Summary of the Workshop
INTRODUCTION
Toxicogenomics is a discipline that has developed with recent advances in toxicology, molecular genetics, and cell biology and holds promise for advancing the scientific basis of risk assessment and other fields of science. To consider the potential contributions of toxicogenomics in risk assessment, the National Research Council convened a 1-day workshop titled “How Toxicogenomics Technologies Could Inform Critical Issues in Carcinogenic Risk Assessment of Environmental Chemicals,” on December 15, 2003, at the request of the standing National Research Council Committee on Emerging Issues and Data on Environmental Contaminants. This standing committee, which is sponsored by the National Institute of Environmental Health Sciences (NIEHS), provides a forum on toxicogenomics and other emerging issues.
The Committee on How Toxicogenomics Could Inform Critical Issues in Carcinogenic Risk Assessment of Environmental Chemicals was formed to plan the workshop and summarize its highlights. This summary covers the presentations and discussion at the workshop and background on risk assessment; it is not a primer on either risk assessment or toxicogenomics. The objective of the workshop was not to evaluate the promise of toxicogenomic technologies but rather to highlight eventual possible toxicogenomic uses of data in risk assessment. These highlights can serve as a basis for discussing what can be realistically expected of
toxicogenomics and for discussing policy challenges associated with the use of the data.
The workshop focused on cancer risk assessment for several reasons: cancer risk assessments are numerous, the many scientific uncertainties lead to controversies on this topic, and there is much scientific research involving toxicogenomics in the field of carcinogenesis. The workshop dealt specifically with cancer risk assessment of environmental exposure to chemicals,1 based on the committee’s understanding of applicable frameworks.
Risk assessment is a set of methods, applied in regulatory and other settings, for estimating the likelihood that exposure to hazardous agents, including chemicals, will harm people or the environment. Many public-health decisions in environmental, occupational, and consumer protection are based on risk assessments of chemicals. Risk assessment is conducted in several steps: hazard identification, the characterization of intrinsic toxic properties of a chemical or the nature of the hazard; the quantitative relationship between exposure and effects, the dose-response assessment; and assessment of potential exposures of human populations to the chemical of concern. A final step is risk characterization, which “combines the assessment of exposure and response under various exposure conditions to estimate the probability of specific harm to an exposed individual or population” (NRC 1994). The first three steps of risk assessment are described in this report; the probabilistic approach of risk characterization was not a focus of the workshop or of this report.
Risk assessments are frequently criticized for their dependence on default values used to deal with uncertainties in the absence of relevant data (NRC 1994). The task of risk assessors is difficult because they are often charged with protecting the public health without adequate scientific understanding of the fundamental causes of cancer and other diseases associated with chemical exposures. New scientific discoveries or technologies that might resolve crucial data gaps and data inconsistencies have the potential to improve risk assessment by providing additional data on toxic effects, increasing understanding of mechanisms and modes of action, and enhancing the reliability of dose response extrapolation.
Toxicogenomics encompasses technologies that enable scientists to measure genetic-sequence variation (genomics), gene transcription (tran-
scriptomics), protein expression (proteomics), and metabolite profiles (metabolomics) in response to chemicals and other stressors. Those “-omics” technologies hold promise for obtaining new information relevant to the scientific requirements of risk assessment. However, exactly how toxicogenomic findings will be incorporated into the regulatory process may not be clear for some time,2 even the general concepts of how toxicogenomics might be incorporated into risk assessment may be unclear to toxicologists and other biologists not working directly in risk assessment, and some risk assessors may not be sufficiently familiar with toxicogenomics to see its eventual impact on their work. In an attempt to bridge that gap and to provide an opportunity for scientists interested in regulatory issues and scientists interested in genomics to discuss the intersection of their interests, the workshop included scientists with expertise in “-omics” technologies, as well as experts in toxicology, risk assessment, epidemiology, and public health.
The workshop began with an overview of how scientific information generally informs risk assessment and an overview of the types of data gaps that make regulatory risk assessments challenging and controversial. It then moved to presentations on types of toxicogenomic studies, focusing largely on studies of gene expression. Next, two case studies were presented to illustrate the nature and extent of challenges in carcinogen risk assessment and to foster discussion of the potential contribution of toxicogenomic technologies to improving cancer risk assessment. The workshop concluded with a discussion of types of research that might be undertaken to move the field forward.
The workshop agenda is included as Appendix A, and biographical sketches of speakers and planning-committee members are presented in Appendix B. Audiofiles and PowerPoint files for most of the presentations are available at http://dels.nas.edu/emergissues.
CANCER RISK ASSESSMENT
Government agencies charged with the protection of the public health, such as the U.S. Environmental Protection Agency (EPA) and the U.S. Food and Drug Administration (FDA), are required to review, quantify, and ultimately regulate chemicals in a manner that will protect and
enhance the public health and the environment. Since the early 1980s, in the United States and increasingly worldwide, regulatory analyses are conducted with an evaluation framework called risk assessment. To understand how toxicogenomics may enhance risk-assessment, it is necessary to understand how risk assessment is generally conducted. This section provides a general discussion of regulatory risk assessment related to cancer risks, as understood by the committee, but it does not consider specific risk assessment protocols (which are often disagreed upon). Further details about the risk-assessment process can be found in such references as EPA's 1986 Guidelines for Carcinogen Risk Assessment, EPA’s 2003 Draft Final Guidelines for Carcinogen Risk Assessment, an EPA staff paper titled “An Examination of EPA Risk Assessment Principles and Practices,” the National Research Council’s Risk Assessment in the Federal Government: Managing the Process (also known as the Red Book), and the National Research Council’s Science and Judgment in Risk Assessment (EPA 1986, 2003, 2004; NRC 1983, 1994).
The goal of a risk assessment is to obtain a reasonable estimate of the likelihood of harm associated with exposure to a toxic chemical on the basis of 1) the hazard (the nature of the chemical), 2) the relationship between dose and effects, and 3) potential exposure. The risk characterization itself combines information on exposure and response assessments (NRC 1994). The calculations and analyses provide risk assessors a quantitative basis for health and environmental regulatory standards and guidelines.
Current Approaches to Risk Assessment
Hazard Identification: Qualitative Determination of Whether the Chemical Causes Cancer
Cancer risk assessments have both qualitative and quantitative components. The key qualitative determination is of whether a chemical has the property of inducing cancer in test animals or in exposed human populations. That determination is based on human data (as from epidemiologic or clinical studies) and experimental data (from in vitro or animal studies). When possible, regulators look beyond empirical tumor data and epidemiologic studies of cancer incidence or prevalence to what EPA refers to as mode-of-action (MOA) data (EPA 2003). MOA data comprise chemical and biologic information on key cellular and bio-
chemical events that are thought to lead to the tumor end point.3 (How toxicogenomics may contribute to risk assessment with MOA data is described later.)
EPA describes the degree of certainty that a chemical may be carcinogenic to humans as outlined in Box 1. The analysis requires considerable scientific judgment and uses several criteria: the quality of the evidence reviewed, the consistency of findings in experimental animals, and, if available, information on effects in humans. Consistently positive findings of well-conducted epidemiologic studies offer the strongest evidence of human cancer risk, and the few chemicals on which there is such information are considered proven human carcinogens. Lacking that information (and it is lacking for most chemicals of regulatory concern), a conclusion that a chemical acts as a carcinogen and may pose a carcinogenic risk to humans can be supported by consistent findings of well-conducted animal studies, especially studies in more than one species, and by an indication that the number of tumors increases with dose (EPA 2003).
