B
Summary of Workshop Presentations
This section summarizes the presentations and discussions by speakers, committee, and participants at the IOM workshop. (See Appendix D for agenda and lists of speakers and participants.) This workshop was useful for understanding how to broaden the topic, identify areas in which there is or is not consensus, and spot issues that need further attention. The focus of the workshop was to address the three questions that comprised the study's statement of task:
-
What areas within the existing portfolio are likely to yield information appropriate to this topic? What are the knowledge gaps that warrant future research?
-
Are there research strategies and priorities for addressing the knowledge gaps?
-
What other strategies, including interagency coordination, might improve the prospect of developing knowledge that will identify gender differences in susceptibility to environmental factors?
In addition to the above three considerations, each panelist was asked to provide suggestions for further research and interagency collaboration.
The workshop was composed of two panels. The first panel examined the overall issue of patterns of environmental exposure among women. The second panel focused on patterns of susceptibility to environmental factors. Speakers on each panel also participated in the general discussion of how current information applies to the three questions above. The balance of the workshop was devoted to a general discussion of federal efforts and resources and the opportunities for collaboration among federal agencies.
Panel I: Patterns Of Exposure
Environmental Exposure in the Workplace1
Research on gender differences in susceptibility to environmental exposures in the workplace must answer a number of questions relating to physiological and hormonal differences, differences in susceptibility and deposition of toxicants, and to metabolic and genetic differences between men and women. These issues will be addressed by other speakers. However, research on environmental exposure and gender susceptibility also requires careful attention to issues of methodology and experimental design. For example, there are no standardized definitions of job titles or job content, making it difficult to compare the actual exposures or outcomes of different groups of workers, male and female. Failure to address these conceptual problems will lead to inaccurate data, misleading findings, and poor policy decisions.
Approximately 20 years ago, General Motors, among other employers, established a so-called ''fetal protection policy" that simply excluded all fertile women from jobs that involved exposure to lead, regardless of the women's intentions with regard to childbearing. Nearly two decades later, the Supreme Court, in a 9-0 decision (UAW v. Johnson Control), ruled that employers could not establish such fetal protection policies, because there was abundant evidence that pregnant women were not the only group of workers vulnerable to the effects of lead. One of the lessons learned from this experience is that research which seeks to explore issues of differential vulnerability to toxins in the workplace should not be narrowly focused on those groups perceived as most vulnerable but should encompass all groups that are "at risk."
When research is carried out on a particular biological process, such as pregnancy, care should be taken to avoid over-extrapolation of the results to broad categories of workers, as this can lead to the development of poor social policy. Conversely, research which is grounded on clear definitions of the limitations to generalizability and on hypotheses that take into account the social and physical realities of the workplace and of employment can provide a sound scientific basis for prevention and control of environmental hazards in the workplace.
A major methodological problem in carrying out such research is that men and women do not encounter the same environmental exposures at work. For the most part, American women remain in "ghettoes of employment." Today there are more women in the workforce, but the traditional employment patterns of women have not changed significantly over the past 50 years (see Figure B-1). Twenty-one percent of all professional women are still engaged in teaching in secondary (i.e., below college-level) education. Women still dominate in
primary education, government, and clerical employment. Men still dominate in industries with the greatest potential occupational exposure to hazards (e.g., construction, manufacturing).
Furthermore, when men and women are employed in the same sectors, the distribution of job titles is different. One example is the health care sector, where 72 percent of the workers are women, but this includes 94 percent of the registered nurses and only 20 percent of the physicians. And even when men and women have the same job title, women, in general, have different tasks than men. In one study of workers in the Canadian poultry industry, for example, women working within the processing sector had an elevated accident rate compared to the male workers. When the data were analyzed according to the actual tasks performed, and when the working conditions were surveyed, it was found that there were substantial differences in the nature of the work and its physical construction that could successfully explain a great deal of this reported rate difference.
For example, the height of the workstation, the design of protective equipment, the rate of work, the number of breaks, and the physical mobility around the plant placed women workers at a disadvantage. In practice, it is very difficult to find examples of work environments where men and women are exposed to the same environmental risks and factors. In "high-exposure" industries, furthermore, there have not been enough women employed, exposed to enough risks, and for enough time to draw statistically meaningful conclusions about differential susceptibility. Inconsistencies in employment statistics collected by various federal agencies create additional problems in data collection and analysis for occupational environmental research.
An even greater difficulty in research on gender and occupational exposure, however, is the complexity of the conceptual framework. In general, research has concentrated on the independent variables (the so-called "host factors") such as reproductive state, toxicological mechanisms, and individual metabolism. In general, the state-of-the-art of occupational health research is not advanced with regard to identifying the full range of relevant variables, such as socioeconomic status and lifestyle and defining how these variables interact. Significantly, these factors can be either independent or intervening variables, thus further complicating the conceptual framework.
Failure to take into account all these interactions in the conceptual framework can lead to incorrect conclusions and poor social policy that may ultimately be detrimental to the well-being of working women and men. Environmental health research often finds itself extrapolating from individual traits into population-based results and conclusions. It is important to remember that women and their exposures should be treated as separate variables; personal characteristics and environmental variables are the building blocks of any conceptual framework, and one cannot simply be considered a proxy for the other.
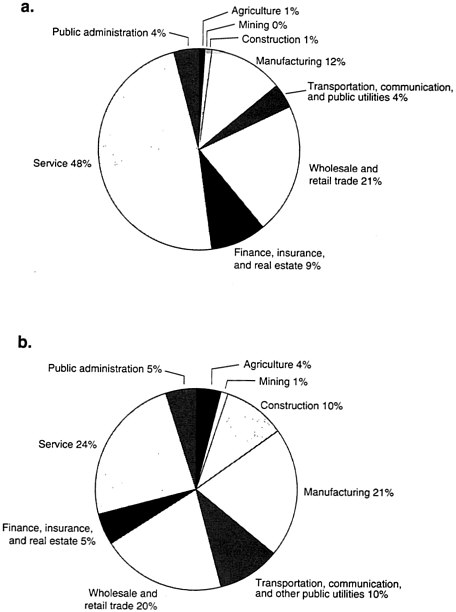
FIGURE B-1 Industrial employment of women and men by sector, 1992.
Historically, the complexities in characterizing "host factors" have led to a simple methodological solution: the wholesale exclusion of women from occupational health studies. A 1993 survey of the literature on cancer outcomes in occupational health found only a handful of studies in which women had not been excluded. In most cases their exclusion was based on a presumption of gender differences, on the idea that women are vulnerable or differentially susceptible and since their numbers were small, they should be excluded from studies. (Such presumptions have lead, as discussed above, to the idea that
women should also be excluded from the actual employment situations themselves: that is, "protected" from the hazards.)
The actual state-of-the-art, however, is that, in fact, comparatively little is known about the actual exposures in the workplace. There are almost no registries with meaningful data on occupational exposures and illnesses; physicians are seldom trained to recognize environmental diseases. Those data which have been painstakingly collected in the past, such as the National Occupational Environmental Survey (NOES) and carried out by the National Institute for Occupational Safety and Health (NIOSH) are out of date. One priority, therefore, might be to provide adequate funding to re-establish a NOES-like survey and to establish a working conditions surveillance system under NIOSH's direction. Improvement of surveillance, one of the priorities in the new National Occupational Research Agenda (NORA), should also be integrated into the activities of other agencies that have an interest in biological responses to environmental exposures. In general, far too few resources have been devoted to the systematic collection and registration of occupational exposures and health outcomes in a way that is useful and conducive to research and establishment of meaningful policy.
Finally, a fundamental methodological issue confronting the panel is the "problem" statement itself: developing knowledge that will identify gender differences in susceptibility to environmental factors. The question of host-factor susceptibility is itself fraught with methodological issues with regard to determining when a problem is a woman's health problem versus when it is a public health problem that women also face. Asked another way, when are the observed differences attributable to gender (i.e., when are women differentially vulnerable?) and when does gender serve as a proxy variable for underlying exposures (i.e., when are women differentially exposed?)? Further, while there are real biological differences between men and women, it is also true that not all women or men are alike, nor is a woman the same biological creature at all stages in her life. Exposure to a given level of lead, for example, may have very different consequences for a healthy young woman than for an older worker with a 25-year lifetime exposure to lead.
