The New England Journal of Medicine
©Copyright, 1994, by the Massachusetts Medical Society
Volume 330 APRIL 7, 1994 Number 14
HANTAVIRUS PULMONARY SYNDROME: A CLINICAL DESCRIPTION OF 17 PATIENTS WITH A NEWLY RECOGNIZED DISEASE
JEFFREY S.DUCHIN, M.D., FREDERICK T.KOSTER, M.D., C.J.PETERS, M.D., GARY L.SIMPSON, M.D., PH.D., BRUCE TEMPEST, M.D., SHERIF R.ZAKI, M.D., PH.D., THOMAS G.KSIAZEK, D.V.M., PH.D., PIERRE E.ROLLIN, M.D., STUART NICHOL, PH.D., EDITH T.UMLAND, M.D., RONALD L.MOOLENAAR, M.D., SUSAN E.REEF, M.D., KURT B.NOLTE, M.D., MARGARET M.GALLAHER, M.D., JAY C.BUTLER, M.D., ROBERT F.BREIMAN, M.D., AND THE HANTAVIRUS STUDY GROUP*
Abstract Background. In May 1993 an outbreak of severe respiratory illness occurred in the southwestern United States. A previously unknown hantavirus was identified as the cause. In Asia hantaviruses are associated with hemorrhagic fever and renal disease. They have not been known as a cause of human disease in North America.
Methods. We analyzed clinical, laboratory, and autopsy data on the first 17 persons with confirmed infection from this newly recognized strain of hantavirus.
Results. The mean age of the patients was 32.2 years (range, 13 to 64); 61 percent were women, 72 percent were Native American, 22 percent white, and 6 percent Hispanic. The most common prodromal symptoms were fever and myalgia (100 percent), cough or dyspnea (76 percent), gastrointestinal symptoms (76 percent), and headache (71 percent). The most common physical findings were tachypnea (100 percent), tachycardia (94 percent), and hypotension (50 percent). The laboratory findings included leukocytosis (median peak cell count, 26,000 per cubic millimeter), often with myeloid precursors, an increased hematocrit, thrombocytopenia (median lowest platelet count, 64,000 per cubic millimeter), prolonged prothrombin and partial-thromboplastin times, an elevated serum lactate dehydrogenase concentration, decreased serum protein concentrations, and proteinuria. Rapidly progressive acute pulmonary edema developed in 15 of the 17 patients (88 percent), and 13 patients, all of whom had profound hypotension, died (case fatality rate, 76 percent). Increases in the hematocrit and partial-thromboplastin time were predictive of death.
Conclusions. Infection with a newly described hantavirus causes the hantavirus pulmonary syndrome, which is characterized by a brief prodromal illness followed by rapidly progressive, noncardiogenic pulmonary edema. (N Engl J Med 1994;330:949–55.)
ON May 14, 1993, the New Mexico Office of the Medical Investigator was notified of the unexplained deaths of a couple living in the same household in rural New Mexico: a 21-year-old woman and a 19-year-old man. Both died of acute respiratory failure—the man within five days after the woman. By May 17, Indian Health Service physicians had reported five deaths from adult respiratory distress syndrome among previously healthy adults. Surveillance was initiated for an influenza-like illness followed by the rapid onset of unexplained respiratory failure. On May 22, the brother of the initial patient had an acute onset of a similar illness, as did his wife five days later; by June 7, 24 cases, including 12 deaths (some identified retrospectively as occurring since March 1993), meeting the clinical case definition had been reported and were under investigation.1 All the subjects lived in or near the Four Corners area of New Mexico, Arizona, Colorado, and Utah.
Illnesses considered in the initial differential diagnosis included pneumonic plague, leptospirosis, inhalational anthrax, rickettsial infections, pulmonary tularemia, atypical bacterial and viral community-acquired pneumonias, legionellosis, meningococcemia and other sepsis syndromes, and illnesses caused by viruses not commonly seen in the United States (flavivirus, arenavirus, and bunyavirus). There was no evidence of exposure to known toxic agents. Laboratory tests for bacterial and viral pathogens and a variety of toxic agents were negative, and the initial autopsy findings suggested that bacterial or parasitic causes were unlikely. The results of laboratory studies of serum and tissue samples from several patients suggest-
|
* |
The members of the Hantavirus Study Group are listed in the Appendix. |
Reprinted with permission from Durchin, et al., The New England Journal of Medicine 330(14):949–55, Copyright 1994, Massachusetts Medical Society
ed an acute infection with a new species of hantavirus. This report describes the first 17 patients with this illness, which was characterized by rapidly progressive respiratory and hemodynamic deterioration due to a strain of hantavirus previously unrecognized in North America.