Evaluation of Dose-Response Relationship: Quantitative Determination of Carcinogenic Potency
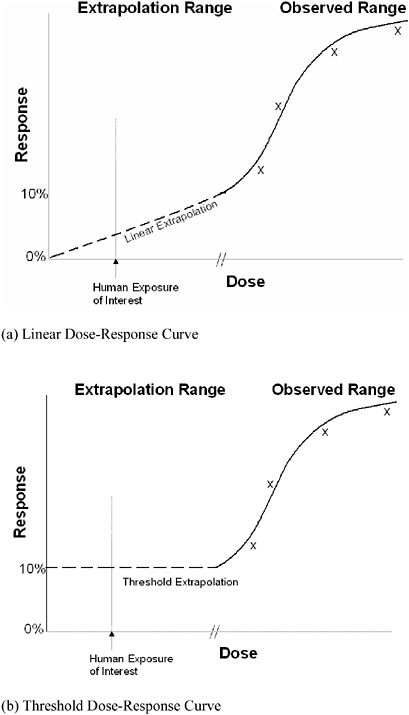
After the qualitative classification of carcinogenic potential, risk assessors work on the quantitative component of the risk-assessment framework to calculate a carcinogenic potency factor for the chemical. The potency factor indicates the extent to which cancer incidence increases with dose or exposure (the dose-response relationship). The dose-response relationship is evaluated by modeling the empirical data (see Figure 1, where x’s indicate observed data). Because only rarely is a dose-response analysis based on epidemiologic studies, data from animal bioassays are generally used to model this relationship in the observable
|
BOX 1 EPA’s Standard Descriptors for Expressing Conclusions About the Weight of Evidence of Human Carcinogenic Potential Different conclusions may apply to a single agent if carcinogenicity is dose or route dependent. This text is from EPA’s 2003 Draft Final Guidelines for Carcinogen Risk Assessment (EPA 2003, pages 2-40 to 2-43): “Carcinogenic to Humans” This descriptor is appropriate when there is convincing epidemiologic evidence of a causal association between human exposure and cancer. Exceptionally, this descriptor is equally appropriate with a lesser weight of epidemiologic evidence that is strengthened by other lines of evidence. It can be used when all of the following conditions are met: a) there is strong evidence of an association between human exposure and either cancer or the key precursor events of the agent’s mode of action but not enough for a causal association, and b) there is extensive evidence of carcinogenicity in animals, and c) the mode(s) of carcinogenic action and associated key precursor events have been identified in animals, and d) the key precursor events that precede the cancer response in animals are anticipated to occur in humans and progress to tumors, based on available biological information. “Likely To Be Carcinogenic to Humans” This descriptor is appropriate when the weight of the evidence is adequate to demonstrate carcinogenic potential to humans but does not reach the weight of evidence for the descriptor “carcinogenic to humans.” Adequate evidence consistent with this descriptor covers a broad spectrum. Although the term “likely” can have a probabilistic connotation in other contexts, its use as a weight of evidence descriptor does not correspond to a quantifiable probability. This is because the data that support cancer assessments generally are not suitable for numerical calculations of the probability that an agent is a carcinogen. The weight of evidence descriptor “likely to be carcinogenic to humans” may be taken loosely to imply that an agent is more likely than not—but is not certain—to cause cancer in humans. “Suggestive Evidence of Carcinogenic Potential” This descriptor of the database is appropriate when the weight of evidence is suggestive of carcinogenicity; a concern for potential carcinogenic effects in humans is raised, but the data are judged not sufficient for a stronger conclusion. This descriptor covers a spectrum of evidence associated with varying levels of concern for |
|
carcinogenicity, ranging from a positive result in the only study on an agent to a single positive result in an extensive database that includes negative studies in other species. Depending on the extent of the database, additional studies may or may not provide further insights. Some examples include:
“Inadequate Information to Assess Carcinogenic Potential” This descriptor of the database is appropriate when available data are judged inadequate for applying one of the other descriptors. Additional studies generally would be expected to provide further insights. Some examples include:
"Not Likely To Be Carcinogenic to Humans” This descriptor is appropriate when the available data are considered robust for deciding that there is no basis for human hazard concern. The judgment may be based on
|
range. Typically, tumor incidence measured in 18- to 24-month animal (rodent) bioassays4 is used.
The reliance on animal bioassays has long raised concerns. First, the use of animal bioassays to determine human risk is complicated by uncertainties in the interspecies extrapolation between rodents and humans and even between mice and rats—the traditional two species used in bioassays for cancer-hazard characterizations may have different responses to a given chemical. Second, relatively high doses are usually used in bioassays5—sometimes several hundred times those expected in environmental situations—and clearly are not optimal for understanding effects at more environmentally relevant doses. Excess doses are used largely for statistical reasons. It is not possible to detect either the presence or absence of a significant increase in tumor rate reliably unless the tumor rate is very high, which requires a high exposure.6
As a result, there are rarely experimental data in the range of exposures that are of concern to risk assessors, and the relationship between dose and response must be extrapolated from study doses down to doses relevant to the general population rather than determined observationally. The dashed curve in Figure 1a illustrates a linear model of extrapolation, and the dashed curve of Figure 1b illustrates an alternative, nonlinear, or threshold dose-response model. The risk assessors’ determination of which model shape appropriately conveys the relationship between dose and response for a particular chemical can be controversial.
Whenever possible, risk assessors evaluate MOA information to draw a conclusion about the most likely shape and interpretation of the dose-response relationship. MOA information may provide insight into the relevant underlying biologic processes important to the specific chemical (such as pharmacokinetics and cellular mechanisms of response). More specific information about how typical and conventional MOA information fits into a risk assessment is described below.
Some inadequacies in the scientific information to be used in hazard characterization for risk-assessment calculations can be reduced by further study, such as additional bioassays or mechanistic studies, but other shortcomings remain. Depending on the adequacy of the data elements presented by these situations, risk assessors may rely on safety factors (often also referred to as uncertainty factors). Safety factors are values used in risk calculations to fill critical information gaps. The intention is to apply them so that the resulting risk calculations err on the side of safety rather than leading to a failure to protect the public health. For example, it is common to see a 10-fold safety factor applied to compensate for differences between humans and animals, on the basis that humans may be more sensitive than the animal species usually tested; that is, the potency is considered to be 10 times greater in humans than in the animal tested. An additional 10-fold safety factor is sometimes applied to account for variability of response within human populations.
Evaluation of Human Exposure
Exposure evaluation is the next element of a risk assessment. Direct measurements of exposure of the population of concern are seldom available, even in occupational settings. Therefore, some risk assessments use data taken on spatial or temporal scales that are substantially different from the scales of the problem under consideration, such as emissions data from stationary sources, and models are used to estimate exposures of populations—and the estimates may or may not adequately represent the actual range of exposures in the populations of concern. Probabilistic analytic techniques may be used in exposure assessment to deal with shortcomings in exposure information. The reliability of those techniques depends on the quality of data used (EPA 1997). The techniques were not discussed at the workshop.
Role of Mode-of-Action Data7
James Bus, of the Dow Chemical Company, provided an overview on the types of conventional (nontoxicogenomic) MOA information in
risk assessment. Examples of the types of MOA information that risk assessors might consider, when available, include absorption, distribution, metabolism, and elimination; DNA and protein adduct formation; receptor binding; promotional effects (such as cell proliferation); inhibition and alteration of cell death (apoptosis); mutagenicity and chromosomal effects; and immune suppression.
Bus noted that the predictive power of animal cancer bioassays is limited by the relatively small numbers of animals that can be evaluated (usually 50 animals/dose) and that MOA information may provide regulatory toxicologists with insights that supplement the analysis of experimental data on dose and tumor development and promote the understanding of the underlying biologic bases of tumor responses. The improved understanding can contribute to improved risks assessments in two fundamental ways: it may facilitate the qualitative categorization of a chemical’s potential human cancer risks based on nonhuman data, and it may guide the selection of models used as quantitative elements of the dose-response relationship.
Qualitatively, risk assessors have used MOA data to draw conclusions on the strength of evidence to support inferences of human cancer risk from animal data. Recent evaluations have led to conclusions that some animal tumors are caused by a mechanism that is not relevant to human biology. While not discussed at the workshop, a classic example is the accumulation of α2u-globulin that occurs only in male rat renal tubular cells after treatment with a wide array of chemicals. Some male rat renal tumors can be attributed solely to this chronic protein accumulation; α2u-globulin is not produced in humans, so these tumors are not considered relevant to human kidney risk (EPA 1991; IARC 1999; EGE 2004). Conversely, MOA information was used by the International Agency for Research on Cancer to strengthen the conclusion that dioxins are human carcinogens in the absence of definitive epidemiologic data (IARC 1997).
Quantitatively, risk assessors use MOA data to determine the most biologically appropriate dose-response model for exposure-effect relationships in humans (threshold vs. linear dose-response modeling at low doses; see Figure 1). The data allow risk assessors to consider the role of such MOAs as mutagenicity, mitogenesis, inhibition of cell death, cytotoxicity with regenerative cell proliferation, and immune suppression in observed tumor response. EPA has proposed that some of the MOAs, such as mutagenicity, are more indicative of linearity between dose and response. Other MOAs, such as cytotoxicity with regenerative cell pro-
liferation, may be operative only at higher doses and thus may be more indicative of a nonlinear or threshold relationship. Of course, chemicals can operate with more than one MOA over a dose range and can operate with different MOAs in different organs or cells. Risk assessors evaluate those complex possibilities before concluding that the carcinogenic risk of a chemical is due to only one particular MOA.
Because of the complexities and uncertainties associated with cancer and the lack of data, it may not be simple for some scientific information to be used in a qualitative assessment of a chemical’s carcinogenicity or a quantitative assessment of its potency.8 That should come as no surprise inasmuch as many of the studies contributing to the basic understanding of cancer causation are in cutting-edge fields of biologic research and are insufficient to challenge existing default assumptions used in risk assessments of specific chemicals. Risk assessors face an enormous challenge in attaching appropriate scientific weight to findings derived from basic science rather than from empirical toxicologic literature.
Bus described two examples to illustrate how conventional (nontoxicogenomic) non-tumor MOA data might be used to enhance the interpretive value of animal bioassay data. He described aniline-induced spleen tumors in rats and suggested that the tumors are probably unrelated to a human risk of spleen tumors because they are probably due to primary toxicity in red blood cells that results only from high doses (Bus and Popp 1987). And he described a cancer bioassay of propylene glycol monomethyl ether (PGME) to illustrate how prior knowledge of its MOA could be used to proactively incorporate special studies into the bioassay design so that both the quantitative and qualitative relevance of any potential tumor outcomes would be better understood. In the case of PGME, researchers hypothesized, on the basis of its molecular structure and structure-activity relationships, that it induces the α2u-globulin synthesis mechanism, which is generally accepted as irrelevant to humans. Given knowledge about PGME’s possible MOA, they incorporated end points into the bioassay to detect signs of α2u-globulin synthesis. When tumors were observed, they were able to say that they were most likely to be mediated through an α2u-globulin protein MOA, generally regarded as irrelevant to humans (Spencer et al. 2002).
