2
The National Library of Medicine's Toxicology and Environmental Health Information Program
In 1966, at a time of increased concern over the potential health effects of chemicals, the President's Science Advisory Committee examined the state of information in the growing science of toxicology and concluded that ''there exists an urgent need for a much more coordinated and more complete computer based file of toxicological information than any currently available and, further, that access to this file must be more generally available to all those legimately needing such information" (PSAC, 1966). That recommendation led to the establishment of the Toxicology Information Program (TIP) at the National Library of Medicine (NLM), which was retitled in 1994 as the Toxicology and Environmental Health Information Program (TEHIP) to more accurately reflect the scope of the program. The TEHIP program currently encompasses 16 databases offering a wide range of toxicology and environmental health information of importance to health professionals, the general public, scientists, and policymakers.
This chapter provides a brief overview of NLM and the Division of Specialized Information Services (SIS), which oversees the TEHIP program. To inform readers unfamiliar with the breadth and depth of the TEHIP program's information resources, the main focus of this chapter is a description of the TEHIP program and of each of the TEHIP databases. The chapters that follow provide an assessment of the TEHIP program and address the toxicology and environmental health information needs of health professionals. For definitions of the acronyms refer to the glossary included at the end of the report.
NATIONAL LIBRARY OF MEDICINE
Begun in the early 1800s as a part of the Office of the Surgeon General of the Army, NLM has expanded its mission and scope to become one of the country's three national libraries1 and the primary collector of medical information (Miles, 1982). NLM's collection exceeds 5 million books, journals, and audiovisuals on health and medicine. The library also provides access to more than 40 online databases through its Medical Literature Analysis and Retrieval System (MEDLARS) (NLM, 1995). One of NLM's most well-known achievements is the bibliographic database MEDLINE, which is internationally respected as a source of citations and abstracts to the world's medical literature. Approximately 300,000 citations are added to the MEDLINE database annually.
The Lister Hill National Center for Biomedical Communications and the National Center for Biotechnology Information are the research and development arms of NLM. Through these centers, NLM conducts a range of intramural and extramural research to explore new technologies in the fields of medicine, library science, computer science, and informatics.
The National Library of Medicine Act of 1956 (Public Law 84-941) broadly mandated that NLM collect and organize health sciences information "to assist the advancement of medical and related sciences, and to aid the dissemination and exchange of scientific and other information important to the progress of medicine and to public health" (NLM, 1986). The library works with the 4,500 health science libraries of the National Network of Libraries of Medicine (NN/LM) and the eight Regional Medical Libraries (covering all geographic regions of the United States) to provide health professionals and other interested individuals with access to biomedical information. More than 3 million interlibrary loan requests are filled annually by NN/LM.
Organization and Funding
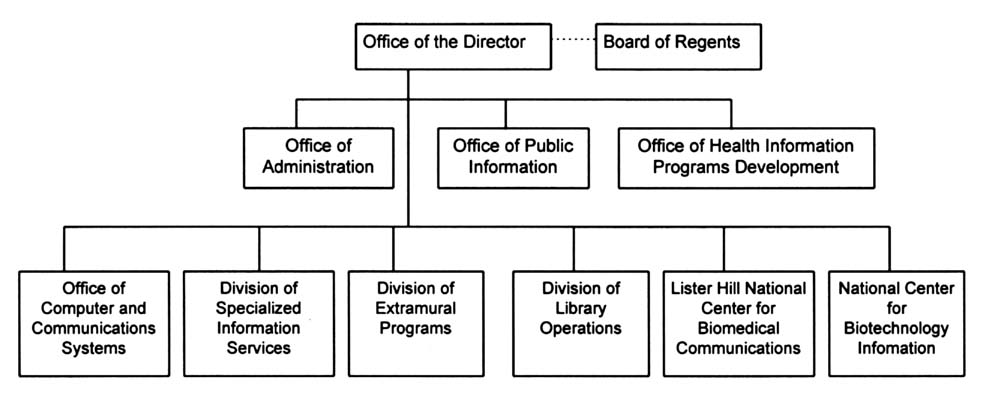
NLM is organized into six divisions (Figure 2.1), the largest of which is Library Operations, responsible for fundamental library services including literature collection, indexing, and cataloging. In fiscal year (FY) 1995, NLM employed 586 full-time equivalent (FTE) personnel.
The majority of NLM's funding comes from its federal budget appropriation, which in FY 1995 was $128 million. Additionally, NLM receives reimbursements for projects with other agencies ($12.9 million in FY 1995). Although user fees are charged for searching the NLM database system,2 these fees do not supplement the NLM budget. Appropriated funds are used to build and maintain the MEDLARS databases, and user fees cover only the added costs associated with accessing this information (e.g., telecommunications charges) (NLM, 1996b,c).
MEDLARS
In the early 1960s, NLM applied emerging computer technologies to establish MEDLARS, which was used to produce bibliographic publications including Index Medicus and to conduct individual literature searches for health professionals (Miles, 1982). Since then MEDLARS has grown to encompass more than 40 bibliographic and factual databases, of which the most well-known and most often searched is the bibliographic database MEDLINE. In 1995, more than 7.3 million searches were performed on the MEDLARS databases (NLM, 1996a). There are a number of specialized databases on MEDLARS including those pertaining to HIV/AIDS (e.g., AIDSLINE and AIDSDRUGS), bioethics (BIOETHICSLINE), and the history of medicine (HISTLINE). Additionally, the toxicology and environmental health databases discussed in this report are part of MEDLARS. Table 2.1 provides a timeline overview of some of the major events and changes occurring within the past 40 years in computer technology, in the emergence and response to environmental health issues, and in the development of NLM's online databases.
MEDLARS databases reside on two separate computer subsystems. NLM's original retrieval system, ELHILL, was designed for bibliographic databases. As a result, in the early 1980s when the records of the TEHIP factual databases became too large for the ELHILL system, it was determined that a new system was needed. TOXNET was developed in 1985 and is the system of networked microcomputers used for database file building, updates, and online searching for most of the TEHIP databases (Van Camp, 1989). A gateway links the ELHILL and TOXNET systems, making the databases available for searching by all NLM users.
