Page 13
1
INTRODUCTION
This report summarizes and analyzes what is known about the medical use of marijuana; it emphasizes evidence-based medicine (derived from knowledge and experience informed by rigorous scientific analysis), as opposed to belief-based medicine (derived from judgment, intuition, and beliefs untested by rigorous science).
Scientific data on controversial subjects are commonly misinterpreted, overinterpreted, and misrepresented, and the medical marijuana debate is no exception. We have tried to present the scientific studies in such a way as to reveal their strengths and limitations. One of the goals of this report is to help people to understand the scientific data, including the logic behind the scientific conclusions, so it goes into greater detail than previous reports on the subject. In many cases, we have explained why particular studies are inconclusive and what sort of evidence is needed to support particular claims about the harms or benefits attributed to marijuana. Ideally, this report will enable the thoughtful reader to interpret new information about marijuana that will continue to emerge rapidly well after this report is published.
Can marijuana relieve health problems? Is it safe for medical use? Those straightforward questions are embedded in a web of social concerns, which lie outside the scope of this report. Controversies concerning nonmedical use of marijuana spill over onto the medical marijuana debate and tend to obscure the real state of scientific knowledge. In contrast
Page 14
with the many disagreements bearing on the social issues, the study team found substantial consensus, among experts in the relevant disciplines, on the scientific evidence bearing on potential medical use. This report analyzes science, not the law. As in any policy debate, the value of scientific analysis is that it can provide a foundation for further discussion. Distilling scientific evidence does not in itself solve a policy problem. What it can do is illuminate the common ground, bringing to light fundamental differences out of the shadows of misunderstanding and misinformation that currently prevail. Scientific analysis cannot be the end of the debate, but it should at least provide the basis for an honest and informed discussion.
Our analysis of the evidence and arguments concerning the medical use of marijuana focuses on the strength of the supporting evidence and does not refer to the motivations of people who put forth the evidence and arguments. That is, it is not relevant to scientific validity whether an argument is put forth by someone who believes that all marijuana use should be legal or by someone who believes that any marijuana use is highly damaging to individual users and to society as a whole. Nor does this report comment on the degree to which scientific analysis is compatible with current regulatory policy. Although many have argued that current drug laws pertaining to marijuana are inconsistent with scientific data, it is important to understand that decisions about drug regulation are based on a variety of moral and social considerations, as well as on medical and scientific ones.
Even when a drug is used only for medical purposes, value judgments affect policy decisions concerning its medical use. For example, the magnitude of a drug's expected medical benefit affects regulatory judgments about the acceptability of risks associated with its use. Also, although a drug is normally approved for medical use only on proof of its ''safety and efficacy,'' patients with life-threatening conditions are sometimes (under protocols for "compassionate use") allowed access to unapproved drugs whose benefits and risks are uncertain. Value judgments play an even more substantial role in regulatory decisions concerning drugs, such as marijuana, that are sought and used for nonmedical purposes. Then policymakers must take into account not only the risks and benefits associated with medical use but also possible interactions between the regulatory arrangements governing medical use and the integrity of the legal controls set up to restrict nonmedical use.
It should be clear that many elements of drug control policy lie outside the realm of biology and medicine. Ultimately, the complex moral and social judgments that underlie drug control policy must be made by the American people and their elected officials. A goal of this report is to
Page 15
evaluate the biological and medical factors that should be taken into account in making those judgments.
How This Study Was Conducted
Information was gathered through scientific workshops, site visits, analysis of the relevant scientific literature, and extensive consultation with biomedical and social scientists. The three 2-day workshops—in Irvine, California; New Orleans, Louisiana; and Washington, D.C.—were open to the public and included scientific presentations and reports, mostly from patients and their families, about their experiences with and perspectives on the medical use of marijuana. Scientific experts in various fields were selected to talk about the latest research on marijuana, cannabinoids, and related topics (listed in Appendix B). Selection of the experts was based on recommendations by their peers, who ranked them among the most accomplished scientists and the most knowledgeable about marijuana and cannabinoids in their own fields. In addition, advocates for (John Morgan) and against (Eric A. Voth) the medical use of marijuana were invited to present scientific evidence in support of their positions.
Information presented at the scientific workshops was supplemented by analysis of the scientific literature and evaluating the methods used in various studies and the validity of the authors' conclusions. Different kinds of clinical studies are useful in different ways: results of a controlled double-blind study with adequate sample sizes can be expected to apply to the general population from which study subjects were drawn; an isolated case report can suggest further studies but cannot be presumed to be broadly applicable; and survey data can be highly informative but are generally limited by the need to rely on self-reports of drug use and on unconfirmed medical diagnoses. This report relies mainly on the most relevant and methodologically rigorous studies available and treats the results of more limited studies cautiously. In addition, study results are presented in such a way as to allow thoughtful readers to judge the results themselves.