The hantavirus genus belongs to the Bunyaviridae family and includes the causative agents of a group of febrile nephropathies known collectively as hemorrhagic fever with renal syndrome, which occurs throughout Europe and Asia.2 The hallmarks of hemorrhagic fever with renal syndrome are hematologic abnormalities, prominent (often severe) renal involvement, and increased vascular permeability.3 Hantaan virus is the prototype hantavirus; isolated in 1977, it occurs predominantly in the Russian Far East, China, and Korea.4 Severe disease associated with the Hantaan virus is characterized by five phases: febrile, hypotensive, oliguric, diuretic, and convalescent. However, 30 to 40 percent of patients have minimal illness, and in only 20 to 30 percent is the illness moderate or severe.5–7 Respiratory symptoms are generally not pronounced, and pulmonary involvement has not been a prominent feature of the known hantaviral syndromes.8 Several species of rodents in the United States have been shown to be infected with hantaviruses. Although seroprevalence studies have detected antibodies to hantaviruses in a small percentage of people in the United States, there were no reports of acute illness resulting from hantavirus infection acquired in North America before the outbreak of cases described in this report.9,10
METHODS
The case definition of acute, unexplained respiratory distress syndrome consisted of either of the following findings in any patient presenting after January 1, 1993: unexplained adult respiratory distress syndrome or radiographic evidence of acute, bilateral, interstitial pulmonary infiltrates with hypoxemia (arterial oxygen saturation, less than 90 percent while the patient is breathing room air), or autopsy findings of unexplained, noncardiogenic pulmonary edema. Physicians in New Mexico, Arizona, Colorado, and Utah were requested to report cases meeting this definition to their state health departments.
Serum samples were tested for antibodies against a panel of heterologous hantaviral antigens, and tissue samples were tested for evidence of hantavirus infection by means of the polymerase chain reaction in frozen lung-tissue specimens or immunohistochemical staining of formalin-fixed specimens11 (and Centers for Disease Control and Prevention [CDC]: unpublished data). A case of unexplained respiratory distress syndrome was considered to be confirmed as hantavirus infection if the results of antibody, polymerase-chain-reaction, or immunohistochemical testing were positive. Thirty-one patients meeting the case definition were identified by surveillance in the four-state area. As of July 11, 1993, 17 of the 31 had confirmed hantavirus infection; medical records were available for 16 of these patients. One additional patient, whose illness began before the surveillance period (November 1992), was identified and is included in the analysis.
Autopsy examinations were performed in 9 of 13 deceased patients (69 percent) at the New Mexico Office of the Medical Investigator. Autopsies were performed in 12 patients elsewhere, and the histopathological findings reviewed at the CDC. Medical records were abstracted by a single reviewer using a standardized data-collection form. Interviews with physicians were conducted to supplement the medical history in the case of two patients whose medical records were incomplete.
Data were stored and analyzed with the use of Epi-Info, version 5.01 (CDC and World Health Organization, Atlanta). A multiple logistic regression with a stepwise procedure was performed with SAS software (SAS Institute, Cary, N.C.).
RESULTS
During the study period, acute hantavirus infection was confirmed in 18 patients, 14 of whom died (78 percent). The median age was 31.0 years (mean, 32.2; range, 13 to 64); 11 of the patients (61 percent) were women. Thirteen patients (72 percent) were Native American, four (22 percent) were white, and one (6 percent) was Hispanic. Twelve patients (67 percent) resided in New Mexico, five (28 percent) in Arizona, and one (6 percent) in Colorado. In 12 patients (67 percent), the onset of illness occurred between May 1, 1993, and June 30, 1993. The medical records of 17 of the 18 patients were available for review.
Sample Case Report
A 19-year-old man living in rural New Mexico presented to an emergency department with a one-day history of fever, myalgia, chills, headache, and malaise; he did not have dyspnea or cough. The patient had been in excellent health and was a marathon runner; his fiancée, with whom he had lived, had died two days earlier of a rapidly progressive respiratory illness. His temperature was 39.4°C, his heart rate was 118 beats per minute, his blood pressure was 127/84 mm Hg, and his respiratory rate was 24 breaths per minute. The physical examination was normal.
The white-cell count was 7100 per cubic millimeter, with 66 percent segmented neutrophils and 10 percent band forms; the hematocrit was 49.6 percent, and the platelet count was 195,000 per cubic millimeter. The serum creatinine level was 1.1 mg per deciliter (100 µmol per liter), the blood urea nitrogen level was 9 mg per deciliter (3.2 mmol per liter), the serum albumin level was 4.8 mg per deciliter, and the serum lactate dehydrogenase level was 195 IU per liter (normal range, 100 to 190). Urinalysis revealed no protein or blood. Oxygen saturation, determined by pulse oximetry, was 91 percent while the patient was breathing room air, and the chest radiograph was normal. The patient was treated with erythromycin, amantadine, and acetaminophen and then discharged.
