Lead Poisoning From Mobilization of Bone Stores During Thyrotoxicosis
Rose H.Goldman, MD, MPH, Roberta White, PhD, Stephen N.Kales, MD, and Howard Hu, MD, MPH, SCD
We describe a case of thyrotoxicosis accompanied by markedly elevated blood lead levels (initially 53 µg/dl) in a 37-year-old woman. No current source of lead exposure was found; the woman gave a history indicative of lead exposure as a child and as an adult 7 years previously, however. In addition, she was found to have markedly elevated bone lead levels, as measured by K-x-ray fluorescence (154±5 in the mid-tibia and 253 ±6 µg/g bone mineral in the patella), and an increased serum osteocalcin level (2.76 nmol/l), reflecting the increased bone turnover that often accompanies hyperthyroidism. During treatment with propylthiouracil, serial observations demonstrated a decline in serum osteocalcin that paralleled a decline in blood lead levels. Bone lead levels did not change appreciably. The patient also continued to have lingering neuropsychological symptoms consistent with chronic lead effects. We suggest that increased bone turnover accompanying thyrotoxicosis led to clinically significant lead poisoning in this patient, due to mobilization of accumulated bone lead stores acquired many years earlier. This phenomenon raises the general issue of more subtle forms of lead exposure from increased bone turnover states (e.g., osteoporosis). © 1994 Wiley-Liss, Inc.
Key words: X-ray fluorescence, occupational exposures, thyroid dysfunction, neuropsychological symptoms, bone lead, lead
INTRODUCTION
After inhalation or ingestion, lead enters the bloodstream and is distributed to soft tissues such as blood, brain, and kidney, and then to bone, leading to adverse health effects such as anemia, central nervous system dysfunction, and reproductive system toxicity [Landrigan, 1989]. After heightened exposure to lead ceases, soft tissue stores of lead deplete readily, predominantly through renal excretion, whereas lead accumulated in bone remains for years [Nilsson et al., 1989]. Concerns have
Reprinted with permission from Goldman, et al., American Journal of Industrial Medicine 25:417–24, Copyright 1994, Wiley-Liss, a subsidiary of John Wiley & Sons, Inc.
been raised regarding the potential of lead toxicity to occur from mobilization of these bone stores during times of increased bone turnover, such as pregnancy, lactation, and other hypermetabolic states [Silbergeld, 1991]. We describe the presentation, evaluation, and treatment of a patient with lead poisoning, no identifiable ongoing lead exposure, high skeletal lead as noted by K-X-ray fluorescence (K-XRF, an in vivo method of accurately quantitating bone stores), and marked hyperthyroidism.
Case Report
Initial presentation. A 37-year-old woman employed as a salesperson in a clothing store experienced persistent fatigue, insomnia, difficult concentrating, abdominal cramps, weight loss, muscle and joint aching, and tremor for several months. After reading newspaper reports on lead poisoning, she wondered if her symptoms might be related to work she last performed 7 years earlier, when she removed paint during the course of renovating houses. She requested a blood lead tests from an otolaryngologist who was treating her for an ear infection. The physician found that her chemistry screen (glucose, liver function tests, blood urea nitrogen, creatinine, calcium, and phosphorus) was normal, but that she had an elevated blood lead level of 2.46 µmol/l (51 µg/dl) and an elevated erythrocyte protoporphyrin (EP) level of 0.78 µmol/l (44 µg/dl; normal <0.62 µmol/l or 35 µg/dl).
She was referred to an occupational medicine specialist (R.H.G.). Detailed questioning did not reveal any sources of recent lead exposure. Her two teenage children who lived with her were asymptomatic and had blood leads less than .19 µmol/l (4 µg/dl).
Seven and 10 years previously she had assisted with the deleading of two homes. For a total of about 6 months she scraped and sanded lead paint, wore no respiratory protection, and smoked and ate at the work site. She also recalled that as a child she lived in an old house, frequently chewed on woodwork, windowsills, paper, and pencils, and had chronic abdominal complaints and anemia. She did not recall having a blood lead test as a child. She was held back for 1 year in school because of “math problems.”
Her medical history was notable only for symptoms of Raynaud’s phenomenon, for which she briefly had taken verapamil without relief. She smoked one pack of cigarettes daily and denied any regular alcohol ingestion.
