tin Tin Htar et al., 2019). As the technology for producing conjugate vaccines improves, the number of serotypes included is set to expand (Lochen et al., 2020). Some evidence suggests that these expanded vaccines could be used to target the bacterial linages that evolve low-level penicillin resistance (Chaguza et al., 2020).
Furthermore, pneumococcal vaccine, like many vaccines, protects against transmissible infection, thereby providing protection that extends beyond the vaccinated population. Vaccinated people harbor less asymptomatic S. pneumoniae in the upper throat and nose, also the site of most exchange of pneumococcal resistance genes (Dagan et al., 2015; Hammitt et al., 2014). By limiting the reservoir of bacteria in this resistance hotspot, the vaccine has the potential to reduce emergence of resistance. Research in Kenyan children (one of few studies of this sort in a low- or middle-income country) found that a 10-serotype conjugate vaccine reduced bacteremic pneumococcal pneumonia by 85 percent and pneumococcal meningitis by 69 percent (Dagan et al., 2015; Hammitt et al., 2014). In this study both vaccinated and unvaccinated people both carried less S. pneumoniae in their nose and throat. This reduction in community-wide disease burden might have been responsible for a decrease in invasive pneumococcal disease among infants too young to be vaccinated and in older age groups (Hammitt et al., 2014).
Immunization against the bacteria that cause cholera and typhoid fever can also decrease antibiotic consumption, have the potential to curb antimicrobial resistance, and may reduce transmission (Gibani et
al., 2019). Cholera vaccination has proven especially valuable in humanitarian emergencies and other settings where access to clean water and sanitation is limited (Hsueh and Waters, 2019). Cholera vaccine can also reduce antimicrobial use in the outbreak, which rapidly selects resistant strains (Okeke, 2009). Two-dose, oral cholera vaccines have been shown to reduce population vulnerability outbreaks for up to 4 years, something that treatment obviously cannot do (Franke et al., 2018). While cholera vaccine is advised for travelers to areas of active cholera transmission, problems with cold chain and other logistical barriers limit the vaccines’ use in the parts of the world where it is most needed (CDC, 2020c; Shaikh et al., 2020). A more serious barrier is cost; the vaccines are expensive, and population-level benefit is unlikely in anything short of a complex humanitarian emergency (Gupta et al., 2016; Teshome et al., 2018). A similar pattern holds with typhoid conjugate vaccines, which are underused in endemic areas, but preventing multidrug-resistant infections is an important reason for adoption (Khan et al., 2017).
Vaccines for Viral Infections
Influenza and other viral respiratory infections are important drivers of antibiotic use, which tends to rise during the influenza season (Martinez et al., 2019; Morris et al., 2017). By disrupting normal protective barriers in the respiratory tract, the influenza virus increases bacterial colonization and predisposes to secondary bacterial infection (MacIntyre et al., 2018; Morris et al., 2017). Influenza vaccines can reduce antibiotic use by preventing these secondary bacterial infections and by avoiding the febrile respiratory infections for which antibiotics are frequently (often inappropriately) prescribed.
Some real-world evidence bears out this effect. Universal influenza vaccination became policy in Ontario, Canada, when policy in the rest of the country was to vaccinate only certain high-risk groups (Kwong et al., 2009). In the years following this policy influenza vaccine coverage rose from 18 to 38 percent, and antimicrobial prescriptions for infections associated with influenza were 64 percent lower in Ontario relative to the rest of the country (Kwong et al., 2009). A similar pattern has been seen in the United States, where a 10-percentage point increase in the statewide influenza vaccination rate is associated with between 6 and 23 percent less antibiotic use after controlling for multiple confounders (Klein et al., 2020). Survey data from low- and middle-income countries show the same trend after introduction of the rotavirus vaccine; by recent estimates this vaccine avoided 13.6 million courses of antibiotics among children under 5 (Lewnard et al., 2020).
There is some trial data, mostly from Europe and North America, on the effect of influenza vaccine on antimicrobial use (Buckley et al., 2019). A
recent systematic review concluded with high certainty that the vaccine has reduced antibiotic use among healthy adults by 28 percent (95% confidence interval: 16.0, 38.4); the same review found evidence of moderate certainty of a reduction in antibiotic use among vaccinated children and in children more broadly, regardless of whether they were vaccinated (Buckley et al., 2019). Nevertheless, the review ultimately concluded that the evidence tying vaccines to reductions in antibiotic use is poor, emphasizing the need for more attention to these outcomes in vaccine trials (Buckley et al., 2019).
In short, the logical argument in favor of wider vaccination as a tool to reduce antimicrobial use is clear, and there is plausible evidence that vaccines control the emergence and spread of resistant bacteria. But the relationship is not well studied or understood (Buckley et al., 2019; Lewnard et al., 2020; Malarski et al., 2019). As with outcomes research on diagnostic tests, the data showing the effect of vaccines on antimicrobial use or emergence of resistance would come from large, multidisciplinary, and long-term studies, which are costly to run and difficult to manage. At the same time, incorporating questions about antimicrobial use or resistance into ongoing vaccine trials could be done with relatively little additional effort or expense. As research and development for vaccines, including vaccines that target antimicrobial-resistant pathogens, are going on all over the world there is considerable opportunity to study this relationship (BCG, 2018). Adding measures of resistance to immunization trials would be a relatively minor additional effort that could yield a disproportionate payoff in terms of understanding this tool for infection prevention.
Recommendation 5-5: The National Institutes of Health and the Centers for Disease Control and Prevention should provide supplemental research funding to track antimicrobial use and antimicrobial resistance in immunization trials and large cohort studies to measure the indirect benefits vaccines provide and to provide evidence to enhance vaccine deployment as a tool to mitigate antimicrobial resistance.
Given the plausible logical argument and promising epidemiological evidence presented in this chapter, it is likely that vaccines for a number of bacterial and viral infections will reduce antibiotic use and curb the escalation of resistance in low- and middle-income countries. A recent WHO framework has called for the same, emphasizing expanding access to vaccines shown to reduce antimicrobial resistance (Vekemans et al., 2021). It is likely that there are many vaccines that meet this criteria, and further research could establish which ones those are. It is also likely that vaccines for animals would have the same preventative effects on the emergence of resistance, a topic discussed more in Chapters 6 and 8.
Nevertheless, there are multiple, often complex pathways by which vaccines influence the emergence of resistance, making it difficult to measure the full value of investment in a vaccine (Kingwell, 2018; Malarski et al., 2019). Better quality evidence, ideally from randomized, controlled trials would clarify these benefits and provide estimates of their magnitude. The size of the potential reduction in antibiotic use will be influenced by the incidence of infection and the uptake and efficacy of the vaccine. Potential benefits may be accrued only to specific demographic groups or in certain geographic areas (e.g., typhoid or cholera vaccines) or could affect the global population if infections are widespread (e.g., pneumococcal, Hib, and influenza vaccines).
This information would be valuable in considering what immunizations countries should recommend for their national immunization programs. These decisions are made by national or regional technical advisory groups charged with weighing the potential benefit of a vaccine against its cost and the ease of deployment (NITAG, 2019; WHO, 2014). The ability of vaccines to control resistance is not a criterion that enters their review (WHO, 2014). But if better evidence were available regarding such indirect benefits of vaccines, these review criteria could change. Cost is an important consideration in evaluating a vaccine, especially in middle-income countries transitioning away from international support for their immunization programs (Wellcome, 2020). Capturing the full public health and economic value of vaccines is imperative for decision makers in these countries. Evidence that a vaccine could prolong the useful life of inexpensive antimicrobial medicines would be a strong financial argument in its favor.
In low- and middle-income countries, febrile illness is often treated with broad-spectrum antibiotics because the cause of the infection can be difficult to confirm. Empiric treatment for potential typhoid fever is common, especially among children in typhoid-endemic areas (Gibani et al., 2018; Veeraraghavan et al., 2018). Partly for this reason, resistant strains of Salmonella Typhi are becoming more common, especially in South and Southeast Asia (Gibani et al., 2018). These resistant bacteria no longer respond to oral antibiotics and require expensive parenteral antibiotic treatments, not readily available or affordable in typhoid-endemic countries (Gibani et al., 2018). Increasing azithromycin-resistant Salmonella Typhi in South Asia has prompted calls for wider use of a new typhoid conjugate vaccine (Bhutta, 2020; Carter et al., 2020). Nevertheless, country adoption of the vaccine has been slow (Jamka et al., 2019). More information on its ability to control resistance might help persuade relevant immunization councils of its value and give a needed support for coverage.
In general, the decision to introduce or expand immunization in low- and middle-income countries is based on evidence that the vaccine in question prevents severe disease and the cost to deploy it would be man-
ageable (Ott et al., 2013). Wider cost savings and indirect benefits are not necessarily part of this evaluation. Partly for this reason, global coverage of influenza, pneumococcal conjugate, and rotavirus vaccines are low. In the 149 WHO member states that have introduced pneumococcal conjugate vaccine, coverage is less than half (WHO, 2020). Fewer countries (101) have introduced rotavirus vaccine, and coverage in these countries was around 35 percent in 2018 (Peck et al., 2019). Influenza vaccines are particularly seldom used in low- and middle-income countries; industry data indicate that over 95 percent of influenza vaccines are deployed in Europe, the Americas, and the Western Pacific (Ortiz and Neuzil, 2019). Closing these coverage gaps could have far-reaching benefits, including curbing resistance. By 2016 estimates, universal coverage with pneumococcal conjugate vaccine would avoid 11.4 million days of antibiotic therapy in children under 5 (Laxminarayan et al., 2016).
The Wellcome Trust has recently supported research investigating the effect of vaccines on measures of antimicrobial resistance and use (Wellcome, 2021). The Bill & Melinda Gates Foundation has recently called for more evidence linking antimicrobial endpoints to vaccine use, ideally taking account of differences in local medicines markets and health systems (Srikantiah, 2018). A 2017 Chatham House publication called for the same (Clift, 2017). A recent Wellcome Trust publication pointed to barriers to vaccine uptake in low- and middle-income countries, something better clarity regarding the full public health value of immunization would help overcome (Wellcome, 2020).
There is also a certain urgency to implementing this recommendation now. There are multiple dengue vaccines currently in clinical development (WHO, 2018). The addition of antimicrobial use or resistance measures to these trials could yield invaluable information that could influence countries’ use of the vaccine.