Two days later, the patient presented at a clinic with persistent symptoms plus vomiting and diarrhea. His temperature was 36°C, his heart rate was 80 beats per minute, his blood pressure was 90/70 mm Hg, and his respiratory rate was 22 breaths per minute. The physical examination was normal, with clear lung fields on auscultation; the patient was discharged with no change in the diagnosis or therapy. A cough that produced copious yellow sputum, sometimes blood-tinged, and progressive shortness of breath subsequently developed. The day after discharge, the patient had acute respiratory failure and cardiopulmonary arrest and could not be resuscitated. The
white-cell count was 65,300 per cubic millimeter, with 45 percent segmented neutrophils and 27 percent band forms. Seven metamyelocytes and 2 myelocytes per 100 cells were noted on review of the differential cell count. The hematocrit was 60.2 percent, and the platelet count was 42,000 per cubic millimeter. The serum creatinine concentration was 2.5 mg per deciliter (220 µmol per liter), the blood urea nitrogen concentration was 32 mg per deciliter (11.4 mmol per liter), the serum lactate dehydrogenase concentration was 1486 IU per liter, and the serum creatine kinase concentration during cardiopulmonary resuscitation was 814 U per liter with an MB fraction of 87 U per liter (11 percent). The chest radiograph showed diffuse interstitial and alveolar infiltrates (Fig. 1).
Clinical Presentation
Among the 17 patients, the mean duration of symptoms before hospitalization was 5.4 days (median, 4; range, 2 to 15). There was no relation between the duration of symptoms at admission and survival (P=0.3). The most common symptoms at the time of hospitalization were fever, myalgia, headache, cough, and nausea or vomiting (Table 1). Myalgia was the most frequently reported initial symptom. Shortness of breath or cough was reported by 13 patients (76 percent) at admission; the cough, which was described as productive by 5 patients, typically preceded respiratory distress. Gastrointestinal symptoms (abdominal pain, nausea and vomiting, or diarrhea) were reported by 13 patients (76 percent); abdominal pain was a prominent symptom in 2. No patient had signs of hemorrhage. Although the diagnosis at the time of admission was pneumonia in 7 patients (41 percent), 10 patients (59 percent) had other diagnoses: abdominal pain in 3 (18 percent); adult respiratory distress syndrome in 2 (12 percent); cardiopulmonary arrest in 2 (12 percent); and sepsis, pyelonephritis, and fever in 1 patient each (6 percent).
Seven patients (41 percent) had underlying illnesses: two had asthma, one had chronic obstructive pulmonary disease and atherosclerotic cardiovascular disease and had undergone a splenectomy, one had a history of silo-filler’s disease, two had chronic hypothyroidism, and one had a seizure disorder. Two patients (12 percent) were cigarette smokers, and one had a history of excessive alcohol use. No patients were taking corticosteroids or other immunosuppressive medications.
The most common physical findings were tachypnea and tachycardia (Table 2). Fifty percent of the patients had a respiratory rate of 28 or more breaths per minute, and 50 percent had a heart rate of 120 or more beats per minute. No patient had conjunctival hemorrhage, petechial rash, clinical signs of internal hemorrhage (including a guaiac-positive stool specimen), or peripheral or periorbital edema.
Notable hematologic findings included an elevated white-cell count with increased neutrophils, myeloid precursors, and atypical lymphocytes. Of the 13 pa
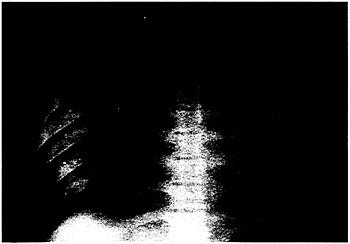
Figure 1. Chest Radiograph Showing Diffuse Interstitial and Alveolar Infiltrates in a Patient with Hantavirus Infection.
tients with differential white-cell counts at the time of admission, 12 (92 percent) had at least 10 percent band forms, 6 (46 percent) had metamyelocytes, and 3 (23 percent) had atypical lymphocytes. Subsequently, metamyelocytes were noted in 11 of 16 patients (69 percent), and atypical lymphocytes in 6 of 16 (38 percent). At the time of admission, the hematocrit was elevated in 13 (76 percent) of the patients (≥50 percent in the men, and ≥45 percent in the women), and the platelet count was less than 150,000 per cubic millimeter in 12 patients (71 percent).
The partial-thromboplastin time was 40 seconds or longer in 8 of the 12 patients (67 percent) tested at the time of admission and in 10 of the 12 (83 percent) subsequently (Table 3). Fibrin split products (or D-dimer) and fibrinogen were measured in seven patients: three had elevated D-dimer levels, and all had normal fibrinogen levels.
