Proceedings of a Workshop
| IN BRIEF | |
|
December 2021 |
The Utility, Feasibility, Security, and Ethics of Verifiable COVID-19 Credentials for International Travel
Proceedings of a Workshop—in Brief
The National Academies of Sciences, Engineering, and Medicine convened a virtual workshop on August 3–5, 2021 to explore effective, feasible, and secure ways to document and provide health information for safe international travel in a way that is ethical and does not exacerbate inequities. Experts considered the use of COVID-19 travel credentials, denoting the traveler’s vaccination, testing, and/or recovery status. This Proceedings of a Workshop—in Brief provides a high-level summary of the discussion on possibilities for employing COVID-19 travel credentials, including how to overcome practical and ethical challenges and their potential role in preventing the spread of disease.
PUBLIC HEALTH AND EPIDEMIOLOGY
Public Health Impacts of International Travel
Kathleen Neuzil, University of Maryland School of Medicine, discussed the public health impact of requiring vaccination or testing for travel. She stated that the goal of travel measures is to minimize the risks of increasing overall infections and introducing variants of concern in the destination country. She explained that early in the pandemic, border closures slowed global dissemination, but as community transmission was established, the benefits of strict border closures in mitigating diseases from travelers diminished.1
At the time of the workshop, eight vaccines were approved for full use, with more than 100 more in the testing/trial stage.2 She noted that performance varies by vaccine type, virus (strain), and host, but protection from serious disease and death is high for all approved vaccines. She said the latest update from the U.S. Centers for Disease Control and Prevention (CDC) was that a similar amount of virus in the nose was found in fully vaccinated breakthrough cases as in unvaccinated/not fully vaccinated/unknown-status patients, but it is reasonable to hypothesize that the duration of viral shedding would be less in vaccinated people.3
Neuzil stated that the goal of testing travelers is to identify infected people who are capable of spreading the virus. The types of tests differ; antigen tests are quicker, lower cost, logistically easier, and less sensitive, while nucleic acid amplification tests, such as polymerase chain reaction (PCR), are more sensitive but may detect a replication incompetent virus for more than 10 days after it is no longer viable.
__________________
1 Linka, K., et al. 2020. Outbreak dynamics of COVID-19 in Europe and the effect of travel restrictions. Computer Methods in Biomechanics and Biomedical Engineering 23(11)710–717. https://doi.org/10.1080/10255842.2020.1759560.
2 Zimmer, C., et al. 2021. Coronavirus vaccine tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html?auth=link-dismiss-google1tap&smid=em-share (accessed August 3, 2021).
3 Brown, C. M., et al. 2021. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. Morbidity and Mortality Weekly Report 70(31):1059–1062.
![]()
She discussed a Yale University study (preprint) in which researchers considered the prevalence, daily incidence, vaccine coverage, immunity, and age demographics of 31 European countries.4 For short-haul flights, when the researchers compared a PCR test on exit from quarantine, rapid antigen test on exit, and rapid antigen test on entry, they found that testing did not make much difference; rather, the biggest risk to a destination country was its own vaccination coverage. The most important factors in this study were the disease prevalence, travel asymmetries between origin and destination countries, and vaccination rates in the destination countries. Neuzil noted that this study highlights the need for safe and effective vaccines that are accessible, affordable, and globally available and that differences in vaccine performance based on host characteristics, outcome, and strain (variants)5 should be expected. She stated that travel needs to be aligned with the goal of balancing public health and political, ethical, social, and economic considerations and new data and assumptions must be incorporated into modeling.
Current Trends and Risk Mitigation Measures
Angie Rasmussen, Vaccine and Infectious Disease Organization, explained how COVID-19 transmission occurs during travel: inhalation (aerosols), direct contact (droplets), and, less commonly, ocular, oral, and indirect (fomite) contact. She stated that planes can be fairly low risk due to air filtration systems, spacing, and capacity limits. However, other parts of the experience can be high risk for transmission—travel to and from the airport (public transport, ride shares), poorly ventilated environments, no ability to physically distance, and poor mask use.
Regarding mitigation measures, Rasmussen suggested that travel testing can be effective, but it depends on implementation, COVID-19 prevalence, and the interval between test and departure. She noted that symptom self-reporting can be difficult due to the wide range of potential symptoms, including none, and temperature screening can be ineffective, given that transmission can occur before symptoms, some people never get a fever, and fever inhibitor medications may mask fever.
Rasmussen noted that despite a decrease in vaccine efficacy to the Delta variant, recent studies suggest no indication that vaccinated people with breakthrough infections of Delta are responsible for much onward transmission, particularly to other vaccinated people,6 and breakthrough infections in vaccinated people are “cleared” much faster than in the unvaccinated.7 She emphasized that to reopen international travel, at this stage, it is best to prioritize populations who have not received vaccines versus providing boosters to those already vaccinated, and vaccination efforts should be combined with mitigating nonpharmaceutical interventions to reduce transmission in highly vaccinated countries.
POLICY DEVELOPMENT
Enacting Policy on Verifiable COVID-19 Credential Requirements
Israel’s vaccine certificate program involves two tiers, the Green Pass for domestic use and the Vaccination Certificate for international use, explained Adam Cutler, Ministry of Health Israel. The Green Pass provides the person’s status (green if the individual is vaccinated, recovered, or recently tested, and red if not), national ID number (for non-citizens: passport number), minor children if applicable, and a quick response (QR) code. The Vaccination Certificate is more detailed and contains the individual’s full name, passport number, date of birth, vaccination details, and a QR code. To travel within Israel, international travelers with a recognized Vaccination Certificate are issued the Green Pass. In practice, Israel verifies foreign vaccine credentials via serological testing to identify antibodies and uses the vaccination record/certificate as supporting evidence. Israel has joined the European Union’s Digital Green Certificate Gateway; at the time of the workshop, it was adjusting its dataset so the QR code is signed digitally to align with the European system.
