4
Tetraphenylborate: In-Tank Precipitation and Small-Tank Precipitation Options
The focus of this chapter is on the tetraphenylborate (TPB) precipitation process, which was developed at Savannah River Site (SRS) in the late 1970s and early 1980s to remove cesium from high-level waste (HLW) supernates. The original design for this process involved the use of an existing underground HLW waste tank at the site; consequently, the process was referred to as in-tank precipitation, or ITP. The in-tank precipitation process was abandoned in 1998 because of technical difficulties, and a hybrid process, referred to as small-tank TPB precipitation, was developed as a potential alternative. This chapter provides a review of these processes and identifies remaining scientific and technical difficulties. Several recommendations on the implementation of the small-tank precipitation process are provided at the end of the chapter.
TETRAPHENYLBORATE PRECIPITATION PROCESS
The TPB precipitation process removes cesium from the supernate by precipitation with sodium tetraphenylborate, Na[B(C6H5)4], through the following reaction:
where the double arrows indicate that the reaction is reversible.
Sodium tetraphenylborate (NaTPB) is a reagent with well-known properties. The low solubility of CsTPB (the solubility product, or Ksp, at 25 °C is 7.84 × 10-10) potentially provides decontamination factors as high as 105 to 106, and the CsTPB precipitate is typically in a form that is easily filtered.
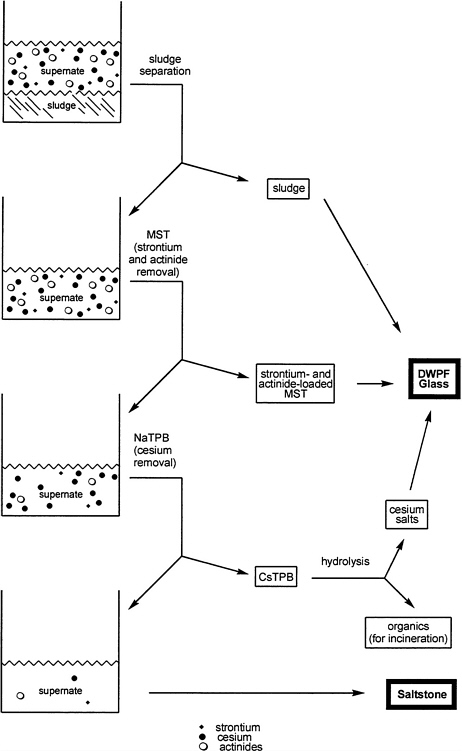
As originally designed for the ITP process, NaTPB and monosodium titanate (MST) (see Chapter 3) were to be added to the HLW supernate to precipitate cesium, strontium, and actinides. The precipitate was to be removed from the tank by filtration and was then to be treated to remove greater than 90 percent of the organic material (i.e., the phenyl [C6H5] groups bound to boron) through a precipitate hydrolysis process using formic acid in the presence of a copper catalyst (Ferrara, Bibler, and Ha, 1992). The products of this reaction are benzene, which can be removed by evaporation and subsequent incineration, and an aqueous solution containing Cs, K, and B(OH)3 ions. This aqueous solution was to be fed to the Defense Waste Processing Facility (DWPF) to be incorporated into glass, and the decontaminated supernate was to be incorporated into grout at the SRS Saltstone Facility. A schematic flow sheet for this process is shown in Figure 4.1.
In-Tank Precipitation Process
In the original design, the TPB precipitation process was to be performed in an existing HLW tank at SRS, and a large-scale test in an actual HLW tank was conducted in 1983 to demonstrate proof-of-principle. The test was conducted in Tank 48, a 1.3-million gallon (5-million liter) underground storage tank in the H-Tank Farm (see Walker et al., 1996). MST and TPB were added to the tank waste, resulting in the generation of 20,000 gallons (76,000 liters) of precipitated slurry containing cesium and other metals. During the wash phase of the test, 183,000 gallons (693,000 liters) of water were added to the tank while the slurry pumps were operating. Benzene generation was noted, and benzene levels in the tank exceeded the maximum instrument readings for a period of 6 hours. The SRS review of the experiment concluded that the test was a success, but recommendations were made that the causes for high benzene release rates be investigated.
