2
Screening Procedure
In response to the increased awareness of concerns about the safety and efficacy of the large tank in-tank precipitation (ITP) process selected for the removal of cesium from the tank waste supernate (U.S. General Accounting Office, 1999; Defense Nuclear Facilities Safety Board, 1996), Westinghouse Savannah River Company (WSRC) undertook a study to identify and evaluate alternatives to ITP. The Savannah River Site (SRS) and WSRC established a procedure1 whereby the cesium separations literature and patents were searched, along with evaluation by panels of experts of cesium removal processes, to select alternative options to replace the current, unacceptable ITP process for high-level waste (HLW) salt removal, treatment, and disposal.
BACKGROUND
The procedure was initiated by a comprehensive literature and patent search. Subsequently, through what WSRC called “Phases I, II, III, and IV” (Figure 2.1), 144 cesium removal process alternatives were identified and evaluated by panels of experts in a sequence of steps that reduced the number to four and finally to one recommended alternative and one backup technology. The selection procedure that identified the 144 process alternatives and subsequently winnowed down the number of alternatives to one recommended and one backup process was based on the expert judgment of representatives from various U.S. Department of Energy (DOE) laboratories and consultants. Although the interim narrowing to one main process and a
|
1 |
The term procedure is used herein to describe the methodology of evaluation, review, selection, ranking, etc., of operations related to the separation of cesium and other elements and the production of waste forms; the termprocess is used herein to refer to the operations, usually chemical engineering related, of carrying out separations and production of waste forms. |
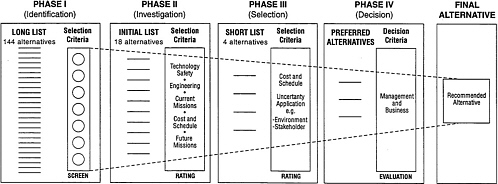
FIGURE 2.1 Schematic outline of the screening procedure used to identify a recommended alternative for cesium removal. SOURCE: Westinghouse Savannah River Company.
backup has been conducted, the committee understands that the current plans by WSRC appear to include an evaluation of several other highly ranked process alternatives.
Literature and Patent Search
The initial literature and patent search, conducted by WSRC (Poirier, Hunt, and Carlson, 1998), interrogated 11 large databases of scientific, engineering, and patent information:
-
Chemical Abstract Services
-
National Technical Information System (NTIS)
-
American Geological Institute's GeoRef Database
-
Nuclear Science Abstract
-
Engineering Information, Inc.
-
Inside Conferences
-
Energy Index
-
Information Services in Physics, Electronics, and Computing (INSPEC)
-
Analytical Abstracts
-
World Patents
-
The U.S. Patent and Trademark Office Homepage
These databases were integrated using seven selected key words containing various phrases related to cesium separations:
-
cesium removal
-
cesium separation
-
cesium extraction
-
cesium precipitation
-
cesium ion exchange
-
cesium filtration
-
cesium concentration
In conducting the search, WSRC focused on identification of separation methods as described by the chemistry of the processes. The resulting assemblage of more than 1,700 citations was grouped into 16 basic process technologies that encompass about 440 varieties of these technologies (Table 2.1).
A ranking of the 16 process technologies uncovered by the literature and patent search revealed that ion exchange, precipitation, adsorption, extraction, filtration, and biological process were the most prominent technologies (over 95 percent) found in terms of numbers of varieties. The largest number of references to varieties of ion exchange was found for crystalline sodium titanate, resorcinol formaldehyde, hexacyanoferrates, and Duolites. Prominent precipitation processes identified by the search included the use
TABLE 2.1 Results of the Literature and Patent Search
|
|
NOTE: Over 1,700 literature and patent references were identified and grouped into 16 “process technologies.” |
of tetraphenylborate and ferrocyanides/ferrates. Clays, zeolites, and ferrocyanides/ferrates were the most numerous adsorption chemicals cited in the literature. The topic of solvent extraction yielded extensive references to the use of crown ethers and calixarenes that have not been used at a plant-size scale. This search also yielded a reference to dicarbollide that has been used at a plant-size scale.