__________________
4 Wells, C. R., et al. 2021. Quarantine and testing strategies for safe pandemic travel. medRxiv. https://doi.org/10.1101/2021.04.25.21256082 (accessed October 14, 2021).
5 At the time of the Wells et al. study, the Delta variant had not yet emerged as a variant of concern.
6 Riemersma, K. K., et al. 2021. Shedding of infectious SARS-CoV-2 despite vaccination when the Delta variant is prevalent—Wisconsin, July 2021. medRxiv. https://doi.org/10.1101/2021.07.31.21261387 (accessed October 14, 2021). See also Sheikh, A., et al. 2021. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. The Lancet 397(10293):P2461–P2462. https://doi.org/10.1016/S0140-6736(21)01358-1 (accessed October 14, 2021). See also Lopez Bernal, J., et al. 2021. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. New England Journal of Medicine 385:585–594. https://www.nejm.org/doi/full/10.1056/NEJMoa2108891 (accessed October 14, 2021).
7 Chia, P. Y., et al. 2021. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: A multicenter cohort study. medRxiv. https://doi.org/10.1101/2021.07.28.21261295 (accessed October 14, 2021). See also Harrison, A. G., et al. 2020. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends in Immunology 41(12):1100–1115. https://doi.org/10.1016/j.it.2020.10.004 (accessed October 14, 2021).
WHO Guidance on Digital Documentation of COVID-19 Certificates
Garrett Livingston Mehl, World Health Organization (WHO), stated that as a neutral party, WHO decided not to select a platform for COVID-19 credentials, but it does offer guidance on digital solutions and how to implement them. He said the aim of digital documentation of COVID-19 certificates (DDCC)8 is to achieve implementable specifications and standards for any application of a certificate for national or cross-border purposes; ensure consistent data representation, exchange, privacy, and security; ensure collective trust across all types of users; and facilitate continuity of care, verifiable proof, and patient empowerment. DDCC supports a paper-first approach, augmented by digital documentation.
DDCC also provides guidance on use cases, workflows, and business process, public key infrastructure, and ethical implementation. Mehl presented a public key infrastructure for verifying and validating certificates to be linked to global trust frameworks (see Figure 1). For example, the EU system, the Digital Green Certificate, shares public keys so that another country may check that the certificate is valid.
Public Acceptance of and Adherence to Guidelines on a Global Scale
According to Scott Ratzan, CUNY Graduate School of Public Health & Health Policy, the public used to trust intermediaries, such as media outlets and politicians, for truth and evidence, but that trust has decreased, particularly as misinformation spreads easily and quickly through social media. Ratzan noted that the public still trusts intermediaries, such as physicians and employers, and emphasized the need for a trusted source for COVID-19 communications, in coordination with the government, the tech sector, and media.9
Ratzan discussed a study (preprint) that involved a series of surveys about COVID-19. He noted the higher levels of acceptance of requiring proof of vaccination for international travel among countries surveyed, compared to other questions (e.g., vaccine uptake) (see Figure 2).
Ratzan emphasized that the name given to the vaccine credential matters. In a U.S. survey that asked the question, “which name would make you most likely to get a vaccine?” the highest percentage of respondents selected “verification” (40 percent).10 He suggested a strategic diplomacy strategy to encourage vaccine credential acceptance and advance vaccine confidence that includes communication, public relations, social marketing, advocacy, policy making, behavioral economics, health literacy, multisector engagement, and social norms.11 This could involve both social and traditional media and engaging the public with updates on privacy, security, equity, and other key issues.
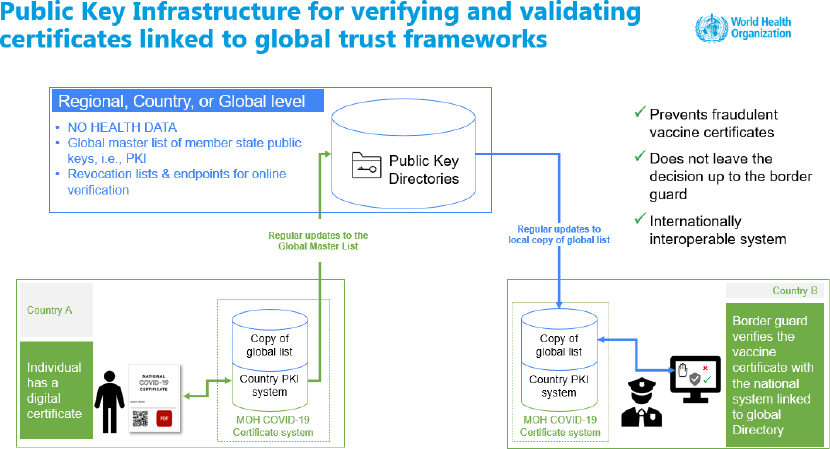
NOTE: PKI = public key infrastructure; MOH = Ministry of Health.
SOURCE: Presented by Garrett Livingston Mehl on August 3, 2021.
__________________
8 WHO (World Health Organization). 2021. Digital documentation of COVID-19 certificates: Vaccination status: Technical specifications and implementation guidance, August 27, 2021. Geneva, Switzerland: WHO. https://apps.who.int/iris/handle/10665/343361 (accessed October 14, 2021).
