Advanced Brain Research, Inc.1
Robin Gaster
North Atlantic Research
EXECUTIVE SUMMARY
ABM is a small company whose research has been funded almost entirely by a series of successful SBIR awards. Currently, ABM is poised to enter Phase III, and is seeking the funding needed to do so successfully.
The company was founded on SBIR awards in 1997, and expanded based on Phase II awards in 1999. It received additional SBIR awards in 2002, and some additional funding from DARPA, during the development of two complementary products: home sleep diagnosis products, and an initial sleep disorder screening product for use in office or other settings.
ABM has received six Phase II NIH awards, and seven Phase I NIH awards, and has been supported almost entirely by $6.3 million in SBIR awards and $700,000 from DARPA.
Primary Outcomes:
-
One product with FDA clearance and a second that has been submitted for clearance, both entering Phase III.
-
Six patents.
-
Publications.
-
Additional employment.
-
Partnerships: Possible pilot program with Waste Management, Inc.
Key SBIR issues:
-
Failure of Fast Track.
-
Better program manager accountability.
-
Commercialization/Phase III support.
-
Commercialization review.
-
Review quality and oversight.
Key recommendations:
-
Optional training program for reviewers.
-
Accelerate shift to electronic submissions. Consider using DoD submission system.
-
Improved program manager assessment using report cards during the Final Report and/or Edison submission processes.
-
Review. Improve commercialization reviews, possibly by instituting two-phase screening system.
-
Phase III. Improve electronic matchmaking by improving online tools at NIH Web site.
BACKGROUND
Advanced Brain Monitoring, Inc., was founded in January 1997 to create low cost, easy-to-use, portable systems to monitor and interpret physiological signals indicating brain activity, and has developed patented data acquisition technology with automated analysis software to measure the brain’s electrical activity (EEG), oxygen levels in the blood and cardiac activity.
ABS used a Phase I award as a founding grant. It opened in 1997 with two full-time and two part-time employees. Phase I awards took the company to January 1999, when it received three Phase II awards. This allowed all three founders to go full time, funded the company’s move to Carlsbad, and paid for three EEG technicians who were hired in June 1999.
The founders have invested about $400,000 on the company, funding primarily used for FDA 510k filings and patent filings, which cannot be delayed while more funding is found. Overall, the company has received more than $6 million
FIGURE App-D-1
SOURCE: Advanced Brain Research.
from NIH in SBIR awards and an additional $700,000 from DARPA under the Augmented Cognition program. ABM has worked with Honeywell and Lockheed in the context of its DARPA-sponsored research.
All current awards will end in March 2005. Company is currently seeking ongoing capital for product rollout.
PRODUCTS
ABM is currently focused entirely on bringing products to market. It has two products that are ready for pilot sales:
-
The Apnea Risk Evaluation System (ARES™) intgrates physiological data acquired in-home with clinical history and anthropomorphic data to quantify level of risk for Obstructive Sleep Apnea (OSA). ARES has three components:
-
ARES Unicorder: a battery powered, self-applied, single site (forehead) physiological recorder that acquires and stores nocturnal data for use in the diagnosis of OSA.
-
ARES Questionnaire (ARES Q): designed to assess pre-existing risk factors for OSA, including age, gender, body mass index (BMI), neck circumference, daytime drowsiness, frequency and intensity of snoring, observed apneas, and history of hypertension, diabetes and cardiovascular disease.
-
ARES Insight Software: automated software to recognize and quantify abnormal respiratory events.
-
The ARES received FDA clearance in October 2004, and its CE mark in February 2005. It must be ordered by a prescription.
ABM sells the AREA system through two channels:
-
Directly to primary care physicians and industrial customers (employers) (as prescribed by a physician).
-
Licensed to larger users. This service includes the technology and training for user staff, and is designed for larger facilities such as hospitals or other bulk purchasers.
-
Alertness and Memory Profiling System (AMP™).The AMP simultaneously acquires data on brain function and cognitive performance during vigilance, attention and memory tests. Its components can be used together or separately:
-
The patented Sensor Headset addresses many of the technical concerns with EEG recordings, including ease of use, comfort, cosmetic acceptability for the workplace, and high quality data acquisition in challenging environments.
-

FIGURE App-D-2
SOURCE: Advanced Brain Research.
-
-
B-Alert®Software. The patented B-Alert software identifies and decontaminates artifacts, monitors changes in the EEG on a second-by-second basis, and classifies each second of brain activity on a continuum from highly vigilant to sleep onset.
-
Neurocognitive test battery. A battery of vigilance, attention, and memory tests that assess and quantify alertness and memory.
-
The Sensor Headset has been submitted for FDA clearance in March 2005, and it received its CE mark in February 2005. The medical application must be ordered by a prescription. There are numerous nonmedical applications for the EEG system.
MARKETS
ABM is addressing two markets:
-
The traditional market for sleep diagnostics, where its lower cost and easier to use system has competitive advantages.
-
New industrial markets for undiagnosed OSA, where companies need better knowledge about employees operating critical equipment.
According to NHLBI, approximately 20 million (6.6 percent) Americans who suffer from OSA, approximately 90 percent are currently undiagnosed.2 The general market is therefore substantial. More specifically, companies whose employees operate critical machinery—e.g., trucks, air traffic controls, trains, etc.—are a very likely market.
ABM faces some significant challenges in marketing its products, even though they address important problems. The ARES system is essentially designed to replace current sleep diagnosis procedures, substituting inexpensive and relatively convenient home diagnosis for expensive and inconvenient sleep studies currently performed in hospitals.
Existing sleep diagnosis labs—potentially a major source of customers—are firmly opposed to in-home studies because it will reduce their own income. Insurance reimbursement for in-home unattended studies is inconsistent. Managed care groups reimburse. The PPOs follow CMS’ lead and either don’t reimburse or at a very low rate. CMS had a review of in-home unattended studies, and—according to ABM—after substantial lobbying of the sleep labs, chose not to categorically reimburse for these studies.
ABM is in discussions with two sleep labs to establish pilot projects that augment rather than cannibalize the sleep labs revenue. ABM has a meeting scheduled with CMS at the end of April to present the results of its study that was funded by NIH (the largest study of its kind for in-home unattended studies).
The AMP system also faces substantial marketing challenges. ABM has established a relationship with Waste Management, Inc., one the country’s largest employers of commercial truck drivers. The pilot—which was to be implemented using a Fast Track since rejected by NIH—involved using the ARES and AMP on Waste Management drivers to 1) determine the level of undiagnosed OSA, and 2) develop a model for incorporating sleep apnea screening into the biannual fitness for duty physicals. The rejected application defunded the pilot, and ABM is now seeking other mechanisms to implement this program. More generally, addressing the problem of undiagnosed sleep apnea potentially opens companies such as Waste Management to significant liability issues. This problem has not yet been resolved.
Despite these difficulties, it is clear that ABM has successfully completed the initial research phase for two complementary products, and is now entering Phase III with both. Its current emphasis is acquiring the funding necessary to implement its marketing strategy.
PATENTS
The company has been awarded 6 patents, funded primarily from founder’s investment and the 7 percent fixed fee received from SBIR awards. All the patents are based on work developed under the NIH SBIR program.
REGULATORY APPROVAL
Both of the company’s products have received the FDA CE mark after completing FDA clinical trials.
PROGRAM MANAGEMENT DIFFERENT ICS
ABM has had dramatically different experiences at different ICs, which it believes are entirely due to the capabilities and approaches of the different program managers. ABM has had a very positive experience with one program manager, but had problems with another who they believe has been, at best, unsupportive, and does not provide the support that reflects NIH guidelines on collaboration between program managers and companies. Short of changing its products and research goals, ABM has not found a way around this program manager, and no way to generate improvement.
ABM’s experience highlights the problem of using program managers as gatekeepers without any tools in place to monitor their effectiveness, or in some cases apparently to train them in relation to new programs.
FAST TRACK
ABM was encouraged by presentations made by Jo Anne Goodnight and started submitting Fast Track applications almost from the start of the program, but has had very mixed experiences at best:
-
Fast Track Application 1. The application received a very high quality review, which recommended splitting the application into Phase I and Phase II. ABM agreed and did so, receiving first a Phase I and then $1.2 million for Phase II, where ABM noted the extensive help from the relevant program manager in preparing a justification for the extra-sized funding.
-
Fast Track 2. This award ran into major administrative problems. The Fast Track was approved in March 2003. The Phase I work was completed in August and a “streamlined noncompeting award process” (SNAP) report was submitted (a short version report designed for projects that are not subject to further competition). This is standard procedure for a Fast Track award and was provided by the program manager in his/her instructions to ABM. However, several problems developed:
-
The total amount of the award was reduced by 5 percent by the review committee because of their opinion that a key consultant was not needed. After discussion with the program manager, the company submitted justification for the payment but the program manager said the review committee’s suggestion was final. If the company needed to pay the consultant, they would have to rebudget form other areas.
-
Even though the program is designed to avoid a gap in funding between Phase I-Phase II, review of the Phase I report was delayed until after October because the Institute needed the new fiscal year to begin in order to have funds for Phase II.
-
-
-
According to ABM, the program manager and the Institute conducted an internal review of the Phase I and turned down the Phase II award due to insufficient detail on what was accomplished in Phase I. (The investigators could easily have written a full Phase I final report but instead provided the amount of information required by the SNAP submission as instructed.) This notification occurred in November, approximately 2.5 months after ABM had notified their program manager that they began the Phase II work that that the pre-award authorization would be used to recapture the funds. The program manager felt that was appropriate because at the time the only delay was due to the new fiscal year. The company wanted to push forward toward commercialization and since the award was noncompetitive and because the company had met its Phase I goals, there was no reason to expect this financial commitment might jeopardize the company’s future.
-
After much negotiations with the NIH program coordinator (which included reviewing with the program coordinator that he/she provided instructions to the company to submit the SNAP, preparation of a full Phase I report, and subsequent re-review), this error was eventually reversed. Because the company had to stop work in November, approximately 12 of the subjects being studied had to be dropped and there was a gap in funding from August when the Phase I ended until the following February.
-
Although the funding was delayed and it interrupted some of the studies, there was no compromise on the part of the program officer about the number of subjects and other research issues. The net result was money was allocated in a manner that reduced the benefits of the large study and reduced the power of the data needed for commercialization.
-
-
Fast Track 3. An application to take the technology developed during earlier SBIR awards and apply it in to the needs of the trucking industry. An agreement for a pilot implementation program was made with Waste Management, Inc., one of the largest operators of commercial trucks.
-
An initial score of 320 meant substantial revisions were needed.
-
ABM resubmitted and was awarded a priority score of 274. Key criticisms included some scientific objections, privacy concerns, issues to do with drivers (social issues), and the lack of women in the study. To address the concern of inadequate female representation, the company had to rewrite the proposal to impose enormous potential costs on ABM including test sites right across the country to increase the number of women in the study. The percentage of female drivers at Waste Management is less that 2 percent of 35,000 drivers. This stringent guideline applied to this unique situation was, in the company’s view, mindless adherence to new
-
-
-
guidelines designed to ensure that projects are not based on male-only research (guidelines which ABM supports in general).
-
ABM resubmitted the application a third time, but in a new year and with an entirely new panel. This time ABM’s review was so poor, it did not receive a priority score at all. One of the lead reviewers simply said that he did not believe that sleep apnea was a widespread medical problem. Because this was the third submission of the application, ABM was forced to give up on this SBIR application.
-
The lessons from this experience seem to be that the Fast Track application is not very well implemented, or at minimum people were not trained prior to implementation. ABM endorses the concept of the Fast Track program. Given the likelihood of obtaining a Fast Track award vs. Phase I and II, the fact that the Phase II dollars are not set aside at the beginning, and misunderstandings about the Fast Track, the company has decided to avoid this program in the future.
REVIEW PROCESS
ABM identified some substantial problems in the review process. The company has noted apparent changes at NIH in how priority scores are calculated, and in the nature of reviewers—notably a pronounced shift toward quasi-commercial concerns. Specifically—
-
Beginning in 2003, the company noticed that reviewer comments (“pink sheets”) no longer tracked closely with the scores.
-
ABM believes that in recent panels, business people may have been over-influencing panel reviews, even when they are not the primary reviewer. The impact of business-based reviews may help to explain the apparent disconnect betweens cores (generated form the panel as whole) and pink sheets (generated primarily from lead reviewers).
-
Study sections often suffer from substantial confusion between the functions and objectives of RO1s and R44s (SBIR awards). Section members who are used to reviewing RO1s are often not prepared for the application-heavy focus of ABM’s applications.
-
Reviewers are sometimes not properly briefed. In one case, for example, a Phase I proposal was sharply criticized for not having a commercialization plan—even though no such plan is required for Phase I.
-
Lead reviewers are sometimes not properly monitored. There appears to be no process for assessing major biases (e.g., the second resubmission on the pilot study).
-
Panel memberships. Letters seeking to affect participants in study sections do not work. ABM knows that in one case it explicitly asked for specific
-
reviewers to be excluded for conflict of interest—and two of those reviewers was the lead reviewer for their application.
-
In a recent review, of both RO1s and R44s, the Committee gave ABM the third highest priority score of 270. The best score was less than 200 and the second highest score was between 200 and 270, both R01s. ABM had the highest R44 score. Over 65 percent of the grants received no priority score.
COMMERCIALIZATION TRAINING
ABM has been a long-time participant in the San Diego Regional Technology Alliance (SDRTA), and is now participating in the NIH commercialization program operated by LARTA. Initial events were not especially helpful, but ABM will be participating in a major technology showcase organized by LARTA in May 2005, for which it has substantial expectations.
LARTA is currently funding a few hours a month from three business consultants, all of whom are viewed fairly positively by ABM, and they have provided some useful market research as well as a contact with Innovex, which provides turn-key national sales forces to sell to physicians, although none has yet provided a real potential partner—which is their primary assigned role.
ABM has also presented posters at the NIH annual conference twice, but in neither case did any business connections result.
PHASE III
SBIR does not permit use of funds for marketing or market research, which makes the transition to Phase III very difficult. ABM did receive CAL-TIP (state) funding of $175,000, which the company said was crucial for the market research necessary to get toward product launch.
AWARD FUNDING LEVELS
ABM’s experience is that applications for more than $1 million get reduced during review.
PROGRAM MANAGEMENT
ABM believes that funding can be delayed when submitting in the April funding cycle: This inevitably means getting caught up in delays in the review process due to summer vacations and the end-of-fiscal year problems at NIH. From a standpoint of counting on an SBIR grant to meet payroll, delay of funding until October can be a significant disruption to a small company that is reliant on the SBIR program as a primary funding source.
However, this contradicts points made in interviews at other companies, who noted that while funding is delayed to October, it does become available as soon as the appropriation is passed, in contrast to funding allocated toward the end of the fiscal year where there may be a liquidity crunch.
SBIR AND VENTURE CAPITAL
ABM has experienced mixed reviews of its SBIR awards from venture capitalists. Some write it off, others view the peer review process as a prohibitive indication of research quality. Receiving more than $6 million in funding from NIH gives ABM immediate legitimacy in discussions with funders, although VCs always discount this funding in the course of valuation.
RECOMMENDATIONS
-
Training program for reviewers (e.g., one-day, on a regional basis). This would not only encourage a more standardized approach, perhaps based on a standard curriculum. It could also encourage some potential participants who might otherwise feel unqualified to become reviewers (e.g., Mr. Levendowski, an MBA with scientific training).
-
Accelerate shift to electronic submissions. ABM is very favorably impressed by the DoD electronic submission process, in comparison to NIH.
-
Improved program manager assessment. ABM felt strongly that final reports and/or Edison submissions should include a report card for the program manager concerned, and that NIH should have review processes in place to improve or eliminate underperforming managers.
-
Review. Commercialization reviews are a problem.
-
ABM suggested that an online questionnaire might help companies answer key commercialization questions, and would also highlight obvious problem areas.
-
ABM supported two phase reviews, with an initial screening by study sections focused entirely on science, and a second level screening of commercialization plans for Phase II. Problems at the second level could then be fixed within a single funding cycle, or applicants could be asked to resubmit for commercialization review only, substantially shortening the entire application process for many awards while improving quality and eliminating many of the current problems with commercialization review.
-
-
Commercialization. NIH could do much more electronic matchmaking. Recommended in particular that NIH implement technology that would permit companies to update their own listings and identify information that is available for review (e.g., business plans, results from Phase I or II, patent applications, etc). Current listings are usually out of date and hence not used much by potential partners.
ADVANCED BRAIN RESEARCH—ANNEX
TABLE App-D-1 Advanced Brain Research NIH SBIR Awards-I
|
Fiscal Year |
Phase Type |
Award Size ($) |
Project Title |
Funding Institute-Center |
|
1996 |
Phase I |
99,980 |
Ambulatory, battery powered, physiological recording |
NS |
|
1999 |
Phase II |
543,000 |
Ambulatory brain monitoring device |
NS |
|
2000 |
Phase II |
204,167 |
Ambulatory brain monitoring device |
NS |
|
1997 |
Phase I |
99,940 |
Alertness quantification system using normative indices |
NS |
|
1999 |
Phase II |
798,773 |
Alertness quantification system using normative indices |
NS |
|
2000 |
Phase II |
276,228 |
Alertness quantification system using normative indices |
NS |
|
1997 |
Phase I |
99,400 |
Portable self-applying drowsiness detection device |
NS |
|
1999 |
Phase II |
365,994 |
Portable drowsiness monitoring device |
NS |
|
2000 |
Phase II |
41,556 |
Portable drowsiness monitoring device |
NS |
|
2000 |
Phase II |
347,762 |
Portable drowsiness monitoring device |
NS |
|
2001 |
Phase I |
125,306 |
In-home sleep apnea risk evaluation system |
HL |
|
2002 |
Phase II |
838,890 |
Validation of In-Home Sleep Apnea Risk Evaluation System |
HL |
|
2003 |
Phase II |
318,195 |
Validation of In-Home Sleep Apnea Risk Evaluation System |
HL |
|
2002 |
Phase I |
139,428 |
Biobehavioral Measurements of Alertness in Sleep Apnea |
HL |
|
2003 |
Phase II |
691,925 |
Automated Detection of Sleep Disordered Breathing |
HL |
|
2003 |
Phase II |
683,352 |
Biobehavioral Measurements of Alertness in Sleep Apnea |
HL |
|
2001 |
Phase I |
99,991 |
Drowsiness Detection: Effects of Feedback Based on EEG |
MH |
|
2001 |
Phase I |
99,994 |
Novel systems to evaluate sleep apnea and vigilance |
HL |
|
NOTE: For a list of codes for National Institutes of Health institutes and centers, see Box App-A-1. SOURCE: Advanced Brain Research. |
||||
TABLE App-D-2 Advanced Brain Research NIH SBIR Awards-II
|
ABM Grant Name |
Description |
Award Size ($) |
Start Date |
Funding Institute-Center or Agency |
|
ABMD—I |
Solid state digital recorder |
99,831 |
11/1/1996 |
NS |
|
Drowsy—I |
Quantify sleep onset |
99,648 |
5/1/1997 |
NS |
|
Alertness—I |
Quantify states of alertness |
99,940 |
3/1/1988 |
NS |
|
Drowsy—II |
Quantify sleep onset—large clinical study |
755,312 |
1/1/1999 |
NS |
|
ABMD—II |
Wireless EEG system |
747,167 |
5/1/1999 |
NS |
|
Alertness—II |
Clinical and validation studies |
1,075,001 |
6/1/1999 |
NS |
|
ARES-A—II |
Prototype ARES and baseline AMP for OSA |
99,994 |
1/1/2001 |
HLB |
|
CAPTIP |
Commercialization and EEG sensor production |
175,000 |
1/1/2001 |
HLB |
|
ARES-B—I |
ARES Questionnaire |
125,306 |
8/1/2001 |
HLB |
|
DMD—I |
Assess real-time recognition of sleep onset |
99,991 |
8/1/2001 |
MH |
|
ARES-A—II |
Development of ARES & clinical studies |
1,157,083 |
1/1/2002 |
HLB |
|
AMP—I |
Development of AMP |
139,428 |
3/1/2002 |
HLB |
|
AMP—II |
AMP Clinical Studies |
968,669 |
4/1/2003 |
HLB |
|
ARES-B—II |
Enclosure, Nasal Pressure, clinical studies |
978,327 |
7/1/2003 |
HLB |
|
|
Total NIH |
6,620,697 |
|
|
|
DARPA—I |
Assess workload |
50,715 |
5/1/2002 |
DARPA |
|
DARPA—II.a |
Assess workload |
100,000 |
1/1/2003 |
DARPA |
|
DARPA II.b |
Assess workload |
250,000 |
1/1/2004 |
DARPA |
|
|
Total DoD |
400,715 |
|
|
|
|
Total grants awarded |
7,021,412 |
|
|
|
NOTE: For a list of codes for National Institutes of Health institutes and centers, see Box App-A-1. SOURCE: Advanced Brain Research. |
||||
Advanced Targeting System, Inc.3
Robin Gaster
North Atlantic Research
EXECUTIVE SUMMARY
Background
ATS is a small biotech company located in San Diego. Unusually, it has had a strong product line since inception in 1994, and currently offers more than 40 products for sale on its Web site.
The company is based on the application of targeted toxins to neuroscience, where the selective approach offered by what the company calls Molecular Neurosurgery offers obvious advantages if successful.
Initial products have been sold to other research companies, but the company is now reaching the clinical trials stage for products aimed at addressing chronic pain. The American Chronic Pain Association (ACPA) estimates that one in three Americans (approximately 50 million people) suffers from some type of chronic pain.
Future research will focus on ways of enhancing the company’s current approach, so as to permit cell modification via cytotoxins, beyond the current tools which allow selective elimination of cells only.
ATS is currently seeking partnerships/funding for clinical trials of chronic pain technologies, partly through participation in the commercialization assistance program operated for NIH by LARTA.
SBIR History and Status
ATS has used SBIR since its inception. It has received two long running Phase II awards, one new Phase II in 2003, and also one of the first CCAs in 2003. ATS has received three additional Phase I awards.
Key Utilization of SBIR
ATS has funded its research primarily through SBIR awards. New funding is now being used for the toxicology/safety testing phase of FDA approval process.
Outcomes:
-
Numerous products (more than 40).
-
Current research supported by SBIR: focused on chronic pain, a major quality of life issue for one in three Americans.
-
Many scientific papers.
-
Two patents.
-
Partnerships with major medical research centers and academics.
-
“Profound addition” to knowledge in the field of chronic pain.
Key issue/concern: resolving the Phase III funding problem.
Recommendations:
-
Phase III: the neuroscience-funding institutes at NIH should collectively fund a research hospital for clinical trials, similar to that funded by NCI.
-
Size/duration. No additional funding for Phase I.
-
Funding cycles. Eliminate the 2-year window for Phase I winners to apply for Phase II.
-
Direct to Phase II. Companies should be allowed to compete directly for Phase II without previous Phase I.
BACKGROUND
ATS was founded in April 1994 by Douglas Lappi, Ph.D. and Ronald Wiley, M.D., Ph.D. (Scientific Advisor), initially for commercial development of ideas and products developed in their academic labs.
ATS is located in an R&D hub (San Diego), is not woman- or minority-owned, is small (9 employees), has been funded internally and by SBIR, has won several SBIR awards but is not a top-20 award winner, and has reached market with its products. ATS has received SBIR awards only from NIH.
FOUNDER/COMPANY HISTORY
Douglas Lappi began work in the field in the 1970s. He worked on targeted toxins focused on cancer, but could not interest previous employers in his ideas for targeting toxins on the brain. He has extensive experience in laboratory work. His two partners are Ron Wylie, Chief of Neurology at the Veteran’s Administration Medical Center, and Professor of Neurology and Pharmacology at Vanderbilt University, Nashville, TN, and Denise Higgins (VP Business Development), previously at the Salk Institute with Lappi.
Wylie’s key insight, according to Lappi, was that “cancer people could learn nothing from us, but we could learn a lot from them.” Essentially, there were many possibilities for applying the science of targeted toxins from cancer to neu-
rological research. The field of targeted toxins and the brain was largely ignored by mainstream research, and the company was started in 1994 with a specific focus on selling targeted agents, especially for neurological research.
Company products were immediately well received by biotech companies. In 1994, at the Society for Neuroscience annual meeting, the ATS poster and booth showed the first use of the 192-Saporin by an independent researcher Dr.Waits at Rutgers University. Lappi says that this was a “thunderbolt” in the field, as researchers had been waiting for this capability for years.
As a result, the first month of product release in 1994 generated “huge” sales, which the company surpassed on a monthly basis only recently.
ATS does not disclose revenues.
TECHNICAL FOCUS
ATS is focused on implementing known techniques in neuroscience by means of new mechanisms. Lesioning of a region by surgical means and observing the effects is a well known and widely used technique in neuroscience research and medicine. ATS aims to provide similar outcomes by application of specific cytotoxins (essentially, using chemistry instead of surgery, with—if successful—much greater specificity and control).
ATS calls the new technique Molecular Neurosurgery (MN). The first ATS MN product (192-Saporin) is now in use in laboratories world-wide.
PRODUCTS
The ATS product line includes targeted toxins, antibodies, and custom services for assisting neuroscientists in studying nervous system function, and brain-related diseases and disorders.
ATS has had products on the market from its first month of operation, and was first to market with cytotoxin research reagents, which are sold to other biotech researchers primarily in the neurosciences. This is a niche market, and as presumably of limited interest to larger companies, although ATS understands a larger company could enter the market at any time. Its original partner, Chemi-Con did compete for a while but has left the market. ATS has protected its position through two patents.
ATS currently offers a large number of products through its online catalog, which lists 20 targeted toxins, four control conjugates, six secondary conjugates, eight proteins and peptides, and four fluorescent conjugates, and more than 25 other neuroscience products, as of March 2005.
EMPLOYMENT
The company began with one full-time employee. The first Phase II award allowed ATS to hire one additional person. Currently, ATS has nine staff members.
COMPANY STRATEGY
ATS is emphatically not a pharmaceutical company. It is too small, and the company does not intend to grow rapidly into a large enough company to pursue drug development on its own.
Accordingly, ATS is seeking development partners, a process in which it has been supported by CAP and the NIMH program officer.
CURRENT AND FUTURE PROJECTS
The general research strategy is to identify a target cell type, place a bioactive molecule inside the cell, determine whether it functions, and then track the results. This is the basis for all products to date.
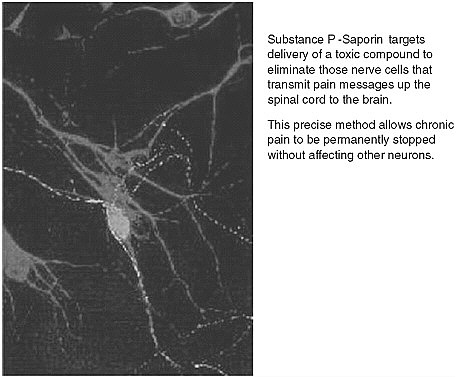
FIGURE App-D-3 Substance P and targeted cell death.
SOURCE: Advanced Targeting System.
Current Research
The company is working on SP-SAP—a patented chemical conjugate composed of the neuropeptide Substance P, and the ribosome-inactivating protein saporin. The project is aimed addressing the problem of chronic pain, a very difficult area to understand, with high levels of complexity and multiple areas of research, including the spinal cord and neuron receptors within the brain.
ATS research has addressed a central question in chronic pain research, namely whether chronic pain can be defined in terms of a unique pathway within the nervous system, or whether it results from some characteristic of the system as whole. ATS determined that chronic pain is related to specific and unique pathways, which could be both identified and disrupted. Lappi claims that this was an enormous breakthrough in the field of chronic pain, and that it is at a minimum a “profound addition” to the field.
Initial research in this area was followed by more work on other screens starting in 1999, developing more chronic pain models in rats. All of these were successful.
This work has potentially profound implications for millions of people who currently endure chronic pain. On the ATS Web site, Lappi notes that—
Many people suffering from intractable chronic pain have exhausted all of their options. Their quality of life is diminished. We envision, in the not too distant future, offering a one-time injection that will end the pain. Chronic pain sufferers won’t need to take a pill every day. Advanced Targeting Systems has excellent preclinical data that leads us to believe that SP-SAP will be safe and effective and compels us to develop SP-SAP for clinical use.
Researchers at UCSD have now completed preliminary toxicology studies with SP-SAP in one of the FDA-required large animal models (funded under 2001 SBIR award from NIMH), and will carry out the full toxicology studies required to address safety issues.4
ATS has submitted its work on chronic pain to the FDA, and its preclinical data have been accepted. Clinical trials are the next step. Toxicology/safety testing will be funded by the SBIR CCA award, and ATS is now actively seeking partnerships or venture funding for this expensive stage of development.
The FDA has advised ATS that SP-SAP may best be developed as an orphan drug for treatment of pain in patients with terminal cancer.
Future Directions
So far, the company’s lead compound, 192-Saporin, has been used to kill selective cells. ATS is now interested in seeing whether a similar delivery mechanism can be used to modify the behavior of cells, instead of just killing them.
Working with an academic—Bob Solveter—the company is working on epilepsy-related problems. Epilepsy is triggered by reduced activity/loss of inhibitor neurons in the hippocampus. This is a fairly well established hypothesis. Animal experiments have shown that the destruction of inhibitor neurons does result in status epilectucus.
The problem then is to increase the activity of inhibitor neurons. ATS is working on using its established delivery systems for inducing enhanced activity among inhibitor cells.
FUNDING
ATS was bootstrapped on the basis of its products and SBIR funding, with no outside funding and minimal investment from founders. It formed an initial partnering agreement with Chemi-Con, which both marketed the first product—192-Saporin—and provided ATS with office and laboratory space.
Subsequently, ATS rented space on favorable terms from Invitrogen, which was seeking further intellectual cross-fertilization at the time.
ATS sought venture capital funding in 2003, but the timing was too difficult. The company is now seeking funding or a corporate partner under NIH auspices via the LARTA program. It is however too soon to tell whether this will lead to anything substantive.
ATS does not see a significant halo with respect to private funding. It believes that while an SBIR award with peer review may help a company get through the door to see a venture capitalist, the latter are focused on economics, not science, and an SBIR award says little about that.
SBIR HISTORY
ATS applied for its first SBIR immediately after being founded in 1994, received a Phase I in 1995, and another in 1996. Both became long-running Phase II awards, with the second running all the way to the first round of CCAs in 2003—the fifth year of support for this project. Three of the six ATS Phase I awards have resulted in Phase IIs.
CCA
ATS received one of the first CCA awards in October 2003—providing $2.4 million over three years—designed to allow ATS to complete toxicology studies and to prepare clinical-grade material for use in human trials.
This CCA award was part of a Program Announcement at NIMH, “Competing Continuation Awards of SBIR Phase II Grants for Pharmacologic Agents and Drugs for Mental Disorders.” ATS notes that “For small businesses like Advanced Targeting Systems, this latest expansion of the SBIR program provides important support at a time when alternative funding is expensive and difficult to find.”
IMPACT OF SBIR
Without SBIR, ATS says that it would have followed much the same development and research trajectory, but at a much slower pace. The existence of markets for its first products would have generated the funding necessary for this lower level of effort. However, the path itself would probably have been different, especially in relation to its relationship with outside academics, who would have been much harder to fund, and whose work with ATS has clearly been critical to the company’s strategy and to its success so far.
SBIR suits ATS for several reasons, according to Lappi:
-
Competition is fair because only other small companies are involved.
-
Many of the other applications are not all that good.
-
SBIR is focused on the same goal as ATS—making a product.
-
Overall, a “tremendously appealing program. SBIR has been a great help, and we appreciate it tremendously.”
PUBLICATIONS
ATS staff have a long history of publications. Lappi himself has more than 70 scientific publications to his credit, and Lappi and Wiley have a book on targeted toxins due out in March 2005.5 However, ATS prefers to focus on the publications generated by other researchers—especially academics—who are using their tools.
The ATS Web site cross references more than 65 papers for 2004 alone that used technologies sold by ATS. Lappi sees scientific publications as “the highest form of advertising.”
Publications have also had an important impact on the company. In the course of its research on chronic pain, ATS submitted a Phase II application that was originally not funded with a score of 220. After publication of the first article on 192-Saporin in Science, the resubmitted application scored 121 (the highest score so far encountered in NRC research at NIH). Reviewers also increased the budget above that originally requested. This research has subsequently received
several additional rounds of funding from NIH under this SBIR award, leading to the CCA award described above.
PATENTS
ATS has received patents on its first two molecules, and has several other patent applications pending. However, applications are expensive (ATS estimates $25,000 per patent), and the company is careful in selecting targets for patenting. Both current patents are based on work funded by SBIR.
UNIVERSITY PARTNERSHIPS
Partnerships appear to have a played a very important role. Academics have acted as first adopters for ATS technology. Subsequent academic/scientific publications then provide the validation necessary for market acceptance, and for further funding from NIH.
This approach is demonstrated in the context of the research on chronic pain currently under way at ATS. Initial pain model studies showing efficacy in rats were performed in the laboratories of University of Minnesota pain expert Dr. Patrick Mantyh. Results from these studies were published in Science in 1997,6 providing enormous validation to the ATS approach. Mantyh’s laboratory published a second Science article in 1999,7 demonstrating the long-term elimination of chronic pain with SP-SAP.
Dr. Tony Yaksh, Professor of Anesthesiology and Pharmacology at the University of California, San Diego, is a leading expert on the administration and pharmacology of drugs in the spinal cord and spinal fluid. His associate, Dr. Jeff Allen, completed preliminary toxicology studies with SP-SAP in one of the FDA-required large animal models. UCSD will carry out the full toxicology studies with funding from the grant awarded to Advanced Targeting Systems.
Management Issues
Phase I-Phase II Gap. Acknowledged by ATS, Lappi notes that, “you can’t run a company on SBIR awards.” He does not see this as a criticism. In fact, he suggests that the gap acts as a kind of guarantee that there is a real business here: that the government is not supporting a business, but is helping an established business do product development.
Differences between ICs. ATS sees significant differences, but based more on scientific prejudices than program management. ATS simply cannot get funded at NCI, despite its strong track record at NIMH. Lappi sees this as stemming from scientific bias—targeted toxicology has not historically worked well in cancer, and as a result reviewers are biased against this approach.
Commercialization assistance program (CAP). ATS is currently involved in the new CAP being operated by LARTA. The company was present at a February 2005 meeting with potential funders in Newport Beach.
RECOMMENDATIONS
-
Size/duration (Phase I): No additional funding for Phase I, even though current levels mean that no business can afford the risk of hiring someone just on the basis of a Phase I Award.
-
Funding cycles. Eliminate the 2-year window for Phase I winners to apply for Phase II. Serves no useful purpose, and sometimes a Phase II application must wait for more data (Lappi has examples).
-
Build a hospital for clinical trials. ATS suggests resolving the Phase III problem in neurological sciences by building a hospital for clinical trials, with the costs shared by multiple ICs. Claims that this approach has been adopted at NCI.
-
Direct to Phase II. Companies should be allowed to compete directly for Phase II without previous Phase I.