REFERENCES
AACTING. 2021. VetStat. https://aacting.org/matrix/vetstat/?lid=1447 (accessed May 4, 2021).
Adibuzzaman, M., P. DeLaurentis, J. Hill, and B. D. Benneyworth. 2017. Big data in healthcare—the promises, challenges and opportunities from a research perspective: A case study with a model database. AMIA Annual Symposium Proceedings 2017:384-392.
Adrizain, R., D. Setiabudi, and A. Chairulfatah. 2019. The inappropriate use of antibiotics in hospitalized dengue virus-infected children with presumed concurrent bacterial infection in teaching and private hospitals in Bandung, Indonesia. PLoS Neglected Tropical Diseases 13(6):e0007438.
Agrawal, A., and T. F. Murphy. 2011. Haemophilus influenzae infections in the H. Influenzae type b conjugate vaccine era. Journal of Clinical Microbiology 49(11):3728-3732.
AHCA/NCAL (American Health Care Association/National Center for Assisted Living). 2020. Five-star quality rating system. https://www.ahcancal.org/Survey-Regulatory-Legal/Pages/FiveStar.aspx (accessed June 24, 2021).
AHRQ (Agency for Healthcare Research and Quality). 2016a. Tool 1. Gather a team. Agency for Healthcare Research and Quality. https://www.ahrq.gov/sites/default/files/wysiwyg/nhguide/3_TK1_T1-Gather_a_Team_final.pdf (accessed September 22, 2021).
AHRQ. 2016b. Tool 2. Roles and responsibilities for antimicrobial stewardship. Agency for Healthcare Research and Quality. https://www.ahrq.gov/sites/default/files/wysiwyg/nhguide/3_TK1_T2-Roles_and_Responsibilities_final.pdf (accessed September 22, 2021).
Apata, I. W., S. Kabbani, A. M. Neu, T. M. Kear, E. M. C. D’Agata, D. J. Levenson, A. S. Kliger, L. A. Hicks, and P. R. Patel. 2021. Opportunities to improve antibiotic prescribing in outpatient hemodialysis facilities: A report from the American Society of Nephrology and Centers for Disease Control and Prevention Antibiotic Stewardship White Paper Writing Group. American Journal of Kidney Diseases 77(5):757-768.
Arensman, K., J. L. Miller, A. Chiang, N. Mai, J. Levato, E. LaChance, M. Anderson, M. Beganovic, and J. Dela Pena. 2020. Clinical outcomes of patients treated for Candida auris infections in a multisite health system, Illinois, USA. Emerging Infectious Diseases 26(5):876-880.
ARLG (Antibacterial Resistance Leadership Group). 2021a. ARLG scientific agenda. https://arlg.org/about-the-arlg/arlg-scientific-agenda (accessed May 24, 2021).
ARLG. 2021b. Who we are. https://arlg.org (accessed May 24, 2021).
ASHA (American Speech-Language-Hearing Association). 2021. Long-term acute care hospitals. https://www.asha.org/slp/healthcare/ltac (accessed May 3, 2021).
ASM (American Society for Microbiology). 2019. CMS final rule on antibiotic stewardship programs. American Society for Microbiology. https://asm.org/Articles/Policy/CMS-Final-Rule-on-Antibiotic-Stewardship-Programs (accessed May 3, 2021).
Augie, B. M., J. Miot, R. L. van Zyl, and P. A. McInerney. 2021. Educational antimicrobial stewardship programs in medical schools: A scoping review. JBI Evidence Synthesis 19(11):2906-2928. https://doi.org/10.11124/JBIES-20-00330.
AVMA (American Veterinary Medical Association). 2020. Antimicrobial resistant pathogens affecting animal health in the United States American Veterinary Medical Association. https://www.avma.org/sites/default/files/2020-10/AntimicrobialResistanceFullReport.pdf (accessed May 24, 2021).
AVMA. 2021a. Antimicrobial stewardship definition and core principles. https://www.avma.org/resources-tools/avma-policies/antimicrobial-stewardship-definition-and-core-principles (accessed May 4, 2021).
AVMA. 2021b. Prescriptions and pharmacies: FAQs for veterinarians. https://www.avma.org/prescriptions-and-pharmacies-faqs-veterinarians (accessed July 13, 2021).
AVMA. 2021c. Veterinary prescription orders. https://www.avma.org/advocacy/state-local-issues/veterinary-prescription-orders (accessed May 4, 2021).
AVMA. 2021d. Veterinary telehealth: The basics. https://www.avma.org/resources-tools/practice-management/telehealth-telemedicine-veterinary-practice/veterinary-telehealth-basics (accessed May 4, 2021).
Bakthavatchalam, Y. D., L. E. Nabarro, and B. Veeraraghavan. 2017. Evolving rapid methicillin-resistant staphylococcus aureus detection: Cover all the bases. Journal of Global Infectious Diseases 9(1):18-22.
Banerjee, R., C. B. Teng, S. A. Cunningham, S. M. Ihde, J. M. Steckelberg, J. P. Moriarty, N. D. Shah, J. N. Mandrekar, and R. Patel. 2015. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clinical Infectious Diseases 61(7):1071-1080.
Barakat, A. J., C. Evans, M. Gill, and D. Nelson. 2019. Rapid strep testing in children with recently treated streptococcal pharyngitis. Pediatric Investigation 3(1):27-30.
Batwala, V., P. Magnussen, and F. Nuwaha. 2011. Antibiotic use among patients with febrile illness in a low malaria endemicity setting in Uganda. Malaria Journal 10(1):377.
Baudoin, F., H. Hogeveen, and E. Wauters. 2021. Reducing antimicrobial use and dependence in livestock production systems: A social and economic sciences perspective on an interdisciplinary approach. Frontiers in Veterinary Science 8:584593.
BCG (Boston Consulting Group). 2018. Vaccines to tackle drug resistant infections: An evaluation of R&D opportunities. Boston Consulting Group. https://vaccinesforamr.org/read-the-report (accessed May 24, 2021).
Beal, S. G., M. Posa, M. Gaffar, J. Reppucci, J. A. Mack, M. J. Gurka, K. Rand, H. Houck, and M. N. Kelly. 2020. Performance and impact of a CLIA-waived, point-of-care respiratory PCR panel in a pediatric clinic. Pediatric Infectious Disease Journal 39(3):188-191.
Beaulac, K., S. Corcione, L. Epstein, L. E. Davidson, and S. Doron. 2016. Antimicrobial stewardship in a long-term acute care hospital using offsite electronic medical record audit. Infection Control and Hospital Epidemiology 37(4):433-439.
Bebell, L. M., and A. N. Muiru. 2014. Antibiotic use and emerging resistance: How can resource-limited countries turn the tide? Global Heart 9(3):347-358.
Bhutta, Z. A. 2020. International travel and the risk of extensively drug-resistant (XDR) typhoid: Issues and potential solutions. Clinical Infectious Diseases 73(11):e4590-e4591. https://doi.org/10.1093/cid/ciaa908.
Bielicki, J. A., and G. Fink. 2020. Measuring antibiotic use in children: Piecing together the puzzle. The Lancet Global Health 8(6):e742-e743.
Bjarnadottir, R. I., C. T. A. Herzig, J. L. Travers, N. G. Castle, and P. W. Stone. 2017. Implementation of electronic health records in US nursing homes. Computers, Informatics, Nursing 35(8):417-424.
Bonislawski, A. 2019. Point-of-care testing hit hard by PAMA cuts. https://www.360dx.com/clinical-lab-management/point-care-testing-hit-hard-pama-cuts#.YUjnQLhKiUl (accessed September 16, 2021).
Bright-Ponte, S. J. 2020. Antimicrobial use data collection in animal agriculture. Zoonoses and Public Health 67:1-5.
Buckley, B. S., N. Henschke, H. Bergman, B. Skidmore, E. J. Klemm, G. Villanueva, C. Garritty, and M. Paul. 2019. Impact of vaccination on antibiotic usage: A systematic review and meta-analysis. Clinical Microbiology and Infection 25(10):1213-1225.
Caliendo, A. M. 2015. Editorial commentary: Rapid blood culture identification: The value of a randomized trial. Clinical Infectious Diseases 61(7):1081-1083.
Carter, A. S., S. P. Luby, and D. O. Garrett. 2020. Introducing typhoid conjugate vaccine in south asia: Lessons from the Surveillance for Enteric Fever in Asia project. Clinical Infectious Diseases 71(Suppl 3):S191-S195.
CDC (Centers for Disease Control and Prevention). 2009. Report of expert consultations on rapid molecular testing to detect drug-resistant tuberculosis in the United States. Atlanta, GA: Centers for Disease Control and Prevention.
CDC. 2012. Guidelines and recommendations: A CDC primer. Atlanta, GA: Office of the Associate Director for Science Centers for Disease Control and Prevention.
CDC. 2015a. Antibiotic prescribing and use in hospitals and long-term care: Costs. https://www.cdc.gov/antibiotic-use/healthcare/evidence/asp-int-costs.htm (accessed May 4, 2021).
CDC. 2015b. The core elements of antibiotic stewardship for nursing homes. Atlanta, GA: Centers for Disease Control and Prevention.
CDC. 2018. Implementation of antibiotic stewardship core elements at small and critical access hospitals. Atlanta, GA: Centers for Disease Control and Prevention, Department of Health and Human Services.
CDC. 2019a. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: Centers for Disease Control and Prevention. http://dx.doi.org/10.15620/cdc:82532.
CDC. 2019b. Antibiotic use in the United States, 2018 update: Progress and opportunities. Atlanta, GA: Centers for Disease Control and Prevention.
CDC. 2019c. Core elements of hospital antibiotic stewardship programs. Atlanta, GA: Centers for Disease Control and Prevention.
CDC. 2020a. Antibiotic stewardship in nursing homes. Atlanta, GA: Centers for Disease Control and Prevention National Center for Emerging Zoonoti.
CDC. 2020b. Antibiotic use in the United States, 2020 update: Progress and opportunities. Atlanta, GA: Centers for Disease Control and Prevention, Department of Health and Human Services.
CDC. 2020c. Cholera—vibrio cholerae infection. https://www.cdc.gov/cholera/travelers.html (accessed May 24, 2021).
CDC. 2020d. Current report: Antibiotic prescribing and use in the U.S. https://www.cdc.gov/antibiotic-use/stewardship-report/current.html (accessed May 3, 2021).