9 Ratzan, S., L. Gostin, N. Meshkati, K. Rabin, and R. Parker. 2020. COVID-19: An Urgent Call for Coordinated, Trusted Sources to Tell Everyone What They Need to Know and Do. NAM Perspectives. Commentary, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/202003a (accessed September 20, 2021).
10 de Beaumont Foundation. 2021. “Vaccine passport” language a turnoff for many Americans. https://debeaumont.org/news/2021/vaccine-passports (accessed October 11, 2021).
11 Ratzan, S. C., et al. 2019. Guiding principles for multisector engagement for sustainable health (MESH). M-RCBG Associate Working
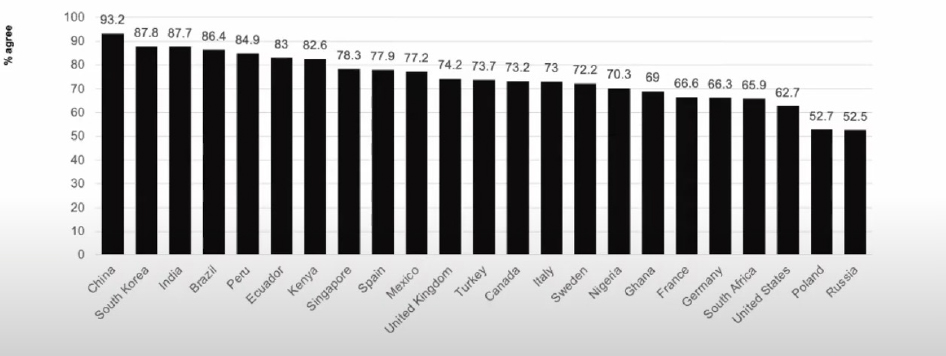
SOURCES: Presented by Scott Ratzan on August 3, 2021; Lazarus, J., et al. 2021. COVID-VAC: The second global study of COVID-19 vaccine acceptance. Research Square. https://doi.org/10.21203/rs.3.rs-780128/v1.
Another approach involves strategically engaging business, civil society, and government bodies to galvanize support for vaccine credentials, such as using a “pledge” and “badge” system in certain industries, such as the Safe Travels or Trusted Traveler programs.
Discussion of Epidemiology, Public Health, and Policy
Saskia Popescu, George Mason University, began the discussion by asking the speakers about the role of travel restrictions in dealing with different variants. Neuzil stated that the two biggest concerns are introducing either the virus in general or a new variant of concern, but vaccination is going to help. Rasmussen added that once a new variant has emerged, it has already been exported elsewhere, and blanket restrictions on whole countries are therefore not very effective. Mehl emphasized that beyond blanket restrictions, it is necessary to consider countries’ access to vaccines and testing and to use other risk reduction measures, such as quarantine.
Regarding the fraudulent use of COVID-19 credentials, Ratzan stated that fraud is a reality, and the CDC card is not a secure document. Cutler said Israel has encountered a relatively high level of fraud in testing. Mehl stated that trust of verifiable COVID-19 credentials could improve through transparency measures, including a federal standard for a minimum dataset and data protection. He noted that the private sector is a very effective partner, but without specifications, it is difficult for companies to align with what is expected. Ratzan emphasized the importance of a trusted, validated source of information and argued that the most reliable sources are not public authorities but rather medical professionals and academics.
ACCURATE, SECURE, VERIFIABLE, AND USABLE DATA
Sources of Credible Data
The purpose of a system of vaccination history is to provide a central repository for consolidating records, to support the functions of immunization programs, said Rebecca Coyle, American Immunization Registry Association.12 She explained that immunization information systems (IIS)13 can be leveraged for disease surveillance—IIS are already pulling data and sending it to CDC. Most IIS data come from electronic health record (EHR)–IIS interfaces in the form of vital statistics, direct data entry by authorized users, automatic flow from EHRs, pharmacy systems, and others. Authorized systems query the IIS and receive consolidated records and immunizations. Certain policies may affect interoperability and/or functionality, including authorized users, consent to opt in or out of policies, and mandates for reporting. External systems can enable the use of secondary databases or partnerships to avoid further load on “live” IIS, such as data lakes and data warehouses.
__________________
Paper No. 106. Cambridge, MA: John F. Kennedy School of Government, Harvard University. https://www.hks.harvard.edu/sites/default/files/centers/mrcbg/working.papers/106_MESH.pdf (accessed October 14, 2021).
12 AIRA is a membership organization that exists to promote the development and implementation of immunization information systems (IIS) as an important tool in preventing and controlling vaccine-preventable diseases. See https://www.immregistries.org/about-aira (accessed October 14, 2021).
13 Coyle defined IIS as confidential, population-based, computerized databases that record all immunization doses administered by participating providers to persons residing within a given geopolitical area.
Data Security, Privacy, and Protection
Josh Mandel, Microsoft, stated that Microsoft is collaborating with Salesforce on designing a platform for the consumer-driven sharing of vaccine credentials with the Vaccine Credential Initiative.14 He noted they grappled with three main issues. The first was how to make sure individuals have access to their own data and cannot access data that they should not. Microsoft has leveraged existing U.S. infrastructure, as some of the most important data flows are through established systems, such as pharmacies’ landing pages/portals and EHRs. The second issue, he said, was how to ensure transparency of user data sharing. He pointed out a need to add human-readable text next to the QR code that mirrors the information shared but avoids oversharing of data. Finally, on the issue of tracking and data flows, the platform and the health information system have no direct communication (no ping back of users’ personal data to health care providers).
