typically smooth transitions from one of the "Roy G. Biv" colors to the next. Rather, fine forests of bright and dark lines  may cut across the rainbow, looking like rows of supermarket bar codes. These features contain a wealth of information about cosmic objects that we cannot learn in any other way. may cut across the rainbow, looking like rows of supermarket bar codes. These features contain a wealth of information about cosmic objects that we cannot learn in any other way.
To understand why these lines exist, we must consider the fundamental nature of light. The German physicist Max Planck  showed in 1900 that light emitted by a hot object must radiate in tiny discrete units, which he called quanta showed in 1900 that light emitted by a hot object must radiate in tiny discrete units, which he called quanta  . "Quantum mechanics" comes from this name. Einstein . "Quantum mechanics" comes from this name. Einstein  further demonstrated the need for quanta further demonstrated the need for quanta  in 1905, proving that light is packaged in quanta in 1905, proving that light is packaged in quanta  that travel in waves. The notion that light travels simultaneously as a particle and a wave was one of the first signs that something was odd in the subatomic realm. that travel in waves. The notion that light travels simultaneously as a particle and a wave was one of the first signs that something was odd in the subatomic realm.
In 1913, the Danish physicist Niels Bohr proposed the idea of a quantized atom  : a system where the electrons orbiting the nucleus could exist only at specific distances and thus only at specific levels of energy. This is akin to being forced to hop down a staircase two or three steps at a time. The theory of quantum mechanics--developed in detail by many physicists in the decades after Bohr's proposal--places explicit limits on the motions and energies of electrons in excited atoms. It's hard to understand why these limits should exist, but as strange as they are, they explain so many aspects of our world perfectly. You might be surprised to learn that the luminous hands on your wristwatch and the glowing stars on the bedroom ceilings of many children are driven by quantum mechanics. Bright light pumps up the energy levels of the electrons in the special materials. Then, when you turn off the lights, the electrons cascade back down. They release photons with a specific energy: that familiar green glow. : a system where the electrons orbiting the nucleus could exist only at specific distances and thus only at specific levels of energy. This is akin to being forced to hop down a staircase two or three steps at a time. The theory of quantum mechanics--developed in detail by many physicists in the decades after Bohr's proposal--places explicit limits on the motions and energies of electrons in excited atoms. It's hard to understand why these limits should exist, but as strange as they are, they explain so many aspects of our world perfectly. You might be surprised to learn that the luminous hands on your wristwatch and the glowing stars on the bedroom ceilings of many children are driven by quantum mechanics. Bright light pumps up the energy levels of the electrons in the special materials. Then, when you turn off the lights, the electrons cascade back down. They release photons with a specific energy: that familiar green glow.
More important for astronomy, the features we see in cosmic spectra depend on quantum mechanics. When electrons in energized atoms bounce from one energy level to another, they absorb or emit photons of light that contain those precise quantities of energy. If enough atoms absorb photons of a particular energy, that part of the spectrum exhibits a dark line. The atoms have swallowed the light. Elements in the atmosphere of a star can play that role. Light from inside the star passes through the elements on its way to Earth. Electrons in the elements subtract light at specific places in the spectrum, leaving dark gaps. Conversely, when enough atoms emit photons of one energy, a bright line appears at that spot in the spectrum. The ensemble (continued)
|
| Decoding Spectral Lines
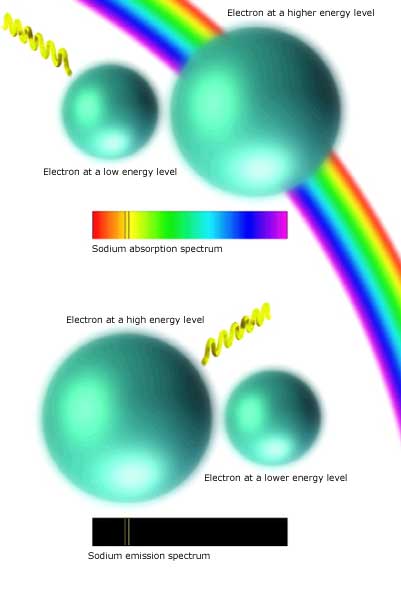
All spectra are the result of specific kinds of discrete energy transactions, gains or losses of energy when an atom is excited and an electron is disturbed from its orbit around the nucleus. Of the three types of spectra, the most familiar is the continuous spectrum. This rainbow band of color is produced by very crowded, energetic conditions, such as in the depths of a star, where the distinctions between energy levels gets smeared. As explained below, the two other types--absorption and emission spectra--are produced by electrons jumping or falling from one energy level to another.
|
When an electron absorbs a passing high-energy photon, it jumps to a higher energy level at a greater distance from the nucleus, represented here by an expanding probability cloud. Many such interactions create gaps in the continuous spectrum. The total effect for all the atoms of a given chemical element is a distinctive pattern of absorbed wavelengths such as the simplified absorption spectrum for sodium at right.

Electrons kicked to higher energy levels will quickly drop back down, giving up a photon whose wavelength matches the energy difference between the two levels. In the case of sodium these emissions produce the characteristic yellow light of sodium streetlamps (above) and the sodium cloud around Jupiter's moon Io (below).
 |
 |
|
|

