1
Introduction
In the universe of addictions, opiate addiction, for which heroin is the primary drug of abuse, is and has been a major part of the general drug abuse problem. For nearly three decades, methadone has been the primary means of treating opiate addiction and its effectiveness for this purpose is well-established. Yet the use of methadone for the treatment of opiate addiction on a long-term (or maintenance) basis has been limited and controversial and remains so. This controversy is reflected in the fact that it has been regulated more extensively in this country than any other therapeutic drug.
Methadone, like all other prescription drugs, is regulated by the Food and Drug Administration (FDA) of the Department of Health and Human Services (HHS) under the Federal Food, Drug, and Cosmetic Act. Because it is classified as a narcotic drug1 with some potential for abuse, methadone is also regulated like, other potent opiates, by the Drug Enforcement Administration (DEA) of the Department of Justice under the Controlled Substances Act. But
unlike any other prescription drug and any other controlled substance, methadone—when used to treat opiate addiction—has also been subjected to a third layer of federal regulations. These regulations govern in great detail how physicians may—and may not—care for opiate-dependent patients and are enforced by federal agents.
The special methadone regulations flow from two statutory requirements that the Secretary of HHS (formerly the Secretary of Health, Education, and Welfare) issue standards of treatment for narcotic addiction. Section IV of Title I of the Comprehensive Drug Abuse Prevention and Control Act of 1970 (Public Law 91-513) charged the Secretary to "determine the appropriate methods of professional practice in the medical treatment of the narcotic addiction of various classes of narcotic addicts."
Section 3 of the Narcotic Addict Treatment Act of 1974 (Public Law 93-281) reiterated this authority and expanded the charge. It required practitioners who dispensed narcotic drugs to individuals for maintenance or detoxification treatment to obtain an annual registration from DEA. DEA was directed to register such applicants if, among other things, they were determined by the Secretary of HHS to be qualified "under standards established by the Secretary" to engage in the treatment activity.
Registration also required a determination by DEA that the applicant would comply with DEA's physical security and record-keeping requirements and with HHS's standards, developed in consultation with DEA, regarding "the quantities of narcotic drugs which may be provided for unsupervised use by individuals in such treatment." "Unsupervised use" refers to take-home medication.
The focus of all these laws and regulations is a pharmaceutical agent. Methadone hydrochloride (6-dimethylamino-4, 4-diphenyl-3-heptanone hydrochloride) is a synthetic opiate developed in Germany during World War II as an alternative to morphine. It was approved by FDA in 1947 as a medication for analgesic and antitussive (relieving or preventing cough) uses.
In the mid-1960s, methadone was shown to be effective in the treatment of opiate addiction and thereafter became widely available for this use. Today, an estimated 115,000 individuals receive methadone treatment for opiate addiction, and many thousands more have received it over the past two decades.
There are two basic types of methadone treatment—detoxification therapy and maintenance therapy. Detoxification therapy involves the use of methadone to reduce the symptoms of acute abstinence (or withdrawal) following cessation of opiate use. Methadone maintenance therapy involves the use of methadone on a sustained basis to reduce or eliminate compulsive opiate use by substituting a drug that produces long-lasting activation of opioid receptors in the brain without causing uncontrolled craving effects or interfering with
normal functioning in society. The term "methadone treatment" is used throughout this report to refer to the use of methadone (the drug) to treat opiate addiction, typically through a combination of dispensing medication and providing counseling and related health services.
The Charge to the Committee
In 1992, the U.S. Public Health Service asked the Institute of Medicine (IOM) to evaluate the standards issued by the Secretary of HHS for narcotic addiction treatment pursuant to the 1970 and 1974 statutes and the regulation of methadone treatment programs pursuant to those standards. This is the report of the IOM committee convened to conduct that study.
The study was supported by the National Institute on Drug Abuse (NIDA) and the Center for Substance Abuse Treatment (CSAT) of the Substance Abuse and Mental Health Services Administration (SAMHSA). The FDA also participated in monitoring the study, and the Office of the Assistant Secretary of Health coordinated liaison between the project and these agencies.
The committee's view of the statement of work evolved over its first three meetings as it deliberated about the nature and purpose of the subject. Consequently, the committee revised its charge at its third meeting as follows:
The committee will study the current Department of Health and Human Services standards for narcotic addiction treatment and the regulation of methadone treatment programs pursuant to those standards: (1) it will evaluate the effects of federal regulations on the provision of methadone treatment services and will explore options for modifying the regulations to encourage optimal clinical practice; (2) it will consider the effects of the regulations on the development of new antinarcotic medications; (3) it will assess the impact of the regulations relative to other factors affecting the provision of treatment services; and (4) it will examine alternatives to the existing regulations.
The above language then guided the committee through the duration of its study. It was subjected to a series of refinements of understanding as a result of the study process.
