Page 83
3
FIRST, DO NO HARM: CONSEQUENCES OF MARIJUANA USE AND ABUSE
Primum non nocere. This is the physician's first rule: whatever treatment a physician prescribes to a patient—first, that treatment must not harm the patient.
The most contentious aspect of the medical marijuana debate is not whether marijuana can alleviate particular symptoms but rather the degree of harm associated with its use. This chapter explores the negative health consequences of marijuana use, first with respect to drug abuse, then from a psychological perspective, and finally from a physiological perspective.
The Marijuana "High"
The most commonly reported effects of smoked marijuana are a sense of well-being or euphoria and increased talkativeness and laughter alternating with periods of introspective dreaminess followed by lethargy and sleepiness (see reviews by Adams and Martin, 1996,1 Hall and Solowij,59 and Hall et al. 60). A characteristic feature of a marijuana "high" is a distortion in the sense of time associated with deficits in short-term memory and learning. A marijuana smoker typically has a sense of enhanced physical and emotional sensitivity, including a feeling of greater interpersonal closeness. The most obvious behavioral abnormality displayed by someone under the influence of marijuana is difficulty in carrying on an intelli-
Page 84
gible conversation, perhaps because of an inability to remember what was just said even a few words earlier.
The high associated with marijuana is not generally claimed to be integral to its therapeutic value. But mood enhancement, anxiety reduction, and mild sedation can be desirable qualities in medications—particularly for patients suffering pain and anxiety. Thus, although the psychological effects of marijuana are merely side effects in the treatment of some symptoms, they might contribute directly to relief of other symptoms. They also must be monitored in controlled clinical trials to discern which effect of cannabinoids is beneficial. These possibilities are discussed later under the discussions of specific symptoms in chapter 4.
The effects of various doses and routes of delivery of THC are shown in Table 3.1.
Adverse Mood Reactions
Although euphoria is the more common reaction to smoking marijuana, adverse mood reactions can occur. Such reactions occur most frequently in inexperienced users after large doses of smoked or oral marijuana. They usually disappear within hours and respond well to reassurance and a supportive environment. Anxiety and paranoia are the most common acute adverse reactions;59 others include panic, depression, dysphoria, depersonalization, delusions, illusions, and hallucinations.1,40,66,69 Of regular marijuana smokers, 17% report that they have experienced at least one of the symptoms, usually early in their use of marijuana.145 Those observations are particularly relevant for the use of medical marijuana in people who have not previously used marijuana.
Drug Dynamics
There are many misunderstandings about drug abuse and dependence (see reviews by O'Brien14 and Goldstein54). The terms and concepts used in this report are as defined in the most recent Diagnostic and Statistical Manual of Mental Disorders (DSM-IV ),3 the most influential system in the United States for diagnoses of mental disorders, including substance abuse (see Box 3.1). Tolerance, dependence, and withdrawal are often presumed to imply abuse or addiction, but this is not the case. Tolerance and dependence are normal physiological adaptations to repeated use of any drug. The correct use of prescribed medications for pain, anxiety, and even hypertension commonly produces tolerance and some measure of physiological dependence.
Even a patient who takes a medicine for appropriate medical indications and at the correct dosage can develop tolerance, physical depen-
Page 85
| TABLE 3.1 Psychoactive Doses of THC in Humans | ||||
| Investigators | THC Delivery | THC Dose | Resulting | Subjects' Reactions |
| Heishman and | One 2.75% | 0.32 mg/kga | 50-100 ng/ml | At higher level, subjects |
| Kelly and | 1-g marijuana | 0.25-0.50 | Not measured | Enough to feel |
| Ohlsson and | 19-mg THC | About 0.22 | 100 ng/ml | Subjects felt "high" |
| 5 mg of THC | About 0.06 | 100 ng/ml | Subjects felt "high" | |
| Chocolate chip | About 0.24 | 8 ng/ml | Subjects rated "high" | |
| Lindgren and | 19-mg THC | 12 mg smoked | 85 ng/ml | Subjects felt "high" |
| 5 mg of THC | 0.06 mg/kg' | 300 ng/ml | Subjects felt maximally | |
| aSubjects' weights and cigarette weights were not given. Calculation based on 85-kg bodyweight and 1-g cigarette weight. Note that some THC would have remained in the cigarettebutt and some would have been lost in sidestream smoke, so these represent maximal possible doses. Actual doses would have been slightly less. bBased on estimated average 85-kg weight of 11 men 18-35 years old. cBased on approximate 80-kg weight of subjects (including men and women). | ||||
Page 86
| Box 3.1 |
| Definitions |
| Addiction. Substance dependence. |
| Craving refers to the intense desire for a drug and is the most difficult aspectof addiction to overcome. |
| Physiologcal dependence is diagnosed when there is evidence of either tolerance or withdrawal; it is sometimes, but not always, manifested in substance dependence. |
| Reinforcement. A drug-—or any other stimulus—is referred to as a reinforcer if exposure to it is followed by an increase in frequency of drug-seeking behavior. The taste of chocolate is a reinforcer for biting into a chocolate bar. Likewise, for many people the sensation experienced after drinking alcohol or smoking marijuana is a reinforcer. |
| Substance dependence is a cluster of cognitive, behavioral, and physiological symptoms indicating that a person continues use of the substance despite significant substance-related problems. |
| Tolerance is the most common response to repetitive use of a drug and can be defined as the reduction in responses to the drug after repeated administrations. |
| Withdrawal. The collective symptoms that occur when a drug is abruptly withdrawn are known as withdrawal syndrome and are often the only evidence of physical dependence. |
dence, and withdrawal symptoms if the drug is stopped abruptly rather than gradually. For example, a hypertensive patient receiving a beta-adrenergic receptor blocker, such as propranolol, might have a good therapeutic response; but if the drug is stopped abruptly, there can be a withdrawal syndrome that consists of tachycardia and a rebound increase in blood pressure to a point that is temporarily higher than before administration of the medication began.
Because it is an illegal substance, some people consider any use of marijuana as substance abuse. However, this report uses the medical definition; that is, substance abuse is a maladaptive pattern of repeated substance use manifested by recurrent and significant adverse consequences.3 Substance abuse and dependence are both diagnoses of pathological substance use. Dependence is the more serious diagnosis and implies compulsive drug use that is difficult to stop despite significant substancerelated problems (see Box 3.2).
Page 87
| Box 3.2 |
| DSM-IV Criteria for Substance Dependence |
| A maladaptive pattern of substance use, leading to clinically significant impairment or distress, as manifested by three (or more) of the following, occurring at any time in the same 12-month period: |
| (1) Tolerance, as defined by either of the following: |
| (a) A need for markedly increased amount of the substance to achieve intoxication or desired effect. |
| (b) Markedly diminished effect with continued use of the same amount of the substance. |
| (2) Withdrawal, as defined by either of the following: |
| (a) The characteristic withdrawal syndrome for the substance. |
| (b) The same (or a closely related) substance is taken to relieve or avoid withdrawal symptoms. |
| (3) The substance is often taken in larger amounts or over a longer period than was intended. |
| (4) There is a persistent desire or unsuccessful efforts to cut down or control substance use. |
| (5) A great deal of time is spent in activities necessary to obtain the substance (e.g., visiting multiple doctors or driving long distances), to use the substance (e.g., chain-smoking), or to recover from its effects. |
| (6) Important social, occupational, or recreational activities are given up or reduced because of substance use. |
| (7) The substance use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance (e.g., current cocaine use despite recognition of cocaine-induced depression or continued drinking despite recognition that an ulcer was made worse by alcohol consumption). |
| Substance abuse with physiological dependence is diagnosed if there is evidence of tolerance or withdrawal. |
| Substance abuse without physiological dependence is diagnosed if there is no evidence of tolerance or withdrawal. |
Reinforcement
Drugs vary in their ability to produce good feelings in users, and the more strongly reinforcing a drug is, the more likely it will be abused (G. Koob, Institute of Medicine (IOM) workshop). Marijuana is indisputably reinforcing for many people. The reinforcing properties of even so mild a
Page 88
stimulant as caffeine are typical of reinforcement by addicting drugs (reviewed by Goldstein54 in 1994). Caffeine is reinforcing for many people at low doses (100-200 mg, the average amount of caffeine in one to two cups of coffee) and is aversive at high doses (600 mg, the average amount of caffeine in six cups of coffee). The reinforcing effects of many drugs are different for different people. For example, caffeine was most reinforcing for test subjects who scored lowest on tests of anxiety but tended not to be reinforcing for the most anxious subjects.
As an argument to dispute the abuse potential of marijuana, some have cited the observation that animals do not willingly self-administer THC, as they will cocaine. Even if that were true, it would not be relevant to human use of marijuana. The value in animal models of drug selfadministration is not that they are necessary to show that a drug is reinforcing but rather that they provide a model in which the effects of a drug can be studied. Furthermore, THC is indeed rewarding to animals at some doses but, like many reinforcing drugs, is aversive at high doses (4.0 mg/ kg).93 Similar effects have been found in experiments conducted in animals outfitted with intravenous catheters that allow them to selfadminister WIN 55,212, a drug that mimics the effects of THC.100
A specific set of neural pathways has been proposed to be a "reward system" that underlies the reinforcement of drugs of abuse and other pleasurable stimuli.51 Reinforcing properties of drugs are associated with their ability to increase concentrations of particular neurotransmitters in areas that are part of the proposed brain reward system. The median forebrain bundle and the nucleus accumbens are associated with brain reward pathways.88 Cocaine, amphetamine, alcohol, opioids, nicotine, and THC144 all increase extracellular fluid dopamine in the nucleus accumbens region (reviewed by Koob and Le Moal88 and Nestler and Aghajanian110 in 1997). However, it is important to note that brain reward systems are not strictly "drug reinforcement centers." Rather, their biological role is to respond to a range of positive stimuli, including sweet foods and sexual attraction.
Tolerance
The rate at which tolerance to the various effects of any drug develops is an important consideration for its safety and efficacy. For medical use, tolerance to some effects of cannabinoids might be desirable. Differences in the rates at which tolerance to the multiple effects of a drug develops can be dangerous. For example, tolerance to the euphoric effects of heroin develops faster than tolerance to its respiratory depressant effects, so heroin users tend to increase their daily doses to reach their desired level of euphoria, thereby putting themselves at risk for respiratory arrest. Because tolerance to the various effects of cannabinoids might
Page 89
develop at different rates, it is important to evaluate independently their effects on mood, motor performance, memory, and attention, as well as any therapeutic use under investigation.
Tolerance to most of the effects of marijuana can develop rapidly after only a few doses, and it also disappears rapidly. Tolerance to large doses has been found to persist in experimental animals for long periods after cessation of drug use. Performance impairment is less among people who use marijuana heavily than it is among those who use marijuana only occasionally,29,104,124 possibly because of tolerance. Heavy users tend to reach higher plasma concentrations of THC than light users after similar doses of THC, arguing against the possibility that heavy users show less performance impairment because they somehow absorb less THC (perhaps due to differences in smoking behavior).95
There appear to be variations in the development of tolerance to the different effects of marijuana and oral THC. For example, daily marijuana smokers participated in a residential laboratory study to compare the development of tolerance to THC pills and to smoked marijuana.61,62 One group was given marijuana cigarettes to smoke four times per day for four consecutive days; another group was given THC pills on the same schedule. During the four-day period, both groups became tolerant to feeling "high" and what they reported as a "good drug effect." In contrast, neither group became tolerant to the stimulatory effects of marijuana or THC on appetite. "Tolerance" does not mean that the drug no longer produced the effects but simply that the effects were less at the end than at the beginning of the four-day period. The marijuana smoking group reported feeling "mellow" after smoking and did not show tolerance to this effect; the group that took THC pills did not report feeling "mellow." The difference was also reported by many people who described their experiences to the IOM study team.
The oral and smoked doses were designed to deliver roughly equivalent amounts of THC to a subject. Each smoked marijuana dose consisted of five 10-second puffs of a marijuana cigarette containing 3.1% THC; the pills contained 30 mg of THC. Both groups also received placebo drugs during other four-day periods. Although the dosing of the two groups was comparable, different routes of administration resulted in different patterns of drug effect. The peak effect of smoked marijuana is usually felt within minutes and declines sharply after 30 minutes68, 95; the peak effect of oral THC is usually not felt until about an hour and lasts for several hours.118
Withdrawal
A distinctive marijuana and THC withdrawal syndrome has been
Page 90
identified, but it is mild and subtle compared with the profound physical syndrome of alcohol or heroin withdrawal.31,74 The symptoms of marijuana withdrawal include restlessness, irritability, mild agitation, insomnia, sleep EEG disturbance, nausea, and cramping (Table 3.2). In addition to those symptoms, two recent studies noted several more. A group of adolescents under treatment for conduct disorders also reported fatigue and illusions or hallucinations after marijuana abstinence (this study is discussed further in the section on "Prevalence and Predictors of Dependence on Marijuana and Other Drugs").31 In a residential study of daily
| TABLE 3.2 Drug Withdrawal Symptoms | ||||
| Nicotine | Alcohol | Marijuana | Cocaine | Opioids (e.g., heroin |
| Restlessness | Restlessness | Restlessness | ||
| Irritability | Irritability | Irritability | Irritability | |
| Impatience, | Mild agitation | |||
| Dysphoria | Dysphoria | Dysphoria | ||
| Depression | Depression | |||
| Anxiety | Anxiety | |||
| Difficulty | ||||
| Sleep | Insomnia | Sleepiness, | Insomnia | |
| Sleep EEG | ||||
| Nausea | Nausea | Nausea | ||
| Cramping | Cramping | |||
| Decreased | Tachycardia, | Bradycardia | ||
| Sweating | Muscle aches | |||
| Seizures | Increased sensitivity | |||
| Alcohol craving | Cocaine craving | Opioid craving | ||
| Increased | Delirium | |||
| Tremor | ||||
| Perceptual | ||||
| aSevere agitation, confusion, visual hallucinations, fever, profuse sweating, nausea, diarrhea, dilated pupils. SOURCE: O'Brien (1996).113 | ||||
Page 91
marijuana users, withdrawal symptoms included sweating and runny nose, in addition to those listed above.62 A marijuana withdrawal syndrome, however, has been reported only in a group of adolescents in treatment for substance abuse problems31 and in a research setting where subjects were given marijuana or THC daily.62,74
Withdrawal symptoms have been observed in carefully controlled laboratory studies of people after use of both oral THC and smoked marijuana.61,62 In one study, subjects were given very high doses of oral THC: 180-210 mg per day for 10-20 days, roughly equivalent to smoking 9-10 2% THC cigarettes per day.74 During the abstinence period at the end of the study, the study subjects were irritable and showed insomnia, runny nose, sweating, and decreased appetite. The withdrawal symptoms, however, were short lived. In four days they had abated. The time course contrasts with that in another study in which lower doses of oral THC were used (80-120 mg/day for four days) and withdrawal symptoms were still near maximal after four days.61,62
In animals, simply discontinuing chronic heavy dosing of THC does not reveal withdrawal symptoms, but the "removal" of THC from the brain can be made abrupt by another drug that blocks THC at its receptor if administered when the chronic THC is withdrawn. The withdrawal syndrome is pronounced, and the behavior of the animals becomes hyperactive and disorganized.153 The half-life of THC in brain is about an hour.16,24 Although traces of THC can remain in the brain for much longer periods, the amounts are not physiologically significant. Thus, the lack of a withdrawal syndrome when THC is abruptly withdrawn without administration of a receptor-blocking drug is probably not due to a prolonged decline in brain concentrations.
