4
Agronomic and Environmental Effects of Genetically Engineered Crops
This chapter examines the evidence on agronomic and environmental effects of currently commercialized genetically engineered (GE) crops. The analysis in this chapter is retrospective, looking at the effects that have occurred between the 1990s, when GE crops were first commercialized, and 2015. Although this chapter mentions general economic effects in a few places, full discussion of this topic is in Chapter 6.
As stated in Chapter 3, the United States was the first country to commercialize GE crops. Roughly half of U.S. land in crop production in 2014 was planted with GE crops—primarily maize (Zea mays), soybean (Glycine max), and cotton (Gossypium hirsutum)—and this area made up 40 percent of the world’s production of GE crops (Fernandez-Cornejo et al., 2014; James, 2015). Given its market share, it is not surprising that much of the research on agronomic and environmental effects of genetic engineering in agriculture has been conducted in the United States. The committee relied primarily on that literature for much of its analysis, but it also drew on studies available from other countries that produce GE crops. Chapter 3 noted that most GE crops in production from the 1990s to 2015 were engineered with resistance to herbicides, resistance to insects, or a combination of the two; this review of agronomic and environmental effects therefore is focused on these traits.1
__________________
1 The committee recognizes that there are other approaches to managing crop pests besides GE crops; many of these, including the implementation of production systems the use agroecological principles to reduce the need for pesticides, were addressed in the 2010 National Research Council report Toward Sustainable Agricultural Systems in the 21st Century (NRC, 2010b). The present committee is aware of the central role that agroecology plays in fostering resilience in agriculture, but its report focuses specifically on the role and effects of GE crops.
The chapter begins with an analysis of the interaction between genetic-engineering technology and crop yield. That is followed by an examination of the agronomic effects of insect-resistant (IR) crops, specifically in terms of crop yield, insecticide use, secondary insect-pest populations, and the evolution of resistance to the GE trait in targeted insect populations. A similar review is conducted for the effects related to herbicide-resistant (HR) crops. There is discussion of the effects on crop yield of herbicide and insect resistance used together. Then the chapter turns to the environmental effects of IR and HR crops on the farm and beyond, including effects on biodiversity in plant and animal communities and diversity of crop species and varieties2 planted on farms and potential effects of GE crops on landscapes and ecosystems. A GE variety’s characteristics are due to a combination of the GE trait and the background germplasm into which the trait is placed. Therefore, the committee has endeavored to be specific about the effect of the trait itself on the crop’s performance and environmental effects. Unless otherwise noted, whenever a difference is noted in this chapter it is statistically significant.
EFFECTS OF GENETIC ENGINEERING ON CROP YIELDS
Over the course of the study, the committee heard from speakers and received public comments that indicated that GE crops and their accompanying technologies were not substantially increasing crop yields; other comments and speakers endorsed genetic engineering as a contributor to yield increase, yield stabilization, or both.3 Before examining the evidence available on the effects on crop yields, it is useful to understand the factors that influence crop yield in general.
Potential versus Actual Yield
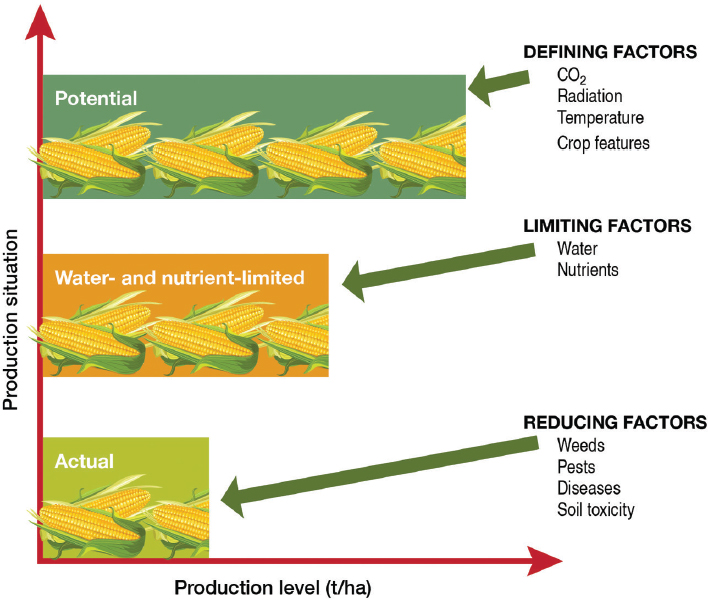
The distinction between potential yield and actual yield has been discussed in an earlier National Research Council report (NRC, 2010a)4 and other studies and reports (Sinclair, 1994; van Ittersum and Rabbinge, 1997; Gurian-Sherman, 2009; Lobell et al., 2009). Potential yield is the theoretical yield a crop genotype can achieve without any limitations of water or
__________________
2 The term variety is used throughout this chapter in its most general sense to encompass varieties, cultivars, and hybrids.