The Institute of Medicine (IOM) appointed a panel of nine experts to advise the study team on technical issues. These included neurology and the treatment of pain (Howard Fields); regulation of prescription drugs (J. Richard Crout); AIDS wasting and clinical trials (Judith Feinberg); treatment and pathology of multiple sclerosis (Timothy Vollmer); drug dependence among adolescents (Thomas Crowley); varieties of drug dependence (Dorothy Hatsukami); internal medicine, health care delivery, and clinical epidemiology (Eric B. Larson); cannabinoids and marijuana pharmacology (Billy R. Martin); and cannabinoid neuroscience (Steven R. Childers).
Page 16
Public outreach included setting up a Web site that provided information about the study and asked for input from the public. The Web site was open for comment from November 1997 until November 1998. Some 130 organizations were invited to participate in the public workshops. Many people in the organizations—particularly those opposed to the medical use of marijuana—felt that a public forum was not conducive to expressing their views; they were invited to communicate their opinions (and reasons for holding them) by mail or telephone. As a result, roughly equal numbers of persons and organizations opposed to and in favor of the medical use of marijuana were heard from.
The study team visited four cannabis buyers' clubs in California (the Oakland Cannabis Buyers' Cooperative, the San Francisco Cannabis Cultivators Club, the Los Angeles Cannabis Resource Center, and Californians Helping Alleviate Medical Problems, or CHAMPS) and two HIV/ AIDS clinics (AIDS Health Care Foundation in Los Angeles and Louisiana State University Medical Center in New Orleans). We listened to many individual stories from the buyers' clubs about using marijuana to treat a variety of symptoms and heard clinical observations on the use of Marinol to treat AIDS patients. Marinol is the brand name for dronabinol, which is D9-tetrahydrocannabinol (THC) in pill form and is available by prescription for the treatment of nausea associated with chemotherapy and AIDS wasting.
Marijuana Today
The Changing Legal Landscape
In the 20th century, marijuana has been used more for its euphoric effects than as a medicine. Its psychological and behavioral effects have concerned public officials since the drug first appeared in the southwestern and southern states during the first two decades of the century. By 1931, at least 29 states had prohibited use of the drug for nonmedical purposes.3 Marijuana was first regulated at the federal level by the Marijuana Tax Act of 1937, which required anyone producing, distributing, or using marijuana for medical purposes to register and pay a tax and which effectively prohibited nonmedical use of the drug. Although the act did not make medical use of marijuana illegal, it did make it expensive and inconvenient. In 1942, marijuana was removed from the U.S. Pharmacopoeia because it was believed to be a harmful and addictive drug that caused psychoses, mental deterioration, and violent behavior.
In the late 1960s and early 1970s, there was a sharp increase in marijuana use among adolescents and young adults. The current legal status of marijuana was established in 1970 with the passage of the Controlled
Page 17
| Medical Marijuana Legislation Among the States |
| The 1996 California referendum known as Proposition 215 allowed seriously ill Californians to obtain and use marijuana for medical purposes without criminal prosecution or sanction. A physician's recommendation is needed. Under the law, physicians cannot be punished or denied any right or privilege for recommending marijuana to patients who suffer from any illness for which marijuana will provide relief. |
| The 1996 Arizona referendum known as Proposition 200 was largely about prison reform but also gave physicians the option to prescribe controlled substances, including those in Schedule I (e.g., marijuana), to treat the disease or relieve the suffering of seriously or terminally ill patients. Five months after the referendum was passed, it was stalled whenArizona legislators voted that all prescription medications must be approved by the Food and Drug Administration, and marijuana is not so approved. In November 1998, Arizona voters passed a second referendum designed to allow physician's to prescribe marijuana as medicine, but this is still at odds with federal law.8 |
| As of summer 1998, eight states—California, Connecticut, Louisiana, New Hampshire, Ohio, Vermont, Virginia, and Wisconsin—had laws that permit physicians to prescribe marijuana for medical purposes or to allow a medical necessity defense.8 In November 1998, five states—Arizona, Alaska, Oregon, Nevada, and Washington—passed medical marijuana ballot initiatives. The District of Columbia also voted on a medical marijuana initiative, but was barred from counting the votes because an amendment designed to prohibit them from doing so was added to the federal appropriations bill; however, exit polls suggested that a majority of voters had approved the measure. |
Substances Act, which divided drugs into five schedules and placed marijuana in Schedule I, the category for drugs with high potential for abuse and no accepted medical use (see Appendix C, Scheduling Definitions). In 1972, the National Organization for the Reform of Marijuana Legislation (NORML), an organization that supports decriminalization of marijuana, unsuccessfully petitioned the Bureau of Narcotics and Dangerous Drugs to move marijuana from Schedule I to Schedule II. NORML argued that marijuana is therapeutic in numerous serious ailments, less toxic, and in many cases more effective than conventional medicines.13 Thus, for 25 years the medical marijuana movement has been closely linked with the marijuana decriminalization movement, which has colored the debate. Many people criticized that association in their letters to IOM and during the public workshops of this study. The argument against the medical use
Page 18
of marijuana presented most often to the IOM study team was that "the medical marijuana movement is a Trojan horse"; that is, it is a deceptive tactic used by advocates of marijuana decriminalization who would exploit the public's sympathy for seriously ill patients.
