6
CHEMICAL SENSORS
The ideal chemical sensor is an inexpensive, portable, foolproof device that responds with perfect and instantaneous selectivity to a particular target chemical substance (analyte) present in any desired medium in order to produce a measurable signal output at any required analyte concentration. Such ideal chemical sensors, however, are far from reality in spite of enormous advances over the past decades. Chemical sensors in actuality are complex devices, generally optimized for a particular application. Appendix F summarizes some of the chemical sensor formats of current interest.
The committee defined chemical sensors as devices or instruments that determine the detectable presence, concentration, or quantity of a given analyte. The complexity of a chemical sensor application is related to the technical difficulties associated with these determinations and with the specific nature (i.e., elemental or molecular) of the chemical substance to be analyzed. Given the huge number (>106) of known molecular substances, molecular sensing typically relies on recognition of molecular structure or associated reactivity; this recognition aspect is called selectivity. Sensitivity and limit of detection relate to the quantity or concentration of the element or molecule to be analyzed (the analyte). The quantity of analyte present in a sample can have a dynamic range of greater than 1023, and chemical sensors are commonly required to detect 10-9 molar concentrations or less. Thus, the challenge of attaining the needed sensitivity in chemical sensing is comparable to that of achieving the needed selectivity.
The sensitivity and selectivity aspects of chemical sensing are affected by the phase, dimensional, and temporal aspects of the desired determination. The analyte can be present in a gas, liquid, or solid phase on various dimensional scales ranging from bulk volumes of liters to picoliters, or surface layers from nanoscopic to monomolecular scale. It may also be persistent or transitory. A further set of requirements can originate from a need for repetitive measurements of the analyte over long times (e.g., days, months) or at multiple and perhaps remote locations, such as in environmental analysis and personal monitoring. 1 The design of chemical sensors also requires appreciation of the needed degree of quantitative reliability (precision or accuracy). Finally, economic resources and constraints can affect the design and strategy of any sensing task in many different ways.
The capability of chemical sensing technology is substantial and has grown steadily over the past several decades, but it has been outpaced by the needs and diversity of chemical measurements. Materials limitations are prominent among the existing limitations of chemical sensors. The following discussion outlines the various chemical sensor types and strategies for further development, with emphasis on those areas in which materials needs are especially evident.
It is not practical within the context of the present report to address the entire known range of
chemical sensing principles, methods, and applications; the general bibliography on chemical sensing (Appendix A) contains a more complete exposition. This chapter develops a taxonomy for this broad sensor class, describes some promising application areas (e.g., the detection of toxic chemicals in the environment) and highlights the key materials challenges. The discussion of chemical sensor types is divided into two sections:
-
direct-reading, selective sensors (e.g., electrochemical sensors, optical fibers); and
-
sensors that use a preliminary chromatographic or electrophoretic sample separation step followed by sensitive, but not necessarily selective, detection.
TRADE-OFFS IN CHEMICAL SENSOR DESIGN
The characteristics of a sensor developed for a given application are strongly shaped by the requirements of the application and by existing sensor science and technology. Using the descriptors developed in Chapter 2, limiting features of many existing chemical sensors include:
A direct-reading chemical sensor functions by detecting and rapidly responding to the presence or concentration of an analyte at some interface between the sensor and the sample matrix containing the analyte. This idealized form of chemical sensor is the most demanding, since the sensor must be selective toward the desired analyte; that is, it must be unresponsive to other, perhaps quite similar, chemical substances (interferants) that may be present in the sample matrix. Considerable potential exists to enhance the selectivity of direct-reading chemical sensors by the use of novel materials. (Requirements and possible future trends are discussed in the following section.)
The large investment involved to achieve high selectivity for large numbers of different analytes with direct-reading chemical sensors is often impractical, and this customarily leads to compromises in the requirements. The trade-offs typically involve constraining the context, or environment, of the sensing application and specifying the normally expected quantity or concentration of the target analyte, as well as that of potentially likely interfering species.
In the absence of adequate selectivity, several different types of compromise are normally encountered:
Sensors with limited selectivity. Such sensors may be acceptable in a controlled environment, such as a manufacturing site, where the nature and range of interferants is known and the normally expected quantities or concentrations of target analyte and interferants can be specified with confidence.
Mathematical deconvolution of multiple responses from arrays of imperfectly selective sensors. An array of sensors can be employed, with each sensor in the array having a different but known (calibrated) level of response to each analyte or interferant in the sample mixture. Then appropriate mathematical approaches (e.g., pattern recognition, a topic of ''chemometrics") may permit extraction of the desired analyte response from the fusion of responses from the multiple sensors, (Newman, 1993; Grate et al., 1993; Haswell, 1992; Brown et al., 1992).
Separation of the analytes from the sample matrix, followed by sensing of individual analytes with sensitive but nonselective sensors. In this case, a large number of different analytes in a sample are separated into pure components. A nonselective detector can then be used to detect the pure components. Separation by modern techniques of chromatography and electrophoresis is an extremely powerful and enabling technology for analysis of complex chemical mixtures. (Manz et al., 1993; Harrison et al., 1993a, b). When combined with a suitable detector and electronic support packages, separation-based sensors (i.e., not truly "direct-reading") can nonetheless potentially provide an analysis system with response times approaching those of many direct reading sensors but with greatly enhanced effective selectivity. Miniaturized, total analytical systems are a relatively new area of research in analytical chemistry, but their development could significantly
supplement the capabilities of existing direct reading sensors. Numerous materials issues in designing and fabricating miniaturized separations-based analytical systems are considered later.
