5
Developing the Test Bed— Linking Integrated Service Delivery Systems
OVERVIEW
Many extensive research networks have been established to conduct clinical, basic, and health services research and to facilitate communication between the different efforts. The scale of these networks ranges from local, uptake-driven efforts to wide-ranging efforts to connect vast quantities of clinical and research information. This chapter explores how various integrated service delivery systems might be better linked to expand our nation’s capacity for structured, real-time learning—in effect, developing a test bed to improve development and application of evidence in healthcare decision making.
In the first paper, Steven I. Katz outlines the efforts of the National Institutes of Health (NIH) Roadmap for Medical Research to accelerate biomedical research on a basic level as well as accelerate translational research by connecting existing research networks and maintaining necessary infrastructure for more efficient conduct of clinical research through the National Electronics and Clinical Trials Research network (NECTAR). Cynthia Palmer then discusses efforts of the Agency for Healthcare Research and Quality (AHRQ) to build on the experience of the Integrated Delivery Systems Research Network and establish a vehicle for translation of research into practice by linking healthcare systems with health services researchers. Through rapid cycle, applied research, AHRQ’s Accelerating Change and Transformation in Organizations and Networks initiative has begun to establish a network that fosters demand-driven research and the uptake of innovative approaches to care.
Eric B. Larson discusses the Health Maintenance Organization (HMO) Research Network as a potential model for a national test bed. Many ongoing activities of these 15 linked integrated delivery systems illustrate the potential for these networks to facilitate needed two-way learning between research and healthcare systems. Finally, Michael Mustille presents the history and progress of the Council for Accountable Physician Practices (CAPP) to demonstrate how this network of multispecialty medical groups has accelerated the redesign of physician practice—improving the uptake of evidence-based approaches to care, the translation of research to practice, and the outcomes and efficiency of care.
NIH AND REENGINEERING CLINICAL RESEARCH
Stephen I. Katz, M.D., Ph.D.
National Institutes of Health
The NIH Roadmap for Medical Research was developed to increase synergy across NIH and accelerate the pace of discoveries and their translation. It was launched in order to identify major opportunities and gaps in biomedical research that the agency as a whole needed to address to have the greatest impact on the progress of medical research. Roadmap dollars represent 0.8 percent of the total NIH budget and are planned to reach $500 million by 2008. The Roadmap project is meant to address the questions that no one of the 27 different institutes or centers could address on its own but that we could address collectively. This is not a single initiative, but more than 345 individual awards were given, in FY 2005: 40 percent for basic, 40 percent for translational, and 20 percent for high-risk research.
The NIH Roadmap Strategy was to build on the paradigm of bringing basic science and discovery to clinical practice. Roadmap initiatives are grouped under three main headings: New Pathways to Discovery, Research Teams of the Future, and Reengineering the Clinical Research Enterprise. These are all initiatives that are meant to facilitate bench-to-bedside translation of research. New Pathways to Discovery addresses the fundamental issues that need to be overcome to accelerate research at the basic level. Most notable here are the molecular libraries and imaging advances that will enable high-throughput screening of molecular pathways that have been identified as of interest. Research Teams of the Future embodies the commitment of NIH to an interdisciplinary research approach and the promotion of high-risk research through Pioneer Awards.
About one-third of the Roadmap awards are in the area of Reengineering the Clinical Research Enterprise. These initiatives include the Clinical and Translational Science Awards (CTSAs) and a number of projects that are investigating the integration of clinical research networks. We have
heard repeatedly that the clinical research enterprise is broken. Some of the responsibility for fixing this enterprise falls to NIH, and this paper considers some of the major issues and focuses in particular on the work of some of our translational initiatives on clinical research informatics and integrated research networks.
One of the central challenges to clinical research lies in the regulatory requirements that govern it. Human subjects protection is one issue, but across the board, whether it is HMOs, pharmaceutical research, or the biotech industry, there is no harmonization of clinical research regulatory requirements. For example, the requirements related to reporting adverse events are not uniform across NIH and certainly not across agencies. It took 2.5 years to get uniformity across the NIH and the Food and Drug Administration (FDA) just in the reporting of adverse events of gene therapy trials. The work continues to extend this uniformity across all government agencies as well as outside the government to industry.
Enhancing clinical research workforce training has also been identified as a key need, prompting NIH to institute loan repayment programs and encourage more workforce training at earlier levels in medical schools. Also lending support for enhanced workforce training are the CTSAs, which represent the largest of our investments from the NIH Roadmap for Medical Research. This investment addresses the question of how to best respond to the demand created by recent biomedical discoveries for the evolution of clinical science. CTSAs will create homes for clinical research, researchers, and trainees and are meant to lower barriers between disciplines and encourage creative, innovative approaches to solve complex medical mysteries. These awards are designed to encourage change on a number of dimensions, including the development of novel clinical and translational methodologies; pilot collaborative translational and clinical studies; biomedical informatics; design, biostatistics, and ethics; regulatory knowledge and support; participant and clinical interactions resources; community engagement; translational technologies and resources, and education and career development. In the first round of applications, CTSAs have been awarded to 12 academic health centers, with 60 institutions expected to be part of this new consortium by 2012. This is clearly a sea change in terms of what can go on at academic medical centers. Most academic research centers remain structured in the same way they were 50 years ago and the Clinical and Translational Science Awards are meant to catalyze change—breaking silos, breaking barriers, and breaking conventions. If this works the way we hope it should work, it will clearly change the research enterprise.
The Clinical Research Networks project is part of the Reengineering the Clinical Research Enterprise Roadmap aimed at promoting and expanding clinical research networks that can rapidly conduct high-quality
clinical studies that address multiple research questions. Specifically, NIH is developing the NECTAR network. While research networks currently exist, this initiative is meant to link those networks so that clinical trials can be conducted more effectively. This is particularly important for the NIH where each institute supports clinical research, but does so independently. As a result, to conduct a clinical study, infrastructure is repeatedly built up and broken down. This continual building up and breaking down of infrastructure is a complete loss of money, not to mention the people and resources needed. This is particularly important, for example, in pediatric research, in which most diseases studied are uncommon and can only be done through linked clinical research networks.
However, there are signs of change. For example, in the area of pediatric rheumatic diseases, the Arthritis Foundation helped to build a network across pediatric rheumatic disease allowing many clinical trials to be done simultaneously, leveraging our resources from one trial to other ongoing and planned trials.