Since NORML's petition in 1972, there have been a variety of legal decisions concerning marijuana. From 1973 to 1978, 11 states adopted statutes that decriminalized use of marijuana, although some of them recriminalized marijuana use in the 1980s and 1990s. During the 1970s, reports of the medical value of marijuana began to appear, particularly claims that marijuana relieved the nausea associated with chemotherapy. Health departments in six states conducted small studies to investigate the reports. When the AIDS epidemic spread in the 1980s, patients found that marijuana sometimes relieved their symptoms, most dramatically those associated with AIDS wasting. Over this period a number of defendants charged with unlawful possession of marijuana claimed that they were using the drug to treat medical conditions and that violation of the law was therefore justified (the so-called medical necessity defense). Although most courts rejected these claims, some accepted them.8
Against that backdrop, voters in California and Arizona in 1996 passed two referenda that attempted to legalize the medical use of marijuana under particular conditions. Public support for patient access to marijuana for medical use appears substantial; public opinion polls taken during 1997 and 1998 generally reported 60-70 percent of respondents in favor of allowing medical uses of marijuana.15 However, those referenda are at odds with federal laws regulating marijuana, and their implementation raises complex legal questions.
Despite the current level of interest, referenda and public discussions have not been well informed by carefully reasoned scientific debate. Although previous reports have all called for more research, the nature of the research that will be most helpful depends greatly on the specific health conditions to be addressed. And while there have been important recent advances in our understanding of the physiological effects of marijuana, few of the recent investigators have had the time or resources to permit detailed analysis. The results of those advances, only now beginning to be explored, have significant implications for the medical marijuana debate.
Several months after the passage of the California and Arizona medical marijuana referendums, the Office of National Drug Control Policy (ONDCP) asked whether IOM would conduct a scientific review of the medical value of marijuana and its constituent compounds. In August 1997, IOM formally began the study and appointed John A. Benson Jr. and Stanley J. Watson Jr. to serve as principal investigators for the study.
Page 19
The charge to IOM was to review the medical use of marijuana and the harms and benefits attributed to it (details are given in Appendix D).
Marijuana and Medicine
Marijuana plants have been used since antiquity for both herbal medication and intoxication. The current debate over the medical use of marijuana is essentially a debate over the value of its medicinal properties relative to the risk posed by its use.
Marijuana's use as an herbal remedy before the 20th century is well documented.1,10,11 However, modern medicine adheres to different standards from those used in the past. The question is not whether marijuana can be used as an herbal remedy but rather how well this remedy meets today's standards of efficacy and safety. We understand much more than previous generations about medical risks. Our society generally expects its licensed medications to be safe, reliable, and of proven efficacy; contaminants and inconsistent ingredients in our health treatments are not tolerated. That refers not only to prescription and over-the-counter drugs but also to vitamin supplements and herbal remedies purchased at the grocery store. For example, the essential amino acid l-tryptophan was widely sold in health food stores as a natural remedy for insomnia until early 1990 when it became linked to an epidemic of a new and potentially fatal illness (eosinophilia-myalgia syndrome).9,12 When it was removed from the market shortly thereafter, there was little protest, despite the fact that it was safe for the vast majority of the population. The 1,536 cases and 27 deaths were later traced to contaminants in a batch produced by a single Japanese manufacturer.
Although few herbal medicines meet today's standards, they have provided the foundation for modern Western pharmaceuticals. Most current prescriptions have their roots either directly or indirectly in plant remedies.7 At the same time, most current prescriptions are synthetic compounds that are only distantly related to the natural compounds that led to their development. Digitalis was discovered in foxglove, morphine in poppies, and taxol in the yew tree. Even aspirin (acetylsalicylic acid) has its counterpart in herbal medicine: for many generations, American Indians relieved headaches by chewing the bark of the willow tree, which is rich in a related form of salicylic acid.
Although plants continue to be valuable resources for medical advances, drug development is likely to be less and less reliant on plants and more reliant on the tools of modern science. Molecular biology, bioinformatics software, and DNA array-based analyses of genes and chemistry are all beginning to yield great advances in drug discovery and development. Until recently, drugs could only be discovered; now they can
Page 20
be designed. Even the discovery process has been accelerated through the use of modern drug-screening techniques. It is increasingly possible to identify or isolate the chemical compounds in a plant, determine which compounds are responsible for the plant's effects, and select the most effective and safe compounds—either for use as purified substances or as tools to develop even more effective, safer, or less expensive compounds.
Yet even as the modern pharmacological toolbox becomes more sophisticated and biotechnology yields an ever greater abundance of therapeutic drugs, people increasingly seek alternative, low-technology therapies.4,5 In 1997, 46 percent of Americans sought nontraditional medicines and spent over 27 billion unreimbursed dollars; the total number of visits to alternative medicine practitioners appears to have exceeded the number of visits to primary care physicians.5,6 Recent interest in the medical use of marijuana coincides with this trend toward self-help and a search for "natural" therapies. Indeed, several people who spoke at the IOM public hearings in support of the medical use of marijuana said that they generally preferred herbal medicines to standard pharmaceuticals. However, few alternative therapies have been carefully and systematically tested for safety and efficacy, as is required for medications approved by the FDA (Food and Drug Administration).2
Who Uses Medical Marijuana?
