Page 243
9—
HAAs and Carcinogenesis in Humans
Laboratory assay systems have been designed to test chemical compounds for their potential estrogenicity, androgenicity, antiestrogenicity, and antiandrogenicity. The systems, the chemical compounds, and the results obtained from the assays are fully elaborated elsewhere in this report. Although the evidence from various in vitro and in vivo studies contributes substantially to our understanding of the action of hormonally active agents (HAAs), that information is not a substitute for actual data obtained from human beings. This chapter reviews the data (primarily epidemiologic) that bear on putative associations between HAAs identified in the environment and risk of various cancers in humans.
This review concentrates on data reported in the published literature. This constraint limits both the environmental agents and the specific cancer sites that are covered. Although a broad range of environmental agents that have been identified in laboratory systems to mimic sex steroid hormone activity, the data that exist for evaluating the relationship between HAAs and human cancers are essentially limited to organochlorines, mostly l,l,l-trichloro-2,2-bis(p)-chlorophenyl) ethane (DDT) (and its metabolite 1,1 -dichloro-2,2-bis(p-chlorophenyl) ethylene (DDE)), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), and polychlorinated biphenyls (PCBs). There are some 209 PCB congeners, but only a few recent epidemiologic studies have distinguished among them.
The mechanism of action of DDT, PCBs, and TCDD and their metabolites are diverse because, as discussed in Chapter 2, those compounds exhibit varying hormonal properties in in vitro systems. The DDT isomer o,p'-DDT has estrogenic properties (Johnson et al. 1988; Soto et al. 1994), while the DDT metabolite p,p'-DDE acts as an antiandrogen (Kelce et al. 1995). There is evidence that PCB mixtures and congeners have both estrogenic and antiestrogenic (Gellert 1978b;continue
Page 244
Moore et al. 1997) properties. TCDD is generally considered to be an antiestrogen (Gallo et al. 1986; Romkes et al. 1987; Astroff and Safe 1990).
The committee focused on cancer sites that are known from ancillary data to have some hormonal dependence. If HAAs operate in humans, the activity should be most evident in tissues that are known targets for endogenous and exogenous sex steroid hormones. Thus, breast and endometrial cancers in women and prostate and testicular cancers in men were selected. Each of the cancers reviewed in this chapter does not receive equal attention. Breast cancer predominates merely because the majority of studies have focused on the breast. Analysis of the incidence of endometrial cancer should be the most informative with respect to HAA effects, because the lining of the uterine fundus (endometrium) is an exquisitely sensitive tissue both to estrogenic and to antiestrogenic effects in women.
Where associations are demonstrated, either positive or negative, between HAAs and a particular cancer site, this does not in itself prove causation. Additional considerations are involved in such a determination, many of which are highlighted in epidemiologic theory and methods. For an overview of epidemiologic research and its strengths and weaknesses, the reader is referred to evaluations presented in other National Research Council reports (NRC 199). Most important for this chapter is the concept of synthesis; that is, the integration of data from the multiple disciplines represented in this report, as well as the relative consistency (or lack of it) in the epidemiologic studies reviewed in this chapter.
One further caution is noted concerning the possible mechanisms responsible for any associations demonstrated. Many of the compounds under study are toxic agents in laboratory systems. Thus, one cannot be confident that a hormonally mediated mechanism accounts for an observed association between an HAA and a hormonally sensitive target tissue. Multiple mechanisms of action could be responsible for the toxic effects of these compounds.
The data in this chapter are introduced in a review of the known risk factors for each cancer site, emphasizing those factors that could indicate hormonal alteration. The chapter focuses on studies that specifically address the question of a relationship between environmental HAAs and cancer in humans. The studies are characterized as to type (case control, occupational cohort, unplanned experiment, or ecologic). Methodologic features that enhance or detract from the validity of various study designs are noted when relevant. The final section of the chapter lists the conclusions that can be drawn from the data presented.
Breast Cancer
Breast-Cancer Risk Profile
Breast cancer is the second most common cancer among women in the world, and in developed countries it is the most common (Parkin et al. 1992).continue
Page 245
Worldwide, cancer of the uterine cervix is the most common. For the other major common cancers-lung, colon, and prostate-age-specific rates increase exponentially with increasing age in developed countries. This is not true for gynecologic cancers, however. By the year 2000, it is estimated that breast cancer will account for 500,000 deaths annually (Pisani et al. 1993). Age-specific incidence rates rise rapidly until about age 50, the approximate age of natural menopause. Thereafter, the rate of increase declines, a phenomenon not seen with other common cancers. This inflection in the age incidence curve is attributed to the precipitous drop in ovarian steroid hormone production at the menopause.
Country of birth has a marked correlation with risk. Rates in developed countries are significantly higher than are those in less-developed countries (Pisani et al. 1993). Japan is an exception; there, breast cancer rates are half as high as are those in North America and northern Europe (Coleman et al. 1993), although they are on the rise. When women migrate from one country to another, their breast cancer rates assume a pattern more similar to the host country's, although that change can require two or three generations. This observation has been well documented in Asian-born migrants and among Asian-American women born in the United States (Standford et al. 1995).
Hormonally mediated endogenous factors are known to affect risk of breast cancer (Hulka et al. 1994). Lifetime risk reduction is evident in women who undergo bilateral oophorectomy at an early age. Risk is increased by early age at menarche, by late age at menopause, by nulliparity, or by late age at first pregnancy (Hulka and Stark 1995). Obesity in postmenopausal women increases risk, whereas prolonged lactation in premenopausal women decreases risk (Hulka and Stark 1995). Increasing cumulative lifetime exposure to endogenous estrogen, supplemented by cyclic progesterone, provides a unifying theory to explain many of these observations (Pike et al. 1993).
It is estimated that in 1997 there would be 180,200 new cases of breast cancer and 43,900 deaths from breast cancer in the United States (Ries et al. 1997). Breast-cancer mortality rates stayed fairly constant during the 1970s and 1980s (Miller et al. 1993), and there was a small decline in the 1990s (Parker et al. 1997). Breast cancer incidence rates have been increasing since the 1940s, with a 23% increase between 1973 and 1994 (Ries et al. 1997). Superimposed on this secular trend in incidence rates was an abrupt increase of more than 2% each year in women older than 50 during the 1980s. No increase was evident in women under 40, and there were only small increases in the 40- to 49-yr-old group. The increased rates have been attributed to the large rise in the use of screening mammography during the early and middle part of the decade (Sondik et al. 1989). This attribution is consistent with increased rates of certain tumors (localized invasive tumors and carcinomas in situ) and with the targeted screening of women age 50 and above. Since 1987, breast-cancer rates have reached a plateau (Hankey et al. 1994), although high use of screening mammography continues. It is likely that the reservoir of prevalent breast-cancer cases has beencontinue
Page 246
depleted through prior screening and that cases being detected in the 1990s are true incident disease.
Many factors could account for the long-term increasing incidence of breast cancer. Changes in reproductive patterns over the past 50 yr, including fewer pregnancies and later age at first pregnancy, could have had some effect (White 1987). Some researchers suggest that increasing cumulative lifetime exposure to estrogens could be responsible (Pike et al. 1993). In addition to endogenous sources of estrogen, hormone-replacement therapy (HRT) and hormonal contraceptives are commonly prescribed. Estimates of oral-contraceptive use vary by age group of respondent, but by age 40, 80% of U.S. women have used them (IOM 1991). For HRT, use patterns vary by geographic region, age, and social class (Matthews et al. 1989). For women ages 50-59 in 1985, use prevalence in the western United States was reported at 43%; it was only 15% on the East Coast (Hemminki et al. 1988). Use of HRT by postmenopausal women is encouraged because of its potential for protection against cardiovascular disease, osteoporosis, and fractures.