Additional analyses on the cause(s) of the benzene generation resulted in an incorrect conclusion in 1983: namely, that benzene generation was due primarily to radiolysis. Additional testing at the University of Florida in the mid-1980s under conditions different from those in Tank 48 provided values for radiolytic production for free and trapped benzene (the latter refers to benzene that is physically held within the waste). In 1987 and again in 1994, Savannah River Technology Center (SRTC) conducted tests under conditions similar to those in Tank 48, but failed to duplicate the phenomenon of trapped benzene (Defense Nuclear Facilities Safety Board, 1997, Section 2.3.2). Additional work at the Georgia Institute of Technology confirmed the SRTC findings that the 1983 conclusion on the benzene generation mechanism was incorrect. Nevertheless, the committee understands that over the next 12 years, no comprehensive studies were initiated to identify the mechanism(s) of benzene generation and release or to examine its potential effects on ITP processing requirements.
From the late 1980s to the early 1990s, chemists at SRS were engaged in NaTPB stability tests, since slow decomposition of vendor-supplied NaTPB had been observed. Although NaTPB is typically supplied as a 0.5 molar solution stabilized by 0.1 molar NaOH, it was established that, even in alkaline solution, copper ions catalyze the decomposition of TPB to benzene, phenol, and boric acid (Barnes, 1990, 1992; Crawford et al., 1999). A soluble copper impurity was identified along with other metals, for example, Pd and Ni, in the vendor-supplied NaTPB. Another research program addressed the problems of waste foaming during the ITP processing, which was of concern because of the potential for clogging transfer pipes and inhibiting phase separation.
In 1995, a large-scale production operation was conducted primarily to assess potential benzene vapor phase mixing, temperature, and oxygen concentration in Tank 48. Approximately 1.4 × 105 liters of solution containing 27,500 kilograms of NaTPB were added to Tank 48 over the period from September 2 to September 29, 1995. These added solutions contained an estimated 14.6 kg of dissolved benzene. Beginning on October 9, three slurry pump tests and a filtration test were conducted. During the third slurry pump test on November 9, a maximum temperature of 52 °C was reached in the tank. On December 1, all four slurry pumps were operated to mix the tank contents, and an alarmingly high concentration of flammable benzene vapor that exceeded ten percent of its lower flammability limit accumulated in the tank headspace. The slurry pumps were shut down to slow benzene release from the tank waste.
Over the next few months, slurry pumps were activated occasionally, and high benzene vapor concentrations were repeatedly noted. An estimated 8,500 kg of benzene was generated during the period November 5, 1995 to April 22, 1996 and was eventually removed from Tank 48. Subsequent analysis indicated that greater than 95 percent of the NaTPB decomposed over the period from November 14 to December 28. Depending on which time is assumed for completion of this decomposition, the TPB decomposition rate was estimated as either 25,000 µg/liter·hr (upper bound) or 12,500 µg/liter·hr (lower bound), both far in excess of that expected from radiolysis (Walker et al., 1996).
Extensive analyses of the contents of Tank 48 led to a satisfactory organic material balance,1 indicating that most of the TPB decomposed to benzene with lesser amounts to phenylboronic acid, phenol, biphenyl, and much less to terphenyl and diphenyl mercury. Time profiles for concentrations of hydroxide, phenylboronic acid, phenol, potassium, cesium, and boron in Tank 48 were developed for the period of the 1995–1996 benzene evolution. After the DNFSB report, a research program also was initiated by SRS to examine the chemistry responsible for the rapid TPB decomposition
|
1 |
That is, the total amount of organic material (i.e., phenyl groups) added to the tank could be accounted for by reaction products (e.g., benzene, phenol, biphenyl) and residual TPB after the excursion. |
in the tank. Since copper-catalyzed decomposition of TPB had already been developed for downstream processing of the TPB sludge (the precipitate hydrolysis process mentioned previously in this chapter), and since copper was identified as a catalyst resulting in slow decomposition of the vendor-supplied NaTPB, copper was an initial suspect as a catalyst responsible for decomposition.
Using Tank 51H sludge and/or Tank 48H filtrate, nine TPB decomposition tests revealed decomposition rates as high as 2,214 µg/liter·hr, and more commonly 30 to 773 µg/liter·hr at 40 °C. At 70 °C, in the presence of oxygen, TPB decomposition displayed a lag time of several days. In the absence of oxygen, decomposition was initiated immediately. However, in none of these tests, including those in which suspected metal ion catalysts were added, were rates observed that were comparable to those seen in the 1995 Tank 48 excursion. Moreover, only a small percentage of the NaTPB was decomposed in the test runs, leading researchers to suspect that most of the benzene evolution in these tests resulted from impurities in the vendor-supplied NaTPB. In no case was complete NaTPB decomposition observed, again in contrast to the excursion observed in the 1995 production operations.
