3
Future Directions in Ocean Sciences
The possibility of and the need for studying the ocean on a global scale provide a major impetus for new partnerships in oceanography. The design and deployment of a global ocean observing system, now being discussed, will be possible only with the cooperation of ocean scientists and governments throughout the world.
THE SCIENCE OF OCEANOGRAPHY
Oceanography, the science of the sea, serves many purposes while deriving impetus from many sources. All of oceanography—physical, chemical, geological, and biological—is driven by scientists interested in advancing basic knowledge. Ocean scientists have made a number of exciting discoveries in the past 30 years that have changed our view of Earth. The discovery of oceanic eddies has been important for an understanding of ocean circulation, propagation of sound in the ocean, fisheries productivity, and other ocean processes. Verification by ocean drilling that Earth's crust is divided into moving plates that are created at mid-ocean ridges and recycled into Earth's interior replaced the traditional view that the surface was essentially static. Discovery of dense colonies of animals and bacteria at some deep-sea hydrothermal vents demonstrated that organisms could thrive in ecosystems based on chemical energy from Earth's interior rather
than directly on energy from the Sun. Study of the combined ocean-atmosphere system has provided sufficient knowledge of interannual climate variations that scientists are now able to forecast El Niño climate disturbances months in advance.
Over the next decade, oceanography will continue to provide exciting discoveries by contributing new understanding of Earth as a system and by helping us understand how humankind is altering the system. It is now essential (and possible) to study ocean processes on a global scale. The oceanography of the next decade will take place in the traditional marine science disciplines and at the boundaries of these disciplines. New partnerships among oceanographers working in different disciplines should lead to new discoveries about the ocean's role in climate change, the function of mid-ocean ridges, and coastal ocean processes.
Additional oceanographic studies in the coming decade will focus on how ecosystems affect global cycles of important chemicals and, conversely, how changes in the global environment affect marine ecosystems. Studies of ecosystems at hydrothermal vents and hydrocarbon seeps will refine our ideas about the conditions under which life is possible and about the origins of life. More of the ocean floor must be explored to determine the extent and nature of deep-ocean vents, their ability to support novel organisms, and their importance in global chemical cycles. Continued study of the ocean's chemistry should bring new understanding of the past state of Earth, how ocean processes operate today, and the contribution of sources and sinks of various chemicals. The study of deep-ocean sediment cores will provide more information about past natural cycles of Earth's climate, with which present climate fluctuations can be compared. Oceanographers will achieve a better understanding of the variability of the circulation of the world ocean. The interaction of climate with this circulation is only poorly known, but there is evidence that the transport of surface water to depth can vary greatly even over as short a time as one decade.
Unlike many other sciences driven by scientific curiosity, aspects of marine science have immediate and obvious practical applications. These include, but are not limited to, the control of climate by ocean circulation, chemical and biological reactions to climate change, understanding fisheries productivity, movement of pollutants, and the problem of coastal development in the face of rising sea level. Oceanographers are fortunate to take part in a science that is fascinating, compelling, and intellectually challenging. Oceanography is also a science whose outcome is of
immediate societal application and in which the financial stakes are potentially immense, for example, the economic impact of a reliable forecast of a sea-level rise. Because the societal implications of the science are readily apparent to policy makers, they may demand answers to purely practical questions in the short term. This pressure can distort the investment in basic science, undermining the quest for basic understanding that remains key to the long-term solution of practical problems. Thus the functioning of oceanography in the United States should focus both on sustenance of the underlying basic science and on specific answers to practical questions of short-term urgency.
This chapter summarizes the concerns of basic scientists, with some focus on the interaction of basic science with more practical problems. Several themes are common throughout the discussion, which is divided by classical disciplines. First is the growing sense that the basic science now encompasses the global ocean scale. This capability and the need to conduct global-scale studies have led to the planning of large-scale, long-term cooperative experiments. Primarily planned and executed with National Science Foundation (NSF) support, they focus the work of many scientists on global ocean research. These large programs are usually managed through national or international consortia that involve many scientists, agencies, and often countries. Such programs will explore new questions and test new mechanisms for working together in the next decade. Global uncertainties are rapidly moving much of oceanography from the capabilities and interests of single or small groups of investigators for a limited time to the involvement of many individuals, institutions, and governments for decades. Mechanisms must be developed for these new large-scale efforts to be sustained in a scientifically and technically sound manner, by coordinating the plans of other nations, federal agencies, academic institutions, and individual scientists.
Second, all sections of this chapter emphasize the dependence of the subject as a whole continued technical developments. The ocean is remarkably difficult to study, given its size, opacity to electromagnetic waves, and general hostility (e.g, its corrosiveness, high pressures, and turbulence). The health of all disciplines depends directly on the continued development of new tools designed to solve their fundamental sampling problems. In the past decade, oceanographic sampling improved through incorporation of new technologies from other fields, such as remote sensing, material science, electronics, and computer science. A fundamental change arising from the use of these new technologies is
an increase in the quality and the volume of data collected. Accompanying this change is a significant increase in each oceanographer's capacity to study ocean phenomena, an increase that raises the costs for each oceanographer's science. As the cost per oceanographer for scientific equipment and facilities has increased, the field has responded with increased sharing of facilities, such as ships and submersibles, and equipment, such as the new accelerator mass spectrometer for carbon-14 measurements. The development and shared use of expensive facilities are likely to continue in the future. Yet even with shared facilities, inflation-adjusted research funding for the ocean sciences has remained nearly constant over the past decade, while the number of Ph.D.-level academic oceanographers has increased by about 50 percent and societal pressures to predict man's effect on the ocean have also increased. The growth in the scientific capacity of each investigator and the number of qualified investigators, coupled with nearly constant funding, has resulted in partial funding for some ocean researchers.
Third, the resolution requirements of oceanographic models and the complexity of model physics have always outstripped the largest computational capability anywhere. As understanding of the ocean becomes more sophisticated, more sophisticated models are required. The nurture of computational capability is reflected across the disciplines.
Fourth, the understanding of the ocean and of the problems of oceanographers has progressed so much in the past several decades that all disciplines are now capable of new accomplishments in a seemingly endless number of areas. The problem is that the potential far exceeds the resources likely to be available, and the difficult task of setting priorities within and across disciplines will be amplified.
The foundation of knowledge about the ocean that is now used in policy decisions was gained largely through Office of Naval Research (ONR) and NSF investments in basic research over the past several decades. Yet the demand for quick answers to purely practical questions sometimes obscures the need for investing in basic science, which remains the key to long-term practical applications. Under pressure to provide immediate solutions, mission agencies may be tempted to focus only on the short term. One example of the importance of basic research is a 1961 study that is now contributing to the debate about climate change—the question of whether ocean circulation has two stable states. Both the geological record and numerical models suggest that, at some times
in the past, ocean circulation was unlike today's and that it could switch rapidly from its present state to a radically different one. When the ocean was in this alternate state, Earth's climate was not at all like today's. This idea dates back to a paper written by Henry Stommel (1961), an academic scientist driven primarily by his own curiosity and supported by ONR and NSF. The paper had little impact for more than 20 years. Now regarded as seminal, it illustrates the need to sustain basic science so that future generations will have a knowledge base from which to develop their policy decisions.
The authors of the following sections were asked to discuss the dominant issues of their disciplines and to lay out the grand themes, providing a scientific underpinning to discussion of the new partnerships. Ten years is probably the outer limit of an attempt to suggest what the major science themes will be. A decadal report written in 1960 would almost surely have missed the revolution in plate tectonics and thus would have been hopelessly wrong in its discussion of some dominant scientific themes in 1970. On the other hand, such a report could have captured accurately the methodologies of work at sea and the human resource requirements. Of course, the central questions of the field did not change either—although an intellectual revolution in the way they could be discussed occurred.
The decision to organize this chapter according to traditional oceanographic disciplines was not arbitrary (the coastal ocean is a special case, discussed below). Anyone who reads each section will perceive exciting and important scientific problems that cut across many or even all disciplines. Examples are the growing importance of paleoceanographic studies that involve geology, geophysics, chemistry, biology, and physical oceanography because of their climate implications. Likewise, the study of ridge crests cuts across geology and geophysics, biology and chemistry, and even slightly, physical oceanography. Nonetheless, the board believes that there is a danger in declaring such interdisciplinary studies as the likely focus of future marine science efforts. Without denigrating the science done on such problems, interdisciplinary studies clearly build on the foundations of chemistry, physics, geology, geophysics, and biology. These, in turn, depend directly on their nonmarine counterparts of physics, mathematics, numerical methods, and other fields that provide the intellectual fertilization of marine studies. The history of ocean sciences suggests that one cannot have good interdisciplinary science without good disciplinary foundations, and it is essential that the traditional
oceanographic disciplines retain their identity and vitality. One needs to encourage scientists working on interdisciplinary problems, but they must first be expert in one or more of the basic disciplines. Just how such fostering should take place is the subject of debate, and the reader will detect a degree of disagreement as to how we should move forward. Working out various combinations of scientists and institutions is a major challenge for our academic institutions in the next decade. The board makes no specific recommendation except to note that the strength of the U.S. scientific community is its ability to tolerate and encourage great diversity in its institutions.
Because the following sections were written by a number of different authors, they differ in style and content. The sections are not meant to be all inclusive but instead to provide a flavor of the excitement of each discipline of oceanography.
The treatment here of coastal oceanography is anomalous because it deals with a geographic region—that is, shorelines, estuaries, bays, and the continental shelf—and not a discipline. The large percentage of the U.S. and world population that lives in the coastal zone, and the multiple human uses and impacts on the coastal ocean, place this area of oceanography much more conspicuously and immediately in the public policy arena. Unlike the participants in deep-water marine science, states, cities, and private enterprise are all prominent players in understanding and using the coastal ocean. The interplay of the basic sciences of fluid flow, chemistry, biology, shoreline physics, and geology with public policy concerns leads to a near-term urgency that cuts across scientific disciplines. However, it is important to recognize, as this report does, that the foundations of understanding must rest firmly on the underlying basic sciences.
DIRECTIONS FOR PHYSICAL OCEANOGRAPHY
Summary
The great volume of water in the ocean exerts a powerful influence on the Earth's climate by absorbing, storing, transporting, and releasing heat, water, and trace gases. The goal of physical oceanography is to develop a quantitative understanding of the ocean's physical processes, including circulation, mixing, waves, and fluxes of energy, momentum, and chemical substances within the ocean and across its boundaries. Addressing such problems will require sustained large-scale observations of the world ocean
aided by advances in measurement and computational technology. Designing and deploying a global ocean observing system are among the most important and difficult tasks for physical oceanography and climate studies for the next decade. Such a system would incorporate existing measurement programs as well as observations that are not yet routine.
Several topics will dominate physical oceanographic research in the coming decade. Research in modeling, ocean mixing, thermohaline circulation, and water mass formation processes will be important. To achieve their scientific objectives and to make more complete ocean observations, physical oceanographers must use both proven methods and new technologies, including acoustic techniques; measurements made from volunteer observing ships; satellite observations and data relay; and measurements of the distributions of trace chemicals.
Introduction
The ocean consists of nearly 1.4 billion cubic kilometers of salty water, about 97 percent of the free water on Earth. In comparison, the atmosphere holds only about 0.001 percent. This volume of water exerts a powerful influence on Earth's climate by transporting heat, water, and other climate-relevant properties around the globe and by exchanging these properties, as well as greenhouse gases (e.g., carbon dioxide, methane, and chlorofluorocarbons), with the atmosphere. Net ocean absorption of greenhouse gases and some greenhouse-induced heat from the atmosphere can delay greenhouse warming of the atmosphere. Predicting future climate conditions depends on learning what controls ocean circulation and water mass formation, and whether the system is predictable, even in principle.
Physical oceanography, like many fields of science, consists of theory, observations, and numerical models. Physical oceanographic theories use the equations of fluid dynamics, modified to account for Earth's rotation and shape (e.g., O'Brien, 1985). A goal of physical oceanography is to develop a quantitative understanding of the ocean circulation, including fluxes—of energy, momentum, and chemical substances—within the ocean and across its boundaries. Physical oceanographers must contribute to the increasing societal emphasis on measuring, predicting, and planning for changes in global climate by improving understanding of the physical factors that maintain the overall physical, chemical, and biological characteristics of the ocean. Advances in measurement and com-
putational technology will continue to contribute to advances in physical oceanography.
Studies of climate change put the skills of oceanographers to a severe test. The time scales are long: interannual, decadal, and beyond. Physical processes are three dimensional and involve interaction of the ocean with the atmosphere. Winds transfer momentum and promote mixing and evaporation. Atmospheric temperature influences the density of ocean surface layers through effects on seawater temperature and salinity (through ice formation and melting), which in turn modify the atmosphere. Development of the physical state of the ocean is difficult to model because it involves the complex interaction of processes that operate on vastly different time and space scales. Nonetheless, progress is being made. Techniques that will permit better and more frequent observations are being developed, and advances in numerical modeling will soon permit representation of the major components of ocean circulation.
Ocean observations reflect the state of the ocean and hence the forces acting on it. Because observations are made in a corrosive, turbulent environment with high pressures at depth, they are difficult and expensive to obtain. Because of the size and variability of the ocean, measurements are always incomplete in space and time. Yet understanding the ocean depends on adequate measurements, and to make them we need to use technologies that permit a view of the global ocean. Technologies based on acoustics, space-based remote sensing, and underway automatic measurements could all be applied to global-scale observations.
Predictions of the ocean can be carried out only when the initial and boundary conditions are provided from observations with an accuracy and precision consistent with the physics present. Because oceanic observations are so expensive, models and theories must be used to help determine the most cost-effective measurements and measurement systems.
Global Ocean Observing System
Physical oceanographic observations and modeling are becoming global, but the resources required to deploy and sustain large-scale observations of the world ocean are enormous. The exact configuration of a global ocean observing system is unknown, but it would probably include existing observations from satellites, moored open ocean sensors, volunteer observing ships, and the global sea-level network, as well as other observations that are
not yet defined or collected routinely. The scientific and technological results from several ongoing large-scale research programs—the Tropical Ocean-Global Atmosphere program, the World Ocean Circulation Experiment (WOCE), and the Joint Global Ocean Flux Study—should be used to design an operational observation system that is effective, affordable, and consistent with our knowledge of the scales of ocean biology, chemistry, and physics. It would be the largest field enterprise ever undertaken by the oceanographic community, and it must have an international and multidisciplinary scope well beyond previous experience. The design and implementation of a global ocean observing system (GOOS) must involve ocean scientists substantially because the design is extremely important to the science itself and depends on firm scientific understanding. Designing and deploying a GOOS is one of the most important and difficult tasks for physical oceanography and climate studies in the next decade. The United States should take a major leadership role in both the research and the operations.
Because of the present paucity of ocean data, numerical models are important in the development of a GOOS. Models will be used to interpret available data for testing possible system designs and, ultimately, to interpret the data from such a system.
Major Research Topics for the Coming Decade
Several topics will dominate physical oceanographic research in the coming decade. The list is incomplete; the topics mentioned received some emphasis during the Ocean Studies Board workshops as representing key research issues and include the following: research in modeling; ocean mixing, including interior mixing and the surface mixed layer; thermohaline circulation; and heat and freshwater fluxes.
Ocean Modeling
The central focus of numerical modeling of the ocean has been, and continues to be, directed toward fluid dynamics, but the models have importance far beyond physical oceanography. For example, communities of organisms in the upper ocean live in a delicate balance, depending on the stability of the water column, its mixing rates, and its large-scale vertical and horizontal fluid movements. Our limited ability to predict the movements of the upper ocean limits understanding of basic biological processes.
Combined atmosphere-ocean simulations at interannual time scales require more accurate ocean models. The present generation of coupled atmosphere-ocean models exhibits unacceptable drifts (Manabe and Stouffer, 1988). Climate forecast models must be free of even small systemic errors that accumulate over long simulated periods, hiding the signals that are sought. To understand and mimic the paleoceanographic record, a major test of global models, one must be able to carry model integrations over time scales corresponding to thousands or tens of thousands of years. It is not clear that ocean and atmosphere behavior is predictable on scales of decades or longer. The limits of predictability are being explored as a research topic.