As a first step toward establishing a broader network, an inventory of existing clinical research networks will explore existing informatics and training infrastructures. By identifying characteristics that promote or inhibit successful network interactivity, productivity, and expansion, this inventory will lead to the dissemination of “best practices” that can enhance the efficiency of clinical research networks. Pilot projects will then explore how best to combine and extend clinical networks. The initial NECTAR inventory provided current status of about 250 clinical research networks and evaluated best practices. NECTAR pilot projects have been developed with a broad coverage, including medical disciplines such as cancer, heart, critical care, psychiatry, and transplants; populations and settings (primary care, rural, minority, HMO); ages (pediatric, adult, geriatric); information systems (data standards, informatics, tools, platforms); and geographic locations (U.S. and global).
Collectively, these initiatives—CTSA, NECTAR pilot projects, and the inventory of networks—are complementary programs. CTSA focuses on the academic institutions, and NECTAR focuses on linking organizations together in an electronic research network. CTSA is intended to build the homes for clinical and translational science, emphasizing internal and interinstitutional nationwide interoperable informatics. The NECTAR inventory will also discover best practices for existing clinical network management, helping to define the needed common language and standards. There is strong consensus on the potential to use these linked systems to build a test bed and enhance learning. What is needed is leadership, commitment, passion, and a commitment to funding. Funding in particular will drive the development of needed linkages and infrastructure for a learning healthcare system. Commitment of long-term funding however cannot just be the
responsibility of the NIH or the Veterans Administration (VA), but must extend to states as well as to other forms of support.
In this respect, the President’s Health Information Technology Strategic Plan has great potential to expand the involvement and improve the efficiency and power of clinical and translational research. To truly transform clinical practice we must interconnect physicians, improve our understanding of population health, and empower patients. Our initiatives will optimize efficiency and productivity of biomedical research, encourage the basic exploration of bioinformatics and computational biology, and accelerate research translation through CTSAs. However developing the needed test bed will require substantial cross-agency and cross-sector work and collaboration.
AHRQ AND THE USE OF INTEGRATED SERVICE DELIVERY SYSTEMS
Cynthia Palmer, M.Sc.
Agency for Healthcare Research and Quality
Often, health services research findings are not implemented in practice and thus fail to improve the quality of health care that Americans receive. With its program Accelerating Change and Transformation in Organizations and Networks (ACTION), the Agency for Healthcare Research and Quality places the responsibility of investing in the implementation of good ideas, once proven, directly on those who produce, use, and fund such research. ACTION is a five-year implementation model of field-based contract research that fosters public-private collaboration in rapid-cycle, applied studies. With a goal of turning research into practice, 15 ACTION partner organizations and approximately 150 collaborating organizations link many of the nation’s largest healthcare systems with its top health services researchers. ACTION provides health services in a wide variety of organizational care settings to at least 100 million Americans. The partnerships span all states and provide access to large numbers of providers, major health plans, hospitals, long-term care facilities, ambulatory care settings, and other care sites. Each partnership includes healthcare systems with large, robust databases, clinical and research expertise, and the authority to implement healthcare interventions. ACTION is the successor to the Integrated Delivery System Research Network (IDSRN), a five-year implementation initiative that was completed in 2005. To maximize the likelihood of uptake of innovation, the ACTION program emphasizes projects that are of interest to the partnerships’ own operational leaders as well as the project funders, that are broadly responsive to user needs and operational interests, and that are expected to be generalizable across a number of settings.
Prior to developing the call for proposals for ACTION participants, the IDSRN was evaluated carefully. AHRQ continues to use the findings of that evaluation, and its own perceptions, to help shape and direct the ACTION program. ACTION is meant to help AHRQ achieve its goals by promoting uptake of innovation to change practice, through the capture of information about how people change the way they behave to achieve higher-quality delivery of care. Knowing the right thing to do, even when we have evidence-based practices, is not enough. Getting people to actually do the right thing is the real dilemma. AHRQ recognizes the value of nurturing “receptor sites,” or “test beds,” where care is actually delivered and is trying to foster user-driven or demand-driven research.
One of the members of AHRQ’s current National Advisory Committee developed a model (Balas and Boren 2000), which is quite telling in terms of one of the key issues, suggesting that it takes typically about 17 years after the first study results are published to turn about 14 percent of that research into the benefit of patient care. This sobering concept suggests the need for a shift toward more demand-driven research—in which we bring the producers of work and the users of work together to study how to change practice. In addition, while publications are acknowledged as important, we place a stronger emphasis and focus on other types of products that are in forms that can be more easily disseminated and utilized in other settings.
IDSRN and ACTION represent networks of healthcare delivery-based partnerships in which hospitals, ambulatory care facilities, long-term care facilities, and health plans work in conjunction with health services consultants and researchers through five-year master contracts. Contractors (partners) compete for task orders on a rolling basis with the idea of sustaining networks through five years of studies, keep those partnerships in place so they can grow and develop their infrastructure, and continue to have opportunities to work together over a reasonable period of time. The focus is on rapid-cycle, applied research of interest to AHRQ, the partnerships’ own operational leaders, and others. Since AHRQ is a small agency with limited funding to support this endeavor, funding is secured from other organizations to conduct some of the studies. For example, other Department of Health and Human Services (HHS) agencies and some foundations have contributed funds for several studies. In 2005, about two-thirds of ACTION’s funding was obtained through interagency agreements or other mechanisms.
The IDSRN and ACTION emphasize demand-driven, practical, applied, rapid-cycle work across a broad range of topics. The 93 task orders issued by the IDSRN between 2000 and 2005 were completed on average in 15 months. Through ACTION, AHRQ will attempt to take implementation and the uptake of innovation to scale. The IDSRN evaluation found evidence in about 60 percent of task orders (projects) of demonstrable uptake of innovation. Most uptake of innovation was local, suggesting that the
organizations that conducted the research used the results to improve the quality of care delivery. Part of ACTION’s charge is to extend that kind of uptake more broadly, even nationwide where possible.
To help with dissemination, ACTION partnerships include firms with expertise in communication, dissemination, marketing, and other areas to improve both dissemination and uptake of innovation by the partnerships themselves. Dissemination projects are accomplished under contract rather than through grants—funding studies to procure tools, products, and strategies that are felt to be generalizable and sustainable within other delivery systems.
ACTION has several strategic advantages. Collectively, the partnerships serve more than 100 million individuals through a variety of providers working in widely diverse settings of care. ACTION also has broad diversity in payer, geographic, and demographic mix. This is important to AHRQ’s goal to focus on priority populations such as children, the elderly, the disabled, minorities, and other underserved individuals. ACTION’s partnerships have access to large robust datasets and nationally recognized academic and field-based researchers with expertise in data manipulation methods and emerging organizational and management issues. The partnerships’ operational leaders are committed to helping set the network’s agenda and using findings of value to their organizations. Perhaps ACTION’s most important strategic advantage is speed; the time between the release of a Request for Proposal and an award is approximately 9 to 12 weeks, and the average project is completed in 15 months. Finally, although ACTION is too new to have tested the impact of project findings, the IDSRN had significant impact, with dozens of local to international examples of uptake of tools and strategies developed and tested in IDSRN projects. This occurred in part because, in addition to publications in peer-reviewed and trade journals, AHRQ asks for deliverables such as presentations to the healthcare operational leadership and at live or web-assisted conferences; scalable, scenario-appropriate models; training curricula, workshops, and workshop tools; and “how-to” guides, workbooks, DVDs, and webcasts.
