6
Methadone Treatment
This chapter describes the characteristics of methadone treatment providers, the financing of treatment, and how several states with large addict populations regulate and finance treatment. It draws on several data bases, each of which has certain limitations. These limits are discussed below as appropriate.
The Provider Community
This section describes the characteristics of providers of outpatient methadone maintenance programs–the number of treatment facilities, the regional distribution of facilities, the number of patients, and the capacity, utilization, waiting time, and ownership.
Methadone is used currently to treat opiate addiction by detoxification and maintenance. Detoxification treatment accounts for 8 percent of all methadone patients nationally (Batten et al, 1993), but for a much higher percentage in California (see below and chapter 3). Methadone maintenance treatment is normally provided by an outpatient treatment program. Programs have four common features: a dispensing unit, counseling offices, examining rooms, and an administrative area (Ball and Ross, 1991). They often vary with regard to methadone dosing, take-home policies, and other treatment factors (General Accounting Office, 1990; Ball and Ross, 1991; D'Aunno and Vaughn, 1992). In addition to dispensing medication, programs also provide counseling and other medical services.
Number of Treatment Facilities
FDA, in its 1994 registry of programs approved to dispense methadone under the Narcotic Addict Treatment Act, documents 780 active programs app-
roved for methadone maintenance and 275 approved for detoxification in calendar year 1993 (Food and Drug Administration, 1993; E. Dory, FDA, personal communication, 1994). The data on maintenance programs may overestimate the total: FDA counts as a ''program" an individual dispensing site, and large programs often may have more than one site; and once approved by FDA, a program remains listed until the agency learns of its closure, which may not occur until it seeks to arrange a biennial inspection.1
The Office of Applied Studies of the Substance Abuse and Mental Health Services Administration (SAMHSA), also conducts an annual (since 1992; biennial during 1987–1992) survey of methadone facilities called the National Drug and Alcoholism Treatment Unit Survey (NDATUS), data are reported by drug abuse and alcoholism treatment providers to their respective state agencies, which forward it to SAMHSA. The response rate has been between 80 and 85 percent. In this survey, methadone "units" are defined at state discretion. The term may refer to an individual facility or to a program consisting of a several separate facilities. This definition was adopted in 1987 to ease the states' reporting burden by allowing them to report data for an entire program, regardless of the number of clinical sites (or facilities), on a single survey form. The data on the number of patients in these units and the capacity and utilization of the units are unaffected by the manner in which units are counted.2
In 1992, NDATUS reported a total of 574 units (D. Melnick, SAMHSA, unpublished data, 1994). This was a decline in its unit count of nearly 10 percent over the period since 1987, from 633 to 574 units, which may be due to the reporting system. In 1992, FDA reported 737 active programs. FDA data show an increase in the number of active dispensing sites of 16 percent from
671 in 1987 to 737 in 1992 (E. Dory, FDA, personal communication, 1994). No reconciliation of these two counts has been attempted.
Regional Variation
The NDATUS data show strong regional variation in the distribution of methadone facilities and patients (based on the 1992 client census). Almost half of all U.S. methadone patients are found in the Northeast (46.6 percent); the West has 24.3 percent of all methadone patients; and the South and Midwest have the smallest percentages, with 16.1 percent and 13 percent, respectively (see Table 6-1). On a per capita basis, the Northeast has the highest rate of methadone patients at 94 per 100,000 population, followed by the West at 46, the Midwest at 22, and the South at 19 (see Table 6-1).
TABLE 6-1 Regional Distribution of Methadone Patients, 1992
|
Region |
Number of Patients |
Percentage of Total Patients |
1992 Resident Populationa |
Patients per 1,000 Populationb |
|
West |
25,124 |
24.3 |
55,108,000 |
0.46 |
|
Midwest |
13,453 |
13 |
60,713,000 |
0.22 |
|
Northeast |
48,230 |
46.6 |
51,118,000 |
0.94 |
|
South |
16,687 |
16.1 |
88,143,000 |
0.19 |
|
Total |
103,494c |
100 |
255,082,000 |
0.41 |
|
a Bureau of the Census, 1993. b This rate is based on the total regional resident population. A rate based on an opiate-addicted population might be different. c This figure excludes Puerto Rico. SOURCE: NDATUS, Office of Applied Studies, SAMHSA. |
||||
Underlying these regional differences are even greater differences by state. In 1992, California and New York, with 18,3313 and 31,730 patient,4 respectively, accounted for almost 50 percent of all methadone patients in the United States. Following these two, the states with the largest client censuses were Illinois, New Jersey, Massachusetts, Florida, Maryland, Michigan, and Texas. By contrast, the following 10 states did not report any methadone patients to NDATUS in 1992: Arkansas, Idaho, Maine, Mississippi, Montana, North Dakota, New Hampshire, South Dakota, Vermont, and West Virginia (D. Melnick, SAMHSA, unpublished data, 1994).
Number of Patients
In 1993, the most recent year for which national estimates of methadone patients were available, an estimated 117,000 patients received methadone treatment (Harwood et al., 1994). This figure is calculated from client counts reported to NDATUS in 1991, adjusted for the 82 percent response rate to the survey. A 1990 survey estimated the national total at 112,943 patients receiving methadone, of whom approximately 92 percent of patients were considered to be in maintenance and 8 percent in detoxification (Batten et al., 1993).
The total number of methadone patients has increased in the past five years, according to NDATUS. Between 1987 and 1992, the total number of patients grew by 27.6 percent (see Table 6-2). The client census was relatively stable between the mid 1970s and the mid 1980s, seesawing between 70,000 and 80,000 patients (IOM, 1990). The growth in patients since 1987 may be attributed to many factors—an increase in the survey response rate from 78 percent in 1987 to 85 percent in 1992, efforts to contain drug-related crime, an expanded public health effort related to the AIDS and tuberculosis epidemics, and to a concern for drug-related infant mortality. In addition, federal block grant expenditures for drug treatment grew during this period, accompanied by mandates to the states to ensure greater access to treatment (see below).
Capacity and Utilization Rate
Paralleling an increasing number of patients was a reported increase of 47.6 percent in the average capacity (number of slots) of the methadone units reporting to NDATUS. The average number of methadone patients per unit (shown in Table 6-3) also increased by 40.8 percent. It is unclear whether these data mean that facilities are expanding to accommodate more patients, or that units are being consolidated administratively for reporting purposes, or both.
TABLE 6-2 Estimated Number of Methadone Patients by Facility Ownership, 1987–1992
|
Facility Ownership |
1987 |
1989 |
1991 |
1992 |
% Rise 1987–1992a |
|
Private, for-profit |
13,278 |
16,625 |
23,697 |
25,565 |
92.5 |
|
Private, not-for-profit |
51,096 |
56,338 |
54,969 |
60,428 |
18.2 |
|
State/local govt. |
15,107 |
17,778 |
14,924 |
16,070 |
6.3 |
|
Federal govt. |
2,371 |
1,493 |
1,696 |
2,440 |
2.9 |
|
Total |
81,852 |
92,234 |
95,286 |
104,503 |
27.6 |
|
NOTE: Point prevalence estimates were taken on September 30, 1987, September 30, 1989, September 30, 1991, and September 1992. Excluded are units that did not report methadone patients and capacity. a These figures must be interpreted with some caution because of some small variation in the nonresponse rate each year and because the number of private programs is incomplete. SOURCE: NDATUS, Office of Applied Studies, SAMHSA. |
|||||
NDATUS provides the only national data about methadone treatment capacity and utilization, important indicators of the availability of treatment.