Such variability in response greatly complicates the problem of how to extrapolate individual differences to differences in populations, particularly when the results of those extrapolations may influence the setting of standards. Standards are generally single values, by design, and as such they cannot capture the great variability in human response, unless they are set to accommodate the most sensitive individual. In some cases such standards would not be practical or feasible; in others such standards would unfairly exclude or discriminate against otherwise qualified individuals.
A variety of statistics illustrate the intervention of socioeconomic variables on women's health. For one thing, health is related to access to health care, which in turn is related to wealth: salary, insurance, pensions. Women are far less wealthy than men and have less access to health care, in general. For example, consider that approximately 80 percent of American women are
employed outside the home, and up to 7 percent of these women work two jobs; but in no occupational category are women's average earnings more than 78 percent of those of men. Fewer women are covered by pensions or by group health insurance than men. Wealth alone, however, cannot explain every observed health difference, and the relationships between socioeconomic factors and health outcomes are complex as well. For example, higher levels of education are associated with a decreased incidence of cervical cancer and with a higher incidence of breast cancer but a lower breast cancer mortality rate.
Such complexity means that observed differences in health outcomes among women may be due to many factors other than "gender susceptibility," or they may be due to differential exposures. To a large extent these are separate issues. Even the term "exposure" is not clearly and consistently defined. When pursuing the topic of differential gender susceptibility, it is important to keep in mind the complexities of the conceptual framework and also the realities of the exposure situations at work. In the fourth edition of the International Labor Organization's Encyclopaedia of Occupational Health and Safety, several instances of gender differences in response are reported, but there is not a single instance of a working condition or exposure in which only females are adversely affected. From an historical perspective it is useful to recall the words of Anna Baetjer in her landmark 1946 study, The Health and Efficiency of Women at Work. She observed that the extent of illness among women workers was not known, largely because of a lack of data. She warned against broad generalizations about the weakness of women, when there were no underlying data to support such conclusions, a warning that is still appropriate today.
Environmental Exposure and Nutrition2
This topic involves many of the same paradigm issues developed in the preceding presentation: do women have different diets from men, as well as (or instead of) different environmental exposures, and does the interaction between diet and exposure increase or decrease their risks of disease? How does a woman's dietary intake influence her exposures to environmental factors? How do the patterns of exposure change over the lifespan, and how does a woman's changing physiology alter her exposure?
This topic does not exist as such in the nutritional literature, nor does it fit well in the priority-setting model of nutrition monitoring (see Figure B-2). This model is designed to identify current or potential public health problems based on levels of nutrient intake. From this perspective, the biggest threats to women are too much fat, cholesterol, and sodium (which increase their risk of cardiovascular disease and hypertension) and too little iron and calcium (which increase their risk of anemia and osteoporosis).
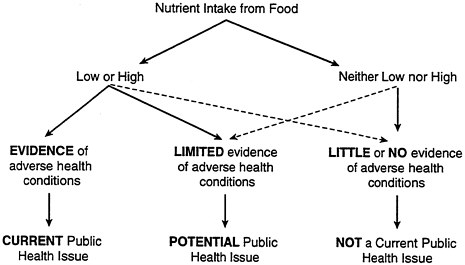
FIGURE B-2 Setting nutrition monitoring priorities.
Interagency cooperation is required to collect and link both kinds of data, but the result is the ability to generate data on dietary intake of contaminants. More effort is needed in this area.
How might researchers use the resulting data for surveillance: that is, how might women's dietary intake influence their exposures to environmental factors? One possibility could be that some foods that are eaten in greater quantities by women might serve as carriers of risk (toxicants or additives) or as carriers of protection (e.g., antioxidants). Or it may be that certain methods of food preparation predispose women to risk. Another possibility is that micronutrient deficiencies can predispose women to disease.
The differences in the recommended nutrient intakes for men and women are relatively small, with the exception of pregnant and lactating women (see Tables B-1 and B-2). Women do eat less food than men, however, which means they can have a harder time getting enough nutrients. Consequently, women's diets need to be better in order to be nutritionally adequate. Unfortunately, recent studies have shown that micronutrient intakes are below recommended levels in a sizable proportion of the population, including some nutrients with known or suspected protective roles against disease, such as vitamins A and E (cancers), vitamin B6 (heart disease), calcium (osteoporosis), and minerals (cardiovascular disease and immune function). Women are more likely than men to have low intakes of iron, calcium, and zinc. There is little information on whether low iron intake exacerbates problems.
TABLE B-1 Recommended Dietary Allowances
|
FOOD AND NUTRITION BOARD, NATIONAL ACADEMY OF SCIENCES—NATIONAL RESEARCH COUNCIL RECOMMENDED DIETARY ALLOWANCES,a Revised 1989 (Abridged1) |
||||||||||||||||||||
|
Designed for the maintenance of good nutrition of practically all healthy people in the United States |
||||||||||||||||||||
|
|
|
|
|
|
|
|
Fat-Soluble Vitamins |
Water-Soluble Vitamins |
Minerals |
|||||||||||
|
|
|
Weightb |
Heightb |
Protein |
Vitamin A |
Vitamin E |
Vitamin K |
Vitamin C |
Thiamin |
Riboflavin |
Niacin |
Vitamin B6 |
Folate |
Vitamin B12 |
Iron |
Zinc |
Iodine |
Selenium |
||
|
Category |
Age (years) or Condition |
(kg) |
(lb) |
(cm) |
(in) |
(g) |
(μg RE)c |
(mg a-TE)d |
(μg) |
(mg) |
(mg) |
(mg) |
(mg NE)e |
(mg) |
(μg) |
(μg) |
(mg) |
(mg) |
(μg) |
(μg) |
|
Infant |
0.0-0.5 |
6 |
13 |
60 |
24 |
13 |
375 |
3 |
5 |
30 |
0.3 |
0.4 |
5 |
0.3 |
25 |
0.3 |
6 |
5 |
40 |
10 |
|
|
0.5-1.0 |
9 |
20 |
71 |
28 |
14 |
375 |
4 |
10 |
35 |
0.4 |
0.5 |
6 |
0.6 |
35 |
0.5 |
10 |
5 |
50 |
15 |
|
Children |
1-3 |
13 |
29 |
90 |
35 |
16 |
400 |
6 |
15 |
40 |
0.7 |
0.8 |
9 |
1.0 |
50 |
0.7 |
10 |
10 |
70 |
20 |
|
|