Interoperability and Verifiability
Dakota Gruener, ID2020,15 stated that dozens of health pass solutions have rushed to the market in recent months, and they vary greatly in terms of privacy, security, and user control over personal data. International agreement on common standards does not yet exist, meaning that the various solutions are not interoperable. Additionally, the operational infrastructure required for interoperability is lacking, such as rules engines, trust registries, and governance frameworks. She noted the lack of global collaboration on COVID-19 credentials has had a real impact on travelers, with chaos and long wait times at borders.
ID2020 has advocated for an equitable approach to health pass solutions, including the need for paper credentials, which would allow proof of testing or vaccination status without relying on a phone or connectivity, and for credentials for proof of testing and of recovery, in addition to proof of vaccination. It is also part of a larger, more than 120 organization effort called the Good Health Pass Collaborative (GHPC), a multisector global initiative designed to establish principles, standards, and a governance framework for digital health passes for international travel.16 GHPC developed an interoperability blueprint, which articulates recommendations for a globally interoperable digital pass and trust ecosystem. Key characteristics include putting the individual at the center of the data exchange with no data flow back to the issuer, using only minimum data for vaccination, testing, and recovery; adhering to the World Wide Web Consortium (W3C) verifiable credentials standard;17 and using the W3C decentralized identified standard.18 GHPC supports purpose-specific selective disclosure, in which a health pass/travel pass contains only the minimum data necessary to prove health status to one verifier.
Discussion on Data Sharing
Abbey Wojno, CDC, began the discussion on data sharing by asking speakers how privacy is maintained if QR codes are used for personal identifiable information and protected health information. Gruener stated that some governments have raised concerns that QR codes on paper credentials fall short of desired privacy goals. Because the QR code is multipurpose, it likely contains more information than should be presented to many verifiers. Concerns also arose that printed QR codes could be easily repurposed or replicated, which undercuts the system’s credibility. While Gruener acknowledged these risks, she stated that with equity considered, governments concerned about paper QR codes have recognized that an all-digital system would not be inclusive enough.
Regarding revoking or suspending a credential, Mandel said they did not build this into the SMART Health Card system and it is up to a verifier to decide how long to trust the credential. However, when an issuer loses control of the signing keys in a security breach, they can regenerate and issue all new keys. Gruener noted that the European Union has been grappling with the issue of revoking keys, and the plan for the EU Digital Green Certificate is to use the credential only during an ongoing public health emergency.
On the question of an expiration date, Gruener explained that rules engines and automated decision making (in line with policies such as an expiration date) can be added by layering it on top of the credential. Coyle explained that vaccinations are never invalidated, but a booster dose is often needed after a specified period. She also noted U.S. legislative attempts to restrict a jurisdiction’s ability to create digital vaccine credentials for consumers, and so it is crucial to take the political/policy piece out of potential future applications and focus on utility instead.
__________________
14 See https://vci.org (accessed October 14, 2021).
15 See https://id2020.org (accessed October 14, 2021).
16 See https://www.goodhealthpass.org (accessed October 14, 2021).
17 W3C definition of verifiable credential: a set of one or more claims made by an issuer that is tamper evident and has authorship that can be cryptographically verified. See https://www.w3.org/TR/vc-data-model (accessed October 14, 2021).
18 W3C definition of decentralized identifier: a portable URL-based identifier associated with an entity.
PANEL: CASE STUDIES AND CURRENT EXAMPLES
The continent of Africa has 55 countries, each with its own policies and data regimes, stated Edem Adzogenu, African Union. With the emergence of COVID-19, the African Union wanted to prevent fake certificates and also provide a way to digitize test certificates. He stated that Africa has a central repository of vaccination records for the region, through which certificates can be certified and verified.19 The system follows the chain of a traveler’s journey and links labs, port authorities, a central repository, airlines, and immigration systems, and it is designed so that if a person does not have the appropriate certificate, it can be looked up. The system also runs a compliance check to ensure that COVID-19 status (e.g., 48-hour negative test) is in line with the country’s policy, and it will generate a code to enable the person to travel. Now the African Union is focused on ensuring interoperability between different countries’ credentials, with the hope of building a centralized system that links up with other regions in the world.
Brian Behlendorf, Linux Foundation, said the Linux Foundation launched its public health initiative 1 year ago. It worked on application programming interfaces (APIs) with Google that alerted people when they were exposed to COVID-19. The foundation found it was important to balance privacy concerns with efficacy, and it worked with the self-sovereign identity community on applications for public health, including exposure notifications, vaccination status, test results, and provider credentialing. He argued that COVID-19 credentials need to be set up based on common standards and requirements, and the foundation has worked with ID2020 on GHPC. The foundation has developed two open-source software packages, called Cardea and MedCreds, which involve three software parts: issuer, wallet (for holders), and verifier.20 He stated that the hope is to drive broad adoption of those common standards, via common software platforms and supported by a large variety of vendors, to provide optionality21 and avoid vendor capture22 in the interest of greater public health and meeting the needs of public health authorities.
According to Sandra Beattie, New York State Division of the Budget, the Excelsior Pass was developed to begin reopening the New York State economy. It is offered as a voluntary alternative to the CDC card, due to equity considerations. At the time of the workshop, nearly 3 million people had obtained the pass since it launched in March 2021. The Excelsior Pass links to a database that includes every person who has been vaccinated or tested in New York State and allows each individual to access their personal health record. The day of the workshop coincided with the soft launch of the Excelsior Pass Plus, which mirrors the information on the CDC card; it can also be used to travel to international destinations, including France, Greece, Italy, Spain, and any other location where VeriFLY partners are located.