Breast Cancer and HAAs
The data relating HAAs in the environment to human cancer are limited to PCBs, DDE, and TCDD. The amount and quality of those data is greatest for breast cancer-a high-incidence, high-profile disease among women. The studies have been varied: They include case-control studies, in which the chemical dose is measured in body adipose tissue or serum; occupational studies of highly exposed individuals, and ecologic analyses. Case-control studies are most likely to provide valid data because of the greater accuracy of the exposure measurement. Data are reviewed below.
Case-Control Studies
Before 1995, at least seven studies were published that contained data on organochlorine concentrations in tissue or sera as shown in Table 9-1 (Adami et al. 1995). The earliest was a 1976 report by Wasserman et al. (1976), in which formalin-fixed breast tissue from nine women with breast cancer and five women in a control group was analyzed for several metabolites of DDT and PCB congeners. They found higher concentrations of several PCB congeners in extracted lipids of malignant breast tissue than in adjacent normal tissue or control tissues. However, the concentrations of p,p'-DDE, the major persistent metabolite of DDT, were significantly higher in control than in case tissues.
Unger et al. (1984) used gas chromatography to analyze PCB and DDE in breast tissue from two sets of cases and controls: a postmortem series (N = 53) and a biopsy series (N = 35). Although there was no significant difference in analyte concentrations between cases and controls in either series, the postmor-soft
Page 247
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Page 248
tem series is difficult to interpret because of the potential for fat or organochlorine loss in the course of progressive, fatal breast cancer. Mussalo-Rauhamaa et al. (1990) analyzed adipose tissue from the breast tissue of 44 living cases and 33 postmortem controls for several organochlorines. No difference was found between cases and controls in either PCBs or DDT metabolites, but the concentrations of ß-hexachlorocyclohexane (ß-HCH) were higher in the tissues of cases than in controls. The use of postmortem controls is not an optimal study-design feature, nor is the practice of excluding individuals with nondetectable concentrations of the analyte from the analysis, which for ß-HCH was approximately half of each group.
Falck et al. (1992) analyzed seven organochlorine compounds in the tissues of women presenting for diagnosis of a breast mass. Among 20 cases and 20 controls, they found higher DDE and PCB concentrations in cases than in controls, although there was no difference in DDE concentrations after adjusting for age and smoking.
Dewailly et al. (1994) attempted to differentiate estrogen-receptor positive (ER+) versus estrogen-receptor negative (ER-) breast-tumor tissues with respect to several organochlorines. In an analysis of 16 compounds in tissues from 17 control subjects and 18 cases, evenly divided between ER+ and ER-, they found mirex (an ant insecticide) and PCB congener 118 to be decreased in ER- cases relative to controls, and DDE and PCB congener 99 elevated in ER+ cases relative to controls. PCB congener 118 has antiestrogenic activity and no estrogenic activity; congener 99 has estrogenic activity (Hansen 1998). Although the number of subjects was too small to produce unequivocal results, a different association between organochlorine concentrations and breast cancer, depending on the ER status of the tumor, would be of interest. In this study, with a small sample size and many analytes being studied, some associations, either positive or negative, are likely to appear by chance alone.
Two studies with stronger epidemiologic design and analytic strategies—and larger numbers of subjects—are those of Wolff et al. ( 1993) and Krieger et al. (1994). Both were case-control studies nested within cohorts. Blood was drawn and a questionnaire was administered at the time of cohort inception. Questionnaire data were used in multivariate models as potential confounders or as effect modifiers of an organochlorine-breast-cancer association. In each study, DDE and PCBs were measured by gas chromatography with electron-capture detection. All PCB congeners were reported as a combined value, and although not specified, the DDE value presumably represents primarily p,p'-DDE.
In the study by Wolff et al. (1993), the cohort (n = 14,290) consisted of women living in the New York City area who attended a mammographic screening clinic between 1985 and 1991 and who agreed to have blood drawn at the time of clinic attendance. Within 6 mo of blood draw, 58 breast-cancer cases were diagnosed. A control group was formed of cancer-free women matched with respect to age, date of blood draw, and menopausal status. There were twocontinue
Page 249
controls for each postmenopausal case and a 4-to-1 ratio for premenopausal cases. The median age was 51, and 80% of the women were white. Higher serum concentrations of PCBs and DDE were observed in cases than were found in controls. Using conditional multiple-logistic regression analysis and retention of first-degree family history, months of lactation, and age at first full-term pregnancy in the model, the odds ratio (OR) was 3.7 (95% confidence interval (CI) 1.01 -13.50) for the top versus the bottom quintile of DDE. The OR for PCBs was elevated in all quintiles, 2-5, relative to the lowest. However, when PCB concentrations were included in the regression equation for DDE effect, the OR for DDE was further increased, and the PCB effect became nonsignificant.
The short interval between blood draw and case diagnosis could be considered a disadvantage of the study, if the carcinogenic process altered lipid and organochlorine concentrations. If such a phenomenon occurred, it would imply that the disease was altering the amount of exposure rather than the exposure causing the disease. Alternatively, organochlorine concentrations present during the period shortly before diagnosis might represent a fairly accurate dose measure of the integrated effects of all prior events and factors influencing dose. That hypothesis is based on the premise that the relevant window of exposure is a few months or years before diagnosis. Because this exposure window has been observed with pharmacologic sex steroid hormones in women, it could also be postulated that the effects of organochlorines could be observed quickly, rather than after a prolonged lag period. If this were the case, adjustments made in the Wolff et al. (1993) study to account for organochlorine excretion via lactation (which has been noted to reduce risk of premenopausal breast cancer (Newcomb et al. 1994)), might have been inappropriate, because lactation would already have been integrated into the biologic dose marker.
The study by Krieger et al. (1994) was based on a cohort of women who received a multiphasic examination and had blood drawn as participants of a California medical-group-practice plan during 1964-1971. Serum specimens were frozen and stored, and the women were followed for the diagnosis of breast cancer until the end of 1990. From among the cancers that developed, 150 cases and 150 controls were selected such that there were 50 cases and 50 matched controls in each of three racial groups: Asian, black, and white. Cases and controls were matched on race and ethnicity, age at multiphasic exam, date of joining the medical-care group, year of multiphasic exam, and length of follow-up. The mean age of the subjects was 45 at blood draw, and the mean follow-up was 14.2 yr. Blood was analyzed for DDE and PCBs.
For the combined group, there was no difference in mean DDE or PCB concentration for cases versus controls. When the racial groups were analyzed separately, white and black cases had higher DDE concentrations than did white and black controls, and Asian cases had lower concentrations than did Asian controls. Multivariate models controlling for body mass index, age at menarche,continue
Page 250
pregnancy, and menopausal status at diagnosis were used to provide risk estimates for tertiles of the organochlorine distributions.
Blacks and whites in the study by Krieger et al. (1994) exhibited some elevations in OR for the second and third tertiles of DDE relative to the first, but all 95% confidence intervals overlapped unity and none of the trend tests was statistically significant at p < .05. When the analysis of PCBs was separated by racial group, Asian and white cases had somewhat lower mean values than did their respective controls. ORs based on tertiles of the PCB distribution were below unity for whites and above unity for blacks, but at nonsignificant levels. Based on the data presented, one can agree with the authors' conclusion that there was no association between the specific serum organochlorine concentrations measured and the risk of breast cancer. However, it is also true that, had the trend in the data persisted with larger numbers of subjects in each racial group, ORs of two- to three-fold could have been interpreted as a positive finding (Savitz 1994).