The principal departure of the revised charge from the initial work statement was to defer, without prejudice, the original suggestion for consideration of an "outcomes" approach to federal regulation. The methadone regulations have been criticized as being "process-oriented" and requiring compliance with administrative procedures that bear no relation to outcomes. Although an ''outcomes" approach might be useful, the committee believes that the current treatment system lacks the institutional infrastructure, including data systems, to support such a far-reaching step. The committee's recommen-
dations, therefore, are ones that can be acted upon now on the basis of existing empirical data; they are made in the interests of improving the current regulations. In addition, the committee has attempted to place the role of regulations and other instruments of public policy—clinical practice guidelines and formal quality assurance systems—in the context of more adequately fulfilling the responsibility of the Secretary for treatment "standards."
In approaching its charge, the committee limited the scope of the study to the special federal regulation of methadone, primarily to FDA regulations and to a lesser extent DEA regulations implemented under the authority of the Secretary of HHS to issue standards of treatment for narcotic addiction. Although the committee also examined how several state governments regulate methadone, it did not evaluate these regulations. Often state and local regulations impose significantly more severe constraints on the delivery of methadone treatment services than does the federal government.
Objectives of Opiate Addiction Treatment
The committee distinguished three objectives that guide the use of methadone in the treatment of opiate addiction. Although the relative importance of these objectives has shifted over time, the committee believes that all three should continue to inform policy.
The first objective of treatment is to reduce the severity of the addiction and the compulsive self-injection of heroin and thus allow the addict to establish or restore and maintain an acceptable level of medical and social functioning. Achieving this objective is complicated by the variability within the addict population with respect to simultaneous use of other addictive substances, other mental health problems, unattended medical problems (including HIV-related illnesses, sexually transmitted disease, multiple drug resistant tuberculosis, violence-related injuries, and malnutrition), low education, high unemployment, and weak family structure.
Heroin-addicted individuals are large consumers of costly emergency health care resources because they attend to their health needs on an episodic, crisis-oriented basis. Many are in poor health, and engage in high-risk behaviors that expose them to communicable diseases and violence. Moreover, they are often uninsured, and so do not attend to routine health problems. As a result, they often utilize hospital emergency services as their primary health care provider, which is costly and inefficient. When successfully treated with methadone, such individuals experience improved general health associated with a reduction in both drug use and high-risk behaviors. However, use of traditional medical resources remains difficult for these individuals, for these individuals, at least partly owing to real or perceived stigmatization of the
addict and the methadone patient. On-site, or easily accessible and accepting, primary health care offers a solution to this problem.
If able to work, untreated heroin addicts are often so preoccupied with satisfying their addiction that they are unable to cope effectively with workplace responsibilities. By contrast, it is clear that many heroin-addicted individuals, when stabilized in methadone treatment programs, are able to obtain and maintain employment, develop access to health care resources and benefits, and care for themselves and their families.
The second objective of treatment is reducing crime and enhancing public safety. Treatment that removes individuals from consumption of illicit drugs also reduces criminal behavior motivated by the addict's need to support his or her drug purchases. Patients in methadone maintenance treatment programs have been shown to have markedly reduced criminal activity compared to their pretreatment behavior (Anglin and McGlothlin, 1985; Anglin and Powers, 1991; Anglin et al., 1989; Ball and Ross, 1991). Compared to drug-free treatment, methadone increases the likelihood of engaging and remaining in treatment, which is in turn correlated with the reduction of criminal activities.
The third objective of treatment is safeguarding public health, including the health of persons who do not abuse drugs, especially with regard to reducing the transmission of the HIV virus (which results in AIDS) and the transmission of other infectious diseases such as hepatitis and tuberculosis. This objective is served to the event that the at-risk addict population ceases or reduces intravenous injections of heroin, needle sharing, and sex-for-drugs transactions, and encounters physicians, nurses, counselors, and other public health workers who are able to treat a range of medical problems and reinforce healthy behaviors in an outpatient setting.
These three concerns—for individual functioning, public safety, and public health—provide the rationale for methadone treatment. They anchor the policy discussion in the recognition that multiple and competing objectives are being pursued. This recognition should help to achieve the appropriate trade-offs that lead to sound and effective public policy.
Effectiveness of Methadone Maintenance Treatment
The effectiveness of methadone treatment of opiate addicts has been examined in many studies conducted over three decades. The early reports of Dole, Nyswander, Cushman, and others established the safety and pharmacological efficacy of methadone in the treatment of opiate dependence (Dole, Nyswander, 1968; Gearing, Schweitzer, 1974; Dole, Nyswander, et al., 1982). Since that time the clinical effectiveness of methadone maintenance has been
evaluated in over 300 published reports (Hubbard, Marsden, et al., 1986; Sells, Demaree, et al., 1979). Even though there has been considerable variability in the methodology and results of these studies, the weight of evidence is that about 25–45 percent of opiate addicts who begin maintenance treatment continue successfully for a year or more (Hubbard, Marsden, et al., 1986; Sells, Demaree, et al., 1979).