Louise Cole, International Air Transport Association (IATA), introduced IATA’s ecosystem, Timatic, which is primarily for airlines to manage passengers to ensure they may enter the country by checking border entry requirements, including passports, visas, and health information (e.g., yellow cards).23 Cole emphasized the fast pace of changes in entry requirements today and the need to provide information directly to passengers. IATA addressed this by developing a rules engine specific to COVID-19. She noted that airlines do not want the responsibility of housing travelers’ personal data, particularly biometric data. She expressed disappointment in the lack of agreement on a global standard for verifiable vaccine credentials, and the various existing systems cannot easily talk to each other.
The IATA Travel Pass Initiative has taken the approach of building a travel pass that can read and verify credentials, store them in the wallet on the user’s device, and allow them to be used as verifiable credentials so they can be turned into consumable data. The app is based on WC3 standards, decentralized identity communication protocols, and it is designed to align with self-sovereign identity principles. IATA is also working on One ID, a biometric digital identity system to facilitate travel, which could one day manage contactless travel internationally.
Alexander Dahl, Akinox, said Akinox is a digital health automation platform, which has been focusing on how to break health care out of silos, to get more patient-centered care.24 At the time of the workshop, 7.3 million people have downloaded the Akinox COVID-19 credential application in Quebec out of the 7.5 million invitations sent. The company developed its wallet and verifier apps to be aligned with the Vaccination Credential Initiative, GHPC, and the work of WHO, the International Civil Aviation Organization (ICAO), the European Union, and others. It was designed with the goals of transparency, privacy, openness, equity, flexibility, and interoperability, and it aims to encourage vendor adoption of open standards and responsible employment (see Figure 3).
California launched a digital vaccine record portal in June 2021, said Rick Klau, California Department of Technology. The state already had an immunization registry, which it leveraged to create the portal. It was a priority and a challenge to preserve privacy and maintain information security. He said that providers prioritized administering vaccines, and the data were not as complete as they hoped. The unintended benefit of the launch for the public was
__________________
19 See https://africacdc.org/trusted-vaccines (accessed October 11, 2021).
20 See https://www.lfph.io/2021/05/25/momentum (accessed October 14, 2021).
21 Refers to having multiple options (in this case, software platforms) from which to choose.
22 Refers to dependence on a single company’s proprietary technology.
23 See https://iata.org.xy2401.com/publications/timatic/Pages/index.aspx.html (accessed October 14, 2021).
24 See https://www.akinox.com (accessed October 14, 2021).
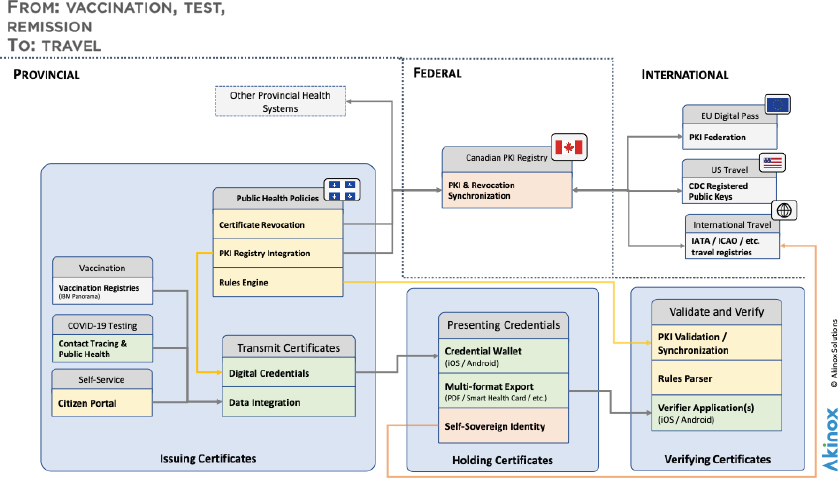
NOTE: CDC = U.S. Centers for Disease Control and Prevention; EU = European Union; IATA = International Air Transport Association; ICAO = International Civil Aviation Organization; PKI = public key infrastructure.
SOURCE: Presented by Alexander Dahl on August 3, 2021.
that suddenly patients and their providers had an incentive to clean up and remediate that data. He stated that California venues were motivated to keep their staff healthy and safe and independently decided to check vaccination status as a condition of entry. He suggested that credentialing and equipping residents with vaccination record access had immediate applications in the local context and can be scaled up for international travel.
Discussion on Case Studies and Current Examples
Brian Anderson, The MITRE Corporation, moderated the discussion session. When asked about the future implications of COVID-19 credentials, Klau identified implications for the portability of data across state lines, conveying other health information (e.g., yellow card), and, in general, the ability to agree on how to convey and interpret health information in a way that is useful to both holder and issuer. Adzogenu stated that the African Union saw this as an opportunity to build an e-health screening system for the continent, and discussions have already begun (as of October 2020) between government officials on how to harmonize. He added that the African Union has already built the capability to issue alerts for Ebola and other diseases in areas where diseases need to be monitored to determine what travel restrictions may be needed.
On the topic of revoking credentials, Cole noted that in the IATA app, if a government decides it will no longer allow people to travel after X months, the user will get a notification that they are “no longer fit to travel” and will have to upload information on additional vaccine dose(s). Beattie explained that New York’s Excelsior Pass mirrors the CDC card, and because the CDC card currently has no expiration date, neither does the Excelsior Pass.
One issue associated with the COVID-19 credentials, Behlendorf stated, is the risk of normalizing the frequent sharing of signed clinical health data. He advocated for the use of health credentials in the future but stated that it is important to build in safeguards and limitations, such as expiration dates.