Conventional analytical chemistry techniques and instruments at a central laboratory site. This traditional mode of chemical analysis remains necessary whenever:4
-
alternative sensors lack the required technical capabilities;
-
correlation of multiple kinds of analytical measurements is required to obtain the needed information; or
-
site-centered sensing is economically infeasible compared with the cost of a central laboratory, due to the large number of sites of interest, short sensor lifetimes, or high sensor unit cost.
The above discussion applies to the sensing of samples in the gas or liquid phase. Chemical sensing within solid samples, and of their surfaces, is particularly difficult and, in the former case, not well-developed technology. As a result, the overwhelming majority of solid-state analytical problems are addressed by traditional laboratory approaches. Chemical sensors adaptable to solid-state problems are a frontier topic of substantial importance and challenge.
DIRECT-READING, SELECTIVE CHEMICAL SENSORS
The operation of a direct-reading, selective chemical sensor is based on the existence of a selective recognition event that results in a change in a measurable parameter. Selected transduction parameters and generic device types are summarized schematically in Figure 6-1.
In most cases, the response of the sensor-sample interface to the presence of analyte within the
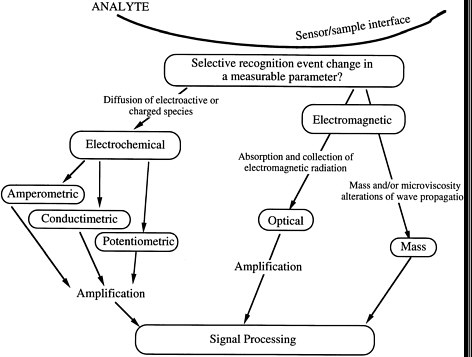
FIGURE 6-1 Direct-reading, selective, chemical sensors.
sample relies on some form of chemical reactivity of the analyte. The design of molecular selectivity for analytes typically involves a delicate choice of the sensing chemistry and associated materials. Chemical reactivity can involve a very wide range of chemical phenomena, including:
-
Recognition of size/shape/dipolar properties of molecular analytes by molecular films, phases, or sites. These can be bioreceptor sites, structures allowing molecular recognition or host-guest interactions, or ceramic or other materials with templated cavities. The molecular recognition leads to selective, strong binding or absorption of analyte to the sensor material.
-
Selective permeation of analyte in a thin-film sensor. If the binding or permeation of analyte is reversible, the sensor film can be re-used (i.e., recycled) in repeated measurements. Irreversible binding of analyte to the sensor, or side reactions with interferants, can stoichiometrically consume the sensor material, shortening its useful lifetime.
-
Catalytic reaction cycle of the sensing materials, which results in analyte consumption.
The most important materials-related factor leading to enhancements of direct-reading chemical sensors is the choice of materials employed to elicit stable selectivity of interaction with the target analyte. Material needs for this class of sensors are summarized in the last section of this chapter.
SENSORS WITH SAMPLE SEPARATION
Chromatographic or electrophoretic separation in chemical analysis has classically not been considered to be a "chemical sensor." However, the difficult analyte selectivity issue of direct-reading sensors can be avoided by using a chromatographic or capillary electrophoretic format. In this case, a mixture of analytes flowing under pressure, or under an electrical gradient, are separated as a result of localized environmental interactions which can then be detected as they flow sequentially past the detector (sensor).
The enormous power of column chromatographic and capillary electrophoretic separations, together with their potential for miniaturization as analytical systems, demands that these areas be considered in any trade-off with chemical sensors. The key issue is the response time scale of chemical sensors relative to the time scale for the separations-based approach. If the chemical separation is not very difficult, it is often possible to design the chromatographic or electrophoretic experiment such that the moving phase segment can be shortened to produce separation and detector response within a few minutes or even fractions of a second. This scenario is analogous to using a direct-reading sensor with a relatively slow response time.
There are wide variations in the sensitivity, selectivity to structure, and cost of the numerous different kinds of detectors employed in column chromatographic and capillary electrophoretic experiments. An important consideration for sensor systems with sample separation is amenability of the detector system (detector, supporting appurtenances, control and measurement electronics) to miniaturization. Detector systems for which miniaturization appears most readily achievable or likely to yield the greatest benefits include:
-
thermal conductivity;
-
electrode, amperometric;
-
fluorescence (laser induced);
-
surface acoustic wave (SAW) device;
-
mass spectrometry; and
-
ionic conductivity.
These detectors are relatively low cost, except for mass spectrometry. However, mass spectrometry is the most powerful of the sensitive and structurally selective detectors, and in combination with a preceding chromatographic or electrophoretic separation step, it is probably the most generally applicable chemical analysis system currently available.
Recalling the definition of an ideal chemical sensor, the requirements of portability, ease of fabrication, and low cost would seem to be incompatible with a sensor requiring a preceding separation step or a complex detector like a mass spectrometer. However, inadequately explored research avenues offer the potential for chromatographic and electrophoretic instruments that are simple, miniature, and portable. This general problem is discussed in the following section.
SENSOR MINIATURIZATION, SIMPLIFICATION, AND PLATFORMS
The foundation for chemical sensing often originates with analytical studies using bulky, complex instruments. Transforming the results of these studies into a useful chemical sensor generally requires some miniaturization of the sensor and supporting instrumentation. Miniaturization frequently involves simplification and some degradation of sensor performance. Examination of low cost, miniaturized platforms on which sensor devices can be fabricated is stimulated by the potential economic advantages that could result from more timely forms of monitoring. Recognition of the potential importance of low-cost, miniaturized sensor platforms has been gradually growing. Both separations-based (chromatographic, capillary zone electrophoretic) and direct-reading (fiberoptic, surface acoustic wave device, amperometric) sensors have been demonstrated in on-chip formats (Terry et al., 1979; Manz et al., 1990, 1991; Harrison, 1993a, b; Murray et al., 1989; Monnig and Jorgenson, 1991; Seitz, 1984; Wolfbeis, 1985; Angell, 1987; White, 1987; Wohltjen, 1984; Grate et al., 1993; Frye and Martin, 1991; Ballantine and Wohltjen, 1989; Morita et al., 1988; Aoki et al., 1988; Chidsey et al., 1986; Tonucci et al., 1992; Seiler et al., 1993) Substantial research investments remain to be made before the significant promise of these approaches can be realized for chemical sensors. Given the advantages of existing lithographic technologies and potential-associated economies of scale, much of the existing work has employed either silicon or silica (SiO2) as platform materials for the sensor system. Polymeric platforms may offer some economic advantages for chemical sensors, but the research base in this area is more limited.
