5
Biological Effects of Oil Releases
|
HIGHLIGHTS This chapter focuses on:
|
Oil in the sea from anthropogenic sources, whether from spills or chronic releases, is perceived as a major environmental problem. Major oil spills occur occasionally and receive considerable public attention because of the obvious attendant environmental damage, including oil-coated shorelines and dead or moribund wildlife, especially oiled seabirds and marine mammals. Acute effects may be of short duration and limited impact, or they may have long-term population- or community-level impacts depending on the timing and duration of the spill and the numbers and types of organisms affected. Oil also enters the sea when small amounts are released over long periods, thus creating chronic exposure of organisms to oil and its component chemical species. Sources of chronic exposures include point sources, such as natural seeps, leaking pipelines, offshore production discharges, and non-point runoff from land-based facilities. In these cases, there may be a strong gradient from a high to a low oil concentration as a function of distance from the source. In other cases, such as with land-based runoff and atmospheric inputs, the origin of the oil is a nonpoint source, and environmental concentration gradients of oil compounds may be weak. Chronic exposures may also result from the incorporation of spilled oil into sediments in which weathering of oil is retarded, and from which nearly-fresh oil may be released to the water column over extended periods. In recent years, it is the long-term effects of acute and chronic pollution that have received increasing attention (Boesch et al., 1987).
What separates short-term from long-term effects is open to debate. Boesch et al. (1987) suggested that effects of duration longer than two years should be considered as long-term. These can be either effects that persist after an initial insult, or effects that result from persistent pollution. We do not know the upper bound for the potential length of a long-term effect. It is likely to be at least the length of a generation of the affected organisms, and it may be longer. An effect can be either direct damage to a resource or damage to the ability of an environment to support a resource. An effect can be said to be over when complete recovery has taken place. The quantification of both effects and recovery are difficult, particularly when they must be measured against a changing marine environment (Figures 5-1A and B) (Wiens,
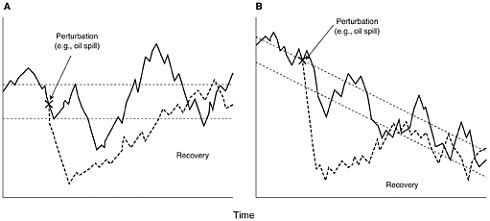
FIGURE 5-1 Hypothetical examples show how the impact of an oil spill and subsequent recovery can be assessed when the system under study undergoes natural variations (solid line). In (A), the system varies in time, but the long-term mean remains unchanged. In (B), there is a long-term decline in the state of the system (e.g., population size). Dashed lines indicate a “window” of normal variation about the mean (e.g., a 95 percent confidence interval). Operationally, “impact” occurs when the system is displaced outside this “window” (from Wiens, 1995, American Society for Testing and Materials).
1995; Spies et al., 1996; Peterson, 2001). Perhaps more difficult than detecting an effect is determining its significance (Boesch et al., 1987) (Figure 5-2). The spatial extent, persistence and recovery potential are all important, as is the perceived or monetary value of the affected resources. All else being equal, damage to a large area is more significant than damage to a small area of similar habitat. Damage to a small area that contains a highly valued resource can be of greater significance than damage to a much larger area devoid of valued resources. These issues are hotly contested after major pollution incidents.
DETERMINING EFFECTS IN A VARIABLE ENVIRONMENT
Oil can kill marine organisms, reduce their fitness through sublethal effects, and disrupt the structure and function of marine communities and ecosystems. While such effects have been unambiguously established in laboratory studies (Capuzzo, 1987; Moore et al., 1989) and after well-studied spills (Sanders et al., 1980; Burns et al., 1993; Peterson, 2001), determining the subtler long-term effects on populations, communities and ecosystems at low doses and in the presence of other contaminants poses significant scientific challenges. Multiple temporal and spatial variables make deciphering the effects extremely difficult, especially when considering the time and space scales at which marine populations and ecosystems change.
Marine ecosystems change naturally on a variety of time scales, ranging from hours to millennia, and on space scales ranging from meters to that of ocean basins. There are many causes of ecological change aside from oil pollution, including human disturbance, physical habitat alteration, other pollution, fishing, alteration of predation patterns, weather, and climate. Time scales at which oil affects the ocean range from days to years or even decades for some spills; chronic pollution occurs over years to decades. Oil spills affect the oceans at spatial scales of tens of square meters to thousands of square kilometers; chronic oil pollution can affect areas as small as a few square centimeters and as large as thousands of square kilometers.
Climatic changes can complicate the interpretation of contaminant impacts, especially if they have different effects on control and impact stations in an experimental design, or if a long time series of data is used to establish the “norm.” Considerable scientific attention has been directed to understanding how climatic forcing affects marine ecosystems and fisheries (Beamish, 1993; Hare and Francis, 1995; McFarlane et al., 2000). Climate change can be cyclical, e.g., the Southern Ocean Oscillation the Pacific Decadal Oscillation (Barnston and Livesy, 1999), the North Atlantic Oscillation (Trenbreth and Hurrell, 1994; Hare and Mantua, 2000), or can be secular e.g., gradual rise in upper ocean temperature.
The biological effects of oil pollution are often referred to as acute or chronic. Spills are commonly thought of as hav
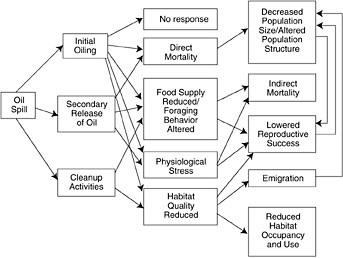
FIGURE 5-2 Schematic representation of oil spill influences on seabirds. The three primary avenues of effects, on population size and structure, reproduction and habitat occupancy, are highlighted (from Wiens, 1995, American Society for Testing and Materials).
ing short-term effects from high concentrations of petroleum. Chronic pollution, such as might occur from urban runoff into coastal embayments, may have continuous effects at low exposures. Not all oil pollution is clearly separable into these two categories. For example, exposure and effects are known to occur for long periods after some spills (Vandermeulen and Gordon, 1976; Sanders et al., 1980; Spies, 1987; Teal et al., 1992; Burns et al., 1993), and chronic exposures can be quite high, as is the case near petroleum seeps (Spies et al., 1980; Steurmer et al., 1982). The reader should bear this in mind during the ensuing discussion of the effects of acute and chronic exposure to oil. Additionally, this report generally focuses on the effects to benthic and wildlife populations, which were found to be most at risk from oil (Boesch et al., 1987).
It is within this complex multi-scale, spatial, and temporal environment that we are challenged to detect change caused by oil in the sea, and to assess the damage at the level of individuals, populations, communities, and ecosystems. Difficulty of detection increases with level of biological organization, with spatial and temporal scales of the affected system, and with the inherent variability of the system. Similarly, determination of complete recovery is complicated by this inherent variability.
The complex mosaic of change in the ocean has two aspects with regard to detecting the effects of oil pollution. First, it poses strategic challenges to determining the impact of oil through gathering observational data, as inevitably we make assumptions about the variability in the ecosystem and that variability can obscure large and continuing impacts. Second, the actual impact of the oil may be more complex than we realize if it interacts with spatially or temporally constrained phenomena.
In the closing decades of the twentieth century it was commonly held that the “balance of nature” has been severely altered by human actions. Consequently, much of our public policy was directed toward maintaining the status quo or returning ecosystems to a more pristine condition. While there is little doubt that human activities have had considerable impact in oceanic ecosystems, there has not been an equally widespread appreciation of how ecosystems change without human interference. The occurrence of several well-developed El Niños in the 1980s and 1990s made strong impacts on the public consciousness about longer-term cycles in the oceans. In Alaska, which has a strong resource-based economy, the rise and fall of salmon stocks in concert with the Pacific Decadal Oscillation (Beamish, 1993; Francis et al., 1998; Beamish et al., 1999) is now well known in the general population. Because public appreciation of ecosystem change seems to be following the growing scientific attention to long-term change in the oceans, the expectation that recovery of a polluted site will result in the return of an ecosystem to the state that it was in at the time of a pollution event is changing.
The observational framework for quantifying impacts involves determining differences based on sets of observations

PHOTO 19 Oil from the Lake Barre spill, May 1997, spill formed a narrow band on the marsh stems, and there was little oiling of the soils. Also, the oil is highly degradable. Thus, most of the marsh vegetation survived. (Photo courtesy of Jacqui Michel, Research Planning, Inc.)
at impacted and putatively non-impacted areas, or at one or a series of sites where before-and-after impact observations are available. Ideally, before-after and control-impact (BACI) observations can be made (Stewart-Oaten et al., 1992; Wiens and Parker, 1995; Peterson et al., 2001). The inherent assumption is that the variability of the ecosystem is sufficiently controlled (in the experimental sense) by these designs, which may or may not be correct. Controlling for impact by comparison of sites that have been affected and not affected allows for a variety of potentially important non-oiling variables to influence the system—such as differences in water temperature, salinity, or substrate type. For example, see Bowman (1978) for a case where high temperatures were documented to have a differential effect on intertidal invertebrate mortality, that might have otherwise been attributed to oil or dispersant toxicity. Usually an attempt is made to find study sites that are as similar as possible in factors suspected to be important. When effects are determined based on comparisons of before-impact conditions and after-impact conditions, it is possible that the ecosystem has changed in ways unknown to the observer. The chances of making errors can be lessened when: (1) multiple sites are used in each of the impacted and non-impacted sites, (2) multiple times are used in the time series, or, even better, (3) when both multiple sites and multiple times are available. Nevertheless, unreported factors not related to oil can interfere with ecosystem processes in ways that disguise the effects of pollution. Of course, with each additional kind of impact that is measured, the chance of making an error (Type I) rises.
At the same time, the mosaic of complex interactions and the resultant changes in ecosystems makes it possible to miss an impact that occurs (Type II error). For example, if an oil spill occurs when the pelagic larval stages of a fish species are developing near the sea surface, many or most of these larvae may die. If these larvae were to be the foundation of what would otherwise have been a strong year class for that fish species and whose population is maintained by infrequent large year classes, then the impact could be much larger than otherwise supposed. That would be a disproportional effect on a process that is temporally constrained. There are also examples of potential impacts on processes that are disproportionate because they are spatially constrained. For instance, a small spill around a seabird habitat where a large proportion of a population is gathered for breeding could have a disproportionately large impact. A good example of this occurred when an estimated 30,000 oiled seabirds washed up along the coasts of the Skagerrak following a small release of oil from one or two ships (Mead and Baillie, 1981). At the other extreme, the wreck of the Amoco Cadiz off the coast of Brittany, France, resulted in the release of 230,000 tonnes of crude oil into coastal waters and the death of less than 5,000 birds (Hope-Jones et al., 1978). These examples help illustrate that the volume of oil is only one factor determining mortality of birds and the
weak empirical relationship between spill volume and bird mortality points out the need to better understand the other sources of uncertainty (such as spill timing).
Assessing recovery after a pollution event is perhaps even more challenging than assessing initial damage. Recovery is further removed in time from the acute phase of the damage, and thus may be occurring in a different environmental framework than that which existed at the time of the accident. If there is variation in time, but the long-term mean remains stable, recovery might be judged by some to have been complete when the environmental variable of concern returns to within the normal range of variation (see Fig 5-1A, Wiens, 1995). In contrast, if the long-term environmental mean is changing, then recovery would occur when the variable of concern returns to within a range of variation around a short-term mean that will be quite different from that when the perturbation occurred (Fig. 5-1B). To assess recovery quantitatively requires either a well designed BACI approach, or one that compares measurements of the environmental variable of interest along a gradient of perturbation (Wiens, 1995). This gradient can be in space or time. One must be certain that, when numbers of organisms are being compared for assessment of recovery, attributes such as age or reproductive potential be taken into account. For example in marine birds, young, inexperienced animals do not have the same value to the population as experienced breeding adults. The natural variability inherent in estimates of populations introduces considerable uncertainty in assessing impact and recovery from pollution events. Confidence limits in excess of 20 percent of the mean size are usual in wildlife censuses. Such variability in the estimated mean makes it certain that population changes will be difficult to detect without a high degree of replication spatially and temporally before and after an event. More importantly, under some circumstances estimates of recovery based on the population returning to a “window” of natural fluctuation could minimize the time to true recovery. Other important considerations in evaluating oil pollution effects are the roles that laboratory studies, mesocosms and impact modeling play in complementing, or, in some cases, replacing the field observations discussed above.
Laboratory studies avoid the aforementioned problem of lack of control, but their improved precision disallows the wide range of possible interactions and indirect effects that can occur in complex ecosystems. Such indirect effects might be substantial. For example, in the Exxon Valdez and Torrey Canyon oil spills, destruction of the algal cover had indirect impacts on limpets and other invertebrates (Southward and Southward, 1978; Peterson, 2001). Such successional, reverberating or cascading indirect effects in a complex ecosystem may be very important, but are not captured by laboratory studies. The bulk of laboratory studies have examined oil impacts on organism mortality and health using dissolved oil or seawater suspensions. Most experiments are conducted for short durations (Capuzzo, 1987), which does not take into account long-term effects.
Field observations and laboratory experiments, as ways of knowing effects, represent two ends of a spectrum. Field observations allow little or no control of interactions between the full complement of ecosystem variables; laboratory experiments allow control of the interaction of single components that have been removed from the ecosystem. Taken together they still may not tell the whole story of oil impact. As a result, efforts have been made to bridge the gap between these two ends of the experimental control-field complexity continuum. Intermediate approaches include: laboratory experiments with multiple species, or communities that include environmental components (micro-and mesocosms); and field experiments, for example that put oiled sediments into the environment to be colonized by natural populations of animals and plants.
The modeling of the impacts of oil spills and their potential effects provides another route for predicting the potential effects of spilled oil. Oil spill impact modeling, which was originally applied to predicting the fate of oil in the environment, has recently been extended to prediction of effects (McCay, 2001).
In this chapter, we provide a brief review of progress in addressing the research recommendations of the 1985 Oil in the Sea report (NRC, 1985). We then examine the acute and chronic effects of oil at the organism, population and community/ecosystem levels. In the review, we single out marine birds and mammals for special attention because of their high visibility in spills and the great public concern for their welfare. It has been our intent to focus on the significant advances in knowledge and perceptions of the effects of oil in the sea, rather than to provide a detailed examination of the many research papers that have been published since the completion of the NRC (1985) or the Boesch and Rabalais (1987) reviews.
Progress Since 1985 Report
Since the major review of oil in the sea conducted by the National Research Council and published in 1985, there have been thousands of individual studies contributing to our overall understanding of the acute and chronic toxicity of oil in the marine environment and the restoration and recovery of oiled habitats. The major recommendations of the 1985 report were:
-
To expand studies of effects of low concentrations of petroleum hydrocarbons on marine organisms, especially larval and juvenile stages;
-
To examine the apparent coincidence of petroleum hydrocarbon exposure with increased prevalence of pollution-related disease in marine organisms;
-
To examine the impacts of petroleum hydrocarbons in polar and tropical habitats;
-
To better integrate laboratory studies with field investigations;

PHOTO 20 (A) Julie N spill of IFO 380 coated the intertidal marshes of the Fore River near Portland, Maine. Photo taken in September 1996. (B)Photo Same area, one year post spill, September 1997. Most of the vegetation had completely recovered. Factors leading to recovery were: the plants were already in senescence when oiled, little or no sediment contamination occurred; large tidal range with good flushing. (Photos courtesy of Jacqui Michel, Research Planning, Inc.)
-
To assess the potential effects of petroleum hydrocarbons at population and ecosystem levels, especially for fish stocks and critical habitats such as mangroves and coral reefs.
Many of the studies conducted since 1985 have addressed these recommendations and have led us to a better understanding of the vulnerability of different habitats and different life history stages of a variety of marine organisms. Field and laboratory investigations have integrated studies of chemical fate and biological effects so that an improved understanding of the recovery process has been defined. In addition, oil spills have been monitored for longer periods of time and across wider far-field conditions to examine the chronic, long-term effects of spills. In their synthesis volume, Long-Term Environmental Effects of Offshore Oil and Gas Development, Boesch and Rabalais (1987) identified several important areas of research needs that complemented those identified in the Oil in the Sea report. Based on detailed consideration of the probability and severity of effects and the potential for resolution of uncertainties, they identified ten categories of potential long-term environmental effects. These were:
High Priority
-
Chronic biological effects resulting from the persistence of medium and high molecular weight aromatic hydrocarbons and heterocyclic compounds and their degradation products in sediments and cold environments.
-
Residual damage from oil spills to biogenically structured communities, such as coastal wetlands, reefs and vegetation beds.
-
Effects of channelization for pipeline routing and navigation in coastal wetlands.
Intermediate Priority
-
Effects of physical fouling by oil of aggregations of birds, mammals, and turtles.
-
Effects on benthos of drilling discharges accumulated through field development rather than from exploratory drilling.
-
Effects of produced water discharges into nearshore rather than open shelf environments.
Lower Priority
-
Effects of noise and other physical disturbances on populations of birds, mammals, and turtles.
-
Reduction of fishery stocks due to mortality of eggs and larvae as a result of oil spills.
-
Effects of artificial islands and causeways in the Arctic on benthos and anadromous fish species.
Many of these concerns have now been fully addressed and are detailed in several synthesis reports written since 1987 (Box 5-1). Those topics not covered in synthesis reports will be addressed in this report.
Toxic Effects of Petroleum Hydrocarbons
The responses of organisms to petroleum hydrocarbons can be manifested at four levels of biological organization: (1) biochemical and cellular; (2) organismal, including the integration of physiological, biochemical and behavioral responses; (3) population, including alterations in population dynamics; and (4) community, resulting in alterations in community structure and dynamics. Impairment of behavioral, developmental, and physiological processes may occur at concentrations significantly lower than acutely toxic levels; such responses may alter the long-term survival of affected populations. Thus, the integration of physiological and behavioral disturbances may result in alterations at the population and community levels.
The effects of petroleum hydrocarbons in the marine environment can be either acute or chronic. Acute toxicity is defined as the immediate short-term effect of a single exposure to a toxicant. Chronic toxicity is defined as either the effects of long-term and continuous exposure to a toxicant or the long-term sublethal effects of acute exposure (Connell and Miller, 1984). Acute and chronic toxicity of petroleum hydrocarbons to marine organisms is dependent upon:
-
concentration of petroleum hydrocarbons and length of exposure,
-
persistence and bioavailability of specific hydrocarbons,
-
the ability of organisms to accumulate and metabolize various hydrocarbons,
-
the fate of metabolized products,
-
the interference of specific hydrocarbons (or metabolites) with normal metabolic processes that may alter an organism’s chances for survival and reproduction in the environment (Capuzzo, 1987), and
-
the specific narcotic effects of hydrocarbons on nerve transmission.
Many of the early studies of acute toxicity focused on the toxicity of individual compounds to marine organisms or the differential toxicity of crude and refined oils (Anderson, 1979). The findings from these types of studies can be summarized as follows: The acute toxicity of individual hydrocarbons is largely related to their water solubility. The acute toxicity of a specific oil type is the result of the additive toxicity of individual compounds, especially aromatic compounds. Narcotic effects of individual petroleum compounds are an important component of acute toxicity and are most closely related to low molecular weight volatile compounds (Donkin et al., 1990). Sublethal effects following acute or chronic exposure to petroleum hydrocarbons include disruption in energetic processes; interference with biosynthetic
|
BOX 5-1 Recent National Research Council Synthesis Reports Addressing Oil in the Sea and Offshore Oil and Gas Development The following list reflects the extensive attention the NRC and government agencies have placed on the effect of petroleum in the environment.
|
processes and structural development; and direct toxic effects on developmental and reproductive stages (Capuzzo et al., 1988).
Weathering processes are extremely important in altering the toxicity of an oil spill. Neff et al. (2000) demonstrated rapid loss of monocyclic aromatic hydrocarbons (e.g., benzene, toluene, ethylbenzene, and xylene) from evaporation and a reduction of acute toxicity of the water-accommodated fraction (WAF) with loss of these compounds (see Box 5-2). With weathering processes and loss of the monoaromatic compounds, the polycyclic aromatic hydrocarbons become more important contributors to the toxicity of weathered oils. Other factors that may contribute to alterations in toxicity include photodegradation and photoactivation (Garrett et al., 1998; Boese et al., 1999; Mallakin et al., 1999; Little et al., 2000).
Barron et al. (1999) examined the chemistry and toxicity of water-accommodated fractions, from three environmentally-weathered middle distillate oils differing in aromatic content to test the hypothesis that the aromatic components of oil are the most toxic fraction. Using short-term growth and survival tests with the mysid, Mysidopsis bahia, they demonstrated that the oil with the lowest aromatic content (expressed as PAH concentration or naphthalene concentration in WAF) had the greatest toxicity. The toxicity of the three weathered oils was consistent with the reported toxicity of unweathered middle distillates tested under similar conditions (Anderson et al., 1974; Markarian et al., 1995) and were more similar to one another when reported as total petroleum hydrocarbons. Therefore, heterocyclic compounds and other soluble components in the water-accommodated fraction of weathered oil may contribute to acute toxicity.
The importance of PAH to weathered oil toxicity depends on the concentrations present, presence of other toxic components, and the degree to which the weathered oil has been degraded by microbial and photooxidation. Neff et al. (2000) provided an estimate of the contribution of different hydrocarbon classes to the toxicity of several Australian oils that had been weathered by evaporation in the laboratory (no microbial or photodegradation). Shelton et al. (1999) showed the importance of microbial degradation on weathered crude oil toxicity. Barron and Ka’aihue (2001) argued that photoenhanced toxicity could contribute to the toxicity of crude oil in the field.
Although a large volume of literature existed in 1985 on the effects of petroleum hydrocarbons on marine organisms in laboratory studies, the majority of studies conducted prior to 1985 were carried out at concentrations higher than is environmentally realistic. Those studies contributed to our understanding of the range of effects that could occur following an oil spill and the potential for long-term consequences, but they could not be used to develop realistic scenarios of the linkages between recovery of organisms and habitats and the degradation/disappearance of hydrocarbons from the habitat. Much progress has been made since the 1985 report addressing these issues. Some of the best examples of acute and chronic toxic effects of oil to marine organisms have been derived from observations in the field following oil spills and in laboratory studies designed to replicate the exposure field of actual spill conditions.
|
BOX 5-2 Benzene, Toluene, Ethyl Benzene, and Xylenes (BTEX) BTEX is the collective name for benzene, toluene, ethyl benzene, and xylenes, the volatile aromatic compounds often found in discharges, and petroleum oils and products (Wang and Fingas, 1996). The behavior of the four compounds is somewhat similar when released to the environment and thus they are usually considered as a group. Most light crude oils contain BTEX usually from about 0.5 up to 5% or more. Gasoline can contain up to 40% BTEX. BTEX compounds are volatile and, if discharged into the sea, will rapidly volatilize into the air, and there is, in fact, a net loss of BTEX compounds. Because of this behavior, the discharges of BTEX were not considered in this study. BTEX compounds are acutely toxic to aquatic organisms if contact is maintained. BTEX compounds are relatively soluble in water, the solubility of benzene is about 1400 mg/L and xylenes about 120 mg/L. Because of the volatility of BTEX, the time exposure to aquatic organisms may be short enough to avoid toxic effects. BTEX are generally neurotoxic to target organisms. Benzene, in particular, has also been found to be carcinogenic to mammals and humans. Gasoline contains large amounts of BTEX. The bulk solubility of gasoline has been found to vary from 100 to 500 ppm, depending on the specific type of gasoline and its constituents. The aquatic toxicity of gasoline is relatively high. The fifty-percent lethal concentration to test organisms over a 48-hour period has been found to be 10 to 50 mg/L for Daphnia magna, the water flea, 5 to 15 mg/L for Artemia, small brine shrimp, and 5 to 10 mg/L for rainbow trout larvae. Produced waters contain a variety of volatile hydrocarbons, including the BTEX series (Rabalais et al., 1991a,b). Produced waters generally have concentrations of dissolved salts much higher than sea water and therefore sink through the water column into which they are disposed. BTEX compounds in produced water discharged to well-mixed open ocean waters are diluted rapidly. Twenty meters down-current from a production platform discharging 11 million L/d of produced water containing an average of 6,410 μg/L total BTEX to the Bass Strait off southeast Australia, the average concentration of BTEX was 0.43 μg/L, a dilution of 14,900-fold (Terrens and Tait, 1996). In well-flushed, dispersive and deeper water environments of the Louisiana coast, the BTEX chemical contaminant signal may be negligible as close as 50-100 m from the point of discharge (Rabalais et al. 1991a,b). In shallower, less dispersive environments the produced water plume along with the BTEX spreads in a thin dense plume across the surface sediments of the receiving environment, and the chemical signature of the produced waters can be detected up to 1000 m from the point of discharge (Rabalais et al., 1991a, b). BTEX were detected in the water overlying the sediment surface near estuarine and coastal environments that were categorized as less dispersive or where the concentration of the BTEX was high in the discharge. Produced waters vary considerably in BTEX concentrations, but produced waters discharged into surface waters of Louisiana ranged from 26—4,700 μg/L benzene, 11—1,300 μg/L toluene, 2.1—75 μg/L ethylbenzene, and 8.8—520 μg/L xylenes. BTEX persisted in the density plume that dispersed across the sediment surface in poorly flushed Louisiana study areas in concentrations up to 86 μg/L benzene, 32 μg/L toluene, 2.3 μg/L ethylbenzene, and 17 μg/L xylenes; in more dispersive environments, they were not detected. BTEX in the overlying water column, if present, along with the more persistent polynuclear aromatic hydrocarbons in the sediments, likely contributed to the mortality of the benthic infauna where diminished benthic communities were documented adjacent to produced water discharges. The mortality could not be attributed to high salinity, because the salinity of the interstitial waters of the sediments examined were within the tolerance range of the euryhaline benthos found in the study area. |
Data gathered from several spills that occurred in the 1970s and 1980s demonstrated that the medium and higher molecular weight aromatic compounds, such as the alkylated phenanthrenes and alkylated dibenzothiophenes, are among the most persistent compounds in both animal tissues and sediments (Capuzzo, 1987). The half-lives of these compounds in marine bivalves following spill conditions can be quite long compared to the relatively rapid decline in monoaromatic compounds and unsubstituted phenanthrenes and naphthalenes (Oudot et al., 1981; Farrington et al., 1982; Anderson et al., 1983; Burns and Yelle-Simmons, 1994). The degree to which the persistence of these compounds in tissues interferes with normal metabolic processes that affect growth, development and reproduction has been the focus of much debate and research. Sublethal effects from hydrocarbon exposure can occur at concentrations several orders of magnitude lower than concentrations that induce acute toxic effects (Vandermeulen and Capuzzo, 1983). Impairment of feeding mechanisms, growth rates, development rates, energetics, reproductive output, recruitment rates and increased susceptibility to disease and other histopathological disorders are some examples of the types of sublethal effects that may occur with exposure to petroleum hydrocarbons (Capuzzo, 1987). Early developmental stages can be especially vulnerable to hydrocarbon exposure, and recruitment failure in chronically contaminated habitats may be related to direct toxic effects of hydrocarbon contaminated sediments (Krebs and Burns, 1977; Cabioch et al., 1980, Sanders et al., 1980; Elmgren et al., 1983).
Several studies have demonstrated the potential for oil residuals on beach sediments to have significant toxic effects on fish eggs and embryos. Heintz et al. (1999) reported embryo mortality of pink salmon with laboratory exposure to aqueous total PAH concentrations as low as 1 ppb total PAH derived from artificially weathered Alaska North Slope crude oil. This is consistent with the field observations of Bue et al. (1996) of embryo mortality of pink salmon in streams traversing oiled beaches following the spill from the
Exxon Valdez. Carls et al. (1999) exposed Pacific herring eggs for 16 days to weathered Alaska North Slope crude oil and observed that exposure to initial aqueous concentrations as low as 0.7 ppb PAH caused developmental malformations, genetic damage, mortality, decreased size at hatching, and impaired swimming. Concentrations as low as 0.4 ppb caused premature hatching and yolk-sac edema. Exposure to less weathered oil produced similar results but at higher exposure concentrations (9.1 ppb).
Other investigators have observed developmental effects on fish and invertebrates exposed to low concentrations of petroleum hydrocarbons (Capuzzo et al., 1988). The high toxicity of weathered oil reported by Heintz et al. (1999) and Carls et al. (1999), however, suggests that higher concentrations of one or more constituents in weathered fractions relative to total PAH contribute to the increased toxicity.
Bioavailability, Bioaccumulation, and Metabolism
The concept of bioavailability is extremely important in understanding and describing the environmental fates and biological effects of petroleum in the marine environment. A concise definition of what is meant in this context by bioavailability is essential. In aquatic toxicology, bioavailability usually is defined as the extent to which a chemical can be absorbed or adsorbed by a living organism by active (biological) or passive (physical or chemical) processes. A chemical is said to be bioavailable if it is in a form that can move through or bind to the surface coating (e.g., skin, gill epithelium, gut lining, cell membrane) of an aquatic organism (Kleinow et al., 1999).
Accumulation of petroleum hydrocarbons by marine organisms is dependent on the biological availability of hydrocarbons, the length of exposure, and the organism’s capacity for metabolic transformations. There are two aspects of petroleum hydrocarbon bioavailability that are important in understanding the behavior of oil in the environment: environmental availability, and biological availability. Environmental availability is the physical and chemical form of the chemical in the environment and its accessibility to biological receptors. Generally, chemicals in true solution in the ambient water are considered more bioavailable than chemicals in solid or adsorbed forms. Petroleum hydrocarbons of the types found in the marine environment may be present in true solution, complexed with dissolved organic matter and colloids, as dispersed micelles, adsorbed on the surface of inorganic or organic particles, occluded within particles (e.g., in soot, coal, or tar), associated with oil droplets, and in the tissues of marine organisms (Readman et al., 1984; Gschwend and Schwarzenbach, 1992). The hydrocarbons in the different phases are exchangeable but, at any given moment, only a fraction of the total hydrocarbons in water, sediments, and biota is in bioavailable forms.
The dissolved hydrocarbons are the most bioavailable, followed by those in tissues of marine organisms (if the organisms are eaten) or associated with liquid, unweathered oil droplets. Thus, bioavailability of PAH from sediments and food is less than that from solution in the water (Pruell et al., 1987). Particulate PAH associated with soot or weathered oil particles (e.g., tarballs) have a low bioavailability (Farrington, 1986; Gustafsson et al., 1997a,b; Baumard et al., 1999). As oil weathers, its viscosity and average molecular weight increase, decreasing the rate of partitioning of higher molecular weight PAH from the oil phase into water in contact with the oil, decreasing the accessibility of these PAH to aquatic organisms (McGrath et al., 2001). Soot-associated PAH are not bioaccumulated in the tissues of aquatic animals. Maruya et al. (1996) showed that sediment-associated animals in San Francisco Bay, CA, were not able to bioaccumulate PAH from the very fine-grained particles (identified as soot) in the sediments. Pruell et al. (1986) showed that the bioaccumulation of PAH from contaminated sediments by mussels correlated with the concentration of dissolved but not particulate PAH in the sediments.
The other aspect of environmental availability is accessibility. Petroleum hydrocarbons that are buried deep in sediments or sequestered in solid, highly weathered oil deposits on the shore are not accessible to marine and terrestrial organisms and, therefore have a low bioavailability. Biological availability depends on the rate at which a chemical is assimilated into the tissues of the organism and accumulates at the sites of toxic action in the organism. This depends on the physical/chemical properties of the chemical in contact with the organism, the relative surface area of permeable epithelia in the organism, and the ability of the organism to excrete or detoxify the chemical. Nonpolar (hydrophobic) organic chemicals such as petroleum hydrocarbons, have a low aqueous solubility and a high lipid solubility. Hydrocarbons in solution in water diffuse down an activity or fugacity gradient from the water phase into lipid-rich tissues of marine organisms in contact with the water. According to equilibrium partitioning theory (Davies and Dobbs, 1984; Bierman, 1990), when an aquatic animal is exposed to a nonpolar organic chemical dissolved in the ambient water, the chemical partitions across permeable membranes into tissue lipids until an equilibrium, approximated by the octanol/ water partition coefficient (Kow) for the chemical is reached. At equilibrium, the rates of absorption into and desorption from the lipid phase of the organism are equal. Toxic responses in the organism occur when the concentration of nonpolar organic chemicals in the tissues reach a critical concentration (McCarty and Mackay, 1993). The log Kow of PAH increases with increasing molecular weight (Neff and Burns, 1996). However, bioavailability, measured as log bioconcentration factor (BCF: concentration in tissues/concentration in water at equilibrium), does not increase in a linear fashion with increasing PAH log Kow (Baussant et al., 2001a,b). The sediment organic carbon-water coefficient, Koc is also useful in predicting uptake of sediment-associated hydrocarbons (Fisher, 1995; Meador et al. 1995; DiToro
et al., 2000; ). The higher molecular weight PAH are less bioavailable than predicted by equilibrium partitioning theory because of limitations on their uptake rates by organisms, their lower solubility in tissue lipids, and rapid metabolism of higher molecular weight PAH in some marine animals. Bioaccumulation factors for pyrogenically derived hydrocarbons are much less than predicted based on Koc and suggest that an additional estimate of the fraction of compound available for equilibrium partitioning may be needed (McGroddy and Farrington, 1995; McGroddy et al., 1996).
Biotransformation is an important factor in examining tissue burdens and biological effects. An organism’s capacity for biotransformation of hydrocarbons has been used in many instances as an estimate of exposure in the absence of measurable hydrocarbon concentrations. Vertebrates have a high capacity for metabolizing aromatic hydrocarbons including PAH through cytochrome P450 1A mediated oxidation (Stegeman, 1989; Stegeman and Lech, 1991; Spies et al., 1996). Elevation of cytochrome P450 1A levels in fish may indicate exposure to some aromatic hydrocarbons, even though tissue levels do not show elevated concentrations. There is a large literature that links elevated P450 1A levels in fish tissues to aromatic contaminants in marine sediments (e.g., Stegeman and Lech, 1991), but it is theoretically possible for some other natural compounds to induce these enzymes as well. Measurement of hydrocarbon metabolites in tissues where elevated cytochrome P450 1A is observed provides further evidence of the relationship of hydrocarbon exposure, metabolism and cytochrome P450 1A activity (Stein et al., 1992; Collier et al., 1993; Wirgin et al., 1994). Metabolism of hydrocarbon mixtures may result in excretion of some compounds but also activation of some compounds to toxic metabolites including DNA adducts (Wirgin et al., 1994).
Long-Term Effects on Benthic Populations
Chronic toxicity of petroleum hydrocarbons after an oil spill is associated with the persistent fractions of oil and individual responses of different species to specific compounds. Alterations in bioenergetics and growth of bivalve molluscs following exposure to petroleum hydrocarbons appear to be related to tissue burdens of specific aromatic compounds (Gilfillan et al., 1977; Widdows et al., 1982, 1987; Donkin et al., 1990). Widdows et al. (1982) demonstrated a negative correlation between cellular and physiological stress indices (lysosomal properties and scope for growth) and tissue concentrations of aromatic hydrocarbons with long-term exposure of Mytilus edulis to low concentrations of North Sea crude oil. Recovery of mussels following long-

PHOTO 21 Oil penetrated deeply into burrows in the muddy sediments on tidal flats and marshes along the Persian Gulf. Note the liquid oil draining out of a burrow in 1993, two years after the spills. (Photo courtesy of Jacqui Michel, Research Planning, Inc.)
term exposure to low concentrations of diesel oil coincided with depuration of aromatic hydrocarbons (Widdows et al., 1987). Donkin et al. (1990) suggested that reductions in scope for growth in M. edulis were related to the accumulation of two- and three-ring aromatic hydrocarbons, as these compounds induced a narcotizing effect on ciliary feeding mechanisms.
Krebs and Burns (1977) observed long-term reductions in recruitment and over-wintering mortality in the fiddler crab Uca pugnax for seven years following the spill of No. 2 fuel oil from the barge Florida. Recovery of crab populations was correlated with the disappearance of naphthalenes and alkylated naphthalenes from contaminated sediments. Similar patterns of long-term changes in recruitment and density of benthic fauna have been observed at sites of other oil spills and in experimental mesocosms (Cabioch et al., 1980; Grassle et al., 1981; Oviatt et al., 1982; Elmgren et al., 1983). Ho et al. (1999) compared the toxicity to the amphipod Ampelisca abdita and chemistry of spilled No. 2 fuel oil in subtidal sediment samples for nine months following the spill from the barge North Cape (Box 4-1). Toxicity to the amphipods decreased as the PAH concentration in sediments decreased over the first six months post-spill (Figure 5-3).
The persistence of PAH in sediments, especially in urban areas with multiple sources of hydrocarbon inputs, is an example of chronic persistence and toxicity beyond the observations made following oil spills (Box 5-3). Meador et al. (1995) reviewed the processes controlling the uptake and persistence of PAH in marine organisms, especially under chronic exposure conditions, highlighting differential mechanisms of uptake, tissue distribution, and elimination. Transfer of contaminants to marine biota and the human consumer and toxicological effects on the ecosystem are dependent on the availability and persistence of these contaminants within benthic environments. The bioaccumulation of
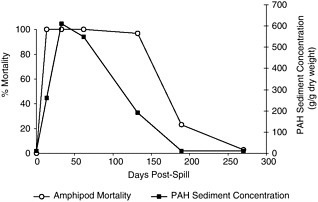
FIGURE 5-3 Amphipod mortality and PAH concentrations in sediments after the North Cape oil spill off Cape Cod, Massachusetts, January 19, 1996 (modified from Ho et al., 1999, Marine Pollution Bulletin).
lipophilic organic contaminants is influenced by chemical factors such as solubility and particle adsorption-desorption kinetics of specific compounds and biological factors such as the transfer of compounds through food chains, the amount of body lipid in exposed organisms, and metabolic transformations. The incidence of tumors and other histopathological disorders in bottom-dwelling fish and shellfish from contaminated coastal areas has suggested a possible link between levels of lipophilic organic contaminants (such as PAH) and the increased incidence of histopathological conditions (Neff and Haensly, 1982; Berthou et al., 1987; Varanasi et al., 1987; Gardner and Pruell, 1988; Moore et al, 1994; McDowell and Shea, 1997).
In addition to possible histopathological damage, sublethal toxic effects of contaminants in marine organisms include impairment of physiological processes that may alter the energy available for growth and reproduction and other effects on reproductive and developmental processes including direct genetic damage (Capuzzo, 1987; Capuzzo et al., 1988). Chronic exposure to chemical contaminants can result in alterations in reproductive and developmental potential of populations of marine organisms, resulting in possible changes in population structure and dynamics. It is difficult to ascertain, however, the relationship between chronic responses of organisms to contaminants and large-scale alterations in the functioning of marine ecosystems or the sustainable yield of harvestable species. Cairns (1983) argued that our ability to detect toxic effects at higher levels of biological organization is limited by the lack of reliable predictive tests at population, community, and ecosystem levels. Much research effort is needed in these areas before environmental hazards as a result of contaminant inputs can be addressed adequately. Koojiman and Metz (1984) suggested that the sublethal effects of contaminant exposure should be interpreted in light of the survival probabilities and reproductive success of populations, thus bridging the gap between individual and population responses. Although a wide range of sublethal stress indices have been proposed for evaluation of chronic responses of organisms to contaminants, few have been linked to the survival potential of the individual organism or the reproductive potential of the population. Rice et al. (2001) reviewed studies on the long-term effects of the Exxon Valdez oil spill on pink salmon, specifically addressing differential effects of low concentrations of oil on specific life history stages. Their results illustrate the complexity of assessing population-level impacts from persistent hydrocarbon residues, even at very low concentrations.
Putative damage to pink salmon as a result of the Exxon Valdez oil spill has been controversial (e.g., Rice et al., 2001). Much of this controversy has focused on the potential damage to embryos incubating in the mouths of streams that were oiled. The potential damage to resident fish embryos in these oiled redds may be long lasting and serious. For example, contrasts between oiled and unoiled streams around
|
BOX 5-3 Boston Harbor Chronic contamination of urban harbors reflects a history of contaminant discharges from a variety of sources. Petroleum hydrocarbons, including polycyclic aromatic hydrocarbons, may be derived from the burning of fossil fuels, accidental oil spills, and chronic inputs from municipal discharges and marinas. Loadings of polycyclic aromatic hydrocarbons to Massachusetts Bay are estimated to be within the range of 2.1 to 13.7 metric tons per year (Menzie-Cura & Associates, 1991). Sites receiving inputs from combined sewer overflows (CSOs) are among the most contaminated sites in Boston Harbor and Massachusetts Bays. Concentrations of total PAH in Boston Harbor sediments are among the highest reported for all coastal sites of the U.S. in the NOAA National Status and Trends program. Among sites examined within the New England region, concentrations of total PAH in sediment samples from Boston Harbor exceeded concentrations in samples from other sites by as much as one to two orders of magnitude (MacDonald, 1991). In addition to sediments, biota from Boston Harbor are highly contaminated with a variety of lipophilic organic contaminants including both low molecular weight and high molecular weight PAH. Concentrations of total PAH in tissues of the blue mussel (Mytilus edulis) are in the upper 15 percent of the most contaminated sites from the U.S. coastline surveyed in the National Status and Trends Program (MacDonald, 1991). The relative abundance of individual PAH in sediments surveyed in Boston Harbor are typical of sediments with highly weathered petroleum inputs mixed with combustion products (McDowell and Shea, 1997). Sediments from Boston Harbor stations are enriched with higher molecular weight PAH indicative of combustion sources and creosote, including fluoranthene, pyrene, and chrysene. McGroddy and Farrington (1995) examined the sediment-porewater partitioning of PAH in three cores from Boston Harbor and found that only a fraction of the total measured sediment PAH concentration was available for equilibrium partitioning and biological uptake. Laboratory desorption experiments demonstrated that only a small fraction of sediment phenanthrene and pyrene were available for equilibrium partitioning (McGroddy et al., 1996). Studies of bioaccumulation of PAH in bivalve mollusks such as the soft-shell clam Mya arenaria and the blue mussel Mytilus edulis also reflect the reduced availability of PAH from Boston Harbor sediments (McDowell et al., 1999). PAH were detected in clam tissues and sediments collected along a gradient of contamination in Boston Harbor and Massachusetts and Cape Cod Bays, but the bioavailability of specific compounds varied at different sites. Estimates of AEP (available for equilibrium partitioning) provided the best predictor of relative bioavailability of pyrogenic PAH. With the presence of high concentrations of contaminants in Boston Harbor sediments and the need for navigational dredging innovative solutions to dredging Boston Harbor had to be developed. The Boston Harbor Navigation Improvement Project was the result of three decades of negotiation involving many stakeholders and considering 312 land-based inland and coastal sites, 21 landfills, and 21 aquatic sites as disposal options (NRC, 1997). Four final management options were identified as acceptable: the Massachusetts Bay Disposal site, the Boston Lightship site, two near-shore borrow pits, and one contained aquatic disposal site. The final selection involves removal of contaminated sediments to allow dredging of highly contaminated sediments, formation of very deep pits, replacement of the contaminated sediment and, finally, placement of clean sand as a sediment cover. Uncontaminated sediments that are removed to form the deep pits will be disposed at the Massachusetts Bay Disposal site. This solution is a good example of meeting both economic and environmental objectives in the management of contaminated sediments. |
Knight Island, Alaska found significantly elevated mortalities of embryos in oiled streams in 1989-1993 (Bue et al., 1998). These findings are indicative of P450 1A induction as measured in oiled streams (Weidmer et al., 1996), as well as with a model of subsurface movement of oil in streams based on intertidal elevations (Rice et al., 2001). These findings were called into question by some subsequent studies on a variety of grounds including questions about study design. Brannon et al. (1995) concluded that oil levels in the redd had no effect on the incubation of fertilized eggs. In a later study, Brannon et al. (2001) claimed that sampling occurred on different time schedules for oiled streams and unoiled streams. Therefore, the authors contended that any damage to eggs was the result of collection and handling, and that oil levels did not negatively impact the embryos. While Rice et al. (2001) clearly showed that their sampling methods had greater power to detect embryo mortality in the field, they were not able to discount the egg-shock hypothesis. However hatchery-raised embryos from parents that were taken from both oiled and unoiled streams had patterns of survival that closely matched those from the field (Bue et al., 1998). Additionally, there was disagreement about damage at other life history stages and laboratory toxicological findings within this species (Brannon and Maki, 1996; Brannon et al., 2001; Rice et al., 2001).