Five patients had metabolic acidosis with an in-
Table 1. Symptoms in 17 Patients with Hantavirus Infection.
|
SYMPTOM |
NO. OF PATIENTS (%) |
|
Fever |
17 (100) |
|
Myalgia |
17 (100) |
|
Headache |
12 (71) |
|
Cough |
12 (71) |
|
Nausea or vomiting |
12 (71) |
|
Chills |
11 (65) |
|
Malaise |
10 (59) |
|
Diarrhea |
10 (59) |
|
Shortness of breath |
9 (53) |
|
Dizziness or lightheadedness |
7 (41) |
|
Arthralgia |
5 (29) |
|
Back pain |
5 (29) |
|
Abdominal pain |
4 (24) |
|
Chest pain |
3 (18) |
|
Sweats |
3 (18) |
|
Dysuria or frequent urination |
3 (18) |
|
Rhinorrhea or nasal congestion |
2 (12) |
|
Sore throat |
2 (12) |
Table 2. Clinical Findings at the Time of Admission in 17 Patients with Hantavirus Infection.
|
SIGN |
PERCENTAGE OF PATIENTS |
MEDIAN (RANGE) |
|
Respiratory rate ≥20/min |
100 |
28 (20–70) |
|
Heart rate ≥100 bpm |
94 |
120 (90–150) |
|
Temperature ≥38.1°C |
75 |
38.8 (35.4–40.4) |
|
Systolic blood pressure ≤100 mm Hg |
50 |
100 (70–130) |
|
Crackles or rales on lung examination |
31 |
|
|
Abdominal tenderness |
24 |
|
|
Cool, clammy, or mottled skin |
18 |
|
|
Injection or suffusion of conjunctiva |
18 |
|
creased anion gap at the time of hospitalization. Although minimal abnormalities of renal function were common, serum creatinine levels did not rise above 2.5 mg per deciliter (220 µmol per liter) in any patient (Table 3). The mean (±SD) specific gravity of urine at the time of admission was 1.024±0.010 (median, 1.024; range, 1.006 to 1.040). Six of 15 patients (40 percent) had proteinuria (≥2+) on admission. Urine dipstick tests for blood were positive in 8 of 14 patients (57 percent) tested at the time of admission; microscopical examination showed 0 to 5 red cells per high-power field in 3 patients and 6 to 30 red cells per high-power field in 4.
The initial chest radiograph showed interstitial or interstitial and alveolar infiltrates in 11 patients (65
Table 3. Results of Laboratory Studies during Hospitalization in Patients with Hantavirus Infection.
|
TEST* |
ADMISSION VALUE |
MAXIMAL [MINIMAL] VALUE |
|
|
median (range) |
|
|
White cells—×10−3/mm3 |
10.4 (3.1–65.3) |
26.0 (5.6–65.3) |
|
Band forms—% |
22 (8–62) |
27 (4–67) |
|
Hematocrit—% |
||
|
Men |
51.3 (49.9–60.0) |
56.3 (49.9–67.6) |
|
Women |
46.4 (35.0–55.8) |
48.5 (36.5–60.3) |
|
Platelets—×10−3/mm3 |
84 (26–320) |
[64] (12–148) |
|
Prothrombin time—sec |
13.0 (11.2–21.1) |
14 (12.6–21.1) |
|
Partial-thromboplastin time—sec |
42.5 (30.0–150.0) |
54.4 (31.0–150.0) |
|
Bicarbonate—mmol/liter |
18 (12–25) |
[14] (8–20) |
|
Lactate dehydrogenase—IU/liter |
362 (209–1525) |
568 (324–1525) |
|
Aspartate aminotransferase— IU/liter |
112 (28–432) |
148 (62–432) |
|
Alanine aminotransferase— IU/liter |
55 (25–148) |
63 (27–149) |
|
Albumin—g/dl |
3.0 (1.5–4.6) |
[2.5] (1.5–3.5) |
|
Blood urea nitrogen—g/dl |
11 (3–23) |
17 (8–32) |
|
Creatinine—mg/dl |
1.1 (0.6–2.5) |
1.4 (0.6–2.5) |
|
Lactate—mmol/liter |
4.4 (2.2–11.0) |
— |
|
Creatine kinase—IU/liter† |
46 (19–1026) |
— |
|
*Maximal normal values: lactate dehydrogenase, 180 to 232 IU per liter; aspartate aminotransferase, 35 to 43 IU per liter; alanine aminotransferase, 35 to 60 IU per liter; lactate, 2.2 mmol per liter; and creatine kinase, 180 to 269 IU per liter. To convert values for blood urea nitrogen to millimoles per liter, multiply by 0.357; to convert values for creatinine to micromoles per liter, multiply by 88.4. †Two of 10 patients tested had elevated creatine kinase concentrations: 1026 IU per liter with an MB fraction of 16 (2 percent) in 1 patient, and 814 IU per liter with an MB fraction of 87 (11 percent) in another patient, who was undergoing cardiopulmonary resuscitation when the sample was obtained. |
||
percent), fluffy alveolar infiltrates in 2 (12 percent), and no abnormalities in 4 (24 percent). Subsequently, 16 patients (94 percent) had rapidly evolving, bilateral, diffuse infiltrates, and 1 patient (6 percent) had interstitial infiltrates confined to the lower lobes. Pleural effusions were noted during the course of the illness in four patients. Eleven of 12 patients (92 percent) who underwent chest radiography and arterial measurement of oxygen saturation at the time of admission had either pulmonary infiltrates or arterial oxygen saturation under 90 mm Hg. In 11 patients, the mean ratio of oxygen tension to inspired oxygen (calculated from the first measurement of arterial blood gas obtained after intubation) was 100 (median, 97; range, 38 to 174).