An outside panel of experts, the Process Chemistry and Mechanisms Panel, was established in January, 1996 to guide an experimental program to determine the mechanism(s) of decomposition of NaTPB and benzene release involved in the 1995 excursion. The panel was composed of Robert Hanrahan (University of Florida), Bruce King (University of Georgia), Edward Lahoda (Westinghouse Science and Technology Center), George Parshall (DuPont Central Research, retired), and Robert Smiley (DuPont, retired), with occasional participation of SRS consultants Russell Drago (University of Florida) and Preetinder Virk (Massachusetts Institute of Technology). The experimental program work was conducted by approximately ten Westinghouse Savannah River Company (WSRC) scientists and engineers, although only six had a primary assignment to this project. Some additional experiments were carried out by other researchers at Georgia Institute of Technology, DuPont, and Pacific Northwest National Laboratory. The experimental work was periodically reviewed and research plans were modified based on discussions with members of the Process Chemistry and Mechanisms Panel, which itself produced 16 reports during its two years of existence (Process Chemistry and Mechanisms Panel, 1996a-k, 1997a-d, 1998).
Most of the tests were conducted with non-radioactive simulants to screen various metal ions for catalytic activity toward TPB decomposition. Based on the analysis of the contents of Tank 48, a simulant slurry recipe was developed for non-radioactive testing. Copper-catalyzed decomposition was suspected during the initial stages of the program (see Crawford et al., 1999), and various tests were conducted using simulated waste and accelerated conditions, such as higher temperature and greater concentrations of suspected metal-ion catalysts. Many essential features of the 1995 event could not be duplicated. Notably, the observed lag time of more than two months preceding rapid TPB decomposition was not duplicated in the tests.
More importantly, the copper catalyst activities were far too low (by at least two orders of magnitude) to account for the rates of benzene released in the 1995 excursion, even at the 52 °C maximum temperature experienced in the 1995 production operations. The tests with simulants revealed benzene release rates that were even lower (by approximately one order of magnitude) than the rates measured with radioactive Tank 51H sludge and/or Tank 48H filtrates.
On August 14, 1996, the Defense Nuclear Facilities Safety Board (DNFSB) recommended that the Department of Energy not proceed with large-scale process testing at the ITP Facility until the mechanisms of benzene generation, retention, and release were better understood and adequate safety measures had been developed to mitigate benzene deflagrations (Defense Nuclear Facilities Safety Board, 1997). Planned operations were put on hold while research on catalytic mechanisms continued.
In the fall of 1996, the Process Chemistry and Mechanisms Panel began to question the possible role of noble metal (e.g., palladium) catalysis. Early in 1997, a significant catalytic influence from the combined presence of noble metals, select organic compounds, and a tetraphenylborate precipitate was noted. As compared with copper catalysis, greater TPB decomposition rates were obtained in simulated tests with palladium additives, but the greatest rates required much higher levels of palladium than have been found in real waste and also required addition of diphenyl mercury, TPB decomposition intermediates, and benzene. Additional features were cited in the Panel's reports for this catalyst system (Process Chemistry and Mechanisms Panel, 1998):
-
activation of the catalytically active species is slowed in the presence of oxygen;
-
temperature, radiation, and copper synergism influence catalyst activation; and
-
tetraphenylborate solids provide a support platform for the palladium catalyst system.
A working mechanism was developed that invokes initial TPB reduction of soluble palladium ions to elemental palladium [Pd2+ to Pd(0)], interaction of Pd(0) with TPB to form a phenylpalladium intermediate that is subsequently protonated by water to benzene and triphenylboron, which regenerates Pd(0). A similar cycle catalyzes hydrolysis of triphenylboron. Copper is postulated to catalyze the subsequent decomposition of tetra-, tri-, and diphenylborons and phenylboronic acid.
At its final (16th) meeting in March, 1998, Process Chemistry and Mechanisms Panel (1998) provided the following summary comments:
-
the key catalyst is palladium metal deposited on a solid support (TPB solids, sodium titanate, or sludge solids);
-
reaction initiation is affected by several parameters which control redox state (oxygen, temperature, intermediates, mercury, TPB, and copper);
-
the TPB decomposition mechanism involves two energies of activation;
-
the decomposition mechanism results from soluble TPB interacting with the catalyst; and
-
the lower TPB solubility in the presence of potassium is most likely due to the formation of a mixed crystalline form.