On physical examination she appeared clinically hyperthyroid, with a regular pulse of 112, blood pressure of 130/60 mm Hg, diffusely enlarged and tender thyroid gland, marked “lid lag,” fine resting tremor of both hands, and symmetric hyperreflexia. She also had difficulty replicating Bender diagrams.
The blood lead level, which was measured by flameless atomic absorption spectroscopy in an OSHA-approved laboratory (Bioran, Inc.), was 2.56 µmol/l (53 µg/dl). The EP level, which was measured by hematofluorometry (ESA Labs, Inc.), was 0.66 µmol/1 (38 µg/dl). The serum thyroxine (T4) was >257 nmol/l (>20 µg/dl; normal 64–154 nmol/l), the triiodothyroxine (T3) uptake was 39.9% (normal 30– 40%), free thyroxine (free T4) was 108 pmol/l (8.4 ng/dl; normal 9.0–22), and thyroxine stimulating hormone was (TSH) <0.05 mIU/l (normal 0.46–3.59). The blood urea nitrogen was 6.1 mmol/l (17 mg/dl), and the serum creatinine 61.9 µmol/l (0.7 mg/dl). The hematocrit was 39.6% and the hemoglobin was 133 g/l (13.3 g/dl). No basophilic stippling was seen on a blood smear. A 24-hour radioactive iodine
uptake scan revealed a diffusely enlarged and hyperactive thyroid, consistent with Grave’s disease. Antithyroid globulin and antithyroid microsomal antibodies were not detected. The serum osteocalcin level was elevated to 2.76 nmol/l (16.0 ng/ml; normal 0.28–1.59 nmol/l or 1.6–9.2 ng/ml), indicating increased bone turnover [Slovik et al., 1984].
Bone lead measurements were taken of the patient’s mid-tibial shaft and patella using a sensitive K-X-ray fluorescence (K-XRF) instrument [Burger et al., 1989] and revealed mid-tibial shaft and patella bone lead levels of 154±5 and 253±6 µg/g bone mineral, respectively (Fig. 1). The levels that would have been expected for a woman of her age who did not have an unusual history of lead exposure are approximately 5 and 10 µg Pb/g bone mineral, respectively [Hu et al., 1990, 1991].
Clinical course. The patient was started immediately on propranolol, which helped control her symptoms of jitteriness, tremor, palpitations, and agitation. One week later she began 800 mg of propylthiouracil daily; 7 weeks after her initial occupational medicine (OM) visit she received 26.1 mCi of I131 for thyroid ablation therapy. Her blood lead levels declined as the hyperthyroidism came under better control (Fig. 1). She became hypothyroid and levothyroxine was begun and gradually increased to a daily dose of 125 µg.
At week 36 of observation, 25 weeks after thyroid ablation therapy, the patient was clinically euthyroid, the blood lead level 0.92 µmol/l (19 µg/dl; Table 1 and Fig. 1), and osteocalcin was within the normal range (1.96 nmol/l or 11.4 ng/ml). Bone lead stores were essentially unchanged at 40 weeks. Yet symptoms of profound fatigue, decreased memory recall and concentration abilities, irritability, and feelings of sadness continued. Neurobehavioral assessment using a neurotoxicological oriented detailed test battery [White et al., 1992] identified problems on cognitive tracking tasks requiring mentally holding and manipulating information and on tests of manual motor manipulation and speed. Visuospatial skills were also inferior to verbal abilities. Minnesota Multiphasic Personality Inventory responses suggested that the patient had many physical symptoms and complaints, and that she was suffering from some anxiety and depression. Overall, the pattern of test findings suggests a deficit in nonverbal processing accompanied by manual motor deficits.
One year after her diagnosis, the patient still complained of fatigue, insomnia, irritability, and decreased ability to concentrate, in addition to low-grade headache and nausea. The T4 was 121 nmol/l with the patient on 150 µg of synthroid daily. Blood lead and EP levels were 0.82 µmol/l (17 µg/dl) and 0.21 µmol/l (12 µg/dl), respectively.
DISCUSSION
This case is important because it demonstrates lead poisoning in the absence of an ongoing source of external lead exposure in a hyperthyroid patient, detects elevated bone lead stores through K-X-ray fluorescence, demonstrates increased bone turnover through elevated serum osteocalcin levels, and documents a decline in blood lead without the use of chelating agents as hyperthyroidism and bone turnover come under control.