TABLE 2.1 Timeline of Events and Changes in Computer Technology and Environmental Health
|
Computer Technology and the MEDLARS Databases |
Environmental Health and the TEHIP Program |
||
|
NLM established |
1956 |
|
|
|
Batch processing; computers adopted for data processing by corporations |
1960s |
|
|
|
Punched card batches used to produce Cumulated Index Medicus |
1961 |
1961 |
Society of Toxicology founded |
|
|
|
1962 |
Publication of Rachel Carson's book Silent Spring |
|
MEDLARS introduced; requested searches were batch processed |
1964 |
|
|
|
|
|
1965 |
IARC established by the World Health Organization |
|
|
|
1966 |
Publication of the President's Science Advisory; Committee's report Handling of Toxicological Information |
|
|
|
1967 |
NLM's TIP established |
|
Time-sharing (sharing of computer services by multiple users introduced) |
1970s |
1970 |
Occupational Safety and Health Act established NIOSH and OSHA |
|
|
|
|
Clean Air Act enacted |
|
Microprocessor developed; enabled the development of the personal computer |
1971 |
|
|
|
MEDLINE introduced online |
|
|
|
|
ARPAnet, the precursor to the Internet, becomes operational |
1972 |
1972 |
TOXLINE developed by NLM |
|
|
|
|
Clean Water Act enacted |
|
MEDLINE tapes leased to commercial vendors |
mid-1970s |
|
|
|
Computer Technology and the MEDLARS Databases |
Environmental Health and the TEHIP Program |
||
|
|
|
1976 |
Toxic Substances Control Act (TSCA) enacted |
|
|
|
1978 |
Toxicology Data Bank developed by NLM |
|
|
|
|
National Toxicology Program established |
|
Desktop computers |
1980s |
1980 |
Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) enacted; ATSDR established |
|
|
|
1983 |
OSHA's Hazard Communication Standard, which covered employees in the manufacturing sector of industry, is enacted |
|
|
|
1985 |
TOXNET developed |
|
Grateful Med developed by NLM |
1986 |
1986 |
Superfund Amendments and Reauthorization Act (SARA) enacted |
|
|
|
1987 |
OSHA's Hazard Communication Standard expanded to include employees in all industries |
|
|
|
1988 |
IOM report Role of the Primary Care Physician in Occupational and Environmental Medicine published |
|
|
|
1989 |
Toxic Chemical Release Inventory (TRI) online database |
DIVISION OF SPECIALIZED INFORMATION SERVICES
Since the 1960s, NLM has had a commitment to the collection and dissemination of toxicology and environmental health information. The Specialized Information Services (SIS) Division of NLM is responsible for the TEHIP program in addition to its responsibilities for the AIDS-related databases (AIDS-LINE, AIDSTRIALS, and AIDSDRUGS) and other designated activities, including outreach programs.
There are 34 FTE personnel working in SIS, making it the third smallest of the NLM divisions (NLM, 1995). SIS is organized into two branches, Biomed
ical Information Services and Biomedical Files Implementation. Personnel in both branches work on the TEHIP program, and many have responsibilities beyond TEHIP.
TOXICOLOGY AND ENVIRONMENTAL HEALTH INFORMATION PROGRAM
Mission and History
The 1966 President's Science Advisory Committee report Handling of Toxicological Information provided the impetus for the development of the Toxicology Information Program (TIP) at NLM (PSAC, 1966). This program began in 1967 with the anticipation of annual funding of several million dollars and staffing of 40 FTEs. However, funding and staffing never reached these anticipated levels. Instead, over the next several years the budgets for TIP were approximately $1 million annually and staffing levels remained at 10 to 18 people (Miles, 1982; NLM, 1993).
TIP undertook a variety of projects, including answering reference queries from the biomedical community. Because staff levels at TIP remained lower than anticipated, NLM initiated an interagency agreement in 1972 with the Atomic Energy Commission to establish a Toxicology Information Response Center at Oak Ridge National Laboratory (ORNL) to handle the reference inquiries (NLM, 1993).3 Additionally, TIP produced numerous publications including the Pesticides Abstracts series—a joint project with the Environmental Protection Agency (EPA).
The primary focus of TIP was the development of online databases, beginning with the TOXLINE database in 1972 (Kissman and Wexler, 1985). TOXLINE was designed as a comprehensive bibliographic resource for scientific literature on toxicology. As described below, TIP developed other databases to meet the needs of users searching for information on chemicals, including the dictionary files CHEMLINE and ChemID and the Hazardous Substances Data Bank (HSDB), an encyclopedic factual database originally developed as the Toxicology Data Bank.
Throughout the program's 29-year history, other databases have been added, many of which originate in other federal agencies, including EPA, the National Cancer Institute (NCI), and the National Institute for Occupational Safety and Health (NIOSH). These databases have been added to the NLM system primarily in response to legislative mandates or because of the agency's interest in making its databases accessible to a wider audience. For example, the
Superfund Amendments and Reauthorization Act of 1986 (SARA) mandated the collection and electronic dissemination of information on the annual release of chemicals into the environment by industrial facilities. As a result, EPA developed the Toxic Chemical Release Inventory database (TRI) and has disseminated the TRI database through NLM since 1989 (beginning with data submitted for TRI87, the first of these annual compilations). The Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA) mandated the establishment of the Agency for Toxic Substances and Disease Registry (ATSDR) and specified that ATSDR maintain an inventory of the health effects of toxic substances. This legislation led to collaborative efforts between NLM and ATSDR in the expansion of what had been the Toxicology Data Bank to become the HSDB. Thus, the evolution of NLM's TEHIP program has been the result of both internal NLM commitments to developing toxicology and environmental health information resources and the interests of other federal agencies in fulfilling their missions and legislative mandates.
In early 1994, following the recommendations of the NLM Long Range Planning Panel (NLM, 1993), the program's focus on environmental health information was made more explicit by renaming the program as the Toxicology and Environmental Health Information Program (TEHIP). The mission of the TEHIP program is to:
-
provide selected core toxicology and environmental health information resources and services,
-
facilitate access to national and international toxicology and environmental health information resources, and
-
strengthen the information infrastructure of toxicology and environmental health (NLM, 1996d).
The TEHIP program encompasses the 16 databases described below. It also offers other services and programs and performs extensive outreach efforts (see Chapter 5). Other aspects of the TEHIP program are addressed throughout the report.
Funding
As with NLM as a whole, the TEHIP program receives funds from two sources: those appropriated to NLM by the U. S. Congress and reimbursements from other agencies of the federal government. When the figures are adjusted for inflation, it can be seen that the budgeted funding for the TEHIP program has remained relatively constant over the past 29 years (in FY 1994, the TEHIP program's appropriated budget in current dollars was approximately $7.4 million [NLM, 1995]; Figure 2.2). However, TEHIP program reimbursements
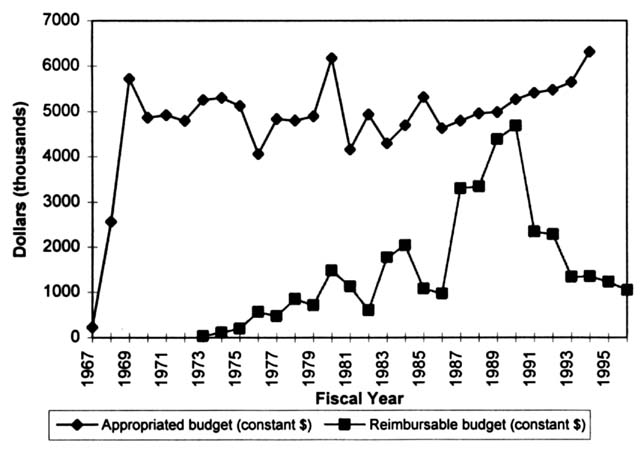
FIGURE 2.2 TEHIP program budget (constant dollars).
from other agencies have fluctuated. Reimbursement funding is the result of collaborative projects with other federal agencies. Recently, the TEHIP program's financial reimbursements from other agencies have dropped significantly.4 In FY 1992, the TEHIP program's total reimbursable budget from other agencies was $2.45 million, whereas in FY 1993 the reimbursable budget dropped by approximately 50 percent to $1.27 million. Since 1993, the reimbursable budget has remained relatively level (the FY 1995 reimbursable budget was $1.23 million).