The Panel was supportive of additional testing to enhance technical understanding of the decomposition mechanism, especially in the areas of temperature effects, induction period, oxygen, and the use of inhibitors.
Also, in early 1998, SRS concluded that both safety and production requirements could not be met using the ITP process, and a decision was made to suspend operations and search for alternatives. At the time of suspension, SRS had spent $489 million to develop and implement the ITP process (U.S. General Accounting Office, 1999, p. 4).
Small-Tank TPB Precipitation
The small-tank TBP precipitation process shares many of the features of the ITP process, except that it is carried out in smaller, purpose-built tanks to provide greater control over precipitation and benzene formation. The process allows for closer temperature control and faster cycling times to reduce the generation of benzene and improved agitation of the liquid to facilitate benzene removal. The process is also designed with secondary containment and positive pressure control so that the processing vessels could be blanketed with nitrogen to reduce explosion hazards and facilitate benzene removal.
Design of the small-tank process has focused primarily on engineering issues: minimization of waste foaming, optimal mixing conditions to minimize the amount of NaTPB needed, measurements of precipitation and dissolution rates, and establishment of the conditions that optimize the recovery of excess NaTPB during washing. Work on many of these issues was underway during this committee study, and the committee received briefings on some of this work during its information-gathering meetings.
Foaming involves the formation of microscopic gas bubbles in the waste during agitation of the waste-TPB slurry. Excessive foaming can disrupt waste processing operations; the foam can inhibit phase separation and disrupt flow through waste-transfer lines. The waste foaming problem was first discovered in laboratory experiments utilizing real samples of tank waste. Current research efforts at the SRTC and Oak Ridge National Laboratory are focused on identifying the causes of foaming, identifying foam-control reagents, testing the radiolytic stability of these reagents, and determining the potential impacts of these reagents on downstream process operations, especially the DWPF.
ANALYSIS
In view of the very low solubility of CsTPB, it was logical for SRS to develop processes based on TPB precipitation for removing cesium from the HLW supernate. In laboratory applications, large decontamination factors have been demonstrated, and the precipitate is typically in a form that is easily filtered. Further, NaTPB is relatively inexpensive, and subsequent acidification of the CsTPB precipitate (the precipitate hydrolysis process discussed elsewhere in this report) allows for controlled decomposition to easily separated benzene that can be destroyed in an existing incinerator, and an aqueous stream containing boric acid and cesium and potassium salts suitable for vitrification at the DWPF.
The implementation of this process at SRS, however, was flawed for a number of reasons. Benzene production at higher-than-measurable levels during the 1983 test in Tank 48 was not correctly analyzed, and incorrect conclusions concerning the generation and release of benzene were reached. As noted in a previous section, work at SRTC and Georgia Institute of Technology called into question the correctness of the postulated benzene generation mechanism as early as 1987. The 1995 production operations in Tank 48, which were conducted to evaluate whether the benzene vapor could be adequately mixed for safe operation and to test filtration procedures, produced an unexpected, rapid TPB decomposition. The causes of this rapid TPB decomposition have not yet been identified. The discovery process is complicated by the fact that the contents of Tank 48 at the time the excursion were highly complex. They included not only the supernate, but also sludge together with much of the tetraphenylborate precipitate and its various decomposition products from the 1983 test.
As was noted earlier in this chapter, early attempts were made to identify the catalyst(s) responsible for the TPB decomposition in Tank 48, and copper catalysis was considered a likely candidate. However, at least two important features could not be reproduced in tests with tank filtrate and/or sludges, even when elevated concentrations of suspected catalytically active ions were added: (1) the rapid rates of benzene formation, (2) complete decomposition of TPB observed in Tank 48. Tests with non-radioactive simulants afforded even lower rates of TPB decomposition, even at higher copper concentrations than those present in Tank 48 (Crawford et al., 1999). Palladium was later found to produce higher TPB decomposition rates, but only in the presence of other phenylated additives, such as diphenyl mercury, TPB decomposition intermediates, and benzene. It is not clear whether these higher rates are sufficient to account for the observed 1995 excursion. The palladium-catalyzed decomposition might be explained by synergism of Pd(0) with the additives, as well as an additional catalytic cycle using copper.