Tissue and blood specimens were obtained from the majority of patients for culture, serologic studies, and fluorescent-antibody testing to detect infectious agents. No patient had evidence of infection with other pathogens (Wachsmuth K: personal communication) .
Clinical Course
In the group of patients who died, the mean number of days from the onset of symptoms to death was 8 (median, 7; range, 2 to 16). Progressive pulmonary edema and hypoxia requiring intubation and mechanical ventilation developed in 15 patients (88 percent) during the first 24 hours after admission. The clinical course of the illness in patients who did not survive was characterized by pulmonary edema accompanied by severe hypotension (systolic blood pressure less than or equal to 85 mm Hg), frequently terminating with sinus bradycardia, electromechanical dissociation, or ventricular tachycardia or fibrillation. None of the surviving patients had severe hypotension. Hypotension did not appear to be a direct consequence of hypoxia, since several patients with adequate oxygenation had progressive hypotension. Tracheal aspirates of pulmonary secretions from two patients were tested for total protein, albumin, and lactate dehydrogenase concentrations; in both patients the levels were elevated, approaching or exceeding serum levels.
In four of the five patients with Swan-Ganz catheters inserted after the onset of pulmonary edema, the wedge pressure was normal or low and the cardiac indexes were markedly decreased; the fifth patient (58 years old, with a history of atherosclerotic cardiovascular disease and myocardial infarction) had an elevated wedge pressure (Table 4). Information on fluid balance was available for nine patients, seven of whom had a positive balance at the time of death. Two surviving patients who were initially in positive balance underwent spontaneous diuresis during their recovery. Hemodynamic and pulmonary deterioration paralleled the development of metabolic acidosis, rising serum lactate dehydrogenase levels, declining serum albumin levels, prolongation of the prothrombin time and the partial-thromboplastin time, and de-
creasing platelet counts. With the exception of microscopical hematuria, there were no signs of hemorrhage in any patient.
Pathological Studies
The pathological findings consistently showed large, serous pleural effusions with severe edema of the lungs. No retroperitoneal effusions were present. Microscopical studies of lung tissue showed intraalveolar edema with scant-to-moderate numbers of hyaline membranes and scant-to-moderate numbers of interstitial lymphoid infiltrates (Fig. 2). In well-preserved tissue specimens, pneumonocytes were largely intact, and neutrophils were notably scarce. There was no evidence of a viral cytopathic effect or viral inclusions.
Mild splenomegaly was present in a few patients, although lymphadenopathy was not seen. However, atypical mononuclear cells were consistently seen in the periarteriolar and red-pulp regions of the spleen and in the paracortex of the lymph nodes. In some patients, atypical mononuclear cells were also found in the hepatic triads; in other patients, the liver appeared to be normal. A few patients had small amounts of gastrointestinal hemorrhage. Other viscera, including the kidneys, heart, and brain, were grossly normal and without major microscopical abnormalities.
Predictors of Mortality
A univariate analysis with the use of a logistic model showed no significant correlation between mortality and either symptoms before admission or laboratory abnormalities or physical findings at the time of admission. However, three multivariate logistic models with measurements obtained during hospitalization showed a significant association between mortality and the following combinations of maximally increased laboratory values: the hematocrit and lactate dehydrogenase level (P<0.005), the hematocrit and partial-thromboplastin time (P<0.002), and the white-cell count and partial-thromboplastin time (P<0.002). All three models predicted mortality with 100 percent sensitivity and specificity.