Capacity, or the number of slots, is defined as the maximum number of active patients who could be enrolled. Utilization is calculated by dividing the number of actual patients by the capacity. These data must be interpreted cautiously, however, because NDATUS's definition of capacity5 does not distinguish between a slot reserved for a private paying client and one for a publicly subsidized client. Private paying patients are believed to be underreported.
Nationwide, 122,442 methadone slots were reported to NDATUS in 1992, an increase from 1987, as can be seen in Table 6-4, paralleling the growth in the number of patients receiving methadone. Because the capacity and the number of patients have grown proportionally, methadone capacity utilization has remained steady at a high rate of 85–90 percent since 1987. Although a useful indicator, NDATUS's capacity utilization rate is not adjusted for differences in unit size. A special analysis of NDATUS's 1991 survey conducted by Lewin-VHI, Inc., yielded a weighted average utilization rate of 91.5 percent (based on 521 methadone treatment units that reported on both patients and capacity) (Lewin-VHI unpublished estimates, 1994).
TABLE 6-3 Size of Methadone Units, 1987–1992
|
|
1987 (633 units) |
1989 (729 Units) |
1991 (527 units) |
1992 (574 units) |
% Increase 1987–1992a |
|
Average capacity per unit |
144.5 |
140.8 |
208.6 |
213.3 |
47.6 |
|
Average number of patients per unit |
129.3 |
126.5 |
180.8 |
182.1 |
40.8 |
|
NOTE: Excludes units that did not report on methadone capacity. a These percentage increases must be interpreted with some caution because the nonresponse rate varied slightly by year and because the number of private programs is incomplete. SOURCE: NDATUS, Office of Applied Studies, SAMHSA. |
|||||
TABLE 6-4 Methadone Patients, Capacity, and Utilization, Nationwide, 1987–1992
|
|
1987 |
1988 |
1991 |
1992 |
|
Patients receiving methadone |
81,852 |
92,234 |
95,286 |
104,503 |
|
Methadone capacitya |
91,495 |
102,646 |
109,906 |
122,442 |
|
Utilization rateb |
89.5 |
89.9 |
86.7 |
85.3 |
|
a Capacity is the maximum number of individuals who could be enrolled as active patients on September 30 of the year of the survey. NDATUS's definition of capacity does not distinguish between slots reserved for private paying patients and publicly subsidized patients. b Rate is number of methadone patients divided by reported capacity. SOURCE: NDATUS, Office of Applied Studies, SAMHSA. |
||||
In California, the utilization rate varies according to whether the slot is reserved for private patients (who have private insurance or pay out-of-pocket) or for subsidized patients (those who receive mostly county, state, federal block grant, or Medicaid funding). California clinic operators identify their authorized slots according to the funding source: they reported in 1989 that 65 percent of the authorized slots were reserved for private patients and about 30 percent of slots were reserved for subsidized patients. The utilization rate (or occupancy rate) for the subsidized slots (95 percent) was higher than that for private slots (83 percent) (Goldstein, 1989), which has resulted in long waiting times for subsidized patients (see below). In New York, by contrast, no difference exists in the utilization rate for private and publicly financed slots, both being uniformly high at about 100 percent in 1994 (V. Fenlon, New York State Office of Alcoholism and Substance Abuse Services, unpublished data, 1994).
In 1992, the average methadone unit in the United States had an unweighted capacity of 213.3 slots and actually treated 182.1 patients, according to NDATUS (see Table 6-3), almost identical to the prior year. When the 1991 data were weighted to adjust for differences in unit size, a different picture emerges about each unit: the average client capacity was 337 slots and the average client census was 308 (Lewin-VHI unpublished
estimates, 1994)6. The large difference between the nonweighted figures reported by NDATUS and those weighted by Lewin-VHI suggests that unit size is not uniformly distributed.
Waiting Lists
Although waiting times for admission to treatment programs led to the inclusion in the ADAMHA Reorganization Act of 1992 of a provision authorizing interim methadone maintenance treatment as a way to increase access to treatment, especially for intravenous drug users at risk of contracting AIDS (see discussion in chapter 5), the national data on waiting lists are not very good.7
Several national surveys on waiting lists suggest strong regional differences.8 In New York City, for example, some facilities do have long waiting lists, which implies administrative or state regulatory limits on slots or program responses to limited financing.
A 1990 survey of client discharge records from drug treatment facilities, including 292 methadone facilities, based on a national sample drawn from NDATUS (Batten et al., 1992), found that 53.9 percent of methadone patients had no waiting time, 6.9 percent had a waiting time of less than seven days, and only 1.5 percent had waiting times of 14 or more days. However, the waiting time was not known or was not mentioned in 36.9 percent of client records. When facility ownership was examined, patients of private for-profit facilities were more likely to have no waiting time compared to those admitted to either private not-for-profit or publicly owned facilities (see Table 6-5).
TABLE 6-5 Waiting Time for Methadone Patients by Facility Type
Waiting times were also addressed in a survey of 172 clinic directors conducted in 1990. The National Drug Abuse Treatment System Survey asked clinic directors to estimate the average waiting time for a client seeking admission to their clinic. Thirty-eight percent of clinics reported no waiting time; less than 7 days' average wait was estimated for 10 percent of the clinics; 7–14 days' average wait was estimated for 19 percent; and 15–95 days for 33 percent (T. D'Aunno, University of Michigan, unpublished data from the Drug Abuse Treatment System Survey). A substantial discrepancy exists between these figures and those in Table 6-5, suggesting the lack of uniform definitions and reporting methodologies.
The General Accounting Office (1990), in a small study of 24 methadone treatment programs, did not find a serious shortage of treatment slots.9 Of the programs they evaluated, 14 did not have waiting lists and the other 10 programs had waits which ranged from one week to three months.
The GAO conclusion is amplified by data from New York and California. These states, which together account for almost 50 percent of U.S. methadone patients, maintain current registries of the number of patients on waiting lists, but not of waiting time. As of January 1994, New York State had 1,122 applicants on waiting lists for all methadone treatment programs, or 3 percent of the 40,292 patients in the state. About one-third of those were waiting for treatment at clinics operated by Beth Israel Hospital in New York City, whereas other facilities had waiting lists of fewer than 100 people (C.V. Fenlon, Office of Alcoholism and Substance Abuse Services, State of New York, unpublished data, 1994). However, New York State regards waiting lists as only one indication of demand for treatment.
California's monthly Drug Abuse Treatment Access Report, in March 1993, listed 2,488 applicants waiting for one of the 13,154 licensed methadone maintenance treatment slots (S. Nisenbaum, California Department of Alcohol and Drug Programs, unpublished data, 1994). Previously, a 1989 survey of all California clinic operators found that 58 percent of clinics kept a waiting list, and 1,077 applicants had waited an average of 6.6 to 7.1 months before entering treatment. Of these applicants, those entering private paying slots waited only 1.5 to 2 months, while those entering publicly funded slots waited 9 to 10 months (Goldstein, 1989). The situation appears to have improved. The state of California now estimates an average wait of 54 days for a subsidized treatment slot and notes that no waiting lists currently exist for private pay slots (S. Nisenbaum, 1994).
These data about waiting lists and times are not easy to interpret. Waiting lists vary regionally and between not-for-profit and for-profit facilities and no uniform definitions guide data collection. Although the data suggest a need for increased treatment capacity, they must be substantially improved in comprehensiveness and quality before they can provide much guidance for policy.