4-6 |
20 |
44 |
112 |
44 |
24 |
500 |
7 |
20 |
45 |
0.9 |
1.1 |
12 |
1.1 |
75 |
1.0 |
10 |
10 |
90 |
20 |
|
|
7-10 |
28 |
62 |
132 |
52 |
28 |
700 |
7 |
30 |
45 |
1.0 |
1.2 |
13 |
1.4 |
100 |
1.4 |
10 |
10 |
120 |
30 |
|
Males |
11-14 |
45 |
99 |
157 |
62 |
45 |
1,000 |
10 |
45 |
50 |
1.3 |
1.5 |
17 |
1.7 |
150 |
2.0 |
12 |
15 |
150 |
40 |
|
|
15-18 |
66 |
145 |
176 |
69 |
59 |
1,000 |
10 |
65 |
60 |
1.5 |
1.8 |
20 |
2.0 |
200 |
2.0 |
12 |
15 |
150 |
50 |
|
|
19-24 |
72 |
160 |
177 |
70 |
58 |
1,000 |
10 |
70 |
60 |
1.5 |
1.7 |
19 |
2.0 |
200 |
2.0 |
10 |
15 |
150 |
70 |
|
|
25-50 |
79 |
174 |
176 |
70 |
63 |
1,000 |
10 |
80 |
60 |
1.5 |
1.7 |
19 |
2.0 |
200 |
2.0 |
10 |
15 |
150 |
70 |
|
|
51+ |
77 |
170 |
173 |
68 |
63 |
1,000 |
10 |
80 |
60 |
1.2 |
1.4 |
15 |
2.0 |
200 |
2.0 |
10 |
15 |
150 |
70 |
|
Females |
11-14 |
46 |
101 |
157 |
62 |
46 |
800 |
8 |
45 |
50 |
1.1 |
1.3 |
15 |
1.4 |
150 |
2.0 |
15 |
12 |
150 |
45 |
|
|
15-18 |
55 |
120 |
163 |
64 |
44 |
800 |
8 |
55 |
60 |
1.1 |
1.3 |
15 |
1.5 |
180 |
2.0 |
15 |
12 |
150 |
50 |
|
|
19-24 |
58 |
128 |
164 |
65 |
46 |
800 |
8 |
60 |
60 |
1.1 |
1.3 |
15 |
1.6 |
180 |
2.0 |
15 |
12 |
150 |
55 |
|
|
25-50 |
63 |
138 |
163 |
64 |
50 |
800 |
8 |
65 |
60 |
1.1 |
1.3 |
15 |
1.6 |
180 |
2.0 |
15 |
12 |
150 |
55 |
|
|
51+ |
65 |
143 |
160 |
63 |
50 |
800 |
8 |
65 |
60 |
1.0 |
1.2 |
13 |
1.6 |
180 |
2.0 |
10 |
12 |
150 |
55 |
|
Pregnant |
|
|
|
|
|
60 |
800 |
10 |
65 |
70 |
1.5 |
1.6 |
17 |
2.2 |
400 |
2.2 |
30 |
15 |
175 |
65 |
|
Lactating |
1st 6 months |
|
|
|
|
65 |
1,300 |
12 |
65 |
95 |
1.6 |
1.8 |
20 |
2.1 |
280 |
2.6 |
15 |
19 |
200 |
75 |
|
|
2nd 6 months |
|
|
|
|
62 |
1,200 |
11 |
65 |
90 |
1.6 |
1.7 |
20 |
2.1 |
260 |
2.6 |
15 |
16 |
200 |
75 |
|
1 NOTE: This table does not include nutrients for which Dietary Reference Intakes have recently been established [see Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride, 1997]. a The allowances, expressed as average daily intakes over time, are intended to provide for individual variations among most normal persons as they live in the United States under usual environmental stresses. Diets should be based on a variety of common foods in order to provide other nutrients for which human requirements have been less well defined. See text for detailed discussion of allowances and of nutrients not tabulated. b Weights and heights of Reference Adults are actual medians for the U.S., population of the designated age, as reported by NHANES II. The median weights and heights of those under 19 years of age were taken from Hamill et al. (1979) (see pages 16-17). The use of these figures does not imply that the height-to-weight ratios are ideal. c Retinol equivalents. 1 retinol equivalent = 1 μg retinol or 6 μg β-carotene. See text for calculation of vitamin A activity of diets as retinol equivalents. d α-Tocopherol equivalents. 1 mg d-α tocopherol = 1 α-TE. See text for variation in allowances and calculation of vitamin E activity of the diet as α-tocopherol equivalents. e 1 NE (niacin equivalent) is equal to 1 mg of niacin or 60 mg of dietary tryptophan. |
||||||||||||||||||||
TABLE B-2 Dietary Reference Intakes
|
FOOD AND NUTRITION BOARD, NATIONAL ACADEMY OF SCIENCES—INSTITUTE OF MEDICINE DIETARY REFERENCE INTAKES, 1997 |
||||||||
|
|
|
Calcium |
Phosphorus |
Magnesium |
Vitamin D |
Fluoride |
||
|
Life-Stage Group |
AIa (mg/day) |
RDAb (mg/day) |
AI (mg/day) |
RDA (mg/day) |
AI (mg/day) |
AI (mg/day) |
||
|
Infants |
||||||||
|
|
0 to 6 months |
210 |
|
100 |
|
30 |
5 |
0.01 |
|
|
6 to 12 months |
270 |
|
275 |
|
75 |
5 |
0.5 |
|
Children |
||||||||
|
|
1 through 3 years |
500 |
460 |
|
80 |
|
5 |
0.7 |
|
|
4 through 8 years |
800 |
500 |
|
130 |
|
5 |
1 |
|
Males |
||||||||
|
|
9 through 13 years |
1,300 |
1,250 |
|
240 |
|
5 |
2 |
|
|
14 through 18 years |
1,300 |
1,250 |
|
410 |
|
5 |
3 |
|
|
19 through 30 years |
1,000 |
700 |
|
400 |
|
5 |
4 |
|
|
31 through 50 years |
1,000 |
700 |
|
420 |
|
5 |
4 |
|
|
51 through 70 years |
1,200 |
700 |
|
420 |
|
10 |
4 |
|
|
> 70 years |
1,200 |
700 |
|
420 |
|
15 |
4 |
|
Females |
||||||||
|
|
9 through 13 years |
1,300 |
1,250 |
|
240 |
|
5 |
2 |
|
|
14 through 18 years |
1,300 |
1,250 |
|
360 |
|
5 |
3 |
|
|
19 through 30 years |
1,000 |
700 |
|
310 |
|
5 |
3 |
|
|
31 through 50 years |
1,000 |
700 |
|
320 |
|
5 |
3 |
|
|
51 through 70 years |
1,200 |
700 |
|
320 |
|
10 |
3 |
|
|
> 70 years |
1,200 |
700 |
|
320 |
|
15 |
3 |
|
Pregnancy |
||||||||
|
|
≤ 18 years |
1,300 |
1,250 |
|
400 |
|
5 |
3 |
|
|
19 through 30 years |
1,000 |
700 |
|
350 |
|
5 |
3 |
|
|
31 through 50 years |
1,000 |
700 |
|
360 |
|
5 |
3 |
|
Lactation |
||||||||
|
|
≤ 18 years |
1,300 |
1,250 |
|
360 |
|
5 |
3 |
|
|
19 through 30 years |
1,000 |
700 |
|
310 |
|
5 |
3 |
|
|
31 through 50 years |
1,000 |
700 |
|
320 |
|
5 |
3 |
|
a AI = Adequate Intake. The observed average or experimentally set intake by a defined population or subgroup that appears to sustain a defined nutritional status, such as growth rate, normal circulating nutrient values, or other functional indicators of health. AI is utilized if sufficient scientific evidence is not available to derive an EAR. For healthy breastfed infants, AI is the mean intake. All other life-stage groups should be covered at the AI value. The AI is not equivalent to a RDA. b RDA = Recommended Dietary Allowance. The intake that meets the nutrient need of almost all (97-98 percent) individuals in a group. c As cholecalciferol. 1µg cholecalciferol = 40 vitamin D. d In the absence of adequate exposure to sunlight. |
||||||||
Significant threats such as environmental toxicants and carcinogens do not appear either in this model or on the list of public health issues related to nutrition, because of an underlying assumption that there are no toxicants and carcinogens in the food system. But while nutrition monitoring systems are not designed to measure contaminants, they do measure intake of the specific foods that might be contaminated, such as fruits and vegetables that might contain pesticides. EPA and FDA do monitor the food system for such contaminants; but the expectation is that if contaminants are discovered, they will be removed from the food system. Calcium intake is considered too low throughout the life cycle, a problem that is associated with increased risk of osteoporosis. Low calcium intake may also be associated with higher levels of lead uptake, but this connection has received very little study. Women are disproportionately affected by iodine deficiencies and goiter as well. Iodine deficiencies can lead to increased uptake of radioactive isotopes, which can be a problem in contaminated areas such as Chernobyl.