The global ocean is so large and its circulation occurs over such a variety of space (tens to thousands of kilometers) and time (days to centuries) scales that ocean circulation modeling has always overwhelmed even the largest supercomputers. This situation will probably remain for some decades to come. Thus it is a major intellectual challenge to design models of ocean circulation with time and space increments small enough to model processes adequately, given foreseeable limitations in computing resources.
Prominent features and processes that must be incorporated more accurately into physical oceanographic models (in a manner consistent with observations) include the effects of complex bottom topography on deep-water masses, deep vertical and horizontal mixing, eddies and fronts in the upper ocean, the interaction of water flow and diffusion of a variety of properties, boundary effects at the seafloor and surface, and the dynamics of shallow and deep boundary currents.
Ocean Mixing
Interior Mixing Large-scale ocean circulation is coupled with, and partially controlled by, small-scale mixing processes. Understanding the places, rates, and mechanisms by which the ocean mixes heat, salt, and momentum is crucial to understanding the circulation of the largest scales and essential to any capability to predict future oceanic states. It is intimidating to realize that to understand the dynamics of large-scale circulation and convective water mass formation, we must also understand the physics acting on the smallest scales (centimeters and millimeters). Heat-
ing, cooling, flow, and mixing processes act together to determine physical properties of the ocean. Changes in any one of these processes can affect the global climate system. Significant progress in the observation of ocean mixing processes and in the interpretation of these observations has been made, but understanding remains inadequate.
The capability to compare direct mixing measurements (through microstructure and purposeful tracer releases) with natural mixing (estimated indirectly from natural tracer distributions) is rapidly accelerating our understanding of mixing processes (Watson and Ledwell, 1988). The results of such comparisons will direct future research. For example, if deep-sea observations confirm that mixing rates are lower than predicted, attention will focus on mixing processes in the benthic boundary layer and continental slopes. If tracer studies indicate significantly more mixing than is seen by direct measurement, double diffusive and other mechanisms, will be explored. With new observational techniques and a clear measurement strategy, significant progress can be expected in the coming decade in the study of ocean mixing.
Surface Mixed Layer The primary production that supports the entire marine food web occurs in the upper sunlit portion of the ocean (the euphotic zone), where photosynthesis occurs. Our growing concern for climate variation makes understanding the uptake of carbon dioxide related to photosynthesis of particular importance. Fortunately, several developments over the last few years in biological oceanography, marine chemistry, and ocean physics promise advances in the study of the biological-chemical cycles in the euphotic zone. Exploitation of new techniques could significantly improve our ability to predict various aspects of global environmental change, including the ocean's role in sequestering carbon dioxide.
The topmost layer of the ocean is called the ''mixed layer" because the waters are mixed by wind, waves, and currents. This layer is often nearly homogeneous in temperature and chemical characteristics, and is bounded by the sea surface and a layer of denser water. The transfer of gases between atmosphere and ocean depends primarily on mixed-layer processes. Understanding the physics of the ocean surface mixed layer, and its coupling with the ocean interior and the atmosphere, is essential if the combined biogeochemical systems of ocean and atmosphere are to be represented correctly in ocean models. Mixed-layer studies are among the endeavors of physical oceanography in which strong
interaction with the fields of biology and chemistry is particularly rewarding. The rates of heat, water, gas, and momentum exchange across the ocean-atmosphere interface must be estimated better from easily measured or calculated environmental parameters (e.g., wind, temperature differences across the air-sea interface, waves, and stability of the mixed layer).
Because photosynthesis involves the conversion of carbon dioxide and nutrients into living material and oxygen, the euphotic zone is an immediate sink for atmospheric carbon dioxide. The flow and mixing of water masses and the transport of nutrients and particles that control phytoplankton populations depend on physical oceanographic processes. Understanding the mixed layer is a complex problem involving studies in marine biology, chemistry, and physics. In a crude sense, the mixed layer can be regarded as controlled by a set of chemical reactions in which biological processes determine most of the reaction rates (nutrient fixation and regeneration) and physical processes (advection, mixing, and particle sinking) determine the rates at which reactants and products are provided to or removed from the system.
The physical oceanographer's approach to studying the surface mixed layer involves measurement of currents and horizontal variations to determine advection, microstructure measurements to study turbulent fluxes, and measurements of deeper properties to infer vertical flows. Chemical oceanographers study the latter process using time-dependent tracers to estimate the vertical path of water masses and observe changes along it. Both methods can be strengthened by a model that integrates the measurements.
Thermohaline Circulation In a few limited regions of the ocean, a combination of low temperature and high salinity produces dense surface water that flows into the deep ocean and spreads laterally to initiate global-scale thermohaline circulation. Deep-reaching convection occurs in the northern North Atlantic Ocean and around Antarctica. These water masses spread throughout the ocean and force deep ocean water, which has been made more buoyant by the downward diffusion of heat, to upwell slowly. Eventually, the upwelled water migrates back to the sinking regions to complete a thermohaline circulation cell (see Gordon et al., 1992). Water masses formed in different regions vary in terms of temperature, salinity, nutrient concentrations, and stored carbon content. The relative contribution from each source region determines the ocean's average temperature, salinity, and other properties, such as carbon storage. In addition, this downward flow of surface water pro-
vides a link between the atmosphere and the deep ocean. Thus a better understanding of the global climate system requires a detailed understanding of the thermohaline circulation, its vulnerability to change, and the processes that govern water mass formation rates. Once these factors are understood, they can be represented in global ocean and climate models.
There is evidence that surface salinity fluctuations in the high-latitude North Atlantic Ocean control the thermohaline circulation by altering North Atlantic Deep Water (NADW) formation. One possible mechanism to slow NADW formation is capping of the ocean surface with low-salinity water, such as Arctic Ocean waters (Weyl, 1968). During the last century, there were at least two episodes of low surface salinity water in the northern North Atlantic Ocean [the latter during the late 1960s and 1970s is referred to as the great salinity anomaly (Dickson et al., 1988)] that drastically reduced or stopped convection and NADW production. Changes in the twentieth century are very small compared to suspected changes in NADW formation rates during the swings between glacial and interglacial periods (Boyle, 1990).
The Indian Ocean is a strongly evaporative ocean. Lacking a northern polar region, the tropical heating of the Indian Ocean cannot be vented by flow to the north. Strong evaporation in the Red Sea and Persian Gulf forms warm salty water that, like the Mediterranean outflow, is small in volume but adds significant heat and salt to the deep ocean. The role of the Indian Ocean in larger-scale thermohaline circulation remains unclear and should be studied in the coming years.
The sea ice cover of the Southern Ocean acts to decouple the ocean from the atmosphere, limiting cooling of the ocean by the polar atmosphere. The insulating blanket of sea ice protects the ocean from the cold atmosphere. The extreme seasonality and rapid spring melting of the Southern Ocean sea ice cover suggest that the heat carried into the surface layer by the upwelling of deep water is a key in understanding the Southern Ocean sea ice budget; the buildup of heat within the mixed layer under the winter ice cover induces melting even before solar radiation melts the ice from above. Ocean heat flux also limits sea ice thickness during the winter to less than 1 meter, in contrast to the 3-meter ice of the more stable Arctic Ocean.
Heat and Freshwater Fluxes The ocean interacts with the atmosphere in affecting the heat and freshwater fluxes that control the climate system. Estimates of the fluxes to and from the ocean
and atmosphere are difficult to obtain with adequate accuracy, and at the present time there are serious conflicts among values estimated from atmospheric models and data, from oceanic models and data, and from the boundary layers in the two media.
The ocean and atmosphere contribute roughly equally to the transport of heat from lower latitudes toward the polar regions—a transport that is required to maintain the global radiation balance at the top of the atmosphere—although the relative importance of the two transport mechanisms varies with latitude. The massive amounts of water moving in the ocean render it a crucial transporter of moisture, but we have little idea of the sizes of freshwater sources and sinks over the ocean (Baumgartner and Reichel, 1975). Their distribution must have profound effects on the distribution of rainfall over land, an important component of the climate in habitable regions of the world. Low surface salinity caps the ocean, attenuating convection and deep-reaching water mass formation. High surface salinity, combined with surface cooling, allows deep convection and ventilation of the interior of the ocean.
We need to monitor changes in ocean surface salinity globally. Satellite sensors monitor sea surface temperature and its anomalies, but monitoring sea surface salinity and its anomalies on a global scale is beyond present capabilities, as is determination of the temperature below the very surface.
Over the last decade, oceanographers have begun making direct estimates of the north-south ocean heat and freshwater transports using transoceanic hydrographic sections and modern measurements of strong boundary currents and then comparing them with the more uncertain indirect estimates based on atmospheric data. To understand heat and freshwater transport fully, oceanographers must describe the general ocean circulation and its variability. It is discouraging, however, that since 1985, only six transoceanic hydrographic sections, the backbone of the observations needed to determine the north-south fluxes, have been carried out: at 47°, 24°, and 10° north latitude in the Pacific; 32° south latitude in the Indian Ocean; and 11° north latitude in the Atlantic. Overcoming organizational and funding obstacles for these long hydrographic sections takes major effort by individual scientists. However, WOCE has plans to make the necessary measurements for determining the ocean heat transport in each ocean basin at several latitudes (WOCE Scientific Steering Group, 1986). This work is a central focus of WOCE and is clearly needed if understanding of how the present climate system works is to progress.
Provision for continuing such observation beyond the end of WOCE is essential.
Methods
To achieve their scientific objectives, physical oceanographers must use both proven methods and new technologies to make more complete ocean observations.
Volunteer Observing Ships The maritime industry is a resource for ocean research and monitoring that can no longer be viewed as simply an adjunct to the academic research fleet. On the contrary, its integration into a global ocean observing system would provide far greater and more frequent access to the ocean than will ever be possible with research vessels alone.
Volunteer observing ships (VOS) offer opportunities to study and monitor the ocean with a coverage and frequency that are unthinkable by any other means. With the advent of new and more sophisticated remote sensing techniques, such as ocean color scanners, altimeters, and scatterometers, it is plausible that the demand for direct observations will increase, for several reasons. First, the need for calibration measurements will grow. Second, the ocean color scanner will observe numerous signals that require in situ samples for identification, interpretation, and analysis. Third, as coverage of the ocean surface improves, a concomitant need for improved subsurface coverage is inevitable.
Without doubt, a major impediment to the use of VOS is the lack of automated instrumentation for nonscientists on moving commercial ships. Most instruments are designed for trained personnel on research vessels equipped with laboratories. A new approach to the VOS concept is needed. It will require discussions and planning with the maritime industry internationally to develop new modes of cooperation. The community must think of VOS as a potential platform, and ship operators must be persuaded that oceanographic work is to their benefit. At the same time, the development of instrumentation optimized for use on VOS must be encouraged, including the following:
-
Modern sensor packages are needed that can be dropped and retrieved repeatedly along a ship's route to measure salinity, oxygen, and fluorescence (primarily from phytoplankton). Data obtained by the sensors could be transferred to a small on-board computer for analysis and transmission to data centers.
-
Disposable free-falling sensor packages should be developed
-
that travel to great depths and transmit their data via acoustic signals or return to the surface and broadcast to satellites. Sensor packages could transmit data for any ocean observation that can be measured in situ. Disposable sensor packages can transmit data via a thin wire that detaches when the sensor reaches its maximum depth, but free-falling sensors could travel much deeper and measure more accurately than present devices.
-
VOS could tow instruments to measure various ocean characteristics. When ship routes and sampling locations coincide, passing ships could retrieve data from moored instruments (e.g., moored current meters and inverted echo sounders) by acoustic signals. At the least, commercial ships equipped with acoustic Doppler current profilers could measure upper ocean currents and heat fluxes routinely.
Remote Sensing by Satellite Satellites observe vast areas of the ocean surface daily, obtaining data at a far faster rate than surface vessels and instruments (e.g., Stewart, 1985). Satellites also aid physical oceanography by transmitting data gathered by in situ ocean sensors combined with highly accurate positioning information. The availability of surface and subsurface instruments has greatly increased the volume of data beyond that possible from ship-based observations, and the instruments provide valuable time-series data.
Satellite sensors are used to map natural infrared and microwave radiation emitted from the sea surface. Infrared radiation provides images of sea surface temperature patterns. Radiation in the microwave frequencies is used to map sea ice distribution in areas of the globe that are otherwise poorly accessible. Phytoplankton blooms can be monitored from space with sensors that respond to the visible and near-infrared radiation reflected by plankton chlorophyll, integrating the effects of temperature, nutrients, and the physical structures and processes of the ocean surface layer. Suspended sediment is also measured by visible sensors. Surface winds are measured by satellite scatterometers. The sea surface slope can be measured from space by radar altimeters with accuracies adequate for determining many features of ocean circulation. This is the only practicable method for global-scale continuous observation of circulation. Synthetic aperture radar on satellites can measure sea state, internal waves, and ice conditions with very high spatial resolution.
Although satellite data yield a nearly continuous view of the ocean, it is important to note that complementary in situ obser-
vations from ships or instrumented drifters are required to improve the mathematical relationships (algorithms) for calibrating satellite observations to in situ values.
A suite of satellites launched by the United States, the European Space Agency, and Japan and with many joint satellite missions should provide many data required by physical oceanographers during the next decade. Advances in physical oceanography are tied closely with satellite views of the ocean and telemetry of data from Earth's surface. Coordination of satellite projects with ocean science objectives and in situ ocean measurements is required. The satellite time series of sea surface temperature, winds, sea ice, ocean color, and ocean height must be continued without interruption to provide a complete record of their variability.
Tracers Distribution of ocean properties can be used to depict the pattern of ocean currents and the effects of mixing within the ocean because water masses from different source regions vary in their physical and chemical signatures. Traditionally, temperature, salinity, oxygen, and nutrient concentrations have been used to track the movement or spreading of ocean water masses. Other more exotic chemicals present in minute concentrations (tracers) are now commonly used to track water masses. For example, chlorofluorocarbons, carbon-14 and tritium from atmospheric nuclear bomb testing, and other natural and synthetic substances with known rates and times of input to the atmosphere have been used as tracers. Tracers average the spreading action of ocean circulation at a variety of scales and integrate the effects of many processes. The infiltration of naturally occurring and synthetic chemical tracers into the ocean provides insight about the time scales of ocean circulation and mixing. The development of baseline time series of tracer concentrations is important.
Acoustic Techniques Because the ocean is transparent to sound but opaque to light, acoustic techniques provide oceanographers with the opportunity to see the interior of the ocean. In a real sense, the hydrophone array serves as the underwater eyes and ears of the oceanographer. The enormous bandwidth of available underwater acoustic instrumentation (10-3 to 106 hertz) allows sound to be used as a probe of structures and processes whose scales range from millimeters to ocean basin scales. The ocean is especially transparent to low-frequency sound. Consequently, underwater sound is becoming an important means of studying the three-dimensional structure of the ocean below its surface. Continuous measurements of current velocity from shipboard acoustic
sensors give a two-dimensional record of ocean currents to several hundred meters. Acoustic techniques are also used to track subsurface floats and transmit information between ships and sensors at depth. Yet, sound is an underemployed tool in oceanography. Significant advances should be made during the next decade in physical, biological, and geological oceanography as a result of thoughtful application of acoustic principles and techniques for direct probing and information transfer. Because of its inherent global nature, the acoustic monitoring of ocean climate is a strong candidate for a global ocean observing system.
The air-sea interface provides a variety of challenges and opportunities to the oceanographer using acoustic techniques. The formation and subsequent collapse of bubbles are important sources of sound whose monitoring could provide an estimate of wave breaking intensity from which gas transfer rates could be inferred. Such measurements give important insight into poorly understood sea surface physics. The passive measurement of rainfall-generated sound is a way to measure precipitation in the open ocean. Direct measurement of precipitation is difficult to obtain and generally inaccurate. Better measurements of precipitation over the ocean are important because of its effect on the global heat and water budgets.
