4
Ground Water Dating and Isotope Chemistry
Fred M. Phillips
Department of Earth and Environmental Science
New Mexico Institute of Mining and Technology
Introduction
Ground water tracers and isotope chemistry of ground water can be considered as subfields of the larger area of environmental tracers in ground water. Environmental tracers are simply chemical or isotopic solutes that are found in ground water as a result of ambient conditions rather than the deliberate activity of a researcher. They are studied mainly for the information they give about the ground water flow regime rather than the nature of the chemical activity in the ground water system. Such tracers have assumed new prominence in the past decade as a result of the refocusing of attention in applied ground water hydrology from questions of ground water supply, which are somewhat independent of the details of the flow path, to questions of ground water contamination, for which understanding the flow path and the nature of solute transport along it are central. Opportunities in the Hydrologic Sciences (NRC, 1991) emphasizes that "environmental isotopes are a key tool in studying the subsurface component of the hydrologic cycle."
Despite recently increased interest in applications of environmental tracers, no clear path of development over the past 5 to 10 years can be laid out. This diffuse and unpredictable nature of development is a direct outcome of the opportunistic nature of the field. Scientific disciplines that have a large theoretical component (e.g., the mathematical description of solute transport in ground water) tend to develop in a coherent way as individual researchers explore and build upon earlier theory, with only occasional correction from experimental results. New developments are driven in large part by intellectual assessment of immedi-
ately preceding work. In contrast, the application of environmental tracers to ground water hydrology has tended to be driven in large part by the introduction of analytical technologies developed by workers in other fields. Although in some cases the systematics of the tracer behavior have been worked out during investigations of ground water systems, more commonly the systematics have been previously well understood from independent investigations and the focus has mainly been on what the tracers can reveal about ground water flow and transport.
Instead of attempting to trace development of the field over the past few years, which might result in a relatively unenlightening catalog of methodologies, a few of the most innovative applications will be highlighted here. Some of the questions raised by Opportunities in the Hydrologic Sciences will then be addressed. That report focuses on the nature of scientific problems in hydrology and the means by which solutions to those problems might be efficiently promoted: "The choice of research problems is occasioned by its level of development within the hierarchy of the science, by the availability of new methods with which to solve it, and by the desire to understand a hydrologic phenomenon more deeply. The solution to the problem advances the development of the science and expands the conceptual framework that gives it meaning... That is the challenge to hydrologic science" (p. 7). Later the report states that "achieving this comprehensive understanding will require the kind of long-term disciplinary and interdisciplinary effort that can be sustained only by a vigorous scientific infrastructure" (p. 13) and, additionally, that "the supporting scientific infrastructure, including distinct educational programs, research grant programs, and research institutions, does not now exist for hydrologic science and must be put in place" (p. 2). Two research problems in environmental tracers will be examined in this paper, both with societally important and immediate applications but with very different histories. The goal is to elucidate how interactions with the "scientific infrastructure'' affected the development of these problems and how characteristics particular to environmental tracer research influenced those interactions.
Recent Developments
Vadose Zone
One major trend in vadose zone hydrology has been a new interest in the behavior of water in arid-region vadose zones, mostly as a result of the need to predict contaminant transport at waste disposal sites. This has resulted in an increased emphasis on environmental tracer methods, partly because tracers are directly relevant to predicting the movement of dissolved contaminants and partly because the time scales for flow in arid vadose zones are often so slow that information from short-term physical monitoring may be difficult to extrapolate to the longer scale appropriate for solute transport.
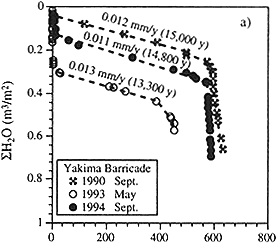
Figure 1 Cumulative water volume as a function of cumulative chloride mass (both per unit area) for three boreholes in the Pasco Basin, Washington. The recharge rates are calculated from the slope of the line and the total chloride accumulation times (in parentheses) from the chloride inventory. Based on the geological history of the site, the actual accumulation time is known to be between 13,000 and 15,000 years. Source: Murphy et al. (1996). © 1996 by American Geophysical Union.