Facility Ownership
There are four general types of facility ownership (DHHS, 1993c): private for-profit facilities owned by an individual, partnership, or corporation; private
not-for-profit facilities owned by a not-for-profit corporation, such as a church, a foundation, or another philanthropic group; state or local government facilities, typically owned by a county or city, although sometimes by a state government; and federal government facilities, most of which are administered by the Department of Veterans Affairs, the Federal prison system, or the U.S. Public Health Service.
NDATUS reports that most of the methadone units are owned by private, not-for-profit organizations. In 1992, there were almost three times as many private, not-for-profit units (321) as State/local units (107) and private, for-profit units (118) (D. Melnick, SAMHSA, unpublished data, 1994).
The share of treatment provided by different ownership types in terms of the number of patients treated is presented above in Table 6-2. The data shown in Table 6-2 yield percentages for 1992 as follows: about 58 percent of the patients were treated in private, not-for-profit units; about 24 percent in private, for-profit units; about 15 percent in state and local government units; and about 2 percent in federally operated units.
On the basis of client census data, the private, for-profit sector has more patients per unit per average than does the not-for-profit sector. This sector has also shown the largest growth of any ownership category in clients served. Table 6-2 shows a pronounced increase of 92.5 percent in the for-profit sector between 1987 and 1992, most of which has occurred in California, Texas, and Florida (see below). More modest growth has occurred in other ownership categories.
Four trends are apparent from this overview. First, the number of clients receiving methadone maintenance is increasing, especially those treated in private, for-profit facilities. Second, the growth in the client population has been accompanied by a growth in treatment capacity, mostly by increases in the size of existing facilities. Third, facility utilization remains high, especially in publicly supported facilities. Fourth, few new programs have been established (owing often to restrictive zoning regulations and community opposition).
Financing of Methadone Treatment
Methadone treatment is financed from a combination of federal, state, and private sources, and the combination varies markedly by state and by program. On the national level, NDATUS collects expenditure data on methadone treatment that are separate from overall alcohol and drug treatment data. Although available on data tapes, these data are not otherwise easily accessible to the public.
Estimates of expenditures for methadone treatment for fiscal year 1993 have recently been made by Harwood and his colleagues at Lewin-VHI (Harwood et al., 1994), using 1991 NDATUS financial information adjusted for inflation. The annual payments from all sources for methadone maintenance treatment is estimated by Harwood et al. at $480 million in FY 1993. This estimate, which does not include inpatient detoxification, assumes that 117,000 patients are receiving outpatient treatment at an average annual treatment cost of $4,100 per patient. Of this amount, the cost of the medication represents a very small fraction, about $4–8 per client per week (P. Coulis, National Institute of Drug Abuse, personal communication, 1994), or about 7 percent of treatment costs. The bulk of treatment costs are for counseling services and other administrative, record-keeping, and regulatory costs. The introduction of LAAM is expected to double the per patient cost of the medication (G. DeVaux, BioDevelopment Corp., personal communication, 1994).
The Lewin-VHI unpublished estimates fiscal year for methadone maintenance for fiscal year 1993 divide the $480 million total cost into three major components (see Figure 6-1). First, public funding accounts for $386 million, or 80 percent of the total, consisting of federal block grant funds, state alcohol and substance abuse funds, Medicaid and local funds. Second, out-of-pocket payments by patients account for $82 million, or 17 percent of the total. Third, private insurance pays for $12 million, or 2.5 percent of the total.
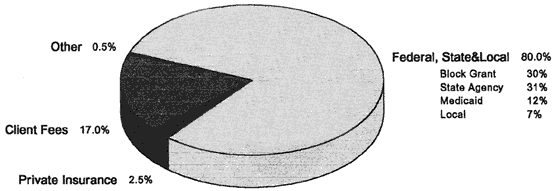
Figure 6-1
Payments for methadone treatment, 1993. Total payments = $480 million. Adapted from 1991 NDATUS by Lewin-VHI. SOURCES: Butynski et al. (1994); Harwood et al. (1994); and DHHS (1993).
Public financing of all substance abuse treatment (for alcohol and drug abuse), which accounts for 80 percent of all financing, includes three components—state agency funding, federal block grants, and Medicaid, all of which are administered by state agencies. Financing from all sources of all state alcohol and drug abuse programs that receive some state funding is surveyed annually in the State Alcohol and Drug Abuse Profile (SADAP), administered since 1987 by the National Association of State Alcohol and Drug Abuse Directors, Inc. (NASADAD). The survey does not include all
programs; it has virtually no data on private for-profit programs, some not-for-profit programs, and Department of Veterans Affairs programs (DHHS, 1993d). Also, data are not disaggregated by treatment modality, such as methadone maintenance. The SADAP survey shows that public financing in fiscal year 1991 accounted for about 78 percent of alcohol and drug abuse expenditures for treatment programs receiving some state support (see data in Table 6-6).
TABLE 6-6 Trends in Financing of State-Supported Alcohol and Other Drug Abuse Services by Largest Funding Sources
|
Year |
Federal Block Grant |
State Alcohol/ Drug Agency |
Private Insurance Client Feesa |
Medicaid and Other Public Sourcesb |
|
1987 |
15% |
45% |
22% |
9% |
|
1988 |
17% |
43% |
20% |
11% |
|
1989 |
20% |
42% |
18% |
12% |
|
1990 |
22.2% |
37.8% |
15.7% |
16.6% |
|
1991 |
29.2% |
34.7% |
15.5% |
13.7% |
|
1992 |
30.2% |
32.8% |
18.1% |
12.3% |
|
NOTE: Data are from the State Alcohol and Drug Abuse Profile (SADAP), administered by the National Association of State Alcohol and Drug Abuse Director, Inc. a Also includes court fines. This column includes the same information as the SADAP category called ''Other Sources." b This column combines the SADAP categories, "Other State Agency" and "Other Federal Government," in order to capture the federal and state shares of Medicaid, which constitute most of these two categories. There are other state and federal funds included in this column, but they do not come from the Federal block grant or from state substance abuse agencies. SOURCE: DHHS, 1993d. |
||||
TABLE 6-7 Federal Block Grant and State Alcohol/Drug Agency Expenditures Reported for State-Supported Alcohol and Other Drug Abuse Services
|
Year |
Federal Block Grant |
State Alcohol/ Drug Agency |
|
1987 |
$272,570,630 |
$819,807,824 |
|
1988 |
$355,025,431 |
$910,134,922 |
|
1989 |
$474,677,106 |
$1,009,099,083 |
|
1990 |
$645,477,081 |
$1,099,253,225 |
|
1991 |
$946,468,801 |
$1,126,634,230 |
|
SOURCE: DHHS, 1993d. |
||
State agency funding, since 1987, has been the largest single component of all state substance abuse expenditures. In 1992, funds from state alcohol and drug agencies accounted for 32.8 percent of all alcohol and drug abuse services according to SADAP (Table 6-6). Valued at about $1.1 billion in 1991 (Table 6-7), this represents the largest single financing component. The state share actually declined from 1987 (45 percent) to 1992 as federal block grants increased more rapidly (Table 6-6).