Beneficial Outcomes
Once established on an adequate dose (usually 60–120 mg per day; see chapter 7 for discussion of dosing), methadone-maintained patients show improvement in a number of outcomes (Dole, Nyswander, 1968; Gearing, Schweitzer, 1974; Sells, Demaree, et al., 1979; Dole, Nyswander, et al., 1982; Hubbard, Marsden, et al., 1986). First, consumption of all illicit drugs declines. There are reductions in the frequency of heroin use to less than 40 percent on average of pretreatment levels during the first treatment year, as some addicts discontinue entirely, others reduce their use only slightly. Further reductions are achieved for patients who continue treatment for two or more years, eventually reaching 15 percent on average of pretreatment levels (Sells, Demaree, et al., 1979; Cummings, 1979; Newton, 1979; Rounsaville, Weissman, et al., 1982; Woody, Luborsky, et al., 1983; Khantzian, Treece, 1985; Wrangle, Corty, Ball, 1987; McNeil-Lehrer, 1988; Ball, Ross, 1993). In addition, crime is reduced, fewer become HIV-positive, and individual functioning is improved.
Three studies discussed below illustrate these points, although several large-scale evaluations of methadone maintenance all show essentially the same results (Dole, Nyswander, 1968; Gearing, Schweitzer, 1974; Hubbard, Marsden, 1986; Sells, Demaree, et al., 1986; Ball, Ross, 1991). In the most detailed examination of methadone maintenance treatment programs to date, Ball and Ross (1991) showed that methadone maintenance was associated with significant reductions in illicit drug use and particularly crime. They evaluated twelve methadone maintenance programs in three northeastern cites, using two samples of newly admitted or stabilized patients, each sample obtained during 12 months over a five-year period.
Although there were differences in methadone treatment effectiveness as a function of the severity of the patient's condition, the number of services provided during treatment, and particularly the medication dose (larger doses producing better outcomes), the overall conclusion was that across all programs, methadone maintenance was associated with significant reductions in use of heroin and non-opiate drugs. There were also substantial reductions in crime from a rate of 237 crime days per year per 100 addicted persons
during an average year of their addiction, to 69 crime days per year per 100 patients during the years of methadone maintenance—a reduction of over 70 percent from pretreatment levels. This is illustrated in Figure 1-1.
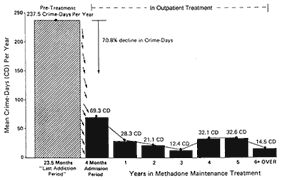
Figure 1-1
Reduction in crime by years in methadone maintenance treatment
SOURCE: Ball and Ross (1991), p. 205.
In a second study, Metzger et al. (1993) show the public health benefits of methadone treatment. They compared the rates of AIDS risk behaviors (particularly injecting drug use and needle sharing) between samples of opiate addicts in methadone maintenance treatment and those not in treatment. This study was interesting for two reasons. First, the two samples were recruited from the same neighborhoods and in fact the same groups social networks: patients in methadone maintenance were asked to refer friends and/or relatives who lived in the same communities and knew the same people, who had been using opiates at least three times per week, and who had been out of opiate treatment for at least the past year. Thus, the two samples were quite comparable with respect to histories of opiate use, demographic characteristics, and peer relationships.
In addition, this study followed both samples every six months for 36 months, achieving a 95 percent contact rate each time. Further, each follow-up evaluation included a blood test for HIV. Results were quite remarkable, as can be seen in Figure 1-2. At baseline, 13 percent of methadone-maintained opiate addicts were HIV-positive, compared with 21 percent of the out-of-
treatment opiate addicts. Over the following three years, an additional 5 percent of methadone-maintained opiate addicts became infected (interestingly, only those who dropped out of treatment). Among out-of-treatment opiate addicts, an additional 26 percent became infected over the same time period. These data do not prove that methadone was the causal agent generating the differences in infection rates, but they do suggest that participation in methadone treatment was at least one factor in the reduction of AIDS risk behaviors. This conclusion does not ignore the fact that self-selection by the treatment population may account for important differences.
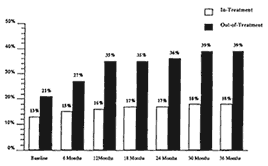
Figure 1-2
Three-year HIV infection rates by treatment status at time of enrollment.
SOURCE: Metzger et al. (1993).
The third study evaluated the contribution of counseling and psychosocial services in methadone maintenance treatment, and shows the effect of these services on outcome. McLellan et al. (1993) randomly assigned newly admitted methadone maintenance patients, all of whom received basically the same methadone dose (the average dose was 65 mg/day and the range was 58–70 mg/day) to receive one of three levels of counseling and other services. (See also chapter 7.)
Level One patients received no other services except emergency counseling. Level Two patients received the same methadone dose plus standard contingency-based counseling.