Some of the oceanographic applications of underwater sound are simple. Others will require improvements in our understanding of the physics of sound propagation in the sea and improved signal processing techniques and instrumentation.
DIRECTIONS FOR MARINE GEOCHEMISTRY
Summary
Studies of chemicals dissolved in seawater, adsorbed on suspended particles, incorporated in living or nonliving organic material, and buried in seafloor sediments have yielded much information about Earth processes and past conditions. Environmental conditions are imprinted on particles that fall to the seafloor and are buried over time. With adequate understanding about processes that affect chemical concentrations and forms after deposition, sediments recovered by seafloor drilling can illuminate Earth's environmental history for millions of years into the past. In addition, modern ocean processes can be studied by measuring the concentrations of trace elements and compounds in seawater. For example, measurement of trace element distributions is a major tool used by physical oceanographers to study ocean circulation.
The ocean is a chemical reactor, with inputs, internal reactions, and outputs. Inputs are received from continents via rivers and airborne transport. Other chemicals enter the ocean from hydrothermal sources, primarily at mid-ocean ridges. As oceanic crust is subducted beneath continents, elements are expelled from both the crust and its overlying sediment layer. Finally, a minor proportion of input to the ocean comes from cosmic sources. Inputs are the least understood part of the reactor.
Elements are redistributed in the ocean by circulation and mixing, and are transformed through chemical reactions and biological activity. Chemicals finally exit the reactor through incorporation into seafloor sediments. The residence time of chemicals in seawater and in the sediments depends on properties of the chemicals, as well as chemical, physical, and biological conditions. Chemical oceanographers seek to understand the present reactor and then, from examination of changes in the outputs over time, determine variations in the reactor's behavior and the compositions and fluxes of inputs in the past. With this information, the limits of future oceanic changes under given climatic and tectonic scenarios can be estimated.
Future studies in chemical oceanography will be aided by new instruments that are capable of analyzing a wide variety of elements and isotopes contained in small samples. Greater knowledge of processes controlling fluxes, redistribution, and removal, and improvements in our ability to read the sedimentary record are likely in the coming decade.
Introduction
Marine geochemistry integrates several oceanographic disciplines. The aims of marine geochemistry are (1) to understand the inputs of elements from the continents, mantle, and cosmic sources into the ocean over time; (2) to understand the process of material removal from the ocean to the sediments and oceanic crust; (3) to understand the process by which elements and their isotopes are redistributed; (4) to determine the mechanisms of chemical coupling between the ocean and the atmosphere, and interpret the sedimentary record of past oceanic change; and (5) to study marine organic compounds both in their relation to the above factors and to the global carbon cycle, and as detectors of oceanographic properties over time.
Oceanic sediments record environmental events over the past 180 million years of Earth's history. Ocean water in which sedi-
ment particles are formed or through which they pass is both an agent of transport and the site where information about environmental conditions is imprinted onto components that eventually become part of marine sediments. Geochemical signatures in the sediments complement the sedimentological and paleontologic record in special ways. Oxygen and carbon isotopes in marine calcareous shells or tests and organic remains provide insight into ocean surface temperatures, ocean circulation, and the extent of ice storage in past glaciations. Trace elements such as cadmium and barium add to our understanding of deep-ocean circulation and upwelling. Isotopes produced by cosmic rays, those originating from uranium decay, and those produced or enhanced by human activities, provide information on chronology, sediment properties, oceanic upwelling, deep-water formation, and transport of elements. The unique properties of the isotope ratios 87Sr/86Sr, 206Pb/207Pb, 143Nd/144Nd, and 187Os/186Os permit insights into the geologic questions of plate tectonics because the isotopic composition of seawater reflects the isotopic composition and relative importance of each source, for example, material derived from the mantle and continental rock weathering.
Although present in greatly diluted concentrations, marine organic substances play a key role in the global carbon cycle as modulators and tracers of oceanic processes. For example, organic remains account for approximately 20 percent of all carbon buried in marine sediments and thus are an important sink for atmospheric carbon dioxide. The burial of organic matter and its subsequent oxidation essentially control atmospheric oxygen levels over geologic time. Dissolved organic matter in seawater may contain a mass of carbon comparable to that in terrestrial biomass and could potentially affect atmospheric carbon dioxide concentrations on a time scale of a thousand years—the ocean's turnover time. In addition, organic molecules in particulate and dissolved forms are important vehicles for the transport of reducing power, nutrients, and trace elements throughout the ocean and across the air-sea and sea-sediment interfaces. Organic molecules in the marine environment are couriers of unique information about the sources, pathways, and histories of the associated particles and water.
The Ocean Reactor
The ocean receives dissolved and particulate material from a variety of sources and pathways. Traditionally, river inputs were
regarded as the major contributor of dissolved material. Wind transport of dust was recognized as adding to the particle flux borne by streams, and the input of altered volcanic material to deep-sea sediments was clearly identified by the 1960s. Within the past 15 years, deep-sea hydrothermal activity, predominantly at spreading centers, was found to be important as both a source and a sink of elements. In addition, a significant flux of dissolved material may be expelled from sedimentary wedges and underlying oceanic crust as they descend into the mantle through subduction zones. Cosmic dust and cosmic ray-produced nuclides are not a major input to the ocean in terms of volume, but are important as tracers and for understanding episodic influxes of significance to the history of our planet.
Elements are redistributed within the ocean in dissolved form by horizontal and vertical advection, diffusive mixing, and incorporation into particles. Chemical species are removed from the ocean as particles settle to the seafloor; they also react directly with the seafloor as the result of diffusion and circulation through sediments and the oceanic crust. Seafloor sediments and altered rocks undergo secondary reactions over time, and their composition may change substantially. From a chemical point of view then, the oceans are a large chemical reactor with multiple feeds and outputs. Chemical oceanographers want to understand the present reactor and then, from examination of changes in the outputs over time, determine variations in the reactor's behavior and the compositions and fluxes of inputs in the past. With this information, the limits of future oceanic changes under given climatic and tectonic scenarios can be estimated.
Geochemical study of the oceanic water column and the output of elements to the sediments is now relatively mature. The descriptive phase is largely complete, and studies of mechanisms are growing more numerous. For inputs of elements to the ocean, however, quantitative research is difficult and, in the case of inputs from the continents, may not correctly reflect disturbances in terrestrial inputs caused by humankind. These disturbances have occurred on time scales of a few years to several centuries, and the ocean has not yet equilibrated to the altered inputs.
Fluxes
Quantitative measurement of river inputs is difficult because measurements of fluid discharge from rivers are uneven in quality, frequency, and distribution. Because the best data are avail-
able from developed regions of the world, they do not necessarily represent areas less impacted by human activities.
Many of these same uncertainties apply to airborne inputs. Transport is strongly seasonal and diffuse, and thus is difficult to measure. Wind erosion rates are sensitive to the nature of the land cover and therefore to changes in land use. Windborne particles efficiently scavenge volatile chemicals released to the atmosphere by volcanism, biomass burning, and industrial activities. Thus the chemical composition of windborne dust is sensitive to pollution. In addition, photochemical reactions occur in the atmosphere, changing the chemistry of the atmosphere and particle-bound chemicals.
Hydrothermal activity in the deep sea is driven by volcanic processes in the oceanic crust, predominantly at spreading centers. Seawater seeps into crustal rocks, entering convection cells in the rocks, and is heated to 300 to 400°C. In the course of this circulation, elements in the seawater react with the hot basaltic rocks; some chemicals are removed (e.g., magnesium, sulfate, and uranium) and others are added (e.g., mobile elements and gases). The fluxes of elements at any one site are difficult to quantify, and it is not feasible to measure the thousands of sites that differ in rock temperature and composition. To account for observed isotope concentrations in seawater (e.g., strontium), a volume of water equal to the world's ocean must circulate through the hydrothermal system at temperatures above 325°C every 10 million years. However, estimates of hydrothermal circulation based on heat lost in the formation of the oceanic crust are about five times lower. If the higher estimate for hydrothermal circulation is correct, this process is as important as rivers for the input of many elements to the ocean. Hydrothermal processes would stabilize seawater composition and thus act as a geochemical flywheel, potentially damping large-scale changes induced by long-term climatic and tectonic changes. Yet, if the lower estimate of hydrothermal circulation is correct, hydrothermal activity is a minor factor in the cycling of ocean elements and is important for only a few. The major inconsistency between the fluxes based on isotopic and thermal constraints, apparent since the first hot springs were found in the deep sea 15 years ago, remains to be resolved.
There is evidence that hydrothermal circulation at relatively low temperatures (a few tens of degrees) away from spreading centers may also be important for fluxes of elements. However, it is not yet possible to calculate even the vaguest estimate of the chemical fluxes involved. Recent seafloor exploration and ocean
drilling show that the ocean crust and its overlying sediment expel water in the subduction process. Given the great compositional and tectonic diversity of active subduction zones, it will be impossible to estimate the geochemical significance of these processes to the oceanic element cycles until much more exploratory work has been done.
Redistribution
All the inputs will react with seawater. Reaction processes in the zone of mixing between river water and the upper ocean are quite well described qualitatively. These processes include desorption of elements from suspended particles, coagulation and precipitation of colloidal material, scavenging by organisms, and vertical transport. Processes associated with the formation of particle plumes above hydrothermal vents have been studied extensively. These particles oxidize rapidly and appear to scavenge both a large proportion of the hydrothermically transported trace metals and a significant component of elements from ambient seawater.
As mixing progresses, ocean circulation is increasingly dominant in dispersing inputs until they cannot be traced directly back to their source. Large rivers can be considered as point sources of material to the ocean superimposed on a diffuse background input from smaller streams. Penetration of the river signal into the deep sea follows complex pathways that are regionally diverse, depending on the current regime and the configuration of the coastline and the continental shelf. Because even unpolluted rivers usually carry elevated nutrient loads relative to coastal seawater, their discharge induces large phytoplankton blooms. The phytoplankton settle toward the seafloor, carrying nutrients and scavenging dissolved substances. In confined systems with high nutrient inputs, settling organic material can fuel bacterial activity and lead to oxygen depletion of bottom waters. The complex coupling of inorganic and biological processes, postdepositional reactions in the sediments, and strong seasonality of inputs produce a system whose chemical transport is difficult to quantify. Estuarine processes make it difficult to estimate river inputs of the more reactive elements to the open ocean. Because of this complexity, chemical oceanography in the coastal ocean has been relatively neglected even though that is the site for some of the most intense biogeochemical interactions in the entire ocean.
High-temperature hydrothermal fluids create great buoyant clouds of fine-grained sulfides and oxides upon their turbulent injection
into the surrounding water column. These clouds rise above the vents until their density equals that of the surrounding seawater. On ridges with high rift walls (e.g., the Mid-Atlantic Ridge), the plumes often stay within the confines of the bounding rift walls. The precipitates and dissolved material they scavenge then accumulate on the walls and floor of the rift valley. On fast-spreading ridges with low walls (e.g., the East Pacific Rise), the plumes rise above the walls and are dispersed in the middepth circulation. Fine-grained vent-derived particles can be transported for thousands of kilometers, slowly settling out to form a compositionally distinctive shadow in the sediments. Unreactive species, such as helium, delineate plume flow.
If redistribution of elements occurred only by the physical dispersal of dissolved material and suspended particles near their sources, geochemical patterns would be related solely to input functions. However, the chemistry of the ocean reactor is determined primarily by organisms and chemical kinetics rather than by thermodynamics. Essential nutrient cycles are controlled largely by the metabolic processes of living organisms. For nonnutrient elements, scavenging by nonliving organic materials is much more important. Settling particles are reactive enough to adsorb and transport a large variety of elements to the bottom. Because bacteria continue to degrade this material, the concentrations of many elements increase with depth and along current flow lines.
Surface productivity shows strong regional variability. The vertical particle flux and the intensity of scavenging and release also vary by region. For geochemical purposes, satellite pictures of ocean productivity must be projected into the vertical dimension to appreciate the fact that strong lateral variations in reactivity occur (reactivity is the intensity of the chemical reactions that are driven by biological activity in the ocean water column). Vast areas, such as the subtropical gyres, are quite unreactive; these are surrounded by a coastal rim of high reactivity. Other zones of high reactivity correspond to areas of physical upwelling.
The combination of lateral and vertical transport and continuous reaction of particles suggests that the water column distribution of elements at a particular location may have little influence on local processes but, instead, reflects the integration of processes over various time scales and distances. The best example of this effect is the deep silica maximum found throughout the Indian and Pacific oceans. It results from strong upwelling and associated high productivity of siliceous plankton in relatively
small areas at these oceans' northern boundaries, and subsequent vertical transport and dissolution of their siliceous tests at depth.
The flux of particles to the seafloor is apparently the most important mechanism by which elements are delivered to the sedimentary reservoir. However, many complex processes, primarily biological, can occur after initial deposition. Particles falling from above are the only source of nutrition for many bottom-dwelling organisms. Thus this material is ingested and excreted repeatedly by a variety of species and is also subject to continuous bacterial degradation, reducing its carbon content and destroying the functional groups responsible for scavenging reactive elements from the water column.
Sinks
The two most important properties of the ocean system that control the uptake of chemical species by the sediments and oceanic crust are its oxidation state and its temperature.
Chemical reactions in sediments involving oxidation and reduction depend on the amount of oxygen present, which in turn depends on metabolic activity and diffusion from the overlying water column through the pore fluids. Where metabolic processes exceed the oxygen supply through diffusion, the sediments become anaerobic. This condition creates diffusion gradients in the oxic-anoxic transition zone from above and below, affecting a range of chemical reactions. The transition zone rises through the sediment column as sediment accumulation progresses. Understanding and quantifying these processes are crucial for studies of global change because of their implications for interpreting the sedimentary record.
The character of the crustal sink for oceanic dissolved material changes with the temperature of the water-rock interactions. At high temperatures, the reaction environment is anoxic. Sulfide-forming elements are precipitated; elements that form insoluble oxides in the reduced state, such as uranium and chromium, are also precipitated. Soluble materials, such as boron and alkali compounds, are completely removed from the rocks. Magnesium is removed but calcium is released. At the very high temperatures (>400°C) associated with recent eruptions, phase separation can occur, producing a dilute aqueous phase and a residual brine. Subsequent mixing of these components appears to be responsible for the large variations in salinity observed in hydrothermal flu-
ids. Details of this process are not understood. A major unknown is the mechanism responsible for maintaining the salinity and temperature of the vent fluids at a given site at stable levels over periods of years.
Low-temperature weathering of the oceanic crust appears to constitute a major sink for alkali compounds and is accompanied by extensive hydration of the rocks. When the crust is subducted, this chemically bound water, along with the elements it can transport, most likely is released and migrates. The water may accelerate melting and participate in the eruption process in volcanic arcs.
Marine Organic Substances
The biochemicals that fuel marine organisms are photosynthesized and then respired in the upper ocean on time scales of hours to days. Only about 20 percent of the photosynthetic product escapes from the sunlit surface ocean as sinking particles, and less than 0.5 percent is ultimately preserved in marine sediments. Living organisms comprise only about 1 percent of the organic matter in the ocean. The remaining organic matter is primarily a dilute solution (about 1 part per million) of ''dissolved" macromolecules (i.e., material that passes through filters with a pore size of 0.5 micrometer). The turnover rate of this dissolved pool is now under discussion; the traditional view is that the pool turns over at a rate of thousands of years. The alternative view is that, because the pool of dissolved organic material contains excess carbon-14 relative to what is expected in thousand-year-old organic material, it must turn over more rapidly. Because of the challenges of isolating or directly characterizing this extremely dilute component of seawater, only about 20 percent of the organic molecules have been described.
A little over a decade ago, a novel suite of organic lipids was first reported in sediments from the Atlantic Ocean and the Black Sea. The component molecules have a linear sequence of 37 to 39 carbon atoms containing one to four double bonds, with an oxygen atom doubly bonded to the second or third carbon in the chain. These long-chain alkenones were found to be produced by the marine coccolithophorid algae Emiliania huxleyi and related species that are widely distributed in tropical and subtropical oceans. The same molecules were also discovered in sediments dating back to the Miocene (about 20 million years ago).