Federal block grant funding has assumed an increasingly prominent role in the overall financing of substance abuse services. Table 6-6 shows that in 1992 block grants accounted for 30.2 percent of all substance abuse funding, up from 1987, when it accounted for 15 percent of the total. Between 1987 and 1991, state agencies reported an increase in block grant revenues from about $273 million to nearly $1 billion (Table 6-7).10 Since the establishment
of the block grant in 1981, federal legislation has changed its underlying philosophy toward a stronger federal role in monitoring how funds are spent and in holding states accountable.11 The federal block grant requires that states give priority to intravenous drugs users, persons of color, ethnic minorities, women, especially women with children.
Medicaid is an important source of funding for methadone treatment, but one that is extremely difficult to measure (IOM, 1990; Solloway, 1992; Horgan et al, forthcoming). States have great discretion in determining substance abuse coverage and they are not required to report substance abuse expenditures, let alone those for methadone, to the Health Care Financing Administration, which oversees Medicaid.
Nevertheless, estimates are available concerning Medicaid's contribution to treatment. According to SADAP, federal and state Medicaid accounts for, at most, 12.3 percent of all alcohol and drug abuse funding (Table 6-6). An estimate of Medicaid financing specifically for methadone treatment has been made by the Drug Services Research Survey (DSRS) of Brandeis University. This two-phase survey of a national sample of drug treatment facilities (phase I) and client discharge records (phase II) revealed that about 8 percent of total revenues for all drug treatments were from Medicaid (Horgan et al., 1994). When methadone client records were examined, Medicaid was listed as the expected source of payment for 27 percent12 of these patients upon admission (Batten et al., 1992). Medicaid-certified methadone facilities received about 30 percent of their revenues from Medicaid, a percentage greater than nonmethadone treatment facilities that were also Medicaid-certified (Horgan et al., 1994).
Department of Veterans Affairs
The Department of Veterans Affairs (VA) sponsors one of the world's largest networks of hospitals and outpatient clinics. There are an estimated 27 million beneficiaries served at 171 hospitals and 220 outpatient facilities nationwide. Although all veterans and their dependents are eligible for care, the highest priority is accorded to those veterans whose illnesses or injuries are a direct result of military service (i.e., "service-connected veterans") or low income veterans who are not "service-connected." Substance abusers are most
likely to become eligible via the low-income requirement, rather than the service-connected requirement. The VA is obligated to provide hospital and some outpatient care to high priority veterans, and, if space is available, they may provide care to other eligible veterans who do not qualify as high priority. In practice, only a small percentage of lower priority veterans receive care.
In the wake of criticism that the VA has insufficient capacity to treat veterans with substance abuse disorders (IOM, 1990), Congress gave the VA additional appropriations in fiscal year 1990 to expand and enhance treatment capacity. Patients diagnosed with alcohol or drug abuse represent one of the most frequently discharged groups of patients from VA medical centers (VAMCs), second only to those with heart disease (Department of Veterans Affairs, 1991). A total of 26 percent of all VA inpatients were diagnosed with a primary or secondary substance abuse disorder (Peterson et al., 1993). Although much is known about the characteristics and costs of the inpatient substance abuse population, the VA did not collect data until recently about the number of outpatient substance abuse patients, including methadone patients, and their cost of care.
In 1993, the first year for which data were available, a total of 5,886 patients nationwide received methadone maintenance treatment at approximately 36 VAMCs (R. Swindle, Program Evaluation Resource Center, Palo Alto VAMC, personal communication, 1994). These patients are approximately 5 percent of all U.S. methadone patients. Trend data are unavailable. Most VA methadone patients are low-income veterans whose opiate dependence is not service-connected, but who may have another ailment that is service-connected.
The VA estimates that the overall cost of outpatient methadone maintenance treatment was about $22 million in fiscal year 1993 (Department of Veterans Affairs, 1993). This estimate is based on an average outpatient visit cost of $36.65 for all types of substance abuse treatment; the average cost of an outpatient methadone visit, though not determined, it is likely to be lower (R. Swindle, Program Evaluation Resource Center, Palo Alto VAMC, personal communication, 1994). There were over 607,000 outpatient methadone visits in 1993.
The VA plays an important role in research on treatments for drug abuse. Through an interagency agreement with the National Institute on Drug Abuse, the VA sponsors large-scale clinical trials for new medications. For example, the VA was a major participant in the clinical trial that led to the approval of LAAM for opiate addiction.
Out-of-Pocket and Private Insurance
Private funding of methadone treatment consists of patient out-of-pocket expenditures and private insurance through a third party carrier or a prepaid plan. Based on 1991 NDATUS data, Harwood estimates that out-of-pocket expenditures constitute 17 percent of the total, private insurance 2.5 percent (Harwood et al., 1994). All private sources make up nearly one-fifth of methadone treatment expenditures (see Figure 6-1). This percentage is quite similar to the SADAP estimate for all reported substance abuse expenditures.
Patient out-of-pocket payments are a source of treatment financing, although data are not readily available. The DSRS survey, phase II, of client records for all types of drug treatment facilities (described earlier) analyzed the expected source of client payments at the time of admission. Although the sample of methadone patients was small, the findings in Table 6-8 show that 30.8 percent of methadone patients in all facilities were expected to pay for treatment out-of-pocket (''self-pay"). The DSRS survey included a sample of private for-profit facilities. When the methadone client data were stratified by facility type, it was found that 77.2 percent of patients in private for-profit facilities were expected to pay out-of-pocket (Table 6-8), a far greater percentage than patients at other types of methadone facilities.
Data reported by a sample of clinic directors in a 1990 survey reflect differences between for-profit and not-for-profit clinics with respect to client out-of-pocket payment. Fifty-seven percent of private for-profit clinics derive 80 percent or more of their revenues from client out-of-pocket payments. In contrast, 73 percent of private not-for-profit clinics obtain 20 percent or less of their revenues from client payments (T. D'Aunno, unpublished data from the Drug Abuse Treatment System Survey). Similarly, based on an analysis of NDATUS, the IOM (1990) found that 75 percent of revenues at private for-profit facilities were from client out-of-pocket payments.
Private insurance appears to represent a very small component of client financing. The DSRS survey found that 4.4 percent of methadone patients at all facilities were expected at admission to be covered by private health insurance and an additional 1.9 percent by an HMO or other prepaid plan (Table 6-8). In comparison to patients receiving other types of drug treatment, methadone patients were the least likely to have private health insurance coverage. For example, approximately 30 percent of patients in hospital inpatient drug abuse programs were expected to be covered by private health insurance (Batten et al, 1992).
TABLE 6-8 Percentage Distribution of Methadone Patients by Primary Source of Payment and Facility Type
<><><><><><><><><><><><>
The previous two sections have examined the characteristics and the financing of the methadone treatment system. The system has been growing over the last several years, in contrast to relative stability from the mid-1970s to the mid-1980s. Increases occurred in the number of patients, the number of slots, and the number of facilities. The growth is most pronounced in the for-profit sector, which nearly doubled the number of patients between 1987 and 1992. The utilization rate remains exceedingly high, giving rise to waiting lists at many clinics, particularly those that receive large public subsidies. The time spent on a waiting list, which is difficult to evaluate on a nationwide basis, has exceeded several months in some studies. Data about facility utilization, waiting time, and the demand for treatment is needed for policymakers, but good national data on these matters do not currently exist.
Most of the estimated $480 million in total treatment expenditures comes from public sources, such as state substance abuse agencies, federal block grants, and Medicaid. Private out-of-pocket payments account for an estimated 17 percent of the total, and private insurance accounts for the smallest portion, only 2.5 percent of the total (Lewin-VHI, unpublished estimates, 1994).