One frequently used tracer in this situation is also one of the simplest—chloride. Chloride inventories with depth are commonly used to estimate net infiltration rates (see Figure 1), and increases in concentration are used to estimate evapotranspiration (Allison et al., 1994). Ancillary tracers include tritium (Scanlon, 1992), 36Cl (Tyler et al., 1996), and the stable isotopes of hydrogen and oxygen (Liu et al., 1995). Although a shallow upper zone of arid-region soils is typically hydraulically active, response time in deep desert vadose zones is on the scale of 103 to over 104 years (Phillips, 1994; Murphy et al., 1996). Even the basics of water flow in arid-region vadose zones are still incompletely understood. This setting constitutes one of the frontiers of hydrology at the present time.
Shallow Aquifers
This setting is currently of great interest because the preponderance of ground water contamination problems are found there. For many years tritium was the tracer of choice. However, the inexorable radio decay of the pulse of tritium released into the environment by the atmospheric nuclear weapons test of the early 1960s has reduced tritium concentrations in most ground water to the point where they are of little use. Instead, attention has focused on the application of
chlorofluorocarbons and combined 3H/3He to understanding shallow flow regimes. These methods are described in the following section.
Deep Aquifers and Regional Flow Systems
Probably the most exciting development in this setting has been the adaptation of solid source mass spectrometric methods originally developed for "hard rock" geochemistry to the investigation of heavy isotope ratios in deep ground water. At great depths the hydraulic properties are generally very poorly known and deep flow systems may as much reflect processes under ancient tectonic and climatic regimes as they do the influence of current conditions. In these circumstances tracers that can yield information on flow paths and rates are invaluable. Musgrove and Banner (1993) and Stueber et al. (1993) have demonstrated the utility of 87Sr/86Sr, 147Sm/144Nd, and other isotopes in unraveling the ancient flow regimes of the American midcontinent (see Figure 2), as have Moldovanyi et al. (1993) for the Gulf Coast Basin.
An area in which tracing studies in deep aquifers have contributed to the broader scientific framework is the increasing use of water in such aquifers (or minerals precipitated from the water) as archives of information about paleo-
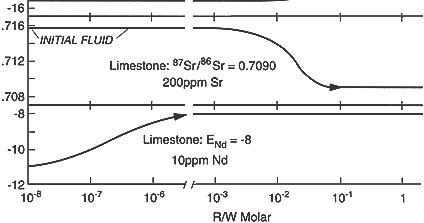
Figure 2 Calculated variations in three isotopic tracers as a function of progres-sive rock-water interaction (moles of rock dissolved and reprecipitated per mole of water) for ground water flowing through a limestone aquifer. The differing rates at which the water compositions respond to reaction with the rock suggest applications as tracers of water source and flow path history. Source: Reprinted, with permis-sion, from Banner et al. (1989). © 1989 from Elsevier Science.
climatic conditions in the aquifer recharge area. The most notable examples are the studies by Stute et al. (1992, 1995) that used noble gas paleothermometry to establish the glacial-to-interglacial temperature reduction and that glacial temperatures were strongly reduced in the tropics. Other studies have focused on the reconstruction of variations in the stable isotope composition of precipitation over time (Winograd et al., 1992; Plummer, 1993; Clark et al., 1997). This is one of a relatively small number of instances where ground water science has made a significant impact on disciplines other than traditional subsurface fields.
Case Studies in the Development of Environmental Tracers
The 1991 National Research Council report Opportunities in the Hydrologic Sciences played a major role in formalizing research support for hydrology as a separate discipline through establishing a separate National Science Foundation (NSF) program focused on hydrology. Ironically, this recognition was achieved just as internal pressure (i.e., the level of competition for research funding) rose to new highs and as the national rationale for funding scientific research was called into question as a result of ending the Cold War and of increased international economic competition. One reaction to the new circumstances was to call for increased emphasis on research that had relatively immediate societal applications. Hydrology clearly falls into this category; indeed, one common criticism of hydrological research is that the direction of research is driven too much by perceived applications. Nevertheless, no expansion of funding for research on hydrologic tracers has resulted from this change in emphasis.
Another reaction to the changing circumstances has been to reevaluate some of the basic assumptions that have served as a rationale for national funding of basic research for the past 40 years. Does "pure" or "curiosity-driven" research really contribute to the national welfare or is it largely a sideshow to research whose results are immediately applicable to societal problems? How are basic research results turned into products that will benefit society? How can the scientific research establishment promote the transfer of basic research results into applications?