3 Some of the comments expressing these views can be found in Appendix F.
4 In the 2010 National Research Council report, potential yield was defined as “the yield that would be realized in the absence of damage caused by pests (i.e., weeds, insects)” (NRC, 2010a:138). That report acknowledged that such weather conditions as wind, rain, drought, and frost could affect yield. In the present report, the definition of potential yield includes more detail to capture those limiting factors.
nutrients and without losses to pests and disease (van Ittersum et al., 2013), given a specified carbon-dioxide concentration, temperature, and incident photosynthetically active radiation (Figure 4-1). Limitations of natural nutrient and water availability cause gaps between the potential yield and actual yield if nutrient and water supplementation are not possible. Actual yield may be further curtailed by “reducing factors,” which can be organized into three main groups:
- Insect pest and diseases, which physically damage crops.
- Weeds, which reduce crop growth by competition for water, light, and nutrients.
- Toxicities caused by waterlogging, soil acidity, or soil contamination.
Genetic improvement of crops can close the gap between actual yield and potential yield or it can increase the overall potential yield. Such change can be accomplished in three ways. First, the potential yield can be increased; for example, the canopy architecture of the plant can be improved
SOURCE: Based on van Ittersum et al. (2013).
to increase the conversion of photosynthetically active radiation through photosynthesis. Second, limitations of water and nutrient availability can be ameliorated by enhancing the efficiency with which water and nutrients are captured and used for crop growth. Third, factors that reduce yield can be mitigated by protecting the crop from pests, including weeds, insects, and disease.
In general, all three kinds of improvement can be accomplished through conventional plant breeding (described in Chapters 2 and 3), genetic engineering, or a combination of the two. For example, conventional plant breeding in the 1960s and 1970s led to the development of semi-dwarf wheat (Triticum aestivum) and rice (Oryza sativa), which had greater potential yields than earlier varieties. Selection and mutagenesis, both conventional plant-breeding techniques, were used to develop varieties of maize, canola (Brassica napus), rice, wheat, and sunflower (Helianthus annuus) that were resistant to imidazolinone herbicides (Tan et al., 2005), thereby reducing competition between crops and weeds for water, light, and nutrients when the herbicide was applied.
As of 2015, most GE crops contained traits that were intended to reduce crop competition with weeds, prevent damage from insects, or both. A few commercialized crops were engineered for protection against viruses and others for environmental (abiotic) stress resistance, but little information was available on the effects of these GE traits on yield (Box 4-1). A 2010 National Research Council report on the impacts of GE crops, which focused on the United States, concluded that “GE traits for pest management have an indirect effect on yield by reducing or facilitating the reduction of crop losses” (NRC, 2010a:138). That is, GE traits for herbicide, insect, and virus resistance have the potential to close yield gaps, but they do not increase the potential yield of a crop. That report found that the yields of HR crops had not increased because of the HR trait and that the yields of IR crops had increased in areas that suffered substantial damage from insects that were susceptible to Bt toxins. That report also concluded that effects of GE crops change with time.