Johnson et al. (2001) reported threshold-sediment PAH concentrations for toxicopathic liver lesions in English sole ranging from 54 to 2,800 ng/g dry weight and a threshold for DNA adducts in liver of 300 ng per g dry weight. These thresholds were based on analyses of fish collected in Puget Sound, Washington. Other effects included inhibited gonadal growth, inhibited spawning, reduced egg viability, and reduced growth, although there were insufficient data to determine a precise threshold. From these analyses, Johnson et al. (2001) proposed a sediment quality guideline of 1000 ppb total PAH (ng/g dry weight) to minimize effects on estuarine

PHOTO 22 MODIS (or Moderate Resolution Imaging Spectroradiometer) satellite imagery (250 m resolution) of New England. Urbanization, visible from space, increases both population density and the percent of paved surface, altering the volume and composition of runoff. (Image courtesy of NASA.)
fish (Figure 5-4). This is consistent with observations made by other investigators for other estuarine species (Mya arenaria, soft shell clam; McDowell and Shea, 1997; Ampelisca abdita, amphipod; Ho et al., 1999). However, toxic effects observed will be dependent on not only the concentration of total PAH but the composition and relative distribution of individual compounds. This makes it very difficult to compare studies unless detailed composition data are also presented.
Birds and Marine Mammals
Marine birds and mammals can be affected by oil in the sea through several pathways (see references in Hunt, 1987; Kajigaya and Oka, 1999; Tsurumi et al., 1999). As air-breathing organisms that obtain much or all of their food from beneath the surface of the sea, marine birds and mammals must frequently pass through the water’s surface. When floating oil is present, they become fouled. Additionally, many species of birds frequent the intertidal zone while foraging and resting, as do seals, sea lions, river otters, and occasionally sea otters. While there, these warm-blooded vertebrates may become coated with oil that has come ashore. The presence of oil on the feathers of a seabird or the pelage of a marine mammal can destroy the waterproofing
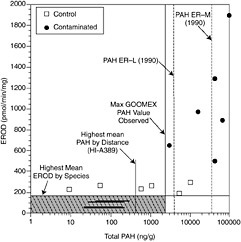
FIGURE 5-4 Correlation between total PAH concentrations in sediments and hepatic EROD activities of fish (from McDonald et al., 1996, Canadian Journal of Fisheries and Aquatic Sciences).
and insulating characteristics of the feathers or fur and lead to death from hypothermia. Seabirds and marine mammals may be poisoned when they ingest oil during the course of trying to remove it from their feathers or pelage, or when it adheres to food items. Likewise, marine mammals (and possibly seabirds) may inhale toxic doses of petroleum vapor when at the surface in the vicinity of an oil spill (Geraci, 1990; Geraci and Williams, 1990; St. Aubin, 1990a), although there appear to be few data indicating that this is an important source of mortality (Figure 5-5). In some cases, these upper trophic level predators may become exposed to oil by ingesting prey that have oil or its metabolites in their tissues. Seabirds can transfer oil from their feathers to the surface of their eggs during incubation. Depending on the type of oil on the feathers and the presence of toxic components, embryos in the affected eggs may fail to develop. Oil can also indirectly affect the survival or reproductive success of marine birds and mammals by affecting the distribution, abundance or availability of prey.
In seabirds, ingestion of oil or oil-contaminated prey may lead to immuno-suppression and Heinz-body hemolytic anemia which compromises the ability of the blood to carry oxygen (Leighton et al., 1983; Fry and Addiego, 1987). This effect persists long after the birds appear to have recovered from exposure (Fry and Addiego, 1987). Diminished oxygen transport capacity in the blood is a particular problem for species of birds that obtain their food by pursuing prey underwater. Although the effects of the anemia have yet to be demonstrated in the field (Nisbet, 1994), seabird survival post-oiling, with or without cleaning, may be compromised. Marine mammals are also vulnerable to the toxic effects of ingested oil, and species of marine mammals such as sea otters that depend on a clean pellage for insulation are also vulnerable to surface oiling (Geraci and St. Aubin, 1987; Geraci, 1990; Geraci and Williams, 1990; St. Aubin, 1990a,b; St. Aubin and Lounsbury, 1990). Effects may be exacerbated by stress resulting from handling during cleaning (Briggs et al., 1996).
The number of seabirds killed and the damage to local populations in a spill is more likely to be determined by location and timing of the spill than by its size (Hunt, 1987; Burger, 1993). Burger found a statistically significant but
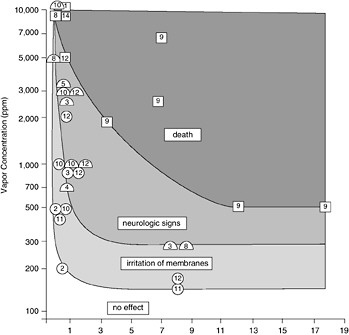
FIGURE 5-5 Summary of the effects on mammals of exposure to an inhalation of various petroleum vapors, principally those of gasoline (from Geraci and St. Aubin, 1987, Academic Press).
weak log-log correlation between the number of birds killed and the volume of oil spilled when he analyzed 45 spills. In another analysis of 98 spills, there was no correlation (Dagmar Etkin, Environmental Research Consulting, personal communication). Large spills that occur over the deep ocean in open water that has little bird life will have a lesser effect on seabirds than a small spill in a critical habitat where high numbers of birds are aggregated on the water. The season in which a spill occurs is also critical (Hunt, 1987). If the spill occurs when birds are aggregated during breeding or migration, the impact will be much greater than if they are widely dispersed at sea. It is likely that the cumulative effect of numerous “small” spills and chronic pollution has had a greater effect on seabird populations than the rarer large spills. Recent evidence, however, suggests that the incidence of seabird mortality from small spills may be declining in the North Sea region (Camphuysen, 1998). This shift may reflect the reduction of small discharges of oil noted earlier in Chapter 3.
There has been a “general-rule-of-thumb” that the body count of birds recovered after a spill represents about 10 percent of the birds killed in a spill (Tanis and Morzer Bruijns, 1969; NRC, 1985). Burger (1993), however, found that there was no justification for this assumption; the mean estimate for 21 spills for which the number of birds found dead and that the overall mortality were determined to be between four and five times the number of birds actually counted. The relationship among the number of carcasses recovered and the total mortality will vary between species, being dependent among other factors on body size, average distance to shore carcass buoyancy, and the prevailing winds during and after the spill.
Few studies of oil spills have provided the information necessary to assess delayed or long-term effects on community-level processes in the affected communities or ecosystems. Where keystone predators are removed, competitive dominants at the next lower trophic level can increase and change the structure of the community. For example, in areas of Prince William Sound where sea otters were removed by oiling, their preferred prey, sea urchins, have increased in some locations (Peterson, 2001). Elsewhere in Alaska where sea urchin populations have been able to increase in the absence of sea otters, severe damage to kelp beds have resulted (Estes, 1995; Estes and Duggins, 1995).
In addition to the strong evidence for the impact of massive contamination associated with an oil spill, there is increasing evidence that chronic, low-level exposures to hydrocarbons in the sea can have a significant effect on the survival and reproductive performance of seabirds and some marine mammals. Sublethal effects of oil on seabirds include reduced reproductive success, and physiological impairment, including increased vulnerability to stress (reviewed in Fry and Addiego, 1987,1988; Hunt, 1987; Briggs et al., 1996). In contrast, in marine mammals, sublethal exposure to petroleum hydrocarbons has been shown to cause minimal damage to pinnipeds and cetaceans (e.g., Geraci, 1990; St. Aubin, 1990b), although sea otters appear to be more sensitive (Geraci and Williams, 1990; Monson et al., 2000). Because both marine birds and marine mammals have the enzymes necessary for the detoxification and elimination of petroleum hydrocarbons, parent compounds of petroleum hydrocarbons are not accumulated and sequestered in tissues as chlorinated hydrocarbons. Toxic metabolites produced by metabolism of polycyclic aromatic hydrocarbons, however, may accumulate and induce toxic effects (Brunstrom et al., 1991; Melancon, 1996, 1995). Chronic pathologies would not be expected once oil ceased to be ingested.
There is mixed evidence that oil pollution can have demonstrable effects on the population trajectories of marine birds and mammals. Spilled oil has had and still poses a potentially devastating effect on African Penguins (Spheniscus demersus) in southern Africa (Westphal and Rowan, 1970; Vermeer and Vermeer, 1975; Clark, 1984; Dagmar Etkin, Environmental Research Consulting, personal communication). At Les Sept Iles, France, declines in the numbers of Common Murres (Urea aalge) attending colonies have been attributed to the effects of oil spilled in the Torrey Canyon and Amoco Cadiz accidents (Nisbet, 1994). In northern Europe between 1915 and 1988, 60-90 percent of beached alcids were oiled (Camphuysen, 1989), and Hudson (1985) found that oiling accounted for between 18 and 28 percent of mortality of banded alcids. In addition, there is conflicting sentiment that populations of sea ducks in the Baltic declined as a result of oil pollution (Lemmetyinen, 1966; Vermeer and Vermeer, 1975; Clark, 1984; vs. Joensen and Hansen, 1977). In the cases of two of the largest recent spills, the Exxon Valdez spill in Prince William Sound, Alaska and the Persian Gulf War (Box 5-4) release of oil in the northern Arabian Sea, the population-level impacts on seabirds are not clear.
There has been considerable variability in the estimates of the number of seabirds killed in the 1989 Exxon Valdez oil spill, which has led to much contention (e.g., Piatt and Lensink, 1989; Parrish and Boersma, 1995a,b; Piatt, 1995; Wiens et al., 1995, 1996; Ford et al., 1996, Piatt and Ford, 1996; Wiens et al., 1996; Day et al., 1997; Murphy et al., 1997; Lance et al., 2001). Piatt et al. (1990) provided an initial estimate that the number of seabirds killed in the spill was on the order of 100,000 to 300,000 birds (Piatt et al., 1990), and later, Piatt and Ford (1996) provided a best estimate of 250,000 birds killed. Even more difficult has been the determination of population-level impacts. Irons (1996) obtained evidence of lower rates of production of young in the surface-foraging black-legged kittiwake (Rissa tridactyla), but this did not translate into a decrease in the size of colonies in Prince William Sound, or even in the oiled portion of the Sound. The Common Murre (Urea aalge) was the species that sustained the highest mortality (Piatt and Anderson, 1996; Piatt and Ford, 1996), and it might have been expected that a population-level effect of this mortality
|
BOX 5-4 Gulf War Spill, Arabian Gulf Over a period of about four months from January-March 1991, crude oil was released into the Arabian Gulf from five tankers, a major tank field, and several offshore terminals, refineries, and battle-damaged tankers as part of the Iraq-Kuwait conflict. Though the actual volume of release will never be known, the best estimate is about 1,770,000 tonnes (520,000,000 gallons) (Tawfiq and Olsen, 1993), making it the largest oil spill in history and three times as large as the next largest spill (the 1979 Ixtoc well blowout in the Gulf of Mexico). Although the massive slicks were initially predicted to spread throughout the Arabian Gulf and out through the Gulf of Hormuz, a seasonal shift in wind patterns held the bulk of the oil along the shoreline between the Kuwait border and Abu Ali Island near Al Jubail, a distance of about 175 km. The oil fate was estimated by Tawfiq and Olsen (1993) as follows: 40 percent evaporated; 10 percent dissolved/ dispersed; 10 percent recovered in Saudi Arabia; 15 percent stranded on shore in Saudi Arabia; and 25 percent unaccounted for. There was concern that a significant portion of the unaccounted for oil sank; however, Michel et al. (1993) did not find evidence for any significant sunken oil in the nearshore subtidal zone during diving surveys (197 dives) offshore the most heavily oiled shorelines and bays in Saudi Arabia. None of the researchers studying the Arabian Gulf after the spill reported large-scale oil contamination of bottom sediments (Price and Robinson, 1993). The spill significantly affected shoreline habitats, with 707 km of shoreline oiled in Saudi Arabia alone, including 124 km of marshes (Gundlach et al., 1993). Very little shoreline cleanup was attempted. An estimated 50-100 percent of the intertidal biota were killed (Jones et al., 1996); in heavily oiled marshes, less than 1 percent of the plants survived (Böer and Warnken, 1996). Followup shoreline surveys in 1992 and 1993 showed that the stranded oil had penetrated up to 40 cm into the sediments, with liquid oil filling burrows in muddy sediments (Hayes et al., 1995). The heavy surface oiling formed persistent pavements along the upper intertidal zone and on the tops of mid-tide bars that showed little evidence of erosion six years after the spill. The surface pavements slowed the rate of subsurface oil weathering and physical removal, effectively sealing the subsurface oil in place. Intertidal species diversity in the lower intertidal zone on sandy and muddy substrates was 50-100 percent of controls by 1994, whereas in the upper intertidal zone, species density and density was 0-70 percent of unpolluted sites (Jones et al., 1996). As of 1997, there was little evidence of recovery of heavily oiled marshes. Much of the heavily oiled shoreline occurred along sheltered bays with little exposure to waves and currents. Thus, natural removal of the stranded oil will be very slow, and full recovery of intertidal communities will likely require decades. Amazingly, no significant long-term impacts to subtidal habitats and communities were observed, including seagrass beds, coral patch and fringing reefs, unvegetated sandy and silty substrates, and rocky outcrops (Kenworthy et al., 1993; Richmond, 1996). Kuwait crude forms a very stable emulsion that resulted in thick surface slicks that stranded onshore rather than mixed into the water column. Impacts to shrimp stocks, however, were severe; in 1992 spawning biomass dropped to 1 percent and total biomass dropped to 27 percent of pre-war levels (Matthews et al., 1993). Causes of this collapse were attributed to a combination of mass mortality of eggs, larvae, and postlarvae resulting from oil exposure during the entire spawning season, emigration of adults out of the oiled areas, mortality of adults, heavy fishing of adults and juveniles thus further reducing the spawning biomass, and decrease in water temperatures and light intensity because of oil fires smoke and haze. At least 30,000 seabirds are estimated to have died as a result of the spill. Although the oil spill killed an estimated 25 percent of the 1991 Saudi Arabian breeding population of the endemic Socotra cormorant, these colonies tripled in population by 1995 (Symens and Werner, 1996). Internationally important breeding tern populations in Saudi Arabia and Kuwait escaped direct oiling impacts in 1991 (70,000 pairs breed on offshore islands in summer), but severe declines in breeding success in 1992 and 1993 resulted from an acute shortage of food that was attributed to the oil impacts on fish recruitment (Symens and Alsuhaibany, 1996). In 1994, breeding success was high. During the spill, shorebird populations were reduced by up to 97 percent; however, it is not known whether the birds avoided the noxious oil or were driven away by a lack of food and found good feeding areas elsewhere, became oiled and died, or died from starvation (Evans et al., 1993). The greatest shorebird impacts, however, were likely the indirect effects of long-term degradation of intertidal habitats and the loss of their food supply. |
would be evidenced by striking changes in the numbers or reproductive performance of murres nesting in the oiled area. Natural variability and the precision of population estimates, however, complicated the determination of impact to Common Murres, and it remained impossible to assign, with certainty, the population-level effects of the spill in this species (Boersma et al., 1995; Piatt and Anderson, 1996). Erikson (1995) also reported no evidence of depressed numbers of murres attending colonies in 1991, as compared to historic data. A lack of up-to-date monitoring in the murre colonies prior to the spill exacerbated the difficulties attendant on determining the effects of the spill. In other species, there was little evidence of significant population-level damage from the spill (Kuletz, 1996; Oakley and Kuletz, 1996; Sharp et al., 1996). Controversy as to the magnitude and duration of the effects of the spill is ongoing (e.g., Irons et al., 2001; Wiens et al., 2001).
In addition, some studies have argued that other sources of PAH in both the east and west Prince William Sound, including vessel traffic and PAH from coal and possibly from oil seeps further south in the Gulf of Alaska, may play a role (Page et al., 1996, 1998, 1999). There has also been consid
erable controversy concerning changes in avian use of nearshore marine habitats within Prince William Sound that might indicate long-term depression of bird populations using these waters (Day et al., 1995, 1997; Wiens, 1995; Wiens et al., 1996; Irons et al., 2000; Lance et al., 2001). Some studies found that, within two years of the spill 23 of 42 species showed no evidence of negative impacts from the spill (e.g., Day et al., 1995), whereas other investigators (Irons et al., 2000) found negative effects in 6 of 14 taxa up to nine years after the spill (Table 5-1). Some of these differences reflect methodologies used, whereas others appear to be matters of interpretation (Day et al., 1997; Murphy et al., 1997; Peterson, 2001). Effects differed between avian species that were apparently chronically exposed to oil residues through their epibenthic prey. For example, in surveys of habitat use by Barrow’s Goldeneye (Bucephala islandica) in 1995, 1996 and 1997, Esler et al. (2000b) were unable to detect a significant effect of oiling history on habitat use, even though concurrent studies (Trust et al., 2000) found elevated levels of the enzyme cytochrome P450 1A in these birds, thus indicating on-going ingestion of oil-contaminated prey. Trust et al. (2000) also found elevated levels of cytochrome P450 1A in Harlequin Ducks (Histrionicus histrionicus) in oiled areas of Prince William Sound. In contrast to Barrow’s Goldeneye, between 1995 and 1998, Harlequin Ducks within oiled areas of the Sound had lower densities (Esler et al., 2000a) and lower over-winter survival than did individuals over-wintering in non-oiled areas of the Sound. A demographic model suggested that the differences in over-winter survival between oiled and unoiled areas was sufficient to account for continued declines in the populations of Harlequin Ducks in the oiled areas. These effects reflect loss of individuals from habitually used wintering or foraging sites. Since it is unclear how these local “subpopulations” relate to biologically defined populations (stocks in fisheries parlance), it remains difficult to assess the “population” effects of this damage.
Wiens et al. (2001), using canonical correspondence analyses, found that although there was a clear effect of the spill, in years subsequent to the spill there was increasing occupancy of previously oiled sites by all species that had exhibited initial spill impacts. However, all species recovered at the same rate, so community composition was affected over time, though the consequences of these effects are unknown.
It is also less than clear that the immense discharges of petroleum into the marine environment during the Persian Gulf War in 1991 had a lasting effect on the populations of seabirds breeding in the northern Arabian Gulf (Case History 5-3). For example, during the war, an estimated 8,000 to 10,000 Socotra Cormorants (Phalacrocorax nigrogularis) were killed, approximately 50 percent of the Saudi Arabian population (Symens and Werner, 1996). As of 1995, the population had rebounded to 30,000 pairs, suggesting that the losses to oil during the war had little population-level effect, except possibly in slowing the rate of post-war increase. In contrast, four species of terns nesting on the offshore islands of the northern Gulf of Arabia showed little evidence of oiling during 1991. Although about 1 percent of the total adult tern population was moderately to heavily
TABLE 5-1 Indirect, chronic, or delayed responses of birds to the Exxon Valdez oil spill (after Peterson, 2001)
|
Species |
Foraging Ecology |
Type of Response |
Period/Duration |
References |
|
Black Oystercatcher |
Intertidal invertebrates |
Numbers declined post 1989 |
1990, with recovery by 1993 |
Klosiewski and Laing, 1994 |
|
Chicks fed oiled mussels required more food for less growth and fledged later |
1990 only |
Andres, 1996, 1997 |
||
|
Laid fewer eggs on renesting |
1990 only |
Andres, 1996, 1997 |
||
|
Nesting disrupted on oiled island as compared to unoiled island |
Recovery by 1993 |
Sharp et al., 1996 |
||
|
Harlequin Duck |
Shallow sub-tidal invertebrates |
Lack of recovery in numbers present in oiled areas |
Not until at least 1991 |
Klosiewski and Laing, 1994; Day et al., 1995, 1997; Irons et al., 2000 |
|
Decline in winter counts in western (oiled) vs. eastern (unoiled) Sound |
Through 1997-1998 |
Rosenberg and Petrula, 1998; Rosenberg, 1999 |
||
|
P450 1A induction |
Tested for in 1998 |
|
||
|
Barrow’s Goldeneye |
Shallow sub-tidal invertebrates, mussels |
Declining numbers in oiled vs. unoiled areas |
Through 1998 |
Holland-Bartels et al., 1999; Irons et al., 2000 |
|
Elevated P450 1A levels |
1996-1997 |
Trust et al., 2000 |
||
|
Cormorants, black-legged kittiwakes, murres, pigeon guillemot, mergansers, and loon |
Shallow subtidal fishes |
Continued depression in census counts along oiled shores vs. expectation |
Through 1998 (except for 1993 for loons) |
Irons et al., 2000 |
oiled, less than 25 percent of the average of the <10 adult terns found dead each year between 1991 and 1994 were oiled (Symens and Alsuhaibany, 1996). Oiling apparently occurred when terns encountered tar balls while plunge diving in pursuit of small fish. Small spots of oil transferred from adults to eggs caused no decline in hatching success (Symens and Alsuhaibany, 1996). There was evidence that the oil spilled during the Persian Gulf War had an indirect effect on tern reproductive success. The clutch sizes of the White-cheeked Tern (Sterna repressa) were reduced in 1992 and 1993, and the breeding success (chicks per pair) of Lesser Crested Terns (Sterna bengalensis), White-cheeked Terns and Bridled Terns (Sterna anaethetus) were less in 1992 and 1993 than those in either 1991 or 1994. This decline in 1992 and 1993 was apparently caused by a lack of small fish on which to forage. Exposure to the massive spills during the Persian Gulf War significantly reduced the abundance of fish eggs and larvae (McCain and Hassan, 1993); Symens and Alsuhaibany (1996) suggest that this mortality of forage fishes resulted in a scarcity of fish prey for the terns in 1992 and 1993. In those two years, the diets of the terns shifted, and one of the larger species, the Swift Tern (Sterna bergii), resorted to eating the chicks of the smaller White-cheeked Terns, and stealing food from Lesser Crested Terns returning to their colonies. Although this example shows effects of an oil spill on the reproductive ecology of marine birds up to two years after the spill, Symens and Alsuhaibany (1996) suggested that this two-year interruption would have a negligible effect on the population biology of these long-lived seabirds.
Among marine mammals, river otters (Lutra lutra) in the British Isles and Alaska, and sea otters (Enhydra lutris) and harbor seals (Phoca vitulina) in Prince William Sound, Alaska, all showed short-term population declines after oiling of their inshore marine habitats (Baker et al., 1981; Spraker et al., 1994; Monson et al., 2000; Peterson, 2001). For some species, these effects may have persisted over ten years (e.g., sea otters, Monson et al., 2000). However, in the case of the Exxon Valdez oil spill in Prince William Sound, Alaska, considerable controversy remains concerning the magnitude of the initial losses and the duration of population-level effects (e.g., Garshelis and Johnson, 1995; Hoover-Miller et al., 2001). These uncertainties stem from the lack of sufficient pre-spill data to characterize the population status of these species and difficulties in obtaining adequate post-spill data to distinguish between local movements of animals and area-wide population effects.
Chronic or delayed responses of marine bird and mammal populations to petroleum hydrocarbons in the sea can occur because of continued ingestion of oil via contaminated prey, or because of failure of prey populations to recover subsequent to injury. In the 10 years since the Exxon Valdez oil spill, several species of birds and marine mammals have demonstrated indirect or delayed responses to the spill. These responses were found in sea ducks and shorebirds, species

PHOTO 23 Spills from coastal facilities such as marine terminals and tank farms make up nearly one quarter of the spills (by volume) associated with the transportation of petroleum. (Photo courtesy of Environmental Research Consulting.)
that forage primarily on intertidal and shallow subtidal invertebrates, as well as in several species that forage on small fish caught in inshore waters (Peterson, 2001). Seabird responses were of three types: reduced use of oiled habitats as compared to use of unoiled habitats for up to nine years post-spill, reduced numbers post-spill as compared to pre-spill, and lower growth and delayed fledging in a species that fed contaminated mussels to its young. Species of ducks with populations that continued to decline post-spill (Harlequin Ducks and Barrow’s Goldeneye) both feed on shallow-water invertebrates, including mussels, and both showed elevated levels of the enzyme cytochrome P450 1A, indicating continued ingestion of petroleum hydrocarbons (Trust et al., 2000).
Marine mammal populations that may have exhibited prolonged effects subsequent to an oil spill include sea otter and harbor seal populations in Prince William Sound (Garshelis and Johnson, 1995; Hoover-Miller et al., 2001; Peterson, 2001). In some regions of the Prince William Sound, sea otter abundance had not recovered as of 1998 (Dean et al., 2000), whereas in other areas, sea otter numbers were as high or
higher than prespill counts (Garshelis and Johnson, 1993). Other results indicating damage to otters that persisted for more than several years include the finding that the over-wintering mortality of juveniles was higher at oiled as compared to unoiled sites in the winters of 1990-91 and 1992-93 (Monson et al., 2000), and that mortality of prime-aged sea otters was higher than normal after the spill (Monson et al., 2000). The presumed cause of the failure of the sea otter population to recover is continued contamination via their prey. Some of the measured increases or decreases in sea otter populations may have resulted from local movements of otters or other behavioral or demographic phenomena, and assessment of long-term population effects of the oil spill to sea otters remains difficult and controversial. In the case of harbor seals, there is some controversy as to whether they have declined in Prince William Sound (Peterson, 2001), or whether the apparent declines are the result of movement of seals that were avoiding or moving away from oil contaminated haulout (Hoover-Miller et al., 2001). Harbor seals were declining before the spill, and if there has been a continued decline, it may be a continuation of the past decline, or it may be the result of a decrease in the abundance of near-shore fish prey, but the available evidence is inconclusive (Peterson 2001). What is important here is that sub-lethal effects can be identified in marine birds and mammals for several years after the acute effects of a spill have passed.
In summary, it has proven difficult, except in a few notable exceptions, to demonstrate population-level effects of oil spills for either marine birds or marine mammals based on censuses. Although many individuals may be killed, it is frequently difficult to demonstrate commensurate declines in local or regional populations, or to show significant demographic effects, because the power of present techniques to detect change is weak. Without more complete knowledge of the structure of populations of marine birds and mammals and their demography, it may remain beyond our reach to assign damage or recovery except in cases where ongoing monitoring provides an adequate basis for comparative studies. The temporal and spatial variability found in ecosystems makes even the most sophisticated statistical approaches open to individual interpretation and controversy (Wiens and Parker, 1995; Day et al., 1997; Irons et al., 2000; Peterson, 2001). As both Nisbet (1994) and Piatt and Anderson (1996) point out, even though we often cannot demonstrate statistically that oil pollution has caused population-level effects in marine birds, given what we know of their life history patterns, including long life spans, low adult mortality, and low rates of reproduction, it is risky to assume that increased rates of mortality are without population-level effect. Total population size, including breeders and non-breeders, has not been determined for any seabird species, and thus it is impossible to determine directly whether pollution is affecting global populations (Nisbet, 1994). Only if the effects of oil pollution are compensatory and not additive to other, natural, causes of mortality can we hope that large removals of individuals are without population-level consequences. The same arguments should hold true for marine mammals.
Modeling the Impacts of Oil
Modeling has been used in many ways to assess the impacts of oil spills on living resources and habitats:
-
To evaluate the impacts of an oil spill using a model, the fate of the oil must first be quantified. Historically, most oil fate models have focused on the trajectory and fate of oil on the water’s surface (e.g., see reviews by Huang and Monastero, 1982; Spaulding, 1995; ASCE, 1996; Reed et al., 1999). Surface trajectory models are used to calculate the intersection of the trajectory path with maps of resources of concern including biota and habitats (e.g., Samuels and Lanfear, 1982; Seip et al., 1991). This approach is appropriate for quantification of impacts to birds, mammals, and shoreline habitats. Bird and mammal impacts have also been modeled by backtracking from locations where oiled animals have stranded on beaches, accounting for losses at sea (Seip et al., 1991; Ford et al., 1996).
-
To evaluate the effects of subsurface oil, subsurface oil must be explicitly tracked (French et al., 1996, 1999). A prime example is the North Cape oil spill of January 1996 that occurred during a severe winter storm (French, 1998a,b,c) (Case History, 4-1).
-
To evaluate impacts on aquatic biota, oil entrainment and dissolution into the water must be simulated. The relevant concentrations are of those components that might have an impact on aquatic organisms and habitats. The concentrations of main concern are the soluble and volatile lower- and intermediate-molecular-weight compounds that are acutely toxic to biota, primarily monoaromatic hydrocarbons (MAHs) and polycyclic aromatic hydrocarbons (PAH) (French, 2000; French McCay, 2001). Other compounds in oil may also contribute to toxicity. Submerged oil and oil smothering on shorelines are also important exposure pathways. Thus, the model must consider the entire fate of the oil and all its components over time, both on and in the water, and in sediments.
Modeling Impacts of Oil on Shoreline Habitats
Oil trajectory models have been used to determine where oil will intersect the shoreline and impacts are presumed if oil reaches a location. The problem with this approach is that impacts are related to the amount and weathering state of the oil. Thus, this simply identifies areas that might be exposed to some amount of oil, but does not quantify an impact.
Computer models of the physical fates, biological effects, and economic damages resulting from releases of oil and
other hazardous materials were developed for use in Natural Resource Damage Assessments (NRDA) under the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA) and the Oil Pollution Act of 1990 (OPA) (French and French, 1989; Reed et al., 1989; French and Reed, 1991; French, 1991; French et al., 1996; Reed et al., 1996). There are presently two models in regulation: the Natural Resource Damage Assessment Model for Coastal and Marine Environments (NRDAM/CME, French et al., 1996) and the Natural Resource Damage Assessment Model for Great Lakes Environments (NRDAM/GLE, Reed et al., 1996).
The NRDA models simulate spreading and shoreline stranding of oil. The amount of oil remaining on the shoreline is a function of oil viscosity and shoreline type. Stranded oil is assumed to be removed by waves and other physical processes at a constant rate. The holding capacities and removal rates are based on data collected after spills. Impacts on intertidal habitats, such as salt marshes, mangroves, macroalgal beds and coral reefs, are assumed to be a 100 percent loss if a threshold thickness (dose) is exceeded for any interval of time. The threshold is based on observational data for salt marsh impacts (French et al., 1996).
Modeling Impacts of Surface Oil to Wildlife
Wildlife (birds, mammals, and reptiles) are primarily impacted by direct exposure to floating oil, ingestion of contaminated prey or depletion of food resources. Impacts via a loss of food resources are included in the NRDAM/CME (French et al., 1996), under the assumption that wildlife are food-limited and a proportionate loss of wildlife biomass would result from lost prey production because of a spill. Models used to assess impacts of oil on wildlife populations are summarized in Table 5-2.
In evaluating the wildlife impacts of the Exxon Valdez, Ford et al. (1996) used experimental bird drift and loss rates to estimate the percent of oiled animals that would reach a beach and be stranded. Oiled and dead birds are scavenged and may sink at sea. The percent stranded is related to the trajectory of the carcasses. Ford et al. (1996) used reverse trajectory modeling to determine where beached animals originated, and the percent loss estimates from the drift experiments, to estimate a total kill.
In the NRDAM/CME (French and French, 1989; French et al., 1996), wildlife, oiled and killed, are a function of area swept by surface oil, dosage, and vulnerability. Wildlife are assumed to move randomly within the habitats they normally use for foraging. The dose is estimated from the oil thickness, path length through the oil, and the width of a (swimming) bird. A portion of wildlife in the area swept by the slick is assumed to die based on the probability of encounter with the slick, dosage, and mortality once oiled. Estimates for these probabilities are derived from information on behavior and field observations of mortality after oil spills.
French and Rines (1997) performed hind-casts on 27 oil spills to validate the wildlife impact model. The results showed that the model is capable of hind-casting the oil trajectory and shoreline oiling, given (1) accurate observed wind data following the spill, and (2) a reasonable depiction of surface currents. Since winds and currents are the primary forcing variables on oil fate, obtaining accurate data on these is very important to the accuracy of any simulation. The accuracy of the impact model is primarily dependent on the accuracy of the wildlife abundance data for the time and location of the event. In the validation study, regional mean abundances from literature sources were assumed.
In nearly all cases, impact information for a spill consists primarily of counts of rescued or dead wildlife. Model validation is necessary to illustrate where the model predicts reasonable estimates of impacts on wildlife. Modeling results show that the wildlife impact algorithm in the model is valid when input data on abundance are accurate (French and Rines, 1997). In a few cases, the model estimated more birds killed than were observed. These cases were for species impacts not normally assessed or reported. Even in cases where large efforts were made to recover oiled wildlife, such as following the Exxon Valdez, it is well recognized that many oiled animals are lost at sea or scavenged and not counted directly as oiled. Small and less visible species and
TABLE 5-2 Models Used to Assess Impacts of Oil on Wildlife
|
Purpose |
Reference |
|
Sea birds—Oil spill trajectory model and oil vulnerability index |
Samuels and Lanfear, 1982 |
|
California sea otter—Sea otter movements and oil spill trajectory model |
Brody, 1988 |
|
Sea birds and marine mammals—Oil slick encounter and subsequent mortality |
Ford et al., 1982; Ford, 1985 |
|
Gray and bowhead whales—Oil spill impacts |
Reed et al., 1987a; Jayko et al., 1990 |
|
Fur seal model—Simulated population processes and mortality due to oiling |
Reed et al., 1987b; French et al., 1989; Reed et al., 1989 |
|
Sea birds—Estimate numbers oiled from strandings of oiled animals on beaches |
Seip et al., 1991; Ford, 1987; Ford et al., 1996 |
|
Exxon Valdez—Experimental bird drift and loss rates to estimate the percent of oiled animals that would reach a beach and be stranded. |
Ford et al., 1996 |
those that remain at sea will be the most under-counted. Thus, it is not possible to verify some of the model estimates of impacts. The model results point to where additional observations are needed to evaluate impacts to these less obvious species (French and Rines, 1997).
Modeling Impacts of Subsurface Oil on Aquatic Biota
Oil toxicity models have been developed to estimate water column toxicity after an oil spill (French, 2000; French McCay, 2001). As discussed above oil toxicity may be attributed to many different compounds. Exposure concentrations of each compound in the mixture, as well as their toxicities, must be estimated to quantify the toxicity of oil to water column organisms (French et al., 1996; Anderson et al., 1987; French, 2000; French McCay, 2001). Typically, for surface releases of fuel and crude oils, only the PAH are dissolved in sufficient quantity and remain in the water long enough for their toxic effects to be significant. The more turbulent the release (i.e., if it is during a storm or from a blowout or pipeline under pressure), the higher the relative concentrations of the more toxic PAH, and the higher the impacts to water column organisms. For a subsurface release deep in the water column or for a gasoline or other product spill where the MAHs and lower molecular weight aliphatics are significant fractions of the oil, all of these compounds may cause significant acute toxic effects (French, 2000; French McCay, 2001).
The biological model in the NRDAM/CME (French et al., 1996), and the updated version of that model, SIMAP (French et al., 1999), which includes the oil toxicity model described above, estimates acute toxic response of aquatic biota. (Because of the widespread use of SIMAP, the approach it represents is fairly widely known. Other, less familiar models may address the challenges of modeling oil spill fates and effects at least as well.) Fish and their eggs and larvae are affected by dissolved contaminant concentration (in the water or sediment). Mortality is calculated using LC50, corrected for temperature and duration of exposure, and assuming a log-normal relationship between percent mortality and dissolved concentration. Movements of biota, either active or by current transport, are accounted for in determining concentration and duration of exposure. Organisms killed are integrated over space and time by habitat type to calculate a total kill. Lost production of plants and animals at the base of the food chain is also computed. Lost production of fish, shellfish, birds, and mammals due to reduction or contamination of food supply is estimated using a simple food web model (French et al., 1996). In addition to the direct kill and food-web losses of eggs and larvae, young-of-the-year fish may be lost via habitat disruption. This is included in the model for wetland and other nursery habitats destroyed by lethal concentrations or oiling. Losses are related to the habitat loss. Thus, recovery of spawning and nursery habitat in wetlands follows recovery of plant biomass and production (French et al., 1996).
Applicability of Modeling
Success of a model simulation is dependent on both the algorithms and the accuracy of the input data. Results of the validation exercises have shown the algorithms provide reasonably accurate results. The most important input data in determining accuracy of results are winds, currents, and biological abundance of the affected species. These data inputs need to be site- and event-specific for an accurate model estimate of impacts of a spill. Thus, the limitations of modeling are largely driven by the availability of these input data (French and Rines, 1997; French, 1998a,b,c).
While oiled wildlife and shoreline habitats may be observed and quantified in the field after a spill, it is difficult and often infeasible to measure directly impacts to aquatic biota in the water column. To characterize fully the impact by field sampling, water and sediment samples would be needed at frequent time intervals over the first few weeks after the release (and especially in the first 24-48 hours), and with enough spatial coverage to characterize the extent of contamination. In addition, comprehensive sampling of each of the species affected is needed in the exposed and unaffected areas. Because marine organisms are patchy in their distribution, large numbers of stations and samples within stations are needed to map abundance accurately. Such extensive sampling of all (or even selected) species affected is often not feasible, given the rapidity at which the evidence disappears (by scavenging of killed organisms and by migration of animals into the impacted area). Modeling may be used in combination with field sampling to quantify oil fate and impacts (French, 1998a,b,c).
The weaknesses of modeling are related to our incomplete scientific knowledge of the impacts of oil spills. Because oil spills are infrequent and unplanned events, which have most of their effects on organisms over a very short time, it is very difficult to obtain quantitative information with which to develop and verify models. The implementation of NRDA regulations under OPA has facilitated the gathering of quantitative data on spills, and provided opportunities for improving and verifying models.
Effects on Communities and Habitats
Effects on communities will be discussed from the standpoint of habitat types in which they occur. Two broad habitat categories are considered: (1) biogenically-structured habitats, and (2) inorganic substrates, such as intertidal rock, sand, and subtidal muds.
Biogenically-structured Communities
Long-term and chronic effects are likely to be expressed as residual damage from oil spills to biogenically-structured communities, such as coastal wetlands, reefs, and vegetation beds. Effects of oiling on biogenically-structured habitats may result from acute damage on habitats such as coral
reefs, live-bottom habitats, mangrove swamps, salt marshes, oyster reefs, and seagrass and kelp beds. Here the concern is that even though oil may not persist following an oil spill, the time required for recovery of damaged populations of organisms that provide the physical structure of the habitat may be many years. In some biogenic habitats, such as mangroves and mussel beds, oil can sometimes penetrate into the lower-energy sediments associated with these habitats and have potentially long-lasting effects. Biological communities that are integrally dependent on physical structures, which are themselves formed by living organisms, may be inherently slow to recover from severe impacts. In some cases where the structure-forming species actually stabilize the habitat, it is conceivable that permanent modification of that habitat could result from an acute incident that kills the key structuring species. Recovery from the effects of an oil spill in a community in which organisms provide the physical structure of the habitat depends on structural damage incurred during cleanup operations, the persistence of contamination, and the inherent ability of the community to recover.
Corals
The 1985 Oil in the Sea report focused extensively on the effects of oil spills on tropical habitats including coral reef ecosystems and mangroves. At the time, there were multiple field studies documenting effects on corals including decreased feeding response, coral colonization and premature expulsion of coral planula. The end result was coral tissue death, coral bleaching, and the loss of an entire year’s larval recruitment class. One lament of the 1985 Oil in the Sea report was the lack of information on concentrations and composition of oil in the water that prevented comparison of spill effects between coral sites.
Since 1985, a wealth of field and laboratory studies have increased our knowledge of the effects of oil on coral reefs. The 1986 Galeta spill into Bahia las Minas, Panama is arguably the most studied oil spill in the tropics. Large amounts of medium weight crude oil (see Box 5-5) spilled into mangroves, seagrass beds, and coral reefs on the Caribbean coast of Panama (Burns and Knap, 1989; Jackson et al., 1989; Guzman and Holst, 1993; Guzman et al., 1994; Box 5-5). Another notable tropical oil spill was the consequence of the Persian Gulf War in 1991 where 1,770,000 tonnes of oil were spilled into the marine environment (Price and Robinson, 1993). Despite a 120-fold difference in total volume of oil spilled, the long-term effects (greater than five years) of oil in Panama were more pronounced and detrimental due likely to repeat inoculation of oil from the surrounding mangroves into the coral ecosystem. In contrast, no long-lasting effects to the coral reef ecosystem were reported from the Persian Gulf War spills (Price and Robinson, 1993).
Corals located in intertidal reef flats are exposed to oil slicks and are more susceptible to damage and death than corals in subtidal reefs. Coral located subtidally or in areas with high wave action are not directly exposed to the marine surface layer where oil slicks can coat them. Instead, only the water-soluble fraction of oil generally affects submerged coral. The water-soluble fraction is primarily composed of benzene, toluene, ethylbenzene, and xylene, which can rapidly evaporate to the atmosphere. One laboratory study found that 15 percent of the benzene and toluene and 80 percent of the xylene were lost after 24 hours of exposure to the atmosphere (Michel and Fitt, 1984).
Acute and chronic exposures of oil on coral have been studied in the laboratory and field (reviewed by Peters et al, 1997). The symbiotic algae associated with coral are affected after 24 hours of exposure to the water-soluble fraction of oil (benzene, toluene, ethylbenzene, and xylene; see Box 5-2). Photosynthetic capacity can recover fully if there is only short-term exposure to oil (less than 72 hours), and no adverse affects were measured for exposure of less than one hour (Michel and Fitt, 1984). Mixtures of dispersants and oil are more toxic to coral than just the oil (Peters et al, 1997). Branching coral (e.g., Acropora palmata) is considered more sensitive to oil exposure than massive coral (e.g. Montastre, Bak, 1987).
Mussels
Mussels often occur in dense intertidal aggregations and their interlocking byssal threads provide a low-energy habitat with protection from the rigors of breaking waves above the bed. The interstices of mussel beds are micro-habitats rich in intertidal life (Ricketts and Calvin, 1948). As with other bivalves, mussels effectively accumulate high concentrations of a variety of contaminants including petroleum hydrocarbons from the water and their food.