It was later demonstrated in the laboratory that the average number of double bonds (extent of unsaturation) in these alkenones
of E. huxleyi and related algal species increased at lower culturing temperatures, apparently a chemical response to maintain membrane fluidity. Brassell et al. (1986) discovered that in natural deposits the average number of carbon double bonds in alkenones from Quaternary sediments of the northeast tropical Atlantic showed a strong inverse correlation, over the past 120,000 years, with the temperature of the near-surface ocean water as inferred from the stable oxygen isotopic composition of coexisting calcium carbonate shells of the planktonic foraminifera Globerigerinoides sacculifer. This observation suggests that the relative changes in water temperature at the ocean surface during at least the past 100,000 years could be inferred from the stratigraphic record of alkenone composition in the underlying sediments. Prahl and Wakeham (1987) calibrated the alkenone paleothermometer for E. huxleyi within ±0.5°C and they provided evidence against the idea of diagenetic alteration of the molecular temperature record in the marine water column.
The alkenone paleothermometer has potential applications beyond simple confirmation of stable oxygen isotopic records of sea surface temperatures. For example, alkenone measurements can be applied readily to bulk sediment samples. The alkenone paleothermometer thus ranks as one of the major contributions in the past decade of marine organic chemistry research to understanding paleoceanography and paleoclimatology.
The application of the alkenone paleothermometer was made possible by the recent development of gas chromatographic ratio mass spectrometers, instruments that measure the stable carbon isotopic compositions of individual organic molecule types separated during the rapid (approximately one hour) gas chromatographic analysis of complex organic mixtures.
Future Directions
Analytical Methods
The chemical processes occurring within the ocean reactor are kinetically controlled except in high-temperature regimes, where thermodynamic equilibria may be inferred. These kinetic processes are driven largely by organisms; they involve chemical reactions that are difficult to reproduce in laboratory experiments because of their complexity and variety. Thus, the general strategy in studying the marine geochemistry of the present ocean and its variations in the geologic past is to pose questions in which
causes are inferred from measured distributions, that is, as an inverse problem. Variations in measured concentrations reflect changes in the relative importance of these causes. This inversion is constrained by different chemical properties of the elements of the periodic table, their valence states, their isotopes, and the compounds they form. It follows that this strategy requires the measurement of a vast array of chemical properties. Although technology development is required for measuring some of these properties, each of the elements and forms is not equally important diagnostically. Extraction of all the information useful in constraining the inversion is a prodigious analytical task.
Until recently, each available instrument could analyze only a few elements at the sensitivity, precision, and accuracy levels necessary for their concentration in natural samples. Thus no laboratory could perform more than a small fraction of possible measurements. Over the past 10 years, the sensitivity of instruments has increased vastly. Accurate and precise multielement analyses on single samples are now feasible, and multiple collector thermal ionization mass spectrometers have increased the sensitivity for a wide range of elements and isotopes. High-energy accelerator mass spectrometry allows the measurement of the cosmogenic radioisotopes in the study of a wide range of geochronological questions; it also allows exploitation of the unique properties of these isotopes in a variety of tracer studies. Plasma source mass spectrometry makes it possible to perform accurate multielement analyses on extremely small amounts of material. This technology eases measurement of refractory elements that, because of their low volatility or high ionization potential, are difficult to measure with conventional techniques.
Mass spectrometry for the measurement of δ18O, δ13C, and other light isotope systems are well established, as are methods for determining radiogenic isotopes. New developments in high-resolution thermal ionization mass spectrometry for the measurement of 230Th, 232Th, 234U, and 238U and the negative thermal ionization mass spectrometric measurement of 187Os/186Os have improved our capacity to use these isotopes in marine geochemical studies.
Improving Our Knowledge of Fluxes
Much more research needs to be done to quantify continental inputs to the oceanic reactor. An important adjunct to studies of fluxes will be long time-series measurements that will allow an
estimation of variability of fluxes. Weathering processes are traditionally regarded separately from oceanography, but oceanic techniques are essential to trace the pathways by which material enters estuarine mixing zones through rivers, progresses across the shelf, and finally moves into the deep sea. For some elements (e.g., iron), delivery to the world ocean by this route is negligible because of their insolubility in seawater. Soluble elements pass through the coastal ocean with little loss. Research on this topic has great societal importance because chemical fluxes substantially alter global climate, coastal pollution, and possibly harmful algal blooms and fisheries production.
The strength of airborne transport of particles is known to vary widely with climatic conditions. Airborne transport is the one direct pathway between the continents and the surface waters overlying the deep ocean, and its importance as a supplier of micronutrients (e.g., iron and selenium) needs to be more firmly established. It is possible that the productivity of some areas of the ocean is controlled partially by the amount of airborne trace metals. This area of research is an active one.
Estimates of the hydrothermal flux range over a factor of five. The mechanisms responsible for the wide salinity variations and the temporal stability of the values at a given site are not understood. Both of these problems can be resolved only by systematic investigation of vent fluids from different sites and by development of additional tracers of both the subsurface reaction processes and the characteristic hydrothermal inputs to the global reactor.
Fluid inputs from the mid-ocean ridge flanks and from subduction zones are perhaps best studied by drilling and pore water sampling because the flow across the seafloor-water interface may be too diffuse for discrete sampling in the water column. Much improved down-hole sampling and measurement capabilities are required. Systematic sampling of representative ridge flanks and subduction zone complexes is needed, using the complete range of modern geophysical tools.
Understanding Redistribution and Removal in the Ocean
The general circulation of the global ocean has been relatively well described. From the geochemical point of view, much remains to be learned about the relative importance of the removal of elements at ocean boundaries versus in situ removal by settling particles. The spatial and temporal variability of processes con-
trolling the vertical flux of elements needs to be characterized. The same is true for the evolution of the chemical properties of sinking particles traveling through the complex food web in the water column and on the seafloor. In the upper waters and thermocline, large horizontal variations in primary productivity and higher levels of food webs have been observed, but little is known about the lateral variability of deep-ocean ecosystems. The effects of ecosystems on the chemistry of particles passing through them are largely unknown. It is important to know whether there is a simple proportionality between surface productivity and the chemistry of the underlying water column or whether the particular faunal assemblages in the water column exercise a major role in controlling element concentrations.
The mechanisms of sediment interactions and diagenesis are well studied for the major constituents, nutrients, and oxygen; much more work is needed on the behavior of minor and trace constituents. Intensive research on the behavior of trace elements in the most reactive upper few meters of the ocean is also necessary. Descriptions of composition changes need to be developed for the various sediment types and environments through all phases from initial burial to subduction.
Reading the Record
Complete decipherment of the proxy record contained in the concentration distributions of trace elements and isotopes in sediments requires an understanding of the pathways of input and the mechanisms of redistribution, removal, and transformation of the elements studied. A given tracer may record a single aspect or some combination of these factors. Ideally, multiple tracers should be used as a check on internal consistency. Because only a small number of tracers are now available, much development work is required.
It is known that the continental inputs of a number of potential tracers are changed markedly by human influence, which makes estimates of their response to environmental changes difficult. River inputs are often strongly mediated by coastal processes and hence are sensitive to sea-level variations that change the size and character of the coastal ocean. Comparative studies of shelf-dominated systems (e.g., the Yangtze and Yukon rivers) with systems in which rivers discharge directly over deep waters (e.g., the Columbia and Congo rivers) may be informative.
The relative importance of physical and chemical redistribu-
tion processes and the mechanisms of sediment uptake must be established for each tracer; this will be difficult experimental work. Postdepositional redistribution processes must be characterized for the range of sedimentary environments. The establishment of reliable proxy records merits high priority because it is the most direct constraint on the modeled mechanisms of previous global changes in environmental processes.
DIRECTIONS FOR MARINE GEOLOGY AND GEOPHYSICS
Summary
The plate tectonic paradigm, first quantitatively described more than 25 years ago, provides an integrated physical and chemical framework for understanding the geological evolution of Earth. Marine geologists and geophysicists played a critical role in the development of this paradigm. By linking marine magnetic anomalies to geomagnetic reversals of Earth's magnetic field, marine geophysicists were able to confirm seafloor spreading and provide quantitative estimates of seafloor spreading rates. Through holes drilled by the Deep Sea Drilling Project, marine geologists were able to extend the geomagnetic reversal time scale back nearly 200 million years, providing a framework within which to reconstruct the past positions of the continents and the opening and closing of ocean basins.
Throughout most of the 1970s, the major emphasis in marine geology and geophysics was on large-scale kinematic descriptions of relative plate motions and their consequences for the geological evolution of ocean basins. However, by the 1980s, the focus of the field had shifted toward more process-oriented studies centered around understanding how oceanic crust and lithosphere are created, how these processes are related to the underlying mantle, and the consequences of seafloor spreading on ancient ocean circulation and climate. Four themes currently dominate research in marine geology and geophysics: (1) the formation of oceanic crust and lithosphere along mid-ocean ridges, and the associated volcanic, hydrothermal, and biological processes; (2) off-ridge processes and their relation to mantle convection; (3) the structure and tectonics of active and passive continental margins; and (4) the record of past climate change and ocean circulation preserved in marine sediments. In addition to these four themes, there is increasing interest in the study of coastal processes including sediment
erosion and transport, and estuarine and delta sedimentation. Coastal processes are discussed elsewhere in this report.
In the 1990s, marine geologists and geophysicists will begin a major, decade-long study of the global mid-ocean ridge system through the Ridge Inter-Disciplinary Global Experiment (RIDGE) program. The long-term goal of this program is to obtain a sufficiently detailed spatial and temporal definition of the global mid-ocean ridge system to construct quantifiable, testable models of how the system works, including the complex interactions among the magmatic, tectonic, hydrothermal, and biological processes associated with crust formation. Among the goals that are achievable in the next decade are a global characterization of the structure and energy fluxes along the entire 50,000-kilometer-long mid-ocean ridge system and the establishment of a permanent seafloor observatory on an active ridge segment to investigate the scales of variability in tectonic, magmatic, hydrothermal, and biological processes associated with the formation of new oceanic crust.
The ancient ocean crust contains unique information about mantle convection and composition. An improved understanding of the chemical and isotopic record of mantle convection and the variation of melt production through time is likely in the next decade. Mapping crustal composition on an ocean basin scale will require hundreds of shallow holes to be drilled into ocean crust. Directly sampling the suboceanic mantle will require the development of new drilling technology beyond that presently available to the ocean drilling community. New seismic tomography techniques for imaging the Earth's mantle will allow marine geologists to begin to relate mantle convection processes to melt production rates, lithospheric stress and intraplate deformation, and the variation in chemical and isotopic composition of the crust. Vastly improved seismic images of the suboceanic mantle are possible if an array of seafloor seismic stations is established in the 1990s to augment the global digital seismic network.
Continental margins are the locus of lithospheric deformation, sediment accumulation, and substantial and chemically distinctive magmatism. Subduction and rifting processing at margins determine the size, shape, and distribution of continents and result in complex and dynamic interactions among oceanic crust, continental crust, and mantle systems. A basic description of the nature and evolution of many margins is available today, but understanding of the dynamics of margin development is still very limited. The development of new technology for probing the deeper structure of continental margins, and new conceptual advances in
areas such as sequence stratigraphy and fault dynamics, provide an opportunity for the development of fundamental new insight into margin structure and evolution. What is needed is a coordinated, interdisciplinary research effort involving both land-based and sea-based research programs over the next decade.
Marine sediments provide an important record of geological processes including past global climates. For example, analysis of marine sediment cores has provided critical information on the importance of Earth's orbit in short-term climate change. Marine sediments also provide a record of global sea-level changes, sea surface and bottom water temperature variations, changes in ocean current patterns, the volume of water locked in polar ice caps, and the effects of a changing physical and chemical environment on the evolution of marine life. Through drilling and coring, especially in high-latitude regions, paleoceanographers are poised to make major advances in our understanding of the natural variability in global climate systems in the coming decade.
Introduction
The plate tectonic paradigm forms an integrated and linked physical and chemical framework for the flow of energy and mass through Earth (Figure 3-1). Radioactive decay of material within Earth's interior produces heat and creates a convective system that transports heat and material from deep within Earth to shallow levels. Upper mantle rocks partially melt, producing basaltic magma. Much of this melt is preferentially focused along the world-encircling mid-oceanic ridge, where oceanic crust is created. In time, the oceanic lithosphere (the oceanic crust and upper mantle) will be recycled into the mantle at convergent margins. The oceanic crust and some sediment are carried back into the mantle, and the crustal components dehydrate, pumping water and gases into the overlying mantle, causing partial melting and fractionation, and creating silica-rich rocks, ore bodies, and explosive volcanism along the overlying volcanic arc.
As oceanic crust ages and moves away from the ridge axis, it modifies Earth's environment. The chemistry of seawater is altered as the oceanic crust cools and exchanges elements with the seawater that circulates through it (see "Directions for Marine Geochemistry"). At convergent margins, some sediment is scraped off the subducting crustal plates, injecting fluids rich in dissolved constituents into the overlying ocean waters. Moreover, the aging oceanic lithosphere serves as a repository for sediments that
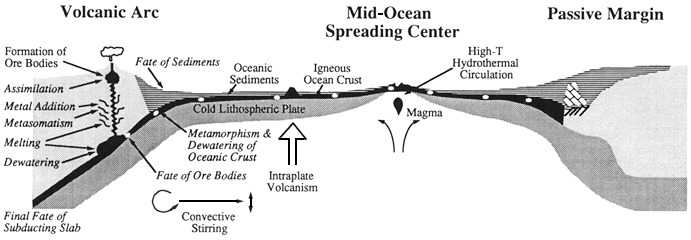
FIGURE 3-1 Cartoon of the solid Earth geochemical cycle showing some of the fluxes, processes that control the fluxes, and the sedimentary reservoirs that provide a record of these processes. Lithosphere is created at mid-ocean spreading centers from upwelling mantle material and is recycled into the mantle at subduction zones. This solid Earth geochemical cycle controls the flux of heat and mass from the Earth's mantle to the hydrosphere, biosphere, and atmosphere.
represent a coherent high-resolution continuous record of environmental changes on time scales from years to millions of years. Given the mobility of crustal plates, the geometry and location of ocean basins and continents have changed through time. This changing plate mosaic has had a profound effect on global sea level and on ocean and atmospheric circulation.
In essence, plate tectonics is the surface expression of a solid Earth geochemical cycle. An understanding of how this cycle has operated in time and space is a fundamental starting point for Earth systems research, and it unifies the field of marine geology and geophysics. The four principal elements of this research are (1) the oceanic ridge and lithosphere, (2) off-ridge processes, (3) ocean margins, and (4) ocean basin sediments.
Oceanic Ridge and Lithosphere
The global mid-ocean ridge is perhaps the most striking feature on the solid surface of our planet. Sections of the ridge extend along the floor of the world's ocean to a length in excess of 50,000 kilometers. The mid-ocean ridge dominates Earth's volcanic flux and creates an average of 20 cubic kilometers of new oceanic crust every year. Two-thirds of the annual heat loss from Earth's interior occurs through the generation and cooling of the oceanic lithosphere, partially by the circulation of seawater through fractures in the hot oceanic crust. This hydrothermal circulation facilitates a major chemical exchange between seawater and oceanic crustal rocks that acts as an important regulator of the chemistry of the ocean and of the volatile content of Earth's interior. The most stunning manifestations of this circulation are the high-temperature hydrothermal vents along the ridge axis.
Many discoveries of ridge phenomena have been made over the past decade, and a number of sophisticated technological tools have been developed for detailed investigation of the seafloor and the subsurface crust. High-temperature hydrothermal vents, for example, were discussed only as theoretical possibilities before their discovery in the Pacific in the late 1970s. High-resolution swath mapping and side-scan sonar imaging systems have only recently begun to provide information on the detailed morphology and structure of ridge systems. Multichannel seismic imaging techniques have advanced and thus have enabled marine geologists to begin imaging the magma chambers that lie below the ridge axis (Detrick et al., 1987). Much of the promise of this new technology remains to be realized. Detailed sampling and map-
ping of the mid-ocean ridge, for example, have been confined to only a small fraction of the total ridge length. The diversity of volcanic and tectonic processes manifested along the ridge axis, as a consequence, has not yet been fully defined. More fundamentally, the complex and linked processes of magmatism, hydrothermal circulation, development of vent ecosystems, and lithospheric evolution are only dimly understood. The dynamics of these processes have not yet been elucidated because of the lack of in situ observations of sufficient duration and diversity to determine the important interactions and time scales.