Few crops that target the yield-limiting factors of nutrient and water availability have been commercialized. A variety of maize with drought tolerance was commercially available when the committee was writing its report. Chang et al. (2014) evaluated the potential for eight drought-tolerant GE maize hybrids to increase grain production in high-water-deficit environments in South Dakota in 2009 and 2010. They found that the trait did not significantly affect yield components, distribution of above-ground to below-ground biomass, or grain yield. Drought-tolerant maize is discussed further in Chapter 8.
The committee could only find one example of yield enhancement, that is, an increase in potential yield through genetic engineering. It in-
volved a single-gene approach; a reported 20-percent increase in biomass yield of eucalyptus (Eucalyptus spp.) trees resulted from the expression of an endoglucanase gene from the small annual plant Arabidopsis thaliana (FuturaGene, 2015). Eucalyptus is grown primarily as a source of cellulose for such products as paper, and expression of the endoglucanase gene causes more cellulose to be deposited in cell walls of the transgenic plants. Transgenic eucalyptus that expresses endoglucanase was approved for cultivation on tree plantations in Brazil in 2015.
Effects of Genetically Engineered Traits versus Conventional Plant Breeding on Yield
The committee heard concerns from the public and from researchers that GE crops commercialized up to 2015 had not contributed to an increase in yield as much or as effectively as conventional plant breeding had (Cotter, 2014; Goodman, 2014; Gurian-Sherman, 2014; Dever, 2015). It has often been difficult to separate the effects of GE traits and conventional breeding on yield over the last two decades because genetic engineering and conventional breeding have been used together in bringing GE traits into commercialization. If more effort is given to the conventional breeding of varieties
with specific GE traits than of those without them, the greater yield of the GE varieties could be due largely to the conventional-breeding component.
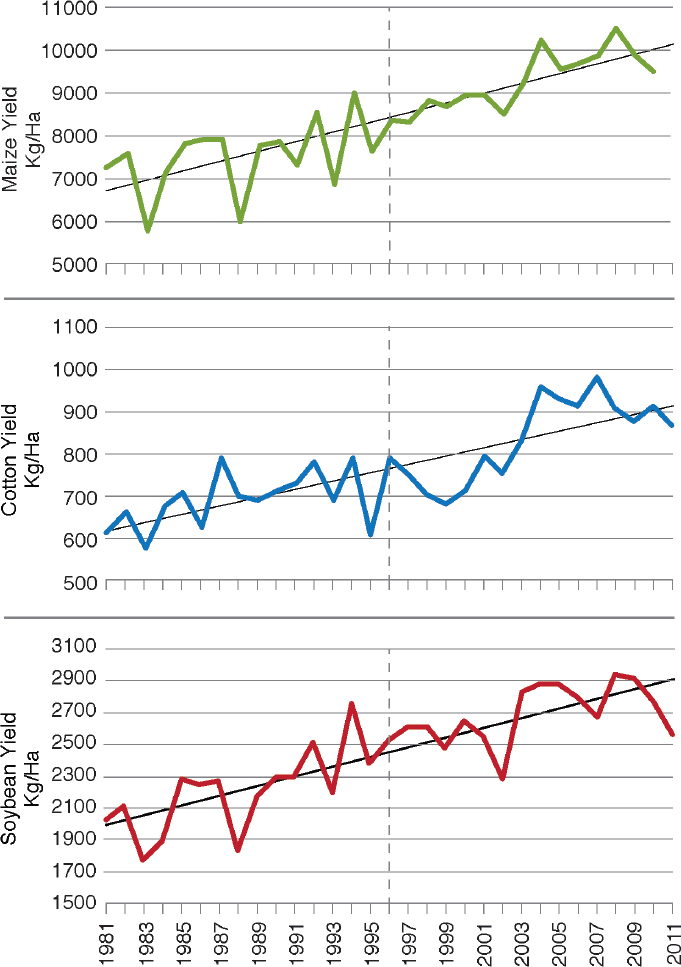
The committee examined data on farm yields of the major crops in the United States that have been engineered since the 1990s in a general attempt to determine whether there is an obvious signature of the genetic-engineering era. In Figure 4-2, Duke (2015) showed the changes in yield of soybean, maize, and cotton in the United States from 1980 to 2011 on the basis of data from National Agricultural Statistics Service (NASS) of the U.S. Department of Agriculture (USDA) and provided a best-fit linear-regression line through the data points. Yield of all three crops has increased dramatically since 1980. If there was a change in the slope of increase in yield since the commercialization of GE varieties (marked by the dashed line), it could be taken as circumstantial evidence but not proof that genetic engineering caused a more rapid increase in yields. However, there is no obvious change in the slope for cotton and maize, which have the Bt and HR traits, or for soybean, which has only the HR trait. One could move beyond the rules of parsimony and hypothesize that, without the introduction of GE traits, the rate of yield increase would have declined. Mechanisms that could support such a hypothesis include a recent decline in conventional-breeding effort, diminution in genetic variation available to conventional breeders, and adverse effects of global climate change. The committee found no evidence of such mechanisms.
