8
RISK CHARACTERIZATION AND PUBLIC HEALTH IMPLICATIONS
THE purpose of this chapter is to present a summary of the findings of the committee concerning the health effects of methylmercury (MeHg), end points of toxicity, the critical studies, exposure and dose metrics, and sources of uncertainty that should be considered by EPA in deriving the reference dose (RfD). It includes a discussion of the relevant health end points and the scientific basis and public-health rationale for selecting neurotoxicity in children exposed in utero as the critical end point for the EPA RfD.
The committee was directed to investigate the toxicological effects of MeHg and to evaluate research relevant to EPA's MeHg RfD. The activities of the committee included the following:
-
An evaluation of the available human epidemiological and animal toxicity data.
-
An examination of the critical studies, end points of toxicity, and uncertainty factors used in the derivation of the RfD.
-
A review of exposure data from the available epidemiological studies focusing on consumption of MeHg in fish.
-
Consideration of new and emerging health-effects data.
-
Identification of knowledge gaps and recommendations for future research.
The committee evaluated the body of evidence that has provided the
scientific basis for the risk assessments conducted by EPA and other regulatory and health agencies. The committee also reviewed new findings that have emerged since the development of the current RfD and met with the investigators of major ongoing epidemiological studies to examine and compare the methods and results.
Mercury (Hg) is pervasive and persistent in the environment, released from a large variety of natural and anthropogenic sources. The serious health impacts of high-level exposures have long been recognized. Between 1950 and 1975, major poisoning episodes in Japan and Iraq resulted in outbreaks of serious neurotoxic effects, including death, and led to the identification of developmental neurotoxicity as the health effect of greatest concern following high-level episodic exposure. As a result of its well-recognized toxicity, widespread industrial use, and environmental persistence, Hg has been extensively studied. Compared with data bases on many other pollutants, there is a robust data base on Hg, which includes environmental fate and transport; examination of toxicokinetics and toxicodynamics; biological and environmental measures of exposure and dose; and in vitro, animal, and human studies for a broad range of toxicity end points.
Historically, epidemiological investigations have focused on high exposures and related health impacts. More recently, large prospective epidemiological studies have been conducted to examine chronic low-level MeHg exposure. These studies examined the association between subtle end points of neurotoxicity and prenatal exposure measured by maternal markers of prior exposure. These markers are presumed to reflect maternal MeHg exposure from fish consumption. The committee focused on these studies because they provide the most comprehensive evidence of low-dose MeHg toxicity and they examine the exposure pathway most relevant to U.S. population exposures, including the sensitive population of children who were exposed to MeHg in utero.
THE CURRENT EPA REFERENCE DOSE
EPA defines an RfD as an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime (EPA 1997a). The
current EPA RfD for MeHg is 0.1 µg/kg-day. The RfD is an important risk-characterization tool that is broadly used as a measure of the “acceptability” of population exposure levels. It is used to guide risk-management decisions and regulatory policies ranging from fish-consumption advisories to air-emission permits. This section provides an overview of the development of the MeHg RfD.
Neurotoxicity in children exposed in utero is the health outcome selected by EPA for the current MeHg RfD. The RfD is based on data from the Iraqi poisoning episode, where the population consumed high levels of MeHg from treated seed grain. The critical study for the RfD conducted by Marsh et al. (1987) identified 81 children who had been in utero during the episode and examined their neurodevelopmental outcomes. Maternal-child pairs were selected from one of five Hg-hair-concentration groups, and the combined incidence of developmental effects (late walking, late talking, mental symptoms, seizures, or increased neurological score) was determined for each group. Exposure levels measured by maternal-hair concentration and combined developmental effects were used to estimate a benchmark dose. The benchmark dose of 11 ppm of Hg in hair was calculated as the 95% lower confidence limit on the maternal-hair concentration corresponding to a 10% extra risk level (Crump et al. 1995). In this report, the lower confidence limit is referred to as the BMDL. The following section describes how EPA derived the current RfD from that value.
A ratio of 250:1 was used to convert hair Hg concentration (mg of Hg/kg of hair) to blood Hg concentration (mg of Hg/L of blood) to derive the RfD critical dose (EPA 1997c):
11 mg/kg of hair would correspond to 11/250 = 44 µg/L of blood.
The following equation was used to obtain a daily dietary intake of MeHg that results in a blood Hg concentration of 44 µg/L:
where
d = daily dietary intake (micrograms of MeHg per kilogram of body weight per day),
C = concentration in blood (44 µg/L),
b = elimination constant (0.014 days-1),
V = volume of blood in the body (5 L),
A = absorption factor (expressed as a unitless decimal fraction of 0.95),
f = fraction of daily intake taken up by blood (unitless, 0.05), and
bw = body-weight default value of 60 kg for an adult female.
Using that equation, the total daily quantity of MeHg ingested by a 60-kg female to maintain a blood Hg concentration of 44 µg/L or a hair Hg concentration of 11 ppm would be
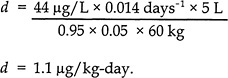
A composite uncertainty factor (UF) of 10 was used in the derivation of the RfD to account for human-population variability, lack of a two-generation reproductive study, and lack of data on sequelae resulting from longer durations of exposure (EPA 1997c):
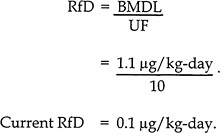
As the calculation shows, the application of UFs has a major influence on the quantification of the final RfD. Although the scientific rationale for the application of these factors is strong, it must be recognized that choosing the ultimate magnitude of the UFs is a policy decision, which is influenced by professional judgment, public-health goals, and the regulatory mandates of EPA.
EVALUATING THE RfD–END POINTS OF MeHg TOXICITY
The committee reviewed human epidemiological results and animal
toxicity data to examine potential human health effects and evaluate the use of neurotoxicity in children exposed in utero as the health end point for the derivation of the RfD. Other end points evaluated are carcinogenicity and immunological, reproductive, renal, and cardiovascular toxicity. Chapter 5 presents an in-depth presentation of the health effects of MeHg. The following is a summary of major findings.
Carcinogenicity
Studies in humans of the carcinogenic effects of MeHg are inconclusive. Although no studies have found an association between MeHg and overall cancer death rates in humans, two studies (Kinjo et al. 1996; Janicki et al. 1987) have found associations between Hg exposure and acute leukemia. Interpretation of these findings is limited because of small study populations and lack of control for other risk factors. Renal tumors have been observed in male mice (Mitsumori et al. 1981; Hirano et al. 1986) but only at or above the maximum tolerated dose. Hg has also been shown to cause chromosomal damage and aneuploidy in a number of in vivo and in vitro systems. On the basis of the available human, animal, and in vitro data, the International Agency for Research on Cancer (IARC) and EPA have classified MeHg as a “possible” (EPA Class C) human carcinogen (EPA 2000).