CDC. 2020e. Dialysis safety. https://www.cdc.gov/dialysis/index.html (accessed May 3, 2021).
CDC. 2020f. Pneumococcal disease (streptococcus pneumoniae). https://wwwnc.cdc.gov/travel/diseases/pneumococcal-disease-streptococcus-pneumoniae (accessed May 19, 2021).
CDC. 2020g. Small and critical access facilities. https://www.cdc.gov/antibiotic-use/core-elements/small-critical.html (accessed May 3, 2021).
CDC. 2021a. Antibiotic prescribing and use: Nursing home facilities. https://www.cdc.gov/antibiotic-use/core-elements/nursing-homes.html (accessed May 4, 2021).
CDC. 2021b. Antibiotic use data and research. https://www.cdc.gov/antibiotic-use/data/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fantibiotic-use%2Fhealthcare%2Fevidence.html (accessed May 3, 2021).
CDC. 2021c. CDC to invest $2.1 billion to protect patients and healthcare workers from COVID-19 and future infectious diseases. https://www.cdc.gov/media/releases/2021/p0917-COVID-19-funding.html (accessed September 24, 2021).
CDC. 2021d. A complex web: Everything is connected food, farms, & animals. https://www.cdc.gov/drugresistance/pdf/CDC-Fights-AR-Food-508.pdf (accessed July 13, 2021).
CDC. 2021e. Core elements of antibiotic stewardship. https://www.cdc.gov/antibiotic-use/core-elements/index.html (accessed May 3, 2021).
CDC. 2021f. National Center for Health Statistics: Nursing home care. https://www.cdc.gov/nchs/fastats/nursing-home-care.htm (accessed May 3, 2021).
Chaguza, C., E. Heinsbroek, R. A. Gladstone, T. Tafatatha, M. Alaerts, C. Peno, J. E. Cornick, P. Musicha, N. Bar-Zeev, A. Kamng’ona, A. Kadioglu, L. McGee, W. P. Hanage, R. F. Breiman, R. S. Heyderman, N. French, D. B. Everett, and S. D. Bentley. 2020. Early signals of vaccine-driven perturbation seen in pneumococcal carriage population genomic data. Clinical Infectious Diseases 70(7):1294-1303.
Chambers, A., S. MacFarlane, R. Zvonar, G. Evans, J. E. Moore, B. J. Langford, A. Augustin, S. Cooper, J. Quirk, L. McCreight, and G. Garber. 2019. A recipe for antimicrobial stewardship success: Using intervention mapping to develop a program to reduce antibiotic overuse in long-term care. Infection Control and Hospital Epidemiology 40(1):24-31.
Chan-Tack, K. M., B. W. Trautner, and D. J. Morgan. 2020. The varying specificity of urine cultures in different populations. Infection Control and Hospital Epidemiology 41(4):489-491.
Chandler, C. I. R., E. Hutchinson, and C. Hutchison. 2016. Addressing antimicrobial resistance through social theory: An anthropologically oriented report. London School of Hygiene & Tropical Medicine. http://www.lshtm.ac.uk/php/ghd/research/app/anthropologyofantimicrobialresistance.html (accessed September 22, 2021).
Childers, C. P., J. Q. Dworsky, G. Kominski, and M. Maggard-Gibbons. 2019. A comparison of payments to a for-profit dialysis firm from government and commercial insurers. JAMA Internal Medicine 179(8):1136-1138.
Chirollo, C., F. P. Nocera, D. Piantedosi, G. Fatone, G. Della Valle, L. De Martino, and L. Cortese. 2021. Data on before and after the traceability system of veterinary antimicrobial prescriptions in small animals at the University Veterinary Teaching Hospital of Naples. Animals (Basel) 11(3):913.
Chitnis, A. S., J. R. Edwards, P. M. Ricks, D. M. Sievert, S. K. Fridkin, and C. V. Gould. 2012. Device-associated infection rates, device utilization, and antimicrobial resistance in long-term acute care hospitals reporting to the National Healthcare Safety Network, 2010. Infection Control and Hospital Epidemiology 33(10):993-1000.
Clark, C. 2017. Veterinary feed directive: What small farming operations need to know. https://www.extension.iastate.edu/smallfarms/veterinary-feed-directive-what-small-farming-operations-need-know-0 (accessed May 4, 2021).
Clark, S., R. Daly, E. Jordan, J. Lee, A. Mathew, and P. Ebner. 2012. Extension education symposium: The future of biosecurity and antimicrobial use in livestock production in the United States and the role of extension. Journal of Animal Science 90(8):2861-2872.
Clift, C. 2017. The value of vaccines in the avoidance of antimicrobial resistance. Chatham House. https://www.who.int/immunization/research/meetings_workshops/PDVAC_2017_AMR_Clift.pdf (accessed May 24, 2021).
CMS (Centers for Medicare & Medicaid Services). 2012. Research & demonstration grant options. https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ResearchDemoGrantsOpt (accessed May 24, 2021).
CMS. 2015. Nursing home data compendium 2015 edition. Centers for Medicare & Medicaid Services, Department of Health and Human Services. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/nursinghomedatacompendium_508-2015.pdf (accessed July 13, 2021).
CMS. 2016. Medicare and Medicaid programs; reform of requirements for longterm care facilities. Washington, DC: Centers for Medicare & Medicaid Services, Department of Health and Human Services.
CMS. 2019a. Medicare and Medicaid programs; regulatory provisions to promote program efficiency, transparency, and burden reduction; fire safety requirements for certain dialysis facilities; hospital and critical access hospital (CAH) changes to promote innovation, flexibility, and improvement in patient care. Washington, DC: Centers for Medicare & Medicaid Services, Department of Health and Human Services.
CMS. 2019b. What are long-term care hospitals? Washington, DC: Department of Health and Human Services.
CMS. 2020. Quality measures. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIQualityMeasures (accessed June 25, 2021).
CMS. 2021a. Fact sheet: Nursing home compare five-star quality rating system. https://www.cms.gov/medicare/provider-enrollment-and-certification/certificationandcomplianc/downloads/consumerfactsheet.pdf (accessed July 13, 2021)
CMS. 2021b. Find & compare nursing homes, hospitals & other providers near you. https://www.medicare.gov/care-compare (accessed June 25, 2021).
CMS. 2021c. Five-star quality rating system. https://www.cms.gov/Medicare/ProviderEnrollment-and-Certification/CertificationandComplianc/FSQRS (accessed June 25, 2021).
CMS. 2021d. Medicare program—general information. https://www.cms.gov/Medicare/Medicare-General-Information/MedicareGenInfo (accessed May 19, 2021).
CMS. 2021e. Proposed local coverage determination (LCD): Moldx: Multiplex nucleic acid amplification test (NAAT) panels for infectious disease testing (dl38988). https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=38987&ver=11&keyword=moldx&keywordType=starts&areaId=all&docType=P&contractOption=all&sortBy=updated&bc=AAAAAAQAAAAA&KeyWordLookUp=Doc&KeyWordSearchType=Exact (accessed September 22, 2021).
CMS. 2021f. Successful return to home & community. https://www.medicare.gov/carecompare/details/long-term-care/092002?city=Washington&state=DC&zipcode=20001 (accessed June 25, 2021).
CMS. 2021g. Your rights and protections as a nursing home resident. https://downloads.cms.gov/medicare/your_resident_rights_and_protections_section.pdf (accessed September 15, 2021).
CMS News and Media Group. 2020. CMS care compare empowers patients when making important health care decisions. https://www.cms.gov/newsroom/press-releases/cms-care-compare-empowers-patients-when-making-important-health-care-decisions (September 22, 2021).
Cohen, C. C., M. Pogorzelska-Maziarz, C. T. Herzig, E. J. Carter, R. Bjarnadottir, P. Semeraro, J. L. Travers, and P. W. Stone. 2015. Infection prevention and control in nursing homes: A qualitative study of decision-making regarding isolation-based practices. BMJ Quality & Safety 24(10):630-636.
Cohen, J. F., N. Bertille, R. Cohen, and M. Chalumeau. 2016. Rapid antigen detection test for group a streptococcus in children with pharyngitis. Cochrane Database of Systematic Reviews 7:CD010502.
Collier, P., and L. J. O’Neill. 2018. Two years on: An update on achievement towards the recommendations of the Antimicrobial Resistance Report. Journal of Applied Microbiology 125(2):308-312.
Cooke, J., C. Llor, R. Hopstaken, M. Dryden, and C. Butler. 2020. Respiratory tract infections (RTIs) in primary care: Narrative review of c reactive protein (CRP) point-of-care testing (POCT) and antibacterial use in patients who present with symptoms of RTI. BMJ Open Respiratory Research 7(1):e000624.
Cooper, S. J. 2020. Overview of CMS rule changes to promote antimicrobial stewardship programs. HHS Listening Session on Combating Antibiotic-Resistant Bacteria, Washington, DC. https://www.hhs.gov/ash/advisory-committees/paccarb/meetings/upcoming-meetings/february-26-2020-hhs-listening-sessions/index.html (accessed July 13, 2021).
Craig, J. 2019. Weekly digest: XDR typhoid fever in Pakistan; flu activity rising in northern hemisphere and South Asia; antibiotic use on Pennsylvania dairy farms. Center for Disease Dynamics, Economics & Policy. https://cddep.org/blog/posts/weekly-digest-jan-04-2019 (accessed May 4, 2021).
Dagan, R., C. Juergens, J. Trammel, S. Patterson, D. Greenberg, N. Givon-Lavi, N. Porat, A. Gurtman, W. C. Gruber, and D. A. Scott. 2015. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible streptococcus pneumoniae. Journal of Infectious Diseases 211(7):1144-1153.
D’Agata, E. M. C., D. Tran, J. Bautista, D. Shemin, and D. Grima. 2018. Clinical and economic benefits of antimicrobial stewardship programs in hemodialysis facilities: A decision analytic model. Clinical Journal of the American Society of Nephrology 13(9):1389-1397.
Damborg, P. 2021. VetCAST susceptibility test breakpoints. Committee on the Long-Term Health and Economic Effects of Antimicrobial Resistance: Meeting 3, Webinar. January 5-8, 2021. https://www.nationalacademies.org/event/01-05-2021/examining-the-long-term-health-and-economic-effects-of-antimicrobial-resistance-in-the-united-states-meeting-3.