THE HMO RESEARCH NETWORK AS A TEST BED
Eric B. Larson, M.D., M.P.H., M.A.C.P.
Group Health Cooperative’s Center for Health Studies for the HMO Research Network1
Learning from experience is something that individuals do continually and automatically. For a country whose health care has been widely
characterized as costly, inefficient, dangerous, and often falling well short of best practices, learning from experience should serve as a driving force in our efforts to cross the quality chasm (IOM 2001). Making this happen, however, remains a tremendous challenge. If we were truly able to improve health care constantly by learning from experience, presumably we would have already solved the problems highlighted in recent Institute of Medicine (IOM) reports on health care and quality (IOM 2007a, 2007b, 2006a, 2006b, 2004a, 2004b, 2004c, 2004d, 2003a, 2003b, 2002). What we need is a setting equipped to conduct observational investigations under normal working conditions. Engineers call this a test bed.
HMORN’s Key Features
The HMO Research Network (HMORN) links 15 integrated delivery systems (Figure 5-1). Collectively and individually, these member organizations are examples of learning healthcare systems that can—and do—learn through direct experience. Over the past decade, HMORN has collected evidence from both its own research and research in general, translating that evidence into both better and best practices. This paper explores some key questions that a learning organization such as HMORN faces, a few of the lessons learned, and several of the priorities and needs required to foster healthcare systems that are truly devoted to learning.
A learning healthcare system such as HMORN faces five key questions:
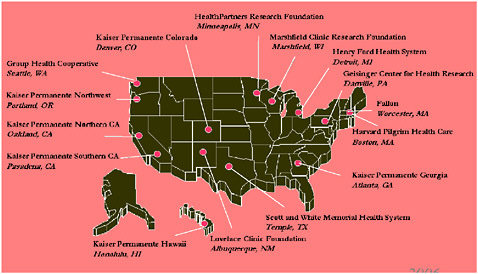
FIGURE 5-1 Sites in the HMO Research Network.
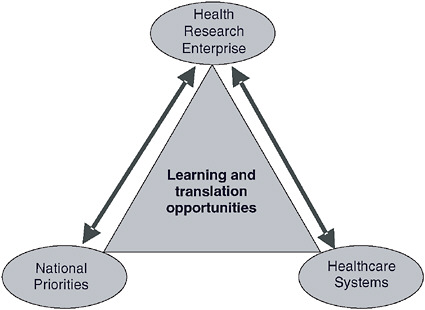
FIGURE 5-2 Reciprocal exchange between research and both healthcare and national priorities.
(1) What is structured real time learning? (2) What are some seminal features of integrated delivery systems? (3) Why are integrated delivery systems well suited to structured real-time learning? (4) Why might linking integrated delivery systems surpass single integrated delivery systems for realtime learning? (5) What is the potential of HMORN, and what are some of the priority areas in which we could accelerate the benefits of such linkages? Answering these questions illuminates the unique features of HMORN and sets the stage for discussing both the network’s potential and the priorities for using it as a real-time test bed, or a learning healthcare system.
Structured Real-Time Learning
The HMORN seeks to achieve two-way (bidirectional or reciprocal) learning between health research and both national priorities and healthcare systems (Figure 5-2). This can occur when researchers take advantage of opportunities to respond to important national research issues in a realworld setting—and also use insights gained through that setting to advance the nation’s research agenda. In this way, a healthcare research enterprise such as HMORN can mediate the exchange of learning and translation between healthcare systems and national priorities. The knowledge exchange
between these two spheres, especially when conducted in a way that promotes implementation of new knowledge, can greatly accelerate advances in health care through opportunities for translation.
Seminal Features of Integrated Delivery Systems
Integrated delivery systems offer close ties between care delivery, financing, administration, and patients. Ideally, this leads to closer alignment of incentives, especially for ongoing efforts to improve quality. HMORN centers have shared administrative claims and clinical databases. Where researchers enjoy effective relationships with administrators, clinicians, and even health plan members, interest in research projects can be truly shared—and research results are more likely to be translated appropriately. Partnerships with the parent organizations can then lead to research teams that include practicing clinicians and HMORN scientific collaborators.
Another key feature is that institutional review boards (IRBs) oversee all research activities. These IRBs typically include administrators, clinicians, local researchers, and members of the public. The local IRB is a critical control point for all research in an integrated delivery system. Unlike other healthcare research, which enrolls participants from the community, HMORN research typically involves health plan members as participants. Therefore, the HMORN IRBs review research protocols that can have real and perceived influences on the members’ relationships with their HMO (Greene and Geiger 2006).
Fit with Structured Real-Time Learning
Healthcare systems can—and in fact do—serve as natural laboratories for applied research questions: thus they have an ongoing role in efforts to optimize health and health care. When these efforts are combined with appropriate measurement strategies designed to answer defined research questions, healthcare systems offer unparalleled learning opportunities. The collective experiences of HMORN members include a wide array of pioneering trials in dozens of clinical and population-based areas, as well as epidemiological, cost-effectiveness, and clinical studies (Raebel et al. 2005; Fishman et al. 2004; Elmore et al. 2005; Geiger et al. 2006; Paasche-Orlow et al. 2006). At this writing, these include research in diabetes prevention, warfarin metabolism, communication around genetic testing, and strategies to increase enrollment in clinical trials. The longest running longitudinal study of aging in the US has operated continuously for over five decades, located in a Seattle HMO (Group Health) (Schaie 1993). That same HMO for the past two decades, now facilitated by real time comprehensive member data, is the setting for ongoing cohort studies of aging and dementia
using representative random samples of a very stable population of persons over age 65. (Larson et al. 2006). This partial list illustrates the variety of cutting-edge clinical research opportunities, which include many other types of studies.
Advantage of Linked over Single Integrated Delivery Systems
This is really the question of the moment. The answer hinges on the fact that the larger and broader the scope of the study, the more heterogeneity one can achieve both in patient populations and in approaches to care and quality. Linked integrated delivery systems offer significant advantages over single ones: heterogeneous patient populations—and heterogeneous approaches to care—combined with an enduring commitment to translate research into practice and improve quality. This heterogeneity provides more insights into patient-, provider-, and system-level factors that affect care. The ability to examine these multiple factors has formed the cornerstone of several HMORN projects (Leyden et al. 2005; Taplin et al. 2004; et al. 2005; Stevens et al. 2005). Multisite studies improve generalizability, especially studies of interventions and evaluations.