Immunotoxicity
Occupational studies suggest that Hg exposure can affect the immune system in humans (Dantas and Queiroz 1997; Moszczynski et al. 1999). In vitro and animal studies have shown that Hg can be immunotoxic. They suggest that exposure to MeHg can increase human susceptibility to infectious diseases and autoimmune disorders by damaging the immune system (Ilbäck et at. 1996). Animal studies have also shown that prenatal and perinatal exposure to MeHg produce long-term effects on the developing immune system (Wild et al. 1997). Immunological studies in animals are summarized in Table 5-3.
Reproductive Effects
The reproductive effects of MeHg exposure have not been evaluated in humans. However, an evaluation of the clinical symptoms and outcomes of over 6,000 MeHg-exposed Iraqi citizens found a low rate of pregnancies (79% reduction) among the exposed population (Bakir et al. 1973). That provides suggestive evidence of an effect of MeHg on human fertility. Animal studies, including work in nonhuman primates, have found reproductive problems, including decreased conception rates, early fetal losses, and stillbirths (Burbacher et al. 1988).
Renal Toxicity
The kidney is sensitive to inorganic Hg exposure, and renal damage has been observed following human ingestion of organic forms of Hg. Renal effects from organic Hg exposure have been observed only at exposure levels that also cause neurological effects. Renal damage was observed in the victims of the Iraqi poisoning, and an evaluation of death rates in an area of Minamata City, which had the highest prevalence of Minamata disease, found an increase in deaths from renal disease among women but not men (Tamashiro et al. 1986). Several reports of animal studies have also described MeHg-induced renal toxicity.
Cardiovascular Effects
The cardiovascular system appears to be a target for MeHg toxicity in humans and animals. Blood-pressure elevations have been observed in occupationally exposed men (Höök et al. 1954) and in children treated with mercurous chloride for medical conditions. More recently, there is evidence that suggests effects at low levels of exposure. A recent study of 1,000 children from the Faroe Islands found a positive association between prenatal exposure to MeHg, and blood pressure and heart rate variability at age 7 (Sørensen et al. 1999). A Finnish cohort study of 1,833 men linked dietary intake of fish and Hg concentrations in hair
and urine with increased risk of acute myocardial infarction (AMI) and coronary heart disease and cardiovascular disease (Salonen et al. 1995). Men who consumed at least 30 g of fish a day had a 2.1 higher risk of AMI. Cardiovascular effects have also been observed in several animal models of MeHg toxicity.
Central-Nervous-System Toxicity
The toxic effects of MeHg in the brain have been well documented in human and animal studies. Although both the adult and fetal brains are susceptible, the developing nervous system is more sensitive to the toxic effects of MeHg than is the developed nervous system. It should be pointed out however, that few studies of MeHg effects in adults have investigated the sensitive and subtle types of neurologic endpoints recently examined in children exposed in utero. Studies of Minamata victims indicate that prenatal exposure caused diffuse damage in the brain and adult exposure caused focal lesions. About 10% of the total body burden of MeHg is found in the brain. After ingestion, MeHg accumulates in the brain where it is slowly converted to inorganic Hg. On the basis of available studies, neurodevelopmental effects appear to be a sensitive end point for MeHg toxicity. There is an extensive human data base on neurodevelopmental effects, including studies of populations following high-dose poisonings and chronic low-level Hg exposure. In general, experimental animal studies have reported a continuum of neurodevelopmental effects similar to those reported in studies of humans exposed to MeHg. Of the three major long-term prospective studies, the Faroe Islands study reported an effect of low-level prenatal exposure on children's performance on neurobehavioral tests particularly in the domains of attention, fine-motor function, confrontational naming, visual-spatial abilities, and verbal memory. Similar effects were not found in the main Seychelles study; however, the smaller New Zealand study found effects on standardized tests of cognitive and neuromotor function that were similar to those administered in the main Seychelles study, and there was preliminary evidence of similar effects in the Seychelles pilot study.
SELECTION OF THE END POINT FOR THE RfD
The findings of the committee regarding the end points of MeHg toxicity support the selection of neurotoxicity in children exposed in utero as a suitable end point for the development of the RfD based on the available data. These effects have been well documented in a number of investigations, including prospective epidemiological studies examining low-dose chronic exposure through consumption of contaminated fish and seafood. Evidence from animal studies is consistent with the neurotoxicity findings in humans.
Given the limits of the available data, developmental neurotoxicity is the most sensitive, well-documented health end point. Therefore, its use as the basis for the RfD should be protective for other adverse effects that occur at higher doses of exposure. However, there is emerging evidence of potential effects on both the immune and cardiovascular systems at low doses of exposure. Although these effects are not well understood, emerging data underscore the need for continued research and raise the possibility of adverse effects to other organ systems at or below the current levels of concern for developmental neurotoxicity.
EXAMINATION OF THE CRITICAL STUDIES FOR THE RfD
The traditional approach to development of an RfD and other public-health-based risk guidance numbers is to select a critical study that is well conducted and provides the most sensitive, or lowest, no-observed-adverse-effect level (NOAEL), lowest-observed-adverse-effect level (LOAEL), or a lower 95% confidence limit on the benchmark dose (BMDL). The relevance of the study exposure levels and pathways to the population of concern should also be considered.
The current EPA RfD is based on developmental neurotoxic effects in children exposed in utero to high-level episodic exposure from bread made with grain treated with MeHg as a pesticide (Marsh et al. 1987). Although that study was judged the most appropriate at the time of the development of the current RfD, a number of recognized sources of uncertainty, including possible selection bias in the cohort, cannot be controlled. In addition, the exposure scenario in Iraq is not comparable
to the low-level chronic exposure that the general population of North America might experience through the consumption of fish.
Since the establishment of the current RfD, results from the prospective studies in the Faroe Islands (Grandjean et al. 1997, 1998, 1999a) and the Seychelles (Davidson et al. 1995a,b, 1998), as well as a peer-reviewed re-analysis of the New Zealand study (Crump et al. 1998), have added substantially to the body of knowledge concerning the developmental neurotoxic effects of chronic low-level exposure to MeHg. Each of these studies was well designed and carefully conducted. They examined the relation of prenatal MeHg exposure to neuropsychological function in childhood. MeHg was significantly associated with poorer performance in the Faroe Islands and New Zealand studies, but not in the main Seychelles study.