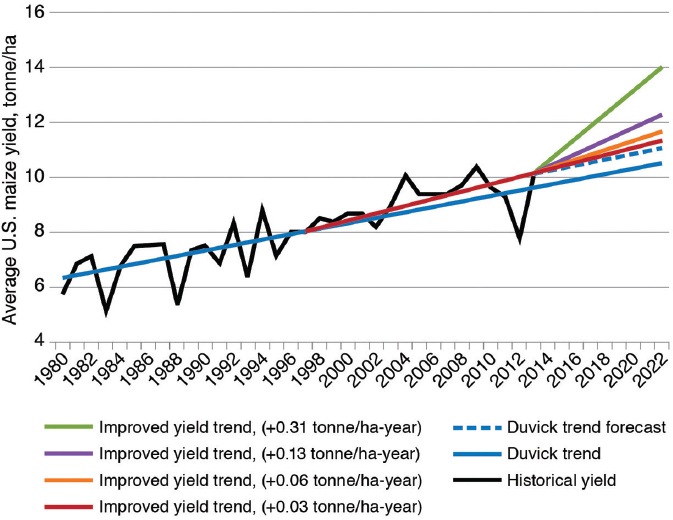
From the same data on maize yield used by Duke (2015), Leibman et al. (2014) argued that the slope of increase in yield has increased since commercialization of GE traits (Figure 4-3), although no statistical-significance value is provided for this change in slope. Leibman et al. speculated on how the more rapid change in yield improvement could change yields in the future. If such a change is significant, it will be important to determine whether it is the result of farming practices, GE traits, increased efforts in conventional breeding or emerging genetic-engineering technologies (see Chapter 7), or some combination thereof. Whatever the causes, yields have been increasing since the commercialization of GE traits, and there is no obvious sign of an increase in the relative variance in yield among years.
FINDING: The nation-wide data on maize, cotton, or soybean in the United States do not show a significant signature of genetic-engineering technology on the rate of yield increase. This does not mean that such increases will not be realized in the future or that current GE traits are not beneficial to farmers.
RECOMMENDATION: To assess whether and how much current and future GE traits themselves contribute to overall farm yield changes, research should be conducted that isolates effects of the diverse environmental and genetic factors that contribute to yield.
SOURCE: Duke (2015).
NOTE: Dashed line indicates when genetically engineered varieties of these crops were first introduced in the United States.
SOURCE: Leibman et al. (2014).
NOTE: Blue solid line indicates trends proposed by Duvick (2005) for historical annual increases in grain yield of 0.10 tonne/ha-year. Purple line indicates trend of larger average annual increases (0.13 tonne/ha-year) in grain yield since adoption of GE maize in 1996. Dotted blue line indicates a return to previous historical average annual increases (0.10 tonne/ha-year) in grain yield in the future. Red, orange, and light green lines represent forecasts of improved yield trends of 0.06–0.31 tonne/ha-year over and above historical average of 0.10 tonne/ha-year.
EFFECTS RELATED TO THE USE OF Bt CROPS
The committee examined the effects of GE insect resistance on crop yield, insecticide use, secondary insect-pest populations, and the evolution of resistance to the GE trait in targeted insect populations.