By contemporary standards of mechanistic understanding in organometallic chemistry, the current proposed mechanistic scheme for TPB decomposition is rather crude, and some of the key proposed steps do not appear to have precedent. A variety of transition metal ions have been shown to stoichiometrically oxidize TPB to triphenylboron and biphenyl: Ce(IV), Ir(IV), Fe(III), and Cu(II) (Eisch and Wicsek, 1974; Turner and Elving,
1965; Geske, 1959, 1962; Abley and Halpern, 1971; Strauss, 1993), or to catalyze TPB hydrolysis (Flaschka and Barnard, 1960). Truly catalytic hydrolytic decomposition of TPB in alkaline solution appears to be confined to copper-based systems (Barnes, 1990, 1992; Crawford et al., 1999) and a palladium/phenylated boron/diphenyl mercury/benzene system discovered by SRS scientists (see above).
The first step in the proposed palladium catalysis system involves what appears to be transfer of a phenyl group from boron to Pd(0). Such transmetallation chemistry is well established for a wide variety of metal ions: Fe(II), Fe(III), Ni(II), Zr(IV), Rh(I), Rh(III), Ru(II), W(IV), Hg(II), Pt(II) (Bianchini et al., 1989; Bonnessen et al., 1989; Legzdins and Martin, 1983; Reed et al., 1979; Sacconi, Dapporto, and Stoppioni, 1976; Haines, and duPreez, 1971; Clark, and Dixon, 1969), and Pd(II) (Cho and Uemura, 1994; Cho, Ohe, and Uemura, 1995; Crociani et al., 1990, 1991; Moreno-Mañas, Pérez, and Pleixats, 1996), but not, to our knowledge, for Pd(0). Also unclear are the mechanisms by which diphenyl mercury and the decomposition intermediates accelerate the palladium-catalyzed decomposition sequence.
The lack of understanding of the details of the palladium catalytic cycle, or for that matter, whether a palladium system is responsible for the TPB decomposition in Tank 48, remain matters of concern, since the possibility exists that another, perhaps even more rapid TPB decomposition scenario could be repeated in a future processing operation. The apparent variability of tank waste composition at SRS (see Chapter 8) raises additional concerns, especially given that the reasons for rapid TPB decomposition in Tank 48 remain unexplained. It seems unlikely that a single mechanism for TPB decomposition will explain the 1995 excursion and, at the same time, will foreshadow all possible decomposition scenarios with waste from the other tanks.
Apart from the lack of mechanistic understanding, the failure to duplicate the high rates of TPB decomposition in any of the tests, with real tank waste or simulants, illustrates the current lack of understanding of the tank waste chemical system. The estimated average rates for TPB decomposition in Tank 48 are based on an assumed steady decomposition over a period of approximately six weeks. However, a very high rate, far in excess of the assumed 25,000 µg/liter·hr (upper bound), but for a much shorter period, cannot be ruled out. Although 25,000 µg/liter·hr rates have not been achieved in any of the tests, the actual rate for the TPB decomposition in Tank 48 is unknown. Moreover, the observed months-long lag between TPB addition and maximum benzene production in Tank 48 in the 1995 production test also has not yet been adequately explained or duplicated in any subsequent tests.
There appears to have been over-reliance on tests with simulants in the past research programs addressing TPB decomposition mechanisms. Early tests with Tank 51 sludge and Tank 48 filtrate produced significantly greater TPB decomposition rates, as compared with tests with simulants. Given the complex compositions of the tank sludge, filtrate and solutions, it is not surprising that the principal components responsible for faster TPB decomposition have probably not been identified.
The rapid decomposition of NaTPB that occurred in the 1995 processing operations has several consequences that weigh against its use for removal of cesium by the ITP process as originally planned. One major difficulty is the generation of large amounts of benzene, which could present safety problems if not properly handled. Although safety issues could presumably be resolved by standard industrial processing controls, the quantity of benzene generated could pose regulatory problems. Another problem is the reduction in decontamination factor. As the soluble TPB decomposes, and hence the concentration of [B(C 6H5)4]-(aq) decreases, the equilibrium would shift by redissolution of precipitated cesium tetraphenylborate as indicated by the equation shown at the beginning of this chapter. Any Cs[B(C6H5)4] that redissolved would lower the decontamination factor, and this could be counteracted only by addition of more NaTPB. Such further addition would be inefficient and would merely revert to an earlier stage of the process, with all of the potential problems of decomposition unchanged. In other words, the 1995 excursion might not represent an extreme rate at all, and even higher rates of TPB decomposition might be possible, especially as tank heels (hard-packed waste at the bottom of the tanks) of varying composition are processed.