Much of the debate over the adverse effects of MeHg and the selection of a critical study for the RfD and other guidance has focused on the similarities and differences between the Faroe Islands and the Seychelles studies. The levels of maternal exposure are similar in both studies, but a number of differences in design and cohort characteristics might contribute to the disparate findings. They used different primary biomarkers of Hg exposure (cord blood versus maternal hair), different types of neurological tests (domain specific versus global), and different ages at testing (7 years versus 5.5 years). In addition, the studies had different patterns of exposure (due to whale consumption in the Faroe Islands). When the New Zealand study is considered, those research design differences seem less determinative. In New Zealand, adverse effects were found with exposure measures and a research design similar to the Seychelles study. These studies are contrasted and discussed in detail in Chapter 6.
The Faroe Islands population was also exposed to PCBs. The initial statistical analyses published by the investigators of the Faroe Islands study suggest that the associations of prenatal exposure with language, memory, and verbal-learning deficits might be attributable to prenatal PCB exposure, although the associations with attention and neuromotor-function deficits were not. However, prenatal Hg exposure was associated with deficits in language development in the Seychelles pilot and New Zealand studies, in which there was no evidence of increased PCB exposure. A re-analysis of the Faroe Islands data showed that the association of Hg exposure with language and verbal deficits was as strong among children with low PCB exposure as among those with
high exposure. Furthermore, a series of sensitivity analyses provided by the Faroe Islands research group (E. Budtz-Jørgensen, Copenhagen University, N. Keiding, Copenhagen University, and P. Grandjean, University of Southern Denmark, unpublished material, June 21, 2000) indicated that the PCB exposures were unlikely to be causing serious bias in BMD estimates. On the basis of these considerations, the committee concluded that the neurodevelopmental sequelae found in the Faroe Islands study were not attributable to PCB exposure and that PCB exposure did not invalidate the use of the Faroe Islands study as the basis of risk assessment.
The committee explored the possibility that differences in power might explain the discrepancies in the findings of the major studies. Five of the eight effects observed in the Faroe Islands study were very small. Despite the large sample size of the Seychelles study, its power to detect such small effects was poor. The Seychelles study had adequate power to detect the effects seen in the New Zealand study; therefore, such power considerations cannot fully explain its failure to detect any adverse effects at 5.5 years of age.
Despite their differences, the Faroe Islands, Seychelles, and New Zealand studies represent exposure scenarios that are more consistent than the Iraqi study with the North American experience. However, their conflicting results present a vexing choice for the development of a revised RfD. A conservative approach would be to derive as a point of departure the lowest BMD from the positive end points in the Faroe Islands study or the New Zealand study. It is possible to derive a lower limit approximation of a NOAEL or BMD from the Seychelles results, as was done by the Agency for Toxic Substances and Disease Registry for its minimal risk level (MRL). However, the choice of a negative study to derive guidance numbers when well-designed, plausible positive studies are available is difficult to defend. The committee recommends a more inclusive approach to developing any future RfD or exposure guidance. Given the availability of well-designed epidemiological studies in which prenatal MeHg levels were within the range of general-population exposures, contemporary exposure standards should consider the findings of all three studies — the New Zealand, Faroe Islands, and Seychelles studies.
To synthesize information from the different studies and outcomes, the committee conducted an integrative analysis to derive and compare estimates of BMDLs. This analysis is described in Chapter 7. The
committee debated whether to include the Seychelles study in the BMD evaluation. It concluded that it would be inappropriate to exclude the data from any well-designed study and that the inclusion of the Seychelles study was important to ensure that the analysis would reflect the full range of effects of MeHg exposure.
BMD CONSIDERATIONS: SELECTING A POINT OF DEPARTURE
The current MeHg RfD is based on a BMD estimation. The selection of a particular BMD for the derivation of the RfD represents a critical decision, influenced by both scientific and policy considerations. The BMDL is defined as a lower confidence limit on the dose corresponding to a given increase in response (e.g., 1%, 5%, or 10%) over the background rate (Crump 1984), the benchmark response (BMR). It is intended to be applied as an alternative to the NOAEL to provide a point of departure for low-dose extrapolation. The BMD represents a refinement over the traditional NOAEL or LOAEL, since it is not constrained to be one of the observed or experimental doses, and uses the full-range of dose-response information inherent in the data. Various terms are used for BMD estimates. In this report, the term BMDL denotes the lower confidence limit on the dose corresponding to the BMR of interest, and BMD is used to denote the point estimate of the dose.
The critical studies of MeHg examined a range of neurodevelopmental outcomes. Selection of the most appropriate BMD requires consideration of the biological significance of the effects, including the sensitivity and severity of the outcomes, consideration of the ability to detect both exposure and effects, and selection of an appropriate dose-response model. To examine and compare the results of the critical studies, BMD calculations were conducted and compared for various end points. These results are presented and discussed in detail in Chapter 7.
Various analyses were conducted as part of the committee's consideration of the overall weight of the evidence of developmental neurotoxic effects from low-level MeHg exposure. It is intended as a bounding exercise to evaluate and present the range of effects, BMDs, and BMDLs for each of the major epidemiological studies. The results provide a range of BMDLs, which should be considered in selecting the critical
BMD for development of a revised RfD. The methods considered included (1) approaches based on selecting a single outcome from a single study, and (2) an integrative analysis that synthesizes information over different studies and outcomes. Because the integrative analysis is exploratory, it would be premature to use this approach as the basis for risk assessment for MeHg. However, the approach was useful for facilitating a weight-of-evidence assessment.
The BMDLs derived from the various end points of the critical studies (with a P0 of 0.05, where P0 denotes the probability that an unexposed individual falls below the cutoff value that defines an adverse effect, and a BMR of 0.05) range from 4 (New Zealand McCarthy Perceptual Performance Test) to 23 (Seychelles Preschool Language Scale Test) in parts per million (ppm) Hg in maternal hair. It should be noted that the choice of P0 and the BMR are, in part, policy decisions. The full range of findings is presented in Table 7-2. Table 8-1 lists the BMDLs derived using the K-power model from Table 7-2. The K-power model was suggested because from a toxicological perspective, it has greater biological plausibility, since it allows the dose response to take on a sublinear form, if appropriate. The K-power model is typically fit under the constraint that K ≥ 1, so that supralinear models are ruled out. As shown in Table 8-1, the data suggest fairly high within-study consistency but high study-to-study variability. However, the ratio between the highest and lowest BMDLs was only 6.
The integrative analysis used a hierarchical model to quantify study-to-study and outcome-to-outcome variability, while smoothing away much of the random variability observed in the original data. Smoothed estimates of the BMDs and BMDL for each study were derived (with a P0 of 0.05 and a BMR of 0.05), and the distribution was examined. Outcome-to-outcome variability is reduced, but substantial study-to-study variability remains. The smoothed mean of the distribution of the various BMDs is 21 ppm, with a lower 5th percentile of 7 ppm.