On the other hand, women (and especially older white women) are more likely than men to take vitamin supplements, which might mitigate these risks but might also introduce new risks. Women also appear to eat more fruits and vegetables than men— the so-called "salad factor"—although the data to support this perception are far from striking. Some of these foods may contain substances that protect against disease, such as antioxidants. However, the same fruits and vegetables may also contain contaminants, and this would expose women to higher risks. Conceptually, however, this type of increased susceptibility is behavioral rather than biological.
Some methods of food preparation, such as grilling or barbecuing, are suspected of inducing potentially dangerous enzymes and mutagens. However, it is difficult to identify major differences in food preparation between men and women. Women prepare most of the food, but in most cases they prepare it the same for themselves as for their families. Weight-consciousness has led men and women alike to avoid many fried foods.
Obesity may be more of a factor than undernutrition in susceptibility to environmental contaminants. Obesity has increased markedly since the 1970s, with one-third of American adults and one-fifth of adolescents now characterized as obese. Because obesity increases the amount of body fat, it provides greater reservoirs for lipophilic (fat-loving) toxicants that tend to reside in adipose tissue. Contrary to popular belief, however, by current definition (body mass index [calculated as weight in kilograms divided by the square of height in meters] of 25 or greater), being overweight is not a greater problem for women than men. Nevertheless, two of the differences between men and women that stand out in the national data are that women diet more often and consume more low-fat foods and beverages.
Women's nutritional status may have its own effect on their environmental exposures. Women are smaller than men on average, but have proportionately more adipose tissue and more cycles of fat gain and loss because of dieting behavior. To the extent that weight loss mobilizes potential toxicants that had
been stored in adipose tissue, it may expose women to greater risk. This may explain why epidemiological data show an association between weight loss (voluntary or involuntary) and mortality.
The same may hold for other changes in body composition during pregnancy and lactation, menstruation, and menopause. Older women also experience greater bone loss than men, which may release metals and other substances that were stored in the bones. Fractures also release potential toxicants from the bones into the bloodstream, and this may expose women to greater risk if osteoporosis leads to more frequent fractures.
Review of the cancer literature reveals very little attention to these aspects of nutritional factors. Articles typically focus on the consumption of fruits and vegetables; to the extent that their antioxidant effects attenuate the risk of lung, cervical, renal cell, and nasopharyngeal cancers, dietary intake of these foods may reduce the risk of cancer. Many studies address environmental factors and many others consider diet, but very few consider both diet and environmental factors.
Multiple Environmental Exposures Across the Lifespan3
The first challenge in this area is to understand what is meant by "multiple exposures." Exposure to potentially harmful substances and stresses occurs not only in environmental and occupational settings but also in residential, recreational, and even medical settings. These separate exposures may have both cumulative and synergistic effects, and their combined consequences will be different at various points in a woman's menstrual cycle, career, and lifespan.
"Environmental" exposures, in the conventional sense, can include such sources as alcohol, water, air pollution, environmental tobacco smoke, and soil (especially for children). A few studies have examined various exposures in terms of differences between men and women. For example, male and female runners are impacted in different ways by the same levels of ozone, and women runners are impacted in different ways by ozone at different points in their menstrual cycle.
In the home, 90 percent of the work is still done by women. Consequently, in addition to their occupational exposures they also experience "residential" exposures. The agents for these exposures include cleaning products, some of which include solvents that can be absorbed through the skin. Other examples include pesticides and painting products. Men and women also have different hobbies; a recent study found that, among the top 10 hobbies for men and women, only one (bowling) was common to both. Hobbies such as gardening
and furniture refinishing can lead to additional exposures, all of them independent of and additive to a woman's occupational exposures.
''Lifestyle" exposures can include such factors as smoking, drinking, and the use of recreational drugs. These exposures, in turn, can be modulated by the hormonal cycle. For example, one study showed that, given the same initial dose of cocaine, male subjects had over double the plasma level found in women. However, women in the follicular stage (before ovulation) have higher plasma levels of cocaine than those in the luteal phase, when estradiol and progesterone levels are higher (Lukas, et al., 1996).
Prescription and nonprescription drugs are used differently by men and women; they are also used differently by women at different times in their lives. This results in additional exposures, but it can also affect how the individual responds to other agents. For example, Tagamet (widely used by both men and women) also affects the p450 enzyme system, on which the body depends to detoxify some substances to which it is exposed. Nonsteroidal anti-inflammatory drugs are more commonly used by women, in whom arthritis is more common, and the effects of these drugs on the liver and kidney may interfere with their ability to detoxify some contaminants. Antidepressants are also used more often by women, but their interactions with other chemicals are unknown. More research is needed on patterns in prescribing drugs and on their interactions with other chemicals, as they relate to gender differences.
Hormonal interactions are different during prepuberty, puberty, adulthood, pregnancy, perimenopause, and menopause. Responses to chemical contaminants can vary accordingly. Artificial variations occur as a result of the use of birth control drugs and hormone replacement therapy. These variations are further complicated by temporal changes that are not captured by cross-sectional data. Between 1960 and 1993, for example, the proportion of women aged 25–29 who had no live births rose from 20 percent to 44 percent. At the same time, the onset of puberty is earlier and earlier. There is also a much higher level of smoking among teenage girls than there was a generation or two ago.
There have also been changes over time in the rates of occupational injuries: they are rising for women, declining for men (see Figure B-3). This is not the result of aging in women; instead, it is most likely due to the fact that, as women move into traditionally "male" occupations, they are also exposed to more risks. That is, their exposure has changed, not their susceptibility.
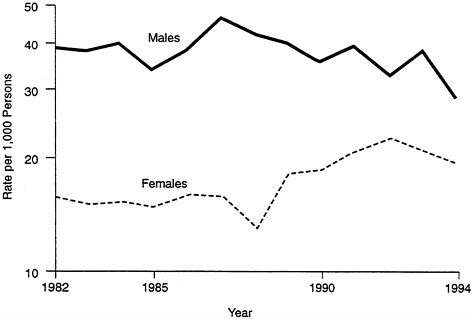
FIGURE B-3 Occupational injuries.
These multiple exposures are important because of the potential for synergy among them. A classic example is the relationship between exposures to smoking and asbestos and the risk of lung cancer (see Table B-3). People with neither exposure have a rate of lung cancer of 11 in 100,000. For asbestos workers the rate goes to 58 (a relative risk 5-fold greater than for those without exposure) and for smokers the rate is 123 (an 11-fold relative risk); but when people were exposed to both smoking and asbestos the rate is not additive (11 + 58 + 23 = 192), but it is over 600. Furthermore, the relative risk is not additive (5 + 11 = 16) but multiplicative (a relative risk of 5 × 11 = 55 times).
TABLE B-3 synergistic Effect of Multiple Agents:
Cigarette Smoking and Occupational Exposure to Asbestos
|
Lung Cancer Death Rate per 100,000 Man-Years |
Nonsmokers |
Smokers |
|
Not exposed to: Asbestos Asbestos workers |
11 58 |
123 602 |
|
SOURCE: Hammond et al., 1979. |
||
Exposures to "environmental factors," in this broader sense, differ not only between men and women but between home and workplace and over the
lifespan. For example, women are more likely to be exposed to environmental tobacco smoke in the home, while men are more likely to be exposed in the workplace (see Figures B-4 and B-5). Similarly, the lifetime prevalence of psychiatric disorders is the same for men and women, but the distribution is different: substance abuse is higher in men, while anxiety and depression are higher in women. Presumably the drugs they are given are different as well. Men also respond to stress and competition by producing higher levels of epinephrine; this may also have significance in workplace challenges.
Because of these differences in the sources, levels, and combinations of exposures between men and women, estimates of the risks associated with chemical exposure cannot be based solely on adult male cohort studies. For example, current estimates of the risk of benzene exposure are based on such studies, but evidence from animal studies points to differential impacts on blood-forming tissues in male and female fetal, adult, and pregnant rats exposed to benzene and/or ethanol (see Table B-4). In adult males, all three exposure regimes suppressed the formation of erythroid colony-forming units (CFUs, the precursors of erythroblasts and erythrocytes). In females, on the other hand, no exposure had any effect on CFU formation in nonpregnant adults, but exposure to ethanol increased CFU formation in pregnant females, and the combination of ethanol and benzene increased CFU formation in females that had been exposed in utero. In this case, gender differences may actually be protective for women.