Blood for the study was drawn before either DDT or PCBs were withdrawn from the U.S. market, so blood concentrations were high by comparison with the study by Wolff et al. (1993). The geographic locations of the studies also might have figured into the different concentrations found. Whereas in the study by Wolff et al. (1993) the controls' mean serum DDE was 7.7 ng/mL, the comparable numbers from Krieger et al. (1994) were 35.0 for whites, 43.4 for blacks, and 50.8 for Asians. Black women and Asian women had higher concentrations than did white women.
Overall, these studies published prior to 1995 do not support an association between DDT metabolites or PCBs and risk of breast cancer. Additional studies, published since that time, are summarized in Table 9-2. The table is divided into two groups. In the first group, organochlorine compounds were measured in adipose tissue; in the second group, concentrations were obtained from serum. Except for the small cohort by Sutherland et al. (1996), the studies are all case-control in design.
The first of the studies employing adipose tissue was a large case-control study from five European countries in which DDE concentrations were related to risk of breast cancer (van't Veer et al. 1997). Postmenopausal breast-cancer cases were identified from hospitals, and age-matched controls were obtained from population registries or from the register of the general practitioner of the case; 265 and 341 controls were included. Adipose tissue was removed by fine-needle aspiration from the buttocks within 1 wk of the case's diagnosis. Gas chromatography and electron-capture methods were used to measure DDE. Conditional logistic regression allowed for matching of age and sex, adjustment for body mass index, age at first full-term pregnancy, and alcohol consumption. ORs were calculated by quartiles of the controls' DDE distribution, with the lowest quartile being the reference group. The OR for the highest relative to the lowest quartile was 0.73 (95% CI 0.44-1.21) in a model, without adding covariates.continue
Page 251
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Page 252
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(table continued on next page)break
Page 253
(table continued from previous page)break
|
Page 254
|
(table continued on next page)break
Page 255
(table continued from previous page)break
|
Page 256
Addition of relevant covariates to the equation resulted in an OR of 0.48 (95% CI 0.25-0.95) with a p for trend in ORs of .02. Although DDE concentrations varied by country, overall they were low—1.9 µg/g in controls. The authors (van't Veer et al. 1997) equate this level with a serum concentration of 3.1 ppb. The latter number is less than half the mean serum value in the study by Wolff et al. (1993), and it is more than 10 times lower than the mean control value for white women in the study by Krieger et al. (1994). DDT use has been restricted in Europe since the early 1970s. These data could be interpreted to suggest an inverse association between body burden of DDE and risk of breast cancer. The authors stated that the data provided no support for the suggestion that exposure to DDE increased the risk of breast cancer.
The Swedish (Liljegren et al. 1998) and German (Guttes et al. 1998) studies were similar in that various compounds in breast adipose tissue were analyzed from women with invasive carcinoma (cases) and women with benign breast lesions. In both studies multiple compounds were measured in a small number of subjects. Whereas Guttes et al. (1998) found higher concentrations of DDE and PCBs numbers 1 18 and 153 in the fat of cases than controls, Liljegren et al. (1998) found no such associations. After reviewing case-control studies from numerous countries in which total DDT and PCBs concentrations had been analyzed in breast fat, Guttes et al. (1998) concluded that ''there is no correlation between concentrations of these substances in the human body and breast cancer."
Studies based on serum concentrations of various compounds include the Charleston Heart Study cohort of 405 white and black women who had blood drawn and analyzed for DDE in 1974-1975 (Sutherland et al. 1996). During follow-up that lasted until 1994, 20 women developed breast cancer. Proportional hazard regression models, including relevant covariates, provided no evidence of increasing breast-cancer risk with increasing serum concentrations of DDE. Although the numbers are small, the data are of interest because the average DDE concentration in 1974-1975 was 32.0 ng/mL—approximately the same as for white subjects in the study by Krieger et al. (1994).
More recently a publication appeared from the Nurses Health Study, a cohort of about 120.000 nurses followed since 1976 (Hunter et al. 1997). The authors compared blood levels of DDE and PCBs in 240 incident cases of breast cancer and equal numbers of paired, matched control women. Bloods were drawn in 1989 or 1990 and cases were diagnosed through May 1992. The results can be summarized briefly. For DDE, mean values (ng/ml) in cases were 6.01 and in controls 6.97. For PCBs, the mean values were 5.08 and 5.16 in cases and controls, respectively. The multivariate adjusted relative risk for breast cancer comparing the fifth (highest) with the first (lowest) quintile of DDE was 0.72, 95% CI 0.37-1.40. The analogous risk estimate for PCBs was 0.66, 0.32-1.37. Those findings are convincingly consistent with no association between plasma organochlorine levels and risk of breast cancer, with the best estimate of risk for both compounds being less than one.break
Page 257
Because most reports have been based on U.S. or European populations, a publication from Mexico, where DDT is still used for malaria control, should reflect the breast-cancer experience in a more highly exposed population (Lopez-Carrillo et al. 1997). A case-control study based on 141 newly diagnosed cases and equal numbers of controls from three referral hospitals in Mexico City between 1994 and 1996 produced findings very similar to those reported in other recent studies. Mean serum DDE levels (ng/mL) were 4.75 in cases and 4.07 in controls, not a statistically significant difference. When DDE levels for all subjects were divided into tertiles, the multivariate adjusted odds ratio for breast cancer for the highest relative to the lowest tertile was 0.76 and for the second versus the first tertile was 0.60. Confidence intervals of 95%, including unity, surrounded each estimate. The unexpected and not fully explained finding in this study was the relatively low blood levels of DDE among all study subjects. The levels were similar to those reported in the Nurses Health Study, which encompasses many geographic areas in the United States and the Wolff et al. (1993) study from New York City.
The small hospital-based case-control study by Schecter et al. (1997) examines DDT/DDE concentrations in the serum of women in Vietnam where DDT has been heavily used in the recent past. The p,p' DDE levels were more than twice as high (16.7 ng/mL in controls) as those characteristic of United States or European women. Little can be said about the lack of case-control differences given the small sample size.
The recent report by Høyer et al. (1998) was based on the population of women in the Copenhagen City Heart Study. From the sample of 10,317 women identified in 1967, bloods were drawn and stored on 7,712. This cohort of women was followed until 1993 through linkage to the Danish Cancer Registry to identify the occurrence of breast cancer. The 268 women who developed invasive breast cancer provided the cases for a nested case-control study in which two controls were matched to each case on age, date of examination, and vital status at the time of the case diagnosis. Serum samples were available for 240 cases and 477 controls (Høyer et al. 1998).
Using gas chromatographic techniques, serum samples were analyzed for 18 different pesticides or their metabolites and 28 different PCB congeners. The data analysis provided odds ratios estimated by conditional multiple logistic regression, with the reference group based on the lowest quartile of the frequency distribution of the particular compound in the controls. Data were presented on four of the 46 individual compounds that were subjected to laboratory analysis in addition to total PCBs and total DDT. There were no associations between any of the specific DDT isomers or metabolites or any of the individual PCB congeners and breast cancer. Of the 46 compounds analyzed, however, only dieldrin showed a positive association with breast cancer risk: odds ratios of 1.96 and 2.05 in the top two quartiles of the distribution of values. Whether this is a biolgically significant or a chance occurrence, given the 46 different compounds analyzed and the multiple compari-soft
Page 258
sons, is difficult to know. However, the observation is sufficiently interesting to warrant additional epidemiologic and laboratory studies.