A number of separation and sensor systems have been built on potentially mass-producible platforms:
-
gas liquid chromatography and high-performance liquid chromatography separation systems;
-
capillary-zone electrophoresis separation systems;
-
fiberoptic sensor systems;
-
piezoelectric effect-based mass sensors, such as SAW devices; and
-
electrochemical, amperometric sensors.
Some of these systems have received little or no attention with respect to microfabrication, despite the potentially substantial dividends that would result from miniaturization. A number of general fabrication/miniaturization issues have been identified by the committee; information and relevant references are summarized in Table 6-1.
Mass spectrometry is perhaps the most prominent of the tools that could potentially revolutionize separation-based, miniaturized chemical sensor systems. Improvements in inexpensive mass analyzers with a truly portable format have the potential to revolutionize approaches to chemical sensing based on combinations with high-speed gas or liquid chromatography or with capillary electrophoresis. Miniaturization would almost certainly degrade the mass analyzer resolution, but even a low-resolution capacity would find many applications and would offer benefits in multiplexing. Key issues include:
-
miniaturization of vacuum systems for the mass analyzer and of supporting electronics for detector read-out, mass scanning, and data reading; and
-
fabrication of an on-wafer mass analyzer, such as a quadrupole or ion trap device, that uses lithographic or micromachining technology.
SENSORS FOR TOXIC CHEMICALS
Applications of sensors to detect chemical toxins fall into a variety of contexts. Toxicity commonly refers to potential consequences of exposure on human health and safety. Toxins are generally taken as being fabricated chemicals, and this assumption is reflected in regulations governing toxin monitoring. However, there is a growing awareness, felt keenly in risk-assessment issues, of the potential importance of "natural" toxins (Ames et al., 1990). Toxicity can also refer to effects on any biological system (not necessarily human) and to influences on nonbiological systems, such as the ozone layer.
TABLE 6-1 Chemical Sensors: Miniaturization/Fabrication Trends
|
SENSOR TYPE |
REQUIREMENTS/REMARKS |
|
Gas liquid chromatography |
Examples known (Terry et al., 1979; Manz et al., 1990) of silicon wafer with lithographically defined sample introduction, column, and detector; 2 times 6 mm liquid chromatography detector channels |
|
High performance liquid chromatography |
Micropumps and valves reported (Shoji et al., 1988) Adaptable to injection flow analysis (Ruzicka and Hansen, 1984) Miniaturized liquid chromatography requires micropump and microsample injectors to accommodate small sample sizes Simple on-wafer mass analyzer device possible for molecular mass-sensitive detection? |
|
Capillary zone electrophoresis |
Examples known of channels etched in silicon with oxide, nitride coatings (Harrison et al., 1993a); also, 10 mm × 10 mm × 2 cm long channels micromachined into glass wafers (Harrison et al., 1993b) with >40,000 plates and separation times ~ 10 seconds Small sample volume injection possible with crossing channels and independent electric field gradient control (Harrison et al., 1993b) Most work done with silica microcapillaries which are readily used in shortened forms for high speed separations (Monig and Jorgenson, 1991) Multiple channels, combination of CZE with flow injection analysis, and reagent derivatization channels possible (Manz et al., 1991; Manz et al., 1993) Submicron channels may be possible (Tonucci et al., 1992) Requires electrically insulated channel, since samples move under electric field gradient Improve micromachining for reagent derivatization tracks, avoiding cross-channel leakage and improving control of surface chemical interactions with sample; (separation can be enhanced by interactions between sample and stationary phase) |
|
Fiberoptics |
Submicron fibers and photopolymerized molecular coatings possible (Tan et al., 1992; Barnard and Walt, 1991) Spatially defined sensor positions |
|
Piezoelectric effect-based mass sensors |
Deposition of surface acoustic wave electrode patterns becoming standard Possible exploration of new forms of mechanical excitation of piezoelectric material |
|
Electrochemical amperometric |
Examples known of three and four Au and Pt electrode electrochemical cells and interdigitated arrays lithographically defined on silicon, with micron-scale electrodes and electrode-electrode spacings (Morita et al., 1988; Aoki et al., 1988; Chidsey et al., 1986) Many amperometric formats are improved by miniaturization, close electrode spacing (Morita et al., 1988) Fabrication of microelectrodes (micron and nanometer scale electrodes) necessary (Wightman and Wipf, 1989) Need to improve low cost, disposable electrochemical cell-on-chip designs |
Monitoring of toxic chemicals for benefits to human health and safety is illustrated by two examples: (1) workplace or occupational environmental monitoring and (2) on-site monitoring of chemical warfare agents, precursors, and degradation products under the Chemical Weapons Convention.5 These examples serve to illustrate differences in design and selection of chemical sensors and existing materials constraints within the general context of toxic chemical monitoring. Many of these ideas and technologies for improved chemical sensing can be applied to other types of environmental monitoring.
Environmental Monitoring
The following discussion shows that the requirements identified for general chemical sensing (i.e.,
improved chemical selectivity and miniaturization of separations-based sensor systems) apply equally to environmental monitoring. However, two additional factors significantly influence the development of chemical sensors for environmental monitoring: the requirement for a complex monitoring strategy covering the transport of toxic chemicals from source to human exposure and the influence of regulatory requirements.
