| arise. Models of solar-system formation suggest that dense Earth-like planets probably form in the warm environment close to the parent star. Gas giants, whose elements are more prone to being boiled off by a hot young star, prefer a cooler setting farther away. Recent discoveries of Jupiter-sized planets orbiting close to other stars may conflict with that model. Alternatively, it could mean that over time giant planets can migrate inward toward their parent stars.
When a planet coalesces from the debris around a star, it starts as a thorough mixture of the matter in that part of the disk. But the densest matter quickly sinks to the center of the new planet, a process similar to pulp settling to the bottom of a glass of freshly squeezed orange juice. In Earth's case those materials were iron and nickel. This segregation of dense elements released a tremendous amount of heat, keeping Earth molten for much of its early history. The less dense elements, such as oxygen, stayed near the surface. They eventually formed the floating slabs of rocky crust on which we live. As evidence of that process, Earth's solid surface is nearly half oxygen by weight.
Another source of heat within Earth remains important today: radioactivity. For quantum mechanical reasons, most combinations and sums of protons and neutrons in an atomic nucleus are unstable. Atoms containing such combinations will eject a helium nucleus of two protons and two neutrons (an "alpha particle") or change a neutron into a proton and an electron (a "beta particle"). These decays are mediated by the weak nuclear force. Over time they transmute a radioactive substance into other elements, atom by atom. For example, radioactive uranium decays to lead, a stable element, via a chain of other radioactive elements, including thorium, radium, and radon. If dangerous levels of radon gas build up in your basement, you know that this process is happening in the soil and rocks around your home. There's also a clear benefit from radioactivity: Without it, Earth's interior would be much colder. The planet would be less active and, perhaps, less hospitable to life.
We cannot predict when a particular radioactive atom will decay. Rather, we use statistical averages to calculate overall rates of decay for large numbers of atoms. The time it takes half of any given amount of an element to decay is called its half-life. After two half-lives have passed, one-quarter of the original element is left. After 10 half-lives, only a thousandth remains. These intervals of time can range from fractions of a second to billions of years, depending on the element. |
| At the
Star's Core
With no more hydrogen left to fuse into helium, nuclear
reactions halt, gravity squeezes atoms closer together, and the core
heats up. Radiating heat expands the outer envelope to 100 times its
original diameter. |
Alchemy
by Supernova
From
the study of hundreds of supernovas in distant galaxies, scientists
have identified two main varieties of these cosmic cataclysms. Type
I supernovas are thought to involve gravitationally bound pairs
of stars. The slightly more common Type II explosion typically involves
a single high mass star--anywhere from eight to 20 times the mass
of our Sun. Fusing hydrogen atoms at the rate of some 20 trillion
tons per second, such a star reaches the end of its supply in only
about 10 million years.
As
long as fusion lasts at the core, its outward pressure counteracts
the inward gravitational pull exerted by the star's enormous mass.
As soon as fusion ends, however, the countdown to the explosion
begins. Illustrated here and on the following page is the process
known as core collapse, which frees enough nuclear energy to blow
up the star's outer layers. The process also creates all the elements
heavier than hydrogen and helium, seeding the universe with matter
to make up not only the next generation of stars, but also planets,
moons, comets, and all living things.
|
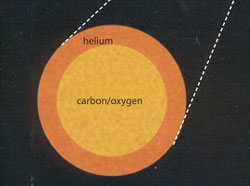 One Million Years to Blast Off
Gravitational collapse in the core pushes temperatures over 170 million degrees. Helium atoms fuse to form carbon and oxygen.
|

A Thousand Years to Go
Once helium in the inner shell is used up, the core begins to contract again, in alternating cycles of fusion and contraction. Carbon fuses to neon and magnesium. |

Seven Years Left
As temperatures reach 1.5 billion degrees in the stellar core, neon atoms fuse to form more oxygen and magnesium. |
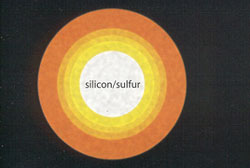
A Year to Go
Temperatures in the collapsing core top 2 billion degrees. Compressed oxygen atoms fuse to form silicon and sulfur. |
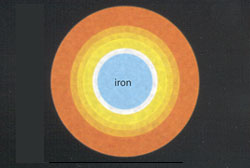 A Few Days Left
At 3 billion degrees, silicon and sulfur fuse to form a compressed ball of iron. This is the last reaction that can take place. |
 Only Tenths of a Second Left
At nearly 45,000 miles per second, the iron core crushes in on itself, packing an Earth-sized object into a ball 10 miles across. Astrophysicists call this "maximum scrunch." |
|