Many recent writers on this topic have attempted to generalize answers to these questions. This paper will take a different approach and examine two case studies from environmental tracer research in hydrology. These cases raise their own questions because of widely contrasting histories of development. In one case a technique that was demonstrated as feasible and that had obvious application to both immediate societal problems of ground water contamination and fundamental problems of transport theory sat "on the shelf" for almost 15 years before it was put into practice, at which point it was widely hailed. In the other case a truly "off the wall" curiosity-driven research idea with no evident application to societal problems was funded and, even before publication of the results,
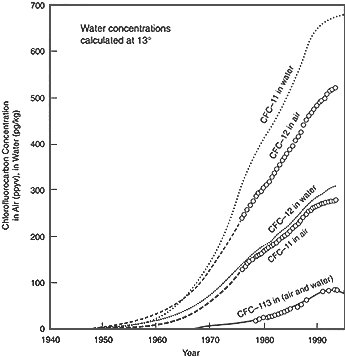
Figure 3 Histories of CFC concentrations in air and in water equilibrated with air, showing how measured concentrations in ground water can identify the date of recharge. Source: Szabo et al. (1996). © 1996 by the American Geophysical Union.
taken up and used to solve a major applied hydrology problem. Lessons can be learned from these examples, in the context of the fundamental nature of environmental tracer research.
Chlorofluorocarbons: Tracer Redivivus
Chlorofluorocarbons (CFCs) have many industrial uses, especially as working fluids in refrigeration units. Rates of atmospheric degradation are very slow. Thus, CFCs that have escaped after utilization have steadily accumulated in the atmosphere over the past 50 years (see Figure 3). They have only slight tendencies to adsorb on solid surfaces and are hence reasonably conservative in ground water. The key to their application as tracers is that, despite very low solubility in water, they are measurable at very low concentrations owing to their high electron affinity, which results in very sensitive responses in electron capture detectors attached to gas chromatographs.
Appreciation of the potential role of CFCs as ground water tracers was first developed in the early 1970s through collaboration between John Hays, a geochemist with a strong analytical orientation; Stanley N. Davis, a hydrogeologist; and Glen Thompson, a geology graduate student—all at the University of Indiana (Thompson et al., 1974). The work progressed, as Thompson's Ph.D. research, from the initial suggestion to methods development and limited field application (Thompson, 1976; Thompson and Hayes, 1979). Further development work continued until Thompson switched from ground water research to private consulting in the early 1980s. After that time virtually nothing was heard about CFCs as ground water tracers until the technique was revived by Niel Plummer and Eurybiades Busenberg of the U.S. Geological Survey (USGS) in the early 1990s (Busenberg and Plummer, 1991, 1992).
During this same period, the emphasis in ground water hydrology shifted from water supply to water contamination and solute transport. CFC tracing is now seen as a superb tool for understanding the shallow flow systems most susceptible to contamination. It can delineate recharge areas and rates, and flow directions and velocities, helping to establish sources and paths of contamination and predict future transport (Bohlke and Denver, 1995). During the 1980s, the focus of ground water theoretical development changed to solute (i.e., contaminant) transport, and elaborate tracer tests were conducted in order to test the theories. In retrospect, much of the same information could have been obtained by examining natural flow systems and applying CFC dating to provide a framework for the interpretation of the transport of environmental tracer inputs, such as the bomb tritium pulse (Szabo et al., 1996; see Figure 4). The widespread and rapid appreciation of the potential of the CFC method was demonstrated by the bestowal of the O. E. Meinzer Award to Plummer in 1993.
Why should a technique that was demonstrated as feasible, if not perfected, and was so immediately applicable to the burning questions of the day have been allowed to wither on the vine? Some of the answers to this question are clearly circumstantial. The departure of Thompson derailed the progress of the research. Bureaucratic factors also played a role. CFC samples are extremely subject to contamination and loss into or on container walls. Thompson overcame this difficulty by transporting his gas chromatographs into the field. However, the electron capture detector contained a small radioactive source, and during the 1980s regulatory controls on such sources virtually limited their use to laboratory settings. This problem was ultimately solved by Plummer through stringent sampling protocols and selection of container materials.