The general pathways for transport of toxic materials from their sources to produce human exposure, with potential ensuing absorption, metabolism, and adverse health effects, are summarized in Figure 6-2. This figure provides a framework for design of an environmental sensing strategy and assessment of the hazard level. Specifically, sensing can be aimed at determining the level or activity of toxins in the emission source; in the media into which the toxin is incorporated en route to human exposure (the transport medium); and at the point of human exposure, including possible consequences of exposure. Monitoring of the emission source, the transport medium, and the point of human exposure may be necessary for a comprehensive plan designed both to assess hazard and to exert control on the emission sources in order to achieve hazard reduction. Typical problems in occupational exposure monitoring include:
-
dermal exposure monitoring;
-
air-purifying respirator end-of-service-life indicators;
-
personal monitors for aerosols;
-
biological monitoring (blood, urine, breath);
-
personal/area monitors for gases and vapors; and
-
soil/groundwater monitoring.
For a given toxin analyte, chemical sensors differing in sensitivity, selectivity, or other characteristics may be required to monitor the emission source, the transport medium, and individual exposure. The toxin concentration is typically greater, at the source than after dispersal in a transport medium; and the complexity of, and potential interferences in, the transport matrix (e.g., air, water, soil, skin, biological fluid sample) can vary widely. The physical and chemical properties of the analyte and its immediate environment (airborne vapor, contained in solid or liquid aerosol, chemically or photochemically reactive and decaying into substances of differing toxicity, radioactive, ionic, acidic, lipophilic, etc.) are also influential in the choice and design of a suitable sensor.
Regulatory requirements influence environmental chemical sensing design, particularly at the point of human exposure. Such requirements can specify detection of the approach to some designated exposure limit over a short time span, over a
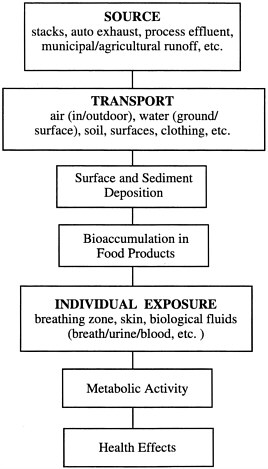
FIGURE 6-2 Schematic pathway for human exposure to toxic materials.
typical work day, or over some longer period. Regulatory requirements are set by governmental agencies having differing jurisdictions and differing approaches to monitoring, including the Environmental Protection Agency, the Food and Drug Administration, the Occupational Safety and Health Administration, and the American Conference of Governmental Industrial Hygienists.
There emerge from the regulatory requirements detailed lists of toxic chemical exposure limits, time scales permitted for such exposure, and often a recommended analytical method or reference method (see, for example, Environmental Protection Agency, National Ambient Air Quality Standards). Overlaid on such requirements is the prioritization imposed by the need to monitor different types of hazards in the face of finite economic resources. In a practical sense, the materials needs of environmental chemical sensors are substantially influenced by the nature of governmental regulation. Success in meeting regulatory needs at low cost will reduce the need to make hard-pressed choices among regulatory priorities. Despite the pressure of regulatory requirements, not all research on environmental chemical sensors is driven by regulation. A significant amount of basic work on environmental sensing is under way in universities and in industrial laboratories.
Thus sensing of environmental chemicals is a substantial, complex challenge that dictates several key requirements that affect sensor technology:
-
The difficulties in attaining adequate selectivity of sensor response to the desired toxic analyte apply to environmental monitoring as well as to other types of chemical sensing.
-
Environmental sensing requirements further justify the emphasis on miniaturization of chemical sensors, particularly for use in mass-producible formats. Small size and weight, together with low cost, offer a number of advantages, including:
-
attaching the sensor to the individual (lapel, belt) to provide a more accurate and realtime indication of worker exposure to particularly severe toxins;
-
redundant sensors to compensate for sensor failure or poisoning;
-
improved portability; and
-
lower power requirements, resulting in longer unattended field use and easier access in certain sampling situations.
-
Chemical Weapons Detection
Implementation of any chemical weapons treaty depends upon the existence of the necessary technology to monitor and verify compliance. A critical issue is the need for on-site inspection of factories and facilities suspected of making chemical warfare agents or precursors (Ember, 1993), and the associated requirement to detect the presence and amount of specified toxic chemicals. In contrast to the case of occupational environmental monitoring discussed above, sensor units for chemical weapons treaty verification will likely be custom units produced in very small numbers (e.g., tens of units). A unit acquisition cost on the order of $10,000 would probably be acceptable. However, the issues of selectivity and sensitivity are similar for chemical weapons detection and workplace monitoring. For example, Schedule II compounds (chemicals with limited dual use) are complex organic molecules without a distinctive characteristic that allows easy and rapid identification. In addition, these compounds can be masked by, or mistaken for, other organic compounds that may be present in higher concentrations (NRC, 1993). Limits of detection on the order of 1 part per billion are required (i.e., high sensitivity) together with high selectivity in order to avoid incorrect sensor response to common, legitimate chemicals, such as pesticides.
An example specification for the detection of Schedule II compounds is presented in Table 6-2 in terms of the descriptors identified in Chapter 2. The committee identified a number of candidate sensor types that could potentially meet the requirements presented in the table:
-
gas chromatograph-mass spectroscopy;
-
ion mobility spectrometer;
-
patch chemical reactions showing color change;
-
fiberoptic and related methods;
-
electrochemical sensors;
-
acoustic-wave chemical sensors; and
-
immunochemical assays.