Mussels can be affected by the accumulation of petroleum compounds. Low concentrations of petroleum hydrocarbons can interfere with cellular and physiological processes like cellular immunity (McCormick-Ray, 1987; Dyrynda et al., 1997), lysosome characteristics (Pelletier et al., 1991), byssus attachment (Linden et al., 1980), growth (Widdows et al., 1987, 1989), and ability to tolerate air exposure (Thomas et al., 1999). Thus, there is a basis for expecting population impact under some conditions. Oil exposure or vigorous cleanup of the intertidal zone results in damage to these beds, and it may take years for the beds to re-establish their former richness. At the same time mussel beds effectively trap oil and under some circumstances allow the oil to persist for years after a spill. For example, after a 7,000 tonnes spill into a tropical estuary with mangrove habitats, damage to mussels was apparent one year after the spill (Garrity and Levings, 1993). A spill of Bunker C fuel oil, spilled from a collision of two tankers in San Francisco Bay in 1971, resulted initially in smothering of intertidal invertebrates. Five years after the spill, there was no evidence of long-lasting effects of the oil spill on recruitment patterns of intertidal invertebrates in high energy environments (Chan, 1977).
|
BOX 5-5 Galeta Tank Spill, Bahia Las Minas, Panama On April 27, 1986 a storage tank at a refinery at Bahia Las Minas, Panama ruptured, releasing an estimated 14,300 tonnes (4,200,000 gallons) of a blend of 70 percent Venezuelan and 30 percent Mexican crude oil (API gravity = 27) into the sea (Keller and Jackson, 1993). Onshore winds kept the oil trapped in deep bays near the release site for six days, but shifting winds and rainfall runoff caused the slicks to spread to adjacent areas. Dispersants were applied (21,000 L) starting nine days after the spill. Eventual impacts resulting from dispersant application could not be separated out from other factors. About 82 km of coastline were heavily oiled, including more than 1,000 ha of mangrove forests, intertidal reef flats, and subtidal flats and seagrass beds. These habitats received extremely heavy dosing of a medium-heavy crude oil. There was some shoreline cleanup on beaches and rocky shores, and channels were dug into mangroves in an effort to increase oil flushing from interior areas. Large expanses of mangrove forest were inaccessible, however, and no oil removal was conducted there. Approximately 69 ha of mangrove forest (dominated by the red mangrove, Rhizophora mangle) were killed; sublethal impacts affected approximately 308 ha (Duke et al., 1997). The spill affected a biological preserve at the Smithsonian Tropical Research Institute, where biological baseline studies had been conducted since 1970, sixteen years pre-spill. Because of these extensive baseline data, the U.S. Minerals Management Service funded studies of the fate and effect of the oil in this tropical ecosystem for five years (Keller and Jackson, 1993); many important findings have resulted. Oil in surficial soils degraded within six months; however, pools of oil trapped in mangrove soils showed little degradation, and chronic re-oiling of adjacent areas occurred for at least five years (Burns et al., 1994). Oil concentrations in bivalves were 5-15 times background five years post-spill, with seasonal highs associated with periods of oil remobilization. The mangrove fringe along the outer coast, lagoons, and tidal creeks was frequently re-oiled, resulting in high prop root mortality and severe impacts on attached populations and communities that was most severe five years later (Garrity et al., 1994). Where the oil floated over the reef flats, there was little mortality. The spill occurred, however, during a period of low tides, and oil was trapped on the seaward borders of the reef flat. Wherever the reef flat was in direct contact with the oil, there was extensive mortality, and the effects persisted for over five years for sessile species (Cubit and Connor, 1993). Mortality to intertidal communities and organisms was not a widespread, toxic effect of oil mixed in water, because the oil had weathered prior to stranding. A primary factor in the recovery rate for sessile biota on reef flats was also how much of the plants and animals survived the spill and cleanup, and then vegetatively spread or washed in from nearby habitats afterwards—an important factor in cleanup design. In most areas, subtidal seagrass beds (Thalassia) showed sublethal impacts but recovered within eight months. The exception was the shoreward margins of the beds that died off in a band 20-90 cm wide. The fauna of oiled seagrass beds remained highly altered for 2-3 years post-spill (Marshall et al., 1993). As mangrove forest and seagrass beds died back, oiled sediments were exposed and eroded, providing a chronic source of oiled sediment for re-deposition in adjacent habitats. Subtidal reef corals (Diplora clivosa, Porites asteroides, and Siderastrea siderea) were affected to water depths of 6m, with a strong correlation between effects and oil concentrations in subtidal sediments (Guzman et al., 1993). Affected coral populations had not started recovery after five years, as demonstrated by reduced sexual reproduction and larval recruitment, reduced populations of grazing fish, and very low recruitment of most formerly dominant coral species. Minimal estimates of the time required for equivalent populations to become established were10-20 years (Guzman et al. 1993). Studies among the habitats showed consistent patterns in recovery rates; that is, species with high reproductive potential, planktonic stages, and immigration or wave transport of fragments of surviving sessile species from adjacent habitats recovered more quickly, whereas those with low dispersal abilities and low reproductive potentials recovered more slowly. Habitats where heavily oiled sediments persisted or where they were exposed to chronic re-oiling also recovered slowly. This spill provided some of the best evidence of the complexity of the tradeoffs of natural recovery versus the impacts of cleanup in sensitive environments. |
In the Exxon Valdez spill, mussel beds were contaminated with oil, and it was decided not to disturb the mussel beds during cleanup operations. This decision was based partly on the food value of mussels to sea ducks, shorebirds, and sea otters (A. Weiner, Alaska Department of Environmental Conservation, personal communication). As a consequence, oil persisted in these less energetic habitats within the intertidal zone. In these environments, oil was retained in the sediments underneath the mussel beds in an unweathered state for many years after the spill and would be expected to continue to persist (Babcock et al., 1997; Carls et al., 2001). There were at least 50 such mussel beds identified in western Prince William Sound, and it is likely that oil will only slowly decrease in these environments without intervention. Since it appears that some species of fish, sea birds and sea otters are still exposed to low levels of oil in western Prince Williams Sound 11 years after the spill, and some of the highest remaining concentrations of oil are found in mussel beds, these beds might be contributing to the continuing contamination of higher-trophic-level species (e.g., Duffy et al., 1996). Boehm et al. (1996) examined the distribution of PAH in mussels collected at various sites within Prince William Sound and observed wide spatial variation in PAH concentrations in mussels from different habitats and predicted that concentrations would fall to background levels within a few years. There is no consensus yet on which choice is best: immediate cleanup with destruction of mussel beds that may take many years to re-establish, or leaving them alone to
naturally weather and risk uncertain effects at higher trophic levels over long periods.
Subtidal Vegetation
Studies of oil effects on sea grass (e.g., Thalassia sp., Halophila sp., Zostera sp.) are limited to short- and long-term effects from particular oil spills. Little evaluation of chronic or acute damage from laboratory studies exists. Eelgrass meadows in the tidal zone are generally directly exposed to oil and die-off in the first year of an oil spill. In the subtidal areas, damage is limited to dying leaves. After the initial mortality in the first year, long-term effects of eelgrass are mixed. Long term (> 5 years) effects at the Exxon Valdez spill were inferred by decreased mean density of shoots and flowering shoots in the oiled area. Biomass, however, was the same between oiled and non-oiled areas (Dean et al., 1998). In the Persian Gulf War spills, no difference between oiled or non-oiled seagrass meadows could be detected after one year (Kenworthy et al., 1993). Reasons for resilience of eelgrass are speculative but are likely a result of life history patterns. Some species such as Zostera marina in Alaska propagate by lateral root growth, not by producing germinating seeds, and are less susceptible to oil in the sediment. Environmental parameters such as time of year of the spill relative to germination may also be important, but remains unexplored.
Subtidal kelps are apparently not particularly vulnerable to petroleum hydrocarbons. Around shallow-water natural petroleum seeps, the large kelp Macrocyctis pyrifera (in the sporophyte stage) does not accumulate petroleum hydrocarbons to very high concentrations (Straughn, 1976), and these kelp beds are well-developed despite continual inundation with surface oil. Laboratory and field studies indicate that gametophytes of this species may be more sensitive than mature plants (Reed et al., 1994). Following the Exxon Valdez spill, some large subtidal kelps had different size distributions in oiled areas compared to non-oiled areas, but it is uncertain if this was a spill effect (Dean et al., 1996). In the Nakhodka oil spill in Japan, no effects on subtidal kelp were reported from field surveys (Hayashi et al., 2000).
Like mussels, which retain oil in their byssus threads, kelp holdfasts are also low-energy environments that can retain oil for years after a spill. For example, a small spill of diesel oil at Macquarie Island in the sub-Antarctic resulted in contamination of holdfasts of kelp that lasted for at least five years and inhibited the full recovery of the kelp-associated invertebrate community from the effects of the oil (Smith and Simpson, 1998).
Intertidal Vegetation
Estuaries in many areas of the world are susceptible to exposure by oil because of the location of petrochemical industries in the coastal zone and transport of oil products, either by vessel or via pipelines, that either pass closely by or through estuaries. Spills or operational discharges can potentially cause damage to intertidal vegetated habitats, including salt marshes and mangroves. These types of vegetation may occur separately or in combination with each other. Oil spills are known to cause severe and long-term damage to mangrove and salt marsh ecosystems (e.g., Teal et al., 1992; Burns et al., 1993; Duke et al., 1997; Mille et al., 1998). The vegetation and the structure that salt marshes and mangroves provide may be affected, sediments may be contaminated, and ecosystem functions may be impaired with regard to utilization by organisms, including important fisheries species, geochemical cycling, and stabilization of sediments. The rate of degradation of the oil in the sediments is influenced by the sediment type, oxygen content and bacterial community of the sediment, availability and level of nutrients in the sediments and at the oil/sediment interface, and the depth to which the oil has penetrated. Oiling effects may be limited or negligible and short-term when the oil exposure is minimal, the vegetative structure is not impacted (either by the oiling or various cleanup procedures), and residual oil levels are minimal or rapidly weathered. Oiling effects are particularly great when oil coats the vegetation or is incorporated deeply in the sediments beneath the vegetation.
Salt Marshes
The negative effects of oil on marsh vegetation are dependent on the type of oil (constituents, viscosity), the amount of oil, the amount of plant coverage, the depth of penetration of the oil into the marsh sediments, the season, and the type and effectiveness of any cleanup or remedial actions (reviewed by Webb, 1996; Pezeshki et al., 2000) (Table 5-3). Lighter and more refined oils such as No. 2 fuel oil are extremely toxic to smooth cordgrass (Spartina alterniflora). Crude oils and heavy fuel oils are generally the same in overall effects on plants, i.e., little toxic effects to plants occur unless the oil penetrates into the sediments and chronic toxicity to the plants occurs as roots are continuously exposed to oil. The aboveground portion of smooth cordgrass is generally killed only when oil covers all plant surfaces. Regrowth from roots will occur soon after death of the aboveground portions of the plants. If sediments are heavily contaminated by oil, then production of new shoots is problematic and plant recovery is diminished. Oil spills are more damaging to smooth cordgrass during the spring growing season than in fall when the plants are beginning their dormancy. Regrowth the following spring after a fall oil spill does not appear to be greatly reduced. Oil coverage of 1 to 2 l/m2 was generally dispersed with the tides, leaving little to penetrate sediments and cause long-term damage. When high levels of crude and heavy fuel oils accumulate in the sediments or remain within the marsh for long periods, the result is complete death of large areas of smooth cordgrass.
TABLE 5-3 Data from Field Studies on Impacts to Marsh Vegetation from Experimental, Single-dose Oiling (from Hoff et al., 1996)
|
Location |
Vegetation |
Oil Type |
Time of Oiling |
Cleanup |
Recovery Time |
|
Galveston Bay, Tex.a |
Spartina alterniflora |
Arabian crude |
Nov. |
none |
1 yr |
|
Lybian crude |
1 yr |
||||
|
No. 6 fuel |
1 yr |
||||
|
No. 2 fuel |
2 yr |
||||
|
Louisianab |
Spartina alterniflora |
S. La. crude |
June |
none |
3 mos |
|
flushing |
3 mos |
||||
|
cutting |
2-5 yr |
||||
|
York River, Va.c |
Spartina alterniflora |
S. La. crude fresh/weathered |
Sept. |
none |
|
|
St. Louis Bay, Miss.d |
Juncus roemerianus |
Empire Mix crude |
March |
none |
>1 yr |
|
Saudi crude |
1-3yr |
||||
|
aWebb et al., 1985 bDeLaune et al., 1984 cBender et al., 1977 dde la Cruz et al., 1981 |
|||||
In a series of experimentally oiled salt marsh plots, cleanup techniques implemented 18 to 24 h after the application were not effective in removing oil that had penetrated the surface (Kiesling et al., 1988). When oil remained on the sediment surface, flushing techniques were most effective at removal, reducing levels of oil by 73 to 83 percent. When dispersants were added to the water during flushing, oil removal was only slightly enhanced. Clipping of vegetation followed by sorbent pad application to sediments was moderately effective, reducing added oil by 36 to 44 percent. Burning had a negative effect on oil removal; oil increased in sediments of burned plots compared to controls. Consideration should be given to natural microbial breakdown of the oil that can be facilitated with fertilizers. When large amounts of oil are present on a marsh, damage from trampling during cleanup can be severe, causing damage to plants and forcing oil into the sediments (Webb, 1993).
Densities of animals in salt marshes may be reduced by acute, short-term toxic effects of crude oil that sharply increase mortality rates (Anderson et al., 1974; Sanders et al., 1980; McDonald et al., 1991; Nance, 1991; Widbom and Oviatt, 1994), or cause avoidance by mobile organisms (Moles et al., 1994). Oil may persist in marsh sediments for many years (Teal and Howarth, 1984; DeLaune et al., 1990; Teal et al., 1992) and may continue to affect habitat use. Populations of opportunistic infaunal organisms, such as capitellid and spionid polychaetes and nematodes, are often enhanced in oiled sediments if the concentrations are not high enough to be toxic (DeLaune et al., 1984; Rozas et al., 2000). Many of these organisms in highly urbanized estuaries and in estuaries near petrochemical installations or petroleum production facilities may be acclimated to hydrocarbons (Smith et al., 1984; Carman et al., 1995).
Chronic contamination was studied in marsh areas of upper Galveston Bay, Texas that receive episodic oil from spills in the San Jacinto River and the Houston Ship Channel (Rozas et al., 2000). Marsh sediments were contaminated with low levels of petroleum hydrocarbons, but there were few statistically significant negative relationships between animal density (fish and decapod crustaceans) and hydrocarbon concentration (Rozas et al., 2000). Further, hydrocarbon concentration was not important among the environmental variables measured in explaining animal densities. The conclusions of Rozas et al. (2000) were that background levels of weathered oil in marsh sediments did not affect habitat use by most estuarine organisms; however, they noted that the longer-term effects of continued exposure that might result in chronic, sublethal effects were not known. The background levels found in the Galveston Bay marshes were similar to those found in other highly urbanized estuaries (Overton et al., 1986; Bomboi and Hernandez, 1991). The low concentrations of weathered petroleum hydrocarbons in the Galveston Bay marsh study were one to two orders of magnitude lower than the average value of 2-5 mg/g (produced water versus oil) that Nance (1991) reported was needed to depress populations of benthic organisms in a small bayou connected to the Galveston Bay estuary. Even though oil may initially reduce the use of intertidal habitats by aquatic organisms (Sanders, 1978; Burns and Teal, 1979; Maccarone and Brzorad, 1995), habitat use may return to normal levels after the oil has undergone sufficient weathering (Barber et al., 1995).
One of the most detailed post-spill studies was carried out on the long-term effects of No. 2 fuel oil spilled in Buzzards Bay, Massachusetts that affected a 8-km stretch of salt marsh shoreline (Sanders et al., 1980). The oil had its greatest effect, and persisted the longest, in the Wild Harbor marsh (versus a control in Sippewissett marsh 4 km to the south). Marsh grasses contaminated with oil died. Recovery of the Wild Harbor marsh was well along five years after the spill,
but probably not complete. The population of fiddler crabs in the Wild Harbor marsh was reduced relative to that in Sippewissett marsh for at least seven years (Krebs and Burns, 1977). Behavioral effects, abnormal burrow shapes, and reduced female-to-male ratios were seen in Wild Harbor. Crab density was negatively correlated with aromatic hydrocarbon concentrations within the marsh, as was the density of newly settled juveniles.
Other sources of oils that may directly affect salt marshes or mangroves are produced water discharges. Results from Louisiana estuaries indicate that discharges of produced waters directly onto salt marshes will kill the vegetation, but discharges into receiving waters do not affect the peripheral marsh vegetation (Boesch and Rabalais, 1989a). Within the Nueces Bay estuary of Texas, however, Caudle (1995) identified extensive marsh areas in the bay that were denuded of vegetation due to long-term exposure to produced water.
Documented recovery times (return to some precursor percent cover of vegetation, diversity, or height and biomass of plants) for oiled marshes range from a few weeks to decades (reviewed by Hoff, 1996). There are several well-studied marsh sites where recovery times ranged from five years to greater than 20 years, including two sites in Buzzard’s Bay. Massachusetts, the Miguasha spill in Quebec, the Metula spill in Chile, and the Amoco Cadiz in France (Table 5-4). The reasons for longer recovery times were related to the following characteristics: (1) northern, temperate, cold environments, (2) the high organic content of the peaty soils, (3) sheltered location, (4) heavy oiling, (5) spills of fuel oils (bunker C or No. 2 fuel), and (6) physical disturbance during response activities, particularly for the Amoco Cadiz.
In contrast, recovery times of three years or less have been documented for sites at several locations in the Gulf of Mexico: Neches River, Texas (Esso Bayway), Harbor Island, Texas pipeline, and a pipeline rupture in southeastern Louisiana (Table 5-5). These marshes exhibiting quicker recovery share the following characteristics: (1) warm climate, (2) more mineral-rich soils, (3) light to moderate oiling, (4) spills of light to medium crude oil, and (5) variety of cleanup methods that were less intrusive. In many instances, cleanup techniques delayed recovery time, from physical disruption of roots, flushing of soils, thus lowering the soil surface below levels where vegetation could re-establish, and activities that mix oil deeper into the marsh soils.
Mangroves
There are numerous documentations of the death, defoliation, genetic, and other damage to mangroves and their associated communities after exposure to oil (e.g., Proffitt et al., 1995). Damage to mangrove forests varies with the amount and toxicity of the spilled oil product(s) with or with
TABLE 5-4 Examples of Oil-impacted Marshes with Recovery Times of Five Years or More, Documented by Follow-up Studies (from Hoff et al., 1996).
|
Location |
Vegetation |
Oil Type |
Time of Oiling |
Cleanup |
Recovery Time |
|
Chile |
Salicornia ambigua |
Arabian crude |
Aug. 1974 |
none |
> 20 yrs |
|
Metulaa |
Suaeda argentinensi |
Bunker C |
|
|
|
|
Quebec |
Spartina alterniflora |
Bunker C |
Sept. 1974 |
sediment |
> 11 yrs |
|
Miguashab |
Spartina patens |
|
|
removal |
|
|
|
|
|
|
manual |
|
|
|
|
|
|
burning |
|
|
|
|
|
|
digging |
|
|
Brittany, France |
Salicornia |
Arabian light |
March 1978 |
sediment |
5 − > 8 yrs |
|
Amoco Cadizc |
Suaeda |
Iranian light |
|
removal |
|
|
|
Halimione |
Crude |
|
|
|
|
|
|
Bunker C |
|
|
|
|
|
|
No. 2 fuel |
|
|
|
|
West Falmouth, Mass. |
Spartina alterniflora |
No. 2 fuel |
Sept. 1969 |
? |
> 8 yrs |
|
Floridad |
Salicornia europaea |
|
|
|
|
|
|
Spartina patens |
|
|
|
|
|
Buzzard’s Bay, Mass. |
Spartina alterniflora |
|
Oct. 1974 |
? |
> 3 yrs |
|
Bouchard 65e |
Salicornia virginica |
|
|
|
|
|
aBaker et al., 1993 bVandermeulen and Jotcham, 1986 cBaca et al., 1987 dBurns and Teal, 1979; Teal et al., 1992 eHampson and Moul, 1978 |
|||||
TABLE 5-5 Examples of Oil-impacted Marshes with Recovery Times of 3 Years or Less, Documented by Follow-up Studies (from Hoff et al., 1996)
|
Location |
Vegetation |
Oil Type |
Time of Oiling |
Cleanup |
Recovery Time |
|
Hackensack estuary, N.J. |
Spartina alterniflora |
No. 6 fuel |
May 1976 |
none |
? |
|
Wellen tank farma |
|
|
|
cutting |
|
|
Galveston Bay, Tex. Bayou |
Spartina alterniflora |
light crude |
Jan. 1984 |
none |
8 mos – > 2-5 yrs |
|
pipelineb |
Juncus roemerianus |
|
|
sorbents |
|
|
|
|
|
|
flushing |
|
|
Harbor Island, Tex. |
Spartina alterniflora |
crude oil |
Oct. 1976 |
none |
6 mos − > 6 mos |
|
Am Petrofina pipelinec |
Avicennia germinans |
|
|
sorbents |
|
|
|
|
|
|
burning |
|
|
|
|
|
|
clipping |
|
|
Aransas River, |
Spartina alterniflora |
S. Texas light crude |
Jan. 1992 |
burning |
> 2 yrs |
|
Chiltipin Creekd |
|
|
|
|
|
|
Neches River, Tex. |
Spartina patens |
Arabian crude |
Jan. 1979 |
none |
7 mos |
|
Esso Baywaye |
|
|
|
sorbents |
|
|
|
|
|
|
flushing |
|
|
|
|
|
|
burning |
> 7 mos |
|
|
|
|
|
cutting |
|
|
Neches River, Tex. |
Spartina alterniflora |
light crude |
April 1993 |
none |
? |
|
Unocalf |
|
|
|
sorbents |
|
|
Nairn, La. |
Spartina patens |
Louisiana crude |
April 1985 |
flushing |
< 1-5 yrs |
|
Shell pipelineg |
Spartina alterniflora |
|
|
trampling |
|
|
|
Distichlis spicata |
|
|
|
|
|
aMattson et al., 1977 bAlexander and Webb, 1987 cHolt et al., 1978 dTunnell and Hicks, 1994 eMcCauley and Harrel, 1981; Meyers, 1981; Neff et al., 1981 fNOAA, 1993 gMendelssohn et al., 1990; Fischel et al., 1989 |
|||||
out dispersants (Getter et al., 1985), tidal height and range, oil residence time, and season of the oiling. Oiling effects on mangroves differ with life history state and may affect the growth forms of young trees (Getter, 1982; Devlin and Proffitt, 1996). A primary cause of death in oiled mangroves is reported to be the disruption of gas exchange when aerial roots are coated with oil and can no longer supply oxygen to root tissues below ground in hypoxic soils (Teas et al., 1993). Oil can also be taken up by the root system, translocated to leaves, accumulate in the stomata, thereby interrupting transpiration (Getter et al., 1981, 1985). Oil can also disrupt root membranes and allow lethal concentrations of salt to accumulate in mangrove tissues (Page et al., 1985).
Oiling of mangroves following spills can lead to the death of those plants and ultimately unstable habitats and sediment erosion (Nadeau and Berquist, 1977; Duke and Pinzon, 1993; Garrity et al., 1994). Following the death of large numbers of mangrove trees after the Galeta oil spill in Panama, many trees rotted and fell, seagrass rhizome mats disappeared, and sediments from these habitats eroded at rates up to several centimeters per day (Jackson et al., 1989; Keller and Jackson, 1993; Box 5-5). The eroded sediments and oil in various stages of degradation were deposited in neighboring habitats including seagrass beds and coral reefs, which had not been contaminated in the original spill. In many instances the residence times of oil in these deep mud habitats have stretched to decades, which prolongs ecosystem recovery.
The degree of impact to mangroves is a function of the oil type, spill volume, duration of re-oiling, extent of oil coverage on exposed roots, and degree of substrate oiling. Light, refined products can be acutely toxic, for example the jet fuel spill in Puerto Rico that killed 5.5 ha of adult trees (Ballou and Lewis, 1989). Heavier types of oil can lead to eventual death by smothering. Slicks passing though forests at high water often leave a band of oil at the water line, with minimal impacts to the trees. Greatest impacts occur where sediments are contaminated, such as along intertidal berms (Getter et al., 1981). Black mangroves are most sensitive to oil because they osmoregulate by passing materials through the roots and the vascular system, and then out of the leaves
through specialized glands on the leaf surface. When black mangroves are oiled, this osmoregulatory process facilitates the uptake of oil (Getter et al., 1985). Impacts can be spatially variable (healthy trees adjacent to dead trees) and delayed for years. Because of the potential for damage during cleanup and to the difficulty of access into mangrove forests, intrusive cleanup is considered only under very heavy oiling conditions (e.g., the Galeta spill in Panama).
Recovery of oiled mangroves depends on the initial and residual oil loading as well as damages resulting from cleanup efforts. Physical and chemical weathering of oil may be fairly rapid, occurring over a few months to a year, or gradual and long-term (Burns et al., 2000; Figure 5-6).
Two researchers have attempted to predict the rates of recovery of oiled mangrove habitats; each of these analyses is summarized in Table 5-6. The earliest effort to describe the phases of recovery of oiled mangroves was by Lewis (1981). He proposed the generalized response stages shown in Table 5-6, based on his experience at spills in Florida (T/V Howard Star) and Puerto Rico (T/V Zoe Colocotronis) and a synthesis of the literature at that time. Lamparelli et al. (1997) conducted a nine-year study of a crude oil spill site along a tidal channel in Brazil with a tidal range of 1-5 m. They measured leaf area and herbivory, tree density, basal area, and tree height for Rhizophora mangle, Laguncularia racemosa, and Avicennia schaueriana.
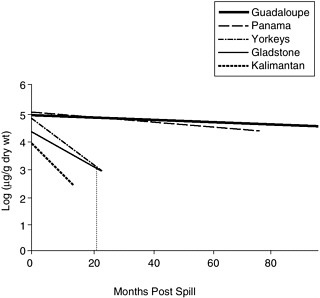
FIGURE 5-6 Loss of oil from Pacific region mangrove sediments. Dotted line indicates time predicted for beginning of recovery of benthic fauna at Gladstone (from Burns et al., 2000, Marine Pollution Bulletin).
TABLE 5-6 Proposed Stages of Impact and Recovery of Oiled Mangroves
|
Author |
Stage/Phase |
Response |
|
Lewis, 1981 |
Acute |
|
|
|
0-15 days |
Death of birds, turtles, fish, and invertebrates |
|
|
15-30 days |
Defoliation and death of small (<1m) mangroves; loss of aerial root community |
|
|
Chronic |
|
|
|
30 days−1 year |
Defoliation and death of medium (<3m) mangroves; tissues damage to aerial roots |
|
|
1-5 years |
Death of larger (>3m) mangroves; loss of oiled aerial roots and regrowth of new ones (sometime deformed); recolonization of oil-damaged areas by new seedlings |
|
|
1−10 years? |
Reduction in litter fall, reduced reproduction, and reduced survival of seedlings; death or reduced growth of young trees colonizing oiled site? increased insect damage? |
|
|
10−50 years? |
Complete recovery |
|
Lamparelli et al., 1997 |
Initial Effect 0-1 year |
Seedlings and saplings die; no structural alterations can be measured |
|
|
Structural Damage 1-4 years |
High mortality is observed, and the oil impact can be measured in terms of major structural alterations |
|
|
Stabilization 4-9 years |
No or few additional alterations to the structural parameters; sapling growth is observed |
|
|
Recovery > 9 years |
It is possible to measure improvements in the structural tree parameters; ecosystem may not recover fully to its original state |
Intertidal Shores
Rocky intertidal shores are quite susceptible to damage by oil spills depending on the amount and characteristics of the oil to which they are exposed. The 1985 Oil in the Sea report adequately characterized the damage of shorelines to spills and stressed the critical role of geomorphology in the recovery of these shorelines. We reiterate here the importance of the interactions of wave and tidal energy with shoreline geomorphology in determining recovery and punctuate this with lessons from more recent studies.
The persistence of the oil and the time to recovery are a function of the energetic fluxes where oil is deposited. If the initial oiling from a spill is an outer, exposed coast, and the rocky substrate is continuous without substantial low energy interstices, then oil will not persist long and recovery will be relative quick (e.g., see Chan, 1977 for an account of recovery on heavily oiled rocky coasts after the San Francisco Bay spill). If the shoreline is relatively sheltered or there are significant interstices where the oil can enter and be sheltered from the energetic fluxes of waves and tides, then oil will persist and recovery may take substantially longer.
The degree of impact and recovery from a spill on the rocky intertidal is very much a function of the circumstances of a spill. Not only is the aforementioned geological structure of the shoreline important, but the type of oil, the weather conditions following the spill, the thickness and lateral continuity of the slick, the time of year, and the recent history of disturbance of the biological communities are all important factors affecting severity of impact. One example of how low energy environments can retain oil and effects can persist is a southern ocean spill at Macquarie Island. In this spill, most intertidal components appear to have recovered within several years after the spill occurred, but in the holdfasts of kelp, which is an environment not unlike mussel beds, oil was retained for years and the fauna of this microhabitat has not recovered (Smith and Simpson, 1995).
By far the greatest acute injury to intertidal communities as a whole arises from direct contact with oil. Heavy deposits of oil essentially smother intertidal organisms. Toxicity also occurs from elevated concentrations of the soluble components of oil in small pools of water, in wetted surfaces and in the water of rising tides. The common organisms found on rocky intertidal shores of North America—Fucus, mussels, periwinkles, starfish, and barnacles—are all susceptible to the toxic effects of oil (Chan, 1977; Stekoll et al., 1993). Recovery of these components can be quite substantial within a year or two, or nearly complete. Subtle long-term effects are possible, however (Peterson, 2001). In the Exxon Valdez spill, the aggressive washing of the intertidal rock shores resulted in loss of a significant amount of silt from the rock interstices and the associated bivalve fauna has not been fully re-established and may not be until these sediments have been replenished by natural processes (Driskell et al., 1996).
The above caveats about the nature of the oil, the thickness and extent of the slick and the weather conditions determining impact also apply to softer substrates. Of particular note is the stranding of oil in protected, low-energy environments, such as bays and harbors. If oil arrives in one of these otherwise low-energy environments under storm conditions and gets worked into the substrate, it will likely be there for years and possibly decades. Two examples are the Florida spill in West Falmouth, Massachusetts in 1969 (Burns and Teal, 1979) and some areas affected by the Amoco Cadiz spill in France in 1978 (Dauvin and Gentil, 1990). It was clear at the time of the Oil in the Sea report (NRC, 1985), that the combinations of circumstances resulting in acute effects can also result in recovery times of years and even decades.
In the last 17 years there has been more focus on chronic contamination by PAH, the sensitivity of meiofauna, and indirect effects mediated by changes in predator-prey relationships, as well as by the direct toxic impacts. In particular, chronic exposure of fauna and potential effects have been studied over more realistic time scales and concentrations. Microcosm experiments where realistic doses of PAH are maintained in sediments to provide a chronic exposure regime have been particularly valuable. For example, in salt marsh sediments in Louisiana concentrations of high molecular PAH (up to 16 ppm) were found to decrease the biomass of epibenthic diatoms and cyanobacteria after 4-day exposures, with some indications that snails from high exposure treatments lost weight after initial gains (Bennett et al., 1999). Such experimental results point to the need to examine more closely estuarine food webs where concentrations of PAH in this range can be found.
The spatial scale of the affected sand or mud shoreline area will determine the rebound of the affected area. A practical example of this is the impact of the Amoco Cadiz oil spill on benthic crustaceans. Failure to recover in some subtidal habitats was due to the fractionated distribution pattern of favored habitat by some species of amphipods (Dauvin and Gentil, 1990). Nevertheless, the populations were able to recover; densities on the impacted site attained high values similar to those found before the spill within 15 years (Dauvin, 1998).
Subtidal Areas
Oil can arrive in the subtidal by two mechanisms. Surface oil can weather, lose buoyancy and eventually sink, and it can associate with particulate matter suspended in the water and eventually sink, thereby affecting the benthic community (Elmgren et al., 1983). A second route of oil to the benthos is the transport of oil or contaminated particles from nearby oiled beaches.
As with the intertidal fauna, the most sensitive organisms in the subtidal benthos appear to be the crustaceans. Major effects on the crustacean fauna were documented in the
Tsesis spill (Elmgren et al., 1980), the Florida barge spill (Sanders et al., 1980), the Amoco Cadiz spill (Dauvin and Gentil, 1990), the Exxon Valdez spill (Jewett et al., 1999), and the 1996 North Cape oil spill where 8 to 9 million American lobsters were killed subtidally from a fuel oil spill (McCay, 2001). In addition, the rhepoxiniid amphipods, which appear to be particularly susceptible, are one of the few severely depressed faunal components in the benthic communities in areas of moderate petroleum seepage in the Santa Barbara Channel (Davis and Spies, 1980).
Not all spills demonstrated adverse effects in subtidal habitats. A study of the possible effects of tar residues from the Haven oil spill in Italy revealed no discernable differences between tar-affected and non-affected benthic communities (Guidetti et al., 2000). Exxon Valdez oil was generally not discernable below 40 meters in most portions of Prince William Sound and was never found in measurable quantities below 100 m depth. It is not surprising then that a study of deep benthic communities found no differences between various areas that could be attributed to oil from the spill (Feder and Feder, 1998).
During the Braer spill off the Shetland Islands, 84,700 tonnes of a light Gullfaks crude oil were released from the grounded vessel during hurricane-force winds, and an estimated 35 percent of the oil was deposited on the seabed in water depths from 2-100 m in an area of 4,000 km2 (Kingston, 1999). The sedimented oil provided a long-term pathway for exposure to benthic fisheries. For example, burrowing Norway lobster (Nephrops) remained contaminated for over five years, whereas epibenthic lobsters (Homarus) eliminated petroleum contaminants to background levels of PAH in one month (Kingston, 1999).
Effects Associated with Various Sources
As discussed in Chapter 3, petroleum enters the marine environment from a variety of sources, at different rates, and in diverse settings. Understanding how the environment responds to releases associated with specific sources is an important aspect of understanding the overall impact of widespread extraction, handling, and use of petroleum hydrocarbons.
Lessons from Natural Seeps
Natural petroleum seeps occur in many parts of the ocean, and can be utilized to understand the effects of oil contamination (Spies et al., 1980). As petroleum enters the ocean from the seabed, it is relatively unweathered in comparison to many other sources of oil that reaches the bottom (Reed and Kaplan, 1977; Steurmer et al., 1982). There are some significant consequences to this difference that limit the usefulness of oil seeps as effects models for other sources of oil in which weathering occurs before the oil is deposited in bottom sediments. Also, the possibility must be kept in mind that, with a history of thousands of years, animals living near seeps might have unique adaptations. Biological studies of seeps have concentrated on the extensively contaminated benthos (Spies and Davis, 1979; Spies et al., 1990).
There are two aspects to the effects of fresh seeping petroleum on benthic ecosystems. First, fresh petroleum, being a highly reduced source of energy, is readily oxidized by microbes (Bauer et al., 1988), which, in turn, can serve as a supplementary food source for benthic food webs in shallow water (Spies and DesMarais, 1983; Bauer et al, 1990). In the case of seeps in deep water, it can be a nearly exclusive carbon source. Second, at sufficiently high concentrations, the aromatic components of seep petroleum are toxic to marine organisms (Davis et al., 1981). There is also an interaction between toxicity of oil and microbial metabolism of petroleum. The decrease in oxygen in the surface layers of the sediments that results from microbial metabolism of petroleum is a limiting factor to benthic organisms. The oil, while supporting microbial growth that acts as a food source, may also be toxic to other organisms or indirectly decrease habitat quality through oxygen deficiency (Spies et al., 1989; Steichen et al., 1996). Microbial transformations of aromatic hydrocarbons may alter hydrocarbon composition and various oxidized products may be formed (Bartha and Atlas, 1987). Natural biogeochemical tracers indicate that both the petroleum carbon, particularly the lighter fractions, and sulfur from sulfide is incorporated into benthic meiofauna and macrofauna (Spies and DesMarais, 1983; Bauer et al., 1990). Circumstantial evidence for damage to gill tissues in bottom-feeding surf perches are linked to oil exposure through cytochrome P450 1A induction and aromatic petroleum metabolites in bile (Spies et al., 1996).
The most detailed investigations of petroleum seepages have been carried out in the Santa Barbara Channel off the coast of southern California. The following summarizes the findings of studies conducted at a depth of 20m in one of these oil seep areas, the Isla Vista seep. Starting with the fresh oil and gas in the sediments of a petroleum seep, several related phenomena occur. Bacterial populations, as measured by ATP content or by direct microscopic counts, are elevated several fold over surrounding sediments (Spies et al., 1980; Bauer et al., 1988). The sediments are highly reducing, oxygen is undetectable in sediments below a very thin surface layer, sulfate oxidizing activity is markedly elevated, hydrogen sulfide is abundant, and sulfide-oxidizing bacteria (Beggiatoa) are abundant at the surface of sediments, often forming prominent white mats.
The heavy seepage areas where the Beggiatoa mats form support a low-diversity benthic community consisting of large numbers of nematodes, a few polychaete worms (e.g., Capitella capitata), some oligochaete worms, and a limited number of harpacticoid copepod species (Spies et al., 1980; Montagna et al., 1987, 1989, 1995). Porewater concentrations of aromatic hydrocarbons within a few centimeters of an active seep were approximately 1 ppm. The nematodes
form a halo of high abundance, often only a few centimeters from the most active sources of seepage. Within several meters of the very active seeps, and where a small amount of seepage is still found, a diverse benthic community occurs of mainly detrital feeders. This community is similar in composition to the surrounding community that occupies much of the inner continental shelf in southern California. There are some key differences: oligochaetes are a significant component of the community, and some rhepoxiniid amphipods that are particularly sensitive to oil are missing but are found outside the seepage area (Davis and Spies, 1980). Also, in comparison to a nearby station, this community has a consistently larger number of organisms per unit area of sandy bottom.
Based on these findings the following is a conceptual model of the shallow water seep system in the Santa Barbara Channel. The lighter fractions of seeping petroleum are metabolized by microbes, and the energy in the petroleum is partially converted into microbial biomass. Rapid utilization of oxygen by microbial petroleum oxidizers shifts sediment biochemistry to sulfate oxidation and the resulting product, hydrogen sulfide, is utilized by sulfide oxidizers (Beggiatoa). At some distance from areas of active seepage, where pore water aromatic hydrocarbon and sulfide concentrations drop to tolerable levels and oxygen can penetrate further into the surface layer of the sediment, the diversity of organisms increases. Bacteria that assimilate and oxidize petroleum are utilized by meio- and macrofauna as an additional source of energy to supplement the usual contemporary photosynthetic sources of carbon for nearshore benthic communities. This pattern within the benthic community is broadly similar to that described for other sources of organic enrichment in the ocean, e.g., sewage and paper mill wastes (Pearson and Rosenberg, 1978).
In very deep water hydrocarbon seeps, most of the carbon utilized by mussels can originate from petroleum, because little surface water primary production is available. Various microbial chemosynthetic endosymbionts may be present in association with macrofauna of deepwater seeps. These microbes can oxidize either hydrogen sulfide or methane. Methane oxidizers then become carbon sources for their hosts. Methane oxidation has been little investigated in shallow water benthic systems, but could be an important process.
Since the shallow water seeps in southern California occupy only a small fraction of the continental shelf and seep benthic communities are composed almost predominantly of species with wide dispersal mechanisms (particularly in the larval stage), the studies of these “open” communities leave unresolved the issue of multi-generational effects of oil exposure.
Production Fields as Tutoring Grounds
Production fields offer an opportunity for uncovering long-term, chronic effects of petroleum hydrocarbons in the marine environment. Production fields are common in U.S. estuaries, primarily in the northwestern Gulf of Mexico, but are also increasing globally. In the United States, offshore oil and gas production has been limited primarily to continental shelf waters but continues to progress into deeper waters seaward of the shelf break. Regulations regarding the use of oil-based drilling fluids differ globally, as do regulations concerning the discharge of produced waters within U.S. state and federal waters and globally, so that practices that would contribute to the long-term accumulation of contaminants differ regionally as well as with time as regulations change. Still, practices that are currently disallowed in many coastal environments may continue to be allowed in other areas, and as oil and gas production expands globally into developing countries permitting regulations may allow practices there that are currently disallowed in U.S. waters. Thus, lessons learned from various practices, even if currently disallowed, provide the tutoring ground for further production globally.
Because of the continued inputs of drilling fluids and produced water discharges into estuarine, coastal and marine receiving waters, production fields are likely to illustrate long-term, low-level, chronic biological effects in sedimentary habitats resulting from the persistence of metals and medium and high molecular weight aromatic hydrocarbons, heterocyclics and their degradation products. Chronic contamination may result from continuous or intermittent discharges (produced waters, drilling fluids, deck washings) or from repetitive, accidental spills (numerous small spills and/ or a small number of major spills during the life of a field). The effects of these effluents are complicated by the addition of drilling discharges accumulated during field development (as opposed to exploratory drilling).
Drilling Fluids and Produced Waters
The major discharges associated with exploratory and development drilling are drilling cuttings and drilling fluids. Drill cuttings are particles of crushed sedimentary rock produced by the action of the drill bit as it penetrates the formation. Their accumulation on the ocean floor alters the benthic sedimentary environment. Drilling fluids are mixtures of natural clays and/or polymers, weighting agents and other materials suspended in a water or oil-based material. Oil-based drilling fluids have never been permitted for discharge to U.S. state or federal waters. Discharges of oil-based mud cuttings were permitted to waters of Canada, the North Sea countries, Australia, and several other offshore regions in the world until recently. Their ocean discharge has been banned in most of the world. Several metals of environmental concern found in drilling fluids are arsenic, barium, chromium, cadmium, copper, iron, lead, mercury, nickel, and zinc.
During production of oil or gas from a platform, produced water from the formation may be discharged to the environment. Besides being more saline than sea water, produced
waters contain elevated concentrations of radionuclides, metals, volatile organic aromatic compounds, monoaromatic hydrocarbons, light alkanes, higher molecular weight aromatic hydrocarbons, ketones, phenols, and organic acids. The environmental effects that may result from oil and gas production in a field depend greatly on the characteristics of the receiving environment. For example, there was a decreased abundance of fouling organisms, particularly barnacles, from the surface to a depth of about 3 m on a platform leg immediately below the produced water discharge located 1 m above the water surface (Howard et al., 1980). Produced water discharges, however, are usually dispersed to some degree. If discharged into the ocean, the produced water dilutes rapidly so that no impacts are ascribed to salinity. In more confined estuarine waters, produced water discharges form dense, saline plumes that move along the bottom sediments, but the resulting elevated water column and interstitial sediment salinity levels are within the range of tolerance of euryhaline estuarine organisms. In shallow, more confined areas with high suspended sediment loads or fine-grained sediments, medium molecular weight hydrocarbons and metals can absorb to particles and be deposited. Measurable effects are most likely in shallow waters, areas of restricted flow and dispersion, water with a high concentration of suspended particulates, and areas of fine-grained anaerobic sediments.
Effects of Production Discharges in Estuarine Waters
U.S. regulations now prohibit most discharges of produced waters from platforms to state waters of Texas and Louisiana, although the phase-out is not yet complete and some exceptions are provided, for example the highly dispersive distributaries of the Mississippi and Atchafalaya Rivers. The discharge of treated produced water from several offshore platforms at shore-based facilities is still permitted in Upper Cook Inlet, Alaska. The discharge of produced waters into estuaries and shallow coastal waters continues globally in developing fields (e.g., Nigeria, Angola, China, Thailand), and the effects of produced water discharges may still linger where the practice has been discontinued (Rabalais et al., 1998).
The effects of produced water discharges in estuaries have been studied extensively in Texas and Louisiana. For example Mackin (1971) surveyed estuarine benthic communities in eight Texas bays receiving produced water effluents. He reported no effects in two bays, minor localized effects in several other bays, and a zone of severely depressed fauna up to 106 m from submarine outfalls in Trinity Bay, Texas and a zone of enhanced faunal abundance and diversity down-current from there. Mackin (1971), however, conducted no chemical analyses. Armstrong et al. (1979) repeated these studies in Trinity Bay to correlate the benthic community effects with the distribution of hydrocarbons in sediments. In shallow waters of 2-3 m, they demonstrated the impacts of high concentrations of hydrocarbons, in this case sediment naphthalene concentrations of 4 to 8 ppm up to 1200 m from the platform, with corresponding severely depressed benthic fauna.
It was not until the mid to late 1980s that more extensive, systematic studies of the effects of produced water discharges in estuarine waters were conducted. While most surface water disposal was terminated on January 1, 2000, except within the distributary channels of Louisiana’s major rivers (the Mississippi and Atchafalaya), it is prudent to review the results of these studies for several reasons. First, this disposal method was practiced in coastal Louisiana and Texas and at one time accounted for 2,500,000 bbl/d of discharges into estuarine waters with the potential in some areas for long-term accumulation of contaminants and subsequent reintroduction to the environment (Boesch and Rabalais, 1989b; Rabalais et al., 1991a,b; Rabalais et al., 1998). Second, accidents associated with current disposal methods (pipelines and barges) will have similar results. Third, surface water disposal in estuarine waters still occurs elsewhere in the world.