Dargatz, D. A., M. M. Erdman, and B. Harris. 2017. A survey of methods used for antimicrobial susceptibility testing in veterinary diagnostic laboratories in the United States. Journal of Veterinary Diagnostic Investigation 29(5):669-675.
Denyer Willis, L., and C. Chandler. 2019. Quick fix for care, productivity, hygiene and inequality: Reframing the entrenched problem of antibiotic overuse. BMJ Global Health 4(4):e001590.
Do, N. T. T., H. T. L. Vu, C. T. K. Nguyen, S. Punpuing, W. A. Khan, M. Gyapong, K. P. As-ante, K. Munguambe, F. X. Gómez-Olivé, J. John-Langba, T. K. Tran, M. Sunpuwan, E. Sevene, H. H. Nguyen, P. D. Ho, M. A. Matin, S. Ahmed, M. M. Karim, O. Cambaco, S. Afari-Asiedu, E. Boamah-Kaali, M. A. Abdulai, J. Williams, S. Asiamah, G. Amankwah, M. P. Agyekum, F. Wagner, P. Ariana, B. Sigauque, S. Tollman, H. R. van Doorn, O. Sankoh, J. Kinsman, and H. F. L. Wertheim. 2021. Community-based antibiotic access and use in six low-income and middle-income countries: A mixed-method approach. The Lancet Global Health 9(5):e610-e619.
Doron, S., and L. E. Davidson. 2011. Antimicrobial stewardship. Mayo Clinic Proceedings 86(11):1113-1123.
DVFA (Danish Veterinary and Food Administration). 2017. The Yellow Card Initiative on antibiotics. https://www.foedevarestyrelsen.dk/english/Animal/AnimalHealth/Pages/The-Yellow-Card-Initiative-on-Antibiotics.aspx (accessed May 4, 2021).
Ehren, K., A. Meissner, N. Jazmati, J. Wille, N. Jung, J. J. Vehreschild, M. Hellmich, and H. Seifert. 2020. Clinical impact of rapid species identification from positive blood cultures with same-day phenotypic antimicrobial susceptibility testing on the management and outcome of bloodstream infections. Clinical Infectious Diseases 70(7):1285-1293.
Ekakoro, J. E., M. Caldwell, E. B. Strand, and C. C. Okafor. 2019. Drivers, alternatives, knowledge, and perceptions towards antimicrobial use among Tennessee beef cattle producers: A qualitative study. BMC Veterinary Research 15(1):16.
Eljaaly, K., S. Elarabi, S. Alshehri, and D. E. Nix. 2018. Impact of requiring re-authorization of restricted antibiotics on day 3 of therapy. Journal of Antimicrobial Chemotherapy 73(2):527-530.
EMA (European Medicines Agency). 2020. Advice on implementing measures under article 57(4) of regulation (EU) 2019/6 on veterinary medicinal products: Report on the format of the data to be collected on antimicrobial medicinal products used in animals. Amsterdam, The Netherlands: Committee for Medicinal Products for Veterinary Use.
Espinosa-Gongora, C., L. R. Jessen, O. J. Dyar, A. Bousquet-Melou, B. Gonzalez-Zorn, C. Pulcini, G. Re, S. Schwarz, D. Timofte, P. L. Toutain, L. Guardabassi, The PREPARE-VET Working Group, ESCMID Study Group for Veterinary Microbiology (ESGVM), and ESCMID Study Group for Antimicrobial Stewardship (ESGAP). 2021. Towards a better and harmonized education in antimicrobial stewardship in European veterinary curricula. Antibiotics (Basel) 10(4):364.
EUCAST (European Committee on Antimicrobial Susceptibility Testing). 2021. The EUCAST Veterinary Subcommittee on Antimicrobial Susceptibility Testing (VETCAST). https://www.eucast.org/organization/subcommittees/vetcast (accessed May 19, 2021).
Eyler, R. F., and K. Shvets. 2019. Clinical pharmacology of antibiotics. Clinical Journal of the American Society of Nephrology 14(7):1080-1090.
FAO (Food and Agriculture Organization of the United Nations). 2019. Monitoring and surveillance of antimicrobial resistance in bacteria from healthy food animals intended for consumption. Bangkok, Thailand: Food and Agriculture Organization of the United Nations.
FAO and Denmark Ministry of Environment and Food. 2019. Tackling antimicrobial use and resistance in pig production: Lessons learned from Denmark. Rome, Italy: Food and Agriculture Organization of the United Nations and Denmark Ministry of Environment and Food–Danish Veterinary and Food Administration.
FDA (Food and Drug Administration). 2020a. Antibacterial susceptibility test interpretive criteria. https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria (accessed May 19, 2021).
FDA. 2020b. Center forVeterinaryMedicine. https://www.fda.gov/about-fda/fda-organization/center-veterinary-medicine (accessed May 24, 2021).
FDA.2020c.FDAannounces2020fundingopportunitytohelpdefinedurationsofuseforcertainmedically important antimicrobial drugs for food animals. https://www.fda.gov/animal-veterinary/cvm-updates/fda-announces-2020-funding-opportunity-help-define-durations-use-certain-medically-important (accessed September 20, 2021).
FDA. 2020d. FDA announces FY 2020 funding opportunity for collection of antimicrobial use data in dogs and cats. https://www.fda.gov/animal-veterinary/cvm-updates/fda-announces-fy-2020-funding-opportunity-collection-antimicrobial-use-data-dogs-and-cats (accessed May 4, 2021).
FDA. 2020e. FDA releases annual summary report on antimicrobials sold or distributed in 2019 for use in food-producing animals. https://www.fda.gov/animal-veterinary/cvm-updates/fda-releases-annual-summary-report-antimicrobials-sold-or-distributed-2019-use-food-producing (accessed May 4, 2021).
FDA. 2020f. Learn about FDA advisory committees. https://www.fda.gov/patients/about-office-patient-affairs/learn-about-fda-advisory-committees (accessed May 19, 2021).
FDA. 2021a. Extralabel use and antimicrobials. https://www.fda.gov/animal-veterinary/antimicrobial-resistance/extralabel-use-and-antimicrobials (accessed May 4, 2021).
FDA. 2021b. FDA seeks public comment on potential approach for defining durations of use for certain medically important antimicrobial drugs for food animals. https://www.fda.gov/animal-veterinary/cvm-updates/fda-seeks-public-comment-potential-approach-defining-durations-use-certain-medically-important (accessed September 20, 2021).
FDA. 2021c. Real-world evidence. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (accessed August 11, 2021).
FDA Center for Veterinary Medicine. 2018. Supporting antimicrobial stewardship in veterinary settings: Goals for fiscal years 2019–2023. https://www.fda.gov/files/animal%20&%20veterinary/published/Supporting-Antimicrobial-Stewardship-in-Veterinary-Settings--Goals-for-Fiscal-Years-2019-2023.pdf (accessed September 24, 2021).
Feldstein, D., P. D. Sloane, and C. Feltner. 2018. Antibiotic stewardship programs in nursing homes: A systematic review. Journal of the American Medical Directors Association 19(2):110-116.
Ferrante di Ruffano, L., C. J. Hyde, K. J. McCaffery, P. M. Bossuyt, and J. J. Deeks. 2012. Assessing the value of diagnostic tests: A framework for designing and evaluating trials. BMJ 344:e686.
Franke, M. F., R. Ternier, J. G. Jerome, W. R. Matias, J. B. Harris, and L. C. Ivers. 2018. Long-term effectiveness of one and two doses of a killed, bivalent, whole-cell oral cholera vaccine in haiti: An extended case-control study. The Lancet Global Health 6(9):e1028-e1035.
Frost, I., J. Craig, J. Joshi, K. Faure, and R. Laxminarayan. 2019. Access barriers to antibiotics. Washington, DC: Center for Disease Dynamics, Economics & Policy.
FTF CARB (Federal Task Force on Combating Antibiotic-Resistant Bacteria). 2020. National action plan for combating antibiotic-resistant bacteria 2020-2025. Washington, DC: U.S. Department of Health & Human Services. https://aspe.hhs.gov/system/files/pdf/264126/CARB-National-Action-Plan-2020-2025.pdf (accessed July 1, 2021).
Fu, C. J., E. Mantell, P. W. Stone, and M. Agarwal. 2020. Characteristics of nursing homes with comprehensive antibiotic stewardship programs: Results of a national survey. American Journal of Infection Control 48(1):13-18.
Genome Web. 2012. Update: Biofire ramping up filmarray adoption, test development. https://www.genomeweb.com/pcr/update-biofire-ramping-filmarray-adoption-test-development#.YNNAjuhKiUl (accessed June 25, 2021).
Gerding, D. N. 2001. The search for good antimicrobial stewardship. Joint Commission Journal on Quality Improvement 27(8):403-404.
Gibani, M. M., C. Britto, and A. J. Pollard. 2018. Typhoid and paratyphoid fever: A call to action. Current Opinion in Infectious Diseases 31(5):440-448.
Gibani, M. M., M. Voysey, C. Jin, C. Jones, H. Thomaides-Brears, E. Jones, P. Baker, M. Morgan, A. Simmons, M. A. Gordon, V. Cerundolo, V. E. Pitzer, B. Angus, M. M. Levine, T. C. Darton, and A. J. Pollard. 2019. The impact of vaccination and prior exposure on stool shedding of Salmonella Typhi and Salmonella Paratyphi in 6 controlled human infection studies. Clinical Infectious Diseases 68(8):1265-1273.
Gliklich, R. E., N. A. Dreyer, and M. B. Leavy. 2014. Registries for evaluating patient outcomes: A user’s guide, 3rd ed. Rockville, MD: Agency for Healthcare Research and Quality. 13(14):EHC111.
Goebel, M. C., B. W. Trautner, and L. Grigoryan. 2021. The five Ds of outpatient antibiotic stewardship for urinary tract infections. Clinical Microbiology Reviews e0000320.
Goggs, R., J. M. Menard, C. Altier, K. J. Cummings, M. E. Jacob, D. F. Lalonde-Paul, M. G. Papich, K. N. Norman, V. R. Fajt, H. M. Scott, and S. D. Lawhon. 2021. Patterns of antimicrobial drug use in veterinary primary care and specialty practice: A 6-year multiinstitution study. Journal of Veterinary Internal Medicine 35(3):1496-1508.