DISCUSSION
Illness resulting from infection with this newly described member of the genus hantavirus is typically severe, characterized by prodromal fever, myalgia, and other symptoms followed by pulmonary edema and hypotension. Because of the characteristically prominent pulmonary involvement, we have designated this illness the hantavirus pulmonary syndrome. The main distinguishing feature of this syndrome is noncardiogenic pulmonary edema. Al though an infrequent complication of previously described hantavirus infections, pulmonary edema has been associated with a high rate of mortality.12,13 The case fatality rate was 76 percent in our series (83 percent of the deaths occurred within nine days after
Table 4. Results of Initial Hemodynamic and Pulmonary Studies in Five Patients.*
|
PATIENT No. |
CARDIAC INDEX |
SYSTEMIC VASCULAR RESISTANCE |
PCWP |
PEAK INSPIRATORY PRESSURE |
PULMONARY-ARTERY PRESSURE† |
OUTCOME |
|
|
liters/ min/m2 |
dyn · sec · cm−5 |
mm Hg |
cm H2O |
mm Hg |
|
|
1 |
1.9 |
1268 |
8 |
32 |
29/16 |
Survived |
|
2 |
3.5 |
812 |
2 |
33 |
11/6 |
Survived |
|
3 |
1.6 |
2701 |
7 |
31 |
38 (mean) |
Died |
|
4 |
1.9 |
1857 |
5 |
NA |
46 (mean) |
Died |
|
5 |
1.8 |
1598 |
28 |
30 |
33/16 |
Died |
|
*PCWP denotes pulmonary-capillary wedge pressure, and NA not available. †Pulmonary-artery pressure is expressed as systolic/diastolic pressure. |
||||||
the onset of symptoms), as compared with 5 to 15 percent for severe hemorrhagic fever with renal syndrome and 1 percent for disease due to the Puumala virus.14
The most likely explanation for the pulmonary findings is an increased permeability of the pulmonary capillaries. An immunohistochemical analysis revealed widespread endothelial distribution of viral antigen in the lungs, kidneys, heart, pancreas, adrenal glands, and skeletal muscle (CDC: unpublished data). The pulmonary manifestations of hantavirus pulmonary syndrome may be due to a direct cellular effect of viral infection, the presence of viral antigen in pulmonary-capillary endothelium, or a virus-induced immune-mediated response. There is evidence of a widespread increase in vascular endothelial permeability in patients with hemorrhagic fever with renal syndrome.15,16 Whether the pulmonary edema associated with hantavirus pulmonary syndrome is a manifestation of a localized pulmonary process or of a more widespread increase in vascular permeability is unclear. The absence of edema in other tissue on clinical examination and at autopsy suggests
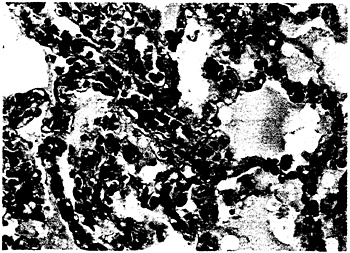
Figure 2. Lung-Tissue Specimen from a Patient with Hantavirus-Associated Interstitial Pneumonitis.
The specimen shows minimal or moderate interstitial lymphoid-cell infiltrates, congestion, and intraalveolar edema (hematoxylin and eosin, ×285).
that the process is localized. Findings that are consistent with a systemic process, however, include the distribution of viral antigen and atypical mononuclear cells throughout the body and the occurrence of proteinuria and hematuria, suggesting renal vascular involvement.
The absence of a marked local response of the pulmonary inflammatory cells distinguishes this syndrome from a variety of other infectious processes. Although the pulmonary findings meet the current criteria for adult respiratory distress syndrome,17,18 the atypical histopathologic features, including minimal numbers of neutrophils within the alveolar or interstitial spaces, minimal alveolar epithelial disruption, and limited hyaline-membrane formation, may indicate a distinct pathogenesis.19,20 Although all the patients with severe disease had abnormal oxygenation, hemodynamic deterioration and death occurred in several patients whose arterial oxygen tension had been adequately maintained.
Many early symptoms of hantavirus pulmonary syndrome are also seen in hemorrhagic fever with renal syndrome. Abdominal pain, which can mimic an acute abdomen, is a prominent feature of hemorrhagic fever with renal syndrome and was present in four of the patients with hantavirus pulmonary syndrome. In patients who have hemorrhagic fever with renal syndrome, severe pain in the abdomen, back, and costovertebral angle is thought to be due to extensive extravasation of plasma into the retroperitoneal and peritoneal spaces,13 which was not seen at postmortem examination in the patients we studied. In two of our patients with abdominal pain, computed tomographic scanning or ultrasound examination did not show abnormal fluid collection in the retroperitoneal space or elsewhere. Renal involvement was minimal, and none of our patients had the marked proteinuria followed by oliguria and renal insufficiency that is characteristic of severe hemorrhagic fever with renal syndrome.5,13 Similarly, our patients did not have erythematous flushing, periorbital edema, severe costovertebral-angle tenderness, or such hemorrhagic manifestations as petechial and conjunctival hemorrhage, all of which are commonly seen in patients who have hemorrhagic fever with renal syndrome.
Laboratory features common to hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome include leukocytosis with increased myeloid elements and atypical lymphocytes, hemoconcentration, thrombocytopenia, coagulopathy, decreased serum protein concentrations, and proteinuria.21,22 In hemorrhagic fever with renal syndrome, coagulopathy has been associated with disseminated intravascular coagulation and hemorrhage.23 Although three of the patients with hantavirus pulmonary syndrome had evidence of increased fibrinolysis, their fibrinogen levels were normal, and with the exception of microscopical hematuria, signs of hemorrhage were not observed. Elevated serum lactate dehydrogenase and aminotransferase concentrations were common in our series and have been reported previously in patients with hantavirus infection.12
Currently, no combination of symptoms, signs, or routine laboratory tests can reliably establish or eliminate the diagnosis of hantavirus infection in people with characteristic clinical signs and symptoms of that illness. Although an elevation of the serum lactate dehydrogenase concentration can be a nonspecific indicator of cellular death, an elevated hematocrit and a prolonged partial-thromboplastin time may be more directly related to the pathophysiology of hantavirus infection.