ADVANCED TARGETING SYSTEMS—ANNEX
Table App-D-3 Advanced Targeting Systems NIH SBIR awards
|
Fiscal Year |
Phase Type |
Award |
Institute-Center |
Project Title |
|
1995 |
Phase I |
$96,844.00 |
NS |
Specific tool for modeling neuronal degeneration |
|
2001 |
Phase II |
$282,235.00 |
NS |
A specific tool for targeting neurodegeneration |
|
2002 |
Phase II |
$237,928.00 |
NS |
A specific tool for targeting neurodegeneration |
|
2003 |
Phase II |
$245,067.00 |
NS |
A specific tool for targeting neurodegeneration |
|
|
|
$862,074.00 |
|
|
|
1999 |
Phase I |
$113,227.00 |
MH |
Mabs to target specific neuronal populations |
|
2003 |
Phase II |
$341,281.00 |
MH |
Monoclonal antibodies to target neuronal populations |
|
|
|
$454,508.00 |
|
|
|
1996 |
Phase I |
$99,997.00 |
MH |
New tool for basic neurobiological research |
|
1998 |
Phase II |
$404,567.00 |
MH |
New tools for basic neurobiological research |
|
1999 |
Phase II |
$344,750.00 |
MH |
New tools for basic neurobiological research |
|
2001 |
Phase II |
$397,984.00 |
MH |
Toxicology/safety studies of a chronic pain therapeutic |
|
2002 |
Phase II |
$276,089.00 |
MH |
New tools for basic neurobiological research |
|
2003 |
Phase II |
$799,709.00 |
MH |
Drug development of a chronic pain therapeutic |
|
|
|
$2,323,096.00 |
|
|
|
1999 |
Phase I |
$100,000.00 |
NS |
Tools for the dissection of pain transmission pathways |
|
2000 |
Phase I |
$100,000.00 |
DA |
A tool to study the diverse behavior effects of galanin |
|
2001 |
Phase I |
$133,547.00 |
DE |
Targeting neurons involved in chronic pain transmission |
|
Total |
|
$3,973,225.00 |
|
|
|
NOTE: For a list of codes for National Institutes of Health institutes and centers, see Box App-A-1. SOURCE: Advanced Targeting Systems. |
||||
Bioelastics Research, Ltd.
Paula Stephan
Georgia State University
DESCRIPTION OF THE FIRM
Bioelastics Research, Ltd. (Bioelastics) was founded by Dan W. Urry, Ph.D., at the University of Alabama (UAB) Birmingham in 1989. The company suspended operations October 31, 2004. At the time the firm was founded, Dr. Urry was a professor of molecular biophysics at UAB. Dr. Urry currently is on the faculty of University of Minnesota (department of chemical engineering and material science) teaching courses from January to April each year, having retired from UAB. After retiring from UAB, and prior to the suspension of operations, Dr. Urry continued to spend time in Birmingham each year. Before it suspended operations in the fall of 2004, the firm had four employees. Its average annual revenue was under $700,000. At its largest, the firm employed eight people. The firm resided in incubator space at UAB. During the first few years, the firm paid rent that was under market but in subsequent years the firm paid the market rate.
The company’s technology evolved around polymers made from elastic sequences in the body. This technology provided a basis for producing materials that would prevent adhesion, deliver drugs, and provide acoustic prevention (sound deadening) as well as a number of other applications including tissue reconstruction that is applicable to urinary incontinence and spinal injuries. Initial work on this technology was done at University of Alabama Birmingham in the lab of the founder, Dr. Dan W. Urry.
The firm received an initial investment of $333,000 from three local investors. UAB provided funding for initial patent applications before Bioleastics was founded. The firm was required to pay back the amount UAB spent and Bioelastics has born all the patent cost forward since then. In addition, Bioelastics was required to pay a $50,000 per year due-diligence payment to UAB. Patent costs are still being generated and are to be paid by the current holding company or whoever eventually acquires the technology.
The initial impetus to found the firm was the availability of cost-sharing funds for small businesses from the Office of Naval Research (ONR) which Dr. Urry found out about while working on research funded by ONR in the late 1980s at UAB. The firm was started using the $333,000 investment money noted above to set up the firm and then applied to ONR for a project directed towards wound repair technology.
INPUTS
The firm had 18 SBIRs from NIH: 12 Phase I and 6 Phase II. Almost all of the SBIR awards were to facilitate the development of products based on bioelastic material. Examples include: ingestible implants to correct urinary incontinence; materials for strabismus and retinal surgery, development of an artificial pericardia.
SBIRs did not play a role in the founding of the company, but the company began applying for SBIR awards at an early stage, and from 1991 on “SBIRs were very important to the company’s financial position.” The company received no funding from the state of Alabama. In addition to the SBIR awards, and the ONR award referred to above, the company received federal funding from DoD and DARPA as well as some other, non-SBIR funds, from ONR.
The only angel/VC money that the company received was the initial $333,000 noted above. The inability to attract other funding arguably relates to the Birmingham location of the firm. There was very little VC money in Birmingham at the time and what was available had been invested in several high profile companies that “soaked up the local money and provided no return.” This hindered Bioleastics ability to raise local venture funding. Moreover there was little interest at the time Bioleastics started up from VC companies operating out of state. “You don’t get the interest from the Carolinas and Atlanta to come over here. They [VCs] didn’t really like having it in Birmingham.” Moving, which could have opened up the opportunity for VC, was problematic for the company. Some of the primaries did not want to leave the Birmingham area and the initial investors wanted the company to stay. There was, to quote Mr. Parker, a hometown spirit of “make this benefit Alabama.”8
The SBIR awards helped the company to raise many of the contracts that the company had with firms, allowing the company to expand the research that it was doing and grow the research to a particular application. By way of example, one of the SBIR awards dealt with adhesions. As a result of this research, Bioelastics established a research contract with a firm to develop a product in which the firm was potentially interested. While they were able to do that successfully, the stumbling block was the lack of a production facility. This “chicken-and-egg” problem plagued the company throughout its entire tenure and eventually played a key role in causing the company to suspend operation. In essence, the contracting companies did not want to take on the initial expense, estimated at approximately $10 million, of creating a facility to produce the polymers but would have readily bought the polymers if they were available and reasonably priced for the application.
The company had four employees when it received its first SBIR award in the early 1990s. At its largest, the company employed eight individuals. SBIR
awards impacted company hiring in the sense that in several instances individuals were hired on after the grant was awarded. For example, Dr. Asima Pattanaik was hired as a result of an SBIR award and remained with the company for eight years, becoming a PI on several SBIR awards. To quote Mr. Parker, “SBIR funding allowed the company to maintain a “core group.”
KEY OUTCOMES FROM SBIR
Commercial Outcomes
The company never had a product that generated revenue. It did, as noted above, have research contracts with commercial companies to explore the development of bioelastic material and viable products were produced. The key constraint in producing a viable commercial product was the lack of a production facility capable of producing the material. According to Mr. Parker, the initial investors did not believe that they needed to have a production company. They “expected someone to walk up and pick it [the company] up for a big chunk of change and move on with it but no one wants to put that type of capital into a company that … needed a production company and a huge initial investment.” Stated somewhat differently, as long as the firm focused on research it was successful. But when the initial investors pushed for commercialization and got control of the company, problems emerged. “The firm was good at doing research, but when they [the initial investors] turned it over to someone else to move it from a research company to a production company the transition was not successful.”
The company never licensed a product but in several instances option payments were obtained from firms interested in taking a look at the company’s technology.
Noncommercial Outcomes
The company has been awarded ten patents. The last patent was awarded in March of 2004; it had been applied for in April 2001. A few of these patents were based on research that was funded by SBIR grants. Most of the SBIR grants furthered the advance of technology for which a patent had already been applied.
Scientists working at the firm published a considerable number of papers. A list of publications is provided at the end of this paper, representing the scholarly work accomplished during the existence of Bioelastics. A number of these papers can be attributed to SBIR awards. Some of the publications represent basic research papers; some proposed the basis for what became an SBIR proposal from work done with other agencies and some resulted from work done with other companies.
Dr. Urry has established a considerable scientific reputation based on his
work in the field of bioelastics and is a frequent speaker at international conferences and symposia. The SBIRS helped open up a whole new frontier: “We now believe we can understand how the body works—the reactions of how proteins change in the body. How they will release, conform and reattach and the nanoscale processes they undergo. Also, Dr. Urry can now explain how the internal motors work and describe the forces that drive them. No one else in the world developed or conceived of this. It’s really a Nobel Prize type application.”
IMPACT OF SBIR ON THE FIRM
The SBIR program was very important to the company’s financial position from 1991 on. It provided a good deal of the funding for the research that the company did. The SBIR program also influenced the research direction of the company to the extent that the company would respond to special SBIR initiatives. The SBIR program allowed the company to hire additional researchers and maintain a core group. But the company could only maintain a core group and that was a problem. “You cannot go out and hire new people if there is only six months of funding; for two years you can afford to go out and hire people but there is not a six-month job market. If you are going to grow on a Phase II you better have a clear exit strategy.”
Participation in the SBIR program affected the firm’s commercialization strategy by allowing the firm to take a longer view of commercialization. “A problem the company had from inception is that it didn’t have a rock solid business plan. It was a spin out—first company to spring out from UAB. In so doing, the university put a lot of restrictions on the inventor…. It required an annual due diligence payment in order to keep the technology of $50,000 at minimum; it held a 7 percent ownership stake and a 50 percent stake in any patent that developed in the company.” This made it more difficult to commercialize. “SBIRs were not a constraint; the business plan, the structure of the company were the big constraints.”
SBIR PROGRAM ADMINISTRATION
The firm first became aware of SBIRs as a result of a program solicitation that was distributed in the early 1990s. At the time, Mr. Parker was working in Dr. Urry’s lab at UAB.
The firm managed the delay between Phase I and Phase II awards by having multiple concurrent ongoing projects.
Mr. Parker found the size of the SBIR awards to be “decent.” But the time frame to be “short.” Unless you are working on a project full force, for example, it is hard to accomplish Phase I research in a six month period. They learned to live with the lag time between submission and receipt of funding. But, if it “took a year for a project to be funded it could be hard to keep all of that [personnel and
equipment] together. It really takes an established company that has a product to support bringing in money during that period of time.”
Mr. Parker answered affirmatively in terms of whether it would be beneficial to increase the duration of the award, adding that if “not increasing duration, being careful when you fund people with a broad scope, which has trouble fitting in a six month period.” He added that broad scope projects also have difficulty fitting into the time allowed for a Phase II. “Six month SBIRs turned into a year; Phase IIs turned into three years.”
The company did not find the paperwork to be severe. “It took some time but it was not overly demanding.” Mr. Parker went on to say that “I think it would be useful if they provided a few more guidelines as to what they want in reports. It might be helpful to them to have more uniform reporting.”
The primary recommendation for change that Mr. Parker offered was to demand a better plan as to how the research will be commercialized. “If a company goes in with the sole purpose of doing research and getting the information, that’s fine … but also need to have a plan of what to do with it once they get it. Will they be able to market it? Will they be able to get additional funding? Is additional funding available?” He believes that one would see a higher success rate coming out of SBIR funding if the agency paid more attention to the business plan.
Mr. Parker also recommended that NIH consider requiring grantees to submit an annual report that is directed at sharing information with other SBIR awardees. He compared the current NIH reporting requirements to reporting required by DoD for certain non-SBIR awards. “DoD requires a PowerPoint presentation, summing up your program and stating where you are going…. This is presented and you have to at least develop it to the point where you can get somebody’s attention with it.” These DoD reports, presented annually, brought people up to speed concerning what was going on and drove collaborations. The experience made participants aware that they were “part of a group; you all are striving to overcome something. It brings the community together.”
CROSS-CUTTING RESEARCH QUESTIONS
Firms are inhibited from getting an SBIR award if they are not well established, having neither facilities, equipment, nor personnel in place. He saw a number of companies in the incubator space at UAB that were not as established as Bioelastics. “One person in one room with one piece of equipment. They got a Phase I and they scaled up. But there was no way to survive beyond except to enter into the Phase II.”
One benefit that a firm gains from an SBIR award that is not available through many other programs is the ability to “take advantage of connections with other people” by going to meetings organized for SBIR recipients at NIH. The company found attending such meetings and interacting with other researchers to be helpful in building collaborations that helped to move projects forward.
“Especially in small companies it is the collaborations that strengthen you and builds you … it’s these meetings that really bring you together.”
Note: the interview was conducted February 18, 2005 by Paula Stephan, with Tim Parker, in Pell City, Alabama. Mr. Parker was Manager of Research for Bioelastics and had worked with the company for thirteen years. Prior to joining the company, Mr. Parker worked for five years in Dr. Urry’s lab at the University of Alabama Birmingham.
REFERENCES
Alkalay, Ron N., David H. Kim, Dan W. Urry, Jie Xu, Timothy M. Parker, and Paul A. Glazer. 2003. “Prevention of Postlaminectomy Epidural Fibrosis Using Bioelastic Materials.” Spine. 28:1659-1665.
Daniell, H., C. Guda, D. T. McPherson, X. Zhang, and D. W. Urry. 1996. “Hyper Expression of a Synthetic Protein Based Polymer Gene.” Pp. 359-371 in Methods in Molecular Biology Vol. 63: Recombinant Proteins: Protocol Detection and Isolation. R. Tuan, ed. Totowa, NJ: Humana Press, Inc.
Gowda, D. Channe, Timothy M. Parker, R. Dean Harris, and Dan W. Urry. 1994. “Synthesis, Characterizations and Medical Applications of Bioelastic Materials.” Pp. 81-111 in Peptides: Design, Synthesis, and Biological Activity. Channa Basava and G. M. Anantharamaiah, eds. Boston: Birkhäuser.
Gowda, D. C., T. M. Parker, C. M. Harris, R. D. Harris and D. W. Urry. 1994. “Design and Synthesis of Poly-tricosapeptides to Enhance Hydrophobic-induced pKa Shifts.” Pp. 940-943 in Peptides: Chemistry, Structure and Biology. R. S. Hodges and J. A. Smith, eds., Proceedings of the Thirteenth American Peptide Symposium, Edmonton, Alberta, Canada.
Gowda, D. Channe, Chi-Hao Luan, Raymond L. Furner, ShaoQing Peng, Naijie Jing, Cynthia M. Harris, Timothy M. Parker, and Dan W. Urry. 1995. “Synthesis and Characterization of Human Elastin W4 Sequence.” International Journal of Peptide and Protein Research. 46:453-463.
Guda, C., X. Zhang, D. T. McPherson, J. Xu, J. H. Cherry, D. W. Urry, and H. Daniell. 1995. “Hyper Expression of an Environmentally Friendly Synthetic Polymer Gene.” Biotechnology Letters. 17, 745-750.
Herzog, R. W., N.K. Singh, D. W. Urry, H. Daniell. 1997. “Expression of a Synthetic Protein-based Polymer (Elastomer) Gene in Aspergillus Nidulans.” Applied Microbiology & Biotechnology. 47:368-372.
Hoban, Lynne D., Marissa Pierce, Jerry Quance, Isaac Hayward, Adam McKee, D. Channe Gowda, Dan W. Urry, and Taffy Williams. 1994. “The Use of Polypenta-peptides of Elastin in the Prevention of Postoperative Adhesions.” Journal of Surgical Research. 56:179-183.
Jing, Naijie. Kari U. Prasad, and Dan W. Urry, “The Determination of Binding Constants of Micellar-packaged Gramicidin A by 13C and 23Na NMR,”Biochem. Biophys. Acta, 1238, 1-11, 1995.
Jing, Naijie and Dan W. Urry. 1995. “Ion Pair Binding of Ca++ and C1− Ions in Micellar-packaged Gramicidin A.” Biochem. Biophys. Acta. 1238(1):12-21.
Kemppainen, B. W., D. W. Urry, C.-X. Luan, J. Xu, S. F. Swaim and S. Goel. 2004. “In vitro skin penetration of dazmegrel delivered with a bioelastic matrix.” International Journal of Pharmaceutics. 271:301-303.
Kemppainen, B., N.-Z. Wang, S. Swaim, D. W. Urry, C.-X. Luan, J. Xu, E. Sartin, R. Gillette, S. Hinkle, and S. Coolman. 2004. “Bioelastic Membranes for Topical Application of Thromboxane Synthetase Inhibitor for Protection of Skin from Pressure Injury: A Preliminary Study.” Wound Repair and Regeneration. 12.
Luan, Chi-Hao and Dan W. Urry. 1999. “Elastic, Plastic, and Hydrogel Protein-based Polymers.” Pp. 78-89 in Polymer Data Handbook. James. E. Mark, ed. New York: Oxford University Press.
Manno, M., A. Emanuele, V. Martorana, P. L. San Biagio, D. Bulone, M. B. Palma-Vitorelli, D. T. McPherson, J. Xu, T. M. Parker, and D. W. Urry. 2001. “Interaction of processes on different time scales in a bioelastomer capable of performing energy conversion.” Biopolymers. 59:51-64.
McPherson, David T., Jie Xu, and Dan W. Urry. 1996. “Product Purification by Reversible Phase Transition Following E. coli Expression of Genes Encoding up to 251 Repeats of the Elastomeric Pentapeptide GVGVP.” Protein Expression and Purification 7:51-57.
Nicol, Alastair, D. Channe Gowda, Timothy M. Parker, and Dan W. Urry. 1994. “Cell Adhesive Properties of Bioelastic Materials Containing Cell Attachment Sequences.” Pp. 95-113 in Biotechnol. Bioactive Polym. Charles G. Gebelein and Charles E. Carraher, Jr., eds. New York: Plenum Press.
Nicol, Alastair, D. Channe Gowda, Timothy M. Parker, and Dan W. Urry. 1993. “Elastomeric Polytetrapeptide Matrices: Hydrophobicity Dependence of Cell Attachment from Adhesive, (GGIP)n, to Non-adhesive, (GGAP)n, Even in Serum.” J. Biomed. Mater. Res. 27:801-810.
Patkar, Anant, Natarajan Vijayasankaran, Dan W. Urry, and Friedrich Srienc. 2002. “Flow Cytometry as a Useful Tool for Process Development: Rapid Evaluation of Expression Systems.” Journal of Biotechnology. 93:217-229.
Strzegowski, Luke A., Manuel Bueno Martinez, D. Channe Gowda, Dan W. Urry, and David A. Tirrell. 1994. “Photomodulation of the Inverse Temperature Transition of a Modified Elastin Poly (Pentapeptide),” Journal of the American Chemical Society. 116:813-814.
Urry, D. W., A. Nicol, D. C. Gowda, L. D. Hoban, A. McKee, T. Williams, D. B. Olsen, and B. A. Cox. 1993. “Medical Applications of Bioelastic Materials.” Pp. 82-103 in Biotechnological Polymers: Medical, Pharmaceutical and Industrial Applications. Charles G. Gebelein, ed. Atlanta: Technomic Publishing Company, Inc.
Urry, D. W., D. C. Gowda, S. Q. Peng, T. M. Parker, and R. D. Harris. 1992. “Design at Nanometric Dimensions to Enhance Hydrophobicity-induced pKa Shifts.” Journal of the American Chemical Society. 114:8716-8717.
Urry, Dan W., Larry C. Hayes, D. Channe Gowda, Cynthia M. Harris, and R. Dean Harris. 1992. “Reduction-driven Polypeptide Folding by the ∆Tt Mechanism.” Biochem. Biophys. Res. Comm. 188:611-617.
Urry, Dan W. 1993. “Bioelastic Materials as Matrices for Tissue Reconstruction.” Pp. 199-206 in Tissue Engineering: Current Perspectives. Eugene Bell, ed. New York: Birkhäuser Boston, Div. Springer-Verlag.
Urry, Dan W., Larry C. Hayes, Timothy M. Parker and R. Dean Harris. 1993. “Baromechanical Transduction in a Model Protein by the ∆Tt Mechanism.” Chem. Phys. Letters. 201:336-340.
Urry, Dan W., ShaoQing Peng and Timothy M. Parker. 1993. “Delineation of Electrostatic-and Hydrophobic-Induced pKa Shifts in Polypentapeptides: The Glutamic Acid Residue.” Journal of the American Chemical Society. 115:7509-7510.
Urry, Dan W., D. Channe Gowda, Betty A. Cox, Lynne D. Hoban, Adam McKee and Taffy Williams. 1993. “Properties and Prevention of Adhesions Applications of Bioelastic Materials.” Mat. Res. Soc. Symp. Proc. 292:253-264.
Urry, Dan W., ShaoQing Peng, Timothy M. Parker, D. Channe Gowda, and Roland D. Harris. 1993. “Relative Significance of Electrostatic- and Hydrophobic-Induced pKa Shifts in a Model Protein: The Aspartic Acid Residue.” Angew. Chem. (German). 105:1523-1525; Angew. Chem. Int. Ed. Engl. 32:1440-1442.
Urry, Dan W., D. Channe Gowda, Cynthia M. Harris and R. Dean Harris. 1994. “Bioelastic Materials and the ∆Tt-Mechanism in Drug Delivery.” Pp. 15-28 in Polymeric Drugs and Drug Administration. Raphael M. Ottenbrite, ed., American Chemical Society Symposium Series 545:15-28.
Urry, Dan W., D. Channe Gowda, ShaoQing Peng, Timothy M. Parker, Naijie Jing, and R. Dean Harris. 1994. “Nanometric Design of Extraordinary Hydrophobicity-induced pKa Shifts for Aspartic Acid: Relevance to Protein Mechanisms.” Biopolymers. 34:889-896.
Urry, Dan W., Shaoqing Peng, D. Channe Gowda, Timothy M. Parker, and R. Dean Harris. 1994. “Comparison of Electrostatic- and Hydrophobic-induced pKa Shifts in Polypentapeptides: The Lysine Residue.” Chemical Physics Letters. 225:97-103.
Urry, Dan W. 1994. “Conversion of Available Energy Forms into Desired Forms by a Biologically Accessible Mechanism.” Pp. 629-636 in Nondestructive Characterization of Materials VI. Robert E. Green, Jr., ed. Proceedings of the Sixth International Symposium on Nondestructive Characterization of Materials, Oahu, Hawaii. New York: Plenum Press.
Urry, Dan W. 1994. “Biophysics of Energy Converting Model Proteins.” Mat. Res. Soc. Symp. Proc. 321-332.
Urry, Dan W., Alastair Nicol, David T. McPherson, Jie Xu, Peter R. Shewry, Cynthia M. Harris, Timothy M. Parker, and D. Channe Gowda. 1995. “Properties, Preparations and Applications of Bioelastic Materials.” Pp. 1619-1673 in Encyclopedic Handbook of Biomaterials and Bioengineering—Part A—Materials, Vol. 2. New York: Marcel Dekker, Inc.
Urry, Dan W., David T. McPherson, Jie Xu, D. Channe Gowda, and Timothy M. Parker. 1995. “Elastic and Plastic Protein-based Polymers: Potential for Industrial Uses,” Pp. 259-281 in Industrial Biotechnological Polymers. Chas. Gebelein and Chas. E. Carraher, Jr., eds. Lancaster, PA: Technomic Publshing Co.
Urry, D. W. 1994. “Postulates for Protein (Hydrophobic) Folding and Function.” International Journal of Quantum Chemistry. 21:3-15.
Urry, Dan W. and Chi-Hao Luan. 1995. “A New Hydrophobicity Scale and Its Relevance to Protein Folding and Interactions at Interfaces.” Pp. 92-110 in Proteins at Interfaces 1994. Thomas A. Horbett and John L. Brash, eds. American Chemical Society Symposium Series: Washington, DC.
Urry, Dan W. 1995. “Elastic Biomolecular Machines: Synthetic chains of amino acids, patterned after those in connective tissue, can transform heat and chemical energy into motion.” Scientific American. January, 64-69.
Urry, Dan W., Larry C. Hayes, and D. Channe Gowda. 1994. “Electromechanical Transduction: Reduction-driven Hydrophobic Folding Demonstrated in a Model Protein to Perform Mechanical Work.” Biochemical and Biophysical Research Communications. 204:230-237.
Urry, Dan W., Chi-Hao Luan, and ShaoQing Peng. 1995. “Molecular Biophysics of Elastin Structure, Function and Pathology.” Pp. 4-30 in Proceedings of The Ciba Foundation Symposium No. 192. The Molecular Biology and Pathology of Elastic Tissues. Sussex, UK: John Wiley & Sons, Ltd.
Urry, Dan W., D. T. McPherson, J. Xu, H. Daniell, C. Guda, D. C. Gowda, Naijie Jing, and T. M. Parker. 1996. “Protein-Based Polymeric Materials: Syntheses and Properties” Pp. 7263-7279 in The Polymeric Materials Encyclopedia: Synthesis, Properties and Applications. Boca Raton, FL: CRC Press.
Urry, D. W., C.-H. Luan, C. M. Harris, and T. Parker. 1997. “Protein-based Materials with a Profound Range of Properties and Applications: The Elastin Tt Hydrophobic Paradigm.” Pp. 133-177 in Proteins and Modified Proteins as Polymeric Materials. Kevin McGrath and David Kaplan, eds. Birkhauser Press.
Urry, D. W., Cynthia M. Harris, Chi Xiang Luan, Chi-Hao Luan, D. Channe Gowda, Timothy M. Parker, ShaoQing Peng, and Jie Xu. 1997. “Transductional Protein-based Polymers as New Controlled Release Vehicles,” Part VI: New Biomaterials for Drug Delivery. Pp. 405-437 in Controlled Drug Delivery: The Next Generation. (Kinam Park, ed. Am. Chem. Soc. Professional Reference Book.
Urry, Dan W., D. Channe Gowda, ShaoQing Peng, and Timothy M. Parker. 1995. “Non-linear Hydrophobic-induced pKa Shifts: Implications for Efficiency of Conversion to Chemical Energy.” Chemical Physics Letters. 239:67-74.
Urry, Dan W., Larry C. Hayes, D. Channe Gowda, ShaoQing Peng, and Naijie Jing. 1995. “Electrochemical Transduction in Elastic Protein-based Polymers.” Biochem. Biophys. Res. Commun. 210:1031-1039.
Urry, Dan W. and ShaoQing Peng. 1995. “Non-linear Mechanical Force-induced pKa Shifts: Implications for Efficiency of Conversion to Chemical Energy.” Journal of the American Chemical Society 8478-8479.
Urry, Dan W. and Chi-Hao Luan. 1995. “Proteins: Structure, Folding and Function.” Pp. 105-182 in Bioelectrochemistry: Principles and Practice. Giorgio Lenaz, ed. Basel, Switzerland: Birkhäuser Verlag AG.
Urry, Dan W., Asima Pattanaik, Mary Ann Accavitti, Chi-Xiang Luan, David T. McPherson, Jie Xu, D. Channe Gowda, Timothy M. Parker, Cynthia M. Harris, and Naijie Jing. 1997. “Transductional Elastic and Plastic Protein-based Polymers as Potential Medical Devices.” Pp. 367-386 in Handbook of Biodegradable Polymers. Domb, Kost, and Wiseman, eds. Chur, Switzerland: Harwood Academic Publishers.
Urry, Dan W., Larry C. Hayes, and Shao Qing Peng. 1996. “Designing for Advanced Materials by the Tt-Mechanism.” SPIE—The International Society for Optical Engineering Smart Structures and Materials. San Diego, CA: Smart Materials Technologies and Biomimetics 2716, 343-346.
Urry, Dan W. 1996. “Engineers of Creation.” Pp. 39-42 in Chemistry in Britain.
Urry, Dan W., ShaoQing Peng, Jie Xu, and David T. McPherson. 1997. “Characterization of Waters of Hydrophobic Hydration by Microwave Dielectric Relaxation.” Journal of the American Chemical Socitey. 119:1161-1162.
Urry, Dan W. 1997. “On the Molecular Structure, Function and Pathology Of Elastin: The Gotte Stepping Stone.” Pp. 11-22 in The Structure, Function and Pathology of Elastic Tissue. Potenza, Italy: Tip. Mario Armento & Co. Publisher.
Urry, Dan W. and Asima Pattanaik. 1997. “Elastic Protein-based Materials in Tissue Reconstruction.” Annals of the New York Academy Sciences. 831:32-46.
Urry, D. W., S. Q. Peng, L. C. Hayes, D. T. McPherson, Jie Xu, T. C. Woods, D. C. Gowda, and A. Pattanaik. 1998. “Engineering Protein-based Machines to Emulate Key Steps of Metabolism (Biological Energy Conversion).” Biotechnology and Bioengineering. 58:175-190.
Urry, Dan W. 1997. “Physical Chemistry of Biological Free Energy Transduction as Demonstrated by Elastic Protein-based Polymers.” J. Phys. Chem. 101:11007-11028.
Urry, Dan W. 1999. “Five Axioms for Protein Engineering: Keys for Understanding Protein Structure/Function?” Pp. 75-78 in Proceedings of the Fifteenth American Peptide Symposium, Peptides: Frontiers of Peptide Science. James P. Tam and Pravin T. P. Kaumaya, eds. Boston: Kluwer Academic Publishers.
Urry, Dan W., ShaoQuing Peng, Chi-Hao Luan, Chi-Xiang, Luan, Asima Pattanaik, Jie Xu, David T. McPherson. 1998. “Hydrophobic Hydration in Protein Models for Muscle Contraction: Calcium Ion, Thermal, Stretch and pH Activation.” Scanning Microscopy International.
Urry, Dan W. 1998. “Five Axioms for the Functional Design of Peptide-Based Polymers as Molecular Machines and Materials: Principle for Macromolecular Assemblies.” Biopolymers (Peptide Science) 47:167-178.
Urry, Dan W., Asima Pattanaik, Jie Xu, T. Cooper Woods, David T. McPherson, and Timothy M. Parker. 1998. “Elastic Protein-based Polymers in Soft Tissue Augmentation and Generation.” J. Biomater. Sci. Polymer Edn. 9:1015-1048.
Urry, Dan W. 1999. “Elastic Molecular Machines in Metabolism and Soft Tissue Restoration.” TIBTECH. 17:249-257.
Urry, Dan W., Larry Hayes, Chixiang Luan, D. Channe Gowda, David McPherson, Jie Xu, and Timothy Parker. 2001. “∆Tt-Mechanism in the Design of Self-Assembling Structures.” Pp. 323-340 in Self Assembling Peptide Systems in Biology, Medicine and Engineering. Amalia Aggeli and Neville Boden, eds. Netherlands: Kluwer Academic Publishers.
Urry, D. W., T. Hugel, M. Seitz, H. Gaub, L. Sheiba, J. Dea, J. Xu and T. Parker. 2002. “Elastin: A Representative Ideal Protein Elastomer.” Phil. Trans. R. Soc. Lond. B. 357:169-184.
Urry, D. W., T. Hugel, M. Seitz, H. Gaub, L. Sheiba, J. Dea, J. Xu, L. Hayes, and T. Parker. 2002. “Ideal Protein Elasticity: The Elastin Model.” In P. Shewry and A. Bailey, eds. Cambridge: Cambridge University Press.
Urry D. W. and T. M. Parker. 2002. “Mechanics of Elastin: Molecular Mechanism of Biological Elasticity and its Relationship to Contraction, Special Issue: Mechanics of Elastic Biomolecules.” Journal of Muscle Research and Cell Motility, 23, 543-559, 2002.
Urry, D. W., T. C. Woods, L. C. Hayes, J. Xu, D. T. McPherson, M. Iwama, M. Furuta, T. Hayashi, M. Murata, and T. M. Parker. 2004. “Elastic Protein-Based Biomaterials: Elements of Basic Science, Controlled Release and Biocompatiblity.” Pp. 31-54 in Tissue Engineering and Novel Delivery Systems. New York: Marcel Dekker, Inc.
Urry, D. W., J. Xu, W. Wang, L. Hayes, F. Prochazka, and T. M. Parker. 2003. “Development of Elastic Protein-based Polymers as Materials for Acoustic Application.” Mat. Res. Soc. Symp. Proc. 774:81-92.
Wang, N. Z., D. W. Urry, S. F. Swaim, R. L. Gillette, C. E. Hoffman, S. H. Hinkle, S. L. Coolman, C.-X. Luan, J. Xu, and B. W. Kemppainen. 2004. “Skin concentrations of thromboxane synthetase inhibitor after topical application with bioelastic membrane.” J. Vet. Pharmacol. Therap. 27:37-43.
Zhang, X., C. Guda, R. Datta, R. Dute, D. W. Urry, and H. Daniell. 1995. “Nuclear Expression of an Environmentally Friendly Synthetic Protein-based Polymer Gene in Tobacco Cells.” Biotech Letters. 17(12):1279-1284.
Zhang, X., D. W. Urry, and H. Daniell. 1996. “Expression of an Environmentally Friendly Synthetic Protein-based Polymer Gene in Transgenic Tobacco Plants.” Plant Cell Reports. 16:174-179.
Cambridge NeuroScience9
Paula Stephan
Georgia State University
DESCRIPTION OF THE FIRM
Cambridge NeruoScience (CNS) was incorporated in Delaware in December 1985 and began operations in January 1986 in Cambridge, MA where it remained until 2000, when the firm relocated to Norwood, MA.
The company’s focus involved the discovery and development of pharmaceutical products to treat a variety of severe neurological and psychiatric disorders. Product development was focused in three areas: neuroprotective compounds for the treatment of acute neurological disorders such as stroke and traumatic brain injury, novel antipsychotic compounds for the treatment of mental illnesses such as schizophrenia, and growth factors for the treatment of neurodegenerative diseases such as diabetic peripheral neuropathies, and ALS (Prospectus, Initial Public Offering, June 6, 1991, page 10). As the company grew and advanced its technology, research and development programs in multiple sclerosis and neuropathetic pain were added to the portfolio.
The company had a number of collaborations with academic institutions at the time that it went public in 1991. These included, for example, the Ludwig Institute for Cancer Research in London, the University of Oregon, Cornell University, the Medical College of Virginia, the University of Toledo, Columbia University and the Massachusetts Institute of Technology.
The company raised venture capital from Warburg, Pincus Capital Partners and Aeneas Venture Corporation and, as noted above, made an initial public offering in 1991 which generated $22,320,000 in proceeds for the company. Warburg, Pincus provided the initial funds (approximately $5 million) to start the company.
The company states in its prospectus that it had 44 full-time employees at the time of its initial public offering in 1991; 36 of these employees were engaged in research and development.
The company faced considerable competition both from start-ups and from fully integrated pharmaceutical companies in the neuroscience pharmaceutical market. At the same time, the neurological market has significant growth potential. For example, the number of cases of Alzheimer’s disease is rapidly growing
as life expectancy increases; and the population of individuals suffering from strokes is large and growing. Moreover, there are virtually no products that successfully treat strokes. Strokes are, according to Dr. Marchionni, “a graveyard for compounds in development.”
The Cambridge location was chosen because of the location of the three co-founders: Joe Martin, M.D., Ph.D., Chairman of Neurology, Massachusetts General Hospital and Professor of Neurology at Harvard Medical School at the time the company was founded; Howard M. Goodman, Ph.D., Chief of the Department of Molecular Biology at Massachusetts General Hospital and Professor of Genetics at Harvard Medical School; and Rod Moorhead, of Warburg, Pincus Capital Partners. Dr. Goodman was a board member of Warburg, Pincus Capital partners at the time the company was started.
Cambridge NeuroScience was acquired by CeNeS Pharmaceuticals, Inc., a company listed on the London Stock Exchange, in December of 2000; CeNeS closed operations of Cambridge NeuroScience in January of 2002.
INPUTS
The company received at least 13 SBIRs during its 15 years in existence. With but one exception, the company followed up each Phase I with a successful Phase II. In one instance, the company chose not to follow up with a Phase II application due to the limited success in demonstrating feasibility of the approach in Phase I. The first SBIR award was applied for soon after the company was founded. The company was in the process of being awarded an SBIR Phase II at the time that it was acquired by CeNeS but did not receive the actual funding because, by the time it received the Notice of Grant Award, it had been acquired by a foreign-owned company.