This patient’s bone lead levels were very high. Her history suggests that they probably derived from remote childhood exposures and 6 months of adult exposure as a lead paint remover. However, her tibia lead levels are comparable to those seen
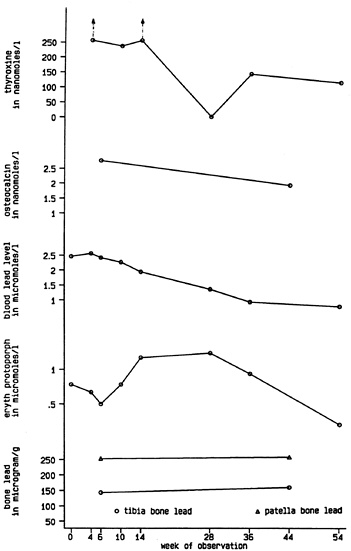
Fig. 1. Serial studies of laboratory data. Therapy with propylthiouracil was begun on week 4; radioactive thyroid ablation was performed on week 11. Normal upper limit levels: thyroxine=154 nmol/l; osteocalcin=1.59 nmol/l; erythrocyte protoporphyrin (eryth protoporph)=0.62 µmol/l; patella lead= 30 µg/g, tibia lead=20 µg/g. There are no “normal” levels of lead, a xenobiotic. The Centers for Disease Control has established 0.48 µmol/l (10 µg/dl) as the tolerable upper limit for lead in a child’s blood, whereas the Occupational Safety and Health Administration does not allow a worker to return to work until his/her blood lead level is below 1.93 µmol/l (40 µg/dl).
TABLE I. Serial Laboratory Values in a 37-Year-old Woman
|
Laboratory |
Normal range |
Week of observation |
||||||||
|
0 |
4 |
6 |
10 |
14 |
28 |
36 |
44 |
54 |
||
|
Thyroxine (nmol/l) |
64–154 |
— |
>257 |
— |
238 |
>257 |
<3.7 |
147 |
— |
121 |
|
Osetocalcin (nmol/l) |
0.28–1.59 |
— |
2.76 |
— |
— |
— |
— |
— |
1.96 |
— |
|
Blood lead (µmol/l) |
<1.21 |
2.46 |
2.56 |
2.41 |
2.26 |
1.93 |
1.35 |
0.92 |
— |
0.82 |
|
Eryth. Protop. (µmol/l) |
<0.62 |
0.78 |
0.66 |
0.50 |
0.80 |
1.17 |
1.24 |
0.94 |
— |
0.21 |
|
Tibia bone lead (µg/g) |
<20 |
— |
— |
154 |
— |
— |
— |
— |
160 |
— |
|
Patella bone lead (µg/g) |
<30 |
— |
— |
253 |
— |
— |
— |
— |
258 |
— |
|
Eryth. protop.=erythrocyte protoporphyrin. |
||||||||||
among male workers with several decades of lead exposure [El-Sharkawi et al., 1986; Hu et al., 1991]. This raises the possibility that the patient’s lead exposure occurred when she was undergoing rapid bone deposition (e.g., the skeletal growth of adolescence), thereby leading to unusually high bone lead levels. Clarification of this issue will await additional studies of bone lead and exposure.
We believe that the patient’s high blood lead levels resulted from increased bone turnover and mobilization of her bone lead stores due to hyperthyroidism. Thyroxine and triiodothyronine stimulate osteoclastic activity and bone resorption, although their precise mode of action is unknown [Mundy, 1990]. Hyperthyroidism, whether endogenous or exogenous, has been found to cause osteoporosis [Fallon, 1983]. This reasoning prompted us to treat this patient’s hyperthyroidism (and increased bone turnover) to lower her blood lead, without the use of additional chelating agents, particularly since a blood lead of 50 µg/dl is usually associated with little or no clinical symptoms in adults.
A case has previously been described of lead poisoning in a young patient who had a retained bullet in his leg for over 3 years [Cagin et al., 1978]. Upon developing hyperthyroidism, he was found to have a blood lead level >100 µg/dl. Bone lead content measured by chemical analysis of a bone biopsy sample was 207 µg/g (wet bone, presumably). This level decreased as his hyperthyroidism and lead poisoning were treated with propylthiouracil and chelating agents.
Case reports have also described lead poisoning, in the absence of ongoing lead exposure, associated with other physiologic states that are accompanied by increased bone turnover, such as pregnancy [Thompson et al., 1985], chemotherapy [El-Sharkawi et al., 1986; Tothill et al., 1989; Beaney et al., 1990], and tumorous infiltration of bone [Brown and Tompsett, 1945].