Gotterson, F., K. Buising, and E. Manias. 2021. Nurse role and contribution to antimicrobial stewardship: An integrative review. International Journal of Nursing Studies 117:103787.
Gould, C. V., R. Rothenberg, and J. P. Steinberg. 2006. Antibiotic resistance in long-term acute care hospitals: The perfect storm. Infection Control and Hospital Epidemiology 27(9):920-925.
Government of Ireland. 2021. Webinar series for EU regulations on veterinary medicinal products, 2019/6, and the manufacture, placing on the market and use of medicated feed, 2019/4. https://www.gov.ie/en/publication/2dffa-webinar-series-for-eu-regulation-on-veterinary-medicinal-products-20196-and-the-manufacture-of-medicated-feed-20194/#the-futureof-prescribing-eu-regulations-and-what-they-mean-for-you (accessed May 4, 2021).
Gupta, S. S., K. Bharati, D. Sur, A. Khera, N. K. Ganguly, and G. B. Nair. 2016. Why is the oral cholera vaccine not considered an option for prevention of cholera in India? Analysis of possible reasons. Indian Journal of Medical Research 143(5):545-551.
Hammitt, L. L., D. O. Akech, S. C. Morpeth, A. Karani, N. Kihuha, S. Nyongesa, T. Bwanaali, E. Mumbo, T. Kamau, S. K. Sharif, and J. A. Scott. 2014. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of streptococcus pneumoniae and non-typeable haemophilus influenzae in Kilifi, Kenya: Findings from cross-sectional carriage studies. The Lancet Global Health 2(7):e397-e405.
Hampton, L. M., M. M. Farley, W. Schaffner, A. Thomas, A. Reingold, L. H. Harrison, R. Lynfield, N. M. Bennett, S. Petit, K. Gershman, J. Baumbach, B. Beall, J. Jorgensen, A. Glennen, E. R. Zell, and M. Moore. 2012. Prevention of antibiotic-nonsusceptible streptococcus pneumoniae with conjugate vaccines. Journal of Infectious Diseases 205(3):401-411.
Hanson, K. E., M. M. Azar, R. Banerjee, A. Chou, R. C. Colgrove, C. C. Ginocchio, M. K. Hayden, M. Holodiny, S. Jain, S. Koo, J. Levy, T. T. Timbrook, and A. M. Caliendo. 2020. Molecular testing for acute respiratory tract infections: Clinical and diagnostic recommendations from the IDSA’s diagnostics committee. Clinical Infectious Diseases 71(10):2744-2751.
Harris-Kojetin L. D., M. Sengupta, E. Park-Lee, R. Valverde, C. Caffrey, V. Rome, and J. Lendon. 2016. Long-term care providers and services users in the United States: Data from the National Study of Long-Term Care Providers, 2013-2014. Vital Health Stat 3. (38):1-105.
Haverkate, M. R., S. Weiner, K. Lolans, N. M. Moore, R. A. Weinstein, M. J. Bonten, M. K. Hayden, and M. C. Bootsma. 2016. Duration of colonization with klebsiella pneumoniae carbapenemase-producing bacteria at long-term acute care hospitals in Chicago, Illinois. Open Forum Infectious Diseases 3(4):ofw178.
Hemarajata, P., S. Yang, O. O. Soge, R. M. Humphries, and J. D. Klausner. 2016. Performance and verification of a real-time PCR assay targeting the gyra gene for prediction of ciprofloxacin resistance in neisseria gonorrhoeae. Journal of Clinical Microbiology 54(3):805-808.
Henriksson, P. J. G., A. Rico, M. Troell, D. H. Klinger, A. H. Buschmann, S. Saksida, M. V. Chadag, and W. Zhang. 2018. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: A review from a systems perspective. Sustainibility Science 13(4):1105-1120.
Holz, M., S. Naavaal, S. Stilianoudakis, C. Carrico, B. E. Byrne, and G. L. Myers. 2021. Antibiotics and antimicrobial resistance: Evaluation of the knowledge, attitude, and perception among students and faculty within us dental schools. Journal of Dental Education 85(3):383-391.
Horton, G., J. Roach, and S. Scheffler. 2020 (unpublished). Understanding measures, star ratings, and quality outcomes. Centers for Medicare & Medicaid Services. https://www.cms.gov/files/document/dialysis-facility-compare-january-2020-national-provider-call-presentation.pdf (accessed September 22, 2021).
Horumpende, P. G., T. B. Sonda, M. van Zwetselaar, M. L. Antony, F. F. Tenu, C. E. Mwanziva, E. R. Shao, S. E. Mshana, B. T. Mmbaga, and J. O. Chilongola. 2018. Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi municipality, Kilimanjaro region in Tanzania: A simulated clients approach. PloS One 13(11):e0207465.
Hsueh, B. Y., and C. M. Waters. 2019. Combating cholera. F1000Res 8:F1000. Faculty Rev-589. https://doi.org/10.12688/f1000research.18093.1.
Humphries, R. M., A. N. Abbott, and J. A. Hindler. 2019. Understanding and addressing CLSI breakpoint revisions: A primer for clinical laboratories. Journal of Clinical Microbiology 57(6):e00203-19.
ISU (Iowa State University). 2021. Diagnostic tests. https://vetmed.iastate.edu/vdl/diagnostic-tests (accessed May 19, 2021).
Jacob, J., A. Morace, J. Park, and N. Renzi. 2019. 1169. Preventing central line-associated bloodstream infections in long-term acute care. Open Forum Infectious Diseases 6(Suppl 2):S418-S419.
Jacobs, T. G., J. Robertson, H. A. van den Ham, K. Iwamoto, H. Bak Pedersen, and A. K. Mantel-Teeuwisse. 2019. Assessing the impact of law enforcement to reduce over-the-counter (otc) sales of antibiotics in low- and middle-income countries; a systematic literature review. BMC Health Services Research 19(1):536.
Jamka, L. P., K. W. Simiyu, A. D. Bentsi-Enchill, A. J. Mwisongo, H. Matzger, A. A. Marfin, A. J. Pollard, and K. M. Neuzil. 2019. Accelerating typhoid conjugate vaccine introduction: What can be learned from prior new vaccine introduction initiatives? Clinical Infectious Diseases 68(Suppl 2):S171-S176.
Jansen, K. U., C. Knirsch, and A. S. Anderson. 2018. The role of vaccines in preventing bacterial antimicrobial resistance. Nature Medicine 24(1):10-19.
Joint Commission. 2016. Approved: New antimicrobial stewardship standard. Joint Commission Perspectives 36(7):1-3.
Kadri, S. S. 2020. Key takeaways from the U.S. CDC’s 2019 antibiotic resistance threats report for frontline providers. Critical Care Medicine 48(7):939-945.
Kahlmeter, G., C. G. Giske, T. J. Kirn, and S. E. Sharp. 2019. Point-counterpoint: Differences between the European Committee on Antimicrobial Susceptibility Testing and Clinical and Laboratory Standards Institute recommendations for reporting antimicrobial susceptibility results. Journal of Clinical Microbiology 57(9):e01129-01119.
Kahn, M. G., M. A. Raebel, J. M. Glanz, K. Riedlinger, and J. F. Steiner. 2012. A pragmatic framework for single-site and multisite data quality assessment in electronic health record-based clinical research. Medical Care 50(Suppl):S21-S29.
Kapadia, S. N., E. L. Abramson, E. J. Carter, A. S. Loo, R. Kaushal, D. P. Calfee, and M. S. Simon. 2018. The expanding role of antimicrobial stewardship programs in hospitals in the united states: Lessons learned from a multisite qualitative study. Joint Commission Journal on Quality and Patient Safety 44(2):68-74.
Kasabova, S., M. Hartmann, N. Werner, A. Kasbohrer, and L. Kreienbrock. 2019. Used daily dose vs. defined daily dose-contrasting two different methods to measure antibiotic consumption at the farm level. Frontiers in Veterinary Science 6:116.
Katz, M. J., A. P. Gurses, P. D. Tamma, S. E. Cosgrove, M. A. Miller, and R. L. P. Jump. 2017. Implementing antimicrobial stewardship in long-term care settings: An integrative review using a human factors approach. Clinical Infectious Diseases 65(11):1943-1951.
Kelloway, C. 2018. Mars buys another veterinary network, as a candy-company turned pet care giant furthers its influence over animal health. Food & Power. https://www.foodandpower.net/latest/2018/06/21/mars-buys-another-veterinary-network-as-a-candy-company-turned-pet-care-giant-furthers-its-influence-over-animal-health (accessed May 19, 2021).
Khan, M. I., C. Franco-Paredes, S. Sahastrabuddhe, R. L. Ochiai, V. Mogasale, and B. D. Gessner. 2017. Barriers to typhoid fever vaccine access in endemic countries. Research and Reports in Tropical Medicine 8:37-44.
Kingwell, K. 2018. Vaccines take a shot at antimicrobial resistance. Nature Reviews Drug Discovery 17(4):229-231.
Klein, E. Y., E. Schueller, K. K. Tseng, D. J. Morgan, R. Laxminarayan, and A. Nandi. 2020. The impact of influenza vaccination on antibiotic use in the United States, 2010-2017. Open Forum Infectious Diseases 7(7):ofaa223.
Klugman, K. P., and S. Black. 2018. Impact of existing vaccines in reducing antibiotic resistance: Primary and secondary effects. Proceedings of the National Academy of Sciences of the United States of America 115(51):12896-12901.
Koper, L. M., C. Bode, A. Bender, I. Reimer, T. Heberer, and J. Wallmann. 2020. Eight years of sales surveillance of antimicrobials for veterinary use in Germany—what are the perceptions? PloS One 15(8):e0237459.
Kwong, K. L., D. Lung, S. N. Wong, T. L. Que, and N. S. Kwong. 2009. Influenza-related hospitalisations in children. Journal of Paediatrics and Child Health 45(11):660-664.
LaBore, K. 2014. Minnesota nursing home abuse and neglect attorney: Residents have free choice to determine physician. https://www.mnnursinghomelaw.com/physician-2 (accessed September 15, 2021).
Laxminarayan, R., P. Matsoso, S. Pant, C. Brower, J. A. Rottingen, K. Klugman, and S. Davies. 2016. Access to effective antimicrobials: A worldwide challenge. Lancet 387(10014):168-175.