Although SBIRs played no role in the founding of the company, “right off the bat” the company applied for SBIR funding. This was facilitated by the fact that from the beginning, with seed money from venture capitalists, the company was able to hire a team of fairly experienced staff scientists. While none had SBIR experience, all had worked in postdoc positions and understood something about the culture of grant writing.
The company received no funding from the state of Massachusetts. In addition to receiving SBIR support from the federal government, one of its scientists, Dr. Stanley Goldin, was able to transfer an R01 from NIH to Cambridge NeuroScience when he came to Cambridge NeuroScience from Harvard Medical School. Since his area of research was of interest to the company, this arrangement was of mutual benefit and he was able to continue his research program with the aid of NIH support.
SBIRs played a role in external partners’ decisions to provide funding in two ways. First, the award of an SBIR was treated by the company as a marketing tool, with the company issuing a press release at the time of the award. Second,
and more meaningfully, SBIR grant proposals were often given to potential partners through confidentiality agreements to provide a detailed description of the science that was being done at Cambridge NeuroScience, allowing the potential partner to do due diligence on scientific elements. A funded grant, according to Dr. Marchionni, “says here is a group of disinterested reviewers who have decided to say we are going to invest tax payers money to try to move this forward; we think it has a reasonable chance of success. The independent endorsement carries some weight. It’s probably worth more than what you get paid to do the research.” It should also be noted that SBIR funding was discussed in the prospectus for the IPO as a source of revenue.
The company had an extremely aggressive hiring plan when it started, and had 16 to 18 staff scientists by the time it received its first SBIR award.
SBIRs definitely helped the company grow in terms of hiring. Dr. Marchionni, for example, was able to add people to his group because of the grant. “It helped fortify a group that would have been half the size if it did not have the grant.” Moreover, the grants allowed for “growing” a work force, not only in size, but also in skill, by permitting the company to bring individuals on board and then invest in training them. “Because they were on board I could train them in advanced molecular skills.” This meant that when they came to another project in which the company had a greater corporate investment and which went beyond the grant, “these people were ready to go.” As a result, “we were able to compete in a high-stakes game; we were able to compete against Genentech in essence. If I had had to wait until the time that we got into the growth factor work to hire people, the whole project would have been taken over by Genentech before we were ever able to get into it.”
The hiring and training that result from SBIRs allow the company to take a longer-run view than it would have taken without such funding. While company management focuses on immediate returns to shareholders, SBIRs permit the company to focus on things that don’t necessarily have an immediate return to shareholders. The grant allows the company to “hire on new employees who will have an impact that goes long beyond the period of the grant.” It provides the company enough flexibility to hire individuals and create a critical mass of a sort.
KEY OUTCOMES FROM SBIR
Commercial Outcomes
The company never had a product that generated revenue through sales. However, the ion channel patents were bought by Scion and, as part of the deal, an SBIR Phase II award relating to ion channels moved, with the PI, from Cambridge NeuroScience to Scion. Scion also bought the chemical library of CNS.
The company had one drug that made it to Phase III Clinical Trials for the
treatment of stroke and TBI. The drug (CERESTAT, or aptiganel hydrachloride) did not show efficacy during Phase III and the trials were terminated, both for the treatment of stroke and for TBI. “It was really close … it is possible that if the trial were rerun and using more narrow inclusion criteria, it might work.” SBIR funding was involved at some early stage of the work that developed CERESTAT.
Many biotech companies choose to focus on niche markets. The stroke market, by way of contrast, is not a niche market and if the trials had proved successful the company could have anticipated considerable commercial success. “We appeared on local television news; we were on the verge of making a major breakthrough in treatment for stroke. It really looked like it was going to work.” The company’s stock fell by something like 80 percent when the trail was discontinued. “Once that happened, the company died a slow death.”
A consistent theme during the interview was that SBIR awards are not appropriate for the “front runner of the company.” Those projects should be funded primarily by the company. But SBIR awards permit the company to enhance the projects it is doing, providing the opportunity for greater depth, thus improving the chances of success. SBIRs also allow a company to diversify. Stated somewhat differently, companies are under pressure to convert their dollars to a profit; the pace of research on the “front runner” is too fast to permit for the grant approach. The SBIR program is relatively slow: eight months at least before the money comes in. “You can’t wait that long; things happen too fast; you risk losing out to your competition if it is the lead program in the company.”
Dr. Marchioni elaborated on this theme, saying that SBIRs provide a relatively successful company two specific benefits. First, SBIR awards allow a company to round out research on the lead program that it cannot fully support. “You write a grant for something you will be needing six months from now; that will require adding things; you can round out your effort through the grant by making it a fuller program with a greater chance of success. But the grant funding is not the main source of support. The grant is not driving the company. You have to have other sources of funding to make it work. You use the grant to embellish and enhance the effort, but it’s not the sole source of support.” Second, the SBIR program allows relatively successful companies to explore alternatives that the company might otherwise not be able to explore, providing for diversification. For example, the company acquires an option to technology from a university; the university researchers continue to work on it in the laboratory. The company then can begin to participate directly in this technology by applying for SBIR funding. If successful in obtaining this grant support, then the company is in a strong position to convert the option into a license and start working on it internally in a new area. This approach will grow the scientific base of the company’s technology and accrue benefit to the university as well—technology originally discovered there can be developed further and eventually be commercialized. Diversification provides the company some hedge against risk and creates jobs.
Venture capitalists, to paraphrase Dr. Marchionni, cannot necessarily fund the complete diversification of a company that needs to diversify. Neither do they want to. “Venture Capitalists want you to be extremely focused and often give you one chance to succeed.”
Noncommercial Outcomes
Cambridge NeruoScience had many patents; it is difficult to determine which ones specifically related to SBIR grants. An example of a patent that was clearly based on SBIR research is patent 6,232,061 issued May 15, 2001 for homology cloning. Scientists working at CNS published a considerable number of scientific papers; some were based on research funded through the SBIR program.
IMPACT OF SBIR ON THE FIRM
The SBIR program clearly contributed to the research productivity of Cambridge NeuroScience. It allowed the company to hire and train additional researchers, engage in more in-depth research for front runner programs and diversify its research efforts.
SBIR PROGRAM ADMINISTRATION
Although none of the scientific staff had prior SBIR experience, from the time the company started the research scientists were aware of the SBIR program.
The company did not experience problems related to the delay between Phase I and Phase II awards. Dr. Marchionni indicated that they were aware that the SBIR grant did not pay for everything and that they realized they had to have other sources of income to support research. He also noted that once the project took on “front burner” status in the company, the company was no longer interested in using grants to support the research because the time required to write the grant application detracted from the research effort.
He expressed the view that the perfect SBIR company is a Series A company or a company with angel backing. Such companies have sufficient funds to take advantage of the grant and make full use of it, but not be overly concerned about what is going to happen when they lose it for a period of time. “If SBIR income is the only income you have, SBIR money is being wasted.”
Dr. Marchionni viewed much of the review and selection process to be fair. The company chose a strategy of including detailed data in the grant applications. They knew they were competitive and they didn’t want to provide an excuse for not getting funding. They also thought that the chances that disclosure would cause a problem were “pretty minimal.” “In the end, there was never a single thing that we disclosed that someone else took over and started working on.”
Dr. Marchionni saw serious problems in two aspects of the review process:
the composition of the panel, which is largely made up of academics, and the panel’s interest in the proposed budget. Academic reviewers often employ criteria that apply to RO1 proposals in reviewing SBIRs, which is not appropriate. Second, many of them fail to fully appreciate the entire process of (bio)technology development, even though many serve as consultants to industry. Many have expressed concerns that were completely out of sequence with standard industry practices. For example, some issues that might come into play in the design of clinical studies simply do not need to be addressed until you begin to get there by overcoming many other, more immediate, hurdles along the way. To use a baseball analogy, a first-base coach does not have to be concerned about whether a runner should slide into third on a triple. As the runner approaches third base, he will get instructions from the third-base coach.
Moreover, some academics have a bias towards SBIR proposals that are written by fellow academics and try to “create the rules so that the funding goes to academics.” Dr. Marchionni recommends including more individuals from industry in the review process. While he recognizes that this could create a potential conflict of interest, in today’s world it is reasonably common for academic reviewers to have ties to industry and thereby they, too, could have a conflict of interest. He noted that there is not that big a divide between what it takes to be a successful scientist in a company and a successful scientist in academe.
A second concern that Dr. Marchionni expressed relates to the fact that it is not uncommon to get comments back from review relating to the appropriate size of the submitted budget. He argues that this is inappropriate. Just as only the direct portion of the budget is subject to review for R01 grants, only the direct portion of costs should be considered for review in the SBIR proposal. The other costs should be made “invisible,” just as indirect costs are made invisible on R01 proposals.
Dr. Marchionni had two specific recommendations for changes to the SBIR program. The first concerns the recent VC rule which states that a company that is majority-owned by VC is not eligible for SBIR awards. This rule excludes firms that, in his view, are strong candidates for SBIR awards, not needing to rely exclusively on SBIR awards for funding, but using the SBIR awards to enhance and diversify the research of the company. Both activities result in the creation of new jobs. Dr. Marchionni points out that if the VC rule had been in effect at the time that Cambridge NeuroScience was founded, the company would never have received an SBIR award. (See comments by Dr. Marchionni at <http://www.zyn.com/sbir/articles/vc/vc-8.htm> and reprinted in the annex.) Neither would it have created the number of research jobs that it did during the late 1980s and early to mid 1990s.
He also questions the ruling that the company was ineligible for SBIR funding once it had been bought by a British firm, noting that the proposed research was to be done in the United States. In his view, SBIR funding contributes to
the employment of life scientists who, especially in recent times, have found it difficult to find employment in research environments.
CROSS-CUTTING RESEARCH QUESTIONS
Dr. Marchionni sees the SBIR program as providing a “signal” to external players, such as firms that the company may be working with to form an alliance. He consistently made the case that the SBIR program affects firm performance through the enhancement of its front-burner programs and the ability to diversity its research portfolio. Moreover, it allows the firm to hire new scientists, thereby creating jobs and providing training consistent with the firm’s research agenda. He also noted that certain research areas within the firm were not appropriate for SBIR funding, moving at too fast a pace to write a grant for the research.
CAMBRIDGE NERUOSCIENCE—ANNEX
Statement by Mark Marchionni, Ph.D.
I wish to submit the following comments on the SBIR eligibility requirements pertaining to company ownership by individuals.
The SBIR program has made possible the growth and survival of emerging companies for decades. In helping to create new jobs and advance innovative technology this program has been an essential part of growing and maintaining a vibrant and competitive economy in the United States.
The National Institutes of Health has administered an SBIR program for decades, and this source of funding has been instrumental to emerging companies in a growing biotechnology industry. Many of these companies have been owned in part or were started by venture capital firms. From my personal experience, I was one of the first scientific staff members to join Cambridge NeuroScience, Inc. (CNSI) in 1987, shortly after the company was started by the venture capital firm Warburg Pincus. Prior to the initial public offering, Warburg retained majority ownership of CNSI and we submitted and were funded for more than 10 NIH SBIR grants (five Phase I and five Phase II). I was the Principal Investigator on two such grants. These funds were critical in creating the jobs to grow the company from 18 (when I joined) to more than 60 at the time of the IPO. By virtue of this SBIR support, several product candidates advanced into clinical trails or were partnered with major pharmaceutical companies as part of Phase III commercialization. Thus, not only has it been accepted practice for the NIH SBIR program to support the growth of emerging biotechnology companies, but it has helped to accomplish the mission of the NIH and create new jobs as well.
At the current time, I submit that the Small Business Administration and the NIH need to support the growth of new jobs in this essential economic sector—biotechnology. Many have lost their jobs in the recent economic decline
and tax revenues need to be spent wisely to stimulate job growth. Biotechnology represents a sector of the economy where the U.S. has a clear competitive advantage, and provides an important opportunity to revitalize economic growth. Further, a very sizeable percentage of all drugs produced in the past decade have been discovered at small companies. Since companies that are controlled by venture capital firms often represent some of the more innovative and competitive start-ups, policies that support collaboration between these companies and government agencies would represent prudent and productive use of tax revenues. It is these very companies that have the greatest chance of advancing their technology through to commercialization. Therefore, I offer my very strong support for producing a wording of the eligibility requirements that would enable the NIH to include in the SBIR program emerging companies that are majority owned by venture capital firms.
CryoLife10
Paula Stephan
Georgia State University
DESCRIPTION OF THE FIRM
CryoLife was founded in 1984 by Steven G. Anderson and Robert McNally. Prior to founding the company, Anderson worked at Intermedics, which is now Guidant. CryoLife was founded to commercialize the cryopreservation of human allograft heart valves. The initial technology was “out there,” coming from the University of Alabama Birmingham, which had a cryopreservation lab for allograft heart valves, but was not trying to distribute valves or make the technology widely available. The company has had revenue almost from its inception and had a profit beginning around 1986. Anderson serves as Chairman, President and Chief Executive Officer of the company; McNally is no longer with the company.
CryoLife is located in Kennesaw, Georgia. It is situated in a dedicated building, the second phase of which was completed approximately three years ago. The firm, though incorporated in Florida, has only operated in Georgia. A major factor in the decision to locate in the Atlanta area was the need to be near a large, busy airport, given that the firm deals with human tissue.
CryoLife employs approximately 325 individuals, 10 of whom have a Ph.D. Sales for the year ending 2003 were approximately $60 million; sales had been approximately $88 million for the year ending 2001 (see discussion below).
The firm has developed proprietary processes for the preservation of human heart valves, veins and connective tissue. The firm has developed a tissue engineered heart valve and vascular graft replacement called SynerGraft® Valve and Synergraft® Vascular; the firm also has developed a bioadhesive product, BioGlue® Surgical Adhesive (hereafter referred to as “BioGlue”).
The firm faces some competition in the human tissue business, from both the profit and the not-for-profit sector. BioGlue® is approved for sales in approximately 50 countries. “In August of 2002 the firm received an order from the Atlanta district office of the FDA regarding the nonvalved cardiac, vascular, and
orthopedic tissue processed by the company since October 3, 2001.”11 Nonvalved cardiac and vascular tissues processed after September 5, 2002 were not subject to the FDA order. Revenue from tissue declined subsequent to this order.
INPUTS
The firm has received an undetermined number of SBIR awards. One database (which counts awards since 1993) says 13; another indicates 12 and excludes awards after 1997. The company reports that they have received several awards with dates subsequent to 1997. Of the 12 in the NAS database, seven were Phase I and five were Phase II. The last SBIR award that the company received was in 2002; the company currently has a Phase I award application pending. Two of the awards have been to the company’s subsidiary, AuraZyme. All SBIR awards have been from NIH although not all awards have come from the same institute at NIH.
The SBIR program did not play a role in the founding of the company. The company was six years old and had 50 to 70 employees at the time that it received its first SBIR award. The current Director of Tissue Technologies, Dr. Steven Goldstein, was hired in 1991 to work on the first SBIR award. It is unclear how the company initially found out about the SBIR program, although it is thought that the Director of Research at that time knew about the program. He is no longer with the company.
The company has received funding from several sources in addition to the SBIR program. Specifically, prior to going public, the company had private investors. It has also received a contract from the Office of Naval Research and NIST funding through the ATP program. The company has received no support from the State of Georgia.
The firm went public in 1993, raising approximately $15,500,000 at that time. The company currently trades on the New York Stock Exchange. SBIR funding contributed to the success of the public offering in the sense that it helped the company initiate development of a diversified product mix by the time the company went public. For example, SBIR funding helped in the development of the vascular product that the company had at the time it went public.
KEY OUTCOMES FROM SBIR
The company reports that the SBIR program has proven key to funding the research and development behind almost all new products of the company, and the company continues to see SBIR funding as an important source of research funding. To quote one of the scientists, “SBIR funding has basically been our external source of funding when we want to build a new product.”
The company has used the SBIR program to develop proprietary property that it has purchased. BioGlue® is a case in point. The BioGlue® patent was purchased but CryoLife used SBIR funds to develop concepts from the patent. AuraZyme provides another example. While the patents for AuraZyme technologies were filed before the SBIR grants were awarded, the grants have provided resources to solidify the technology and provide in vivo testing results for proof of principle.
The company holds a number of patents. While some were received subsequent to receipt of SBIR funding, in other instances SBIR funding provided the resources to “solidify the technology” and verify the facts, as noted above. The company currently has at least one pending patent application related to an SBIR award.
Company scientists have a history of publishing. Examples of articles and presentations that have been published as a result of SBIR funding include:
-
Paper: Gilbert CW, McGowan EB, Seery GB, Black KS, Pegram MD. Targeted prodrug treatment of HER-2-positive breast tumor cells using trastuzumab and paclitaxel linked by A-Z-CINN Linker. J. Exp. Ther Oncol. 2003, Jan-Feb: 3(1), 27-35; supported by NCI grant 1R43CA95937-01.
-
Poster: from 1R43CA95937-01; Proceeding of the American Association for Cancer Research 43, #2061, pg. 414, 2002, Gilbert CW, McGowan EB, Black KS, Pegram MD, Seery GB. “Efficacy testing of targeted drug delivery using A-Z-CINN Linker in vivo: a model for a single treatment cancer cure; supported by NCI grant 1R43CA95937-01.
-
Poster, American Heart Association 4th Annual Conference on Arterio-sclerosis, Thrombosis, and Vascular Biology, Washington, DC; May 8-10, 2003, Poster P112. McGowan EB, Gilbert CW, Black KS. AZ-Plasmin, a Novel Thrombolytic Agent for Treatment of Vascular Occlusions.
A list of published scientific papers and abstracts presented related to SynerGraft SBIR grants are given in an annex at the end of this paper.
The company holds four trademarks (BioGlue®, Synergraft®, AZ-CINN™, AuraZyme™). In three instances the trademarks are associated with products for which SBIR funding has been received. In no instance was SBIR funding used for the initial research. The fourth trademark is the name of a wholly owned subsidiary of CryoLife.
IMPACT OF SBIR ON THE FIRM
The company estimates that SBIR funding has provided 20 percent to 25 percent of all research revenue from external sources. Alternative sources for research include revenue from company sales and NIST ATP awards. The company sees SBIR awards as a continuing source of research and development support.
SBIR PROGRAM ADMINISTRATION
The company has not always chosen to follow up a Phase I with a Phase II application. In the case of BioGlue®, for example, it was decided not to apply for Phase II because the application might have required the company to disclose too much. In another instance, the research objective changed in the middle of the Phase I award. In still other instances, the aggressive timeline of the company requires that they get the project finished before a Phase II grant would become available. As was noted several times in the discussion, the company has a revenue stream from sales which helps to support research and development. It was also noted that there have been instances where the company has submitted a Phase II grant that has not been funded.
Research scientists expressed reasonable satisfaction with the way in which the SBIR program is currently structured, which now allows for larger and longer Phase Is. They see these changes as a significant improvement. A concern was expressed, however, by one scientist that Phase I awards involving animal studies are difficult to accomplish in a short period of time given the amount of paper work that is required to conduct animal studies. Another scientist stated that the duration of Phase I awards, while generally not a problem for a company the size of CryoLife, was really too short for a one or two-person company. AuraZyme is a two-person company that is fortunate to be “nested” within CryoLife’s corporate structure, but still suffers from these restraints. The short time frame “pushes them to the edge.” Small companies simply don’t have the time to get the space and the equipment and perform the research in the traditional Phase I time frame of six months. Another scientist expressed frustration over the amount of time required between submission and the time of the award. To quote the scientist, “You can have a baby in the amount of time it takes.”
One of the PIs indicated that she had benefited from the advice, and Web site, of Dr. Gregory Milman, Director, Office for Innovation and Special Programs, National Institute of Allergy and Infectious Diseases, NIH. Dr. Milman has developed a video entitled “Advice on SBIR and STTR Applications” that one can access at: <http://www.niaid.nih.gov/ncn/sbir/advice/>. The video takes about an hour to listen to. He also provides annotated examples of outstanding Phase I and Phase II applications at: <http://www.niaid.nih.gov/ncn/sbir/app>.
At least one of the PIs has had RO1 experience in the past and commented that the SBIR program would be enhanced if one could receive extension funds like one could with RO1s.
The interviewees viewed the award process as fair; on the other hand they are aware that one is at a disadvantage in writing grants that relate to proprietary information in the sense that the grant application must be vague. Reviewers pick up on this and the scores reflect this. This can be especially a problem in moving forward from a Phase I award to a Phase II application.
Finally, some frustration was expressed that the SBIR program is becoming increasingly competitive as the number of applications from university faculty
members’ labs increases. The hypothesis expressed was that faculty are increasingly creating companies to apply for SBIR funding to support the research of postdocs and research scientists working directly with the faculty on research. Applications from these “garage-model firms” more closely resemble RO1 grant applications and receive higher scores than do SBIR applications from established companies that are working to develop proprietary information. While it was recognized that some successful companies start out as “garage-model firms,” the argument was that in many instances the SBIR program is providing funding for the faculty member’s lab, rather than for a viable commercial enterprise.
CROSS-CUTTING RESEARCH ISSUES
Issues related to disclosure can inhibit a firm’s participation in the SBIR program, or can affect the prospects of a favorable review. The firm also recognizes that at times the SBIR program is too slow to accommodate the aggressive timeline of the firm. As noted above, the SBIR program has proven key to funding the research and development behind almost all new products of the company and the company continues to see SBIR funding as an important source of research funding.
CRYOLIFE, INC.—ANNEX
PUBLISHED SCIENTIFIC PAPERS SYNERGRAFT
IN PREPARATION
10 Year Results with the CryoLife O’Brien Valve. The Journal of Heart Valve Disease. Prof. Hvass.
2004
A Cautionary Case: The Synergraft Vascular Prosthesis. Eur J Vasc Endovasc Surg. 27:42-44. M.A. Sharp, D. Phillips, I. Roberts, L. Hands.
Cellular Remodeling of Depopulated Bovine Ureter Used as an Arteriovenous Graft in the Canine Model. Journal of the American College of Surgeons. 198(5):778-783. J. Matsuura, K. Black, C. Davenport, C. Goodman, N. Pagelsen, A. Levitt, D. Rosenthal, E. Wellons, M. Fallon, J. Ollerenshaw
2003
Congenital Abdominal Aortic Aneurysm: A Case Report. Journal of Vascular Surgery. 38(1):190-193. P. Bell, C. Mantor, M. Jacocks. [Article featuring use of a SynerGraft allograft.]
Decellularized Pulmonary Homograft (SynerGraft) for Reconstruction of the Right Ventricular Outflow Tract: First Clinical Experience. Z Kardiologie. 92(1):53-59. H. Sievers, U. Stierle, C. Schmidtke, M. Bechtel.
Early Failure of the Tissue Engineered Porcine Heart Valve SYNERGRAFT in Pediatric Patients. Eur J Cardiothorac Surg. 23(6):1002-1006. P. Simon, M. Kasimir, G. Seebacher, G. Weigel, R. Ullrich, U. Salzer-Muhar, E. Wolner.
Evaluation of the Decellularized Pulmonary Valve Homograft SynerGraft™ The Journal of Heart Valve Disease. 12:734-740. J. Bechtel, M. Muller-Steinhardt, C. Schmidtke, A. Brunswik, U. Stierle, H.H. Sievers.
Immunogenicity of Decellularized Cryopreserved Allografts in Pediatric Cardiac Surgery: Comparison with Standard Cryopreserved Allografts. J Thorac Cardiovasc Surg. 126(1):247-252. J. Hawkins, N. Hillman, L. Lambert, J. Jones, G. Di Russo, T. Profaize, T. Fuller, L. Minich, R. Williams, R. Shaddy.
Mechanical Heart Valve Prosthess: Identification and Evaluation (Erratum). Cardiovasc Pathol. 12(6):322-344. J. Butany, M. Ahluwalia, C. Monroe, C. Fayet, C. Ahn, P. Blit, C. Kepron, R. Cusimano, R. Leask.
2002
Decellularized Pulmonary Homograft (SynerGraft) For Reconstruction of the Right Ventricle Outflow Tract: First Clinical Experience. Z Kardiol. 92:53-59. H. Sievers, U. Stierle, C. Schmidtke, M. Bechtel.
Decellularized Cadaver Vein Allografts Used for Hemodialysis Access Do Not Cause Allosensitization or Preclude Kidney Transplantation. American Journal of Kidney Diseases. 40(6):1240-1243. R. Madden, G. Lipkowitz, B. Benedetto, A. Kurbanov, M. Miller, L. Bow.
Tissue Restoration and Repair—Application of Cutting-Edge Technologies to Surgical Products. Business Briefing: Medical Device Manufacturing & Technology (Reference Section) [CD-Rom]; K. Black.
2001
Cardiac Tissue Engineering: New Life for Ailing Hearts. The Cardiovascular Watch. 1(8). Remarks by K. Black. March 16.
Decellularized Human Valve Allografts. Ann Thorac Surg. 71:S428-432. R. Elkins, P. Dawson, S. Goldstein, S. Walsh, K. Black.
Humoral Immune Response to Allograft Valve Tissue Pretreated with an Antigen Reduction Process. Seminars in Thoracic and Cardiovascular Surgery. 13(4—Suppl 1):82-86. R. Elkins, M. Lane, S. Capps, C. McCue, P. Dawson.
Recellularization of Heart Valve Grafts (SynerGraft) by a Process of Adap-
tive Remodeling. Seminars in Thoracic and Cardiovascular Surgery. 13(4—Suppl 1):87-92. R. Elkins, S. Goldstein, C. Hewitt, S. Walsh, P. Dawson, J. Oller.
2000
Advances in Heart Valve Surgery. Cardiac Surgery. November-December. [Article featuring SynerGraft Heart Valve.] Source: John E. Mayer, Jr., M.D.
Tissue Engineered Heart Valves. The Journal of Biolaw & Business. 3(2). K. Black.
Transpecies Heart Valve Transplant: Advanced Studies of a Bioengineered Xeno-Autograft. Ann Thorac Surg. 70:1962-1969. S. Goldstein, D. Clarke, S. Walsh, K. Black, M. O’Brien.
1999
The SynerGraft Valve: A New Acellular (Nonglutaraldehyde-Fixed) Tissue Heart Valve for Autologous Recellularization First Experimental Studies Before Clinical Implantation. Seminars in Thoracic and Cardiovascular Surgery. 11(4—Suppl 1):194-200. M. O’Brien, S. Goldstein, S. Walsh, K. Black, R. Elkins, D. Clarke.
1998
Tissue Modifications. Transplantation Proceedings. 30:2729-2731. K. Black, S. Goldstein, J. Ollerenshaw.
1997
Acellular Porcine Pulmonary and Aortic Heart Valve Bioprostheses (book chapter). Pp. 225-233 in Stentless Bioprostheses Second Edition, Isis Medical Media. D. Ross, J. Hamby, S. Goldstein, K. Black.
1994
Tissue-Based Heart Valve Grafts—New Developments. Cardiac Chronicle. 8(3). S. Goldstein, S. Harris.
ABSTRACT PRESENTATIONS SYNERGRAFT
2005
Superior Durability of Synergraft Decellularized Pulmonary Allografts Compared to Standard Cryopreserved Allografts (poster presentation). Soci-
ety of Thoracic Surgeons. Z. Tavakkol; S. Gelehrter; C. S. Goldbert; E. L. Bove; E. J. Devaney; R. G. Ohye.
2004
Aortic Root Replacement with a Novel Decellularized Cryopreserved Aortic Homograft: Postoperative Immunoreactivity and Early Results [poster presentation]. Advances in Tissue Engineering and Biology of Heart Valves. K. Zehr, M. Yagubyan, H. Connolly, S. Nelson, H. Schaff.
Biomechanical Properties of SynerGraft Treated Human Heart Valves and Vascular Grafts [poster presentation]. Advances in Tissue Engineering and Biology of Heart Valves. S. Walsh, P. Dawson, K. Black.
Clinical Outcomes of a Depopulated Bovine Ureter (SynerGraft Vascular Graft Model 100) Used as an Arteriovenous Access Graft [poster presentation]. Advances In Tissue Engineering and Biology of Heart Valves. C. Darby, A. Cornall.
Decellularized Allograft Heart Valves (CryoValve SG)—Early Clinical Results from a Multicenter Registry. Advances in Tissue Engineering and Biology of Heart Valves. D. Clarke, R. Elkins, D. Doty, J. Tweddell.
In Vivo Remodeling and Tissue Engineering of a Novel Decellularized Bovine Ureter Following Implantation as an Arteriovenous Graft [poster presentation]. Advances in Tissue Engineering and Biology of Heart Valves. C. Hewitt, S. Marra, A. DelRossi.
Mid-Term Findings on Echocardiography and Computed Tomography After RVOT-Reconstruction: Comparison of Decellularized (SynerGraft) and Conventional Homografts. Third EACTS/ESTS Joint Meeting. J. M. Bechtel, J. Gellisen, A. W. Erasmi, M. Petersen, U. Stierle, H. H. Sievers.
Multicenter Clinical Outcomes with a Decellularized Porcine Pulmonary Heart Valve (SynerGraft Heart Valve, Model 700) for Reconstruction of the Right Ventricular Outflow Tract. Advances in Tissue Engineering and Biology of Heart Valves. R. Chard, G. Gargiulo, U. Hvass, H. Lindberg, I. Mattila, M. Redmond, L. Segadal, P. Simon.
Performance of Decellularized Bovine Ureter as a Peripheral Vascular Graft in the Dog [poster presentation]. Advances in Tissue Engineering and Biology of Heart Valves. S. Goldstein, J. Matsuura, C. Ponce, K. Sylvester, D. Fronk, K. Black.
2003
A Xenograft for Vascular Access: A New Start to An Old Idea? [poster pre-
sentation]. 3rd International Congress, Vascular Access Society. A. Cornall, C. Darby.
An Underestimated Resource for Difficult Patients. The Lower Limb A-V Fistulas. 3rd International Congress Vascular Access Society. L. Berardinelli, C. Beretta, M. Carini.
Bovine Ureter Grafts: Our Intial Experience. The European Society for Cardiovascular Surgery 52nd Congress. G. Esposito, T. Castrucci, A. Nicoletti, G. Canu, M. Fusari.
CryoLife O’Brien Stentless Aortic Porcine Valve at 10 Years. Society for Heart Valve Disease 2nd Biennial Meeting. U. Hvass, F. Baron, A. Elsebaey, D. Nguyen, E. Flecher.
Early Performance of CryoValve SG Pulmonary Heart Valve Used for the Ross Procedure. Society for Heart Valve Disease 2nd Biennial Meeting. J. Oury, P. Wojewski, D. Doty, D. Oswalt, R. Elkins.
Up to 8 Years Experience with the Pulmonary Autograft in Subcoronary and Root Inclusion Technique. Society for Heart Valve Disease 2nd Biennial Meeting. H. Sievers, U. Stierle, G. Dahmen, C. Schmidtke.
2002
Cellular Remodeling of Depopulated Bovine Ureter Used as an Arteriovenous Graft in the Canine Model. American College of Surgeons 88th Annual Clinical Congress/Surgical Forum. J. Matsuura, E. Wellons, K. Black, J. Ollerenshaw.
CryoVein®SG & CryoArtery®SG: Tissue Engineered Vascular Allografts for AV Access [poster presentation]. American Association of Tissue Banks 26th Annual Meeting. K. Sylvester, S. Capps, D. Fronk.
Depopulated Femoral Vein Allograft as an Arteriovenous Graft in High Risk Patients for Infection. European Society for Cardio-Vascular Surgery. J. Matsuura.
Depopulated Venacaval Homograft: A New Venous Conduit. American Association of Thoracic Surgeons. M. Malas, C. Baker, S. Quardt, M. Barr, W. Wells.
Early Clinical Experience with SynerGraft®for Hemodialysis Access and Peripheral Vascular Disease. European Society for Cardio-Vascular Surgery. B. Yoffe, E. Harah, Y. Leonty.
Immunogenicity of Decellularized Cryopreserved Allografts in Pediatric Cardiac Surgery: Comparison with Standard Cryopreserved Allografts. American Association of Thoracic Surgeons. J. Hawkins, J. Jones, N. Hillman, L. Lamberg, G. DiRusso, T. Profaizer, T. Fuller, R. Shaddy.
In Vivo Resistance to Calcification of SynerGraft Tissue Engineered Heart Valve Grafts. American Association of Thoracic Surgeons. R. Elkins, S. Goldstein, S. Walsh, K. Black.
Use of a Bioengineered Vascular Tissue Graft for Use in Battlefield Injuries. 23rd Army Science Conference. K. Black, J. Matsuura, C. Davenport, C. Goodman, N. Pagelsen, J. Ollerenshaw.
2001
A Tissue Engineered Heart Valve: In Vitro Performance of the SynerGraft Heart Valve. The Society for Heart Valve Disease First Biennial Meeting. S. Walsh, S. Goldstein, C. Bair, K. Black.
Humoral Immune Response to Allograft Valve Tissue Pretreated with an Antigen Reduction Process. Stentless Bioprostheses 4th Annual Symposium. R. Elkins, M. Lane, S. Capps, C. McCue, P. Dawson.
In Vivo Performance of an Unfixed Composite Aortic Xenograft [SynerGraft] as Aortic Root Replacement in the Sheep [poster presentation]. The Society for Heart Valve Disease. R. Elkins, S. Goldstein, S. Walsh, K. Black.
New Technologies in Heart Valve Surgery. Scandinavian Association for Thoracic Surgery 59th Annual Meeting. S. Goldstein.
Recellularization of Heart Valve Grafts [SynerGraft] by a Process of Adaptive Remodeling. Stentless Bioprostheses 4th Annual Symposium. R. Elkins, S. Goldstein, S. Walsh, J. Ollerenshaw, C. Hewitt, K. Black, D. Clarke, M. O’Brien.
Results of 13-Year Follow-Up Study on Aortic Heart Valve Replacements Utilizing the Ross Procedure. Western Thoracic Surgical Association. R. C. Elkins, M. F. O’Brien.
SynerGraft: The First Successful Reconstruction and Regeneration Tissue Products. Tissue Engineering for Heart Valve Substitutes Symposium. K. Black.
SynerGraft Vascular Conduit as a Hemodialysis Access Graft in the Canine Model. 2nd International Congress Vascular Access Society. J. Matsuura, K. Black, E. Wellons, C. Davenport, C. Goodman, K. Greene, J. Ollerenshaw.
SynerGraft Vascular Conduit as a Hemodialysis Access Graft in the Canine Model. Eastern Vascular Society 15th Annual Meeting. J. Matsuura, K. Black, E. Wellons, C. Davenport, C. Goodman, K. Greene, J. Ollerenshaw.
SynerGraft Vascular Conduit as a Hemodialysis Access Graft in the Canine Model [poster presentation]. SVS/AAVS Joint Meeting. J. Matsuura, K. Black, E. Wellons, C. Davenport, C. Goodman, K. Greene, J. Ollerenshaw.
2000
Advances in Tissue Processing. 24th Annual Meeting American Association of Tissue Banks. K. Black.
Decellularized Human Valve Allografts. VIII International Symposium Cardiac Bioprosthesis. R. Elkins, K. Black, P. Dawson, S. Goldstein, S. Walsh.
In Vivo Arterialization of SynerGraft Processed Non-Vascular Xenogeneric Conduit. 2nd International Meeting of the Onassis Cardiac Surgery Center. R. Hanley, R. Lust, Y. Sin, K. Black, J. Ollerenshaw.
In Vivo Arterialization of SynerGraft®Processed Non-Vascular Xenogeneic Conduit. American Heart Association Scientific Sessions.