TEHIP DATABASES
The TEHIP program has 16 online databases (Table 2.2). These databases originate in several different federal agencies, a fact that has complicated attempts to standardize the databases and improve access. One of the features of the TEHIP databases unfamiliar to many users is the inclusion of both bibliographic and factual databases.5
Bibliographic databases are fairly standard in format and are organized to have one record per article citation (Figure 2.3). Each record includes the reference information needed to identify a journal article or other document (e.g., author, title, source, volume, and page numbers) and an abstract if one is available. To enhance the precision of online searching, Medical Subject Headings (MeSH) indexing terms are often included in NLM bibliographic records (Lowe and Barnett, 1994). Many health professionals are familiar with MeSH from their searches of MEDLINE. MeSH is a controlled vocabulary that is used to describe the subject content of the journal article.
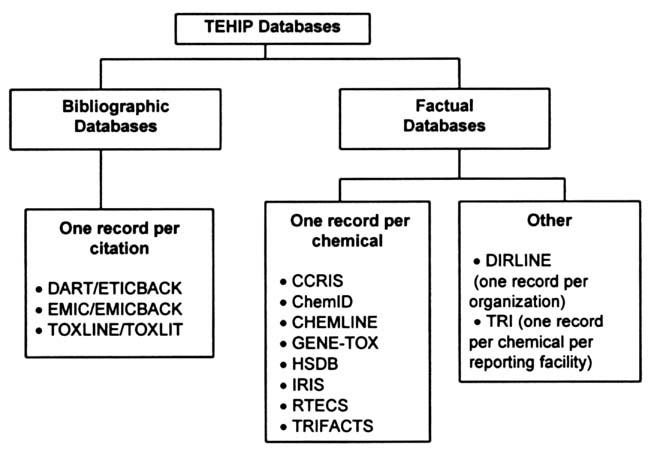
FIGURE 2.3 Organization of the TEHIP databases.
The common feature of factual databases is their presentation of factual data (Table 2.3); however, this information can vary widely in content and form and may include original data from scientific studies, summary statements, dictionary-type information (e.g., names and synonyms), or directory-type information (e.g., addresses and phone numbers). Most of the TEHIP factual databases are organized by chemical record (i.e., each record in that database contains the information on one specific chemical). Exceptions to this are the TRI and DIRLINE databases, whose unique features are described below.
The following sections provide brief descriptions of the TEHIP databases—their subject content, organization, and review and update procedures. Further detailed information on each database, including descriptions of each of the searchable fields, is available in the NLM publication Reference Materials for the Toxicology Information Program Online Services (NLM, 1994). Technical information can be obtained from the responsible federal agency.
TEHIP FACTUAL DATABASES
Ten of the 16 TEHIP databases are factual databases and range from presenting original experimental data to summaries reflecting scientific judgments of the toxicity and risks associated with exposure to chemical substances.
ChemID and CHEMLINE
Information on chemicals can be difficult to locate because of the numerous synonyms and trade names by which chemicals are known and the complex nomenclature used to name chemicals, including the use of numerals and Greek letters. The Chemical Abstracts Service (CAS) Registry Number (RN) is a unique identifying number assigned by CAS to each chemical and is a searchable field in all of the TEHIP databases except DIRLINE. By using the Registry Number, the searcher ensures that the information pertains to the specified chemical, thereby relieving the searcher from knowing all the synonyms or the precise nomenclature.
The ChemID and CHEMLINE databases, both developed and maintained by NLM (CHEMLINE with CAS assistance), are dictionary-type files that provide the searcher with the CAS Registry Number or other information needed to hone a search on a specific chemical. Once that information is obtained the searcher can comprehensively search other TEHIP databases.
TABLE 2.2 TEHIP Databasesa
|
Database |
Sponsoring Agencies |
Factual or Bibliographic |
Number of Records |
Subject Content |
Review and Update |
|
CCRIS |
NCI |
Factual |
>7,100 |
Results of carcinogenicity, mutagenicity, tumor production, tumor inhibition studies |
Studies selected by experts in respective fields, updated monthly |
|
ChemID |
NLM |
Factual |
>294,000 |
Chemical dictionary, synonyms, CAS Registry Number, molecular formula, SUPERLIST |
Information from nonproprietary sources chosen by NLM, updated quarterly |
|
CHEMLINE |
NLM |
Factual |
>1.4 million |
Chemical dictionary, synonyms, CAS Registry Number, molecular formula |
Information from Chemical Abstracts and other sources, updated bimonthly |
|
DART |
NLM, NIEHS, FDA, EPA |
Bibliographic |
>29,000 |
Literature on teratology and many aspects of reproductive toxicology |
Citations from MEDLINE and other sources, updated monthly |
|
DIRLINE |
NLM |
Factual |
>17,000 |
Directory of health information resources including organizations, software, and databases |
Eleven sources of information, updated quarterly |
|
EMIC |
NLM, EPA, NIEHS |
Bibliographic |
>14,000 |
Literature since 1991 published on substances tested for genotoxic activity |
Information from ORNL, updated monthly |
|
EMICBACK |
NLM, EPA, NIEHS |
Bibliographic |
>75,000 |
Pre-1950 through 1991 literature on substances tested for genotoxic activity |
Information from ORNL, closed file |
|
Database |
Sponsoring Agencies |
Factual or Bibliographic |
Number of Records |
Subject Content |
Review and Update |
|
ETICBACK |
NLM |
Bibliographic |
>49,000 |
Teratology literature from 1950–1989, continued by DART |
Information from ORNL, closed file |
|
GENE-TOX |
EPA |
Factual |
>2,900 |
Results from expert review of scientific literature on chemicals tested for mutagenicity |
Reviewed by panels of external scientists, updates subject to EPA revisions |
|
HSDB |
NLM (previously ATSDR) |
Factual |
>4,500 |
Peer-reviewed summaries of the toxicology of potentially hazardous substances |
Peer-reviewed by panel of external reviewers |
|
IRIS |
EPA |
Factual |
>660 |
EPA health risk and regulatory information, includes carcinogenic and noncarcinogenic risk assessment data |
Reviewed by two working groups of EPA scientists, updated monthly |
|
RTECS |
NIOSH |
Factual |
>133,000 |
Toxic effects including skin and eye irritation, carcinogenicity, mutagenicity, and reproductive consequences |
Information selected by NIOSH staff, updated quarterly |
Organization and Content
The ChemID and CHEMLINE databases are organized by chemical record. Each record consists of up to 18 fields of data, including the CAS Registry Number, synonyms (e.g., the aspartame record lists 22 synonyms for the chemical including its trade name, Nutrasweet), molecular formula, MeSH headings, and number of rings and ring size. The classification code field lists the general categories (e.g., insecticide, vasodilator, or steroid) to which a chemical belongs. Another useful field found in both databases is the locator field, which lists all of the NLM databases6 (including MEDLINE) that contain information (either factual or bibliographic) on the specific chemical (Box 2.1). Thus, a searcher could verify that an exhaustive search was done by checking that all databases with information on the chemical had been searched.