The mean of the distribution is in close agreement with the unsmoothed mean of the BMDs from the Faroe Islands study (22 ppm). The integrative analysis does not permit the direct calculation of a BMDL. However, the lower 5th percentile of the theoretical distribution of true BMD values is analogous to a BMDL; that value is 8 ppm.
The examination of the BMDs suggests a number of ways to select a point of departure for the derivation of the RfD. The most sensitive end
TABLE 8-1 BMDLs for Study End Points (ppm Hg in maternal hair, BMR = 0.05)
|
BMDL (K power) |
Study |
Age |
End Point |
|
Seychellesa |
66 months |
Bender Copying Errors |
25 |
|
Child Behavior Checklist |
17 |
||
|
McCarthy General Cognitive |
23 |
||
|
Preschool Language Scale |
23 |
||
|
WJ Applied Problems |
22 |
||
|
WJ letter/word Recognition |
22 |
||
|
Faroe Islandsb |
7 years |
Finger Tapping |
12 |
|
CPT Reaction Time |
10 |
||
|
Bender Copying Errors |
15 |
||
|
Boston Naming Test |
10 |
||
|
CVLT: Delayed Recall |
14 |
||
|
New Zealandc |
6-7 years |
TOLD Language Development |
6 |
|
WISC-R:PIQ |
6 |
||
|
WISC-R:FSIQ |
6 |
||
|
McCarthy Perceptual Performance |
4 |
||
|
McCarthy Motor Test |
6 |
||
|
aData from Crump et al. 1998, 2000. bData from Budtz-Jorgensen et al. 1999. cData from Crump et al. 1998, 2000. |
|||
point from the most sensitive study is the McCarthy Perceptual Performance from New Zealand (BMD, 8 ppm; BMDL, 4 ppm). The Faroe Islands study represents the central tendency of the three studies, and a central BMD from this study could provide a reasonable point of departure (median BMDL value, 12 ppm). The central tendency of the
integrative analysis (BMD, 21 ppm) or lower 5% limit (7 ppm) might also be considered. The Seychelles study, because of the lack of positive findings, does not provide an appropriate point of departure for risk assessment. Although the Seychelles study is a well-conducted study, the cohort appeared to be less sensitive than those of the New Zealand and Faroe Islands studies for reasons that are still not understood.
Abbreviations: BMDL, lower 95% confidence limit on the benchmark dose; BMR, benchmark response; WJ, Woodcock-Johnson Tests of Achievement; CPT, Continuous Performance Test; CVLT, California Verbal Learning Test; TOLD, Test of Language Development; WISC-R:PIQ, Wechsler Intelligence Scale for Children-Revised performance IQ; WISC-R:FSIQ, Wechsler Intelligence Scale for Children-Revised Full-Scale IQ.
SELECTION OF THE CRITICAL STUDY AND POINT OF DEPARTURE FOR THE REVISED RfD
The committee conducted an in-depth examination of the methods, strengths, uncertainties, and outcomes of each of the major studies. It included an examination of findings and comparison of BMDs and BMDLs. On the basis of its consideration of the body of evidence, the committee concluded that a well-designed study with positive effects provides the most appropriate public-health basis for the RfD. When the two studies with positive effects are compared, the strengths of the New Zealand study include an ethnically heterogeneous sample, in which the observed effects cannot be attributed to the particular vulnerability of a genetically homogenous ethnic group, and the use of developmental end points with greater predictive validity for school performance than that of the discrete neuropsychological tests used in the Faroe Islands study. The advantages of the Faroe Islands study over the New Zealand study include a larger sample size, the use of two biomarkers of exposure, and more extensive scrutiny in the epidemiological literature. In addition, the Faroe Islands data have undergone extensive re-analysis in response to questions raised by panelists at the NIEHS (1998) workshop and by this committee in the course of its deliberations. Therefore, the committee recommends the Faroe Islands study as the critical study for the revision of the RfD. For that study, dose-response data based on Hg concentrations in cord blood should be used to estimate the BMD. Because the data on the most sensitive end point — the Continuous Performance Test — were analyzed for only half the sample, the committee recommends that the BMDL based on the next most sensitive end point — the Boston Naming Test — be considered as a reasonable and representative point of departure for a revised RfD.
SOURCES OF UNCERTAINTY: CONSIDERATION FOR UNCERTAINTY FACTORS
Evaluation of the sources of uncertainty is essential for the development of a RfD. Some uncertainty is inherent in any experimental or epidemiological study. To address these uncertainties in the derivation of the RfD, the NOAEL or BMDL may be divided by one or more uncertainty factors. Uncertainty factors were originally termed safety factors and were used in the derivation of acceptable daily intakes (ADIs) to account for recognized uncertainties by incorporating an additional margin of safety on the NOAEL (NRC 1994). Traditionally, uncertainty factors and modifying factors of 10 or 3 have been applied to address well-recognized issues, which reflect potentials for additional sensitivities or adverse effects not addressed in the dose-response analysis. These issues include variation in sensitivity among humans, animal-to-human extrapolation, extrapolation from subchronic-to-chronic exposure, LOAEL-to-NOAEL extrapolation, and incomplete data to address all possible outcomes. A modifying factor, based on professional judgment, may also be applied to address uncertainties in the data base or critical study (Dourson et al. 1996). Traditional default uncertainty factors of 10 have been acknowledged to be somewhat arbitrary since they were proposed by O.G. Fitzhugh and A. Lehman in the early 1950s (NRC 1994).
At the present time, there is no consistent approach in the application of uncertainty factors across the various regulatory and public-health agencies. The selection and application of uncertainty factors represents a scientific policy judgment that has a major influence on the determination of the RfD or other risk-management guidance numbers. For example, application of large uncertainty factors might overshadow the moderate differences among the various study findings and their NOAELS or BMDs in determining the magnitude of the RfD. That possibility is particularly relevant to the MeHg RfD, because the body of evidence examined by the committee indicates a general convergence of the lower doses associated with neurodevelopmental effects. Given the relatively small differences in BMDLs, a more consistent approach to the application of uncertainty factors could reduce the current inconsistencies between the EPA RfD and other risk guidance numbers.
To identify sources of uncertainty in deriving the current MeHg RfD, EPA conducted an analysis of uncertainties (EPA 1997b, Vol. VI Appen-
dix) in relation to the critical study of neurodevelopmental effects from the 1971 Iraqi MeHg poisoning incident (Marsh et al. 1987). Major sources of uncertainty were identified as the variability in susceptibilities within the Iraqi cohort, population variability in the pharmaco-kinetic processes, and response classification error. An additional concern was the applicability of a risk assessment based on a grain-consuming population to the U.S. population for which fish consumption is the primary source of MeHg exposure. A composite uncertainty factor of 10 was applied in the derivation of the RfD to account for several uncertainties, including human-population variability, lack of a two-generation reproductive study, and lack of data on sequelae resulting from longer durations of exposure (EPA 1997c). Although the rationale for the composite uncertainty factor applied in the current RfD is well described, it is not possible to quantitatively validate that it adequately addresses the combined uncertainties in the Iraqi data because some of them have been described only in qualitative terms.