Successful Use of Natural Revitalizing XenoGraft Connective Tissue Matrices in Animal and Human Heart Valve Replacement [poster presentation]. International Society for Applied Cardiovascular Biology. S. Goldstein, S. Walsh, K. Black, M. O’Brien.
SynerGraft Heart Valve: Reconstitution of an Unfixed Acellular Xenograft In Vivo [received AHA Citation]. American Heart Association. R. Elkins, K. Black, M. O’Brien, S. Goldstein, S. Walsh, D. Clarke, S. Bode, J. Hamby.
SynerGraft Tissue Conduit is Adopted by Host in Aortic Reconstruction. VIII International Symposium Cardiac Bioprosthesis. D. Clarke, R. Lust, Y. Sun, K. Black, J. Ollerenshaw.
SynerGraft®Treatment of Valve Allografts [poster presentation]. 24th Annual Meeting American Association of Tissue Banks. P. Dawson, S. Goldstein, S. Walsh, K. Black.
SynerGraft Vascular Tissue Conduit is Rapidly Recellularized Following Peripheral Bypass. European Association for Cardio-Thoracic Surgery 14th Annual Meeting. D. Clarke, M. Tillson, K. Black, J. Ollerenshaw.
Tissue Heart Valve Engineering: Experience with an Autologous Engineered Xenograft. Peripheral Vascular Surgical Society. M. O’Brien.
Transpecies Heart Valve Transplant: Advanced Studies of a Bioengineered Xeno-Autograft. The Society of Thoracic Surgeons 36th Annual Meeting. S. Goldstein, D. Clarke, S. Walsh, K. Black, M. O’Brien.
Use of Decellularized Cadaver Allograft (SYN) Does not Cause Allosensitization in Hemodialysis Patients and is Safe for Use in Potential Transplant Recipients. 2001 ASN/ISN World Congress of Nephrology. G. Lipkowitz, B. Benedetto, R. Madden, A. Kurbanov, L. Bow, M. Miller, J. Matsuura.
1999
A New Era in Health Care. Cambridge Health Care Institute Meeting on Tissue Engineering. S. Goldstein.
A Simple Tissue Implant Model to Study Xenogeneic and Allogeneic Rejection [poster presentation]. Association for Academic Surgery. H. Tran, M. Puc, N. Patel, S. Goldstein, J. Ollerenshaw, K. Black, A. DelRossi, C. Hewitt.
Acellular Porcine Heart Valve Leaflets Do Not Mineralize in the Ovine RVOT; Stentless Bioprosthesis Third International Symposium. S. Goldstein, K. Black, D. Clarke, E.C. Orton, M. O’Brien.
Advanced Tissue / Cellular Engineering. European Medical & Biological Engineering Conference. K. Black.
Advances in Tissue Processing. 24th Annual Meeting American Association of Tissue Banks. K. Black.
Decellularized Human Valve Allografts. VIII International Symposium Cardiac Bioprosthesis. R. Elkins, K. Black, P. Dawson, S. Goldstein, S. Walsh.
In Vivo Arterialization of SynerGraft Processed Non-Vascular Xenogeneric Conduit. 2nd International Meeting of the Onassis Cardiac Surgery Center. R. Hanley, R. Lust, Y. Sin, K. Black, J. Ollerenshaw.
In Vivo Arterialization of SynerGraft®Processed Non-Vascular Xenogeneic Conduit. American Heart Association Scientific Sessions.
Inflammatory Responses to Uncrosslinked Xenogeneic Heart Valve Matrix. World Heart Valve Disease Symposium. S. Goldstein, K. Black, D. Clarke, E.C. Orton, M.F. O’Brien.
Performance of an Acellular, Composite Porcine Heart Valve Bioprosthesis in the Ovine RVOT. 34th Congress of the European Society for Surgical Research. S. Goldstein, S. Walsh, K. Black, E.C. Orton, D. Clarke.
Successful Trans-Species Implant of a Tissue Engineered Heart Valve. First Satellite Symposium on Tissue Engineering for Heart Valve Bioprostheses. K. Black.
Transpecies Heart Valve Transplant: Advanced Studies of a Bioengineered Autograft. The European Association for Cardio-Thoracic Surgery. S. Goldstein, S. Walsh, K. Black, D. Clarke, E.C. Orton, M.F. O’Brien.
1998
Meniscal Transplantation to Tissue Engineering: CryoLife’s Vision of Orthopedics. Osteochondral Autograft Transfer System 1998 Meniscus and Cartilage Transplantation Study Group on Meniscus Reconstruction. K. Black.
1997
Acellular Porcine Pulmonary and Aortic Heart Valve Bioprostheses. 2nd Intl Stentless Bioprostheses Symposium. D. Ross, J. Hamby, S. Goldstein, K. Black.
Effects of Cryopreservation on Biomechanical Properties of Tissue Engineering Matrices. Workshop on Biomaterials and Tissue Engineering. S. Goldstein, J. Hamby.
The Ross Operation in Children: 10-Year Experience. 33rd Annual Meetingof the Society of Thoracic Surgeons. R. Elkins, C. Knott-Craig, K. Ward, M. Lane.
1995
Age-Dependent Alterations in Collagen Cross-Links in Porcine Aortic Heart Valve Leaflets. American Society for Biochemistry and Molecular Biology. S. Goldstein, M. Yamauchi.
Modulation of Human Dermal Fibroblast Remodeling of Porcine Heart Valve Leaflet Matrix. American Society for Artificial Internal Organs. S. Goldstein, D. Fronk.
1994
Development of a Chimeric Heart Valve: Effects of Cell Removal upon Leaflet Mechanics and Immune Responses in a Xenogeneic Model. VI International Symposium Cardiac Bioprostheses. S. Goldstein, K. Brockbank.
Localization of Epitopes Involved in Hyperacute Rejection in Porcine Heart Valve Xenografts. Strategies for Xenotransplantation. S. Goldstein.
1993
Effects of Cell Removal Upon Heart Valve Leaflet Mechanics and Immune Responses in a Xenogeneic Model. 2nd Intl Congress on Xenotransplantation. S. Goldstein, K. Brockbank.
Illumina12
Robin Gaster
North Atlantic Research
EXECUTIVE SUMMARY
Illumina is a successful venture funded biomedical company selling tools for researchers in genomics. Located on a new campus in the San Diego biomedical cluster, the company was expected to reach break-even in 2005.
Illumina is a classic example of a venture-funded biomedical company, one that has gone public and appears poised to reach profitability in 2006, while providing cutting edge technology in an area of critical importance for the large-scale analysis of genetic variation and function. Because Illumina received venture funding during its first year in existence, and subsequently received further rounds before a successful IPO, SBIR was never the primary source of research funding.
However, according to its founder and one of its key initial researchers, Dr. Mark Chee, SBIR did provide funding for projects that would not have been funded in the normal course of company business—and these projects turned out to be of critical importance for the development of core Illumina product lines.
The Illumina case therefore shows that even where companies are well funded, SBIR can have an important impact in funding alternative or complementary lines of business that might not fit within a company’s top research priorities, or might not meet projected internal hurdle rates.
Comments from Dr. Chee about the SBIR program focused on the extended funding cycle and time lags, and selection and review procedures.
COMPANY HISTORY AND OBJECTIVES
Illumina was founded in April 1998 by David Walt, Ph.D., CW Group (Larry Bock), John Stuelpnagel, D.V.M., Anthony Czarnik, Ph.D. and Mark Chee, Ph.D., based on core technology developed at—and then exclusively licensed from—Tufts University. The first substantial venture capital funding (about $8.6 million) came in November 1998.
Illumina completed a $28 million Series C financing in December 1999, and an IPO at the end of July 2000, raising just over $100 million.
Illumina’s mission is to develop tools for the large-scale analysis of genetic
FIGURE App-D-4
SOURCE: Illumina.
variation and function, which in turn will support an understanding of variation and function at the cellular level, critical for achieving the broad social goal of personalized medicine.
Illumina’s tools convert data generated from human genome sequencing into medically relevant information, linking genetic variation and genetic function to specific diseases, improving the community’s ability to discover drugs, and permitting diseases to be detected earlier, and with greater accuracy and specificity.
Massive quantities of raw genetic data have flowed from the successful sequencing of the human genome. This has driven demand for tools that can assist researchers in processing the billions of tests necessary to convert raw data into medically valuable information. Such tools must perform functional analysis of highly complex biological systems. Illumina’s technology platform has been developed into a line of products that can address the scale of experimentation and the breadth of functional analysis required.
ILLUMINA TECHNOLOGY
BeadArray Technology
Illumina has developed a proprietary array technology that enables the large-scale analysis of genetic variation and function. BeadArray technology combines microscopic beads and a substrate in a simple proprietary manufacturing process to produce arrays that can perform many assays simultaneously.
This approach provides a combination of high throughput, cost effectiveness, and flexibility:
-
High throughput is achieved by using a high density of test sites per array, and by formatting arrays in either a pattern arranged to match the wells of standard microtiter plates or in various configurations in the format of standard microscope slides, allowing throughput levels of up to 150,000 unique assays per plate. Laboratory robotics are also used to speed processing time.
-
Cost effectiveness is maximized by reducing consumption of expensive reagents and valuable samples, and through low-cost manufacturing processes that exploit cost reductions generated by advances in fiber optics,
FIGURE App-D-5 Bead-based technology on a chemically etched fiber optic strand.
SOURCE: Illumina.
-
digital imaging, and bead chemistry technologies. Per-sample running costs, including labeling, will typically range between $80 and $200, comparable in cost to just the sample labeling steps using other microarray platforms.
-
The flexibility needed to address multiple markets segments is provided by varying the size, shape, and format of the well patterns, and creating specific bead pools or sensors for different applications.
BeadArray technology is deployed by Illumina in two different array formats, the Array Matrix and the BeadChip. Illumina’s first bead-based product was the Array Matrix, which incorporates fiber optic bundles, manufactured to Illumina specifications, cut into lengths of less than one inch. Each bundle contains approximately 50,000 individual fibers.
Ninety-six bundles are placed into an aluminum plate which forms an Array Matrix. BeadChips are fabricated in microscope slide-shaped sizes with varying
|
BOX App-D-1 Genetic Variation and Function Every person inherits two copies of each gene, one from each parent. The two copies of each gene may be identical, or they may be different. These differences are referred to as genetic variation. Examples of the physical consequences of genetic variation include differences in eye and hair color. Genetic variation can also have important medical consequences, including predisposition to disease and differential response to drugs. Genetic variation affects diseases, including cancer, diabetes, cardiovascular disease and Alzheimer’s disease. In addition, genetic variation may cause people to respond differently to the same drug. Some people may respond well, others may not respond at all, and still others may experience adverse side effects. The most common form of genetic variation is a Single Nucleotide Polymorphism, or SNP. A SNP is a variation in a single position in a DNA sequence. It is estimated that the human genome contains between three and six million SNPs. While in some cases a single SNP will be responsible for medically important effects, it is now believed that the genetic component of most major diseases is the result of the interaction of many SNPs. Therefore, it is important to investigate many SNPs together in order to discover medically valuable information. Current efforts to understand genetic variation and function have primarily centered around SNP genotyping and gene expression profiling. SNP Genotyping SNP genotyping is the process of determining which SNPs are present in each of the two copies of a gene, or other portion of DNA sequence, within an individual or other organism. The use of SNP genotyping to obtain meaningful statistics on the effect of an individual SNP or a collection of SNPs, and to apply that information to clinical trials and diagnostic testing, requires the analysis of millions of SNP genotypes and the testing of large populations for each disease. For example, a single large clinical trial could |
numbers of sample sites per slide. Both formats are chemically etched, to create tens of thousands of wells for each sample site.
In a separate process, Illumina create sensors by affixing a specific type of molecule to each of the billions of microscopic beads in a batch. Different batches of beads are coated different specific types of molecule. The particular molecules on a bead define that bead’s function as a sensor. For example, Illumina creates a batch of SNP sensors by attaching a particular DNA sequence to each bead in the batch. Batches of coated beads are combined to form a pool specific to the type of array. A bead pool one milliliter in volume contains sufficient beads to produce thousands of arrays. This technology permits the creation of universal arrays for SNP genotyping. By varying the reagent kit, users can still use the array to test for any combination of SNPs.
To form an array, a pool of coated beads is brought into contact with the array surface where they are randomly drawn into wells, one bead per well. The tens of thousands of beads in the wells comprise individual arrays. Because the
|
involve genotyping 200,000 SNPs per patient in 1,000 patients, thus requiring 200 million assays. Using previously available technologies, this scale of SNP genotyping was both impractical and prohibitively expensive. Large-scale SNP genotyping will be used for a variety of applications, including genomics-based drug development, clinical trial analysis, disease predisposition testing, and disease diagnosis. SNP genotyping can also be used outside of healthcare, for example in the development of plants and animals with desirable commercial characteristics. These markets will require billions of SNP genotyping assays annually. Gene Expression Profiling Gene expression profiling is the process of determining which genes are active in a specific cell or group of cells and is accomplished by measuring mRNA, the intermediary between genes and proteins. Variation in gene expression can cause disease, or act as an important indicator of disease or predisposition to disease. By comparing gene expression patterns between cells from different environments, such as normal tissue compared to diseased tissue or in the presence or absence of a drug, specific genes or groups of genes that play a role in these processes can be identified. Studies of this type, used in drug discovery, require monitoring thousands, and preferably tens of thousands, of mRNAs in large numbers of samples. Once a smaller set of genes of interest has been identified, researchers can then examine how these genes are expressed or suppressed across numerous samples, for example, within a clinical trial. The high cost of current gene expression methods has limited the development of the gene expression market. As gene expression patterns are correlated to specific diseases, gene expression profiling is becoming an increasingly important diagnostic tool. Diagnostic use of expression profiling tools is anticipated to grow rapidly with the combination of the sequencing of various genomes and the availability of more cost-effective technologies. SOURCE: Illumina. |
beads assemble randomly into the wells, a final procedure called decoding is used in order to determine which bead type occupies which well in the array. Decoding also validates each bead in the array—a further quality control test. By using multiple copies of each bead type, the reliability and accuracy of the resulting data is improved via statistical processing of results from identical beads.
An experiment is performed on the Array Matrix by preparing a sample, such as DNA from a patient, and introducing it to the array. The Matrix is dipped into a solution containing the sample, and molecules in the sample bind to matching molecules on the coated bead. The BeadArray Reader detects the matched molecules by shining a laser on the fiber optic bundle. Measuring the number of molecules bound to each coated bead, results in a quantitative analysis of the sample.
Oligator Technology
Genomic applications require many different short pieces of DNA that can be made synthetically, called oligonucleotides (single-stranded DNA). For example, SNP genotyping typically requires three to four different oligonucleotides per assay. An SNP genotyping experiment analyzing 10,000 SNPs may therefore require 30,000 to 40,000 different oligonucleotides, contributing significantly to the expense of the experiment.
Illumina’s Oligator technology is designed for the parallel synthesis of many different oligonucleotides. Each synthesizer can produce up to 3,072 oligos in parallel, using very small amounts of material.
PRODUCT ROLLOUT AND COMMERCIAL RESULTS
In 2001, Illumina launched its commercial genotyping service product line, combining BeadArray technology with an automated process controlled by a laboratory information management system to provide high throughput identification of the most common form of genetic variation, known as single nucleotide polymorphisms, or SNPs.
In 2002, Illumina launched BeadLab, an integrated turnkey system built around BeadArray technology. The BeadLab can routinely produce up to 1.4 million genotypes per day.
In 2003, Illumina launch several new products, including 1) a new array format, the Sentrix BeadChip; 2) a gene expression product line on both the Sentrix Array Matrix and the Sentrix BeadChip that allows researchers to analyze a focused set of genes across 8 to 96 samples on a single array; and 3) a benchtop SNP genotyping and gene expression system, the BeadStation, for performing moderate-scale genotyping and gene expression using our technology.
As of end-2004, nine BeadLabs were in use, along with 42 BeadStations.
TABLE App-D-4 Revenue and Expenses Trends at Illumina, 2000-2004
|
|
Year |
||||
|
2004 |
2003 |
2002 |
2001 |
2000 |
|
|
Total Revenue ($) |
50,583 |
28,035 |
10,040 |
2,486 |
1,309 |
|
Total Costs and Expenses ($) |
56,096 |
54,657 |
51,895 |
32,805 |
24,544 |
|
Net Loss ($) |
(6,225) |
(27,063) |
(40,331) |
(24,823) |
(18,606) |
|
SOURCE: Illumina. |
|||||
In 2005, Illumina bought CyVera, whose digital-microbead platform is highly complementary to Illumina products and services.
The systematic rollout of these technologies has provided a firm base for rapidly expanding revenues at Illumina. The 2005 annual report shows that revenues have increased dramatically in 2003 and 2004, and that the company seemed poised for profitability in 2005.
This strongly positive trend is reflected especially in revenue growth, as shown in Figure App-D-6.
THE ROLE OF SBIR
Dr. Chee is the source for SBIR related activities at Illumina, as he was the principal investigator on most early SBIR awards, and has been a strong champion of the program within the company.
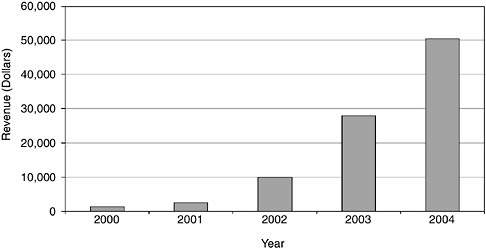
FIGURE App-D-6 Illumina revenue growth 2000-2004.
SOURCE: Illumina.
According to Dr. Chee, SBIR was a very positive experience for working with VCs, as it provided a significant technical validation of the technology. While the initial round of funding came before the first SBIR award, the latter was very important for the second round of financing a year later. However, it would not be accurate to say even here that SBIR made the difference between VC funding or not.
The real importance of SBIR at Illumina was that it provided flexibility in pursuing projects outside the mainstream of immediate research objectives. As such, it provided a key counter-balance to the tendency to over-focus, which is perhaps inevitable in a small company. At Illumina, SBIR definitely promoted a more diverse approach to R&D. And of course, additional funding is always useful to researchers.
The additional opportunities provided by SBIR almost all paid off in commercially successful, revenue-generating products.
(1)
Genotyping system.
There was a lack of demand from customers at that time—mainly because they were genotyping at a very much smaller scale (by a couple of orders of magnitude or thereabouts). Although this lack of immediate demand would probably not have blocked the project receipt of SBIR funding allowed this research to progress more rapidly.
The genotyping project became the technical foundations for a critical product line, which in turn became the base for Illumina’s work with The International HapMap Project, a highly prestigious international genotyping project. The Illumina technology has been enormously important for cutting end-user costs, which is especially important for some of the developing nations participating in the project—for example China, which is using the technology to meet its commitments to the HATMAP project.
SBIR funding was used to start work on the foundation research used to determine the best way to implement design of the array-based system. Positive results during the SBIR-funded research quickly led to substantial subsequent company investment. According to Dr. Chee, SBIR funding for this project lies somewhere between necessary and really useful.
(2)
The pyro-sequencing project.
The pyro-sequencing project was another nonmainstream project that would not have been attempted without SBIR support. In this case, while the technical results from the research were good, the project was eventually abandoned for business reasons.
(3)
Gene expression profiling.
This project constitutes an important SBIR success story, leading to significant Illumina products. The profiling project was a lower priority for Illumina, partly because this appeared to be an effort that would compete directly with the much better funded and established Affymetrix.
SBIR allowed the company to project its thinking into the next wave of technology, and this worked out very well. It took about 3 years to develop this tech to the marketplace. That technology created the base for Illumina’s product lines covering whole genome expression arrays. These arrays generate superb data, and are a highly successful commercial product. They would not have been possible without SBIR.
Overall, it is clear that SBIR was important at Illumina because it allowed Dr. Chee to successfully champion projects that would not normally have been funded by the company, projects with high-risk/high-return characteristics. SBIR funding was not needed for core company projects and research, but within the company—like any small company, even a well funded start-up, there was limited funding for projects outside the immediate research stream. As a result, the projects funded by SBIR would not have been funded in the normal course of business.
SBIR at Illumina should not be understood as focusing on peripheral research; instead, it allowed a focus on higher-risk research that was positioned further from the market projects that resulted in dramatic improvements in the core technology.
There has been a very big pay-off from SBIR projects at Illumina:
-
Genotyping.
-
Parallel arrays.
-
Gene expression profiling.
The first two are integral parts of Illumina’s main product lines. They have returned many times the original investment in revenues for the company.
It is also worth noting that Illumina’s experience in some ways confirms the very short product cycle identified in many Phase II Recipient Survey responses. For example, Illumina’s parallel array processor, which addresses multiple arrays at the same time, was originally seen as developed of a technology platform. Research went very fast, and Illumina was able to start selling a commercial system before the end of Phase II.
RECOMMENDATIONS FOR CHANGES TO THE SBIR PROGRAM
The following comments are from the interview with Dr. Chee, as he has been the primary SBIR contact at Illumina.
Dr. Chee said that in general, he believed the SBIR program was highly successful. He had some observations about areas of possible improvement.
Selection/Review
Overacademic Reviews
Like a number of other applicants in his experience, Dr. Chee said that he often received comments that reflected lack of understanding of the differences between commercial and academic R&D. One example of the differences could be found in his work on gene expression profiling
Because the company was building work in this area without a preexisting base, technology was at every stage of the project extremely crude. Initial results of the research were as a result “awful.” However, that research also suggested that the theory on which the research was based was valid, and there were some very preliminary indications that the technology would in the end work well.
Reviewer comments seemed to indicate expectations that by end of Phase I, there would be a system in place that was performing well. In Dr. Chee’s view, this indicated a very naïve understanding of how new technology gets invented in a company, as opposed to in an academic setting, where preliminary successful research results are usually required before substantial grants are awarded.
This misunderstanding of company-based research led to problems with overacademic reviews. Dr. Chee pointed out that RO1 applications typically came from universities with existing labs already in place for preliminary experiments, a system of research, and staff and grad students. The result was usually lots of good preliminary data.
In contrast, Illumina started with three people at a conference table. Work was literally conducted sitting on the floor, writing on notepads. Everything was built from scratch: the Illumina team wrote their own software, and mixed their own reagents. Nothing worked well during the initial period, and even later, good results often took significant amounts of time. As a result, Illumina’s applications “looked pretty sketchy” in the initial period. Reviewers of these early applications wanted an R01 type approach, and clearly did not understand the corporate research environment of a start-up.
Commercial Review
Dr. Chee observed that academic review for commercial potential was likely to be a futile exercise for Phase I, and that pressure to develop a complete commercialization plan at this stage was likely to be more trouble than help to the company. He also noted that presenting more material on commercialization did not always work to the company’s advantage in review, as it presented more material for reviewers to criticize. Instead of the focus on commercialization
planning, Dr. Chee suggested that NIH focus on commercialization potential—commercialization track record and other funding.
In general, Dr. Chee thought that the concept of a commercialization consultant attached to the review section might be worth exploring, but he did not think that a separate two-stage review which separated commercialization and technical review would be a good idea, as it could add additional delays.
Delays/Funding Cycle
Dr. Chee emphatically noted that the primary problem with the SBIR program was the funding cycle and the long delays between application and funding. In his recent experience, applications were likely to be “in limbo for a year even if the application was eventually successful.”
Anything that could reduce the length of the funding cycle was worth exploring, and Dr. Chee was very positive about the possibility of offering applicants a chance to provide a short written “rebuttal” to the comments of the lead reviewer during the first. This in his view fit well with the new system implemented at CRS about 2 years ago whereby lead reviewers prepared comments before the meeting of the study section.
Similarly, Dr. Chee strongly favored “instant scoring”—the notion that the actual score should be developed very quickly after the meeting of the review panel, and also that scores once assigned should be released electronically to applicants immediately, well before final pink sheet comments could be available. He also favored any methods for accelerating pink sheet distribution itself.
Conflict of Interest
Dr. Chee said that in the real world it is difficult to avoid conflicts completely and still get good reviewers. However, his approach was not to worry too much about potential conflicts, trusting to the system to sort that out. He has tried to point out direct competitors (e.g., for Illumina applications, Affymetrix) who should not act as reviewers.
However, Dr. Chee also recognized that the review process is intrinsically difficult, and that the SBIR program does this work reasonably well, in comparison with other NIH selection panels with which he has been involved.
Award Size
In general, Dr. Chee noted that there was room for much better cost-benefit analysis by NIH, and that one size (award) did not and should not fit all applicants. He believed that if different size awards became the norm, it would be especially important for NIH to developed procedures for assessing the relative costs and likely benefits of applications.
Specially, he saw merit in testing approaches that would weight applications inversely for the funding required, so that applications that were especially expensive would have to provide correspondingly greater benefits.
He observed that the very small size of Phase I actually worked against startups that had no other resources on which to draw and possibly no infrastructure, while being required to present feasible and exciting projects. However, he believed that the current size of the Phase I award at NIH was appropriate and should not be increased.
Inhibitex13
Paula Stephan
Georgia State University
DESCRIPTION OF THE FIRM
Inhibitex was founded in 1994 at Texas A&M where the co-founders, Dr. Joseph M. Patti and Dr. Magnus Höok, were on the faculty. The firm moved to Atlanta in 1998 when it received funding from Alliance Technology Ventures, which has a mandate to build biotech in Atlanta. Inhibitex hired its first employee soon after arriving in Atlanta. During its early years in Atlanta the firm worked out of lab space at Georgia State University; the firm got dedicated space after hiring its fifth employee. It currently has approximately 75 employees and will relocate to new space, financed in part by the state of Georgia, in 2005. Dr. Patti has been full-time with the firm since 1998. He currently serves as Vice President, Preclinical Development and Chief Scientific Officer. He is also a director of the firm. Dr. Höok remains on the faculty of Texas A&M and serves on the Scientific Advisory Board of the company.
The scientific platform for the company is MCSRAMM; the research underpinning this platform came out of Texas A&M. The company has two products in clinical trails: Veronate and Aurexis. Vernoate is in Phase III clinical trials as an anti-infectious drug to prevent hospital-associated infections in very low birth weight infants (VLBW infants). There are approximately 60,000 VLBW infants born each year in the United States and studies indicate that 30 to 50 percent develop at least one hospital-associated infection while in the neonatal intensive care unit, resulting in significant mortality and morbidity. Veronate has been awarded Fast Track and Orphan Drug status by the FDA. Clinical trials started in 2002. Aurexis is designed to combat S. aureus blood stream infections in hospitalized patients (staph). It is a leading cause of hospital-associated infections and related mortality. It is estimated that there were approximately one million cases of hospital-associated S. aureus infections worldwide in 2002. Aurexis is designed to be used in tandem with standard antibiotic treatments. Aurexis has recently completed a 60-patient Phase II clinical trial.14 The company has three additional product candidates in preclinical development. The company has a collaborative agreement with Wyeth for global development of vaccines targeting staphylococcal infections. The company also has a co-collaboration agreement with Dyax, a company based in Cambridge, MA, for the development of human monoclonal antibodies targeting enterococci.
The company has raised approximately $174 million since 1998: $85 from private funding, which includes venture capital from New Enterprise Associates and Alliance Technology Ventures, and $39 million from its IPO in June of 2004 and $50 million from a PIPE financing in November 2004. The company’s stock is traded on NASDQ under the ticker “INHX.”
Since inception the company has not generated any revenue from the sale of products and does not expect to until it receives regulatory approval for commercialization of products. Its current revenue (approximately $1 million in 2003) comes from the amortization of an up-front license fee, quarterly research and development support payments received in connection with a license and collaboration agreement with Wyeth and a grant received from the FDA’s Office of Orphan Products Development.
INPUTS
The company has had one SBIR, Phase I. It was awarded 9/15/1999, for $99,350; Joseph Patti was the PI. The company, according to Dr. Patti, applied for three other SBIR Phase Is which were unfunded. The funded study, according to Dr. Patti, was designed to look at the potential of a multicomponent S. aureus vaccine. The company had fewer than five employees at the time the SBIR award was received.
The co-founders of the firm were both affiliated with Texas A&M at the time the company was founded. Joseph M. Patti was assistant professor at Texas A&M Institute of Biosciences and Technology (1994-1998) and co-founder Magnus Höok was Regents Professor and Director for the Center of Extracellular Matrix Biology. Prior to his appointment as an assistant professor, Dr. Patti was a post-doc in the lab of Dr. Höok. The SBIR award played no role in the creation of the firm.
In addition to SBIR funds, the firm received government funds from the FDA as a result of Veronate being awarded orphan drug status. The company has a collaborative agreement, noted above, for the global development of vaccines against staphylococcal infections. The company received venture funding and had an initial public offering in 2004 (see discussion above).
The company does not see the SBIR program as playing a role in the decision of external partners to provide funding. Indeed, Dr. Patti expressed the view that SBIR funding could potentially be a drawback to the receipt of VC money because VCs might look unfavorably on the reporting obligations associated with an SBIR award. He also noted that disclosure can be an issue: “The IP people get kind of antsy when you start talking about some of these grants, whether they are considered confidential or not confidential. Who’s reviewing it? Do they have an alliance? Are they competitors?”
KEY OUTCOMES FROM SBIR
According to Dr. Patti, although preliminary, the data generated from the SBIR award were “interesting and prompted us to continue the work, albeit with a slightly different focus.” Dr. Patti went on to say that “one could argue that it [SBIR] helped get our Wyeth deal, although even in the absence of this funding, we would have pursued the vaccine approach.” The collaboration agreement with Wyeth was executed prior to the company going public and was described in the IPO prospectus.
The company has a number of patents, and Inhibitex scientists have published papers in scientific journals. None of the patents or publications relate directly to the SBIR award.
IMPACT OF SBIR ON THE FIRM
The company does not see the SBIR award as playing a key role in the company’s strategy and currently does not anticipate applying for further SBIR awards. “I have struggled with the applicability of this program to a growing firm; useful if you have a side project—useful to look at noncore areas—but how could you survive if you were asking SBIR funding for the core?” According to Dr. Patti, even the larger grants of several million dollars that are currently being awarded are too small to run a small Phase II clinical trial. The Inhibitex 60-person Phase II clinical trial for Aurexis cost approximately $5 million. SBIRs can be helpful in funding exploratory science, but “you can’t grow your organization based off of them.”
Dr. Patti expressed the opinion that SBIRs were not compatible with the life span of an early stage biotech company. He estimates the length of time between writing the proposal and receiving SBIR money to be approximately a year, while the life span of an early stage biotech company is at most two years. Moreover, the amount of money (at least when Phase Is were limited to $100,000) was insufficient to support research unless the firm is in an academic lab and does not have to pay overhead, etc. If the firm is in dedicated space, the amount of funding is insufficient and is “not compatible with the expectations of the investors that you are going after.”
SBIR ADMINISTRATION
The company sees the SBIR program as being well publicized. Other than venture capital and participation in the ATP program, “this is it.”
The company has not participated in any business/commercialization support activities sponsored by (a) the funding SBIR agencies, (b) the states related to SBIR opportunities.
The company clearly sees the size and duration of the SBIR awards (at least as they existed in the late 1990s and early 2000s) to be insufficient for a dedi-
cated biotech firm. The greatest drawback, from the company’s point of view, is the speed of the SBIR process. The 18 months that elapse between writing the proposal and receipt of the money is too long. Increasing turnaround takes precedence over increased funding: “Raising the amount is good but turning it around faster is really important.”
The company sees the award selection process as biased toward the NIH mentality of “show me the data and then I’ll fund it.” While this is a workable model for established PIs at universities, it is inconsistent with an early stage biotech company that is “pushing the envelope to find out what the data is.” Companies need the money before the study has been conducted; not after it has already been done. Yet the review process (and resulting priority score) is biased towards proposals that provide data and “have all the answers.”
Dr. Patti suggests that NIH have representatives from biotech companies as part of the review process. People who work in the biotech world on a daily basis understand the issues faced by a biotech company while university-based scientists appear to have less appreciation for such issues. NIH priority scores often reflect this lack of understanding.
There was a considerable amount of paperwork associated with the grants and at the time the company had the SBIR there was a fairly obtuse financial reporting system that required one to report via a dial in system. The reporting period extended for two-to-three years even though that grant was for one year.
CROSS-CUTTING RESEARCH ISSUES
The company expressed the view that two factors inhibit participation in the SBIR program: VC-related issues and the slowness of turnaround. The turnaround issue was discussed above. The VC-related issues cut both ways. First, as noted above, venture capitalists may see the reporting requirements associated with the SBIR award as detracting from the desirability of investing in the firm. Second, a company is now excluded if VC has more than a 50 percent stake in the company. This means that the most attractive companies with the most potential are now excluded from SBIR eligibility. If this rule had existed when Inhibitex applied, they would not have been able to get an SBIR award. “If you are successful, VC owns lots of your company.”
SBIRs provide peer recognition of quality science. SBIR awards are noted in BioWorld as well as trade magazines. The receipt of an award sends a signal comparable to that of having a paper published. In some cases one may be able to leverage this recognition and associated funding with an early investor. But there are also downsides, as noted above.
JP Laboratories
Andrew Toole
Rutgers University
COMPANY AND FOUNDER BACKGROUND
JP Laboratories, Inc. is a privately-held research and development (R&D) company located in Middlesex, NJ. The company was founded by Dr. Gordhan N. Patel in 1983 to invent products based on his research experience and emerging knowledge in the fields of chemistry, physics, and biology. Dr. Patel founded the company using an initial $100,000 investment of personal funds. Over the last twenty-two years, he has implemented a successful business strategy that has allowed JP Laboratories (Labs) to remain a small—never more than five employees—but innovative firm. His business strategy focuses on inventing products and licensing them to larger firms for commercialization. According to Dr. Patel, his company has invented twenty products and successfully licensed ten of these for commercialization, SBIR funds, other governmental funds, and the royalty streams from these licenses have allowed the company to expand its R&D capabilities and continue the invention process.
In 2003, following the invention of their Self-indicating Instant Radiation Alert Dosimeter (SIRAD), Dr. Patel decided to expand the company’s business strategy to include manufacturing and sales for their SIRAD product. This product, which is described more fully in the next section, evolved from decades of Dr. Patel’s research and discovery activities related to various types of indicator devices. SBIR awards from DoD and NIH supported part of the research underlying SIRAD. JP Labs and Dr. Patel received several major awards and recognitions for developing SIRAD: (1) in 2003, Dr. Patel was invited to testify to a congressional subcommittee about SIRAD’s use in counterterrorism, <http://reform.house.gov/UploadedFiles/Patel%20Testimony.pdf>; (2) in 2004, JP Labs received the Frost & Sullivan Excellence in Technology Award in the field of homeland security for this product; and (3) in 2005, JP Labs received R&D-100 award (see the photo at the end of this document). Dr. Patel’s decision to expand the business strategy of JP Labs is likely to dramatically change their corporate profile going forward. The next several years will be a critical transition period involving additional private investment, facilities expansion, new hiring, and internal corporate restructuring.
Dr. Patel is a good example of a “scientist-inventor-entrepreneur.” Born and raised in Manund (Gujarat state), a small village in India, Dr. Patel developed a strong scientific background as a university student and research scientist. He earned undergraduate and graduate degrees in chemistry and physics from Sardar Patel University in Vidyanagar. In 1970, having just completed his Ph.D. in phys-
ics on the crystallization of polymers, he joined the research lab of Dr. Andrew Keller at the University of Bristol, UK. After three years at Bristol, he spent a short time in a research position at Baylor University in Waco, Texas. As a scientist, Dr. Patel published over 65 research papers in peer-review journals. From there, he joined Allied Corp. and worked as a bench-level scientist for nearly ten years. While at Allied, Dr. Patel was an inventor and co-inventor on numerous patents on polymers, crystals, and time-temperature indicators. According to the U.S. Patent and Trademark Office, Dr. Patel is an inventor on 38 different U.S. patents since 1975. In 1983, when he lost his job due to downsizing at Allied, Dr. Patel became an entrepreneur by founding JP Laboratories.