The initial clinical experience suggests that the key components of therapy for patients with hantavirus infection are maintenance of adequate oxygenation and careful monitoring and support of hemodynamic functioning. Pressor or inotropic agents should be administered in combination with careful volume replacement to treat symptomatic hypotension or shock while avoiding volume expansion and overhydration.
An understanding of the pathophysiology of hantavirus infection, especially the mechanisms underlying the increase in pulmonary and systemic endothelial permeability and vascular tone, may lead to targeted therapeutic interventions. Although data are being collected on the role of immunologic therapy and mediators of inflammation and vascular function in patients with the adult respiratory distress syndrome and septic shock,24 little information has been published on this approach to the treatment of hantavirus infection.25,26 In one controlled study of patients who had hemorrhagic fever with renal syndrome, intravenous administration of the antiviral agent ribavirin reduced mortality when the drug was given early in the course of the illness.27 Since June 4, 1993, intravenous ribavirin has been available through an investigational protocol to treat patients with possible hantavirus infection.
Hantaviruses have been isolated from several species of rodents found in both rural and urban settings in the United States.28–30 Human infection with these viruses has also been documented,31 although the seroprevalence data suggest that humans in the United States have a low risk of infection.32,33 Cases of hanta-virus infection continue to be identified, however, and the geographic area where infections occur is expanding. As of December 31, 1993, 53 cases of hantavirus pulmonary syndrome in 14 states have been confirmed.34 Until the epidemiology of this infection is better understood, prevention strategies, such as behavioral or lifestyle modification and control of rodents, will continue to be based on the epidemiology of other hantavirus infections.35
We are indebted to the Special Pathogens Laboratory, Division of Viral and Rickettsial Diseases, Centers for Disease Control and Prevention, for contributions to this study; to the physicians and nurses in New Mexico, Arizona, Colorado, and Utah who participated in the care of the patients; to H.Lipman for statistical support; and to L.McIntyre for editorial assistance.
APPENDIX
*The Hantavirus Study Group includes M.Burkhart, N.Kalishman, R.Voorhees, J.Voorhees, M.Samuel, M.Tanuz, L.Hughes, S.Wictor, G.Oty, L.Nims, S.Castle, B.Bryt, and C.M.Sewell— New Mexico Department of Health; P.Reynolds and T.Brown— New Mexico Environment Department; L.Sands, K.Komatsu, C.Kioski, K.Fleming, J.Doll, C.Levy, T.M.Fink, P.Murphy, B.England, M.Smolinski, B.Erickson, W.Slanta, and G.Gellert —Arizona Department of Health Services; P.Schillam and R.E. Hoffman—Colorado Department of Health; S.Lanser and C.Nichols—Utah Department of Health; L.Hubbard-Pourier— Division of Health, Navaho Nation; J.Cheek, A.Craig, R.Haskins, B.Muneta, S.John, J.Kitzes, J.Hubbard, M.Carroll, R.Wood, C.North, P.Bohan, and N.Cobb—Indian Health Service; R.Zumwalt and P.McFeely—New Mexico Office of the Medical Investigator; H.Levy, G.Mertz, S.Young, K.Foucar, B.Hjelle, J.McLaughlin, S.Allen, and S.Simpson—University of New Mexico Hospital; T.Merlin—Lovelace Medical Center; and M.Schmidt, L.Simonsen, C.Vitek, C.Dalton, R.Helfand, P.Ettestadt, J.Tappero, A.Khan, L.Chapman, R.Pinner, K.Wachsmuth, A.Kaufmann, J.Wenger, and J.McDade—Centers for Disease Control and Prevention.
REFERENCES
1. Outbreak of acute illness—southwestern United States, 1993. MMWR Morb Mortal Wkly Rep 1993;42:421–4.
2. Haemorrhagic fever with renal syndrome; memorandum from a WHO meeting. Bull World Health Organ 1993;61:269–75.
3. McKee KT Jr, MacDonald C, LeDuc JW, Peters CJ. Hemorrhagic fever with renal syndrome—a clinical perspective. Mil Med 1985;150:640– 7.
4. Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean Hemorrhagic fever. J Infect Dis 1978;137:298–308.
5. Lee HW. Korean hemorrhagic fever. Prog Med Virol 1982;28:96–113.
6. Antoniadis A, Le Duc JW, Daniel-Alexiou S. Clinical and epidemiological aspects of hemorrhagic fever with renal syndrome (HFRS) in Greece. Eur J Epidemiol 1987;3:295–301.
7. Gligic A, Dimkovic N, Xiao SY, et al. Belgrade virus: a new hantavirus causing severe hemorrhagic fever with renal syndrome in Yugoslavia. J Infect Dis 1992;166:113–20.