TABLE 6-2 Sensor System Needs for Verification of a Chemical Weapons Treaty (Schedule II Compounds)
|
DESCRIPTORS |
SENSOR SPECIFICATIONS |
|
CHARACTERISTICS |
|
|
Response time |
Can be > 10 seconds; < 1-minute response and recycle time desirable |
|
Sensitivity |
Detection of toxins at nonlethal levels (approximately 1 ppb in gas phase)a |
|
Resolution |
Approximately 1 ppb in air for selected compounds |
|
Range |
From 1 ppb to 1 part per thousand |
|
Linearity |
Sensor nonlinearities can be corrected by microprocessor |
|
Limit of detection |
1 ppb |
|
Selectivity |
High: must not give incorrect response to common and legitimate chemicals (e.g., pesticides) |
|
Accuracy |
Detection at 1-ppb level may be sufficient |
|
Repeatability (precision) |
Repeatability of measurement must be such that the sensor system does not indicate the presence of target compound when it is there in concentrations lower than 0.1 ppb |
|
CONSTRAINTS |
|
|
Packaging |
|
|
Size |
Hand held, less than 1 cubic foot |
|
Weight |
Less than 5 pounds |
|
Hermeticity |
Must survive rain; "sports-type cassette player" level of packaging |
|
Isolation |
|
|
Thermal |
-10 °C to +50 °C |
|
Electromagnetics |
Human ambient (power lines, transformers) |
|
Mechanical |
Must survive drop test from 10 feet |
|
Chemical |
Will be hand held, sampling vapors; must survive normal human environments of humidity, air, environmental chemicals, and poisons |
|
Optical |
Not applicable |
|
ECONOMIC |
|
|
Acquisition |
Unit cost could be fairly high (~$10K), since only a few custom units (about 10) will be madeb |
|
Development |
In the absence of suitable commercial products, up to $10M will be spent over 5 years to develop sensor system |
|
Manufacturability |
Not applicable |
|
Life cycle |
Total number of units too small for problem |
|
RELIABILITY |
|
|
Lifetime |
2 years, multiuse on inspection tours, hundreds of cycles |
|
Calibration |
Once per month or before each country tour.c |
|
ACQUISITION (data) |
Discrete, with about 10 seconds between readings; internal data storage, possibly with encryption, with data sent to a microcomputer later |
|
IMPLEMENTATION |
|
|
Scale |
Array or alternative detector scheme for measurement over a large area |
|
Format |
Chemical sensor array or other detector scheme will feed information to microprocessord |
|
Mode |
Hand held unit with small pump for sampling vapor |
|
TRANSDUCTION |
No specification made |
|
TRANSDUCTION MODE |
No specification made |
|
MEASUREMENT SCALE |
No specification made |
|
aA detailed list of compounds and required sensitivities is not yet available. There may be a requirement that no chemical interpretation need be made by the operator in the field; that is detection of a pattern of chemical responses and data reduction into a pattern recognition program would provide a judgment that chemical weapons are being manufactured in the vicinity or that the treaty is being violated in some other way. bOther treaty scenarios include the possibility that industrial plants will require permanently installed units to avoid potential loss of proprietary information. Only information about detected violations would leave the site. cExpensive recalibration, including factory recalibration, is acceptable, given the small number of units and the political implications of poor sensor performance. dA decision will be made on whether to challenge an inspected facility based on some chemical information score, such as a ten-step indicator, with zero indicating no possibility of chemical weapons production and 10 indicating sublethal concentrations of the chemical agent itself. |
|
A discussion of the use of the communication tool in identifying candidate technologies for chemical weapons detection is presented in Appendix G. The matrix shown in Table 6-3 summarizes the results of comparing specification requirements and attributes of candidate sensing technologies using the descriptors. Anticipated problems in meeting specification requirements with available technologies have been marked "X," and particularly desirable features of the candidate systems have been marked with a "+." Use of the descriptor terminology and approach developed in Chapter 2 is extremely useful in conducting a comparative evaluation of different technologies.
An analysis of the data in Table 6-3 indicates that:
-
no single candidate technology can fulfill all the specified requirements for detection of Schedule II compounds;
-
existing sensor technologies are inadequate in several areas, most notably selectivity; and
-
there may be a very fine distinction between desirable attributes.
The framework tool developed in Chapter 2 can be useful in identifying general areas (sensitivity, selectivity, etc.) for development of sensors to meet specific application needs and in highlighting potentially significant development costs. However, the committee noted that, for a given application, additional descriptors may be needed, such as one that summarizes the status of sensor development. Such a descriptor would permit the identification of technology deficiencies in particular areas, allowing the user to make a better estimation of the likely cost to bring a particular sensor technology up to the required performance level.
TABLE 6-3 Candidate Sensing Technologies for Detection of Chemical Warfare Agents
|
|
CANDIDATE SENSING TECHNOLOGIES |
||||||
|
DESCRIPTOR |
GC-MS |
Ion Mobility Spectrometer |
Patch Test |
Fiberoptic |
Electrochemical |
Acoustic Wave Chemical |
Immunochemical Assays |
|
Response time |
|
|
X |
|
|
|
X |
|
Sensitivity |
+ |
X |
|
X |
|
X |
+ |
|
Resolution |
|
|
X |
|
|
|
+ |
|
Range |
|
|
X |
|
|
|
|
|
Linearity |
|
|
|
|
|
|
|
|
Detection limit |
|
X |
|
|
|
X |
|
|
Selectivity |
+ |
+ |
|
|
X |
X |
+/X |
|
Accuracy |
+ |
|
X |
|
|
X |
|
|
Repeatability |
+ |
|
|
|
|
|
|
|
Constraints |
|
|
|
|
|
|
|
|
Packaging |
X |
X |
|
+ |
|
|
+ |
|
Isolation |
|
|
|
+ |
|
|
|
|
Economic |
|
|
|
|
|
|
|
|
Acquisition |
X |
|
|
|
|
|
X |
|
Development |
|
|
|
X |
|
X |
X |
|
Lifecycle |
|
|
|
|
|
|
|
|
Reliability |
|
|
|
|
|
|
|
|
Lifetime |
|
|
X |
|
X |
|
X |
|
Calibration |
|
|
|
|
X |
X |
X |
|
Acquisition (data) |
|
|
|
|
|
|
|
|
Implementation |
|
|
|
|
|
|
|
|
Scale |
|
|
|
|
|
|
|
|
Format |
|
|
|
|
+ |
+ |
|
|
Key: X (potential) problem in meeting specification; + desirable attribute |
|||||||
MATERIALS DEVELOPMENT OPPORTUNITIES FOR CHEMICAL SENSORS
Most chemical sensor applications have been based on a broad background of measurement principles and chemical reactivity developed through research in analytical and other branches of chemistry. Many fundamental ideas, devices, and materials have been adapted from other sciences and technologies (Murray et al., 1989):
-
Inexpensive optical fibers from the communications industry have been applied in spectroscopically based direct-reading sensors and near-field microscopy (Betzig et al, 1991).