Similar evidence for the interaction of multiple exposures over the lifespan can be seen in a recent study of the link between smoking and breast cancer (Ambrosone et al., 1996). Previous epidemiological studies had been ambiguous; but this study focused on N-acetyltransferase-2 (NAT-2), an enzyme that is assumed to break down the aromatic amines in tobacco smoke, chemicals that are known to induce mammary cancers in animals and to cause DNA damage in human mammary cells.
FIGURE B-4 Environmental tobacco smoke in the home.
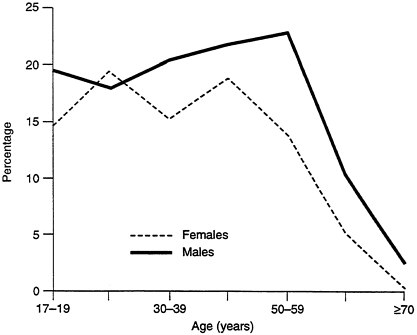
FIGURE B-5 Environmental tobacco smoke in the workplace.
TABLE B-4 influences of Gender, Development, and Ethanol Consumption on Benzene's Effect on Erythroid Colony-Forming Units
|
Exposed Mice, 10-day Exposure |
Benzene, 10 ppm |
Ethanol, 5% |
Benzene, Ethanol |
|
Adult male |
Reduced |
Reduced |
Reduced |
|
Fetal male (days 6-15) |
Reduced |
— |
— |
|
Adult male exposed in utero |
Reduced |
Reduced |
— |
|
Adult female |
— |
— |
— |
|
Fetal female (days 6-15) |
— |
— |
— |
|
Adult female exposed in utero |
— |
— |
Increased |
|
Pregnant female (days 6-15) |
— |
Increased |
Increased |
|
SOURCE: Corti and Snyder, 1996. |
|||
Investigators learned that there are competing pathways for the metabolism of these compounds. The usual pathway is acetylation by NAT-2, which detoxifies them; individuals with low levels of NAT-2 are "slow acetylators" who clear these compounds more slowly and thus have higher residual levels. The competing pathway is oxidation by CYP-1A2, which can lead to a reactive
N-hydroxy metabolite that enters the circulation and is activated when it bonds to DNA in the target tissue. This would mean that subjects with low levels of NAT-2 would also have higher levels of this metabolite and thus be at even greater risk.
When investigators stratified their human subjects into those with high or low levels of NAT-2 (i.e., as rapid or slow acetylators), there was a much higher risk of cancer among postmenopausal women who were heavy smokers and slow acetylators (see Figure B-6). A slow acetylator who smokes a pack of cigarettes per day has an odds ratio for breast cancer that is 5.1 times that of a nonsmoker. Paradoxically, the odds ratio for a pack-a-day fast acetylator is less than for a nonsmoker, an apparent protective effect that deserves further study. The negative effects of smoking were also greatest among those who started smoking early, and for those who started before age 16 the odds ratio of slow to rapid acetylators was 4.5. The latter finding was consistent with the hypothesis that breast tissue is most sensitive to environmental factors during this time of development.
In a study of multiple exposures in the semiconductor industry, researchers followed 3,200 subjects in eight companies, equally divided between men and women and between fabrication and nonfabrication workers. Subjects were evaluated for 15 chemical agents, two physical agents, and numerous ergonomic stressors. Investigators found that fabrication workers (those in protective gear in the "clean-room" assembly areas) were more likely to be exposed to chemical and electromagnetic stressors than nonfabrication workers, regardless of gender. However, female fabrication workers were far more likely than males to be exposed to certain ergonomic stressors, such as the use of equipment with eyepieces (e.g., microscopes) and awkwardly placed vacuum wands. By using cluster analysis, researchers also found that fabrication workers were likely to be exposed to certain combinations of chemical agents. Given the possible interactions among these agents, cluster analysis techniques should be used more widely in epidemiological studies involving multiple exposures.
In conclusion, there are three dimensions in which exposure can change over the lifespan and which might warrant further research. First, there are variations in the chemicals to which we are exposed in a variety of settings. Second, there may be variations in our ability to absorb those chemicals. For example, gastrointestinal absorption of lead is twice as high in children as in adults. And third, there may be changes in our susceptibility to damage by these chemical agents. In utero, in particular, the DNA repair mechanisms, immune system, and blood-brain barrier are all poorly developed, leaving the fetus more vulnerable than an adult to many toxic and mutagenic compounds.
PANEL II: PATTERNS OF SUSCEPTIBILITY
The second panel addressed questions about the factors that evoke a response to exposure, the molecular and cellular processes involved in this
response, and genetic differences in susceptibility to both exposure and response. Panelists also addressed the differences between men and women and among women in their responses to the same exposures. The first presentation focused on a disease that affects the human organism, while the others described laboratory studies that highlight the kind of basic research currently under way.
Epidemiology, Gender, and Environmental Influences on Multiple Sclerosis4
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system that attacks the myelin sheath surrounding the spinal cord. There are 350,000 cases in the United States, making it (after trauma) the second most common neurological cause of disability in young adults. The typical patient is an otherwise healthy, young white woman, although the disease also occurs in men and less frequently in all racial groups. The incidence of MS appears to be rising, especially among women. There are new techniques for diagnosing the disease, but there is no specific laboratory test for MS. As a result, there may be a large number of patients who have MS and are never diagnosed properly. Autopsies suggest that perhaps 25 percent of all cases are "silent."
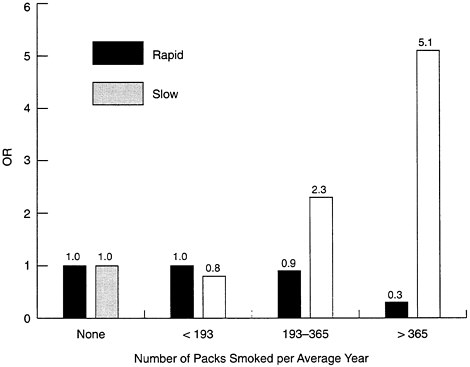
FIGURE B-6 Smoking and the risk of cancer among postmenopausal women.
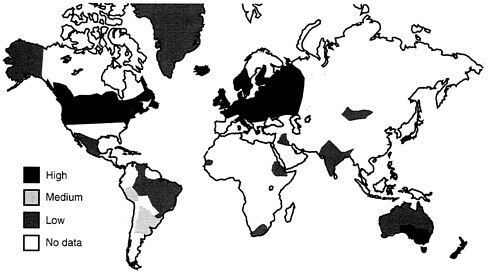
FIGURE B-7 Geographic localization of multiple sclerosis in the world.
Data suggest that the risk of developing MS is higher in white women as compared to white men by a factor of almost two; but white men have a higher risk than black men, and black women have a lower risk than white women (Kurtzke, et al., 1979; Kurtzke, 1977). In general, whites have a higher risk than blacks. Worldwide, there is a very low rate of multiple sclerosis in Africa and in Asia (See Figure B-7). There is a distinct localization of MS in the temperate latitudes: Europe (especially Scandinavia), North America, and Australia. In the United States, the highest incidence is in the northern tier of states, possibly because of the high concentration of Scandinavian immigrants in the northern states, plus some unknown environmental factor. Other known risk factors include average temperature, dairy products, meat, and sanitation. Common characteristics associated with higher risk of MS are urban residence, high socioeconomic status (SES), Swedish ancestry, and high education attainment (Kurtzke and Page, 1997). A man with all these risk factors might have up to 119 times the risk of contracting MS as an average American would; a woman with these risk factors, perhaps 200 times the risk.
