|
|
|
|
|
|
|
|

|
|||||||
 |
| Matter | Pages 72-73 | See Linked Version | |
| that comes along. A well-known chart called the periodic table of the elements helps students of chemistry figure out which elements can react, how many atoms each element must contribute to do so, and what the properties of the resulting compound will be. The rows and columns of the periodic table are not especially artistic. Rather, its patterns--the periodicity of its design--nicely illustrate the origins of chemical behaviors on Earth and in the cosmos that otherwise might seem random. To understand why the periodic table works so well, we must turn again to quantum mechanics. Newton's laws of motion don't work well on atoms. Electrons in particular pose a special problem. They are so tiny and move so fast that classical physics fails utterly in describing them. Many physicists helped explain this failure by devising the tenets of quantum mechanics in the early twentieth century. But the one rule that explained the periodic table most convincingly was the Pauli exclusion principle, named for the Austrian physicist Wolfgang Pauli. This rule states that no two electrons can inhabit the same space at the same time and move in exactly the same way. Instead, electrons fill a series of "shells" around the nucleus of an atom. If one of an atom's shells isn't completely full, the atom can either donate electrons to other atoms or accept electrons from them. The combined atoms then form a stable molecule. The configuration of the periodic table shows exactly which such bonds can occur. We can (continued) | |
| |
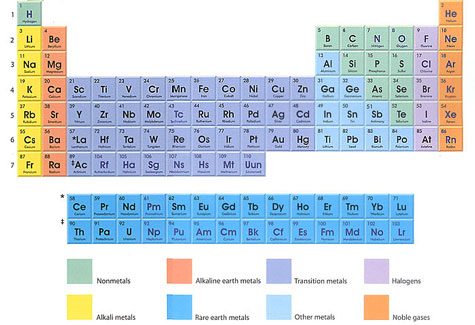
The Periodic Table By the late nineteenth century chemists realized that if they arranged the elements in increasing order of atomic weight, the chemical properties of the elements would then repeat in a periodic manner. If the elements were listed horizontally by increasing atomic weight, groups of elements with similar chemical properties fell in vertical columns. For example, the alkali metals lithium, sodium, and potassium (below left) have similar properties as do the halogens fluorine, chlorine, bromine, and iodine (below right). The scheme allowed for the prediction of elements not yet discovered. A better understanding of why certain elements share certain properties came with the advent of quantum mechanics. Atoms of different elements contain different numbers of electrons. Hydrogen, for example, has just one electron, helium has two, and carbon has six. These fast-moving subatomic particles fill the atom's orbital shells to different degrees. With one electron the hydrogen atom has a vacancy in the first orbital shell; it therefore combines easily with itself and with other elements. By contrast, helium's two electrons fill its first orbital shell; like all the noble gases, it remains inert and nonreactive. The modern periodic table arranges the 110 known elements in order of increasing atomic number, which is the number of protons in the nucleus and also therefore the number of electrons surrounding the nucleus. Hydrogen, with one proton and one electron, is at top left. Helium, with two protons, is at top right. These two elements make up the first period, with other elements in rows 2 through 7 listed in increasing order of atomic weight. As seen below, element 110 has a temporary designation. It and several other more recently discovered elements (111, 112, 114, 116, and 118) await official nomenclature. | |
|