However, in addition to these fairly obvious obstacles, certain difficulties that are characteristic of environmental geochemistry research played a major role. The first of these is the issue of ''art." "Art" refers to the range of skills and techniques that do not really fall within the domain of science but rather of craft. Geochemical research typically involves a great deal more art than many other fields in hydrology. Although the principles of a technique such as
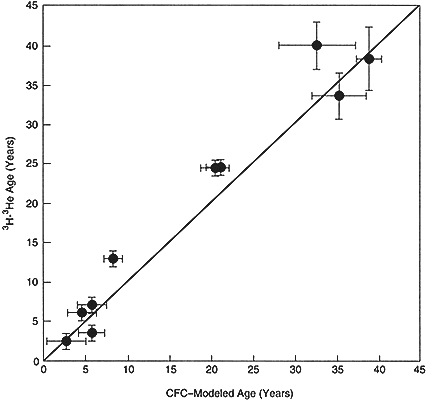
Figure 4 Comparison by Szabo et al. (1996) of the CFC and 3H/3He ages of shallow ground water in the Kirkwood-Cohansey aquifer system of southern New Jersey. The very good agreement between the two tracers indicates very minor dispersive mixing in the system. © 1996 by the American Geophysical Union.
analysis of very low levels of CFCs in natural water may be well understood, being able to reliably and reproducibly measure samples at a rapid enough rate for routine research applications may take years of tinkering with detector settings, column packings, valve assemblies and materials, carrier gases, data reduction programs, and literally hundreds of other experimental variables. Such art is rarely recorded; rather it is assimilated through apprenticeship. Any experimentalist knows that once such a technique is dropped, although the principles may be easily recovered, there will have to be an enormous investment of time to recover the art. This loss of expertise, in the case of CFC analysis, raised a barrier that forestalled most researchers who considered carrying on the method.
A second major impediment was presented by the nature of the research funding process. Developing the art as well as the science of the analytical method is time consuming; the (at that time standard) two-year grant required almost yearly new proposals, often before much demonstrable progress had been made. Without research funding programs directed specifically at hydrology, the proposals for continued funding had to compete with a very wide range of geochemical research projects. Geochemical analysis is instrumentation intensive, and some laboratories have succeeded in building up facilities worth millions of dollars. At least some of these laboratories took the viewpoint that the topics they researched were fundamentally so much more important than hydrological tracing that, in comparison, the new method did not deserve support. Expressed through the medium of proposal reviews, these opinions were successful in preventing continued funding for the research.
In this regard the USGS team led by Plummer had something of an advantage. Without being constrained by having to provide immediate results and without having to regularly run the gauntlet of hostile reviewers from other disciplines, they had the opportunity to systematically address the development of the method and present it to the hydrological community in a fairly mature state. The final factor that must also be mentioned is vision. Once a new geochemical method fails to reach fruition, even if this is due largely to circumstance rather than its merits, the huge investment of time and labor necessary to reinvent it raises a high barrier. It takes a researcher with a strong vision of the potential reward to take the risk of losing that investment.
Chlorine-36 in Fossil Rat Urine: Tracer Serendipitous
In the early 1990s I received a telephone call from an external reviewer for the U.S. Department of Energy plan of investigations at Yucca Mountain. The reviewer told me about the planned collection of 36Cl samples from the exploratory shaft under the mountain. The basic idea was that samples would be collected at intervals as the shaft progressed and the 36Cl/Cl ratio measured and compared with the modern atmospheric ratio, which is about 500 × 10-15. Ratios higher than this value were considered indicative of very rapid infiltration of 36Cl fallout from nuclear weapons testing in the 1950s. I had no connection with the Yucca Mountain project, but the reviewer had contacted me as an independent authority on hydrological applications of 36Cl. When asked why she was so concerned about this particular aspect of the proposed investigations, she replied that it was considered the single most critical component of the entire range of research at the site, since the widespread presence of bomb 36Cl would show that unsaturated fluxes were much greater than anticipated.
In fact, when the 36Cl results came in (Fabryka-Martin et al., 1993), they showed that most of the ratios were above the modern atmospheric value (see Figure 5). However, instead of indicating that bomb 36Cl was entering every-
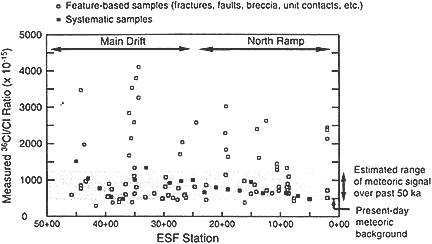
Figure 5 36Cl/Cl ratio as a function of distance (100-m increments) along the Exploratory Studies Facility (ESF) tunnel at the proposed high-level nuclear waste repository site, Yucca Mountain, Nevada. The present-day 36Cl/Cl ratio is approximately 500 × 10 -15. Samples with 36Cl/Cl higher than 1,250 × 10-15 are thought to contain bomb 36Cl. Source: Reprinted, with permission, Levy et al. (1997). © Los Alamos National Laboratory.
where, these were interpreted as indicating transport times in excess of 10,000 years (although it should be noted that some high ratios are still thought to indicate infiltration of fallout). Why the new interpretation? It was because a long-term history of much higher cosmogenic 36Cl production had in the meantime been established through an entirely independent and quite unconventional research project.