SBIR AND INVENTION AT JP LABS
The SBIR Program provided vital financial support for product innovation at JP Labs from the very beginning of the company. Their first SBIR award, granted by the U.S. Army, was received in the year the company was founded, 1983. Since that time Dr. Patel has successfully won seventeen SBIR awards from a variety of agencies including the DoD, NSF, NIH, and EPA. Twelve of these awards were for Phase I feasibility studies and five were for Phase II product development. The awards total over $2.6 million (in nominal dollars) through 2005 (their last SBIR award was in 2001). Figure App-D-7 shows the time profile of awards to JP Labs.
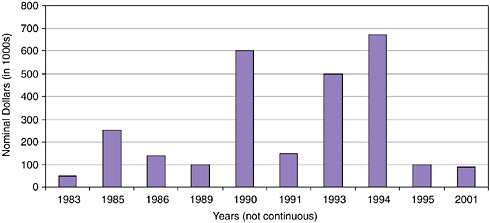
FIGURE App-D-7 JP Labs SBIR awards, 1983-2004.
SOURCES: U.S. Small Business Administration, Department of Defense, National Institutes of Health, and National Science Foundation.
These SBIR awards have supported the R&D activities at JP Laboratories in four major research areas (SBIR funding agencies in parentheses):
-
Color changing indicators for perishable goods and sterilization (Army, NIH, NSF, USDA).
-
Radiation sensing devices (DARPA, Navy, NIH).
-
Synthetic lipids and blood (NIH).
-
Etching and metallization of plastics (EPA).
JP Labs has successfully licensed many of the products discovered in these four research areas. In the area of color changing indicators, Dr. Patel developed a sticker that is a time-temperature indicator for use with perishable items like foods and medicines. He licensed this technology to Rexam PLC, a UK-based firm focused on beverage and plastic packaging. In another example, the EPA supported research into a system for etching plastics for plating. The new method Dr. Patel developed is less expensive and environmentally safer than the prevailing technology, Chromic acid. Four of JP Labs’ U.S. patents are related to this technology and it was successfully licensed to Enthone, Inc., a specialty chemical firm in New Haven Connecticut. Six of the products, indicators for monitoring sterilization of medical supplies are licensed to NAMSA, Northwood, OH.
Research funded through the SBIR program also played an important role in the development of their Self-indicating Instant Radiation Alert Dosimeter (SIRAD) product. The last funding of almost a million dollars for development SIRAD for first responders was provided by Technical Support Working Group, Arlington, VA (funded in part by the Department of Homeland Security, the Department of State, the Department of Defense, and the Department of Justice). As shown in Figure App-D-8, SIRAD is a credit card sized badge that detects radiation levels instantaneously and indicates the radiation level using a color changing strip. It can be used as an inexpensive but accurate dosimeter in situations where radiation exposure is likely.
While the discovery of SIRAD draws on decades Dr. Patel’s research and experience, the SBIR program helped finance some the practical research underlying its invention. In 1985, DARPA funded a Phase I study into “monitoring radiation with conductive polymers.” At the end of 1988, the NIH funded a Phase I, and subsequently a Phase II, study into the use of a radiographic film dosimeter to examine the dose, dose rate, and energy of neutrons. Finally, the Navy funded a Phase II development study for a radiation dosimeter that evolved directly into the SIRAD product.
KEY SBIR HIGHLIGHTS
Dr. Patel was very positive about the role and contribution of the SBIR Program to the success of JP Laboratories. The key outcomes from SBIR participa-
FIGURE App-D-8 SIRAD.
NOTE: Photos of SIRAD badges before (left) and after (right) irradiation with 100 rads of 100 KVP X-ray. Dose is estimated by comparing the color of the sensing strip with the color reference bar printed on each side of the strip. The closest match indicates the dose in rad.
SOURCE: JP Laboratories Web site, <http://www.jplabs.com/html/what_is_sirad.html#SIRAD>.
tion are numerous new patents, several new products, and royalty payments from licensing agreements. Dr. Patel said SBIR is a “great program” and that JP Labs “would not have survived without SBIR.” Two of the most important benefits for JP Laboratories from participation in the SBIR Program were:
(1)
Key Source of Early-stage Financing
Dr. Patel used his personal savings to start JP Labs. At that time, he also worked as a consultant for his old employer Allied Corp. and this provided some cash flow. Nevertheless, additional investment was needed to keep the company going. Venture capital sources were either not interested or demanded control of the company, an option Dr. Patel did not find attractive. As he searched for funding sources, he learned about the newly started SBIR program and decided to apply. The Army funded his first SBIR feasibility study in 1983. The SBIR program became a critical source of R&D funding over the next twelve years and enabled a significant portion of the inventive activity that led to licensing revenue for JP Labs.
(2)
Helpful for Business Deals
Having invented a potential product, Dr. Patel indicates that the SBIR Program serves as a recognizable source of credibility. Dr. Patel is more of a scientist

FIGURE App-D-9
SOURCE: JP Laboratories.
than a businessman. When searching for licensees, Dr. Patel found it helpful to let people know that the research was backed by a particular U.S. government agencies through the SBIR Program. This would draw the attention of firms and facilitate the licensing process.
ISSUES WITH THE CURRENT SBIR PROGRAM
Dr. Patel does not see any significant problems with the SBIR Program. His experience has been very positive. He noted two points. First, the funding gap between Phase I and Phase II awards was not a major problem. He was able to adjust largely because the licensing revenues from prior inventions began to flow. Second, his research focuses mainly on the chemistry and physics of materials, which is relatively less expensive and involves shorter research lags than product innovation related to biopharmaceutical medicines.
Nanoprobes
Andrew Toole
Rutgers University
COMPANY BACKGROUND
Nanoprobes, Inc., is a privately-held research and development company located in Yaphank, NY. The company was founded in 1990 by James Hainfeld and Frederic Furuya to commercialize research products based on a new method for labeling biological molecules. The founders had discovered a new way to design gold labels to increase the label’s effectiveness as a molecular detection tool. Even before the company was fully operational, it had identified its first commercial product based on this technology, which was later introduced as Nanogold.
Over the past fifteen years, the managers of Nanoprobes have successfully grown the company. In 1990, Nanoprobes started with one full-time scientist and a business manager working in the basement of the life sciences building at the State University of New York at Stony Brook. In 1992, the company became one of the first occupants in a new facility constructed for the Long Island High Technology Incubator (LIHTI) at Stony Brook. Over the next few years, Nanoprobes expanded to eight full-time employees. In 2000, the company “graduated” from LIHTI and moved to its current research facility in Yaphank, NY. Today, Nano-probes has 15 employees, 13 of them full-time, engaged primarily in research and product fulfillment activities.
The business evolution of Nanoprobes reflects its scientific orientation. Nanoprobes’ product innovation and improvement depend heavily on successful laboratory-style research. An explicit part of its business strategy is to expand its scientific capabilities in order to broaden and deepen its scientific knowledge surrounding gold labeling technologies. This requires expertise in fields like chemistry, biophysics, biology, and microscopy. With over half its employees engaged in research activities, Dr. Powell notes that Nanoprobes has an “academic culture” that supports discovery, publication, and involvement in professional societies such as the Microscopy Society of America. In fact, it is commonplace for Nanoprobes to subcontract research with academic scientists at universities and other research institutions. This strategy has worked well. Nanoprobes currently offers numerous product variations within about ten separate product categories. Some of these categories are Nanogold conjugates, Nanogold labeling reagents, FluoroNanogold, Ni-NTA-Nanogold, Undecagold reagents, negative stains, silver and gold enhancers, etc.
Building an outstanding scientific reputation is also critical for marketing and sales at Nanoprobes. Researchers are the primary buyers of Nanoprobes’ prod-
ucts. Reaching these customers requires active participation in research networks and professional organizations. For example, Nanoprobes will be displaying and discussing staining procedures in a poster session at the United States and Canadian Academy of Pathology meetings in February 2006. Also, publishing in top journals is necessary for illustrating the value of their products and building credibility among researchers. Dr. Powell notes that their Nanogold probes have been cited in over 150 publications.
NANOPROBES’ TECHNOLOGY, SBIR, AND INNOVATION
Gold labeling is the core technology at Nanoprobes. The company scientifically investigates, innovates, and markets a variety of forms of this labeling technology which have a number of potential uses. Most of the company’s revenue stream is produced by various forms and enhancements of its primary product, Nanogold.
Nanogold is a larger gold cluster compound (1.4 nm in diameter) that is an uncharged separate molecule in solution that does not interfere with antibody binding. When coupled with Fab’ fragments, it is the smallest gold-antibody probe commercially available. It offers improved labeling density and greater staining of hard-to-reach antigens. Visualization is further intensified when combined with visual enhancing methods such as immunogold silver staining. The pictures in Figure App-D-10 are pictures of Nanogold-Fab’ conjugate using a scanning transmission electron microscope (STEM) and a transmission electron microscope (TEM).
The SBIR program provides vital financial support for product improvement and innovation at Nanoprobes. Soon after the company was founded, it received its first SBIR award, granted by the U.S. Department of Energy in 1991. Since that time the scientists at Nanoprobes have successfully won thirty-five SBIR project awards, mostly from the U.S. National Institutes of Health (NIH). Twenty-five these awards were for Phase I feasibility studies, seven were for product development in Phase II, and three were Fast Track (combined Phase I and II). The awards total over $9 million (in nominal dollars) through 2005. Table App-D-5 lists the date, agency, topic, and phase for Nanoprobes’ SBIR awards (Fast Track awards are identified in the topic field).
According to Dr. Powell, the management at Nanoprobes decided to maintain a steady stream of SBIR grants. This steady stream has been valuable to the company’s success in a variety of ways. The grants have contributed to the firm’s patenting activity. Nanoprobes currently has ten patents and several patent applications pending approval from the U.S. Patent and Trademark Office. The grants have contributed to the maintenance of their academic research ties through subcontracting. They have contributed to the firm’s publication and conference activity, which is vital for establishing credibility with potential partners and investors, marketing, and sales. And, they have contributed to the firm’s
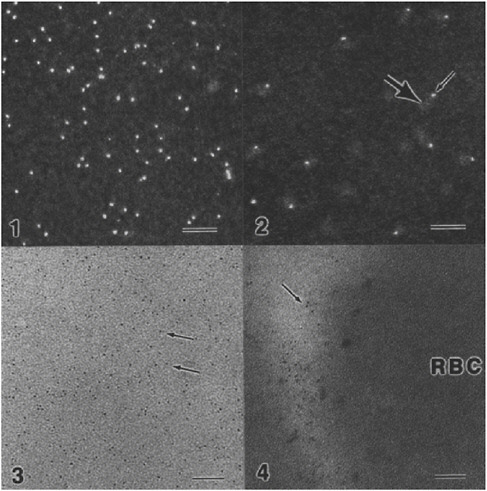
FIGURE App-D-10 (1) STEM darkfield micrograph of 1.4 nm gold particles (bright dots). Full width 128 nm, 500,000 X mag. Bar=20 nm.; (2) STEM darkfield micrograph of Fab’s (thick arrow) with 1.4 nm gold particles (thin arrow) attached. Full width 128 nm, 500,000 X mag. Bar=20 nm.; (3) TEM brightfield micrograph of 1.4 nm gold particles (arrows). 300,000 X. Bar=30 nm.; (4) TEM micrograph of human red blood cell (RBC) with Fab’-1.4 nm gold particles attached (arrow). Magnification=300,000 X. Bar=30 nm.
SOURCE: Nanoprobes Web site, <http://www.nanoprobes.com/MSA92ng.html>.
internal research capabilities by allowing Nanoprobes to broaden and accelerate its R&D process.
These SBIR contributions have impacted the company’s product offerings. Dr. Powell notes that SBIR funds supported modifications and reformulations of the company’s core product, Nanogold. SBIR funds supported part of its work on Undecagold reagents. For this product category, the company actually responded to an SBIR solicitation. For their FluoroNanogold product, Dr. Powell notes
TABLE App-D-5 Nanoprobes’ SBIR Awards
|
Nanoprobes, Inc., SBIR Award History |
||||
|
Year |
Funding Agency |
Topic |
Phase I |
Phase II |
|
1991 |
DoE |
Ultrasensitive detection system for DNA sequencing |
YES |
NO |
|
1991 |
NIH |
Amplification of silver-gold immuno and DNA probes |
YES |
NO |
|
1992 |
NIH |
Combined fluorescent and gold immunoprobes |
YES |
YES |
|
1993 |
NIH |
Gold coupled nucleotides for ultra sensitive probes |
YES |
NO |
|
1993 |
NIH |
Large metal cluster and cluster-polymer immunoprobes |
YES |
YES |
|
1993 |
NIH |
Caged metal cluster and colloid immunoprobes |
YES |
YES |
|
1994 |
NSF |
Molecular cytoskeletal microscopy probes and biological wires |
YES |
NO |
|
1995 |
NSF |
Gold & combined fluorescent/gold labels for solid-phase DNA synthesis |
YES |
NO |
|
1996 |
NSF |
Metal cluster and combined fluorescent/gold labels for peptide synthesis |
YES |
NO |
|
1996 |
NIH |
Nickel chelating polyhistidine specific molecular probe |
YES |
NO |
|
1997 |
NIH |
Heavy atom clusters for membrane protein derivatization |
YES |
NO |
|
1997 |
NIH |
Fluorescent and large metal cluster combination probes (FAST TRACK) |
YES |
YES |
|
1998 |
NIH |
Signal amplification by catalytic nanogold deposition |
YES |
NO |
|
1999 |
NSF |
Autometallographic fabrication of copper interconnects |
YES |
NO |
|
1999 |
NIH |
Luminescent lanthanide chelates labels for immunoassays |
YES |
NO |
|
1999 |
NIH |
Large covalent gold labels and probes (FAST TRACK) |
YES |
YES |
|
2000 |
NIH |
Nonradioactive southern blotting detection system |
YES |
NO |
|
2000 |
NIH |
Metal enhanced radiation therapy |
YES |
NO |
|
2000 |
NIH |
Gold quenched molecular beacons |
YES |
YES |
|
2001 |
NIH |
Enzymatic metallography for biological detection |
YES |
YES |
|
2001 |
NIH |
Durable emitters for nanospray mass spectrometry |
YES |
YES |
|
2002 |
NIH |
Live cell correlative imaging probes |
YES |
NO |
|
2002 |
NIH |
Improved MRI contrast agents |
YES |
YES |
|
2003 |
NIH |
Reiterative signal amplification by gold deposition |
YES |
NO |
|
2004 |
NIH |
Correlative chromogenic gene and protein assessment |
YES |
NO |
|
2004 |
NIH |
Gold enhanced angiography (FAST TRACK) |
YES |
YES |
|
2005 |
NIH |
Nanogold enhanced head & neck cancer radiotherapy |
YES |
N/A |
|
2005 |
NIH |
Correlative Enzymatic and gold probes |
YES |
N/A |
|
SOURCES: U.S. Small Business Administration; National Institutes of Health; and National Science Foundation. |
||||
that it “owes the most to the SBIR program.” In 1992, the NIH funded Phase I research into combined fluorescent and gold immunoprobes. This research was further supported by a Phase II grant with financing in 1994 and 1995.
SBIR has also enabled Nanoprobes to investigate the applications of its core technologies to improved public health. One such technology, “Enzyme Metallography” has proven to be a better detection method for pathological assessment of human biopsy material. In collaboration with the Cleveland Clinic, this has been used to develop a test for Her-2/neu breast cancer, an important marker for
aggressive malignant behavior that occurs in about 30 percent of breast cancer cases. Due to the improved detection, this technology was recently licensed to Ventana Medical Systems, Inc., which makes automated tissue staining equipment used in many hospitals and testing labs worldwide. This test is expected to be released in the next 1-2 years worldwide and should result in improved detection and management of this condition by helping to identify patients suitable for treatment with the very promising therapeutic, Herceptin. The SBIR program played a key part by enabling the research to be carried out for this development.
Nanoprobes is also investigating the use of gold nanoparticles in vivo (at the animal stage) as X-ray contrast agents for better visualizing coronary disease and tumors. Gold absorbs X-rays more strongly than current iodine agents and stays in the blood longer, allowing better images to be obtained and potentially enabling the noninvasive assessment of coronary arteries and earlier detection of tumors. In addition, Nanoprobes is investigating the use of gold nanoparticles to enhance radiotherapy. Since gold absorbs x-rays, its presence in tumors can increase the specific dose. This approach has yielded promising results in mice, where one study resulted in 86 percent long term (>1 year) survival, vs. 20 percent without gold. The SBIR program has been absolutely necessary to provide capital for these high-risk, early-stage studies.
KEY SBIR HIGHLIGHTS
Dr. Powell is very positive about the role and contribution of the SBIR Program to the success of Nanoprobes. He says it is a “tremendously good program” that has allowed the company to become “more sophisticated and successful.” Dr. Powell highlights the following three benefits of the SBIR program for Nanoprobes:
(1)
Key Source for Building Research Capabilities
In the beginning, personal funds were used to get Nanoprobes started. However, for small science-intensive companies, it is important to achieve a critical mass of research personnel and equipment to sustain the firm going forward. SBIR funds are an important source of capital for this process, especially Fast Track awards. Fast Track awards decrease the uncertainty the firm faces by providing a longer period of continuous funding. They know in advance how much money will be coming and for how long and can plan more ambitious and longer-term projects.
(2)
SBIR Financing Provides Flexibility
SBIR funds are more flexible than other sources. Dr. Powell notes that these grants provide a “degree of creative freedom that one does not normally get with venture capital funding.” This is important since opportunities change as research moves forward. Another aspect of this flexibility is that SBIR projects allow the firm to explore higher risk research avenues.
(3)
An Important Source of Credibility
Small firms, especially start-ups, must somehow achieve credibility in the marketplace. Potential buyers and business partners are skeptical about the capabilities of small firms and the claims they make about their products. Over time, the business strategy at Nanoprobes is shifting toward licensing and partnering. SBIR helps overcome the credibility barrier.
ISSUES WITH THE CURRENT SBIR PROGRAM
Dr. Powell does not see any significant problems with the SBIR Program. He made the following point about the current program.
Onerous Application Process
Over time, the SBIR application process has become more complicated. A complete Phase I application can now be up to sixty-four pages long. It requires too much time to prepare. Further, the recent movement to the “grants.gov” online application system was very demanding.
Neurocrine, Inc.15
Robin Gaster
North Atlantic Research
EXECUTIVE SUMMARY
Background
Neurocrine is a drug development/biotech company, and one of the largest companies in the study. Publicly traded on the NASDAQ, and with a market cap of about $1.5 billion, Neurocrine has approximately 450 employees, has been heavily backed by venture capital from inception in 1992, and recently moved into a brand new purpose-designed campus. It has no products on the market but several in the pipeline, two very close to market.
Neurocrine is located in an R&D hub (San Diego), is not woman or minority owned, was venture backed, and while a multiple winner generates only a small percentage or R&D funds from SBIR.
SBIR History and Status
Since 1992, Neurocrine has received 22 Phase I awards and 14 Phase II awards, generating a total of just over $10 million in SBIR funding. (See annex.) In 2004, Neurocrine became ineligible for further SBIR funding in light of the recent interpretation of ownership regulations, as Neurocrine is 89 percent owned by institutional investors.
Key Utilization of SBIR
Neurocrine does not use SBIR for projects within the company’s critical development path. SBIR is however seen as an important source of discretionary funding that allows for more speculative or longer-term research, or research on alternative mechanisms for achieving critical path results. This research has in some cases subsequently led to internally funded research and to integration into Neurocrine’s primary product pipeline.
Key Issue/Concern
The new interpretation of SBA guidelines, which Neurocrine continues to vigorously contest (Neurocrine in fact continues to apply for SBIR awards). Also, Neurocrine is concerned about a perceived shift in the Phase II application process, where it believes reviewers now require much more detailed technical information, which constitutes a risk to critical company intellectual property (IP).
Outcomes:
-
Products: none yet.
-
Commercial pipeline: products on the way, some with significant input from SBIR.
-
Knowledge: at least 30 papers and several patents based directly on SBIR.
-
Employment: dramatic expansion, not directly related to SBIR.
Recommendations/Comments:
-
Eliminate commercialization plans from review process from both Phase I and Phase II.
-
Reconsider VC and large drug company participation on panels.
-
Reconsider demands for increasingly detailed IP during Phase II application process.
-
Increase size/duration of Phase I awards to $300,000, for one year.
-
Increase size of Phase II to $1 million per year.
-
Reduce duration of Phase II awards: two year maximum, with second year entirely conditional on meeting year one milestones.
Additional Lessons Learned
Neurocrine’s story appears to indicate that there is considerable confusion at NIH and in reviewer panels about the focus of SBIR within the product development cycle. On the one hand, increased focus on commercialization inevitably means pressure to move downstream toward products; commercialization plans based on very early basic research are not defensible. On the other, Neurocrine had an otherwise high scoring proposal rejected as being too close to the market.
BACKGROUND
Neurocrine started with $5 million in venture funding in 1992-1993, raised $50 million though a series B offering in 1993-1994, made three major partner-
ship deals amounting to well over $100 million in funding in 1994-1995, and completed an IPO in 1996. Today, Neurocrine is a public company more than 89 percent owned by institutional investors, with a market capitalization of approximately $1.5 billion on the NASDAQ, and cash reserves of more than $300 million, against debt of approximately $66 million. The company recently moved to a large new custom built campus in San Diego.
Employment
Neurocrine has always been a much larger company than the norm for SBIR. By 1993, a year after founding, it already employed 25-30 people, reaching 100 in 1996. Currently, Neurocrine employs about 450 people, and will soon be above the SBIR limit of 500 for small companies.
Products
To date, the company has no products that have reached market, although a recent filing problem with FDA is being resolved and Indiplon, a new drug for insomnia, is expected to reach the market in approximately 14 months. Indiplon has two formulations that have completed all clinical trials with apparent success.
The outcomes described above help to define both the importance of SBIR to Neurocrine and some limitations. Of the 11 programs now making their way toward or through clinical trials, 5 received significant support from SBIR. As Conlon noted, the primary pipeline is not dependent on SBIR, but at the same time SBIR has opened the door to research that is clearly now part of that primary pipeline.
TABLE App-D-6 Research and Development Pipeline
|
Program |
Targeted Indication |
Status |
SBIR |
|
Indiplon IR |
Insomnia |
Filed |
NO |
|
Indiplon MR |
Insomnia |
Filed |
NO |
|
GnRH Antagonist—56418 |
Endometriosis |
Phase I |
YES |
|
Altered Peptide Ligand |
Multiple Sclerosis |
Phase II |
YES |
|
Altered Peptide Ligand |
Type 1 Diabetes |
Phase II |
NO |
|
D2 Receptor Agonist |
Sexual Dysfunction |
Phase II |
NO |
|
Urocortin II |
Cardiovascular/Endocrine |
Phase I |
NO |
|
CRF R1 Antagonist |
Anxiety |
Phase I |
YES |
|
Melanocortin Receptor Agonist/Antagonist |
Obesity/Cachexia |
Preclinical |
YES |
|
Melanin Concentrating Hormone Antagonist |
Obesity |
Preclinical |
YES |
|
Sleep Program |
Insomnia |
Preclinical |
NO |
|
SOURCE: Neurocrine, Inc. |
|||
PHASES IN NEUROCRINE’S USE OF SBIR
SBIR never had a real financial impact at Neurocrine, where financial resources were substantial even in the mid-1990s.
Neurocrine’s use of SBIR changed over time, falling into three distinct time periods:
-
Stage 1. Initial Use, 1993-1996. During this period, Neurocrine was at least initially still a relatively small company (about 25 employees in 1994), and was still seeking its intellectual way. While focused on the intersection of biology and chemistry at the cellular level, it had not yet tightened its focus on small molecule bioscience.
Neurocrine received ten Phase I awards between 1994 and 1997, of which eventually became Phase II awards. Paul Conlon, now VP research and development and Principal Investigator on the first awards, noted that they played a key role in allowing Neurocrine to test ideas and explore possible directions for the company.
They also gave Neurocrine valuable credibility when exploring partnerships with other much bigger and more established companies: “Validation was important—being able to say that our work was being funded by the National Institutes of Health, and that it had been approved by a peer review panel, provided tremendous credibility.”
That credibility may have a made a key difference for Neurocrine. In 1994-1995, Neurocrine made deals with three major pharmaceutical companies, including a $70 million agreement with Ciba Geigy. These deals in turn provided the evidence of progress on which to base Neurocrine’s public offering in 1996.
-
Stage 2. Consistent Success. During 1997-2002, Neurocrine “figured out the SBIR application process.” They were now consistently putting together good applications, and their success rate rose substantially. They understood what review panels wanted to see, and they also found that their cutting edge work on small molecules was being well received. The result was a string of Phase I and successor Phase II awards.
These awards now filled a somewhat different function at Neurocrine. With the new focus on small molecules, the earlier search for scientific identity was largely concluded; now SBIR was being used much more directly to explore promising offshoots of core research. For example, awards for work on the GNRH receptor.
-
Stage 3. Difficulties, 2003-2005. In 2003, Neurocrine suddenly found its long string of SBIR successes under pressure, from two directions.
-
First, the application process changed. Neurocrine found that work programs and descriptions that had been sufficiently detailed for success during stage 2 were now challenged by panel members seeking much more specific detail. Neurocrine strongly believes that this change accompanied changes in the composition of review panels, with the introduction of members from major pharmaceutical companies. Essentially, Neurocrine believes that this change puts its crown jewels of intellectual property at risk. (see box App-D-2). After substantial negotiations, a compromise was reached in the case of one application, but it became moot for reasons described immediately below.
-
Second, Neurocrine was ruled ineligible for further SBIR awards as a public company that was more than 50 percent owned by institutional investors (Neurocrine is in currently owned 89 percent by institutions). This resulted from the new interpretation of existing SBA statutes and regulations, implemented at NIH in 2003.
-
The changing application process is taken very seriously at Neurocrine, for which protection of its IP is central. Neurocrine notes that it makes no sense to jeopardize major potential partnership agreements for $50 million or more, to pursue a $1 million SBIR award. Neurocrine would walk away from SBIR altogether before risking its IP.
Clearly, Neurocrine is different from the majority of small and poorly funded SBIR companies, who may not have any alternatives to SBIR funding. However, even if smaller companies have little alternative to accepting these new demands from reviewers, they may still be unfair, and they may still pose long-term problems for the NIH SBIR program. Other interviews may help to determine whether this is an unusual case, or whether there has been a real change in requirements from review panels. Neurocrine is arguing from two cases in 2003.
STRATEGIC ROLE OF SBIR AT NEUROCRINE
Neurocrine has clearly used SBIR to its advantage. However, Neurocrine sees SBIR as filling a very specific function, and one that is not at the very core of Neurocrine’s research program. SBIR provides discretionary funding to allow research into promising offshoots and alternatives that likely would not otherwise get done.
But this funding is and must be unprogrammed within the company precisely because it is high risk. According to Conlon, no company can afford to place SBIR at the heart of its research program because the money is unpredictable (although of course many smaller companies do precisely that). So for companies
that do have other resources—and Neurocrine has many, not least $300 million in cash in the bank—SBIR is useful funding that allows interesting and sometimes important work (at least in retrospect) , but does not fund core research of critical strategic importance to the company.
Example 1: CRF research funding through SBIR allowed exploration of a range of possible applications, for anxiety, IBS, depression. And further SBIR funding allowed the company to explore R1 and R2 receptors, identifying ways to separate out the different R1 and R2 receptor sites. This exploration would not have been possible without SBIR.
Example 2. Neurocrine’s core MC4 program was focused on researching agonists for use in treating endometriosis. SBIR funding allows the company to explore an alternative mechanism—antagonists—which resulted in a new program focused on treating a related disease, Cachexia. Again, SBIR funding supported new research directions.
Example 3. SBIR funding paid for upgrading Neurocrine’s proprietary library of GPCR molecules. While core work is based on that library, its function is to provide better leads for small molecule efforts. However, even with better leads, it takes two years to turn a lead into a drug candidate.
In several cases, Neurocrine has made significant use of SBIR even when not receiving Phase II awards (and in one case, Neurocrine withdrew its application for Phase II funding after being given an award, as the company changed strategy), (e.g., Nonpeptide Antagonists of CCR-7 for Immunosuppression. The Phase II was funded, but not included in the list of awards, as Neurocrine declined the monies.
Legitimation Effects. Conlon believes not only that SBIR lent important legitimacy when talking to potential partners, but that receiving their first Phase II —after a number of awards ended at Phase I—provided important internal validation that the company was on the right track (and given the early involvement of venture capital, probably helped validate the company with funders as well).
COMMERCIALIZATION AND THE REVIEW PROCESS
Neurocrine is deeply dissatisfied with the actual impact of efforts to improve commercialization review at NIH, and in fact now recommends that on balance commercialization review should be eliminated, and NIH should return to simply funding the best science.
Neurocrine offered a number of objections to the current approach:
-
Timing. Even Phase II is too early for an effective commercialization plan. For Neurocrine, Phase II is about narrowing down candidates for further drug development. At Phase II application, the market may be 8-12 years of further development away—a period which will require a
-
partnership with a major drug company and many millions of dollars. It is hard to see what value any commercialization plan might have at this stage.
-
Reviewer Capacity and Conflicts of Interest. Few academic reviewers have any effective capacity to review commercial plans. And Neurocrine was clearly very perturbed about potential conflicts of interest stemming from the addition of reviewers from major drug companies.
-
Less Focus on the Quality of Science. The new emphasis on commercialization means by definition that less attention is paid to the quality of the science.
-
Phasing. If commercialization plans for Phase II are impractical, Phase I plans are even less realistic. Neurocrine thought they should be eliminated.
Example
Neurocrine’s Phase I award to research development of TCR antagonists to MBP-reactive t-cells generated an extraordinarily high score of 131 at Phase I. After successfully completing Phase I, Neurocrine submitted a Phase II application. This was rejected primarily (according to Neurocrine) on commercialization grounds—reviewers claimed that the project was too development oriented, too close to market. However, within months, Neurocrine had reached agreement to develop the project further through a $ partnership worth approximately $70 million with Ciba Geigy. Neurocrine sees this as evidence that the commercialization assessment process is fatally flawed. However, it is also possible to argue that the reviewer was correct—and that the Ciba Geigy deal provides that the product was indeed sufficiently developed to receive fully commercial funding.
Neurocrine has also noted considerable confusion at NIH about the relationship of SBIR awards to product development cycles. In the example above, the product is still years away from the market, so it is hard to see how it could be too commercial.
OTHER ISSUES
In discussing SBIR with Conlon and Maki, it became apparent both that the company is very experience with and sophisticated about SBIR. Maki has served on NIH study sections, but only for RO1s, not SBIR. However, understanding of the new competing continuation awards was still limited.
Neurocrine saw no significant differences between the ICs from the perspective of applicants; had generally had excellent experiences with program staff at all ICs.
A final note. Neurocrine’s experience confirms once again that resubmis-
sion is a normal part of the NIH SBIR process; Conlon expects to resubmit on a regular basis.
RECOMMENDATIONS
Neurocrine had a number of recommendations for improving the SBIR program:
-
Commercialization plan. Even though Neurocrine scores reasonably well on commercialization plans, as it has a large business development unit that writes these plans, it believes that they in the end simply randomize panel review results. The difficulties of getting good commercialization reviews in their view much more than outweigh the benefits, and Neurocrine believes that panels should go back to simply reviewing the science. This would also resolve important difficulties with the make-up of panels.
-
Study sections. If commercialization reviews are not eliminated, Neurocrine believes changes should be made in the composition of review panels, and that venture capitalists should be excluded (and possibly representatives from large drug companies).
-
Track record. Neurocrine rejected possible mechanisms for ensuring that past track record would be taken into account during panel discussions.
-
Size/Duration (Phase I). Neurocrine would be willing to trade off significantly larger Phase I awards for significantly fewer of them: $300K awards, with 1/3 as many would be appropriate.
-
Size/Duration (Phase II). Neurocrine is not at all convinced about the need for longer awards, but believes both in larger awards and in more pay for performance. It suggested that awards should be $1 million per year for two years, with the second year being completely conditional on achieving specific research milestones.