The authors did not show the actual concentrations of dieldrin for either cases or controls. But for all subjects combined, dieldrin was not detectable in 22% of samples, a higher percentage than for any of the other compounds shown. Median blood concentrations for dieldrin were also significantly lower than those for other compounds.
Several studies have been conducted of workers employed in the production of dieldrin, settings that would allow for higher exposures than those experienced by the Copenhagen City Heart Study population. In these occupational studies, no type of cancer has been reported in excess (Van Raalte 1977; Ribbens 1985: de Jong 1991). Furthermore, the evidence for estrogenic activity of dieldrin is limited to testing in in vitro systems (the E-screen).
The Moysich et al. (1998) study is based on a subset of women from a case-control study of breast cancer conducted in western New York State from 1986 to 1991. DDE, HCB, mirex, and 73 PCB congeners were analyzed by gas chromatography with electron capture. Although the overall findings for individual and grouped compounds were essentially negative, a subgroup analysis of parous women who had never lactated revealed small elevations in the odds ratios for several compounds including mirex. Lactation appears to provide a means for reducing the body burden of various fat-soluble chemicals including the organochlorines under study.
Olaya-Contreras et al. (1998) conducted a hospital-based case-control study in Bogota, Colombia and, unlike the other studies in Table 9-2, found an association between DDE levels and risk of breast cancer. Mean and median concentrations in both cases and controls appeared low, and there is little information provided that helps to clarify this discrepant observation.
Occupational Studies
Studies are often conducted in occupational settings where workers are exposed to relatively high concentrations of chemicals used in the products and processes of manufacturing. Occupational studies are sometimes designed to use retrospective cohorts of employees working at a plant or in another occupational setting who are identified from employment records and characterized for likely exposure to the agent of interest. Exposure measurement is usually based on job title, industrial hygiene surveys of the ambient environment, measurements of air concentrations of chemicals, and, occasionally, sampling of blood concentrations. The cause-specific mortality experience of the cohort can be identified through death certificates and medical records up to a recent point in time. An unexposed reference population must be chosen from which to obtain the expected number of deaths or disease events. Analyses are then performed to identify differential mortality rates, or observed-to-expected deaths, by amountcontinue
Page 259
of exposure. The advantage of occupational studies over studies of the general population is the higher exposure encountered and the fact that some of the subjects will have been employed at the study site for long periods. Studies on occupational PCB and TCDD exposures are reviewed below.
Studies of occupational PCB exposures have been reported from Italy and the United States. Bertazzi et al. (1987) reported on the cancer mortality of workers engaged in the manufacture of capacitors impregnated with PCBs. Workers employed for at least 1 wk between 1946 and 1978 were enrolled and followed for the occurrence of cancer deaths from 1946 to 1982. PCB exposure was high, as shown by monitoring of air and surface areas. Blood concentrations in a selected sample of 37 workers averaged 282.8 ppb or 142.8 ppb, depending on the chlorine content of the PCBs. Several cases of chloracne were reported in 1954 and 1977, indicating TCDD exposure as well. Among the 1,556 women in the cohort, the 12 deaths from cancer and four deaths from hematologic neoplasms were significantly higher than the number expected, based on local population rates. It was later reported (Adami et al. 1995) that two of the cancer deaths were from breast cancer, where 1.99 would be expected in the local population.
In a retrospective cohort mortality study from two plants manufacturing electrical capacitors in New York and Massachusetts, 1,318 women were followed through 1982 for a total of 30,492 person-years (Brown et al. 1987). Forty-five deaths from malignancies occurred, compared with 48.3 expected based on U.S. general population rates. Of those deaths, nine were breast cancer deaths; 11.6 deaths would have been expected. The four deaths attributed to biliary tract cancers were noted to be in excess.
A cohort of 3,588 electrical-transformer and capacitor workers was followed from 1957 to 1986 (Sinks et al. 1992); 846 were women. Estimates of exposure were based on environmental sampling and the distance employees worked from the impregnation ovens. For cancer deaths of men and women combined, the standardized mortality ratio (SMR) was 0.8, using U.S. population rates as the reference. There was a suggested increase in deaths from malignant melanoma and brain cancer. No deaths from breast cancer were noted.
2,3,7,8-Tetrachlorodibenzo-p-dioxin functions by binding the aryl hydrocarbon (Ah) receptor and regulating the induction of specific forms of cytochrome P450, specifically aryl hydrocarbon hydroxylase (Skene et al. 1989). Ah receptor agonists, including TCDD, inhibit E2-induced responses in rodent models and in human mammary cell lines, suggesting antiestrogenic rather than estrogen agonist effects. TCDD is frequently a contaminant in other complex chemical mix-soft
Page 260
tures and the production of phenoxy herbicides. It has been shown to be carcinogenic in some laboratory animal species (IARC 1997).
Manz et al. ( 1991 ) reported on cancer mortality of a cohort of workers in a West German herbicide plant that was heavily contaminated with TCDD. A cohort of 1, 184 men and 399 women hired between 1952 and 1984 was followed until 1989, during which time malignant neoplasm was the underlying cause of death for 93 men and 20 women. Individuals were classified into three exposure groups (high, medium, and low), according to the production departments in which they had worked. German national data provided the reference rates for calculating SMRs. Among women, the SMR for all malignant neoplasms was 0.94 (0.58,1.45); the SMR for carcinoma of the breast was 2.15 (0.98,4.09), based on nine deaths. Interpretation of this possible excess is difficult: Only 7% of the women worked in high-exposure departments, compared with 40% of the men. Furthermore, some processes in the plant involved exposure to other carcinogens, such as benzene. No data are provided for women to indicate whether risk was associated with duration of employment or inclusion in the high-exposure group. In an occupational cohort of phenoxy herbicide workers in Denmark, which included 1,069 women, there was no excess of breast cancer (Lynge 1985). However, the herbicide (4-chloro-2-methyl-phenoxyacetic acid) was not thought to be contaminated with significant concentrations of TCDD.
An international registry of workers occupationally exposed to chlorophenoxy herbicides, chlorophenols, and dioxins has been established by the International Agency for Research on Cancer (IARC) and the National Institute of Environmental Health Sciences (NIEHS). Most reports have concentrated on men or on men and women combined (Saracci et al. 1991). Kogevinas et al. (1993) used data from the registry to report on incidence and mortality from malignant neoplasms in 634 exposed women with an accumulated 10,782 person-years at risk. Cause-specific national death rates and cancer-incidence rates were used as the referents. Exposure classification was based on job history; no blood concentrations of any chemicals were available for women. Cancer risk for all neoplasms combined and for breast cancer specifically was not increased; the standardized incidence ratios (SIRs) were 96 and 91, respectively. Only one of the seven incident breast-cancer cases had probable (versus unlikely) exposure to TCDD. The SMR for all malignant neoplasms combined was also low (66). The SIR and SMR were elevated for the subset of workers who were most probably exposed to TCDD. Attribution of risk is tenuous, however, because these workers were exposed to many other toxic chemicals. Three of the nine cancer deaths were malignant melanomas among New Zealand residents, for whom ultraviolet radiation was likely to be a prominent exposure.
The occupational cohorts add little information either to support or to refute an association between PCBs or TCDD and risk of breast cancer. Primarily, they are unsatisfactory because of the small cohort sizes and few deaths, resulting in low power to detect true effects and a significant opportunity for reporting effectscontinue
Page 261
that are merely chance events. In the years covered by these studies, 1950s to 1980s, few women were employed in manufacturing, and of those, fewer still were in the most heavily exposed occupations.