Chemicals are selected for inclusion in ChemID or CHEMLINE when they are listed in any one or more of the other NLM databases (including MEDLINE) or in the EPA Toxic Substances Control Act (TSCA) Inventory of Chemical Substances. Additionally, CHEMLINE contains records for chemicals on the European Inventory of Existing Commercial Chemical Substances.
ChemID has records on more than 294,000 chemical compounds. One of the unique and useful features of ChemID is the SUPERLIST field, which specifies the scientific and regulatory lists on which the chemical appears. Information is currently available for 19 listings including the EPA Pesticide List, the Occupational Safety and Health Administration (OSHA) Toxic and Hazardous Substances List, and the National Toxicology Program (NTP) Carcinogen List. ChemID specifies the particular spelling of the chemical name or the synonym used by each list and includes information on each list including a list description, contact information for the list producer, and references.
CHEMLINE has records on more than 1.4 million chemical substances and was the first of the two chemical dictionary databases to be included in the NLM online system. The data in CHEMLINE are primarily supplied by CAS with NLM augmenting the file with nonproprietary data. The royalty charges for the CAS data result in significantly higher connect fee charges for CHEMLINE than for ChemID (see Chapter 6). In 1989, concerned that the royalty charges would discourage access to CHEMLINE, NLM developed ChemID from nonroyalty sources and provides it to users at regular MEDLARS rates.
TABLE 2.3 Types of Information Available in the TEHIP Databases
|
|
Factual |
Bibliographic |
||
|
Databases |
Original/Experimental Data |
Summary Statements/ Data Excerpts |
Dictionary or Directory Information |
Bibliographic Citations and Abstracts |
|
CCRIS |
|
x |
|
|
|
ChemID |
|
|
x |
|
|
CHEMLINE |
|
|
x |
|
|
DART/ETICBACK |
|
|
|
x |
|
DIRLINE |
|
|
x |
|
|
EMIC/EMICBACK |
|
|
|
x |
|
GENE-TOX |
x |
x |
|
|
|
HSDB |
|
x |
|
|
|
IRIS |
x |
x |
|
|
|
RTECS |
|
x |
|
|
|
TOXLINE/TOXLIT |
|
|
|
x |
|
TRI |
x |
|
|
|
|
TRIFACTS |
|
x |
|
|
|
BOX 2.1 Locator Field in the ChemID Database NM Aspartame RN 22839-47-0 LO AIDSLINE; CANCERLIT; CCRIS; DART; EINECS; EMICBACK; ETICBACK; HSDB; MEDLINE; MED75; MED80; MED85; MED90; MESH; RTECS; TOXLINE; TOXLINE65; TOXLIT; TOXLIT65; TSCAINV; SUPERLIST NOTE: NM = name; RN = Registry Number; and LO = locator field. The acronyms for the databases are listed in the glossary. |
A computer-based tutorial, CHEMLEARN, has been developed by SIS to instruct librarians, information specialists, and scientists on effective searching strategies for the ChemID and CHEMLINE databases.
Hazardous Substances Data Bank
In 1978, NLM began development of the Toxicology Data Bank to provide an online source for evaluated toxicology data (Kissman and Wexler, 1985). This database has evolved into HSDB, a peer-reviewed database with a wide range of information on more than 4,500 chemicals. Funding for HSDB has been provided, in part, by ATSDR.
The user has three options for searching HSDB: direct searching (i.e., using the command language), menu searching, or using Grateful Med, a front-end software package developed by NLM. Direct searching requires the user to have knowledge of specific field names and the complex command language, whereas menu and Grateful Med searching provide useful entry points for infrequent users of the database.
Organization and Content
HSDB is organized by chemical record, and each record may contain more than 150 fields of information. The scope of this database broadly encompasses toxicology information, and HSDB records are often quite extensive. Standard texts, monographs, and other tertiary sources are the major sources for HSDB information, which are augmented by primary literature and with information from other databases (e.g., POISINDEX®7). NLM's decision to use authoritative
tertiary sources was based on the fact that these sources had already undergone a selection and evaluation process prior to being published (Kissman and Wexler, 1985). The Source Evaluation Team at NLM selects the HSDB sources on the basis of a number of criteria including peer review, quality, and conciseness (NLM, 1994).
Eleven major categories of information are available in each record (Box 2.2), each of which has numerous fields of information. As with any TOXNET database the searcher can retrieve the entire record for a chemical, look at all the information contained in a major category (e.g., toxicity or biomedical effects), or retrieve just individual fields of information (e.g., emergency medical treatment).
|
BOX 2.2 Major Categories of HSDB Data
|
The substance identification section includes the CAS Registry Number, molecular formula, and synonyms. HSDB includes extensive information on manufacturing and other uses for the chemical, including the most current U.S. production figures for that chemical. Information on chemical and physical properties includes 19 fields of information such as boiling point, molecular weight, spectral properties, and viscosity.
Extensive information on safety and handling that may be useful for emergency response teams is available. Information in this category includes the U.S. Department of Transportation (DoT) Emergency Guidelines, information on respirators and other protective equipment and clothing, clean-up and disposal methods, and facts on storage conditions.
HSDB includes a section on toxicity and biomedical effects that contains available human and animal data. NLM has a reciprocal agreement with Micromedex, Inc., in which information from the Micromedex database POISINDEX® is included in HSDB (e.g., information on emergency medical treatment including clinical effects, laboratory tests, treatment overview, and range of toxicity). In return, HSDB is made available as a subfile of Micromedex's
TOMES Plus® database (Toxicology, Occupational Medicine, and Environmental Series). This section of HSDB also draws from other databases and reports and has separate fields for data from the NTP Reports, the International Agency for Research on Cancer (IARC) Summary and Evaluation, and the TSCA Test Submissions (industry reports submitted to EPA).
Extended information is also available on the chemical's pharmacokinetics, pharmacology (if it is a drug), environmental fate and exposure potential (including food survey values, probable routes of human exposure, and human body burden information), monitoring and analysis methods (e.g., analytic and clinical laboratory methods), additional references, and the new information that has been added to the HSDB record (included in the express data field). The major federal exposure standards and regulations on the chemical are a part of the HSDB record and include the OSHA Standards, the NIOSH Recommendations, the CERCLA Reportable Quantities, and the Water Standards.