Those two examples showed the value of conventional MOA data in improving risk assessments. However, MOA data are often incomplete or contradictory, and that poses difficulties for interpretation. The
advent of toxicogenomic technologies might improve both the pace of development and the quality of conventional MOA information and further refine the understanding of toxicologic responses. It could also lead, as in the PGME example, to improved bioassay study designs that provide more rapid access to critical MOA data for use in evaluating potential human health risks.
Critical Data Needs in Conventional Risk Assessment
John Moore, of Hollyhouse Consulting, and Linda Greer, of the Natural Resources Defense Council, led discussions about what they viewed as the most crucial information gaps and inconsistencies (that is, where study results may seem to provide conflicting information) that complicate cancer risk assessments (see Box 2). Greer and Moore aimed to inform workshop participants about the gaps and the current regulatory responses to them to frame discussion of how toxicogenomics might fill them.
|
BOX 2 Typical Information Gaps and Inconsistencies That Limit Conventional Risk Assessment
|
Lack of Screening or Other Data (Untested Compounds)
Greer described the most common challenge in cancer risk assessment as the lack of data (for example, in vitro or animal-screening data9 or epidemiologic data) for evaluating the potential carcinogenicity of a chemical. This presents a conundrum to risk assessors who may be concerned about exposures to these compounds for other reasons. Chemicals may be tested with inexpensive short-term indicator methods to evaluate if they might be involved in cancer, and the results are referred to as “screening” data. Those screening methods, which range from relatively simple in vitro bacterial mutagenicity tests to short-term whole-animal assays, may include, for example, tests to identify chemicals that damage genetic material (that is, genotoxic chemicals).
Risk assessors do have other options when experimental data are lacking—for example, looking at relationships between chemical structure and biologic activity (structure-activity relationships)—but the lack of sufficient screening data constitutes an information gap that impedes risk assessment. Greer noted that people generally overestimate the extent to which chemicals in commerce, even those manufactured in high volumes, have been tested for carcinogenicity or other forms of toxicity, such as teratogenicity, reproductive toxicity, and neurotoxicity.
Insufficiencies and Uncertainties in Human Data, Including Exposure Levels
For cancer risk assessment, human data, such as results of epidemiologic studies, are the most sought-after data; use of such data eliminates the uncertainties in extrapolating effects from other animals to humans. EPA’s cancer guidelines indicate that human data are used whenever possible in carcinogenicity evaluations (EPA 2003). However, Moore pointed out that epidemiologic studies of chemical exposure and cancer
risk have various limitations that often reduce their value for risk assessment. Many epidemiologic studies are retrospective and thus may rely on estimates, rather than measurements of exposure. Poor exposure data in both exposed and control groups hamper epidemiologic investigations, and misclassification of exposure is a major contributor to the insensitivity of many epidemiologic investigations. In addition, many epidemiologic studies lack sufficient power to detect low-dose effects. The number of persons studied may be small or might not have been collected throughout the lifetime of the exposed cohort, which is particularly important for detecting cancer because disease can occur decades after exposure. Finally, the exposed cohort may have been exposed to many other agents, such as cigarette smoke, which would confound the analysis.
Relevance of Animal Data to Humans
Even chemicals that have been tested in standard animal bioassays may pose difficulties in interpretation for risk assessment. Tumors in laboratory animals are not always predictive of cancer in humans, and questions about the relevance of animal data to humans (interspecies extrapolation) can arise. For example, humans sometimes have metabolic pathways different from those of laboratory animals, which would generate different metabolites and pose different levels of risk. Interspecies extrapolation may also be complicated by the high doses used in bioassays.
Paucity of Information on the Relationship Between Dose and Response, Especially at Low Doses
Because animal bioassays are conventionally conducted at relatively high doses to produce tumors in the small number of animals tested, questions about appropriate interspecies extrapolation are often tied to questions about the relationship between dose and response. High doses might create responses that would not be evident at more environmentally or occupationally realistic doses. Moore pointed out that even when metabolic pathways are similar in humans and test animals, testing
at high doses in animals can saturate or activate pathways that may not be relevant in humans exposed to lower doses.
Risk assessors default to a low-dose linear model in the absence of sufficient MOA data that indicate a threshold dose below which the MOA would not be active. Thus, the risk assessment is conducted as though MOAs that operate at relatively high doses also operate at low doses.
Inconsistencies in Animal Data
The carcinogenic effects of a chemical are sometimes not consistent among laboratory test species. Species differences, such as between rats and mice, raise questions about which species best predicts a carcinogenic effect in humans. In addition, chemicals may cause tumors in different organs in different species (Haseman and Lockhart 1993). Differences in species responses affect a chemical’s carcinogenicity classification in risk assessment. Chemicals that cause cancer in two animal species are considered more likely to be carcinogenic in humans than chemicals that cause cancer in only one animal species (EPA 2003).
Exposure at Different Life Stages
Frederica Perera, of Columbia University, discussed how responses to chemicals can depend on age or the state of development, and so potential risk will differ depending on whether the exposure occurs during fetal development, early childhood, adolescence, etc. She did not focus on cancer alone, but she thought that the lack of exposure data obtained at different life stages was a limitation in risk-assessment datasets. Standard animal bioassays begin dosing in early adulthood (for example, when rodents are 6- to 8-weeks-old) and typically do not include exposures during perinatal periods of development. That protocol has been raised as a potentially important limitation of animal studies because epidemiologists and toxicologists are concerned about different effects of exposure during critical windows of development on the onset of disease in humans. Whether cancer is affected by early exposure was not discussed at the workshop.
Coexposures
Standard animal bioassays of a specific chemical do not investigate coexposures to other chemicals or the nutritional status of the animal.10 Such coexposures can confound epidemiologic studies inasmuch as humans are rarely exposed to only one chemical. For example, many of the epidemiologic studies of 1,3-butadiene workers have been complicated by the coexposure of many workers in this industry to styrene. The inability to study 1,3-butadiene exposure alone creates uncertainties in the hazard assessment of this chemical. In addition to the difficulties in distinguishing the effects of individual chemicals, datasets may fail to detect the role of chemical interactions in increasing cancer risk (Silbergeld 2003). Risk assessors generally acknowledge the possibility of additive or synergistic coexposures but do not generally alter calculations in the risk assessment on the basis of them.
Human Variability in Susceptibility
An important scientific gap in risk assessment is the paucity of information on human variability in susceptibility to chemically induced cancer. For example, polymorphisms11 in genes associated with metabolism of carcinogens may increase or decrease a person’s risk of cancer. In his presentation on arsenic (below), David Eaton, of the University of Washington, discussed the possible significance of polymorphisms in genes involved in methylation of arsenic. Those genes, studied by Silbergeld and colleagues (Loffredo et al. 2003; Marnell et al. 2003), may be factors in individual susceptibility to arsenic carcinogenicity (NRC 1999).
The developing literature on polymorphisms and variation in individual susceptibility to cancer is not known to have been incorporated into risk assessment apart from EPA’s use of a standard uncertainty factor of 10, or a variation of it, to account for unspecified variations in human response.
APPLICATION OF TOXICOGENOMIC APPROACHES IN RISK ASSESSMENT
In the second phase of the workshop, some toxicogenomic experiments were reviewed to familiarize the risk-assessment community with the general experimental approaches and the types of data produced.
What Is Toxicogenomics?
Toxicogenomics has been described as a discipline combining expertise in toxicology, genetics, molecular biology, and environmental health to elucidate the response of living organisms to stressful environments. Scientists in the field use new technologies, such as microarrays, to simultaneously assess the coordinated expression of thousands of genes in response to a particular chemical exposure. They look at how individual and species differences in underlying DNA sequences can result in different responses to the environment. Although the “-omics” part of toxicogenomics encompasses other types of profiling technologies, including protein profiling (proteomics) and metabolite profiling in a cell or tissue (metabonomics), most discussion in this workshop was on “transcriptomics,” or changes in transcriptional profiles, the changes in a cell’s or tissue’s messenger RNA (mRNA) in response to a chemical perturbation. Consideration of underlying DNA-sequence variability was also discussed with respect to the usefulness of toxicogenomic data.
Toxicogenomic Techniques
Toxicogenomic researchers profile transcriptional responses by using DNA microarrays, sometimes referred to as gene chips, which are glass slides or membrane filters to which thousands of unique DNA “target” sequences are affixed. After exposure of a sample (such as tissue, cells, or an animal) to a chemical or other condition, messenger RNA is extracted from the sample and converted to complementary DNA (cDNA) and then applied to the microarray. The cDNA from different samples is labeled with red and green fluorescent or radioactive markers to visualize where it binds to the target gene sequences on the microarray. The relative intensity of the fluorescent or radioactive signals is interpreted as reflecting changes in mRNA levels.