Yield Effects of Genetically Engineered Insect Resistance
As of 2015, IR traits had been incorporated into maize, cotton, eggplant, and poplar. This section relies in part on past reviews of the literature, but these reviews typically do not provide the reader with an understanding of technical caveats associated with the reviewed studies. There is continu-
ing controversy over claims about yield effects of GE insect-resistant crops, and the committee received a number of public comments related to this. Therefore, in addition to relying on review articles, the committee also examined a substantial number of original research articles to carefully assess the quality of data that has been used to support various claims.
Bt Maize
Meta-analyses and summary reports that included an examination of Bt maize production in different parts of the world were reviewed. Areal et al. (2013) compared yields of Bt maize with those of the non-Bt counterparts. On the basis of data collected from the Philippines, South Africa, the United States, Spain, Canada, and the Czech Republic, Areal and colleagues found that Bt maize yielded 0.55 tonne/hectare more than maize without Bt. Areal et al. (2013:27) were careful to point out that “although it cannot be discerned whether the advantages of cultivating [genetically modified] GM crops were due to the technology itself or to farmers’ managerial skills (GM adopter effect), the GM adopter effect is expected to diminish as the technology advances” because early adopters are typically farmers with better managerial skills (and resources), and over time farmers with a mix of managerial skills will use the technology.
A review by Fernandez-Cornejo et al. (2014) found a yield advantage of Bt maize over non-Bt maize in all the surveys (four) and experiments (five) that they examined that were conducted in 1997–2003 in the United States. However, a different analysis that used yield data from South Africa, Germany, and Spain collected from 2002 to 20075 did not identify a difference between the yields of Bt and non-Bt maize overall or in each of the three countries separately (Finger et al., 2011). Neither the Bt trait used nor the insect pest targeted was specified in the studies because they looked at findings from a number of locations.
Gurian-Sherman (2009) reviewed results of studies conducted in the United States and Canada on effects of GE traits on maize and soybean yield. With regards to maize, he reviewed six studies published in 1997–2004 and concluded that the Bt traits to resist European corn borer (Ostrinia nubilalis) closed the yield gap by 7–12 percent in locations where infestation by the insect was high.6 On the basis of review of three additional studies in Iowa published in 2005–2008, he concluded that the Bt trait targeting corn rootworm (Diabrotica spp.) substantially decreased the yield gap in situations where insect-pest pressure was high and water
__________________
5 Yield data from Spain in 1997 were also included.
6Gurian-Sherman (2009) estimated that high levels of European corn borer infestation occurred on 12–25 percent of U.S. maize acres, on the basis of Rice and Ostlie (1997).
availability was low. In situations without those constraints, the Bt traits for either pest did not have an effect on yield. Gurian-Sherman (2009) estimated that Bt maize production in general brought U.S. maize 3–4 percent closer to its potential yield. Klümper and Qaim (2014) conducted a large meta-analysis on the effects of GE crops, including those with Bt traits. They did not segregate their results by crop, but in aggregate they found that yields of maize and cotton were 22 percent greater when a Bt trait was present (n=353).
The reviews and meta-analyses cited above make clear that benefits associated with crops with Bt traits vary substantially and depend on insect-pest abundance in the area of the survey or experiment. It is not clear from the broad literature surveys whether differences in yields are due solely to the Bt traits’ reducing of insect-pest damage or to differences among the farmers who use the varieties and other agronomic differences among the varieties (see also Box 6-1). Therefore, the committee looked carefully at studies of maize with and without Bt traits that were conducted since initial commercialization because these studies allow one to assess the role of insect-pest abundance and the genetic background of the crop varieties tested. The committee includes here a set of specific studies that it found to be most informative regarding the factors that influence if and how much the Bt trait decreases yield gaps.
Bowen et al. (2014) compared seven pairs of Bt maize hybrids and their non-Bt counterparts at several sites in Alabama. They also included a comparison between a maize hybrid with one Bt trait and its isogenic7 counterpart with two Bt traits. The study was conducted from 2010 to 2012. In southern parts of the United States, the insect targets of Bt hybrids are primarily corn earworm (Helicoverpa zea) and fall armyworm (Spodoptera frugiperda). Infestations of corn earworm and fall armyworm were variable over that time. Bowen and colleagues found that yields were greater in Bt maize than in non-Bt maize in 2 of the 3 years. However, there was not a clear relationship between the amount of insect-pest damage and the yield improvements due to Bt because the year with the greatest pest damage (2010) had intermediate yield improvements.