Any refinement of the current RfD will require consideration of sources of uncertainty. The committee has evaluated the body of evidence, focusing on the prospective epidemiological studies of neurotoxicity in children exposed in utero. Refinement of the current RfD based on results from these studies will require both quantitative and qualitative analysis of uncertainties to guide the application of uncertainty factors.
Not all sources of uncertainty require the addition of uncertainty factors in the derivation of the RfD. When the MeHg prospective epidemiological studies provide the basis for the RfD, uncertainty factor adjustments are potentially required only for the following reasons:
-
If the uncertainty could result in underestimation of the adverse effects of MeHg exposure on human health.
-
If there is reason to suspect that the U.S. population is more sensitive than the study populations to the adverse effects of MeHg.
Although there are multiple sources of uncertainty in the quantitative derivation of the RfD, not all result in an RfD that is insufficiently protective. Table 8-2 lists sources of uncertainty identified by the committee.
Individual responses to MeHg exposure are variable and a key source of uncertainty. Factors that might influence susceptibility include age,
TABLE 8-2 Sources of Uncertainty in Key Epidemiological Studies
|
Susceptible subpopulations
Measures of exposure
Lack of consideration of other key or most-sensitive health end points
|
gender, genetics, health status, nutritional influences including dietary interactions, and interindividual toxicokinetic and toxicodynamic variability. For example, data from Iraq indicate that although some individuals were sensitive to low levels of exposure, some members of the cohort were not sensitive to extremely high levels of exposure. That finding suggests a wide interindividual variability in sensitivity. Development of the RfD must consider this individual variation; in particular, any biomarker-based measure should account for the toxicokinetic variability in the population. At present, there is no clear evidence that the U.S. population is more sensitive than any of the key study populations. However, in any given population, there might be sensitive subpopulations whose sensitivity to MeHg is not adequately represented in the dose-response assessment. That possibility could represent an additional source of uncertainty.
Limitations in the evaluation of exposures also represent a source of uncertainty. Of particular concern is the uncertainty in the linkage between the time and the intensity of exposure to critical periods of
brain development. Each dose metric provides different information about exposure. Dietary-recall data might be useful in stratifying exposure levels, but appropriate dietary data were not collected in the key studies. Measurement of cord blood does not detect temporal variability in exposure and reflects exposure during a period late in gestation. Therefore cord-blood concentrations might not correspond to the periods of greatest fetal sensitivity to Hg neurotoxicity. Similarly, average concentrations of Hg in hair provide no information on peak exposures and, because of variation in length and growth rate, might not reflect comparable periods of gestation.
In any experimental or epidemiological data, there is also some uncertainty on whether the measured effects represent the true most sensitive or critical effects. Neurodevelopmental effects are the most extensively studied sensitive end point for MeHg exposure, but there remains some uncertainty about the possibility of other health effects at low levels of exposure. In particular, there are indications of immune and cardiovascular effects, as well as neurological effects emerging later in life, that have not been adequately studied.
A number of additional sources of uncertainty are not possible to quantify but might contribute to the differences in study findings and BMDLs for the various outcomes. Those might include differences in nutritional and dietary confounders and effect modifiers such as beneficial effects from eating fish. Differences in population susceptibilities and unmeasured coexposure to other pollutants, including other forms of Hg, might introduce uncertainty.
On the basis of an evaluation of the sources of uncertainty in the key epidemiological studies, the committee identified two major categories of uncertainty, which should be considered in the determination of uncertainty factors for the revision of the RfD:
-
Interindividual toxicokinetic variability in dose reconstruction (see Chapter 3).
-
Data-base insufficiency (i.e., because of consideration of possible low-dose sequelae and latent effects, and immunotoxicity and cardiovascular effects) (see Chapter 5).
On the basis of the analysis presented in Chapter 3, the committee believes that an uncertainty factor of 2-3 for dose reconstruction from
hair Hg concentrations or an uncertainty factor of about 2 for dose reconstruction from blood Hg concentrations is objective and appropriate. Despite ongoing work to provide a data-based and probabilistic basis for uncertainty-factor adjustments in the derivation of the RfD (e.g., Hattis et al. 1999), the choice of values for most categories of uncertainty other than toxicokinetics, and for the aggregate uncertainty-factor adjustment remains, in part, a policy decision. That is particularly the case for the uncertainty factor category of data-base insufficiency. The choice of values for most uncertainty-factor categories (e.g., animal to human) can be related to extant (although limited) analyses of empirical data. In the case of data-base insufficiency, however, the uncertainty-factor value is intended to address the possibility that more accurate or complete information might result in a lower NOAEL or BMD or might result in a more sensitive end point. If data were available to assess such a possibility adequately and quantitatively, such data might well lead to a more appropriate RfD rather than to an uncertainty-factor adjustment. Thus, the selection of an appropriate uncertainty-factor value for data-base insufficiency is inherently uncertain. Nonetheless, the committee believes that there is a reasonable possibility that significant immunotoxicity and cardiovascular effects, as well as neurotoxic sequelae, might occur at exposure levels below the dose corresponding to the neurodevelopmental BMD identified by the committee. Therefore, given the relatively unambiguous starting point for variability in dose reconstruction, the committee believes that an overall uncertainty-factor adjustment of no less than 10 is necessary and appropriate to provide an adequate margin of protection.
IMPLICATIONS FOR PUBLIC HEALTH AND RISK MANAGEMENT
The RfD provides critical guidance for a broad range of public-health and regulatory initiatives aimed at reducing Hg exposures and preventing adverse health impacts. The goal of the RfD is to estimate a level of daily exposure without adverse public health impacts even for sensitive individuals.
EPA has estimated from food consumption surveys that 7% of women
nationwide exceed the RfD. From a food consumption survey in New Jersey it was estimated that 21% of women of childbearing age exceed the current RfD (Stern et al. 1996). EPA has calculated that a hair Hg concentration of 1.0 ppm would approximately result from an intake of MeHg at the current EPA RfD (see calculations in the Current EPA Reference Dose section in this chapter). Although estimates of hair and blood concentrations in the U.S. population are sparse, when that hair Hg concentration (1.0 ppm) is compared with available data, it is again seen that more highly exposed subpopulations frequently exceed the current RfD (EPA 1997c; Stern et al. 2000).