Cheryl Walker, of the University of Texas, introduced the techniques at the heart of the development of genomics data to the workshop. When looking at transcript expression data, the scientist determines whether the gene is upregulated (more transcripts are produced) or downregulated (fewer transcripts are produced). Microarray data are usually expressed quantitatively as “-fold” changes in gene expression; for example, a two-fold change or a three-fold change. Usually scientists arbitrarily select a two-fold change in expression as upregulation or downregulation. The red-green “heat map” shown in Figure 2 is an example of the type of analysis commonly used to illustrate patterns of gene expression observed in microarray experiments. These patterns of gene expression are sometimes referred to as a signature or a fingerprint, conveying that the patterns seem peculiar to or diagnostic of different chemicals or groups of chemicals. The term signature is used for the remainder of this summary. In addition, hierarchic clustering can be used to identify relevant patterns of expression; the data generated by these types of higher-order analyses are largely qualitative. In the latter type of analysis, genes are grouped or “clustered” on the basis of their assumed functional relationships to identify patterns representative of exposed samples compared with control samples. Genes are clustered when a group of genes exhibiting similar transcriptional profiles discriminates a subset of samples (for example, discriminates treated vs. control samples). That type of information is considered more biologically relevant than transcriptional responses in individual genes. Researchers use statistical techniques to evaluate the patterns and determine whether they are similar or different in compared samples. Pattern recognition has become a cornerstone of global12 gene-expression analysis with microarray technology because it provides statistically interpretable information, but more-sophisticated methods of analysis have also been developed.
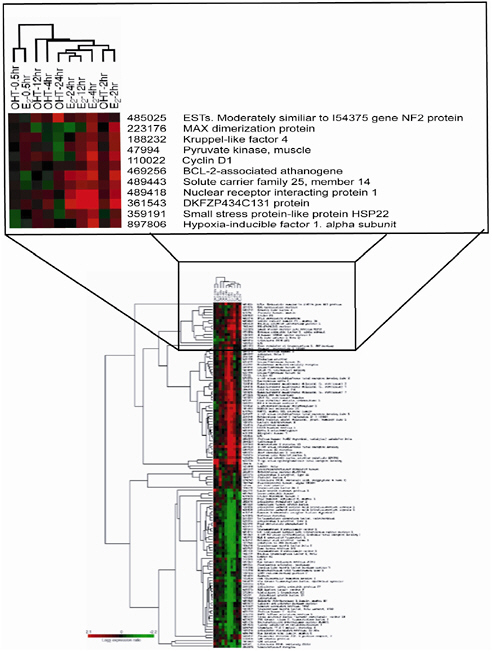
FIGURE 2 The red-green heat map, an example of the type of analysis commonly used to illustrate patterns of gene expression observed in microarray experiments. The heat map illustrates the expression pattern of genes upregulated (red) and downregulated (green) in response to treatment with estrogen (E2) or 4-hydroxytamoxifen (OHT), the active metabolite of tamoxifen. Source: Hodges et al. 2003. Reprinted with permission; copyright 2003, American Association for Cancer Research.
An important concept in toxicogenomics is the correlation of particular gene-response patterns with phenotypes, including particular diseases or other health end points. That prospect is exciting, but difficult questions remain. For example, Walker described the challenge of determining how “good” a correlation needs to be—that is, how predictive it needs to be to be considered valid or useful. Are there core components of a signature that are required to predict a biologic end point? Another issue is tissue heterogeneity. If a toxicant targets a specific cell type in a tissue, will profiling the entire tissue be predictive of relevant biologic changes, or will the target cells need to be separated from other cell types? Isolation of target cells would be more laborious and expensive, but if it is not done, nonspecific responses from other cells in the tissue may create “noise.”
Some workshop participants pointed out that it is in principle important to distinguish adverse responses to exposures, which indicate biologic changes that can lead to disease, from adaptive responses. Greer noted that while this challenge calls for careful judgment, risk assessors have experience in distinguishing adverse effects from other biologic responses even in their traditional analyses at the physiologic or biochemical level.
Richard Paules, of NIEHS, expressed optimism that signatures predictive of some precancer changes might be in hand in the not too distant future and noted that NIEHS is keenly interested in such changes. Several other participants agreed that the use of the technology to detect preneoplastic changes indicative of future disease would be of great value.
Mechanistic Insight
In addition to the possibility of using signature patterns of gene expression to assess similarities among chemicals and to predict adverse effect outcomes, toxicogenomic experiments can generate insight into the biologic mechanisms by which a compound causes an adverse effect. Understanding a chemical’s mechanisms may contribute to the MOA components of risk analysis.
Kenneth Ramos, of the University of Louisville, described how gene-expression changes can improve scientists’ understanding of how chemicals act in a cell and contribute to pathogenesis. He discussed how looking at global patterns of gene expression with microarray analysis,
rather than studying only one gene at a time, can reveal various previously unsuspected intracellular phenomena. For example, Ramos studied the carcinogen benzo[a]pyrene and its ability to activate aryl hydrocarbon receptor (Ahr) signaling and cause oxidative stress. He found that the genes involved in the regulation of immune function were critical but unexpected biologic targets of the toxic activity of this chemical.
Ramos also described how patterns of gene expression can be analyzed to learn more about cell circuitry—not only how a chemical affects various genes but, more broadly, how the genes “talk to” or interact with each other in the cell. He suggested that the response of a single gene, or even several hundred genes, may not be biologically relevant but that important biologic knowledge might emerge from focusing on how genes interact with one another. Figure 3 (from Johnson et al. 2004) shows the type of information that can be derived from circuitry analysis.
The data come from experiments in which Ramos and colleagues worked to identify genes whose transcriptional changes best predict the impact of Ahr ligands on five genes of interest. Interesting biologic relationships among the genes emerged from their experiments. For example, the osteopontin gene, which is a stress-regulated gene involved in phenotypic control, emerged as one that interacted closely with the Ahr.
In the discussion with workshop participants, Ramos pointed out that cells have a number of critical “behaviors” that are associated with a tumor response: apoptosis, cell cycle and quiescence, proliferation, and differentiation. Carcinogens are being examined for their effects on those critical behaviors with techniques other than toxicogenomics, and risk assessors then attempt to assign a mode of carcinogenic action to chemicals. Studies of the “cell circuitry” behind the MOAs could contribute to the basic understanding of cancer development, although it may be a long time before these mechanisms are well understood. Some workshop participants recognized that such insight is one way that toxicogenomics could contribute to risk assessment, in addition to making major contributions to scientists’ understanding of basic cell biology.
Preneoplastic Changes
In conventional toxicity studies, empirical evidence of tumors or other morphologic evidence of chronic toxicity often takes months or years to appear. However, genomic techniques may allow for the identi-
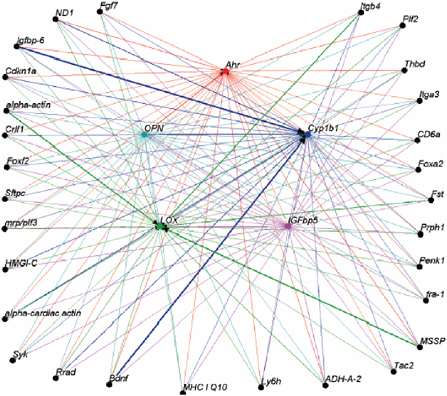
FIGURE 3 Gene network diagram derived from DNA microarray experiments using circuitry analysis, a bioinformatics approach using the Osprey network visualization system (Breitkreutz et al. 2003). These data come from experiments in which Ramos and colleagues sought to identify associations between the expression patterns of individual genes regulated by ligands of the aryl hydrocarbon receptor (Ahr). Several target genes affected by the Ahr ligands were examined: Ahr, cytochrome P450 1B1 (Cyp1b1), insulin-like growth factor-binding protein-5 (IGFbp5), lysyl oxidase (LOX), and osteopontin (OPN). The genes listed on the outside of the figure were identified as the best predictors of the five target genes in the center of the figure. The lines denote predictive connection among the genes in the network. The weight of each line conveys the strength of the prediction (for example, the correlation is stronger between IGFbp-6 and Cyp1b1 than between IGFbp-6 and Ahr). Source: Johnson et al. 2004.
fication of characteristic preneoplastic gene-expression profiles that can be detected within hours or days of exposure and foretell disease.
Possible Applications of Toxicogenomic Information to Risk Assessment
After Walker and Ramos explained the nature of some toxicogenomic data, the discussion turned to how such information might be useful in risk assessment. Presentations by Walker and Ramos and further discussion from workshop participants identified some topics through which toxicogenomics might contribute to risk assessment:
-
New ways of screening untested compounds.
-
Providing more accurate information on human exposure levels.
-
Better understanding of the relevance of animal data to humans.
-
Improved understanding of dose-response relationships, particularly at low doses.
-
Improved interpretation of animal-bioassay data that are inconsistent across species.
-
New approaches to examining the effects of different exposure regimens, particularly during development and early life stages.
-
Providing new methods to study coexposures to multiple chemicals.
-
Improved understanding of human variability and its effects on response.
Using Gene Expression to Screen Chemicals
Workshop participants discussed at length how gene-expression patterns might aid risk assessors in predicting the carcinogenicity of chemicals by providing a potential method for systematically screening chemicals. Specifically, chemicals that evoke similar patterns of gene expression could be grouped into categories on the basis of their signatures. The gene-expression pattern of a compound that is not well characterized could be compared with signatures of better-understood compounds to make an initial determination of whether the unknown chemical might pose a carcinogenic hazard. The gene-expression pattern of a less-understood compound could be refined, for example, by testing it at different doses, examining effects in different tissues, and testing it in
different species. The results could provide useful “first-cut” information about the possible adverse effects of the chemical.