The HMORN offers an unparalleled opportunity to study rare disorders in a more natural setting; by contrast, when studies are based only in referral centers, their samples are often highly selective. The scale of the population base (more than 15 million), plus electronic medical records, accurate diagnostic databases, and engaged scientists, should facilitate unique opportunities for studying rare disorders (as well as treatment effects in various subpopulations and common conditions).
A growing number of multisite studies, involving various combinations of HMORN, demonstrate the utility and value of linking integrated delivery systems:
-
The HMO Cancer Research Network (CRN) has been funded by NCI since 1999 to study the effectiveness of cancer control interventions across the spectrum of cancer from prevention to palliative care (Hornbrook et al. 2005; Wagner et al. 2005). The CRN was recently highlighted as an example of a “best-practice” research consortium at the 2006 National Leadership Forum on clinical research networks (Inventory and Evaluation of Clinical Research Networks 2006).
-
The Vaccine Safety Datalink is a collaborative project between the National Immunization Program of the Centers for Disease Control and Prevention (CDC) and eight large HMOs (DeStefano 2001). The project began in 1990 with the primary purpose of rigorously evaluating concerns about the safety of vaccines.
-
The Centers for Education and Research on Therapeutics (CERT) is a national demonstration program funded by AHRQ to conduct research and provide education that advances the optimal use of therapeutics (drugs, medical devices, and biological products) (Platt et al. 2001).
-
The DEcIDE Network (Developing Evidence to Inform Decisions about Effectiveness) is also funded by AHRQ. A collaborative research and practice-based network program, it helps AHRQ and other federal agencies to implement Section 1013 of the Medicare Modernization Act of 2003.
-
The NIH Roadmap Initiative on Reengineering the Clinical Research Enterprise, under which HMORN formed the Coordinated Clinical Studies Network (CCSN), is devoted to improving the systems and processes that support our many multisite studies (Greene et al. 2005a).
HMORN, Priority Areas, and Potential
The considerable potential of HMORN is illustrated by the priority areas in which we could accelerate the benefits of such linkages:
Natural experiments in health services research are ongoing: How do benefit changes, new health insurance products, and organization and policy shifts affect outcomes? How do increased prescription copayments affect adherence? When clinicians receive faxed alerts because their patients fail to renew prescriptions, how are outcomes affected? How does the use of “carve-out” disease management systems compare with that of integrated disease management systems that maintain intact primary care relationships? What happens when we provide physicians with performance feedback? What happens when new electronic medical records offer opportunities for more personalized care and real-time evaluation, including asynchronous direct communication with doctors and healthcare teams? How do patient behavior and healthcare utilization change when patients have direct access to specialty care?
Surveillance of the enrollee populations in the network is another general area in which HMORN can meet immediate needs: from biosafety to more focused post-marketing surveillance. The network offers size, data systems, and persons with programming, biostatistical, epidemiologic, and clinical expertise. In therapeutics, the CDC’s Vaccine Safety Datalink (eight HMORN plans) is the world’s foremost source of vaccine safety data. Ten HMORN health plans make up the largest component of the FDA’s population-based drug safety surveillance program. In infectious diseases, five plans have developed methods for early identification and reporting of acute illness clusters that might represent outbreaks of influenza, severe
acute respiratory syndrome (SARS), or bioterrorist events. CDC’s national BioSense initiative has adopted these methods. Members of HMORN have developed automatic reporting systems for notifiable diseases (including tuberculosis, Lyme disease, hepatitis C, and Chlamydia). In one intriguing example, a surveillance case study involved researchers and clinicians, working in real time, to address an urgent public health concern: at Kaiser Permanente Colorado, researchers compared the ability to detect influenza A through syndromic surveillance by providers, sentinel provider diagnosis, and laboratory-confirmed cases. The study concluded that syndromic surveillance can be useful for detecting clusters of respiratory illness in various settings (Ritzwoller et al. 2005).
The HMORN is creating the largest observational test bed for IOM’s aims for patient-centered health information technology (IOM 2001). Several plans have functioning web-based patient portals, and others are in development. These respond to the IOM care redesign rules that encourage transparency, free flow of information, and patient control. This is an area ripe for discovery and close examination. Web-based patient portals, secure messaging between patients and providers, and the accompanying transformation of the doctor-patient interaction could lead to dramatic changes in cost and quality of care and market dynamics. HMORN is uniquely positioned to study the impact. HMORN plans have conducted randomized trials and quasi-experimental studies of computerized physician order entry (CPOE) systems, including studies of different kinds of alerts and academic detailing to reduce prescribing errors. Additionally, two HMORN randomized controlled trials (RCTs) are testing tailored health behavior change interventions (for fruit and vegetable consumption and smoking cessation) that could eventually be integrated into these portals. Finally, at the levels of both health plans and research centers, we are participating in the dialogue about the structure, function, and standards that would compose a national healthcare infrastructure. Interoperability of healthcare data (allowing data sharing among disparate systems) will influence—and, we hope, enhance—the depth and breadth of research that HMORN could undertake.
The HMORN is extremely well-suited to developing and implementing clinical trials based either on individual or on “cluster” randomization (i.e., intact groups of individuals are assigned randomly to receive different interventions). Of particular relevance in HMORN are practical clinical trials (Tunis et al. 2003), marked by heterogeneous populations and practice settings and their ability to study a broad range of clinically meaningful health outcomes. Information systems will routinely include data on all aspects of care useful for screening populations for eligible participants. In stable HMOs, these systems can provide opportunities for automated, less resource intensive, and longer follow-up, providing real-world outcomes
over time. A newly funded HMORN study will examine ways to reduce barriers to trial accrual (Somkin et al. 2005), focusing on the effect of patient- and provider-level notification systems that can flag potentially eligible patients for specified cancer clinical trials. In systems that enjoy high levels of trust from patients and healthcare professionals, clinical trials can have high enrollment rates and excellent follow-up rates. The HMORN’s NIH Roadmap project has developed infrastructure and tools designed to determine easily how feasible a given clinical trial is in subject availability, budget, more efficient coordination of human subjects review processes, enrollment manuals, data collection processes, adherence to Health Insurance Portability and Accountability Act (HIPAA) rules, and coordinated close-out processes. This project is now working on improving the readability of consent forms and other subject materials and improvements in cluster randomized trial methodologies. These efforts should greatly facilitate the ability to perform clinical trials in HMORN and may lower the costs of launching these trials.