Reay-Jones and Reisig (2014) conducted field studies in which corn earworm was the target pest. At two sites, one in North Carolina and one in South Carolina, they planted near isolines of non-Bt maize and maize with one to three Bt traits in 2012 and 2013. They did not find differences in yields between near isolines with and without Bt traits. They noted
__________________
7 An isogenic line has closely related genotypes of a crop that differ by one or a few genes and are therefore expected to perform similarly on farms. Near isogenic lines (or near isolines) are more vaguely defined and can have multiple genes differing between them and thus may have differences in performance under some farm conditions.
that similar results were found in the southeastern United States in maize planted at recommended times (Buntin et al., 2001, 2004; Allen and Pitre, 2006; Reay-Jones et al., 2009; Reay-Jones and Wiatrak, 2011). Because the targeted insect did not cause much damage in the region, they concluded that Bt traits aimed at corn earworm may not close the gap between actual yield and potential yield.
In experiments conducted soon after Bt maize commercialization in Canada and in the Midwest and Northeast growing areas of the United States where European corn borer populations typically caused yield losses in non-Bt maize, increased Bt maize yields were shown to be clearly associated with decreased insect-pest damage. Baute et al. (2002) concluded that in the Canadian Midwest, European corn borer infestations resulted in 6- and 2.4-percent reductions in yield for 1996 and 1997. In experiments in four to six locations per year in Pennsylvania and Maryland in 2000, 2001, and 2002, Dillehay et al. (2004) found that the average yields of Bt maize varieties and their non-Bt isolines were 9.1 and 8.6 tons/hectare, respectively (a 5.8-percent difference). Yield per plant in the non-Bt isolines was reduced by 2.37 percent per corn borer tunnel in the plant.
Between the 1996 introduction of Bt maize and 2009, European corn borer populations and the damage that they cause decreased dramatically, as documented by Hutchison et al. (2010). The decline appears to have continued to a point where the European corn borer adults can be difficult to find in the Midwest (Box 4-2). In a study throughout Pennsylvania in 2010, 2011, and 2012, at 16, 10 and 3 farm sites, respectively, Bohnenblust et al. (2014) planted Bt and non-Bt varieties. The populations of European corn borer was low throughout the area studied; in only three of the sites were insect densities great enough to cause a 3-percent yield loss in non-Bt hybrids. Overall, yield of the Bt varieties was 1.9 percent greater than that of the non-Bt varieties. Some of the small difference could be explained by insect pest pressure, but some of the difference could also have been due to differences in other characteristics of the varieties. As with the experiments in Pennsylvania, most current differences in yield between Bt and non-Bt varieties in the Midwest are unlikely to be caused by this once important insect pest.
Field experiments in Nebraska in 2008, 2009, and 2010 compared glyphosate-resistant maize hybrids that also had Bt traits targeting European corn borer and corn rootworm with genetically similar hybrids without the Bt traits “in environments with no detectable infestation [of European corn borer or corn rootworm] based upon visual observations in-season and during harvest” (Novacek et al., 2014:94). Therefore, any differences in yield could not be attributed to effects of the Bt toxins. The density of maize plants in the different test plots was 49,300–111,100 plants/hectare. The hybrids with Bt traits yielded about 5 percent more than their counter-
parts in 2008, but no difference in yield was observed in 2009 or 2010. The increased yield in 2008 was not explained by damage to the non-Bt hybrids caused by the target insect pests to the non-Bt hybrids.