Boesch and Rabalais (1989a), Neff et al. (1989), St. Pé (1990), Rabalais et al. (1991a,b), Steimle & Associates, Inc. (1991), Mulino et al. (1996) studied the effects of produced water discharges in estuarine waters of Louisiana. Where suitable measurements were made, the eventual fate of the dispersed produced water and the effects on benthic infauna could be explained by the volume of the discharge, the concentration of the various constituents, and the sedimentary regime, physical structure, and hydrology of the receiving environment (Boesch and Rabalais, 1989; Rabalais et al., 1991a). Dilution of water-soluble contaminants was influenced primarily by the volume of the receiving waters, the current velocity, and the potential for resuspension of sediments. Dispersion of sediment-adsorbed contaminants was influenced by the bed shear stress, sedimentation rates, and the grain size distribution of the surface sediments. The dilution potential of the environment was high for erosional environments with high current speeds and low for depositional environments, with intermediate potential for environments with periodic resuspension (storm-related) and deposition. There were no documented effects on the benthic community due to elevated salinities, because the overlying water and sediment interstitial salinities were within the range of the euryhaline organisms found in these habitats. Volatile hydrocarbons in the water column density plume that disperses across the sediment bed varied from nil to as high as 130 mg/L; alkylated PAH in bottom sediments reached concentrations from 2 to 40 ppm with one value of 100 ppm (Rabalais et al., 1991a,b). The potential for accumulation to depth in depositional environments exists (some sediments contained 30 ppm alkylated PAH at 35 cm depth) (Rabalais et al., 1991a). Produced water source contaminants persisted in surface sediments for two years after cessation of the effluent, as did benthic community effects, and persisted for as
long as nine years in vertical sediment cores (Rabalais et al., 1998).
The effects of produced water discharges in estuarine systems include toxicity to various organisms and, at the community level, the reduction of infaunal abundance and diversity (reviewed by St. Pé, 1990; Rabalais et al., 1991a). The persistent elevation of sediment hydrocarbon and metal concentrations and modification of benthic communities occurred from within a few hundred meters to up to a kilometer from the discharge.
In the Lake Barre field, Louisiana (one out of five such studies), oysters placed in trays adjacent to a produced water discharge suffered mortality as far as 23 m from the outfall and showed decreased growth rates between 23 and 46 m from the outfall (Menzel, 1950; Menzel and Hopkins, 1951, 1953). Similarly deployed oysters near produced water discharges in Pass Fourchon and Bayou Rigaud, Louisiana, resulted in mortality to oysters, reduced growth in others, and bioaccumulation of alkylated PAH and total hydrocarbons 3 to 18 times above background level (Rabalais et al., 1992). The potential for oysters to take up and accumulate contaminants originating in produced water occurred both in close proximity to the discharge and to great distances (350 m at Bayou Rigaud and 1000 m at Pass Fourchon).
Production Effects in Continental Shelf Waters
Several production field studies have been conducted in U.S. offshore waters, in the Norwegian and British sectors of the North Sea field, and on the Dutch continental shelf. [Continental shelf is defined from subtidal barrier shoreface to shelfbreak, usually 200 m water depth.] Because continental shelf ecosystems are complex, open and dynamic, there are fundamental problems in identifying the nature and extent of environmental effects and in determining causality. Any studies conducted in production fields must be designed and interpreted within the context of natural variability and other environmental factors that are important in shaping the physical environment and biological communities. In addition, there is a range of biological, chemical and statistical techniques that can be applied to any of these studies, often suitably, but just as often not. The complicating factors of natural variability have plagued many studies of oil and gas production effects in the northern Gulf of Mexico where one would be most likely to expect to find demonstrable effects on marine ecosystems. Two studies—the Offshore Ecology Investigation (OEI) and the Central Gulf Platform Study—were conducted in coastal Louisiana where extensive oil production activity might make this area a worse case scenario for effects. The locations just west of the Mississippi River delta, however, confounded possible oil-related effects with ecological factors of salinity variability, turbidity, high organic loading, sediment type, and seasonal bottom-water hypoxia (low dissolved oxygen concentrations).
Following 25 years of oil production in both Timbalier Bay and the area directly offshore on the continental shelf in 5 to 25 m water depth, the OEI study (reviewed by Neff, 1987; Spies, 1987) was designed to examine both localized platform effects, and the overall “health” of the ecosystems in the study area. The conclusions of the study (Menzies et al., 1979) were that there were no effects and that (1) natural phenomena in the area were a greater impact than petroleum-related activities, (2) petroleum contamination in the area was low and could not be tied to platform sources, and (3) the region was in “good ecological health.” The OEI study was criticized by Sanders (1981) who pointed out faults in study design, laboratory procedures, insufficient contaminant data, and inappropriate statistical treatment of benthic data. A second group of scientists independent of the OEI effort concluded that chemical contaminant data were insufficient to draw conclusions and that the benthic data indicated that the communities were more likely controlled by estuarine- and riverine-influenced salinity and turbidity than by adverse effects of oil (Bender et al., 1979).
The Central Gulf Platform study (water depths of 9 to 98 m; reviewed by Neff, 1987 and Spies, 1987) corrected many of the shortfalls of the OEI study by employing a larger variety of analyses, but was again situated in an area influenced by the Mississippi River, with confounding effects of turbidity, fluctuating salinity, seasonal hypoxia, and potential additional anthropogenic inputs of petroleum (Bedinger et al., 1981). In addition, a major tropical storm likely affected the benthic communities mid-way through sampling. Meiofaunal and macrofaunal benthic data analyzed with clustering techniques identified groups of fauna that were most similar with regard to depth, salinity, distance from shore (= sedimentary characteristics) and dissolved oxygen. Chemical analyses revealed both low and high molecular weight hydrocarbons often in very high concentrations, but there were no consistent patterns with regard to the amount of production from the platform, the age of the platform, or distance from the platform. Conclusions that the Mississippi River was the probable principal source of hydrocarbons to the study area were not supported by the data. The implications in the study conclusions that hydrocarbons were having a chronic sublethal effect on the fauna of the study area were criticized by Spies (1987), because of extrapolation of laboratory toxicity literature to concentrations of hydrocarbons found in the field. That is, most of the toxic effects documented in the literature were from relatively low molecular weight aromatic hydrocarbons, and the sediment hydrocarbons in the Central Gulf Platform study were dominated by highly-weathered mixtures.
Rabalais et al. (1993) tested specifically for the differences in hypoxia versus effects of petroleum production at two production platforms in 20 m water depth. Hydrocarbon concentrations, in general, were low for both study sites (even where sediments were silty) and were characterized as weathered petrogenic or biogenic in nature. There were no
consistent patterns of benthic community structure with distance from either discharge nor were there any relationships with petroleum indicators. As found in other studies, dissolved oxygen concentration, bottom water temperature and salinity, and sediments were the important environmental factors that explained the variation in benthic community parameters of species richness and abundance.
In a shallower area of the continental shelf (2 m) just offshore of the lower end of Atchafalaya Bay where uniformly silty sediments dominated and the environment was expected to be dispersive, a clear signal of produced water-associated contaminants and effects on benthic biota were observed to at least 200 m and 300 m, respectively (Rabalais et al., 1991a). The shallow water column at this site and flushing potential of Atchafalaya River discharge were expected to dilute and transport the produced water effluents away from the area. The high silt content of the sediments, the large volume of produced water discharged (21,000 bbl/d), and the high concentrations of volatile hydrocarbons may have been factors in the pronounced produced-water-effect at this station. By contrast, a nearby station in 8-m water depth, where the discharge of produced water was an order of magnitude less, was contaminated only within 20 m of the discharge where benthic fauna were also impacted (Neff et al., 1989).
Several production platforms in southern California were assessed for oil and metal contamination and affected marine communities (18 to 30 m water depth; reviewed by Neff, 1987). There were some elevated levels of production contaminants in sediments directly under and adjacent to platforms, but no concentrations of metals and petroleum hydrocarbons in selected fish and mussels. The platforms, piles of cuttings, and biofouling organisms both on the platforms and those sloughed to the bottom functioned as artificial reefs, providing habitats for a wider variety of marine animals than occurred on nearby hard and soft bottoms. In a study of a produced water discharge outfall in a high energy subtidal (10-12 m) environment off southern California, Osenberg et al. (1992) indicated that benthic infaunal community effects were localized within 100 m of the outfall.
In offshore waters around production platforms in the Gulf of Mexico, there was little evidence of bioaccumulation of produced water contaminants in edible tissues of resident fishes and invertebrates (Continental Shelf Associates, Inc., 1997). For the southern California produced water outfall in a high energy subtidal zone, Osenberg et al. (1992) found that the effects on outplanted mussels were more widespread than on the benthic infauna, between 500 and 1000 m from the outfall, as opposed to within 100 m. The observed effects on the mussels were reduction in growth, condition, and tissue production and varied inversely with relative exposure of the mussels to the produced water plume.
Two major studies have been conducted on the Texas continental shelf to examine the ecological effects of chronic contamination as well as sublethal impacts. These studies were conducted away from the influence of the Mississippi River and focused on near-field effects with a closer link between biological and chemical analyses. The Buccaneer Gas and Oil Field study (20 m depth; Middleditch, 1981) documented persistent accumulation of sediment hydrocarbons only within about 100 m of the platforms, but did not provide a thorough analysis of the chemical constituents present. A widespread effect on the benthos, including reduced numbers of individuals and species around the platforms, was apparent, but there were also areas well away from the platforms with similar benthic communities.
The Gulf of Mexico Offshore Operations Monitoring Experiment (GOOMEX) was designed to test and evaluate a range of biological, biochemical and chemical methodologies to detect and assess chronic sublethal biological impacts in the vicinity of long-duration activities associated with hydrocarbon production (Kennicutt et al., 1996b). The study was located in a gas field in the western Gulf of Mexico continental shelf and as removed as possible from confounding effects of Mississippi River discharge. The three platforms were in progressively deeper water, 29, 80 and 125 m. Sediments close (< 100 m) to the three platforms studied were enhanced in coarse-grain materials primarily derived from discharged muds and cuttings. Hydrocarbon and trace metal (Ag, Ba, Cd, Hg, Pb, and Zn) contaminants were associated with these coarse-grain sediments (Kennicutt et al., 1996a). Contaminants were asymmetrically distributed around each platform in response to the prevailing currents. The positive relationship between sand content and contaminant levels is contrary to the view of contaminants being associated with finer-grain sediments (Peterson et al., 1996).
The hydrocarbons occurred in concentrations that seemed too low to be important contributors to the observed toxicological effects. PAH were generally less than 100 ng/g, which was an order of magnitude lower than what Spies (1987) suggested was needed to induce biological response. At a few locations close to one platform, trace metal (i.e., Cd, Hg, Pb, and Zn) concentrations exceeded levels thought to induce biological effects. In deeper water (> 80 m), sediment trace metal contaminant patterns were stable over time frames of years. A few metals (Pb, Cd) exhibited evidence of continued accumulation in sediments over the history of the platform at the deeper water sites (> 80 m) immediately after cessation of drilling cf. 5-10 years after the last discharges. The chemical contaminants principally originated from the original drilling mud discharge and perhaps from produced waters during production (Kennicutt et al., 1996b).
Sediment chemical analyses and porewater toxicity tests with sea urchin fertilization and embryological development assays from the GOOMEX study (Carr et al., 1996) indicated toxicity near four of the five platforms, the majority collected within 150 m of a platform and those with the highest concentrations of contaminants. There was agreement among results of porewater tests with three species (sea urchin embryological development, polychaete reproduction,
and harpacticoid nauplii survival). Samples from the deepest site (> 80 m, HI-A389 near the Flower Gardens), which contained the highest contaminant concentrations, were the most toxic samples of the sites. Repeatability of toxicity between seasons demonstrated the persistence of the toxicity.
The meiofauna and macrofauna effects (Montagna and Harper, 1996) were localized within 100-200 m from the platforms (Table 5-7). The patterns of community change were increases in deposit-feeding polychaetes and nematodes that indicated organic enrichment, while density declines of harpacticoid copepods and amphipods indicated toxicity. The increase in annelids closer to the platforms occurred despite the steep gradient in sand content; total anne
TABLE 5-7 Responses of Biological and Ecological Indicators to Distance from Platforms in the Gulf of Mexico (compiled by P. Montagna)
|
|
Platform |
||
|
Indicator |
MAI-686 29 m depth |
MU-A85 80 m depth |
HI-A389 125 m depth |
|
Macroinfauna |
|||
|
Total Abundance |
0 |
↑70 percent |
↑3-5× |
|
Polychaete (P) Abundance |
0 |
↑90 percent |
↑3-5× |
|
Amphipod (A) Abundance |
↓4-12× |
↓4-5× |
↓3-5× |
|
P:A ratio |
↑6-10× |
↑8-10× |
↑20-30× |
|
Meiofauna |
|||
|
Total Abundance |
↓2-3× |
0 |
↑50 percent |
|
Nematode (N) Abundance |
↓2× |
↓2× |
↑60-80 percent |
|
Harpacticoid (H) Abundance |
↓2× |
0 |
↓3× |
|
N:H ratio |
0 |
0 |
↑4-5× |
|
Nematode Production |
↓49× |
↓135× |
↓2-3× |
|
H. Gravid Females |
↑3× |
0 |
↑3× |
|
H. Clutch Size |
0 |
↓10 percent |
↓14 percent |
|
H. Genetic Diversity |
↓2× |
↓1-5× |
↓2-3× |
|
Megafauna |
|||
|
Catch Per Unit Effort |
0 |
0 |
↑ |
|
Size |
0 |
↓ |
↑ |
|
Histopathology |
0 |
↑ |
0 |
|
Sex Ratio |
0 |
0 |
↑Female |
|
Toxicity & Biomarkers |
|||
|
Pore Water Toxicity |
↑ |
0 |
↑ |
|
Invertebrate AHH |
0 |
0 |
0 |
|
Fish: EROD, PAH in Bile, P4501A mRNA |
0 |
0 |
0 |
|
Summary of results for macrofauna and meiofauna (Montagna and Harper, 1996), nematode production (Montagna and Li, 1997), genetic diversity (Street and Montagna, 1996), harpacticoids reproduction (Montagna, unpublished data), megafauna (Ellis et al., 1996), toxicity (Carr et al., 1996) and biomarkers (McDonald et al., 1996). Table symbols represent percent increase (↑), no change (0), or decrease (↓) from near-field (< 100 m) to far-field stations (100 m to 3 km). 1x = 100 percent. |
|||
lids would be expected to be more abundant in finer sediments, not coarser. In contrast with annelids and oligochaetes, amphipod abundances were depressed around all platforms, with effects confined to 50 to 100 m. This was also consistent with literature on modest pollution, and is suggestive of a toxic response. Sea stars were reduced near the platform, but that pattern did not hold for ophiuroids. Changes in meiofaunal responses were most noticeable within 50 m of platforms. Harpacticoid abundance, community diversity, genetic diversity, reproductive success and survivability declined nearer the platforms with an increasing contaminant gradient at all study sites. On the other hand, total nematodes were enhanced. Patterns were absent at the shallowest site (29 m, MAI-686) where the relatively high-energy physical environment has led to more extensive dispersion of materials discharged. The other sites were in 80 m (MU-A85) and 125 m (HI-A389). They concluded that patterns of response to sedimentary contamination were detectable at higher taxonomic levels, and that these responses were driven by intrinsic physiological and ecological characteristics of higher taxa. Crustaceans (especially amphipods and harpacticoid copepods) and echinoderms are sensitive to toxics whereas polychaetes, oligochaetes, and nematodes (especially nonselective deposit feeders) are enhanced by organic enrichment (either from hydrocarbons or biologically produced materials falling from the platform structure). They concluded that metals drove the toxicity effects, and that the dual effects of toxicity and organic enrichment resulted in readily detectable responses in benthic meiofauna and macrofauna to 100-200 m.
The GOOMEX studies also focused on chronic, sublethal effects. Various physiological (McDonald et al., 1996) and genetic results (Montagna and Harper, 1996) provided evidence that crustaceans around the platforms were exhibiting sublethal responses to contaminant exposure. The percentage of gravid female harpacticoid copepods was greater and the percentage of juveniles was reduced within 50m of the platforms. In addition, reproductive effort for female harpacticoids carrying eggs was reduced. These responses could be explained as sublethal physiological reactions of these organisms to stress related to exposure to toxicants. The demonstration in multiple species of harpacticoids that genetic diversity was significantly reduced near the platforms and associated with increased contaminants as compared with the far-field sites suggested detection of a sublethal response of these sensitive organisms to some aspect of the platform-associated environment.
Several studies have been conducted in the North Sea (reviewed by Neff, 1987; Spies, 1987). The Ekofisk Oilfield study (70 m water depth) was designed to assess the impacts of ballast water discharge from a one-million barrel oil storage tank placed on the sea bottom, as well as impacts from production platforms. The Ekofisk study was complicated by a well blowout, and it is not known whether drilling employed oil-based fluids. Results indicated elevated concen
trations of oil constituents around some platforms at Ekofisk. Some faunal change around the platforms could be related to elevated hydrocarbon content, but were more readily explained by the changed sedimentary conditions and associated total organic carbon content surrounding the platforms (Spies, 1987). The Forties Oilfield, developed using only water-based drilling muds, is situated in 100 to 125 m water depth and represents deeper conditions than most studies on the Louisiana and Texas continental shelves. Impacts of oil production activities there were localized, primarily within 450 m of the platforms, and were of low magnitude. Only water-based drilling fluids were used in the Buchan field, and no produced water was discharged. No biological impacts of the platform or production activities were detected.
Oil-based drilling fluids were used extensively in the North Sea, and the amounts of petroleum hydrocarbons discharged with drill cuttings and their subsequent accumulation have been documented by several authors, (reviewed by Davies et al., 1983; summarized by Neff, 1987). There are four zones of chemical and biological impact around platforms discharging contaminated cuttings. Zone I, extending out to 250 m and exceptionally to 500 m from the platform, is characterized by hydrocarbon concentrations 1000 times above background and severely impoverished and modified benthos. Zone II extends from 200 to 2000 m from the platform with sediment concentrations 10-700 times above background, modified species diversity, and increased abundance of opportunistic polychaetes. Zones III and IV have normal benthic communities and decreasing gradients of hydrocarbon contamination. At the time of the Davies et al. (1983) review, none of the areas had been studied long enough to determine benthic recovery.
In contrast to the implication of metals over the long-term being the agent of benthic effects in a production field offshore Texas, U.S., the implicated contaminants where oil-based drilling fluids were used over many decades in oil and gas production in the North Sea are the hydrocarbons in those drilling fluids (Grant and Briggs, 2002). In toxicity studies of sediments from around the North West Hutton platform in the North Sea, sediments from 600 m from the platform remained acutely toxic to the amphipod Corophium and acutely toxic to the same organism at 100 m from the platform when 3% contaminated sediment was mixed with clean sediment. Metals toxicity was only a factor immediately adjacent to the platform.
Production Fields
There are clear effects of produced water discharges on waters, sediments, and living resources in estuarine production fields where the receiving environment is not conducive to the dispersion of the effluent plume. In shallow shelf waters, hydrocarbons from produced water accumulate in bottom sediments and benthic fauna may be depressed up to 300 m from the outfall. Measurable effects occur around offshore platforms, but except for artificial reef effects, sedimentary changes or changes brought about by a cuttings pile, such effects are usually localized. Beyond some contamination of organisms by petroleum, there is little convincing evidence of significant effects from petroleum around offshore platforms in deeper water. Where oil-based drill cuttings are discharged, there are readily evident effects of sediment contamination and benthic impacts to much greater distances from the platforms (up to 1 to 2 km).
While directed studies have identified some specific sublethal effects of long-term oil and gas development, the most significant unanswered questions remain those regarding the effects on ecosystems of chronic long-term, low-level exposures resulting from discharges and spills caused by development activities. Ultimately, we must determine whether the potential for effects from production fields are significant with regard to the geographic scale, what the cumulative effects are, whether ecosystem integrity is compromised, and whether there are significant impacts to resources that humans value, such as fisheries, marine mammals, endangered species, or rare or aesthetically pleasing environments.
Deep Sea Communities
Unfortunately, our knowledge base for the effects of chemicals or habitat perturbation is the most meager for the deep sea. It is unexpected, however, that ecological processes in the deep sea are fundamentally different from those of the continental shelf. There are additional environmental and physical parameters at work in the deep sea that make populations and communities there unique. What is not known are the sensitivities to contaminants; rates and mechanisms for population control, biological interactions, and recruitment; or rates or potential for recovery from impact.
Unique features of the deep sea and the fauna make them more susceptible to certain types of chemical spills. Increased turbidity from a spilled chemical such as a drilling mud could impact animals adapted to low light (including possibly, those with bioluminescent capabilities) by increased turbidity from deep plumes of low transmission water and indirectly through biological light interactions. Spills of chemicals with labile carbon may alter the local balance of oxygen consumption and result in hypoxia or anoxia, especially in oxygen minimum zones. Microhabitat diversity is a key to deep-sea diversity, and any chemical spill that alters deep habitats will likely have an impact. Chemical spills that disrupt the accessibility of fluxed detrital material for the dominant deposit-feeding organisms will affect feeding and subsequently the health of the organism(s). Chemicals that affect mortality, population levels, biological interactions, recruitment, growth rates, through either acute or chronic, sublethal toxicity or habitat alteration or both are likely to affect soft-bottom benthos in the deep sea similarly to continental shelf organisms. Basic biological information for most deep-sea organisms (e.g., feeding type, reproduc
tion, life span, growth rates, predators, and community ecology), however, is nonexistent.
Chemosynthetic seep communities are considered prevalent between 300 and 1000 m water depth on the northern Gulf of Mexico slope. Commonality, however, is not a reason for relaxing criteria for acceptable impact without knowledge of the ability of undamaged or damaged fauna to ultimately repopulate any impacted areas. Some organisms that inhabit the cold seep communities may be extremely old, and damaged communities would be slow or unlikely to recover. Hard bottom communities with highly diverse biogenically-structured communities are afforded protection from drilling operations in the Gulf of Mexico, and any chemical spills that approximate these types of effects would be expected to produce similar harm to live-bottom communities.
Summary
Since the compilation of the 1985 NRC report, Oil in the Sea, great progress has been made in identifying the toxic effects of petroleum hydrocarbons in a wide variety of organisms. We have also gained considerable knowledge of the effects of oil on various marine habitats through laboratory experiments, mesocosm experiments and practical experience with spills. Our knowledge of the effects of produced waters has expanded for inshore and offshore production fields and for multiple mixtures of oil and other contaminants in confined water bodies such as harbors. We now have first-hand experience with spills in coral reefs, mangroves, seagrass beds, and high-latitude cold-water environments. We are now in a better position to assess risks to individual organisms and habitats from the production, transport and consumption of petroleum than we were in 1985.
Assessing the effects of any particular spill and recovery from its effects has proven more complicated than was anticipated in 1985. We know that the natural variability of marine ecosystems and the open nature of marine communities, in which recruitment of young may be dependent on planktonic larvae transported from great distances, creates a substantial challenge in assessing both the effects of a spill and recovery from those effects. Although we now know much more about the toxicity and sublethal effects of petroleum hydrocarbons to organisms, we still have great difficulty in assessing the population, community, or ecosystem effects of pollution events. To assess the effects of oil in the sea and recovery from impacts, we need new information on the population structure of these marine organisms that is critical for the function of their communities. In addition, appreciation of the influence of decadal-scale and longer climate change means that we cannot expect communities or ecosystems to return to the state in which they were at the time of a pollution incident. Given the various time-scales of ecosystem change, before-after and control-impacted (BACI) designs for assessing damage are valuable, but they are no substitute for an up-to-date time series from a well-designed monitoring program. Based on an improved understanding of change in the marine environment, there is great value in having time series for detecting change and for pointing to processes critical for understanding change.
The effects of oil in the sea depend greatly on the season, place, and the types of organisms present. Although for a given habitat at a given time, a large amount of oil is likely to create more damage than a small amount, small amounts in sensitive environments or where there are populations at risk can have devastating effects.
Reducing the Threat to the Marine Environment
Ecosystems and their components vary at time-scales from seasons to decades and longer. Therefore, in the absence of on-going monitoring it is exceedingly difficult to quantify the effects of oil in the sea, or to establish when recovery from a pollution event is complete. Establishment of monitoring programs in selected regions with an elevated risk of petroleum spills or discharges would enhance the ability to determine effects and recovery, and to understand the processes controlling ecosystem responses to pollution. Existing databases on the distribution, frequency and size of petroleum spills and existing petroleum and distribution routes could be used to identify locations most appropriate for monitoring. Federal agencies, especially the USGS and EPA, should work with state and local authorities to establish or expand efforts to monitor vulnerable components of ecosystems likely to be exposed to petroleum releases.
There are demonstrable effects of acute oiling events at both small and large spatial-scales. These effects result from physical fouling of organisms and physiological responses to the toxic components of oil. Although there is now considerable information on the toxicological effects of individual components of oil, there is a lack of information about the synergistic interactions within organisms between hydrocarbons and other classes of pollutants. This problem is particularly acute in areas subject to chronic pollution, e.g., urban runoff. Research on the cumulative effects of multiple types of hydrocarbons in combination with other types of pollutants is needed to assess toxicity and organism response under conditions experienced by organisms in polluted coastal zones. Federal agencies, especially the USGS, NOAA, and EPA, should work with industry to develop or expand research efforts to understand the cumulative effects of multiple types of hydrocarbons in combination with other types of pollutants on marine organisms. Furthermore, such research efforts should also address the fates and effects of those fractions that are known or suspected to be toxic in geographic regions where their rate of input is high.
There are demonstrable sublethal physiological effects of long-term, chronic releases of hydrocarbons into the marine environment. These have been found in areas affected by
urban runoff, in areas where oil has been incorporated in sediments and is then released back to the water column, and in production fields. Chronic sources of hydrocarbon pollution remain a concern, and their effects on populations and ecosystems need further assessment. Federal agencies, especially the USGS, EPA, and NOAA should work with state and local authorities and industry to implement a comprehensive laboratory and field based investigation of the impact of chronic releases of petroleum hydrocarbons.
Biogenically-structured habitats, such as salt marshes and mangrove forests, are subject to destruction or alteration by acute oiling events. Because the structure of these habitats depends upon living organisms, when these are killed, the structure of the habitat, and sometimes the substrate on which it grows, is lost. Depending upon the severity of oiling and particularly if oil is incorporated in the sediments or structure of the habitat, recovery of the habitat and the organisms dependent on it may be exceptionally slow. In areas of sensitive environments or at-risk organisms, federal, state, and local entities responsible for contingency plans should develop mechanisms for higher level of prevention, such as avoidance, improved vessel tracking systems, escort tugs, and technology for tanker safety.
Although there is now good evidence for the toxic effects of oil pollution on individual organisms and on the species composition of communities, there is little information on the effects of either acute or chronic oil pollution on populations or on the function of communities or ecosystems. The lack of understanding of population-level effects lies partly in the fact that the structure of populations of most marine organisms is poorly known because of the open nature of communities and the flow of recruits between regions. Also, in some populations, (e.g., bony fish), the relationships between numbers of juveniles produced and recruitment to the spawning adult population are unknown. The U.S. Departments of Interior and Commerce should identify an agency, or combination of agencies, to develop priorities for continued research on:
-
the structure of populations of marine organisms and the spatial extent of the regions from which recruitment occurs,
-
the potential for cascades of effects when local populations of organisms that are key in structuring a community are removed by oiling, and
-
the basic population biology of marine organisms may lead to breakthroughs in understanding the relationship between sublethal effects, individual mortality, and population consequences.
There is a tremendous need for timely dissemination of information across state, federal, and international boundaries about the environmental effects of oil in the sea. Although the United States has experience that might benefit the international community, the United States might benefit greatly from lessons learned in other countries with offshore oil production, heavy transportation usage, and diffuse inputs of petroleum from land- and air-based sources. Therefore, the federal agencies identified above, in collaboration with similar international institutions, should develop mechanisms to facilitate the transfer of information and experience.
References
Abdullah, A. R., N. M. Tahir, and L. K. Wei. 1994. Hydrocarbons in seawater and sediment from the west coast of peninsular Malaysia. Bulletin of Environmental Contamination and Toxicology 53:618-626.
Abdullah, A. R., W. C. Woon, and R. A. Baker. 1996. Distribution of oil and grease and petroleum hydrocarbons in the Straits of Johor, Malaysia Peninsula. Bulletin of Environmental Contamination and Toxicology 57:155-162.
Achman, D. R., K. C. Hornbuckle, and S. J. Eisenreich. 1993. Environmental Science and Technology 27:75-86.
Alexander, S. K. and J. W. Webb, Jr. 1987. Relationship of Spartina alterniflora growth to sediment oil content following an oil spill. In Proceedings: 1987 Oil Spill Conference. American Petroleum Institute. Washington, D.C., pp. 445-449.
American Petroleum Institute (API). 2001a. Basic Petroleum Data Book. Washington, D.C.
American Petroleum Institute (API). 2001b. National Ocean Industries Association, and Offshore Operators Committee. 2001. Comments on draft document (unpublished).
American Society for Testing and Materials. 1999. Annual Book of A.S.T.M. Standards. West Conshohocken, PA.
American Society of Civil Engineers. 1996. State-of-the-art review of modeling transport and fate of oil spills. Journal of Hydrologic Engineering. Pp. 594-609.
Anderson, J. W.1979. An assessment of knowledge concerning the fate and effects of petroleum hydrocarbons in the marine environment. In W. B. Vernberg, F. J. Vernberg, A. Calabrese and F. P. Thurberg (Eds.). Marine Pollution: Functional Responses. Academic Press, NY, pp. 3-22.
Anderson, J. W., J. M. Neff, B. A. Cox, H. E. Tatem, and G. H. Hightower. 1974. Characteristics of dispersions and water-soluble extracts of crude and refined oils and their toxicity to estuarine crustaceans and fish. Marine Biology 27:75-88.
Anderson, J. W., R. G. Riley, S. L. Kiesser, and J. Gurtisen. 1987. Toxicity of dispersed and undispersed Prudhoe Bay crude oil fractions to shrimp and fish. Proceedings 1987 Oil Spill Conference: (Prevention, Behavior, Control, Cleanup), Tenth Biennial, American Petroleum Institute, Washington, DC, pp. 235-240.
Anderson, J. W., R. G. Riley, S. L. Kiesser, B. L. Thomas, and G. W. Fellingham. 1983. Natural weathering of oil in marine sediments: Tissue contamination and growth of the littleneck clam, Protothaca staminea. Canadian Journal of Fisheries and Aquatic Sciences 40 (Suppl. No. 2):70-77.
Andres, B. A. 1996. Consequences of the Exxon Valdez oil spill on black oystercatchers inhabiting Prince William Sound, Alaska. Ph.D. thesis, Ohio State University, Columbus, OH.
Andres, B. A. 1997. The Exxon Valdez oil spill disrupted the breeding of black oystercatchers. Journal of Wildlife Management 61:1322-1328.
Apa, P. D., and L. Zheng. 1997. Simulation of oil spills from underwater accidents I: Model development. Journal of Hydraulic Research: IAHR 35(5):673-687
Armstrong, H. W., K. Fucik, J. W. Anderson and J. M. Neff. 1979. Effects of oilfield brine effluent on sediments and benthic organisms in Trinity Bay, Texas. Marine Environmental Research 2:55-69.
Atlas, R. M., and C. E. Cerniglia. 1995. Bioremediation of petroleum pollutants. BioScience 45:332-338.
Atlas, R. M., and R. Bartha, 1992. Hydrocarbon biodegradation and oil spill bioremediation. K. C. Marshall [ed.]. Advances in Microbial Ecology. Plenum Press, New York, pp. 287-238.
Atwood, D. K., F. J. Burton, J. E. Corredor, G. R. Harvey, A. J. Matajimenez, A. Vasquezbotello, and B. A. Wad. 1987a. Results of the CARIPOL petroleum monitoring project in the Wider Caribbean. Marine Pollution Bulletin 18:540-48.
Atwood, D. K., F. J. Burton, J. E. Corredor, G. R. Harvey, A. J. Matajimenez, A. Vasquezbotello, and B. A. Wade. 1987b. Petroleum pollution in the Caribbean. Oceanus 30:28-32.
Audunson, T., H. K. Celius, O. Johansen, P. Steinbakke, and S. Sörstrom, 1984. The experimental oil spill on Haltenbanken. 1982. Continental Shelf Institute, Trondheim, Norway.
Babcock, M. M., P. M. Harris, and S. D. Rice. 1997. Restoration of oiled mussel beds in Prince William Sound, Alaska, five years after the Exxon Valdez oil spill. Journal of Shellfish Research Volume 6.
Baca, B. J., T. E. Lankford, and E. R. Gundlach. 1987. Recovery of Brittany coastal marshes in the eight years following the Amoco Cadiz incident. Proceedings 1987 Oil Spill Conference. American Petroleum Institute, Washington, D.C., pp. 459-464.
Bak, R. P. M. 1987. Effects of chronic oil pollution on a Caribbean reef. Marine Pollution Bulletin 18:534-539.
Baker J. E., and S. J. Eisenreich. 1990. Concentrations and fluxes of polycyclic aromatic compounds and polychlorinated biphenyls across the air water interface of Lake Superior. Environmental Science and Technology 24:343-352.
Baker, J. 2001. Personal communication on April 6, 2001. Chesapeake Biological Laboratory, Solomons, MD.
Baker, J. M., L. M. Guzman, P. D. Bartlett, D. I. Little, and C. M. Wilson. 1993. Long-term fate and effects of untreated thick oil deposits on salt marshes. Proceedings 1993 Oil Spill Conference. American Petroleum Institute, Washington, D.C., pp. 395-399.
Baker, J. M. 1983. Impact of oil pollution on living resources. The Environmentalist: 3(Suppl. 4):1-48.
Baker, J. M., M. Spalding, and J. Moore, 1995. Sensitivity mapping worldwide: harmonization and the needs of different user groups. In: Proc. 1995 International Oil Spill Conference. American Petroleum Institute, Washington, D.C., pp. 77-81.
Baker, J. R., A. M. Jones, T. P. Jones and H. C. Watson. 1981. Otter Lutra lutra L. mortality and marine oil pollution. Biological Conservation 20:311-321.
Ball, W. P., and P. V. Roberts. 1991. Long-term sorption of halogenated organic chemicals by aquifer materials—Part II. Intraparticle diffusion. Environmental Science and Technology 25(7):1237-1249.
Ballou, T. G. and R. R. Lewis III. 1989. Environmental Assessment and Restoration Recommendation for a Mangrove Forest Affected by Jet Fuel. 1989 Oil Spill Conference Proceedings: American Petroleum Institute, Washington, D.C., pp. 407-412.
Bamford, H. A., J. H. Offenberg, R. K. Larsen, F. Ko, and J. E. Baker. 1999a. Diffusive exchange of polycyclic aromatic hydrocarbons across the air-water interface of the Patapsco River, an urbanized subestuary of the Chesapeake Bay. Environmental Science and Technology 33:2138-2144.
Bamford, H. A., D. L. Poster and J. E. Baker. 1999b. Temperature dependence of the Henry’s Law constants of thirteen polycyclic aromatic hydrocarbons between 4ºC and 31ºC. Environmental Toxicology and Chemistry 18(9):1905-1912.
Bamford, H. A., D. L. Poster, and J. E. Baker. 2000. Henry’s Law constants of polychlorinated biphenyl congeners and their variation with temperature. Journal of Chemical and Engineering Data 49:1069-1074.
Barakat, A. O., A. R. Mostafa, J. Rullkötter, and A. R. Hegaz. 1999. Application of multi-molecular marker approach to fingerprinting petroleum pollution in the marine environment. Marine Pollution Bulletin 38:534-544.
Barber, W. E., L. L. McDonald, W. P. Erickson, and M. Vallarino. 1995. Effect of the Exxon Valdez oil spill on intertidal fish, a field study. Trans. Amer. Fish. Soc. 124:461-476.
Barnston, A. G., and R. E. Livesy. 1987. Classification, seasonality and persistence of low frequency atmospheric circulation patterns Monthly Weather Review 115:1089-1112.
Barron, M. G., and L. Ka’aihue. In review. Potential for photoenhanced toxicity of spilled oil in Prince William Sound and Gulf of Alaska waters. Marine Pollution Bulletin.
Barron, M. G., T. Podrabsky, S. Ogle and R. W. Ricker. 1999. Are aromatic hydrocarbons the primary determinant of petroleum toxicity to aquatic organisms? Aquatic Toxicology 46:253-268.
Bartha, R., and R. M. Atlas. 1987. Transport and transformations of petroleum: biological processes. Long-term environmental effects of offshore oil and gas development. D. F. Boesch and N. N. Rabalais, (eds.). Elsevier Applied Science, London, pp. 287-341.
Barton, P. J., and J. Fearn. 1997. Study of two and four stroke outboard marine engine exhaust emissions sing a total dilution sampling system. SAE Technical Paper, 1294 pp.
Bauer, J. E., P. A. Montagna, R. B. Spies, D. H. Hardin and M. Prieto. 1988. Microbial biogeochemistry and heterotrophy in sediments of a marine hydrocarbon seep. American Society of Limnology and Oceanography 33:1493-1513.
Bauer, J. E., R. B. Spies, J. S. Vogel, D. E. Nelson, and J. R. Southon. 1990. Radiocarbon evidence of fossil-carbon cycling in sediments of a nearshore hydrocarbon seep. Nature 348:230-232.
Baumard, P., H. Budzinski, P. Garrigues, T. Burgeot, X. Michel, and J. Bellocq. 1999. Polycyclic aromatic hydrocarbon (PAH) burden of mussels (Mytilus sp., in different marine environments in relation with sediment PAH contamination, and bioavailability. Marine Environmental Research 47:415-439.
Baussant, T., S. Sanni, A. Skadsheim, G. Jonsson, J. F. Børseth, and B. Gaudebert. 2001b. Bioaccumulation of polycyclic aromatic compounds: II. modeling bioaccumulation in marine organisms chronically exposed to dispersed oil . Environmental Toxicology and Chemistry 20:1185-1195.
Baussant, T., S. Sanni, G. Jonsson, A. Skadsheim, and J. F. Børseth. 2001a. Bioaccumulation of polycyclic aromatic compounds: I. comparison of bioconcentration in two marine species and in semipermeable membrane devices during laboratory-simulated chronic exposure to dispersed crude oil. Environmental Toxicology and Chemistry 20:1175-1184.
Beamish, R. J. 1993. Climate and exceptional fish production off the west coast of North America. Canadian Journal of Fisheries and Aquatic Science 50:2270-2291.
Beamish, R. J., D. J. Noakes, G. A. McFarlane, L. Klyashtorin, V. V. Ivanov and V. Kurashov. 1999. The regime concept and natural trends in the production of Pacific salmon. Canadian Journal of Fisheries and Aquatic Science 56:516-526.
Becker, P. R., and C. A. Manen. 1988. Natural oil seeps in the Alaskan marine environment. Final Report, Outer Continental Shelf Environmental Assessment Program, U.S. Department of Commerce, Technical Information Service, PB88-235965, p. 114.
Bedinger, Jr., C. A., R. E. Childers. J. W. Cooper, K. T. Kimball, and A. Kwok. 1981. Part 1. Background, program organization and study plan. Pages 1-53 in C. A. Bedinger (ed.). Ecological Investigations of Petroleum Production Platforms in the Central Gulf of Mexico. Vol. 1, Pollutant Fate and Effects Studies. Southwest Research Institute, San Antonio, TX.
Bence, A. E., K. A. Kvenvolden, and M. C. Kennicutt II. 1996. Organic geochemistry applied to environmental assessments of Prince William Sound, Alaska, after the Exxon Valdez oil spill—a review. Organic Geochemistry 24:7-24.
Ben-David, M., T. M. Williams and O. A. Ormseth. 2000. Effects of oiling on exercise physiology and diving behavior of river otters: a captive study. Canadian Journal of Zoology 78:1380-1390.
Bender, M. E., D. J. Reish, and C. H. Ward. 1979. Independent appraisal. Re-examination of the Offshore Ecology Investigation. In C. H. Ward, M. E. Bender, and D. J. Reish (eds.). The Offshore Ecology Investigation: Effects of Oil Drilling and Production in a Coastal Environment. Rice University Studies 65:1-589.
Bender, M. E., E. A. Shearls, R. P. Ayres, C. H. Hershner and R. J. Huggett. 1977. Ecological effects on experimental oil spills on eastern coastal plain estuarine ecosystems. Proceedings 1977 Oil Spill Conference. American Petroleum Institute, Washington, D.C., pp. 505-509.
Bennett, A., T. S. Bianchi, J. C. Means, and K. R. Carman. 1999. The effects of polycyclic aromatic hydrocarbon contamination and grazing on the abundance and composition of microphytobenthos in salt marsh sediments: (Pass Furchon, LA) I. A microcosm experiment. J. Exp. Marine Biology. Ecology 242:1-20.
Berridge, S. A., R. A. Dean, R. G. Fallows and A. Fish. 1968. The Properties of Persistent Oils at Sea. Journal of the Institute of Petroleum: Vol. 54. The Institute of Petroleum, London, pp. 300-309.
Berthou, F., G. Balouet, G. Bodennec, and M. Marchand. 1987. The occurrence of hydrocarbons and histopathological abnormalities in oysters for seven years following the wreck of the Amoco Cadiz in Brittany (France). Marine Environmental Research 23:103-133.
Bertrand, J. C., M. Bianchi, M. Almallah, M. Aquaviva, and G. Mille. 1993. Hydrocarbonoclastic bacterial communities composition grown in sea-
water as a function of sodium chloride concentration. Journal of Environmental Marine Biology and Ecology 168(1):125-138.
Bestari, K. T. J., R. D. Robinson, K. R. Solomon, T. S. Steele, K. E. Day, and P. K. Sibley. 1998. Distribution and composition of polycyclic aromatic hydrocarbons within experimental microcosms treated with liquid creosote. Environmental Toxicology and Chemistry 17(12), 2359-2368.
Bidleman, T. F., A. A. Castleberry, W. T. Foreman, M. T. Zaranski, and D. W. Wall. 1990. Petroleum Hydrocarbons in the Surface Water of Two Estuaries in the Southeastern United States. Estuarine Coastal and Shelf Science 30:91-109.
Bierman, V. J., Jr. 1990. Equilibrium partitioning and biomagnification of organic chemicals in benthic animals. Environmental Science and Technology 24:1407-1412.
Bishnoi, P. R., and B. B. Maini. 1979. Laboratory study of behavior of oil and gas particles in salt water, relating to deepwater blowouts. Spill Technology Newsletter 4:24-36.
Blenkinsopp, S., Z. J. Wang, D. W. S. Foght, G. Westlake, M. Sergy, M. Fingas, L. Sigouin, K. Semple. 1997. Assessment of the freshwater biodegradation potential of oils commonly transported in Alaska. ASPS# 95-0065, Contract # 18-8002-51. Environment Canada, Ottawa.
Bodkin, J. L., B. E. Ballachey, T. A. Dean, A. K. Fukuyama, S. C. Jewett, L. McDonald, D. H. Monson, C. E. O’Clair, and G. R. VanBlaricom. 2001. Sea otter Enhydra lutris perspective: Mechanisms of impact and potential recovery of near shore vertebrate predators following the 1989 Exxon Valdez oil spill. Part A. Sea otter population recovery. Pp. 3A. 1-3A. 28 in L. E.
Boehm, P., and D. Fiest. 1982. Subsurface distributions of petroleum from an offshore well blowout. The IXTOC Blowout, Bay of Campeche. Environmental Science and Technology 16(2):67-74.
Boehm, P. D. 1980. Evidence for the decoupling of dissolved, particulate and surface microlayer hydrocarbons in northwestern Atlantic continental shelf waters, Marine Chemistry 9:255-281.
Boehm, P. D., P. J. Mankewiecz, P. J. Hartung, J. M. Neff, D. S. Page, J. E. O’Reilly, K. R. Parker. 1996. Characterization of mussel beds with residual oil and the risk to foraging wildlife 4 years after the Exxon Valdez oil spill. Environmental Toxicology and Chemistry 15:1289-1303.
Boehm, P. D.; Page, D. S.; Gilfillan, E. S.; Stubblefield, W. A.; Harner, E. J. 1995. Shoreline ecology program for Prince William Sound, Alaska, following the Exxon Valdez Oil Spill: Part 2—Chemistry and Toxicology . Pages 347−397 In: P. G. Wells, J. N. Butler, and J. S. Hughes, Eds., Exxon Valdez Oil Spill: Fate and Effects in Alaskan Waters. ASTM Special Technical Publication 1219. American Society for Testing and Materials, Philadelphia, PA.
Böer, B. and J. Warnken. 1996. Flora of the Jubail Marine Wildlife Sanctuary, Saudi Arabia. In: A Marine Wildlife Sanctuary for the Arabian Gulf. Environmental Research and Conservation Following the 1991 Gulf War Oil Spill. F. Krupp, A.H. Abuzinada, and I.A. Nader (eds.). Frankfurt & Riyadh. Pp. 290-301.