Other problems pervade the studies to make them less informative than the case-control studies previously reviewed. The exposures are generally complex. Although the analysis is focused on PCBs or TCDD, many organic solvents and other toxicants, including carcinogens, are involved in the manufacturing processes. Therefore, even if an excess cancer risk were identified, it might be impossible to attribute it to a single chemical. Exposure assessment is frequently sketchy, based on ecologic information about the workplace rather than on individual exposure measurements. Interindividual variability in metabolism and excretion of chemicals is rarely measured. Information on potential confounders of the association between exposure and disease is usually lacking. For breast cancer, that is probably less critical than it would be for a disease such as lung cancer, for which smoking has a large effect on disease risk that could be differentially distributed among the exposure groups. The choice of reference population from which the expected number of cases is derived will influence the SMR or SIR. Use of general population rates tends to reduce the magnitude of the SMR because of the "healthy-worker" effect: People who are employed and able to work are, in general, healthier and at lower risk of death than the population at large, which includes many ill and less-fit persons. Also, the use of different reference populations for obtaining rates makes it impossible to compare SMRs or SIRs directly among studies.
Natural Experiments
An industrial accident at a chemical plant near Seveso, Italy, in 1976 provides information on cancer effects in a population exposed to TCDD (Bertazzi et al. 1989). The area surrounding the plant was divided into zones (A, B, and R) based on decreasing mean concentrations of TCDD soil contamination. Subject exposure classification was based on area of residence. The referent cohort lived in an uncontaminated area. A 10-yr mortality analysis revealed no clear association between site-specific cancer deaths and area of residence. In men and women, the risks of death from all malignancies combined were not elevated—irrespective of zone or time after the accident. None of the 193 people with confirmed chloracne died during the study period. Residents of the contaminated zones appeared to be at decreased risk of death from breast cancer.
A report on cancer incidence after the Seveso accident appeared in 1993 (Bertazzi et al. 1993). As with the mortality study, relative risks and 95% confidence intervals were reported separately for each of the three exposure zones. The authors stated that there was a deficit of breast cancer, because the relativecontinue
Page 262
risk declined with increasing exposure. In zone R, with 106 cases, the relative risk was 1.1 (95% CI 0.9-1.3); zone B had ten cases, and relative risk was 0.7 (0.4-1.4); zone A had one case, and relative risk was 0.5 (0.1-3.3). For zones A and B, the estimates were imprecise because of the small number of events.
Increased relative risk was observed for several neoplasms: hepatobiliary cancers; hematologic malignancies including lymphosarcoma, multiple myeloma, myeloid leukemia, and non-Hodgkin's lymphoma; and soft tissue tumors. Most of those diagnoses were elevated in only one sex or exposure zone. However, the pattern of results supports the prior literature on TCDD, showing an excess of soft tissue sarcomas and lymphomas, and possibly of malignancies, at other sites. No mention is made of other chemical contaminants released during the accident or their possible role in causing cancer.
An accidental poisoning of the food supply with polybrominated biphenyls (PBBs) occurred in Michigan in 1973. A cohort of 3,653 individuals who consumed contaminated foodstuffs was followed until 1991 for cause of death (Sinks et al. 1996). PBB blood concentrations were assayed from blood samples drawn in 1976. Mortality from all causes and all cancers was less than expected. The SMR for breast cancer also was reduced, based on the five deaths observed. Only for cancer of the stomach was there a suggested increased risk of death. An earlier incidence study based on the same cohort of subjects had reported higher risks of breast cancer among women with PBB blood concentrations >2 ppb compared with those with blood concentrations <2 ppb. The analysis was based on 20 breast-cancer cases, and 95% CIs for all risk estimates encompassed unity (Henderson et al. 1995).
Ecologic Data
Overall, existing epidemiologic data provide little support for a positive association between the HAAs discussed in this chapter and the risk of breast cancer (Longnecker et al. 1997). Any positive association suggested in earlier studies becomes less tenable in view of more recent data from large, well-designed investigations. Although the analytic epidemiologic data previously presented provide little support for this supposition, ecological analyses could be useful to indicate whether the hypothesis is plausible. Trends in exposure and disease should be positively correlated, taking into account a biologically relevant lag time between them. Human adipose concentrations of DDT, p,p'-DDE and PCBs were at their peak in the United States in the 1970s. Since then, serum and adipose concentrations have declined, consistent with the declining concentrations in the environment and in food sources (Kutz et al. 1991). The decline has been 5-fold or more for serum concentrations of p,p'-DDE, a stable DDTcontinue
Page 263
metabolite (Krieger et al. 1994; Wolff et al. 1993). Products that contain PCBs are still in use, although they are not newly manufactured, so human exposure to PCB congeners is declining more slowly. It seems unlikely that a declining exposure would be responsible for an increasing incidence of cancer.
There are other uncertainties in establishing a relationship between specific HAAs measured in the above studies and breast cancer. Specifically, after age 40, breast-cancer incidence rates are consistently higher in whites than they are in blacks, and the disparity increases with age. Conversely, serum concentrations of p,p'-DDE and PCBs are consistently reported to be higher in blacks than in whites (Krieger et al. 1994; Sutherland et al. 1996; Schildkraut et al. 1999). However, those differences might reflect differences in sensitivity, perhaps due to genetic or phenotypic differences. Differences in the distributions of the polymorphisms for various metabolizing enzymes by racial group that could affect serum concentrations are not well characterized (Millikan et al. 1995).
Endometrial Cancer
It was estimated that in 1997 there would be 34,900 new cases and 6,000 deaths from cancer of the uterus (Ries et al. 1997). The 1990-1994 average age-adjusted incidence rate was 21.5 per 100,000 women. Between 1973 and 1994, the incidence of uterine cancer decreased by 27.9%.
If some HAAs have either an estrogenic or an antiestrogenic effect in humans, that effect should be most easily observable in the endometrium. Unfortunately, there are few data on the topic. One case-control study of endometrial cancer reported on women from five geographic regions of the United States (Sturgeon et al. 1998). A subset of 90 cases and 90 matched community controls had blood drawn for analysis of serum organochlorine concentrations. Laboratory analyses were conducted to identify four DDT-related compounds, 27 PCB congeners, and 13 other organochlorine compounds. Results, corrected for serum lipid concentrations, were shown for a portion of the compounds tested; three of four DDT metabolites, PCBs grouped by their likely estrogenic, antiestrogenic, or enzyme-inducing properties, and six of the 13 other compounds. Many of the compounds were non-detectable in 40% or more of the subjects tested. Relative risks were calculated based on quartiles or tertiles of the distribution of values in the control subjects. No statistically significant increases in relative risks were observed for "high" versus "low" levels of any individual compound or group of compounds.
The retrospective cohort studies in occupational settings are uninformative. Neither Sinks nor Bertazzi mentioned uterine cancer with respect to PCBs (Bertazzi et al. 1987; Sinks et al. 1996). Brown and CDC (1987) reported an SMR for "female genital organs" of 85. The most common cancer of the female genital organs arises from the uterine cervix, and it has a epidemiologic risk profile that is different from that for carcinoma of the endometrium.break
Page 264
The TCDD occupational data are only slightly more informative. Manz et al. (1991) provided no information on cancer-specific causes of death in women except for breast cancer. Saracci et al. (1991 ) noted an SMR of 94 for "female genital organs." The Lynge (1985) study of phenoxy herbicides in Denmark provided a corpus uteri relative risk of 0.67 for exposed women based on two observed cases. However, this cohort was thought not to be heavily exposed to TCDD. In white populations, more than 95% of cancers of the corpus uteri are endometrial; the remainder are sarcomas of the uterine muscle and supporting tissues. None of the 9 reported cancer cases or deaths in the combined international cohort of women exposed to TCDD were endometrial or "corpus uteri" cancers (Kogevinas et al. 1993).