For a better understanding of mid-ocean ridge processes and their impacts on the chemical, physical, and biological evolution of the oceanic mantle, crust, and hydrosphere, specific aspects of the ridge system will require focused research efforts. Some are discussed below.
Mantle Flow, Melt Generation, and Magma Transport Beneath Mid-Ocean Ridges
Plate spreading and the generation of new oceanic crust and lithosphere along oceanic spreading centers involve a variety of complex and interrelated geodynamic processes: upwelling and horizontal divergence of the solid mantle beneath spreading centers, pressure-release melting of this upwelling mantle and segregation of the partial melt from the deforming solid matrix, the emplacement and solidification of melt at shallow depths to create the oceanic crust, and the cooling of the crust and mantle to form the oceanic lithosphere. These processes are still among the more poorly understood aspects of the seafloor spreading process. Two of the most important questions are (1) the pattern of mantle flow beneath mid-ocean ridges, and (2) the geometry of the melting region in the mantle and how melt migrates to the ridge axis. Simple plate-driven flow, due to viscous coupling of the asthenosphere to the separating lithospheric plates, predicts a simple two-dimensional upwelling pattern more than several hundred kilometers in width. Pressure-release melting of this upwelling mantle is thus expected to occur over a very broad region beneath mid-ocean ridges. One of the first-order paradoxes in our present understanding of mid-ocean ridge geodynamics is how partial melt formed over such broad regions beneath ridges migrates to the extremely narrow (1-to 5-kilometer-wide) zone of eruption observed at mid-ocean ridges. We also have only a very crude un-
derstanding of how this two-dimensional plate-driven flow develops into a more three-dimensional upwelling pattern along some ridges and how this flow affects, or is affected by, the observed segmentation of oceanic spreading centers.
By deploying an array of ocean bottom seismometers across a section of the mid-ocean ridge and recording a sufficient number of seismic events at different ranges and angles, it should be possible to improve the resolution of the seismic structure of the shallow mantle beneath a ridge crest. A major goal for the next decade is to carry out one or more of these studies on the mid-ocean ridge.
Processes That Transform Magma into Oceanic Crust
The transformation of magma into oceanic crust at spreading centers has fundamental implications for the mechanisms of heat and material transport from deep within Earth to the lithosphere, hydrosphere, and biosphere. The important processes that transform mantle melt into oceanic crust and the role of crustal chambers are poorly understood. The global distribution and physical properties of magma chambers at oceanic ridges and their temporal and spatial variability should be determined. Internal dynamics of magma chambers are important factors that must be understood, along with their effects on the structure and composition of the crust, the transfer of heat from the magma chamber, and the physical and chemical processes occurring at the interface between the magma chamber and the overlying region of seawater circulation.
Processes That Control the Segmentation and Episodicity of Lithospheric Accretion
The use of new technology, such as satellites, swath mapping, and side-scan sonar, has revealed that the global rift system is segmented and that the pattern of segmentation varies temporally and spatially. It is essential to understand the physical processes controlling segmentation and its temporal and spatial variation as well as the processes causing episodic production along individual segments and their boundary zones. Melt migration and eruption, faulting, fissuring, and stretching must also be better understood so that the individual processes and their possible interactions can be studied and interpreted.
Physical, Chemical, and Biological Processes Involved in Interactions Between Circulating Seawater and the Lithosphere
Hydrothermal plumes that issue from seafloor vents link the oceanic lithosphere, hydrosphere, and biosphere through complex physical, chemical, and biological interactions (Rona et al., 1986). A detailed understanding of the individual processes that constitute a hydrothermal system will provide insight into many problems in biological, chemical, geological, and physical oceanography. Although present research on seafloor hydrothermal circulation has begun to address a few of these problems, new approaches and a more focused effort will be required to achieve an interdisciplinary view.
Distribution and Intensity of Mid-Ocean Hydrothermal Venting
The character of hydrothermal plumes is determined by both crustal processes and the oceanic environment. Changes in the plume can reflect events with diverse spatial and temporal scales, such as magma chamber evolution, changes in the subsurface hydrothermal plumbing, and shifting bottom currents. To understand these complex interactions, we must study hydrothermal plumes over a wide range of scales in time and space: from the scale of the individual vent plume fluctuating over a period of seconds up to the 1,000-kilometer scale of the large ocean-basin plumes estimated to contain the integrated output from 100 years of hydrothermal venting. An important new research direction is to move from the realm of general observation to the quantification of rates and processes in hydrothermal plumes.
In summary, an improved understanding of the mid-ocean ridge system will require focused efforts. Present technologies are relatively well developed for establishing the occurrence of spatial variations within the ridge system, but obtaining observations of temporal change will be challenging. Global-scale reconnaissance surveys can help in selecting sites for more focused regional studies in which coordinated experiments would involve a range of long-term measurements. A common requirement of many of the recommended studies is accurate age information on time scales between a decade and a million years. Innovative approaches to dating hydrothermal fluids, rocks, and biological materials will be necessary to meet this requirement.
Off-Ridge Processes
Off-ridge processes can be studied to determine how Earth functioned in the past and whether there are additional temporal variability and forcing functions that will not be discovered by studying the geological present. The seafloor contains a record of the creation of the oceanic lithosphere. In addition, important questions concern changes in the oceanic crust as it ages. The older oceanic crust also contains information concerning mantle convection and composition. Several important investigative themes can be identified.
Chemical and Isotopic Record of Mantle Convection
Because the mantle is overlain by the crust, it is not possible at present to sample the suboceanic mantle directly, except in tectonically anomalous areas (e.g., oceanic fracture zones). The basalts that are derived from the mantle, however, are indirect mantle samples that have been modified by partial melting and partial crystallization. Because the oceanic crust is thin and its composition is similar to the magma, and because the spreading center provides a relatively permeable and free pathway to the surface, ocean ridges are the sites where the magma is least modified. Thus ocean ridge basalts typically provide the least adulterated record of mantle composition and temperature. Mapping crustal composition can provide quantitative information about the size, distribution, and composition of mantle reservoirs and the efficiency of convective stirring. This information is a record and an opportunity to map indirectly the composition and temperature of the mantle.
Variation of Melt Production (Convective Vigor) Through Time
There is strong evidence that plate separation rates and basaltic magma production rates along ridges and within plates are not constant. For example, a 50 to 75 percent increase in the rate of formation of oceanic crust and a doubling in the production rate of basaltic magmas between 120 and 80 million years ago (Figure 3-2; Larson, 1991) has been documented. The changes may be due to a large mantle-derived super plume that may have lifted off the core-mantle boundary and have been responsible for increased seafloor spreading and large-scale oceanic plateau production (e.g., Ontong Java and Kerguelen plateaus). It has been suggested that the super
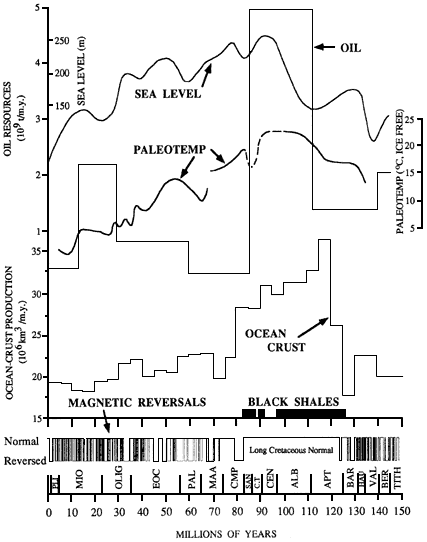
FIGURE 3-2 Combined plot of magmatic reversal stratigraphy; world crustal production; high-latitude, sea surface paleotemperatures; long-term eustatic sea level; times of black shale deposition; and world oil resources plotted on the geologic time scale. Note that increased volcanic activity in the Cretaceous is associated with eustatic sea levels, high sea surface temperatures, and black shale production. Thus, this may be a link between mantle processes (volcanism) and global climate. (Compiled from a variety of sources; Larson, 1991.)
plume had major geological consequences, including considerable increases in eustatic sea level, paleotemperature, oil generation, black shale deposition, and species diversification of phytoplankton and zooplankton. Such an event has profound implications for our understanding of mantle dynamics, oceanic plateau formation, and global environmental change.
Structure and Composition of Oceanic Crust
An understanding of crust formation cannot be achieved in the absence of better knowledge of the composition of the total ocean crust. This information would allow solution of a host of long-standing controversies, including the relationship between crustal structure and spreading rate, the origin of the seismically defined stratigraphy of the oceanic crust, the total magnetization of the crust and how it is distributed with depth, and the depth and nature of hydrothermal interaction in the crust.
Knowledge of Stresses Acting on Oceanic Lithosphere and Intraplate Deformation
The observational basis for plate motions is well established, but the relative importance of the forces (ridge-push, trench-pull, and plate-drag) that act on the plates and cause them to move is unresolved. In addition, the stresses that act upon the oceanic lithosphere at or near plate boundaries are poorly understood. Determining the stresses required to create these structures is key to understanding the tectonics of these environments (Zoback et al., 1985).
Ocean Margins
Continental margins are a principal site of lithospheric deformation, sediment accumulation, and mass flux on Earth and the site of substantial and chemically distinctive magmatism. Understanding their nature and origin will provide knowledge of the history of the ocean basins, and because the margins are progressively incorporated into the continental mass by plate interactions, the knowledge is also essential to our understanding of the mechanisms of continental evolution. In the next several years, the opportunity exists for researchers to develop a fundamentally new understanding of margins, a leap that may parallel that brought about by the plate tectonic revolution some 25 years ago.
A basic description of the nature and evolution of many continental margins is available today. However, understanding of the dynamics of margin development has not grown at the same rate. Knowledge of continental margin structure and motion has progressed, but understanding of the margins is limited by the need to understand the basic physical processes that accompany margin formation. Continental margins research must embrace a unified approach that emphasizes the important role of process-oriented interdisciplinary programs. Further, margins cross the shoreline, and such efforts will further enhance the developing synergism with Earth scientists involved in land and ocean research.
Fault Dynamics and Lithospheric Deformation
Any approach to the problems associated with lithospheric deformation demands the use of many tools. A wide-ranging field-mapping program is needed specifically to establish the link between surface deformation and deformation in the lower crust. For example, the configuration of large faults at depth in the crust and the structural fabric associated with distributed deformation in the upper mantle must be established by large-scale seismic imaging and tomographic techniques. Laboratory studies are also essential to establishing the constitutive laws for frictional behavior of both large deformation zones and discrete fault zones and defining their variability with strain, strain rate, and fluid content. Models of fluid flow in deforming porous media and methods to relate the models to observable quantities for field observation are required.
Mantle Dynamics and Extension
Understanding the interaction between mantle processes and lithospheric extension will require a focused multidisciplinary effort. Wide-ranging field-mapping programs must be designed to establish specific links between surface deformation and deformation in the lower crust. Large geophysical experiments will be needed to define the modern structure of rifts and margins and the thermal and dynamic state of mantle beneath young, presently active rifts. This work must be combined with geological mapping and thorough geochemical studies of magmatic systems to determine the nature of mantle sources, the history of melting, and fractionation. The development of shear-wave techniques
and electromagnetic methods to increase their sensitivity to the detection of fluids will be particularly relevant.
Recycling
Mass flux studies needed to understand convergent margins as dynamic systems will involve integrated field, analytical, experimental, and theoretical studies that draw from geochemistry, petrology, and geophysics. Such projects require marine as well as land-based studies and careful integration of results from the two. Sampling must include fluids, melts, sediments, crust, and gases from the entire subduction zone, the subducting plate to the back-arc zone. Theoretical and experimental studies are an essential addition to these geochemical and geophysical programs. Existing studies have related only to isolated aspects of mass fluxes at convergent margins. However, the results of these studies can be used in conjunction with samples from existing and planned Deep Sea Drilling Project (DSDP) and Ocean Drilling Program (ODP) holes drilled offshore of various trenches to formulate an integrated, multidisciplinary plan for studying these complex systems.
Ocean Basin Sediments
Marine sediments provide important records for important Earth processes. For example, marine sediments furnish a history of regional and global volcanic activity, a record of the long-term changes in Earth's magnetic field, and a tool for studying large-scale tectonic processes, such as continental accretion and rifting. An active area of research is the study of past global climates.
Evidence of global environmental change comes from the paleoclimate record, which is the only long-term record available. The paleoceanographic record provides information necessary to understand environmental changes (see ''Directions for Marine Geochemistry"). The paleoceanographic record also places observations of the present ocean in a historical context of long-term environmental variability. It affords a unique opportunity to test our understanding of the climate system as represented by numerical models of the ocean-atmosphere system. If models of the present ocean are capable of hindcasting oceanographic conditions, then we should have more confidence in their predictive capability. Whereas satellites provide a global means to observe the ocean surface, ocean sediments and the proxy indicators of oceano-
graphic processes defined by paleoceanographers provide a global array of sensors to monitor processes within the ocean over long time scales. However, we must understand the relationships between these proxy data and modern processes to use this global information effectively.
With sampling resolution ranging from annual to interannual to millions of years, study of the marine sediment record allows the study of past climates on a wide range of time scales. Six specific research themes need to be addressed to improve significantly our understanding of global climate change and its effects; they are described briefly below.
Short-Term Spatial and Temporal Variability in the Climate System
It is desirable to characterize the natural variability in the climate system on annual and interannual time scales over spans of thousands of years. With this information, the significance of instrumentally observed climate changes can be assessed and the variations related to human influences. Marine sediment records already analyzed provide a qualitative, although spatially limited, picture of variability over the past 1,000 years, which appear to have contained several intervals of colder than normal climate (e.g., the Little Ice Age) as well as possible warmer time intervals (e.g., the Medieval Warm Period). Information on the magnitude and frequency of short-term variability should make possible a substantially improved assessment of the degree to which present trends are associated with increasing greenhouse gas influences.
Geological Record of the Carbon System
The objective is to identify and understand the role of the carbon system in past climatic change by isolating the response of global climate to natural changes in atmospheric carbon dioxide and other greenhouse gases. Studies of ice cores spanning the past 160,000 years now provide direct evidence that atmospheric carbon dioxide has changed over a large range (180 to 300 parts per million) during this period. Geologic evidence for the more remote geologic past suggests that atmospheric carbon dioxide may have been as high as four to eight times its present level. Knowledge of these large atmospheric carbon dioxide changes on geologic time scales presents an opportunity to understand global climate change and to test model estimates. For the period of ice
core records, there is a well-documented forcing of climate changes by the distribution of solar radiation owing to Earth's orbital parameters. The response of climate to these forcing functions can be quantified through study of the geologic records. Thus the task is to document the interaction between the oceanic and terrestrial carbon cycle and atmospheric carbon dioxide as well as the pattern of climate change.
Instabilities in Ocean-Atmosphere Circulation in Earth History
Evidence strongly suggests that ocean circulation is sensitive to climate change, and changes in ocean circulation in turn influence the nature of the climate equilibrium (Kennett, 1977). The geologic record provides evidence for rapid, short-term transitions in deep-water circulation, associated changes in surface circulation and upwelling, climate changes during recent geologic history, and several abrupt reorganizations in ocean circulation over the past 60 million years. The ability to characterize the transitions in ocean circulation and to define, independently, the nature of the changes in the atmosphere will provide the means to describe case studies of the links between the ocean and the atmosphere.
Historical evidence confirms ocean response times on the order of decades, even for deep water, and a close link among climate and moisture fluxes, salinity, and deep-water circulation. Modeling studies indicate the potential for abrupt transitions between modes of deep-water circulation associated with little or no change in external forcing or with implied changes in surface moisture fluxes.