Review and Updates
HSDB is peer-reviewed; HSDB data initially undergo review by the NLM Quality Control group, a function provided to NLM under contract, in which checks are made for missing data, errors, lack of clarity, and so forth. Data that have passed this level of review are labeled as quality-control reviewed. The more extensive peer review process is conducted by the Scientific Review Panel, a group of 16 scientists (including 3 physicians) who are selected by NLM. The panel meets three times a year to examine new or revised HSDB records for accuracy and completeness. Each data statement passing this process is labeled as peer-reviewed. Data in HSDB are also labeled with an unreviewed tag. This information includes data that do not lend themselves to review, such as industry data (NLM, 1994).
Chemical Carcinogenesis Research Information System
The National Cancer Institute's (NCI) database, the Chemical Carcinogenesis Research Information System (CCRIS), provides test results from scientifically-evaluated carcinogenicity, mutagenicity, tumor production, and tumor inhibition studies.
Organization and Content
The CCRIS database is organized by chemical record and contains information on more than 7,100 chemicals. Each record has two information sections.
The substance identification section of the record includes the CAS Registry Number, as well as a classification of the chemical's major use (e.g., chelating agent, refrigerant, or surfactant) for each chemical with a commercial application. The most extensive section of the record contains the information on the scientific studies done on carcinogenicity, tumor promotion, mutagenicity, and tumor inhibition. Multiple studies are listed for each outcome. For each study, information is provided on the species, strain, and sex of the animal (data from human epidemiologic studies are included), the route and dose of exposure, the target tissue and type of lesion (as applicable), the test results, and the bibliographic reference. The CCRIS database brings together the major scientific studies on each chemical and gives extensive information on the study and the study results.
Review and Updates
Sources for CCRIS are selected by NCI scientists from the primary literature, special reviews, NCI reports (e.g., NCI/National Toxicity Program Carcinogenesis Technical Reports and NCI Short Term Test Program reports), and other authoritative sources (e.g., IARC monographs and EPA's GENE-TOX Program Reports). Studies must contain clearly positive or negative results and meet criteria for being adequately conducted. Monthly updates are performed, primarily to add additional information to existing records.
GENE-TOX
The GENE-TOX database, sponsored by EPA, contains data on mutagenicity testing for more than 2,900 chemicals. In 1979, EPA began a multiphase effort to review and evaluate the scientific literature on methodologies for genetic toxicology. In the first phase of this effort (1979–1986), 196 scientists from government, academia, and industry selected 23 assays and evaluated the published scientific literature on studies in which those assays were used (Auletta et al., 1991; Waters, 1994). Review articles were published by the work groups detailing the results of the literature review and assay evaluation. Phase II of the project updated the literature reviews for selected mutagenicity assays (e.g., the Ames assay) and established and evaluated the GENE-TOX database, which provides the results of the evaluations. Because of the costs involved, it was decided to include only the qualitative results and not the extensive supporting documentation (Waters, 1994). Phase III continues to update the information and to add data on selected new assays. As expected, assays such as the Ames/Salmonella test have been conducted with numerous chemicals (more
than 2,000), whereas some bioassays have been conducted with fewer than 10 compounds (Lu and Wassom, 1992).
Organization and Content
The GENE-TOX database is organized by chemical record and is maintained by EPA. Each record contains two major sections of data: substance identification (including CAS Registry Number, chemical name, and synonyms) and results of mutagenicity studies.
For each chemical, details are given for the mutagenicity studies reviewed by the GENE-TOX panels. The information on each study includes the assay type, species and cell type, dose-response data, study results, and references to the GENE-TOX expert panel report for this assay. Additionally, the reference field specifies the five-digit number that can be searched in the EMIC database (see the description of EMIC below) to retrieve the complete citation. References for the particular study in the primary literature are also provided.
Review and Updates
As discussed above, all GENE-TOX records have received extensive evaluation. The database is updated when new peer-reviewed data become available from EPA.
Integrated Risk Information System
The Integrated Risk Information System (IRIS) provides EPA with health risk and regulatory information on more than 660 chemicals including carcinogenic and noncarcinogenic risk assessments for oral and inhalation routes of exposure. The database was initially created by EPA in 1986 as an internal resource that could be used to develop and access agency-wide EPA consensus judgments on the human health effects of chemicals (Tuxen, 1992). The database was added to the TOXNET system in 1992.
Organization and Content
Each IRIS database record has information on one chemical, which is then organized into eight major categories of data. The chemical identification fields allow the database to be searched by chemical name, CAS Registry Number, chemical synonyms, or molecular formula.
The categories of carcinogenic and noncarcinogenic assessment provide extensive technical information describing animal and human studies on each chemical. The noncarcinogenic fields provide reference doses for oral and inhalation exposures to the chemical. Reference doses are estimates of the daily amount of a chemical that humans can be exposed to throughout their lives without the risk of suffering adverse effects (NLM, 1994). The carcinogenicity assessment category describes the unit risk (the upper-level lifetime risk of contracting cancer when exposed to specific concentrations of the chemical). Carcinogenicity assessments are provided, when available, for both oral and inhalation exposures. EPA currently classifies the carcinogencity of a chemical into one of the following five classification codes: A (human carcinogen), B (probable human carcinogen), C (possible human carcinogen), D (not classifiable as to human carcinogenicity), and E (no evidence of carcinogenicity for humans) (NLM, 1994). Both the noncarcinogenic and carcinogenicity assessment categories provide descriptions of studies, references to the EPA source document for the assessment and other studies cited, the data that the data were most recently reviewed, and an EPA contact person.
Additionally, IRIS contains the advisories from the EPA Office of Drinking Water and exposure standards and regulations including the requirements of the Clean Air, Clean Water, and Safe Drinking Water Acts.
Review and Updates
IRIS is updated monthly by EPA. Updates include the addition of new records, revisions of existing records, or record deletions. All information in the IRIS database undergoes scientific review by two agency-wide work groups of EPA scientists, the Oral Reference Dose/Inhalation Reference Concentration Work Group and the Carcinogen Risk Assessment Verification Endeavor Work Group. Information is incorporated into the IRIS database after the work group has reached consensus on the health effects or dose-response assessment (Tuxen, 1992). Each record includes the revision history field, which provides the date on which that IRIS record most recently completed EPA scientific review.
Registry of Toxic Effects of Chemical Substances
The National Institute for Occupational Safety and Health (NIOSH) has developed and updated the Registry of Toxic Effects of Chemical Substances (RTECS) database since its inception in 1971. RTECS provides toxic effects and regulatory information on more than 133,000 chemicals. Mandated by the Occupational Safety and Health Act of 1970, the original edition was called the
Toxic Substances List and included information on approximately 5,000 chemicals. RTECS was added to the NLM online system in 1977 and is also available through license agreements with NIOSH in CD-ROM and online formats from a number of database vendors. Publication of print and microfiche versions of RTECS has recently ceased.