-
Direct access to Phase II. Neurocrine strongly agreed that companies should be allowed to apply directly for Phase II awards, without going through Phase I first. They pointed out that by definition this would bring better quality projects now excluded into the program.
|
BOX App-D-2 Patenting Neurocrine has filed numerous patents during the past ten years. It regards protection of IP very seriously. However, the new demands for specific structural details of proposed molecules to be developed during Phase II projects are difficult to handle because the company is not yet in a position to file patent applications for several reasons:
From Neurocrine’s perspective, these new demands would mandate withdrawal from the SBIR program were they to be fully implemented (i.e., were detailed structures to become a necessary part of SBIR filing). Of course, Neurocrine has other resources. Many smaller companies do not. |
NEUROCRINE—ANNEX
TABLE App-D-7 Neurocrine SBIR Awards and Outcomes
|
Year |
Fiscal Year |
Phase Type |
Award Size ($) |
Project Title |
Funding Institute-Center |
Notes/Outcomes |
|
Phase II |
||||||
|
1 |
1994 |
Phase I |
77,250 |
COMBINATORIAL ORGANIC SYNTHESIS OF A CRF-R ANTAGONIST |
NS |
Research assisted in the development of a CRF-1 Receptor antagonist that showed efficacy in depressed patients in the clinic. However, the compound was discontinued due to toxicological findings. Additional CRF-1 antagonists are presently being evaluated in the clinic. |
|
2 |
1996 |
Phase II |
384,159 |
COMBINATORIAL ORGANIC SYNTHESIS OF A CRF-R ANTAGONIST |
NS |
|
|
3 |
1997 |
Phase II |
365,804 |
COMBINATORIAL ORGANIC SYNTHESIS OF A CRF-R ANTAGONIST |
NS |
|
|
1 |
1994 |
Phase I |
77,250 |
CRF BINDING PROTEIN AND DEMENTIA |
NS |
Effort discontinued in preclinical studies due to lack of progress. |
|
02A1 |
1997 |
Phase II |
375,000 |
CRF BINDING PROTEIN AND DEMENTIA |
NS |
|
|
3 |
1998 |
Phase II |
375,000 |
CRF BINDING PROTEIN AND DEMENTIA |
NS |
|
|
1 |
1995 |
Phase I |
100,000 |
NOVEL CRF—RECEPTOR |
NS |
CRF-2 was identified, characterized, and is still an area of active interest in our company. To date however, no small molecule compounds have progressed into clinical trials. |
|
2 |
1998 |
Phase II |
398,300 |
NOVEL CRF RECEPTOR |
NS |
|
|
3 |
1999 |
Phase II |
351,700 |
NOVEL CRF RECEPTOR |
NS |
|
|
Year |
Fiscal Year |
Phase Type |
Award Size ($) |
Project Title |
Funding Institute-Center |
Notes/Outcomes |
|
1 |
1996 |
Phase I |
100,000 |
CRF BINDING PROTEIN AND OBESITY |
DK |
Effort discontinued in preclinical studies due to lack of progress. |
|
2 |
1998 |
Phase II |
595,586 |
CRH BINDING PROTEIN AND OBESITY |
DK |
|
|
3 |
1999 |
Phase II |
570,105 |
CRH BINDING PROTEIN AND OBESITY |
DK |
|
|
01A1 |
1997 |
Phase I |
100,000 |
CRF RECEPTOR ANTAGONISTS IN NEUROPROTECTION |
NS |
Research assisted in the development of a CRF-1 Receptor antagonist that showed efficacy in depressed patients in the clinic. However, the compound was discontinued due to toxicological findings. Additional CRF-1 antagonists are presently being evaluated in the clinic. |
|
2 |
2000 |
Phase II |
635,649 |
CRF RECEPTOR ANTAGONISTS IN NEUROPROTECTION |
NS |
|
|
3 |
2001 |
Phase II |
666,805 |
CRF RECEPTOR ANTAGONISTS IN NEUROPROTECTION |
NS |
|
|
1 |
2000 |
Phase I |
240,527 |
A SCREENING LIBRARY FOR PEPTIDE-ACTIVATED GPCRs |
GM |
An acitve area of research within the company to improve the quality and quantity of small molecule leads. |
|
2 |
2002 |
Phase II |
889,087 |
A Screening Library for Peptide-Activated GPCRs |
GM |
|
|
3 |
2003 |
Phase II |
638,359 |
A Screening Library for Peptide-Activated GPCRs |
GM |
|
Year |
Fiscal Year |
Phase Type |
Award Size ($) |
Project Title |
Funding Institute-Center |
Notes/Outcomes |
|
1 |
2000 |
Phase I |
99,931 |
NOVEL NON-PEPTIDE ANTAGONIST OF THE GNRH RECEPTOR |
HD |
Research assisted in the development of a GnRH Receptor antagonist that has recently shown efficacy in Phase I studies. |
|
2 |
2001 |
Phase II |
471,580 |
Novel Non-Peptide Antagonists of the GnRH Receptor |
HD |
|
|
3 |
2002 |
Phase II |
471,580 |
Novel Non-Peptide Antagonists of the GnRH Receptor |
HD |
|
|
1 |
2001 |
Phase I |
138,733 |
NOVEL NON-PEPTIDE ANTAGONIST OF THE MCH RECEPTOR |
DK |
Research assisted in the development of a MCH Receptor antagonist that is still being evaluated preclinically. |
|
2 |
2003 |
Phase II |
466,776 |
Novel non-peptide antagonist of the MCH receptor |
DK |
|
|
|
|
8 |
8,589,181 |
|
|
|
|
Phase I Only |
||||||
|
1 |
1994 |
Phase I |
75,000 |
DEVELOPMENT OF TCR ANTAGONISTS TO MBP-REACTIVE T-CELLS |
NS |
Research assisted in the development of an APL that is in Phase II clinical studies. |
|
01A1 |
1995 |
Phase I |
95,025 |
CRF RECEPTOR ANTAGONISTS FOR TREATMENT OF COCAINE ABUSE |
DA |
Research assisted in the basic understanding of CRF-1 Receptor antagonist. |
|
01A1 |
1995 |
Phase I |
100,000 |
ANTI INFLAMMATORY/EFFECTS OF CRF BINDING PROTEIN |
AR |
Effort discontinued in preclinical studies due to lack of progress. |
|
1 |
1996 |
Phase I |
99,634 |
NOVEL TYPE III INTERLEUKIN 1 RECEPTOR |
AI |
Effort discontinued in preclinical studies due to lack of progress. |
|
Year |
Fiscal Year |
Phase Type |
Award Size ($) |
Project Title |
Funding Institute-Center |
Notes/Outcomes |
|
1 |
1997 |
Phase I |
100,000 |
IGFBP 3 LIGAND INHIBITORS FOR THE TREATMENT OF DIABETES |
DK |
Effort discontinued in preclinical studies due to lack of progress. |
|
01A1 |
2000 |
Phase I |
133,067 |
DEVELOPMENT OF A TOXIC FUSION PROTEIN FOR BRAIN TUMORS |
CA |
Research assisted in the development of a Fusion Toxin that has been through Phase II clinical studies. |
|
01A1 |
2000 |
Phase I |
99,390 |
DEVELOPMENT OF NEUROPROTECTIVE DRUGS FOR RETINAL DISEASE |
EY |
Effort discontinued in preclinical studies due to lack of progress. |
|
1 |
2001 |
Phase I |
207,018 |
Melanocortin-4 Receptor Antagonists |
CA |
Research assisted in the development of a MC-4 Receptor antagonist that is still being evaluated preclinically. |
|
1 |
2002 |
Phase I |
133,540 |
CRF-Type-2 Receptor Antagonists for Anxiety |
MH |
Research assisted in the basic understanding of CRF-2 Receptor antagonist. It is still an active area of interest, however no small molecule antagonists have been identified to date. |
|
01A1 |
2002 |
Phase I |
137,959 |
Nonpeptide Antagonists of CCR-7 for Immunosuppression |
NS |
Effort discontinued in preclinical studies due to lack of progress. |
|
01A1 |
2002 |
Phase I |
130,048 |
Novel CRF antagonists for inflammation and pain |
AR |
Research assisted in the basic understanding of CRF-1 Receptor antagonist. |
|
Year |
Fiscal Year |
Phase Type |
Award Size ($) |
Project Title |
Funding Institute-Center |
Notes/Outcomes |
|
1 |
2002 |
Phase I |
139,336 |
Novel non-peptide agonists of the melanocortin receptor |
DK |
Research assisted in the development of a MC-4 Receptor agonist that is still being evaluated preclinically. |
|
1 |
2002 |
Phase I |
139,497 |
Selective CRF Antagonists for Bowel Disorders |
DK |
Research assisted in the basic understanding of CRF-1 Receptor antagonist. |
|
1 |
2002 |
Phase I |
170,479 |
Corticotropin Releasing Factor Receptor Function in CNS |
MH |
Research assisted in the basic understanding of CRF-1 Receptor antagonist. |
|
|
|
14 |
1,759,993 |
|
|
|
|
NOTE: For a list of codes for National Institutes of Health institutes and centers, see Box App-A-1. SOURCE: Neurocrine, Inc. |
||||||
Optiva, Inc.16
Robin Gaster
North Atlantic Research
COMPANY HISTORY
Optiva was founded in 1988 by David Giuliani, an entrepreneur formerly in management at Hewlett Packard,17 and two faculty members at the University of Washington in Seattle, Drs. David Engel and Roy Martin.
The company (originally called GEMTech) was founded to pursue the idea of a dental hygiene device using a piezoelectric multimorph transducer that worked on sonic principles, using sound waves to dislodge plaque. Such an approach could have significant advantages, for example, in addressing plaque below the gum line.
Early financing came from the founders, and was used to develop the original technical ideas. However, three years of effort and prototypes convinced the team that the original technology could not be made into a commercial product. Instead, they adopted an alternative technology based on activating water in the mouth by use of sonic technology somewhat akin to a tuning fork. This approach was compatible with another objective—putting all the moving parts in the head of the toothbrush, so that the more expensive body could be sealed against water leakage.
After considerable experimentation, the team determined that when tuned to 520 vibrations per second, the vibrating brush head generated fluid dynamics that would erode plaque beyond the reach of the brush itself.
To commercialize the product, Giuliani raised $500,000 from 25 private investors, and also benefited from an NIH SBIR award which effectively doubled the size of the investment.
The company faced both very substantial opportunities, and significant challenges. Almost all Americans suffer from periodontal disease at some point, so the potential market was very large. Even the existing market was substantial—12 percent of Americans used electric toothbrushes, generating a total market of $125 million annually.
However, that existing market was dominated by very strong brands owned by large companies—Braun, Bausch and Lomb, Teledyne. Also, the Sonicare® toothbrush was more expensive to make, and would have to sell for considerably more than standard electric toothbrushes ($129, vs. $50-70 for other brands).
Optiva therefore decided to focus on dentists as the critical intermediary between the company and consumers.
The Sonicare® toothbrush was launched at a periodontal convention in Florida in 1992. According to the company history, the first dentist to visit the booth bought 36, and the company sold 70 altogether. Optiva hired a sales manager.
This approach proved very successful. Using studies (some funded by SBIR) that demonstrated the benefits of Sonicare® technology, dentists proved interested; 98 percent of those who tried the product recommended it to their patients,

FIGURE App-D-11
SOURCE: Optiva.
and some even signed on a resellers. Optiva began advertising in dental journals, and formed a small sales force to reach out to dentists.
This was a taste of the very rapid success to come. Again according to the company, by 1995 more than one-third of dentists in the U.S. were recommending Sonicare®. Consumer-direct marketing was also expanding rapidly, as Shaper Image featured Sonicare® products in their catalog and in their stores. GEMTech changed its name to Optiva.
The company’s sales strategy focused on dentists, partly mandated by its situation as small under-capitalized company without distribution agreements. For Giuliani, leverage was key: he developed the company’s core strategic thrust of “borrowing other assets and using them to build the company’s reputation. The reputation of dentists was what we leveraged. This approach took advantage of the dentists’ patient contacts and patients’ trust in their dentists.” Giuliani went on to note that “Borrowed reputation had to be returned with interest—through a product that dentists were confident in and proud to be related to.”
Starting in 1992, the company sought to move beyond its dentist-based sales strategy, into direct consumer marketing. Initial efforts at direct mail failed resoundingly (the product was too complex to explain in a page), but in 1993 Sharper Image ordered 4,000 units and then 16,000 more a few months later—a huge sale at the time, which stretched the company’s manufacturing and fulfillment operation to the limit.
Following the Sharper Image sale, consumer word of mouth purchases expanded rapidly, bolstered by a strong endorsement from Oprah Winfrey on her TV show.
In 1994, Optiva received a patent for «high-performance acoustical cleaning apparatus for teeth.»18 This effectively blocked competitors such as Teledyne (which had to settle a subsequent patent infringement case).
Optiva also boosted both manufacturing capacity—including redesigns which cut manufacturing costs by 60 percent—and sales capacity,. It added 50 manufacturer reps working on commission to pursue sales at Costco and other high volume outlets, as well as specialty stores like Brookstone. Approximately 25,000 retail stores were stocking the product. Optiva also continued to focus on dentists, claiming that by the end of 1995, one-third of all U.S. dentists were recommending Sonicare®.
By 1996, Optiva and Sonicare® were being recognized as a major U.S. success story: Giuliani was invited to breakfast at the White House in May 1996, where the company was cited for its exemplary employment practices and employee benefits. Giuliani and was named SBA’s Small Business Person of the Year in 1996.
In 1999, Optiva Corporation relocated its headquarters and manufacturing operations from Bellevue, Washington, to a new 176,000-square-foot, state-of-
the-art facility in Snoqualmie, Washington. The company also launched its television ad campaign featuring a decidedly unconventional Tooth Fairy, and it ended the year with more than 600 employees.
In October 2000, Philips Domestic Appliances and Personal Care (DAP), a division of Royal Philips Electronics acquired Optiva Corporation. In January 2001, Optiva Corporation changed its name to Philips Oral Healthcare, Inc. With the combined resources of the former Optiva Corporation and its new owners, Philips DAP, the company set forth to leverage its tremendous research and development capabilities to create the next generation of power toothbrushes. By the end of 2001, the company produced its 10 millionth Sonicare® and became the #1 rechargeable power toothbrush in the United States.
According to Giuliani, the sale to Philips substantially benefited the former owners, who were able to cash out, the new owners who gained a proved and market leading technology which has become a center piece for their dental product line, and the asset itself, where the company’s limited ability to maximize commercial value was dramatically improved by Philips which brought to the table major international marketing capabilities. For founders and company employers, this also provided significant emotional return on the original investment. Overall, some years after the sale, Giuliani continues to see this as an excellent outcome for the company. As he observed, “You want your child to marry well.”
OUTCOMES
Sonicare® has been a huge commercial success. It currently serves about one-third of the U.S. market for electric toothbrushes. By 1996, it was selling 1 million brushes annually, and generating revenues of more than $70 million.
In October 1997, Optiva was named the fastest growing private company in the country by Inc Magazine, topping the Inc 500 list. By January 2001, shortly after the sale to Philips in 2000, the company was generating approximately $200 million annually in revenues, and employed more than 600 people in Snoqualmie, Washington, where the company’s headquarters and manufacturing facilities are located.19 Although the size of the acquisition transaction was not publicly revealed, industry sources estimated that it was worth more than $1 billion.
Philips has strongly backed the product line, continuing to introduce new products, and has retained the Snoqualmie operation as world headquarters for the division. Philips has also leveraged its international capabilities—noting for example that after successful launches, more than 65 percent of UK dentists recommend Sonicare®, and that the product had captured 21 percent of the Dutch market four months after launch in the Netherlands.20
|
19 |
“Sonic toothbrush maker takes acquirer’s name,” Puget Sound Business Journal, January 8, 2001. |
|
20 |
<http://www.homeandbody.philips.com/sonicare/gb_en/03d-story.asp>. |
The product has also clearly had a significant impact on public health. It is in use in a very substantial number of homes, and studies show that it provides better results than a manual toothbrush (e.g., it removes about 40 percent more plaque21).
SONICARE®TECHNOLOGY
The technology behind Sonicare® has been validated in extensive academic studies. According to Philips Sonicare® Division, there have been 85 published studies by 119 researchers at 40 universities.22 These studies have covered a wide range of topics related to Sonicare®, including:
-
Plaque removal.
-
Gum health.
-
Biofilm removal.
-
Dental hypersensitivity.
-
Stain removal.
-
Dry mouth.
According to Sonicare®, the technology achieves its bristle velocity through a combination of high frequency and high amplitude bristle motion. This velocity generates dynamic fluid action, which is gentler on dentin than a manual or an oscillating toothbrush. The cleaning power of dynamic fluid action, coupled with the specially designed bristle orientation, results in deep penetration of interproximal spaces, where the “shear force” of the fluids help dislodge the biofilm.23
Thus like other electric toothbrushes, the primary mode of cleaning produced by a sonic toothbrush is created by the scrubbing action of the brush’s bristles on the surfaces of teeth. However, Sonicare® also produces a secondary cleaning action founded in the intense speed at which the bristles of the sonic toothbrush vibrate. This vibratory motion imparts energy to the oral fluids that surround teeth (such as saliva). The motion of these agitated fluids can dislodge dental plaque, even beyond where the bristles of the toothbrush actually touch.
The brush head of the toothbrush is designed to vibrate at over 30,000 brush strokes per minute. This high speed brushing motion creates movements in the fluids that surround the teeth, creating fluid pressure and shear forces. These fluid dynamics can dislodge dental plaque in hard-to-reach areas between teeth and below the gum line. The cleaning effect of these fluid forces has been measured to occur at distances of up to 4 millimeters (slightly more than one-eighth of an inch) beyond where the bristles of the sonic toothbrush actually touch.
|
21 |
K. Moritis, M. Delaurenti, M. R. Johnson, J. Berg, and A. A. Boghosian, “Philips Oral Healthcare,” American Journal of Dentistry, 15 (Special Issue): 23B-25B, 2002. |
|
22 |
|
|
23 |
<http://www.sonicare.com/dp/why_reco/why_reco_superior.asp>. |
It is worth noting that this secondary action is considerably less important overall than the actual brush impact (which is shared with all toothbrushes, although nonsonic brushes typically generates less than 10,000 brush strokes per minute, or less than one-third the level of Sonicare®). Also there are no studies about long term impacts in terms of plaque removal. However, the unique technology of the Sonicare® product has clearly been the primary differentiator, and is the basis for the product’s very substantial commercialization success.
The Sonicare® technology is still apparently regarded as the gold standard for technology in this area, and has not been successfully replicated by other companies (partly because it is patent-protected).
THE IMPACT OF SBIR FUNDING
Even though Optiva received only one SBIR award (for both Phase I and Phase II), from two applications, Optiva’s need to create a product matched well with the commercialization focus of the SBIR program. (Giuliani noted that the rejected application was focused on methods, not products, and was therefore correctly rejected for being insufficiently commercial).
Phase I SBIR funding had some immediate and significant validation effects for the company, in particular in relation to third-party investors. Giuliani noted that “Once we got notice of our award, it provided important validation of scientific merit for potential investors. Even the Phase I award helped us to close investment deals. And our Investor presentations afterwards included the pink comment sheet from NIH.”
Phase II funding came at a critical time for the company. Just as the company was moving toward productization, significant clinical research and validation was required. SBIR funding helped specifically meet that need. Without SBIR funding, Giuliani believed that the company “Would have had less success; SBIR money and the SBIR process played a significant role in the company’s early success.”
The SBIR provided substantial additional benefits to the project, beyond the funding. It focused the research team onto being able to present a cogent explanation of its product and the latter’s potential unique benefits to society. According to Giuliani, the SBIR application “forced us to take our existing, less coherent approach and mold it into a better project.”
The SBIR award also played in important role in helping the company to gain the trust of its core market—the dentists. Giuliani had researched the dental care industry, and noted that “no new tech had ever succeeded in home dental care without backing of dental community.” Dentists became the critical intermediary between the company and the marketplace, and the NIH award was once again an important step in validating the product.
In addition, the SBIR application process generated very important advice and contacts. Most unusually, the study section visited both the company and
the University of Washington. This brought the company into contact with very experienced people with “phenomenal capabilities.” The company prepared very extensively for the site visit, and found the feedback from the visitors hugely helpful.
Giuliani believes that this input as extremely important: “We could not have paid people to do what the visitors did, especially as we had—at the time—no money and no useful contacts. They provided insights that money literally could not buy.”
The visitors brought both genuine interest and also a certain degree of healthy skepticism to the visit. According to Giuliani, the visit had been arranged because the study section was impressed by the potential of the project, and believed that they could help tune company plans through a visit.
EXPERIENCE WITH SBIR
Giuliani had had experiences with SBIR from several perspectives.
Although the impact of SBIR has been highly positive at Optiva, the company subsequently had two or three “bad experiences” with the application process, where applications were poorly understood, or, on the opinion of the applicants, rejected on unsubstantiated or inappropriate grounds. As a result, Optiva ceased applying for SBIR funding (and is of course on longer eligible after its purchase by Philips).
Giuliani has continued to pursue SBIR funding for his new company, Pacific Bioscience, which seeks to adapt sonic technology to skin cleaning. These applications have also been rejected, again on grounds that Giuliani found unconvincing. As a result, he is a strong supporter of the notion that better communication between applicants and reviewers might generate better results and consequently more enthusiasm for the program.
Giuliani also has experience working as a reviewer on study sections. Overall, he was quite impressed. He found the materials were well prepared, that proposers were in general doing a good job of presenting their projects effectively, and that the study section was well organized and reviewers took their work seriously. He noted that there was a considerable amount of group discussion, which was in general not dominated by one person. However, he also noted that there would always be some degree of arbitrariness in the process. There was also a range of quality among the reviews, and that it was clear to him that at least one person—and maybe more—had not read any of the materials in preparation for the study section meeting.
RECOMMENDED IMPROVEMENTS IN SBIR
Most of Giuliani’s comments focused on the selection process, where he was especially concerned by the overall quality of the review, and also by problems with cycle time partly caused by slow responses to review from NIH.
Review for Commercialization
Giuliani noted that there were considerable difficulties in reviewing applications for commercial merit. Specifically, academic reviewers were poorly suited for this function. However, he believed that adding additional layers of review—on the NSF model—while it might improve commercial review would also provide additional burdens for applicants, and might also therefore tend to slow the application process still further.
Instead, he suggested that NIH consider hiring a “genuine business person” as a consultant to the study section. These consultants could read each application for commercial context, and would provide a report to the study section. Such a consultant could provide further support by ranking applications, or even triaging them from a potential investor’s point of view. Such an approach would be educational for the study section, and could increase the comparability of reviews across the entire set of SBIR applications.
Cycle Time
Giuliani was concerned about the slow cycle time, and in particular the delays in receiving reviews. He believed that two improvements might be especially useful:
-
Rebuttal, whereby applicants would be provided with an electronic copy of the lead reviewer’s written summary some period before the meeting of the study section, and would be able to submit a short rebuttal. Giuliani suggested that this approach might help to clarify issues concerning technical aspects of the application, as well as providing some significant additional incentives for reviewers to improve the quality of their reviews.
-
Shortened Response Time. Giuliani was especially concerned that current response times (the time required between the study section meeting and delivery of the pink sheet with comments and scoring to applicants) was still much too long, and that NIH had taken insufficiently advantage of electronic communications tools to shorten the cycle time. He offered two suggestions:
-
Electronic communications replacing written responses.
-
Immediate communication of raw scores to applicants, which would improve company planning around applications.
-
Award size
Giuliani did not favor any increase in funding, either for Phase I or Phase II, nor did he favor the option of allowing the company to apply directly for Phase II skipping Phase I).
Role of Venture Capital in SBIR
Optiva did not receive significant VC funding. According to Giuliani, some had been received rather late in the company’s independent life. An IPO had been planned but the company chose not to pursue it. Overall, venture funders owned less than 10 percent of the company at the time of its sale to Philips. In general, Giuliani supported the current SBA interpretation which excludes companies more than 50 percent owned by institutional investors.
OSI Pharmacueticals, Inc.
Andrew Toole
Rutgers University
COMPANY BACKGROUND
OSI Pharmaceuticals (henceforth: OSI) is a biotechnology company focused on the discovery, development, and commercialization of pharmaceutical products intended to extend life or improve the quality-of-life for cancer and diabetes patients.
OSI was originally founded in 1983 under the name “Oncogene Science, Inc.” to focus on cancer therapeutics. At that time the company was backed by venture capital investors. Oncogene Science went public in 1985 and eventually changed its name to OSI Pharmaceuticals to reflect the expansion of its drug discovery and development activities beyond cancer indications. As of December 2004, OSI employs 452 people. Over 80 percent of these employees are located within the United States and about 34 percent are primarily engaged in research activities.
OSI owns facilities in the United States and the United Kingdom. Its U.S. operations are divided between Colorado and New York. Colorado is home to its drug development group while New York hosts its corporate headquarters and its drug discovery operations. Somewhat uniquely, its drug discovery facility is part of the Broad Hollow Bioscience Park on the campus of Farmingdale State University. This park is a collaborative effort between Cold Spring Harbor Laboratory and Farmingdale State University intended to grow the bioscience industry on Long Island. OSI’s early collaborations with Cold Spring Harbor made it a good candidate for the science park. As for their overseas facility, OSI owns a subsidiary called Prosidion that focuses on diabetes and obesity research. Prosidion was spun out from OSI in 2003.
OSI has been successful at discovering and developing novel pharmaceutical agents through its research activities supported by large pharmaceutical partners, the SBIR program, and other sources of private investment. They now have three FDA-approved drugs on the market. Tarceva is their flagship product and is the first drug they took from concept to market. To date, it is the only epidermal growth factor receptor (EGFR) inhibitor to have demonstrated the ability to improve survival from non-small cell lung cancer and it may have therapeutic activity against certain forms of pancreatic cancer. OSI also markets Novantrone (mitoxantrone concentrate for injection) for approved oncology indications and Gelclair for the relief of pain associated with oral mucositis.
Over time, OSI evolved from a contract research firm to a fully integrated pharmaceutical company. Their contract research experience helped them build
strong capabilities in High-throughput Screening (HTS), chemical libraries, medicinal and combinatorial chemistry, and automated drug profiling technology platforms. To this research base OSI added sales and marketing capabilities as well as a regulatory affairs group. According to Hoovers corporate information, OSI had $42.1 million in sales in 2004, a 32.1 percent increase over 2003 sales.
OSI’s CEO is Colin Goddard, Ph.D., who was appointed in October 1998. Before joining the company, Dr. Goddard spent four years at the National Cancer Institute in Bethesda, Maryland. He was trained as a cancer pharmacologist in Birmingham, U.K., and received his Ph.D. from the University of Aston in September 1985.
TECHNOLOGIES AND INTELLECTUAL PROPERTY
One of OSI’s core research strengths is High-throughput Screening (HTS). HTS works by testing hundreds of thousands of compounds to identify hits against a biological target. These hits are subsequently developed into drug leads through medicinal chemistry. A state-of-the-art HTS operation is located at their Farmingdale, NY, research facility. OSI has compiled a library of more than 300,000 compounds which are formatted in plates in a manner that allows them to be accessed and mobilized quickly for screening. Assays are developed using advanced technologies, mostly involving fluorescence-based readouts that are easily miniaturized for processing in a 384-well plate. The screens themselves are run at very high throughput on robotic screening systems that are capable of running in unattended mode for extended periods. The screening operation can test an entire library against a biological target in about two weeks. A companywide data management system stores all the data generated throughout the discovery cascade. A series of querying and data mining tools enables OSI researchers to examine the data and decide which compounds to synthesize and which compounds should be taken to preclinical and clinical testing.
Intellectual Property
OSI owns approximately 90 U.S. patents and about 150 foreign patents. They have about 100 U.S. patent applications and 200 foreign patent applications pending. Pfizer is a long-standing collaborative partner of OSI Pharmaceuticals in the area of cancer research. This relationship led to co-ownership of about 600 U.S. and foreign granted patents and patent applications in about 50 patent families. Two of these co-owned patent families cover the method of preparation for OSI’s leading product, Tarceva. Moreover, OSI jointly owns some patents and patent applications with North Carolina State University.
IMPORTANCE OF SBIR
OSI received 51 SBIR grants between 1983 and 2002. Of these, 17 proposals progressed to Phase II awards. The Phase I grants totaled US$2.92 million (nominal dollars) while the Phase II grants totaled US$8.96 million (nominal dollars).
Dr. Haley was very positive about the role and contribution of the SBIR Program to the success of OSI. However, he stressed the difficulty of assessing the SBIR program using commercialization outcomes and readily quantifiable measures. Pharmaceutical innovation takes an average of 12-15 years from concept to market and most SBIR awards are facilitating concept and preclinical research. Even with SBIR awards contributing to research success, there are numerous factors and other inputs that cloud any clear picture of SBIR’s individual contribution. So, while OSI has no commercialization outcomes resulting directly from SBIR grants, Dr. Haley said the program added value to the firm and its technology development in a variety of other ways. He says, “We developed a lot of technology using SBIR grants and generated a lot of hits.” Four of the most important benefits for OSI from participation in the SBIR Program were:
(1)
Key Source of Early-stage Financing
OSI actively pursued SBIR financing in its early years. The company submitted between one and three project proposals per quarter. Most of these proposals were intended to explore highly uncertain and risky research possibilities. Even though many of these SBIR projects created knowledge, patents, and academic publications, private investors would never have backed these projects because they were “too innovative,” exploring too far beyond the known technological frontier. Thus, Dr. Haley believes that several of OSI’s research achievements might not have happened without SBIR funding. He stated many of the SBIR-backed research projects supported some of the most creative work that has ever been undertaken at OSI. More broadly, Dr. Haley noted “This program does have a big impact on early-stage biotechnology companies. They all rely on it for funding early-on.”
(2)
Helpful for Obtaining Follow-on Funding and Business Deals
Specific technological and scientific knowledge generated with SBIR funding laid the foundation for new contract research projects for big pharmaceutical firms that provided an important source of additional funds for OSI. The SBIR knowledge base helped to create opportunities for additional projects with companies like Pfizer, Aventis, Novartis and others. Thus, SBIR funding did not lead directly to products but to valuable research services and business deals. In another example, OSI was exploring two alternative approaches to the discovery of its flagship product, Tarceva. One approach, which turned out not to be suc-
cessful, was supported by an SBIR award from the National Cancer Institute. The other approach was not initially funded and OSI was having a difficult time convincing Pfizer, a long-term research partner, to finance that research. Dr. Haley recalled that government funding for the project made Pfizer nervous and leveraged them to go forward. The Pfizer-backed approach led to the discovery of Tarceva.
(3)
Facilitated Hiring of Employees
Dr. Haley emphasizes the importance of the SBIR awards for making it possible to hire additional scientists. He suggests “it was the development of a critical mass of experienced technologically savvy people all in one spot that made lots of things happen.” Even hiring two employees can have a big impact by allowing the firm to reach a critical mass of people involved in knowledge creation. The SBIR Program contributed to a more stable employment environment that ended up having multiple benefits for OSI.
(4)
Facilitated Cross-fertilization Between Research Programs
Cross-fertilization is one of the most significant benefits resulting from strategic hiring and sustained research on multiple projects. OSI had scientists working on different projects, some SBIR supported and some supported through private financing sources. Dr. Haley was clear to point out that the synergies flowing from this multiple project model “had a big impact on us.” And this relates back to his comments on the difficulty of measuring the impact of SBIR awards. Dr. Haley says that SBIR research frequently resulted in “tangential impacts” that happen through cross-fertilization mechanisms. It could be as simple as two scientists discussing research problems and solutions. These effects can be seen as spillovers between research projects that result in economies of scope in scientific discovery. In Dr. Haley’s opinion tangential effects are not exceptions occurring through SBIR but frequent events that push research forward.
ISSUES WITH THE CURRENT SBIR PROGRAM
Dr. Haley highlighted a few issues of concern about the SBIR Program at NIH. His perspective is based on many years of experience with the program beginning in the late 1980s. He also serves on NIH review panels that evaluate new SBIR proposals for funding.
Before mentioning the issues of concern, Dr. Haley noted that the SBIR Program has evolved in a positive and useful direction since its inception. Prior to the 1991 reauthorization of the SBIR Program, the funding levels and the intellectual property rights (IPR) provisions were inadequate. Quite simply, the $50,000 for Phase I and $500,000 for Phase II did not provide enough money
to get anything done. However, the current funding levels are sufficient. He also stated that it is also important that private firms be allowed to retain exclusive rights to their discoveries made possible by SBIR funds. While this is currently the case, government’s emergency march-in rights are still a concern for private firms. Because of this, firms do not want to link their discoveries directly to government funding. By blurring this link, firms can protect their IPR from government march-in.
With regard to concerns about the program, Dr. Haley made the following three points:
(1)
“Funding Gap“ Between Phase I & Phase II Awards
The funding gap between Phase I completion and Phase II approval, which can be six months or more, creates an unstable employment environment. Stable employment of scientific staff is critical for small firms, especially in the early-years when there are very few sources of capital. The funding gap can induce key scientific personnel to leave the firm and force the firm to abandon that line of research. This is an unintended but significant problem with the current SBIR approval and funding system.
(2)
Funds Tied Too Tightly to the Project’s Specific Aims
Biomedical research is notoriously unpredictable. SBIR funding is not nimble enough to change as research opportunities evolve within a project. SBIR funds are tied immutably to the specific aims laid out in the initial proposal. There should be a mechanism in place that allows some flexibility in the SBIR award to address new and unanticipated avenues that are not explicitly part of the grant’s specific aims. This is particularly important in highly competitive research areas where the extra time and effort required to initiate a new SBIR cycle is overly burdensome.
(3)
Proposal Reviewers Tend to Favor Less Risky Projects
While Dr. Haley is generally pleased with the NIH SBIR review system, he still sees a tendency for reviewers to favor less risky projects that might have a good chance of attracting private investment. Sometimes the most innovative and risky projects are also the ones with the highest social returns. Private investors may not see these as attractive opportunities and the SBIR Program, in principal, can fund these higher risk projects. This problem is intertwined with the SBIR Program’s focus on commercialization. High risk projects, by definition, have a lower chance of commercialization but may produce other intermediate outcomes of value via patenting, publication, etc. Greater weight could be placed on these intermediate outcomes in the proposal review and approval process.
Retractable Technologies Inc.—RTI
Robin Gaster
North Atlantic Research
OVERVIEW
The case of Retractable Technology, Inc., (RTI) illustrates the difficulties that can confront companies even if their research and development effort is entirely successful. Especially in health care, where the final consumers are rarely the purchasers of medical goods and services, and where skewed incentives often generate negative outcomes. The existence of extensive middleman interventions has in some cases—as RTI demonstrates—meant that even products that are technically successful, effectively manufactured, and backed by a strong entrepreneurial team can still face substantial difficulties in the marketplace.
COMPANY HISTORY
RTI was founded by Thomas Shaw, an engineer, in 1994, after he was inspired by a TV program about a doctor who had contracted HIV from an accidental needlestick injury. The company focused on building a retractable needle that would essentially eliminate needlestick injuries altogether.
The objective was and remains important. As of 2005, CDC estimates that 800,000 needlesticks occurred annually in the United States, with about 385,000 in hospitals24 (where the true number was likely much higher, according to another CDC study.25) About 1,000 nurses and health care workers contracted hepatitis C from needlesticks—a potentially deadly disease—and about 35 contracted HIV.
Needle safety is not just a U.S. problem. In developing countries, retractable needles could be a critical tool in the fight against deadly diseases such as AIDS, as these needles prevent the re-use of needles by multiple patients, a procedure that can have a devastating accelerating impact on the propagation of diseases.
In January 1992, Shaw received a $50,000 Phase I SBIR grant from NIH to develop one of his syringe prototypes. In October 1993, he received a $600,000
Phase II award to further develop and commercialize one of his designs, and to produce 10,000 samples for clinical trials.
The current company, RTI, was founded in 1994, and in 1997 RTI completed work on a state of the art manufacturing facility in Little Elm, Texas, capable of manufacturing approximately 50 million retractable syringes annually.
Initial trials were greeted very enthusiastically by local doctors and hospitals; a number of doctors became investors in the company, as it raised $42 million in investment funding: “I was immediately impressed when I saw the prototype,” recalls Dr. Lawrence Mills, former chief of thoracic surgery at Presbyterian Hospital in Dallas. “I thought the biggest problem was the company wouldn’t be able to make them fast enough to keep up with the demand.” (Now retired, Dr. Mills is a shareholder in Retractable.)26
THE ROLE OF SBIR AT RTI
RTI is in one sense a classic SBIR story: one Phase I, one Phase II, a product in clinical trials at the end of Phase II, and a successful product in the marketplace within two years thereafter.
It is also clear that SBIR had a significant role to play in the evolution of RTI; while the company was not founded specifically on the basis of the SBIR funding, its primary product (sole product for some years) was derived directly from the SBIR-funded project. In an interview, the founder, Tom Shaw, said that the funding from SBIR had come at a critical time in the development of the project.
However, Shaw noted that product development is one thing; being able to break into the medical market in the U.S. is something else entirely, and here the SBIR award had minimal impact.
BACKGROUND ON MEDICAL PURCHASING IN THE UNITED STATES
Most U.S. hospitals and large clinics belong to group purchasing organizations (GPOs). These organizations were originally designed to work on behalf of hospitals, to aggregate demand for medical products, and hence to position hospitals to get a better deal from the large manufacturers who largely dominate the health care market place.
These GPOs have developed a range of business practices designed to ensure on the one hand that hospitals purchase medical services and other medical services only through the GPO, and on the other that manufacturers supplying the GPO do not sell directly to hospitals, thus by-passing the GPO.
These arrangements include:27
-
Exclusive dealing agreements, whereby the hospital agrees to purchase all medical devices through the GPO, except where the GPO does not offer the goods or services needed.
-
Near-exclusive agreements, where the hospital agrees to purchase to fixed amount—90-95 percent perhaps—of a given commodity through the GPO. Because standardization is now the rule with a given hospital—it is documented to save lives—a commitment at this level is effectively a commitment to purchase 100 percent through the GPO.
-
Bundling agreements, whereby a number of products are bundled into a single contract. As in DoD, bundling has the effect of excluding potential competitors for a single good or service.