In 1990 Edouard Bard had shown, by dating submarine corals using both U/Th and 14C, that 14C ages became systematically younger than the actual ages by several thousand years in the period from 12,000 to 20,000 years ago (Bard et al., 1990). This shift was attributed to increased 14C production during that period, resulting from an increased cosmic-ray flux, which in turn was attributed to a weakened dipole geomagnetic field (Mazaud et al., 1991). However, this was difficult to demonstrate because 14C activity in the atmosphere is also affected by shifts between the atmosphere/biosphere/marine reservoirs. After reading that paper, I realized that 36Cl could potentially address the problem because it has a very short residence time in the atmosphere and essentially falls directly out onto the land surface. A record of 36Cl deposition should therefore be much closer to a direct record of variations in cosmogenic production.
The difficulty was finding an archive in which 36Cl deposition would be preserved, inasmuch as it is extremely mobile in the presence of water. Finally, I
realized that fossil rat urine, preserved in ancient packrat middens of the southwestern. United States (Betancourt et al., 1990), would preserve the chlorine that the rats ingested and excreted, in a context where the 36Cl/Cl ratio could be assigned an age by means of 14C dating of the urine itself. This experiment was proposed to the NSF and, after some initial difficulties, was funded. Midden samples from western and southern Nevada were obtained through the cooperation of Peter Wigand at the Desert Research Institute in Reno. The initial results were successful, and in a few years a sketchy preliminary history of 36Cl deposition was reconstructed. The major feature of this reconstruction was a pattern of relatively low ratios during the Holocene (i.e., the past 10,000 years) compared to ratios higher by almost a factor of two prior to 13,000 years ago (Plummer et al., 1997; see Figure 6).
The significance of this result was immediately apparent to those involved in the Yucca Mountain project. Most of the unexpectedly high 36Cl/Cl ratios beneath Yucca Mountain probably were a fingerprint of recharge prior to 10,000 years ago, rather than since 1950. The right information happened to appear just
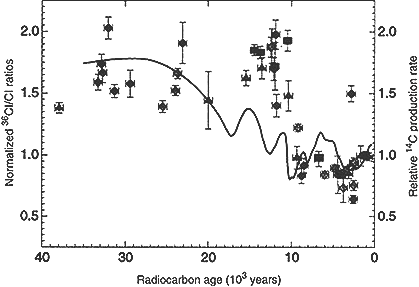
Figure 6 Measurements of the 36Cl/Cl ratio in fossil packrat urine as a function of radiocarbon age. Chlorine-36 ratios are normalized to the modern ratio. The solid line indicates the history of variation in production of cosmogenic nuclides inferred from the record of variations in atmospheric radiocarbon activity. Source: Reprinted, with permission, from Plummer et al. (1997). © 1997 from the American Association for the Advancement of Science.
at the right time to prevent a major misinterpretation of some of the most critical data from one of the largest public works projects planned in the United States. Furthermore, it is obvious that this temporal variation in 36Cl fallout can serve as a hydrological tracer in many other similar circumstances.
The contrast with the development of the CFC tracer method could hardly be more striking. Why was the secular variation 36Cl tracer springing into existence just as it was needed, while the CFC tracer sat on the shelf for years after its capabilities had been highlighted? The most obvious answer has to do with research funding. Although the basic idea would undoubtedly have made a fine target for one of Senator Proxmire's ''Golden Fleece" awards were he still alive, reviewers and program managers were able to see that it was a means to obtain critical data that could not be had otherwise. Other factors played a role. The "art" of 36Cl analysis was maintained through long-term funding of the PRIME Lab accelerator mass spectrometry NSF facility at Purdue University and at my own laboratory, overcoming the inevitable start-up problems that stall the acquisition of real results.