Evidence from the Faroe Islands suggests that some infectious agent is involved in the etiology of MS (Kurtzke, et al., 1995), and several viral diseases demonstrate similar geographical patterns. Multiple sclerosis is a disease of humans; however, for research purposes, the animal model for MS is experimental autoimmune encephalomyelitis (EAE). Transgenic mice that develop spontaneous EAE are less likely to develop the disease in a germ-and virus-free environment, indicating that an infectious agent may be a cofactor.
Evidence for some genetic contribution to MS comes from its greater incidence among Caucasians as opposed to African Americans; its association with certain histocompatibility subtypes (e.g., HLA subtype DR2); and the way it is manifested among twins: 25 percent of identical twins also develop the disease, versus 4 percent of fraternal twins. Although these studies indicate some genetic contribution, the major susceptibility factor seems to be environmental.
Like most autoimmune diseases, MS is more common among women than men: approximately two-thirds to three-quarters of all MS patients are women. The age of onset is also earlier among women than men. However, among the men who do contract MS, the disease is more severe than among women, and the 10-year mortality rate is higher.
Sex hormones appear to have an important effect in MS. Pregnancy has a major effect, with a marked decrease in the number of relapses in the third trimester, at a time when female sex hormones are very high. Estrogen may be a protective factor. There may also be a role for other hormones, such as pituitary and adrenal gland hormones, that are different between men and women. In the animal model, for example, the suppression of prolactin (a pituitary hormone which is secreted in higher levels in females) alleviates the severity of EAE.
The effect of gender on susceptibility is confounded by environmental factors in complex ways, in both humans and animal models, and gender differences often disappear with changes in environment. Like breast cancer, MS is more prevalent in high-SES women; while lifestyle factors are difficult to isolate, there does seem to be an association between MS and a diet high in dairy and meat.
Estrogen Receptor Knockout Mouse Studies and Implications for Differences in Susceptibility5
Researchers at the National Institute of Environmental Health Sciences (NIEHS) have focused on the basic mechanism of action of estrogen, in order to understand where environmental estrogen might disrupt normal physiological processes. Estrogen produces a wide range of responses in a variety of sites, including the cardiovascular system and bones, in the male as well as the female. A variety of compounds in the environment can have estrogen-like effects on the body.
Endocrinologists believe that the intracellular action of estrogen—whether synthetic, endogenous, or environmental—is a receptor-mediated pharmacological reaction. At the molecular level, estrogen may also operate through nonreceptor-mediated action, with productive or antagonistic effects relative to the genomic action. By developing an estrogen-receptor knockout (ERKO) mouse model, researchers have been able to differentiate and study the receptor-mediated actions of estrogen. A second estrogen receptor, ER-beta, has recently been discovered; ER-beta is still present in the ERKO mouse but its independent role has yet to be studied.
When treated with three different types of estrogen agonists (e.g., stilbene, steroidal, and triphenylethylene), the classic uterotropic bioassay shows that ERKO mice are unresponsive to the hormone treatment. This also demonstrates that the uterine hyperemia measured by this assay is a receptor-mediated response. The receptor itself is a ligand-activated transcription factor. Another very sensitive marker of response is the stimulation of lactipherin, whose gene response and mRNA increase about 300-fold in response to a single injection of hormone; but ERKO mice are totally unresponsive, again demonstrating the total lack of functional estrogen receptors.
The ovary is dramatically affected by the absence of estrogen receptors. In the ERKO animals, follicular genesis does not proceed past the secondary follicular stage, and the ERKO ovaries never ovulate. As a result, the ERKO animals are infertile. Current studies indicate that the granulosa cells in the follicles undergo increased apoptosis, causing the follicle to degenerate and be resorbed prior to maturation. This may provide an animal model of polycystic ovary syndrome, a clinically interesting possibility that researchers will evaluate further.
Estrogen is also involved in the expression of the progesterone receptor, which is implicated in mammary tumor and breast cancer studies. However, the ERKO mouse shows no stimulation of the progesterone receptor, which at least in the ovary appears to be totally dependent on a functional estrogen receptor. In the uterus, on the other hand, there is both estrogen-dependent and estrogen-independent expression of the progesterone receptor. For some reason, the regulation of this receptor is different in these two reproductive tract tissues.
Hormone levels are dramatically altered in ERKO females. They have extremely high circulating levels of estradiol, because they lack negative feedback systems. Castration ablation experiments in males show that their gonadotropins go up as well, but in intact ERKO males they remain in the normal range for wild-type animals. This suggests that there may be a difference in regulation of gonadotropin between males and females, as well as a difference in the specific gonadotropin secretion: that is, serum LH levels are elevated but serum FSH is not.
Because the ERKO female does not develop mammary gland tissue, this model allows us to examine the role of the estrogen receptor in the development of breast cancer. Researchers have done this by crossing the ERKO mouse with the WNT-1 mouse, which has a high susceptibility to mammary cancer. The
results show that the rudimentary duct tissue of the ERKO mouse is still susceptible to the action of the oncogene. That is, the WNT oncogene does not require a functional estrogen receptor to produce its phenotype. A surprising result was that ERKO females had a 58 percent incidence of tumors, while wild-type males had a 49 percent incidence, which may indicate that, in this model and with this oncogene, the female has increased susceptibility or the male has some protective effect. Further testing with other oncogene crosses is currently in progress to evaluate this finding.
In the ERKO male, there is extensive dismorphogenesis and swelling of the testes and a lack of sperm cells over time. Sperm count and sperm motility both decrease, and the remaining sperm are incapable of in vitro fertilization. In addition, the ERKO male's brain has tyrosine hydroxylase levels comparable to a wild-type female, indicating that the brain has also been reprogrammed. ERKO males have also lost their aggressive behavior; and the reason may be that male androgens, chemically transformed to estrogen, are responsible for male behaviors. The loss of these behaviors in ERKO males suggests that chemical transformation requires a functional estrogen receptor to produce male behavioral phenotypes.
Researchers have just begun to examine the effect of environmental estrogens. Because both male and female ERKO mice are infertile, researchers must generate the recessives from inbreeding of heterozygotes. This takes a lot of time, and researchers are only now getting a large enough pool of animals to do further treatment studies. Preliminary data indicates that Genistein may produce growth effects through the estrogen receptor in the uterus, but the regulation of LH negative feedback may involve a nonestrogen receptor mechanism.
Gender Differences in Metabolism and Susceptibility to Environmental Exposures6
Recent data indicate that the relatively small gender-specific differences in the metabolism of xenobiotics do not fully explain the gender-specific adverse effects of toxicants. Nor does the current literature indicate that gender-specific differences in the induction of catabolic enzymes by toxicants are responsible for the gender-different toxic effects that are observed. More importantly, gender differences are observed in isolated cells which possess little or no capacity to metabolize xenobiotics. This is not to say that gender differences in metabolism or enzyme induction do not exist, but rather to demonstrate the basis for a hypothesis that there are gender-specific differences in sensitivity and in the mechanism of toxic action, which are related to the primary rather than a secondary effect of a compound. On this basis, a hypothesis has been tested, using a well-defined toxicant, the herbicide 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, a known
carcinogen and hormone disrupter in rats). TCDD's induction of adverse effects in adipose cells, a gender nonspecific tissue, was then studied. Metabolism is not the primary issue in these studies, since TCDD, with a half-life of several years in humans, never clears the system during these studies in either gender, and studies show no pharmacokinetic differences between young and old animals.
The following data are consistent in rat, hamster, and monkey models; they support the concept of a gender-specific difference in the sensitivity to some xenobiotics as well as a gender-specific difference in the mechanism of toxicity. These data also suggest that adipose tissue should be added to the list of sex-steroid-hormone target tissues. These data also predict that some toxicants should have a greater effect on lower vertebrates, which are more sensitive to sex steroids in terms of somatic development.