-
Rebates or discounts, where GPOs do not mandate an exclusive agreement, but instead offer substantial rebates for reaching targets—e.g., 90-95 percent of product purchases through the GPO. Rebates may be even more effective than exclusionary deals, as they may be easier to enforce. Sometimes all past rebates remain conditioned on continued meeting of the objective—creating golden handcuffs.
-
Nonvolume payments from the GPO, which can include stock options or other investment opportunities in favored manufacturers for senior hospital staff including purchasing managers. Other fixed fees appear to be used as a means of encouraging loyalty to the GPO program.
Together, these incentives and arrangements have allowed GPOs to dominate the market for sales to hospitals in the United States. And in turn, GPOs have developed close relationships with the manufacturers whose products they carry. While GPOs charge hospitals a membership fee, they also have developed arrangements whereby manufacturers pay large sums of money in “fees” of various kinds—for appearing at GPO-sponsored events, for example. While in most areas of the economy these arrangements would be illegal, in this case they are not:
“Most troubling is that some GPOs are funded by suppliers rather than solely by hospitals. The fees that suppliers pay, which would normally be considered illegal kickbacks, are allowed by the 1986 amendment to the Social Security Act. Thus, buying groups may serve the interests of the suppliers that provide their funding, not providers, thereby undermining value-based competition. While the extent of this bias is contested, the potential for conflict of interest is indisputable.
To enable value-based competition, every buying-group practice should be consistent with open and fair competition. There is no valid reason for buying groups to accept financing or any payments from suppliers: if a buying group adds value, the customers (hospitals) should voluntarily pay for it.”28
RTI AND THE GPOS
RTI has been forced to confront the GPO issue head-on since very soon after its foundation. Early positive reviews from local oppositely staff did not result in sales, or even the opportunity to make sales. At the hospital where the syringe was initially tested, Presbyterian Hospital of Dallas, Dr. Edward L. Goodman, then Presbyterian’s director of infection control, wrote that the new syringe was “essential to the safety and health of our employees, staff, and patients” and urged management to buy it. (Goodman was an early RTI shareholder, too.) Mr. Shaw claims that hospital officials told him that the institution could not purchase from RTI because of an exclusionary agreement with Premier, one of the two largest U.S. GPOs.29
RTI notes on its Web site that “Often our salespeople are not even allowed to show our products to hospital materials managers because restrictive GPO contracts effectively preclude their purchase of our products, regardless of their effectiveness in preventing needlestick injuries.”30
QUALITY AND COST
RTI has always claimed that its technology was fundamentally better than that of its competitors. It provided numerous documents to bolster its case, and there has been some analysis in the industry. In October 1999, a nonprofit agency which publishes the medical industry’s widely circulated counterpart to Consumer Reports, published its thorough evaluation of safety needle devices. RTI’s VanishPoint syringe was the only syringe to receive the agency’s highest possible rating.31 The editors of Health Devices went to some pains to object to this characterization of their research, but it appears accurate. Cost concerns have also been an issue. RTI’s retractable technology was initially somewhat more expensive than competing products (although Shaw noted that these competing products were considered ineffective by users and hence could not be viewed as strictly comparable).
In June 2002, RTI signed a long-term contract with Double Dove Co., Ltd., one of the largest syringe makers in China. Double Dove supplies syringes to RTI at an average unit cost of 8.5 cents, fully packaged, sterilized, and ready for use; this allows RTI to price its needles below competitors even in markets in developing countries.
MARKETS AND SALES
Despite the barriers presented by the GPO structure, RTI has had some significant commercial success. In 2002, it delivered its 100 millionth VanishPoint syringe; in July 2002 it recorded its first profitable quarter.
This success, however, was based only on the exploitation of niche markets within the U.S. that were not dominated by the GPO structure. These included federal prisons, Indian reservations, the Mississippi health department, the Veterans’ Administration, as well as some foreign markets such as South Africa. RTI also worked with the Service Employees International Union, the largest union of health care workers in the U.S., which had concerns about large numbers needlestick injuries, particularly in California the center of the AIDS crisis.
RTI has also had important symbolic wins. Its syringe has been selected for use in the U.S. government Global HIV/AIDS Initiative in Africa, winning three successive contracts. The CDC chose the VanishPoint syringe and blood collection tube holder for a large four-year study on the safety and effectiveness of an anthrax vaccine. In August 2005, RTI signed a licensing agreement with BTMD, a Chinese government-designated medical device manufacturer, for distribution of RTI’s safety needle devices in China.
Despite these limited successes (RTI is still not making an operating profit as of 2006, although sales reached a record $25 million for the year), the difficulties
FIGURE App-D-12 The RTI VanishPoint® syringe.
SOURCE: Retractable Technologies, Inc.
RTI experienced in reaching U.S. markets led RTI to file antitrust lawsuits against both Becton Dickinson, the largest U.S. syringe manufacturer, and large GPOs, including the two biggest, Novation and Premier.
LAWSUITS AND REGULATORY REFORM
Pretrial proceedings lasted six years, during which RTI’s Dallas factory operated at 50 percent capacity for much of the time. In 2003, as the trial date neared, Becton Dickinson, Tyco International, and the other defendants offered substantial settlements—but no change in their business practices.
Ultimately the GPOs did agree to change their business practices, although what they promised exactly remains confidential. Premier and Novation, along with syringe manufacturer Tyco, paid about $50 million to settle the case. Finally, under pressure from lawyers and shareholders, RTI accepted a settlement of $100 million from Becton Dickinson.
The issue of obstructive business practices has been taken up elsewhere. The U.S. Senate antitrust subcommittee has held hearings on whether hospitals’ buying practices are stifling competition. The U.S. Department of Justice opened a criminal investigation of hospital purchasing last year. The New York State attorney general’s office is investigating Becton Dickinson’s sales practices.32
The settlements have funded further expansion in RTI’s marketing, but in the view of RTI, they have not substantially affected the tight relationships between hospitals, GPOs and large manufacturers.
On the regulatory front, the problem of needlestick injuries generated some significant changes. In September 1998, California became the first state to pass a law requiring the use of safety needles. Cal-OSHA enforces this law. Many other states have passed similar laws.
In November 1999, both the Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH), a component of the Centers for Disease Control and Prevention (CDC), issued documents insisting on the use of safety needle devices.
In November 2000, President Clinton signed into law the Needlestick Safety and Prevention Act (Public Law 106-430). As a result, OSHA’s revised Occupational Exposure to Bloodborne Pathogens rule became effective in 2001. Employers are required to identify, evaluate, and implement the use of safer medical devices. They must also maintain a sharps injury log and involve frontline health care workers in the evaluation and selection process for safety devices.
RTI was involved in this legislation, but believes that its implementation has been too weak to change business practices among hospitals.
CURRENT STATUS
RTI is concerned that the long view for their company is dark. The original patents will expire in 2015, at which point many of RTI’s technical advantages over its competitors will disappear. Unless the market opens dramatically before then, RTI will continue to be overwhelmed by the huge resources available to its competitors and a marketplace that is structured in ways that sharply tilt the competitive edge away from outsiders like RTI.
CONCLUSIONS
RTI can be seen in some ways as company with severe Phase III problems, somewhat analogous to companies with successful products trying to negotiate the tortuous twists and turns of the DoD acquisition community. Yet unlike DoD, it does not appear that there are institutional forces anywhere in the health care sector working on behalf of the smaller firms with innovative technologies that are trying to reach the market.
For RTI, the years spent fighting for the right to deploy VanishPoint technology in the marketplace have left scars. In an interview, Tom Shaw expressed considerable concern at what he saw as the lack of support from the Congress once VanishPoint technology moved from the lab into the marketplace. As Shaw put it, “While the SBIR award made it possible to develop the VanishPoint technology and to make RTI potentially successful, it is inexplicable that the Congress would fund good research but simply lack the courage to stand up against large corporations’ anticompetitive practices. The current situation does not support the financial and medical interests of America’s taxpayers.”33
SAM Technologies
Robin Gaster
North Atlantic Research
BACKGROUND AND HISTORY
Dr. Gevins founded SAM in 1986, at about the same time that he founded its sister nonprofit research organization, the San Francisco Brain Research Institute, founded by Gevins in 1980 and previously part of the University of California School of Medicine in San Francisco. Dr. Gevins was focused on a project he had conceived while a freshman at MIT, to build a technology that could measure the intensity of mental work in the brain—reflecting in real time the concentration and attention capacity of the user.
Since 1986, SAM has consistently pursued this single goal, using all its SBIR and other awards to help build a prototype to measure signals in the brain that reflect attention and memory. This is, in short, a case study in how multiple SBIR and other awards can help to support a visionary and very high risk project in long-term biomedical research.
Dr. Gevins had received RO1 grants at UCSF, where he was offered a tenured position in the psychology department. However, RO1 reviewers were not in the mid-1980s friendly to technology-oriented projects, and Dr. Gevins found that SBIR was a better channel for his engineering activities.
Over the past 30 years, Dr. Gevins has received continuous federal support from the Air Force, the Navy, DARPA, NASA, NSF, and seven NIH institutes. These awards have been used to maintain a core staff working on the central project of the company. To fund such a complex and long-term project, Dr. Gevins systematically divided it into essential individual subprojects, and sought funding for them through unsolicited federal basic research and SBIR awards. This minimized overall risk, and SAM’s work has been supported by many SBIR awards from many agencies, as the project covers many possible applications of the technology. For example, SAM has received significant support from support from the National Institute on Ageing, because SAM’s assessment and analysis technology could have a very large impact on seniors facing performance deficits of many kinds.
SAM has developed both the hardware that measures brain signals and transmits that signal to a processing device (now a PC), as well as the software used to integrate different kinds of brain stimulation signals. One early SBIR was designed to build the meters necessary to capture the EEG signals SAM intended to work with, as these meters were not then available elsewhere.
In 2005-2006, SAM completed the first commercial product in the MM line, the world’s first medical test that directly measures brain signals regulating at-
tention and memory, the SAM Test (Sustained Attention & Memory Test). The SAM Test is covered by four U.S. patents and by a number of trade secrets. The test is designed to fill an urgent need for an objective measure of how a patient’s cognitive brain functioning is affected by a disease, injury, or treatment in a wide range of areas including head injuries, sleep disorders, mild cognitive impairment of aging, attention deficit hyperactivity disorder, epilepsy, and depression.
The next proposed product, the Online Mental Meter, is designed for widespread use beyond medical care as a computer peripheral that provides continuous information about the user’s state of alertness and mental overload or underload, by measuring mental activity in real time while people perform everyday tasks at a computer. The Online MM constitutes a substantial technical leap from the SAM Test, which requires that a subject perform a standardized repetitive psychometric test of sustained focused attention and memory. SBIR projects 17 and 18 (see Table App-D-8) have paved the way for this advance.
Continuous real-time measurement of mental effort could become a key enabling technology for a wide variety of advanced adaptive systems that will vary the sharing of tasks between a human and a computer in an optimal manner depending on the user’s cognitive state. SAM believes that such systems may well be ubiquitous in the future.
SAM aims to become the gold standard for medical testing in neurology. Currently, the brain measurement component of psychological testing requires a PET or MRI scene, which is inconvenient and very costly ($4,000 or more each). In addition, existing performance-based tests can be misleading, as they fail to measure brain activity directly. For example, early Alzheimer’s patients often produce acceptable memory and brain performance, because these patients are able to compensate for their initial problems. Direct brain measurement would reveal what performance analysis obscures—the actual problem at the neuron level.
Federal funding has allowed SAM to reject overtures from venture capital companies. According to Dr. Gevins, venture companies have “a different agenda, timescale, and process.” In contrast, SBIR supports a transition from basic research to the next step.” Dr. Gevins observed that venture capital companies in general have declining interest in truly innovative work, because such work often takes too long to get to market for venture capital timescales.
SAM is currently working with consultants under the LARTA commercialization support program to develop a strategic alliance with a large corporation in order to make the SAM Test commercially available as a fee-for-service medical test. The partner will need to undertake independent clinical trials, FDA registration, approval for third-party reimbursement and a major marketing and sales campaign, activities that could take at least 3 years and cost in excess of $15 million.
TABLE App-D-8 SAM Technologies SBIR Awards
|
|
Funding-Institute-Center or Agency |
Year |
|
Description |
|
1 |
NIH |
1988 |
R44-RR03553 |
Removal of Distortion from Magnetic Resonance Images |
|
2 |
NIMH |
1989 |
R44-MH42725 |
Active Electrode Hat for EEG Imaging of Schizophrenics |
|
3 |
AFOSR |
1989 |
F49620-89-C-0049 |
Software Tools for Signal Identification using Neural Networks |
|
4 |
AFRL |
1989 |
F33615-89-C-0605 |
Flight Helmet EEG System |
|
5 |
NIMH |
1991 |
R44-MH43075 |
EEG ArtifactDetection |
|
6 |
AFOSR |
1991 |
F49620-92-C-0013 |
Physiological Indices of Mental Workload |
|
7 |
NINDS |
1992 |
R44-NS27392 |
Neurofunctional Research Workstation |
|
8 |
NIAAA |
1993 |
R44-AA08680 |
Cognitive Performance Assessment for Alcohol Intoxication |
|
9 |
NIMH |
1993 |
N44-MH30023 |
128 Channel Automated EEG Recording System |
|
10 |
NINDS |
1994 |
R44-NS28623 |
Multimodality Workstation for Seizure Localization |
|
11 |
NINDS |
1995 |
R44-NS32241 |
Functional Brain Imaging of Unconstrained Subjects |
|
12 |
NASA |
1995 |
NAS9-19333 |
Spacecrew Testing and Recording System |
|
13 |
AFRL |
1995 |
F41624-95-C-6000 |
Decontamination of Physiological Signals of Mental Effort |
|
14 |
NIMH |
1996 |
N44-MH60027 |
Neurocognitive Experiment Authoring Tool |
|
15 |
NIAAA |
1997 |
R44-AA11702 |
Attention & Alertness Neurometer |
|
16 |
NINDS |
1998 |
N44-NS-0-2394 |
Assessment of Alertness in Patients with Sleep Disorders |
|
17 |
AFOSR |
1998 |
F49620-98-C-0049 |
Sustained Attention Meter for Monitoring Cognitive Load |
|
18 |
AFOSR |
1998 |
F49620-96-C-0021 |
Brain Automatization Monitor |
|
19 |
AFOSR |
1998 |
F41624-98-C-6007 |
Operator State Classifier Developer’s Toolkit |
|
20 |
AFRL |
1998 |
F41624-97-C-6030 |
Rapid Application Cutaneous Electrode (RACE) System |
|
21 |
AFRL |
1999 |
F41624-99-C-6007 |
An Ambulatory Neurophysiological Monitoring System |
|
22 |
NIMH |
2001 |
R44-MH60053 |
Neurocognitive Assessment Meter For Psychiatric Drugs |
|
23 |
NIDA |
2001 |
R44-DA12840 |
Neurocognitive Index of Cannabis Effects |
|
24 |
NIA |
2002 |
R44-AG17397 |
Neurocognitive Assessment of the Elderly |
|
25 |
NICHD |
2002 |
R44-HD37728 |
|
|
26 |
NINDS |
2003 |
R44-NS42992 |
|
|
27 |
DARPA |
2003 |
DAAH01-03-C-R292 |
Multicompartment Neuroworkload Monitor |
|
28 |
NHLBI |
2004 |
R44-HL065265 |
|
|
29 |
ONR |
2004 |
N00014-04-C-0431 |
Multitasking Personnel Selection Test |
|
SOURCE: SAM Technologies. |
||||
Staff
SAM has 13 scientists, engineers, and associates, and several outside consultants, covering a range of disciplines. The 8 most senior staff members have been with SAM an average of 11 years. Collaborations with scientists and doctors at universities, medical schools, and government labs are used to leverage internal research efforts, and SAM has made distribution agreements with medical device companies which account for most product sales. SAM is an FDA registered Medical Device Manufacturer.
OUTCOMES
Commercial Products
Six of the SBIR-funded projects (#3, 7, 9-12) have to date resulted in two commercial products.
Image VueTM
Image Vue™ is a softare package for visualizing brain function and structure by fusing EEG data with MRI (Magnetic Resonance Images), using patented algorithms to integrate functional and structural information about the brain, in order to localize epileptic seizures in a patient’s brain. A wizard-driven software system running under Windows XP, it co-registers EEGs with MRIs, performing patented DEBLURRING™ spatial enhancement and sveral types of source localization analysis, and provides interactive 3-D graphics visualization. The patented XCALIPER™ hardare and associated software facilitates rapid measurement of EEG electrode positions needed for co-registration with MRIs.
The product is used primarily to visualize and localize the origin and spread of epileptic seizures in the human brain in planning neurosurgical treatment of complex partial seizure disorders that are refractory to treatment with antiepileptic drugs.
Image Vue™ is FA-registered and is sold by Nicolet Biomedical Inc. (a subsidiary of Viasys Healthcare, Inc), the world’s largest supplier to the clinical neurology market. Nicolet has purchased approximately 100 systems from SAM to date, from which they have generated about $2,000,000 in revenues. A number of competing products worldwide have been modeled on Image Vue™.
MANSCAN®
MANSCAN® evolved from basic research completed under prior NIH R01s, which with the aid of SBIR awards has been turned into robust algorithms embodied in a convenient, integrated system to enable research on human brain function that would not otherwise be commercially available.
FIGURE App-D-13
SOURCE: SAM Technologies.
MANSCAN® is an integrated software and hardware system for performing brain function research via high-resolution EEG and event-related potential (ERP) studies, and for integrating the results with magnetic resonance images. MANSCAN® It was the first system to integrate the high time resolution of EEG with the high anatomical resolution of MRI, and the first to allow subsecond measurement of rapidly shifting functional cortical networks. It enabled a new generation of research, and a number of significant advances in understanding attention, memory and other basic cognitive brain functions have been made with it.
Results of these studies provide unique views of structural and functional neuroanatomy. MANSCAN® analysis and visualization functions quickly and easily quantify features from EEGs and ERPs, leading neuroscience toward the goal of uniting brain electrical activity with brain anatomy.
MANSCAN®’s hardware includes quick application electrode caps, an efficient device called XCALIPER™ for measuring electrode positions, and an advanced digital amplifier called MICROAMPS™. MANSCA® software is fully integrated with the Microsoft NT/W2000/XP operating system running on a PC.
Thirty MANSCAN® systems have been sold to qualified scientists at U.S. universities, medical schools and government labs, where it is helping them to perform advanced research. MANSCAN® has generated approximately $650,000 of revenue. Several competing products worldwide have been modeled on MANSCAN®. MANSCAN® is also specifically designed as a step toward the MM.
Knowledge Effects
SAM aims to produce commercial products—clearly its entire mission is focused on commercial outcomes in the long run. However, there are been significant knowledge effect benefits during the course of this high risk research. Nine of the projects listed below led to over 50 peer-reviewed scientific and engineering publications. Thirteen of the projects led to 18 U.S. patents. More widely, SAM staff have published more than 150 peer-reviewed publications including five papers in Science.
SBIR ISSUES AND CONCERNS
The Selection Process
In recent years, SAM has been criticized as being “insufficiently innovative,” possibly because the field is catching up with SAM. Dr. Gevins notes that the recent drive for closer attention to commercialization is impacting SBIR reviews at NIH, but also that reviewers from academia have a better understanding of more basic research and are likely to be somewhat biased toward it. He also notes that academic reviewers are not themselves unbiased, in that they tend to focus on whether outputs from the project in question will be useful in their own research. Review quality and outcomes also vary very substantially by study section.
Conflict of Interest
Dr. Gevins is very concerned about potentially major conflicts of interest stemming from the use of industry participants on study sections. He procedures for addressing such conflicts are “pathetic”: Section members are handed a written conflict of interest description immediately before the panel meets, and are then on the honor system to disqualify themselves. No NDA is signed, and little attention is paid to the process. Dr. Gevins believes that the current approach is just designed to protect NIH from awkward questions, rather than to provide real protections to applicants.
SAM always reviews membership of review panels, and not infrequently requests that the SRA exclude a panel member from reviewing a SAM proposal. This veto process mostly works, according to Dr. Gevins, but is not foolproof. There is clearly some element of risk involved in releasing internal plans to outsiders (Dr. Gevins pointed out that this risk is endemic to the funding process—and that venture capitalists never sign NDAs, so there are also risks involved in working with venture capitalists).
In short, Dr. Gevins argues that while formal protections are in place, no effort is made at NIH to define or verify the absence of conflicts of interest, even though SRAs are in general honest and conscientious.
Recommendation. NIH must take the conflict of interest problem much more seriously. It should implement its own conflict of interest policies more effectively, and should consider mechanisms for auditing reviewer activities, at least on a random basis.
Expertise
Dr. Gevins sees a disconnect between the SRA running the selection process, and the IC which will eventually fund the project, and which has technical expertise in the subject area. This contrasts with funding at DoD, NSF, where a single point of contact essentially determines funding and manages the award.
Recommendation. It should be mandatory that the primary reviewer should have technical competence in the field covered by the proposal.
Commercial Review
Dr. Gevins sees substantial room for improvement in addressing commercial-izing concerns. He does not support the commercialization index in use at DoD, which he regards as highly oversimplified and biased toward short-cycle projects. He did agree that a commercialization review could be useful, but though there might be helpful ways to separate out technical/scientific review from commercialization, which could be addressed by a separate perhaps permanent panel of experts, and where problems could be addressed within a single funding cycle rather than requiring full resubmission, which means at least one and possibly two funding cycles delay.
Reviewer Evaluation
CSR manages this function and does so fairly effectively. Dr. Gevins believes that the key motivation for reviewer participation is to follow activity near the cutting edge in a particular field.
Overly Random Scoring
Like many interviewees, Dr. Gevins noted the substantial random element in the review process. In particular, he believed that that there are quite substantial differences in scoring tendency between different review panels.
Recommendation. Scores should be normalized across study sections, just as they are for RO1s. Otherwise it is perfectly possible—indeed likely—that one study section will tend to systematically provide higher scores than another. As all scores are integrated into a single priority score list for a given IC, this would inevitably generate a bias toward projects that were reviewed by the higher scoring study section.
Funding Issues
Size of Awards
Dr. Gevins said that in his experience, over-limit applications were always discussed beforehand with the program manager. SAM makes a point of mentioning this in the application, to ensure that reviewers know that the relevant program manager is in the loop. Extra-large awards can sometimes be held up for one or more funding cycles by the program manager, even if they are technically inside the Payline. This is a less formal procedure, but similar to that in place at NIH for RO1 awards.
Recommendation. SAM supports increasing the size of Phase I awards and reducing the number of awards. Some program announcements already call for Phase Is in the vicinity of $500,000. SAM also supports increasing the size of Phase II awards and reducing the number. SAM would recommend a three year Phase II award for $1 million, possibly requiring prior approval from the program manager.
Commercialization Support
SAM has actively participated in the new LARTA-led program. It sees the program as useful, particularly because it forces companies to focus on commercialization. However, at the time of the interview, SAM had received almost no useful time from the consultants, who appeared to have too many companies in their portfolios (20 or more each).
In general, the basic outline of the LARTA support program met SAM’s needs, which focused not on preparing for public presentations, but on moving steadily through the steps of developing a good commercialization plan, focused on strategic alliances. SAM had received a steady flow of reminder/check-up calls, pushing the company to focus on the commercial element of the business.
Recommendation. NIH should consider allowing LARTA to focus its resources more tightly on fewer companies, providing them with more resources.
Program Managers
Dr. Gevins wondered what role SBIR program managers or liaisons play at the various institutes. As they did not appear to manage the financial and reporting aspects of individual grants, and did not substantially influence selection, he was unclear as to their role. He saw program managers as providing no value added for recipients, and added that in some cases they could be highly destructive (a point also made by other interviewees). He believed that some managers clearly had their own research agendas, and sought to impose these on the SBIR application process. Dr. Gevins also noted that being a program manager with responsibilities for many SBIR awards was not a plum job at NIH; as a result, it was often handed off to the least senior staff member.
Recommendation. SAM suggested adding a program manager review section to the final report for each project, which would allow NIH to gather better feedback about program manager performance.
Funding Gap Issues
SAM handles the gap by operating with multiple overlapping project. Current work has taken six years on a specific stage of the overall brain measurement project.
Dr. Gevins also noted that ICs do not always fund projects immediately—the latter can be delayed for one or more funding cycle. SAM believes that April applications are likely to be funded fastest, because they show up at the beginning of the fiscal year and require least juggling from the IC. For example, SAM had a project that was approved during a September Council meeting, but had still not been funded by the IC as of the following March.
Other Concerns and Recommendations
SAM offered a range of other concerns and suggestions:
-
Believes awards are too short. Six months for Phase I is “a joke,” as Phase I research always takes about a year. SAM has never completed a two-year Phase II award in the standard two years.
-
Does not support Fast Track, partly because reviewers tend to split Fast Track applications in two anyway, and also because there are too few advantages in this process for companies, in comparison to the additional uncertainty.
-
Supports direct access to Phase II, without prior Phase I. SAM believes that the time required to apply for and complete a Phase I is the problem, as this can add a year or more to a project.
-
Supports the view that drug development funding could be distorting the overall shape of the SBIR program.
-
Supports competing continuation awards.
Sociometrics Corporation
Robin Gaster
North Atlantic Research
EXECUTIVE SUMMARY
Sociometrics is a woman- and minority-owned company of approximately 16 employees that develops commercial products and services based on state-of-the-art behavioral and social research. Located in California’s Bay Area R&D hub, it has received funding from diverse government and private sources, generating revenues of more than $28 million since its foundation in 1983. By introducing the concept of packaged replication programs to the practice of social behavior change, Sociometrics has changed the nature of the field.
Sociometrics illustrates well one type of successful SBIR company. Winner of an NIH SBIR award during the first year of the program in 1984, Sociometrics has gone on to continue winning awards with consistency. Between 1992-2002, the company received 19 Phase I awards from NIH (see annex); 18 of these have become Phase II projects, a very high 94 percent conversion rate. Since 2003, six new Phase I projects have been awarded; two additional Phase I applications have received priority scores at the fundable level. Sociometrics will be submitting eight Phase II applications from these projects at the appropriate time.
Consistent with the goals of the SBIR program, Sociometrics has become a product-oriented company. Every Phase II it has conducted has generated a highly marketable, commercial product. Sociometrics has now developed several product lines, secured a distribution agreement with a major electronic publishing house, and had products chosen by the Centers for Disease Control and Prevention (CDC) for its public health initiatives. In 2002, the majority of the firm’s profits shifted from contract and grant research fees to product sales, a testament to the firm’s SBIR-related success. This success has been due in part to the cumulative and strategic nature of Sociometrics’ efforts. New projects and products build on previous ones, adding one or more innovations in the process. The company has also leveraged the ubiquity of the World Wide Web to increase its products’ reach and public access, designing most products for download or interactive use on the Internet.
Despite Sociometrics’ own success, its market niche—the development of behavioral and social science-based commercial products—remains under-resourced with private funds. In part, this situation obtains because typical customers for such products are nonprofit organizations that appreciate and use the resources but cannot afford to pay very much for them. Responding to this important need, Sociometrics has kept its product pricing at close to production cost, leveraging instead good business practice with a sense of public service.
For these reasons the company continues to rely on SBIR funding to provide the necessary development support to create new products and expand its range of services. While SBIR grants remain an important revenue stream for many small companies in the program, Sociometrices involvement has distinguished itself with: a) a wide set of well-regarded and widely-used products; b) a profitable business-model, distinguishing it from many other behavioral and social science-focused SBIR firms; and c) Phase II funding as sufficient support to bring the company’s products to market, in contrast to many biotech- and pharmaceutical-oriented companies.
Motivated once again by both business and public service concerns, Sociometrics staff members have published extensively in peer-reviewed journals. The company does not develop patentable products.
PRIMARY OUTCOMES
-
Four major product lines, with a fifth under development;
-
Consistent profitability from inception;
-
Industry standard for topically focused social science data and program archives;
-
Distribution agreement for data products with world-leading provider of authoritative reference information solutions;
-
CDC adoption of program archive products for nationwide distribution;
-
Product line with social impact: effective program replication kits have changed the way behavioral practitioners operate in community settings; and
-
More than 60 peer-reviewed publications based on SBIR-funded projects.
KEY SBIR ISSUES
Sociometrics has found the SBIR program a very productive platform for its work. The program has allowed Sociometrics to: a) take state-of-the-art social and behavioral research in its topical areas of expertise (reproductive health, HIV/AIDS, drug abuse, and mental health); b) use Scientist Expert Panels to assess the research and identify the best available data, practices, and knowledge; and c) develop commercial products and services based on the panels’ selections. Sociometrics’ products are aimed at a diverse set of target audiences. For example, its data archives and evaluation instruments are meant for use by researchers, faculty members, and students. Its effective program replication kits, evaluation publications, and program development and evaluation training workshops are intended for use by health practitioners in schools, clinics and community-based organizations. Its forthcoming Web-based behavioral and social science information resources, summarizing state-of-the-art research knowledge in select topical
areas in nonscientist language, will be aimed at both academic and practitioner audiences.
BACKGROUND
Sociometrics Corporation was established in September 1983 as a corporation in the State of California. Within a year of its founding, Sociometrics had applied for its first SBIR project. Dr. Josefina Card, Sociometrics founder and CEO, was encouraged to found the company as a for-profit organization by Dr. Wendy Baldwin, then a program manager at NIH. (Dr. Baldwin later went on to become Deputy Director of NIH for Extramural Research.) Dr. Baldwin suggested that Sociometrics incorporate as a for-profit entity in order to benefit from the newly created SBIR program without precluding the possibility of obtaining basic and applied research grants.
Sociometrics’ goals are:
-
To conduct applied behavioral and social research to further our understanding of contemporary health and social problems;
-
To promote evidence-based policymaking and intervention program development;
-
To conduct evaluation research to assess the effectiveness of health-related prevention and treatment programs;
-
To facilitate data sharing among social scientists as well as public access to exemplary behavioral and social data; and
-
To help nonexperts utilize and benefit from social science and related technologies and tools.
In carrying out its mission, several areas of corporate expertise have been developed:
-
The design and operation of machine-readable, topically focused data archives;
-
The development of powerful, yet user-friendly, software for search and retrieval of information in health and social science databases;
-
The harnessing of state-of-the-art developments in computer hardware and software to facilitate access to, and use of, the best data in a given research area;
-
Primary and secondary analysis of computer data bases using a variety of commercially available statistical packages as well as custom-designed software;
-
The design, execution, and analysis of program evaluations;
-
The design, execution, and analysis of health and social surveys;
-
The collection and analysis of social and psychological data using a variety of modes (mail; telephone; focus groups; in-person interviews);
-
The collection and dissemination of social intervention programs with demonstrated promise of effectiveness; and
-
The provision of training and technical assistance on all the above topics.
Sociometrics currently has 16 employees, 6 of whom have Ph.D.s and 5 of whom have Masters degrees. Expertise of its staff spans a diverse set of behavioral and social science fields, including: sociology, social psychology, clinical psychology, demography, linguistics, education, and public administration. In June 2005, a seventh Ph.D., specializing in political science and international relations, will be joining the firm.
Sociometrics has been the recipient of several awards including:
-
A Medsite award for “quality and useful health-related information on the Internet”;
-
A U.S. Small Business Administration Administrator’s Award for Excellence “in recognition of outstanding contribution and service to the nation by a small business in satisfying the needs of the federal procurement system”; and
-
A certificate of recognition for Project HOT (Housing Options for Teachers) by the California State Senate, the California State Assembly, and the Palo Alto Council of PTAs “in appreciation for service supporting Palo Alto Unified School District’s teachers and staff.”
PRODUCTS
Sociometrics currently provides four research-based product lines:
-
Data archives and analysis tools;
-
Replication kits for effective social and behavioral intervention programs;
-
Evaluation research; and
-
Training and technical assistance services.
Sociometrics staff members are currently developing a fifth product line, online behavioral and social science-based information resources, to facilitate “distance learning.”
Data Archives and Analysis Tools
The Sociometrics data archives are collections of primary research data. Each collection is focused on a topic of central interest to an NIH Institute or Center (IC). The data sets comprising each collection are selected and vetted by high-level scientist advisory boards to ensure that each collection is best-of-breed. Data sets are acquired from their holders, packaged and documented in standard fashion, and then made publicly available both online and on CD-ROM. Each successive data archive has leveraged features of previous archives that promote ease of use and has then developed new features of its own. This cumulative development effort has resulted in a digital library consisting of several hundred topically focused data sets that are easy to use, even by novices such as students and early-career researchers. Data in the Sociometrics’ archives are accompanied by standard documentation, SPSS and SAS analytic program statements, and the company’s proprietary search and retrieval tools. By providing high-quality data resources, and adding features facilitating appropriate and easy use, Sociometrics has created a niche of standardized, quality data products that complement the larger, though not universally standardized, data resources offered by other major data providers such as the University of Michigan. Currently, Sociometrics publishes nine data archives, with a tenth data archive on childhood problem behaviors under development. The nine collections are disseminated both as single
|
BOX App-D-3 The Sociometrics Data Archives: Topical Foci and Scope
|
data sets (by Sociometrics) as well as via institutional subscriptions to the entire collection known as the Social Science Electronic Data Library (SSEDL).
Sociometrics’ first data archive on adolescent pregnancy and pregnancy prevention was funded as part of the very first cohort of SBIR awards at NIH. More than 20 years after its initial release, this archive continues to be highly relevant, utilized, and regularly updated by Sociometrics. The archive was originally published on mainframe tapes, and has since been delivered to its customers using ever-changing computer data storage technologies including 5¼ and 3½ inch floppy disks and CD-ROM. It is now available 24/7 on the World Wide Web, where it has been accessible for the past 8 years.
A year ago, Sociometrics entered into a five-year distribution agreement for the Social Science Electronic Data Library (SSEDL) with Thomson Gale, a world-leading provider of authoritative reference information solutions. The agreement calls for Thomson Gale to market SSEDL via subscription to its wide range of academic and research library customers, while allowing Sociometrics to continue selling individual data sets from its own Web site. Sociometrics receives a portion of the Thomson Gale subscription sales in royalties. Several thousand SSEDL data sets have been downloaded from the Sociometrics Web site over the last three years, some by pay-as-you-go customers who execute a secure credit card transaction, others by faculty members and students able to download the data sets at no charge because their university is an SSEDL subscriber. Sales of the data archives and associated products have yielded approximately $125,000 in profits over the last three years. This figure does not yet include royalties from the Thomson Gale agreement which came into effect at the close of the 2003-2004 fiscal year, the latest date for which figures are available.
Competition
The Inter-University Consortium for Political and Social Research (ICPSR) data archive at the University of Michigan provides the main competition for Sociometrics’ data archives. Despite ICPSR’s much larger collection of data sets, Sociometrics has maintained a specialized niche, leveraging organization around selected health-related topics, careful selection of exemplary data by Scientist Expert Panels standardized documentation, ease of use of data sets, and value-added SPSS and SAS data analytic statements into a specialized collection tailored to data novices such as students and early-career researchers, that complements the ICPSR collection.
Replication Kits
Having developed the Data Archives, Sociometrics realized in 1992 that additional public service would occur if it extended and adapted its work beyond selection, packaging, and distribution of exemplary data to selection, packaging,
and distribution of practices shown by such data to be effective in changing unhealthy or problem behaviors. Diverse health issues with important behavioral determinants (adolescent pregnancy, STD/HIV/AIDS, and substance abuse) were selected to showcase the new product line. Prior to 1992, information on effective programs was limited to brief descriptions in scientific journals often not read by health practitioners, a serious barrier to their widespread use. Using SBIR funding, Sociometrics sought to overcome this barrier by adapting to this new product line the time-tested methods it used to establish its data archives.