In this group, patients' drug use was monitored by the counselor using weekly urine screenings. Patients who showed no drug use were not required
to meet regularly while patients with positive urine screens were required to meet more frequently. No other services were provided. Level Three patients received the same counseling as the level Two patients but, in addition, had the availability of on-site medical care, family therapy, employment counseling, and psychiatric treatment.
Results of the six-month study indicated that level One patients did reduce their opiate use ''as measured by urine screenings" but only by half as much as the other two groups. In fact, 69 percent of the level One group had eight consecutive positive urines. This was defined a priori as treatment failure and was used as a criterion for removing patients from the methadone-only condition and transferring them to standard treatment (i.e., methadone plus counseling). Only forty-one percent of level Two patients and 19 percent of level Three patients met this definition of treatment failure.
Figure 1-3 shows level One patients had the poorest outcomes in nearly all areas measured, especially in cocaine use, crime, and unemployment. Level Two patients showed significantly better outcomes than Level One patients in opiate and cocaine use and needle sharing. Level Three patients had significantly better outcomes than level Two patients relative to reduced cocaine use and needle sharing, and greater employment. These data indicate that although the dosage of methadone is extremely important and that adequate doses by themselves can produce significant reductions in pre-treatment levels of opiate use, greater reduction of heroin use was obtained through counseling and other services, and medication in conjunction with counseling and other psychosocial services produces much greater social rehabilitation.
These studies demonstrate the benefits of methadone maintenance. Still, two major factors limit its effectiveness. These are, first, the multiple health and social problems of methadone maintenance patients, and second, the variability in the quality of treatment program management and services.
Factors Limiting Effectiveness
Multiple Problems of Methadone Maintenance Patients
Although drug use (especially use IV drug) is the major focus of methadone treatment, it is only one of many complications seen in patients applying for methadone treatment. For example, studies by Rounsaville et al. (1982), Khantzian and Treece (1985), and Woody et al. (1983) have documented the high proportion of psychiatric diagnoses among methadone patients. Metzger and Platt (Ball, Ross, 1991; Metzger, Woody, et al., 1993) have shown the extreme frequency of unemployment and deficits in job-seeking skills among a large proportion of these individuals. Finally, studies by Stanton
and his colleagues have documented the serious family and personal difficulties found in opiate-dependent patients being treated with methadone (McLellan, Arndt, et al., 1993).
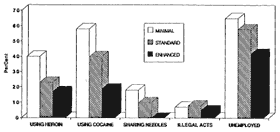
Figure 1-3
Methadone services: Target behaviors at six months by level of service. Minimal = methadone only, counseling in emergencies; standard = methadone plus standard counseling; and enhanced = standard plus psychosocial services. SOURCE: McLellan et al. (1993).
The multiple complexities of the methadone patient population significantly affect the course and overall results of treatment (Hubbard, Marsden, 1986; Sells, Demaree, et al., 1979; McLellan, Arndt, et al., 1993; Woody, McLellan, et al., 1987). For example, opiate addicts who have been stabilized by methadone maintenance but have experienced little improvement in their psychiatric, family, and employment situation will always remain in danger of relapse to injecting drug use. This of course has potentially large economic implications for any administrative decisions regarding the expansion of methadone treatment services. For example, a program of methadone maintenance alone, without counseling and other services, may be the least expensive way of effecting at least some improvement in drug use and AIDS-related behaviors. But, it is also possible that a more enhanced and expensive program, providing effective treatment for the medical, psychiatric, family, and employment difficulties of these patients, may be more cost-effective than the less expensive program in reducing AIDS related behaviors, encouraging rehabilitation, reducing crime, and controlling demands for welfare benefits and expensive medical services.
Variability among Methadone Maintenance Programs
The second factor limiting the effectiveness of methadone maintenance treatment is the variability among programs. Ball and Ross (1991) showed dramatic differences among methadone treatment programs in such fundamental outcome measures as proportion of opiate-positive urines and number of visits to the program. In general, the patients treated in these programs were quite similar in terms of their demographic characteristics and treatment problems at the time of admission to methadone maintenance across the different geographic sites surveyed. In contrast, the data gathered on the programs themselves revealed major differences in almost all areas of organization and service provision; and these program variables have been closely associated with patient outcome. For example, with respect to methadone dosage, one program had an average methadone dose of 20–25 mg while other programs averaged 55–60 mg per day. Moreover, the data show a clear inverse relationship between level of methadone dose and level of heroin consumption in the prior 30 days. Programs also differed sharply in the provision of access to medical care for their patients, use of ancillary psychotherapeutic medications (e.g., antidepressants and antipsychotics), uniformity of enforcement of rules, counselors' case loads, psychiatric input, quality of inservice training and physician facilities.