Some workshop participants believed that those approaches could be useful even when the specific mechanisms that might underlie observed gene-expression patterns are not known. Their usefulness would be enhanced if particular signatures were linked empirically (with experimental data) to cancer or precancer conditions even when the full biology underlying the gene-expression pattern was not understood. For example, if a signature correlated with an increase in liver damage, the empirical linkage would be valuable information—a biomarker—even if the underlying biologic mechanism of liver damage were not understood.13
Although pattern-recognition experiments may hold considerable promise, technical problems limit their usefulness at present. For example, artifactual variation (as opposed to biologic variation) resulting from variability in sample preparation and the type of microarray used will need to be overcome before widespread signature experiments are begun. It can be a challenge to distinguish interlaboratory or technical variability from inherent biologic variability. As an example of variability due to microarray type used, Walker described results from Mah et al. (2004). Two microarray systems often used to study mRNA expression profiles—one cDNA based and one oligonucleotide based—were compared in an experiment that attempted to control for known sources of variability. Researchers looked at sequence-verified genes that were present in both microarray systems and found that only 64% of the genes were identified as either “present” by both systems or “absent” by both systems. In another comparison of variability, the researchers compared the rank order of expression of 13 genes quantified by real-time PCR (polymerase chain reaction), Affymetrix oligonucleotide microarray, and cDNA-based (clone) microarray. Figure 4 shows that the rank order correlation between the cDNA-based and oligonucleotide microarray systems was weak. That gene-expression patterns may differ depending on the microarray systems used (as illustrated in those two examples) or differ depending on the group conducting the experiment poses complications for the use of this type of data in risk assessment. However, at least some participants thought that many of the complications were due to the early stage of the technology and were optimistic that they would soon be overcome.

FIGURE 4 Comparison of the expression of 13 genes in patient samples using two microarray systems, the olignonucletide-based (Affymetrix) and cDNA-based (clone) systems, relative to rank-order gene expression verified by quantitative real-time polymerase chain reaction (PCR). The rank-order correlation between the cDNA-based and oligonucleotide microarray systems was weak. Source: Mah et al. 2004. Reprinted with permission; copyright 2004, American Physiological Society.
Human Exposure Levels
The diverse “-omics” technologies underpinning toxicogenomics could be used to quantify and integrate human exposures—a key problem in epidemiologic investigations—and perhaps even to quantify cumulative exposure over time. Although every person presumably has unique characteristics with respect to dose-response manifestation, toxicogenomic data could be used to classify individuals, local communities, and larger populations more accurately with respect to exposure status. For example, microarray responses could indicate that an organism had been exposed to a given chemical by showing a gene-expression pattern characteristic of the chemical—a pattern that would not necessarily need to be indicative of an adverse effect. Biomarker development is not limited to detection of gene-expression changes but could also include re-
search on metabolite and protein changes in response to exposures. And biomarkers could be used to determine the effectiveness of interventions to lower human exposure.
Relevance of Animal Data to Humans
“-Omics” data may assist in cross-species extrapolation. Because genes and genetic pathways are often conserved across species, the same events may be measured in human and experimental-animal cells. That could facilitate the comparison of cross-species observations at the same levels of exposure.14 Likewise, “-omics” data may assist in identifying unique, species-specific responses that could provide information valuable for demonstrating that a response in the model species (such as rats or mice) should not be used to predict human response.
Dose-Response Relationships
The dose-response relationship is the core quantitative component of a regulatory risk assessment. Toxicogenomic techniques might be useful in elucidating different responses at different doses and differences due to changes in frequency and duration of exposure. Herman Gibb, of EPA, noted that the EPA was particularly interested in using “-omics” technologies to gain information on responses at low doses.
Walker pointed out that although a continuous range of exposures often occurs in the environment, genomic researchers anticipate that patterns of gene expression and even individual expression of specific genes may sometimes be discontinuous in response to changes in dose because of thresholds in the modulation of gene expression. In fact, she noted, gene-expression patterns may change in response to increasing dose in a disproportionate way, as well as discontinuously. Consequently, a researcher might see one expression pattern over a low-dose range, a second pattern over a medium-dose range, and a third over a high-dose range. Relating those changes in gene expression to empirical evidence of tumors at a given dose range could be challenging.
Interpreting Animal-Bioassay Data That Are Inconsistent Across Species
When a chemical produces different conventional toxic effects in different laboratory animal species, comparing gene-expression patterns from each laboratory animal species to gene-expression patterns in human cells may provide insight into which laboratory animal species is most relevant to humans for a particular toxic response. Ellen Silbergeld, of Johns Hopkins University, noted that toxicogenomic techniques show promise in this area. When it is unclear whether a response observed in a laboratory species may also occur in humans, a systematic study to compare gene-expression patterns could be conducted in target tissues of interest, particularly in easily obtainable tissues, such as skin, bladder, and lung, and in blood. Such studies would help in interpreting more traditional toxicologic findings. John Groopman, of Johns Hopkins University, and Patricia Buffler, of the University of California, Berkeley, discussed the practicalities of obtaining tissue from both diseased people (cases) and controls, noting that it will be necessary to collaborate with the clinical community to obtain tissue samples from biopsies and surgery with, of course, proper informed consent of the study participants.
Exposures During Different Life Stages
Gene-expression profiling can be used to investigate responses to a chemical that depend on life stage. Experiments could be done to investigate, for example, whether a profile at one time would be predictive of a profile at another time. Walker and others pointed out that over the timeframe of fetal and neonatal development, patterns or networks of response to a chemical may change because of developmental differences in gene activation and signal-transduction pathways.
Coexposures to Multiple Chemicals
Humans are typically exposed to mixtures of chemicals; such exposures are difficult to evaluate systematically with traditional toxicologic methods. At least one workshop participant thought that toxicogenomics could improve the quantitation of exposure including exposure to multiple chemicals. In addition, the high-data producing capability of gene-
expression profiling might be useful for evaluating the synergistic, additive, or antagonistic toxic effects of concurrent exposures to more than one chemical by permitting many exposure combinations to be assessed more efficiently. However, it was also mentioned that, given how difficult it is to interpret “-omics” responses to even a single compound, substantial challenges are likely in interpreting “-omics” responses to mixtures.
Human Variability
As understanding of polymorphisms improves, toxicogenomic technologies hold promise for the identification of susceptible people. Just as in conventional animal bioassays that use outbred strains in which not every exposed animal develops tumors in response to a carcinogen, not every person exposed to a carcinogen develops cancer. Many smokers, for example, do not develop lung cancer despite the strong statistical association. The heterogeneity of response to toxic substances could be studied by comparing genotypes of exposed persons and animals that develop cancer with genotypes of exposed persons and animals that do not develop cancer. That type of work has begun in a handful of epidemiologic studies that are using molecular techniques, as epidemiologists are increasingly interested in the possible genetic bases of differences in individual response to chemical exposures.
Scientists have already found that key enzymes involved in the metabolic pathways of many chemicals are polymorphic in their ability to catalyze metabolic reactions. For the general population, the effects of the differences may be subtle; Eaton and others anticipated that it will be a long time before human sensitivity can be predicted with genotyping although some predictions are already being made. One reason it may take a long time is that most chronic diseases are multifactorial—multiple genetic components and multiple environmental factors contribute to a given disease. The combinations of genetic and environmental factors are almost limitless. Samuel Wilson, of NIEHS, noted that the NIEHS initiative to identify haplotypes15 indicative of individual variability in response to chemical exposure is still in the early stages.
USE OF “-OMICS” EXPERIMENTS TO IMPROVE RISK ASSESSMENT: LESSONS FROM CASE STUDIES
Workshop participants considered two chemicals, 1,3-butadiene and arsenic, as a basis for exchanging ideas about specific toxicogenomic experiments that could contribute to the reduction of uncertainties. Those chemicals were selected as promising examples for discussion because they are both relatively well studied, yet data gaps and inconsistencies have long complicated their risk assessments. Presenters interpreted information on 1,3-butadiene and arsenic risk assessments to provide background for discussing toxicogenomic applications. The highlights of their presentations are discussed here. It should be noted that additional data may have been published after the risk assessments that the presenters discussed and that others may interpret the risk assessments or data differently from the presenters.
1,3-Butadiene
Ramos provided information on the challenges in assessing the risks posed by 1,3-butadiene on the basis of his review of EPA’s risk assessment (EPA 1998a). 1,3-Butadiene is an aliphatic hydrocarbon that is present in gasoline exhaust and is used in the manufacture of synthetic rubber and various polymers. It is a relatively well-studied compound, and extensive toxicologic bioassays and epidemiologic studies are available, as is considerable information on its metabolism and cellular and biochemical effects.