The HMORN has consistently proved its ability to conduct studies designed to enhance the quality of patient care. Such studies, which have direct application in delivery systems, run the gamut. Case-control studies of late-stage breast and cervical cancer showed that failure to screen ever, or within the past three years, explained more than half of all cases of delayed diagnoses (Leyden et al. 2005; Taplin et al. 2004). This, in turn, led to systematic redesign of screening outreach programs among HMOs participating in this study. Studies of clinicians’ use of the “five As” framework (ask, advise, assist, agree, arrange) to encourage patients to quit smoking resulted in two sites changing their tobacco cessation programs (Solberg et al. 2004). Perhaps the most notable example of an HMO-based effort to improve the quality of patient care was the development of the Chronic Care Model (Greene et al. 2005b). This model, which has been widely adopted throughout HMORN and worldwide, is distinguished by its strong evidence base and comprehensive attention to the many drivers that influence the quality and outcome of the patient-practitioner interaction. Finally, physician-focused interventions have demonstrated how techniques such as computerized physician order entry reminders, “academic detailing,” or physician feedback (comparing physicians with their peers) can improve appropriate prescribing in older patients and compliance with guidelines (Simon et al. 2006; Smith et al. 2006).
Improving the HMORN Test Bed
To realize fully an ongoing system of structured real-time learning, several structural innovations are needed. These innovations must facilitate two-way knowledge transfer and the ability to share and apply research
lessons that will benefit not only members of HMORN but U.S. health care overall. Today, the leadership of HMORN has developed mechanisms for HMO researchers and clinicians to create and implement new studies. Currently, these mechanisms are somewhat ad hoc but can be memorialized in everyday processes and procedures. Task order funding models allow development of rapid-cycle research proposals and projects. However, the rapid cycle is challenging, both for smaller centers with less infrastructure and for multisite studies. Ideally, these funding mechanisms would include some allowance for infrastructure support and even the resources and time to embed lessons in the delivery system. That support would then cover staff needed for enhanced processes and procedures across the network.
A durable infrastructure in a network-based setting would greatly reduce inefficiencies and speed the cycle of research and translation. One of the key findings of the national study of clinical research networks was the amount of redundancy and reinvention that takes place in collaborative research (Inventory and Evaluation of Clinical Research Networks 2006). By now, there should be ways to simplify and institutionalize many processes that are common to all studies. Through the NIH Roadmap’s CCSN, HMORN has begun to develop new approaches to coordinate IRB reviews of multisite studies, which can be among the more time-consuming aspects of collaborative projects. It would be mutually beneficial for NIH, the Office for Human Research Protections (OHRP), and other bodies responsible for harmonizing regulations and developing new guidance for the protection of human subjects to work with groups such as HMORN to ensure that innovations to minimize redundancy and rework (which plague multisite studies today) are feasible and, if so, incorporated into national models for research review.
The HMORN has, at best, informal—and, at worst, inconsistent—systems to collect, disseminate, and translate innovations and best practices among its members and to help meet its mission as a nonproprietary, public interest research group (Greene, Hart, and Wagner 2005b). If the United States continues to rely on private “market forces” to render efficiencies in its healthcare system, an invaluable adjunct would be a mechanism to collect, disseminate, and translate innovations and best practices (especially those funded by public and not-for-profit foundation sources). The HMORN might be ideally suited to take on this important task as a demonstration project. The general inclination of leaders in HMORN is to support the general good, rather than the private good, through their research efforts and results. However, in our current market-driven system, and in the absence of such a demonstration project, researchers are exposed to understandable conflicts in their need to balance proprietary and national interests.
An ongoing challenge that all HMORN centers face is how to involve
operational staff, including physicians and other professionals, in research. Experience has shown that when front-line staff participate in design and implementation, such research is more likely to be relevant and “field-ready” for translation. However, it is usually difficult to justify costs for such persons in today’s research budgets. Funding agencies should consider including front-line staff as a specification in certain Requests for Applications (RFAs)—particularly those that emphasize translation and dissemination. Learning organizations such as the members of HMORN would likely be able to demonstrate or at least respond to such specifications, because integrated delivery systems typically take an organized approach to determining the applicability of research findings and translating research into practice.
In summary, we believe the HMO Research Network affords the possibility of a structured real-time learning test bed that approaches the ideal. This paper emphasizes that HMORN is uniquely poised to take advantage of opportunities for two-way learning, and we have described several areas in which the Network and its members have conducted—and can further conduct—research that will improve the development and application of evidence to improve healthcare quality and effectiveness. Figure 5-3 illustrates what this might look like. The figure’s important features are its reliance on reciprocity and its ability to link real-world care and research to public health goals. If we are to learn from experience about approaches to improving the health of the public, the accumulated experiences of HMORN are a logical place to begin.
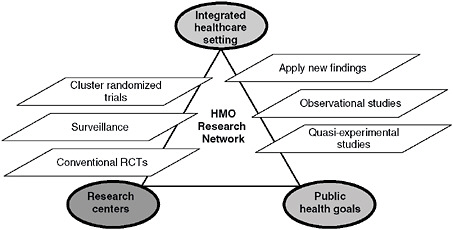
FIGURE 5-3 Idealized intersections among priorities in health care, research, and public health.
COUNCIL OF ACCOUNTABLE PHYSICIAN PRACTICES
Michael A. Mustille, M.D.
The Permanente Federation
The healthcare sector is beset by a constellation of perceived failures. Cost inflation threatens the affordability of care not only for individuals but for businesses, government entities, and ultimately, for the nation as a whole. Quality of care appears mediocre in general and does not seem to be substantially better now than it was 15 years ago (McGlynn et al. 2003). What is known of the science of medicine is too often not accomplished in the practice of medicine. Acquisition, management, and sharing of medical information are inhibited by a combination of anachronistic paper record keeping and scarce, idiosyncratic automated systems that are designed primarily for financial, not clinical, transactions. Underlying these shortcomings is a payment structure too often poorly aligned, and sometimes contradictory, with regard to incentives and priorities that would lead to improvements in care quality and cost.
These failures are complex in origin, and the corrections needed to remedy them will not be simple. However, one element of the solution that offers promise has largely been absent from public debates on health sector problems—namely, care delivery system redesign and, in particular, redesign of physician practice. There are clear examples of physician group practices that have been very successful in delivering high-quality care at reasonable cost despite the general health sector failures outlined above. A recent paper in Health Services Research examined multispecialty medical groups (MSMGs) and showed the positive relationship between delivery system organization and Health Plan Employer and Data Information Set (HEDIS) Effectiveness of Care measure scores (Gillies et al. 2006). This cross-sectional, multivariate regression analysis examined the impact of health plan organizational characteristics (i.e., tax status, size, age, type of system used to deliver care) on clinical process and patient satisfaction measures. The analysis of HEDIS scores showed that delivery systems organized around multispecialty staff or group practices outperformed other less integrated delivery models on clinical process measures, while member satisfaction as measured by Consumer Assessment of Health Care Providers and Systems (CAHPS) was no different. These organized delivery systems have succeeded for years, and in many cases for decades, in dealing with issues of cost, quality, and trust. A physician voice from these systems could point to characteristics and features of their groups that would be helpful to those seeking solutions for the complex problems plaguing the healthcare sector.