Why Methadone is Regulated Differently from Other Drugs
If methadone is effective, why it is regulated so highly and so differently from other drugs? That question requires us to consider how it is actually regulated. First, the manufacturing, labeling, and dispensing of methadone are subject to FDA's general requirements for establishing the safety, effectiveness, and consistent quality of virtually all prescription pharmaceuticals. Second, because methadone is a narcotic, its production, distribution, and dispensing are also subject to the DEA requirements applied to schedule II controlled substances to prevent diversion and illicit use. These are the most rigorous requirements imposed on legitimate medicines that have a potential for abuse. Many useful and important therapeutic agents are subject to this dual system of control, including morphine, codeine, medicinal cocaine, amphetamines, and short-acting barbiturates.
In the case of methadone, however, a unique third tier of regulation is imposed: Special standards have been established prescribing how, and under what circumstances, methadone may be used to treat narcotic addiction (21 CFR 291.501 and 291.505). These standards are set and implemented by FDA,
under authority vested in the Secretary of HHS. (Under a delegation of authority from the Secretary, FDA exercised its authorities in the issuance of regulations jointly with NIDA during the 1980s and, in 1993, with SAMHSA.) The regulations, issued by FDA in December 1972, were designed to create a special "closed system" of distribution and use of methadone, effectively restricting distribution to hospital pharmacies and to physicians registered with both FDA and DEA who could authorize dispensing of the medication in a treatment program only. This three-tiered system of federal regulation has continued for over 20 years and has recently been extended to a methadone analog, levo-alpha-acetyl-methadol (LAAM).
It is also essential to note that methadone treatment of opiate addiction is restricted still further by many state governments. Although a model uniform state statute for controlled substances abuse was promoted in the 1970s, individual states rejected or deviated from this model when enacting their own statutes in ways that permitted a fourth tier of regulation on use of methadone, often significantly more restrictive than federal regulation. The report summarizes state rules in five jurisdictions—New York, California, Massachusetts, Illinois, and Florida. Unfortunately, a comprehensive description of the authorities and agencies of the states that govern medications that may be used for treatment of opiate addiction does not exist. To complicate the picture even further, a fifth tier of regulatory authority over methadone treatment programs is sometimes found at the county and municipal level.
No other medication is so highly regulated. There are three basic reasons for this unique regulatory status. First, methadone (and, by extension, LAAM) is the only opiate authorized to be used to treat opiate addicts. Therefore, its intended use creates a potential for abuse different from that of other controlled substances insofar as providing methadone to patients on a regular basis creates special opportunities for diversion. The initial regulations were written in part, to respond to real abuses and to the perceived threat of diversion of methadone into illicit channels. For reasons spelled out below, the committee believes that the benefits both to the individual patient and to society at large from authorizing greater clinical discretion in methadone treatment far outweigh the risks from diversion, but we recognize the existence of both a real threat and considerable public fear of diversion.
Second, once regulations are adopted, bureaucratic inertia inhibits revision. Initially adopted in 1972, the regulations were modified in 1980, again in 1989, and changed twice in 1993. There does not appear, however, to have been any effort to reexamine the underlying assumptions or long-term consequences of the regulations, until this committee review was requested.
Third, there is the matter of attitudes. Neither public nor professional attitudes nor those of the addict community have provided strong support for methadone treatment. In this connection, the federal regulations have served
function of providing local communities and society at large with some assurance that public and professional reservations about methadone were being addressed. In short, the regulations exist, in part, to garner support for methadone treatment.
The committee believes it is important to be realistic about the matter of attitudes if recommended policies are to be implemented effectively. We therefore expand somewhat on this point, as follows:
With respect to public opinion, a substantial segment of public opinion over the years has opposed the use of methadone for the treatment of opiate addiction, and another segment is ambivalent about its use. Public attitudes toward addiction of any type, but particularly heroin addiction, are overwhelmingly negative. The debate over the extent to which addiction is a disease or a moral failure remains unsettled in the public mind. The stereotypes of addicts are of individuals engaged in criminal activity, predatory toward others, and unable or unwilling to respect the norms of acceptable social behavior or participate in the work force. The public's fear of opiate addicts creates a reluctance to spend ''treatment" dollars on them; it also creates sympathy for a criminal justice response.
Public attitudes have strongly affected the number and location of treatment clinics. The effort to open a methadone treatment clinic often arouses intense local opposition from the prospective neighbors, both poor and middle class. Instances abound of local community groups barring the opening of such clinics, and forcing clinics to close or move out of neighborhoods. In 1993, for example, a new methadone treatment program opened in New York was one of only three the state has opened in the last 20 years.
The attitudes of the public—and those of drug treatment professionals are also divided regarding the pharmacologic treatment of addiction on a maintenance basis. Negative attitudes towards maintenance pharmacotherapy are being tempered somewhat, the committee believes, by two factors. One is that few individuals in the general population have escaped some encounter with drug addiction, either in themselves or through a family member, friend, neighbor, or work-place associate. The other factor is that, over time, there has been a growing acceptance of the use of medications on a long-term basis for medical and psychological conditions as diverse as high blood pressure, chronic depression, or diabetes.