The toxicity of 1,3-butadiene results from its metabolism by cytochrome P450 enzymes, which create reactive epoxides that bind to DNA and other macromolecules to form adducts.16 1,3-Butadiene has several reactive metabolic products (see Figure 5). One—1,2:3,4-diepoxybutane—is thought to be the most important on the basis of comparative genotoxicity studies of each mutagenic intermediate.
|
|
somes, but tend to be associated with one another. Because of this tendency, relatively few of the theoretically possible haplotypes are observed at significant frequency.” |
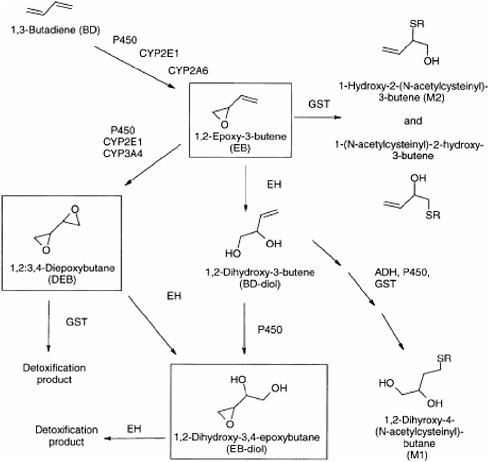
FIGURE 5 1,3-Butadiene metabolism. Abbreviations: ADH, alcohol dehydrogenase; CYP2E1, cytochrome P450, isozyme 3E1; CYP2A6, cytochrome P450, isozyme 2A6; CYP3A4, cytochrome P450, isozyme 3A4; EH, epoxide hydrolase; GST, glutathione S-transferase. Source: Albertini et al. 2003. Reprinted with permission; copyright 2003, Elsevier.
Ramos highlighted several key findings in the 1,3-butadiene literature that create uncertainties in characterizing and quantifying the human cancer risk posed by exposure to 1,3-butadiene:
-
Animal bioassay data. Mice are more sensitive than rats in 1,3-butadiene bioassays, apparently because mice form reactive epoxides more readily than rats. A current hypothesis is that humans are more like rats in 1,3-butadiene sensitivity, although additional evidence at the cellular or biochemical level is needed to confirm this. Unlike male rats,
-
female rats develop mammary tumors when exposed to 1,3-butadiene. In humans, epidemiologic studies of workers exposed to 1,3-butadiene have not included women so there is no information on differential sensitivity between the sexes.
-
Poor information on exposure. Epidemiologic studies on 1,3-butadiene exposure have important shortcomings. For example, high-quality exposure data are not available on 1,3-butadiene workers who have been the subjects of retrospective epidemiologic work. Many workers may have been misclassified as to exposure to 1,3-butadiene.
-
Coexposures to other chemicals. Most 1,3-butadiene workers are coexposed to styrene in the workplace, and this possibly confounds the results. However, exposure conditions are not well quantified, and it has not been possible to eliminate or control for possible styrene confounding in epidemiologic studies.
-
Variability in individual susceptibility. Detoxification enzymes are highly polymorphic in humans. For example, there is evidence that people who lack the glutathione S-transferase theta-1 enzyme involved in biotransformation of reactive epoxides (GSTT1null people) show increased DNA damage in lymphoid cells, a possible precursor to cancer (Norppa et al. 1995). However, studies have not been conducted to determine whether exposed workers with this polymorphism are more likely to develop disease than those without it.
Arsenic
Eaton provided information on the National Research Council’s arsenic risk assessment (NRC 2001). Arsenic is a naturally occurring toxic metal that causes a number of adverse health effects, including cancer. Exposure of concern in the United States is primarily through drinking water, which occurs in “hot spots” across the United States and abroad.
Eaton explained that although arsenic carcinogenicity is relatively well studied empirically in humans, its mechanism of action is poorly understood. Toxicologically, the impact of arsenic on key cell functions is also relatively well studied. Hundreds of papers address such processes as arsenic induction of cell-cycle arrest and apoptosis, alterations in cell growth and proliferation, inhibition of the energy cycle, induction of oxidative stress, alterations in DNA damage and repair, and alterations in gene expression. However, arsenic is unusual among chemicals of concern in that it does not cause tumors in common laboratory animal mod-
els, although it clearly does so in humans. Thus, for arsenic, inferences related to mechanisms that could lead to carcinogenicity cannot be interpreted fully from studies in animal cells.
Arsenic is known to induce oxidative stress, which may be a key element in its carcinogenicity. Although it does not appear to be directly genotoxic, it does affect the accuracy and efficiency of DNA repair and clearly causes alterations in gene expression. The importance of biotransformation pathways of arsenic is not well understood; arsenic is methylated in the body, but it is unclear whether this is a detoxification reaction. Furthermore, there are both organic and inorganic forms of arsenic. The differential toxicity and carcinogenicity of the forms are not well understood.
The National Research Council’s arsenic risk assessment is based primarily on epidemiologic studies in Taiwan, Chile, and Utah (NRC 1999, 2001). Those studies show a strong association between exposure to arsenic in drinking water and cancer of the skin, lung, and bladder and possible associations with kidney and liver cancer. However, the epidemiologic studies suggest that exposure to arsenic differs in its cardiovascular and cancer-causing effects between different populations. Although the differences in health effects were initially hypothesized to be caused by differences in diet and nutrition among the populations studied, it has been suggested that they may reflect polymorphisms in arsenic detoxification enzymes in these human populations. Three such polymorphisms have been identified (Marnell et al. 2003; Thomas et al. 2004).
Despite many studies, there remain key uncertainties in the arsenic data that have complicated arsenic risk assessment. Eaton described the uncertainties as follows:
-
Different responses in different species. Although the human data are generally regarded as sufficient to classify arsenic as a carcinogen (EPA 1998b; IARC 1987), that arsenic is not generally carcinogenic in rodents means that the nontumor experimental data from animal studies (for example, to elucidate metabolism and distribution) are of questionable relevance to humans. What makes rodents insensitive to arsenic carcinogenicity is not known.
-
Epidemiologic studies. There have been disagreements about which of the several epidemiologic studies is preferred for modeling the arsenic dose-response relationship. Some Taiwanese studies may have
-
had exposure misclassification and other possible confounders, but the Taiwanese studies were the most robust.
-
Dose-response relationship. The shape of the arsenic dose-response curve is controversial, and there is some evidence of a threshold. Experimentation in this field has been limited by the lack of animal models of arsenic carcinogenicity.
-
Toxicity of different arsenic forms. Questions exist about the relative potency of different forms of arsenic (such as, inorganic arsenic of different valences, monomethyl arsenic, and dimethyl arsenic).
-
Coexposures to other chemicals. Two of the principal cancers associated with arsenic lung cancer and bladder cancer—are also associated with smoking. Coexposure to carcinogens in tobacco smoke is probably important and possibly a confounder or potentiator of arsenic effects.
-
Individual variability in susceptibility to arsenic. Genetic polymorphisms in methylation reactions of arsenic have been identified, but their consequences are not clear.
Possible Directions for Future Toxicogenomic Investigations
The 1,3-butadiene and arsenic case studies illustrate data gaps and uncertainties that occur in many risk assessments. 1,3-Butadiene is relatively well studied, but there are still uncertainties as to its MOA. In particular, different responses in rats and mice, the quality of exposure data from epidemiologic studies, and the lack of epidemiologic data in women maintain the controversy about the risk that 1,3-butadiene poses to workers and the general population.
Arsenic is an interesting contrast to 1,3-butadiene because its risk assessment is based on human data. Although extensive human exposure data are available, a lack of understanding of the cancer MOA in humans, coupled with a lack of an animal model of cancer, has complicated EPA’s estimate of the carcinogenic potency of arsenic. The difficulties in quantifying exposure in epidemiologic studies and the possibility of confounding factors that also lead to cancer complicate the arsenic risk evaluation further.
These two chemicals also have a number of common critical data gaps and uncertainties, such as species differences and the effects of coexposure to chemicals with which they commonly co-occur. On the basis of the case studies and the general data on risk assessment described
earlier by Greer and Moore, workshop participants identified several types of toxicogenomic inquiries that might be particularly useful for the two chemicals and others about which there are similar uncertainties. The purpose of this exercise was not to determine how uncertainties regarding the specific chemicals could be resolved but to illustrate how toxicogenomics might be useful in resolving uncertainties about chemicals in general.
Species Differences and Gaining a Better Understanding of Mode of Action
Species differences in response to a chemical are critical for both 1,3-butadiene and arsenic. Workshop participants thought that such differences presented a prime opportunity for toxicogenomic experimentation.
As explained above, there are large differences in how rats and mice react to 1,3-butadiene and great uncertainty as to which species is more relevant to humans. Patterns of gene-expression changes in target tissues (such as lung, bladder, and skin) of rats and mice could be compared to determine whether the differential sensitivity in tumor incidence is mirrored in unique patterns of gene-expression changes in the two species. The patterns could then be compared with those in humans. A comparison of the three species—two known to be quite distinct in their tumor response—could help to determine whether humans more closely resemble rats than mice in sensitivity to 1,3-butadiene, as is currently hypothesized. Such work might also confirm the proposed MOA or provide evidence of alternative MOAs.
In the case of arsenic, humans appear to be more sensitive to carcinogenic effects than laboratory animals are, but there is no biologic understanding of that. Although there are a number of hypotheses, the MOAs involved in the tumors seen in humans are not known. Patterns of gene-expression changes in rats, mice, and humans exposed to arsenic could be compared to identify the changes in humans that are not seen in the animal models. That would provide a starting point for toxicologists to begin studying the MOA of the chemical in humans and perhaps to identify potential key events that contribute to the specific cancer sensitivity.