Reflections
The state of research on environmental tracers in ground water hydrology is flourishing. New tracers are being developed, tracer results are being applied more and more widely as integral parts of hydrological investigations, and results of environmental tracer studies are being applied to fields outside hydrology. What can be done to promote continued growth of the field? Two case studies with remarkably contrasting histories were examined in order to reflect upon this question. Two studies cannot be presumed to yield any definitive patterns, but some common threads can be discerned:
- The first point, and by far the most important, is a reaffirmation of the societal value of basic scientific research. This idea is certainly nothing new, but given the recent spate of attacks on this proposition, it is worth reemphasizing. Who could have predicted that the apparently straightforward and obviously applicable CFC tracer would not be successful for almost 20 years after it was first proposed, while the esoteric investigation of fossil rat urine would pay immediate practical dividends? The best criterion for predicting the utility of research directions is not an attempt to second-guess their end use, but rather some estimate of their fundamental scientific worth.
- The long technique development times and the large investment in the geochemical art need to be recognized when supporting hydrological tracer research. Promising techniques may be killed by demanding immediate results. The trend toward longer NSF grant periods is a very promising development in this regard.
- Proposed advances in environmental tracers need to be evaluated within
- the context of the discipline of scientific hydrology. Possession of impeccable credentials in other areas of geochemistry does not qualify proposal reviewers from outside the field. The establishment of a separate NSF program in hydrology is a major step forward from the situation at the time CFC research temporarily died. For this step we owe a great deal to the Opportunities in the Hydrologic Sciences report and to those who invested their time in writing it.
References
Allison, G. B., G. W. Gee, and S. W. Tyler. 1994. Vadosezone techniques for estimating ground-water recharge in arid and semiarid regions. Soil Sci. Soc. Am. J. 58:6–14.
Banner, J. L., G. J. Wasserberg, P. F. Dubson, A. B. Carpenter, and C. H. Moore. 1989. Isotopic and Trace Element Constraints on the Origin and Evolution of Saline Ground Waters from Central Missouri. Geochim. Cosmochim. Acta 53:383–398.
Bard, E., B. Hamelin, R. G. Fairbanks, and A. Zindler. 1990. Calibration of the 14C timescale over the past 30,000 years using mass spectrometric U-Th ages from Barbadoscorals. Nature 345:405–410.
Betancourt, J. L., T. P. Van Devender, and P. S. Martin. 1990. Packrat Middens: The Last 40,000 Years of Biotic Change. Tucson: University of Arizona Press.
Bohlke, J. K., and J. M. Denver. 1995. Combined use of groundwater dating, chemical, and isotopic analyses to resolve the history and fate of nitrate contamination in two agricultural watersheds, Atlantic coastal plain, Maryland. Water Resour. Res. 31:2319–2340.
Busenberg, E., and L. N. Plummer. 1991. Chlorofluorocarbons (CCl3F and CCl2F2): Use as an Age Dating Tool and Hydrologic Tracer in Shallow Ground-Water Systems. U.S. Geological Circular 1992.
Busenberg, E., and L. N. Plummer. 1992. Use of chlorofluorocarbons (CCl3F and CCl2F2) as hydrologic tracers and age-dating tools: The alluvium and terrace system of central Oklahoma. Water Resour. Res. 28:2257–2284.
Clark, J. F., M. Stute, P. Schlosser, S. Drinkard, and G. Bonani. 1997. A tracer study of the Floridan aquifer in southeastern Georgia: Implications for groundwater flow and paleoclimate. Water Resour. Res. 33:281–290.
Fabryka-Martin, J. T., S. J. Wightman, W. J. Murphy, M. P. Wickham, M. W. Caffee, G. J. Nimz, J. R. Southon, and P. Sharma. 1993. Distribution of chlorine-36 in the unsaturated zone at Yucca Mountain: An indicator of fast transport paths. Pp. 58–68 in Proceedings of FOCUS '93: Topical Meeting on Site Characteristics and Model Validation. September 26–29, 1993, Las Vegas, Nevada.. La Grange Park, III.: American Nuclear Society.
Levy, S. S., D. S. Sweetkind, J. T. Fabryka-Martin, P. R. Dixon, J. L. Roach, L. E. Wolfsberg, D. Elmore, and P. Sharma. 1997. Investigations of Structural Controls and Mineralogic Associations of Chlorine-36 Fast Pathways in the ESF. Report LA-EES-l-TIP-97-004. New Mexico: Los Alamos National Laboratory.