-
Lithographic patterning technology widely used in the manufacture of modern microelectronics has been exploited to fabricate miniaturized electro-chemical devices such as interdigitated array electrodes, microelectrodes, and chemically sensitive field-effect transistors and to form patterned electrodes on surface acoustic wave (SAW) devices. 6 (Kepley et al., 1992; Ricco and Martin, 1992; Martin et al., 1990).
-
Materials research has resulted in advanced piezoelectric materials that are employed as micro-positioners; these materials have enabled new forms of microscopy, like scanning electrochemical, scanning tunnelling, and atomic force microscopy. The availability of these micropositioners is also critical for chemical sensing on an extremely small dimensional scale (Snyder and White, 1992).
-
Ultrasensitive light detection using charge coupled devices, which were developed for astronomy, is under active consideration for detection of laser-induced fluorescence from extremely small populations of molecules.
Research in the above areas involves applying new technologies to analytical chemistry and chemical sensor research. It is intrinsically multi-disciplinary, with contributions from analytical chemists, materials scientists, electrical engineers, and professionals in other fields. At the initial stages of research, the interest is generally focused on exploring and proving the principles by which a new technology can be applied to measure a chemical substance. Open access to specialized equipment and facilities, such as those required for lithographic patterning, can be crucially important to foster interest and progress as applications to specific practical analytical and chemical sensing measurements start to appear.
As previously mentioned, the most important materials-related opportunities to improve direct-reading chemical sensors involve the choice of materials employed to elicit stable selectivity of interaction with the target analyte. Table 6-4 summarizes materials needs for direct-reading chemical sensors. Nearly all the requirements are presented in terms of material functionality rather than material type (e.g., ceramic, polymer, semiconductor) to avoid inappropriate assumptions based on existing solutions.
Limitations of the existing chemistry or technology can become apparent at any stage during sensor development. Table 6-5 summarizes some key materials challenges for various chemical sensor technologies. The most frequent materials limitation for chemical sensors probably relates to the chemistry required to fashion an adequately selective response to the target analyte. Considerable potential exists to enhance the selectivity of direct-reading chemical sensors by the use of novel materials. One strategy to address this is the development of miniaturized high-speed separations-based sensors. These have the potential for avoiding difficulties in molecular selectivity but present major challenges in improving detector sensitivity.
Miniaturized total analytical systems are a relatively new area of research in analytical chemistry, but their development could greatly supplement the capabilities of existing direct reading sensors. The numerous materials issues in designing and fabricating low-cost, miniaturized separations-based analytical systems include:
-
coatings and films with improved properties for enhanced sensor performance (e.g., chemical selectivity, chromatographic efficiency, stability under electric field gradients, electrocatalysis efficiency);
-
materials that enhance detector sensitivity and increase performance range (fiber optics);
TABLE 6-4 Materials Needs for Selective Direct-Reading Chemical Sensors
|
Material Forms |
Applications |
Functional Requirements |
Possible Mechanisms |
|
Membranes |
Amperometric, conductimetric, potentiometric electrochemical sensors |
Analyte selectivity Stability |
Analyte binding or partitioning Permselectivity Catalytic reactivity Sensing electrode arrays |
|
Coatings/Thin Films |
Amperometric, conductimetric, potentiometric electrochemical sensors Optical fibers and waveguides Piezoelectric devices Surface acoustic waves |
Analyte selectivity Stability |
Analyte binding or partitioning Enzyme or antibody properties Sensing electrode, optical fiber, waveguide arrays Permselectivity Electrocatalytic activity Changes in light propagation or luminescence Viscoelastic changes |
|
Bulk Materials |
Amperometric and electrochemical sensors |
Analyte selectivity Stability |
Solid or polymer electrolytes with selective binding sites |
|
Fibers (optical) |
Optical fibers and waveguides |
Extended operational wavelength range |
Improved near- and extended-infrared transparency and reflection |
-
fiberoptic materials with improved performance in the near-infrared and infrared spectral regions;
-
technologies for cost-effective miniaturization of sensor systems;
-
on-chip formats for practical applications of miniaturized sensor systems; and
-
chemical sensor systems with increased ruggedness, reliability, and control.
Research efforts directed at determining which chemical sensing technologies are practical and should be developed for high-volume home and personal wellness applications are expected to have particularly high payback.
New materials can lead to improvements in the selectivity of direct chemical sensors. The development of fast, miniaturized chromatographic and capillary electrophoresis systems with detectors that are senstitive to chemical structure is important for both general chemical sensing and for the more specific case of environmental monitoring. In the latter case, the requirement to monitor a given analyte over a wide range of concentrations and in a variety of environments places particularly stringent requirements on chemical sensor sensitivity and selectivity. The need to meet and possibly redefine regulatory requirements for monitoring toxins is also an important driver in the development of environmental chemical sensors. Mass-producible sensor formats are particularly important for occupational environmental monitoring in view of the need for low-cost compliance with regulatory requirements.