In addition, arsenic offers a unique opportunity to use genomic information to explore which biologic responses that are not tumors themselves are potentially critical to tumor formation. Because arsenic does not cause cancer in laboratory animals, it would be enlightening to use
the response patterns in microarray experiments to identify gene changes that are not precancerous in animals. Such changes could be cataloged for later study of effects of unknown chemicals.
In another approach for improving the understanding of MOAs, participants discussed opportunities to study gene-expression profiles in human populations before and after arsenic exposure. Drinking water is the primary route of arsenic exposure, and this allows accurate exposure measurements. In the United States, populations are exposed to a wide range of arsenic concentrations because only some drinking-water supplies are being upgraded to remove arsenic. Populations that use the latter supplies could be followed to detect changes in gene-expression patterns or proteins before and after arsenic exposure is reduced. The genes and proteins whose expression reverses with decreased arsenic exposure could be indicators of cellular processes involved in arsenic’s effects.
Differences Between Male and Female Responses
The differential sensitivity of males and females to 1,3-butadiene is poorly understood. Like differences between species, sex differences could be studied by comparing gene-expression changes.
Toxicity of Metabolic Products
Differences in gene-expression patterns between inorganic parent and methylated forms of arsenic could be compared to shed light on whether methylation is an activation or detoxification mechanism in humans. Similarly, other organic and inorganic forms of arsenic could be compared to understand their relative toxicities.
In the case of 1,3-butadiene, the gene-expression pattern of the parent compound could be compared with that of the key metabolites to challenge or add weight to the hypothesis that 1,2:3,4-diepoxybutane is the most potent and important 1,3-butadiene metabolite.
Dose-Response Relationship
Gene-expression experiments conducted over a continuum from high to realistic (low) doses of both chemicals might allow researchers to distinguish adaptive responses that are not indicative of toxicity or car-
cinogenicity from responses that signal precancer and other adverse responses. Gene-expression changes could be measured over a range of doses that would include exposures of the general population and in the workplace. Once particular types of responses are identified, experiments could indicate whether threshold exposures to either compound exist.
Coexposure to Other Chemicals
Few, if any, conventional toxicologic experiments have been conducted to study the effects of coexposure to chemicals that commonly occur in occupational or environmental settings. Yet many chemicals are known to co-occur with a few other toxicologically relevant substances, such as tobacco smoke.
1,3-Butadiene and styrene coexposure is common in the workplace and has complicated the interpretation of several epidemiologic studies of occupational exposure. Microarray experiments with coexposure to 1,3-butadiene and styrene would be particularly important if they helped to tease out the effects of coexposure to styrene on toxicity of 1,3-butadiene at the cellular level. It would also be helpful to examine the effects of coexposure to 1,3-butadiene and gasoline components, such as benzene, xylene, and toluene.
Whether smoking potentiates arsenic toxicity is a key question in epidemiologic studies. It is particularly difficult to answer because of the lack of animal models of arsenic carcinogenicity. However, microarray experiments with human tissues might be able to elucidate the effect of confounders, such as smoking, on cancer outcomes of exposure to arsenic.
Individual Variability in Susceptibility
Individual human variability in metabolizing enzymes could be compared. Studies that compare traditional toxic end points in animals with different metabolic enzymes (some of which mimic human polymorphisms) could reveal information about the effects of human variability. Similarly, studies with human cells that express different metabolizing enzymes could be useful. For arsenic in particular, the effects of genotype on gene expression could be studied in humans directly because the target tissues of interest—skin, lung, and bladder—yield cells that can be obtained noninvasively.
MOVING FORWARD
The evaluation of chemicals in risk assessment has progressed from empirical pathologic assessment of tumors to the incorporation of biochemical and cellular information about how chemicals exert their toxic effects. Risk assessment now attempts to include information about a chemical’s MOA and encourages the consideration of a wide variety of data. But despite the advances in risk assessment, data gaps and uncertainties in connection even with well-studied chemicals complicate risk-assessment efforts.
For the field of toxicogenomics to deliver on its potential, it will be important to identify the types of specific data gaps and inconsistencies of greatest importance to the risk-assessment process and to set research priorities accordingly. Achieving that goal may be hampered by the general unfamiliarity of many toxicogenomic researchers with the risk-assessment framework and the key technical issues of controversy underlying regulatory decisions on individual compounds. Similarly, some risk assessors may be unfamiliar with the rapidly developing world of toxicogenomics and thus lack the insight to understand what toxicogenomic research could deliver in the short and long term.
In the final session of the workshop, participants considered the presentations and discussions, brought up new ideas, and highlighted topics that are advancing rapidly. There was no consensus process, nor were the ideas fully developed. But the following paragraphs provide some insight into how research relevant to risk assessment might move forward in the coming years.
Screening of Chemicals
The paucity of data on the possible carcinogenicity of many chemicals invites contributions from toxicogenomic research. Risk assessors must either defer action or make decisions on poorly tested or untested compounds by using structure-activity relationship (SAR) models to infer likely effects. Some workshop participants expressed enthusiasm for using gene-expression patterns to supplement current SARs, and several believed that such experiments could be accomplished in a relatively short period.
“Training sets” could be developed by looking at the geneexpression patterns generated by chemicals that have relatively well-known adverse effects. Researchers could develop testable hypotheses
about gene-expression patterns by using the training sets and use the training sets to test the hypotheses against other, less well-understood chemicals. One participant noted that researchers have already tested what they have learned with some chemicals on chemicals whose effects are somewhat understood. Using blind tests, they have successfully used patterns of gene expression to predict the biology of unknown chemicals.
Individual Sensitivity
One participant suggested that a simple way to start investigating individual sensitivity is to compare how various types of mice respond to given toxicants; for example, transgenic or mutant mice that have polymorphisms in particular genes could be compared with wild-type mice. The results might explain how polymorphisms influence heterogeneity of response. Enhancements in consistency of results from different microarray systems would improve that kind of experiment, as they would improve other types of experiments also described here, by decreasing artificial variability (variability not related to biologic differences). At present, there may be too much variability to develop insight into polymorphism influence on response. At a minimum, it would be necessary to sift through a lot of artificial variability to develop insights.
Better Understanding of a Chemical’s Mode of Action
One view is that for toxicogenomics to contribute substantially to risk assessment, links between gene expression and disease or other adverse outcomes must be established—a process sometimes referred to as phenotypic anchoring. Even without those linkages, analysis of gene-expression changes can provide clues about which biologic pathways may be involved in the MOA of a toxic chemical. Workshop participants noted the importance of conducting toxicogenomic studies over ranges of dose and time. The concern that the MOA of a chemical may be dose- or time-dependent is a key issue in regulatory risk assessment.
Master Switches
In discussing the value of information on preneoplastic changes for both cancer biologists and public-health officials, participants noted the
merits of focusing research attention on the effects of chemicals on master switches in a cell. Master switches are thought to consist of a relatively few loci important for cancer. Thus, rather than looking at thousands of potential gene interactions, investigations of effects on cell circuitry and gene-gene interactions relevant to suspected master switches could have high priority. Some participants suggested that it might be valuable to study gene interactions related to master switches during key stages of development while keeping an eye on the responses of the entire genome.
Study of Preneoplastic Changes
Some participants suggested that researchers set priorities among studies of signatures of preneoplastic changes, pointing out that the few key events involved in preneoplastic changes might narrow the search for the highest-priority signatures. Specifically, it might be valuable to look at gene-expression patterns that correlate with the detection of known preclinical markers, that is, intermediate end points that have been validated as predictive of the disease before the disease manifests. One participant pointed out the value of looking at transcriptional profiling prospectively in people known to have been exposed to a compound to see whether there are early markers that will prove predictive of development of disease. Such markers would be particularly valuable if they enable invasive procedures to be replaced with procedures using surrogate cells or surrogate tissue to study chemical MOAs.
CONCLUSION
This workshop provided a forum for discussion of some of the ways that toxicogenomics might eventually contribute to improving the science of risk assessment. The discussion allowed different communities of experts, including those conducting “-omics” work and those working in the policy arena, to see where their fields might intersect in the future. The workshop provided a baseline of shared understanding or future discussions on, for example, the scientifically appropriate use of toxicogenomic data in risk assessment and the nonscientific challenges involved in using such information.
REFERENCES
Albertini, R., H. Clewell, M.W. Himmelstein, E. Morinello, S. Olin, J. Preston, L. Scarano, M.T. Smith, J. Swenberg, R. Tice, and C. Travis. 2003. The use of non-tumor data in cancer risk assessment: Reflections on butadiene, vinyl chloride, and benzene. Regul. Toxicol. Pharmacol. 37(1):105-132.
Breitkreutz, B.J., C.S. Stark, and M. Tyers. 2003. Osprey: A network visualization system. Genome Biology 4(3): R22.
Bus, J.S., and J.A. Popp. 1987. Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally-related compounds. Food Chem. Toxicol. 25(8):619-626.