Boersma, P. D., J. K. Parrish, A. B. Kettle. 1995. Common Murre abundance, phenology, and productivity on the Barren Islands, Alaska: The Exxon Valdez oil spill and long-term environmental change. In: Wells, P. G., Butler, J. M., Hughes, J. S. (eds). Exxon Valdez oil spill: Fate and effects in Alaskan Waters. ASTM STP 1219, America Society for Testing and Materials. Philadelphia, PA. Pp. 820-853.
Boesch, D. F., and N. N. Rabalais (eds.). 1989a. Produced Waters in Sensitive Coastal Habitats: An Analysis of Impacts, Central Coastal Gulf of Mexico. OCS Report/MMS 89-0031. U.S. Dept. of the Interior, Minerals Management Service, Gulf of Mexico, OCS Regional Office, New Orleans, LA, 157 pp.
Boesch, D. F., and N. N. Rabalais (eds.). 1989b. Environmental Impact of Produced Water Discharges in Coastal Louisiana. Report to The Louisiana Division of the Mid-Continent Oil and Gas Association. Louisiana Universities Marine Consortium, Chauvin, LA, 287 pp.
Boesch, D.F. and N.N. Rabalais. 1987. Long-term environmental effects of offshore oil and gas development. Elsevier Applied Science, New York. 708 pp.
Boesch, D. F., J. N. Butler, D. A. Cacchione, J. R. Geraci, J. M. Neff, J. P. Ray and J. M. Teal. 1987. An assessment of the long-term environmental effects of U.S. offshore oil and gas development activities: future research needs. D. F. Boesch, and N. N. Rabalais [eds.]. Long-Term Environmental Effects of Offshore Oil and Gas Development. Elsevier Applied Science, London and New York, pp. 1-53.
Boese, B. L., R. J. Ozretich, J. O. Lamberson, R. C. Swartz, F. A. Cole, J. Pelletier, and J. Jones. 1999. Toxicity and phototoxicity of mixtures of highly lipophilic PAH compounds in marine sediment: Can the capital sigma PAH model be extrapolated? Archives of Environmental Contamination and Toxicology 36:270-280.
Bomboi, M. T., and A. Hernandez. 1991. Hydrocarbons in urban runoff, their contribution to wastewaters. Water Res. 25:557-565.
Bowman, R. S. 1978. Dounreay Oil Spill: major implications of a minor incident. Marine Pollution Bulletin 9, 269-273.
Bowman, T. D. 1993. Bald eagles: after the spill. Alaska Wildlife 25:13-14.
BP Amoco Statistical Review of World Energy. 2000. British Petroleum Amoco, London, UK.
BP Statistical Review. 2002. Statistical review of world energy 2002—oil. Available online: www.bp.com/worldenergy.
BP Statistical Review of World Energy. 1997. British Petroleum Amoco, London, UK. 43pp.
Bragg, J. R., and E. H. Owens. 1995. Shoreline cleansing by interactions between oil and fine material particles. Proceedings of the International Oil Spill Conference: American Petroleum Institute, Washington, D.C. Pub. No. 4620:216-227.
Brannon, E. J., L. L. Moulton, L. G. Gilbertson, A. W. Maki, and J. R. Skalski. 1995. An assessment of oil spill effects on pink salmon populations following the Exxon Valdez oil spill—Part 1: Early life history. In: P. G. Wells, J. N. Butler, and J. S. Hughes, Eds., Exxon Valdez Oil Spill: Fates and Effects in Alaskan Waters. ASTM STP 1219. American Society for Testing and Materials, Philadelphia, PA, pp. 548-584.
Brannon, E. L., and A. W. Maki. 1996. The Exxon Valdez oil spill: analysis of impacts on the Prince William Sound pink salmon. Review of Fisheries Science 4:289-337.
Brannon, E. L., K. C. M. Collins, L. L. Moulton and K. R. Parker. 2001. Resolving allegations of oil damage to pink salmon eggs in Prince William Sound. Canadian Journal of Fisheries and Aquatic Science 58:1070-1076.
Briggs, K. T., S. H. Yoshida, and M. E. Gershwin. 1996. The influence of petrochemicals and stress on the immune system of seabirds. Regulatory Toxicology and Pharmacology 23:145-155.
Brody, A. 1988. A simulation model for assessing the risks of oil spills to the California sea otter population and an analysis of the historical growth of the population. D. B Siniff and K. Ralls [eds.]. Population Status of California Sea Otters. Report to U.S. Department of the Interior, Minerals Management Service, Pacific Outer Continental Shelf Region, Los Angeles, CA. Contract No. 14-12-001-30033, pp. 191-274.
Brookman, G. T., M. Flanagan, and J. O. Kebe. 1985. Literature Survey: Hydrocarbon Solubilities and Attenuation Mechanisms. American Petroleum Institute, Publication Number 4414, Washington, D.C.
Brown, J. S., T. C. Sauer, Jr., M. J. Wade, and J. M. Neff. 1992. Chemical and toxicological characterization of produced water freon extracts. In Ray, J. P., and F. R. Engelhart, eds. Produced Water. Plenum Press, New York and London, pp. 113-131.
Brunstrom, B., D. Broman, and C. Naf. 1991. Toxicity and EROD-inducing potency of 24 polycyclic aromatic hydrocarbons (PAH) in chick embryos. Archives of Toxicology 65:485-489.
Bue, B. G., S. Sharr and J. E. Seeb. 1998. Evidence of damage to pink salmon populations inhabiting Prince William Sound, Alaska, two gen
erations after the Exxon Valdez oil spill. Transactions of the American Fisheries Society 127:35-43.
Bue, B. G., S. Sharr, S. D. Moffitt, and A. K. Craig. 1996. Effects of the Exxon Valdez oil spill on pink salmon embryos and preemergent fry. Proceedings Of The Exxon Valdez Oil Spill Symposium, American Fisheries Society Symposium 18:619-627.
Buist, I. A., and S. G. Potter. 1987. Oil submergence: wind/wave tank tests and modeling. Proceedings of the Tenth Arctic Marine Oilspill Program Technical Seminar, Environment Canada, Ottawa, Ontario, pp. 1-20.
Burger, A. E. 1993. Estimating the mortality of seabirds following oilspills; effects of spill volume. Marine Pollution Bulletin 26:140-143.
Burns, K. A., and J. M. Teal. 1979. The wet Falmouth oil spill, hydrocarbons in the salt marsh ecosystem. Estuarine, Coastal and Shelf Science 8:349-360.
Burns, K. A., and A. H. Knap. 1988. The Bahia Las Minas Oil Spill: Hydrocarbon uptake by reef building corals. Marine Pollution Bulletin 20 (8):391-398.
Burns, K., and A. Saliot. 1986. Petroleum hydrocarbons in the Mediterranean Sea: A mass balance. Marine Chemistry 20:141-157.
Burns, K. A., and A. H. Knap. 1989. The Bahia las Minas Oil Spill: Hydrocarbon Uptake by Reef Building Corals. Marine Pollution Bulletin 20(8):391-398.
Burns, K. A., and L. Yelle-Simmons. 1994. The Galeta oil spill. 4. Relationship between sediment and organism hydrocarbon loads. Estuarine, Coastal and Shelf Science 38:397-412.
Burns, K.A., S. Codi, and N.C. Duke. 2000. Gladstone, Australia Field Studies: Weathering and degradation of hydrocarbons in oiled mangrove and salt marsh sediments with and without the application of an experimental bioremediation protocol . Marine Pollution Bulletin 41:392-402.
Burns, K. A., S. D. Garrity, and S. C. Levings. 1993. How many years until mangrove ecosystems recover from catastrophic oil spills? Marine Pollution 26:239-248.
Burns, K. A., S. D. Garrity, D. Jorissen, J. MacPherson, M. Stoelting, J. Tierney and L. Yelle-Simmons. 1994. The Galeta oil spill. II. Unexpected persistence of oil trapped in mangrove sediments. Estuarine, Coastal, and Shelf Science 38:349-364.
Butler, J. N., and B. F. Morris. 1974. May 13-17, 1974. In: Quantitative monitoring and variability of pelagic tar in the North Atlantic: Proceedings of the Marine Pollution Monitoring (Petroleum) Symposium, IOC/ WMO/U.S. Department of Commerce, Gaithersburg, MD.
Butler, J. N., B. F. Morris, and J. Sass. 1973. Pelagic Tar from Bermuda and Sargasso Sea: Bermuda Biological Station. Special Publication No. 10:p. 346.
Butler, J. N., P. G. Wells, S. Johnson, and J. J. Manock. 1998. Beach tar on Bermuda: recent observations and implications for global monitoring. Marine Pollution Bulletin 36:458-463.
Cabioch, L. 1980. Pollution des sediments subtidaus et perturbation des communautés animales benthiques. Ambio 9:294-296.
Cabioch, L., J. C. Dauvin, J. Mora Bermudez, and C. Rodriguez Babio. 1980. Effects of the Amoco Cadiz oil spill on the sublittoral benthos, north of Brittany. Helgolander Meeresuntersuchungen: Hamburg, 33:192-208.
Cairns, J. 1983. Are single species tests alone adequate for estimating environmental hazard? Hydrobiologia 100:47-57.
Camphuysen, C. J. 1989. Beached bird surveys in the Netherlands: 1915-1988. Seabird mortality in the southern North Sea since the early days of oil pollution. Amsterdam: Vogelbescherming Werkgroep Nordzee (Technisch Rapport 1).
Camphuysen, C. J. 1998. Beached bird surveys indicate a decline in chronic oil pollution in the North Sea. Marine Pollution Bulletin 36:519-526.
Canadian Environmental Assessment Agency (C.E.A.A.). 1995. Military Flying Activities in Labrador and Quebec, Ottawa.
Capuzzo, J. McDowell, M. N. Moore and J. Widdows. 1988. Effects of toxic chemicals in the marine environment: Predictions of impacts from laboratory studies. Aquatic Toxicology 11:303-311.
Capuzzo, J. M. 1987. Biological effects of petroleum hydrocarbons: Assessments from experimental results. In: Long-term Environmental Effects of Offshore Oil and Gas Development. D. F. Boesch and N. N. Rabalais [eds.]. Elsevier Applied Science, London, pp. 343-410.
Carey, J. H., E. D. Ongley, and E. Nagy. 1990. Hydrocarbon transport in the Mackenzie River, Canada. The Science of the Total Environment 97/ 98:69-88.
Caribbean Environment Programme. 1994. Regional overview of land-based sources of pollution in the wider Caribbean region: CEP Technical Report No. 33. United Nations Environment Programme. Web site: www.cep.unep.org/pubs/techreports/tr33en/index.html.
Carls, M. G., G. D. Marty, T. R. Meyers, R. E. Thomas, and S. D. Rice. 1998. Expression of viral hemorrhagic septicemia virus in prespawning Pacific herring (Clupea pallasi). Canadian Journal of Fisheries and Aquatic Sciences 55:1-10.
Carls, M. G., M. M. Babcock, P. M. Harris, G. V. Irvine, J. A. Cusick, and S. D. Rice. 2001. Persistence of oiling in mussel beds after the Exxon Valdez oil spill. Marine Environmental Research 51:67-190.
Carls, M. G., S. D. Rice, and J. E. Hose. 1999. Sensitivity of fish embryos to weathered crude oil: Part I. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval Pacific herring (Clupea pallasi). Environmental Toxicology and Chemistry 18:481-493.
Carman, K. R., J. W. Fleeger, J. C. Means, S. M. Pomarico, and D. J. McMillin. 1995. Experimental investigation of the effects of polynuclear aromatic hydrocarbons on an estuarine sediment food web. Marine Environmental Research 40:289-318.
Carman, K. R., T. S. Bianchi, and F. Kloep. 2000. Influence of grazing and nitrogen on benthic algal blooms in diesel fuel-contaminated saltmarsh sediments. Environmental Science and Technology 14:107-111.
Carr, R. S., D.C. Chapman, B. J. Presley, J. M. Biedenbach, L. Robertson, P. Boothe, R. Kilada, T. Wade and P. Montagna. 1996. Sediment porewater toxicity assessment studies in the vicinity of offshore oil and gas production platforms in the Gulf of Mexico. Canadian Journal of Fisheries and Aquatic Sciences 53:2618-1628.
Caudle, C. 1995. Impact Assessment of Produced Water Discharges to Nueces Bay—August 1993. Publ. No. AS-49/SR, Texas Natural Resources Conservation Commission, Austin, Texas, 30 pp.
Chaffee, C., and R. B. Spies. 1982. The effects of used ferrochrome lignosulfonate drill muds from a Santa Barbara Channel oil well on the development of starfish embryos. Marine Environmental Research 7:265-278.
Chan, G. 1977. The five-year recruitment of marine life after the 1971 San Franciso oil spill. In: Proceedings 1977 Oil Spill Conference, American Petroleum Institute, Washington, D.C.
Chen, F., and P. D. Yapa. 2001. Modeling gas separation from a bent deepwater oil and gas jet/plume. Proceedings of the 5th Intr. Marine Environmental Modeling Seminar, October 9-2001. New Orleans, LA.
Chiou, C. T., S. E. McGroddy, and D. E. Kile. 1998. Partition characteristics of polycyclic aromatic hydrocarbons on soils and sediments. Environmental Science and Technology 32:264-269.
Clark, B., J. Parsons, C. Yen, B. Ahier, J. Alexander, and D. Mackay. 1987. A study of factors influencing oil submergence, EE-90, Environment Canada, Ottawa, Ontario, 77 p.
Clark, R. B. 1984. Impact of oil pollution on seabirds. Environmental Pollution 33:1-22.
Clester, S. M., S. J. Hornafius, U. Scepan, and J. E. Estes. 1996. Quantification of the relationship between natural gas seepage rates and surface oil
volume in the Santa Barbara Channel. Trans. American Geophysical Union, 77(46), Supplement, F420.
Clewell, H. J. 1980a. The effect of fuel composition on groundfall from aircraft fuel jettisoning. Air Force Engineering and Services Laboratory Report, ESL-TR-81-13. Tyndall Air Force Base, FL.
Clewell, H. J. 1980b. Fuel jettisoning by U.S. Air Force aircraft. Volume 1: Summary and analysis. Air Force Engineering and Services Laboratory Report, ESL-TR-80-17. Tyndall Air Force Base, FL.
Clewell, H. J. 1983. Ground contamination by fuel jettisoned from aircraft in flight. Journal of Aircraft 18 (4):382-384.
Cline, P. V., J. J. Delfino, and P. S. C. Rao. 1991. Partitioning of aromatic constituents into water from gasoline and other complex mixtures. Environmental Science and Technology 25:914-920.
Coates, J. D., R. T. Anderson, J. C. Woodward, E. J. P. Phillips, and D. R. Lovley. 1996. Anaerobic hydrocarbon degradation in petroleum-contaminated harbor sediments under sulfate-reducing and artificially imposed iron-reducing conditions. Environmental Science and Technology 30:2784-2789.
Cole, R. H., R. E. Frederick, R. P. Healy, and R. G. Rolan. 1984. Preliminary findings of the priority pollutant monitoring project of the Nationwide Urban Runoff Program. Journal Water Pollution Control Federation 56(7):898-908.
Collier, T., Margaret M. Krahn, Cheryl A. Krone, Lyndal L. Johnson, Mark S. Myers, Sin-Lam Chan, and Usha Varanasi. 1993. Oil exposure and effects in subtidal fish following the Exxon Valdez oil spill. In: Proceedings 1993 International Oil Spill Conference (Prevention, Preparedness, Response), March 29-April 1, 1993, Tampa, FL. Pp. 301-305.
Collins, K. M., E. L. Brannon, L. L. Moulton, M. A. Cronin, and K. R. Parker. 2000. Hydraulic Sampling Protocol to Estimate Natural Embryo Mortality of Pink Salmon. Transactions of the American Fisheries Society 129:827-834.
Connell, D. W. 1982. An approximate petroleum hydrocarbon budget for the Hudson Raritan Estuary, New York. Marine Pollution Bulletin 13(3):89-93.
Connell, D. W., and G. J. Miller. 1984. Chemistry and Ecotoxicology of Pollution. John Wiley & Sons, New York. 444 pp.
Conover, R. J. 1971. Some relations between zooplankton and bunker C oil in Chedabucto Bay, following the wreck of the tanker Arrow. Journal of Fisheries Research Board of Canada 28:1327-1330.
Continental Shelf Associates, Inc. 1997. Gulf of Mexico Produced Water Bioaccumulation Study. Definitive Component Technical Report. Prepared for Offshore Operators Committee, by Continental Shelf Associates, Inc., Jupiter, FL.
Cordah. 1998. Review of drill cuttings piles in the North Sea. Report for the Offshore Decommissioning Communications Project. Cordah Environmental Consultants, Aberdeen, Scotland. 90 pp.
Cotham W. E., and T. F. Bidleman. 1995. Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in air at an urban site near Lake Michigan. Environmental Science and Technology 29:2782.
Cross, Jeffrey N., John T. Hardy, Jo Ellen Hose, G. Patrick Hershelman, Liam D. Antrim, Richard W. Gossett, and Eric A. Crecelius. 1987. Contaminant Concentrations and Toxicity of Sea-surface Microlayer near Los Angeles, California. Marine Environmental Research 23:307-323.
Cross, N. L., and R. G. Picknett. 1973. Ground contamination by fuel jettisoned from aircraft. NATO discussion paper from AGARD workshop held April 1973, pp. 12. 1-12. 9.
Crunkilton, Ronald L., and William M. DeVita. 1997. Determination of aqueous concentrations of polycyclic aromatic hydrocarbons (PAH) in an urban stream. Chemosphere 35(7):1447-1463.
Cubit, J. D., and J. L. Connor. 1993. Effects of the 1986 Bahia Las Minas oil spill on reef flat sessile biota, algal-turf infauna, and sea urchins. Keller, B. D. and J. B. C. Jackson (eds.). Long-term assessment of the oil spill at Bahia Las Minas, Pamana. Volume II, Technical Report. Minerals Management Service, Gulf of Mexico OCS Region, New Orleans, LA. OCS Study MMS 93-0048, pp. 131-242.
Culliton, Thomas J., Maureen A. Warren, Timothy R. Goodspeed, Davida G. Remer, Carol M. Blackwell, and John J. McDonough, III. 1990. Fifty Years of Population Change Along the Nation’s Coasts: 1960-2010. Coastal Trends Series. Rockville, MD. National Oceanic and Atmospheric Administration.
Daisey, J. M., R. J. Hershman, and Kneip. 1982. Ambient levels of particulate organic matter in New York City in winter and summer. Atmospheric Environment 16:2161-2168
Daisey, J. M., R. J. McCaffrey, and R. A. Gallagher. 1981. Polycyclic aromatic hydrocarbons and total extractable particulate organic matter in Arctic aerosol. Atmospheric Environment 15(8):1353-1363.
Dale, B. A., G. M. Narahara, and R. M. Stevens. 2000. Case history of reservoir subsidence and wellbore damage management in the South Bellridge Diatomite Field . SPE Production & Facilities 15 (1):50-57.
Dale, M. S., B. Koch, R. F. Losee, E. W. Crofts and M. K. Davis. 2000. MBTE in Southern California Water. AWWA 92:42-51.
Dauvin, J. C. 1987. Evolution à long terme (1978-1986) des populations d’amphipodes des sables fins de la Pierre Noire (Baie de Morlaix, Manche Occidentale) après la catastrophe de l’Amoco Cadiz. Marine Environmental Research 21:247-273.
Dauvin, J. C. 1998. The fine sand Abra alba community of the bay of Morlaix twenty years after the Amoco Cadiz oil spill. Marine Pollution Bulletin 36:669-676.
Dauvin, J. C., and F. Gentil. 1990. Conditions of the pericarid populations of sub-tidal communities in northern Brittany ten years after the Amoco Cadiz oil spill. Marine Pollution Bulletin 21:123-130.
Davies, J. M., J. Addy, L. Blackman, J. Blanchard, J. Ferbrache, D. Moore, H. Sommerville, A. Whitehead and T. Wilkinson. 1983. Environmental Effects of Oil Based Mud Cuttings. Report of Joint Working Group of UKOOA Clean Seas and Environmental Committee/Dept. of Energy/ Dept. of Agriculture and Fisheries for Scotland/Ministry of Agriculture, Fisheries and Food. Great Britain, 24 pp.
Davies, R. P., and A. J. Dobbs. 1984. The prediction of bioconcentration in fish. Water Resources Bulletin 18:1253-1262.
Davis, P. H., and R. B. Spies. 1980. Infaunal benthos of a natural petroleum seep: a study of community structure. Marine Biology 59:31-41.
Davis, P. H., T. W. Schultz and R. B. Spies. 1981. Toxicity of Santa Barbara seep oil to starfish embryos II. The growth bioassay. Marine Environmental Research 5:287-294.
Davis, S. 2000. Transportation Energy Data Book: Edition 20, Oak Ridge National Laboratory, Department of Energy. ORNL6959.
Dawbarn, R., K. W. Nutt, and C. W. Pender. 1975. A study of the jettisoning of JP-4 fuel in the atmosphere. Arnold Engineering Development Centre Report: AEDC-TR-75-49, Tennessee.
Day, R. H., S. M. Murphy, J. A. Wiens, C. G. Harner, and L. N. Smith. 1997. Effects of the Exxon Valdez oil spill on habitat use by birds in Prince William Sound, AK. Ecological Applications 7:593-613.
Day, R. H., S. M. Murphy, J. A. Wiens, G. D. Hayward, E. J. Harner, and L. N. Smith. 1995. Use of oil-affected habitats by birds after the Exxon Valdez oil spill. Exxon Valdez oil spill: fate and effects in Alaskan waters. P. G. Wells, J. N. Butler, and J. S. Hughes [eds.]. ASTM-STP 1219, American Society for Testing and Materials, Philadelphia, PA, pp. 726-761.
de la Cruz, A. A., C. T. Hackney, and B. Rajanna. 1981. Some effects of crude oil on a Juncus tidal marsh. Journal of the Elisha Mitchell Scientific Society 97(1):14-28.
Dean, T. A., Bodkin, J. L., Jewett, S. C., Monson, D. H., and Jung, D.2000. Changes in sea urchins and kelp following a reduction in sea otter den
sity as a result of the Exxon Valdez oil spill. Marine Ecology Progress Series 199:281-291.
Dean, T. A., M. S. Stekoll, and R. O. Smith. 1996. Kelp and oil: the effects of the Exxon Valdez oil spill on subtidal algae. In: S. D. Rice, R. B. Spies, D. A. Wolfe, and B. A. Wright, editors. Proceedings of the Exxon Valdez oil spill symposium. American Fisheries Society Symposium 18:412-423
Dean, T. A., M. S. Stekoll, S. C. Jewett, R. O. Smitha and J. E. Hose. 1998. Eelgrass (Zostera marina L.) in Prince William Sound, Alaska: effects of the Exxon Valdez oil spill. Marine Pollution Bulletin 36:201-210.
Degens, E., and V. Ittekkot. 1983. Dissolved organic carbon: An overview. In Degens, Egon T., Stephan Kempe, and Hassan Soliman, ed. Transport of Carbon and Minerals in Major World Rivers, Part 2. Heft 55 SCOPE/UNEP Sonderband, Hamburg, Germany, pp. 21-38.
Degens, E., S. Kempe, and J. Richey. 1991. Summary: biogeochemistry of major world rivers. Degens, E. T., S. Kempe, and J. E. Richey [eds.]. Biogeochemistry of Major World Rivers. John Wiley and Sons, Chichester, NY, pp. 323-347.
DeLaune, R. D., C. J. Smith, W. H. Patrick, Jr., J. W. Fleeger, and M. D. Tolley. 1984. Effect of oil on salt marsh biota, methods for restoration. Environmental Pollution 36:207-227.
DeLaune, R. D., R. P. Gambrell, J. H. Pardue, and W. H. Patrick, Jr. 1990. Fate of petroleum hydrocarbons and toxic organics in Louisiana coastal environments. Estuaries 13:72-80.
DeLeon, I., C. Byrne, E. Peuler, S. Antoine, J. Schaeffer, and R. Murphy. 1986. Trace organic and heavy metal pollutants in the Mississippi River. Chemosphere 15(6):795-805.
DeLuca, M., and L. LeBlanc. 1997. Offshore forecast—1998. Offshore Magazine, December 1997.
Delvigne, G. A. 1993. Natural dispersion of oil by different sources of turbulence. Proceedings of the 1993 International Oil Spill Conference. American Petroleum Institute, Washington, DC, pp. 415-419.
Delvigne, G. A., J. A. van der Stel, and C. E. Sweeney. 1987. Final report: Measurement of vertical turbulent dispersion and diffusion of oil droplets and oiled particles, Delft Hydraulics Laboratory Report.
Delvigne, G. A. L., and C. E. Sweeney. 1998. Natural dispersion of oil. Oil and Chemical Pollution. Vol. 4. Pp. 281-310.
Devlin, D. J., and C. E. Proffitt. 1996. Experimental analyses of the effects of oil on mangrove seedlings and saplings and the mangrove gastropod Melampus coffeus L. Pages 3-23 in C. E. Proffitt and P. F. Roscigno (eds.). Symposium Proceedings: Gulf of Mexico and Caribbean Oil Spills in Coastal Ecosystems: Assessing Effects, Natural Recover, and Progress in Remediation Research. OCS Study MMS 95-0063, U.S. Dept. of Interior, Minerals Management Service, New Orleans, LA.
Dickhut, R. M., and K. E. Gustafson. 1995. Atmospheric washout of polycyclic aromatic hydrocarbons in the southern Chesapeake Bay region. Environmental Science and Technology 29:1518-1525.
DiToro, D. M., J. A. McGrath, and D. J. Hansen. 2000. Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria: I. Water and Tissue. Environmental Toxicology and Chemistry 19(8):1951-1970.
DOE (U.S. Department of Energy). 1999. Petroleum Supply Annual, 1999, Volume 1. on the World Wide Web: http://www.eia.doe.gov/pub/oil_gas/petroleum/data_publications/petroleum_supply_annual/psa_volume1/historical/1999/txt/table_14.txt
Donkin, P., J. Widdows, S. V. Evans, and M. D. Brinsley. 1991. QSARs for sublethal responses of marine mussels (Mytilus edulis). Sci. Tot. Environ. 109/110:461-476.
Donkin, P., J. Widdows, S. V. Evans, C. M. Worrall and M. Carr. 1990. Quantitative structure-activity relationships for the effect of hydrophobic chemicals on rate of feeding by mussels (Mytilus edulis). Aquatic Toxicology 14:277-294.
Doskey P. V., and A. W. Andren. 1986. Particulate- and Vapor-phase n-alkanes in the Northern Wisconsin Atmosphere. Atmospheric Environment 20:(9) 1735-1744.
Driscoll, S. K., and A. E. McElroy. 1996. Bioaccumulation and metabolism of benzo [a] pyrene in three species of polychaete worms. Environmental Toxicology and Chemistry 15:1401-1410.
Driskell, W. B., A. K. Fukiyama, J. P. Houghton, D. C. Lees, A. J. Mearns, and G. Shigenaka. 1996. Recovery of Prince William Sound intertidal infauna from Exxon Valdez oiling and shoreline treatments: 1989 through 1992. Pp. 362-378. In: S. D. Rice, R. B. Spies, D. A. Wolfe and B. A. Wright, [eds.]. Proceedings of the Exxon Valdez oil spill symposium. American Fisheries Society Symposium 18.
Duce, R. A., and R. B. Gagosian. 1982. The Input Of Atmospheric N-C10 to N-C30 Alkanes to the ocean. Journal of Geophysical Research— Oceans and Atmospheres 87 (NC9): 7192-7200.
Duffy, L. K., R. T. Bowyer, J. W. Testa, and J. B. Faro. 1996. Acute phase proteins and cytokines in Alaskan mammals as markers of chronic exposure to environmental pollutants. In: Rice, S. D., R. S. Spies, D. A. Wolfe , and B. A. Wright. In: Proceedings of the Exxon Valdez oil spill symposium. American Fisheries Society Symposium 18, pp. 809-813.
Duke, N. C., and Z. S. Pinzon. 1993. Mangrove forests. Pp. 447-533 in B.D. Keller and J. B. C. Jackson, Long-Term Assessment of the Oil Spill at Bahia las Minas, Panama, Vol. II, Synthesis Report. OCS Study Minerals Management Service, 93-0047, United States Department of the Interior, Minerals Management Service, New Orleans, LA.
Duke, N. C., Z. S. Pinzon, and M. C. Prada T. 1997. Large-scale damage to mangrove forests following two large oil spills in Panama. Biotropica 29:2-14.
Dyrynda, EA, R. J. Law, P. E. J. Dyrynda, C. A. Kelly, R. K. Pipe, and N. A. Ratcliffe. 1997. Changes in immune parameters of natural mussel Mytilus edulis populations following a major oil spill (Sea Empress, Wales, UK). Marine Ecology Progress Series 206:155-170.
Eastcott, L., W. Y. Shiu, and D. Mackay. 1988. Environmentally relevant physical-chemical properties of hydrocarbons: a review of data and development of simple correlations. Oil and Chemical Pollution 4:191-216.
Eganhouse, R., and I. Kaplan. 1981. Extractable organic matter in urban stormwater runoff: 1. Transport dynamics and mass emission rates . Environmental Science and Technology 15(3):310-315.
Eganhouse, R., B. Simoneit, and Isaac R. Kaplan. 1981. 1. Extractable organic matter in urban stormwater runoff; 2. Molecular Characterization. Environmental Science and Technology 15(3):315-326.
Eganhouse, R. P., and I. R. Kaplan. 1982. Extractable organic matter in municipal wastewaters: 1. Petroleum hydrocarbons: temporal variations and mass emission rates to the oceans. Environmental Science and Technology 16:180-186.
Eley, F. J. 1988. Proceedings of the workshop on evaporation and evapo-transpiration processes. Canadian Climate Center Report Vol. 82:108.
Elliot, A.J., N. Hurford, and C.J. Penn. 1986. Shear diffusion and the spreading of oil slicks. Marine Pollution Bulletin 17: 308-313.
Elliott, A. J. 1986. Shear diffusion and the spread of oil in the surface layers of the North Sea. Deutsche Hydrographishe Zeitschrift 39:113-137.
Elliott, A. J., and D. C. Wallace. 1989. Dispersion of surface plumes in the southern North Sea. Dt. Hydrogr. Zeit., Vol 42:1016.
Ellis, M. S., E. A. Wilson-Ormond and E. N. Powell. 1996. Effects of gas-producing platforms on continental shelf macroepifauna in the northwestern Gulf of Mexico: Abundance and size structure. Canadian Journal of Fisheries and Aquatic Sciences 53:2589-2605.
Elmgren, R., G. A. Vargo, J. F. Grassle, J. P. Grassle, D. R. Heinle, G. Longelis and S. L. Vargo. 1980. Trophic interactions in experimental marine ecosystems perturbed by oil. Pages 779-800 in J. P. Giesy (ed., Microcosms in Ecological Research. United States Department of Energy, Washington, D.C.
Elmgren, R., S. Hanson, U. Larson, B. Sundelin, and P. D. Boehm. 1983. The Tsesis: Acute and long-term impact on the benthos. Marine Biology 73:51-65.
Energy Information Agecny. 1999. International Energy Review. [Online]. Available: http://www.eia.doe.gov
Environment Canada. 2000. A Field Guide to the Documentation and Description of Oiled Shorelines. Second Edition. Alberta, Canada. 108 pp.
Environmental Science and Technology 32:3719-3723.
Erikson, D. E. 1995. Surveys of murre colony attendance in the northern Gulf of Alaska following the Exxon Valdez oil spill. In: Wells, P. G., J. M. Butler, J. S. Hughes, (eds). Exxon Valdez oil spill: Fate and effects in Alaskan Waters. ASTM STP 1219, America Society for Testing and Materials. Philadelphia, PA. Pp. 780-853.
Esler, D., T. D. Bowman, T. A. Dean, C. E. O’Clair, S. C. Jewwett, and L. L. McDonald. 2000a. Correlates of harlequin duck densities during winter in Prince William Sound, AK, Condor 102:920-926.
Esler, D., T. D. Bowman, C. E. O’Clair, T. A. Dean, and L. I. McDonald. 2000b. Densities of Barrow’s Goldeneyes during winter in Prince William Sound, Alaska in relation to habitat, food and history of oil contamination. Waterbirds 23:423-429.
Esler, D. J. A. Schwartz, R. L. Jarvis, and D. M. Mulcahy. 2000c. Winter survival of adult female harlequin ducks in relation to history of contamination by the Exxon Valdez oil spill. Journal of Wildlife Management, 64.
Estes, J. A., and D. O. Duggins. 1995. Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecological Monographs 65:75-100.
European Commission. 1996. Pollution from Aircraft Emissions in the North Atlantic Flight Corridor (POLINAT): European Commission, Environmental Research Programme, report no. Eur 16978 EN.
Evans, M. I., P. Symens, and C. W. T. Pilcher. 1993. Short-term damage to coastal bird populations in Saudi Arabia and Kuwait following the 1991 Gulf war marine pollution. Marine Pollution Bulletin 27:157-161.
Fallah, M. H., and R. M. Stark. 1976. Literature review: Movement of spilled oil at sea. Marine Technology Society Journal 10:3-18 .
Fam, S., M. K. Stenstrom, and G. Silverman. 1987. Hydrocarbons in runoff. Journal of Environmental Engineering 113(5):1032-1046.
Fannelop, T. K., and K. Sjoen. 1980. Hydrodynamics of underwater blowouts. AIAA 8th Aerospace Sciences Meeting, January 14-16, AIAA paper: 80 pp. Pasadena, California.
Farrington, J. W. 1986. Fossil fuel aromatic hydrocarbon biogeochemistry in the marine environment: research challenges. In: C. S. Giam and H. J. M. Dou, eds. Strategies and Advanced Techniques for Marine Pollution Studies: Mediterranean Sea. Springer-Verlag, Berlin.
Farrington, J. W., A. C. Davis, N. M. Frew, and K. S. Rabin. 1982. No. 2 Fuel Oil Compounds in Mytilus edulis: Retention and Release after an Oil Spill. Marine Biology, 66:15-26.
Fay, J.A. 1969. The spread of oil slicks on a calm sea–oil on the sea. Plenum, New York. Pp 53-64.
Feder, H. M., and M. Blanchard. 1998. The deep benthos of Prince William Sound, Alaska, 16 months after the Exxon Valdez oil spill. Marine Pollution Bulletin 36:118-130.
Fellin, P., L. A. Barrie, and D. Muir. 1997. Monitoring of selected organochlorine and polycyclic aromatic hydrocarbon compounds in the Arctic. J. E. Baker [eds.]: Atmospheric Deposition of Contaminants to the Great Lakes and Coastal Waters. SETAC Press, Pensacola, FL, pp. 259-276.
Fellin, P., L. A. Barrie, D. Dougherty, D. Toom, D. Muir, N. Grift, L. Lockhart, and B. Billeck. 1996. Air Monitoring in the Arctic: Results for selected persistent organic pollutants for 1992, Environmental and Toxicological Chemistry 15(3):253-261.
Fenchal, T., and T. H. Blackburn. 1979. Bacteria and Mineral Cycling. National Library of Canada, 225 pp.
Fingas, M. 2000. Basics of oil spill cleanup. Second edition. CRC Press, LLC. Boca Raton, FL. 233 pp.
Fingas, M. F. 1993. The behaviour of oil in ice: Combatting marine oil spills in ice and cold conditions. National Board of Waters and the Environment, Helsinki, Finland, pp. 5-22.
Fingas, M. F. 1995. A literature review of the physics and predictive modeling of oil spill evaporation. Journal of Hazardous Materials 42:157-175. Fingas, M. F. 1999. The evaporation of oil spills: Development and implementation of new prediction methodology. Proceedings of the 1999 International Oil Spill Conference. American Petroleum Institute, Washington, DC, pp. 281-287.
Fingas, M. F., and B. Hollebone. 2001. The fate and behavior of oil in freezing environments. In: Marine Oil Spills in Ice and Cold Conditions. National Board of Waters and Environment, Helsinki, Finland, 14pp.
Fingas, M. F., B. Fieldhouse, and J. V. Mullin. 1996. Studies of water-in-oil emulsions: The role of asphaltenes and resins. Proceedings of the Nineteenth Arctic and Marine Oil Spill Program Technical Seminar, Environment Canada, Ottawa, Ontario, pp. 73-88.
Fingas, M. F., B. Fieldhouse, J. Lane, and J. V. Mullin. 2000. Studies of water-in-oil emulsions: Long-term stability, oil properties, and emulsions formed at sea. Proceedings of the Twenty-Third Arctic and Marine Oil Spill Program Technical Seminar. Environment Canada, Ottawa, Ontario, pp. 145-160.
Fischel, M., W. Grip and I. A. Mendelssohn. 1989. Study to determine the recovery of a Louisiana march from an oil spill. In Proceedings 1989 Oil Spill Conference. American Petroleum Institute, Washington, D.C., pp. 383-387.
Fischer, P. J. 1978. Natural gas and oil seeps, Santa Barbara Basin, California. The State Land Commission. 1977: California Gas, Oil, and Tar Seeps, pp. 1-62.
Fisher, S. W. 1995. Mechanisms of Bioaccumulation in Aquatic Systems. In Reviews of Environmental Contamination and Toxicology 142:87-117.
Flores, H., A. Andreatta, G. Llona, and I. Saavedra. 1998. Measurements of oil spill spreading in a wave tank using digital image processing. R. Garcia-Martinez, and C. A. Brebbia [eds.]. Oil and Hydrocarbon Spills, Modeling, Analysis and Control. Computational Mechanics Publications Southampton, UK, pp. 165-173.
Ford, R. G. 1985. A risk analysis model for marine mammals and seabirds: a southern California Bight scenario. Final Report to United States Department of the Interior, Minerals Management Service, Pacific OCS Region, Los Angeles, CA, MMS 85-0104, May 1985, p. 236.
Ford, R. G. 1987. Estimating mortality of seabirds from oil spills. Proceedings of 1987 Oil Spill Conference, Baltimore, MD, American Petroleum Institute (API), pp. 547-551.
Ford, R. G. 1991. Simulation of oil spill trajectories and fates of seabirds killed by oil spilled from the Apex Houston. Report prepared by Ecological Consulting, Inc., Portland, OR, 21pp.
Ford, R. G., J. A. Wiens, D. Heinemann, and G. L. Hunt. 1982. Modeling the sensitivity of colonially breeding marine birds to oil spills: Guillemot and Kittiwake populations on the Pribilof Islands, Bering Sea. Journal of Applied Ecology 19:1-31.
Ford, R. G., M. L. Bonnell, D. H. Varoujean, G. W. Page, H. R. Carter, B. E. Sharp, D. Heinemann, and J. L. Casey. 1996. Total direct mortality of seabirds from the Exxon Valdez oil spill. American Fisheries Society Symposium 18:684-711.
Foreman, W. T., and T. F. Bidleman. 1990. Semi-Volatile Organic Compounds in the ambient air of Denver, Colorado. Atmospheric Environment. 24A:2405-2416.
Francis, R. C., S. R. Hare, A. B. Hollowed, and W. S. Wooster. 1998. Effects of interdecadal climate variability on the oceanic ecosystems of the NE Pacific. Fisheries Oceanography 7:1-21.
Frankel, E. 1995. Ocean Environmental Management: Englewood Cliffs. Prentice Hall PTR, p. 381.
Fraser, M. P., G. R. Cass, B. R. T. Simoneit, and R. A. Rasmussen. 1998. Air quality model evaluation data. 5. C5-C22 Nonpolar and semipolar aromatic compounds. Environmental Science and Technology 32:1760-1770.
Fraser, M. P., G. R. Cass, B. R. T. Simoneit, and R. A. Rasmussen. 1997. Air quality model evaluation data for organics. 4. C2-C36 Non-Aromatic Hydrocarbons. Environmental Science and Technology 31:2356-2367.
Freedman, B. 1989. Environmental ecology: The impacts of pollution and other stresses on ecosystem structure and function. Academic Press, San Diego, CA, 424pp.
French McCay, D. 2001. Development and Application of an Oil Toxicity and Exposure Model: Oil Toxicity and Exposure: Final Report to NOAA Damage Assessment Center, Silver Spring, MD, January 2001, 50pp. + appendixes.
French, D. P. 1991. Estimation of exposure and resulting mortality of aquatic biota following spills of toxic substances using a numerical model, Aquatic Toxicology and Risk Assessment: Fourteenth Volume, ASTM STP 1124. M. A. Mayes, and M. G. Barron [eds.]. American Society for Testing and Materials: Philadelphia, PA, pp. 35-47.
French, D. 1998a. Modeling the impacts of the North Cape oil spill. Proceedings: Twenty-First Arctic and Marine Oilspill Program (AMOP) Technical Seminar, June 10-12, 1998, Edmonton, Alberta, Canada, pp. 387-430.
French, D. P. 1998b. Estimate of injuries to marine communities resulting from the North Cape oil spill, based on modeling of fates and effects. Report to NOAA Damage Assessment Center, Silver Spring, MD.
French, D. P. 1998c. Updated estimate of injuries to marine communities resulting from the North Cape oil spill based on modeling of fates and effects. Report to NOAA Damage Assessment Center, December, 1998, Silver Spring, MD.
French, D. P. 2000. Estimation of oil toxicity using an additive toxicity model. Proceedings of the Twenty-Third Arctic and Marine Oil Spill Program Technical Seminar, Vancouver, BC, Canada, pp. 561-600.
French, D. P., and F. W. French, III. 1989. The biological effects component of the natural resource damage assessment model system. Oil and Chemical Pollution 5:125-163.
French, D. P., and M. Reed. 1990. Potential impact of entanglement in marine debris on the population dynamics of the northern fur seal, Callorhinus ursinus. R. S. Shomura and M. L. Godfrey [eds.]: Proceedings of the Second International Conference on Marine Debris, April 2-7, 1989, Honolulu, HA, pp. 431-452.
French, D. P., and M. Reed. 1991. Computer modeling of physical fates, biological impacts and natural resource damages resulting from discharges of oils and hazardous substances, Dangerous Properties of Industrial Materials Report, Van Nostrand Reinhold Co., NY. Vol. 11, No. 5/6, pp. 416-422.
French, D. P., and H. Rines. 1997. Validation and use of spill impact modeling for impact assessment, Proceedings of 1997 International Oil Spill Conference, Fort Lauderdale, Florida, American Petroleum Institute (API) Publication 4651, pp. 829-834.
French, D. P., M. Reed, J. Calambokidis, and J. Cubbage. 1989. A simulation model of seasonal migration and daily movements of the northern fur seal, Callorhinus ursinus, Ecological Modeling 48:193-219.
French, D. P., M. Reed, K. Jayko, S. Feng, H. Rines, S. Pavignano, T. Isaji, S. Puckett, A. Keller, F. W. French III, D. Gifford, J. McCue, G. Brown, E. MacDonald, J. Quirk, S. Natzke, R. Bishop, M. Welsh, M. Phillips and B. S. Ingram. 1996. The CERCLA type A natural resource damage assessment model for coastal and marine environments (NRDAM/ CME), Technical Documentation, Vol. I—Model Description, Final Report: submitted to the Office of Environmental Policy and Compliance, Contract No. 14-0001-91-C-11. April, 1996. U.S. Dept. of the Interior, Washington, DC.
French, D., H. Schuttenberg, and T. Isaji. 1999. Probabilities of oil exceeding thresholds of concern: examples from an evaluation for Florida Power and Light. Proceedings of the Twenty-Second Arctic and Marine Oil Spill Program AMOP 99 Technical Seminar, June 2-4. Calgary, Alberta, Canada, pp. 243-270.
Frost, K. J., L. F. Lowry, E. Sinclair, J. Ver Hoef, and D. McAllister. 1994a. Impacts on distribution, abundance, and productivity of harbor seals. In: Marine mammals and the Exxon Valdez. T. R. Loughlin [ed.]. Academic Press. San Diego, CA, pp. 97-118.