Episodes of Moderate to Extreme Warmth
Several intervals during the past 100 million years were significantly warmer than the present. Proxy evidence and the results of preliminary global circulation model sensitivity studies suggest carbon dioxide levels significantly higher than today's as the likely explanation of the global warmth during most of these episodes.
Geological Record of Global Sea-Level Change
The geological record contains widespread stratigraphic evidence of sea-level rises and falls (Shackleton, 1987), but further
studies are needed to determine the magnitudes, rates, and causes of sea-level changes. Before the mechanisms of sea-level change can be addressed meaningfully, better estimates of the magnitude and rates of sea-level changes during the preglacial and glacial past must be obtained. It is especially important to determine upper limits to the rate at which the sea level can rise. The constraints provided by better-magnitude estimates could eliminate several postulated causes of sea-level change and help focus on the most relevant possibilities.
Effects of a Changing Physical-Chemical State on the Evolution of Marine Life
The fossil record preserved in the ocean is the best source of information on evolutionary dynamics, as well as a powerful tool in forecasting the biological effects of global change. It provides an exceptionally detailed picture of the distributions of fossil species and the climatic conditions in which they lived. Many extinct marine fossils have living counterparts that can be studied for knowledge of the ecology and genetics of extinct species.
Were organisms able to acclimate to the new environmental conditions and, if so, how? The biological effects of extreme shocks to the biosphere can also be examined, such as that imparted by the asteroid impact with Earth at the close of the Cretaceous. These events permit us to evaluate both how organisms respond to the threat of extinction and how survivors set about repopulating the vacated environment. Many of these events can also be studied in the terrestrial fossil record. However, the higher resolution of the oceanic record permits a far more complete analysis of the forces that underlie evolutionary processes on a global scale than can ever be accomplished by using terrestrial organisms. This resolution permits us to evaluate the role of climate change as a driving force behind the production of new species, the extinction of existing species, and geographic shifts in populations. We can forecast the biological consequences of human-caused changes in the environment by examining similar events in the fossil record.
Research Approaches
Study of oceanic crust and sediments has been aided over the past 30 years by a number of new techniques, whose application
will continue to yield information about the seafloor, a region of Earth that still remains largely unknown. Rock and sediment cores obtained through the DSDP/ODP have provided glimpses of the structure, composition, and the processes that formed these materials. Continued systematic drilling will be required to obtain a complete picture of the structure of the ocean crust and particularly the chemical composition and hydrothermal alteration processes. Drilling also allows geophysical and geochemical instruments to be placed within the drill holes to measure temperature, chemical fluxes, crustal strain, and other variables important for understanding geological, geochemical, and geophysical processes.
The technique of seismic tomography began when it was learned that by studying the propagation of seismic waves, both in terms of speed and path, through the Earth, features of Earth's structure could be discerned. Later, explosives and noise generated by "air guns" were used to generate sound that can be transmitted some distance into the seafloor and reflected back to acoustic receivers. These techniques have been used to gain a more detailed picture of the upper seafloor, particularly the sediment layer overlying the crust. The newest seismic technique has been to drill holes deep into the seafloor, placing acoustic sources in some holes and receivers in others, to produce a horizontal seismic tomograph of the intervening sediments and crust.
These methods provide a snapshot of structure and composition from which processes and fluxes may be inferred. As with other oceanographic disciplines, the importance of time-series observations for observing dynamic processes is critical. Scientists that study marine geology and geophysics will increasingly use time-series measurements of changing features, through repeat cruises, rapid-response measurement techniques, and particularly sensors moored on the seafloor. The area of fluxes is one in which chemical oceanography and marine geology and geophysics interact, because measurement of benthic chemical fluxes is important for both fields. Finally, the concept of "seafloor observatories" is being implemented through the RIDGE program and through research sponsored by the Office of Naval Research. These "laboratories" are actually areas of the seafloor where repeated intensive observations are made. For example, ONR has designated sites on the Mid-Atlantic Ridge (a slow-spreading ridge) and the East Pacific Rise (a fast-spreading ridge) as natural laboratories for comparative studies.
DIRECTIONS FOR BIOLOGICAL OCEANOGRAPHY
Summary
In the next decade, biological oceanography will emphasize the effects of ecosystems on global cycles of important elements, such as carbon, nitrogen, and oxygen, and conversely the effects of global environmental changes on marine ecosystems. Of timely interest are climate change and population dynamics of marine organisms. In addition to climate change that may be accelerated by carbon dioxide and other greenhouse gas emissions, overfishing, eutrophication, introduced species, and other anthropogenic changes affect marine populations, although impacts vary regionally.
The complexity of biological systems and their variability in both time and space pose practical problems for designing programs and setting research priorities. Potentially important approaches include both studies focused on regions or times of the year with clearly distinguishable food-web structures and intensive examination of areas where geochemical measurements have identified inconsistencies or contradictions. There is also an urgent need to initiate and strengthen long time-series studies of the biology and chemistry of key oceanographic regimes. In addition, concerted effort must be applied to increasing understanding of the basic ecology, physiology, and molecular biology of key marine species.
For the foreseeable future, biological oceanographers will need ships to collect seawater, sediments, and organisms and to prepare and process samples at sea. Thus oceanographic vessels will remain the primary facility for advancing basic knowledge of marine ecosystems. However, the use of other technologies and approaches could lead to important breakthroughs. They include satellite and aircraft remote sensing; numerical modeling; molecular biological techniques; optical, acoustical, and sample collection instrumentation and in situ data acquisition systems, including bottom landers; and remotely operated vehicles.
Introduction
Studying marine communities is difficult without an understanding of their associated physical, geological, and chemical environments. It is likely that biological oceanographers will strengthen interdisciplinary collaboration in the 1990s to include more atmospheric chemists, meteorologists, sedimentologists, paleontol-
ogists, and other Earth scientists. At the same time, advances in biological oceanography will contribute information critical to studies in chemical oceanography and related disciplines. The ocean is a biochemical system, and the biotic and abiotic components of seawater coevolved, resulting in a distribution of elements in the world's ocean that is dictated by biological processes in the sunlit surface waters.
Biological oceanographers study the regulation of plant, animal, and bacterial production; the mechanisms affecting the way production is partitioned among trophic levels and individual species; and the dynamics of marine populations. They use various approaches to study these phenomena. Some biological oceanographers measure concentrations of carbon, nitrogen, calories, and other basic constituents of life and the rates at which they are transferred through the food web and to the seafloor as sinking particles. Others explore the physiology, behavior, genetic diversity, and abundance of individuals within populations and use this knowledge to develop conceptual models of the nature and regulation of marine communities. In recent years, molecular biology tools have contributed to these measurements. Research in the next 10 years will emphasize how biota affect global cycles of carbon, nitrogen, phosphorus, oxygen, and other key elements and, conversely, how climate and other ocean environment changes affect marine ecosystems. During the 1990s and beyond, studies of marine ecosystems are likely to be central to resolving controversies surrounding the key issues of global change.
There is general agreement that the ocean is a significant sink in the global carbon cycle (and related cycles of nitrogen, phosphorus, silicon, and other biologically important elements) and thus is an important modulator of the greenhouse effect caused by the buildup of atmospheric carbon dioxide. Carbon dioxide in the surface mixed layer of the ocean is generally within 30 percent of saturation, whereas it is supersaturated in the deep ocean by as much as 300 percent with respect to the present atmospheric carbon dioxide level. The concentration gradient is maintained by the "biological pump" in the surface waters, which through biological fixation, packaging, and transfer results in a net downward flow of carbon to the deep sea. The ocean is a carbon sink because some of the organic matter synthesized by organisms in the sunlit upper ocean (the euphotic zone) settles to the seafloor, and some small fraction of that reaching the seafloor is eventually buried in marine sediments, where it may remain for millions of years. Annual carbon burial in marine sediments is 0.5 × 1015
grams globally making this process the largest biotic sink of the global carbon cycle (Moore and Bolin, 1986).
One of the major uncertainties in the models of the global carbon cycle is the role of marine organisms in the ocean carbon budget. During the spring bloom in the North Atlantic, the air-sea carbon dioxide flux is strongly controlled by biological activity. However, the comparative magnitude of the ocean and terrestrial sinks of carbon is in dispute (Tans et al., 1990), owing primarily to lack of knowledge about air-sea gas exchange rates, the variability of carbon dioxide saturation of surface waters, and the effects of food webs on the production, reoxidation, sedimentation, and burial of carbon.
The rate at which dissolved or particulate matter passes through the horizontal plane at any particular depth in the ocean is called vertical flux, whereas lateral flux refers to flux through a vertical plane. In the ocean, the vertical flux of organic material (as well as the lateral flux of organic material between estuaries and waters above continental shelves and between shelf and oceanic waters) and its burial rate in ocean sediments are not simple linear functions of primary production. The structures of marine food webs (the number and type of organisms at various feeding levels and the feeding relationships among the organisms) in the euphotic zone, in mid waters, deep waters, and at the seafloor are key variables affecting vertical and lateral fluxes of biologically important elements.
As indicated above, marine food webs affect global biogeochemical cycles, and marine populations, in turn, are affected by changes in global climate and human-induced changes in ocean environments. Some of the best examples of climate effects on marine organisms come from European fisheries, for which long time series exist for fish catch and abundance in relation to key physical and biological variables. An extraordinary event occurred during the 1960s in the North Sea, where the abundance of codlike fish exploded as the herring population declined. This major change probably occurred in response to a period of cooling that decreased the abundance of certain zooplankton species during the time of the year when young herring require zooplankton as food (Cushing, 1982). The impact of El Niño on South American anchoveta populations is another well-known example.
Human activities also affect marine populations, particularly in estuarine and coastal waters, although anthropogenic effects are difficult to distinguish from highly variable natural cycles. Of particular concern are the long-term effects of nutrient enrich-
ment (eutrophication) resulting from altered land use, and waste disposal as a reason for the low oxygen concentrations on continental shelves. An additional concern is the deliberate or accidental transport of species from one ocean to another by shipping or other activities, leading to outbreaks of the introduced species. Documenting the causes and effects of changes in marine populations is difficult, but new techniques and approaches will make this research possible in the future.
Effects of the Food Web on Global Biogeochemical Cycles
Phytoplankton, macroalgae, and sea grasses use energy derived from sunlight to incorporate inorganic carbon, nitrogen, phosphorus, and other elements into organic molecules that are the building blocks of life and sources of energy for nonphotosynthetic organisms that consume these plants. Some bacteria also synthesize organic matter using chemical energy (chemosynthesis) rather than sunlight as a primary energy source. In the ocean, most organic carbon produced by photosynthesis and chemosynthesis is ingested by zooplankton and higher animals, oxidized for energy, and ultimately respired as carbon dioxide in the upper few hundred meters of the water column. At the same time, nutrient elements, such as nitrogen, phosphorus, and some trace metals, are recycled and reused by phytoplankton. A variable fraction of organic matter is not recycled in surface waters; instead, it settles out of the upper ocean layers, thereby contributing to vertical fluxes. Thus fluxes of carbon, nitrogen, oxygen, phosphorus, sulfur, and other biologically important elements are controlled by food-web processes. A major research theme for the 1990s will be to describe the effects and possible controls that food-web structure and function have on fluxes from the euphotic zone to middepth and deep waters, to the ocean sediments, and into the geological record.
Episodic Export of Material from the Surface
The simplest description of the effects of marine food webs on vertical flux involves only the size and species of phytoplankton and whether the phytoplankton sink before being ingested. For example, the spring diatom bloom in the North Atlantic is thought to sink without significant predation, whereas where cyanobacteria are the dominant primary producers, sinking of organic material from the upper ocean is largely mediated by food-web processes.
In the latter case, vertical flux is small, mainly because of the number of steps in the cyanobacteria-based food webs, which lead to more recycling. In either case, the importance of the downward fluxes to the biota is that food resources are no longer available to the community from which they exit but seem to fuel successively deeper communities.
Most particles in the ocean are small and sink slowly. Particles that account for most of the transfer of material to the seafloor are the rarer, large particles that have both high mass and high sinking rates. They include the fecal pellets produced by large zooplankton, large aggregates of detritus and plant debris (marine snow), and living organisms. Zooplankton can increase vertical flux by repackaging and concentrating organic matter from small, slowly sinking phytoplankton and microorganisms into fecal pellets and mucous feeding structures that sink much faster than individual particles. Sinking flux varies by an order of magnitude among food webs. Food webs dominated by large zooplankton consumers may export a much greater percentage of consumed primary production than food webs in which phytoplankton are initially consumed by smaller protozoans and zooplankton, owing to the relative sizes and sinking rates of fecal pellets. Episodic zooplankton swarms could dominate the long-term average export of organic matter from surface ocean communities, but such swarms are often missed by short-term studies.
The activities of marine animals in breaking apart and consuming large aggregates on their way to the seafloor may also be significant, and as yet poorly quantified, factors in controlling particle flux. Many of these particles are consumed by animals as they sink and are converted into smaller fecal pellets, new animal growth, respired carbon dioxide, and dissolved organic matter.
Dissolved Organic Material
The measurement of dissolved organic material (DOM) is also of great interest to biological oceanographers and is an area of overlap between the disciplines of biological and chemical oceanography. The size, average age, and biological availability of the DOM pool are controversial, but the pool could be significant in global fluxes of carbon, nitrogen, and other biologically important elements. Furthermore, a major unresolved question is the degree to which DOM provides nutrition for the ubiquitous microbial community, which may use organic carbon at 10 to 40 percent of the rate at which phytoplankton use it.
Benthos
Deep-sea benthic organisms receive a slow nonseasonal rain of fecal pellets and dead organisms. Recent studies in the North Atlantic show that additional large pulses of organic particles arrive at the bottom within weeks to months following the spring phytoplankton bloom, probably accelerated by formation of marine snow particles. A complementary study in the same general area indicated that benthic organisms grow faster than previously believed, with maximum growth rates following the annual deposition of phytoplankton detritus from the spring bloom (Lampitt, 1990). An open question is the extent to which benthic organisms rely on these episodes of rich input. Certain large animals may metabolically cache food resources.
Ocean Margins
The role of coastal areas in global ocean carbon and nutrient cycles is controversial. Several issues remain, such as the percentage of seasonal and annual coastal production that is exported to the deep sea, the percentage of global productivity that takes place in the coastal ocean, and the extent to which the coastal ocean functions as a net carbon sink because of the massive inputs of nutrients. Interdisciplinary studies will be required to resolve the controversies regarding lateral exchanges between estuaries and the coastal ocean, and between coastal and deeper waters. This point is developed further in ''Directions for Coastal Ocean Processes."
Biology of Hydrothermal Vent and Hydrocarbon Seep Habitats
Most oceanic food webs are based on photosynthetic productivity occurring in the upper regions of the ocean. A little more than a decade ago, it was discovered that dense bacterial and animal communities, which rely largely on in situ chemosynthetic activity, thrive at deep-sea hydrothermal vents and at hydrocarbon seep zones. Carbon fixation in these habitats is driven by highly reduced substances, such as hydrogen sulfide, that are exploited by both free-living bacteria and bacteria living within animal tissues.
The role of deep-sea hydrothermal vent systems in generating and dispersing fixed carbon is an area of active study. Although it is unlikely that carbon fixation at the hydrothermal vents is a
major factor in total global carbon fixation, carbon fixation in the deep sea deserves study because of its uniqueness. Characterization of the global extent of these systems, the rates at which their free-living and symbiotic bacteria fix carbon dioxide, and the extent to which organic materials at vents are distributed to other regions of the oceans will be key areas of research for the next decade. Beyond understanding the biogeochemical role of these communities, studies of vent communities will give insight into the evolution and functioning of nutritious and detoxifying mutualism among organisms. Support for this work has a broad international base, such as through the RIDGE program, which supports multidisciplinary investigations of the biology, geochemistry, and geophysics of mid-ocean ridge-crest systems.
Study of these diverse ecosystems in which chemosynthetic processes replace or complement photosynthetic productivity is necessary to understand the complex nature of marine food webs and the full suite of exchanges and transformations that constitute the global carbon cycle.