In 2002, a number of physician leaders from successful MSMGs across the United States gathered to discuss the issue of physician practice redesign
and founded a not-for-profit organization called the Council of Accountable Physician Practices. A look at the roster of CAPP members shows that they are located throughout the nation and indicates that MSMGs have been successful in a variety of different geographies and settings:
Austin Regional Clinic, Texas
Billings Clinic, Montana
Cleveland Clinic, Ohio
Dean Health System, Wisconsin
Duluth Clinic, Minnesota
Everett Clinic, Washington
Fallon Clinic, Massachusetts
Geisinger Clinic, Pennsylvania
Group Health Permanente, Idaho, Washington
Harvard Vanguard Medical Associates, Massachusetts
HealthCare Partners Medical Group, Southern California
HealthPartners, Minnesota
Henry Ford Medical Group, Michigan
Intermountain Health Care, Utah
Jackson Clinic, Tennessee
Lahey Clinic, Massachusetts
Marshfield Clinic, Wisconsin
Mayo Clinic, Arizona, Florida, Minnesota
Mayo Health System, Iowa, Minnesota, Wisconsin
Nemours, Delaware, Florida, Maryland, New Jersey, Pennsylvania
Ochsner Clinic, Louisiana
Palo Alto Medical Foundation, Northern California
Permanente Federation, Northern and Southern California, Colorado, District of Columbia, Georgia, Hawaii, Maryland, Ohio, Oregon, Virginia, Washington
Scott and White, Texas
Sharp Rees-Stealy Medical Group, Southern California
Virginia Mason Clinic, Washington
Wenatchee Valley Medical Center, Washington
Since 2002, CAPP has gathered evidence from its members and from the medical literature about the achievements of multispecialty medical groups and is supporting further research to refine the understanding of key success factors. CAPP’s array of MSMGs offers an opportunity to study the design elements and characteristics of successful physician practice and can serve as a model for delivery system redesign.
An example serves to illustrate the potential benefits in studying MSMGs. Physicians from Group Health Cooperative of Puget Sound were
able to rapidly translate the benefits of groundbreaking heart disease research into improved medical treatment for diabetics, with a great reduction in cardiovascular complications over the expected result. Less than two years after publication of the Heart Protection Study on the use of statins (Gurm and Hoogwerf 2003) and the preceding Heart Outcomes Prevention Evaluation (HOPE) trial on angiotensin-converting enzyme (ACE) inhibitor use in patients with diabetes (Yusuf et al. 2000), Group Health had developed new clinical practice recommendations for medication management in diabetes based on these studies, embedded them into its population care management system, developed educational materials for primary care clinicians and patients, and redesigned important roles and tasks in care teams to ensure their implementation. By comparison, some estimate that it generally takes 17 years to translate evidence-based care practices from the literature into general use in medical practice (Balas and Boren 2000). Knowing the critical elements that enabled this advance in care and having some insight as to how they were put in place would greatly advance physician practice redesign.
CAPP’s 35 MSMGs share a common vision as learning organizations dedicated to the improvement of clinical care. Their features include physician leadership and governance; commitment to evidence-based care management processes; well-developed quality improvement systems; team-based care; the use of advanced clinical information technology; and the collection, analysis, and distribution of clinical performance information. These features are congruent with the IOM recommendations on key elements needed to redesign delivery systems. The Chasm report (IOM 2004a) envisions a delivery system capable of meeting six challenges: (1) evidence-based care processes; (2) effective use of information technology; (3) knowledge and skills management; (4) development of effective teams; (5) coordination of care across patient conditions, services, and settings over time; and (6) use of performance and outcome measurement for continuous quality improvement and accountability.
Physician governance is a key characteristic of MSMGs. These are physician-led organizations that are guided by ethical and medical principles important to physicians, creating a professional and scientific approach to governance that is deeply rooted in the quality improvement processes of peer review and information sharing, thus cultivating an environment that fosters care improvement. Group responsibility is a clearly articulated commitment. Physicians in MSMGs are responsible not only for the individual and unique patient they are currently treating, but for all of the patients cared for by the practice. Colleagues in group practice have adopted performance management practices to hold one another accountable to provide the best quality of care possible and to contribute to the improvement of the quality of care over time.
MSMG processes and infrastructure characteristics include an evidence-based approach to care. One of the areas of general health sector failure involves the identification and dissemination of successful practices. MSMGs have an organized quality improvement structure that supports identification of important literature contributions to care improvement, analyzes physician and group care practices in light of the science base, can widely disseminate and implement needed changes to care practices based on the evidence, and can monitor physician implementation of the successful practices. Physicians practicing together using a shared medical record, a single organizational structure, and a common payment and incentive system are better able to improve not only the quality of care but also the efficiency of resource use. Because processes and outcomes can systematically be tracked in such a practice, administrative and clinical redundancy and waste are easily recognized and can effectively be eliminated.
Effective knowledge management tools are central to successful learning systems, and in the case of health care, clinical information technology is a critical component. Multispecialty medical groups are at the forefront of using health information technology (HIT) and electronic health records (EHR) to support advanced systems of care. Since MSMGs typically share a common, often automated, medical record, they have access to every part of the patient’s experience from ambulatory through hospital to convalescent and end-of-life care. Providing services across the full continuum of care enables them to collect data across this continuum. Such a depth of timely, accurate, clinically relevant information, shared among all providers and available for detailed analysis and reporting, not only supports high-quality, efficient care, but is also a key resource for identifying practice redesign elements.
Common information systems complement team-based care because all team members have access to a shared medical record as they collaborate to manage both illness and health. Collaboration is critical in treating patients with chronic conditions, especially those with multiple, comorbid conditions. The chronic care model (Wagner 1998) provides a clear framework for understanding how shared information is central to success in treating these patients. The team has access to timely and accurate clinical data, and to the extent that information is effectively shared with the patient and the family, all team members are well prepared to collaborate in developing and executing the treatment plan. Shared systems enable the coordination of care within the group and can be used to personalize care through shared decision making such that the patient becomes the focal point of care.