In the Seveso industrial accident, cancer of the uterine corpus was reported only from zone R, the most heavily populated but the least heavily exposed of the three exposure zones (Bertazzi et al. 1993). In zone R, the relative risk for corpus cancer was 0.5 CI (0.2-1.0), based on 9 cases. That apparent risk reduction is consistent with the antiestrogenic effects of TCDD described by in vitro assays and animal models.
Endogenous and Exogenous Hormones and Their Effects in Women
The relevance of data on therapeutic exogenous hormones (e.g., oral contraceptives and HRT) to HAAs in the environment is not known. Although exposure to HAAs could be continuous, analogous to the administration of hormonal drugs, the potency of the HAAs is orders of magnitude lower than that of the endogenous steroid hormones present in premenopausal women or of exogenous HRT exposure in postmenopausal women (Adami et al. 1995).
If chemicals exert hormonal actions in humans, one of the most responsive tissues that would demonstrate the effects of such actions is the lining of the uterine cavity. Endometrial tissue proliferates, becomes secretory, and then sloughs as the result of stimulation by ovarian estrogens followed by progesterone in the normal menstrual cycle. Glandular tissue of the breast also undergoes changes in response to cyclical endogenous hormones. However, the endometrium is distinctive in its response to exogenous sex steroid hormones. Estrogens alone, when given as replacement therapy after the menopause, have been shown to cause cellular proliferation, hyperplasia, and carcinoma. The time required for these changes to occur could be a few months or years. One to 3 yr of unopposed estrogen use increases risk for the development of carcinoma; after 10 yr of use, the relative risk estimate approaches 10-fold. For this reason. progestins have been added to the hormone replacement regimen for women with intact uteri. Progestins block the induction of cell proliferation by estrogens and cause cellular differentiation in the uterus, thereby protecting the endometrium from cancer development. Progestins can be given continuously with estrogen orcontinue
Page 265
for 10 d or more of a monthly cycle. With the latter regimen, bleeding occurs monthly and the endometrial cells sluff. Combined treatment reduces endometrial cancer risk, although a small elevation above nonuser concentrations still remains (Weiss et al. 1979; Hulka et al. 1980; Brinton and Hoover 1993).
Modern oral contraceptives contain both an estrogen and a progestin in every dose. In the past, sequential oral contraceptives, which contained only estrogen in a portion of the monthly pill supply, were available. When endometrial cancer occurred in women of reproductive age using these products, sequential products were taken off the market (Piper and Kennedy 1987).
The latency period between exposure to a cancer-inducing agent and cancer diagnosis must be considered. Most of the experimental data from in vitro systems and animal models indicate that estrogen agonists have a proliferative effect on cells and tissues. In models of carcinogenesis, this is considered a promotional action and its effect on cancer production is rapid. Thus, one would expect to see the effects of these compounds on human cancer risk within a few years.
A human example of this model system exists for estrogen-replacement therapy and endometrial cancer. In the late 1960s and early 1970s, endometrial cancer incidence rates had taken an abrupt upward turn (Weiss et al. 1976). Although not recognized at the time, the rise was consistent with the increasing use of estrogens, unopposed by progestins, for HRT. Publication of the first epidemiologic studies showing a strong association between estrogen-replacement therapy and endometrial cancer appeared in December 1975 (Smith et al. 1975: Ziel and Finkle 1975). Those reports had a rapid effect on sales of noncontraceptive estrogens, which dropped by about one-third between 1975 and 1980 (Kennedy et al. 1985). The decline was associated with a rapid decline in the frequency of endometrial-cancer reported to the SEER registries. In California, the decline in endometrial cancer incidence was almost concurrent with the decline in prescribing estrogen for replacement therapy (Austin and Roe 1982). After the estrogen-induced epidemic of the 1970s, age-adjusted incidence rates for endometrial cancer declined from 28.4 per 100,000 women in 1973 to 21.6 per 100,000 in 1992 (Kosary et al. 1995). If HAAs in the environment are acting as estrogen agonists, their cancer-inducing effects should be evident in the endometrium.
Hormonal effects on breast tissue are different from those observed in the uterus. Endogenous estrogens cause glandular cells to proliferate, but the addition of progesterone in the latter half of the cycle not only produces maturation effects but causes structural changes in the glands such that they become more complex and extensive. The cancer-causing effects of exogenous hormones on the breast have been studied extensively in epidemiology studies. Still, major studies of HRT are not in full agreement on its effect. If one accepts the data from the Nurses Health Study (Colditz et al. 1995), the relative risk of breast cancer is 1.3 with estrogen alone and 1.4 with estrogen plus progestin. Thesecontinue
Page 266
risks are higher for women in their 60s, who are current hormone users of at least 5 yr duration. It seems likely that both recency of use and duration of use affect the risk estimates. Most researchers agree that adding a progestin to the estrogen does not reduce the risk of breast cancer as it does for endometrial cancer.
The risk of breast cancer from the use of oral contraceptives has been studied extensively (IOM 1991). One recent publication compiles information from almost all of the epidemiology studies on this topic published in recent years (Collaborative Group on Hormonal Factors in Breast Cancer 1996). A complete reanalysis was done from the original data, providing more than 50,000 cases and 100,000 control women. Small increments in risk (1.2-1.5) were observed for current and recent use; no increase in risk was observed after 10 yr or more since last use.
The use of diethylstilbestrol (DES) to reduce spontaneous abortion is frequently cited as a model for the effects of HAAs in the environment. Adenocarcinoma of the vagina has been documented in the female offspring of treated mothers, occurring at a rate of about 1 per 1,000 treated pregnancies. However, follow-up of the mothers themselves for the development of breast cancer has revealed surprisingly little. Relative risks of up to 1.5 have been reported, and some studies have shown no risk elevation (Colton et al. 1993). It should be noted that DES is an extremely potent synthetic estrogen; it was given in doses ranging from 5 mg/d in the first trimester to 150 mg/d in the third trimester of pregnancy (Mittendorf 1995). It is not clear how this unfortunate human experience, based on high doses of a potent estrogen taken orally for a limited time. relates to exposure to HAAs in the environment.
Testicular Cancer
It was estimated that in 1997 there would be 7,200 new cases and 350 deaths from testicular cancer in the United States (Parker et al. 1997). The disparity between cases and deaths indicates the effectiveness of treatment and the excellent prognosis for those diagnosed with the disease. Testicular cancer is uncommon in white men and rare in black men. The 1990-1994 average age-adjusted incidence rates were 5.3 per 100,000 for white men and 0.7 per 100,000 for black men in the United States. Secular trends in incidence rates differ for whites and blacks. Between 1973 and 1994, white men exhibited an average annual increase of 2.4%, whereas black men experienced an annual decrease of 0.2%. Both groups showed a decline in mortality rates that averaged 5.6% annually (Ries et al. 1997). Figure 9-1 shows incidence and mortality trends for white and black men in the United States. The peak incidence rates are in the 25- to 39-yr age groups: for whites ages 30-34, 16.7 per 100,000; for blacks ages 25-29, 1.9 per 100,000, and for blacks ages 34-39, 1.9 per 100,000. Testicular cancer is unlike most major cancers with respect to its rarity (particularly in blacks), its excellent response to treatment, and its age incidence curve, where young adults are atcontinue
Page 267
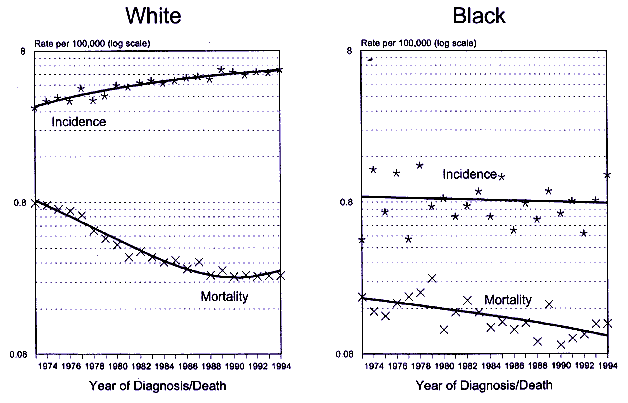
Figure 9-1.