SOURCE: Poirier, Hunt, and Carlson (1998); Poirier (1998).
Initial Selection of Process Alternatives (Phase I)
The WSRC procedure for identifying and evaluating cesium removal processes throughout the rest of this procedure was guided by a list of critical needs and boundary conditions and constraints that the alternatives must meet (Piccolo, 1999, p. 3 (Table 2.2).
In selecting the initial list of cesium removal alternatives (designated by WSRC as Phase I), a team of experts (Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1998a, 1998e) began by
TABLE 2.2 Minimum Critical Needs and Boundary Conditions and Constraints
|
Critical Needs
Boundary Conditions and Constraints
|
|
|
SOURCE: Piccolo (1999, p. 3). |
|
grouping the alternative processes into 11 categories.2 Distributed within these were 144 specific processes3(the ‘Long List') (Table 2.3). It should be noted, however, that these 11 categories do not necessarily correspond to the processes identified in the initial literature compilation.
TABLE 2.3 Initial Selection of Cesium Removal Process Alternatives (Phase I—‘Long List')
|
|
2 |
These included such options as geologic disposal that were less dependent on separations processing than others. |
|
3 |
Although the references cited earlier listed 126 specific processes, the committee was notified on March 9, 2000, by R. Jones, WSRC, that “The activity to create the cesium removal ‘Long List' of alternatives began during Phase I and continued throughout Phase III.” After publication of the referenced documents concerning Phase I, an additional 18 specific process were identified and added to the list. |
The 144 process alternatives on the ‘Long List' were apparently obtained from sources other than directly from the literature search, although the WSRC team received a review of the search and identification of technologies for potential inclusions in the evaluation (Poirier, 1998). Each process input to the procedure was designated as a pro forma and was reviewed by the Savannah River Site High Level Waste Salt Disposition Systems Engineering Team (1998b) for relevance, even in the absence of process details. The process outlines, required functions, and corresponding criteria for these candidate processes were submitted and accepted in the usually terse pro forma format.
Once the categories of processes that were considered to be viable for the required mission had been identified, the list of specific candidate processes was narrowed to 83 by formally rejecting 52 of the initial ‘Long List.' The reduction of 144 processes to 83 alternatives was largely accomplished by rejecting those that failed to provide a satisfactory end state for the high-level waste, those that had an inadequate scientific base, or those that had obvious and detrimental safety implications. The list of 83 was further reduced by combining some of the process variations and those pro formas that were later submitted (Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1998e) into a group of 28 alternatives. This group was reduced to 18 (Table 2.4) by combinations, modifications, or hybrids of the 28 alternatives using screening criteria similar to those mentioned in the note to Table 2.3.
The screening criteria used for this part of the selection process included scientific and engineering maturity, implementation feasibility, safety and licensability, response to the Defense Nuclear Facilities Safety Board (DNFSB) Recommendation 96-1 (1996), and feasibility of obtaining the re-
quired permits for disposal of the final waste forms (Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1998c).
The reduction of the 28 process alternatives to 18 (the ‘Initial List') was achieved by ranking, in each of the process categories, the 28 alternatives and generally, but not always, retaining the uppermost ranked processes (Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1998a) (Table 2.4). The ranking was determined on the basis of process robustness, technical maturity, and potential for implementation. Although these terms seem somewhat subjective, WSRC provided numerical guidelines throughout the procedure to quantify the evaluations into ratings. Of the 18 alternatives, 2 solvent extraction processes, 1 selective crystallization process, 2 vitrification processes, 1 grout disposal process, 6 ion exchange processes (of which 3 use crystalline silicotitanate), 1 electrochemical process, and 5 tetraphenylborate processes remained to be evaluated in more detail.