Organization and Content
RTECS is organized by chemical record, and each record contains four major categories of information: substance identification (including the CAS Registry Number), toxicity and biomedical effects, toxicology and carcinogenicity review, and exposure standards and regulations. Like CCRIS, RTECS provides results from scientific studies and does not evaluate or summarize that information. The section of the RTECS record on toxicity and biomedical effects provides information on scientific studies on mutagenicity, carcinogenicity, skin and eye irritation, general toxicity, and reproductive effects. For each study, information is provided on the type of test or study including species of test animal; the route, dose, and duration of exposure; target tissues; study results; and brief citations. RTECS uses a controlled vocabulary developed by NIOSH to facilitate searching by standardizing the descriptions of health effects, cell types, species, etc.
The toxicology and carcinogenicity review section of the RTECS record provides relevant IARC reviews, threshold value limits, and NIOSH Recommended Exposure Limits for the specified chemical.
The final RTECS section summarizes agency standards and regulations and provides a federal program status field listing the activities of government agencies and programs (e.g., the Carcinogenesis Bioassay Program) for that particular chemical.
Review and Updates
Information in RTECS is selected under the direction of NIOSH staff from key scientific journals, government reports, and other technical documents. The data come directly from the publication and do not undergo additional review by NIOSH. The RTECS database at NLM is maintained and updated by NLM with information provided by NIOSH (NLM, 1994). RTECS is updated quarterly, a process that involves adding additional records, as well as adding and deleting information from existing records.
Toxic Chemical Release Inventory
The Emergency Planning and Community Right-to-Know Act of the Superfund Amendments and Reauthorization Act of 1986 (SARA) mandated that facilities in the United States meeting its criteria must annually report information on environmental release of chemicals (due either to routine release as part of business operations or by accident) to the EPA. The Act further specified that all the emissions information be made available in a computer file format (Bronson, 1991). The end result is the Toxic Chemical Release Inventory database (TRI), which is developed and maintained by EPA and accessed through NLM's TOXNET system.
Facilities are required to report emissions if, among other criteria, they manufacture or process more than 25,000 pounds of the chemical per year. Each facility completes and submits an EPA Form R for each chemical meeting the criteria. More than 300 chemicals are on the list for mandated reporting. Separate TRI files are available for each year beginning with 1987, and the databases can be searched individually or as a group.
Organization and Content
Each TRI record corresponds to one EPA Form R submission (one chemical from one reporting facility). The TRI94 file with data on 1994 emissions contains more than 75,000 records. The major categories of data in the TRI records are facility identification (including facility name and address), substance identification (including the CAS Registry Number and manufacturing and processing uses), and environmental release of the chemical (including the amounts of air emissions, water discharges, releases to underground injection, waste treatment, and off-site waste transfer). The geographic information available in the TRI files (including zip code, city, county, or state) make these databases a valuable resource for community and other groups interested in assessing potential environmental hazards in their region.
Similarly to HSDB, the searcher of the TRI databases has three search options: direct searching (i.e., using the command language), menu searching, or using Grateful Med. Menu searching offers screens to prompt the user through the process of developing the search strategy and is useful for the wide range of users, including the general public. Grateful Med also provides similar ease of use.
TRI data can be searched and manipulated by the database through ranging and calculating operations. A search, for example, could be performed on all facilities in Virginia with total air release of ethylbenzene greater than 2,000 pounds per year. Additionally, the TRI databases can perform a number of cal
culating and statistical functions (e.g., average, standard deviation, mean, median, and mode).
TRIFACTS
A companion database to TRI is TRIFACTS, a factual database with information on the health effects, ecological effects, safety, and handling of the more than 300 TRI chemicals. The information in TRIFACTS is intended to provide the lay person with summary information on the TRI chemicals. When the searcher logs onto TRIFACTS, he or she is informed that TRIFACTS summaries ''should be supplemented with technical literature to answer in-depth questions."
TRIFACTS summaries are adapted from the State of New Jersey Hazardous Substance Fact Sheets. The Fact Sheets were originally mandated by New Jersey's Worker and Community Right to Know Act and were developed for all chemicals on New Jersey's Right to Know Hazardous Substance List. EPA, through an agreement with the State of New Jersey, adapted the Fact Sheets for use as an online database and added EPA ecological data to the file. The TRIFACTS database was added to the TOXNET system in 1992.
Organization and Content
The TRIFACTS database has one record per chemical for most all of the over 300-plus TRI chemicals. Within each record the major categories of data are substance identification (including CAS Registry Number), chemical and physical properties, and safety and handling (including recommendations on personal protective equipment and clothing; the DoT Emergency Guidelines for firefighters, police, or emergency workers; and the National Fire Protection Association's Hazard Classification of flammability). Another major category of data includes summaries of the chemical's human toxicity and biomedical effects (Box 2.3). Information is included on emergency medical treatment procedures and acute and chronic (including cancer and reproductive) effects of the chemical on humans. Additionally, TRIFACTS provides ecological information (acute and chronic effects on aquatic and terrestrial life) and summaries of the exposure standards and regulations (including OHSA standards and NIOSH recommendations).
|
BOX 2.3 Excerpt from the TRIFACTS Record on Toluene NAME Toluene RN 108-88-3 ACUTE The following acute (short term) health effects may occur immediately or shortly after exposure to toluene:
|
Directory Of Information Resources Online
The DIRLINE (Directory of Information Resources Online) database is unique among the TEHIP databases in providing directory information that not only covers the fields of toxicology and environmental health but also encompasses information resources throughout all fields of health and biomedicine. Descriptions and contact information for more than 17,000 biomedical and health-related organizations, databases, software programs, and other information resources are available through DIRLINE. This database was developed by SIS staff to provide an alternate source for answering information requests and was designed to be used by health professionals, information specialists, and the general public (NLM, 1994).
Organization and Content
The content of the DIRLINE database is merged from the following sources of directory information:
-
Centers for Disease Control and Prevention National AIDS Clearinghouse
-
Directory of Biotechnology Information Resources
-
Health Services Research Information (National Information Center for Health Services Research)
-
Maternal and Child Health Information (produced by the National Center for Education in Maternal and Child Health)
-
Maternal and Child Health Information (produced by the National Center for Education in Maternal and Child Health)
-
National Institutes of Health Research Resources
-
NLM (list of organizations developed by the Library of Congress)
-
NLM History of Medicine Division
-
ODPHP Health Information Center Databases (sponsored by the Office of Disease Prevention and Health Promotion, U.S. Department of Health and Human Services)
-
Poison Control Centers (data provided by the American Association of Poison Control Centers)
-
Regional Alcohol and Drug Awareness Resource Network (produced by the National Clearinghouse on Alcohol and Drug Information)
-
Self-Help Clearinghouses (produced in collaboration with the Surgeon General's Initiative in Self-Help and Public Health)
Each DIRLINE record provides information on one information resource (Box 2.4). DIRLINE records include contact information (including address and telephone number), a summary description of the resource, and MeSH terms. Because of the multiple sources making up the DIRLINE database, duplicate records may be included.