Craving
Craving, the intense desire for a drug, is the most difficult aspect of addiction to overcome. Research on craving has focused on nicotine, alcohol, cocaine, and opiates but has not specifically addressed marijuana. 115 Thus, while this section briefly reviews what is known about drug craving, its relevance to marijuana use has not been established.
Most people who suffer from addiction relapse within a year of abstinence, and they often attribute their relapse to craving.58 As addiction develops, craving increases even as maladaptive consequences accumulate. Animal studies indicate that the tendency to relapse is based on changes in brain function that continue for months or years after the last use of the drug. 115 Whether neurobiological conditions change during the manifestation of an abstinence syndrome remains an unanswered question in drug abuse research.88 The "liking" of sweet foods, for example, is
Page 92
mediated by opioid forebrain systems and by brain stem systems, whereas "wanting" seems to be mediated by ascending dopamine neurons that project to the nucleus accumbens.109
Anticraving medications have been developed for nicotine and alcohol. The antidepressant, bupropion, blocks nicotine craving, while naltrexone blocks alcohol craving."l5 Another category of addiction medication includes drugs that block other drugs' effects. Some of those drugs also block craving. For example, methadone blocks the euphoric effects of heroin and also reduces craving.
Marijuana Use and Dependence
Prevalence of Use
Millions of Americans have tried marijuana, but most are not regular users. In 1996, 68.6 million people —32% of the U.S. population over 12 years old—had tried marijuana or hashish at least once in their lifetime, but only 5% were current users.132 Marijuana use is most prevalent among 18- to 25-year-olds and declines sharply after the age of 34 (Figure 3.1).77,132 Whites are more likely than blacks to use marijuana in adolescence, although the difference decreases by adulthood.132
Most people who have used marijuana did so first during adolescence. Social influences, such as peer pressure and prevalence of use by peers, are highly predictive of initiation into marijuana use.9 Initiation is not, of course, synonymous with continued or regular use. A cohort of 456 students who experimented with marijuana during their high school years were surveyed about their reasons for initiating, continuing, and stopping their marijuana use.9 Students who began as heavy users were excluded from the analysis. Those who did not become regular marijuana users cited two types of reasons for discontinuing. The first was related to health and well-being; that is, they felt that marijuana was bad for their health or for their family and work relationships. The second type was based on age-related changes in circumstances, including increased responsibility and decreased regular contact with other marijuana users. Among high school students who quit, parental disapproval was a stronger influence than peer disapproval in discontinuing marijuana use. In the initiation of marijuana use, the reverse was true. The reasons cited by those who continued to use marijuana were to "get in a better mood or feel better." Social factors were not a significant predictor of continued use. Data on young adults show similar trends. Those who use drugs in response to social influences are more likely to stop using them than those who also use them for psychological reasons.80
The age distribution of marijuana users among the general popula-
Page 93
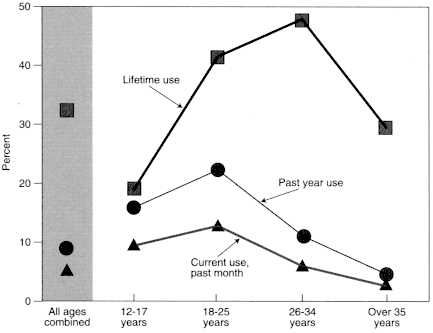
Figure 3.1
Age distribution of marijuana users among the general population.
tion contrasts with that of medical marijuana users. Marijuana use generally declines sharply after the age of 34 years, whereas medical marijuana users tend to be over 35. That raises the question of what, if any, relationship exists between abuse and medical use of marijuana; however, no studies reported in the scientific literature have addressed this question.
Prevalence and Predictors of Dependence on Marijuana and Other Drugs
Many factors influence the likelihood that a particular person will become a drug abuser or an addict; the user, the environment, and the drug are all important factors (Table 3.3).114 The first two categories apply to potential abuse of any substance; that is, people who are vulnerable to drug abuse for individual reasons and who find themselves in an environment that encourages drug abuse are initially likely to abuse the most readily available drug—regardless of its unique set of effects on the brain. The third category includes drug-specific effects that influence the abuse
Page 94
| TABLE 3.3 Factors That Are Correlated with Drug Dependence |
| Individual Factors |
| · Pharmacological effects of the drug |
| · Gender |
| · Age |
| · Genetic factors |
| · Individual risk-taking propensities |
| · Prior drug use |
| Environmental Factors |
| · Availability of the drug |
| · Acceptance of use of that drug in society |
| · Balance of social reinforcements and of punishments for use |
| · Balance of social reinforcements and of punishments for abstinence |
| SOURCE: Crowley and Rhine (1985).32 |
liability of a particular drug. As discussed earlier in this chapter, the more strongly reinforcing a drug is, the more likely that it will be abused. The abuse liability of a drug is enhanced by how quickly its effects are felt, and this is determined by how the drug is delivered. In general, the effects of drugs that are inhaled or injected are felt within minutes, and the effects of drugs that are ingested take a half hour or more.
The proportion of people who become addicted varies among drugs. Table 3.4 shows estimates for the proportion of people among the general population who used or became dependent on different types of drugs. The proportion of users that ever became dependent includes anyone who was ever dependent—whether it was for a period of weeks or years—and thus includes more than those who are currently dependent. Compared to most other drugs listed in this table, dependence among marijuana users is relatively rare. This might be due to differences in specific drug effects, the availability of or penalties associated with the use of the different drugs, or some combination.
Daily use of most illicit drugs is extremely rare in the general population. In 1989, daily use of marijuana among high school seniors was less than that of alcohol (2.9% and 4.2%, respectively).76
Drug dependence is more prevalent in some sectors of the population than in others. Age, gender, and race or ethnic group are all important.8 Excluding tobacco and alcohol, the following trends of drug dependence are statistically significant:8 Men are 1.6 times as likely than women to become drug dependent, non-Hispanic whites are about twice as likely as blacks to become drug dependent (the difference between non-Hispanic
Page 95
| TABLE 3.4 Prevalence of Drug Use and Dependencea in the General Population | ||
| Drug Category | Proportion That Have | Proportion of Users That |
| Tobacco | 76 | 32 |
| Alcohol | 92 | 15 |
| Marijuana (including hashish) | 46b | 9 |
| Anxiolytics (including sedatives and hypnotic drugs) | 13 | 9 |
| Cocaine | 16 | 17 |
| Heroin | 2 | 23 |
| aDiagnosis of drug dependence used in this study based on DSM-III-R criteria.2 | ||
| bThe percentage of people who ever used marijuana is higher than that reported by the National Household Survey on Drug Abuse (32%), probably due to different survey methods (for discussion, see Kandel, 199276). | ||
| SOURCE: Adapted from Table 2 in Anthony and co-workers (1994).8 | ||
and Hispanic whites was not significant), and people 25-44 years old are more than three times as likely as those over 45 years old to become drug dependent.
More often than not, drug dependence co-occurs with other psychiatric disorders. Most people with a diagnosis of drug dependence disorder also have a diagnosis of a another psychiatric disorder (76% of men and 65% of women).76 The most frequent co-occurring disorder is alcohol abuse; 60% of men and 30% of women with a diagnosis of drug dependence also abuse alcohol. In women who are drug dependent, phobic disorders and major depression are almost equally common (29% and 28%, respectively). Note that this study distinguished only between alcohol, nicotine and "other drugs"; marijuana was grouped among "other drugs." The frequency with which drug dependence and other psychiatric disorders co-occur might not be the same for marijuana and other drugs that were included in that category.
A strong association between drug dependence and antisocial personality or its precursor, conduct disorder, is also widely reported in children and adults (reviewed in 1998 by Robins126). Although the causes of the association are uncertain, Robins recently concluded that it is more likely that conduct disorders generally lead to substance abuse than the reverse.126 Such a trend might, however, depend on the age at which the conduct disorder is manifested.
Page 96
A longitudinal study by Brooks and co-workers noted a significant relationship between adolescent drug use and disruptive disorders in young adulthood; except for earlier psychopathology, such as childhood conduct disorder, the drug use preceded the psychiatric disorders.18 In contrast with use of other illicit drugs and tobacco, moderate (less than once a week and more than once a month) to heavy marijuana use did not predict anxiety or depressive disorders; but it was similar to those other drugs in predicting antisocial personality disorder. The rates of disruptive disorders increased with increased drug use. Thus, heavy drug use among adolescents can be a warning sign for later psychiatric disorders; whether it is an early manifestation of or a cause of those disorders remains to be determined.
Psychiatric disorders are more prevalent among adolescents who use drugs—including alcohol and nicotine—than among those who do not.79 Table 3.5 indicates that adolescent boys who smoke cigarettes daily are about 10 times as likely to have a psychiatric disorder diagnosis as those who do not smoke. However, the table does not compare intensity of use among the different drug classes. Thus, although daily cigarette smoking among adolescent boys is more strongly associated with psychiatric disorders than is any use of illicit substances, it does not follow that this comparison is true for every amount of cigarette smoking.79
Few marijuana users become dependent on it (Table 3.4), but those who do encounter problems similar to those associated with dependence on other drugs. 19,143 Dependence appears to be less severe among people
| TABLE 3.5 Relative Prevalence of Diagnoses of Psychiatric Disorders Associated with Drug Use Among Childrena | ||
| Relative Prevalence Estimatesb | ||
| Drug Use | Boys | Girls |
| Weekly alcohol use | 6.1 | 1.6 (n.s.) |
| Daily cigarette smoking | 9.8 | 2.1 (n.s.) |
| Any illicit substance use | 3.2 | 5.3 |
| aSubjects were from 9 to 18 years old (average, 13 years old). | ||
| bAn estimate of 1 means that the relative prevalence of the disorder is equal in those who do and those who do not use the particular type of drug; that is, there is no measurable association. An estimate greater than I indicates that the factor is associated. Substance abuse was excluded because the subjects were already grouped by high drug use. Except where noted (n.s.), all values are statistically significant. | ||
| SOURCE: Data from Table 4 in Kandel and co-workers (1997).79 | ||
Page 97
who use only marijuana than among those who abuse cocaine or those who abuse marijuana with other drugs (including alcohol).19, 143
Data gathered in 1990-1992 from the National Comorbidity Study of over 8,000 persons 15-54 years old indicate that 4.2% of the general population were dependent on marijuana at some time.8 Similar results for the frequency of substance abuse among the general population were obtained from the Epidemiological Catchment Area Program, a survey of over 19,000 people. According to data collected in the early 1980s for that study, 4.4% of adults have, at one time, met the criteria for marijuana dependence. In comparison, 13.8% of adults met the criteria for alcohol dependence and 36.0% for tobacco dependence. After alcohol and nicotine, marijuana was the substance most frequently associated with a diagnosis of substance dependence.
In a 15-year study begun in 1979, 7.3% of 1,201 adolescents and young adults in suburban New Jersey at some time met the criteria for marijuana dependence; this indicates that the rate of marijuana dependence might be even higher in some groups of adolescents and young adults than in the general population.71 Adolescents meet the criteria for drug dependence at lower rates of marijuana use than do adults, and this suggests that they are more vulnerable to dependence than adults25 (see Box 3.2).
Youths who are already dependent on other substances are particularly vulnerable to marijuana dependence. For example, Crowley and coworkers31 interviewed a group of 229 adolescent patients in a residential treatment program for delinquent, substance-involved youth and found that those patients were dependent on an average of 3.2 substances. The adolescents had previously been diagnosed as dependent on at least one substance (including nicotine and alcohol) and had three or more conduct disorder symptoms during their life. About 83% of those who had used marijuana at least six times went on to develop marijuana dependence. About equal numbers of youths in the study had a diagnosis of marijuana dependence and a diagnosis of alcohol dependence; fewer were nicotine dependent. Comparisons of dependence potential between different drugs should be made cautiously. The probability that a particular drug will be abused is influenced by many factors, including the specific drug effects and availability of the drug.
Although parents often state that marijuana caused their children to be rebellious, the troubled adolescents in the study by Crowley and coworkers developed conduct disorders before marijuana abuse. That is consistent with reports that the more symptoms of conduct disorders children have, the younger they begin drug abuse,127 and that the earlier they begin drug use, the more likely it is to be followed by abuse or dependence.125
Genetic factors are known to play a role in the likelihood of abuse for
Page 98
drugs other than marijuana,7,129 and it is not unexpected that genetic factors play a role in the marijuana experience, including the likelihood of abuse. A study of over 8,000 male twins listed in the Vietnam Era Twin Registry indicated that genes have a statistically significant influence on whether a person finds the effects of marijuana pleasant.97 Not surprisingly, people who found marijuana to be pleasurable used it more often than those who found it unpleasant. The study suggested that, although social influences play an important role in the initiation of use, individual differences—perhaps associated with the brain's reward system—influence whether a person will continue using marijuana. Similar results were found in a study of female twins.86 Family and social environment strongly influenced the likelihood of ever using marijuana but had little effect on the likelihood of heavy use or abuse. The latter were more influenced by genetic factors. Those results are consistent with the finding that the degree to which rats find THC rewarding is genetically based.92
In summary, although few marijuana users develop dependence, some do. But they appear to be less likely to do so than users of other drugs (including alcohol and nicotine), and marijuana dependence appears to be less severe than dependence on other drugs. Drug dependence is more prevalent in some sectors of the population than others, but no group has been identified as particularly vulnerable to the drug-specific effects of marijuana. Adolescents, especially troubled ones, and people with psychiatric disorders (including substance abuse) appear to be more likely than the general population to become dependent on marijuana.
If marijuana or cannabinoid drugs were approved for therapeutic uses, it would be important to consider the possibility of dependence, particularly for patients at high risk for substance dependence. Some controlled substances that are approved medications produce dependence after long-term use; this, however, is a normal part of patient management and does not generally present undue risk to the patient.
Progression from Marijuana to Other Drugs
The fear that marijuana use might cause, as opposed to merely precede, the use of drugs that are more harmful is of great concern. To judge from comments submitted to the IOM study team, it appears to be of greater concern than the harms directly related to marijuana itself. The discussion that marijuana is a "gateway" drug implicitly recognizes that other illicit drugs might inflict greater damage to health or social relations than marijuana. Although the scientific literature generally discusses drug use progression between a variety of drug classes, including alcohol and tobacco, the public discussion has focused on marijuana as a "gateway"
Page 99
drug that leads to abuse of more harmful illicit drugs, such as cocaine and heroin.
There are strikingly regular patterns in the progression of drug use from adolescence to adulthood. Because it is the most widely used illicit drug, marijuana is predictably the first illicit drug that most people encounter. Not surprisingly, most users of other illicit drugs used marijuana first.81,82 In fact, most drug users do not begin their drug use with marijuana—they begin with alcohol and nicotine, usually when they are too young to do so legally.82,90
The gateway analogy evokes two ideas that are often confused. The first, more often referred to as the ''stepping stone'' hypothesis, is the idea that progression from marijuana to other drugs arises from pharmacological properties of marijuana itself.82 The second is that marijuana serves as a gateway to the world of illegal drugs in which youths have greater opportunity and are under greater social pressure to try other illegal drugs. The latter interpretation is most often used in the scientific literature, and it is supported, although not proven, by the available data.