Recently, attention has focused on the availability and sharing of accurate performance information, a process that has come to be called “transparency.” Measuring and sharing performance among providers is a critical first step to achieving transparency, and because of shared clinical infor-
mation systems, the MSMGs are designed to enable consistent individual physician performance monitoring. Transparency among physicians within the group is the central characteristic of MSMG peer review. It provides the opportunity to compare individual physicians against group benchmarks, permits even broader transparency in comparisons outside the group, and ultimately enables reporting to patients, payers, and the general public. This process of acquiring and reporting accurate information on performance supports a consumer’s decisions when choosing a physician practice or a treatment team, carrying out treatment plans, and assuming responsibility for self-management, thus finally linking the chain of transparency to informed and effective action to improve health.
Multispecialty medical groups can fill what are currently research gaps between clinical trial research, epidemiological research, and the real-world delivery of care. The multispecialty medical group is a practically oriented healthcare delivery system that translates what is known from research into what is done in practice and, ultimately, what improves the outcomes and efficiency of care. Studying the factors that enhance or impair this process will be illuminating. CAPP, as an example, is currently sponsoring research that examines the impact of the electronic health record on the management of diabetes and explores the correlation between various care management practices supported by the EHR and geographic area variation in medical quality outcomes and resource use. Such studies are generally not possible in non-integrated delivery models where inconsistency in practice and data capture inhibits accurate observation.
The Institute of Medicine can play a critical role in enhancing our ability to learn from the successes of MSMGs. The IOM has already enunciated principles that serve to point the direction to accomplish the task. In 2003, the IOM identified 20 Priority areas for national action. The 20 areas included not only complex chronic conditions, such as diabetes, that greatly benefit from integrated care, but also what the IOM termed “cross-cutting” areas, such as care coordination and self-management or health literacy (IOM 2003c). Coordinated care is at the heart of MSMG systems of care.
Further elaboration came in 2005 with the Performance Measurement report that envisioned an organized research agenda, jointly sponsored by federal and private stakeholders (IOM 2006b):
Recommendation 5 (IOM, 2006b): The National Quality Coordination Board should formulate and promptly pursue a research agenda to support the development of a national system for performance measurement and reporting. The board should develop this agenda in collaboration with federal agencies and private-sector stakeholders. The agenda should address the following:
-
Development, implementation, and evaluation of new measures to address current gaps in performance measurement.
-
Applied research focused on underlying methodological issues, such as risk adjustment, sample size, weighting, and models of shared accountability.
-
Design and testing of reporting formats for consumer usability.
-
Evaluation of the performance measurement and reporting system.
Several issues critical to improvement of the care delivery system could well be examined in such a research agenda by formulating studies using multispecialty medical groups as the test bed. The IOM should make recommendations in these specific areas:
-
Encourage studies using common measurement sets across multiple delivery system models in a way that can compare their impact on coordination of care, clinical quality, patient satisfaction, and efficiency of resource use. Studies must consider both MSMGs and nonintegrated practices by incorporating measures and methods to compare practice-level and group-level performance in addition to individual physician performance. The AQA (Ambulatory Care Quality Alliance) has recently endorsed a set of quality measures that are intended to be applied to all physicians regardless of practice type and will eliminate the gap in performance data from non-group and small-group practices that has hampered such comparisons in the past (Crosson 2005).
-
Encourage physician leaders to design and create more integrated care systems. Most physician leaders have historically reacted defensively to the quality and cost challenges we face in the healthcare sector, rather than proactively redesigning the system of care. In contrast, MSMG leaders have generally demonstrated greater innovation and success in meeting these challenges. The IOM should recommend programs, incentives, and legislation that encourage more leaders interested in designing and developing such organizations to come forward.
-
Encourage studies to elucidate the most effective method to incentivize physicians to improve quality and efficiency. The impact of rewarding individual physician performance versus physician group performance is likely quite different, and there is little evidence available on how to balance incentives at different levels to achieve the most effective care system and the best care outcomes. Pay-for-performance programs for physicians are proliferating in the healthcare sector and should be designed by using an evidence-based approach.
REFERENCES
Balas, E, and S Boren. 2000. Managing clinical knowledge for healthcare improvements. In Yearbook of Medical Informatics, edited by V Schatauer. Stuttgart, Germany: Schattauer Publishing.
Crosson, F. 2005. The delivery system matters. Health Affairs 24(6):1543-1548.
DeStefano, F. 2001. The Vaccine Safety Datalink project. Pharmacoepidemiology and Drug Safety 10(5):403-406.
Elmore, J, L Reisch, M Barton, W Barlow, S Rolnick, E Harris, L Herrinton, A Geiger, R Beverly, G Hart, O Yu, S Greene, N Weiss, and S Fletcher. 2005. Efficacy of breast cancer screening in the community according to risk level. Journal of the National Cancer Institute 97(14):1035-1043.
Fishman, P, M Hornbrook, R Meenan, and M Goodman. 2004. Opportunities and challenges for measuring cost, quality, and clinical effectiveness in health care. Medical Care Research and Review 61(3 Suppl.):124S-143S.
Geiger, A, C West, L Nekhlyudov, L Herrinton, I Liu, A Altschuler, S Rolnick, E Harris, S Greene, J Elmore, K Emmons, and S Fletcher. 2006. Contentment with quality of life among breast cancer survivors with and without contralateral prophylactic mastectomy. Journal of Clinical Oncology 24(9):1350-1356.
Gillies, R, K Chenok, S Shortell, G Pawlson, and J Wimbush. 2006. The impact of health plan delivery system organization on clinical quality and patient satisfaction. Health Services Research 41(4 Pt. 1):1181-1199.
Greene, S, and A Geiger. 2006. A review finds that multicenter studies face substantial challenges but strategies exist to achieve Institutional Review Board approval. Journal of Clinical Epidemiology 59(8):784-790.
Greene, S, E Larson, D Boudreau, K Johnson, J Ralston, R Reid, and P Fishman. 2005a. The Coordinated Clinical Studies Network: a multidisciplinary alliance to facilitate research and improve care. The Permanente Journal 9(4):33-35.
Greene, S, G Hart, and E Wagner. 2005b. Measuring and improving performance in multicenter research consortia. Journal of the National Cancer Institute Monographs (35):26-32.
Gurm, H, and B Hoogwerf. 2003. The Heart Protection Study: high-risk patients benefit from statins, regardless of LDL-C level. Cleveland Clinic Journal of Medicine 70(11):991-997.
Hornbrook, M, G Hart, J Ellis, D Bachman, G Ansell, S Greene, E Wagner, R Pardee, M Schmidt, A Geiger, A Butani, T Field, H Fouayzi, I Miroshnik, L Liu, R Diseker, K Wells, R Krajenta, L Lamerato, and C Neslund Dudas. 2005. Building a virtual cancer research organization. Journal of the National Cancer Institute Monographs 35:12-25.
IOM (Institute of Medicine). 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press.