In general, members of the medical profession often share many of the negative attitudes of the general public. Many are either indifferent or hostile to its use for the treatment of heroin addiction. Ignorance about its effectiveness may stem from the fact that methadone maintenance historically has been poorly linked to the provision of primary and specialized medical care and to mental health services, both of which are often needed by patients.
To complicate matters still further, drug treatment providers themselves are often divided philosophically on the value of methadone maintenance. For example, an antagonistic relationship exists between methadone maintenance and those with a drug-free or substance-free philosophy. Indeed, the ranks of methadone treatment providers include those who support use of maintenance treatment and others who use methadone only for detoxification followed by drug-free therapies. These different approaches to treatment have not been reconciled, and are not easily addressed on strictly scientific grounds.
Finally, the addict population itself is ambivalent toward methadone treatment programs. Participation in methadone treatment involves a great loss of autonomy. The continued dependence on methadone makes the patient vulnerable to threats or actual withholding of the drug for valid or invalid reasons. Many addicts are unwilling to give up such autonomy until they reach a situation so desperate that they feel they have no choice. Others, especially those with jobs or other scheduled responsibilities, find it too burdensome to attend the clinic daily, as present regulations require.
The Committee's View
The current regulations are designed to restrict distribution of methadone intended for therapeutic use, and to establish and enforce standards of medical care utilizing methadone, in order to prevent diversion of methadone into nonmedical channels and to exclude inappropriate persons from methadone treatment. In the committee's view, these regulations are predicated on a belief that the societal risks are so great, and the societal benefits so limited, that extraordinary controls are necessary above and beyond those applicable to any other therapeutic drug in the United States. This belief might have been valid and useful in the late 1960s and early 1970s, when experience with methadone was not extensive and the social risks associated with heroin use seemed largely confined to the addicts themselves.
Having studied the issues during a 18 month period (described below), the committee concludes that contemporary circumstances are dramatically different from those of 20 or more years ago. We know a lot more today about the risks and potential misuse of methadone. Although the drug can be abused, it is rarely a primary drug of abuse. Its therapeutic effectiveness has been clearly demonstrated, alone and in conjunction with other treatment activities. Untreated opiate addiction has been shown to be closely linked with other major public health and safety problems, including violent crime, AIDS, drug resistant tuberculosis, and hepatitis, disorders that can then jeopardize the health of those who have never used illicit drugs. Methadone treatment has been shown to reduce these health problems which are endemic in the addict
community. Thus, methadone is no longer seen as a treatment designed to alleviate only the addict's problems, but also as a partial solution to a complex set of public health and safety issues.
In light of these considerations, the committee urges reassessment of the appropriate balance between the risks of methadone and its benefits. The current regulations foster situations where addicts cannot obtain a treatment program tailored to their individual circumstances, physicians are unable to exercise professional judgment in treating individual patients, programs are isolated from mainstream medical care (thus depriving patients of important ancillary services), and significant economic costs are incurred in assuring compliance with regulatory requirements—costs that are shared by programs, insurers, patients, and taxpayers.
We have concluded that there is no compelling medical reason for regulating methadone differently from all other medications approved by FDA, including schedule II controlled substances. Nevertheless the committee is not recommending abolition of the methadone regulations. The regulations serve important functions, not the least of which is to maintain community support for methadone treatment programs by assuring that the programs maintain standards and are subject to outside review.
What the committee does recommend is a careful readjustment of the regulatory controls so that communities can attain the full potential benefit of this effective means of treating opiate addiction and its associated problems of crime, disease, and disorder, without increasing the risks of diversion and misuse. In sum, current policy puts too much emphasis on protecting society from methadone, and not enough on protecting society from the epidemic of addiction, violence, and infections that methadone can help reduce.
Report Organization
The report is organized around the questions that occupied the study committee. These questions pertain mainly to the use of methadone and, to some extent LAAM, for the treatment of opiate addiction. In addition, the use of methadone for analgesic purposes is discussed in Chapter 7 in the context of the unanticipated effects of the regulations on pain management. Chapter 2 begins by addressing the question "what is methadone?" describes the drug, the physiology of opiate addiction, and the pharmacology, safety, and rationale of the use of methadone in treating such addiction.
Chapter 3 presents an epidemiological perspective on the heroin addict population, the dominant group among opiate addicts, and deals with the characteristics of methadone-maintained individuals, who are drawn from this larger pool. The chapter, in essence, asks, "Who are the direct beneficiaries of
methadone maintenance treatment?" As indicated above, the committee believes that society—in terms of public safety and public health—benefits indirectly from the effectiveness of methadone in helping opiate addicts directly. The report sets forth the benefits of methadone treatment in this chapter and in chapter 2. Although chapter 3 presents extensive data on New York and California, the scope of the research informing the study and the committee members' own knowledge and experience was far broader.
In chapter 4, the risks of diversion of methadone are discussed. The attention devoted to this subject derives from the fact that concern for diversion has loomed large historically and continues to play an important part in policy discussions about the use of methadone for opiate addiction treatment.