The stepping stone hypothesis applies to marijuana only in the broadest sense. People who enjoy the effects of marijuana are, logically, more likely to be willing to try other mood-altering drugs than are people who are not willing to try marijuana or who dislike its effects. In other words, many of the factors associated with a willingness to use marijuana are, presumably, the same as those associated with a willingness to use other illicit drugs. Those factors include physiological reactions to the drug effect, which are consistent with the stepping stone hypothesis, but also psychosocial factors, which are independent of drug-specific effects. There is no evidence that marijuana serves as a stepping stone on the basis of its particular physiological effect. One might argue that marijuana is generally used before other illicit mood-altering drugs, in part, because its effects are milder; in that case, marijuana is a stepping stone only in the same sense as taking a small dose of a particular drug and then increasing that dose over time is a stepping stone to increased drug use.
Whereas the stepping stone hypothesis presumes a predominantly physiological component of drug progression, the gateway theory is a social theory. The latter does not suggest that the pharmacological qualities of marijuana make it a risk factor for progression to other drug use. Instead, the legal status of marijuana makes it a gateway drug.82
Psychiatric disorders are associated with substance dependence and are probably risk factors for progression in drug use. For example, the troubled adolescents studied by Crowley and co-workers31 were dependent on an average of 3.2 substances, and this suggests that their conduct disorders were associated with increased risk of progressing from one drug to another. Abuse of a single substance is probably also a risk factor
Page 100
for later multiple drug use. For example, in a longitudinal study that examined drug use and dependence, about 26% of problem drinkers reported that they first used marijuana after the onset of alcohol-related problems (R. Pandina, IOM workshop). The study also found that 11% of marijuana users developed chronic marijuana problems; most also had alcohol problems.
Intensity of drug use is an important risk factor in progression. Daily marijuana users are more likely than their peers to be extensive users of other substances (for review, see Kandel and Davies78). Of 34- to 35-year-old men who had used marijuana 10-99 times by the age 24-25, 75% never used any other illicit drug; 53% of those who had used it more than 100 times did progress to using other illicit drugs 10 or more times. 78 Comparable proportions for women are 64% and 50%.
The factors that best predict use of illicit drugs other than marijuana are probably the following: age of first alcohol or nicotine use, heavy marijuana use, and psychiatric disorders. However, progression to illicit drug use is not synonymous with heavy or persistent drug use. Indeed, although the age of onset of use of licit drugs (alcohol and nicotine) predicts later illicit drug use, it does not appear to predict persistent or heavy use of illicit drugs.90
Data on the gateway phenomenon are often overinterpreted. For example, one study reports that "marijuana's role as a gateway drug appears to have increased."55 It was a retrospective study based on interviews of drug abusers who reported smoking crack or injecting heroin daily. The data from the study provide no indication of what proportion of marijuana users become serious drug abusers; rather, they indicate that serious drug abusers usually use marijuana before they smoke crack or inject heroin. Only a small percentage of the adult population uses crack or heroin daily; during the five-year period from 1993 to 1997, an average of three people per 1,000 used crack and about two per 1,000 used heroin in the preceding month.132
Many of the data on which the gateway theory is based do not measure dependence; instead, they measure use—even once-only use. Thus, they show only that marijuana users are more likely to use other illicit drugs (even if only once) than are people who never use marijuana, not that they become dependent or even frequent users. The authors of these studies are careful to point out that their data should not be used as evidence of an inexorable causal progression; rather they note that identifying stage-based user groups makes it possible to identify the specific risk factors that predict movement from one stage of drug use to the next—the real issue in the gateway discussion.25
In the sense that marijuana use typically precedes rather than follows initiation into the use of other illicit drugs, it is indeed a gateway drug.
Page 101
However, it does not appear to be a gateway drug to the extent that it is the cause or even that it is the most significant predictor of serious drug abuse; that is, care must be taken not to attribute cause to association. The most consistent predictors of serious drug use appear to be the intensity of marijuana use and co-occurring psychiatric disorders or a family history of psychopathology (including alcoholism).78,83
An important caution is that data on drug use progression pertain to nonmedical drug use. It does not follow from those data that if marijuana were available by prescription for medical use, the pattern of drug use would be the same. Kandel and co-workers also included nonmedical use of prescription psychoactive drugs in their study of drug use progression.82 In contrast with the use of alcohol, nicotine, and illicit drugs, there was not a clear and consistent sequence of drug use involving the abuse of prescription psychoactive drugs. The current data on drug use progression neither support nor refute the suggestion that medical availability would increase drug abuse among medical marijuana users. Whether the medical use of marijuana might encourage drug abuse among the general community—not among medical marijuana users themselves but among others simply because of the fact that marijuana would be used for medical purposes—is another question.
Link Between Medical use and Drug Abuse
Almost everyone who spoke or wrote to the IOM study team about the potential harms posed by the medical use of marijuana felt that it would send the wrong message to children and teenagers. They stated that information about the harms caused by marijuana is undermined by claims that marijuana might have medical value. Yet many of our powerful medicines are also dangerous medicines. These two facets of medicine—effectiveness and risk—are inextricably linked.
The question here is not whether marijuana can be both harmful and helpful but whether the perception of its benefits will increase its abuse. For now any answer to the question remains conjecture. Because marijuana is not an approved medicine, there is little information about the consequences of its medical use in modern society. Reasonable inferences might be drawn from some examples. Opiates, such as morphine and codeine, are an example of a class of drugs that is both abused to great harm and used to great medical benefit, and it would be useful to examine the relationship between their medical use and their abuse. In a "natural experiment" during 1973-1978 some states decriminalized marijuana, and others did not. Finally, one can examine the short-term consequences of the publicity surrounding the 1996 medical marijuana campaign in California and ask whether it had any measurable impact on marijuana con-
Page 102
sumption among youth in California; the consequences of "message" that marijuana might have medical use are examined below.
Medical Use and Abuse of Opiates
Two highly influential papers published in the 1920s and 1950s led to widespread concern among physicians and medical licensing boards that liberal use of opiates would result in many addicts (reviewed by Moulin and co-workers106 in 1996). Such fears have proven unfounded; it is now recognized that fear of producing addicts through medical treatment resulted in needless suffering among patients with pain as physicians needlessly limited appropriate doses of medications.27,44 Few people begin their drug addiction problems with misuse of drugs that have been prescribed for medical use.114 Opiates are carefully regulated in the medical setting, and diversion of medically prescribed opiates to the black market is not generally considered to be a major problem.
No evidence suggests that the use of opiates or cocaine for medical purposes has increased the perception that their illicit use is safe or acceptable. Clearly, there are risks that patients will abuse marijuana for its psychoactive effects and some likelihood of diversion of marijuana from legitimate medical channels into the illicit market. But those risks do not differentiate marijuana from many accepted medications that are abused by some patients or diverted from medical channels for nonmedical use. Medications with abuse potential are placed in Schedule II of the Controlled Substances Act, which brings them under stricter control, including quotas on the amount that can be legally manufactured (see chapter 5 for discussion of the Controlled Substances Act). That scheduling also signals to physicians that a drug has abuse potential and that they should monitor its use by patients who could be at risk for drug abuse.
Marijuana Decriminalization
Monitoring the Future, the annual survey of values and lifestyles of high school seniors, revealed that high school seniors in decriminalized states reported using no more marijuana than did their counterparts in states where marijuana was not decriminalized.72 Another study reported somewhat conflicting evidence indicating that decriminalization had increased marijuana use.105 That study used data from the Drug Awareness Warning Network (DAWN), which has collected data on drugrelated emergency room (ER) cases since 1975. There was a greater increase from 1975 to 1978 in the proportion of ER patients who had used marijuana in states that had decriminalized marijuana in 1975-1976 than in states that had not decriminalized it (Table 3.6). Despite the greater
Page 103
| TABLE 3.6 Effect of Decriminalization on Marijuana Use in Emergency Room (ER) Cases | ||||
| Total Reports of Drug Use per ERa | ||||
| States That | States That Did Not | |||
| Periodt' | ||||
| Marijuana use | 1975 | 0.8 | 1.5 | |
| 1978 | 2.7 | 2.5 | ||
| Other drug use | 1975 | 47 | 55 | |
| 1978 | 55 | 70 | ||
| aData are based on patient self-reports. | ||||
| bStates that decriminalized marijuana did so after 1975 and before 1978. The 1975 values reflect ER marijuana reports before or in the first months of decriminalization, whereas 1978 values reflect ER reports when decriminalization laws had been in effect at least a year. The 1978 levels are median values for quarters in 1978 and are derived from Figures I and 2 in Model (1993).105 | ||||
| SOURCE: Adapted from Figures 1 and 2 in Model (1993).105 | ||||
increase among decriminalized states, the proportion of marijuana users among ER patients by 1978 was about equal in states that had and states that had not decriminalized marijuana. That is because the non-decriminalized states had higher rates of marijuana use before decriminalization. In contrast with marijuana use, rates of other illicit drug use among ER patients were substantially higher in states that did not decriminalize marijuana use. Thus, there are different possible reasons for the greater increase in marijuana use in the decriminalized states. On the one hand, decriminalization might have led to an increased use of marijuana (at least among people who sought health care in hospital ERs). On the other hand, the lack of decriminalization might have encouraged greater use of drugs that are even more dangerous than marijuana.
The differences between the results for high school seniors from the Monitoring the Future study and the DAWN data are unclear, although the author of the latter study suggests that the reasons might lie in limitations inherent in how the DAWN data are collected.105
In 1976, the Netherlands adopted a policy of toleration for possession of up to 30 g of marijuana. There was little change in marijuana use during the seven years after the policy change, which suggests that the change itself had little effect; however, in 1984, when Dutch "coffee shops" that sold marijuana commercially spread throughout Amsterdam, marijuana
Page 104
use began to increase.98 During the 1990s, marijuana use has continued to increase in the Netherlands at the same rate as in the United States and Norway—two countries that strictly forbid marijuana sale and possession. Furthermore, during this period, approximately equal percentages of American and Dutch 18 year olds used marijuana; Norwegian 18 year olds were about half as likely to have used marijuana. The authors of this study conclude that there is little evidence that the Dutch marijuana depenalization policy led to increased marijuana use, although they note that commercialization of marijuana might have contributed to its increased use. Thus, there is little evidence that decriminalization of marijuana use necessarily leads to a substantial increase in marijuana use.
The Medical Marijuana Debate
The most recent National Household Survey on Drug Abuse showed that among people 12-17 years old the perceived risk associated with smoking marijuana once or twice a week had decreased significantly between 1996 and 1997.132 (Perceived risk is measured as the percentage of survey respondents who report that they "perceive great risk of harm" in using a drug at a specified frequency.) At first glance, that might seem to validate the fear that the medical marijuana debate of 1996—before passage of the California medical marijuana referendum in November 1997—had sent a message that marijuana use is safe. But a closer analysis of the data shows that Californian youth were an exception to the national trend. In contrast to the national trend, the perceived risk of marijuana use did not change among California youth between 1996 and 1997.132* In summary, there is no evidence that the medical marijuana debate has altered adolescents' perceptions of the risks associated with marijuana use.132
Psychological Harms
In assessing the relative risks and benefits related to the medical use of marijuana, the psychological effects of marijuana can be viewed both as unwanted side effects and as potentially desirable end points in medical treatment. However, the vast majority of research on the psychological effects of marijuana has been in the context of assessing the drug's intoxicating effects when it is used for nonmedical purposes. Thus, the litera-
*Although Arizona also passed a medical marijuana referendum, it was embedded in a broader referendum concerning prison sentencing. Hence, the debate in Arizona did not focus on medical marijuana the way it did in California, and changes in Arizona youths' attitudes likely reflect factors peripheral to medical marijuana.
Page 105
ture does not directly address the effects of marijuana taken for medical purposes.
There are some important caveats to consider in attempting to extrapolate from the research mentioned above to the medical use of marijuana. The circumstances under which psychoactive drugs are taken are an important influence on their psychological effects. Furthermore, research protocols to study marijuana's psychological effects in most instances were required to use participants who already had experience with marijuana. People who might have had adverse reactions to marijuana either would choose not to participate in this type of study or would be screened out by the investigator. Therefore, the incidence of adverse reactions to marijuana that might occur in people with no marijuana experience cannot be estimated from such studies. A further complicating factor concerns the dose regimen used for laboratory studies. In most instances, laboratory research studies have looked at the effects of single doses of marijuana, which might be different from those observed when the drug is taken repeatedly for a chronic medical condition.
Nonetheless, laboratory studies are useful in suggesting what psychological functions might be studied when marijuana is evaluated for medical purposes. Results of laboratory studies indicate that acute and chronic marijuana use has pronounced effects on mood, psychomotor, and cognitive functions. These psychological domains should therefore be considered in assessing the relative risks and therapeutic benefits related to marijuana or cannabinoids for any medical condition.
Psychiatric Disorders
A major question remains as to whether marijuana can produce lasting mood disorders or psychotic disorders, such as schizophrenia. Georgotas and Zeidenberg52 reported that smoking 10-22 marijuana cigarettes per day was associated with a gradual waning of the positive mood and social facilitating effects of marijuana and an increase in irritability, social isolation, and paranoid thinking. Inasmuch as smoking one cigarette is enough to make a person feel "high" for about 1-3 hours,68,95,118 the subjects in that study were taking very high doses of marijuana. Reports have described the development of apathy, lowered motivation, and impaired educational performance in heavy marijuana users who do not appear to be behaviorally impaired in other ways. 121,122 There are clinical reports of marijuana-induced psychosis-like states (schizophrenia-like, depression, and/or mania) lasting for a week or more.112 Hollister suggests that, because of the varied nature of the psychotic states induced by marijuana, there is no specific "marijuana psychosis." Rather, the marijuana experience might trigger latent psychopathology of many types.66
Page 106
More recently, Hall and colleagues60 concluded that "there is reasonable evidence that heavy cannabis use, and perhaps acute use in sensitive individuals, can produce an acute psychosis in which confusion, amnesia, delusions, hallucinations, anxiety, agitation and hypomanic symptoms predominate." Regardless of which of those interpretations is correct, the two reports agree that there is little evidence that marijuana alone produces a psychosis that persists after the period of intoxication.
Schizophrenia
The association between marijuana and schizophrenia is not well understood. The scientific literature indicates general agreement that heavy marijuana use can precipitate schizophrenic episodes but not that marijuana use can cause the underlying psychotic disorder.59,96,151 As noted earlier, drug abuse is common among people with psychiatric disorders. Estimates of the prevalence of marijuana use among schizophrenics vary considerably but are in general agreement that it is at least as great as that among the general population.134 Schizophrenics prefer the effects of marijuana to those of alcohol and cocaine,35 which they seem to use less often than does the general population.134 The reasons for this are unknown, but it raises the possibility that schizophrenics might obtain some symptomatic relief from moderate marijuana use. But overall, compared with the general population, people with schizophrenia or with a family history of schizophrenia are likely to be at greater risk for adverse psychiatric effects from the use of cannabinoids.