PRIVACY ISSUES AND LEGAL CONSIDERATIONS
U.S. Law and Border Control
According to Meryl Chertoff, Georgetown University Project on State and Local Government, the United States has a protectionist approach to COVID-19 travel restrictions that depends on testing, not vaccination.25 The nonreciprocal provisions per Presidential Proclamation 9984 have suspended air travel into the country by non-U.S. citizens/permanent residents who had been in 1 of 33 countries listed in the past 14 days.26 She argued that a “COVID colonialism sys-
__________________
25 After the workshop, the United States announced a new policy lifting some restrictions for foreign nationals starting November 2021. See Shepardson, D., and A. Shalal. 2021. U.S. to relax travel restrictions for vaccinated foreign air travelers in November. Reuters. https://www.reuters.com/world/uk/us-relax-travel-restrictions-passengers-uk-eu-november-source-2021-09-20 (accessed October 14, 2021).
26 See https://travel.state.gov/content/travel/en/us-visas/visa-information-resources/covid-19-travel-restrictions-and-exceptions.html (accessed October 14, 2021). See previous footnote for updates on this policy.
tem” has been established in which countries that need tourism and the money it brings in are forced to open borders, but the countries that are better situated financially are able to exclude visitors.
She explained that multiple federal authorities are involved in administering COVID-19 protocols: CDC is relying on private airlines to carry out regulations, and airports rely on the Transportation Security Administration (TSA) and the Federal Aviation Administration to create their own regulations. This means that when you leave the TSA envelope, you are under state government jurisdiction. Legal questions remain about who is in charge, for example, on the issue of who has the authority to mandate quarantine.27
Today, CDC cannot impose masking rules or vaccination requirements. It initially placed conditional sailing orders restricting the cruise industry from operating, but in a case brought by Florida, the Eleventh Circuit ultimately decided that CDC exceeded its authority and failed to comply with rulemaking requirements.28 Chertoff suggested a review of the Public Health Services Act, 42 Sections 70 and 71.
Privacy Protections and Legal Challenges in Implementing Vaccine Information Sharing
I. Glenn Cohen, Harvard Law School, stated that much of the debate about digital health passes are really debates about related but separate questions about whether mandating vaccination is justifiable and which exemptions should be permitted. He said there is a clear legal way for building a digital health pass system. One option is that the U.S. Congress could build the infrastructure for a digital health pass system for U.S. residents and visitors; another option is for the executive branch to build a digital health pass system through healthcare.gov to empower CDC to track diseases or the U.S. Department of Health and Human Services to administer immigration. Some states prohibit vaccine verification by entities such as universities and businesses, and this may continue without a contrary federal law. The U.S. Department of Homeland Security governs ports of entries for immigration, which are federal instrumentalities, but outside those spaces, state law governs.
On the issue of privacy, Cohen emphasized that the Health Insurance Portability and Accountability Act does not prohibit building a vaccine verification program or a system that stores the relevant information. However, the U.S. Federal Trade Commission’s fair information practice principles concept of “data minimization” is to collect only what you need, and collecting information such as vaccination status, dates, and type requires justification. Implementers, he noted, also need to consider data centralization versus decentralization; blockchain and distributed systems are relevant options. Another concern is function creep, which could be combated through data minimization, destroying data after a particular period, providing rights to withdraw data, and/or penalizing the misuse of data.
Europe and the General Data Protection Regulation
The European Union has had to grapple with the tensions of the principle of free movement and economic considerations versus public health, stated Jean McHale, University of Birmingham. It considered the relevant privacy, transparency, and data protection principles, including those under its Charter of Fundamental Rights (Articles 7 and 8) and General Data Protection Regulation. The Digital Green Certificate follows Regulation (EU) 2021/953 on a framework for issuing, verifying, and accepting interoperable COVID-19 vaccination, test, and recovery certificates. Its “phase-in” began July 1 to August 12, 2021.
The Digital Green Certificate is issued by national authorities and is valid across all EU countries, and member states are to accept vaccines that are approved by the European Medicines Agency (EMA).29 Member states agreed upon a validity period of 72 hours for PCR tests and 48 hours for rapid antigen tests. The certificate operates via a QR code and a digital signature to ensure it is authentic. The information is stored in a secure database in the country of origin, and the certificate is not retained by the destination country. McHale explained the “emergency brake mechanism” in which further restrictions can be added by member states, due to uncertainties that remain regarding reinfection and duration of vaccine efficacy. Some of the remaining issues include streamlining and consistency across member states; concerns related to fraud and security; and the question of longer-term facilitation of the transfer of health information.
Discussion of Privacy and Legal Considerations
Lawrence Gostin, Georgetown University, moderated the discussion and began by asking speakers if they envision this as an area for international law to provide a solution for the inconsistencies between requirements in different locations and different platforms. Cohen responded that he doubted the political will is strong enough to address global incon-
__________________
27 See Price, P. J. 2016. Quarantine and liability in the context of Ebola. Public Health Reports 131(3):500–503. https://doi.org/10.1177/003335491613100316 (accessed November 22, 2021).
28 See State of Florida v. Becerra.
29 This included four such vaccines at the time of the workshop: Comirnaty (BioNTech, Pfizer), Moderna, Vaxzevria (previously COVID-19 Vaccine AstraZeneca, Oxford), and Janssen (Johnson & Johnson).
sistencies. Citing the United States as an example, Cohen noted a lack of uniformity in government action and regulation. When asked for insights on the situation in Asia and Latin America, Cohen responded that Asia has generally more openness and enthusiasm to tracking information, which would strike Americans as not preferable. Chertoff stated that the situation is difficult in Latin America, and many countries are not letting a lot of people leave or enter.