8. Giles RB, Sheedy JA, Ekman CN, et al. The sequelae of epidemic hemorrhagic fever: with a note on causes of death. Am J Med 1954;16:629– 38.
9. Tsai TF, Bauer SP, Sasso DR, et al. Serological and virological evidence of a Hantaan virus-related enzootic in the United States. J Infect Dis 1985; 152:126–36.
10. LeDuc JW, Smith GA, Johnson KM. Hantaan-like viruses from domestic rats captured in the United States. Am J Trop Med Hyg 1984;33:992–8.
11. Nichol ST, Spiropoulou CF, Morzunov S, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 1993;262:914–7.
12. Bruno P, Hassell LH, Brown J, Tanner W, Lau A. The protean manifestations of hemorrhagic fever with renal syndrome: a retrospective review of 26 cases from Korea. Ann Intern Med 1990;113:385–91.
13. Sheedy JA, Froeb HF, Batson HA, et al. The clinical course of epidemic hemorrhagic fever. Am J Med 1954;16:619–28.
14. Johnson K. Hantaviruses. In: Evans AS, ed. Viral infections of humans: epidemiology and control. 3rd ed. New York: Plenum Press, 1989:341–50.
15. Earle DP. Analysis of sequential physiologic derangements in epidemic hemorrhagic fever: with a commentary on management. Am J Med 1954;16:690–709.
16. Lukes RJ. The pathology of thirty-nine fatal cases of epidemic hemorrhagic fever. Am J Med 1954;16:639–50.
17. Petty TL. Adult respiratory distress syndrome: definition and historical perspective. Clin Chest Med 1982;3:3–7.
18. Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg 1982;144:124–30.
19. Simon RH, Ward PA. Adult respiratory distress syndrome. In: Gallin JI, Goldstein IM, Snyderman R, eds. Inflammation: basic principles and clinical correlates. 2nd ed. New York: Raven Press, 1992:999–1016.
20. Rinaldo JE, Rogers RM. Adult respiratory-distress syndrome: changing concepts of lung injury and repair. N Engl J Med 1982;306:900–9.
21. Gajdusek DC. Virus hemorrhagic fevers: special reference to hemorrhagic fever with renal syndrome (epidemic hemorrhagic fever). J Pediatr 1962;60: 841–57.
22. Lee HW. Hemorrhagic fever with renal syndrome in Korea. Rev Infect Dis 1989;11:Suppl 4:S864–S876.
23. Lee M, Kim BK, Kim S, et al. Coagulopathy in hemorrhagic fever with renal syndrome (Korean hemorrhagic fever). Rev Infect Dis 1989;11:Suppl 4:S877–S883.
24. St John RC, Dorinsky PM. Immunologic therapy for ARDS, septic shock, and multiple-organ failure. Chest 1993;103:932–43.
25. Obukhova GG. Kinen system components and blood serum proteinase inhibitors in hemorrhagic fever with renal syndrome. Vopr Med Khim 1980;26:118–20. (In Russian.)
26. Yang CW, Bang BK. Changes of serum levels of tumor necrosis factor-alpha in patients with hemorrhagic fever with renal syndrome. J Cathol Med Coll 1992;45:819–30. (In Korean.)
27. Huggins J, Hsiang C, Cosgriff T, et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis 1991;164:1119–27.
28. Childs JE, Korch GW, Glass GE, LeDuc JW, Shah KV. Epizootiology of Hantavirus infections in Baltimore: isolation of a virus from Norway rats, and characteristics of infected rat populations. Am J Epidemiol 1987;126: 55–68.
29. Lee PW, Amyx HL, Yanagihara R, Gajdusek DC, Goldgaber D, Gibbs CJ Jr. Partial characterization of Prospect Hill virus isolated from meadow voles in the United States. J Infect Dis 1985;152:826–9.
30. Tsai TF, Bauer SP, Sasso DR, et al. Preliminary evidence that Hantaan or a closely related virus is enzootic in domestic rodents. N Engl J Med 1992;307:623–4.
31. Yanagihara R, Gajdusek DC, Gibbs CJ Jr, Traub R. Prospect Hill virus: serologic evidence for infection in mammalogists. N Engl J Med 1984;310: 1325–6.
32. Yanagihara R. Hantavirus infection in the United States; epizootiology and epidemiology. Rev Infect Dis 1990;12:449–57.
33. Update: outbreak of hantavirus infection—southwestern United States, 1993. MMWR Morb Mortal Wkly Rep 1993;42:441–3.
34. Hantavirus pulmonary syndrome—United States, 1993. MMWR Morb Mortal Wkly Rep 1994;43:45–8.
35. Hantavirus infection—southwestern United States: interim recommendations for risk reduction. MMWR Morb Mortal Wkly Rep 1993;42(RR-11):1–13.