Cognition
As discussed earlier, acutely administered marijuana impairs cognition.60,66,112 Positron emission tomography (PET) imaging allows investigators to measure the acute effects of marijuana smoking on active brain function. Human volunteers who perform auditory attention tasks before and after smoking a marijuana cigarette show impaired performance while under the influence of marijuana; this is associated with substantial reduction in blood flow to the temporal lobe of the brain, an area that is sensitive to such tasks.116,117 Marijuana smoking increases blood flow in other brain regions, such as the frontal lobes and lateral cerebellum.101,155 Earlier studies purporting to show structural changes in the brains of heavy marijuana users22 have not been replicated with more sophisticated techniques.28,89
Nevertheless, recent studies14,122 have found subtle defects in cognitive tasks in heavy marijuana users after a brief period (19-24 hours) of marijuana abstinence. Longer term cognitive deficits in heavy marijuana
Page 107
users have also been reported.140 Although these studies have attempted to match heavy marijuana users with subjects of similar cognitive abilities before exposure to marijuana use, the adequacy of this matching has been questioned.133 The complex methodological issues facing research in this area are well reviewed in an article by Pope and colleagues.121 Care must be exercised so that studies are designed to differentiate between changes in brain function caused the effects of marijuana and by the illness for which marijuana is being given. AIDS dementia is an obvious example of this possible confusion. It is also important to determine whether repeated use of marijuana at therapeutic dosages produces any irreversible cognitive effects.
Psychomotor Performance
Marijuana administration has been reported to affect psychomotor performance on a number of tasks. The review by Chait and Pierri23 not only details the studies that have been done but also points out the inconsistencies among studies, the methodological shortcomings of many studies, and the large individual differences among the studies attributable to subject, situational, and methodological factors. Those factors must be considered in studies of psychomotor performance when participants are involved in a clinical trial of the efficacy of marijuana. The types of psychomotor functions that have been shown to be disrupted by the acute administration of marijuana include body sway, hand steadiness, rotary pursuit, driving and flying simulation, divided attention, sustained attention, and the digit-symbol substitution test. A study of experienced airplane pilots showed that even 24 hours after a single marijuana cigarette their performance on flight simulator tests was impaired.163 Before the tests, however, they told the study investigators that they were sure their performance would be unaffected.
Cognitive impairments associated with acutely administered marijuana limit the activities that people would be able to do safely or productively. For example, no one under the influence of marijuana or THC should drive a vehicle or operate potentially dangerous equipment.
Amotivational Syndrome
One of the more controversial effects claimed for marijuana is the production of an "amotivational syndrome." This syndrome is not a medical diagnosis, but it has been used to describe young people who drop out of social activities and show little interest in school, work, or other goal-directed activity. When heavy marijuana use accompanies these symptoms, the drug is often cited as the cause, but no convincing data demon-
Page 108
strate a causal relationship between marijuana smoking and these behavioral characteristics.23 It is not enough to observe that a chronic marijuana user lacks motivation. Instead, relevant personality traits and behavior of subjects must be assessed before and after the subject becomes a heavy marijuana user. Because such research can only be done on subjects who become heavy marijuana users on their own, a large population study—such as the Epidemiological Catchment Area study described earlier in this chapter—would be needed to shed light on the relationship between motivation and marijuana use. Even then, although a causal relationship between the two could, in theory, be dismissed by an epidemiological study, causality could not be proven.
Summary
Measures of mood, cognition, and psychomotor performance should be incorporated into clinical trials evaluating the efficacy of marijuana or cannabinoid drugs for a given medical condition. Ideally, participants would complete mood assessment questionnaires at various intervals throughout the day for a period before; every week during; and, where appropriate, after marijuana therapy. A full psychological screening of research participants should be conducted to determine whether there is an interaction between the mood-altering effects of chronic marijuana use and the psychological characteristics of the subjects. Similarly, the cognitive and psychomotor functioning should be assessed before and regularly during the course of a chronic regimen of marijuana or cannabinoid treatment to determine the extent to which tolerance to the impairing effects of marijuana develops and to monitor whether new problems develop.
When compared with changes produced by either placebo or an active control medication, the magnitude of desirable therapeutic effects and the frequency and magnitude of adverse psychological side effects of marijuana could be determined. That would allow a more thorough assessment of the risk:benefit ratio associated with the use of marijuana for a given indication.
CONCLUSION: The psychological effects of cannabinoids, such as anxiety reduction, sedation, and euphoria, can influence their potential therapeutic value. Those effects are potentially undesirable in some patients and situations and beneficial in others. In addition, psychological effects can complicate the interpretation of other aspects of the drug's effect.
Page 109
RECOMMENDATION: Psychological effects of cannabinoids, such as anxiety reduction and sedation, which can influence medical benefits, should be evaluated in clinical trials.
Physiological Harms: Tissue and Organ Damage
Many people who spoke to the IOM study team in favor of the medical use of marijuana cited the absence of marijuana overdoses as evidence that it is safe. Indeed, epidemiological data indicate that in the general population marijuana use is not associated with increased mortality.138 However, other serious health outcomes should be considered, and they are discussed below.
It is important to keep in mind that most of the studies that report physiological harm resulting from marijuana use are based on the effects of marijuana smoking. Thus, we emphasize that the effects reported cannot be presumed to be caused by THC alone or even in combination with other cannabinoids found in marijuana. It is likely that smoke is a major cause of the reported effects. In most studies the methods used make it impossible to weigh the relative contributions of smoke versus cannabinoids.
Immune System
The relationship between marijuana and the immune system presents many facets, including potential benefits and suspected harms. This section reviews the evidence on suspected harms to the immune system caused by marijuana use.
Despite the many claims that marijuana suppresses the human immune system, the health effects of marijuana-induced immunomodulation are still unclear. Few studies have been done with animals or humans to assess the effects of marijuana exposure on host resistance to bacteria, viruses, or tumors.
Human Studies
Several approaches have been used to determine the effects of marijuana on the human immune system. Each has serious limitations, which are discussed below.
Assays of Leukocytes from Marijuana Smokers. One of the more common approaches has been to isolate peripheral blood leukocytes from people who have smoked marijuana in order to evaluate the immune
Page 110
response of those cells in vitro—most often by measuring mitogen-induced cell proliferation, a normal immune response. Almost without exception, this approach has failed to demonstrate any reduction in leukocyte function. The major problem with the approach is that after blood samples are drawn from the study subjects the leukocytes must be isolated from whole blood before they are tested. That is done by high-speed centrifugation followed by extensive washing of the cells, which removes the cannabinoid; perhaps for this reason no adverse effects have been demonstrated in peripheral blood leukocytes from marijuana smokers.75,91,123,160
Leukocyte Responses to THC. Another approach is to isolate peripheral blood leukocytes from healthy control subjects who do not smoke marijuana and then to measure the effect of THC on the ability of these cells to proliferate in response to mitogenic stimulation in vitro. One important difference between leukocytes isolated from a marijuana smoker, as described above, and leukocyte cell cultures to which THC has been added directly is in the cannabinoid composition. Marijuana smoke contains many distinct cannabinoid compounds of which THC is just one. Moreover, the immunomodulatory activity of many of the other cannabinoid compounds has never been tested, and it is now known that at least one of those—cannabinol (CBN)—has greater activity on the immune system than on the central nervous system,64 so it is unclear whether the profile of activity observed with THC accurately represents the effects of marijuana smoke on immune competence. Likewise, the extent to which different cannabinoids in combination exhibit additive, synergistic, or antagonistic effects with respect to immunomodulatory activity is unclear. The issue is complicated by the fact that leukocytes express both types of cannabinoid receptors: CB1 and CB2.
An additional factor that might affect the immunomodulatory activity of cannabinoids in leukocytes is metabolism. Leukocytes have very low levels of the cytochrome P-450 drug-metabolizing enzymes,20 so the metabolism of cannabinoids is probably different between in vivo and in vitro exposure. That last point is pertinent primarily to investigations of chronic, not acute, cannabinoid exposure.
Human-Derived Cell Lines. A third approach for investigating the effects of cannabinoids on human leukocytes has been to study human-derived cell lines.* As described above, the cell lines are treated in vitro with cannabinoids to test their responses to different stimuli. Although cell lines
*Cell lines are created by removing cells from an organism and then treating them so they are ''immortalized,'' meaning they will continue to divide and multiply indefinitely in culture. Cellular processes can then be studied in isolation from their original source.
Page 111
are a convenient source of human cells, the problems described above apply here as well. In addition, the cell lines might not be the same as the original cells. For example, cell lines do not necessarily have the same number of cannabinoid receptors as the original human cells.
Rodent Studies
The most widely used approach is to evaluate the effects of cannabinoids in rodents, using rodent-derived cells in vitro. The rationale is that the human and rodent immune systems are remarkably similar, and it is assumed that the effects produced by cannabinoids on the rodent immune system will be similar to those produced in humans. Although no substantial species differences in immune system sensitivity to cannabinoids have been reported, the possibility should be considered.
Summary
The complete effect of marijuana smoking on immune function remains unknown. More important, it is not known whether smoking leads to increased rates of infections, tumors, allergies, or autoimmune responses. The problem is how to duplicate the "normal" marijuana smoking pattern while removing other potential immunomodulating lifestyle factors, such as alcohol and tobacco use. Epidemiological studies are needed to determine whether marijuana users have a higher incidence of such diseases, as infections, tumors, allergies, and autoimmune diseases. Studies on resistance to bacterial and viral infection are clearly needed and should involve the collaboration of immunologists, infectious disease specialists, oncologists, and pharmacologists.
Marijuana Smoke
Tobacco is the predominant cause of such lung diseases as cancer and emphysema, and marijuana smoke contains many of the components of tobacco smoke.69 Thus, it is important to consider the relationship between habitual marijuana smoking and some lung diseases.
Given a cigarette of comparable weight, as much as four times the amount of tar can be deposited in the lungs of marijuana smokers as in the lungs of tobacco smokers.162 The difference is due primarily to the differences in filtration and smoking technique between tobacco and marijuana smokers. Marijuana cigarettes usually do not have filters, and marijuana smokers typically develop a larger puff volume, inhale more deeply, and hold their breath several times longer than tobacco smokers.119 However, a marijuana cigarette smoked recreationally typically is not packed
Page 112
as tightly as a tobacco cigarette, and the smokable substance is about half that in a tobacco cigarette. In addition, tobacco smokers generally smoke considerably more cigarettes per day than do marijuana smokers.
Cellular Damage
Lymphocytes: T and B Cells.
Human studies of the effect of marijuana smoking on immune cell function are not all consistent with cannabinoid cell culture and animal studies. For example, antibody production was decreased in a group of hospitalized patients who smoked marijuana for four days (12 cigarettes/day), but the decrease was seen in only one subtype of humoral antibody (IgG), whereas two other subtypes (IgA and IgM) remained normal and one (IgE) was increased.108 In addition, T cell proliferation was normal in the blood of a group of marijuana smokers, although closer evaluation showed an increase in one subset of T cells161 and a decrease in a different subset (CD8).157 It appears that marijuana use is associated with intermittent disturbances in T and B cell function, but the magnitude is small and other measures are often normal.87
Macrophages.
Alveolar macrophages are the principal immune-effector cells in the lung and are primarily responsible for protecting the lung against infectious microorganisms, inhaled foreign substances, and tumor cells. They are increased during tissue inflammation. In a large sample of volunteers, habitual marijuana smokers had twice as many alveolar macrophages as nonsmokers, and smokers of both marijuana and tobacco had twice as many again.11 Marijuana smoking also reduced the ability of alveolar macrophages to kill fungi, such as Candida albicans;* pathogenic bacteria, such as Staphylococcus aureus; and tumor target cells. The reduction in ability to destroy fungal organisms was similar to that seen in tobacco smokers. The inability to kill pathogenic bacteria was not seen in tobacco smokers.10 Furthermore, marijuana smoking depressed production of proinflammatory cytokines, such as TNF-I and IL-6, but not of immunosuppressive cytokines.10 Cytokines are important regulators of macrophage function, so this marijuana-related decrease in inflammatory cytokine production might be a mechanism whereby marijuana smokers are less able to destroy fungal and bacterial organisms, as well as tumor cells.
The inability of alveolar macrophages from habitual marijuana smokers without apparent disease to destroy fungi, bacteria, and tumor cells
*Candida albicans is a yeast infection that is particularly prevalent among people whose immune systems are suppressed, such as in AIDS patients.
Page 113
and to release proinflammatory cytokines, suggests that marijuana might be an immunosuppressant with clinically significant effects on host defense. Therefore, the risks of smoking marijuana should be seriously weighed before recommending its use in any patient with preexisting immune deficits-including AIDS patients, cancer patients, and those receiving immunosuppressive therapies (for example, transplant or cancer patients).
Bronchial and Pulmonary Damage
Animal Studies.
A number of animal studies have revealed respiratory tract changes and diseases associated with marijuana smoking, but others have not. Extensive damage to the smaller airways, which are the major site of chronic obstructive pulmonary disease (COPD),* and acute and chronic pneumonia have been observed in various species exposed to different doses of marijuana smoke.41,42,128 In contrast, rats exposed to increasing doses of marijuana smoke for one year did not show any signs of COPD, whereas rats exposed to tobacco smoke did.67
Chronic Bronchitis and Respiratory Illness.
Results of human studies suggest that there is a greater chance of respiratory illness in people who smoke marijuana. In a survey of outpatient medical visits at a large health maintenance organization (HMO), marijuana users were more likely to seek help for respiratory illnesses than people who smoked neither marijuana or tobacco.120 However, the incidence of seeking help for respiratory illnesses was not higher in those who smoked marijuana for 10 years or more than in those who smoked for less than 10 years. One explanation for this is that people who experience respiratory symptoms are more likely to quit smoking and that people who continue to smoke constitute a set of survivors who do not develop or are indifferent to such symptoms. One limitation of this study is that no data were available on the use of cocaine, which when used with marijuana could contribute to the observed differences. Another limitation is that the survey relied on self-reporting; tobacco, alcohol, and marijuana use might have been underreported (S. Sidney, IOM workshop).
When marijuana smokers were compared with nonsmokers and tobacco smokers in a group of 446 volunteers, 15-20% of the marijuana smokers reported symptoms of chronic bronchitis, including chronic
*COPD is a slow progressive obstruction of the airways, loss of their elasticity, and loss of lung volume, characterized by chronic shortness of breath, chronic bronchitis, and reduced oxygenation of blood.
Page 114
cough and phlegm production,146 and 20-25% of the tobacco smokers reported symptoms of chronic bronchitis. Despite a marked disparity in the amount of each substance smoked per day (three or four joints of marijuana versus more than 20 cigarettes of tobacco), the difference in the percentages of tobacco smokers and marijuana smokers experiencing symptoms of chronic bronchitis was statistically insignificant.146 Similar findings were reported by Bloom and co-workers,15 who noted an additive effect of smoking both marijuana and tobacco.
Bronchial Tissue Changes.