The committee conducted a margin-of-exposure (MOE) analysis to examine the margin of safety between available estimates of population exposure and BMDLs derived from the major epidemiological studies. The MOE approach provides a method of characterizing risks and is being used increasingly to examine potential population risks, particularly for noncancer end points. The MOE approach has been recommended by The Presidential/Congressional Commission on Risk Assessment and Risk Management (1997) as a common metric to be used by both environmental-protection and public-health agencies for assessing and comparing health risks. The MOE is the ratio of the critical dose (NOAEL or BMDL) to the estimated population exposure level. The smaller the ratio, the greater the cause for concern. Because the BMDLs are not adjusted by uncertainty factors, MOEs less than 10 indicate that population exposures might be approaching levels of public-health concern. Table 8-3 presents the results of the MOE analysis. The analysis compared available estimates of the range of population Hg concentration in hair to BMDLS from the major studies: the cord-blood-derived BMDL for the lowest reliable end point (the Boston Naming Test) from the Faroe Islands study; the 5% lower bound BMD from the committee's integrative analysis; and the Iraq study BMDL, which is the point of departure for the current RfD.
MOEs for the estimates of mean population levels range from 7.5 (New Zealand, most sensitive end point) to 77.3 (Seychelles, median end point). Those results indicate that the risk of adverse health impacts from the current exposure level in the majority of the population is low. However, for those at the high end of the population exposure distribution (95th percentile), the MOEs indicate that the margin of safety for the
TABLE 8-3 Population Margins of Exposure (MOE)a for Selected BMDLs and Exposure Estimates (ppm of Hg in Maternal Hair or Estimated Equivalent to Maternal Hair)
|
MOE |
|||||||
|
Estimated MeHg Exposure in Selected Populations |
|||||||
|
New Jersey Pregnant Women b |
EPA Region V Populationc |
U.S. Women of Childbearing Aged |
|||||
|
Study |
Selected BMDL (value, ppm) |
Mean (0.53) |
95th Percentile (2.0) |
Mean (0.29) |
95thPercentile (1) |
Mean (0.36) |
95th Percentile (2.4) |
|
New Zealand |
Most sensitive (4) |
7.5 |
2.0 |
13.8 |
4 |
11.1 |
1.7 |
|
Faroe Islands |
Most sensitive (10) |
18.9 |
5.0 |
34.5 |
10 |
27.8 |
4.2 |
|
Faroe Islands |
Most-sensitive-reliable, cord-blood derived (12) |
22.6 |
6.0 |
41.4 |
12 |
33.3 |
5.0 |
|
Seychelles |
Median (22) |
41.5 |
11 |
77.3 |
22 |
61.1 |
9.2 |
|
New Zealand, Faroe Islands, and Seychelles (integrative analysis) |
Lower 5% (7) |
13.2 |
3.5 |
24.1 |
7 |
19.4 |
2.9 |
|
Iraq |
(11)e |
20.8 |
5.5 |
37.9 |
11 |
30.6 |
4.6 |
|
aMOE, BMDL/exposure estimate. bData from Stern et al. 2000. cData from Pellizzari et al. 1999. dData from Smith et al. 1997. eCurrent RfD basis. Abbreviations: BMDL, lower 95% confidence limit on the benchmark dose; RfD, reference dose. |
|||||||
most highly exposed is consistently below 10. That indicates that the exposure levels of high-end consumers are close to those at which there are observable adverse neurodevelopmental impacts.
To further characterize the risks of MeHg, the committee developed an estimate of the number of children born annually to women most likely to be highly exposed through high fish consumption (highest 5% estimated to consume 100 g per day). Available consumption data and current population and fertility rates indicate that over 60,000 newborns annually might be at risk for adverse neurodevelopmental effects from in utero exposure to MeHg.
The MeHg-associated performance decrements on the neuropsychological tests administered in the Faroe Islands and New Zealand studies suggest that prenatal MeHg exposure is likely to be associated with poorer school performance. In the Faroe Islands sample, MeHg-related deficits were seen across a broad range of specific domains, including vocabulary, verbal learning, visuospacial attention, and neuromotor function. Deficits of the magnitude reported in these studies are likely to be associated with increases in the number of children who have to struggle to keep up in a normal classroom or who might require remedial classes or special education.
Revision of the RfD for MeHg can have far-reaching implications for public health and environmental protection. Currently, 40 states have issued advisories concerning consumption of certain freshwater fish. Any revision of the RfD will have implications for the market for fish and seafood and the dietary choices of Americans. Regulatory impacts might also be substantial, because federal and state agencies use the RfD to develop water-quality criteria and set limits on Hg releases in air and water. Additionally, there are implications for industrial use of Hg and Hg-containing materials, as well as decisions about disposal methods and recycling options.
Ideally, the application of the RfD in risk management should provide a margin of safety for all of the population. The application of the RfD to guide regulatory and risk-management policies must also consider risk tradeoffs, economic and technological limitations, as well as cultural and political influences. It must be recognized that the refinement of the RfD might not eliminate agency differences in risk management. However, improving the scientific basis for decision-making represents an important step forward in developing a cohesive strategy to prevent adverse effects from MeHg.
COMMITTEE FINDINGS AND RECOMMENDATIONS
-
Hg is pervasive and persistent in the environment. Its use in products and emission from industrial processes and combustion have resulted in global circulation and atmospheric deposition. There have been well-documented instances of population poisonings, highly exposed occupational groups, and worldwide chronic low-level environmental exposures. The bioaccumulation of MeHg can lead to high concentrations in many species of fish and result in unacceptable levels of exposure and risk to highly exposed or susceptible subpopulations.
-
The weight of the evidence of developmental neurotoxic effects from exposure to MeHg is strong. There is a strong data base, which includes multiple human studies and experimental evidence in animals and in vitro tests. Human studies include both high-exposure scenarios and evaluations of effects of chronic low-level exposure. The epidemiological studies also include well-established biomarkers to evaluate exposure levels in study populations.
-
The weight of evidence from multiple epidemiological studies supports the selection of neurotoxicity in children exposed in utero as the most sensitive well-documented effect and a suitable end point for the derivation of the BMD. However, emerging evidence of other potential effects should also be considered in the calculation and the implementation of the EPA RfD.
-
Given the availability of results from large prospective epidemiological studies, the Iraq study results should no longer be considered the critical study for the EPA RfD. The exposure scenarios in Iraq are not comparable to the low-level chronic exposures in North America. In addition, there are well-recognized uncertainties concerning exposure and response classification in the Iraq study.
-
The New Zealand, Faroe Islands, and Seychelles studies are well-designed epidemiological investigations in which prenatal MeHg exposures were within the range of at least some U.S. population exposures. Any revision of the RfD or other exposure standards should consider the findings of these studies.