Liu, B., F. Phillips, S. Hoines, A. R. Campbell, and P. Sharma. 1995. Water movement in desert soil traced by hydrogen and oxygen isotopes, chloride, and chlorine-36, Southern Arizona. J. Hydrol. 168:91–110.
Mazaud, A., C. Laj, E. Bard, M. Arnold, and E. Tric. 1991. Geomagnetic field control of 14C production over the last 80 ky: Implications for the radiocarbon time scale. Geophys. Res. Lett. 18:1885–1888.
Moldovanyi, E. P., L. M. Walter, and L. S. Land. 1993. Strontium, boron, oxygen, and hydrogen isotope geochemistry of brines from basal strata of the Gulf Coast sedimentary basin, USA. Geochim. Cosmochim. Acta 57:2083–2099.
Murphy, E. M., T. R. Ginn, and J. L. Phillips. 1996. Geochemical estimates of paleorecharge in the Pasco Basin: Evaluation of the chloride mass balance technique. Water Resour. Res. 32:2853–2868.
Musgrove, M. L., and J. L. Banner. 1993. Regional ground-water mixing and the origin of saline fluids: Midcontinent, United States. Science 259:1877–1882.
National Research Council. 1991. Opportunities in the Hydrologic Sciences. Washington, D.C.: National Academy Press.
Phillips, F. M. 1994. Environmental tracers for water movement in desert soils of the American Southwest . Soil Sci. Soc. Am. J. 58:15–24.
Plummer, L. N. 1993. Stable isotope enrichment in paleowaters of the Southeast Atlantic Coastal Plain, United States. Science 262:2016–2020.
Plummer, M. A., F. M. Phillips, J. Fabryka-Martin, H. J. Turin, P. E. Wigand, and P. Sharma. 1997. Chlorine-36 in fossil rat urine: An archive of cosmogenic nuclide deposition during the past 40,000 years. Science 277:538–541.
Scanlon, B. R. 1992. Evaluation of liquid and vapor water flow in desert soils based on chlorine-36 and tritium tracers and nonisothermal flow simulations. Water Resour. Res. 28:285–298.
Stueber, A. M., L. M. Walter, T. J. Huston, and P. Pushkar. 1993. Formation waters from Mississippian-Pennsylvanian reservoirs, Illinois Basin, U.S.A.: Chemical and isotopic constraints on evolution and migration. Geochim. Cosmochim. Acta 57:763–784.
Stute, M., M. Forster, H. Frischkorn, A. Serejo, J. F. Clark, P. Schlosser, W. S. Broecker, and G. Bonani. 1995. Cooling of tropical Brazil (5°C) during the last glacial maximum . Science 269:379–383.
Stute, M., P. Schlosser, J. F. Clark, and W. S. Broecker. 1992. Paleotemperatures in the southwestern United States derived from noble gases in ground water. Science 256:1000–1002.
Szabo, Z., D. E. Rice, L. N. Plummer, E. Busenberg, S. Drinkard, and P. Schlosser. 1996. Age dating of shallow groundwater with chlorofluorocarbons, tritium/helium 3, and flow path analysis, southern New Jersey coastal plain. Water Resour. Res. 32:1023–1038.
Thompson, G. M. 1976. Trichloromethane: A New Hydrologic Tool for Tracing and Dating Ground Water. Ph.D. thesis, University of Indiana.
Thompson, G. M., and J. M. Hayes. 1979. Trichlorofluoromethane in ground-water. A possible tracer and indicator of groundwater age. Water Resour. Res. 15:546–554.
Thompson, G. M., J. M. Hayes, and S. N. Davis. 1974. Fluorocarbon tracers in hydrology. Geophys. Res. Lett. 1: 177–180.
Tyler, S. W., J. B. Chapman, S. H. Conrad, D. P. Hammermeister, D. O. Blout, J. J. Miller, M. J. Sully, and J. M. Ginanni. 1996. Soil-water flux in the southern Great Basin, United States: Temporal and spatial variations over the last 120,000 years. Water Resour. Res. 32:1481–1499.
Winograd, I. J., T. B. Coplen, J. M. Landwehr, A. C. Riggs, K. R. Ludwig, B. J. Szabo, P. T. Kolesar, and K. Revesz. 1992. Continuous 500,000-year climate record from vein calcite in Devils Hole, Nevada. Science 258:255–260.














