Stimulating Light
Light waves with lengths ranging from the thickness of a dime to that of a dime-store novel are called microwaves. In nature, microwaves are emitted by clouds of interstellar gas and also by the gaseous shrouds of newly formed stars. These interstellar clouds are largely made up of diatomic hydrogen, two atoms of hydrogen joined in a single molecule. When the clouds are surrounded by energetic plasma or radiation fields, the water molecules in the cloud can be stimulated to emit microwaves as well. A feedback loop can result (right), producing powerful beams of microwave radiation shooting into space. This phenomenon is called "microwave amplification by stimulated emission of radiation," or "maser" for short. |
In interstellar space, a diatomic molecule of hydrogen (red), collides with a water molecule, shown here with a blue oxygen atom and red hydrogen atoms.  |
The collision transfers energy to the water molecule, causing it to jump to a higher energy state. The hydrogen molecule loses energy in the process.  |
When the excited water molecule drops to a lower energy level, it emits a photon (yellow). Rather than dropping to the lowest ground state, the molecule shown here has dropped to an intermediate metastable state, where it can remain for some time.  |
When a passing photon strips the metastable molecule of its energy, it drops to the ground state and emits a photon of the same wavelength, traveling in the same direction as the incoming photon. On encountering other metastable molecules, the two photons set off a chain reaction of emissions. 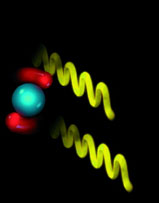 |