Levin, D. I., T. Lingam, and N. J. Janiga. 2020. 2020 outlook: Dialysis clinics and ESRD. https://healthcareappraisers.com/2020-outlook-dialysis-clinics-and-esrd (accessed May 4, 2020).
Lewinsohn, D. M., M. K. Leonard, P. A. LoBue, D. L. Cohn, C. L. Daley, E. Desmond, J. Keane, D. A. Lewinsohn, A. M. Loeffler, G. H. Mazurek, R. J. O’Brien, M. Pai, L. Richeldi, M. Salfinger, T. M. Shinnick, T. R. Sterling, D. M. Warshauer, and G. L. Woods. 2017. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clinical Infectious Diseases 64(2):e1-e33.
Lewnard, J. A., N. C. Lo, N. Arinaminpathy, I. Frost, and R. Laxminarayan. 2020. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature 581(7806):94-99.
Lin, M. Y., R. D. Lyles-Banks, K. Lolans, D. W. Hines, J. B. Spear, R. Petrak, W. E. Trick, R. A. Weinstein, M. K. Hayden, Centers for Disease Control, and Prevention Epicenters Program. 2013. The importance of long-term acute care hospitals in the regional epidemiology of klebsiella pneumoniae carbapenemase-producing enterobacteriaceae. Clinical Infectious Diseases 57(9):1246-1252.
Lipsitch, M., and G. R. Siber. 2016. How can vaccines contribute to solving the antimicrobial resistance problem? mBio 7(3):e00428-00416.
Livorsi, D. J., R. Nair, B. C. Lund, B. Alexander, B. F. Beck, M. Goto, M. Ohl, M. S. Vaughan-Sarrazin, M. B. Goetz, and E. N. Perencevich. 2021. Antibiotic stewardship implementation and antibiotic use at hospitals with and without on-site infectious disease specialists. Clinical Infectious Diseases 72(10):1810-1817.
Lochen, A., N. J. Croucher, and R. M. Anderson. 2020. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Scientific Reports 10(1):18977.
Lovegreen, O., D. Riggs, M. A. Staten, P. Sheehan, and A. G. Pittas. 2018. Financial management of large, multi-center trials in a challenging funding milieu. Trials 19(1):267.
Lubbers, B. 2021. Addressing challenges in veterinary diagnostics. Committee on the Long-Term Health and Economic Effects of Antimicrobial Resistance: Meeting 3, Webinar. January 5-8, 2021. https://www.nationalacademies.org/event/01-05-2021/examining-the-long-term-health-and-economic-effects-of-antimicrobial-resistance-in-the-united-states-meeting-3 (accessed June 4, 2021).
Luo, R., J. Sickler, F. Vahidnia, Y. C. Lee, B. Frogner, and M. Thompson. 2019. Diagnosis and management of group a streptococcal pharyngitis in the United States, 2011-2015. BMC Infectious Diseases 19(1):193.
Lynch, J. 2021. Telehealth and antimicrobial stewarship. Committee on the Long-Term Health and Economic Effects of Antimicrobial Resistance: Meeting 3, Webinar. January 5-8, 2021. https://www.nationalacademies.org/event/01-05-2021/examining-the-long-term-health-and-economic-effects-of-antimicrobial-resistance-in-the-united-states-meeting-3 (accessed June 4, 2021).
MacIntyre, C. R., A. A. Chughtai, M. Barnes, I. Ridda, H. Seale, R. Toms, and A. Heywood. 2018. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infectious Diseases 18(1):637.
Makam, A. N., T. Tran, M. E. Miller, L. Xuan, O. K. Nguyen, and E. A. Halm. 2019. The clinical course after long term acute care hospital admission among older Medicare beneficiaries. Journal of the American Geriatrics Society 67(11):2282-2288.
Malarski, M., M. Hasso-Agopsowicz, A. Soble, W. Mok, S. Mathewson, and J. Vekemans. 2019. Vaccine impact on antimicrobial resistance to inform Gavi, the vaccine alliance’s 2018 vaccine investment strategy: Report from an expert survey. F1000Res 8:1685.
Martinez, E. P., M. Cepeda, M. Jovanoska, W. M. Bramer, J. Schoufour, M. Glisic, A. Verbon, and O. H. Franco. 2019. Seasonality of antimicrobial resistance rates in respiratory bacteria: A systematic review and meta-analysis. PloS One 14(8):e0221133.
Mayo Clinic. 2020. C. Difficile infection. https://www.mayoclinic.org/diseases-conditions/cdifficile/symptoms-causes/syc-20351691 (accessed May 3, 2021).
MedPAC (Medicare Payment Advisory Commission). 2020. Long-term care hospital services. In Report to the Congress: Medicare Payment Policy. Medicare Payment Advisory Commission. https://www.medpac.gov/wp-content/uploads/import_data/scrape_files/docs/default-source/reports/mar20_medpac_ch11_sec.pdf (accessed May 4, 2021).
Meeker, D., J. A. Linder, C. R. Fox, M. W. Friedberg, S. D. Persell, N. J. Goldstein, T. K. Knight, J. W. Hay, and J. N. Doctor. 2016. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: A randomized clinical trial. JAMA 315(6):562-570.
Mendelson, M., and M. P. Matsoso. 2015. The World Health Organization global action plan for antimicrobial resistance. South African Medical Journal 105(5):325.
Messacar, K., S. K. Parker, J. K. Todd, and S. R. Dominguez. 2017. Implementation of rapid molecular infectious disease diagnostics: The role of diagnostic and antimicrobial stewardship. Journal of Clinical Microbiology 55(3):715-723.
Moeller, G., S. Buchsbaum, P. Wilson, A. Shepherd, P. Kaspar, M. Giovanni, L. Penny, M. Urdea, P. Vickerman, C. Dye, J. Cunningham, S. Reed, C. Hanson, P. Small, M. Perkins, R. Allan, A. Magill, T. Taylor, R. Guerrant, J. Agosti, L. von Seidlein, J. Hughes, M. Miller, C. Mason, P. Tarr, C. Wilfert, K. Holmes, P. Mwaba, N. Hellmann, R. Peeling, D. C. Hay Burgess, J. Wasserman, C. A. Dahl, F. Girosi, S. S. Olmsted, E. B. Keeler, Y-W. Lim, J. E. Aledort, M. E. Rafael, and K. A. Ricci. 2007. Estimating the global health impact of improved diagnostic tools for the developing world. Santa Monica, CA: RAND Corporation.
Morley, P. S., M. D. Apley, T. E. Besser, D. P. Burney, P. J. Fedorka-Cray, M. G. Papich, J. L. Traub-Dargatz, J. S. Weese, and American College of Veterinary Internal Medicine. 2005. Antimicrobial drug use in veterinary medicine. Journal of Veterinary Internal Medicine 19(4):617-629.
Morris, D. E., D. W. Cleary, and S. C. Clarke. 2017. Secondary bacterial infections associated with influenza pandemics. Frontiers in Microbiology 8:1041.
Morris, Z. S., S. Wooding, and J. Grant. 2011. The answer is 17 years, what is the question: Understanding time lags in translational research. Journal of the Royal Society of Medicine 104(12):510-520.
Munoz-Price, L. S. 2009. Long-term acute care hospitals. Clinical Infectious Diseases 49(3):438-443.
Muri-Gama, A. S., A. Figueras, and S. R. Secoli. 2018. Inappropriately prescribed and over-the-counter antimicrobials in the brazilian amazon basin: We need to promote more rational use even in remote places. PloS One 13(8):e0201579.
Mushtaq, A., R. A. Awali, S. Chandramohan, A. Krishna, C. Biedron, O. Jegede, and T. Chopra. 2017. Implementing an antibiotic stewardship program at a long-term acute care hospital in Detroit, Michigan. American Journal of Infection Control 45(12):e157-e160.
Nasr, Z. G., D. M. Abbara, and K. J. Wilby. 2021. A scoping review of antimicrobial stewardship teaching in pharmacy education curricula. American Journal of Pharmaceutical Education 85(6):8415.
NIA (National Institute on Aging). 2017a. Residential facilities, assisted living, and nursing homes. https://www.nia.nih.gov/health/residential-facilities-assisted-living-and-nursing-homes (accessed May 19, 2021).
NIA. 2017b. What is long-term care? https://www.nia.nih.gov/health/what-long-term-care (accessed May 19, 2021).
NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases). 2012. Livertox: Clinical and research information on drug-induced liver injury, vancomycin. https://www.ncbi.nlm.nih.gov/books/NBK548881 (accessed May 4, 2021).
NIMSS (National Information Management & Support System). 2017. NC1206: Antimicrobial resistance. https://www.nimss.org/projects/view/mrp/outline/18361 (accessed July 13, 2021).
NITAG (National Immunization Technical Advisory Groups). 2019. Network. https://www.nitag-resource.org/network/vision-mission (accessed May 19, 2021).
NLM (National Library of Medicine). 2020. Strep a test. https://medlineplus.gov/lab-tests/strep-a-test (accessed July 13, 2021).
O’Fallon, E., S. Gautam, and E. M. D’Agata. 2009. Colonization with multidrug-resistant gram-negative bacteria: Prolonged duration and frequent cocolonization. Clinical Infectious Diseases 48(10):1375-1381.
Okeke, I. N. 2009. Cholera vaccine will reduce antibiotic use. Science 325(5941):674.
Okeke, I. N., R. W. Peeling, H. Goossens, R. Auckenthaler, S. S. Olmsted, J. F. de Lavison, B. L. Zimmer, M. D. Perkins, and K. Nordqvist. 2011. Diagnostics as essential tools for containing antibacterial resistance. Drug Resistance Updates 14(2):95-106.
O’Neill, J. 2016. Tackling drug-resistant infections globally: Final report and recommendations. 2016. London, UK: HM Government and Wellcome Trust. https://wellcomecollection.org/works/thvwsuba (accessed April 8, 2021).
Ortiz, J. R., and K. M. Neuzil. 2019. Influenza immunization in low- and middle-income countries: Preparing for next-generation influenza vaccines. Journal of Infectious Diseases 219(Suppl 1):S97-S106.
Ott, J. J., J. Klein Breteler, J. S. Tam, R. C. Hutubessy, M. Jit, and M. R. de Boer. 2013. Influenza vaccines in low and middle income countries: A systematic review of economic evaluations. Human Vaccines & Immunotherapeutics 9(7):1500-1511.