Frost, K. J., C. A. Manen, and T. L. Wade, 1994b. Hydrocarbon contaminants in harbor seals in Prince William Sound and the Gulf of Alaska. In: Marine mammals and the Exxon Valdez. T. R. Loughlin, [ed]. Academic Press. San Diego, CA, pp. 331-358.
Frost, K. J., L. F. Lowry, and J. M. Ver Hoef. 1999. Monitoring the trend of harbor seals in Prince William Sound, Alaska, after the Exxon Valdez oil spill. Marine Mammal Science 15:494-506.
Fry, D. M., and L. A. Addiego. 1987. Hemolytic anemia complicates the cleaning of oiled seabirds. Wildlife Journal 10(3):3-8.
Fry, D. M., and L. A. Addiego. 1988: Effects of oil exposure and stress on seabird endocrine systems. In: Proceedings 1988 International Association of Aquatic Animal Medicine 19:60-67.
Fulton, M. H., G. I. Scott, A. Fortner, T. F. Bidleman, and B. Ngabe. 1993. The effects of urbanization on small high salinity estuaries of the southeastern United States. Archives of Environmental Toxicology 25:476-484.
Gabche, C. E., J. Folack, and E. C. Yongbi. 1998. Tar ball levels on some beaches in Cameroon. Marine Pollution Bulletin. 36:535-539.
Gabele, P. A. and S. M. Pyle. 2000. Emissions from two outboard engines operating on reformulated gasoline containing MBTE. Environmental Science and Technology 34:368-372.
Garcia-Martinez, R., Mata, L. J. and Flores-Tovar, H. A. 1996. Correction to the Mackay oil spreading formulation. Proc of the Nineteenth Arctic and Marine Oil Spill Program Technical Seminar, Environment Canada, Ottawa, Canada, pp. 1627-1635.
Gardner, G. R., and R. J. Pruell. 1988. A histopathological and chemical assessment of winter flounder, lobster and soft-shelled clam indigenous to Quincy Bay, Boston Harbor and an in-situ evaluation of oysters including sediment (surface and cores) chemistry. U.S. Environmental Protection Agency, Environmental Research Laboratory, Narragansett, RI.
Garrett, R. M., I. J. Pickering, C. E. Haith, and R. C. Prince. 1998. Photooxidation of crude oils.
Garrity, S., S. Levings, and K. A. Burns. 1994. The Galeta oil spill I, long term effects on the structure of the mangrove fringe. Estuarine, Coastal and Shelf Science 38:327-348.
Garrity, S. D., and S. C. Levings. 1991. Effects of an oil spill on some organisms living on mangrove roots in low wave-energy habitats in Carribean Panama. Marine Environmental Research 35:251-271.
Garrity, S.D., and S. C. Levings. 1993. Effects of an Oil Spill on Some Organisms Living on Mangrove (Rhizophora mangle L.) Roots in Low Wave-Energy Habitats in Caribbean Panama. Marine Environmental Research 35: 251-271.
Garrott, R. A., L. L. Eberhardt, and D. M. Burn. 1993. Mortality of sea otters in Prince William Sound following the Exxon Valdez oil spill. Marine Mammal Science 9:343-359 and 15:494-506.
Garshelis, D. L., and C. B. Johnson. 2001. Sea otter population dynamics and the Exxon Valdez oil spill: disentangling the confounding effects. Journal of Applied Ecology 38:19-35.
Geraci, J. R. 1990. Physiologic and toxic effects on cetaceans. In: Sea Mammals and Oil: Confronting the Risks. J. R. Geraci, and D. J. St. Aubin [eds.]. Academic Press. San Diego, CA, pp. 167-197.
Geraci, J. R., and D. J. St. Aubin. 1987. Effects of offshore oil and gas development on marine mammals and turtles. In Long Term Environmental effects of offshore oil and gas development. D. F. Boesch and N. N. Rabalais [eds.]. Elsevier Applied Science Publishers, London, UK and New York, pp. 587-617.
Geraci, J. R., and D. J. St. Aubin (Eds.). 1990. Sea Mammals and Oil: Confronting the Risks. Academic Press, San Diego, 282pp. Geraci, J. R., and D. J. St. Aubin (Eds.). 2000. Sea mammals and oil, confronting the risks. Academic Press, San Diego, CA, XVI + 282pp.
Geraci, J. R., and T. D. Williams. 1990. Physiologic and toxic effects on sea otters. In: Sea Mammals and Oil: Confronting the Risks. J. R. Geraci, and D. J. St. Aubin [eds.]. Academic Press. San Diego, CA, pp. 211-221.
Getter, C. C. 1982. Oil spills and mangroves: a review of the literature, field and lab studies. P. J. Rand (ed.) Land and Water Issues Related to Energy Development. Ann Arbor Science, Ann Arbor, MI, pp. 303-318.
Getter, C. D., G. I. Scott, and J. Michel. 1981. The effects of oil spills on mangrove forests: a comparison of five spill sites in the Gulf of Mexico and the Caribbean Sea. 1981 Oil Spill Conference Proceedings: American Petroleum Institute, Washington, D.C. Pp. 535-540.
Getter, C. D., T. G. Ballou, and C. B. Koons. 1985. Effects of dispersed oil on mangroves: synthesis of a seven-year study. Marine Pollution Bulletin 16:318-324.
Geyer, R. A., and C. P. Giammona. 1980. Naturally occurring hydrocarbons in the Gulf of Mexico and Caribbean Sea. R. D. Geyer [ed.]. Marine Environmental Pollution I. Hydrocarbons. Elsevier, New York, pp. 37-106.
Gigliotti, C. L., J. Dachs, E. D. Nelson, P. A. Brunciak, and S. J. Eisenreich. 2000. Polycyclic aromatic hydrocarbons in the New Jersey Coastal Atmosphere. Environmental Science and Technology 34:3547-3554.
Gilfillan, E. S., D. W. Mayo, D. S. Page, D. Donovan and S. Hanson. 1977. Effects of varying concentrations of petroleum hydrocarbons in sediments on carbon flux in Mya arenaria. In: Physiological Responses of Marine Biota to Pollutants. F. J. Vernberg, A. Calabrese, F. P. Thurberg and W. B. Vernberg (eds.). Academic Press, New York, NY. Pp. 299-314.
Gleick, P. H., [ed.]. 1993. Water in crisis: A guide to the world’s fresh water resources. Oxford University Press, New York. 473pp.
Grant, A., and A. D. Briggs. 2002. Toxicity of sediments from around a North Sea oil platform: are metals or hydrocarbons responsible for ecological impacts? Marine Environmental Research 53:95-116.
Grassle, J. F., R. Elmgren, and J. P. Grassle. 1981. Response of benthic communities in MERL experimental ecosystems to low level, chronic additions of No. 2 fuel oil. Marine Environmental Research 4:279-297.
Group of Experts on the Scientific Aspects of Marine Pollution (GESAMP). 1993. Impacts of oil and related chemicals and wastes in the marine environment . GESAMP Reports and Studies: No. 50 International Marine Organization. London, UK.
Gschwend, P. M., and R. P. Schwarzenbach. 1992. Physical chemistry of organic compounds in the marine environment. Marine Chemistry 39:187-207.
Guidetti, P., M. Modena, G. La Mesa and M. Vacchi. 2000. Composition, abundance and stratification of macrobenthos in the marine area impacted by tar aggregates derived from the Haven oil spill (Ligurian Sea, Italy), Marine Pollution Bulletin 40:1161-1164.
Gundlach, E. R., J. C. McCain, and Y. Fadlallah. 1993. Distribution of oil along the Saudi Arabian coastline (May/June 1991) as a result of the Gulf War oil spills. Marine Pollution Bulletin 27:93-96.
Gupta, M. K., R. W. Agnew, D. Gruber, W. Kreutzberger. 1981. Constituents of highway runoff. Volume IV: Characteristics of highway runoff from operating highways. Research Report: FHWA/RD-81/045: p. 171.
Gustaffson, M. C. U., M. Hansson, C. G. Kannangara, and L. Hederstedt. 1997. Isolated Bacillus subtilis HemY has coproporphyrinogen III to coproporphyrinogen III oxidase activity. Biochimica et Physica Acta-Protein Structure and Molecular Enzymology 1340(1):97-104 .
Gustafson, K. E., and R. M. Dickhut. 1997a. Gaseous Exchange of Polycyclic Aromatic Hydrocarbons Across the Air-Water Interface of Southern Chesapeake Bay, Environmental Science and Technology, Vol. 31, pp. 1623-1629.
Gustafson, K. E., and Rebecca M. Dickhut. 1997b. Distribution of polycyclic aromatic hydrocarbons in southern Chesapeake Bay surface water: Evaluation of three methods for determining freely dissolved water concentrations. Environmental Toxicology and Chemistry 16(3):452-461.
Gustafsson, Ö., F. Haghseta, C. Chan, J. MacFarlane, and P. M. Gschwend. 1997a. Quantification of the dilute sedimentary soot phase: implications for PAH speciation and bioavailability. Environmental Science and Technology 31:203-209.
Gustafsson, Ö., P. M. Gschwend and K. O. Buesseler. 1997b. Using 234th disequilibria to estimate the vertical removal rates of polycyclic aromatic hydrocarbons from the surface ocean. Marine Chemistry, Vol. 57:11-23.
Guzmán, H. M., and I. Holst. 1993. Effects of chronic oil-sediment pollution on the reproduction of the Caribbean coral Siderastrea siderea. Marine Pollution Bulletin 26:276-282
Guzmán, H. M., J. B. C. Jackson, and I. Holst. 1993. Changes and recovery of subtidal reef corals . In: Keller, B. D. and J. B. C. Jackson (eds., Long-term assessment of the oil spill at Bahia Las Minas, Pamana. Volume II, Technical Report. Minerals Management Service, Gulf of Mexico OCS Region, OCS Study MMS 93-0048, New Orleans, LA, pp. 361-446.
Guzmán, H.M., K.A.Burns, and J.B.C. Jackson. 1994. Injury, regeneration and growth of Caribbean reef corals after a major oil spill in Panama. Marine Ecology Progress Series 105: 231-241.
Hale, D. 2000. Personal communication. Louisiana Department of Environmental Quality, Baton Rouge, LA.
Hall, K., and B. Anderson. 1988. The toxicity and chemical composition of urban stormwater runoff. Canadian Journal of Civil Engineering 15:98-106.
Halls, J., J. Michel, S. Zengel, J. Dahlin, and, J. Petersen. 1997. Environmental sensitivity index guidelines, Version 2. 0: Prepared for the Hazardous Materials Response and Assessment Division, NOAA. Seattle, WA. National Oceanic and Atmospheric Administration. Tech. Memo. NOS ORCA 115, 79pp. + app.
Hallsall C. J., L. A. Barrie, P. Fellin, D. C. G. Muir, B. N. Billeck, L. Lockhart, F. Y. A. Rovinsk, E. Y. A. Kononov, and B. Pastukhov. 1997. Spatial and temporal variation of polycyclic aromatic hydrocarbons in the Arctic. Environmental Science and Technology 31:3593-3599.
Hampson, G. R., and E. T. Moul. 1978. No. 2 fuel oil spill in Bourne, Massachusetts: immediate assessment of the effects on marine invertebrates and a year study of growth and recovery of a salt marsh. Journal of Fisheries Research Board of Canada 35:731-734.
Hare, S. R., and N. J. Mantua. 2000. Empirical evidence for North Pacific regime shifts in 1977 and 1989. Progress in Oceanography 47:103-146.
Hare, S. R., and R. C. Francis. 1995. Climate change and salmon production in the northeast Pacific Ocean. In: Beamish, R. J. (ed.). Climate change and northern fish populations. Canadian Special Publication in Fisheries and Aquatic Science 121:357-372.
Harman-Fetcho, J. A., L. L. McConnell, C. P. Rice, and J. E. Baker. 2000. Wet deposition and air-water gas exchange of currently used pesticides to a sub-estuary of the Chesapeake Bay. Environmental Science and Technology 34:1462-1468.
Hayashi, I. K., Toshinori and H. Yamakawa. 2000. Distributional characteristics of benthic organisms in sublittoral rocky areas of Mikuni, Fukui Prefecture: part of the survey on the effects of the Nakhodka oil spill. Bulletin Japan Sea National Fisheries Research Institute 50:42-137.
Hayes, M. O., and J. Michel. 2001. A primer for response to oil spills on gravel beaches. Proceedings of the 2001 International Oil Spill Conference. American Petroleum Institute, Washington, D.C.
Hayes, M. O., E. R. Gundlach, and C. D. Getter. 1980. Sensitivity ranking of energy port shorelines. In: Proceedings of the Specialty Conference on Ports ’80. American Society of Civil Engineers, New York, pp. 697-709.
Hayes, M. O., J. Michel, T. M. Montello, D. V. Aurand, T. C. Sauer, A. Al-Mansi, and A. H. Al-Momen. 1995. Distribution and weathering of oil from the Iraq-Kuwait conflict oil spill within intertidal habitats–two years later. In: Proceedings 1995 International Oil Spill Conference, API Publ. No. 4620, American Petroleum Institute, Washington, D. C, pp. 443-451.
Heider, J., A. M. Spormann, H. R. Beller, and F. Widdel. 1999. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiology Reviews 22:459-473.
Heintz, R. A., J. W. Short, and S. D. Rice. 1999. Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon (Onchohynchus gorbuscha) embryos incubating downstream from weathered Exxon Valdez crude oil . Environmental Toxicology and Chemistry 18:494-503.
Heintz, R. A., S. D. Rice, A. C. Wertheimer, R. F. Bradshaw, F. P. Thrower, J. E. Joyce, and J. W. Short. 2000. Delayed effects on growth and marine survival of pink salmon Onchohynchus gorbuscha of exposure to crude oil during embryonic development. Marine Ecology Progress Series 208:205-216.
Hibbs, D. E., J. S. Gulliver, V. R. Voller, and Y-H Chen. 1999. An aqueous concentration model for riverine spills. Journal of Hazardous Materials Vol A 64:37-53.
Hiffe, T. M., and A. H. Knap. 1979. The fate of stranded pelagic tar on a Bermuda beach. Marine Pollution Bulletin 10:203-205.
Ho, K., L. Patton, J. S. Latimer, R. J. Pruell, M. Pelletier, R. McKinney, and S. Jayaraman. 1999. The chemistry and toxicity of sediment affected by oil from the North Cape spilled into Rhode Island Sound. Marine Pollution Bulletin 38:314-323.
Hoff, R. Z. 1996. Responding to oil spills in marshes: the fine line between help and hindrance. Pages 146-161 in C. E. Proffitt and P. F. Roscigno (eds., Symposium Proceedings: Gulf of Mexico and Caribbean Oil Spills in Coastal Ecosystems: Assessing Effects, Natural Recovery, and Progress in Remediation Research. OCS Study MMS 95-0063. Dept. of the Interior, Minerals Management Service, New Orleans, LA.
Hoff, R. M., and K. W. Chan. 1987. Measurement of polycyclic aromatic in air along the Niagara River. Environmental Science and Technology 21:556-561
Hoffman, E. J., Gary L. Mills, James S. Latimer, and James G. Quinn. 1983. Annual input of petroleum hydrocarbons to the coastal environment via urban runoff. Canadian Journal of Fisheries and Aquatic Sciences 40(Suppl. 2):41-53.
Hoffman, E. J., and J. G. Quinn. 1987a. Chronic hydrocarbon discharges into aquatic environments. I. Municipal treatment facilities. In: Vandermeulen, John H., and Steve E. Hrudey [eds.]. Oil in Freshwater: Chemistry, Biology, Countermeasure Technology. Proceedings of the Symposium on Oil Pollution in Freshwater, Alberta, Canada. Pergamon Press, New York, pp. 97-113.
Hoffman, E. J., and J. G. Quinn. 1987b. Chronic hydrocarbon discharges into aquatic environments. II. Urban runoff and combined sewer overflows. In: Vandermeulen, John H, and Steve E. Hrudey [eds.]. Oil in Freshwater: Chemistry, Biology, Countermeasure Technology. Proceedings of the Symposium on Oil Pollution in Freshwater, Alberta, Canada. Pergamon Press, New York, pp. 114-137.
Hoffman, E. J., G. L. Mills, J. S. Latimer, and J. G. Quinn. 1984. Urban runoff as a source of polycyclic aromatic hydrocarbons to coastal waters. Environmental Science and Technology 18:580-587.
Hoffman, E. J., J. S. Latimer, C. D. Hunt, G. L. Mills, and J. G. Quinn. 1985. Stormwater runoff from highways. Water, Air, and Soil Pollution 25:349-364.
Holland-Bartels, L. (ed., Mechanisms of impact and potential recovery of nearshore vertebrate predators following the 1989 Exxon Valdez oil spill. Exxon Valdez Oil Spill Trustee Council Restoration Project Final Report (Restoration Project 95025-99025). U.S. Geological Survey, Alaska Biological Science Center, Anchorage.
Holland-Bartels, L., B. Ballachey, M. A. Bishop, J. Bodkin, T. Bowyer, T. Dean, L. Duffy, D. Esler, S. Jewett, L. McDonald, D. McGuire, C. O’Clair, A. Rebar, P. Snyder, and G. VanBlaircom. 1999. Mechanisms of impact and potential recovery of nearshore vertebrate predators. Exxon Valdez Oil Spill Restoration Project Final Report (Restoration Project 98025). US Geological Survey, Biological Resources Division, Anchorage, AK.
Hollebone, B., D. Perman, and M. Fingas. 2001. The fate and behaviour of oil in freezing environments. Environment Canada Manuscript Report: Ottawa, Ontario.
Holt, S., S. Rabalais, N. Rabalais, S. Cornelius, and J. S. Holland. 1978. Effects of an oil spill on slat marshes at Harbor island, Texas: I: Biology. In: Proceedings of Conference on Assessment of Ecological Impacts of Oil Spills. American Institute of Biological Sciences, Washington, D.C., pp. 344-352.
Hoover-Miller, A., Parker, K. R., and Burns, J. J. 2001. A reassessment of the impact of the Exxon Valdez oil spill on harbor seals (Phoca vitulina richardsi) in Prince William Sound, Alaska. Marine Mammal Science 17:111-135.
Hope-Jones, P., J.-Y. Monnat, C. J. Cadbury, and T. J. Stowe. 1978. Birds oiled during the Amoco Cadiz incident—an interim report. Marine Pollution Bulletin 9:307-310.
Hornafius, J. S., D. Quigley, and B. P. Luyendyk. 1999. The world’s most spectacular marine hydrocarbon seeps (Coal Oil Point, Santa Barbara Channel, California: quantification of emissions. Journal of Geophysical Research 104(C9):20703-20711.
Hornbuckle, K. C., C. W. Sweet, R. F. Pearson, and D. L. Swackhamer. 1995. Environmental Science and Technology 29:869-877 .
Hornbuckle, K. C., J. D. Jeremiason, C. W. Sweet, and S. J. Eisenreich. 1994. Environvironmental Science and Technology 1491-1501.
Horsfall, Jr., M., Fred E. Ogban, and Ayebaemi I. Spiff. 1994. Petroleum hydrocarbon pollution: The distribution in sediment and water of the New Calabar River, Port Harcourt, Nigeria. The Science of the Total Environment 141:217-221.
Howard, R. L., G. S. Boland, B. J. Gallaway, and G. D. Dennis. 1980. Effects of gas and oil field structures and effluents on fouling community production and function. In Environmental Assessment of the Buccaneer Gas and Oil Field in the Northwestern Gulf of Mexico, 1978-1979. Vol. V. NOAA Technical Memo. NMFS-SEFC-39. 287
Howlett, E., K. Jayko, and M. L. Spaulding. 1993. Interfacing real time information with Oil Map. Proceedings of the 16th Arctic and Marine Oil Spill Program (AMOP), Technical Seminar, June 7-9, 1993, Edmonton, Alberta, Canada, pp. 539-548.
Huang, J. C., and F. C. Monastero. 1982. Review of the state of the art oil spill simulation models. Final Report submitted to the American Petroleum Institute, Raytheon Ocean Systems Company, East Providence, RI.
Huang, W. L., H. Yu, and W. J. Weber. 1998. Hysteresis in the sorption and desorption of hydrophobic organic contaminants by soils and sediments—1. A comparative analysis of experimental protocols. Journal of Contaminant Hydrology31:129-148.
Hudson, P. J. 1985. Population parameters for the Alcidae. In: Nettleship,
D. N. and Birkhead, T. R. [eds.]. The Atlantic Alcidae, Academic Press, Orlando, FL.
Hugi, C. 1993. Modelluntersuchungen von Blasenstrahlen fuer die Seebelueftung, Ph.D. Thesis. Inst. f. Hydromechanik u. Wasserwirtschaft, ETH, Zurich, 233-261 pp.
Hunt, G. L., Jr. 1987. Offshore oil development and seabirds: The present status of knowledge and long-term research needs. In: Long-Term Environmental Effects of Offshore Oil and Gas Development. D. F. Boesch and N. N. Rabalais [eds.] London and New York, Elsevier Applied Science Publishers, pp. 539-586.
Hunter, J. V., T. Sabatino, R. Gomperts, and M. J. MacKenzie. 1979. Contribution of urban runoff to hydrocarbon pollution. Journal Water Pollution Control Federation 51(8):2129-2138.
Intergovernmental Oceanographic Commission (IOC), UNESCO. 1984. Manual for monitoring oil in dissolved/dispersal petroleum hydrocarbons in marine waters and on the beaches. In: Procedures for the Petroleum Component of the IOC Marine Pollution Monitoring System (MARPOLMON-P). IOC Manuals and Guides 13. Tar Sampling on Beaches, 13-14pp.
International Association of Oil and Gas Producers, 2000. http://www.ogp.org.uk
International Maritime Organization (IMO). 1990. Petroleum in the marine environment. MEPC30/INF. 13. Sept.
International Maritime Organization (IMO). 2000. Evaluation of IMO Greenhouse Gas Emissions Study, BLG 6/12.
International Petroleum Encyclopedia. 1980. Tulsa, OK. Penn Well Publishing13:454. As cited in National Research Council (1985).
INTERTANKO. 2000. The Critical Behaviour of Crude Oil Influencing its Carriage by Sea.
Irons, D. B. 1996. Size and productivity of Black-legged Kittiwake colonies in Prince William Sound before and after the Exxon Valdez oil spill. American Fisheries Society Symposium 18:738-747.
Irons, D. B., Kendall, S. J., Erickson, W. P., McDonald, L. L., and Lance, B. K. 2001. A brief response to Wiens et al., Twelve years after the Exxon Valdez oil spill. Condor 103:892-894.
Irons, D. B., S. J. Kendall, W. P. Erickson, L. L. McDonald, and B. K. Lance. 2000. Nine years after the Exxon Valdez oil spill: effects on marine bird populations in Prince William Sound, AK, Condor 102:723-737.
Ishaq, A. 1992. Surface and subsurface drainage of metropolitan city in arid zone. Journal of Irrigation and Drainage Engineering 118 (1):19-35.
Ishaq, A., and R. Alassar. 1999. Characterizing urban storm runoff quality in Dhahran City in Saudi Arabia. Water International 24(1):53-58.
Iwata, H., S. Tanabe, N. Sakai, and R. Tatsukawa. 1993. Environmental Science and Technology 27:1080-1098.
Jackson, J., J. Cubit, V. Batista, K. A. Burns, H. Caffey, R. Caldwell, S. Garrity, C. Getter, C. Gonzalez, H. Guzman, K. Kaufmann, B. Keller, A. Knap, S. Levings, M. Marshall, R. Steger, R. Thompson, and E. Weil. 1989. Effects of a major oil spill on Panamanian coastal marine communities. Science 234:37-44.
Jansen, G., and M. Sklar. 1998. Geographic Allocation of State Level Nonroad Engine Population Data to the County Level. U.S. EPA Report: No. NR-014. Office of Mobile Sources, Ann Arbor, MI.
Jayko, K., M. Reed and A. Bowles. 1990. Simulation of interactions between migrating whales and potential oil spills. Environmental Pollution 63:97-127.
Jensen, K., and K. Jørgensen. 1984. Sources and effects of petroleum hydrocarbon pollution in the Baltic Sea. Ophelia (Suppl. 3):61-68.
Jewett, S. C., T. A. Dean, R. O. Smith, and A. Blanchard. 1999. Exxon Valdez oil spill: Impacts and recovery in the soft-bottom benthic community in and adjacent to eelgrass beds. Marine Ecology Progress Series 185:59-83.
Joensen, S. R., and E. B. Hansen. 1977. Oil pollution and seabirds in Denmark 1971-1976. Danish Review of Game Biology 10:1-31.
Johansen, O. 2000. DeepBlow—a lagrangian plume model for deepwater blowouts. Spill Science & Technology Bulletin 6:103-111.
Johansen, O., H. Rye, and C. Cooper. 2001. DeepSpill—Field Study of a simulated oil and gas blowout in deep water, Proceedings Intern. Marine Environmental Modeling Seminar. New Orleans, LA.
Johnsen, S., T. I. Røe, G. Durell, and M. Reed. 1998. Dilution and bioavailability of produced water components in the northern North Sea. A combined modeling and field study. SPE 46578. Pages 1-11 In: 1998 SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production. Society of Petroleum Engineers, Richardson, TX.
Johnson, C. B. and D.L. Garshelis. 1993. Sea Otter abundance distribution, and pup production in Prince William Sound following the Exxon Valdez oil spill, Atlanta, GA. April 26-28, 1993, Exxon Valdez oil spill: fate and effects in Alaskan waters. Philadelphia, PA. Pp. 894-932.
Johnson, L. L., M. R. Arkoosh, T. K. Collier, M. M. Krahn, J. P. Meador, M. S. Myers, W. R. Reichert and J. E. Stein. 2001. The effects of polycyclic aromatic hydrocarbons in fish from Puget Sound Washington. In: The Toxicology of Fishes. R. Di Giulio and D. Hinton [eds.]. Taylor & Francis, New York and London (in review).
Jones, D. A., I. Watt, J. Plaza, T. D. Woodhouse, and M. Al-Sanei. 1996. Natural recovery of the intertidal biota within the Jubail Mainre Wildlife Sanctuary after the 1991 Gulf War oil spill. In Krupp, F., A. H. Abuzinada, and I. A. Nader, eds. A Marine Wildlife Santuary for the Arabian Gulf. Environmental Research and Conservation Following the 1991 Gulf War Oil Spill. National Commission for Wildlife Conservation and Development, Riyadh, Kingdom of Saudi Arabia and Senchenberg Research Institute, Frankfurt a. M., Germany, pp. 138-158.
Jones, R. K. 1997. A Simplified Pseudo-Component Oil Evaporation Model. In: Proceedings of the Twentieth Arctic Marine Oil Spill Program Technical Seminar. Environment Canada, Ottawa, Ontario, pp. 43-61.
Jüttner, F., D. Backhaus, U. Mathias, U. Essers, R. Greiner and B. Mahr. 1995. Emissions of Two- and Four-Stroke Outboard Engines-I. Quantification of Gas and VOC. Water Resources Bulletin 29:1976-1982.
Kagan, B. 1995. Ocean-Atmosphere Interaction and Climate Modeling. Cambridge University Press Cambridge, UK, 377pp.
Kajigaya, H., and N. Oka. 1999. Physical effects of oil pollution in birds. J. Yamashina [ed.] Inst. Ornithol 31:16-38. (In Japanese, with English abstract).
Kan, A. T., G. Fu, M. Hunter, W. Chen, C. H. Ward, and M. B. Tomson. 1998. Irreversible sorption of neutral hydrocarbons to sediments: Experimental observations and model predictions. Environmental Science and Technology 32:892-902.
Kan, A. T., W. Chen, and M. B. Tomson. 2000. Desorption kinetics of neutral hydrophobic organic compounds from field-contaminated sediment. Environmental Pollution 108:81-89.
Karickhoff, S. W. 1984. Organic pollutant sorption in aquatic systems. Journal of Hydraulic Engineering-ASCE 110(6):707-735.
Keller B. D., and J. B. C. Jackson. 1993. Long-term assessment of the oil spill at Bahia Las Minas, Pamana Synthesis Report. Volume I: Executive Summary and Volume II, Technical Report. Minerals Management Service, Gulf Of Mexico OCS Region, OCS Study MMS 93-0048, 1017pp.
Kennicutt, M. C. II, P. N. Boothe, T. L. Wade, S. T. Sweet, R. Rezak, F. J. Kelly, J. M. Brooks, B. J. Presley, and D. A. Wiesenburg. 1996a. Geochemical patterns in sediments near offshore production platforms. Canadian Journal of Fisheries and Aquatic Science 53:2554-2566.
Kennicutt, M. C. II, R. H. Green, P. Montagna and P. F. Roscigno. 1996b. Gulf of Mexico Offshore Operations Experiment (GOOMEX) Phase I:
Sublethal responses to contaminant exposure—introduction and overview. Canadian Journal of Fisheries and Aquatic Science 53:2540-2553.
Kenworthy, W. J., M. J. Durako, S. M. R. Fatemy, H. Valavi, and G. W. Thayer. 1993. Ecology of seagrasses in northeastern Saudi Arabia one year after the Gulf War oil spill. Marine Pollution Bulletin 27:213-222.
Kerr, J. M., H. R. Melton, S. J. McMillen, R. I. Magaw, and G. Naughton. 1999. Polyaromatic hydrocarbon content in crude oils around the world. SPE 52724. Paper presented at the 1999 SPE/EPA Exploration and Production Environmental Conference, Austin, TX. Society of Petroleum Engineers, Richardson, TX, 10pp.
Kiesling, R. W., S. K. Alexander, and J. W. Webb. 1988. Evaluation of alternative oil spill cleanup techniques in a Spartina alterniflora salt marsh. Environmental Pollution 55:221-238.
Kingston, P.1999. Recovery of the marine environment following the Braer spill, Shetland. In Proceedings 1999 Oil Spill Conference, Seattle, Washington, March 8-11, 1999. American Petroleum Institute, Washington, D.C., pp. 103-109.
Kleinow, K., J. Baker, J. Nichols, F. Gobas, T. Parketron, D. Muir, G. Monteberdi, and P. Mastrodone. 1999. Exposure, uptake, and disposition of chemicals in reproductive and developmental states of selected oviparous vertebrates. Pages 9-111 In: R. T. Di Giulio and D. E. Tillitt, Eds., Reproductive and Developmental Effects of Contaminants in Oviparous Vertebrates. SETAC Press, Pensacola, FL.
Klosiewski, S. P., and K. K. Laing. 1994. Marine bird populations of Prince William Sound, Alaska, before and after the Exxon Valdez oil spill. Exxon Valdez Oil Spill State/Federal Natural Resource Damage Assessment Final Report, U.S. Fish and Wildlife Service, Anchorage, AK.
Knap, A. H., J. M. Hiffe, and J. N. Butler. 1980. Has the amount of the tar on the open ocean changed in the past decade? Marine Pollution Bulletin 11:161-164.
Kneip, T. J., N. H. Cutshall, R. Field, F. C. Hart, P. J. Lioy, J. Mancini, J. A. Mueller, C. Sobotowski, and J. Szeligowski. 1982. Management of nonpoint sources. Pp. 145-161 in Garry F. Mayer [ed.]. Ecological Stress and the New York Bight. Science and Management. In: Proceedings of a Symposium on the Ecological Effects of Environmental Stress, New York, NY. June 10-15, 1979. Estuarine Research Foundation Columbia, SC, pp. 175-161.
Knowles, R. S. 1983. The first pictorial history of the American oil and gas industry 1859-1983. Ohio University Press, Athens, OH. 169pp.
Koojiman, S. A. L. M. and J. A. J. Metz. 1984. On the dynamics of chemically stressed populations: The deduction of population consequences from effects on individuals. Ecotoxicology and Environmental Safety 8:254-274.
Krauss, M., W. Wilcke, and W. Zech. 2000. Availability of polycyclic aromatic hydrocarbons (PAH) and polychlorinated biphenyls (PCBs) to earthworms in urban soils. Environmental Science and Technology.
Krebs, C. T., and K. A. Burns. 1977. Long-term effects of an oil spill on populations of the salt marsh crab, Uca pugnax. Science 197:484-487.
Kuletz, K. J. 1996. Marbled Murrelet abundance and breeding activity at Naked Island, Prince William Sound, and Kachemak Bay, Alaska, before and after the Exxon Valdez oil spill. American Fisheries Society Symposium 18:770-784.
Kvenvolden, K. A., and B. R. T. Simoneit. 1990. Hydrothermally derived petroleum: Examples from Guaymas Basin, Gulf of California, and Escanaba Trough, Northeast Pacific Ocean. American Association of Petroleum Geologists Bulletin 74:223-237.
Kvenvolden, K. A., and J. W. Harbaugh. 1983. Reassessment of the rates at which oil from natural sources enters the marine environment. Marine Environmental Research 10:223-243.
Kvenvolden, K. A., F. D. Hostettler, P. R. Carlson, J. B. Rapp, C. N. Threlkeld, and A. Warden. 1995. Ubituitous tar balls with a California-source signature on the shorelines of Prince William Sound, Alaska. Environmental Science and Technology 29:2684-2694.
Lacaza, J. C., and O. Villedon de NeVde. 1976. Influence of illumination on phototoxicity of crude oil. Marine Pollution Bulletin, 7. 73-76 .
Lamoureux, E. M., and B. J. Brownawell. 1999. Chemical and biological availability of sediment-sorbed hydrophobic organic contaminants. Environmental Toxicology and Chemistry 18:1733-1741.
Lamparelli, C. C., F. O. Rodeigues, and D. Orgler de Moura. 1997. Long-term assessment of an oil spill in a mangrove forest in Sao Paulo, Brazil. In: B. Kjerfve, L. Drude de Lacerda, and W. H. Salif Diop [eds.]. Mangrove Ecosystem Studies in Latin America and Africa. UNESCO, Paris, France, pp. 191-203.
Lance, B. K., D. B. Irons, S. J. Kendall, and L. L. McDonald. 2001. An evaluation of marine bird population trends following the Exxon Valdez oil spill, Prince William Sound, Alaska. Marine Pollution Bulletin 42:298-309
Landes, K. K. 1973. Mother nature as an oil polluter. American Association of Petroleum Geologists Bulletin 57:637-641.
Landrum, P. F., D. C. Gossiaux, and J. Kukkonen. 1997. Sediment characteristics influencing the bioavailability of nonpolar organic contaminants to Diporeia spp. Chemistry Speciation Bioavailability 9:43-55.
Larsen, R. A., L. L. Hunt, and D. W. Ablankenship. 1977. Environmental Science and Technology 11:492-496.
Latimer, J., and J. Quinn. 1998. Aliphatic petroleum and biogenic hydrocarbons entering Narragansett Bay from tributaries under dry weather conditions. Estuaries 21(1):91-107.
Latimer, J., E. Hoffman, Gerald Hoffman, James L. Fasching, and James G. Quinn. 1990. Sources of petroleum hydrocarbons in urban runoff. Water, Air, and Soil Pollution 52:1-21.
Laws, E. 1993. Aquatic Pollution: An Introductory Text. Second Edition. John Wiley & Sons, New York, 611pp.
Leahy, J. G., and R. R. Colwell. 1990. Microbial degradation of hydrocarbons in the environment. Microbiology Review 54:305-315.
Lee, K., and N. E. Levy. 1987. Enhanced biodegredation of light crude oil in a sandy beach. Preceedings, 1987 Oil Spill Conference. pp. 411-416.
Lee, R. F., and D. S. Page. 1997. Petroleum Hydrocarbons and Their Effects in Subtidal Regions after Major Oil Spills. Marine Pollution Bulletin 34:928−940.
Lee, S. C., D. Mackay, F. Bonville, E. Joner, and W. Y. Shiu. 1989. A study of the long-term weathering of submerged and overwashed oil. Proceedings of the Twelfth Arctic Marine Oilspill Program Technical Seminar, Environment Canada, Ottawa, Ontario, pp. 101-111.
Leech, M., M. Walker, M. Wiltshire, and A. Tyler. 1993. OSIS: A windows 3 oil spill information system. Proceedings of the 16th Arctic Marine Oilspill Program Technical Seminar, Calgary, Canada, pp. 549-572.
Leenheer, J. 1982. United States Geological Survey data information service. E. T. Degens [ed.]. Transport of Carbon and Minerals in Major World Rivers, Pt. 1. Sonderband 52. Hamburg: SCOPE/UNEP, pp. 355-356.
Lehr, W. J., and D. Simecek-Beatty. 2001. The relation of Langmuir circulation processes to the Standard Oil spill spreading, dispersion, and transport algorithms. Spill Science & Technology Bulletin 6(3/4):247-253.
Lehr, W. J., H. M. Cekirge, R. J. Fraga, and M. S. Belen. 1984. Empirical studies of the spreading of oil spills, Oil and Petrochemical Pollution 2:7-11.
Leighton, F. A., D. B. Peakall, and R. G. Butler. 1983. Heinz-body hemolytic anemia from the ingestion of crude oil: a primary toxic effect in marine birds. Science 209:871-873.
Leister, D.L. and J.E. Baker. 1994. Atmospheric deposition of organic contaminants to the Chesapeake Bay. Atmospheric Environment 28: 1499-1520.
Lemmetyinen, R. 1966. Damage to water fowl caused by waste oil in the
Baltic area. Translation Bureau, Dept. of State, Canada, translation of Suomen Riista 19:63-71.
Letter Clarifying VOC Definition Policy Signed by: G. T. Helms. Signature Date: January 26, 1996 Available: http://www.epa.gov/ttn/caaa/t1/memoranda/reply.pdf
Levins, P., J. Adams, P. Brenner, S. Coons, G. Harris, C. Jones, K. Thrun, A. Wechsler. 1979. Sources of toxic pollutants found in influents to sewage treatment plants. VI. Integrated interpretation. EPA Contract No. 68-01-3857. U.S. Environmental Protection Agency, Washington, D.C., 118 pp.
Levy, E. M. 1978. Visual and chemical evidence for a natural seep at Scott Inlet, Baffin Island, District of Franklin. Geological Survey of Canada, Current Research Paper: 78-1B, 21-26.
Levy, E. M., and M. Ehrhardt. 1981. Natural seepage of petroleum at Buchan Gulf, Baffin Island. Marine Chemistry 10:355-364.
Lewis, R. R. III. 1981. Impact of oil spills on mangrove forests. Unpubl. report by Mangrove Systems, Inc., Tampa, FL, 32 pp.
Ligocki, M. P., and J. F. Pankow. 1989. Measurements of the gas/particle distributions of atmospheric organic compounds. Environmental Science and Technology 23:75-83.
Linden, O., M. Foberg, J. J. Kineman, R. Elmgren, and S. Hansson [eds.]. 1980. Laboratory studies carried out in connection with the spill: measurements of byssus formation by the mussel Mytilus edulis. In: The Tsesis oil spill. Report of the first year scientific study (October 26, 1977 to December 1978)—A cooperative international investigation. Stockholm Univ., Asko Lab., Dep. Zool. and Bot. Inst., Sweden.
Little, E. E., L. Cleveland, R. Calfee and M. G. Barron. 2000. Assessment of the photoenhanced toxicity of weathered oil to the tidewater silverside. Environmental Toxicology and Chemistry 19:926-932.
Lloyds Register. 1999. World Fleet Statistics. Table 6, Shiptype Categories—Age Profile/Completions During the Year.
Lopes, T., and S. Dionne. 1998. A review of semivolatile and volatile organic compounds in highway runoff and urban stormwater: Open-File Report 98-409. U.S. Geological Survey, Denver, CO, 67pp.
Lorentz, W. P., J. Hall, J. Kern, H. Finley, J. Hanifen, B. Goatcher, D. Hamilton, L. Pace, R. Markarian, T. Penn, and C. Piehler. 2001. The Lake Barre oil spill NRDA: From response to restoration. Proceedings 2001 International Oil Spill Conference, API Publ. No. 14710, American Petroleum Institute Washington, D.C., pp. 667-670.
Loveley, D. R., J. D. Coates, J. C. Woodward, and E. J. P. Phillips. 1995. Beznene oxidation coupled to sulfate reduction. Applied and Environmental Microbiology 61:953-958.
Maccarone, A. D., and J. N. Brzorad. 1995. Effects of an oil spill on the prey populations and foraging behavior of breeding wading birds. Wetlands 15:397-407.
MacDonald, D. A. 1991. Status and trends in concentrations of selected contaminants in Boston Harbor sediments and biota. NOAA Technical Memorandum: NOS-OMA 56, Seattle, WA.
MacDonald, I. R. 1998. Natural oil spills. Scientific American 279:56-61.
MacDonald, I. R., D. Buthman, W. W. Sager, B. B. Peccini, and N. L. Guinasso, Jr. 2000. Pulsed discharge from a mud volcano. Geology 28:907-910.
MacDonald, I. R., J. F. Reilly Jr., W. E. Best, R. Venkataramaiah, R. Sassen, N. S. Guinasso Jr., and J. Amos. 1996. Remote sensing inventory of active oil seeps and chemosynthetic communities in the northern Gulf of Mexico. Hydrocarbon Migration and its Near-surface Expression. D. Schumacher and M. A. Abrams [eds.]. American Association of Petroleum Geologists Memoir 66:27-37.
MacDonald, I. R., N. L. Guinasso Jr., S. G. Ackleson, J. F. Amos, R. Duckworth, R. Sassen, and J. M. Brooks. 1993. Natural oil slicks in the Gulf of Mexico visible from space. Journal of Geophysical Research 98(C9):16,351-16,364.
MacDonald, S. J., K. L. Willett, Jane Thomsen, Karla B. Beatty, Kevin Connor, Tumkur R. Narasimhan, Cynthia M. Erickson, and Stephen H. Safe. 1996. Sublethal detoxification responses to contaminant exposure associated with offshore production platforms. Canadian Journal of Fisheries and Aquatic Sciences 53:2606-2617.
Mackay, D, W., Y. Shiu, K. Hossain, W. Stiver, D. McCurdy, and S. Peterson, 1982. Development and calibration of an oil spill behavior model: Report No. CG-D-27-83. U.S. Coast Guard Research and Development Center, Groton, CT.
Mackay, D., A. Chau, B. Clark, C. Yen, J. Parsons, and B. Ahier. 1986. The dispersion and submergence of oil spills. Proceedings of the Ninth Arctic Marine Oilspill Program Technical Seminar. Environment Canada, Ottawa, Ontario, pp. 101-111.
Mackay, D., and P. J. Leinonen. 1977. Mathematical model of the behavior of oil spills on water with natural and chemical dispersion, Prepared for Fisheries and Environment Canada. Economic and Technical Review Report: EPS-3-EC-77-19.
Mackay, D., and R. S. Matsugu. 1973. Evaporation rates of liquid hydrocarbon spills on land and water, Canadian Journal of Chemical Engineering 51:434-439.
Mackay, D., H. Puig and L. S. McCarty. 1992. An equation describing the time course and variability in uptake and toxicity of narcotic chemicals to fish. Environmental Toxicology and Chemistry 11:941-951.
Mackay, D., S. Paterson, and K. Trudel. 1980. A mathematical model of oil spill behavior. Department of Chemical and Applied Chemistry, University of Toronto, Canada.
Mackay, D., W. Y. Shiu, A. Maiganen, and S. Feenstra. 1991. Dissolution of non-aqueous phase liquids in groundwater. Journal of Contaminant Hydrology 8:23-42.
Mackay, D., W. Y. Shiu, and K. C. Ma. 1992. Illustrated handbook of physical chemical properties and environmental fate for organic chemicals, vol. 1. Monoaromatic hydrocarbons, chlorobenzenes and PCBs; also vol. II Polynuclear aromatic hydrocarbons, polychlorinated dioxins and dibenzofurans , Lewis Publishers, Boca Raton, FL.
Mackay, D. 1986. A natural gradient experiment on solute transport in a sand aquifier. Water Resources Research 22:2017-2067 .
MacKenzie, M., and J. Hunter. 1979. Sources and fates of aromatic compounds in urban stormwater runoff. Environmental Science and Technology 13(2):179-183.
Mackin, J. G. 1971. A Study of the Effect of Oilfield Brine Effluents on Benthic Communities in Texas Estuaries. Texas A&M Research Foundation, Project 735. Texas A&M University, College Station, TX.
Makepeace, D., D. Smith, and St. Stanley. 1995. Urban stormwater quality: Summary of contaminant data. Critical Reviews in Environmental Science and Technology 25(2):93-139.