In Illinois, Haegele and Below (2013) compared two sets of locally adapted maize hybrids with the same general genetic backgrounds in the growing seasons of 2008 and 2009. In each set, one hybrid had GE resistance to glyphosate and the other had the GE trait for glyphosate resistance and Bt traits for resistance to European corn borer and corn rootworm. Each hybrid was grown with several rates of nitrogen fertilization. Root damage was measured in 2008 and inferred in 2009. The authors stated that “based on these low levels of apparent root injury, few differences
in grain yield or agronomic performance between non-Bt and Bt hybrids might be expected” (Haegele and Below, 2013:588). Nevertheless, averaged over all rates of nitrogen fertilization, yields were greater in the Bt hybrids than in the comparable non-Bt hybrids: about a 7-percent difference in one set and an 18-percent difference in the other.
Another study in Illinois examined insect resistance as one of five factors that might contribute to yield of maize (Ruffo et al., 2015). Maize with only glyphosate resistance was compared with its near isoline that contained Bt targeting European corn borer and corn rootworm. The other four factors tested were density of maize plants per unit area, strobilurincontaining fungicide, application of a combined phosphorus–sulfur–zinc fertilizer, and application of nitrogen fertilizer. In field trials on two sites during the 2009–2010 growing season, they compared effects of near isolines with and without the Bt toxins. When all other factors were maximal for increased yield, the Bt hybrids had 8.7 percent greater yield. When none of the other factors was optimized for yield the Bt hybrids had 4.5 percent greater yield. The authors hypothesized that adult corn rootworm feeding on silks may have influenced kernel formation and affected yield, but insect data were not presented, so it was difficult to determine whether corn rootworm had any effect. European corn borer numbers in Illinois were very small in 2009 and 2010 (Hutchison et al., 2010; Box 4-1).
Nolan and Santos (2012) compiled results of maize hybrid trials conducted by land-grant universities in the 10 leading maize-producing U.S. states from 1997 to 2009. For hybrids that had herbicide resistance, they found yield increases for maize with Bt resistance to European corn borer and for maize with Bt targeting corn rootworm compared with non-GE hybrids. Maize with Bt targeting European corn borer yielded 6 percent more than non-GE hybrids on the basis of data from 1999–2009 (fixed-effects model); maize with Bt targeting corn rootworm yielded 7.4 percent more on the basis of data from 2005–2008. The yield difference was 7.1 percent when the two traits were present in the same variety on the basis of data from 2005–2009. No data were presented on rate of insect-pest infestation, although the authors stated that infestations of European corn borer were decreasing, which is consistent with surveys in the region.
Shi et al. (2013) used a time-series analysis of experimental data on small plots in Wisconsin (1990–2010) to assess changes in yield and variability of yield. They found that the average yield in all years for maize with a Bt trait targeting European corn borer was greater (410 kilograms/hectare) than for non-GE maize. However, the average yield was less (765 kilograms/hectare) for Bt maize with resistance to corn rootworm. The Bt trait for European corn borer reduced yield in the early years of the survey but increased yield in later years even though the population of the pest had declined. Shi et al. (2013) concluded that for some traits there
is yield drag initially, but with continued breeding the effect is decreased or reversed. A reversal could explain results of other experiments described above in which a hybrid with a Bt trait outperformed a non-Bt hybrid even without insect-pest pressure. Although yield was adversely affected by the Bt trait for rootworms during the period through 2010 examined by Shi et al. (2013), this may no longer be the case.
In the Brazilian state of Santa Catarina, Ozelame and Andreatta (2013) found a maize hybrid with Bt targeted at corn earworm and several other pests to yield 6.89 percent better than the non-Bt near isoline, but no statistical analysis was conducted. The study was conducted in the harvest of 2010–2011. A study in the Philippines in the wet season of 2010 reported that yields in the Isabela province did not differ statistically between Bt and non-Bt maize (Afidchao et al., 2014). Gonzales et al. (2009) conducted surveys of Bt and non-Bt maize in the Philippines and conclude that Bt maize had yield increases of 4–33 percent. Because no statistical analysis was provided, it was not possible to quantitatively assess the results.