Gostin pointed out that the United States will need to grapple with the question of which vaccines to accept and noted that if this is based on U.S. Food and Drug Administration approval, AstraZeneca would not be included at the time of the workshop. He noted that EMA excluded the AstraZeneca vaccine manufactured in India, and McHale responded that this is due to approval processes despite the emergency. She explained that this does not mean the vaccines will not be accepted eventually, but the process takes time.
ETHICAL CONSIDERATIONS
Global Justice and Barriers to Access
Vardit Ravitsky, University of Montreal, described a possible scenario in which citizens of rich countries have had two or more doses of the best COVID-19 vaccines and can travel anywhere in the world. Meanwhile, many citizens in low-income countries have received no or a less reliable vaccine, the infection rate in their country is such that they are considered “high-risk” visitors, and they are therefore unable to travel internationally. She stated that citizens of the Global South already face additional barriers to international travel, and vaccine inequity is an unprecedented ethical challenge of global distribution and production that must be solved at the global level. She argued that pressure must be applied to national governments to pursue vaccine equity. Based on the principle of free movement, she said, vaccination status ought to be supplemented with alternatives when possible, such as COVID-19 testing and antibody testing.
Fairness and Vaccine Credentials: Problems with Ignoring Differential Risk
According to Govind Persad, Sturm College of Law, University of Denver, responding to unequal access by ignoring real differences (especially in risk) between vaccinated and unvaccinated people is a mistaken approach; it hurts vaccinated people in low- and middle-income countries. He outlined the pros and cons of three scenarios. (1) Border closure treats entrants identically and limits the spread, but it excludes vaccinated people and does not follow the “least restrictive means” to protect public health. (2) Open entry treats everyone identically, but it increases spread (which causes great and unequally distributed harms) and generates pressure to exclude based on nationality. (3) A vaccine requirement avoids the unequal harms of closure and spread, but it treats people differently based on vaccine status.
Persad outlined the practicalities of different options for international travel. He said that testing and quarantine does not protect from later infection and spread, but one option could be to vaccinate upon arrival. He argued that if vaccines are similar in protection, they should be treated the same, but those that are different may be treated differently. Persad noted that vaccine requirements should not be a pretext to exclude travelers based on nationality. One opportunity to increase access could be to establish an entry fee to countries, similar to the September 11 Security Fee, and use the funds directly to make vaccination more accessible to all.
Vaccine Manufacturing in the Global South: Paradoxically Fueling Inequity
Keymanthri Moodley, Centre for Medical Ethics and Law, Stellenbosch University, noted the differential vaccine access in the Global North and Global South and stated that the obvious solution is to increase vaccine access in the latter. She provided two examples: South Africa and India. South Africa is licensed to produce the Johnson & Johnson and Pfizer vaccines. India is licensed for the AstraZeneca vaccine under the name “Covishield” at the Serum Institute of India. She stated that Europe does not recognize this vaccine, which reinforces prejudice against vaccines produced in the Global South. She explained that South Africa and India have both approached the World Trade Organization about limitations around patents and intellectual property with respect to vaccine manufacturers, and at the time of the workshop, it had been deliberating for 9 months. She emphasized concerns that if the rules are applied differently to vaccines produced in the Global South, there will be continued discrimination of residents there, and that this requires urgent intervention by an international organization, such as WHO.
Vaccination Certificates: A Public Health Ethics Approach
According to Carla Saenz, Pan American Health Organization, for better ethics analysis, the moral value of public health needs to be discussed. The question of whether “vaccine certificates” are effective is an integral part of this, and a transparent ethics analysis must be communicated to the public. WHO issued its judgment against a vaccine certifi-
cate, but the International Health Regulations Emergency Committee met and discussed the lack of harmonization of documentation requirements for international travel,30 which she suggested should be expedited. Other considerations she mentioned include the cost of COVID-19 tests, low vaccination rates (just 5 percent in some countries), and the different vaccines available. She emphasized that low-income countries are particularly vulnerable to the consequences of COVID-19, including death, disease, unemployment, and other socioeconomic issues. She stated that many low- and middle-income countries rely on tourism as a source of income, so their perspectives must be taken into account.
Discussion on Ethical Considerations
Ezekiel Emanuel, University of Pennsylvania, moderated the discussion. As an answer to the question of when it would be appropriate and ethical to use vaccine certificates for international travel, Moodley responded that a useful marker could be herd immunity at a recommended level, with the caveat that some experts today suggest that herd immunity is a fallacy.
In response to the question on the possibility of shutting down international travel, Ravitsky advocated for a nuanced approach that includes a certificate in conjunction with other measures, such as testing, quarantine, vaccination, infection rate in the country of origin, and/or public health protection measures. Persad also suggested a nuanced approach instead of closure or nonclosure, which should incorporate accommodations for unvaccinated travelers. He said it is important not to just look at the viral rate in the country of origin, because inequalities could emerge for people who are vaccinated with a high-efficacy vaccine traveling from a country with a lot of spread.
Saenz noted that it would be useful to develop a more robust credentialing system, to leverage that for other similar applications, such as adapting the yellow card system. Ravitsky noted that it is more palatable in some cultural contexts to develop the system as temporary and limited to COVID-19, due to concerns about rights, freedom, and privacy. Moodley emphasized that the priority should be to build a comprehensive system for COVID-19, to capture the date of vaccination, doses, and specific vaccine received.