The manner in which methadone is regulated, that is, the way in which the balance has been struck between the benefits and risks of methadone treatment, is addressed in chapter 5. Historically, treatment standards have been implemented by exclusive reliance on regulations; the chapter describes the evolution of the current regulations.
Chapter 6 examines how methadone treatment is being delivered today. Attention is given to the providers of treatment, to treatment financing, and to the activities of five state substance abuse agencies.
Chapter 7 presents the changes the committee believes should be made in existing regulation to improve treatment. To describe the characteristics of good treatment, the chapter focuses on clinical concerns such as access to treatment, comprehensive services, adequate dosing, integration with primary care and other social services, flexibility to respond to changes in drug use within the patient population and to changing requirements for service intensity of individual patients, and respect for patient rights.
Chapter 8 examines alternative ways that standards might be implemented. These ways include regulations and the associated issues of enforcement, clinical practice guidelines and formal quality assurance systems. The chapter also considers federal leadership in research, federal-state relations, financing, and policy guidance. The committee emphasizes that it is not enough simply to recommend changes in the regulations without also addressing these interrelated issues of funding, access, quality assurance, mainstreaming of care, and greater acceptance of methadone maintenance treatment by insurers, the medical community, politicians, and the public. Regulations are not sufficient to ensure good service delivery or good medical care for patients; other commitments are necessary.
Study Methods
The committee used a number of approaches to inform itself about the issues in this study. It met five times over a eighteen-month period to hear presentations by government officials, to give its members an opportunity to report on their own experiences, and to discuss the issues raised in this volume. All meetings, save the last, were two days in length. Project staff and a number of committee members attended the National Methadone Conference of November 1992 in Orlando, Florida, and the National Methadone Conference of April 1994 in Washington, D.C. The latter afforded an opportunity for approximately half of the committee to hold a brief evening meeting—the "sixth," strictly speaking. Except for this last occasion and the fifth meeting, all meetings, were open and were attended by representatives of the government agencies involved and other interested parties.
The committee chairman and several members attended and participated in the June 1993 meeting of the College on Problems of Drug Dependence, in Toronto. Site visits involving committee members and project staff were made to methadone clinics in Washington, D.C., Baltimore, New York, Boston, and Cambridge, Massachusetts.
In addition, half-day meetings were held between a small group of IOM committee members and IOM staff and representatives of various government agencies. One such meeting was with the staff of the FDA's inspection, compliance, and enforcement effort to familiarize the committee with these aspect of the regulations. Another such meeting was held with representatives of the DEA. This meeting, in turn, resulted in a subsequent visit to DEA offices by the IOM study director for follow-up information. A third meeting was held with the officials of the NIDA Medications Development Division. Project staff also had regular interaction with officials of NIDA, SAMHSA/CSAT, FDA, and DEA. The committee chairman and the study director met once with Mr. Peter Edelman, Counsel to the Secretary of HHS responsible for substance abuse policy, and once with Dr. Lee Brown, Director of the Office of National Drug Control Policy.
One paper was commissioned, which became the basis for chapter 6. Computer runs of data used in chapters 3, 4, and 6 were provided to the committee by the Drug Use Forecasting group of the National Institute of Justice, of the Department of Justice, by the Community Epidemiology Branch of NIDA, by the Office of Applied Studies of SAMHSA, and by the New York Office of Alcoholism and Substance Abuse Services. A series of special computer runs dealing with heroin addiction were performed using the data of the Epidemiological Catchment Area study, arranged by Dr. Lee Robins, a committee member.
Visits by the study director, accompanied by one or several committee members, to New York and Boston resulted in meetings with methadone program administrators in these states. The IOM requested that the New York Committee of Methadone Program Administrators (COMPA) provide it with a commentary on the regulations, which COMPA did to the committee's great benefit. Similarly, through Commissioner Marguerite Saunders, of the New York Office of Alcoholism and Substance Abuse Services, and a committee member, the state methadone authorities were asked for comment and complied in about half the cases.
A Note on Terminology
As chapter 3 in this report points out, there is no official psychiatric diagnosis termed "opiate addiction," and instead Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), offers four related diagnoses: opiate dependence, opiate abuse, opiate intoxication, and opiate withdrawal; several symptoms whose presence may warrant a diagnosis of dependence, according to DSM-IV, are described. A detailed definition of addiction may be found, however, in Dorland's Medical Dictionary, as chapter 3 also points out; this definition specifies four symptoms that are characteristic of addiction.
Notwithstanding these and other variations to be found among contemporary authoritative sources, this committee employs the concept of addiction and related concepts, as follows:
Addiction is a condition distinguished by a lack of control and compulsion that leads to illicit or inappropriate drug-seeking behavior. It can occur with or without physical dependence. Opiate addiction has been operationally defined as at least one year of daily repeated opiate self-administration, with development of tolerance, physical dependence, and drug-seeking behavior (see chapter 2). Chapter 3, whose purpose is to describe the recipients of methadone treatment, lays stress on that aspect of addiction whereby the addict finds it impossible to stop using opiates without help.