Significantly increased blood lead levels have been noted in epidemiological studies of postmenopausal women [Silbergeld et al., 1988]. Given the size of her lead burden, the patient in this case may be at risk for lead intoxication again when she enters the postmenopausal period, possibly exacerbated by l-thyroxine therapy.
This case also raises the issue of long-term health effects associated with lead poisoning. The deficits seen in this patient’s test performance were determined to be longstanding because the visuospatial deficits seen occurred across tasks (not just on complex constructional tests such as Block Designs, which might be seen in adult lead exposure) [White et al., 1990] and because the patient’s performance on arithmetic knowledge closely paralleled that seen on visuospatial tasks, a correlation often seen developmentally [Rourke, 1985]. In addition, the identification of manual motor
and cognitive abnormalities despite normalization of thyroid status is consistent with a longstanding rather than acute process.
Lead exposure was thought to be the etiology of the deficits observed in this patient for several reasons. This patient’s results, which included problems with all types of visuospatial tasks and cognitive tracking but not short-term memory or attention, are typical of those seen in children with acute lead exposure and adults with histories of childhood plumbism [White and Proctor, 1992; Feldman and White, 1992]. In addition, bilateral manual motor deficits were seen, which are more consistent with lead as an etiology than other diagnostic possibilities in this patient (such as an idiopathic learning disability). Thyroid dysfunction does not explain the behavioral findings because thyroid function was stable at the time of testing and because her capacity for consistent sustained attention was intact. Finally, test results cannot be explained on the basis of depression because she did not show any of the performance problems which explain deficits in neurobehavioral performance among depressives (i.e., generalized slowing across all tasks, inconsistent attention, or hypoarousal).
Finally, this case demonstrates the clinical utility of a K-XRF instrument for the in vivo measurement of bone lead stores when one suspects a significant internal source of lead exposure. This instrument, recently developed for in vivo measurements, uses a 109Cd gamma-ray source to provoke the emission of fluorescent photons from target tissue; these photons are detected and counted in a back-scatter geometry [Hu et al., 1989]. The number of lead fluorescent photons is compared with the number of photons from the coherent scatter signal, which comes principally from calcium hydroxyapatite; thus the unit of measurement is micrograms of lead per gram of bone mineral (µg/g). This method of normalization renders the measurement insensitive to variations in bone shape, size, density, and histomorphometry, overlying tissue thickness, and movement [Somervaille et al., 1985]. Validation studies of the current instrument in comparison to chemical analysis in cadaveric studies have indicated a high degree of precision and accuracy [Burger et al., 1990; Hu et al., 1990].
This patient remembered pica as a child, but was not known to have had childhood plumbism. X-ray fluorescence was critical in establishing very high bone stores suggestive of childhood lead poisoning. In a study of adults with medical documentation of hospitalization for childhood lead poisoning, 37% were unaware of this history [Hu, 1991].
Given the toxic potential of bone lead stores demonstrated by this case study, the continued lowering of the amount of lead exposure that has been associated with significant health effects in recent research, and the continued widespread nature of lead exposure in the U.S. [ATSDR, 1988], some combination of K-XRF and blood lead testing may eventually become an important screening procedure for select populations of individuals such as patients with thyroid disorders, women contemplating pregnancy, and patients with clinical syndromes suggestive of a low-level lead effect.
ACKNOWLEDGMENTS
K-XRF measurements were performed by Scott Slater and Sudha Kotha. Many thanks to Melanie Brundt, MD, for comments regarding the endocrine section. This
research was supported, in part, by National Institute of Environmental Health Sciences (NIEHS) Occupational and Environmental Health Center Grant 2 P30 ES 00002 and NIEHS ES 05257–01 A1. Dr. Hu was supported, in part, by the Agency for Toxic Substances and Disease Registry Clinical Fellowship Program in Environmental Medicine. Dr. Kales was supported by an Occupational Physician Scholarship Fellowship from the American College of Occupational and Environmental Medicine and National Institute for Occupational Safety and Health Educational Resource Center 5T15 OH 07096. The K-XRF instrument used in this work was developed by ABIOMED, Inc. of Danvers, MA, with support from a National Institutes of Health/ Small Business Innovation and Research grant (2R44 ES03918–02). Many thanks to Dr. Doug Burger for his technical assistance with the instrument.