There have been no comprehensive surveys of the demographics and medical conditions of medical marijuana users, but a few reports provide some indication. In each case, survey results should be understood to reflect the situation in which they were conducted and are not necessarily characteristic of medical marijuana users as a whole. Respondents to surveys reported to the IOM study team were all members of "buyers' clubs," organizations that provide their members with marijuana, although not necessarily through direct cash transactions. The atmosphere of the marijuana buyers' clubs ranges from that of the comparatively formal and closely regulated Oakland Cannabis Buyers' Cooperative to that of a "country club for the indigent," as Denis Peron described the San Francisco Cannabis Cultivators Club (SFCCC), which he directed.
John Mendelson, an internist and pharmacologist at the University of California, San Francisco (UCSF) Pain Management Center, surveyed 100 members of the SFCCC who were using marijuana at least weekly. Most of the respondents were unemployed men in their forties. Subjects were paid $50 to participate in the survey; this might have encouraged a greater representation of unemployed subjects. All subjects were tested for drug use. About half tested positive for marijuana only; the other half tested positive for drugs in addition to marijuana (23% for cocaine and 13% for
Page 21
| TABLE 1.1 Self-Reported Disorders Treated with Marijuana by Members of San Francisco Cannabis Cultivators Club | |
| Disorder | No. of Subjects |
| HIV | 60 |
| Musculoskeletal disorders and arthritis | 39 |
| Psychiatric disorders (primarily depression) | 27 |
| Neurological disorders and nonmusculoskeletal pain syndromes | 9 |
| Gastrointestinal disorders (most often nausea) | 7 |
| Other disorders | |
| Glaucoma, allergies, nephrolithiasis, and the | |
| skin manifestations of Reiter syndrome | 7 |
| Total disorders | 149 |
| Total number of respondents | 100 |
amphetamines). The predominant disorder was AIDS, followed by roughly equal numbers of members who reported chronic pain, mood disorders, and musculoskeletal disorders (Table 1.1).
The membership profile of the San Francisco club was similar to that of the Los Angeles Cannabis Resource Center (LACRC), where 83% of the 739 patients were men, 45% were 36-45 years old, and 71% were HIV positive. Table 1.2 shows a distribution of conditions somewhat different from that in SFCCC respondents, probably because of a different membership profile. For example, cancer is generally a disease that occurs late in life; 34 (4.7%) of LACRC members were over 55 years old; only 2°/ of survey respondents in the SFCCC study were over 55 years old.
Jeffrey Jones, executive director of the Oakland Cannabis Buyers' Cooperative, reported that its largest group of patients is HIV-positive men in their forties. The second-largest group is patients with chronic pain.
Among the 42 people who spoke at the public workshops or wrote to the study team, only six identified themselves as members of marijuana buyers' clubs. Nonetheless, they presented a similar profile: HIV/AIDS was the predominant disorder, followed by chronic pain (Tables 1.3 and 1.4). All HIV/AIDS patients reported that marijuana relieved nausea and vomiting and improved their appetite. About half the patients who reported using marijuana for chronic pain also reported that it reduced nausea and vomiting.
Note that the medical conditions referred to are only those reported to the study team or to interviewers; they cannot be assumed to represent complete or accurate diagnoses. Michael Rowbotham, a neurologist at the UCSF Pain Management Center, noted that many pain patients referred
Page 22
| TABLE 1.2 Self-Reported Disorders Treated with Marijuana by Members of Los Angeles Cannabis Resource Center (LACRC), According to Center Staffa | ||
| Treated Disorder | No. of Subjects | % of Subjects |
| HIVb | 528 | 71 |
| Cancer | 40 | 5.4 |
| Terminal cancer | 10 | 1.4 |
| Mood disorders (depression) | 4 | 0.5 |
| Musculoskeletal (multiple sclerosis, arthritis) | 30 | 4.1 |
| Chronic pain and back pain | 33 | 4.5 |
| Gastrointestinal | 7 | 2.3 |
| Neurological disorders (epilepsy, Tourette syndrome, brain trauma) | 7 | 0.9 |
| Seizures or migrainesc | 13 | 1.8 |
| Glaucoma | 15 | 2.0 |
| Miscellaneous | 42 | 5.7 |
| Total number | 739 | 100 |
| aResults are based on a review of 739 individual records by LACRC staff members. In contrast with Mendelson's survey of San Francisco Cannabis Cultivators Club (Table 1.1), only the primary disorder is indicated here. Membership in LACRC is contingent on a doctor's letter of acknowledgment, but diagnoses are not independently confirmed. | ||
| bHIV patients use marijuana to control nausea, increase appetite (to combat wasting), and relieve gastrointestinal distress caused by AIDS medications. These uses are not indicated separately. | ||
| cAs described by LACRC staff, some of these cases might also be neurological disorders. | ||
to that center arrive with incorrect diagnoses or with pain of unknown origin. At that center the patients who report medical benefit from marijuana say that it does not reduce their pain but enables them to cope with it.