Maldonado, C., J. M. Bayona, and L. Bodineau. 1999. Sources, distribution, and water column processes of aliphatic and polycyclic aromatic hydrocarbons in the northwestern Black Sea water. Environmental Science and Technology 33(16):2693-2702.
Malins, D. C., Ed. 1977. Effects of petroleum in arctic and subarctic marine environments and organisms. Academic Press, New York, 321 pp.
Mallakin, A., B. J. McConkey, G. Miao, B. McKibben, V. Snieckus, D. G. Dixon and B. M. Greenberg. 1999. Impacts of structural photomodification on the toxicity of environmental contaminants: Anthracene photooxidation products. Ecotoxicology and Environmental Safety 43:204-212.
Markarian, R. K., J. P. Nicolette, T. Barber and L. Giese. 1995. A critical review of toxicity values and an evaluation of the persistence of petroleum products for use in natural resource damage assessments. American Petroleum Institute, Washington, DC, Publication Number 4594.
Marshall, M. J., V. Batista, and D. Matias. 1993. Effects of the 1986 Bahia Las Minas, Panama oil spill on plants and animals in seagrass communities. Keller, B. D. and J. B. C. Jackson (eds.). Long-term assessment
of the oil spill at Bahia Las Minas, Pamana. Volume II, Technical Re- port. Minerals Management Service, Gulf of Mexico OCS Region, New Orleans, LA. OCS Study MMS 93-0048, pp. 793-850.
Maruya, K. A., R. W. Risebrough, and A. J. Horne. 1996. Partitioning of polynuclear aromatic hydrocarbons between sediments from San Francisco Bay and their porewaters. Environmental Science and Technology 30:2942-2947.
Mastran, T., A. Dietrich, D. Gallagher, and T. Grizzard. 1994. Distribution of polyaromatic hydrocarbons in the water column and sediments of a drinking water reservoir with respect to boating activity. Water Resources Bulletin 28(11):2353-2366.
Masutani, S. M., and E. E. Adams. 2000. Experimental study of multi-phase plumes with application to deep ocean oil spills. Report to Minerals Management Service: Contract 1435-01-98-CT-30964, Herndon, VA. Matthews, C. P., S. Kedidi, N. I. Fita, A. Al-Yahya, and K. Al-Rasheed. 1993. Preliminary assessment of the effects of the 1991 Gulf War on Saudi Arabian prawn stocks. Marine Pollution Bulletin 27:251-271.
Matthews, C. P., S. Kedidi, N. I. Fita, Al Al-Yahya, and K. Al-Rasheed. 1993. Preliminary assessment of the effects of the 1991 Gulf War on Saudi Arabian prawn stocks. Marine Pollution Bulletin, Vol 27, pp. 251-271.
Mattson, C., N. Vallario, D. Smith, S. Anisfield, and G. Potera. 1977. Hackensack estuary oil spill: cutting oil-soaked marsh grass as an innovative damage control technique. Proceedings 1977 Oil Spill Conference. American Petroleum Institute, Washington, D.C., pp. 243-246.
Mauseth, G. S., C. A. Martin, and K. Whittle. 1997. Closing and reopening fisheries following oils spills: three different cases with similar problems. In: Proceedings of the 20th Arctic and Marine Oil Spill Program Technical Seminar, Environment Canada, Ottawa, 1283-1301pp.
Mazurek, M. A., G. R. Cass, and B. R. T. Simoneit. 1991. Biological input to visibility reducing aerosol particles in remote arid southwestern United States. Environmental Science and Technology 25:684-694.
McCain, J. C., and M. Hassan. 1993. Assessment of ichthyoplankton in Saudi Arabian Gulf waters before and after the 1991 Gulf oil spill. Arabian Journal of Science and Engineering 18:269-291.
McCarthy, J. J. 2000. The evolution of the Joint Global Ocean Flux Study project. Hanson, Roger B., Hugh W. Ducklow, and John G. Field [eds.]. The Changing Ocean Carbon Cycle. Cambridge University Press, Cambridge, UK, pp. 3-15.
McCarthy, L. H., T. G. Williams, G. R. Stephens, J. Peddle, K. Robertson, and D. J. Gregor. 1997. Baseline studies in the Slave River, NWT, 1990-1994: Part I. Evaluation of the chemical quality of water and suspended sediment from the Slave River (NWT). The Science of the Total Environment 197:21-53.
McCarty, L. S., and D. Mackay. 1993. Enhancing ecotoxicological modeling and assessment, Journal of Environmental Science and Technology Vol. 27, No. 9:1719-1728.
McCauley, C. A. and R. C. Harrel. 1981. Effects of oil spill cleanup techniques on a salt marsh. Proceedings, 1981 Oil Spill Conference. American Petroleum Institute, Washington, D. C, pp. 401-407.
McCay, D. P. F.2001. Modeling oil, chemical spill impacts. Sea TechApr. 2001, pp. 43-49.
McClary, K. 2000. Personal communication, Texas Natural Resources and Railroad Commission, Austin, TX.
McCormick-Ray, M. G.1987. Hemocytes of Mytilus edulis affected by Prudhoe Bay crude oil emulsion. Marine Environmental Research 22:107-122.
McCourt, J., and L. Shier. 2001. Preliminary findings of oil-solids interaction in eight Alaskan rivers. Proceedings of the 2001 Oil Spill Conference, Washington, D.C. American Petroleum Institute, pp. 845-849.
McDonald, S. J., T. L. Wade, J. M. Brooks, and T. J. McDonald. 1991. Assessing the exposure of fish to a petroleum spill in Galveston Bay, Texas. C. Worbel and C. A. Brebbia (eds.). Water Pollution, Modeling,
Measuring, and Prediction. Computational Mechanics Publications, Southampton and Elsevier Applied Science, London, pp. 707-718.
McDonald, S. J., K. L. Willett, J. Thomsen, K. B. Beatty, K. Connor, T. R. Narasimhan, C. M. Erickson, and S. H. Safe. 1996. Sublethal detoxification responses to contaminant exposure associated with offshore production platforms. Canadian Journal of Fisheries and Aquatic Sciences 53(11):2606-2617.
McDougall, T. J. 1978. Bubble plums in stratified environments. Journal of Fluid Mechanics.
McDowell, J. E. and D. Shea. 1997. Population processes of Mya arenaria from contaminated habitats in Massachusetts Bays. Final Report to the Massachusetts Bays Program: U.S. Environmental Protection Agency, Boston, MA.
McDowell, J. E., B. A. Lancaster, D. F. Leavitt, P. Rantamaki, and B. Ripley. 1999. The effects of lipophilic organic contaminants on reproductive physiology and disease processes in marine bivalve mollusks. Limnology and Oceanography 44:903-909.
McFall, J., S. R. Antoine, and I. R. DeLeon. 1985. Organics in the water column of Lake Ponchartrain. Chemosphere 14(9):1253-1265 .
McFarlane, G. A., J. R. King, and R. J. Beamish. 2000. Have there been recent changes in climate? Ask the fish. Progress in Oceanography 47: 147-169.
McGrath, J. A., E. L. Hellweger, W. A. Stubblefield, A. W. Maki, and D. M. Di Toro. 2001. Predicting the effects of non-weathered and weathered crude oil using narcosis theory. Abstract No. 364. SETAC 22nd Annual Meeting Abstract Book. Society of Environmental Toxicology and Chemistry, Pensacola, FL, p. 78.
McGroddy, S. E., and J. W. Farrington. 1995. Sediment porewater partitioning of polycylic aromatic hydrocarbons in three cores from Boston Harbor, MA. Environmental Science and Technology 29:1542-1550.
McGroddy, S. E., J. W. Farrington, and P. M. Gschwend. 1996. Comparison of the in-situ and desorption sediment—water partitioning of polycyclic aromatic hydrocarbons and polychlorinated biphenyls. Environmental Science and Technology 30:172-177.
McLean, J. D., P. M. Spiecker, A. P. Sullivan, and P. K. Kilpatrick. 1998. The role of petroleum asphaltenes in the stabilization of water-in-oil emulsions. O. C. Mullins and E. Y. Sheu [eds.]. Structure and Dynamics of Asphaltenes . Plenum Press, New York, pp. 377-422.
McVeety B. D., and R. A. Hites. 1988. Atmospheric deposition of polycyclic aromatic hydrocarbons to water surfaces: a mass balance approach. Atmospheric Environment 22:511-536.
McWilliams, J. C., and P. P. Sullivan. 2001. Vertical mixing by Langmuir circulations. Spill Science and Technology Bulletin6(3/4):225-237. Mead, C., and S. Baillie. 1981. Seabirds and oil: the worst winter. Nature, London 292:10-11.
Meador, J. P., J. E. Stein, W. L. Reichert, and U. Varanasi. 1995. Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Reviews Environmental Contamination and Toxicology 143:79-165.
Melancon, M. J.1995. Bioindicators used in aquatic and terrestrial monitoring. Handbook of Ecotoxicology, Hoffman, D. J., A. G. Burton, B. A. Rattner and J. Cairns, Jr. (eds.). Lewis Publishers, p 220-239.
Melancon, M. J.1996. Development of cytochrome P450 in avian species as a biomarker for environmental contaminant exposure and effect: Procedures and baseline values, Environmental Toxicology and Risk Assessment: Biomarkers and Risk Assessment (5th. Volume), ASTM STP 1306, David A. Bengtson and Diane S. Henshel, [eds.]. American Society for Testing and Materials, Philadelphia, PA, pp. 95-108.
Mendelssohn, A. A., M. W. Hester, C. Sasser, and M. Fischel. 1990. The effect of a Louisiana crude oil discharge from a pipeline break on the
vegetation of a Southeast Louisiana brackish marsh. Oil and Chemical Pol○lution 7:1-15.
Menon, N. N., and N. R. Menon. 1999. Uptake of polycyclic aromatic hydrocarbons from suspended oil borne sediments by the marine bivalve Sunetta scripta. Aquatic Toxicology 45:63-69.
Menzel, R. W. 1950. Report on Oyster Studies in Caillou Island Oil Field, Lake Pelto Oil Field, Dog Lake Oil Field, Lake Felicity, and Bayou Bas Bleu, Terrebonne Parish, Louisiana. Texas A&M Research Foundation, Project 9. Texas A&M University, College Station, TX, 300pp.
Menzel, R. W., and S. H. Hopkins. 1951. Report on Experiments to Test the Effects of Oil Well Brine or Bleedwater on Oysters at Lake Barre Oil Field. Texas A&M Research Foundation, Project 9. Texas A&M University, College Station, TX.
Menzel, R. W., and S. H. Hopkins. 1953. Report on Oyster Experiments at Bay Ste. Elaine Oil Field . Texas A&M Research Foundation, Project 9. Texas A&M University, College Station, TX.
Menzie and Cura Associates. 1991. Sources and Loadings of Pollutants to the Massachusetts Bays. Final Report to the Massachusetts Bays Program. U.S. Environmental Protection Agency, Boston, MA.
Menzies, R. J., J. P. Morgan, C. H. Oppenheimer, S. Z. El-Sayed, and J. M. Sharp. 1979. Design of the Offshore Ecology Investigation. In: C. H. Ward, M. E. Bender, and D. J. Reish (eds.). The Offshore Ecology Investigation: Effects of Oil Drilling and Production in a Coastal Environment. Rice University Studies 65:1-589, pp. 19-32.
Meybeck, M. 1988. How to establish and use world budgets of riverine materials. Lerman, A., and M. Meybeck [eds.]. Physical and chemical weathering in geochemical cycles. Kluwer. As cited in Spitzy and Ittekkot (1991), pp. 247-272.
Meyers, R. J. 1981. Response to the Esso Bayway oil spill. Proceedings 1981 Oil Spill Conference. American Petroleum Institute, Washington, D.C., pp. 409-412.
Michael, A. 1982. The potential contribution of petroleum hydrocarbons to changes in benthic communities of the New York Bight. In Garry F. Mayer [ed.]. Ecological Stress and the New York Bight: Science and Management: Proceedings of a Symposium on the Ecological Effects of Environmental Stress, New York, New York, June 10-15, 1979. Columbia, SC. Estuarine Research Foundation, 715pp.
Michel, J. 2001. Personal communication on April 13, 2001. Research Planning, Inc., Columbia, SC.
Michel, J., F. Csulak, D. French, and M. Sperduto. 1997. Natural resource impacts from the North Cape oil spill. Proceedings of 1997 International Oil Spill Conference, American Petroleum Institute, Washington, D.C., pp. 841-859.
Michel, J., M. O. Hayes, and P. J. Brown. 1978. Application of an oilspill vulnerability index to lower Cook Inlet, Alaska. Environ. Geology, Vol. 2(2), pp. 107-117.
Michel, J., M. O. Hayes, R. S. Keenan, T. C. Sauer, J. R. Jensen, and S. Narumalani. 1993. Contamination of nearshore subtidal sediments of Saudi Arabia from the Gulf War oil spill: Marine Pollution Bulletin 27:109-116.
Michel, J., M. O. Hayes, W. J. Sexton, J. C. Gibeaut, and C. Henry. 1991. Tends in Natural Removal of the Exxon Valdez Oil Spill in Prince William Sound from September 1989 to May 1990. Pages 181-187 In: Proceedings of the 1991 International Oil Spill Conference. Prevention, Behavior, Control, Cleanup. American Petroleum Institute, Washington, DC.
Michel, W.C. and W.K. Fitt. 1984. Effects of a water-soluble fraction of crude oil on a coral reef hydroid: Feeding, growth and algal symbionts. Marine Biology. 175: 143-154.
Middaugh, D. P., M. E. Shelton, C. L. McKenney, Jr., G. Cherr, P. J. Chapman, and L. A. Courtney. 1998. Preliminary observations on responses of embryonic and larval Pacific herring, Clupea pallasi, to neutral fraction biodegradation products of weathered Alaska North Slope oil. Archives of Environmental Toxicology 34:188-196.
Middleditch, B. S. (ed.). 1981. Environmental Effects of Offshore Oil Production. The Buccaneer Gas and Oil Field Study. Plenum Press, New York.
Mikolaj, P. G., A. A. Allen, and R. S. Schlueter. 1972. Investigation of the nature, extent, and fate of natural oil seepage off Southern California. Fourth Offshore Technology Conference: OTC 1549:I-367 to I-380.
Milgram, J. H. 1983. Mean flow in round bubble plumes. Journal of Fluid Mechanics 133:345-376.
Milgram, J. H., and J. J. Burgess. 1984. Measurements of the surface flow above round bubble plumes. Applied Ocean Research. 6:40-44.
Mille, G., D. Munoz, F. Jacquot, L. Rivet, and J.-C. Bertrand. 1998. The Amoco Cadiz oil spill: Evolution of petroleum hydrocarbons in the Ile Grande salt marshes (Brittany) after a 13-year period. Estuarine, Coastal and Shelf Science 47:547-559.
Minerals Management Service Website. [Online]. Available: http://www.mms.gov/stats/
Mitchell, R., I. R. MacDonald, and K. A. Kvenvolden. 1999. Estimation of total hydrocarbon seepage into the Gulf of Mexico based on satellite remote sensing images. Transactions, American Geophysical Union 80(49): Ocean Sciences Meeting Supplement, OS242.
Moles, A., S. Rice, and B. L. Norcross. 1994. Non-avoidance of hydrocarbon laden sediments by juvenile flatfishes. Netherland Journal of Sea Research 32:361-367.
Monson, D. H., D. F. Doak, B. E. Ballachey, A. Johnson, and J. L. Bodkin. 2000. Long-term impacts of the Exxon Valdez oil spill on sea otters, assessed through age-dependent mortality patterns. Proceedings of the National Academies of Science 97:6562-6567.
Montagna P. A., J. E. Bauer, J. Toal, D. H. Hardin and R. B. Spies. 1987. Temporal variability and the relationship between benthic meiofaunal and microbial populations in a natural coastal petroleum seep. J. Mar. Res. 45:761-789.
Montagna, P., J. E. Bauer, D. Hawrdin and R. B. Spies. 1995. Meiofaunal and microbial interactions in a natural submarine petroleum seep. Vie et Millieu 45:17-25.
Montagna, P. A., and D. E. Harper, Jr. 1996. Benthic infaunal long-term response to offshore production platforms. Canadian Journal of Fisheries and Aquatic Sciences 53:2567-2588.
Montagna, P. A., and J. Li. 1997. Modeling contaminant effects on deposit feeding nematodes near Gulf of Mexico production platforms. Ecological Modeling 98:151-162.
Montagna, P. A., J. E. Bauer, J. Toal, D. H. Hardin and R. B. Spies. 1989. Vertical distribution of meiofauna in the sediment of a natural hydrocarbon seep. Journal of Marine Research 47:657-680.
Montagna, P. A., J. E. Bauer, M. C. Prieto, D. H. Hardin and R. B. Spies. 1986. Benthic metabolism in a natural coastal petroleum seep. Marine Ecology Progress Series 34:31-40.
Moore, M. J., R. M. Smolowitz, D. F. Leavitt, and J. J. Stegeman. 1994. Evaluation of chemical contaminant effects in the Massachusetts Bays. Final Report to the Massachusetts Bays Program, Boston, MA.
Moore, M. N., D. R. Livingstone and J. Widdows. 1989. Hydrocarbons in marine molluscs: Biological effects and ecological consequences. Pages 291-328 in U. Varanasi [ed.]. Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. CRC Press, Boca Raton, FL.
Morales, R. A., A. J. Elliott and T. Lunel. 1997. The influence of tidal currents and wind on mixing in the surface layer of the sea. Marine Pollution Bulletin 34:15-25.
Mulino, M. M., M. F. Rayle, J. C. Francis and M. A. Poirrier. 1996. Delineation of benthic impact and recovery at two produced water discharge sites in inshore Louisiana. In M. Reed and S. Johnsen, eds., Produced
Water 2: Environmental Issues and Mitigation Technologies, Plenum Press, New York, pp. 177-194.
Murphy, S. M., R. H. Day, J. A. Wiens, and K. R. Paker. 1997. Effects of the Exxon Valdez oil spill on birds: comparisons of pre- and post-spill surveys in Prince William Sound, Alaska. Condor 99:299-313.
Nadau, R. J., and E. T. Berquist. 1977. Effects of the March 18, 1973 oil spill near Cabo Rojo, Puerto Rico, on tropical marine communities. In Proceedings of the 1977 Oil Spill Conference. American Petroleum Institute, Washington, D. C, pp. 535-538.
Naes, K., E. Oug, and J. Knutzen. 1998. Source and species-dependent accumulation of polycyclic aromatic hydrocarbons (PAH) in littoral indicator organisms from Norwegian smelter-affected marine waters. Marine Environmental Research 45:193-207.
Naes, K., J. Axelman, C. Naf, and D. Broman. 1998. Role of soot carbon and other carbon matrices in the distribution of PAH among particles, DOC, and the dissolved phase in the effluent and recipient waters of an aluminum reduction plant. Environmental Science and Technology 32:1786-1792.
Nance, J. M. 1991. Effects of oil/gas field produced water on the macrobenthic community in a small gradient estuary. Hydrobiologia 220: pp. 189-204.
National Energy Development Group. 2001. National Energy Policy: Reliable, Affordable, and Environmentally Sound Energy for America’s Future. U.S. Government Printing Office, 170 pp.
National Energy Policy Development Group. 2001. National Energy Policy, Report of the National Energy Policy Development Group. May 2001; ISBN 0-16-050814-2.
National Oceanic and Atmospheric Administration (NOAA), Rhode Island Department of Environmental Protection , and U.S. Department of the Interior. 1998. Damage assessment and restoration plan for the North Cape Oil Spill. NOAA Damage Assessment Center, Silver Spring, MD.
National Oceanic and Atmospheric Administration (NOAA). 1987. Narragansett Bay: Issues, Resources, Status and Management. Proceedings of a Seminar held January 28, 1985. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Washington, D.C., 171pp.
National Oceanic and Atmospheric Administration (NOAA). 1992. Oil Spill Case Histories, 1967-1991. Report No. HMRAD 92-11. Hazardous Materials Response and Assessment Division, NOAA, Seattle, WA.
National Oceanic and Atmospheric Administration (NOAA). 1997. Integrating physical and biological studies of recovery from the Exxon Valdez oil spill: case studies of four sites in Prince William Sound, 1989-1994. NOAA Tech. Memorandum NOS OECA 114.
National Oceanic and Atmospheric Administration (NOAA). 2000. Shoreline Assessment Manual. Third Edition. Seattle: Hazardous Materials Response and Assessment Division. 86 pp. + appendixes.
National Oceanic and Oceanic Administration (NOAA). Hazardous Materials Response and Assessment Division (HAZMAT). 1993. Vegetation cutting along the Delaware River following the Canadian Liberty oil spill, post cutting field report, 9 April 1993. 7600 Sand Point Way, NE, BIN C 15700, Seattle, WA 98115, 37 pp.
National Petroleum Council. 1981. Environmental conservation in the oil and gas industry. National Petroleum Council, Washington, D.C. In: National Research Council (1985), 80 pp.
National Research Council (NRC). 1975. Petroleum in the Marine Environment. National Academy Press, Washington, D.C.
National Research Council (NRC). 1985. Oil in the Sea: Inputs, Fates, and Effects. National Academy Press, Washington, D.C.
National Research Council (NRC). 1992. Rethinking the Ozone Problem in Urban and Regional Air Pollution. National Academy Press, Washington, D.C.
National Research Council (NRC). 1995a. Expanding Metropolitan National Highways: Implications for Air Quality and Energy Use— Special Report 245. National Academy Press, Washington, D.C.
National Research Council (NRC). 1995b. Mexico City’s Water Supply: Improving the Outlook for Sustainability. National Academy Press, Washington, D.C.
National Research Council (NRC). 1997. Contaminated sediments in ports and waterways. Cleanup strategies and technologies. National Academy Press, Washington, D.C. 295 pp.
National Research Council (NRC). 1998. Double-Hull Tanker Legislation: An Assessment of the Oil Pollution Act of 1990. National Academy Press, Washington, D.C.
National Research Council (NRC). 1999a. Ozone-Forming Potential of Reformulated Gasoline. National Academy Press, Washington, D.C.
National Research Council (NRC). 1999b. Spills of Nonfloating Oils, Risk and Response. National Academy Press, Washington, D.C.
Neff, J. M. 1987. Biological effects of drilling fluids, drill cuttings and produced waters. In Boesch, D. F. and N. N. Rabalais (eds.). 1987. Long-term environmental effects of offshore oil and gas development. Elsevier Applied Science Publishers, London, pp. 469-538.
Neff, J. M. 1990. Composition and fate of petroleum and spill-treating agents in the marine environment. Pages 1-33 In: J. R. Geraci and D. J. St. Aubin, Eds., Sea Mammals and Oil: Confronting the Risks. Academic Press, San Diego.
Neff, J. M., R. S. Carr and W. L. McCulloch. 1981. Acute toxicity of a used chrome lignosulfonate drilling fluids to several species of marine invertebrate. Marine Environmental Research 4:251-266.
Neff, J. M., T. C. Sauer, and N. Maciolek. 1989. Fate and Effects of produced Water Discharges in Nearshore Marine Waters. API Publication No. 4472, American Petroleum Institute, Washington, D.C., 300pp.
Neff, J. M., and W. A. Burns. 1996. Estimation of polycyclic aromatic hydrocarbon concentrations in the water column based on tissue residues in mussels and salmon: an equilibrium partitioning approach. Environmental Toxicology and Chemistry 15:220-2254.
Neff, J. M., and W. E. Haensly. 1982. Long-term impact of the Amoco Cadiz oil spill on oysters, Crassostrea gigas, and plaice, Pleuronectes platessa, from Aber-Benoit and Aber-Wrach, Brittany, France. Pages 269-328 in Ecological Study of the Amoco Cadiz Oil Spill. NOAA-CNEXO Report.
Neff, J. M., S. Ostazwski, W. Gardiner and I. Stejskal. 2000. Effects of weathering on the toxicity of three offshore Australian crude oils and a diesel fuel to marine animals. Environmental Toxicology and Chemistry 19:1809-1821.
Nelson, E., L. L. McConnell, and J. E. Baker. 1998. Diffusive exchange of gaseous PAH and PAH across the air-water interface of the Chesapeake Bay. Environmental Science and Technology 32:912-919.
Netherlands Oil and Gas Exploration and Production Association (NOGEPA). 1997. Annual Report 1997. [Online]. Available: http://nogepa.nl/report.html.
Newton, J., 2001. A Century of Tankers: The Tanker Story. Intertanko. Oslo, Noway. 256pp.
Nisbet, I. C. T. 1994. Effects of pollution on marine birds. Nettleship, D. N., burger, J. and Gochfeld, M. [eds.]. Seabirds on Islands: Threats, Case studies, and action plans. Birdlife Conservation Series No. 1, Cambridge, U. K, pp. 8-25.
Norwegian Oil Industry Association (NOIA). 1998. Emissions to air and discharges to sea from the Norwegian offshore petroleum activities 1997. [Online]. Available: http://www.olf.no/en/rapporter/miljorap/1997/index.html.
O’Connor, T. P., and J. F. Paul. 2000. Misfit between sediment toxicity and chemistry. Marine Pollution Bulletin 40:59-64.
Oakley, K. A., and K. J. Kuletz. 1996. Population, reproduction, and foraging of Pigeon Guillemots at Naked Island, Alaska, before and after the
Exxon Valdez oil spill. American Fisheries Society Symposium 18:759-769.
Odokuma, L., and G. Okpokwasili. 1997. Seasonal influences of the organic pollution monitoring of the New Calabar River, Nigeria. Environmental Monitoring and Assessment 45:43-56.
Offenberg, J. H. 1998. Semi-Volatile Organic compounds in Urban and Over-Water Atmospheres. Ph. D. Thesis. University of Maryland, College Park, MD.
Office of Technology Assessment. 1987. Wastes in Marine Environments. Office of Technology Assessment, Washington, D.C., 313 pp.
Oil and Gas Producers (OGP) formerly E&P Forum. 1994. Methods for estimating atmospheric Emissions from E&P operations , 25-28 Old Burlington St, London W1X 1LB.
Ollivon, D., M. Blanchard, and B. Garban. 1999. PAH fluctuations in rivers in the Paris region (France): impact of floods and rainy events. Water, Air, and Soil Pollution 115:429-444.
Osenberg, C. W., R. J. Schmitt, S. J. Holbrook, and D. Canestro. 1992. Spatial scale of ecological effects associated with an open coast discharge of produced water. In J. P. Ray and F. R. Engelhard, Produced Water, Plenum Press, New York, pp. 387-402.
Oudot, J., P. Fusey, M. VanPraet, J. P. Feral, and F. Gaill. 1981. Hydrocarbon weathering in seashore invertebrates and sediments over a two-year period following the Amoco Cadiz oil spill: Influence of microbial metabolism. Environmental Pollution Series A. 26:93-110.
Overton, E. B., M. H. Schurtz, K. M. St. Pé, and C. Byrne. 1986. Distribution of trace organics, heavy metals, and conventional pollutants in Lake Pontchartrain, Louisiana. Pages 247-270 in M. L. Sohn (ed.). Organic Marine Geochemistry. American Chemical Society, Washington, D.C.
Oviatt, C., J. Fruthsen, J. Gearing and P. Gearing. 1982. Low chronic additions of No. 2 fuel oil: Chemical behavior, biological impact and recovery in a simulated estuarine environment. Marine Ecology Progress Series 9:121-136.
Owe, M., P. Craul and H. Halverson. 1982. Contaminant levels in precipitation and urban surface runoff. Water Resources Bulletin 18(5):863-868.
Page, D. S., E. S. Giliffan, J. C. Foster, J. R. Hotham, and L. Gonzalez. 1985. Mangrove leaf tissue sodium and potassium ion concentrations as sublethal indicators of oil stress in mangrove trees. Pages 391-393 in Proceedings of the 1985 Oil Spill Conference. American Petroleum Institute, Washington, D.C.
Page, D. S., P. D. Boehm, G. S. Douglas, A. E. Bence, W. A. Burns, and P. J. Mankiewicz. 1997. An estimate of the annual input of natural petroleum hydrocarbons to seafloor sediments in Prince William Sound, Alaska. Marine Pollution Bulletin 34:744-749.
Page, D. S., P. D. Boehm, G. S. Douglas, A. E. Bence, W. A. Burns, and P. J. Mankiewicz. 1996. The natural petroleum hydrocarbon background in subtidal sediments of Prince William Sound, Alaska, USA . Environmental Toxicology and Chemistry 15:1266-1281.
Page, D. S., P. D. Boehm, G. S. Douglas, A. E. Bence, W. A. Burns, and P. J. Mankiewicz. 1998. Petroleum sources in the western Gulf of Alaska/ Shelikoff Strait area. Mar. Pollut. Bull 36:1004-1012.
Page, D. S., P. D. Boehm, G. S. Douglas, A. E. Bence, W. A. Burns, and P. J. Mankiewicz. 1999. Pyrogenic polycyclic aromatic hydrocarbons in sediments record past human activities: a case study of Prince William Sound, Alaska. Marine Pollution Bulletin 38:247-260.
Page, D. S., Gilfillan, E. S., Stoker, S. W., Neff, J. M., Boehm, P. D.1999. 1998 Shoreline Conditions in the Exxon Valdez Oil Spill Zone in Prince William Sound. Pages 119–126 In: Proceedings of the 1999 International Oil Spill Conference. Beyond 2000—Balancing Perspectives. American Petroleum Institute, Washington, DC.
PanCanadian Petroleum Limited. 1999. East Coast Operations, 1999 Discharge Summary Cohasset Project. Report submitted to the Canada-Nova Scotia Offshore Petroleum Board. 12pp.
Panzer, D. 2000. Personal communication in March, 2000. Minerals Management Service, Washington, DC.
Paris Commission (PARCOM). 1986. Eight Annual Report on the Activities on the Paris Commission. Paris Commission, London.
Parker C. A., M. Freegarde and G. C. Hatchard. 1971. The effect of some chemical and biological factors on the degredation of crude oil at sea. In: Water pollution by oil. P. Hepple, Ed. Institute of Petroleum, London, pp. 237-244.
Parrish, J. K., and P. D. Boersma. 1995a. Muddy waters. American Scientist 83:112-115.
Parrish, J. K., and P. D. Boersma. 1995b. Letters to the editors. American Scientist 83:396-400.
Patton, J. S., M. W. Rigler, P. D. Boehm, and D. L. Feist. 1981. Ixtoc-1 Oil-Spill—Flaking
Payne, J. R. and C. R. Phillips 1985. Photochemistry of petroleum in water. Environmental Science and Technology 19:569-579.
Payne, J. R., B. E. Kirstein, J. R. Clayton, Jr., C. Clary, R. Redding, D. G. McNabb, Jr. and G. H. Farmer. 1987. Integration of Suspended Particulate Matter and Oil Transportation Study, Mineral Management Service Contract No. 14-12-0001-30146. Mineral Management Service, Environmental Studies Branch, Anchorage, AK 216pp.
Payne, J. R; G. D. McNabb. 1984. Weathering of petroleum in the marine environment. Marine Technology Society Journal 18(3):24-42. Pearson, T. H., and R. Rosenberg. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology Annual Review 16:229-311.
Pelletier, E., S. Ouellet, and M. Paquet. 1991. Long-term chemical and cytochemical assessment of oil contamination in estuarine intertidal sediments. Marine Pollution Bulletin 22:273-281.
Perry, R., and A. E. McIntyre. 1986. Impact of motorway runoff upon surface water quality. Solbé, J. F. de L. G., (ed.). Effects of Land Use on Fresh Waters: Agriculture, Forestry, Mineral Exploitation, Urbanisation. Ellis Horwood Limited, Chichester, UK, pp. 53-67.
Perry, R., and A. E. McIntyre. 1987. Oil and polynuclear aromatic hydrocarbon contamination of road runoff: A comparison of treatment procedures. In: Vandermeulen, John H., and Steve E. Hrudey, [ed.] Oil in Freshwater: Chemistry, Biology, Countermeasure Technology. Proceedings of the Symposium on Oil Pollution in Freshwater, Alberta, Canada. Pergamon Press, New York, pp. 474-484.
Peters, E. C. 1997. Diseases of coral-reef organisms. In: Birkeland, C., ed., Life and death of coral reefs. Chapman and Hall Publishers, pp. 114-136.
Peters, E. C., N. J. Gassman, J. C. Firman, R. H. Richmond, and E. A. Power. 1997. Ecotoxicology of tropical marine ecosystems. Environmental Toxicology and Chemistry, Vol 16 (1), pp. 12-40.
Peterson, C. H. 2001. The Exxon Valdez oil spill in Alaska: Acute, indirect, and chronic effects on the ecosystem. Advances in Marine Biology 39:1-103.
Peterson, C. H., M. C. Kennicutt II, R. H. Green, P. Montagna, D. E. Harper, Jr., E. N. Powell, and P. F. Roscigno. 1996. Ecological consequences of environmental perturbations associated with offshore hydrocarbon production: a perspective from study of long-term exposures in the Gulf of Mexico. Canadian Journal of Fisheries and Aquatic Sciences 53:2637-2654.
Peterson, C. H., L. L. McDonald, R. H. Green, and W. P. Erickson. 2001. Sampling design begets conclusions: the statistical basis for detection of injury to and recovery of shoreline communities after the Exxon Valdez oil spill. Marine Ecology Progress Series 210:255-283.
Petroleos Mexicanos (PEMEX). 2000. PEMEX Report 1999 Safety, Health, and Environment. 47 pp.
Petty, J. D., BC Poulton, C. S. Charbonneau, J. N. Huckins, S. B. Jones, J. T. Cameron, and H. F. Prest. 1998. Determination of bioavailable contaminants in the Missouri River following the flood of 1993. Environmental Science and Technology 32(7):837R-842R.
Pezeshki, S. R., M. W. Hester, Q. Lin, and J. A. Nyman. 2000. The effects of oil spill and clean-up on dominant US Gulf coast marsh macrophytes: a review. Environmental Pollution 108:129-139.
Pham, T. T., and S. Proulx. 1997. PCBs and PAHs in the Montreal urban community (Quebec, Canada) wastewater treatment plant and in the effluent plume in the St. Lawrence River. Water Resources Bulletin 31(8):1887-1896.
Pham, T. T., S. Proulx, C. Brochu, and S. Moore. 1999. Composition of PCBs and PAHs in the Montreal urban community wastewater and in the surface water of the St. Lawrence River (Canada). Water, Air, and Soil Pollution 111:251-270.
Piatt, J. 1995. Letters to the editors. American Scientist 83:396-398.
Piatt, J. F., and R. G. Ford, 1996. How many seabirds were killed by the Exxon Valdez oil spill? American Fisheries Society Symposium 18:712-719.
Piatt, J. F., and C. J. Lensink. 1989. Exxon Valdez bird toll. Nature 342:865-866.
Piatt, J. F., and P. Anderson. 1996. Response of Common Murres to the Exxon Valdez oil spill and long-term changes in the Gulf of Alaska marine ecosystem. American Fisheries Society Symposium 18:720-737.
Piatt, J. F., and R. G. Ford. 1996. How many seabirds were killed by the Exxon Valdez oil spill?. American Fisheries Society Symposium 18:712-719.
Piatt, J. F., C. J. Lensink, W. Butler, M. Kendziorek, and D. R. Nysewander. 1990. Immediate impact of the Exxon Valdez oil spill on marine birds. Auk 107:387-397.
Plummer, P. S. 1996. Origin of beach-stranded tars from source rock indigenous to Seychelles. American Association of Petroleum Geologists Bulletin 80:323-329.
Plutchak, N. B., and Kolpak, R. L. 1981. Numerical simulation of oil spreading on water. Proc. de La Mecanique des Nappes d?Hydrocarbures, Assoc. Amicalle des Ingenieurs, Paris.
Pocklington, R., and F. Tan. 1983. Organic carbon transport in the St. Lawrence River. Pp. 243-251 in Degens, Egon T., Stephan Kempe, and Hassan Soliman, eds. Transport of Carbon and Minerals in Major World Rivers, Part 2. Heft 55. SCOPE/UNEP Sonderband, Hamburg, Germany.
Poster, D.L. and J.E. Baker. 1996. Influence of submicron particles on hydrophobic organic contaminants in precipitation. 1. Concentrations and distributions of organic contaminants in rainwater. Environmental Science Technology 30.
Price, A. R. G., and J. H. Robinson (eds.). 1993. The 1991 Gulf War: Coastal and Marine Environmental Consequences. Marine Pollution Bulletin 27, 380 pp.
Prince, R. C. 1993. Petroleum spill bioremediation in marine environments. Critical Reviews in Microbiology 19:217-239.
Proffitt, C. E., D. J. Devlin, and M. L. Lindsey. 1995. Effects of oil on mangrove seedlings grown under different environmental conditions. Marine Pollution Bulletin 30:788-793.
Pruell, R. J., J. G. Quinn, J. L. Lake, and W. R. Davis. 1987. Availability of PCBs and PAHs to Mytilus edulis from artificially resuspended sediments. In: J. M. Capuzzo and D. R. Kester, Eds., Oceanic Processes in Marine Pollution. Vol. 1. Robert Krieger Publisher, Malabar, FL, pp. 97-108.
Pruell, R. J., J. L. Lake, W. R. Davis, and J. G. Quinn. 1986. Uptake and depuration of organic contaminants by blue mussels, Mytilus edulis, exposed to environmentally contaminated sediment. Marine Biology 91:497-505.
Quackenbush, T. R., M. E. Teske, and C. E. Polymeropoulos. 1994. A model for assessing fuel jettisoning effects. Atmospheric Environment 28 (16):2751-2759.
Quigley, D. D., J. S. Hornafius, B. P. Luyendyk, R. D. Grancis, J. Clark, and L. Washburn. 1999. Decrease in natural marine hydrocarbon seepage near Coal Oil Point, California, associated with offshore oil production. Geology 17:1047-1050.
Rabalais, N. N., B. A. McKee, D. J. Reed and J. C. Means. 1991a. Fate and Effects of Nearshore Discharges of OCS Produced Waters. Volume II. Technical Report. OCS Study/MMS 91-0005 U.S. Dept. of the Interior, Minerals Management Service, Gulf of Mexico OCS Regional Office, New Orleans, LA, 337pp.
Rabalais, N. N., B. A. McKee, D. J. Reed and J. C. Means. 1991b. Fate and Effects of Nearshore Discharges of OCS Produced Waters. Volume III. Appendixes. OCS Study/MMS 91-0006. U.S. Dept. of the Interior, Minerals Management Service, Gulf of Mexico OCS Regional Office, New Orleans, LA, 225pp.
Rabalais, N. N., L. E. Smith, C. B. Henry, Jr., P. O. Roberts and E. B. Overton. 1998. Long-term Effects of Contaminants from OCS Produced-water Discharges at Pelican Island Facility, Louisiana. OCS Study Mineral Management Service 98-0039. United States Department of the Interior. Minerals Management Service, Gulf of Mexico OCS Region. New Orleans, LA, 88pp.
Rabalais, N. N., L. E. Smith, E. B. Overton, and A. L. Zoeller. 1993. Influence of hypoxia on the interpretation of effects of petroleum production activities. OCS Study/MMS 93-0022. U.S. Dept. of the Interior, Minerals Management Service, Gulf of Mexico OCS Region, New Orleans, LA, 158pp.
Rabalais, N. N., B. A. McKee, D. J. Reed and J. C. Means. 1992. Fate and effects of produced water discharges in coastal Louisiana, Gulf of Mexico, USA. Pages 355-369 in J. P. Ray and F. R. Engelhardt, Produced Water, Plenum Press, New York.
Radler, Marilyn. 1999. 1999 World refining survey. Oil & Gas Journal 97(51):45-90.
Rainey, Gail. 2000. Personal communication. Minerals Management Service, Department of Interior, New Orleans, LA.
Readman, J. W., R. F. C. Mantoura, and M. M. Read. 1984. The physicochemical speciation of polycyclic aromatic hydrocarbons (PAH) in aquatic systems. Z. Anal. Chem. 219:126-131.
Reed, D.C., R. J. Lewis, M. Anghera. 1994. Effects of open-coast oil production outfall on patterns of giant kelp (Macrocystis pyrifera) recruitment. Marine Biology. 120, 25-31.
Reed, M. 1992. State-of-the art summary: Modeling of physical and chemical processes governing fate of spilled oil. Proceedings of the ASCE Workshop on Oil Spill Modeling, Charleston, SC.
Reed, M., and E. Gundlach. 1989. A coastal zone oil spill model: Development and sensitivity.
Reed, M., C. Turner, and A. Odulo. 1994. The role of wind and emulsification in modeling oil spill and surface drifter trajectories. Spill Science & Technology Bulletin 1(2):143-157.
Reed, M., D. P. French, J. Calambokidis and J. Cubbage. 1987b. Simulation modeling of the effects of oil spills on population dynamics of northern fur seals, OCS-MMS 86-0045, Final Report to U.S. Department of the Interior, Minerals Management Service, Alaska OCS Region, Anchorage, AK, Contract No. 14-12-0001-30145, 158pp.
Reed, M., D. P. French, J. Calambokidis and J. Cubbage. 1989. Simulation modeling of the effects of oil spills on population dynamics of northern fur seals. Ecological Modeling 49:49-71.
Reed, M., D. P. French, S. Feng, F. W. French III, E. Howlett, K, Jayko, W. Knauss, J. McCue, S. Pavignano, S. Puckett, H. Rines, R. Bishop, M. Welsh, and J. Press, 1996. The CERCLA type a natural resource damage assessment model for the Great Lakes environments (NRDAM/
GLE), Vol. I—Technical Documentation, Final Report, Submitted to Office of Environmental Policy and Compliance, U.S. Department of the Interior, Washington, DC, by Applied Science Associates, Inc., Narragansett, RI, Contract No. 14-01-0001-88-C-27, April 1996.
Reed, M., D. P. French, T. Grigalunas and J. Opaluch. 1989. Overview of a natural resource damage assessment model system for coastal and marine environments. Oil and Chemical Pollution 5:85-97.
Reed, M., K. Jayko, A. Bowles, E. Anderson, S. Leatherwood and M. L. Spaulding. 1987a. Computer simulation of the probability that endangered whales will interact with oil spills, OCS Study 86-0044, Minerals Management Service, Anchorage, AK.
Reed, M., N. Ekrol, H. Rye. 1999. Oil spill contingency and response (OSCR) analysis in support of the environmental impact assessment offshore Namibia. Spill Science & Technology Bulletin 5(1):29-38.
Reed, M., O. Johansen, P. J. Brandvik, P. Daling, A. Lewis, R. Fiocco, D. Mackay, and R. Prentki. 1999. Oil spill modeling towards the close of the 20th century: Overview of the state of the art. Spill Science & Technology Bulletin 5(1):3-16.
Reed, W. E., and I. R. Kaplan. 1977. The chemistry of marine petroleum seeps. Journal of Marine Biology 7(2):255-293.
Rice, S. D., R. E. Thomas, M. G. Carls, R. A. Heintz, A. C. Wertjeimer, M. L. Murphy, J. W. Short, and A. Moles. 2001. Impacts to pink salmon following the Exxon Valdez oil spill: Persistence, toxicity, sensitivity and controversy. Reviews in Fisheries Science 9:165-211.
Richmond, M. D. 1996. Status of subtidal biotopes of the Jubail Mainre Wildlife Sanctuary with special reference to soft-substrata communities. In Krupp, F., A. H. Abuzinada, and I. A. Nader, eds. A Marine Wildlife Santuary for the Arabian Gulf. Environmental Research and Conservation Following the 1991 Gulf War Oil Spill. National Commission for Wildlife Conservation and Development, Riyadh, Kingdom of Saudi Arabia and Senchenberg Research Institute, Frankfurt a. M., Germany, pp. 159-176.
Ricketts, E.G. and J. Calvin. 1948. Between Pacific Tides. Stanford University Press.
Rifai, H. S., C. J. Newell, and P. B. Bedient. 1993. Getting to the nonpoint source with GIS . Civil Engineering 63(6):44-46.
Riva, J. P., Jr. 1995. World oil production after year 2000: business as usual or crises? The National Council for Science and the Environment. Washington, DC. 17 pp.