State Substance Abuse Agencies13
An important role in the overall substance abuse treatment system, including methadone treatment, is played by state governments. The states are engaged in the regulation and financing of methadone treatment; the licensing of practitioners; quality assurance; data collection; and various activities related to the control of illicit drugs, the general use of controlled substances, and the control of the diversion of methadone.
State government substance abuse activities are quite variable. Although states must adhere to the federal methadone regulations, they may also mandate additional, more restrictive, measures. Some of the most common measures adopted by states concern admission criteria, staffing ratios, take-home policies, dosing limits, treatment duration, and patient rights. Not surprisingly, the patterns vary substantially from state to state.
Variation across the states is also apparent in how treatment is financed. States can and do exercise much latitude in public financing of methadone treatment, using an array of federal, state, and local funds, as discussed in the
previous section. The states are not permitted to use federal block grant funds to finance treatment at private, for-profit clinics, however, nor are they usually permitted to use their own state agency funds for this purpose. (These clinics receive most of their revenues from client fees and Medicaid.)
In addition to variability, there is a notable absence of systematic data on state substance abuse programs. Therefore, in this section, we examine how methadone is regulated and financed by five states with large methadone client populations: New York, California, Illinois, Florida, and Massachusetts. (Data for these five states for the period 1987–1992 are shown in Table 6-9.) How these States will regulate and finance LAAM, a newly approved treatment for opiate addiction, is not yet known, as these states have yet to take all of the necessary regulatory steps towards its introduction.
New York
As of October 1994, the state of New York had over 40,000 methadone maintenance patients, the largest patient population in the U.S., and double that of California, the next largest. In New York this patient population is treated in 148 methadone treatment facilities, of which 114 are voluntary (private), not-for-profit clinics and 34 are for-profit clinics. (There are no state-owned or -operated clinics.) One not-for-profit clinic specializes in the treatment of pregnant addicts and another one in the treatment of adolescent addicts. All clinics are licensed and regulated by the New York State Office of Alcoholism and Substance Abuse Services (OASAS). Apparently because of community opposition, only 3 new methadone clinics have been licensed in New York State in the past 20 years (personal communication, Marguerite Saunders, Commissioner, Office of Alcoholism and Substance Abuse Services).
In January 1994, the not-for-profit clinics had a patient census of 30,662, and the for-profits, 9,265 (V. Fenlon, New York State Office of Alcoholism and Substance Abuse Services, unpublished data, 1994.) The average clinic census is 269 patients; in New York City, five clinics have a license capacity greater than 550.
Financing
New York State compiles financing information for voluntary, not-for-profit clinics, for which total expenditures in 1992–1993 were about $139.5 million (see Table 6-10).
TABLE 6-9 Patients Receiving Methadone, Methadone Capacity, and Capacity Utilization Rate in Five States
|
|
Patients Receiving Methadone |
Methadone Capacitya |
Utilization Rateb |
|||||||||
|
|
1987 |
1989 |
1991 |
1992 |
1987 |
1989 |
1991 |
1992 |
1987 |
1989 |
1991 |
1992 |
|
California |
12,052 |
12,828 |
18,352 |
18,331 |
14,139 |
15,129 |
22,426 |
23,812 |
85.2 |
84.8 |
81.8 |
77.0 |
|
Florida |
1,445 |
1,770 |
2,767 |
2,712 |
1,734 |
2,199 |
3,746 |
4,141 |
83.3 |
80.5 |
73.9 |
65.5 |
|
Illinois |
3,170 |
3,250 |
4,717 |
4,775 |
3,945 |
3,657 |
5,452 |
5,436 |
80.4 |
88.9 |
86.5 |
87.8 |
|
Massachusetts |
390 |
1,389 |
3,576 |
3,814 |
502 |
1,876 |
4,144 |
4,397 |
77.7 |
74.0 |
86.3 |
86.7 |
|
New Yorkc |
34,374 |
33,363 |
29,159 |
31,730 |
34,059 |
34,003 |
30,595 |
33,520 |
100.9 |
98.1 |
95.3 |
94.7 |
|
a Capacity is the maximum number of individuals who could be enrolled as active patients on September 30 of each year when the survey was conducted. b Methadone capacity utilization rate is obtained by dividing the number of methadone patients by the capacity. c These figures underestimate the total by approximately 20 percent, according to the associate commissioner for health and planning of the New York State Office of Alcoholism and Substance Abuse Services. For example, the 1992 figure is estimated at 40,000 patients receiving methadone. SOURCE: NDATUS, Office of Applied Studies, SAMHSA. |
||||||||||||
TABLE 6-10 Financing of Methadone Treatment in Publicly Funded Clinics in New York State, Fiscal Year 1992–1993
|
Revenue Source |
Amount |
Percent of Total |
|
OASAS state aida |
$ 20,441,600 |
14.6 |
|
Federal block grant |
$ 20,964,500 |
15.0 |
|
Medicaid |
$ 87,142,500 |
62.4 |
|
Patient fees |
$ 3,630,900 |
2.6 |
|
Local tax |
$ 2,940,800 |
2.1 |
|
All other |
$ 4,427,400 |
3.3 |
|
Total Financing |
$139,547,700 |
100 |
|
NOTE: Publicly funded clinics are private not-for-profit clinics. Data on private insurance revenues are not included, but are considered to be negligible by state administrators. a Office of Alcoholism and Substance Abuse Services. SOURCE: Addie Corradi, Associate Commissioner, Health and Planning Services Division, New York State OASAS. |
||
Medicaid accounts for 62.4 percent of this total, far higher than the national average of 12 percent, followed by the federal block grant at 15 percent and the OASAS state aid at 14.6 percent. Patient fees accounted for an estimated 2.6 percent of treatment costs. Although data were not available on private insurance, it is considered negligible by state administrators. The average cost of treatment in New York State is estimated at $4,500 per client per year.
New York has liberal eligibility requirements for Medicaid and financing comes from federal, state, and local governments. Consequently, Medicaid expenditures for methadone maintenance are not evenly divided between the state and the federal government. In fact, state and local funds constitute 80 percent of all Medicaid expenditures because many patients are not eligible for federal matching funds but are eligible under more generous state criteria.
Patients in treatment programs are generally eligible for federal Medicaid dollars when they qualify for aid for dependent children (AFDC), supplemental security income (SSI), and Medically Needy programs. Yet, because of strict
categorical and income criteria, many patients are ineligible for these programs.
In New York, however, patients may also obtain Medicaid coverage by qualifying for Home Relief, which provides state-only Medicaid funds. Sixty percent of Medicaid-enrolled methadone patients in New York State receive Home Relief, which also provides cash assistance for low-income people and is designed to maintain them in the community.
Recognizing that the New York share of total Medicaid expenditures is unusually high, state administrators are seeking through a pilot program to shift their costs to federal Medicaid. The pilot program helps disabled patients receiving Home Relief to qualify for SSI and thereby become eligible for federal Medicaid.
All voluntary, not-for-profit clinics qualify for state agency funds, for whom the state is the ''payer of last resort." Once Medicaid, client fees, and private insurance have been obtained, the remaining costs at these publicly funded clinics are covered by state Office of Alcoholism and Substance Abuse Services funds and federal block grant funds so they do not operate at a deficit.
Regulations
New York State methadone regulations are similar to the federal regulations, but diverge in four important areas: staffing ratios, urine testing, client rights, and central registries. The state also provides certain additional services: since 30 percent of patient admissions to methadone clinics are HIV-positive, and thus at high risk for tuberculosis, New York sponsors active methadone clinic-based programs for the onsite delivery of tuberculosis medication.