Tolerance is the condition that results from continued use of an opiate drug (or of any substance) and that makes it necessary to use increasing amounts of the drug to produce the desired physiological and psychological effect.
Physical dependence results from repeated use of a drug, such as an opiate; the physiological systems adapt to such use to the point that they actually require the drug just to maintain physiological equilibrium. Although physical dependence is associated with tolerance, the latter can occur without
causing physical dependence. The withdrawal symptoms associated with dependence are described in chapter 2.
An important distinction made in this report is that tolerance and physical dependence do not equal addiction. Chapter 7 discusses this distinction and the related definitions, and in addiction defines several other terms that belong to the lexicon of methadone treatment.
Finally, another distinction made in this report is between the terms ''narcotic" and "opiate." Because "narcotic" refers to a stupor-inducing drug and is a legal term that includes cocaine (see detailed footnote earlier in this chapter), the committee reserves its use for discussion having a legal or statutory context. In all other discussion we use the term "opiate.'' The committee finds the distinction an important one and incorporates it into its recommendations.
References
Anglin, MD, and McGlothin, WH. 1985. Methadone maintenance in California: A decade's experience. In L. Brill and C. Winick (eds.), The Yearbook of Substance Use and Abuse 3:219–280.
Anglin, MD, and Powers, KI. 1991. Methadone treatment and legal supervision: individual and joint effects on the behavior of narcotics addicts. Journal of Applied Behavioral Science 27(4):515–531.
Anglin, MD, Speckart, GR, Booth, MW and Ryan, TM. 1989. Consequences and costs of shutting off methadone. Addictive Behaviors, 14:307–326.
Ball, JC, and Ross, A. 1991. The Effectiveness of Methadone Maintenance Treatment: Patients, Programs, Services, and Outcomes. New York: Springer-Verlag.
Comments of Representative Rangle C (D-NY). 1988. McNeil-Lehrer Report, Public Broadcasting System (February 9).
Corty, E and Ball, JC. 1987. Admissions to methadone maintenance: Comparison between programs and implications for treatment. Journal of Substance Abuse Treatment 4(3):181–187.
Cummings, N. 1979. Turning bread into stones: Our modern anti-miracle. American Psychologist 34:276–239.
Dole, VP, Nyswander, M. 1968. Successful treatment of 750 criminal addicts. Journal of the American Medical Association 206:2708–2710.
Dole, VP, Nyswander, M, and Des Jarlais D. 1982. Performance-based rating of methadone maintenance programs. New England Journal of Medicine 306:169–172.
Gearing, FR and Schweitzer, MD. 1974. An epidemiological evaluation of long-term methadone maintenance. American Journal of Epidemiology 100:101–105.
Hubbard, RL and Marsden, ME. 1986. Relapse to use of heroin, cocaine and other drugs in the first year after treatment. In Relapse and Recovery in Drug Abuse. NIDA Research Monograph 72, USGPO, Rockville, Md.
Khantzian, EJ and Treece, K 1985. DSM-III psychiatric diagnosis of narcotic addicts. Archive of General Psychiatry 42:1067–1071.
McLellan AT, Arndt LO, Woody GE, Metzger D. 1993. Psychosocial Services in Substance Abuse Treatment? A dose-ranging study of psychosocial services. The Journal of the American Medical Association 269(15):1953–1959.
Metzger DS, Woody GE, McLellan AT, Druley P, DePhillips D, O'Brien C, Stolley P and Abrutyn E. 1993. HIV seroconversion among in and out of treatment intravenous drug users: An 18-month Prospective Follow-up. AIDS 6(9):1049–1056.
Newton, J. 1979. Methadone promise is unfulfilled. Journal of Drug/Alcohol Abuse (December 1):1–2.
Rounsaville, BJ, Weissman, MM, Wilber, CH. 1982. The heterogeneity of psychiatric diagnosis in treated opiate addicts. Archive of General Psychiatry 39:161–166.
Sells, SB, Demaree, RG and Hornick, CW. 1979. Comparative Effectiveness of Drug Abuse Treatment Modalities, NIDA Services Research Administrative Report, Washington, D.C.
Woody GE, Luborsky L, and McLellan AT, et al. 1983. Psychotherapy for opiate addicts: Does it help? Archive of General Psychiatry 40:639–645.
Woody, GE, McLellan, AT, Luborsky, L, et al. 1987. Psychotherapy for opiate dependence: A 12 month follow-up. American Journal of Psychiatry 145:109–114.
Woody, GE, McLellan, AT, Luborsky, L, et al. 1984. Severity of psychiatric symptoms as a predicator of benefits from psychotherapy: The Veterans Administration-Penn Study. American Journal of Psychiatry 141:(10)1172–1177.




