Habitual marijuana smoking is associated with changes in the lining of the human respiratory tract. Many marijuana or tobacco smokers have increased redness (erythema) and swelling (edema) of the airway tissues and increased mucous secretions.43,56 In marijuana smokers the number and size of small blood vessels in the bronchial wall are increased, tissue edema is present, and the normal ciliated cells* lining the inner surface of the bronchial wall are largely replaced by mucous-secreting goblet cells. The damage is greater in people who smoke both marijuana and tobacco.130 Overproduction of mucus by the increased numbers of mucous-secreting cells in the presence of decreased numbers of ciliated cells tends to leave coughing as the only major mechanism to remove mucus from the airways; this might explain the relatively high proportion of marijuana smokers who complain of chronic cough and phlegm production.148
A 1998 study has shown that both marijuana and tobacco smokers have significantly more cellular and molecular abnormalities in bronchial epithelium cells than nonsmokers; these changes are associated with increased risk of cancer.12 The tobacco-only smokers in that study smoked an average of 25 cigarettes per day, whereas the marijuana-only smokers smoked an average of 21 marijuana cigarettes per week. Although the marijuana smokers smoked far fewer cigarettes, their cellular abnormalities were equivalent to or greater than those seen in tobacco smokers. This and earlier studies have shown that such abnormalities are greatest in people who smoke both marijuana and tobacco; hence, marijuana and tobacco smoke might have additive effects on airway tissue.12,43,56 Tenant150 found similar results in U.S. servicemen who suffered from respiratory symptoms and were heavy hashish smokers. (Hashish is the resin from the marijuana plant.)
Chronic Obstructive Pulmonary Disease.
In the absence of epidemiological data, indirect evidence, such as nonspecific airway hyperrespon-
*Ciliated cells have hair-like projections that function to transport mucus toward the mouth by rapid wave-like motion.
Page 115
siveness and measures of lung function, offers an indicator of the vulnerability of marijuana smokers to COPD.154 For example, the methacholine provocative challenge test, used to evaluate airway hyperresponsiveness, showed that tobacco smokers develop more airway hyperresponsiveness. But no such correlation has been shown between marijuana smoking and airway hyperresponsiveness.
There is conflicting evidence on whether regular marijuana use harms the small airways of the lungs. Bloom and co-workers found that an average of one joint smoked per day significantly impaired the function of small airways.15 But Tashkin and co-workers146 did not observe such damage among heavier marijuana users (three to four joints per day for at least 10 years), although they noted a narrowing of large central airways. Tashkin and co-workers' long-term study, which adjusted for age-related decline in lung function (associated with an increased risk for developing COPD), showed an accelerated rate of decline in tobacco smokers but not in marijuana smokers.147 Thus, the question of whether usual marijuana smoking habits are enough to cause COPD remains open.
Conclusion.
Chronic marijuana smoking might lead to acute and chronic bronchitis and extensive microscopic abnormalities in the cells lining the bronchial passageways, some of which may be premalignant. These respiratory symptoms are similar to those of tobacco smokers, and the combination of marijuana and tobacco smoking augments these effects. At the time of this writing, it had not been established whether chronic smoking marijuana causes COPD, but there is probably an association.
HIV/AIDS Patients
The relationship between marijuana smoking and the natural course of AIDS is of particular concern because HIV patients are the largest group who report using marijuana for medical purposes. Marijuana use has been linked both to increased risk of progression to AIDS in HIV-seropositive patients and to increased mortality in AIDS patients.
For unknown reasons, marijuana use is associated with increased mortality among men with AIDS but not among the general population.138 (The relative risk of AIDS mortality for current marijuana users in this 12-year study was 1.90, indicating that almost twice as many marijuana users died of AIDS as did noncurrent marijuana users.) Never-married men used twice as much marijuana as married men and accounted for 83% of the AIDS deaths in the study. The authors of the study note that, while marital status is insufficient to adjust for lifestyle factors—particularly, homosexual behavior—a substantial proportion of the never-married men with AIDS were probably homosexuals or bisexuals. That raises the pos-
Page 116
sibility that the association of marijuana use with AIDS deaths might be related to indirect factors, such as use of other drugs' or high-risk sexual behavior, both of which increase risks of infection to which AIDS patients are more susceptible. The higher mortality of AIDS patients who were current marijuana users also raises the question of whether this was because patients increased their use of marijuana at the endstages of the disease to treat their symptoms. However, the association between marijuana use and AIDS deaths was similar even when the subjects who died earliest in the first five years of this 12-year study, and who were presumably the most sick, were excluded from the analysis. In summary, it is premature to conclude what the underlying causes of this association might be.
For the general population, the mortality associated with marijuana use was lower than that associated with cigarette smoking, and tobacco smoking was not an independent risk factor in AIDS mortality. The authors of the study described above concluded that therapeutic use of marijuana did not contribute to the increased mortality among men with AIDS.
Marijuana use has been associated with a higher prevalence of HIV seropositivity in cross-sectional studies,84 but the relationship of marijuana to the progression to AIDS in HIV-seropositive patients is a reasonable question. It remains unclear whether marijuana smoking is an independent risk factor in the progression of AIDS in HIV-seropositive men. Marijuana use did not increase the risk of AIDS in HIV-seropositive men in the Multicenter AIDS Cohort Study, in which 1,795 HIV-seropositive men were studied for 18 months,84 or in the San Francisco Men's Health Study, in which 451 HIV-seropositive men were studied for six years.34 In contrast, the Sydney AIDS Project in Australia, in which 386 HIV-seropositive men were studied for 12 months,152 reported that marijuana use was associated with increased risk of progression to AIDS. The results of the Sydney study are less reliable than those of the other two studies noted; it was the shortest of the studies and, according to the 1993 definition of AIDS, many of the subjects probably already had AIDS at the beginning of the study.*
The most compelling concerns regarding marijuana smoking in HIV/ AIDS patients are the possible effects of marijuana on immunity.111 Reports of opportunistic fungal and bacterial pneumonia in AIDS patients who used marijuana suggest that marijuana smoking either suppresses the immune system33 or exposes patients to an added burden of patho-
*In 1993 the diagnosis of AIDS was expanded to include anyone with a CD4 count of less than 200. Prior to 1993 this alone would have been insufficient for a diagnosis of AIDS.
Page 117
gens.21 In summary, patients with preexisting immune deficits due to AIDS should be expected to be vulnerable to serious harm caused by smoking marijuana. The relative contribution of marijuana smoke versus THC or other cannabinoids is not known.
Carcinogenicity
The gas and tar phases of marijuana and tobacco smoke contain many of the same compounds. Furthermore, the tar phase of marijuana smoke contains higher concentrations of polycyclic aromatic hydrocarbons (PAHs), such as the carcinogen benzopyrene. The higher content of carcinogenic PAHs in marijuana tar and the greater deposition of this tar in the lung might act in conjunction to amplify the exposure of a marijuana smoker to carcinogens. For those reasons the carcinogenicity of marijuana smoke is an important concern.
It is more difficult to collect the epidemiological data necessary to establish or refute the link between marijuana smoke and cancer than that between tobacco smoke and cancer. Far fewer people smoke only marijuana than only tobacco, and marijuana smokers are more likely to underreport their smoking.
Case Studies.
Results of several case series suggest that marijuana might play a role in the development of human respiratory cancer. Reports indicate an unexpectedly large proportion of marijuana users among people with lung cancer 141, 149 and cancers of the upper aerodigestive tract—that is, the oral cavity, pharynx, larynx, and esophagus—that occur before the age of 45.36,39,149 Respiratory tract cancers associated with heavy tobacco and alcohol consumption are not usually seen before the age of 60,154 and the occurrence of such cancers in marijuana users younger than 60 suggests that long-term marijuana smoking potentiates the effects of other risk factors, such as tobacco smoking, and is a more potent risk factor than tobacco and alcohol use in the early development of respiratory cancers. Most studies lack the necessary comparison groups to calculate the isolated effect of marijuana use on cancer risk. Many marijuana smokers also smoke tobacco, so when studies lack information regarding cigarette smoking status, there is no way to separate the effects of marijuana smoke and tobacco smoke.
Epidemiological Evidence.
As of this writing, Sidney and co-workers139 had conducted the only epidemiological study to evaluate the association between marijuana use and cancer. The study included a cohort of about 65,000 men and women 15-49 years old. Marijuana users were defined as those who had used marijuana on six or more occasions. Among the 1,421
Page 118
cases of cancer in this cohort, marijuana use was associated only with an increased risk of prostate cancer in men who did not smoke tobacco. In these relatively young HMO clients, no association was found between marijuana use and other cancers, including all tobacco-related cancers, colorectal cancer, and melanoma. The major limitation associated with interpreting this study is that the development of lung cancer requires a long exposure to smoking, and most marijuana users quit before this level of exposure is achieved. In addition, marijuana use has been widespread in the United States only since the late 1960s; therefore, despite the large cohort size there might not have been a sufficient number of heavy or long-term marijuana smokers to reveal an effect.
Cellular and Molecular Studies.
In contrast with clinical studies, cellular and molecular studies have provided strong evidence that marijuana smoke is carcinogenic. Cell culture studies implicate marijuana smoke in the development of cancer. Prolonged exposure of hamster lung cell cultures to marijuana smoke led to malignant transformations,94 and exposure of human lung explants to marijuana smoke resulted in chromosomal and DNA alterations.154 The tar from marijuana smoke also induced mutations similar to those produced by tar from the same quantity of tobacco in a common bacterial assay for mutagenicity.158
Molecular studies also implicate marijuana smoke as a carcinogen. Proto-oncogenes and tumor suppressor genes are a group of genes that affect cell growth and differentiation. Normally, they code for proteins that control cellular proliferation. Once mutated or activated, they produce proteins that cause cells to multiply rapidly and out of control, and this results in tumors or cancer.* When the production of these proteins was evaluated in tissue biopsies taken from marijuana, tobacco, and marijuana plus tobacco smokers, and nonsmokers, two of them (EGFR and Ki67) were markedly higher in the marijuana smokers than in the nonsmokers and the tobacco smokers. Moreover, the effects of marijuana and tobacco were additive.131 Thus, in relatively young smokers of marijuana, particularly those who smoke both marijuana and tobacco, marijuana is implicated as a risk factor for lung cancer.
DNA alterations are known to be early events in the development of cancer, and have been observed in the lymphocytes of pregnant marijuana smokers and in those of their newborns.4 This is an important study
*Some of the genes involved in the development of lung cancer include those that encode for Ki-67 (a nuclear proliferation protein responsible for cell division), the p53 tumor suppressor (a protein that normally suppresses cell growth), and epidermal growth factor receptor (EGFR) (a receptor found on a variety of cell types, especially epithelial cells, that promotes cellular growth and proliferation when bound to epidermal growth factor).
Page 119
because the investigators were careful to exclude tobacco smokers—a problem in previous studies that cited mutagenic effects of marijuana smoke.26,53,63,142 The same investigators found similar effects in previous studies among tobacco smokers,5,6 so the effects cannot be attributed solely to THC or other cannabinoids. Although it can be determined only by experiment, it is likely that the smoke contents—other than cannabinoids—are responsible for a large part of the mutagenic effect.
Preliminary findings suggest that marijuana smoke activates cytochrome P4501A1 (CYPlAI), the enzyme that converts PAHs, such as benz[a]pyrene, into active carcinogens.99 Bronchial epithelial cells in tissue biopsies taken from marijuana smokers show more binding to CYP1A1 antibodies than do comparable cells in biopsies from nonsmokers (D. Tashkin, IOM workshop). That suggests that there is more of CYP1A1 itself in the bronchial cells of marijuana smokers, but different experimental methods will be needed to establish that possibility.
Conclusions
There is no conclusive evidence that marijuana causes cancer in humans, including cancers usually related to tobacco use. However, cellular, genetic, and human studies all suggest that marijuana smoke is an important risk factor for the development of respiratory cancer. More definitive evidence that habitual marijuana smoking leads or does not lead to respiratory cancer awaits the results of well-designed case control epidemiological studies. It has been 30 years since the initiation of widespread marijuana use among young people in our society, and such studies should now be feasible.
The following studies or activities would be useful in providing data that could more precisely define the health risks of smoking marijuana.
1. Case control studies to determine whether marijuana use is associated with an increased risk of respiratory cancer.
Despite the lack of compelling epidemiological evidence, findings from the biochemical, cellular, immunological, genetic, tissue, and animal studies cited above strongly suggest that marijuana is a risk factor for human cancer. What is required to address that hypothesis more convincingly is a population-based case control study of sufficiently large numbers of people with lung cancer and upper aerodigestive tumors (cancers of the oral cavity and pharynx, larynx, and esophagus), as well as noncancer controls, to demonstrate a statistically significant association, if one exists. Because of the long period required for induction of human carcinomas and the infrequent use of marijuana in the general U.S. population before 1966, no epidemiological studies so far have been extensive enough to measure the
Page 120
association between marijuana and cancer adequately. However, epidemiological investigation of this association is probably possible now in that some 30 years have elapsed since the start of widespread marijuana use in the United States among teenagers and young adults.
2. Molecular markers of respiratory cancer progression in marijuana smokers.
If an epidemiological association between marijuana use and risk of respiratory cancer is demonstrated, studies would be warranted to explore the presence of molecular markers—such as TP53, p16, NATZ, and GSTML—that could be predictive of genetically increased risk of carcinogenesis in marijuana users.
3. Prospective epidemiological studies of populations with HIV seropositivity or at high risk for HIV infection.*
Because HIV/AIDS patients constitute the largest group that reports smoking marijuana for medical purposes and they are particularly vulnerable to immunosuppressive effects, there is a pressing need for a better understanding of the relative risk posed by and the rewards of smoking marijuana. Such studies should include history of marijuana use in the analysis of potential risk factors for seroconversion and acquisition of opportunistic infections or progression to AIDS. The studies could be carried out in the context of any federally approved clinical trials of medical marijuana in immuno-compromised patients and should provide a follow-up period long enough to capture potential adverse events.
4. Regularized recording of marijuana use by patients.
Although marijuana is the most commonly used illicit drug, medical providers often do not question patients about marijuana use and rarely document its use.102 Among 452 Kaiser Permanente patients who reported daily or almost daily marijuana use, physicians recorded marijuana use in only 3% of their medical records (S. Sidney, IOM workshop).
5. Additional cellular, animal, and human studies to investigate the effects of THC and marijuana on immune function.
The effects studied should include effects on proinflammatory versus immunosuppressive cytokines and on the function of leukocytes that present antigen to T cells.
The question that needs to be addressed is whether THC or marijuana is a risk factor for HIV infection, for progression to more severe stages of
*A prospective study is one in which a group of subjects is identified and then studied over the course of time. Such a study allows an experimenter to balance different factors that may contribute to the study outcome. For example, age, family history, and smoking are risk factors for lung cancer. In a prospective study, these factors can be balanced to measure how much smoking increases the risk of lung cancer. A retrospective study is one in which people with a particular disease are identified and their histories are studied. Such studies are easier and less expensive to conduct, but they generally lack the explanatory power of prospective studies.
Page 121
AIDS, or for opportunistic infection among HIV-positive patients. Studies are needed to determine the effects of marijuana use on the function of alveolar macrophages. It would be important to compare the HIV infectivity and replication of alveolar macrophages harvested from habitual marijuana users with those harvested from nonusers or infrequent marijuana users. Cell culture studies could be used to compare the susceptibility of HIV-infected alveolar macrophages to additional infection with opportunistic pathogens. Similarly, further studies on cell cultures of peripheral blood mononuclear cells could be used to assess the effects of exposure to THC on HIV infectivity and replication.