|
BOX 2.4 DIRLINE Record for the Association of Occupational and Environmental Clinics SI NLM/30011 NA Association of Occupational and Environmental Clinics AC AOEC AD 1010 Vermont Ave., NW, Suite 513, Washington, DC 20005 TEL (202) 347-4976 TEL (202) 347-4950 (FAX) AB The AOEC was established in 1987 to enhance the practice of occupational and environmental medicine through information sharing, education, and research. The growing member network now includes 39 clinics, and over 230 individuals. The AOEC aids in identifying, reporting, and preventing occupational and environmental health hazards and their effects. It encourages provision of high quality clinical services for people with work or environmentally related health problems. Its members receive reports based on databases being developed by AOEC, and on other AOEC research and conferences. The AOEC answers inquiries and holds workshops and seminars on Multiple Chemical Sensitivity (MCS). It acts as a resource for patient referrals for ATSDR, NIOSH, and others. NOTE: SI=Secondary Source ID; NA=Name; AC=Acronym; AD=Address; TEL=Telephone Number; and AB=Abstract. |
Review and Updates
DIRLINE is provided free of charge as part of NLM's efforts to increase the availability of HIV/AIDS-related information to the scientific community
and the general public. Access to DIRLINE does not require an NLM user code since DIRLINE is available through NLM's Locator Internet site (telnet locator.nlm.nih.gov) in addition to its availability as part of MEDLARS (NLM, 1995). DIRLINE is updated quarterly.
TEHIP BIBLIOGRAPHIC DATABASES
Many health professionals are familiar with NLM's bibliographic databases, particularly MEDLINE. Six of the TEHIP databases are bibliographic and provide references to the vast range of toxicology and environmental health literature. Obtaining the full text of the journal article or document may require using a variety of mechanisms. For toxicology and environmental health citations listed in MEDLINE, the full text document can be ordered through NLM's online ordering system, LOANSOME DOC. However, retrieving the full text of other citations may require utilizing other scientific libraries and resources.
TOXLINE/TOXLIT
A great diversity of scientific disciplines is involved in the fields of toxicology and environmental health. As a result, the scientific literature is dispersed among numerous journals and is indexed in a variety of sources. In 1972, NLM developed TOXLINE with the goal of having a single bibliographic database that would cover the field of toxicology (Kissman and Wexler, 1985). TOXLINE was designed to incorporate the relevant records from other indexing and abstracting sources and originally was composed of records from Index Medicus, Biological Abstracts, Chemical Abstracts, and International Pharmaceutical Abstracts. As shown in Table 2.4, the bibliographic records in TOXLINE are drawn from 18 sources; additionally, the companion file, TOXLIT, provides access to records from certain royalty sources, currently only Chemical Abstracts (see Chapter 6 for discussion of costs).
Records from the 18 subfiles are not significantly altered or enhanced before being imported into TOXLINE. As a result, there is no single controlled vocabulary, and searchers must use a number of synonyms and similar expressions to search TOXLINE comprehensively. Additionally, the secondary sources that make up TOXLINE overlap to some extent in their coverage (e.g., MEDLINE, BIOSIS, and Developmental and Reproductive Toxicology [DART]) and may result in duplicate, although not identical, citations in TOXLINE (NLM, 1994).
TABLE 2.4 TOXLINE Subfiles
|
TOXLINE Subfile |
Subfile Description |
|
Aneuploidy |
Collection of bibliographic citations prepared by EMIC on numerical chromosomal abnormalities |
|
Developmental and Reproductive Toxicology (DART)a |
DART database on teratology and many aspects of reproductive toxicology |
|
Environmental Mutagen Information Centera |
EMIC database on substances tested for genotoxic activity |
|
ETICBACK database covering the 1950–1989 teratology literature |
|
|
Epidemiology Information Systemb |
Epidemiology Information System database, developed by FDA's Center for Food Safety; citations cover literature published from 1940 to 1988 on the distribution and health effects of food contaminants |
|
Federal Research in Progress (FEDRIP) |
Subset of the FEDRIP database produced by NTIS and describing current federal research and development projects |
|
Hazardous Materials Technical Center (HMTC)b |
HMTC Abstract Bulletin on hazardous wastes, published by the Department of Army's Hazardous Materials Technical Center |
|
International Labour Office |
CIS Abstracts; toxicology-related material produced by the International Labour Office's International Occupational Safety and Health Information Centre |
|
International Pharmaceutical Abstracts (IPA) |
Subset of the IPA database on development and use of drugs; produced by the American Society of Health System Pharmacists |
|
NIOSHTIC |
Subset of the NIOSH's NIOSHTIC database on occupational safety and health literature |
|
Pesticides Abstractsb |
EPA publication on the epidemiological effects of pesticides; the publication was terminated in 1981 |
|
Poisonous Plants Bibliographyb |
Pre-1976 citations to literature on poisonous plants |
Organization and Content
Because of the size of the database files (TOXLINE and TOXLIT files contain more than 4 million records), the pre-1981 records from TOXLINE and TOXLIT (which primarily cover the literature from 1965 to 1980) have been put into separate databases (TOXLINE65 and TOXLIT65). All four files (TOXLINE, TOXLINE65, TOXLIT, and TOXLIT65) are organized as traditional bibliographic databases with fields for basic bibliographic information including author, title, source, language, publication type, and abstract (when available).
MeSH indexing terms are available only for the TOXBIB, BIOSIS, and DART subfiles of TOXLINE; additionally, the keyword field provides access to the indexing terms used by the subfile producer (e.g., FEDRIP). All of the indexing terms are used, along with terms from other fields, to provide text word searching capabilities. The CAS Registry Number is found in many of the TOXLINE records.
Additionally, there are fields for specialized information to accommodate the information from the various subfiles (e.g., the Award Type field is used in the Toxicology Research Projects and FEDRIP subfiles to indicate whether the research project is intramural, contract, fellowship, or grant).
Review and Updates
TOXLINE and TOXLIT are updated monthly, with new records added from various subfiles. More than 140,000 records are added annually and the file is rebuilt each year to provide updated MeSH indexing.
Developmental and Reproductive Toxicology
Two bibliographic databases provide citations and abstracts to the scientific literature on teratology and developmental and reproductive toxicology. The ETICBACK (Environmental Teratology Information Center Backfile) database was produced by ORNL and covers the teratology literature from 1950 to 1989. A decision to expand the database to more fully cover the developmental and reproductive toxicology literature resulted in the closing of the ETIC file and the introduction of the DART database, which covers literature from 1989 to the present. Funding constraints, however, have prevented the comprehensive coverage of the scientific literature on lactation effects and on some aspects of male and female reproductive toxicology.
Organization and Content
Both DART and ETICBACK are bibliographic databases and provide searchable fields for basic bibliographic information including author, source, language, publication type, and, when available, the abstract. Substance identification fields in both databases provide searchable access to the CAS Registry Number and other identification information. MeSH terms have been added to the DART records, although some of the MeSH searching commands (e.g., explode or pre-explode) are not yet available in the TOXNET system. More than 60 percent of DART records come from MEDLINE; the others are identified by screening the literature not covered by MEDLINE (including meeting abstracts and government reports) (NLM, 1994). ETICBACK records include a number of specialized fields including assay method, experimental conditions, and maternal effects. Both databases currently receive or have received funding from National Institute of Environmental Health Sciences (NIEHS), EPA, NLM,
and the Food and Drug Administration's National Center for Toxicological Research.