Reduction of Process Alternatives to Four (Phase II)
During Phase II of the screening procedure, the reduction in the number of alternatives from 18 processes to 4 (the ‘Short List') was accompanied by an increase in the detailed examination of each of the remaining
TABLE 2.4 Initial Selection of Cesium Removal Process Alternatives (Phase I—‘Initial List')
|
|
SOURCE: Savannah River Site High Level Waste Salt Disposition SystemsEngineering Team (1998b). |
options (Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1999b and 1998f) (Table 2.5). The substantive steps used to evaluate the initial list of 18 candidate process alternatives included flow sheet analysis, risk analysis, preliminary life cycle cost estimates, reexamination of evaluation criteria, and sensitivity analysis.
TABLE 2.5 Reduction of Process Alternatives to Four (Phase II—‘Short List')
Process flow sheet calculations and the assumptions and bases for the models used to estimate the flow sheet parameters were described in a study that used a preliminary outline of the processes (Westinghouse Savannah River Company, 1998a). The high-level waste (HLW) feed to each of the process models was averaged from all of the tanks, and the end state was assumed to be Defense Waste Processing Facility (DWPF) glass4 and the saltstone grouting process product. The base case was the ITP process yielding saltstone grout and DWPF glass and was described in some detail.
A preliminary “risk assessment” with adjusted risk values (Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1998g), using a general guide to the procedure for the assessment (Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1998d) was issued for the 18 process alternatives. The procedure employed by WSRC appeared to be a quantification of potential risks, both technological and regarding implementation, to which were assigned likelihood values and numeric consequence estimates. Numeric values were assigned to each part of the assessment and to the final scores for each candidate process. Where members of the evaluation team identified high risks, mitigation of the risks was evaluated and a modified numerical risk and consequence ranking assigned. For each of the 18 ‘Initial List ' categories, WSRC identified what it called the “highest risks.” Those categories having the most high risks were the crystalline silicotitanate process, acid side solvent extraction, small tank tetraphenylborate (TPB), direct grouting, and fractional crystallization. Based on the total number of identified risks of all types, the proc-
|
4 |
In some cases, ceramic or special glasses were assumed as the end state. |
esses having the most included small tank TPB precipitation, fractional crystallization, direct grouting, catalyst removal in-tank precipitation, and crystalline silicotitanate.
Presentations by WSRC personnel (Piccolo, 1999, p.8; see also Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1999a) indicated that three factors—weighted process evaluation criteria, the preliminary process risk compilation, and flow sheet analysis—were used by the team in deciding which candidate processes to delete from further consideration. On the basis of the scores for technology, four processes were selected to represent the final alternatives, namely direct grouting (score = 22.54), small tank tetraphenylborate precipitation (19.32), zeolite ion exchange (18.86), and crystalline silicotitanate ion exchange (18.86). If only process engineering (construction, operations, reliability, availability, maintainability, and inspectability) weighted evaluation scores are used, caustic side solvent extraction (15.50), acid side solvent extraction (14.25), and direct vitrification (14.00) become the finalists. However, total weighted evaluation scores for all of the final four are probably not significantly different (direct grouting–83.86; small tank tetraphenylborate precipitation–72.88; crystalline silicotitanate ion exchange–69.16; and caustic side solvent extraction–—69.14). Zeolite ion exchange received a total weighted evaluation score of 70.65 that would have rated it the number three candidate, but it was rejected in favor of the flexibility of crystalline silicotitanate ion exchange with DWPF vitrification. The direct grout process was excluded from further consideration for non-technical reasons such as the time required to provide for public approval, and regulatory approval (Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1999b, p. 40).
Selection of Recommended Process Alternative (Phases III and IV)
The final stage of the procedure (Phases III and IV) for evaluation of processes for removal of cesium resulted in a recommended alternative process (small tank tetraphenylborate) and one back-up process (crystalline silicotitanate ion exchange) (Savannah River Site High Level Waste Salt Disposition Systems Engineering Team, 1998h, 1999a, and 1999b) (Table 2.6). Included in this stage were a more detailed life cycle cost estimate and an uncertainty evaluation to define programmatic risks, and several iterations with other evaluation criteria.
ANALYSIS AND FINDINGS
Based on its review of the documents provided and presentations by and discussions with SRS and its contractors, the committee made the following observations about the screening procedure.