The underlying hypothesis of our recent research is that some signal transduction receptors and pathways evolved prior to sexual reproduction: that is, they are ''pregender." These ancient transduction pathways have been readapted to other functions after sexual reproduction arose. This concept fits with the observations that hormones do not change as much in evolution as does the use to which they are adapted. Such re-adaptation may have paralleled and played a role in the expression of receptors and transduction pathways which became central for sex-steroid-hormone transduction and reproductive processes. We speculate and attempt to provide evidence that some toxicant-sensitive transduction pathway, overlap with sex-steroid-hormone-induced transduction pathways. Our data support the concept that some toxicants interrupt the mechanism by which sex-steroid-hormones program cells to be gender-differentiated and to function in predicted gender-specific ways. Some of our experimental data indicate that toxicant transduction pathways overlap with steroid and growth factor pathways, and this overlap may represent the basis of gender-specific differences in susceptibility to toxicants.
Pivotal to our general hypothesis is the observation that some currently accepted transduction pathways for toxicants evolved prior to expression of sexual reproduction. The eight-cell mouse embryo demonstrates that the AH receptor, which is a receptor for TCDD-like toxicants, exists prior to the estrogen receptor (Peters and Wiley, 1995). This observation is consistent with the concept that the AH receptor evolved prior to the estrogen receptor and prior to sexual reproduction. This lays the foundation for understanding gender differences, which is expanded below.
When intact monkey granulosis cells are exposed in vitro, TCDD decreases the level of MAP-2 kinase (which transduces growth factor pathways). In contrast, exposure to estrogen increases MAP-2K, and TCDD blocks this action of estrogen. When the nucleus is removed from the cells, the effects of TCDD and estrogen on the nucleus-free cell system are the same. Tamoxifen (an antiestrogen that acts at the level of the estrogen receptor) blocks the effect of estrogen but not the effect of TCDD, suggesting that TCDD is not operating through the estrogen receptor but through the AH receptor. These data also imply the existence of prenuclear (cytosolic) effects of both TCDD and estrogen
which may be estrogen-receptor independent, and that cytosolic pathways may be involved in some gender-specific effects.
In whole-animal studies, TCDD decreases the growth rate of immature female rats but not the growth rate of mature females or castrated males treated with estrogen, suggesting that mature females are protected from the negative effects of TCDD by estrogen. These data alone do not prove that sex steroids such as estrogen determine gender-specific toxicities; but this model makes it possible to address questions about the mechanistic basis for (1) gender differences in sensitivity to environmental factors, (2) the increased sensitivity of dams to some toxicants during pregnancy, (3) steroid hormone disruption by environmental factors, and, if one extends the concept, (4) the underlying basis for differences in species: that is, sensitivity in the induction of developmental defects. There is little hard evidence to support the concept that overlapping pathways are the basis for the observed developmental defects, but it is often overlooked that the development of fish, reptiles, and birds are more susceptible to environmental and steroid inducers than are mammals.
Existing data make it possible to test the following specific hypothesis: sex steroid hormones and growth factor transduction pathways are shared by toxicant-induced pathways. Sex-steroid (and possibly growth factor) modulation of these pathways determines the effect of the toxicant. These pathways can originate in the cytoplasm and involve phosphorylation/phosphatase activities.
Our current data demonstrate that many of the observed gender differences in response to toxicants are qualitative (i.e., mechanistic) rather than quantitative (sensitivity alone) differences. These differences may have been previously overlooked because males are often used in mechanistic studies to avoid the confounding affect of cyclic steroid hormone levels in females. In addition, not all environmental toxicants in the same class of compounds (e.g., pesticides including arylhydrocarbons, chlorinated hydrocarbons) will have different gender-specific adverse effects, because receptor-ligand interactions may be as structure-specific for toxicants as they are for sex steroids. While both gender-specific steroids and toxicants ultimately exert a portion of their effects through the interaction at the nuclear level, many of these actions are initiated and dependent on cytosolic events, but some are nuclear independent. While some of these cytosolic actions depend on cytosolic receptors, it is possible that some do not. Evidence to support the prenuclear nature of these pathways comes from studies of the effect of estrogen and TCDD on three cytosolic enzymes: tyrosine kinase, MAP-2 kinase, and PKA. It is clear that nuclear pathways are affected downstream of these cytosolic pathways, as shown by studies of the effect of estrogen and TCDD on levels of AP-1, and underscore how toxicants may disrupt steroid hormone and growth factor transduction pathways.
These and other observations lead to a second hypothesis: estrogen has both immediate and long-term effects on cytosolic signal transduction pathways. Depending on cell type, estrogen will enhance or dampen the adverse effect of the toxicants that employ these pathways. The positive and negative effects of estrogen are time-and dose-related and appear to require the presence of the
estrogen receptor. The latter point is worth stressing: low levels of estrogen, unopposed for a prolonged time period, may have the same effect as a higher dose for a short interval. This effect is demonstrated in studies of castrated female rats, in which exogenous estrogen replacement (simulating the long-term effect of low levels of estrogen seen with maturation) protects the animals against the adverse effect of TCDD (e.g., the loss of body weight; see Figure B-8) which is seen in males and untreated females. These data also provide evidence that transduction pathways, altered by estrogen in a relatively short period of time, interact with the mechanisms associated with the adverse effects of the toxicant.
Androgens also appear to modulate the effects of toxicants, either directly (through the androgen receptor) or by antagonizing the action of estrogen. As evidence of this, TCDD causes an increase in tyrosine kinase activity in the intact mature male rats, but this effect disappears in castrated males, which look strikingly similarly to intact females (see Figure B-9). This suggests that testosterone enhances some effects of TCDD, while estrogen protects or attenuates them. Similar results have been obtained in guinea pigs and suggest that the modulatory effects of androgens may be as important, if not more important, as estrogens; however, genomic differences cannot be ignored and caution should be exercised in interpreting data from experiments in which either gender is treated with sex-steroid hormones to elicit a specific response.
Taken together, these results permit the posing of a third hypothesis: one type of hormone disruption occurs when toxicants and hormones (and perhaps growth hormones as well) share a critical transduction pathway and have opposing effects on that pathway. Evidence to support this hypothesis comes from studies of granulosa cells, in which polyaromatic hydrocarbons—TCDD in this case—decrease the cell's ability to produce estradiol and also reduce estrogen receptor levels, thus demonstrating two different "hormone disrupting" mechanisms. Consequently, TCDD-like compounds can disrupt both estrogen production and estrogen action in the same or different cells. If the presence of estrogens and the ability for them to act are protective in terms of some adverse effects, then we can expect, and will find, delayed effects of some toxicants in females which are similar to the acute effects seen in males. These results suggest that TCDD-like compounds interfere not only with the transduction pathway but also with signaling pathways and have an important time-dependency in terms of adverse effects.
Finally, with regard to the adverse effects of environmental toxicants on development, data thus far point to a fourth specific hypothesis: lower vertebrates are more sensitive to the adverse effects of toxicants on development, because they remain more susceptible to steroid hormone-induced developmental change. Some lower vertebrates may be more hormonally programmed and less genetically programmed to develop normally, compared to mammals. For example, the sex ratio in alligators depends on the temperature at which the eggs are incubated, and when bird eggs are given testosterone the entire clutch develops as males. In mammals, on the other hand, if the SRY gene is present, the individual will be programmed to differentiate as a male. A consequence of
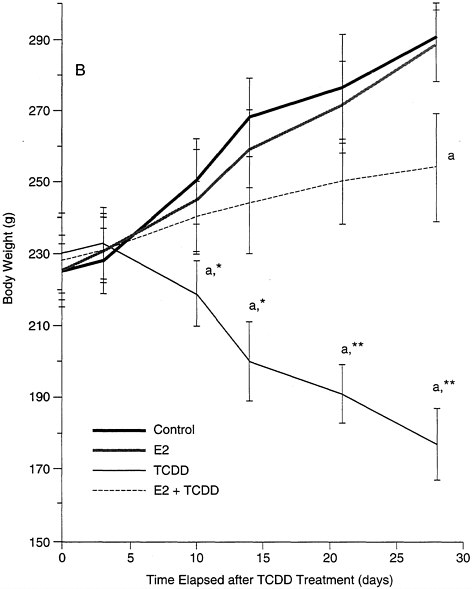
FIGURE B-8 Estrogen protective effects against TCDD.