-
After considering the weight of evidence and range of results from the three major epidemiological studies, the committee concludes
-
that a positive study will provide the strongest public-health basis for the RfD and recommends the Faroe Islands study as the critical study. Within that study, the lowest BMD for a neurobehavioral end point considered to be sufficiently reliable is the Boston Naming Test. The BMDL estimated from that test is 58 ppb Hg in cord blood (approximately corresponding to 12 ppm Hg in hair). That value should be considered a reasonable point of departure for the development of the revised RfD.
-
An MOE analysis using available estimates of population exposure levels indicates that average U.S. population risks from MeHg exposure are low. However, those with high exposures from frequent fish consumption might have little or no margin of safety.
-
The population at highest risk is the offspring of women of child-bearing age who consume large amounts of fish and seafood. The committee estimates that over 60,000 children are born each year at risk for adverse neurodevelopmental effects due to in utero exposure to MeHg.
-
There is a critical need for improved characterization of population exposure levels to improve estimates of current exposure, track trends, and identify high-risk subpopulations. Characterization should include improved nutritional and dietary exposure assessment and improved biomonitoring for all population groups. Exposure to other chemical forms of Hg, including exposure to elemental Hg from dental amalgams, should also be investigated.
-
The application of uncertainty factors in the revision of the RfD should be based on a thorough quantitative and qualitative evaluation of the full range of uncertainties and limitations of the critical studies. Uncertainty factors applied in the development of a revised RfD should include data-base insufficiency and interindividual toxicokinetic variability in dose reconstruction. As a starting point, an uncertainty factor of 2-3 should be applied to a central tendency estimate of dose derived from maternal hair, or a factor of about 2 should be applied to a central tendency estimate of dose derived from cord blood to account for interindividual pharmacokinetic variability in dose reconstruction. The choice of an uncertainty factor for data-base insufficiency is, in part, a policy decision; however, given the data indicating possible low-dose sequelae and latent effects and immunotoxicity and cardiovascular effects, the
-
committee concludes that an overall composite uncertainty factor of no less than 10 is needed.
-
Concurrent with the revision of the RfD, harmonization efforts should be undertaken to establish a common scientific basis for the establishment of exposure guidance and reduce current differences among agencies. Harmonization efforts should address the risk-assessment process and recognize that risk-management efforts reflect the differing mandates and responsibilities of these agencies.
-
Recent studies have found associations between exposure to MeHg and impairments of the immune, reproductive, and cardiovascular systems. Immune and cardiovascular effects have been observed following both prenatal and adult exposures. MeHg exposure levels associated with those effects are comparable to and in some cases lower than those known to cause neurodevelopmental problems. Additional research should be done using animal models and human populations that have chronic, low-dose exposure to MeHg. Effects of exposure during fetal development through the entire life span is needed. Further research is also needed to evaluate MeHg-induced chromosomal aberrations and cancer.
-
The committee recommends that results from the Boston Naming Test in the Faroe Islands study be used in the calculation of the RfD. For that study, dose- response data based on Hg concentrations in cord blood should be modeled using the K-power model (K ≥ 1). On the basis of that study, that test, and that model, the committee 's preferred estimate of the BMDL is 58 parts per billion (ppb)1 of Hg in cord blood (approximately corresponding to 12 ppm Hg in hair). To estimate this BMDL, the committee's calculations involved a series of steps, each involving one or more assumptions and related uncertainties. Alternative assumptions could have an impact on the estimated BMDL value. In selecting a single point of departure, the committee followed established public-health practice of using the lowest value for the most sensitive, relevant end point.
|
1 |
The BMDL of 58 ppb is calculated statistically and represents the lower 95% confidence limit on the dose (or biomarker concentration) that is estimated to result in an increased probability that 5% of the population will have an abnormal score on the Boston Naming Test. |
-
The BMDL of 12 ppm is nearly identical to the BMDL currently used by EPA (11 ppm). Given the toxicokinetic variability and uncertainties in the data, an uncertainty factor of at least 10 is supported by the committee. Therefore, on the basis of its analysis of the available data, the committee finds that the value of EPA's current RfD for MeHg (0.1 µg/kg per day) is scientifically justifiable for the protection of public health.
REFERENCES
Bakir, F., S.F. Damluji, L. Amin-Zaki, M. Murtadha, A. Khalidi, N.Y. al-Rawi, S. Tikriti, H.I. Dhahir, T.W. Clarkson, J.C. Smith, and R.A. Doherty. 1973. Methylmercury poisoning in Iraq. Science 181(96):230-241.
Budtz-Jørgensen, E., N. Keiding, and P. Grandjean. 1999. Benchmark Modeling of the Faroese Methylmercury Data. Research Report 99/5. Prepared at the University of Copenhagen, Denmark, for the U.S. Environmental Protection Agency.
Burbacher T.M., M.K. Mohamed, and N.K. Mottett. 1988. Methylmercury effects on reproduction and offspring size at birth . Reprod. Toxicol 1(4):267-278.
Crump, K.S. 1984. A new method for determining allowable daily intakes. Fundam. Appl. Toxicol. 4(5):854-871.
Crump, K.S., T. Kjellström, A.M. Shipp, A. Silvers, and A. Stewart. 1998. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 18(6):701-713.
Crump, K.S., C. Van Landingham, C. Shamlaye, C. Cox, P.W. Davidson, G.J. Myers, and T.W. Clarkson. 2000. Benchmark concentrations for methylmercury obtained from the Seychelles child development study. Environ. Health Perspect. 108(3):257-63.
Crump K, J. Viren, A. Silvers, H. Clewell 3rd, J. Gearhart, and A. Shipp. 1995. Reanalysis of dose-response data from the Iraqi methylmercury poisoning episode. Risk Anal. 15(4):523-532.
Dantas, D.C., and M.L. Queiroz. 1997. Immunoglobulin E and autoantibodies in mercury-exposed workers. Immunopharmacol. Immunotoxicol. 19(3): 383-92.
Davidson, P.W., G.J. Myers, C. Cox, C. Shamlaye, O. Choisy, J. Sloane-Reeves, E. Cernchiari, D.O. Marsh, M. Berlin, M. Tanner, and T.W. Clarkson. 1995a. Neurodevelopmental test selection, administration, and performance in the main Seychelles child development study. Neurotoxicology 16(4):665-676.
Davidson, P.W., G.J. Myers, C. Cox, C.F. Shamlaye, D.O. Marsh, M.A. Tanner, M. Berlin, J. Sloane-Reeves, E. Cernichiari, O. Choisy, A. Choi, and T.W. Clarkson. 1995b. Longitudinal neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from maternal fish ingestion:outcomes at 19 and 29 months. Neurotoxicology 16(4):677-688.