TABLE 2.6 Phases III and IV and Decision Phase
|
Results
Evaluation Criteria
|
|
SOURCE: Savannah River Site High Level Waste Salt Disposition SystemsEngineering Team (1998g, 1999b). |
Literature and Patent Search
The initial literature search for process alternatives to separate cesium from the highly alkaline supernate was documented. It appears that a sufficient sample of the pertinent chemistries for the separation of cesium from tank waste supernate is represented. The committee did not receive a summary or analysis of the large amount of written material that resulted from this search procedure, nor was it made aware that such an analysis was available. The committee concluded that, while the scope of the chemistries that were found in the search appeared adequate, it is not clear that the extent of the application of technologies was equally well ascertained. It is unlikely that a common set of key words would satisfy the retrieval of technologies and chemistries from all of the data bases that were searched. Further, some chemistries that have been used on a plant-size scale resulted in only a few references (e.g., cobalt dicarbollide). That circumstance may have been due to the proprietary nature of some information or incomplete information on work that preceded computer archiving. A more comprehensive search may have revealed more of the pertinent information.
Initial Selection of Process Alternatives (Phase I)
The committee concluded that the procedures used to arrive at the ‘Initial List' of 18 from a group of 144 processes in the ‘Long List' are not obvious, having been obscured in the many volumes of documents containing individual comments and evaluations of members of the expert teams. The value of the extensive global literature and patent search also is not clear, based on the fact that all but one of the technologies (bioremediation, subsequently rejected as not being feasible) were identified by the expert judgment of the Savannah River Site High Level Waste Salt Disposition Systems Engineering Team and its consultants during this part of the procedure. Inclusion of the previously favored processes (the large tank ITP or a variation of it) was maintained throughout the screening procedure; however, the committee was not aware of any additional, substantive information to warrant such retention that might have appeared subsequent to the DNFSB recommendation. It is not clear from the documents provided to the committee how the results of the literature and patent search were incorporated into this step or the subsequent steps of the screening procedure.
The committee concluded that the generation of 18 process alternatives cannot be described as a transparent procedure. Several important separations processes for cesium (e.g., use of cyanoferrates and carbollide extractants) were omitted from the ‘Long List'; an internal WSRC note from the leader of the literature and patent search recommended consideration of at least one of them—hexacyanoferrate (Poirier, 1998, p. 2. However, the committee also concluded that the ‘Initial List' of 18 alternatives contains processes that probably could be successful in meeting the general objectives of the process within many of the constraints that WSRC has enumerated. Elsewhere in this report, the committee calls attention to the fact that successful application of such separation processes must be based on a rigorous and disciplined program of research and development (R&D).
A comparison between the major chemical processes identified in the literature search (discussed earlier) and the ‘Long List' of 144 processes, from which an ‘Initial List' of 18 was derived shows significant differences. One of the reasons for these differences was that the literature search was focused on the chemical nature of the separations and primarily identified the central chemical on which the separations rests. The topical array leading ultimately to the final 18 processes does not allow such a ready comparison since it is organized by process (e.g., unit operation) and is often devoid of specific identification of the underlying chemistry.
The ‘Long List' included some process alternatives that were obviously not pertinent (e.g., geologic disposal). Further, some of the categories into which the 144 processes were divided were not sought during the literature search (e.g., vitrification); on the other hand, some alternatives (e.g., electrochemical) were included in the ‘Long List' of 144 processes even though their presence in the global literature search was trivial. Finally, and most importantly, some of the processes identified in the literature search as extensively described and even applied on a plant scale were not present in
the ‘Long List'. The committee found no information that identified the reasons for these differences.
The explication of the 18 processes was characterized by WSRC to the committee as truncated. Because of time constraints, the unavailability and uncertainty of some detailed information, especially on the scientific bases for some of the processes, reduced the data base that supported the evaluation.