Effects of Climate Change on Populations of Marine Organisms
The characteristics of a region that determine its suitability for any given organism include not only the availability of food and the abundance of predators but also the dynamic physical features (mixing and circulation) of the local environment that influence the success of recruitment, efficiency of feeding, and susceptibility of organisms to predation. Global change could affect oceanic animal populations by changing physical processes of significance to the planktonic organisms. At present, it is not possible to predict definitively the impacts of global change on the physical parameters of the ocean and the atmosphere. However, the effects of climate change can be partly anticipated by examining similar effects on shorter time scales, such as seasonal freshwater pulses, El Niños, and other infrequent oceanographic phenomena. Three examples illustrate how global climate change could affect the physical features and processes of the sea that influence the abundance, distribution, and production of marine planktonic animals.
High-latitude marine ecosystems may be more susceptible to global change than low-latitude marine ecosystems. If precipitation patterns change as estimated and global warming triggers the rapid melting of previously persistent ice fields and the retreat of
glaciers, the volume of fresh water that enters polar waters (e.g., the Gulf of Alaska) is likely to increase substantially. The input of fresh water can be critical to coastal currents, as seen by the effects of the Mississippi and St. Lawrence rivers. Changes in precipitation (temporal and spatial) and the amount of ice melt could shift the direction and change the magnitude of coastal currents. Such physical changes will affect fish populations by affecting transport of eggs and larvae.
The effects of El Niño events on eastern boundary current ecosystems in the Pacific Ocean could serve as a model of the possible effects of global warming (McGowan, 1990) in terms of decreased primary and secondary production. In addition, an increased temperature differential between land and ocean could enhance coastal winds and hence wind-induced transport of surface water away from the shore, reducing the reproductive success of species that spawn offshore but rely on coastal habitats later in their life cycles. Stronger winds would also increase turbulence in the surface mixed layer, dispersing patches of planktonic food, and thereby making the food less available for fish.
The third example involves the impacts of a changing sea level. If sea level rises at a rate of 1 to 3 millimeters per year over the coming 50 to 100 years, profound impacts on nearshore habitats would result. In areas with broad, flat coastal plains, the width of the inner continental shelf may be expected to increase greatly. This change would wipe out many coastal habitats. In addition, distribution of the wave energy over a wider continental shelf may substantially modify the transport of planktonic organisms to shore, affecting the success of larval recruitment and the transition of organisms from larval to juvenile stages.
Other Anthropogenic Influences
Other human-induced environmental changes also affect marine populations, although they vary regionally and their extent is disputed. For example, McGowan (1990) reported no detectable change of pelagic species or of ecosystem structure in the California Current ecosystem despite extensive harvesting (fishing) of top predators and vastly increased inputs of pollutants. In contrast, the Baltic Sea ecosystem has changed significantly in the past 50 years in response to eutrophication (Kullenberg, 1986).
The incidence of unusual, and sometimes harmful, phytoplankton. blooms is increasing in coastal waters around the world. The evidence is particularly compelling in European and Japanese wa-
ters, where long-term water quality monitoring programs exist. The precise causes of any bloom event are difficult to ascertain, but there is increasing evidence that unusual phytoplankton blooms are related to changes in nitrogen-silicon ratios caused by eutrophication. The food-web consequences of the global epidemic of noxious phytoplankton blooms could be severe in some areas.
Fishing activity also changes the structure of marine ecosystems, although the effects of overfishing are often difficult to resolve from. long-period cycles in organism abundance. Overfishing of the Georges Bank off the northeastern United States has changed the composition of fish species. From the mid-1960s until the early 1970s, herring and haddock declined by about a factor of 10 due to severe fishing pressure. At the same time, squid, dogfish, and sand lance increased, probably because they filled the ecological niches of depleted haddock and herring stocks (Sissenwine, 1986).
Long-term studies of some coastal benthic communities suggest that they have changed significantly owing to eutrophication. A 20-year time series of benthic species abundance data at a station in Puget Sound suggests that eutrophication may be causing shifts in the dominant species as well as increasing the magnitude of population fluctuations (Nichols, 1985). Yet, even with decadal time series, cause and effect are difficult to ascribe unambiguously, in part because anthropogenic effects are difficult to distinguish from natural changes.
Research Strategies
The complexity of biological systems and their variability in time and space pose practical problems for designing programs and setting research priorities. Research based on the theme that food-web variability controls variability in fluxes of biologically important elements in the global ocean could take many forms; efforts must then focus on a subset of key questions and approaches.
One possibility is to take a comparative approach and focus studies on regions or times of the year with clearly distinguishable food-web structures, and to examine processes in the euphotic zone and in deeper waters. A second possible strategy is to plan biological studies to resolve seeming inconsistencies or contradictions obtained from geochemical measurements and models. For example, recent interest in vertical fluxes in the North Atlantic (Altabet, 1989) were inspired in part by geochemical studies indicating that conventional views of productivity and particle
flux in nutrient-poor waters were inconsistent with geochemical data.
A third possible strategy is to study regions of the ocean that are anomalous vis-à-vis standard paradigms. What controls productivity in nutrient-rich areas of the sea, such as the subarctic Pacific and the equatorial Pacific? If this question could be answered unequivocally, it would indicate significant progress toward a general understanding of oceanic productivity.
In support of all three strategies is continued research on the ecology, physiology, and molecular biology of representative species from specific oceanographic regimes. Without an understanding of the basic biology of individual organisms, one cannot hope to understand how the marine food web works or to predict how the ecosystem will respond to change.
Technologies and Approaches for the 1990s
The pace of scientific progress is often closely coupled with the development and application of new technologies. Several technologies and approaches will aid the study of marine ecosystems in the 1990s and could lead to important breakthroughs.
Satellite Remote Sensing
By the mid-1990s, three variables will be measured simultaneously by satellite for routinely characterizing ecosystems and related environmental factors. The three variables are sea surface temperature; sea surface and near-surface ocean color to determine chlorophyll and water clarity; and sea surface wind fields for estimating rates of vertical mixing, air-sea gas exchange rates, and other wind-related processes, such as the seasonal changes in the depth of the surface ocean mixed layer.
Numerical Modeling
Two developments in modeling should make significant contributions to ecosystem studies in the 1990s. First, models are being developed that can be used to help form hypotheses regarding the role of oceanic biota in global nutrient budgets. These models ultimately will merge basic mathematical descriptions of biogeochemical cycles with general circulation models and, from given starting conditions, will attempt to predict the evolution of fluxes over time. Global models will be particularly useful con-
ceptual tools as techniques are developed to incorporate tracer distributions and satellite data into modeling procedures. For studies of marine populations, models based on individual organisms show promise because they allow treatment of biological variability at the species level.
Molecular Biology
Marine organisms in general have not been studied as extensively as their terrestrial counterparts; relatively little is known about the biota of the world ocean. Barriers to rapid advancements in biological oceanography include the inability of conventional technology to distinguish rapidly among marine taxa and to resolve important questions related to marine community structure, flow dynamics, and their interrelationships. Similarly, advances in marine biology and biological oceanography are limited by the paucity of fundamental knowledge of the genetics, molecular biology, biochemistry, and physiology of marine organisms.
A new suite of elegant and sophisticated technologies and instruments for molecular biology has been developed in the past two decades that could greatly facilitate studies of marine organisms. The technologies of molecular genetics are now applicable to ocean science. These technologies, which allow one to manipulate and probe the most fundamental life processes in ways that were not previously imagined, will revolutionize knowledge of the processes and mechanisms that regulate population, species, and community structures in ocean ecosystems.
In general, molecular biology will aid the study of marine ecosystems in two ways: it will help to determine the physiological state of organisms, and it will help to identify and characterize the genetic structure of marine populations. Work in these areas will help both to identify the causes of biological variability in the ocean and to understand the implications of this variability for the stability and ecological balance of human-impacted ecosystems. For example, these techniques were used to discover archaebacteria and prochlorophyte phytoplankton, to study the role of marine viruses, to determine the diversity of marine bacteria, and to study the enzyme activity of marine organisms. Research in these areas is in its infancy, and new techniques of molecular biology will undoubtedly continue to play an important role in future research.
Acoustics
Sound is an extremely useful tool in biological and fisheries oceanography. The scattering of sound by organisms at many different trophic levels can be used for a variety of purposes. Schools of fish and patches of plankton can be located and tracked acoustically. It may eventually be possible to distinguish living from nonliving scatterers and to identify the biological scatterers by species. It may soon be possible to estimate biomass acoustically as a function of trophic level in the ocean. Sound scattering has been used commercially since the 1930s to locate fish schools, but only recently have multifrequency systems been available for quantitative study of animal plankton. The Multi-Frequency Acoustic Profiling System is capable of profiling zooplankton in the size range of 0.2 to 10 millimeters. General application of acoustical technology will require the development of inexpensive equipment and techniques to analyze and use the large volumes of data generated.
Bio-optics
Fluorometers, transmissometers, and spectroradiometers are used to measure phytoplankton populations, the turbidity of the water column, and the amount and wavelength of light that penetrate beneath the ocean surface at a given site. Correlating site measurements with measurements from satellite ocean color sensors provides the means to extrapolate phytoplankton measurements to a global scale. Mooring optical instruments together with current meters and temperature and salinity sensors provides a technique for collecting long (months) and highly resolved (minutes to hours) time-series measurements, permitting biological oceanographers to study what physical factors control phytoplankton populations. Moorings contribute data on variation over time and depth, whereas satellite sensors provide information on variation over the global ocean surface. Flow cytometry is another optically based technology that is extremely useful for characterizing the size and pigment composition of phytoplankton and bacteria and for sorting populations based on these and other criteria.
Imaging for Organism Enumeration
New techniques for imaging organisms in situ, now available, show promise for widespread application in the 1990s. These
technologies include schlieren video systems and holography, which have been used in the laboratory to study zooplankton feeding behavior. When successfully applied in study, three-dimensional analyses of individual organisms and their spatial relations will be possible on scales sufficient to resolve the behavior of individual organisms.
Time Series
The concept of acquiring long time series of key ecosystem variables at important locations in the global ocean is certainly not new. Yet, with the possible exception of tide-gauge stations, the routine collection of temperature data by commercial ships, and a few simple physical measurements, time-series measurement programs are rare. A notable example of long time-series biological measurements is the Continuous Plankton Recorder Surveys of marine plankton in the North Atlantic Ocean. Ongoing programs measuring biological variables (e.g., the California Cooperative Oceanic Fisheries Investigation) are generally poorly funded. Virtually all recent planning reports stress the importance of long time series to resolve key global change issues and to describe the fundamental attributes of marine ecosystem dynamics. Satellite sensors and bio-optical moorings provide one level of information, but many more in situ observations are needed. Federal agencies recently have recognized the importance of financially supporting long-term measurement programs. For example, NSF supports time series stations at Bermuda and Hawaii, and the National Oceanic and Atmospheric Administration and the Office of the Oceanographer of the Navy are planning the U.S. contribution to a global ocean monitoring system. These time-series stations could be considered the beginning of the biological portion of a global ocean observing system.
Ideas and technologies are in place to make significant progress during the next 10 years in determining the role of marine ecosystems in global ocean biogeochemical cycles and the effects of global change on marine ecosystems. Available technologies range from molecular probes to satellite sensors. The ideas cover a comparable range of scales, from hypotheses about predator-prey encounters at centimeter-length scales to those about interannual variability in global ocean primary production. During the past five years, biological oceanographers have conducted a number of workshops and issued a large number of planning documents and reports (Appendix III). The field is obviously not idea limited.
DIRECTIONS FOR COASTAL OCEAN PROCESSES
Summary
In the coming decade, coastal research will be more interdisciplinary than it is now. Understanding coastal processes will require interdisciplinary studies of biological, chemical, physical, and geological processes. There will probably be considerable progress on the exchange of materials across continental shelves and between the ocean and the atmosphere. For example, physical mechanisms for cross-shelf exchange and their interactions with nonphysical mechanisms should be thoroughly studied. By the end of the decade, estimations of air-sea fluxes of momentum, heat, and gases in a nonequilibrium. sea state (typical for the coastal ocean) should be possible. Further, the understanding of biological, chemical, and geological processes that affect these fluxes should have advanced substantially. Considerable progress should have been achieved in understanding the complex inner shelf (water depths of 3 to 30 meters), where measurements are difficult to make and processes difficult to model because of the many more factors that influence the system, compared to the open ocean. In the same vein, a more predictive understanding of the flux of materials through estuaries will emerge' as our knowledge of the interactions of biological, geochemical, and physical processes improves.
Cross-shelf exchange and its related biological, chemical, geological, and meteorological components will be an active research area. So too will ocean fronts and their implications for biology, chemistry, and meteorology. Fronts are distinct boundaries between water masses and are nearly ubiquitous in the coastal ocean. The mechanisms that create them offer their own sampling and modeling difficulties.
Other areas of research are the global implications of coastal nutrient, carbon, and trace metal cycles and the study of ecosystem structures, which affect chemical cycles. Because of the diversity of coastal ocean regions, significant progress in understanding coastal processes may be achieved in some areas, but it is unlikely that results from all coastal regions can be integrated within the decade. Emphasis on toxic algal blooms, ecosystem structure changes, the invasion or dominance of nuisance species, and other human-induced biological effects may well increase.
In terms of facilities, it is likely that the demand for research aircraft will increase over the decade. The deployment of moored
instrument systems will probably increase, especially with newly developed sensors, such as complete meteorological and wave measurements and underwater sensors capable of measuring biologically, chemically, and geologically relevant variables. Such comprehensive packages already exist or are under development, but their use will become more routine as the decade progresses. The need to study small-scale dynamic features, such as fronts on the continental shelf and wind-forced mixing in estuaries, will create a greater demand for towed devices that can sample rapidly and repeatedly in three dimensions. These too are becoming available but will be used more frequently in the future. Similarly, there will probably be a need for new sediment samplers that can also measure near-bottom currents and sedimentary conditions. Advances in remote sensing of coastal areas will benefit the field.
Introduction
The coastal ocean lies at the junction between land and the open ocean and includes estuaries and embayments. By virtue of its location, it is a setting of unusual societal importance. Most of the world's population centers are located near the ocean, so that pollution, recreation, and shipping impact the coastal environment and are likewise affected by coastal processes. As the U.S. population continues to shift toward the ocean, these considerations will become increasingly important. Economically, the coastal ocean is also of great importance, for example, in terms of mineral (especially petroleum) exploitation, recreation, and fisheries. The conflicts among uses of the coastal region have heightened the public's awareness of the region—and of the need to study it in detail.
The coastal region is defined here as the portions of ocean and atmosphere extending seaward from the surf zone and the heads of tidal estuaries and overlaying the continental shelf, slope, and rise. Geologically, this region of the continental margin forms the transition between the thick continental crust and the thinner oceanic crust, both of which float on the underlying mantle. The continental shelf is essentially the submerged edge of the continental crust. Broadly speaking, continental margins are of two types (Figure 3-3.). Those on the leading edges of crustal plate motions (often near trenches) tend to be characterized by narrow shelves, (e.g., the West Coast of the United States). Margins on the trailing, relatively inactive edges of continents tend to be characterized by broad, relatively flat shelves (e.g., the U.S. East
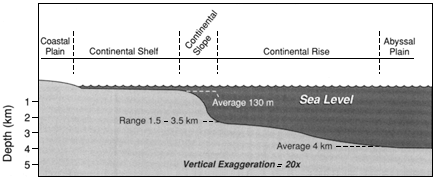
FIGURE 3-3 Typical profiles of two common types of continental margins. Upper panel: A collision margin typical of the Pacific coast of South America. The presence of a submarine trench, a narrow continental shelf, and a landward mountain range characterize this type of margin. Lower panel: A trailing-edge margin typical of much of the Atlantic Ocean. The presence of a continental rise, a broad continental shelf, and a coastal plain are characteristic of this type of margin.
Coast). The margins are often greatly modified by erosion and sediment deposition, processes that tend to carve out submarine canyons and fill in basins, respectively.