Most—not all—people who use marijuana to relieve medical conditions have previously used it recreationally. An estimated 95% of the LACRC members had used marijuana before joining the club. It is important to emphasize the absence of comprehensive information on marijuana use before its use for medical conditions. Frequency of prior use almost certainly depends on many factors, including membership in a buyers' club, membership in a population sector that uses marijuana more often than others (for example, men 20-30 years old), and the medical condition being treated with marijuana (for example, there are probably relatively fewer recreational marijuana users among cancer patients than among AIDS patients).
Page 23
| TABLE 1.3 Summary of Reports to IOM Study Team by 43 Individuals | |||
| Symptoms | Dominant Disease | Symptoms | Dominant Disease |
| Anorexia, | AIDS | Pain | Migraine |
| nausea, | AIDS | Injury | |
| vomiting | AIDS | Injury | |
| AIDS | Epilepsy and postpolio | ||
| syndrome | |||
| AIDS | Trauma and epilepsy | ||
| AIDS | Degenerative disk disease | ||
| AIDS | Rheumatoid arthritis | ||
| AIDS and cancer | Nail-patella syndrome | ||
| Cancer | Reflex sympathetic dystrophy | ||
| Testicular cancer | Gulf War chemical exposure | ||
| Cancer and multiple | Multiple congenital | ||
| sclerosis | cartilaginous exostosis | ||
| Thyroid conditiona | Histiocytosis X | ||
| Migraine | |||
| Wilson's disease | Muscle | Spasticitya | |
| spasticity | Multiple sclerosis | ||
| Mood | Depression | Multiple sclerosis | |
| disorders | Depression | Multiple sclerosis | |
| Depression and anxiety | Paralysis | ||
| Depression and anxiety | Spinal-cord injury | ||
| Manic depression | Spasmodic torticollis | ||
| Manic depression | Intraocular | Glaucoma | |
| Posttraumatic stress | pressure | ||
| Premenstrual syndrome | |||
| Diarrhea | Crohn's disease | ||
| aNot specified. | |||
| NOTE: This table lists the people who reported to the IOM study team during the public workshops, or through letters, that they use marijuana as medicine; it should not be interpreted as a representative sample of the full spectrum of people who use marijuana as medicine. Each dominant disease represents an individual report. | |||
Patients who reported their experience with marijuana at the public workshops said that marijuana provided them with great relief from symptoms associated with disparate diseases and ailments, including AIDS wasting, spasticity from multiple sclerosis, depression, chronic pain, and nausea associated with chemotherapy. Their circumstances and symptoms were varied, and the IOM study team was not in a position to make medical evaluations or confirm diagnoses. Three representative cases presented to the IOM study team are presented in Box 1.1; the stories have been edited for brevity, but each case is presented in the patient's words and with the patient's permission.
Page 24
| TABLE 1.4 Primary Symptoms of 43 Individuals Who Reported to IOM Study Team | |||||
| Symptom Frequency | Multiple Symptoms | ||||
| No. Who | |||||
| Reported | %/ of Those | ||||
| '', of Total | (primary) | Who Reported | |||
| Primary | No. of | Symptoms | Additional | Primary | |
| Symptom | Reportsa | Reported | Symptoms | Symptoms | |
| Anorexia, nausea, | |||||
| vomiting | 21 | 31 | 13 | 62 | |
| Diarrhea | 4 | 6 | 3 | 75 | |
| Intraocular pressure | 2 | 3 | 1 | 50 | |
| Mood disorders | 12 | 18 | 7 | 58 | |
| Muscle spasticity | 12 | 18 | 7 | 58 | |
| Pain | 16 | 24 | 13 | 81 | |
| Total | 67 | 44 | 66 | ||
| aForty-three persons reporting; 20 reported relief from more than one symptom. | |||||
The variety of stories presented left the study team with a clear view of people's beliefs about how marijuana had helped them. But this collection of anecdotal data, although useful, is limited. We heard many positive stories but no stories from people who had tried marijuana but found it ineffective. This is a fraction with an unknown denominator. For the numerator we have a sample of positive responses; for the denominator we have no idea of the total number of people who have tried marijuana for medical purposes. Hence, it is impossible to estimate the clinical value of marijuana or cannabinoids in the general population based on anecdotal reports. Marijuana clearly seems to relieve some symptoms for some people—even if only as a placebo effect. But what is the balance of harmful and beneficial effects? That is the essential medical question that can be answered only by careful analysis of data collected under controlled conditions.