With respect to staffing, one full-time doctor is required for every 300 patients. Two full-time nurses are required for the first 300 patients, but thereafter another full-time nurse is required for each additional 100 patients or fraction thereof. One full-time case worker is required to counsel every 50 patients.
Urine testing is required on a weekly basis for the first three months in treatment. If the testing reveals no indication of drug abuse, patients may be placed on a monthly urine testing schedule. In contrast, the Federal regulations require eight random urine tests during the first year of treatment.
Patients have protections against involuntary termination from treatment. Patients must be informed in writing of the reasons for their discharge and of their right to request a review of the termination decision. They may appeal the decision before the program is allowed to begin the discharge process,
according to OASAS regulations. This discharge process typically involves detoxification treatment with decreasing doses of methadone. During detoxification, the dosage may not be lowered more than 10 mg every three days.
Finally, the regulations mandate a central registry system, which is designed to prevent enrollment of a patient in more than one methadone program. Before an applicant can enroll in a program, the program is required to submit information to the central registry. The registry also maintains up-to-date information about patient discharges and transfers.
California
In California, all state financing, regulatory, and licensing functions related to alcohol and substance abuse are carried out by the Department of Alcohol and Drug Programs. The state of California has the second largest methadone client population in the United States. In 1994, about 20,000 methadone maintenance patients were treated at any one time, including those in temporary treatment slots authorized by the state to respond to the AIDS epidemic. The number of patients receiving methadone grew substantially between 1987 and 1992, increasing by 52 percent (see Table 6-9).
The treatment population size is much smaller than the estimated opiate addict population. Earlier research estimates were that California enrolled fewer than 8 percent of its opiate addicts in treatment (Anglin and McGlothlin, 1985; Goldstein, 1991). More recently, the state of California estimates that approximately 15 percent of opiate addicts are in the treated population (S. Nisenbaum, State of California, unpublished data, 1992).
As of November 1994, the state of California licensed 117 facilities to dispense methadone. Within these facilities are 222 licensed programs, each facility usually having more than one program (defined under FDA regulations as a dispensing site). Among the 222 programs, 117 are outpatient methadone maintenance, 104 are outpatient detoxification, and one is an inpatient detoxification program.
The large proportion of outpatient detoxification programs is unique because in many other states detoxification is usually provided on an inpatient basis or at the end of maintenance treatment. California regulations and financing have encouraged outpatient detoxification programs, in which patients receive tapered methadone doses to bring them to a drug-free state within 21 days, while also undergoing periodic medical evaluation and counseling. Between July 1, 1992, and June 30, 1993, a total of 63,833 episodes of methadone detoxification were provided in approximately 5,500 state-licensed slots (J. Jarfors, State of California, unpublished data, 1994).
State officials are exploring the feasibility of revising its 21-day detoxification regulation to conform to federal regulations that permit long-term detoxification up to 180 days.
California providers include many private, for-profit facilities and programs. Of the 117 methadone facilities in the state, over half are private, not-for-profit (57 percent), but almost a third are private, for-profit (31 percent). A small proportion, 12 percent, are publicly operated by counties; the state does not own or operate any facilities. According to state officials, most new programs are private, not-for-profit.
Financing
While the Department of Alcohol and Drug Programs maintains information on the public funding allocated to methadone programs, a considerable portion of overall expenditures for methadone treatment is not included in the department's figures because as many as 75 percent of treatment slots are fee-for-service, payments for which are not required to be reported to the state. Licensed clinics do report their monthly slot fees in their annual renewal applications. In July 1993, these averaged about $200 a month for methadone maintenance and $248 per 21-day detoxification episode.
Public funding for drug treatment in California has traditionally come from state general funds, federal block grants, and Drug MediCal. The former two sources of funds are combined into a funding stream known as Drug Allocation Money, which is allocated among California counties according to a complex formula and requires a 10 percent match from county revenues. Counties may spend their drug allocation on drug treatment and prevention in any way they choose, provided that they meet any categorical funding stipulations attached to such funds. Thus, counties determine which types of treatment they will provide under their drug allocation money; a number of counties have chosen not to fund methadone programs. Even in counties that do fund methadone treatment, subsidized slots have in recent years largely been limited to patients who are HIV-positive, pregnant women, and other specified categories.
The other major source of public funding for methadone treatment has been Drug MediCal, a program that counties may "opt into" in order to provide drug treatment for individuals who are eligible for MediCal. Drug MediCal differs from regular MediCal. Under regular MediCal, the state matches federal Medicaid dollar for dollar to pay for the medical care of eligible individuals. Under Drug MediCal, before July 1, 1994, the state provided counties with federal Medicaid allocations, which the counties were required to match dollar for dollar from their state generated allocations. As an
incentive to the counties to "opt into" Drug MediCal, the state does not require 10 percent matching on the portion of a county's State Drug Allocation Money that the county uses to match Federal Medicaid funds. Again, the counties have decided which types of treatment will be funded under Drug MediCal.
In 1994, Drug MediCal policies were undergoing change owing to a lawsuit in which the court ruled that persons who qualify for MediCal are entitled to receive methadone maintenance if they otherwise meet eligibility requirements for admission, and a certified program had available capacity. While the details of this new financing system are still being worked out, currently state general funds are being made available to the counties to pay for the treatment of MediCal recipients, including those who qualify for methadone treatment; any funds left over can be used to fund treatment slots for other people in need of treatment who are not MediCal eligible. The full impact of this change will not be known for several years, but it promises to be far-reaching.
Regulations
The state of California has numerous treatment regulations, in effect since 1973, which cover admission criteria, the authorized number of treatment slots, take-home policies, detoxification treatment, staffing, treatment planning, multiple registration, urinalysis, maximum dosage levels, and duration of treatment, all of which are described below.
In order to be admitted into a program, prospective patients must be currently addicted and have a two-year addiction history. This two-year history requirement contrasts with the federal and New York requirement of a one-year history. Potential patients must also have evidence of two prior failures in withdrawal treatment with regimens other than methadone maintenance (California Code of Regulations, Title 9). In response to concerns about HIV transmission, however, California regulations now permit waiver of state admission criteria and allow the use of federal criteria, on a program-by-program basis.
Under the same regulations, the state limits the number of permanent and temporary slots for methadone maintenance and detoxification. A facility can only be licensed for up to 150 permanent detoxification and 300 permanent maintenance slots, and may exceed licensed capacity by 10 percent for emergency admissions. In January 1994, the state licensed 17,639 permanent maintenance and 2,455 temporary maintenance slots. With respect to methadone detoxification, the state licensed 5,337 permanent and 525 temporary slots.
California regulations permit temporary increases in capacity on a program-by-program basis. The licensure of temporary slots and/or the waiver of state admission criteria are recent regulatory responses designed to expand access to treatment primarily because of the HIV and AIDS epidemic. The only difference between a permanent and temporary slot is that the latter has provision for HIV counseling. Patients in temporary slots benefit from the same staffing ratio and counseling requirements as those in permanent slots. (Temporary slots licensed by the state are not the same as slots for ''interim methadone maintenance," which were authorized under the ADAMHA Reorganization Act of 1992 in FDA regulations of January 1993.)
California's regulations are more stringent than federal regulations regarding take-home policies. After the first three months of treatment, federal regulations allow a maximum two-day take-home supply of methadone; California, by comparison, allows a one-day supply. The maximum take-home supply under federal regulations is for six days, if the client has satisfactorily adhered to treatment rules for three consecutive years. In contrast, the California maximum is three days, reached only after two years of treatment.