Reduction of Process Alternatives to Four (Phase II)
The results of the flow sheet analyses are given in terms of rates of use of resources, production of products, or environmental releases. The cited reference (Westinghouse Savannah River Company, 1998a) did not provide conclusions drawn from the flow sheet analyses and assumptions that accompanied the models. Further, the use of monosodium titanate (MST) as a front-end step for many of the alternatives, including the final four processes, apparently was not explicitly assessed in a manner comparable to that used for the alternative processes or integrated with the alternatives. However, the use of titanium in reagents posed questions about the equivalence of titanium in MST and crystalline silicotitanate (CST) in glass chemistry. There appear to be no data to clarify the matter.
The flow sheet calculations for the narrowing of the alternatives from 18 to 4 were claimed to be accurate to ±25 percent, an optimistic claim considering the nature of some of the assumptions used. Neither the flow sheet analysis nor the risk assessment documents provided conclusions that related to the selection of the final four alternatives. Assumptions for the semi-quantitative flow sheets were often invoked and thus indicated a significant absence of reliable and comprehensive data for many of the process steps. Assumptions that appear to be somewhat speculative include (a) reactions proceed to completion, (b) reactions rates for monosodium titanate reach equilibrium in 24 hours for uranium and plutonium (not well known or documented) based on an assumed analogy with the rates for strontium, (c) activity coefficients for tetraphenylborate reactions were calculated but do not seem to be based on experimental data, and (d) concentrations of catalysts, such as copper and palladium, are part of rate equations. In short, the identification of assumptions and uncertainties needed by the expert evaluators provide a general picture of the status of the knowledge for the processes under consideration for alternatives.
The committee notes that this status apparently was not the basis of the risk assessment procedure used by WSRC. Risk assessment is normally used for analysis of the impact of waste disposal or other fuel cycle operations, and its role in this evaluation process was not clear to the committee other than as a guideline for expert judgment. The starting assumptions for the risk assessment were not comparable for each the alternatives (S.F. Piccolo, WSRC, personal communication to committee, November 21, 1999). Unfortunately, the relation between these uncertainties, including those that
were brought out during the presentations to the committee, and the final decision matrixes that reduced the number of alternatives to the final four, were difficult to understand.
The committee, through its knowledge of the general literature, as well as from the presentations and the documents provided by WSRC, made an effort to understand how the final four processes were selected and to understand the overall procedure. Although the procedure did not appear to support the processes ultimately selected, this may have been due to the difficulty in understanding and following the details of the procedure. The committee concluded that the steps and procedures for avoiding the potential for less-than-objective analyses were not adequately covered in documents describing the ‘qualitative' risk assessment processes used in this phase of the screening process. The selection of the final four alternative processes on the basis of the numeric criteria had little bearing on the extensive literature developed for the analyses. In short, a logical trail that allowed an objective evaluation of the processes used for the selection of the final four candidates in the ‘Short List' was not evident. The claim that WSRC has selected the final four alternatives in an obviously objective manner does not seem to be sustainable by the information provided to the committee. The committee concluded, however, that on a technical basis each of the four process alternatives, namely small tank tetraphenylborate precipitation, crystalline silicotitanate ion exchange, direct grout, and caustic side solvent extraction, could be implemented with modest risk if a viable R&D program is carefully planned and successfully executed to address the uncertainties for each. Such a program for each of the four ‘Short List' process is discussed in Chapter 4, Chapter 5, Chapter 6, Chapter 7 of this report.
Selection of Recommended Process Alternative (Phases III and IV)
The relatively high score assigned to the technology rating (science and engineering maturity and process simplicity) for small tank TPB precipitation (78, as compared with the score for the other three process alternatives—49, 58, and 86) and the low score for caustic side solvent extraction (49) in this last step of the screening procedure that narrowed the alternatives to one or two, is at variance with responses provided to the committee during presentations by WSRC personnel. Finally, the technology scoring results appear to be largely insensitive to uncertainties and variations among the candidate processes for process simplicity and engineering maturity. The Savannah River Site High Level Waste Salt Disposition Systems Engineering Team (1999b) introduced the business perspective evaluation, including the entire DOE complex, to arrive at the selection of the small tank ITP as the recommended process, and crystalline silicotitanate in a back-up role. This portion of the procedure was difficult to evaluate.