The detection of chemical weapons is a specific type of environmental sensing. The following materials areas have been identified by the committee as important in developing candidate sensor technologies to meet requirements for chemical weapons detection:
-
fiberoptic coatings with improved chemical selectivity, for example, selective analyte absorption (a polysiloxane film has been shown to respond
TABLE 6-5 Materials Challenges for Chemical Sensors with Sample Separation
|
SENSOR TYPE |
MATERIALS NEEDS |
|
Gas liquid chromatography |
Stationary phase coatings with improved chromatographic efficiency on silicon channel walls |
|
High performance liquid chromatography |
Materials to enhance sensitivity of detectors for gas and solution column eluent Improved design and materials for fusing of microchannel roof |
|
Capillary zone electrophoresis |
Improved design and materials for fusing of microchannel roof Materials for better and miniaturized detectors Improved breakdown and insulation characteristics of silica and of oxide and nitride coatings on silica |
|
Fiber optics |
Improved fiber materials for near-infrared and infrared regions Materials to enhance detector performance in near-infrared and infrared regions Improved fiber coatings for enhanced selectivity to target sample species Improved solid-state lasers for laser-induced fluorescence detection |
|
Piezoelectric-based mass sensors |
Materials for improved coating selectivity Better understanding of mass response of alternative mechanical excitations in contact with liquid and viscous media |
|
Electrochemical amperometric sensors |
Achieve selectivity through molecular coatings, film coatings on electrodes, or chemically modified electrodes (Murray, 1992) Electrode coatings with improved electrocatalytic rate, selectivity, and stability Improved chemical ruggedness of metal electrode patterns |
-
to di-isopropyl-methylphosphonate, but the sensitivity, resolution, and detection limit are inadequate, and selectivity is unsatisfactory, since the material responds to most organic solvents);
-
new membranes and electrode coatings to obtain improved chemical selectivity with electro-chemical sensors; and
-
chemically selective films that undergo changes in mechanical and electrical properties following analyte sorption (SAW devices).
These materials requirements are very similar to some identified previously as being important in improving the selectivity of other direct-reading chemical sensors. It should be noted that selectivity is specific to a particular compound or class of compounds. Thus, specialized materials are required for detection of Schedule II compounds, and these materials will likely differ from those developed to detect toxic chemicals encountered in environmental health monitoring.
Little potential for dual-use applications (or other secondary applications) is anticipated for coatings developed for the detection of chemical warfare agents. Nonetheless, the general lessons learned in developing chemically selective materials (understanding the role of electrical and chemical forces on surface and interfacial phenomena, molecular characterization of ion-specific membranes and modified surfaces with catalytic or enzymatic properties, etc.) can be broadly applicable and should be of help in designing materials to meet particular functional requirements.
The possibility exists of leveraging generic miniaturization techniques, including materials and processing technologies developed for mass market applications, in order to further develop compact, lightweight hand-held sensor systems for chemical weapons detection. Miniaturization techniques of particular interest include methods relating to supporting electronics and protective packaging.
REFERENCES
Ames, B.N., M. Profet, and L.S. Gold. 1990. Dietary pesticides (99.99-percent all natural). Proceedings of the National Academy of Sciences 87(19):7777–7781.
Angell, S.M. 1987. Optrodes: Chemically selective fiber-optic sensors. Spectroscopy 2(4):38–46.
Aoki, K., M. Morita, O. Niwa, and H. Tabei. 1988. Quantitative-analysis of reversible diffusion-controlled currents of redox soluble species at interdigitated array electrodes under steady state conditions. Journal of Electroanalytical Chemistry 256(2):269–282.
Ballantine, D.S., and H. Wohltjen. 1989. Surface acoustic wave devices for chemical analysis. Analytical Chemistry 61(June 1):704A–706A.
Barnard, S.M., and D.R. Walt. 1991. A fibre-optic chemical sensor with discrete sensing sites. Nature 353(6342):338–340.
Betzig, E., J.K. Trautman, T.D. Harris, J.S. Weiner, and R.L. Kostelak. 1991. Breaking the diffraction barrier: Optical microscopy on a nanometric scale. Science 251(5000):1468–1470.
Brown, S.D., R.S. Bear, and T.B. Blank. 1992. Chemometrics. Analytical Chemistry 64(12):PR22–R49.
Chidsey, C.E.D., B.J. Feldman, C. Lundgren, and R.W. Murray. 1986. Micrometer-spaced platinum interdigitated array electrode: Fabrication, theory, and initial use. Analytical Chemistry 58:601–607.
Ember, L.R. 1993. Chemical arms treaty makes unprecedented demands of industry. Chemical and Engineering News Record 71(23):7–18.
Frye, G.C., and S. J. Martin. 1991. Materials characterization using surface acoustic-wave devices. (Review). Journal of Applied Spectroscopy Reviews 26(1–2):73–149.
Grate, J.W., S. L. Rose-Pehrsson, and D. L. Venezky. 1993. Smart sensor system for trace organophosphorous and organosulfur vapor detection employing a temperature-controlled array of surface acoustic wave sensors, automated sample preconcentration, and pattern recognition. Analytical Chemistry 65(July 15):1868–1881.
Harrison, D.J, A. Manz, and P.G. Glavina. 1993a. Towards miniaturized electrophoresis and chemical-analysis systems on silicon—An alternative to chemical sensors. Sensors and Actuators 10(2):107–116.