Røe Utvik, T. I., and S. Johnsen. 1999. Bioavailability of polycyclic aromatic hydrocarbons in the North Sea. Environmental Science and Technology 33:1963-1969.
Røe Utvik, T. I., G. S. Durell, and S. Johnsen. 1999. Determining produced water originating polycyclic aromatic hydrocarbons in North Sea waters: comparison of sampling techniques. Marine Pollution Bulletin 38:977-989.
Røe, Utvik, T. I. 1999. Chemical characterization of produced water from four offshore oil production platforms in the North Sea. Chemosphere 39(15):2593-2606.
Roesner, L. A. 1982. Quality of urban runoff. Kibler, David F., ed. Urban Stormwater Hydrology. American Geophysical Union, Washington, D.C., pp. 161-187.
Rogers, P. 1994. Hydrology and water quality. Meyer, William B., and B. L. Turner, III, ed. Changes in Land Use and Land Cover: a Global Perspective. Cambridge University Press, Cambridge, UK, pp. 231-257.
Rogge, W. F., Mazurek M. A., Hildemann L. M., Cass G. R., Simoeit B. R. T. 1993. Quantification of urban aerosols at a molecular level: Identification, abundance and seasonal variation. Atmospheric Environment. 27A(8):1309-1330.
Rosenberg, D. H. 1999. Harlequin duck restoration monitoring project. Exxon Valdez Oil Spill Restoration Project Annual Report. Alaska Department of Fish and Game, Division of Wildlife Conservation. Anchorage, AK.
Rosenberg, D. H., and M. J. Petrula. 1998. Status of harlequin ducks in Prince William Sound, Alaska after the Exxon Valdez oil spill, 1995-1997. Exxon Valdez Oil Spill Restoration Project 97427 Final Report. Alaska Department of Fish and Game, Division of Wildlife Conservation. Anchorage, AK.
Royal Commission on Environmental Pollution. 1981. Oil Pollution of the Sea. London. 307 p. As cited in National Research Council (1985). Rozas, L. P., T. J. Minello, and C. B. Henry. 2000. An assessment of potential oil spill damage to salt marsh habitats and fishery resources in Galveston Bay, Texas. Marine Pollution Bulletin 40:1148-1160.
Rye, H. 2001. Probable effects of Langmuir circulation observed on oil slicks in the field. Spill Science & Technology Bulletin 6(3/4):263-271.
Rye, H., and P. J. Brandvik. 1997. Verification of subsurface oil spill models. Proceedings, 1997 International Oil Spill Conference, pp. 551-557.
Rye, H., P. J. Brandvik, and M. Reed. 1996. Subsurface oil release field experiment-observations and modeling of subsurface plume behavior. Proceedings, 19th Arctic and Marine Oil Spill Program (AMOP) Technical Seminar 2:1417-1435.
Samuels, W. B., and K. J. Lanfear. 1982. Simulations of seabird damage and recovery from oil spills in the northern Gulf of Alaska. Journal of Environmental Management 15:169-182.
Sanders, H. L. 1978. Florida oil spill impact on the Buzzard’s Bay benthic fauna, West Falmouth. Journal of Fisheries Research Board of Canada 35:717-730.
Sanders, H. L. 1981. Environmental effects of oil in the marine environment. In: Safety and Offshore Oil: Background Papers of the Committee on Assessment of Safety of OCS Activities. National Research Council, National Academy Press, Washington, D.C., pp. 117-146.
Sanders, H. L., J. F. Grassle, G. R. Hampson, L. S. Morse, S. Garner-Price and C. C. Jones. 1980. Anatomy of an oil spill: Long-term effects from the grounding of the barge Florida off West Falmouth, Massachusetts. Journal of Marine Research, 38:265-380.
Schiff, K., and M. Stevenson. 1996. San Diego regional storm water monitoring program: Contaminant inputs to coastal wetlands and bays. Bulletin of the Southern California Academy of Sciences 95(1):7-16.
Schiff, K. C., D. J. Reish, J. W. Anderson, and S. M. Bay. 1992. A comparative evaluation of produced water toxicity. In Ray, J. P. and F. R. Engelhart, eds. Produced Water. Plenum Press, New York and London, 199-207pp.
Schlesinger, William H. 1997. Biogeochemistry: An Analysis of Global Change. Academic Press, San Diego, CA.
Schramm, L. L. 1992. Petroleum Emulsions: Basic Principles, Advances in Chemistry Series, Vol. 231, American Chemical Society, Washington, D.C., pp. 1-49.
Schramm, L.L. (Ed.). 2000. Surfactants: Fundamentals and Applications in the Petroleum Industry, Cambridge University Press, Cambridge, U.K. 621 pp.
Schwarzenbach, R. P., P. M. Gschwend and D. M. Imaboden. 1993. Environmental Organic Chemistry. Wiley Interscience, New York, pp. 436-484.
Seip, K. L., E. Sandersen, F. Mehlum and J. Ryssdel. 1991. Damages to seabirds from oil spills: comparing simulation results and vulnerability indexes. Ecological Modeling 53:39-59.
Shaheen, Donald G. 1975. Contributions of urban roadway usage to water pollution. EPA 600/2-75-004. U.S. Environmental Protection Agency, Washington, DC.
Sharp, B. E., M. Cody, and R. Turner. 1996. Effects of the Exxon Valdez oil spill on the Black Oystercatcher. American Fisheries Society Symposium 18:748-758.
Shelton, M. E., P. J. Chapman, S. S. Foss, and W. S. Fisher. 1999. Degrada
tion of weathered oil by mixed marine bacteria and the toxicity of accumulated water-soluble material to two marine crustacea. Archives of Environmental Toxicology 36:13-20.
Shiu, W. Y., M. Bobra, A. M. Bobra, A. Maijanen, L. Suntio, and D. Mackay. 1990. The water solubility of crude oils and petroleum products. Oil and Chemical Pollution 7:57-84.
Siegenthaler, U., and J. L. Sarmiento. 1993. Atmospheric carbon dioxide and the ocean. Nature 365:119-25. As cited in McCarthy (2000).
Sigman, M. E., P. F. Schuler, M. M. Gosh, and R. T. Dabestani. 1998. Environmental Science and Technology 32:3980-3985.
Simoneit B. R. T., Mazurek M. A. 1982. Organic matter of the troposphere—II. Natural background of biogenic lipid matter in aerosols over the rural Western United States Atmospheric Environment. 16(9):2139-2159
Sjöblom, J., H. Førdedal, T. Skodvin, and B. Gestblom. 1999. Emulsions characterized by means of time domain dielectric measurements (TDS): Technical applications. Journal of Dispersion Science and Technology 30(3):921-943.
Smith, C. J., R. D. DeLaune, W. H. Patrick, Jr., and J. W. Fleeger. 1984. Impact of dispersed and undispersed oil entering a Gulf coast salt marsh. Environmental Toxicology and Chemistry 3:609-616.
Smith, G. A., J. S. Nickles, R. J. Bobbie, N. L. Richards, D.C. White. 1982. Effects of oil and gas well-drilling fluids on the biomass and community structure of microbiota that colonizes sands in running sea-water. Archives of Environmental Contamination and Toxicology Vol. 11, Issue 1, pp. 17-23.
Smith, S. D. A., and R. D. Simpson. 1995. Effects of the Nella Dan oil spill on the fauna of Durvillaea antarctica holdfasts. Marine Ecology Progress Series 121:73-89.
Smith, S. D. A., and R. D. Simpson. 1998. Recovery of benthic communities at Macquarie Island (sub-Antarctic) following a small oil spill. Marine Biology. 131, 567-581.
Smith, S. R., and A. H. Knap. 1985. Significant decrease in the amount of tar stranding on Bermuda. Marine Pollution Bulletin 16:19-21.
Socolofsky, S. A., and E. E. Adams. 2001. Detrainment fluxes for multi-phase plumes in quiescent stratification. International Symposium on Environmental Hydraulics (ISEH and IAHRR).
Southward, A. J., and E. C. Southward. 1978. Recolonization of rocky shores in Cornwall after use of toxic dispersants to clean up the Torrey Canyon spill. Journal Fisheries Research Board of Canada 35:682-706.
Sparling, L. C., and M. R. Schoeberl. 1995. Mixing entropy analysis of dispersal of aircraft emissions in the lower stratosphere. Journal of Geophysical Research 100(D8):16,805-16,812.
Spaulding, M. 1995. Oil spill trajectory and fate modeling: State-of-the-art review. Proceedings of the Second International Oil Spill Research and Development Forum, International Maritime Organization, London, United Kingdom, pp. 508-516.
Spaulding, M. L., P. R. Bishnoi, E. Anderson, and T. Isaji. 2000. An integrated model for prediction of oil transport from a deepwater blowout. 23rd AMOP technical seminar 2000, June 14-16, Vancouver, BC 2:611-635
Speight, J. G. 1991. The Chemistry and Technology of Petroleum. Marcel Dekker, New York.
Sperduto, M., C. Hebert, J. Myers, and G. Haas. 1998. Estimate of total acute mortality to birds resulting from the North Cape oil spill, South Kingstown, Rhode Island, January 19, 1996. Report by U.S. Fish and Wildlife Service and Rhode Island Department of Fish, Wildlife, and Estuarine Resources.
Spies, R. B. 1987. The biological effects of petroleum hydrocarbons in the sea: Assessments from the field and microcosms. Long-Term Environmental Effects of Offshore Oil and Gas Development. D. F. Boesch and N. N. Rabalais, (eds.). Elsevier Applied Science, London, pp. 411-467.
Spies, R. B. 1989. Sediment bioassays, chemical contaminants and benthic ecology: New insights or just muddy water? Marine Environmental Research 27:73-75. (editorial)
Spies, R. B., and D. J. DesMarais. 1983. Natural isotope study of trophic enrichment of marine benthic communities by petroleum seepage. Marine Biology. 73, 67-71.
Spies, R. B., and P. H. Davis. 1979. The infaunal benthos of a natural oil seep in the Santa Barbara Channel. Marine Biology 50:227-237.
Spies, R. B., and P. H. Davis. 1982. Toxicity of Santa Barbara seep oil to starfish embryos. III. Influence of parental exposure and the effects of other crude oils. Marine Environmental Research 6:3-11.
Spies, R. B., and P. H. Davis. 1979. The infaunal benthos of a natural oil seep in the Santa Barbara Channel. Marine Biology 50:227-237.
Spies, R. B., and D. J. DesMarais. 1983. Natural isotope study of trophic enrichment of marine benthic communities by petroleum seepage. Marine Biology 73:67-71.
Spies, R. B., D. D. Hardin and J. P. Toal. 1989. Organic enrichment or toxicity? A comparison of the effects of kelp and crude oil in sediments on the colonization and growth of benthic infauna. Journal of Experimental Marine Biology and Ecology 124, 261-282.
Spies, R. B., J. E. Bauer and D. H. Hardin. 1989. A stable isotope study of sedimentary carbon utilization by Capitella spp.: effects of two carbon sources and geochemical conditions during their diagenesis. Marine Biology 101:68-74.
Spies, R. B., J. J. Stegeman, D. E. Hinton, B. Woodin, M. Okihiro, R. Smolowitz and D. Shea. 1996. Biomarkers of hydrocarbon exposure and sublethal effects in embiotocid fishes fram a natural petroleum seep in the Santa Barbara Channel. Aquatic Toxicology 34, 195-219.
Spies, R. B., J. J. Stegeman, D. W. Rice, Jr., B. Woodin, P. Thomas, J. E. Hose, J. Cross and M. Prieto. 1990. Sublethal responses of Platichthys stellatus to organic contamination in San Francisco Bay with emphasis on reproduction. In: Biological Markers of Environmental Contamination. Lewis Publishers, Chelsea, MI, pp. 87-122.
Spies, R. B., J. S. Felton and L. J. Dillard. 1982. Hepatic mixed-function oxidases in California flatfish are increased in contaminated environments and by oil and PCB ingestion. Marine Biology 70:117-127.
Spies, R. B., P. H. Davis and D. Stuermer. 1980. Ecology of a petroleum seep off the California coast. In: Marine Environmental Pollution. R. Geyer, (Ed.). Elsevier, Amsterdam, pp. 229-263.
Spitzy, A., and V. Ittekkot. 1991. Dissolved and particulate organic matter in rivers. Pp. 5-17 in Mantoura, R. F. C., H.-M. Martin, and R. Wollast, eds. Ocean Margin Processes in Global Change. John Wiley and Sons, New York.
Sporsol, S., N. Gjos, R. G. Lichtenthaler, K. O. Gustavsen, K. Urdal, F. Oreld, and J. Skel. 1983. Source identification of aromatic hydrocarbons in sediments using GC/MS. Environmental Science and Technology 17:282-286.
Spraker, T. R., L. F. Lowry, and K. J. Frost. 1994. Gross necropsy and histopathological lesions found in harbor seals. In: Marine mammals and the Exxon Valdez. Loughlin, T. R. (ed). Academic Press, San Diego, CA, pp. 281-311.
St. Aubin, D. J. 1990a. Physiologic and toxic effects on polar bears. In Sea mammals and oil, confronting the risks. (Geraci, J. R. and St. Aubin, D. J., Eds). Academic Press, San Diego, California, pp. 235-239.
St. Aubin, D. J. 1990b. Physiologic and toxic effects on pinnipeds. In Sea mammals and oil, confronting the risks. (Geraci, J. R. and St. Aubin, D. J., Eds). Academic Press, San Diego, CA, pp. 103-127.
St. Aubin, D. J., and V. Lounsbury. 1990. Oil effects on Manatees: Evaluating the risks. In Sea mammals and oil, confronting the risks. (Geraci, J. R. and St. Aubin, D. J., Eds). Academic Press, San Diego, CA, pp. 241-251.
St. Pé, K. M. 1990. An Assessment of Produced Water Impacts to Low-Energy, Brackish Water Systems in Southeast Louisiana. Louisiana
Department of Environmental Quality, Watter Pollution Control Division, Baton Rouge, LA, 199pp.
Standley, L. J. 1997. Effect of sedimentary organic matter composition on the partitioning and bioavailability of dieldrin to the oligochaete Lumbriculus variegatus. Environmental Science and Technology 31:2577-2583.
Stangroom, S. J., J. N. Lester, and C. D. Collins. 2000. Abiotic behaviour of organic micropollutants in soils and the aquatic environment. A review: I. Partitioning . Environmental Technology 21:845-863.
Statistics Canada. 1997. Shipping in Canada. Statistics Canada, p 19. Statistics Canada. 2000. Statistics Canada’s internet site. [Online]. Available: http://www.statcan.ca/english [2000, June 27].
Stegeman, J. J. 1989. Cytochrome P450 forms in fish: Catalytic, immunological and sequence similarities. Xenobiotica 19: 1093-1110.
Stegeman, J. J. and J. J. Lech. 1991. Monooxygenase systems in aquatic species: Carcinogen metabolism and biomarkers for carcinogen exposure. Environmental Health Perspective 90: 101-109.
Steichen DJ Jr, S.J. Holbrook, and C.W. Osenberg. 1996. Distribution and abundance of benthic and demersal macrofauna within a natural hydrocarbon seep. ISSN: 0171-8630. Copyright Inter-Research, Oldendorf/ Luhe, Marine Ecology Progress Series 138:71-82
Steimle & Associates, Inc. 1991. Produced Water Impacts on Louisiana Wetlands. Health and Environmental Sciences, API Publication No. 4517, Washington, D.C., 132 pp.
Stein, J. E., T. K. Collier, W. L. Reichert, E. Castillas, T. Hom, and U. Varanasi. 1992. Bioindicators of contaminant exposure and sublethal effects: studies with benthic fish in Puget Sound, Washington. Environmental Toxicology and Chemistry 11:701-714.
Stekoll, M. S., L. Deysher, and T. A. Dean. 1993. Seaweeds and the Exxon Valdez oil spill. Pages 135-140, in Proceedings of the 1993 International Oil Spill Conference: Prevention, preparedness and response. American Petroleum Institute Publication 4580. Washington, D.C.
Stenstrom, M. K., G. S. Silverman, and T. A. Bursztynsky. 1984. Oil and grease in urban stormwaters. Journal of Environmental Engineering 110(1):58-72.
Stenstrom, M. K., S. Fam, and G. S. Silverman. 1987. Analysis of oil and grease components to assess the quality of urban runoff. Vandermeulen, John H., and Steve E. Hrudey, ed. Oil in Freshwater: Chemistry, Biology, Countermeasure Technology. Proceedings of the Symposium on Oil Pollution in Freshwater, Alberta, Canada. Pergamon Press, New York, pp. 138-148.
Steurmer, D. H., R. B. Spies and P. H. Davis. 1981. Toxicity of Santa Barbara seep oil to starfish embryos. I. Hydrocarbon composition of test solutions and field samples. Marine Environmental Research 5:275-286.
Steurmer, D. H., R. B. Spies, P. H. Davis, D. J. Ng, C. J. Morris, and S. Neal. 1982. The hydrocarbons in the Isla Vista marine seep environment. Marine Chemistry 11:413-426.
Stewart-Oaten, A., J. Bence, and C. Osenberg. 1992. Assessing effects of unreplicated perturbations: no simple solutions. Ecology 73(4): 1396-1404.
Stiver, W., and D. Mackay. 1984. Evaporation rate of spills of hydrocarbons and petroleum mixtures. Environmental Science and Technology 18:834-840.
Straughan, D. 1976. Sublethal effects of natural petroleum in the marine environment. American Petroleum Institute 4280:1-120.
Straughan, D., and B. C. Abbott. 1971. The Santa Barbara oil spill: ecological changes and natural oil leaks. Water Pollution by Oil. P. Hepple (ed.). Institute of Petroleum, London, pp. 257-262.
Street, G. T., and P. A. Montagna. 1996. Loss of genetic diversity in Harpacticoida near offshore platforms. Marine Biology 126:271-282.
Sugiura, K. M. Ishihara, T. Shimauchi. 1997. Physicological properties and biodegradability of crude oil. Environmental Science Technology 31 (1):45-51.
Sutton, O. G. 1934. Wind structure and evaporation in a turbulent atmosphere, Proceedings of the Royal Society of London, A 146:701-722.
Swannell, R. P., K. Lee, and M. McDonagh. 1996. Field evaluation of marine oil spill bioremediation. Microbiological Reviews 60:342-365.
Symens, P., and A. H. Alsuhaibany. 1996. Status of the breeding population of terns (Sternidae) along the eastern coast of Saudi Arabia following the 1991 Gulf War. In: Krupp, F., A. H. Abuzinada, and I. A. Nader, eds. A Marine Wildlife Santuary for the Arabian Gulf. Environmental Research and Conservation Following the 1991 Gulf War Oil Spill. National Commission for Wildlife Conservation and Development, Riyadh, Kingdom of Saudi Arabia and Senchenberg Research Institute, Frankfurt a. M., Germany, pp. 404-420.
Symens, P., and M. Werner. 1996. Status of the Socotra cormorant in the Arabian Gulf after the 1991 Gulf War oil spill, with an outline of a standardized census technique. In Krupp, F., A. H. Abuzinada, and I. A. Nader, eds. A Marine Wildlife Santuary for the Arabian Gulf. Environmental Research and Conservation Following the 1991 Gulf War Oil Spill. National Commission for Wildlife Conservation and Development, Riyadh, Kingdom of Saudi Arabia and Senchenberg Research Institute, Frankfurt a. M., Germany, pp. 390-403.
Tanis, J. J. C., and M. F. Morzer Bruijns. 1969. The impact of oil-pollution on sea birds in Europe. In International Conference on oil pollution of the sea. Report of proceedings. Advisory Committee on Oil Pollution of the Sea, London, pp. 67-113.
Tawfiq, N. I., and D. A. Olsen. 1993. Saudi Arabia’s response to the 1991 Gulf oil spill. Marine Pollution Bulletin 27:333-345.
Teal, J. M., and R. W. Howarth. 1984. Oil spill studies, a review of ecological effects. Environmental Management 8:27-44.
Teal, J. M., J. W. Farrington, K. A. Burns, J. J. Stegeman, B. W. Tripp, B. Woodin, and C. Phinney. 1992. The West Falmouth oil spill after 20 years: Fate of fuel oil compounds and effects on animals. Marine Pollution Bulletin 24:607-614.
Teas, J. H., R. R. Lessard, G. P. Canevari, C. D. Brown, and R. Glenn. 1993. Saving oiled mangroves using a new non-dispersing shoreline cleaner. In Proceedings, Conference on Assessment of Ecological Impacts of Oil Spills. American Institute of Biological Sciences, Washington, D.C., pp. 147-151.
Telang, S. A., G. W. Hodgson, and B. L. Baker. 1981. Occurrence and distribution of oxygen and organic compounds in mountain streams of the Marmot Basin. Journal of Environmental Quality 10(1):18-22.
Terrens, G. W., and R. D. Tait. 1996. Monitoring ocean concentrations of aromatic hydrocarbons from produced formation water discharges to Bass Strait, Australia. SPE 36033. In: Proceedings of the International Conference on Health, Safety & Environment. Society of Petroleum Engineers, Richardson, TX, pp. 739-747.
Tesseraux, I., B. Mach, and G. Koss. 1998. Aviation fuels and aircraft emissions risk characterization based on data of the Hamburg airport. Zentralblatt fuer Hygiene und Umweltmedizin 201:135-151
Thomann, R. V., and J. Komlos. 1999. Model of biota-sediment accumulation factor for polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry 18:1060-1068.
Thomas, R.E., P. M. Harris, and S. D. Rice. 1999. Survival in air of Mytilus trossulus following long-term exposure to spilled Exxon Valdez crude oil in Prince William Sound Comp . Biochem. Physiol, C: 122C, 147-152.
Thorpe, S. A. 2001. Langmuir circulation and the dispersion of oil spills in shallow seas. Spill Science & Technology Bulletin 6(3/4):213-223.
Tomlinson, Richard D., Brian N. Bebee, Andrew A. Heyward, Sydney G. Munger, Robert G. Swartz, Steven Lazoff, Dimitris E. Spyridakis, Michael F. Shepard, Ronald M. Thom, Kenneth K. Chew, Richard R.
Whitney. 1980. Fate and effects of particulates discharged by combined sewers and storm drains. EPA-600/2-80-111. U.S. Environmental Protection Agency, Cincinnati, OH.
Topham, D. R. 1984. The formation of gas hydrates on bubbles of hydrocarbon gases rising in seawater. Chemical Engineering Science 39(5):821-828.
Topham, D. R. 1975. Hydrodynamics of an oil well blowout. Beaufort Sea Technical Report, Institute of Ocean Sciences. Sidney, B. C. 33.
Topham, D. R. 1984. The formation of gas hydrates on bubbles of hydrocarbon gases rising in seawater. Chemical Engineering Science, 39 (5):821-828.
Trenbreth, K. E., and J. W. Hurrell. 1994. Decadal atmospheric-ocean variations in the Pacific. Climate Dynamics 9, 303-309.
Trust, K. A., D. Esler, B. R. Woodin, and J. J Stegeman. 2000. Cytochrome P450 1A induction in sea ducks inhabiting near shore areas of Prince William Sound, Alaska. Marine Pollution Bulletin 40:397-403.
Tsurumi, M., N. Oka, and K. Ono. 1999. Bibliography of world oil pollution with special reference to seabirds mainly since 1978. J. Yamashina Inst. Ornithol. 31:142-200.
Tunnell, J. W., and D. W. Hicks. 1994. Environmental impact and recovery of the Exxon pipeline oil spill and burn site, upper Copano Bay, Texas. Unpublished second quarterly report FY 1994. U.S. Army Corps of Engineers Navigation Data Center. 1997a. United States Waterway Data.
U.S. Army Corps of Engineers (ACOE). 1997b. Waterborne Commerce of the United States. 1997. Part 5-National Summaries, Section 2 Compiled under the supervision of the Water Resources Support Center. Fort Belvoir, VA.
U.S. Bureau of the Census. 1998. State and Metropolitan Area Data Book 1997-98. 5th edition. U.S. Bureau of the Census, Washington, DC.
U.S. Coast Guard, Marine Casualty and Pollution Database on CD-ROM, US Coast Guard (G-MOA). Washington, D.C.
U.S. Department of Energy (DOE). 1999. Petroleum Supply Annual, 1999 Volume 1. [Online]. Available: http://www.eia.doe.gov/pub/oil_gas/petroleum/data_publications/petroleum_supply_annual/psa_volume1/historical/1999/txt/table_14.txt
U.S. Environmental Protection Agency (U.S. EPA). 1991. Nonroad Engine and Vehicle Emission Study Report (EPA-21A-2001).
U.S. Environmental Protection Agency, Office of Compliance. 1996a. Profile of the oil and gas industry. EPA 310-R-99-006. U.S. Environmental Protection Agency, Washington, D.C.
U.S. Environmental Protection Agency (USEPA). 1996b. Development document for the final effluent limitations guidelines and standards for the coastal subcategory of the oil and gas extraction point source category. EPA 821/R-96-023.
U.S. Environmental Protection Agency, Office of Water. 1996c. The quality of our nation’s water: 1996. U.S. Environmental Protection Agency, Washington, D.C.
U.S. Environmental Protection Agency, Office of Water. 1998. National water quality inventory: 1996 report to Congress. EPA 841-F-97-003. U.S. Environmental Protection Agency, Office of Water, Washington, D.C.
United Kingdom Offshore Operators Association (UNOOA). 1999. 1999 Environmental Report. [Online]. Available: http://www.ukooa.co.uk/issues/1999report/enviro99_water.htm.
United Nations. 1998. Demographic Yearbook 1995. United Nations publication, Sales No. E/F. 97. XIII. 1. [Online]. Available: http://www.un.org/unsd/demog/index.html.
United States Air Force. 1975. Air Force Fuel Dumping: October 1974 to March 1975, Air Force Engineering and Services Laboratory Report . AFCEC-TR-75-21, Tyndall Air Force Base, FL.
Utvik, T.I.R. and S. Johnson. 1999. Bioavailability of polycyclic hydrocarbon in the North sea. Environmental Science and Technology 33: 1963-1969.
van Oudenhoven, J. A. C. M., V. Draper, G. P. Ebbon, P. D. Holmes, and J. L. Nooyen. 1983. Characteristics of petroleum and its behaviour at sea. Den Haag, Belgium. 46pp.
Van Vaeck L., Broddin G, and K. Van Cauwenberghe. 1979. Differences in particle size distributions of major organic pollutants in ambient aerosols in urban, rural and seashore areas, Environmental Science and Technology 13:1494-1502.
Van Vleet, E. S., and J. G. Quinn. 1978. Contribution of chronic petroleum inputs to Narragansett Bay and Rhode Island Sound sediments. Journal of the Fisheries Research Board of Canada 35:536-543.
Vandermeulen, J. H., and J. R. Jotcham. 1986. Long-term persistence of bunker C fuel oil and revegetation of a north-temperate saltmarsh: Miguasha 1974-1985. In: Proceedings of the Ninth Annual Arctic and Marine Oil Spill Program Technical Seminar, Environment Canada, June 10-12, 1986, Edmonton, Canada.
Vandermeulen, J. H., and D. C. Gordon, Jr. 1976. Re-entry of five year old stranded Bunker C fuel oil from a low-energy beach into the water, sediments, and biota of Chedabucto Bay, Nova Scotia. Journal of Fisheries Research Board of Canada 33:2002-2010.
Vandermeulen, J. H., and J. M. Capuzzo. 1983. Understanding sublethal pollutant effects in the marine environment. Paper No. 9 in Ocean Waste Management: Policy and Strategies. Background Papers of Symposium, May 2-6, 1983, University of Rhode Island, Kingston.
Varanasi, U., J. E. Stein, M. Nishimoto, W. L. Reichert, and T. K. Collier. 1987. Chemical carcinogenesis in feral fish: Uptake, activation, and detoxication of organic xenobiotics.
Vermeer, K., and R. Vermeer. 1975. Oil threats to birds on the Canadian west coast. Can. Field Nat. 89:278-298.
Wakeham, S. G. 1977. Hydrocarbon budgets for Lake Washington. Limnology and Oceanography 22:952-957.
Walker, W. J., R. P. McNutt, and C. A. K. Maslanka. 1999. The potential contribution of urban runoff to surface sediments of the Passaic River: sources and chemical characteristics. Chemosphere 38(2):363-377.
Wang, Z. 1994a. Analysis results of alkylated PAH homologues for remote sensing test samples. Technical Report 94-04. Environment Canada, Ottawa.
Wang, Z. 1994b. Analysis results of ten biodegradation oil samples from fresh water standard inoculum experiments (Part I). Technical Report 94-07. Environment Canada, Ottawa.
Wang, Z. 1994c. Analysis results of ten biodegradation oil samples from fresh water standard inoculum experiments (Part II). Technical Report 94-07. Environment Canada, Ottawa.
Wang, Z. 1995. Analysis results of the Ile-de-la-Madeleine incinerator burn samples. Unpublished. Environment Canada, Ottawa.
Wang, Z. 1998b. Study of 25-year-old Metula oil spill samples: degradation and persistence of stranded oil at the sheltered and low-energy “Puerto Espora” location. Special Report 98-7. Environment Canada, Ottawa.
Wang, Z. 1999. TPH analysis results of Fco soil samples. Technical Report 99-06. Environment Canada, Ottawa.
Wang, Z. 2000. Analysis results of 98 mobile burn water, diesel, and residue samples. Technical Report 2000-3. Environment Canada, Ottawa.
Wang, Z. 1998a. Hydrocarbon analysis results of legal samples—identification and matching of an unknown spilled oil from Canal Lachine, Quebec. Special Report 98-02. Environment Canada, Ottawa.
Wang, Z., and M. Fingas. 1998. BTEX quantitation in oils by GC/MS, in Encyclopedia of Environmental Analysis and Remediation, Vol. 2, Robert A. Meyers, Ed., John Wiley and Sons, New York, pp. 829-852.
Wang, Z., and M. Fingas. 1996. Separation and characterization of petro
leum hydrocarbons and surfactant in orimulsion dispersion samples. Proceedings of the Nineteenth Arctic Marine Oilspill Program Technical Seminar. Environment Canada, Ottawa, Ontario, pp. 115-135.
Wang, Z., M. Fingas, and D. S. Page. 1999a. Oil spill identification (review). Journal of Chromatography A, 843:369-411.
Wang, Z., M. Fingas, and K. Li. 1994. Fractionation of a light crude and identification and quantitation of aliphatic, aromatic, and biomarker compounds by GC-FID and GC-MS, part II. Journal of Chromatographic Science 32:367-382.
Wang, Z., M. Fingas, E. H. Owens, L. Sigouin. 2000c. Study of long-term spilled Metula oil: degradation and persistence of petroleum biomarkers. Proceedings of the 23rd Arctic and Marine Oil Spill Program (AMOP) Technical Seminar. Environment Canada, Ottawa, pp. 99-122.
Wang, Z., M. Fingas, E. H. Owens, L. Sigouin. In press. Long-term fate and persistence of the spilled Metula oil in a marine salt environment: degradation of petroleum biomarkers. Journal of Chromatography A.
Wang, Z., M. Fingas, L. Sigouin. 2000b. Characterization and source identification of an unknown spilled oil using fingerprinting techniques by GC-MS and GC-FID. LC-GC 10:1058-1068.
Wang, Z., M. Fingas, M. Landriault, L. Sigouin, P. Lambert. 2000a. Differentiation of PAHs in burn residue and soot samples and differentiation of pyrogenic and petrogenic PAHs—the 1994 and 1997 Mobile Burn study. Hsu, C. S., I. Mochida, C. Song (eds.). Chemistry of Diesel Fuel. Taylor and Francis Publishing Company, New York, pp. 237-254.
Wang, Z., M. Fingas, M. Landriault, L. Sigouin, S. Grenon, D. Zhang. 1999b. Source identification of an unknown spilled oil from Quebec (1998) by unique biomarkers and diagnostic ratios of “source-specific marker” compounds. Environmental Technology 20:851-862.
Wang, Z., M. Fingas, M. Landriault, L. Sigouin, Y. Feng, J. Mullin. 1997b. Using systematic and comparative data to identify the source of an unknown oil on contaminated birds. Journal of Chromatography A 775:251-265.
Wang, Z., M. Fingas, S. Blenkinsopp, G. Sergy, M. Landriault, L. Sigouin, J. Foght, K. Semple, D. W. S. Westlake. 1998a. Comparison of oil composition changes to biodegradation and physical weathering in different oils. Journal of Chromatography A 809:89-107.
Wang, Z., M. Fingas, S. Blenkinsopp, G. Sergy, M. Landriault, L. Sigouin, P. Lambert. 1998b. Study of the 25-year-old Nipisi oil spill: persistence of oil residues and comparisons between surface and subsurface sediments. Environmental Science and Technology 32:2222-2232.
Wang, Z., P. Jokuty, M. Fingas, L. Sigouin. 2001. Characterization of Federated oil fractions used for the PTAC project to study the petroleum fraction-specific toxicity to soils. Proceedings of the 24th Arctic and Marine Oil Spill Program (AMOP) Technical Seminar. Environment Canada, Ottawa, pp. 79-98.
Wang, Z., S. Blenkinsopp, M. Fingas, G. Sergy, M. Landriault, L. Sigouin, J. Foght, K. Semple, D. W. S. Westlake. 1997a. Chemical composition changes and biodegradation potentials of nine Alaska oils under freshwater incubation conditions. Preprints of Symposia. American Chemical Society 43(3):828-835.
Wania, F., and D. Mackay. 1996. Tracking the distribution of persistent organic pollutants. Environmental Science and Technology 30:A390-A396.
Warnken, J. 1993. Salt-marshes and intertidal habitats of the Jubail Marine Wildlife Sancturary: extent of oil-impacted area and estimated losses of above-ground plant biomass following the 1991 Gulf War oil spill. In Krupp, F., A. H. Abuzinada, and I. A. Nader, eds. A Marine Wildlife Santuary for the Arabian Gulf. Environmental Research and Conservation Following the 1991 Gulf War Oil Spill. National Commission for Wildlife Conservation and Development, Riyadh, Kingdom of Saudi Arabia and Senchenberg Research Institute, Frankfurt a.M., Germany, pp. 177-185.
Weaver, D. W. 1969. Geology of the northern Channel Islands. Pacific Sections AAPG and SEPM Special Publications, 200pp.
Webb, J. W. 1993. Final Report: Oil Spill Impacts and Restoration Evaluation of Marrow Marsh Resulting from the Apex Barge Spill in Galveston, Bay. Texas A&M University, Galveston, TX, 51pp.
Webb, J. W. 1996. Effects of oil on salt marshes. Pages 55-64 in C. E. Proffitt and P. F. Roscigno (eds.). Symposium Proceedings: Gulf of Mexico and Caribbean Oil Spills in Coastal Ecosystems: Assessing Effects, Natural Recovery, and Progress in Remediation Research. OCS Study MMS 95-0063. Dept. of the Interior, Minerals Management Service, New Orleans, LA.
Webb, J. W., S. K. Alexander and J. K. Winters. 1985. Effects of autumn application of oil on Spartina alterniflora in a Texas salt marsh. Environmental Pollution (A)38:321-337.
Weems, L. H., I. Byron, J. O’Brien, D. W. Oge, and R. Lanier. 1997. Recovery of LAPIO from the bottom of the lower Mississippi River. Proceedings of the 1997 Oil Spill Conference. American Petroleum Institute, Washington, D.C., pp. 773-776.
Weidmer, M.M, J. Fink, J.J. Stegeman, and R. Smolowitz. 1996. Cytochrome P-450 induction and histopathology in preemergent pink salmon from oiled spawning sites in Prince William Sound. In Proceedings: S.D. Rice, R.B. Spies, D.A. Wolfe, and B.A. Wright (Eds.) Exxon Valdez Oil spill symposium. American Fisheries Society Symposium No. 18.
Wertheimer, A. C., and A. G. Celewycx. 1996. Abundance and growth of juvenile pink salmon I oiled and unoiled locations of western Prince William Sound after the Exxon Valdez oil spill., in: S. D. Rice, R. B. Spies, D. A. Wolfe and B. A. Wright (eds.). Proceedings of the Exxon Valdez oil spill symposium. American Fisheries Society Symposium 18, pp. 518-532.
Weston, D. P., and L. M. Mayer. 1998. In vitro digestive fluid extraction as a measure of the bioavailability of sediment-associated polycyclic aromatic hydrocarbons: Sources of variation and implications for partitioning models. Environmental Toxicology and Chemistry 17:820-829.
Westphal, A., and M. K. Rowan, 1970. Some observations on the effects of oil pollution on the Jackass Penguin. Ostrich (Suppl., 8:521-526.
Whipple, W., Jr. and J. Hunter. 1979. Petroleum hydrocarbons in urban runoff. Water Resources Bulletin 15(4):1096-1105.
Widbom, B., and C. A. Oviatt. 1994. The ‘World Prodigy’ oil spill in Narragansett Bay, Rhode Island, acute effects on marcobenthic crustacean populations. Hydrobiology 291:115-124.
Widdows, J., D. Dixon, P. Donkin, S. V. Evans, I. McFadzen, D. Page, P. N. Salkeld, and C. M. Worrall. 1989. Sublethal biological effects monitoring in the region of Sullom Voe, Shetland. Shetland Oil Terminal Environmental Advisory Group, Aberdeen (UK), 21pp.
Widdows, J., P. Donkin and S. V. Evans. 1987. Physiological responses of Mytilus edulis during chronic oil exposure and recovery. Marine Environmental Research 23:15-32.
Widdows, J., P. Donkin, M. D. Brinsley, S. V. Evans, P. N. Salkeld, A. Franklin, R. J. Law, and M. J. Waldock. 1995. Scope for growth and contaminant levels in North Sea mussels Mytilus edulis. Marine Ecology Progress Series 127:131-148.
Widdows, J., T. Bakke, B. L. Bayne, P. Donkin, D. R. Livingstone, D. M. Lowe, M. N. Moore, S. V. Evans and S. L. Moore. 1982. Responses of Mytilus edulis on exposure to the wateraccommodated fraction of North Sea oil. Marine Biology 67:15-31.
Wiens, J. A. 1995. Recovery of seabirds following the Exxon Valdez oil spill: an overview. Wells, P. G., Butler, J. N., Hughes, J. S. (Eds.). Exxon Valdez oil spill: fate and effects in Alaskan waters. STP 1219, American Society for Testing and Materials, Philadelphia, PA, pp. 824-893.
Wiens, J. A., and K. R. Parker. 1995. Analyzing the effects of accidental environmental impacts: approaches and assumptions. Ecological Applications 5:1069-1083.
Wiens, J. A., R. H. Day, S. M. Murphy and K. R. Parker. 2001. Drawing conclusions nine years after the Exxon Valdez oil spill. Condor, 103:886-892.
Wiens, J. A., T. O. Crist, R. H. Day, S. M. Murphy, G. D. Hayward. 1996. Effects of the Exxon Valdez oil spill on marine bird communities in Prince William Sound, Alaska. Ecological Applications 6:828-841.
Wilkinson, E. R. 1971. California offshore oil and gas seeps. California Oil Fields—Summary of Operations 57(1):5-28.
Willette, M. 1996. Impacts of the Exxon Valdez oil spill on migration, growth, and survival of juvenile pink salmon in Prince William Sound. In: S. D. Rice, R. B. Spies, D. A. Wolfe, and B. A. Wright, Eds., Proceedings of the Exxon Valdez Oil Spill Symposium. American Fisheries Society Symposium 18, Bethesda, MD, pp. 533-550.
Wilson, D., Y. C. Poon, and D. Mackay. 1986. An exploratory study of the buoyancy behaviour of weathered oils in water, EE-85, Environment Canada, Ottawa, Ontario 50pp.
Wilson, R. D., P. H. Monaghan, A. Osanik, L. C. Price, and M. A. Rogers. 1973a. Natural marine oil seepage. Science 184:857-865.
Wilson, R. D., P. H. Monaghan, A. Osanik, L. C. Price, and M. A. Rogers. 1973b. Estimate of annual input of petroleum to the marine environment from natural marine seepage. Trans., Gulf Coast Association of Geological Societies 23:182l-193.
Wirgin, I., C. Grunwald, S. Courtenay, G. Kreamer, W. L. Reichert, and J. E. Stein. 1994. A biomarker approach to assessing xenobiotic exposure in Atlantic tomcod from the North American Atlantic Coast. Environmental Health Perspectives 102:764-770.
Wolfe, D. A., M. J. Hameedi, J. A. Galt, G. Watabayashi, J. Short, C. O’Clair, S. Rice, J. Michel, J. R. Payne, J. Braddock, S. Hanna, and D. Sale. 1994. The fate of the oil spilled from the t/v Exxon Valdez. Environmental Science and Technology 28(13):560A-568A.
Wolfe, D. A., K. J. Scott, J. R. Clayton, Jr., J. Lunz, J. R. Payne, and T. A. Thompson. 1995. Comparative toxicities of polar and non-polar organic fractions from sediments affected by the Exxon Valdez oil spill in Prince William Sound, Alaska. Chemistry and Ecology 10:137-156.
World Resources Institute. 1998. World Resources 1998-99. Oxford University Press, New York, 369pp.
Wu, S. C., and P. M. Gschwend. 1988. Numerical Modeling of Sorption kinetics of organic-compounds to soil and sediment particles. American Geophysical Union, Washington 24 (8):1373-1383.
Yamane, A., I. Nagashima, T. Okubo, M. Okada, and A. Murakami. 1990. Stormwater runoff of hydrocarbons in the Tama River basin in Tokyo (Japan) and their fate in the river. Water Science and Technology 22(10/ 11):119-126.
Yapa, P. D., and L. Zheng. 1997. Simulation of oil spills from underwater accidents I: Model development. Journal of Hydraulic Research, IAHR 35(5):673-687
Yaroch, G. N., and G. A. Reiter. 1989. The tank barge MCN-5: lessons in salvage and response guidelines. In: Proceedings of the 1898 Oil Spill Conference. American Petroleum Institute, Washington, D.C., pp. 87-90.
Yerkes, R. F., H. C. Wagner, and K. A. Yenne. 1969. Petroleum development in the region of the Santa Barbara Channel. Geology, Petroleum Development, and Seismicity of the Santa Barbara Channel Region, California. U.S. Geological Survey Prof. Paper 679:13-27.
Youssef, M., and M. Spaulding. 1993. Drift current under the action of wind and waves. Proceedings of the Sixteenth Arctic and Marine Oil Spill Program Technical Seminar, Environment Canada, Ottawa, Ontario, pp. 587-615.
Yunker, M., and R. MacDonald. 1995. Composition and origins of polycyclic aromatic hydrocarbons in the Mackenzie River and on the Beaufort Sea shelf. Arctic 48(2):118-129.
Yunker, M., R. MacDonald, B. Fowler, W. Cretney, S. Dallimore, and F. McLaughlin. 1991. Geochemistry and fluxes of hydrocarbons to the Beaufort Sea shelf: a multivariate comparison of fluvial imports and coastal erosion of peat using principal components analysis. Geochimica et Cosmochimica Acta 55:255-273.
Zakaria, M. P., A. Horinouchi, S. Tsutsumi, H. Takada, S. Tanalse, and A. Ismal. 2000. Oil pollution in the Straits of Malacca: In: application of molecular markers for source identification. Environmental Science and Technology 34:1189-1196.
Zeng, E., and C. Vista. 1997. Organic pollutants in the coastal environment off San Diego, California. 1. Source identification and assessment by compositional indices of polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry 16(2):179-188.
Zhang, H., S. J. Eisenreich, T.; Franz, J. E. Baker, and J. Offenberg. 1999. Evidence for the increased gaseous PCB fluxes to Lake Michigan from Chicago. Environmental Science and Techology 33:2131-2137.
Zheng, L., and P. D. Yapa. 1998. Simulation of oil spills from underwater accidents II: Model verification. Journal of Hydraulic Research, IAHR 36:(1).
Zitka, R. G., and W. J. Cooper. 1987. Photochemistry of environmental aquatic systems. ACS Symposium Series 327. American Chemical Society, Washington, D.C., 288 pp.


































