In terms of staffing, the state requires one physician for every 200 patients and one counselor for every 40 patients. There are no specific licensure requirements for counselors, but they are required to receive ongoing training. Programs are required to review patient treatment plans every 90 days "with the objective of reducing the dosage level." Under federal regulations, treatment plans are required to be reviewed every 90 days during the first year; thereafter, only a twice-a-year review is needed.
To prevent multiple enrollments, and thus limit the possibility of methadone diversion, California mandates a two-tiered process: (1) Upon admission, urinalysis is conducted to identify whether methadone, or its metabolite, are present. If the results are positive, program administrators must contact other methadone programs within a 50-mile radius. (2) Each client is assigned a number that is checked against a statewide data base to ensure that he or she has only enrolled once.
Once admitted to treatment, patients are required to undergo urinalysis every 30 days, a frequency greater than the federal requirement of at least eight times a year. By statute, providers are not permitted under any circumstances to exceed a daily methadone dose of 180 mg. Although the state imposes a two-year limit on maintenance treatment unless a physician certifies that continued treatment is medically justified, almost all patients who reach this limit are evaluated and, on the basis of a determination by the physician, continue treatment beyond two years. If patients are involuntarily discharged from a program, state regulations ensure a fair hearing.
Florida
In Florida, the state Alcohol, Drug Abuse, and Mental Health Office is responsible for technical assistance, oversight, and policy formulation pertaining to methadone treatment at its central office, but all licensing and distribution of public funds are handled by 15 state district offices. There are approximately 2,700 methadone patients in Florida, according to the 1992 NDATUS (see Table 6-9).
A total of 22 methadone clinics operate in Florida, one of which is publicly owned; the remainder are privately owned. Sixteen of the latter are private, for-profit, and five are not-for-profit. For-profit facilities have increased from six in 1986 to 16 in 1994.
Financing
State funds and federal block grant funds finance only not-for-profit clinics, and no for-profit ones. Of the $1.9 million in revenues received by not-for-profit clinics from July 1, 1992, through June 30, 1993, client fees and insurance accounted for 36 percent, one-fifth (19.9 percent) was from state agency and block grant funds, 17.7 percent from local matching funds, and 12.7 percent from Medicaid.14 Medicaid pays for all methadone services, including a dispensing fee, but not for the prescription itself.
Some private, for-profit clinics accept only client fees as payment for services; they do not accept private insurance or Medicaid, and they are ineligible to receive state agency and block grant funding.
The average annual cost of treatment in Florida in private, not-for-profit units ranges from about $2,600 to 3,200. Private for-profit providers charge approximately $9.00 a day, but the state does not compile funding figures for these clinics since they receive no state funds.
Regulations
Florida regulates certain aspects of methadone treatment more stringently than the federal regulations. Within the next several months, however, the state plans a complete revision of these regulations. Current regulations require one staff member for every 45 patients and restrictive take-home policies. Florida also requires a central registry and strict quality assurance. Finally, current
regulations prohibit clinics from turning away applicants who meet federal admission criteria; if space is unavailable, they are placed on a waiting list.
Illinois
In 1994, approximately 6,000 patients received methadone maintenance treatment in Illinois (see Table 6-9). Illinois has 36 methadone programs, of which 28 are private, not-for-profit and eight are private, for-profit. In the past several years, 80 percent of newly licensed programs have been for-profit. Most clinics in Illinois have waiting lists for patients wishing to enter treatment. Waiting times vary from a few day to several months. All licensing, monitoring, and program coordination are orchestrated by the Illinois Department of Alcoholism and Substance Abuse.
Financing
The 29 private, not-for-profit clinics received $10.7 million from state agency and block grant funds and $6.03 million from Medicaid in fiscal 1993, but state administrators had no information on client fees and private insurance. Medicaid funding in Illinois has expanded over the past 3–4 years, initially covering only medication and counseling costs and now covering dispensing fees, medical examinations, and urine testing. In the future, state officials expect the Medicaid program to impose limits on the amount of methadone services it covers during a one-year period. Private, for-profit clinics are not permitted to receive state agency and block grant funding. The average client cost in not-for-profit facilities in Illinois ranges from $2,500 to 3,000 per year.
Regulations
Illinois has extensive state regulations for all alcoholism and substance abuse programs, with which methadone clinics must comply. These regulations cover such areas as client rights, quality assurance, and research. For example, each program must have a written statement that describes patient rights, including the route of appeal available when a client objects to a facility's decisions, policies, or procedures.
Illinois methadone regulations require programs to comply with the federal methadone regulations and add only one additional requirement: a client is not allowed to receive more than a 3-day take-home supply of medication without a written exemption from the Department of Alcoholism and Substance Abuse.
Massachusetts
In 1992, there were about 3,800 methadone patients in the state of Massachusetts (see Table 6-9). In the state, 14 agencies operate a total of 29 licensed methadone facilities, including 21 sites, 2 satellites, and 7 medication units. These agencies hold 12 outpatient and two inpatient licenses. Neither the state nor the counties own methadone facilities. Private, for-profit clinics are eligible to receive state funds.
The Massachusetts Bureau of Substance Abuse Services licenses each methadone facility to administer methadone. Separate licensing is required to provide counseling at these facilities, as is true for all substance abuse and mental health treatment centers in the state.
Financing
Providers are paid by the Bureau of Substance Abuse Services and Medicaid at a rate of $9.61 per day per patient for medical assessment, drug dispensing, and case management. Individual counseling is reimbursed at a rate of $51 for a 60-minute session; group counseling is reimbursed at $20 per person for a 90-minute session.
Massachusetts permits the use of its substance abuse funds, but not its block grant funds, to finance treatment at private, for-profit facilities. Although the number of for-profit facilities is small, this is a significant departure from many other states. The average cost of treatment in Massachusetts is $5,000 per year or $2,500 for a 180-day episode.
Medicaid contributes a large percentage (53 percent) of the $13 million in revenues that methadone providers reported to the state. This reliance on Medicaid is similar to that of New York State, in which Medicaid contributes 60 percent of treatment costs at publicly funded clinics, but unlike most states, for which Medicaid accounts for only 12 percent of treatment costs. Massachusetts Medicaid expenditures are evenly matched between federal and state sources. The Bureau of Substance Abuse Services contributes $5 million or 38.5 percent of the total, of which 75 percent comes from legislative appropriations and 25 percent from federal block grant funds. Client fees account for $0.8 million or 6 percent of the total, and private insurance for
$0.3 million or 2.3 percent of the total. The Bureau is the "payer of last resort," as is New York State.
Regulations
Methadone providers in Massachusetts must adhere to treatment requirements under both the Massachusetts Code of Regulations and the contract performance standards that were established as conditions of Medicaid and Bureau funding. State regulations require clinics to be open seven days a week and two hours outside of normal working hours. They also require more frequent treatment reviews than do the federal regulations: Massachusetts patients receive a treatment review and evaluation every 90 days for the entire duration of treatment, whereas federal regulations require treatment plans to be updated every quarter only during the first year of treatment and then twice a year thereafter. Finally, Massachusetts mandates strict patient protections in client termination procedures. Patients must be notified in writing of the reasons for proposed termination and of their opportunity to request a hearing to appeal the decision. Hearing procedures are carefully specified in the regulations.