BUSINESS AND ECONOMIC ISSUES
Impact on International Business and Trade
David Turner, Organisation for Economic Co-operation and Development (OECD), stated in the final session that the pandemic has made a major macroeconomic impact, and the countries in which travel and tourism are most important are those that have suffered the biggest hits to economic growth. He emphasized that as more people are vaccinated, restrictions can be removed/relaxed, which benefits the economy. Turner stated that some evidence suggests that requiring a vaccine certificate can help overcome vaccine hesitancy, although this does raise some potentially difficult civil liberty issues. For example, in France, polls have long suggested vaccine hesitancy was high, and this seemed to be confirmed as vaccine rates showed signs of plateauing over the summer. However, the country saw an uptick in vaccination rates, particularly among younger people (under 50), when President Macron announced a series of new policies in which a mandatory health certificate (indicating vaccination or proof of a negative test) is required for entry to restaurants, cinemas, trains, etc.31 He stressed the importance of coordinating restrictions across countries, particularly for international travel.
Zoritsa Urosevic, UN World Tourism Organization (UNWTO), outlined some UNWTO data on economic losses incurred since January 2020, including a 73 percent reduction in international tourist arrivals, a $1.1 trillion loss in export revenues from international tourism, and 100 million direct tourism jobs at risk.32 She said that the UNTWO panel of experts expects a return to normal travel in 3–4 years, and the difficulty is largely due to travel restrictions. Many countries are maintaining a policy of complete or partial border closures, with the most severe travel restrictions in countries in Asia and the Pacific.
Urosevic outlined the ICAO strategic guidance on promoting safe travel on four key areas: resume safe cross-border travel, promote safe travel at all points of the journey, restore travelers’ confidence, and provide liquidity to companies and protect jobs. She stated that despite global dialogue on these guideposts, at the country level, it is
__________________
30 WHO (World Health Organization). 2021. Statement on the eighth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. https://www.who.int/news/item/15-07-2021-statement-on-the-eighth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)pandemic (accessed October 14, 2021).
31 Corbet, S. 2021. France’s virus pass now required in restaurants, trains. AP News. https://apnews.com/article/europe-business-health-france-coronavirus-pandemic-655d8451d7494f8663ce2072e64cf7a6 (accessed October 14, 2021).
32 UNWTO (World Tourism Organization). 2021. COVID-19 and tourism. https://www.unwto.org/covid-19-and-tourism-2020 (accessed October 14, 2021).
unclear who is in charge. Another challenge is that CDC and EU evaluations are completely different, highlighting the need for countries to agree on a harmonized risk assessment protocol. She outlined three pillars to restoring safe cross-border mobility: (1) common risk assessment criteria (notification rate, test positivity rate, and testing rate); (2) harmonized travel protocol; and (3) digitization of travel documents. She also mentioned that UNWTO and IATA have created a destination tracker, which conveys information on what is required for travel to a particular destination.
In the United States, domestic leisure travel is back, but international travel will not return to prepandemic levels until 2024 or 2025, stated Erik Hansen, U.S. Travel Association. He noted that the United States still bans travel from several countries, and it can be difficult to remove restrictions. He advocated for a risk-based road map to reopen the United States and outlined three guiding principles: (1) reserve entry restrictions for only the highest-risk countries, (2) replace all other blanket travel restrictions with a framework of risk-based entry protocols (country-level risk assessment, implement entry protocols based on individual risk profile), and (3) ensure the framework is easy to understand, communicate, and implement.
Discussion on Business and Economic Issues
Mark Pearson, OECD, moderated the discussion; he noted the economic costs of travel restrictions are serious and need to be loosened. Hansen argued that reciprocity should be implemented. Turner agreed that there should be reciprocity in terms of reopening between countries, but the rules should be simplified to improve confidence levels. Urosevic stated she believes in the adaptability of people today, and although it is almost impossible to plan any trip abroad, the ability to cancel flights and accommodations allows hopeful travelers to plan international trips. ◆◆◆
DISCLAIMER: This Proceedings of a Workshop—in Brief was prepared by Nicole Cervenka, Julie Pavlin, and Claire Biffl as a factual summary of what occurred at the workshop. The statements made are those of the rapporteur or individual workshop participants and do not necessarily represent the views of all workshop participants, the planning committee, or the National Academies of Sciences, Engineering, and Medicine.
*The National Academies of Sciences, Engineering, and Medicine’s planning committees are solely responsible for organizing the workshop, identifying topics, and choosing speakers. The responsibility for the published Proceedings of a Workshop—in Brief rests with the rapporteur and the institution. The planning committee for this workshop consisted of Saskia Popescu (Chair), Brian Anderson, Ezekiel Emanuel, Lawrence Gostin, Margaret A. Hamburg, Kathleen Neuzil, Mark Pearson, Megan Walklet-Tighe, and Abbey Wojno.
REVIEWERS: To ensure that it meets institutional standards for quality and objectivity, this Proceedings of a Workshop—in Brief was reviewed by Meghan Benton, Migration Policy Institute, and Abbey Wojno, U.S. Centers for Disease Control and Prevention. Leslie Sim, National Academies of Sciences, Engineering, and Medicine, served as the review coordinator.
For additional information regarding the workshop, visit https://www.nationalacademies.org/event/08-03-2021/workshop-on-the-utility-feasibility-security-and-ethics-of-verifiable-covid-19-credentials-for-international-travel.
Suggested citation: National Academies of Sciences, Engineering, and Medicine: 2021. The utility, feasibility, security, and ethics of verifiable COVID-19 credentials for international travel: Proceedings of a workshop—in brief. Washington, DC: The National Academies Press. https://doi.org/10.17226/26409.
Health and Medicine Division
Policy and Global Affairs

Copyright 2021 by the National Academy of Sciences. All rights reserved.