Development of a small tank TPB alternative appears to be an attempt to “engineer around” the problems observed with the ITP process. Although the final design is not yet complete, SRS believes that improved mixing and temperature control would permit much shorter residence times for the CsTPB precipitate (estimated currently as several days), as compared with the 200-day processing and up to two-year storage time for the batch-type ITP process. These significantly reduced processing times, of course, similarly reduce the possibility that a TPB catalytic decomposition catalyst system might evolve. Moreover, some TPB decomposition is assumed in the current design of the small tank process: slower feed rates and recycle of off-spec material to reprecipitate any soluble cesium are available contingencies. Nevertheless, the lack of a mechanistic understanding of the TPB decomposition process or empirical bounds on decomposition rates present significant hurdles to the successful implementation of TPB precipitation in the proposed small-tank process.
FINDINGS AND CONCLUSIONS
Based on the foregoing analysis, the committee identified five findings and conclusions with respect to cesium removal using the small tank TPB process:
-
Given the very low solubility of CsTPB, it is understandable that SRS has explored TPB precipitation as a strategy for removing cesium from the HLW supernates at the site. Large decontamination factors are possible in laboratory applications, and the precipitate typically exists in a form that is easily filtered. Further, NaTPB, as is available at this time, is relatively inexpensive and pure, and subsequent acidification of the CsTPB precipitate al-
-
lows for controlled decomposition to benzene, which can be incinerated, and an aqueous stream containing boric acid and cesium and potassium salts suitable for vitrification in the DWPF.
-
Many of the scientific, technical, and regulatory issues associated with TPB precipitation have been identified. The regulatory issues center primarily on maintaining benzene release at or below regulatory limits and below its flammability limit. The scientific and technical issues include the following:
-
MST adsorption and ion exchange kinetics (see Chapter 3).
-
Decontamination factors for cesium under small-batch semicontinuous or continuous process operation.
-
Acceptance criteria for the ultimate glass waste form (see Chapter 8).
-
Precipitate washing and recycle of NaTPB.
-
Excessive foaming of stirred radioactive waste slurries.
-
Cycle times and products associated with the decomposition of CsTPB during precipitate hydrolysis processing.
-
-
The formation of foam in stirred laboratory experiments with NaTPB and HLW and its implications with respect to clogging of transfer pipes and poor phase separation may be a major impediment to the use of this technology in the small tank TBP process.
-
Despite conclusions to the contrary (Process Chemistry and Mechanisms Panel, 1998), the causes of rapid TPB decomposition in the 1995 production operations in Tank 48 remain uncertain. SRS has not achieved a level of understanding sufficient to prevent TPB decomposition, choosing instead to “engineer around” the problem. Although the completed research on the copper-catalyzed TPB decomposition scheme (Crawford et al., 1999) is a valuable contribution, the studies have not really addressed the key issues relevant to ITP or the proposed small tank variant. Copper is one of many potential catalysts, and the tank waste is sufficiently complex and heterogeneous that a mechanistic understanding of catalysis is probably not possible given the time and resources available to this project.
Although the design of the small tank TPB process appears to considerably reduce the likelihood of an event analogous to the 1995 excursion, it may not be possible to entirely prevent future rapid TPB decomposition. Since the contents of individual tanks are not homogeneous and contents can vary substantially from tank to tank, some of the waste could have much higher concentrations of the catalyst(s) for TPB decomposition than were present in Tank 48.
RECOMMENDATIONS
Based on these findings and conclusions, the committee offers the following four recommendations. They are directed primarily to the decision makers and research managers responsible for the cesium separation program:
-
If small-tank TPB precipitation remains as a contending process for removing cesium from HLW supernates at SRS, considerable effort should be made to (i) identify TPB catalytic decomposition mechanisms, (ii) establish probable bounding rates for TPB catalytic decomposition, and (iii) address the other scientific and technical issues listed in finding 2 above.
-
As part of its efforts to bound catalytic decomposition rates, SRS should develop robust testing protocols to process moderately sized samples of real waste from each of the tanks using MST and TPB. These protocols are needed to provide information that can be used to develop a predictive capability for process performance for TPB decomposition rates, temperature excursions, foaming, and filterability.
-
Tests on moderately sized samples of real waste should be implemented as soon as possible under this protocol to help assess the viability of the small tank TPB precipitation option. By using real waste from different tanks with different compositions, this testing will allow process performance to be systematically established under conditions that bracket, with safety margins, the acceptable conditions of planned processing operations.
-
If the small-tank TPB processing option is selected for implementation at SRS, samples of each of the waste batches to be processed should be subjected to the testing protocols described above. This will help ensure that unknown synergistic effects of waste blending from different tanks are better understood prior to full-scale processing.