GENERAL CONCLUSIONS
-
The procedure for identifying alternative processes for separating cesium from HLW was cumbersome, complex, and lacked transparency. The committee concluded that the “winnowing” procedure was sufficiently opaque as presented in the voluminous documentation as to defy ready evaluation of objectivity and completeness. A previous study by a committee of the National Research Council (1998) of systems engineering as applied at the Hanford Site in Washington made a similar observation about the importance of simplifying documentation to improve clarity for reviewers and other interested parties. Nevertheless, the procedure uncovered and, to some extent carried forward, most of the processes that the committee could envision would provide suitable alternatives for cesium removal. There are some alternative processes and varieties not carried forward in the selection procedure because of the emphasis on cesium separations chemistry, but the committee concluded that they do not represent alternatives having a significantly greater likelihood of success or that could be developed much more rapidly than the processes that were selected.
-
The mode of an objective selection of the final process and its backup is not obvious to the committee. The committee concluded that the final phase of the selection process resulting in small tank TPB as the remaining candidate could not have considered the deficiencies in the process brought out during the presentations to the committee. These deficiencies will be discussed further in Chapter 4, but knowledge of them during the selection procedure might have ameliorated the final result.
-
The procedure used to define and narrow the alternative processes for cesium separations raised further concerns:
-
While the numeric (quantified) approach to evaluation seems to have been evenly applied, it leaves an impression of more precision, accuracy, and objectivity than warranted by a procedure depending primarily on expert judgment.
-
More timely attention in the screening procedure to uncovering potential process risks and their scientific and technical uncertainties than was evident for at least the final four cesium separation process alternatives could have lead to recommendations for the R&D necessary to bring each to a point where more rigorous evaluation and final selection could be accomplished. Resolution of important uncertainties that contribute to the technical risk should be identified early in the screening procedure to decrease the need for extensive and impenetrable documentation to support selection decisions for process alternatives.
-
SRS and its contractors appeared to provide inadequate attention in the screening procedure to the role of the cesium separations process selection on the entire HLW system, particularly the steps preceding cesium separations. Evaluation of the explicit interfaces of unit operations (e.g., strontium and plutonium removal) on the risks associated with candidate processes for cesium removal appeared to be incomplete. The committee con-
-
-
cluded that a more disciplined systems engineering approach to the entire high-level waste operations at SRS could have clarified many of the issues that remain unresolved (discussed further in Chapter 8 of this report). Further, the impact of qualitative criteria derived from such evaluation factors as perceived future missions at SRS (e.g., weapons-grade plutonium disposition; see National Research Council, 1999a) and site limitations (e.g., availability of tank space) was not clarified in terms of the effect on the selection procedure outcomes. The committee concluded that the overall quality of the outcome of the procedure to select alternatives to the in-tank precipitation was not commensurate with the effort expended to carry it out.
RECOMMENDATIONS
-
SRS should proceed with an R&D program for the four final processes selected unless important barriers arise or until enough information on uncertainties is available to conduct a rigorous but more visible basis for selection.
-
When using qualitative expert judgment, one should not rely on tools such as the numeric (quantified) evaluation procedures that tend to give the false impression of precision, accuracy, and objectivity.
-
In response to the committee task, “Was an appropriately comprehensive set of cesium partitioning alternatives identified and are there other alternatives that should be explored? ”, the committee recommends that no further effort be expended at this time in alternative identification.
-
In response to the committee task, “Was the process used to screen the alternatives technically sound and did its application result in the selection of appropriate preferred alternatives?”, as noted previously the committee concludes that the screening procedure was cumbersome, complex, and lacked transparency to document the technical soundness of an evaluation and selection of appropriate preferred alternatives based primarily on the best judgment of experts using many qualitative factors. The committee recommends that future such evaluations, depending on expert judgment, be documented in a clear, easily understandable and traceable manner to allow for viable reviews. Although the screening procedure did result in the selection in the ‘Short List' of what the committee believes are at least four appropriate preferred alternatives, further reduction of the alternatives will have to await completion of adequate R& D on each.