this is that levels of toxicants that produce major developmental changes in lower vertebrates have less effect on the reproductive development of rats. This concept may help explain why wildlife biologists see environmental estrogens as a much greater threat than do experimental toxicologists. The mechanisms for these adverse effects, however, may be similar in lower and higher vertebrates, with the primary difference being the degree of genetic programming for development and differentiation. We hypothesize that the major pathway for these interactions appears to be MAP-2 kinase, a very early cytosolic pathway that overlaps with pathways that, later in evolution, are used for sexual
development and differentiation. This explanation relies on the previously speculated interactions between TCDD-like compounds and estrogen and also on interactions at the level of helper proteins in the nucleus and factors that control cell cycles. These same concepts can be extrapolated from developmental defects to interactions between and among steroid hormones, growth factors, and toxicants and to changes in the proliferative potential of certain cell types and the induction of precancerous states. Cells may be likely to be more (or differentially) sensitive to toxicants early in their development and become less sensitive to toxicants as they reach their end-point differentiation. Comparative studies may be useful for examining these issues, as somatic cells from lower vertebrates have more plasticity in terms of responding to steroid induction than do somatic cells from higher vertebrates.
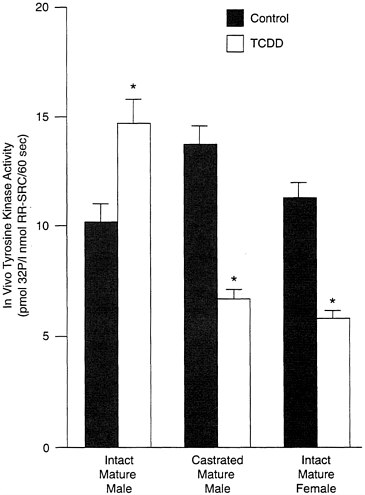
FIGURE B-9 TCDD and tyrosine kinase activity.
Molecular Markers of Carcinogenesis: Gender Differences7
Traditionally, environmental epidemiology has relied entirely on measurement of external exposure. Molecular epidemiology, on the other hand, has allowed a fuller understanding of the complex biochemical and genetic changes that occur as a result of exposure, up to and including clinical disease (see Figure B-10). The goal is to provide prevention and intervention measures that will reduce the likelihood of disease—in this case, cancer. These techniques also raise a number of socioethical concerns, such as discrimination in employment or health insurance, that must also be addressed.
These techniques include not only markers to measure the internal dose but also markers of actual biological effects, notably changes in DNA structure. This follows the paradigm that genotoxicity is the hallmark or driving mechanism of cancer. Assays developed in cell or animal studies have now been applied to humans to provide markers of early biological effects that will lead to cancer.
Researchers are also focusing on the role of oncogenes and tumor suppressor genes as markers of susceptibility. These are being studied not only for their role in carcinogenesis but also for racial, ethnic, and gender differences. Research should also address the effect of endogenous agents (e.g., steroidal hormones) in modulating the activities of these genes.
The availability of these genetic markers complicates the design of epidemiological studies by adding new levels of complexity with regard to exposure, disease, and genetic susceptibility. Even this level of variables is rather simplistic, given the polymorphism of human genes, but these considerations have not always been considered in ongoing studies. Sibling-paired twin studies could narrow the regions and chromosomes on which we look for a polymorphic gene that may or may not be responsible for disease. The pursuit of the BRCA-1 gene is a classic example of this approach. Once the population impact of gene expression is discovered (or determined) by epidemiologic studies, however, it falls to the laboratory scientist to look for functional mutations and to discover the mechanism by which the gene leads to disease.
Animal studies have demonstrated gender differences in susceptibility to cancer in specific nongonadal tissues and in response to specific categories of carcinogens. Genetic factors also appear to play a role, as in the ERKO mouse described earlier. Mechanism studies suggest that both hormones and receptors play a role in these differences, and that exogenous hormonal mimics can modulate both endocrine and metabolic pathways.
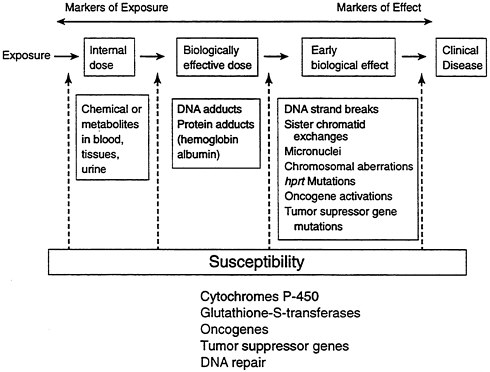
FIGURE B-10 Changes that occur as a result of exposure.
Human studies have also shown gender differences in susceptibility to some environmental exposures, in terms of both odds ratio and target organ (see Table B-5). The tobacco data in particular are disturbing, given the recent increase in tobacco usage by adolescent females. The role of pesticides and other xenoestrogens is also being investigated in several laboratories. The epidemiologic studies that showed association between organochloride pesticides and breast cancer were necessarily limited by their retrospective study designs. Estimates of individual exposure had to be reconstructed from historical or imputed data and all possible risk factors for breast cancer could not be accounted for. Therefore, these studies need to be followed up by prospective epidemiology studies as well as laboratory investigations of the associations.
Studies of molecular biomarkers of susceptibility have found some gender differences in baseline cytogenetic markers and DNA adducts, particularly with regard to tobacco and lung cancer. Researchers found race-but not gender-related differences in the frequency of polymorphisms in genes related to Phase I metabolism, which activates procarcinogens into carcinogens. However, females do have a higher incidence of polymorphisms in certain genes related to Phase II metabolism, which has a role in detoxifying or eliminating carcinogens, and in the p53 tumor suppressor gene. Collectively, these studies show clear gender differences in both susceptibility and the frequency of markers of susceptibility, with racial and ethnic differences among women with regard to
markers. In particular, they also suggest that women have a higher susceptibility than men to lung cancer following exposure to tobacco.
DES has been used as a model of environmental estrogen, and there are reports of reproductive abnormalities in the male offspring of DES subjects, but there has been little tissue-specific study of the effects on the male. It may be that there are "windows of susceptibility" during development, and new studies will address susceptibility at different stages of pregnancy. In general, males appear to be less sensitive to estrogenic compounds, at least initially, but they have a very steep dose-response curve and an abrupt response at higher levels. This may be because androgen is protective in the male, whereas estrogen exaggerates adverse effects.
This review suggests five areas where further research is needed:
-
inclusion of women in occupational studies and further identification of environmental risk factors;
-
further clarification of gender differences in frequency of known markers of genetic susceptibility;
-
evaluation of steroid receptor variants and susceptibility to environmental cancers;
-
vigorous application of animal models to study underlying regulation of environmental carcinogenesis; and
-
identification of biological causality between genetic susceptibility markers and gender-related human cancers.
TABLE B-5 Gender Differences in Cancer Susceptibility: Human Studies/Environmental Exposures
|
• |
Tobacco-related cancers (ORs) (Zang and Wynder, 1996) Lung (bronchogenic carcinoma)—female: 8.1, male: 4.6 Oral—female: 5.0, male: 2.0 |
|
• |
Dioxin-related cancers (Seveso, Italy) (Landi et al., 1997). —Men: Leukemia, esophageal, rectal —Women: Liver, stomach, colon (decrease in breast cancer) —Hodgkin's disease: Women > men |
|
• |
Pesticide exposure (Zahm et al., 1994) —Non-Hodgkin's lymphoma: Women < men —Soft-tissue sarcomas: Women < men |
|
• |
Pesticide-breast cancer controversy |





