REFERENCES
ATSDR (July 1988): “The Nature and Extent of Lead Poisoning in Children in the United States: A Report to Congress.” Atlanta Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services.
Beaney RP, Buxton EJ, El-Sharkawi AM, Todd AC, Braithwaite RA, Somervaille LJ, Chettle DR, Scott MC, Jones SJ, Hainsworth IR, Hainsworth IR, Evetts I, Morgan WD, Evans CJ (1990): Cisplatin invoked lead mobilisation studies. Br J Cancer 61:169–170.
Brown A, Tompsett SL (1945): Poisoning due to mobilization of lead from the skeleton by leukaemic hyperplasia of bone marrow. Br Med J 2:764–765.
Burger D, Morsillo P, Adams B, Hu H, Milder FL (1990): Automated instrument for making K-X-ray fluorescence measurements in human bone. Basic Life Sci 55:287–293.
Cagin CR, Diloy-Puray M, Westerman MP (1978): Bullets, lead poisoning, and thyrotoxicosis. Ann Intern Med 89:509–511.
El-Sharkawi AM, Cobbold S, Evans CJ, Chettle DR, Morgan WD, Jaib MBM, Somervaille LJ, Scott MC (1986): Unexpected mobilisation of lead during cisplatin chemotherapy. Lancet 1:249–250.
Fallon MD, Perry HM III, Bergfeld M, Droke D, Teitelbaum SL, Avioli LV (1983): Exogenous hyperthyroidism with osteoporosis. Arch Intern Med 143:442–444.
Feldman RG, White RF (1992): Lead neurotoxicity and disorders of learning and attention. J Child Neurol 7:354–359.
Hu H (1991): Knowledge of diagnosis and reproductive history among survivors of childhood plumbism. Am J Publ Health 81:1070–1072.
Hu H, Milder F, Burger DE (1989): X-ray fluorescence: Issues surrounding the application of a new tool for measuring lead burden. Environ Res 49:295–317.
Hu H, Milder F, Burger DE (1990): X-ray fluorescence measurements of lead burden in subjects with low-level community lead exposures. Arch Environ Health 45:335–341.
Hu H, Pepper L, Goldman R (1991): Effect of repeated lead exposure, cessation of exposure, and chelation on levels of lead in bone. Am J Ind Med 20:723–735.
Landrigan PJ (1989): Toxicity of lead at low dose. Br J Ind Med 46:593–596.
Mundy GR (1990): Bone resorbing cells. In Favus MJ (ed): “Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism.” Kelseyville, CA: American Society for Bone and Mineral Research, pp 18–22.
Nilsson U, Attewell R, Christoffersson J-O, Schütz A, Ahlgren L, Skerfving S, Mattsson S (1991): Kinetics of lead in bone and blood after end of occupational exposure. Pharm Toxicol 69:477–484.
Rourke BP (1985): “Neuropsychology of Learning Disabilities.” New York: Guilford.
Silbergeld EK (1991): Lead in bone: Implications for toxicology during pregnancy and lactation. Environ Health Persp 91:63–70.
Silbergeld EK, Schwartz J, Mahaffey K (1988): Lead and osteoporosis: Mobilization of lead from bone in postmenopausal women. Environ Res 47:79–94.
Slovik DM, Gundberg CM, Neer RM, Lian JB (1984): Clinical evaluation of bone turnover by serum osteocalcin measurements in a hospital setting. J Clin Endo Metab 59:228–230.
Somervaille LJ, Chettle DR, Scott MC (1985): In vivo measurement of lead in bone using x-ray fluorescence. Phys Med Biol 30:929–943.
Thompson GN, Robertson EF, Fitzgerald S (1985): Lead mobilization during pregnancy. Med J Aust 143:131.
Tothill P, Matheson LM, McKay K, Smyth JF (1989): Mobilisation of lead by cisplatin. Lancet 2:1342.
White RF, Feldman RG, Travers PH (1990): Neurobehavioral effects of toxicity due to metals, solvents, and insecticides. Clin Neuropharmacol 13:392–412.
White RF, Diamond R, Proctor SP, Morey C, Hu H. (1992): Residual cognitive deficits 50 years after lead poisoning during childhood. Br J Ind Med, in press.
White RF, Proctor SP (1992): Research and clinical criteria for the development of neurobehavioral test batteries. J Occup Med 34:140–148.