CancerWEB Project. 2005. Online Medical Dictionary. Definition of Adduct [Online]. Available: http://cancerweb.ncl.ac.uk/cgi-bin/omd?query=adduct [accessed April 25, 2005].
EDF (Environmental Defense Fund). 1997. Toxic Ignorance: The Continuing Absence of Basic Health Testing for Top-Selling Chemicals in the United States. Environmental Defense Fund, Washington, DC [Online]. Available: http://www.environmentaldefense.org/documents/243_toxicignorance.pdf [accessed Feb. 23, 2005].
EGE (Ethylene Glycol Ethers Panel). 2004. IARC Finds Inadequate Evidence of Carcinogenicity in Humans and Limited Evidence of Carcinogenicity in Animals for Two Glycol Ethers. June 2004 [Online]. Available: http://www.egep.org/glycol_ethers_update.htm [accessed Nov. 10, 2004].
EPA (U.S. Environmental Protection Agency). 1986. Guidelines for Carcinogen Risk Assessment. EPA/630/R-00/004. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC.
EPA (U.S. Environmental Protection Agency). 1991. Alpha-2u-globulin: Association with Chemically Induced Renal Toxicity and Neoplasia in the Male Rat. EPA/625/3-91/019F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC.
EPA (U.S. Environmental Protection Agency). 1997. Guiding Principles for Monte Carlo Analysis. EPA/630/R-97/001. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [Online]. Available: http://www.epa.gov/ncea/raf/montecar.pdf [accessed Nov. 5, 2004].
EPA (U.S. Environmental Protection Agency). 1998a. Health Risk Assessment of 1,3-Butadiene. External Review Draft. EPA/600/P-98/001A. NCEA-W-0267. National Center for Environmental As-
sessment, Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC [Online]. Available: http://www.epa.gov/ncea/pdfs/but/butadiene.pdf [accessed Nov. 9, 2004].
EPA (U.S. Environmental Protection Agency). 1998b. Arsenic, Inorganic. CASRN 7440-38-2. Integrated Risk Information System (IRIS) [Online]. Available: http://www.epa.gov/iris/subst/0278.htm [accessed Feb. 24, 2005].
EPA (U.S. Environmental Protection Agency). 1999. Guidelines for Carcinogen Risk Assessment. Review Draft. NCEA-F-0644. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [Online]. Available: http://www.epa.gov/ncea/raf/pdfs/cancer_gls.pdf [accessed Nov. 5, 2004].
EPA (U.S. Environmental Protection Agency). 2002. Interim Policy on Genomics. Science Policy Council. U.S. Environmental Protection Agency. Washington, DC [Online]. Available: http://www.epa.gov/osa/spc/htm/genomics.pdf [accessed April 13, 2005].
EPA (U.S. Environmental Protection Agency). 2003. Draft Final Guidelines for Carcinogen Risk Assessment (External Review Draft, February 2003). EPA/630/P-03/001A. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [Online]. Available: http://www.epa.gov/ncea/raf/cancer2003.htm [accessed Nov. 5, 2004].
EPA (U.S. Environmental Protection Agency). 2004. An Examination of EPA Risk Assessment Principles and Practices. Staff Paper Prepared for the U.S. Environmental Protection Agency by Members of the Risk Assessment Task Force. EPA/100/B-04/001. Office of the Science Advisor, U.S. Environmental Protection Agency , Washington, DC [Online]. Available: http://www.epa.gov/osa/ratf-final.pdf [accessed Nov. 5, 2004].
GAO (U.S. General Accounting Office). 1994. Toxic Substances Control Act: Preliminary Observations on Legislative Changes to Make TSCA More Effective: Statement of Peter F. Guerrero, Director, Environmental Protection Issues, Resources, Community, and Economic Development Division, before the Subcommittee on Toxic Substances, Research, and Development, Committee on Public Works, U.S. Senate. GAO/T-RCED-94-263. Washington, DC: U.S. General Accounting Office.
Hamadeh, H.K., P.R. Bushel, S. Jayadev, O. DiSorbo, L. Bennett, L. Li, R. Tennant, R. Stoll, J.C. Barrett, R.S. Paules, K. Blanchard, and C.A. Afshari. 2002a. Prediction of compound signature
using high density gene expression profiling. Toxicol. Sci. 67(2):232-240.
Hamadeh, H.K., P.R. Bushel, S. Jayadev, K. Martin, O. DiSorbo, S. Sieber, L. Bennett, R. Tennant, R. Stoll, J.C. Barrett, K. Blanchard, R.S. Paules, and C.A. Afshari. 2002b. Gene expression analysis reveals chemical-specific profiles. Toxicol. Sci. 67(2):219-231.
Hodges, L.C., J.D. Cook, E.K. Lobenhofer, L. Leping, L. Bennett, P.R. Bushel, C.M. Aldaz, C.A. Afshari, and C.L. Walker. 2003. Tamoxifen functions as a molecular agonist inducing cell cycle-associated genes in breast cancer cells. Mol. Cancer Res. 1:300-311.
Haseman, J.K., and A.M. Lockhart. 1993. Correlations between chemically related site-specific carcinogenic effects in long-term studies in rats and mice. Environ. Health Perspect. 101(1):50-54.
IARC (International Agency for Research on Cancer). 1987. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Vols. 1–42, Supplement 7. Lyon, France: IARC Press.
IARC (International Agency for Research on Cancer). 1997. Polychlorinated Dibenzo-para-dioxins and Polychlorinated Dibenzofurans. IARC Monographs on the Evaluation on Carcinogenic Risk to Humans Vol. 69. Lyon, France: IARC Press.
IARC (International Agency for Research on Cancer). 1999. Some Chemicals that Cause Renal or Urinary Bladder Tumors in Rodents, and Some Other Substances IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 73 (13–20 October 1998) [Online]. Available: http://www-cie.iarc.fr/htdocs/announcements/vol73.htm [accessed Nov. 15, 2004].
Johnson, C.D., Y. Balagurunathan, M.G. Tadesse, M.H. Falahatpisheh, M. Brun, M.K. Walker, E.R. Dougherty, and K.S. Ramos. 2004. Unraveling gene-gene interactions regulated by ligands of the aryl hydrocarbon receptor. Environ. Health Perspect. 112(4):403-412.
Kier, L.D., R. Neft, L. Tang, R. Suizu, T. Cook, K. Onsurez, K. Tiegler, Y. Sakai, M. Ortiz, T. Nolan, U. Sankar, and A.P. Li. 2004. Applications of microarrays with toxicologically relevant genes (tox genes) for the evaluation of chemical toxicants in Sprague Dawley rats in vivo and human hepatocytes in vitro. Mutat. Res. 549(1-2):101-113.
Loffredo, C.A., H.V. Aposhian, M.E. Cebrian, H. Yamauchi, and E.K. Silbergeld. 2003. Variability in human metabolism of arsenic. Environ. Res. 92(2):85-91.
Mah, N., A. Thelin, T. Lu, S. Nikolaus, T. Kühbacher, Y. Gurbuz, H. Eickhoff, G. Klöppel, H. Lehrach, B. Mellgård, C. M. Costello, and S. Schreiber. 2004. A comparison of oligonucleotide and cDNA-based microarray systems. Physiol. Genomics 16:361-370.
Marnell, L.L., G.G. Garcia-Vargas, U.K. Chowdhury, R.A. Zakharyan, B. Walsh, M.D. Avram, M.J. Kopplin, M.E. Cebrián, E.K. Silbergeld, and H.V. Aposhian. 2003. Polymorphisms in the human monomethylarsonic acid (MMAV) reductase/hGSTO1 gene and changes in urinary arsenic profiles. Chem. Res. Toxicol. 16(12):1507-1513.
NIEHS (National Institute of Environmental Health Sciences). 2003. Polymorphism and Genes, Haplotype. Environmental Genome Project [Online]. Available: http://www.niehs.nih.gov/envgenom/haplotyp.htm [accessed Nov. 9, 2004].
NIH (National Institutes of Health). 2005. Talking Glossary. Definition of Polymorphism. National Human Genome Research Institute, National Institutes of Health [Online]. Available: http://www.genome.gov/glossary.cfm?key=polymorphism [accessed Feb. 25, 2005].
Norppa, H., A. Hirvonen, H. Jarventaus, M. Uuskula, G. Tasa, A. Ojajarvi, and M. Sorsa. 1995. Role of GSTT1 and GSTM1 genotypes in determining individual sensitivity to sister chromatid exchange induction by diepoxybutane in cultured human lymphocytes. Carcinogenesis 16(6):1261-1264.
NRC (National Research Council). 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, DC: National Academy Press.
NRC (National Research Council). 1994. Science and Judgment in Risk Assessment. Washington, DC: National Academy Press.
NRC (National Research Council). 1999. Arsenic in Drinking Water. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Arsenic in Drinking Water: 2001 Update. Washington, DC: National Academy Press.
Silbergeld, E.K. 2003. Facilitative mechanisms of lead as a carcinogen. Mutat. Res. 533(1-2):121-133.
Spencer, P.J., J.W. Crissman, W.T. Stott, R.A. Corley, F.S. Cieszlak, A.M. Schumann, and J.F. Hardisty. 2002. Propylene glycol monomethyl ether (PGME): Inhalation toxicity and carcinogenicity