Harrison, D.J., K. Fluri, K. Seiler, Z. Fan, C. S. Effenhauser, and A. Manz. 1993b. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science 261(5123):895–897.
Haswell, S.J. 1992. Practical Guide to Chemetrics. New York: M. Dekker.
Kepley, L.J., R.M. Crooks, and A.J. Ricco. 1992. Selective surface acoustic wave-based organophosphonate chemical sensor employing a self-assembled composite monolayer: A new paradigm for sensor design. Analytical Chemistry 64(24):3191–3193.
Manz, A., Y. Miyahara, J. Muira, Y. Watanabe, H. Miyagi, and K. Sato. 1990. Design of an open-tubular column liquid chromatograph using silicon chip technology. Sensors and Actuators Bulletin B1(1–6):249–255.
Manz, A., J.C. Fettinger, E. Verpoorte, H. Ludi, H.M. Widmer, and D.J. Harrison. 1991. Micromachining of moncrystalline silicon and glass for chemical analysis systems—A look into next century technology or just a fashionable craze. Trends in Analytical Chemistry 10(5):144–149.
Manz, A., D.J. Harrison, E. Verpoorte, and H.M. Widmer. 1993. Planar chip technology for miniaturization of separation systems. Advances in Chromatography. Vol. 33:1–66. New York: M. Dekker.
Martin, S.J., A.J. Ricco, T.M. Niemczyk, and G.C. Frye. 1990. Characterization of SH-acoustic plate mode-liquid sensors. Sensors and Actuators 20(3):253–268.
Monig, C.A., and J.W. Jorgenson. 1991. On-column sample grating for high-speed capillary zone electrophoresis. Analytical Chemistry 63(April 15):802–807.
Morita, M., M.L. Longmire, and R.W. Murray. 1988. Solid-state voltammetry in a three electrode electrochemical cell-on-chip with a microlithographically defined micro-electrode. Analytical Chemistry 60(December 15): 2770–2775.
Murray, R.W., R.E. Dessy, W.R. Heineman, J. Janata, and W.R. Seitz, eds. 1989. Chemical Sensors and Microinstrumentation. Proceedings of Symposium. American Chemical Society National Meeting held in Los Angeles, California, September, 1988. ACS Symposium Services, Vol. 403. Washington, D.C.: American Chemical Society.
Murray, R.W., ed. 1992. Molecular Design of Electrode Surfaces. New York: John Wiley and Sons.
NRC (National Research Council). 1993. Alternative Technologies for the Destruction of Chemical Agents and Munitions. Board on Army Science and Technology, NRC. Washington, D.C.: National Academy Press.
Newman, A.R. 1993. Review of pattern recognition in acoustic wave devices. Analytical Chemistry 63:585A–588A.
Ricco, A.J., and S.J. Martin. 1992. Real Time, Submonolayer Monitoring of Electrochemical Processes Using Acoustic Plate-Mode Devices. Chapter 7 in New Trends and Approaches in Electrochemical Technology. Tokyo: VCH/ Kodansha Scientific.
Ruzicka, J., and E.H. Hansen. 1984. Integrated microconduits for flow injection analysis. Analytica Chimica Acta 161(July):1–25.
Seitz, W.R. 1984. Chemical sensors based on fiber optics. Analytical Chemistry 56(1):16.
Seiler, K., D.J. Harrison, and A. Manz. 1993. Planar glass chips for capillary electrophoresis: Repetitive sample injection, quantitation, and separation efficiency. Analytical Chemistry 65(May 15, 1993):1481–1488.
Shoji, S., M. Esashi, and T. Masuo. 1988. Prototype miniature blood-gas analyzer fabricated on a silicon-wafer. Sensors and Actuators 14(2):101–107.
Snyder, S.R., and H.S. White. 1992. Scanning tunneling microscopy,
atomic force microscopy and related techniques. Analytical Chemistry 64(June 15):116R–134R.
Tan, W., Z.-Y. Shi, and R. Kopelman. 1992. Development of submicron chemical fiber optic sensors. Analytical Chemistry 64(Dec. 1):2985–2990.
Terry, S.C., J.H. Jerman, and J.B. Angell. 1979. A gas chromatographic air analyzer fabricated on a silicon wafer, IEEE Transactions on Electron Devices, ED-26(12):1880–1866.
Tonucci, R.J., B.L. Justus, A.J. Campillo, and C.E. Ford. 1992. Nanochannel array glass. Science 258(5083):783–785.
White, R.M., 1987. New prospects for acoustic sensors: An overview. Pp. 333–338 in Proceedings of the 41st Annual Frequency Control Symposium, Philadelphia, Pennsylvania; May 27–29, 1987. New York: IEEE.
Wightman, R.M., and D.O. Wipf. 1989. Voltammetry at ultramicroelectrodes. Electroanalytical Chemistry 15:267–353.
Wohltjen, H. 1984. Mechanism of operation and design considerations for surface acoustic-wave device vapor sensors. Sensors and Actuators 5(4):307–325.
Wolfbeis, O.S. 1985. Fluorescence optical sensors in analytical chemistry. Trends in Analytical Chemistry 4(7):184–188.
NOTES
|
1. |
This may require portable sensors that add another aspect of complexity to the problem. |
|
2. |
Response times are commonly in the millisecond-to-second range for direct-reading sensors. Response times for separation-based systems are longer but can be less than 1 minute. |
|
3. |
Within a sensor system, the sensor element is frequently smaller than the supporting electronics. |
|
4. |
It is likely that a large fraction of chemical sensing needs will continue to be met by traditional analysis. |
|
5. |
International Convention on Prohibition of the Development, production, Stockpiling, and Use of Chemical Weapons, and on Their Destruction. |
|
6. |
Appendix F describes in more detail several chemical sensor platforms based on this technology. |
