———. 2002. Guidance for the National Healthcare Disparities Report. Washington, DC: The National Academies Press.
———. 2003a. Financing Vaccines in the 21st Century: Assuring Access and Availability. Washington, DC: The National Academies Press.
———. 2003b. Describing Death in America: What We Need to Know. Washington, DC: The National Academies Press.
———. 2003c. Priority Areas for National Action: Transforming Health Care Quality. Washington, DC: The National Academies Press.
———. 2004a. 1st Annual Crossing the Quality Chasm Summit: A Focus on Communities. Washington, DC: The National Academies Press.
———. 2004b. Health Literacy: A Prescription to End Confusion. Washington, DC: The National Academies Press.
———. 2004c. Insuring America’s Health: Principles and Recommendations. Washington, DC: The National Academies Press.
———. 2004d. Quality Through Collaboration: The Future of Rural Health. Washington, DC: The National Academies Press.
———. 2006a. Improving the Quality of Health Care for Mental and Substance-Use Conditions: Quality Chasm Series. Washington, DC: The National Academies Press.
———. 2006b. Performance Measurement, Accelerating Improvement. Washington, DC: The National Academies Press.
———. 2007a. Hospital-Based Emergency Care: At the Breaking Point. Washington, DC: The National Academies Press.
———. 2007b. Preventing Medication Errors: Quality Chasm Series. Washington, DC: The National Academies Press.
Inventory and Evaluation of Clinical Research Networks. 2006 (July 28). IECRN Best Practices Study Profile of Networks. Available from http://www.clinicalresearchnetworks.org/documents/BPNetworkProfiles.pdf. (accessed November 26, 2006).
Larson, E, L Wang, J Bowen, W McCormick, L Teri, P Crane, and W Kukull. 2006. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Annals of Internal Medicine 144(2):73-81.
Leyden, W, M Manos, A Geiger, S Weinmann, J Mouchawar, K Bischoff, M Yood, J Gilbert, and S Taplin. 2005. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. Journal of the National Cancer Institute 97(9):675-683.
McGlynn, E, S Asch, J Adams, J Keesey, J Hicks, A DeCristofaro, and E Kerr. 2003. The quality of health care delivered to adults in the United States. New England Journal of Medicine 348(26):2635-2645.
Paasche-Orlow, M, D Schillinger, S Greene, and E Wagner. 2006. How health care systems can begin to address the challenge of limited literacy. Journal of General Internal Medicine 21(8):884-887.
Platt, R, R Davis, J Finkelstein, A Go, J Gurwitz, D Roblin, S Soumerai, D Ross-Degnan, S Andrade, M Goodman, B Martinson, M Raebel, D Smith, M Ulcickas-Yood, and K Chan. 2001. Multicenter epidemiologic and health services research on therapeutics in the HMO Research Network Center for Education and Research on Therapeutics. Pharmacoepidemiology and Drug Safety 10(5):373-377.
Quinn, V, V Stevens, J Hollis, N Rigotti, L Solberg, N Gordon, D Ritzwoller, K Smith, W Hu, and J Zapka. 2005. Tobacco-cessation services and patient satisfaction in nine nonprofit HMOs. American Journal of Preventive Medicine 29(2):77-84.
Raebel, M, E Lyons, S Andrade, K Chan, E Chester, R Davis, J Ellis, A Feldstein, M Gunter, J Lafata, C Long, D Magid, J Selby, S Simon, and R Platt. 2005. Laboratory monitoring of drugs at initiation of therapy in ambulatory care. Journal of General Internal Medicine 20(12):1120-1126.
Ritzwoller, D, K Kleinman, T Palen, A Abrams, J Kaferly, W Yih, and R Platt. 2005. Comparison of syndromic surveillance and a sentinel provider system in detecting an influenza outbreak—Denver, Colorado, 2003. MMWR Morbidity and Mortality Weekly Report 54(Suppl.):151-156.
Schaie, K. 1993. The Seattle Longitudinal Study: a thirty-five-year inquiry of adult intellectual development. Zeitschrift fur Gerontologie 26(3):129-137.
Simon, S, D Smith, A Feldstein, N Perrin, X Yang, Y Zhou, R Platt, and S Soumerai. 2006. Computerized prescribing alerts and group academic detailing to reduce the use of potentially inappropriate medications in older people. Journal of the American Geriatrics Society 54(6):963-968.
Smith, D, N Perrin, A Feldstein, X Yang, D Kuang, S Simon, D Sittig, R Platt, and S Soumerai. 2006. The impact of prescribing safety alerts for elderly persons in an electronic medical record: an interrupted time series evaluation. Archives of Internal Medicine 166(10):1098-1104.
Solberg, L, V Quinn, V Stevens, T Vogt, N Rigotti, J Zapka, D Ritzwoller, and K Smith. 2004. Tobacco control efforts in managed care: what do the doctors think? American Journal of Managed Care 10(3):193-198.
Somkin, C, A Altschuler, L Ackerson, A Geiger, S Greene, J Mouchawar, J Holup, L Fehrenbacher, A Nelson, A Glass, J Polikoff, S Tishler, C Schmidt, T Field, and E Wagner. 2005. Organizational barriers to physician participation in cancer clinical trials. American Journal of Managed Care 11(7):413-421.
Stevens, V, L Solberg, V Quinn, N Rigotti, J Hollis, K Smith, J Zapka, E France, T Vogt, N Gordon, P Fishman, and RG Boyle. 2005. Relationship between tobacco control policies and the delivery of smoking cessation services in nonprofit HMOs. Journal of the National Cancer Institute Monographs (35):75-80.
Taplin, S, L Ichikawa, M Yood, M Manos, A Geiger, S Weinmann, J Gilbert, J Mouchawar, W Leyden, R Altaras, R Beverly, D Casso, E Westbrook, K Bischoff, J Zapka,, and W Barlow. 2004. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? Journal of the National Cancer Institute 96(20):1518-1527.
Tunis, S, D Stryer, and C Clancy. 2003. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. Journal of the American Medical Association 290(12):1624-1632.
Wagner, E. 1998. Chronic disease management: what will it take to improve care for chronic illness? Effective Clinical Practice 1(1):2-4.
Wagner, E, S Greene, G Hart, T Field, S Fletcher, A Geiger, L Herrinton, M Hornbrook, C Johnson, J Mouchawar, S Rolnick, V Stevens, S Taplin, D Tolsma, and T Vogt. 2005. Building a research consortium of large health systems: the Cancer Research Network. Journal of the National Cancer Institute Monographs (35):3-11.
Yusuf, S, P Sleight, J Pogue, J Bosch, R Davies, and G Dagenais. 2000. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New England Journal of Medicine 342(3):145-153.


