Ouedraogo, F., M. Salois, B. Bain, C. Hansen, and B. Dutton. 2018. 2018 AVMA report on the market for veterinary services. Colorado State University Libraries. https://mountainscholar.org/handle/10217/195430 (accessed May 4, 2021).
Papich, M. 2020. Antibiotic use in companion animals. Committee on the Long-Term Health and Economic Effects of Antimicrobial Resistance: Meeting 2, Webinar. November 9-11, 2020. https://www.nationalacademies.org/event/11-09-2020/examining-the-long-term-health-and-economic-effects-of-antimicrobial-resistance-in-the-united-states-meeting-2 (accessed June 4, 2021).
Patel R., C. R. Polage, J. D. Bard, L. May, F. M. Lee, V. Fabre, M. K. Hayden, S. D. B. Doernberg, D. A. Haake, B. W. Trautner, L. Grigoryan, E. L. Tsalik, and K. E. Hanson. Envisioning Future UTI Diagnostics. Clinical Infectious Diseases ciab749. Epub ahead of print. https://doi.org/10.1093/cid/ciab749.
PCAST (President’s Council of Advisors on Science and Technology). 2020. National action plan for combating antibiotic-resistant bacteria, 2020-2025. Washington, DC: The White House: Federal Task Force on Combating Antibiotic-Resistant Bacteria.
PCORI (Patient-Centered Outcomes Research Institute). 2014. Our story. https://www.pcori.org/about-us/our-story (accessed July 23, 2021).
Peck, M., M. Gacic-Dobo, M. S. Diallo, Y. Nedelec, S. V. Sodha, and A. S. Wallace. 2019. Global routine vaccination coverage, 2018. Morbidity and Mortality Weekly Report 68(42):937-942.
Petrak, R. M. 2014. Infectious diseases specialists vital in LTC facilities. https://www.mcknights.com/blogs/guest-columns/infectious-diseases-specialists-vital-in-ltc-facilities (accessed May 3, 2021).
Pierce, J., A. Apisarnthanarak, N. Schellack, W. Cornistein, A. A. Maani, S. Adnan, and M. P. Stevens. 2020. Global antimicrobial stewardship with a focus on low- and middle-income countries. International Journal of Infectious Diseases 96:621-629.
Pinto Ferreira, J. 2017. Why antibiotic use data in animals needs to be collected and how this can be facilitated. Frontiers in Veterinary Science 4:213.
Pollack, L. A., K. L. van Santen, L. M. Weiner, M. A. Dudeck, J. R. Edwards, and A. Srinivasan. 2016. Antibiotic stewardship programs in U.S. Acute care hospitals: Findings from the 2014 National Healthcare Safety Network annual hospital survey. Clinical Infectious Diseases 63(4):443-449.
Prestel, C., E. Anderson, K. Forsberg, M. Lyman, M. A. de Perio, D. Kuhar, K. Edwards, M. Rivera, A. Shugart, M. Walters, and N. Q. Dotson. 2021. Candida auris outbreak in a COVID-19 specialty care unit—Florida, July-August 2020. Morbidity and Mortality Weekly Report 70(2):56-57.
Pulcini, C., L. Pauvif, A. Paraponaris, P. Verger, and B. Ventelou. 2012. Perceptions and attitudes of French general practitioners towards rapid antigen diagnostic tests in acute pharyngitis using a randomized case vignette study. Journal of Antimicrobial Chemotherapy 67(6):1540-1546.
Resman, F. 2020. Antimicrobial stewardship programs; a two-part narrative review of stepwise design and issues of controversy part I: Step-wise design of an antimicrobial stewardship program. Therapeutic Advances in Infectious Disease 7:2049936120933187.
Review on Antimicrobial Resistance. 2015. Rapid diagnostics: Stopping unnecessary use of antibiotics. Review on Antimicrobial Resistance. https://amr-review.org/#:~:text=%E2%80%9CThe%20Review%20on%20Antimicrobial%20Resistance,actions%20to%20tackle%20it%20internationally (accessed April 8, 2021).
Roope, L. S. J., R. D. Smith, K. B. Pouwels, J. Buchanan, L. Abel, P. Eibich, C. C. Butler, P. S. Tan, A. S. Walker, J. V. Robotham, and S. Wordsworth. 2019. The challenge of antimicrobial resistance: What economics can contribute. Science 364(6435):eaau4679.
Sanchez, G. V., K. E. Fleming-Dutra, R. M. Roberts, and L. A. Hicks. 2016. Core elements of outpatient antibiotic stewardship. Morbidity and Mortality Weekly Report 65(RR-6):1–12. http://dx.doi.org/10.15585/mmwr.rr6506a1.
Sanders, P., W. Vanderhaeghen, M. Fertner, K. Fuchs, W. Obritzhauser, A. Agunos, C. Carson, B. Borck Hog, V. Dalhoff Andersen, C. Chauvin, A. Hemonic, A. Kasbohrer, R. Merle, G. L. Alborali, F. Scali, K. D. C. Stark, C. Muentener, I. van Geijlswijk, F. Broadfoot, L. Pokludova, C. L. Firth, L. P. Carmo, E. G. Manzanilla, L. Jensen, M. Sjolund, J. Pinto Ferreira, S. Brown, D. Heederik, and J. Dewulf. 2020. Monitoring of farm-level antimicrobial use to guide stewardship: Overview of existing systems and analysis of key components and processes. Frontiers in Veterinary Science 7:540.
Sarnak, M. J., and B. L. Jaber. 2000. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney International 58(4):1758-1764.
Sears, C. 2018. IDSA comments to CMS re pama implementation and diagnostics reimbursement. https://www.idsociety.org/globalassets/idsa/policy--advocacy/current_topics_and_issues/diagnostics/agency-efforts/110718-idsa-comments-to-cms-re-pama-implementation-and-diagnostics-reimbursement.pdf (accessed September 16, 2021).
Shaikh, H., J. Lynch, J. Kim, and J. L. Excler. 2020. Current and future cholera vaccines. Vaccine 38(Suppl 1):A118-A126.
Shenoy, E. S., J. Kim, E. S. Rosenberg, J. A. Cotter, H. Lee, R. P. Walensky, and D. C. Hooper. 2013. Discontinuation of contact precautions for methicillin-resistant staphylococcus aureus: A randomized controlled trial comparing passive and active screening with culture and polymerase chain reaction. Clinical Infectious Diseases 57(2):176-184.
Shively, N. R., M. A. Moffa, K. T. Paul, E. J. Wodusky, B. A. Schipani, S. L. Cuccaro, M. S. Harmanos, M. S. Cratty, B. N. Chamovitz, and T. L. Walsh. 2020. Impact of a telehealth-based antimicrobial stewardship program in a community hospital health system. Clinical Infectious Diseases 71(3):539-545.
Singh, A. K., and A. K. Bhunia. 2019. Animal-use antibiotics induce cross-resistance in bacterial pathogens to human therapeutic antibiotics. Current Microbiology 76(10):1112-1117.
Singleton, D. A., A. Rayner, B. Brant, S. Smyth, P. M. Noble, A. D. Radford, and G. L. Pinch-beck. 2021. A randomised controlled trial to reduce highest priority critically important antimicrobial prescription in companion animals. Nature Communications 12(1):1593.
Sloane, P. D., K. Huslage, C. E. Kistler, and S. Zimmerman. 2016. Optimizing antibiotic use in nursing homes through antibiotic stewardship. North Carolina Medical Journal 77(5):324-329.
Smieszek, T., K. B. Pouwels, F. C. K. Dolk, D. R. M. Smith, S. Hopkins, M. Sharland, A. D. Hay, M. V. Moore, and J. V. Robotham. 2018. Potential for reducing inappropriate antibiotic prescribing in English primary care. Journal of Antimicrobial Chemotherapy 73(Suppl 2):ii36-ii43.
Smith, L., A. Tan, J. D. Stephens, D. Hibler, and S. A. Duffy. 2019. Overcoming challenges in multisite trials. Nursing Research 68(3):227-236.
Srikantiah, P. 2018. The potential of vaccines to prevent AMR. PDVAC Meeting. June 24, 2018. https://terrance.who.int/mediacentre/data/pdvac/PDVAC_2018_Presentations-Day2.pdf (accessed May 24, 2021).
St Cyr, S., L. Barbee, K. A. Workowski, L. H. Bachmann, C. Pham, K. Schlanger, E. Torrone, H. Weinstock, E. N. Kersh, and P. Thorpe. 2020. Update to CDC’s treatment guidelines for gonococcal infection, 2020. Morbidity and Mortality Weekly Report 69(50):1911-1916.
StratisHealth. 2020. Antibiotic stewardship implementation: Suggested strategies from high performing CAHs. Natural Rural Health Resource Center. https://krhop.net/wp-content/uploads/2021/04/Georgia-Kansas-ABCs-of-ASPs-for-CAHs-2021-02-10.pdf (accessed May 4, 2021).
Strich, J. R., and S. S. Kadri. 2019. Difficult-to-treat antibiotic-resistant gram-negative pathogens in the intensive care unit: Epidemiology, outcomes, and treatment. Seminars in Respiratory and Critical Care Medicine. 40(4):419-434. https://doi.org/10.1055/s-0039-1696662.
Sulis, G., P. Adam, V. Nafade, G. Gore, B. Daniels, A. Daftary, J. Das, S. Gandra, and M. Pai. 2020. Antibiotic prescription practices in primary care in low- and middle-income countries: A systematic review and meta-analysis. PLoS Medicine 17(6):e1003139.
Tattevin, P., G. Levy Hara, A. Toumi, M. Enani, G. Coombs, A. Voss, H. Wertheim, A. Poda, Z. Daoud, and R. Laxminarayan. 2020. Advocacy for increased international efforts for antimicrobial stewardship actions in low-and middle-income countries on behalf of Alliance for the Prudent use of Antimicrobials (APUA), under the auspices of the International Society of Antimicrobial Chemotherapy (ISAC). Frontiers in Medicine 7:503.
Teshome, S., S. Desai, J. H. Kim, D. Belay, and V. Mogasale. 2018. Feasibility and costs of a targeted cholera vaccination campaign in Ethiopia. Human Vaccines & Immunotherapeutics 14(10):2427-2433.
The Pew Charitable Trusts. 2016. Judicious animal antibiotic use requires drug label refinements. https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2016/10/judicious-animal-antibiotic-use-requires-drug-label-refinements (accessed September 22, 2021).