Review and Updates
ETICBACK is now a closed file, meaning that no new records are being added to the file. DART is updated monthly and is developed and maintained by NLM, with additions and deletions to the database made as necessary. Each month a search profile is run against the SDILINE database (a subset of the MEDLINE database containing only the most recent month's input into MEDLINE), and relevant records are added to DART. Additionally, relevant sources not indexed by MEDLINE are added.
Environmental Mutagen Information Center
EMIC and its backfile, EMICBACK, cover the scientific literature on genetic toxicity testing. In the late 1960s increased public and scientific concern about the mutagenic actions of chemicals led to the establishment of the Environmental Mutagen Information Center (EMIC) at ORNL (Wassom and Lu, 1992). One of the major functions of the Center since its inception has been the collection and organization of the scientific literature on chemical, biologic, and physical agents that have been tested for genetic toxicity. The result is the EMIC database, which was originally produced by ORNL and which is now managed by NLM. Support for EMIC is provided by EPA and NIEHS.
Organization and Content
Similarly to TOXLINE, EMIC has been separated into two bibliographic database files. The EMICBACK file covers the pre-1950 through 1991 literature, and the EMIC file includes the literature published since 1991. The two files contain more than 88,000 citations to the genetic toxicology literature, which can also be accessed as the EMIC subfile of the TOXLINE database.
In addition to the bibliographic fields (e.g., author, title, source, and language), EMIC and EMICBACK contain a number of specialized fields regarding the assay and the study. These fields include keywords describing the test organism, tissue cultured, type of assay, and experimental conditions. EMIC records also contain fields identifying the substances involved in the test including the names of the test, the inducer, and the control agents. As described above, EMIC contains the records of sources used in EPA's GENE-TOX program, including the panel reports of the GENE-TOX reviewers.
Review and Updates
EMIC records are selected and indexed by ORNL. The EMICBACK file is closed (no new records will be added), and the EMIC file is updated monthly. Approximately 3,000 records are added to EMIC each year.
CONCLUSIONS
Although this chapter presents only an overview of each of the TEHIP databases, it is evident that there is a wealth of information available for use by health professionals, researchers, industry, policymakers, and the general public. What is also evident is the complexity of the TEHIP program—a complexity that results from the number of databases, the disparate scope and content of the databases, the diverse sources of information, and the variations in the type of information provided. The following chapters examine these issues and provide the committee's recommendations for facilitating health professionals' use of toxicology and environmental health information resources.
REFERENCES
Auletta AE, Brown M, Wassom JS, Cimino MC. 1991. Current status of the Gene-Tox Program. Environmental Health Perspectives 96:33–36.
Bronson RJ. 1991. Toxic Chemical Release Inventory information. Medical Reference Services Quarterly 10(1):17–34.
Brooks SM, Gochfeld M, Jackson RJ, Herzstein J, Schenker MB, eds. 1995. Environmental Medicine. St. Louis: Mosby.
Campbell-Kelly M, Aspray W. 1996. Computer: A History of the Information Machine. New York: Basic Books.
Kissman HM, Wexler P. 1985. Toxicology information systems: A historical perspective. Journal of Chemical Information and Computer Sciences 25:212–217.
Lowe HJ, Barnett GO. 1994. Understanding and using the Medical Subject Headings (MeSH) vocabulary to perform literature searches. Journal of the American Medical Association 271(14):1103–1108.
Lu P-Y, Wassom JS. 1992. Risk assessment and toxicology databases for health effects assessment. In: U.S. Environmental Protection Agency, Oak Ridge National Laboratory (ORNL). Proceedings of the Symposium on the Access and Use of Information Resources in Assessing Health Risks from Chemical Exposure. Oak Ridge, TN: ORNL.
Miles WD. 1982. A History of the National Library of Medicine. NIH Publication No. 85-1904. Bethesda, MD: National Institutes of Health.
Netscape. 1996. Internet time line. Inside Netscape Navigator 1(4):8–9.
NLM (National Library of Medicine). 1986. Building and Organizing the Library's Collection. Long Range Plan, Report of Panel 1. Bethesda, MD: NLM.
NLM. 1993. Improving Toxicology and Environmental Health Information Services. Report of the Board of Regents Long Range Planning Panel on Toxicology and Environmental Health. NIH Publication No. 94-3486. Bethesda, MD: NLM
NLM. 1994. Reference Materials for the Toxicology Information Program Online Services. Bethesda, MD: NLM.
NLM. 1995. National Library of Medicine Programs and Services, 1994. NIH Publication No. 95-256. Bethesda, MD: NLM.
NLM. 1996a. The National Library of Medicine [http://www.nlm.nih.gov/publications/factsheets/nlm.html]. November
NLM. 1996b. NLM Policy on Database Pricing [http://www.nlm.nih.gov/publications/factsheets/datapric.html]. November.
NLM. 1996c. NLM Online Services Program Policy Statement [http://www.nlm.nih.gov/publications/factsheets/online_serv_policy.html]. November.
NLM. 1996d. Toxicology and Environmental Health Information Program [http://sis.nlm.nih.gov/tehipfs.htm]. November.
PSAC (President's Science Advisory Committee). 1966. Handling of Toxicological Information. Washington, DC: White House.
Rom WN, ed. 1992. Environmental and Occupational Medicine, 2nd ed. Boston: Little, Brown, and Company.
Siegel ER, Cummings MM, Woodsmall RM. 1990. Bibliographic retrieval systems. In: Shortliffe EH, Perreault LE, eds. Medical Informatics: Computer Applications in Health Care. Reading, MA: Addison Wesley.
Tesler LG. 1991. Networked computing in the 1990s. Scientific American 265(3):86–93.
Tuxen L. 1992. Integrated Risk Information System. In: U.S. Environmental Protection Agency, Oak Ridge National Laboratory (ORNL). Proceedings of the Symposium on the Access and Use of Information Resources in Assessing Health Risks from Chemical Exposure. Oak Ridge, TN: ORNL.
Van Camp AJ. 1989. The TOXNET gateway. Online July:70–74.
Wassom JS, Lu P-Y. 1992. Evolution of toxicology information systems. In: U.S. Environmental Protection Agency, Oak Ridge National Laboratory (ORNL). Proceedings of the Symposium on the Access and Use of Information Resources in Assessing Health Risks from Chemical Exposure. Oak Ridge, TN: ORNL.
Waters MD. 1994. Development and impact of the Gene-Tox Program, genetic activity profiles, and their computerized data bases. Environmental and Molecular Mutagenesis 23(Suppl. 24):67–72.
Zenz C, Dickerson OB, Horvath EP, eds. 1994. Occupational Medicine . St. Louis: Mosby.