The contract performance standards that were negotiated between the state and providers require each program to have a licensed physician as the medical director (who need not be full-time), and one full-time equivalent (FTE) physician, nurse practitioner, or physician's assistant for the first 300 admissions. For each additional 300 admissions, one FTE nurse or physician's assistant is required. Medication dispensing must be performed by a registered nurse, a licensed practical nurse, or a pharmacist. An average of 26 urine screens must be performed on each client annually and urine collection must be supervised by a health aide or health care professional.
Summary
The treatment system, as described in this chapter, includes a large number of treatment programs, a complex treatment financing system, and variety of state substance abuse authorities. This system is characterized by regional variation and the absence of data about key questions, such as the number and size of for-profit providers, which have increased more rapidly in recent years than not-for-profit treatment programs.
The effects of federal block grant funding of substance abuse treatment on reducing the availability of data about methadone treatment, for example, as well as the effects of a government-wide reduction in data collection in the
1980s on substance abuse in general and methadone treatment in particular, create problems for policymakers.
The recommendations of the next chapter, which propose to reduce the scope of federal administrative discretion over treatment programs in favor of greater clinical discretion, informed by clinical practice guidelines, are, the committee believes, quite sound. Their application to all treatment programs would be assisted by the availability of better data.
References
Anglin, MD, and McGlothlin. 1985. Methadone maintenance in California: A decade's experience. In: L. Brill, C. Winick, (eds.), The Yearbook of Substance Use and Abuse Vol. 3. New York: Human Sciences Press.
Ball, JC, and Ross, A. 1991. The Effectiveness of Methadone Maintenance Treatment: Patients, Programs, Services, and Outcomes. New York: Springer-Verlag.
Batten H. et al. 1992. Drug Services Research Survey Final Report: Phase II. Contract number 27-1908-319/1. Submitted to the National Institute on Drug Abuse (February 12).
Batten H. et al. 1993. Drug Services Research Survey Revised Final Report: Phase I Non-Correctional Facilities. Contract number 27-1908-319/1. Submitted to the National Institute on Drug Abuse (February 22).
Bureau of the Census. 1993. Statistical Abstract of the United States . Washington, D.C.: U.S. Government Printing Office.
D'Aunno, T, and Vaughn, T. 1992. Variations in methadone treatment practices. Journal of the American Medical Association 267(2)
Department of Health and Human Services. 1991. NDATUS Instruction Manual. DHHS Publication No. (ADM) 91–1838. Rockville, Md.: Alcohol, Drug Abuse, and Mental Health Administration.
Department of Health and Human Services. 1993a. Approval and Monitoring of Narcotic Treatment Programs: A Guide on the Roles of Federal and State Agencies. (Draft) Contract No. 27-091-004. Washington, D.C.: DHH.
Department of Health and Human Services. 1993b. Levo-ALPHA-Acetyl-Methadol (LAAM) in Maintenance; Revision of Conditions for Use in the Treatment of Narcotic Addiction. No. 58(137). Washington, D.C.: DHHS.
Department of Health and Human Services. 1993c. National Drug and Alcoholism Treatment Unit Survey (NDATUS): 1991 Main Findings Report . DHHS Publication No. (SMA) 93-2007. Washington, D.C.: DHHS.
Department of Health and Human Services. 1993d. State Resources and Services Related to Alcohol and Drug Abuse Problems, Fiscal Years 1987, 1988, 1989, 1990, 1991. DHHS Publication No. (SMA) 93-1989. Washington, D.C.: DHHS.
Department of Health and Human Services. 1993e. Substance abuse prevention and treatment block grants. Federal Register, Vol. 58, No. 60, March 31.
Department of Veterans Affairs. 1993. Annual Cost Distribution Report for FY 1993. Washington, D.C.: U.S. Government Printing Office.
Department of Veterans Affairs. 1991. Annual Report of the Secretary of Veterans Affairs: Fiscal Year 1991. Washington, D.C.: U.S. Government Printing Office.
Food and Drug Administration. 1993. Narcotic Treatment Programs Directory . Rockville, Md.: FDA, Regulatory Management Branch.
General Accounting Office. 1990. Methadone Maintenance: Some Treatment Programs Are Not Effective; Greater Federal Oversight Needed. GAO/HRD90–104. Washington, D.C.: U.S. Government Printing Office.
Goldstein, H. 1991. The Effectiveness of Methadone Maintenance Treatment Drug Abuse Information and Monitoring Project, California Department of Alcohol and Drug Programs, White Paper Series 8. Sacramento, CA.
Goldstein, HM. 1989. The Availability of Methadone Maintenance in California: April 1989. Part II of a report to the California Department of Alcohol and Drug Programs. Drug Abuse Information and Monitoring Project, White Paper Series 9. Sacramento, CA.
Health Care Financing Administration. 1991. Medicaid: A Brief Summary of Title XIX of the Social Security Act. Baltimore: HCFA.
Horgan, C, Larson, MJ, and Simon, L. Medicaid Funding for Drug Abuse Treatment: A National Perspective. NIDA Treatment Services Research Monograph, Rockville, Md. forthcoming.
Institute of Medicine. 1990. Treating Drug Problems. D. Gerstein and H. Harwood (eds.). Washington, D.C.: National Academy Press.
Kirn, T. 1988. Methadone maintenance treatment remains controversial even after 23 years of experience. The Journal of the American Medical Association 260(20): 2970–2975.
Merlis, M. 1993a. Medicaid: An Overview. Publication No. 93–144 EPW. Washington, D.C.: The Library of Congress.
Murphy, S, and Rosenbaum, M. 1988. Money for methadone II: Unintended consequences of limited-duration methadone maintenance. Journal of Psychoactive Drugs 20(4).
Norquist, G, Hough, R, Golding, J, and Escobar, J. 1990. Psychiatric disorder in male veterans and nonveterans. Journal of Nervous and Mental Disease 178: 328–335.
O'Sullivan, J. 1992. Medicaid: Eligibility for Families, Children, and Pregnant Women. Publication No. 93-240 EPW. Washington, D.C.: Library of Congress.
Peterson K, Swindle, R, Paradise M, and Moos R. 1993. Substance Abuse Treatment Programming in the Department of Veterans Affairs: Staffing, Patients, Services, and Policies. Palo Alto, Calif.: Program Evaluation Resource Center, Department of Veterans Affairs Medical Center.
Price, R. 1993b. Medicaid: Eligibility for the Aged, Disabled, and Blind. Publication No. 93–40 EPW. Washington, D.C.: Library of Congress.
Price, R, Burke, AC, D'Aunno, TA, et al. 1991. Outpatient drug abuse treatment services, 1988: Results of a national survey. In: R. W. Pickens, C. G. Leukefeld, and C. R. Schuster (eds.), Improving Drug Abuse Treatment. (NIDA Research Monograph 106, Rockville, Md.: Pp. 63–92.
Schmidt, L, and Weisner, C. 1993. Developments in alcoholism treatment. In: M. Galanter (ed.), Recent Developments in Alcoholism, Vol. 11: Ten Years of Progress. New York: Plenum Press.
Solloway, M. 1992. A Fifty-State Survey of Medicaid Coverage of Substance Abuse Services. Intergovernmental Health Policy Project. Washington, D.C. : George Washington University.
Vocci, FJ and Sorer, H. 1992. Pharmacotherapies for treatment of opioid dependence. Journal of Health Care for the Poor and Underserved 3:109–124.


