Cardiovascular System
Marijuana smoke and oral THC can cause tachycardia (rapid heart beat) in humans, 20-100% above baseline.57,85 The increase in heart rate is greatest in the first 10-20 minutes after smoking and decreases sharply and steadily; depending on whether smoked marijuana or oral THC is used, this can last three or five hours, respectively.68,95 In some cases, blood pressure increases while a person is in a reclining position but decreases inordinately on standing, resulting in postural hypotension (decreased blood pressure due to changing posture from a lying or sitting position to a standing position, which can cause dizziness and faintness). In contrast with acute administration of THC, chronic oral ingestion of THC reduces heart rate in humans.13
In animals, THC decreases heart rate and blood pressure.57,156 However, most of the animal studies have been conducted in anesthetized animals, and anesthesia causes hypertension. Thus, those studies should be interpreted as reports on the effects of cannabinoids in hypertensive subjects. The results of the animal and human studies are consistent with the conclusion that cannabinoids are hypotensive at high doses in animals, as well as humans.156
Tolerance can appear after a few days of frequent daily administration (two or three doses per day) of oral THC or marijuana extract, with heart rate decreasing, reclining blood pressure falling, and postural hypotension disappearing.73 Thus, the intensity of the effects depends on frequency of use, dose, and even body position.
The cardiovascular changes have not posed a health problem for healthy, young users of marijuana or THC. However, such changes in heart rate and blood pressure could present a serious problem for older patients, especially those with coronary arterial or cerebrovascular disease. Cardiovascular diseases are the leading causes of death in the United States (coronary heart disease is first; stroke is third), so any effect of mari-
Page 122
juana use on cardiovascular disease could have a substantial impact on public health (S. Sidney, IOM workshop). The magnitude of the impact remains to be determined as chronic marijuana users from the late 1960s enter the age when coronary arterial and cerebrovascular diseases become common. Smoking marijuana is also known to decrease maximal exercise performance. That, with the increased heart rate, could theoretically induce angina (S. Sidney, IOM workshop), so, this raises the possibility that patients with symptomatic coronary artery disease should be advised not to smoke marijuana, and THC might be contraindicated in patients with restricted cardiovascular function.
Reproductive System
Animal Studies.
Marijuana and THC can inhibit many reproductive functions on a short-term basis. In both male and female animals, THC injections suppress reproductive hormones and behavior.107,59 Studies have consistently shown that injections of THC result in rapid, dose-dependent suppression of serum luteinizing hormone (LH).70 (LH is the pituitary hormone that stimulates release of the gonadal hormones, testosterone and estrogen.) Embryo implantation also appears to be inhibited by THC. But it does not necessarily follow that marijuana use will interfere with human reproduction. With few exceptions, the animal studies are based on acute treatments (single injections) or short-term treatments (THC injections given over a series of days). The results are generally observed for only several hours or in females sometimes for only one ovulatory cycle.
Acute treatments with cannabinoids—including THC, CBD, cannabinol, and anandamide—can decrease the fertilizing capacity of sea urchin sperm.135-137 The sea urchin is only a distant relative of humans, but the cellular processes that regulate fertilization are similar enough that one can expect a similar effect in humans. However, the effect of cannabinoids on the capacity of sperm to fertilize eggs is reversible and is observed at concentrations of 6-100 mM,136.,137 which are higher than those likely to be experienced by marijuana smokers. The presence of cannabinoid receptors in sperm suggests the possibility of a natural role for anandamide in modulating sperm function during fertilization. However, it remains to be determined whether smoked marijuana or oral THC taken in prescribed doses has a clinically significant effect on the fertilizing capacity of human sperm.
Exposure to THC in utero can result in long-term changes. Many in utero effects interfere with embryo implantation (see review by Wenger and co-workers159). Exposure to THC shortly before or after birth can result in impaired reproductive behavior in mice when they reach adult-
Page 123
hood: females are slower to show sexual receptivity, and males are slower to mount.107
Although THC can act directly on endocrine tissues, such as the testes and ovaries, it appears to affect reproductive physiology through its actions on the brain, somewhere other than the pituitary. Some of the effects of THC are exerted through its action on stress hormones, such as cortisol.70
Human Studies.
The few human studies are consistent with the acute animal studies: THC inhibits reproductive functions. However, studies of men and women who use marijuana regularly have yielded conflicting results and show either depression of reproductive hormones, no effect, or only a short-term effect. Overall, the results of human studies are consistent with the hypothesis that THC inhibits LH on a short-term basis but not in long-term marijuana users. In other words, long-term users develop tolerance to the inhibitory effect of THC on LH. The results in men and women are similar, with the added consideration of the menstrual cycle in women; the acute effects of THC appear to vary with cycle stage. THC appears to have little effect during the follicular phase (the phase after menses and before ovulation) and to inhibit the LH pulse during the luteal phase (the phase after ovulation and before menses).103 In brief, although there are no data on fertility itself, marijuana or THC would probably decrease human fertility—at least in the short term—for both men and women. And it is reasonable to predict that THC can interfere with early pregnancy, particularly with implantation of the embryo. Like tobacco smoke, marijuana smoke is highly likely to be harmful to fetal development and should be avoided by pregnant women and those who might become pregnant in the near future. Nevertheless, although fertility and fetal development are important concerns for many, they are unlikely to be of much concern to people with seriously debilitating or life threatening diseases. The well-documented inhibition of reproductive functions by THC is thus not a serious concern for evaluating the short-term medical use of marijuana or specific cannabinoids.
The results of studies of the relationship between prenatal marijuana exposure and birth outcome have been inconsistent (reviewed in 1995 by Cornelius and co-workers30). Except for adolescent mothers, there is little evidence that gestation is shorter in mothers who smoke marijuana.30 Several studies of women who smoked marijuana regularly during pregnancy show that they tend to give birth to lower weight babies.46,65 Mothers who smoke tobacco also give birth to lower weight babies, and the relative contributions of smoking and THC are not known from these studies.
Babies born to mothers who smoked marijuana during pregnancy
Page 124
weighed an average of 3.4 ounces less than babies born to a control group of mothers who did not smoke marijuana; there was no statistically significant difference in either gestational age or frequency of congenital abnormalities.164 Those results were based on women whose urine tests indicated recent marijuana exposure. However, when the analysis was based only on self-reports of marijuana use (without verification by urine tests), there was no difference in weight between babies born to women who reported themselves as marijuana smokers and those born to women who reported that they did not smoke marijuana. That raises an important concern about the methods used to measure the effects of marijuana smoking in any study, perhaps even more so in studies on the effects of marijuana during pregnancy, when subjects might be less likely to admit to smoking marijuana. (The study was conducted in the last trimester of pregnancy, and there was no information about the extent of marijuana use earlier in pregnancy.)
For most of these studies, much of the harm associated with marijuana use is consistent with that associated with tobacco use, and smoking is an important factor, so the contribution of cannabinoids cannot be confirmed. However, Jamaican women who use marijuana rarely smoke it; but instead prepare it as tea.37 In a study of neonates born to Jamaican women who did or did not ingest marijuana during pregnancy, there was no difference in neurobehavioral assessments made at three days after birth and at one month.38 A limitation of the study is that there was no direct measure of marijuana use. Estimates of marijuana use were based on self-reports, which might be more accurate in Jamaica than in the United States because less social stigma is associated with marijuana use in Jamaica but still are less reliable than direct measures.
Newborns of mothers who smoke either marijuana or tobacco have statistically significantly higher mutation rates than those of nonsmokers.4,5
Since 1978, the Ottawa Prenatal Prospective Study has measured the cognitive functions of children born to mothers who smoked marijuana during pregnancy.47 Children of mothers who smoked either moderately (one to six marijuana cigarettes per week) or heavily (more than six marijuana cigarettes per week) have been studied from the age of four days to 9-12 years. It is important to keep in mind that studies like this provide important data about the risks associated with marijuana use during pregnancy, but they do not establish the causes of any such association.
The children in the different marijuana exposure groups showed no lasting differences in global measures of intelligence, such as language development, reading scores, and visual or perceptual tests. Moderate cognitive deficits were detectable among these children when they were
Page 125
four days old and again at four years, but the deficits were no longer apparent at five years.
Prenatal marijuana exposure was not, however, without lasting effect. At ages 5-6 years and 9-12 years, children in the same study who were prenatally exposed to tobacco smoke scored lower on tests of language skills and cognitive functioning.48 In another study,49 50 9 to 12 year olds who were exposed to marijuana prenatally scored lower than control subjects on tasks associated with ''executive function,'' a term used by psychologists to describe a person's ability to plan, anticipate, and suppress behaviors that are incompatible with a current goal.50 It was reflected in how the mothers described their children. Mothers of the marijuana-exposed children were more likely to describe their offspring as hyperactive or impulsive than were mothers of control children. The alteration in executive function was not seen in children born to tobacco smokers. The underlying causes might be the marijuana exposure or might be more closely related to the reasons underlying the mothers' use of marijuana during pregnancy.
Mice born to dams injected with the endogenous cannabinoid, anandamide, during the last trimester of pregnancy also showed delayed effects. No effect of anandamide treatment during pregnancy was detected until the mice were adults (40 days old), at which time they showed behavioral changes that are common to the effects of other psychotropic drugs or prenatal stress.45 As with the children born to mothers who smoked marijuana, it is not known what aspect of the treatment caused the effect. The dams might have found the dose (20 mg/kg of body weight) of anandamide aversive, in which case the effect could have resulted from generalized stress, as opposed to a cannabinoid-specific effect. Either is possible. Despite the uncertainty as to the underlying causes of the effects of prenatal exposure to cannabinoid drugs, it is prudent to advise against smoking marijuana during pregnancy.
Summary and Conclusions
This chapter summarizes the harmful effects of marijuana on individual users and, to a lesser extent, on society. The harmful effects on individuals were considered from the perspective of possible medical use of marijuana and can be divided into acute and chronic effects. The vast majority of evidence on harmful effects of marijuana is based on smoked marijuana, and, except for the psychoactive effects that can be reasonably attributed to THC, it is not possible to distinguish the drug effects from the effects of inhaling smoke from burning plant material.
For most people the primary adverse effect of acute marijuana use is diminished psychomotor performance; it is inadvisable for anyone under
Page 126
the influence of marijuana to operate any equipment that might put the user or others in danger (such as driving or operating complex equipment). Most people can be expected to show impaired performance of complex tasks, and a minority experience dysphoria. People with or at risk of psychiatric disorders (including substance dependence) are particularly vulnerable to developing marijuana dependence, and marijuana use would be generally contraindicated for them. The short-term immunosuppressive effects are not well established; if they exist at all, they are probably not great enough to preclude a legitimate medical use. The acute side effects of marijuana use are within the risks tolerated for many medications.
The chronic effects of marijuana are of greater concern for medical use and fall into two categories: the effects of chronic smoking and the effects of THC. Marijuana smoke is like tobacco smoke in that it is associated with increased risk of cancer, lung damage, and poor pregnancy outcome. Smoked marijuana is unlikely to be a safe medication for any chronic medical condition. The second category is that associated with dependence on the psychoactive effects of THC. Despite past skepticism, it has been established that, although it is not common, a vulnerable subpopulation of marijuana users can develop dependence. Adolescents, particularly those with conduct disorders, and people with psychiatric disorders, or problems with substance abuse appear to be at greater risk for marijuana dependence than the general population.
As a cannabinoid drug delivery system, marijuana cigarettes are not ideal in that they deliver a variable mixture of cannabinoids and a variety of other biologically active substances, not all of which are desirable or even known. Unknown substances include possible contaminants, such as fungi or bacteria.
Finally, there is the broad social concern that sanctioning the medical use of marijuana might lead to an increase in its use among the general population. No convincing data support that concern. The existing data are consistent with the idea that this would not be a problem if the medical use of marijuana were as closely regulated as the use of other medications that have abuse potential, but we acknowledge a lack of data that directly address the question. Even if there were evidence that the medical use of marijuana would decrease the perception that it can be a harmful substance, this is beyond the scope of laws regulating the approval of therapeutic drugs. Those laws concern scientific data related to the safety and efficacy of drugs for individual use; they do not address perceptions or beliefs of the general population.
Marijuana is not a completely benign substance. It is a powerful drug with a variety of effects. However, except for the harm associated with smoking, the adverse effects of marijuana use are within the range toler-
Page 127
ated for other medications. Thus, the safety issues associated with marijuana do not preclude some medical uses. But the question remains: Is it effective? That question is covered here in two chapters: chapter 2 summarizes what has been learned about the biological activity of cannabinoids in the past 15 years through research in the basic sciences, and chapter 4 reviews clinical data on the effectiveness of marijuana and cannabinoids for the treatment of various medical conditions.
Three factors influence the safety of marijuana or cannabinoid drugs for medical use: the delivery system, the use of plant material, and the side effects of cannabinoid drugs. (1) Smoking marijuana is clearly harmful, especially in people with chronic conditions, and is not an ideal drug delivery system. (2) Plants are of uncertain composition, which renders their effects equally uncertain, so they constitute an undesirable medication. (3) The side effects of cannabinoid drugs are within the acceptable risks associated with approved medications. Indeed, some of the side effects, such as anxiety reduction and sedation, might be desirable for some patients. As with many medications, there are people for whom they would probably be contraindicated.
CONCLUSION: Present data on drug use progression neither support nor refute the suggestion that medical availability would increase drug abuse. However, this question is beyond the issues normally considered for medical uses of drugs, and it should not be a factor in the evaluation of the therapeutic potential of marijuana or cannabinoids.
CONCLUSION: A distinctive marijuana withdrawal syndrome has been identified, but it is mild and short lived. The syndrome includes restlessness, irritability, mild agitation, insomnia, sleep EEG disturbance, nausea, and cramping.
CONCLUSION: Numerous studies suggest that marijuana smoke is an important risk factor in the development of respiratory disease.
RECOMMENDATION: Studies to define the individual health risks of smoking marijuana should be conducted, particularly among populations in which marijuana use is prevalent.
Page 128
References
1. Adams IB, Martin BR. 1996. Cannabis: Pharmacology and toxicology in animals and humans. Addiction 91:1585-1614.
2. American Psychiatric Association. 1987. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R). 3rd ed., revised. Washington, DC: American Psychiatric Association.
3. American Psychiatric Association. 1994. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed. Washington, DC: American Psychiatric Association.
4. Ammenheuser MM, Berenson AB, Babiak AE, Singleton CR, Whorton Jr EB. 1998. Frequencies of hprt mutant lymphocytes in marijuana-smoking mothers and their newborns. Mutation Research 403:55-64.
5. Ammenheuser MM, Berenson NJ, Stiglich EB, Whorton Jr EB, Ward Jr JB. 1994. Elevated frequencies of hprt mutant lymphocytes in cigarette-smoking mothers and their newborns. Mutation Research 304:285-294.
6. Ammenheuser MM, Hastings DA, Whorton Jr EB, Ward Jr JB. 1997. Frequencies of hprt mutant lymphocytes in smokers, non-smokers, and former smokers. Environmental and Molecular Mutagenesis 30:131-138.