Thompson, J. M., A. L. Zagel, A. B. Spaulding, E. A. Krause, and J. L. Arms. 2021a. Streptococcal pharyngitis: Compliance with national testing guidelines in a pediatric emergency department. Pediatric Emergency Care. https://doi.org/10.1097/pec.0000000000002512.
Thompson, N. D., A. Penna, T. R. Eure, W. M. Bamberg, G. Barney, D. Barter, P. Clogher, M. B. DeSilva, G. Dumyati, E. Epson, L. Frank, D. Godine, L. Irizarry, M. A. Kainer, L. Li, R. Lynfield, J. P. Mahoehney, J. Nadle, V. Ocampo, L. Perry, S. M. Ray, S. S. Davis, M. Sievers, L. E. Wilson, A. Y. Zhang, N. D. Stone, and S. S. Magill. 2020. Epidemiology of antibiotic use for urinary tract infection in nursing home residents. Journal of the American Medical Directors Association 21(1):91-96.
Thompson, N. D., N. D. Stone, C. J. Brown, A. R. Penna, T. R. Eure, W. M. Bamberg, G. R. Barney, D. Barter, P. Clogher, M. B. DeSilva, G. Dumyati, L. Frank, C. B. Felsen, D. Godine, L. Irizarry, M. A. Kainer, L. Li, R. Lynfield, J. P. Mahoehney, M. Maloney, J. Nadle, V. L. S. Ocampo, R. Pierce, S. M. Ray, S. S. Davis, M. Sievers, K. Srinivasan, L. E. Wilson, A. Y. Zhang, and S. S. Magill. 2021b. Antimicrobial use in a cohort of US nursing homes, 2017. JAMA 325(13):1286-1295.
Tin Tin Htar, M., A. H. J. van Den Biggelaar, H. Sings, G. Ferreira, M. Moffatt, C. Hall-Murray, T. Verstraeten, B. D. Gessner, H. J. Schmitt, and L. Jodar. 2019. The impact of routine childhood immunization with higher-valent pneumococcal conjugate vaccines on antimicrobial-resistant pneumococcal diseases and carriage: A systematic literature review. Expert Review of Vaccines 18(10):1069-1089.
Tomczyk, S., R. Lynfield, W. Schaffner, A. Reingold, L. Miller, S. Petit, C. Holtzman, S. M. Zansky, A. Thomas, J. Baumbach, L. H. Harrison, M. M. Farley, B. Beall, L. McGee, R. Gierke, T. Pondo, and L. Kim. 2016. Prevention of antibiotic-nonsusceptible invasive pneumococcal disease with the 13-valent pneumococcal conjugate vaccine. Clinical Infectious Diseases 62(9):1119-1125.
Toutain, P. L., A. Bousquet-Melou, P. Damborg, A. A. Ferran, D. Mevius, L. Pelligand, K. T. Veldman, and P. Lees. 2017. En route towards European clinical breakpoints for veterinary antimicrobial susceptibility testing: A position paper explaining the VetCAST approach. Frontiers in Microbiology 8:2344.
Trevas, D., A. M. Caliendo, K. Hanson, J. Levy, and C. C. Ginocchio. 2021. Diagnostic tests can stem the threat of antimicrobial resistance: Infectious disease professionals can help. Clinical Infectious Diseases 72(11):e893-e900.
University of Michigan Kidney Epidemiology and Cost Center. 2018. Technical notes on the dialysis facility compare quality of patient care star rating methodology for the October 2018 release. Ann Arbor, MI: Centers for Medicare & Medicaid Services.
USDA (U.S. Department of Agriculture). 2014. Antimicrobial resistance: Action plan. U.S. Department of Agriculture. https://www.usda.gov/sites/default/files/documents/usda-antimicrobial-resistance-action-plan.pdf (accessed July 13, 2021).
USDA. 2017. Antimicrobial use and stewardship on U.S. Swine operations, 2017. Fort Collins, CO: U.S. Department of Agriculture, Animal and Plant Health Inspections Service, Veterinary Services, Center for Epidemiology and Animal Health, National Animal Health Monitoring System.
USDA. 2018. Supporting antimicrobial stewardship in veterinary settings: Goals for fiscal years 2019–2023. Washington, DC: Food and Drug Administration Center for Veterinary Medicine.
USDA. 2019. Antimicrobial use and stewardship on US feedlots, 2017. Fort Collins, CO: U.S. Department of Agriculture, Animal and Plant Health Inspections Service, Veterinary Services, Center for Epidemiology and Animal Health, National Animal Health Monitoring System.
UWTASP (University of Washington Tele-Antimicrobial Stewardship Program). 2018. The challenge of antibiotic resistance is here. https://www.uwtasp.org/about (accessed May 3, 2021).
Uyeki, T. M., H. H. Bernstein, J. S. Bradley, J. A. Englund, T. M. File, A. M. Fry, S. Gravenstein, F. G. Hayden, S. A. Harper, J. M. Hirshon, M. G. Ison, B. L. Johnston, S. L. Knight, A. McGeer, L. E. Riley, C. R. Wolfe, P. E. Alexander, and A. T. Pavia. 2019. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clinical Infectious Diseases 68(6):e1-e47.
Van Katwyk, S. R., S. L. Jones, and S. J. Hoffman. 2018. Mapping educational opportunities for healthcare workers on antimicrobial resistance and stewardship around the world. Human Resources for Health 16(1):9.
Veeraraghavan, B., A. K. Pragasam, Y. D. Bakthavatchalam, and R. Ralph. 2018. Typhoid fever: Issues in laboratory detection, treatment options & concerns in management in developing countries. Future Science OA 4(6):FSO312.
Vekemans, J., M. Hasso-Agopsowicz, G. Kang, W. P. Hausdorff, A. Fiore, E. Tayler, E. J. Klemm, R. Laxminarayan, P. Srikantiah, M. Friede, and M. Lipsitch. 2021. Leveraging vaccines to reduce antibiotic use and prevent antimicrobial resistance: A World Health Organization action framework. Clinical Infectious Diseases 73(4):e1011-e1017.
Veterinary Practice News Editors. 2017. Mars to buy VCA clinics, labs in $9.1 billion deal. Veterinary Practice News. https://www.veterinarypracticenews.com/mars-to-buy-vca-clinics-labs-in-9-1-billion-deal (accessed May 19, 2021).
Wang, T. Z., R. P. L. Kodiyanplakkal, and D. P. Calfee. 2019. Antimicrobial resistance in nephrology. Nature Reviews Nephrology 15(8):463-481.
Watts, J. L., M. T. Sweeney, and B. V. Lubbers. 2018. Antimicrobial susceptibility testing of bacteria of veterinary origin. Microbiology Spectrum 6(2).
Weinstein, M. P., and J. S. Lewis, 2nd. 2020. The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, organization, functions, and processes. Journal of Clinical Microbiology 58(3):e01864-19.
Wellcome. 2020. The global response to AMR: Momentum, success, and critical gaps. Wellcome Trust. https://wellcome.org/reports/global-response-amr-momentum-success-and-critical-gaps (accessed May 24, 2021).
Wellcome. 2021. Impact of vaccines on antimicrobial resistance (closed). https://wellcome.org/grant-funding/schemes/impact-vaccines-antimicrobial-resistance-closed (accessed May 24, 2021).
Wemette, M., A. G. Safi, W. Beauvais, K. Ceres, M. Shapiro, P. Moroni, F. L. Welcome, and R. Ivanek. 2020. New York state dairy farmers’ perceptions of antibiotic use and resistance: A qualitative interview study. PloS One 15(5):e0232937.
WHO (World Health Organization). 2014. Principles and considerations for adding a vaccine to a national immunization programme: From decision to implementation and monitoring. https://apps.who.int/iris/handle/10665/111548 (accessed May 19, 2021).
WHO. 2018. Vaccines and immunization: Dengue. https://www.who.int/news-room/q-a-detail/dengue-vaccines (accessed June 30, 2021).
WHO. 2019a. Antimicrobial stewardship programmes in health-care facilities in low-and middle-income countries: A WHO practical toolkit. https://www.who.int/publications/i/item/9789241515481 (accessed May 24, 2021).
WHO. 2019b. Pneumonia. https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed May 19, 2021).
WHO. 2020. Immunization coverage. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed May 24, 2021).
WHO. 2021. Aware. https://aware.essentialmeds.org/list (accessed September 22, 2021).
Yu, H., O. Baser, and L. Wang. 2016. Burden of clostridium difficile-associated disease among patients residing in nursing homes: A population-based cohort study. BMC Geriatrics 16(1):1-7.
Yui, S., G. Bercades, M. Muzslay, E. Blackburn, S. Ali, D. Smyth, A. Macklin, J. H. Ryu, P. Bassett, N. MacCallum, D. Brealey, and P. Wilson. 2020. Assessment of a rapid diagnostic test to exclude bacteraemia and effect on clinical decision-making for antimicrobial therapy. Scientific Reports 10(1):3122.
Zacharioudakis, I. M., F. N. Zervou, P. D. Ziakas, and E. Mylonakis. 2014. Meta-analysis of methicillin-resistant staphylococcus aureus colonization and risk of infection in dialysis patients. Journal of the American Society of Nephrology 25(9):2131-2141.
Zacharioudakis, I. M., F. N. Zervou, P. D. Ziakas, L. B. Rice, and E. Mylonakis. 2015. Vancomycin-resistant enterococci colonization among dialysis patients: A meta-analysis of prevalence, risk factors, and significance. American Journal of Kidney Diseases 65(1):88-97.
Zhou, Y., J. B. Lynch, P. S. Pottinger, J. Scott, L. A. S. Quilter, M. D’Angeli, Z. Kassamali, R. Jain, R. J. Cybulski, and N. Martinez-Paz. 2017. University of Washington Tele-Antimicrobial Stewardship program (UW-TASP/ECHO): Collaboration across Washington State to improve antimicrobial use. Open Forum Infectious Diseases 4(Suppl 1):S271.
Zvonar, R., S. Natarajan, C. Edwards, and V. Roth. 2008. Assessment of vancomycin use in chronic haemodialysis patients: Room for improvement. Nephrology, Dialysis, Transplantation 23(11):3690-3695.
This page intentionally left blank.


























