SEER incidence and U.S. mortality for testicular cancer in men under 65 yr of age by race, 1973-1994. SOURCE: Ries
et al. 1997.
Page 268
highest risk. The latter pattern is suggestive of etiologic factors operating in utero or in childhood, or from genetically transmitted susceptibility.
Most analyses of blood concentrations of PCBs, DDT, and DDE have shown significantly higher concentrations in blacks than in whites (Krieger et al. 1994: Sutherland et al. 1996; Schildkraut et al. 1999). If these particular chemicals were etiologically related to testicular cancer, one might expect the racial differences in incidence rates to be consistent with the differences in their concentrations. Just the opposite was observed, with very low rates of testicular cancer in blacks relative to whites and reasonably stable rates among blacks over time. Interpretation of these results is complicated by genetic differences between populations.
Epidemiologic studies of testicular cancer have identified a single well-established risk factor: cryptorchidism-a condition marked by the failure of the testes to descend into the scrotum-is consistently elevated among cases relative to controls. It has been estimated that cryptorchidism accounts for up to 10% of all cases (Chilvers and Pike 1992). The data suggest that exposure to DES in utero does not increase the risk of testicular cancer in male offspring (Leary et al. 1984).
Incidence rates for testicular cancer are available from cancer registries in ten northern European countries for the past 3-4 decades. Analyses of these data show a large variation in testicular-cancer incidence rates among countries for each decade. However, all countries were consistent in showing a secular trend of continuously increasing rates (Adami et al. 1994). The reason for the increase is not known. Data were available on concentrations of p,p'-DDE in breast milk from four of the Scandinavian countries in this study. The DDE values were similar in all four countries and showed similar declines from 1965 to 1992 (Ekbom et al. 1996). Breast milk is a relevant medium for assessment of exposure to the fetus and to the infant, because it indicates the presence of xenobiotics in blood to which the fetus is exposed and actual intake during breast-feeding. The decline in p,p'-DDE concentrations in breast milk during the same period that testicular-cancer rates have been increasing, including a biologically plausible time lag, seems inconsistent with a causative role for DDT.
Several occupational cohorts, including workers exposed to herbicides contaminated with TCDD or to PCBs in manufacturing processes, have reported site-specific cancer mortality as an outcome. Only from the International Cohort of Workers Exposed to Phenoxy Herbicides and their Contaminants was testicular cancer reported: SMR = 225 (95% CI, 90-464) (Saracci et al. 1991). Testicular cancer was not reported from other occupational studies--either because it is a rare disease that does not occur in excess or because it is diagnosed and successfully treated. Incident testicular-cancer cases were not in excess among individuals exposed to TCDD in the Seveso accident (Bertazzi et al. 1993).
There are no published case-control or cohort studies of testicular cancer in which blood concentrations of any HAAs have been measured. Such studies are clearly needed because exposure assessments derived from estimation procedurescontinue
Page 269
could be poorly correlated with serum concentrations. This was found to be the case for TCDD among Vietnam veterans (CDC 1988). Blood concentrations provide a more accurate assessment of internal dose and potential biologic response.
In a recent ecologic study, a regression analysis was conducted using p,p'-DDE concentrations from human adipose tissue obtained in 1968 under the U.S. EPA Human Monitoring Program to predict testicular cancer mortality among white males in 22 states. (African-American males could not be analyzed because of their low testicular cancer mortality rates (Cocco and Benichou 1998)). Testicular cancer mortality rates were averaged for each 5-yr interval from 1971 to 1990. The coefficients from the regression analysis were negative in five of the six models, indicating no positive association between DDE and testicular cancer mortality some 2 to 22 yr later. Limitations of this study are the lack of exposure information on individual subjects (characteristic of ecologic studies), the limited statistical power, and the ad hoc nature of the population samples for adipose tissue. However, the findings are of interest since p,p'-DDE has been shown to bind to the androgen receptor in male rats producing an anti-androgenic environment.
Prostate Cancer
Prostate cancer is the most commonly occurring cancer among men in the United States, with 334,500 new cases and 41,600 deaths estimated for 1997 (Parker et al. 1997). Incidence rates, averaged over 1990-1994, were 155.8 per 100,000 for whites and 229.3 per 100,000 for blacks (Ries et al. 1997). The age-adjusted mortality rates were 24.3 and 55.5 per 100,000 for whites and blacks, respectively. Age-specific rates in both racial groups start to increase in the late 40s and continue to increase exponentially through the oldest age groups. The reasons for the higher incidence and mortality rates in blacks than in whites have been studied extensively but not fully explained.
Since the mid-1980s age-specific and age-adjusted incidence rates have increased abruptly, and this shift is superimposed on an existing secular trend of gradually rising rates (Ries et al. 1994). An abrupt increase in mortality rates has not occurred, although the steady upward trend continues for blacks and whites. These changes are shown in Figure 9-2. The increase in incidence rates coincides with and is attributed to the increasing clinical acceptance of prostate specific antigen (PSA) as a test for the early detection of prostate cancer. Screening for prostate cancer is a problematic issue because autopsy studies suggest that about 10% of men ages 50-59 harbor latent prostate cancer, and that percentage rises with age (Sheldon et al. 1980): By age 70, the figure is nearer 30%. Although the majority of the latent lesions never progress to clinical cancer, many are detectable through PSA. Thus, the epidemic of prostate cancer arises both from earlier diagnosis of cancers that would otherwise appear at a more advanced stage later in life and from those that would never surface during a lifetime in the absence ofcontinue
Page 270
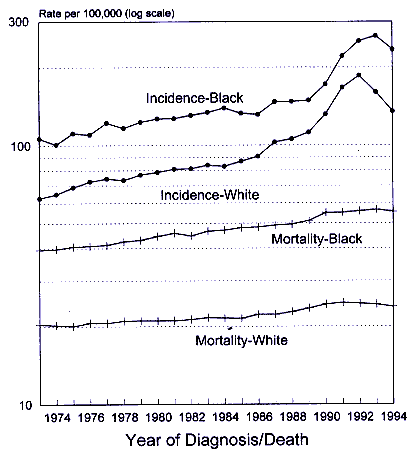
Figure 9-2.
SEER incidence and U.S. mortality for prostate cancer, 1973-1994. SOURCE:
Ries et al. 1997.
screening. The abrupt rise in incidence of prostate cancer since the mid-1980s can be attributed to screening. The 1993-1994 decline may be attributed to the depletion of prevalent cases. But what accounts for the long-term rise in both incidence and mortality is not known. Many factors have been considered, including diet, exposure to infectious agents, reproductive patterns, sexual behavior, lack of exposure to sunlight (inadequate vitamin D), and, currently, exposure to HAAs or other environmental chemicals.