7. Anthenelli RM, Schuckit MA. 1992. Genetics. In: Lowinson JH, Ruiz P, Millman RB, Editors, Substance Abuse: A Comprehensive Textbook. 2nd ed. Baltimore, MD: Williams & Wilkins. Pp. 39-50.
8. Anthony JC, Warner LA, Kessler RC. 1994. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology 2:244-268.
9. Bailey SL, Flewelling RL, Rachal JV. 1992. Predicting continued use of marijuana among adolescents: The relative influence of drug-specific and social context factors. Journal of Health and Social Behavior 33:51-66.
10. Baldwin GC, Tashkin DP, Buckely DM, Park AN, Dubinett SM, Roth MD. 1997. Marijuana and cocaine impair alveolar macrophage function and cytokine production. American Journal of Respiratory and Critical Care Medicine 156:1606-1613.
11. Barbers RG, Gong HJ, Tashkin DP, Oishi J, Wallace J M. 1987. Differential examination of bronchoalveolar lavage cells in tobacco cigarette and marijuana smokers. American Review of Respiratory Disease 135:1271-1275.
12. Barsky SH, Roth MD, Kleerup EC, Simmons M, Tashkin DP. 1998. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. Journal of the National Cancer Institute 90:1198-1205.
13. Benowitz NL, Jones RT. 1975. Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clinical Pharmacology and Therapeutics 18:287-297.
14. Block RI, Ghoneim MM. 1993. Effects of chronic marijuana use on human cognition. Psychopharmacology 110:219-228.
15. Bloom JW, Kaltenborn WT, Paoletti P, Camilli A, Lebowitz MS. 1987. Respiratory effects of non-tobacco cigarettes. British Medical Journal 295:516-518.
16. Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ. 1995. Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metabolism and Disposition (United States) 23:825-831.
17. British Medical Association. 1997. Therapeutic Uses of Cannabis. Amsterdam, The Netherlands: Harwood Academic Publishers.
18. Brook JS, Cohen P, Brook DW. 1998. Longitudinal study of co-occurring psychiatric disorders and substance use. Journal of the American Academy of Child and Adolescent Psychiatry 37:322-330.
Page 129
19. Budney AJ, Radonovich KJ, Higgins ST, Wong CJ. 1998. Adults seeking treatment for marijuana dependence: A comparison with cocaine-dependent treatment seekers. Experimental and Clinical Psychopharmacology 6:419-426.
20. Bums LA, Meade BJ, Munson AE. 1996. Toxic responses of the immune system. In: CD Klaassen, MO Amdur, and J Doul, Editors, Casarett and Doul, Toxicology: The Basic Science of Poisons. 5th ed. New York: McGraw-Hill. Pp. 355-402.
21. Caiaffa WT, Vlahov D, Graham N, Astemborski J, Solomon L, Nelson KE, Mufoz. 1994. Drug smoking, Pneumocystis carinii pneumonia, and immunosuppression increase risk of bacterial pneumonia in human immunodeficiency virus-seropositive injection drug users. American Journal of Respiratory and Critical Care Medicine 150:14931498.
22. Campbell AM, Evans M, Thompson JL, Williams MR. 1971. Cerebral atrophy in young cannabis smokers. Lancet 2:1219-1224.
23. Chait LD, Pierri J. 1992. Effects of smoked marijuana on human performance: A critical review. In: L Murphy and A Bartke, Editors, Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Boca Raton, FL: CRC Press. Pp. 387-424.
24. Charalambous A, Marciniak G, Shiue CY, Dewey SL, Schlyer DJ, Wolf AP, Makriyannis A. 1991. PET studies in the primate brain and biodistribution in mice using (-)-5'-18F-delta 8-THC. Pharmacology Biochemistry and Behavior 40:503-507.
25. Chen K, Kandel DB, Davies M. 1997. Relationships between frequency and quantity of marijuana use and last year proxy dependence among adolescents and adults in the United States. Drug and Alcohol Dependence 46:53-67.
26. Chiesara E, Rizzi R. 1983. Chromosome damage in heroin-marijuana and marijuana addicts. Archives of Toxicology Supplement 6:128-130.
27. Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ. 1994. Pain and its treatment in outpatients with metastatic cancer. New England Journal of Medicine 330:592-596.
28. Co BT, Goodwin DW, Gado M, Mikhael M, Hill SY. 1977. Absence of cerebral atrophy in chronic cannabis users by computerized transaxial tomography. Journal of the American Medical Association 237:1229-1230.
29. Cohen MJ, Rickles Jr WH. 1974. Performance on a verbal learning task by subjects of heavy past marijuana usage. Psychopharmacologia 37:323-330.
30. Cornelius MD, Taylor PM, Geva D, Day NL. 1995. Prenatal tobacco and marijuana use among adolescents: Effects on offspring gestational age, growth, and morphology. Pediatrics 95(5):738-743.
31. Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK. 1998. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug and Alcohol Dependence 50:27-37.
32. Crowley TJ, Rhine MW. 1985. The substance use disorders. In: Simons RC, Editor, Understanding Human Behavior in Health and Illness. 3rd ed. Baltimore, MD: Williams & Wilkins. Pp. 730-746.
33. Denning DW, Follansbee SE, Scolaro M, et al. 1991. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. New England Journal of Medicine 324:654-662.
34. Di Franco MJ, Sheppard HW, Hunter DJ, Tosteson TD, Ascher MS. 1996. The lack of association of marijuana and other recreational drugs with progression to AIDS in the San Francisco Men's Health Study. Annals of Epidemiology 6:283-289.
35. Dixon L, Haas G, Weiden PJ, Sweeney J, Frances AJ. 1991. Drug abuse in schizophrenic patients: Clinical correlates and reasons for use. American Journal of Psychiatry 148:224-230.
36. Donald PJ. 1991. Advanced malignancy in the young marijuana smoker. Advances in Experimental Medicine and Biology 288:33-56.
Page 130
37. Dreher M. 1987. The evolution of a roots daughter. Journal of Psychoactive Drugs 19:165-170.
38. Dreher MC, Nugent K, Hudgins R. 1994. Prenatal marijuana exposure and neonatal outcomes in Jamaica: An ethnographic study. Pediatrics 93:254-260.
39. Endicott JN, Skipper P, Hemandez L. 1993. Marijuana and head and neck cancer. In: Friedman et al., Editors, Drugs of Abuse, Immunity and AIDS. New York: Plenum Press. Pp. 107-113.
40. Fehr K, Kalant H. 1981. Cannabis and health hazards. Proceedings of all ARF/WHO Scientific Meeting on Adverse Health and Behavioral Consequences of Cannabis Use. Fehr K, Kalant H, Editors, Toronto, Canada: Addiction Research Foundation.
41. Fleischman RW, Baker JR, Rosenkrantz H. 1979. Pulmonary pathologic changes in rats exposed to marijuana smoke for one year. Toxicology and Applied Pllarnacology 47:557-566.
42. Fligiel SE, Beals TF, Tashkin DP, Paule MG, Scallet AC, Ali SF, Bailey JR, Slikker WJ. 1991. Marijuana exposure and pulmonary alterations in primates. Pharmacology, Biochemistry and Behavior 40:637-642.
43. Fligiel SEG, Roth MD, Kleerup EC, Barsky SH, Simmons M, Tashkin DP. 1997. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Cllest 112:319-326.
44. Foley K. 1997. Competent care for the dying instead of physician-assisted suicide. Newt England Journal of Medicine 336:54-58.
45. Fride E, Mechoulam R. 1996. Ontogenetic development of the response to anandamide and delta 9-tetrahydrocannabinol in mice. Brain Research, Developmental Brain Research 95:131-134.
46. Fried PA. 1982. Marihuana use by pregnant women and effects on offspring: An update. Neurobehavioral Toxicology and Teratology 4:451-454.
47. Fried PA. 1995. The Ottawa Prenatal Prospective Study (OPPS): Methological issues and findings-it's easy to throw the baby out with the bath water. Life Sciences 56:2159-2168.
48. Fried PA, O'Connell CM, Watkinson B. 1992. 60- and 72-Month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: Cognitive and language assessment. Developmental and Behavioral Pediatrics 13:383-391.
49. Fried PA, Watkinson BSLS. 1997. Reading and language in 9- to 12-year-olds prenatally exposed to cigarettes and marijuana. Neurotoxicology and Teratology 19:171-183.
50. Fried PA, Watkinson B, Gray R. 1998. Differential effects on cognitive functioning in 9- to 12-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology 20:293-306.
51. Gardner EL. 1992. Brain reward mechanisms. In: Lowinson JH, Ruiz P, Millman RB, Editors, Substance Abuse: A Comprehentsive Textbook. 2nd ed. Baltimore, MD: Williams and Wilkins. Pp. 70-99.
52. Georgotas A, Zeidenberg P. 1979. Observations on the effects of four weeks of heavy marijuana smoking on group interaction and individual behavior. Comprehensive Psychiatry 20:427-432.
53. Gilmore DG, Blood AD, Lele KP, Robbins ES, Maximillian C. 1971. Chromosomal aberrations in users of psychoactive drugs. Archives of General Psychiatry 24:268-272.
54. Goldstein A. 1994. Tolerance and Dependence. In: Goldstein A, Editor, Addiction: From Biology to Drug Policy. New York: W.H. Freeman. Pp. 73-84.
55. Golub A, Johnson BD. 1994. The shifting importance of alcohol and marijuana as gateway substances among serious drug abusers. Journal of Studies on Alcohol 55:607614.
Page 131
56. Gong HJ, Fligiel S, Tashkin DP, Barbers RG. 1987. Tracheobronchial changes in habitual, heavy smokers of marijuana with and without tobacco. American Review of Respiratory Disease 136:142-149.
57. Graham JDP. 1986. The cardiovascular action of cannabinoids. In: Mechoulam R, Editor, Cannabinoids as Therapeutic Agents. Boca Raton, FL: CRC Press. Pp. 159-166.
58. Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. 1996. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences, USA 93:12040-12045.
59. Hall W, Solowij N. 1998. Adverse effects of cannabis. The Lancet 352:1611-1616.
60. Hall W, Solowij N, Lemon J. 1994. The Health and Psychological Consequences of Cannabis Use. Department of Human Services and Health, Monograph Series, No. 25. Canberra, Australia: Australian Government Publishing Service.
61. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. 1999. Abstinence symptoms following oral THC administration to humans. Psychopharnacology 141:385-394.
62. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. 1999. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology 141:395-404.
62 a. Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. 1990. Acute and residual effects of marijuana: Profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacology, Biochemistry, and Behavior 37:561-565.
63. Herha J, Obe G. 1974. Chromosomal damage in chronical users of cannabis: In vivo investigation with two-day lymphocyte cultures. Pharmakopsychiatric 7:328-337.
64. Herring AC, Koh WS, Kaminski NE. 1998. Inhibition of the cyclic AMP signaling cascade and nuclear factor binding to CRE and kappa B elements by cannabinol, a minimally CNS-active cannabinoid. Biochemical Pharmacology 55:1013-1023.
65. Hingson R, Alpert JJ, Day N, Dooling E, Kayn H, Morelock S, Oppenheimer E, Zuckerman B. 1982. Effects of maternal drinking and marihuana use on fetal growth and development. Pediatrics 70:539-546.
66. Hollister LE. 1986. Health aspects of cannabis. Pharmacological Reviews 38:1-20.
67. Huber GL, Mahajan VK. 1988. The comparative response of the lung to marihuana or tobacco smoke inhalation. In: Chesher G, Consroe P, Musty R, Editors, Marijuana: An International Research Report: Proceedings of Melbourne Symposium on Cannabis 2-4 September, 1987. National Campaign Against Drug Abuse Monograph Series No. 7 Edition. Canberra: Australian Government Publishing Service. Pp. 19-24.
68. Huestis MA, Sampson AH, Holicky BJ, Henningfield JE, Cone EJ. 1992. Characterization of the absorption phase of marijuana smoking. Clinical Pharmacology and Tlerapeutics 52:31-41.
69. IOM (Institute of Medicine). 1982. Marijuana and Health. Washington, DC: National Academy Press.
70. Jackson AL, Murphy LL. 1997. Role of the hypothalamic-pituitary-adrenal axis in the suppression of luteinizing hormone release by delta-9-tetrahydrocannabinol. Neuroendocrinology 65:446-452.
71. Johnson V. 1995. The relationship between parent and offspring comorbid disorders. Journal of Substance Abuse 7:267-280.
72. Johnston LD, O'Malley PM, Bachman JG. 1989. Marijuana decriminalization: The impact on youth, 1975-1980. Journal of Public Health Policy 10:456.
73. Jones RT, Benowitz NL, Herning RI. 1981. Clinical relevance of cannabis tolerance and dependence. Journal of Clinical Pharmacology 21:143S-152S.
74. Jones RT, Benowitz N, Bachman J. 1976. Clinical studies of tolerance and dependence. Annals of the New York Academy of Sciences 282:221-239.
75. Kaklamani E, Trichopoulos D, Koutselinis A, Drouga M, Karalis D. 1978. Hashis smoking and T-lymphocytes. Archives of Toxicology 40:97-101.
Page 132
76. Kandel DB. 1992. Epidemiological trends and implications for understanding the nature of addiction. In: O'Brien CP, Jaffe JH, Editors, Addictive Studies. New York: Raven Press, Ltd. Pp. 23-40.
77. Kandel DB, Chen KWLA, Kessler R, Grant B. 1997. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug and Alcohol Dependence 44:11-29.
78. Kandel DB, Davies M. 1992. Progression to regular marijuana involvement: phenomenology and risk factors for near-daily use. In: Glantz M, Pickens R, Editors, Vulnerability to Drug Abuse. Washington, DC: American Psychological Association. Pp. 211253.
79. Kandel DB, Johnson JG, Bird HR, Canino G, Goodman SH, Lahey BB, Regier DA, Schwab-Stone M. 1997. Psychiatric disorders associated with substance use among children and adolescents: Findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. Journal of Abnormal Child Psychology 25:121-132.
80. Kandel DB, Raveis VH. 1989. Cessation of illicit drug use in young adulthood. Archives of General Psychiatry 46:109-116.
81. Kandel DB, Yamaguchi K. 1993. From beer to crack: developmental patterns of drug involvement. American Journal of Public Health 83:851-855.
82. Kandel DB, Yamaguchi K, Chen K. 1992. Stages of progression in drug involvement from adolescence to adulthood: Further evidence for the gateway theory. Journal of Studies in Alcohol 53:447457.
83. Kaplan HB, Martin SS, Johnson RJ, Robbins CA. 1996. Escalation of marijuana use: Application of a general theory of deviant behavior. Journal of Health and Social Behavior 27:44-61.
84. Kaslow RA, Blackwelder WC, Ostrow DG, et al. 1989. No evidence for a role of alcohol or other psychoactive drugs in accelerating immunodeficiency in HIV-1 positive individuals: A report for the multicenter AIDS cohort study. Journal of the American Medical Association 261:3424-3429.
85. Kelly TH, Foltin RW, Fischman MW. 1993. Effects of smoked marijuana on heart rate, drug ratings and task performance by humans. Behavioural Pharmacology 4:167-178.