Cannabis and the Cannabinoids
Marijuana is the common name for Cannabis sativa, a hemp plant that grows throughout temperate and tropical climates. The most recent review of the constituents of marijuana lists 66 cannabinoids (Table 1.5).16 But that does not mean there are 66 different cannabinoid effects or interactions. Most of the cannabinoids are closely related; they fall into only 10
Page 25
| TABLE 1.5 Cannabinoids Identified in Marijuana | ||
| Cannabinoid | Common | No. of Known |
| Group | Abbreviation | Variants in Each Group |
| D9-Tetrahydrocannabinol | D9-THC | 9 |
| D8-Tetrahydrocannabinol | D8-THC | 2 |
| Cannabichromene | CBC | 5 |
| Cannabicyclol | CBL | 3 |
| Cannabidiol | CBD | 7 |
| Cannabielsoin | CBE | 5 |
| Cannabigerol | CBG | 6 |
| Cannabinidiol | CBND | 2 |
| Cannabinol | CBN | 7 |
| Cannabitriol | CBT | 9 |
| Miscellaneous types | 11 | |
| Total | 66 | |
groups of closely related cannabinoids, many of which differ by only a single chemical moiety and might be midpoints along biochemical path—ways-that is, degradation products, precursors, or byproducts.16,18 D9 -tetrahydrocannabinol (D9-THC) is the primary psychoactive ingredient; depending on the particular plant, either THC or cannabidiol is the most abundant cannabinoid in marijuana (Figure 1.1). Throughout this report, THC is used to indicate D9-THC. In the few cases where variants of THC are discussed, the full names are used. All the cannabinoids are lipophilic—they are highly soluble in fatty fluids and tissues but not in water. Indeed, THC is so lipophilic that it is aptly described as ''greasy."
Throughout this report, marijuana refers to unpurified plant extracts, including leaves and flower tops, regardless of how they are consumed—whether by ingestion or by smoking. References to the effects of marijuana should be understood to include the composite effects of its various components; that is, the effects of THC are included among the effects of marijuana, but not all the effects of marijuana are necessarily due to THC. Discussions concerning cannabinoids refer only to those particular compounds and not to the plant extract. This distinction is important; it is often blurred or exaggerated.
Cannabinoids are produced in epidermal glands on the leaves (especially the upper ones), stems, and the bracts that support the flowers of the marijuana plant. Although the flower itself has no epidermal glands, it has the highest cannabinoid content anywhere on the plant, probably because of the accumulation of resin secreted by the supporting bracteole
Page 26
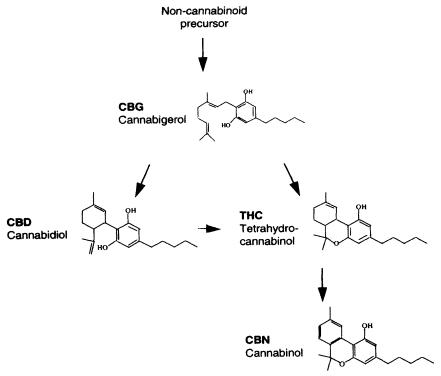
Figure 1.1
Cannabinoid biosynthesis. Arrows indicate cannabinoid
biosynthesis pathway; dark arrows indicate established pathways; the light
gray arrow indicates a probable but not well-established pathway
(R. Mechoulam, Hebrew University, personal communication, 1999).11
The great majority of studies reporting on the effects of cannabinoids
refer to THC; most of the rest are about CBD and CBN. Other cannabinoids
found in marijuana do not appear to play an important role in the drug's effects.
(the small leaf-like part below the flower). The amounts of cannabinoids and their relative abundance in a marijuana plant vary with growing conditions, including humidity, temperature, and soil nutrients (reviewed in Pate, 199414). The chemical stability of cannabinoids in harvested plant material is also affected by moisture, temperature, sunlight, and storage. They degrade under any storage condition.
Page 27
| BOX 1.1 Selected Cases from the Public Sessions |
| G.S. spoke at the IOM workshop in Louisiana about his use of marijuana first to combat AIDS wasting syndrome and later for relief from the side effects of AIDS medications. |
| Skin rashes, dry mouth, foul metallic aftertaste, numbness of the face, swelling of the limbs, fever spikes, headaches, dizziness, anemia, clinical depression, neuropathy so crippling that I could not type, so painful that the bed sheets felt like sandpaper, nausea so severe that I sometimes had to leave the dinner table to vomit, and diarrhea so unpredictable that I dared not leave the house without diapers. |
| These are some of the horrors that I have endured in the past 10 years during my fight for life against the human immunodeficiency virus. But these ravages were not caused by HIV itself, or by any of the opportunistic infections that mark the steady progression of AIDS. Each of these nightmares was a side effect of one of the hundreds of medications I have taken to fight one infection after another on my way to a seemingly certain early grave. |
| Had you known me three years ago, you would not recognize me now. After years of final-stage AIDS, I had wasted to 130 lb. The purple Kaposi's sarcoma lesions were spreading. The dark circles under my eyes told of sleepless nights and half-waking days. I encountered passages of time marked by medication schedules, nausea, and diarrhea. I knew that I was dying. Every reflection shimmered with death, my ghost-like pallor in the mirror, the contained terror on the face of a bus passenger beside me, and most of all the resigned sadness in my mother's eyes. |
| But still I was fortunate because along the way I rediscovered the ancient understanding of marijuana's medicinal benefit. So I smoked pot. Every day. The pot calmed my stomach against handfuls of pills. The pot made me hungry so that I could eat without a tube. The pot eased the pain of crippling neural side effects so that I could dial the phone by myself. The pot calmed my soul and allowed me to accept that I would probably die soon. Because I smoked pot I lived long enough to see the development of the first truly effective HIV therapies. I lived to gain 50 lb., regain my vigor, and celebrate my 35th birthday. I lived to sit on the bus without frightening the passenger beside me. |
| Even at this stage of my recovery I take a handful of pills almost every day and will probably continue to do so for the rest of my life. While I am grateful for the life-saving protease inhibitor therapies, they bring with them a host of adverse reactions and undesirable side effects. Different patients experience different reactions, of course, but almost all patients experience some. Smoking marijuana relieves many of these side effects. I am not one of the exceptional eight patients in the United States with |