The following sections describe processes that make the coastal ocean unique and discuss some scientific issues that will be particularly important over the coming decade. Emphasis is on interdisciplinary aspects because it is likely that most important scientific and societal problems cannot be tackled successfully without a comprehensive approach.
Processes
The lateral boundaries and shelf-slope topography that characterize continental margins substantially determine the nature of coastal currents. For example, on a rotating planet, nearly steady currents are constrained from crossing isobaths (lines of constant depth). As a result, flow in the coastal ocean tends to parallel the coast, and exchange between waters over the continental shelf and the adjacent deep ocean is inhibited. Thus in many cases, distinct shelf water masses form, and the shelf represents a partially closed chemical and biological system. Fronts often mark the boundaries between these coastal and oceanic systems, and these fronts have their own important biological and atmospheric effects.
Wind-driven currents over continental shelves tend to be particularly energetic because the coastline interrupts water transport in the turbulent layer in the upper ocean. This interruption leads to a connection between surface winds and currents deeper in the water column. The resulting currents flowing alongshore below the turbulent surface layer dominate variability in most places over the continental shelves. Wind-driven currents are understood well enough that models are able to predict the speed and direction of coastal currents, as shown by the close agreement between observed and predicted currents shown in Figure 3-4.
Of broader importance to coastal ecosystems is the related onshore-offshore circulation, including the coastal upwelling of cold, nutrient-rich subsurface waters. Their temperature leads to the unusually cool, stable atmospheric conditions that characterize the U.S. West Coast during spring and summer. The upwelled nutrients fuel marine plant growth, leading to high biomass throughout the food web and some of the world's greatest fisheries, including those off the West Coast and off the coast of Peru. Upwelling can also intensify the transfer of organic materials from the surface to the seafloor in such areas. For example, off Peru, as much as one-half of the carbon fixed by phytoplankton production induced by upwelling may be deposited on the bottom. Upwelling in the coastal ocean can also be caused by factors other than wind. For example, upwelling of nutrient-rich water along the inshore edge of the Gulf Stream does much to stimulate productivity off the southeastern coast of the United States, as determined by chlorophyll measurements (Figure 3-5). Whatever its cause, upwelling contributes to the well-known high biological productivity of the coastal ocean (Figure 3-6). Estuaries and coastal embayments, on

FIGURE 3-4 Observed (solid line) and modeled (dashed line) alongshore currents from over the continental shelf off Oregon, summer 1978. Positive velocity denotes northward flow. After Battisti and Hickey (1984).
the other hand, owe their high biological productivity to nutrient inputs from the land and density-driven internal circulation that serves to retain and enhance the recycling of these nutrients.
Sea ice is important in controlling air-sea fluxes in coastal regions when it forms there. Ice cover decreases heat, moisture, and gas fluxes and modifies momentum fluxes. During the formation of ice, salt is excluded, creating saltier adjacent water. These dense water masses can sink, impacting an entire basin through thermohaline circulation (see ''Directions for Physical Oceanography"). Freshwater generated by ice melting stabilizes the water column, thus helping to prompt the spring phytoplankton bloom.
Tidal currents are sometimes enhanced over the continental shelves by physical resonances taking place in bays, such as in the well-known Gulf of Maine-Bay of Fundy example. Strong tidal currents intensify near-bottom mixing that can extend to the sea surface in shallow regions such as Georges Bank. This mixing and the resulting circulation enhance nutrient availability in the upper ocean, cause high primary productivity, enrich fisheries, and increase the transfer of organic material to underlying sediments. Energetic tidal currents can reinforce the many physical processes (including waves and wind-driven currents) that increase sediment resuspension and transport as well as the transport of chemicals that adhere to the particles.
Continental shelves are the transition zone between the land and the ocean and are thus particularly important in processes involving sediment and chemical fluxes. Freshwater outflows propel currents with distinct properties. Sediments from the land are
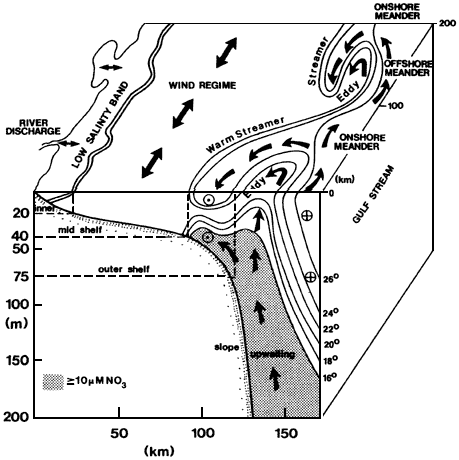
FIGURE 3-5 Upper plate: Schematic of a Gulf Stream frontal eddy (shingle) off the U.S. southeast coast south of Cape Hatteras. Upwelling of nutrient-rich-waters occurs in the cold core of the eddy. Lower plate: Cruise track, surface temperature, chlorophyll a, and salinity across a Gulf Stream frontal eddy off the southwest U.S. coast on April 20–22, 1979. The dashed line in the temperature frame indicates Gulf Stream surface thermal front as determined by satellite-derived frontal analysis on April 20. Note that highest chlorophyll values (>4 mg/m3) are located within upwelled cold core of the eddy. Figures from Lee et al. (1991) and Yoder et al. (1981).
FIGURE 3-6 World ocean primary production according to Koblents-Mishke and coworkers on an equal area projection. Productivity categories are, from low to high, <36, 36–54, 54–90, 90–180, >180 gC/m2/yr. Note that most of the areas of high productivity are located on the ocean margins. From Berger (1988).
often deposited on the continental shelves, although they are sometimes transported to the slopes and deep ocean later. Sedimentary conditions on the shelf are far from static: numerous physical and biological processes can lead to reworking of the sediments and to their eventual transport to other locations. New evidence suggests that the shelf can be a source of particulates that accumulate within estuaries together with sediments delivered to the estuaries by rivers and shoreline erosion. Over geological time scales, the fates of sediments can vary widely with sea level; shelf processes can differ markedly, depending on how much of the shelf (or slope) is exposed above the sea surface. Coastal waters also receive chemicals and particulates weathered from continental rocks and transported to the ocean by rivers, groundwater, and winds. When these chemicals reach the coastal ocean, they are transformed or removed, so that although the properties of the estuarine waters may differ from those of the open ocean, shelf waters closely resemble open ocean water.
Physical processes on the scale of millimeters to kilometers have a major impact on the behavioral responses, feeding rates, interactions, and distributions of plankton, fish, and benthic invertebrates in the coastal ocean. For example, coastal fronts, island wakes, tidal flows, and vertical circulation cells are only a few of the many types of physical phenomena that can aggregate organisms or alter their behavior. Moreover, turbulence and small eddies on the scale of millimeters to meters partially determine the encounter rates of herbivores feeding on passive phytoplankton and bacteria and of predatory interactions among smaller pelagic organisms. An understanding of the effects of water movements on the behavior and distribution of organisms in the ocean will be one of the most challenging aspects of future research, particularly in coastal areas, where both physical processes and organisms are especially diverse and numerous.
Future Directions
The worldwide coastal ocean exhibits vast geographical diversity, depending on the size and openness of bays and estuaries; the width of the continental shelf; the proximity of strong oceanic currents; the strength of tides, winds, river runoff, and surface heat fluxes; and other characteristics. It is clearly impractical to explore fully the biological, chemical, geological, meteorological, and physical structure and variability of every estuary or shelf region of the United States, let alone of the world. One way to proceed is to identify the most significant physical-meteorological processes that to some extent act on all the world shelves and coastal waters. Each physical process and its effects on the biology, chemistry, and geology of the local area could then be studied in a prototypical environment (not limited to U.S. waters) where the process tends to predominate. The results of such interdisciplinary studies could be used to improve our modeling capabilities, enhancing our ability to model more typical shelves or estuaries where a combination of processes interacts. Although this approach is not a panacea, it can at least define the information needed to gain a desired level of understanding of a given coastal region. Within this broad approach to the coastal ocean, a number of important themes will be common to any detailed study of processes.
Air-Sea Interactions
The atmosphere is a major driving force of coastal ocean processes, through both its role in driving currents and its direct and indirect controls on biological and chemical processes. For example, wind-driven coastal upwelling can provide nutrients to the euphotic zone, leading to enhanced primary productivity, and atmospherically generated turbulence can increase predator-prey encounters among plankton (Rothschild and Osborn, 1988). Each of these biological processes results in distinct chemical transformations as well.
Present knowledge of atmospheric effects on the coastal ocean is limited to the effects of large-scale (500-kilometer) atmospheric features. This knowledge is useful for predicting alongshore currents or estimating the transport of dust particles from land to ocean (eolian deposition). Smaller scales in the wind field seem to be more important in determining cross-shelf currents; yet small-scale coastal winds are poorly observed and understood. Interaction of the atmosphere with the coastal ocean on these important scales of tens to hundreds of kilometers is not well-understood.
Air-sea fluxes of momentum and heat, for example, are not adequately characterized in present models, which do not take into account small-scale variability, directional offsets between the wind and waves, limited fetch, and limited water depth (which characterize the coastal environment; Geernaert, 1990). In addition, thermal fronts, which occur throughout the coastal ocean, greatly perturb the atmospheric layer directly above the sea surface and sometimes perturb weather systems. Further, the coastal topography helps to generate small-scale disturbances in the surface winds that can affect currents over the shelf. Air-sea fluxes of particles and chemicals, known to be important, must be a significant part of any study. Until we can quantify the air-sea momentum, heat, and chemical fluxes in this complex environment, we cannot understand the coastal ocean system as a whole.
Air-sea exchange is complex, but answers to the questions must be found. The atmosphere is the basic driving force of many coastal ocean processes. Ocean fluxes, especially heat fluxes, are critical to properties of the atmosphere. Air-sea exchanges that govern the effects of ocean and atmosphere on each other need to be quantified.
Cross-Margin Transport
The interaction of currents with bottom topography tends to isolate continental shelves from the rest of the ocean, although the strength of this isolation is significantly modulated by other processes. Even when the isolation is especially strong, shelf waters resemble the open ocean more than they resemble estuaries. It is difficult to identify which processes determine the cross-margin fluxes of water, particulates, chemicals, and organisms within estuaries, between estuaries and the shelf, on the shelf, and at the shelf-ocean boundary. The relative importance of such factors as wind-driven motions, frontal instabilities, turbulent boundary-layer transports, exchanges through submarine canyons, and the sinking of dense waters has not been evaluated. The difficulty is ultimately their episodic nature in terms of both location and time. Each has distinct effects on biological, chemical, and geological processes, so that interest in them is not limited to physical oceanographers.
Information on cross-margin transport is critical to all subdisciplines of coastal ocean science. Alongshore gradients of most characteristics tend to be small relative to cross-shelf gradients, and alongshore currents are relatively well understood. It is cross-shelf transport, or its absence, that shapes many distributions,
such as those of sediments, that are of scientific interest. Estuarine and cross-shelf exchange is also of interest from a societal standpoint, for example, in determining the fate of riverine inputs of excess nutrients or pollutants. Thus it seems likely that estuarine and cross-shelf exchanges will be a central focus of future efforts in coastal ocean science.
Carbon Cycles
An important and controversial question in oceanography is, What is the role of the coastal ocean in global cycles of carbon, oxygen, nitrogen, and other significant elements? The coastal ocean occupies approximately 20 percent of total ocean area, yet accounts for approximately 50 percent of ocean primary production and approximately 50 percent of global ocean nitrate assimilation by phytoplankton (Walsh, 1991). Describing the mechanisms controlling cycling rates of essential elements has taken on new urgency because of the recently recognized potential for human alteration of global chemical cycles. Biological processes mediate the cycling of many elements and control the fate of numerous materials that enter the ocean. Constructing accurate models of biological controls and predicting their effect on the fate and transformation of dissolved substances and particles in the ocean are severely limited by our lack of understanding of the structure and function of marine ecosystems and their responses to physical and chemical processes. Elucidating these mechanisms is critical to understanding the coastal ocean because of its generally high productivity (and thus its processing capability), its substantial biological variability in space and time, and its role as a conduit between the continents and the deep ocean basins.
A major uncertainty in models of global change, including climate change, is the role of biological processes in mediating and controlling geochemical cycling of important elements. Most scientists agree that biological processes play a key role in the ocean carbon cycle and the cycle of nitrogen, oxygen, and related elements. However, the possible role of marine plants as a sink for carbon dioxide from human activities is highly controversial, and no generally acceptable model has been proposed to explain how the transfer of carbon from the ocean surface to the seafloor (the biological pump) should be working significantly faster now than before the Industrial Revolution. This is an important issue to be considered during the next decade. Understanding ocean margin food webs is of particular interest because they can be altered by eutrophication and other human activities.
In the ocean, the amount of organic material transferred vertically from the surface to the bottom and horizontally from estuaries to shelf waters to the deep ocean is not a simple linear function of primary production; nor are burial rates of organic matter in ocean sediments. The amount of material transported depends on the physical and chemical characteristics of the environment (e.g., rates and mechanisms of nutrient delivery) and on various largely unappreciated characteristics of the species composition and structure of marine food webs in the euphotic zone, deeper in the water column, and in and around the seafloor. Some biogeochemical cycling processes are summarized in Figure 3-7.
Particle Dynamics
Research in several areas needs to be initiated to improve our basic understanding of particle dynamics. Some of these areas have been mentioned, for example, the cross-shelf transport mechanisms and the use of narrow coastal margins with significant sediment inputs to model transport conditions during past times of lower sea level.
Among other research possibilities is the need to test the wide range of theoretical models for sediment transport that evolved in the past two decades. For example, models have been developed to describe the coupling between slowly varying currents and surface gravity waves and to predict resulting sediment transport.
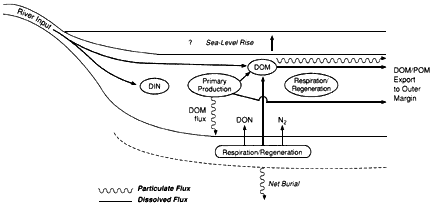
FIGURE 3-7 Schematic of some processes relevant to biogeochemical cycling on the inner continental margin; (DOM = dissolved organic material; DIN = dissolved inorganic nitrogen; DON = dissolved organic nitrogen; POM = particulate organic material). From Mantoura et al. (1991).
However, these models have received little or no testing in the laboratory or through field observations.
Muddy sediments are geologically relevant, and some research has been conducted on the transport of low concentrations of fine-grained sediments. Dense concentrations [>10 grams per liter (i.e., fluid muds)] are also observed in the marine environment, and their transport is poorly understood.
Carbonate sediments are widespread at low latitudes, but the effects of physical processes on their dispersal have not been thoroughly studied. Differences in the particle shapes and densities of carbonate sediments from more common sources make it difficult to extrapolate existing theory of sediment transport.
Theory regarding the formation of sediment layering within the seabed and its dependence on sediment transport and biological activity has evolved rapidly. Additional laboratory and field documentation is needed to link formative mechanisms and preserved strata.
The overall importance of the coastal ocean extends far beyond its relatively small areal extent. An environment of remarkably high biological productivity, this transition zone between land and open ocean is of considerable importance for recreation, waste disposal, and mineral exploitation. Such societal issues as pollution (in its many forms), bioremediation, waste disposal, and risk assessment cannot be addressed adequately until we make substantial advances in our basic understanding of the coastal ocean. A holistic approach to the coastal ocean system, blending marine meteorology with biological, chemical, geological, and physical oceanography, should enable us to progress sufficiently so that we will be better prepared to make the technical and policy decisions facing us over the next decades. Four issues of particular importance are air-sea interactions, cross-margin transport, carbon cycles, and particle dynamics. A balanced program would include studies focused on specific processes, long-term measurements, modeling, and instrumentation development. To take best advantage of the results of these studies, strong working relationships with the applied science communities need to be forged.
Coastal measurements will be an important part of a global ocean observing system because it is at the coasts that most countries, particularly developing nations, will make most of their measurements. Therefore, it is essential that the design of a GOOS include coastal measurements as a critical element of the system.