86. Kendler KS, Prescott CA. 1998. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journal of Psychiatry 155:1016.
87. Klein TW, Friedman H, Specter SC. 1998. Marijuana, immunity and infection. Journal of Neuroimmunology 83:102-115.
88. Koob GF, Le Moal M. 1997. Drug abuse: Hedonic homeostatic dysregulation. Science 278:52-58.
89. Kuehnle J, Mendelson JH, Davis KR, New PF. 1977. Computed tomographic examination of heavy marihuana smokers. Journal of the American Medical Association 237:1231-1232.
90. Labouvie E, Bates ME, Pandina RJ. 1997. Age of first use: Its reliability and predictive utility. Journal of Studies on Alcohol 58:638-643.
91. Lau RJ, Tubergen DG, Barr MJ, Domino EF, Benowitz N, Jones RT. 1976. Phytohemagglutinin-induced lymphocyte transformation in humans receiving delta-9-tetrahydrocannabinol. Science 192:805-807.
92. Lepore M, Liu X, Savage V, Matalon D, Gardner EL. 1996. Genetic differences in delta 9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sciences 58:365-372.
Page 133
93. Lepore M, Vorel SR, Lowinson J, Gardner EL. 1995. Conditioned place preference induced by delta 9-tetrahydrocannabinol: Comparison with cocaine, morphine, and food reward. Life Sciences 56:2073-2080.
94. Leuchtenberger C, Leuchtenberger R. 1976. Cytological and cytochemical studies of the effects of fresh marijuana cigarette smoke on growth and DNA metabolism of animal and human lung cultures. In: Braude MC, Szara S, Editors, The Pharmacology of Marijuana. New York: Raven Press.
95. Lindgren JE, Ohlsson A, Agurell S, Hollister LE, Gillespie H. 1981. Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychopharmacology (Berlin) 74:208-212.
96. Linzen DH, Dingemans PM, Lenior ME. 1994. Cannabis abuse and the course of recent-onset schizophrenic disorders. Archives of General Psychiatry 51:273-279.
97. Lyons MJ., Toomey R, Meyer JM, Green Al, Eisen SA, Goldberg J, True WR, Tsuang MT. 1997. How do genes influence marijuana use? The role of subjective effects. Addiction 92:409-417.
98. MacCoun R, Reuter P. 1997. Interpreting Dutch cannabis policy: Reasoning by analogy in the legalization debate. Science 278:47-52.
99. Marques-Magallanes JA, Tashkin DP, Serafian T, Stegeman J, Roth MD. 1997. In vivo and in vitro activation of cytochrome P4501A1 by marijuana smoke. Synlposiunm on the Cannabinoids of the International Cannabinoid Research Society Program and Abstracts. Stone Mountain, GA, June 1997.
100. Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. 1998. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience 85:327-330.
101. Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE. 1992. Regional cerebral blood flow after marijuana smoking. Journal of Cerebral Blood Flow and Metabolism 12:750-758.
102. Mathre ML. 1998. A survey on disclosure of marijuana use to health care professionals. Journal of Psychoactive Drugs 20:117-120.
103. Mendelson JH, Cristofaro P, Ellingboe J, Benedikt R, Mello NK. 1985. Acute effects of marihuana on luteinizing hormone in menopausal women. Pharmacology, Biochemlistry, and Behavior 23:765-768.
104. Meyer RE, Pillard RC, Shapiro LM, Mirin SM. 1971. Administration of marijuana to heavy and casual marijuana users. American Journal of Psychiatry 128:198-204.
105. Model KE. 1993. The effect of marijuana decriminalization on hospital emergency room drug episodes: 1975-1978. Journal of the American Statistical Association 88:737747.
106. Moulin DE, lezzi A, Amireh R, Sharpe WKJ, Boyd D, Merskey H. 1996. Randomised trial of oral morphine for chronic non-cancer pain. Lancet 347:143-147.
107. Murphy LL, Gher J, Szary A. 1995. Effects of prenatal exposure to delta-9-tetrahydrocannabinol on reproductive, endocrine and immune parameters of male and female rat offspring. Endocrine 3:875-879.
108. Nahas GG, Osserman EF. 1991. Altered serum immunoglobulin concentration in chronic marijuana smokers. Advances in Experimental Medicine and Biology 288:25-32.
109. Nesse RM, Berridge KC. 1997. Psychoactive drug use in evolutionary perspective. Science 278:63-66.
110. Nestler EJ, Aghajanian GK. 1997. Molecular and cellular basis of addiction. Science 278:58-63.
111. Newell GR, Mansell PW, Wilson MB, Lynch HK, Spitz MR, Hersh EM. 1985. Risk factor analysis among men referred for possible acquired immune deficiency syndrome. Preventive Medicine 14:81-91.
Page 134
112. NIH (National Institutes of Health). 1997. Workshop on the Medical Utility of Marijuana. Report to the Director, National Institutes of Health by the Ad Hoc Group of Experts. Bethesda, MD, February 19-20, 1997. Bethesda, MD: National Institutes of Health.
113. O'Brien CP. 1996. Drug addiction and drug abuse. In: Harmon JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, Editor, Goodman and Gillan's The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw-Hill. Pp. 557-577.
114. O'Brien CP. 1996. Recent developments in the pharmacotherapy of substance abuse. Journal of Consulting and Clinical Psychology 64:677-686.
115. O'Brien CP. 1997. A range of research-based pharmacotherapies for addiction. Science 278:66-70.
116. O'Leary DS, Andreasen NC, Hurtig RR, Torres IJ, Flashman LA, Kesler ML, Arndt SV, Cizadlo TJ, Ponto LLB, Watkins GL, Hichwa RD. 1997. Auditory and visual attention assessed with PET. Human Brain Mapping 5:422-436.
117. O'Leary D, Block RI, Flaum M, Boles Ponto LL, Watkins GL, Hichwa RD. 1998. Acute marijuana effects on rCBF and cognition: A PET study. Abstracts-Society for Neuroscience: 28th Annual Meeting. Los Angeles, November 7-12, 1998. Washington, DC: Society for Neuroscience.
118. Ohlsson A, Lindgren J-E, Wahlen A, Agurell S, Hollister LE, Gillespie HK. 1980. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical Pharmacology and Therapeutics 28:409416.
119. Peterson RC. 1979. Importance of inhalation patterns in determining effects of marijuana use. Lancet 1:727-728.
120. Polen MR, Sidney S, Tekawa IS, Sadler M, Friedman D. 1993. Health care use by frequent marijuana smokers who do not smoke tobacco. The Western Journal of Medicine 158:596-601.
121. Pope HG, Gruber AJ, Yurgelun-Todd D. 1995. The residual neuropsychological effects of cannabis: The current status of research. Drug and Alcohol Dependence 38:2534.
122. Pope HG, Yurgelun-Todd D. 1996. The residual cognitive effects of heavy marijuana use in college students. Journal of the American Medical Association 275:521-527.
123. Rachelefsky G, Opelz G, Mickey M, Lessin P, Kiuchi M, Silverstein M, Stiehm E. 1976. Intact humoral and cell-mediated immunity in chronic marijuana smoking. Journal of Allergy and Clinical Immunology 58:483-490.
124. Rickles Jr WH, Cohen MJ, Whitaker CA, Mcintyre KE. 1973. Marijuana-induced statedependent verbal learning. Psycliopharmacologia 30:349-354.
125. Robins LN. 1980. The natural history of drug abuse. Acta Psychiiatrica Scandimnavica Supplement 284:7-20.
126. Robins LN. 1998. The intimate connection between antisocial personality and substance abuse. Social Psychiatry and Psychiatric Epidemiiology 33:393-399.
127. Robins LN, McEvoy LT. 1990. Conduct problems as predictors of substance abuse. In: Robins L, Rutter M, Editors, Straigllt and Devious Pathways from Childhood to Adulthood. New York: Cambridge University Press. Pp. 182-204.
128. Rosenkrantz H, Fleischman RW. 1979. Effects of cannabis on lung. In: Nahas GG, Payton WDH, Editors, Marijuanta: Biological Effects. Oxford, England: Pergamon Press. Pp. 279-299.
129. Rosenzweig MR, Leiman AL, Breedlove SM. 1996. Biological Psycllology. Sunderland, MA: Sinauer Associates.
Page 135
130. Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons MS, Tashkin DP. 1998. Airway inflammation in young marijuana and tobacco smokers. American Journal of Respiratory and Critical Care Medicine 157:1-9.
131. Roth MD, Kleerup EC, Arora A, Barsky SH, Tashkin DP. 1996. Endobronchial injury in young tobacco and marijuana smokers as evaluated by visual, pathologic and molecular criteria. American Review of Respiratory and Critical Care Medicine 153 (part 2):100A.
132. SAMHSA (Substance Abuse and Mental Health Services Administration). 1998. National Household Survey on Drug Abuse: Population Estimates 1997. DHHS Pub. No. (SMA) 98-3250. Rockville, MD: SAMHSA, Office of Applied Studies.
133. Scheier LM, Bovtin GJ. 1996. Cognitive effects of marijuana. Journal of the American Medical Association 275:JAMA Letters.
134. Schneier FR, Siris SG. 1987. A review of psychoactive substance use and abuse in schizophrenia: Patterns of drug choice. Journal of Nervous and Mental Disease 175:641652.
135. Schuel H, Berkery D, Schuel R, Chang MC, Zimmerman AM, Zimmerman S. 1991. Reduction of the fertilizing capacity of sea urchin sperm by cannabinoids derived from marihuana. I. Inhibition of the acrosome reaction induced by egg jelly. Molecular Reproduction and Development 29:51-59.
136. Schuel H, Chang MC, Berkery D, Schuel R, Zimmerman AM, Zimmerman S. 1991. Cannabinoids inhibit fertilization in sea urchins by reducing the fertilizing capacity of sperm. Pharmacology, Biochemistry, and Behavior 40:609-615.
137. Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S. 1994. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor, agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proceedings of the National Academy of Sciences 91:7678-7682.
138. Sidney S, Beck JE, Tekawa IS, Quesenberry CP Jr, Friedman GD. 1997a. Marijuana use and mortality. American Journal of Public Health 87:585-590.
139. Sidney S, Quesenberry CP Jr, Friedman GD, Tekawa IS. 1997b. Marijuana use and cancer incidence (California, United States). Cancer Cause and Control 8:722-728.
140. Solowij N. 1995. Do cognitive impairments recover following cessation of cannabis use? Life Sciences 56:2119-2126.
141. Sridhar JS, Raub WA, Weatherby NL, et al. 1994. Possible role of marijuana smoking as a carcinogen in the development of lung cancer at a young age. Journal of Psychoactive Drugs 26:285-288.
142. Stenchever MA, Kunysz TJ, Allen MA. 1974. Chromosome breakage in users of marijuana. American Journal of Gynecology 118:106-113.
143. Stephens RS, Roffman RA, Simpson EE. 1993. Adult marijuana users seeking treatment. Journal of Consulting and Clinical Psychology 61:1100-1104.
144. Tanda G, Pontieri FE, Di Chiara G. 1997. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common 1l opioid receptor mechanism. Science 276:2048-2049.
145. Tart CT. 1971. On Being Stoned: A Psychological Study of Marijuana Intoxication. Palo Alto, CA: Science and Behavior Books.
146. Tashkin DP, Coulson AH, Clark VA, Simmons M, Bourque LB, Duann S, Spivey GH, Gong H. 1987. Respiratory symptoms and lung function in habitual, heavy smokers of marijuana alone, smokers of marijuana and tobacco, smokers of tobacco alone, and nonsmokers. American Review of Respiratory Disease 135:209-216.
147. Tashkin DP, Simmons MS, Sherrill DL, Coulson AH. 1997. Heavy habitual marijuana smoking does not cause an accelerated decline in FEV1 with age. American Journal of Respiratory and Critical Care Medicine 155:141-148.
Page 136
148. Tashkin E. 1999. Effects of marijuana on the lung and its defenses against infection and cancer. School Psychology International 20:23-37.
149. Taylor FM. 1988. Marijuana as a potential respiratory tract carcinogen: A retrospective analysis. Southern Medical Journal 81:1213-1216.
150. Tennant FS. 1980. Histopathologic and clinical abnormalities of the respiratory system in chronic hashish smokers. Substance and Alcohol Actions/Misuse 1:93-100.
151. Thornicroft G. 1990. Cannabis and psychosis: Is there epidemiological evidence for an association? British Journal of Psychiatry 157:25-33.
152. Tindall B, Cooper D, Donovan B, Barnes T, Philpot C, Gold J, Penny R. 1988. The Sydney AIDS Project: Development of acquired immunodeficiency syndrome in a group of HIV seropositive homosexual men. Australian and New Zealand Journal of Medicine 18:8-15.
153. Tsou K, Patrick SL, Walker JM. 1995. Physical withdrawal in rats tolerant to delta-9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. European Journal of Pharmacology 280:R13-R15.
154. Van Hoozen BE, Cross CE. 1997. Respiratory tract effects of marijuana. Clinical Reviews in Allergy and Immunology 15:243-269.
155. Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. 1996. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Research 67:29-38.
156. Wagner JA, Varga K, Kunos G. 1998. Cardiovascular actions of cannabinoids and their generation during shock. Journal of Molecular Medicine 76:824-836.
157. Wallace JM, Tashkin DP, Oishi JS, Barbers RG. 1988. Peripheral blood lymphocyte subpopulations and mitogen responsiveness in tobacco and marijuana smokers. Journal of Psychoactive Drugs 20:9-14.
158. Wehner FC, Van Rensburg SJ, Theil PF. 1980. Mutagenicity of marijuana and transkei tobacco smoke condensates in the salmonella/microsome assay. Mutation Research 77:135-142.
159. Wenger T, Croix D, Tramu G, Leonardelli J. 1992. Effects of delta-9-tetrahydrocannabinol on pregnancy, puberty, and the neuroendocrine system. In: Murphy L, Bartke A, Editors, Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Boca Raton, FL: CRC Press.
160. White SC, Brin SC, Janicki BW. 1975. Mitogen-induced blastogenic responses of lymphocytes from marijuana smokers. Science 188:71-72.
161. Whitfield RM, Bechtel LM, Starich GH. 1997. The impact of ethanol and marinol/ marijuana usage on HIV+/AIDS patients undergoing azidothymidine, azidothymidine/dideoxycytidine, ordideoxyinosine therapy. Alcoholism Clinical and Experimental Research 21:122-127.
162. Wu TC, Tashkin DP, Djahed B, Rose JE. 1988. Pulmonary hazards of smoking marijuana as compared with tobacco. New England Journal of Medicine 318:347-351.
163. Yesavage JA, Leirer VO, Denari M, Hollister LE. 1985. Carry-over effects of marijuana intoxication on aircraft pilot performance: A preliminary report. American Journal of Psychiatry 142:1325-1329.
164. Zuckerman B, Frank DA, Hingson R, Amaro H, Levenson S, Kayne J, Parker S, Vinci R, Aboagye K, Fried L, Cabral J, Timperi R, Bauchner H. 1989. Effects of maternal marijuana and cocaine use on fetal growth. New England Journal of Medicine 320:762768.






















