Occupational studies of PCB-exposed workers give no hint of an association between PCBs and prostate cancer. Of the studies reviewed, none provided data on prostate cancer deaths (Bertazzi et al. 1987; Brown and CDC 1987: Sinks et al.continue
Page 271
1992). Had deaths occurred, they would most likely have been reported along with the other site-specific cancer deaths.
In the Seveso incidence study, prostate cancers were reported only from zone R (the low-TCDD-exposure zone), and their numbers were consistent with expectations (Bertazzi et al. 1993). However, incidence studies of prostate cancer require special design considerations because of the potential for detection bias due to screening or diagnostic and therapeutic procedures undertaken for benign prostatic hyperplasia.
Mortality studies of prostate cancer can avoid many potential biases. Detection bias is unlikely to occur unless the diagnosis is made at autopsy where the number of histologic sections reviewed correlates with the chances of finding occult cancer. Also, a prostate cancer diagnosis on a death certificate is not unequivocal; it requires verification by medical records and a physician's report.
In the 10-yr mortality follow-up of the Seveso population, three deaths from prostate cancer occurred in zone B, and 16 occurred in zone R. In each area, the numbers were slightly greater than expected, but the variation was not statistically significant (Bertazzi et al. 1989).
Fingerhut et al. (1991) reported on cancer mortality among workers in U.S. chemical companies that made TCDD-contaminated products between 1942 and 1984. Exposure assessments based on years of exposure were validated for a sample of 253 workers from whom serum TCDD concentrations were obtained. In this highly exposed population, the correlation between the logarithm of years exposed to contaminated products and the logarithm of serum concentrations was good (r = 0.72). Among the subcohort that had worked more than a year with at least a 20-yr latency, the SMR for prostate cancer was 152 with 95% CIs of 70-290.
In the cohort study based on the international registry of workers exposed to TCDD-contaminated chlorophenoxy herbicides, there was no increased prostate cancer mortality (Saracci et al. 1991). Worker populations reported from Germany (Becher et al. 1996; Manz et al. 1991) showed a small, statistically insignificant excess in prostate cancer deaths based on seven cases. (There appeared to be duplication in the populations appearing in the two reports.)
These occupational studies were not designed to address the question of which chemicals (if any) are associated with prostate cancer or whether any of the chemicals acts through a hormonally related mechanism. They do provide an incentive to undertake studies with more accurate exposure assessment (serum or adipose-tissue analyses) focused on prostate cancer.
The ecologic study noted previously under Testicular Cancer (Cocco and Benichou 1998) also evaluated prostate cancer mortality rates for the 22 states from which the U.S. EPA's Human Monitoring Program had human adipose tissue. Adipose tissue was analyzed for p,p'-DDE concentrations in 1968. Separate models were produced to predict both African-American and white male mortality rates in each 5-yr time period from 1971 to 1990. The regression coefficients for adipose tissue p,p '-DDE were negative for each race and time period, indicating no positive association between p,p'-DDE and prostate cancer mortality. The usual constraintscontinue
Page 272
of ecologic studies need to be recognized in interpreting these data. In addition, because the primary known mode of action of p,p'-DDE is antiandrogenic these results are not surprising. However, the lack of support for any positive association makes the theory of a positive association less tenable.
Summary and Conclusions
This chapter focuses on breast, endometrial, prostate, and testicular cancers. since these are known to arise in hormonally sensitive issues. If HAAs have a role in cancer formation in humans, their activity should be most evident in tissues that are known targets for endogenous and exogenous sex steroid hormones. The data that exist for evaluating the postulated relationship between HAAs and human cancers are mostly limited to studies involving exposure to DDT. DDE, TCDD, and various PCBs. Other compounds with potential hormonal activity have received little attention. Furthermore, exposure to HAAs and their possible effects during susceptible periods such as fetal life or pregnancy and transgenerational effects have not been evaluated in human studies.
Several recent studies have added to our knowledge concerning a possible association between HAAs and breast cancers. These studies have included large numbers of women and employed internal dose measurements of exposure. In these studies, DDE and PCBs were measured in blood or adipose tissue collected from the subjects at varying time intervals before the diagnosis of breast cancer. thus accounting for varying exposure windows and latency periods, which may be relevant to breast cancer development. Individually and as a group, these studies do not support an association between DDE and PCBs and cancer in humans. However, little is known about the effect of other compounds with hormonal activity in in vivo and in vitro systems.
Endometrial tissue is extremely responsive to the actions of estrogenic and antiestrogenic compounds. Therefore, it should be a sentinel target tissue for HAA actions. The actions should be most readily observable in postmenopausal women in whom endogenous steroid hormone concentrations are low. Decreased frequency of endometrial cancer was suggested in one study of environmental exposure to high TCDD concentrations. No association was found between a variety of organochlorine compounds and risk of endometrial cancer in the one published case-control study.
There are no analytic epidemiologic studies that examine a connection between exposure to HAAs and testicular cancer. Increasing testicular cancer incidence rates in northern European countries and in North America are unlikely to be related to environmental DDT because exposure, as measured by concentrations of DDT metabolites in both serum and breast milk, has declined significantly over the past 40 yr. Although testicular cancer incidence rates have been increasing in the United States in white men, they are extremely low and declining in black men, whereas most analyses of blood concentrations of PCBs, DDE, and DDT are higher in blacks than in whites. Ecologic data alone cannot confirmcontinue
Page 273
an etiologic hypothesis. However, lack of consistency between ecologic data and the hypothesis reduces the plausibility of the hypothesis. Interpretation of these results is complicated by genetic differences between populations.
Epidemiologic data on prostate cancer are derived almost exclusively from occupational and environmental studies that do not include measurements of dose in body fluids or tissues. In occupational studies, exposure to the individual is estimated from job classification, years worked, and environmental monitoring. These studies show no association between PCB exposure and incidence of prostate cancer. However, several studies show small increases in prostate cancer mortality in relation to TCDD exposure, although none of the risk estimates was statistically significant. The antiestrogenic activity of TCDD could alter the functional balance between estrogen and androgen in the prostate resulting in a hormonal environment conducive to cancer formation. However, the currently available data are not adequate to evaluate this possibility.
Recommendations
Based on the committee's evaluation of the available human data on carcinogenicity, the following is recommended:
—Case-control and retrospective cohort studies that use biologic samples (fat or serum) to assay for HAA exposure are needed. If appropriately designed and conducted, such studies can document the presence or absence of associations between HAAs and cancer and whether the associations found are likely to be causal.
—Design considerations in epidemiologic studies must take into account the relevant latency period between exposure and disease, the timing of the exposure with respect to susceptible life stages, and the role of potential confounders that could distort any associations identified.
—Exposure assessment based on biologic markers and/or chemical concentrations in blood or adipose tissue stores should be included in epidemiologic studies. Exposure estimation based on occupational classification, environmental pollution, or dietary intake is rarely accurate for evaluating cancer risks.
—Biologically relevant hypotheses based on HAA toxicologic profiles and activities in animal models need to be incorporated into the epidemiologic studies.
—The biologic potency of HAAs must be related to that of endogenous hormones in premenopausal and postmenopausal women and in men. Additional comparisons should be made with pharmacologic estrogens (hormone-replacement therapy and hormonal contraceptives) and phytoestrogens because large segments of the population are exposed to these compounds.
—Compounds in addition to DDE and PCBs need to be evaluated in human studies of cancer risk.break