| Continued |
Page 28
| legal permission to smoke marijuana. Every day I risk arrest, property forfeiture, fines, ad imprisonment. But I have no choice, you see, just as I have no choice but to endure the side effects of these toxic medications. So, many patients like me are breaking the law to enjoy relief that no other therapy provides. |
| I sit here, I believe, as living proof that marijuana can have a beneficial effect in staving off wasting. every pound was a day. I figured that for every pound of body weight I could maintain, that was another day that I could live in hopes that some effective therapy would emerge. |
| * * * |
| B.D. spoke at the IOM workshop in Louisiana. She is one of eight patients who are legally allowed to smoke marijuana under a Compassionate Use Potocol. She uses marijuana to relieve nausea, muscle spasticity, and pain associated with multiple sclerosis. |
| I was diagnosed with multiple sclerosis in 1988. Prior to that, I was an active person with ballet and swimming. I now have a swimming pool, so I swim each and every day, and smoke marijuana. The government has given me the marijuana to smoke. Each month I pick up a can filled with the marijuana cigarettes rolled by the government. |
| At one time I weighed 85 lb. and I now weigh 105. Twenty pounds is quite a bit to put on. I could not walk. I did not have the appetite. I use a scooter now for distance. I can get around the house. I have a standard poodle who is kind of like an assistant dog. She is good at it. She helps me. |
| When I found out that there was a program to get marijuana from the government, I decided that was the answer. I was not a marijuana smoker before that. In fact, I used to consider the people I knew who smoked the marijuana as undesirables. Now, I myself am an undesirable. |
| But it works. It takes away the backache. With multiple sclerosis, you can get spasms, and your leg will just go straight out and you cannot stop that leg. You may have danced all of your life and put the leg where you wanted It to be, but the MS takes that from you. So I use the swimming pool, and that helps a lot. The kicks are much less when I have smoked a marijuana cigarette. Since 1991, I've smoked 10 cigarettes a day. I do not take any other drugs. Marijuana seems to have been my helper. At one time, I did not think much of the people who smoke it. But when it comes to your health, it makes a big difference. |
| * * * |
| J.H. spoke at the IOM workshop in Washington, D.C. He was seriously injured in an accident, suffers from a form of arthritis associated with abnormal activity of the sympathetic nervous system known as reflexsympathetic dystrophy, and has hepatitis C. He uses marijuana to relieve nausea from liver disease, pain, and muscle spasms. |
| I am 48 years old, married with two children. I am a veteran who served during the Vietnam war. I was exposed to hepatitis C in 1972 by a blood |
Page 29
| transfusion, which I needed because of a motor vehicle accident that broke my back; ruined my right shoulder, my left thumb, and hand; and almost amputated my right leg at the knee. My hepatitis C was not diagnosed until 1997—after the disease had destroyed my pancreas* and I had four heart attacks, one angioplasty, and a minor stroke. In 1989, while at work, I was involved in an accident with a large soil survey auger. My pelvis was crushed, and serious nerve damage was the result. I also have reflex sympathetic dystrophy, which is a neurological disease that has a tremendous amount of pain and muscle spasms. |
| I have reached what the doctors call end-stage liver disease from the hepatitis C. I have lost 85 Ibs. due to the severe bouts of nausea and vomiting. Every time I come home from a hospital stay, my 7 year old asks if I got the liver transplant. I am on a transplant list, but I am not a candidate until I am seven days from death. |
| In October 1997, after trying four different antinausea medications, four of the doctors that I see told me to go to Europe and see a doctor and try medicinal cannabis. My primary care doctor wrote me a letter to carry with my medical records asking that the doctor help me in any way that he could to alleviate the symptoms of the hepatitis C and the reflex sympathetic dystrophy. Those symptoms are severe nausea and pain from the hepatitis C and pain and muscle spasms from the neurological disease. |
| I went to Europe in November 1997, where I saw a doctor of internal medicine. He prescribed me cannabis, 1-2 g a day. I got the medicine and a pipe and tried it. Within two minutes of taking two puffs from the pipe, the nausea was gone. I don't think that I felt the high, although I was quite elated. In about 45 min. I was starving. Normally, I have a fear of eating because I vomit almost always after I eat or take a pill. I forgot about that, and I think I ate more that night than I had eaten in months. I did feel a little nauseated after about four hours, but I smoked two more puffs, and in about two hours I went to bed. The next morning I felt hungry. During my nine-day stay in Europe, I was able to stay free of vomiting and the waves of nausea became less frequent. |
| I had experienced four years of pain control using Tegretol, a drug used by epileptics to control seizures. Now I can't use that medication because of the damage that it causes my cirrhotic liver. When I smoked about 2 g of marijuana a day, the nausea was gone and I was no longer losing weight. The pain was at an acceptable level. Sometimes I still find it necessary to use an opiate painkiller, but only when the pain is at its worst. Surprisingly, I lost an associated high within a few days. I also think the cannabis has an antidepressant effect on me, as I no longer have what I call the "poor me" feelings that I experienced after learning about the hepatitis C. |
| *This is an unlikely consequence of hepatitis C; it is more likely that the patient's liver was damaged. |
Page 30
Organization of the Report
Throughout the report, steps that might be taken to fill the gaps in understanding both the potential harms and benefits of marijuana and cannabinoid use are identified. Those steps include identifying knowledge gaps, promising research directions, and potential therapies based on scientific advances in cannabinoid biology.