The company again worked with Scientist Expert Panels to identify and select effective programs based on their empirical support, collaborated with developers of selected programs to create replication kits for Panel-selected behavior change interventions, and partnered with networks of health professionals to disseminate these kits to schools, clinics, and community-based organizations. Replication kits were conceptualized as boxes containing all the materials required to reimplement the effective intervention. Typical replication kits contain a user’s guide to the program, a teacher’s or facilitator’s manual, a student or participant workbook, one or more videos, and forms for “homework” assignments or group exercises.
The first effective program collection, the Program Archive on Sexuality, Health & Adolescence (PASHA) now comprises 29 replication kits. The newer HIV/AIDS Prevention Program Archive (HAPPA) and the Youth Substance Abuse Prevention Program Archive (YSAPPA) encompass 11 and 12 replication kits, respectively. Despite considerable initial skepticism from academics and some practitioners, the program-in-a-box approach has been received with considerable enthusiasm. PASHA, HAPPA, and YSAPPA have all proven to be social successes, with their programs being implemented in hundreds of schools, clinics, and communities across the country. They have also proven to be commercial successes, generating profits totaling over half a million dollars in the last three years.
Like the Sociometrics Data Archives, the Sociometrics replication kits (known collectively as the Sociometrics Program Archives) are topically-focused, best-of-class collections, selected using clearly defined effectiveness criteria by Scientist Expert Panels, and sold with free technical support for purchasers. This complimentary technical assistance has been lauded as an extremely valuable service by Sociometrics’ customers and the company’s reputation follows, in part, from the excellent product support it provides.
The replication kits have been sold individually from the Sociometrics Web site to such customers as schools, community health and service organizations, and medical clinics. They have also been displayed at exhibit booths at annual meetings of health practitioner professional organizations. Their dissemination is further supported by a company newsletter published three times annually and reaching 30,000 recipients. In 2003, CDC became an important customer for several replication kits, providing “train the trainer” workshops in Atlanta for
hundreds of practitioners in use of selected kits. These new trainers have in turn returned to their hometowns and home organizations to train more staff, resulting in further sales of the replication kits and dissemination of important prevention programs.
Competition
CDC has provided the impetus for the only real competition to Sociometrics in the field of replication kit development for effective teen pregnancy and HIV/AIDS prevention programs. The CDC initiatives have been based on a decentralized distribution model, with CDC funding program developers to publish their programs themselves or to seek out their own commercial distributors. In contrast, the Sociometrics distribution model is centralized, with Sociometrics’ Web site serving as a one-stop-shopping-point for highly effective programs in the areas in which the company operates.
The inevitable delays in implementing a new initiative, plus changes in policy at CDC and substantial budget cuts have put one of the CDC’s two development programs on hold (the one on teen pregnancy prevention), leaving the Program Archive on Sexuality, Health, and Adolescence without significant current competition. The other CDC program on HIV/AIDS prevention, a competitor to Sociometrics’ HIV/AIDS Prevention Program Archive, is using replication kits developed at Sociometrics for some of its selected programs, boosting sales of the Sociometrics HIV/AIDS Prevention Program Archive by an order of magnitude. In this manner Sociometrics Program Archives have complemented the larger CDC efforts, just as the Sociometrics Data Archives have complemented the larger University of Michigan efforts.
Longer-term Challenges and Opportunities
Over the longer term it is possible that commercial challenges may arise from changes in the academic world, where more and more developers of effective programs are deciding to publish their work themselves, releasing kits or parts of kits through their own Web sites or negotiating other arrangements with commercial publishers. Sociometrics is not overly concerned by these developments as it regards its work as complementary to, and supportive of, developers’ efforts to get their effective programs in the public domain. Recent history is supportive. During the initial establishment of Sociometrics’ HIV/AIDS Prevention Program Archive (HAPPA), the advisory panel recommended 18 effective programs for inclusion in the archive; of these one was withdrawn as “obsolete” by its original developer, seven developers had previously decided to use a commercial publisher, and ten were made available through HAPPA. Thus with the help of Sociometrics’ efforts complementing existing efforts, replication kits for almost all effective programs are now publicly available to community-based
organizations striving to prevent HIV. This constitutes an important public service in terms of: (1) packaging the most promising interventions to enhance their usability; (2) facilitating low-cost access to, and widespread awareness of, these interventions; (3) encouraging additional rigorous tests of the interventions’ effectiveness in a variety of populations; and (4) demonstrating the value of, and providing a model for, the research-to-practice feedback loop.
Further opportunities for enhanced product dissemination arise from Sociometrics’ collaboration with other organizations besides CDC. In particular, large nonprofit networks provide many opportunities for partnership. For example, on the teen pregnancy prevention program archive, Sociometrics has worked with the National Campaign to Prevent Teen Pregnancy, Advocates for Youth, and the National Organization for Adolescent Pregnancy, Prevention, and Parenting Inc. These organizations have become bulk purchasers of replication kits. They have provided other marketing support as well; for example, Advocates for Youth placed a link to Sociometrics on its Web site, and marketed Sociometrics’ kits to its constituency from there.
Evaluation Research
Sociometrics has considerable expertise in program evaluation research and technical assistance. Over the last 15 years, the company has conducted many studies and provided technical assistance to many nonprofit organizations to determine whether a particular social intervention program was able to meet its short-term goals and long-term objectives. While most of the company’s work developing its data and program archives has been funded by the SBIR program, Sociometrics’ evaluation work has been funded primarily by state governments (such as California, Minnesota, and Wisconsin), local governments (such as Santa Clara County) and private sources especially foundations seeking an evaluation of the efforts of their grantees (the Packard Foundation, the Mott Foundation, the Northwest Area Foundation, and the Kaiser Family Foundation) and nonprofits seeking an evaluation of the effectiveness of their work. Sociometrics publishes a number of books and resource materials on program evaluation (e.g., Data Management: An Introductory Workbook for Teen Pregnancy Program Evaluators). It also offers at low cost (15 cents per page) evaluation research instruments that have been used in national surveys or in successfully implemented and published evaluation efforts.
Training Services
Sociometrics conducts workshops and courses to familiarize practitioners with the tools and benefits of social science and related technologies. These courses have recently been put online to increase their reach while lowering access costs. Training is offered in a variety of social science areas, particu-
larly in effective program selection, development, and implementation; program evaluation concepts, design, and execution; and data collection, management, and analysis.
Science-Based Information Modules
These new products, still under development, will integrate the research literature in a given topical area, describe what science says in language and format easily understood by nonscientists (eighth grade reading level), and disseminate the information online via the Internet for easy “distance learning” access by all.
Into the Future
Most of Sociometrics’ products are available for 24/7 download (with payment by credit card) on its award-winning Web site at <http://www.socio.com>. Its data archives, collectively known as The Social Science Electronic Data Library (SSEDL), are also available via institutional subscriptions marketed to universities and research libraries by Sociometrics’ dissemination partner Thomson Gale. Sociometrics will continue its development of its Web site as a major product platform. In 2003, this Web site received over 1.7 million hits resulting in 29,729 product downloads. The company will also continue to develop additional subscription products. For example, Sociometrics plans to bundle its HIV and teen pregnancy replication kits, evaluation resources, training courses, and information module products and disseminate these bundled products to academics and health practitioners via online subscriptions. Eventually, mental health resources will be added to the Data Library, HIV, and teen pregnancy subscription resources as a fourth subscription line. Two current and two forthcoming SBIR Phase I grants support expansion into this important topical focus of mental health.
Profits and Revenues
Sociometrics’ gross annual revenues are approximately $2.3 million with approximately 22 percent of this amount being profit (Table App-D-9). Profits from product sales are now substantially larger than profits from SBIR project fees, a testament to the success of Sociometrics as an SBIR firm. However, the profit stream is still insufficient to replace SBIR as the primary funding engine for future development efforts. Sociometrics does not market price its products, as many of its customers are small, community-based nonprofits that cannot afford products fully priced to market. Rather Sociometrics’ products are priced at the cost of production with a small profit mark-up equivalent to a technical assistance retainer. Sociometrics sees this focus on widespread dissemination
TABLE App-D-9 Sources of Revenue and Profit, Sociometrics Corporation, 1984-2004
and use (as opposed to single-minded emphasis on profits alone) as part of its important public service.
REVIEW PROCESS
Sociometrics is generally satisfied with the SBIR proposal review process. It believes that it has “learned to compete successfully on paper.” The company takes a very pragmatic approach to review. It understands that there is a substantial random element in the process (a study conducted by NSF in the early 1990s found that the chance effect for whether a journal article or proposal is accepted by peers is approximately 50 percent). Therefore it believes that the best
approach to unfunded proposals is to consider all reviewer comments seriously and resubmit the proposal whenever these comments can be addressed. Another source of variability is the frequent change in Study Section make-up from one review to the next. As a result, comments made by one panel may be negated by the next panel who have new considerations and concerns. Nevertheless, Sociometrics believes that with tenacity its good proposals will eventually be approved through the current SBIR peer review mechanism. The company estimates that 70 percent of its applications for SBIR Phase I support and 95 percent of its applications for SBIR Phase II support are eventually successful. Forty percent of Phase I applications and 75 percent of Phase II applications are successful at first submission; the other applications require one or two resubmissions before they are eventually funded.
Commercialization has always been a strength for Sociometrics. The company has been pleased that this success criterion has received increased emphasis in recent application guidelines. Consistent with this, Sociometrics staff have observed that reviewer comments have recently praised the company’s strength in this area.
Sociometrics makes sure that its SBIR applications highlight its sales track record as well as its sales and marketing expertise. The company notes that this is very different from R01 research grant applications, for which these capacities are essentially irrelevant.
There needs to be ongoing evaluation of reviewers serving on Study Sections, providing some accountability. Sociometrics supports the concept that “bad reviewers” should be eliminated, but also understands that it is hard to find reviewers. Related to this problem is the concern that reviewers have appropriate expertise for the proposals they evaluate, which is a challenge when Study Sections cover quite broad areas.
OTHER OUTCOMES
Other Funding
Sociometrics has been funded by many NIH agencies and by government agencies outside NIH. Initial SBIR funding came from the Office of Population Affairs under the Deputy Assistant Secretary for Population Affairs, Department of Health and Human Services. Other projects have been funded by the National Science Foundation (NSF), the Centers for Disease Control and prevention (CDC), the Veterans Administration (VA), the National Center for Health Statistics (NCHS), private companies, nonprofit organizations, state and local governments, and private foundations. Sociometrics has never sought venture capital funding because its profits, while impressive for a behavioral and social science firm, are not large enough to make the company sustainable without SBIR funding, a requirement for venture capital funding.
Changing the Field
NIH and CDC officials, among others, regard Sociometrics’ effective-program replication kits as an important innovation helping to bridge the gap between health-related research and practice. The program-in-a-box opened the door for researchers to generate something more than an article or book as an output from their studies, and many researchers were especially pleased to find a way to connect their work to the improvement of practice.
Publications
Sociometrics’ staff members have more than 250 peer-reviewed publications; approximately 60 of these are based on the company’s SBIR work.
Training Effects
Sociometrics is poised for expansion now that younger staff are becoming qualified as PIs in their own right, which relieves some of the PI burden from the two senior managers, who were until recently PIs on all projects.
NIH Institutes and Centers (ICs)
Sociometrics has had the longest relationship with—and is closest to—the National Institute on Child Health and Human Development (NICHD). Sociometrics’ Founder and CEO, Dr. Josefina Card, has served on several NICHD study sections, and has also been on the NICHD National Advisory Council.
SUPPLEMENTAL FUNDING
Supplemental funding procedures vary substantially by IC. Typically, small supplement requests—up to 25 percent of the annual award amount at NIMH—are available at the discretion of the program officer (depending on funding availability). These are referred to as “noncompeting administrative supplements.” Large supplementary funding requests must compete with other similar requests, seeking a “competing supplementary award.” Sociometrics has obtained a few noncompeting administrative supplements. It has also obtained three larger supplement awards by expanding the scope of the funded Phase II grant in a way deemed “high priority” by the funding agency or by competing successfully via another funding mechanism (such as an RFA) with the funding agency then deciding, for administrative simplicity reasons, to add monies to the SBIR grant instead of issuing a new grant award. Examples include:
-
Teen pregnancy prevention program replication kits. Originally funded by NICHD, Sociometrics sought a third year of Phase II support through
-
a supplement to expand the scope of the Program Archive on Sexuality, Health & Adolescence (PASHA) from teen pregnancy prevention alone to teen STD/HIV/AIDS prevention as well. This expansion had been recommended by the PASHA Scientist Expert Panel, in light of the national spotlight on HIV/AIDS and the similar sexual-risk behaviors underlying both unintended pregnancy and STD/HIV/AIDS. The supplement request was forwarded by NICHD to the Deputy Assistant Secretary of Population Affairs who serves simultaneously as Director of the Office of Population Affairs. This political appointee interviewed the Sociometrics PI, and then personally approved the requested additional $750,000 in Phase II funding, transferring the monies to the NICHD grant.
-
Program archive for HIV/AIDS in adults. Initially funded by the National Institute of Allergy and Infectious Diseases (NIAID), supplementary funding was requested for the HIV/AIDS Prevention Program Archive (HAPPA) to expand the project to include programs targeted directly at minorities. In this case, the request was for approximately $575,000 over three years. However, an end-of-year budget underrun at NIAID resulted in the full requested funding being provided over one year, instead the requested three years.
-
Complementary and alternative medicine data archive. Sociometrics had Phase II funding from the National Center on Alternative Medicine (NCAM) to establish the Complementary and Alternative Medicine Data Archive (CAMDA) when it responded to an RFA issued by NCAM encouraging research on minorities and CAM. Sociometrics responded to the RFA by proposing to expand CAMDA to include data sets especially focused on minority populations. Its proposal received a high priority score and NCAM decided to fund the project via an administrative supplement to the Phase II project rather than via a new grant award.
RECOMMENDATIONS
Sociometrics believes that the SBIR program provides an essential resource for generating innovative and effective research-based products in efficient fashion. In response to questions about its support for various issues and trends in the program, Sociometrics makes the following recommendations:
-
Normalization of scores. Scores should be normalized across SBIR study sections.
-
Award size and duration. Phase I duration should be one year, and additional funding (beyond $100,000) should be available with justification. Phase II size and duration limits could remain as they are ($750,000 over two years); Sociometrics has always found it possible to split larger projects into two or more ideas qualifying for separate SBIR funding. While
-
Sociometrics has no a priori objection to “supersized” Phase II awards (awards exceeding the Phase II guidelines of $750,000), it recommends that if such awards are indeed becoming common, then information about them should be fully communicated to applicants and transparency increased. The increasing prevalence of larger Phase II awards might tend to benefit well-established companies and could result in fewer SBIR grants being made. These consequences should be taken into account in approving very large Phase II awards.
-
Direct to Phase II. Phase II competition should be open to all applicants meeting small business qualifications, permitting bypass of Phase I awards (though not of the need to show equivalent results). This might also, however, tend to benefit well-established companies.
-
Resubmission. The one-page Phase I proposal limit for summarizing applicants’ responses to reviewer comments is insufficient. The limit should be increased to two pages or even three, as is the case for Phase II proposals.
-
Evaluation. NIH should develop a program to evaluate the health, social, and economic impact of SBIR projects. Sociometrics would very much like to undertake evaluations either of its own SBIR projects, or of a group of projects that would include some of its own.
-
Chartered study sections for SBIR. Given the now-permanent character of the program, NIH should consider asking Congress to charter what are currently Special Emphasis Panels (SEPs), or should consider changing its guidelines for SEPs to mimic those for chartered study sections. In this manner, the composition of review panels would be more stable from one review round to the next, resulting in better reviews.
SOCIOMETRICS—ANNEX
TABLE App-D-10 Sociometrics’ SBIR Awards, NIH-Sponsored Phase I Projects Started in FY1992-2002
|
Fiscal Year |
Phase Type |
Award Size ($) |
Project Title |
Funding Institute-Center |
|
1994 |
Phase I |
80,991 |
AMERICAN FAMILY DATA CENTER |
HD |
|
1995 |
Phase II |
216,369 |
AMERICAN FAMILY DATA CENTER |
HD |
|
1996 |
Phase II |
222,496 |
AMERICAN FAMILY DATA CENTER |
HD |
|
1998 |
Phase II |
114,998 |
AMERICAN FAMILY DATA CENTER |
HD |
|
1997 |
Phase I |
99,817 |
ARCHIVE—EFFECTIVE YOUTH DRUG ABUSE PREVENTION PROGRAMS |
DA |
|
1998 |
Phase II |
343,632 |
ARCHIVE—EFFECTIVE YOUTH DRUG ABUSE PREVENTION PROGRAMS |
DA |
|
1999 |
Phase II |
396,232 |
ARCHIVE—EFFECTIVE YOUTH DRUG ABUSE PREVENTION PROGRAMS |
DA |
|
2001 |
Phase II |
24,999 |
ARCHIVE—EFFECTIVE YOUTH DRUG ABUSE PREVENTION PROGRAMS |
DA |
|
1999 |
Phase I |
95,825 |
CHILD WELL-BEING & POVERTY: STATISTICAL ABSTRACT & DATA |
HD |
|
2001 |
Phase II |
377,391 |
CHILD WELL-BEING & POVERTY: STATISTICAL ABSTRACT & DATA |
HD |
|
2002 |
Phase II |
371,768 |
CHILD WELL-BEING & POVERTY: STATISTICAL ABSTRACT & DATA |
HD |
|
1999 |
Phase I |
94,833 |
COMPLEMENTARY AND ALTERNATIVE MEDICINE DATA ARCHIVE |
AT |
|
2002 |
Phase II |
375,345 |
COMPLEMENTARY AND ALTERNATIVE MEDICINE DATA ARCHIVE |
AT |
|
2003 |
Phase II |
374,587 |
COMPLEMENTARY AND ALTERNATIVE MEDICINE DATA ARCHIVE |
AT |
|
2004 |
Phase II |
215,070 |
COMPLEMENTARY AND ALTERNATIVE MEDICINE DATA ARCHIVE |
AT |
|
2005 |
Phase II |
258,084 |
COMPLEMENTARY AND ALTERNATIVE MEDICINE DATA ARCHIVE |
AT |
|
1992 |
Phase II |
162,492 |
DATA ARCHIVE ON MATERNAL DRUG ABUSE |
DA |
|
1993 |
Phase II |
174,720 |
DATA ARCHIVE ON MATERNAL DRUG ABUSE |
DA |
|
1993 |
Phase II |
18,336 |
DATA ARCHIVE ON MATERNAL DRUG ABUSE |
DA |
|
1997 |
Phase I |
99,834 |
DATASET DEVELOPMENT SOFTWARE & FAMILY RESEARCH ITEM BANK |
HD |
|
1998 |
Phase II |
382,614 |
DATASET DEVELOPMENT SOFTWARE & FAMILY RESEARCH ITEM BANK |
HD |
|
1999 |
Phase II |
356,870 |
DATASET DEVELOPMENT SOFTWARE & FAMILY RESEARCH ITEM BANK |
HD |
|
Fiscal Year |
Phase Type |
Award Size ($) |
Project Title |
Funding Institute-Center |
|
1995 |
Phase I |
70,770 |
ESTABLISHING A CONTEXTUAL DATA ARCHIVE |
HD |
|
1996 |
Phase II |
338,926 |
ESTABLISHING A CONTEXTUAL DATA ARCHIVE |
HD |
|
1997 |
Phase II |
409,298 |
ESTABLISHING A CONTEXTUAL DATA ARCHIVE |
HD |
|
1993 |
Phase I |
49,975 |
ESTABLISHMENT OF A RESEARCH ARCHIVE ON DISABILITY |
HD |
|
1994 |
Phase II |
236,145 |
ESTABLISHMENT OF A RESEARCH ARCHIVE ON DISABILITY |
HD |
|
1995 |
Phase II |
230,731 |
ESTABLISHMENT OF A RESERCH ARCHIVE ON DISABILITY |
HD |
|
1996 |
Phase II |
31,288 |
ESTABLISHMENT OF A RESEARCH ARCHIVE ON DISABILITY |
HD |
|
1997 |
Phase II |
149,709 |
ESTABLISHMENT OF A RESEARCH ARCHIVE ON DISABILITY |
HD |
|
1992 |
Phase II |
220,955 |
ESTABLISHMENT OF AN AIDS/STD DATA ARCHIVE |
HD |
|
1993 |
Phase II |
236,301 |
ESTABLISHMENT OF AN AIDS/STD DATA ARCHIVE |
HD |
|
1994 |
Phase II |
42,070 |
ESTABLISHMENT OF AN AIDS/STD DATA ARCHIVE |
HD |
|
1995 |
Phase II |
10,000 |
ESTABLISHMENT OF AN AIDS/STD DATA ARCHIVE |
HD |
|
1998 |
Phase II |
350,655 |
HIV/AIDS PREVENTION PROGRAM ARCHIVE |
AI |
|
1999 |
Phase II |
397,449 |
HIV/AIDS PREVENTION PROGRAM ARCHIVE |
AI |
|
2000 |
Phase II |
574,670 |
HIV/AIDS PREVENTION PROGRAM ARCHIVE |
AI |
|
1997 |
Phase I |
99,221 |
INSTITUTE FOR PROGRAM DEVELOPMENT AND EVALUATION |
HD |
|
1999 |
Phase II |
337,465 |
INSTITUTE FOR PROGRAM DEVELOPMENT AND EVALUATION |
HD |
|
2000 |
Phase II |
412,385 |
INSTITUTE FOR PROGRAM DEVELOPMENT AND EVALUATION |
HD |
|
2001 |
Phase II |
49,987 |
INSTITUTE FOR PROGRAM DEVELOPMENT AND EVALUATION |
HD |
|
1993 |
Phase I |
49,494 |
INSTRUMENT ARCHIVE OF SOCIAL RESEARCH ON AGING |
AG |
|
1992 |
Phase II |
223,017 |
MICROCOMPUTER DATA ARCHIVE OF SOCIAL RESEARCH ON AGING |
AG |
|
1993 |
Phase II |
19,106 |
MICROCOMPUTER DATA ARCHIVE OF SOCIAL RESEARCH ON AGING |
AG |
|
2001 |
Phase I |
197,562 |
PROMOTING EVALUATION/TEACHING/RESEARCH ON AIDS (PETRA) |
MH |
|
Fiscal Year |
Phase Type |
Award Size ($) |
Project Title |
Funding Institute-Center |
|
2003 |
Phase II |
420,281 |
PROMOTING EVALUATION/TEACHING/RESEARCH ON AIDS (PETRA) |
MH |
|
2004 |
Phase II |
329,507 |
PROMOTING EVALUATION/TEACHING/RESEARCH ON AIDS (PETRA) |
MH |
|
1994 |
Phase I |
80,991 |
SOCIONET—ONLINE ACCESS TO SOCIAL SCIENCE DATA |
HD |
|
1995 |
Phase II |
446,682 |
SOCIONET: ONLINE ACCESS TO SOCIAL SCIENCE DATA |
HD |
|
1996 |
Phase II |
303,033 |
SOCIONET: ONLINE ACCESS TO SOCIAL SCIENCE DATA |
HD |
|
1998 |
Phase I |
99,340 |
STATISTICS ON DEMAND: DATA ANALYSIS OVER THE INTERNET |
HD |
|
2000 |
Phase II |
381,525 |
STATISTICS USING MIDAS: DATA ANALYSIS OVER THE INTERNET |
HD |
|
1999 |
Phase II |
367,726 |
STATISTICS USING MIDAS: DATA ANALYSIS OVER THE INTERNET |
HD |
|
2002 |
Phase I |
99,011 |
VIRTUAL PROGRAM EVALUATION CONSULTANT (VPEC) |
HD |
|
2003 |
Phase II |
383,357 |
VIRTUAL PRACTIONER EVALUATION CONSULTANT (VPEC) |
HD |
|
2004 |
Phase II |
363,923 |
VIRTUAL PRACTIONER EVALUATION CONSULTANT (VPEC) |
|
|
1993 |
Phase I |
50,000 |
ARCHIVE OF TEEN PREGNANCY PREVENTION PROGRAMS |
HD |
|
1995 |
Phase II |
408,644 |
PROGRAM ARCHIVE ON SEXUALITY, HEALTH, & ADOLESCENCE |
HD |
|
1996 |
Phase II |
987,378 |
PROGRAM ARCHIVE ON SEXUALITY, HEALTH, & ADOLESCENCE |
HD |
|
1997 |
Phase II |
45,000 |
PROGRAM ARCHIVE ON SEXUALITY, HEALTH, & ADOLESCENCE |
OPA |
|
1994 |
Phase I |
79,383 |
ESTABLISHING A STROKE DATA ARCHIVE |
NS |
|
2002 |
Phase I |
199,922 |
PROMOTING CULTURALLY COMPETENT/EFFECTIVE HIV/AIDS PREVENTION PROGRAMS |
AI |
|
2004 |
Phase II |
361,223 |
PROMOTING CULTURALLY COMPETENT/EFFECTIVE HIV/AIDS PREVENTION PROGRAMS |
AI |
|
NOTE: For a list of codes for National Institutes of Health institutes and centers, see Box App-A-1. SOURCE: Sociometrics Corporation. |
||||
VectraMed
Andrew Toole
Rutgers University
COMPANY AND FOUNDER BACKGROUND
VectraMed, Inc., is a biopharmaceutical company located in Plainsboro, NJ. The company was founded by Dr. James Pachence in 1997 to exploit emerging opportunities in the field of drug delivery. VectraMed’s delivery technology promises improvements in both the site specificity and the sustained release capabilities of pharmaceutical agents. Their technology has the potential to improve the efficacy, reduce the toxicity, and reduce the dosage frequency for a variety of existing and novel drug therapies.
While the company remains focused on becoming a leading pharmaceutical firm, it has recently faced significant setbacks. During its first five years of operation, VectraMed was growing. This growth was fueled by a variety of successful efforts to obtain research and development (R&D) funds, hire employees, and advance the scientific basis of their proprietary technology. Early R&D funds were secured from the SBIR Program, angel investors, and corporate partners. With these funds in hand, VectraMed grew from a single founder to twelve employees with eight Ph.D. scientists. Moreover, the results from a number of SBIR funded preclinical studies were very positive. In 2000, they negotiated an exclusive license with Rutgers University and the University of Medicine and Dentistry of New Jersey to develop antifibrotic agents. In January 2001, the company began a joint venture with Elan Pharmaceuticals to develop and commercialize anticancer drugs.
By the end of 2002, however, R&D funds were drying up and it had become clear that the joint venture with Elan needed to be terminated. After Elan’s recent strategic restructuring, they stopped all R&D related activities in their joint ventures. With mounting uncertainty about the financial future of VectraMed, employees left to find more stable environments. Today, VectraMed has completely “scaled down.” Only Dr. Pachence and two other members of his management team are still with the company. Further, the company has sold off all of its physical assets. The intellectual property protecting their drug delivery technology, however, remains with VectraMed.
Dr. James Pachence is a “serial entrepreneur” with a background in academic science. He received his Ph.D. in Biophysics from the University of Pennsylvania in 1980 and spent two years as a Research Professor in the Biochemistry Department at Columbia University. He started his private sector career at Helitrex, Inc., in 1983 to develop novel collagen-based surgical compounds. Catching the entrepreneurial “bug,” Dr. Pachence founded a collagen-focused biomaterials
firm, called MediMatrix, in July 1986. In 1988, MediMatrix merged with Applied Biomedical Sciences, a public company based in Long Beach, CA. A few years later, Dr. Pachence founded Intregra LifeSciences and spent three years as the Vice President of Scientific Affairs. Following a four-year stint as a consultant, Dr. Pachence founded VectraMed in 1997.
VECTRAMED TECHNOLOGY
VectaMed, Inc., has developed a proprietary “Tissue-Activated Drug Delivery” or TADD technology. Their technology attaches pharmaceutical agents to a water-soluble polymer. The polymer has a “comb” structure that allows therapeutic agents to be linked to the “teeth” of the comb using enzyme-cleavable linking groups. This creates a compound with tissue-specific drug release. Figure App-D-14 illustrates the TADD drug and polymer conjugate.
VectraMed has filed several patent applications with the U.S. Patent and Trademark Office. The basis for these applications is the way in which the drug is attached to the polymer. While attached, the drug is not active and is called a “pre-drug.” This inactivity is the key to increased efficacy and reduced toxicity. As the polymer drug conjugate approaches the disease site, a disease-specific trigger “attacks” the cleavable link and releases the pharmaceutical agent.
FIGURE App-D-14 TADD drug and polymer conjugate.
SOURCE: VectraMed, Inc., nonconfidential business plan.
Both the characteristics and versatility of the polymer-based carrier backbone are important to the broad-based applicability of VectraMed’s TADD technology. The polymer is water-soluble and consists of well-defined physiological components. Moreover, with information on disease-specific triggers, a variety of polymer cleavable linking groups can be designed with different circulation times and solubility properties.
VectraMed was pursuing three lines of product development. One of these areas involved cancer drugs through the joint venture with Elan Pharmaceuticals. Prior to Elan’s restructuring, their cancer applications looked promising with increased efficacy and reduced toxicity in animal models. VectraMed is currently in discussions with Elan to terminate the joint venture. In addition to cancer, VectraMed had active programs in chronic inflammatory diseases and fibrotic diseases including pulmonary hypertension, pulmonary fibrosis, and surgical adhesion prevention. While most of the preclinical results were positive, the lack of financial backing has suspended further research.
IMPORTANCE OF SBIR
Dr. Pachence was very positive about the role and contribution of the SBIR Program to the success of VectraMed. While there were no commercialization outcomes, he said that the program has “made an impact” in a variety of ways including contributions to multiple patent applications and multiple papers published in prestigious journals. Four of the most important benefits for VectraMed from participation in the SBIR Program were:
(1)
Key Source of Early-stage Financing
Dr. Pachence used his personal savings to start VectraMed and to license its initial product candidates from Rutgers University and the University of Medicine and Dentistry of New Jersey in 1997. In that same year, VectraMed won two Phase I awards to perform proof of principle animal studies, one for postsurgical adhesions and the other for pulmonary hypertension. Each of these feasibility studies succeeded and VectraMed went on to win Phase II awards for each of these lines of research. SBIR Phase I funds were received in 1997 ($200,000) and the Phase II awards extended over two years, 1999 ($776,699) and 2000 ($846,783). (The SBIR investment into these lines of research totals $1,823,482.) Dr. Pachence noted that these SBIR awards were “key to getting the first stages of development done” and that SBIR was “important for early money.”
(2)
Critical for Follow-on Funding and Business Deals
Given the expense and time required to develop new drug therapies, it was incumbent upon VectraMed to obtain additional private financing beyond the
initial personal funds that Dr. Pachence had invested. To a significant degree, SBIR funds allowed VectraMed to obtain additional follow-on private investment. The SBIR Program financed a large part of the preclinical animal studies. These initial SBIR awards used academic labs as subcontractors to complete the research since VectraMed did not have its own laboratory facilities. The positive results from these studies helped convince corporate partners and angels to invest in VectraMed. This new scientific evidence was combined with previously collected information on the market opportunity to facilitate the completion of a formal business plan. Dr. Pachence noted that “SBIR was super critical for getting the angel financing but also for getting the deals done with the corporate community.”
(3)
Facilitated Hiring of Employees
The SBIR Phase II awards for VectraMed’s pulmonary hypertension and surgical adhesion technologies facilitated the growth of the company. Dr. Pachence noted that “it was critical that I had that Phase II money, otherwise, I would have never hired employees.” The proceeds from these Phase II awards, however, would not have been sufficient by themselves to create and sustain a team of Ph.D. researchers. But the scientific success in Phase I combined with the Phase II award allowed Dr. Pachence to raise some angel financing to complement the SBIR monies. Taken together, VectraMed was allowed to grow its research team from subcontractors to eight full-time scientists.
(4)
Simple Mechanism for Early-stage Collaborative Work
In addition to the initial SBIR grants in the areas of pulmonary hypertension and surgical adhesions, VectraMed received another Phase I award in 1999. This grant supported a collaborative effort between VectraMed and a drug delivery firm called MicroDose. MicroDose, a company whose physical location is near VectraMed, has developed a proprietary delivery system using deep lung inhalation. VectraMed needed a delivery system to administer its molecule and the MicroDose technology looked promising. The SBIR award funded a research effort into this possibility. Unfortunately, the combination was not commercially viable and the research was abandoned after Phase I. Nevertheless, the SBIR-funded research facilitated the collaborative effort and helped to resolve the uncertainty about the most effective route of administration for VectraMed’s polymer molecule.
ISSUES WITH THE CURRENT SBIR PROGRAM
Dr. Pachence highlighted a few issues of concern about the SBIR Program at NIH. His perspective is comparative since he is able to draw on past experi-
ence with the SBIR Program at the Department of Defense and the Advanced Technology Program at the National Institute of Standards and Technology. He made the following four points:
(1)
Obtaining the Award Funds is Difficult at the NIH (Including Phase I & Phase II Delays)
Even prior to VectraMed’s grants, Dr. Pachence won SBIR awards from the NIH back in the late 1980s. Since those early days, NIH SBIR administration has improved quite a bit. In those days, in order to collect his award funds, he would need to travel to the NIH campus to track down the money. While no longer requiring a dedicated trip to the campus, obtaining funds in the current program is still somewhat difficult because it requires an active pursuit of the funds, typically in the form of a phone call to the Study Section leader. This problem extends to the delays between Phase I and Phase II awards. The dispersal of the award funds was more efficiently done by both DoD and APT, although setting up the payment protocols at DoD often took a long time.
(2)
Program Coordination is Poor Within the NIH
In stark contrast to the SBIR at DoD and the ATP at NIST, the SBIR Program at NIH is poorly coordinated. Responsibility for the administration of the SBIR Program within the NIH is shared by different groups. As far as the awardees are concerned, these groups have poor communication and integration. In contrast, ATP at NIST stands out as a highly successful model. There is a “project insider” within the agency that has a sincere interest in the company’s research and actively manages the relationship between the agency and the company. Dr. Pachence says the ATP insider had a real scientific and economic interest, set up quarterly meetings, and produced well thought out reports. SBIR at DoD is also better coordinated than NIH. Perhaps the procurement orientation of the agency’s awards necessitated a deeper agency interest in the firms.
(3)
NIH Study Section Leaders are Too Busy
A critical underlying problem for the administration of the NIH SBIR Program is that study section leaders are too busy. Dr. Pachence noted that these people are “inundated” with programs and responsibilities. As a consequence, a productive relationship cannot be established.
(4)
The NIH Review Process Overemphasizes the Scientific Component of the Proposal
The implementation of the SBIR Program at NIH followed the established organization and procedures traditionally used for purely scientific proposals. As a result, the SBIR proposal review panels are populated with a disproportionate number of academic scientists. Perhaps unintentionally, the science orientation of these individuals created a bias against commercial development grants. While a strong scientific knowledge base is important, understanding medical product development is also a critical component. SBIR proposal review panels should be “balanced” to allow projects with a stronger product development component to be reviewed more favorably.






















































































































