Davidson, P.W., G.J. Myers, C. Cox, C. Axtell, C. Shamlaye, J. Sloane-Reeves, E. Cernichiari, L. Needham, A. Choi, Y. Wang, M. Berlin, and T.W. Clarkson. 1998. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment:outcomes at 66 monts of age in the Seychelles child development study. JAMA 280(8):701-707.
Dourson, M.L., S.P. Felter, and D. Robinson. 1996. Evolution of science-based uncertainty factors in noncancer risk assessment. Regul. Toxicol. Pharmacol. 24(2):108-20.
EPA (U.S. Environmental Protection Agency). 1997a. Mercury Study Report to Congress. Vol. I.: Executive Summary. EPA-452/R-97-003. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, and Office of Research and Development.
EPA (U.S. Environmental Protection Agency). 1997b. Mercury Study for Congress. Volume VI: Characterization of Human Health and Wildlife Risks from Anthropogenic Mercury Emissions in the United States. EPA-452/R-97-008b. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, and Office of Research and Development.
EPA (U.S. Environmental Protection Agency). 1997c. Mercury Study for Congress. Volume VII: Characterization of Human Health and Wildlife Risks from Mercury Exposure in the United States . EPA-452/R-97-009. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, and Office of Research and Development.
EPA (U.S. Environmental Protection Agency). 2000. Methylmercury (MeHg) CASRN 22967-92-6. U.S. Environmental Protection Agency IRIS Substance file. [Online]. Available: http://www.epa.gov/iris/subst/0073.htm. Last Updated: 5 May 1998.
Grandjean, P., P. Weihe, R.F. White, and F. Debes. 1998. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ. Res. 77(2):165-172.
Grandjean, P., E. Budtz-Jørgensen, R.F. White, P.J. Jørgensen, P. Weihe, F. Debes, and N. Keiding. 1999a. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am. J. Epidemiol. 150(3):301-305.
Grandjean, P., P. Weihe, R.F. White, F. Debes, S. Araki, K. Yokoyama, K. Murata, N. Sørensen, R. Dahl, and P.J. Jørgensen. 1997. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 19(6):417-428.
Hattis, D., P. Banati, R. Goble, and D.E. Burmaster. 1999. Human interindividual variability in parameters related to health risks. Risk Anal. 19(4):711-26.
Hirano, M., K. Mitsumori, K. Maita, and Y. Shirasu. 1986. Further carcinogenicity study on methylmercury chloride in ICR mice . Nippon Juigaku Zasshi (Jpn. J. Vet. Sci.). 48(1):127-135.
Höök, O., K.D. Lundgren, and A. Swensson. 1954. On alkyl mercury poisoning: with a description of two cases. Acta Med. Scand. 150(2):131-137.
Ilbäck, N.G., L. Wesslen, J. Fohlman, and G. Friman. 1996. Effects of methyl mercury on cytokines, inflammation and virus clearance in a common infection (coxsackie B3 myocarditis). Toxicol. Lett. 89(1):19-28.
Janicki, K., J. Dobrowolski, and K. Krasnicki. 1987. Correlation between contamination of the rural environment with mercury and occurrence of leukemia in men and cattle. Chemosphere 16(1):253-257.
Kinjo, Y., S. Akiba, N. Yamaguchi, S. Mizuno, S. Watanabe, J. Wakamiya, M. Futatsuka, and H. Kato. 1996. Cancer mortality in Minamata disease patients exposed to methylmercury through fish diet. J. Epidemiol. 6(3):134-8.
Marsh, D.O., T.W. Clarkson, C. Cox, G.J. Myers, L. Amin-Zaki, and S. Al-Tikriti. 1987. Fetal methylmercury poisoning: Relationship between concentration in single strands of maternal hair and child effects. Arch. Neurol. 44(10):1017-1022.
Mitsumori, K., K. Maita, T. Saito, S. Tsuda, and Y. Shirasu. 1981. Carcinogenicity of methylmercury chloride in ICR mice: Preliminary note on renal carcinogenesis. Cancer Lett. 12(4):305-310.
Moszczynski, P., S. Slowinski, J. Rutkowski, S. Bem, and D. Jakus-Stoga. 1995. Lymphocytes, T and NK cells, in men occupationally exposed to mercury vapours. Int. J. Occup. Med. Environ. Health 8(1):49-56.
NIEHS (National Institute of Environmental Health Sciences). 1998. Scientific Issues Relevant to Assessment of Health Effects from Exposure to Methylmercury. Workshop organized by Committee on Environmental and Natural Resources (CENR) Office of Science and Technology Policy (OSTP) The White House, November 18-20, 1998, Raleigh, NC.
NRC (National Research Council). 1994. Science and Judgment in Risk Assessment. Washington, DC: National Academy Press.
Pellizzari, E.D., R. Fernando, G.M. Cramer, G.M. Meaburn, and K. Bangerter. 1999. Analysis of mercury in hair of EPA region V population. J. Expo. Anal. Environ. Epidemiol. 9(5):393-401.
Presidential/Congressional Commission on Risk Assessment and Risk Management. 1997. Risk Assessment and Risk Management in Regulatory Decision-Making . Final Report. Vol.2. Washington, DC: GPO.
Salonen, J.T., K. Seppänen, K. Nyyssönen, H. Korpela, J. Kauhanen, M. Kantola, J. Tuomilehto, H. Esterbauer, F. Tatzber, and R. Salonen. 1995. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction
and coronary, cardiovascular, and any death in Eastern Finnish men . Circulation 91(3):645-655.
Smith, J.C., P.V. Allen, and R. Von Burg. 1997. Hair methylmercury levels in U.S. women. Arch. Environ. Health 52(6):476-80.
Sørensen, N., K. Murata, E. Budtz-Jørgensen, P. Weihe, and P. Grandjean. 1999. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology 10(4):370-375.
Stern, A.H., M. Gochfeld, C. Weisel, and J. Burger. 2000. Mercury and methylmercury exposure in the New Jersey pregnant population . Arch. Environ. Health. In press.
Stern, A.H., L.R. Korn, and B.E. Ruppel. 1996. Estimation of fish consumption and methylmercury intake in the New Jersey population. J. Expo. Anal. Environ. Epidemiol. 6(4):503-525.
Tamashiro, H., M. Arakaki, H. Akagi, K. Hirayama, K. Murao, and M.H. Smolensky. 1986. Sex differential of methylmercury toxicity in spontaneously hypertensive rats (SHR). Bull. Environ. Contam. Toxicol. 37(6):916-24.
Wild, L.G., H.G. Ortega, M. Lopez, and J.E. Salvaggio. 1997. Immune system alteration in the rat after indirect exposure to methylmercury chloride or methylmercury sulfide. Environ. Res. 74(1):34-42.





