Chapter 2 reviews basic cannabinoid biology and provides a foundation to understand the medical value of marijuana or its constituent cannabinoids. In consideration of the physician's first rule, "first, do no harm," the potential harms attributed to the medical use of marijuana are reviewed before the potential medical benefits. Chapter 3 reviews the risks posed by marijuana use, with emphasis on medical use.
Chapter 4 analyzes the most credible clinical data relevant to the medical use of marijuana. It reviews what is known about the physiological mechanisms underlying particular conditions (for example, chronic pain, vomiting, anorexia, and muscle spasticity), what is known about the cellular actions of cannabinoids, and the levels of proof needed to show that marijuana is an effective treatment for specific symptoms. It does not analyze the historical literature; history is informative in enumerating uses of marijuana, but it does not provide the sort of information needed for a scientifically sound evaluation of the efficacy and safety of marijuana for clinical use. Because marijuana is advocated primarily as affording relief from the symptoms of disease rather than as a cure, this chapter is organized largely by symptoms as opposed to disease categories. Finally, chapter 4 compares the conclusions of this report with those of other recent reports on the medical use of marijuana.
Chapter 5 describes the process of and analyzes the prospects for cannabinoid drug development.
References
1. Abel EL. 1980. Marijuana: The First Twelve Thousand Years. New York: Plenum Press.
2. Angell M, Kassirer JP. 1998. Alternative medicine—The risks of untested and unregulated remedies. New England Journal of Medicine 339:839-841.
3. Bonnie RJ, Whitebread II CH. 1974. The Marihuana Conviction: A History of Marihuana Prohibition in the United States. Charlottesville, VA: University Press of Virginia.
4. Eisenberg DM. 1997. Advising patients who seek alternative medical therapies. Annals of Internal Medicine 127:61-69.
5. Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. 1998. Trends in alternative medicine use in the United States, 1990-1997: Results of a follow-up national survey. Journal of the American Medical Association 280:1569-1575.
6. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. 1993. Unconventional medicine in the United States: Prevalence, costs, and patterns of use. New England Journal of Medicine 328:246-252.
Page 31
7. Grifo F, Newman D, Fairfield A, Bhattacharya B, Grupenhoff JT. 1997. The origins of prescription drugs. In: Grifo F, Rosenthal J, Editors. Biodiversity ald Hl1uman Health. Washington, DC: Island Press. Pp. 131-163.
8. Herstek J. 1998. Behavioral Health Issue Briefs. Medical Marijluana. Washington, DC: Health Policy Tracking Service, National Conference of State Legislatures.
9. Kilbourne EM, Philen RM, Kamb ML, Falk H. 1996. Tryptophan produced by Showa Denko and epidemic eosinophilia-myalgia syndrome. Journal of Rhetimiatology Suppl'ImeCit 46:81-88. Comment on: Journal of Rheumatologyl Sluppleme1nt 1996 46:44-58 and 60-72; discussion 58-59.
10. Mathre ML, Editor. 1997. Cannabis in Medical Practice. Jefferson, NC: MacFarland and Co.
11. Mechoulam R. 1986. The pharmacohistory of Cannabis Sativa. In: Mechoulam R, Editor. Cannabinoids as Therapeutic Agents. Boca Raton, FL: CRC Press. Pp. 1-19.
12. Milburn DS, Myers CW. 1991. Tryptophan toxicity: A pharmacoepidemiologic review of eosinophilia-myalgia syndrome. DICP 25:1259-1262.
13. NORML. The Medical Use of Marijuana [WWW document]. URL http://norml.org/ medical/index.html (accessed July 9, 1998).
14. Pate DW. 1994. Chemical ecology of cannabis. Journal of the International Hemp? Association 1:29, 32-37.
15. Peterson K. 15 January 1997. Notes: Weighing in on a medical controversy; USA Today's Baby Boomer Panel. USA Today, p. 12D.
16. Ross SA, Elsohly MA. 1995. Constituents of Cannabis sativa L. XXVIII. A review of the natural constituents: 1980-1994. Zagazig Journal for Pharmaceutical Sciences 4:1-10.
17. Taura F, Morimoto S, Shoyama Y. 1995. First direct evidence for the mechanism of delta 1-tetrahydrocannabinolic acid biosysnthesis. Journal of the American Clhemical Society 117:9766-9767.
18. Turner CE, Elsohly MA, Boeren EG. 1980. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. Journal of Natural Products 43:169-234.




