One of the claims regarding Bt crops is that they would stabilize yield or, more accurately, would limit the risk of a farmer having dramatic yield loss (crop failure). Given that the Bt trait increases yield more when there is high insect pressure, it seems intuitive that it would diminish crop failure under severe insect-pest pressure. The committee was able to find only three peer-reviewed studies specifically focused on quantifying Bt crop contributions to avoiding crop loss. Crost and Shankar (2008) examined variation in farm yields of Bt and non-Bt cotton. In India they found a clear decrease in variance, but in South Africa no difference was shown. Shi et al. (2013) examined maize yields in research plots in Wisconsin, where mean yield was 11,650 kilograms/hectare. They found that varieties with Bt toxins for European corn borer and western corn rootworm had decreased “cost of risk” of 106.5 kilograms/hectare. Working with cotton farmers in India, Krishna et al. (2016) found that lower variance in Bt cotton yield, especially on the low end, increased average yield by 2.5 percent.
In 2008, USDA’s Risk Management Agency concluded that the “Monsanto Company, as a co-submitter of the pilot BYE, has demonstrated that its specific triple-stack genetic traits, when used in combination, provide lower yield risk as compared to non-traited hybrids.”8 The BYE (Biotechnology Yield Endorsement) program provided farmers with discounted crop insurance if they planted maize with Bt traits that targeted Lepidoptera and corn rootworm along with the GE trait for glyphosate resistance. The discount was based on the expectation that there was a lower risk of crop failure with these varieties. The program ended in 2011.
__________________
8 RMA approves BYE for 2008 implementation. January 3, 2008. Available at http://www.rma.usda.gov/news/2008/01/102bye.html. Accessed March 17, 2016.
Bt Cotton
The meta-analysis conducted by Areal et al. (2013) found that on the average cotton containing Bt yielded 0.30 tonne/hectare more than cotton without Bt. Their finding was based on data collected from India, China, South Africa, Argentina, Mexico, and Australia in 1996–2007. They concluded that there is a greater advantage to Bt cotton in developing than in developed countries. The analysis conducted by Finger et al. (2011) used data from the United States, China, Australia, India, and South Africa. Yield data were reported for 1995–2007 from 237 studies that included Bt cotton and 195 studies that included non-Bt cotton. Areal et al. (2013) provided a list of the studies used, but Finger et al. (2011) did not, so it is not possible to know whether they used the same studies. When the studies in Finger et al. (2011) are separated by country, the yield advantage for Bt cotton is different for India, where the yield was 50.8 percent greater. The authors concluded that the reason that India’s yield advantage was much larger than that of the others (particularly the United States and Australia) was that when Bt cotton was commercialized in India in 2002, it introduced insect control to production areas that had had little or none. The authors cautioned that yield advantages within India may depend on the specific location.
Stone (2011) critiqued studies that showed yield increased in India directly after Bt cotton was approved in 2002 and that did not control for the bias whereby early adopters of new technology usually have more assets than later adopters or nonadopters (for more on the assets of early adopters, see section “Income Effect of Early Adoption” in Chapter 6). However, studies performed in years after Bt cotton was introduced and widely adopted9 found yield advantages. In the Indian state of Madhya Pradesh, Forster et al. (2013) compared cotton production over two seasons (2007–2008 and 2009–2010) in four farming systems: Bt, non-Bt, organic, and biodynamic.10 In the 2007–2008 season, the system with Bt had 16-percent higher yield than the isogenic non-Bt system; in the 2009–2010 season, the system with Bt had 13.6-percent higher yield. In this experimental study, the Bt cotton had about 8-percent higher total
__________________
9 In 2006, Bt cotton was grown on 3.8 million hectares of land in India, which was 42 percent of its land in cotton production that year (James, 2006). In 2008, those numbers had grown to 7.6 million hectares, or 82 percent of cotton production (James, 2008); by 2010, Bt cotton was grown on 9.4 million hectares, or 86 percent of the land in cotton production (James, 2010).
10Forster et al. (2013) described biodynamic farming systems this way: “Preparations made from manure, minerals and herbs are used in very small quantities to activate and harmonize soil processes, to strengthen plant health and to stimulate processes of organic matter decomposition. Most biodynamic farms encompass ecological, social and economic sustainability and many of them work in cooperatives.”
nitrogen fertilizer input but was harvested earlier, in accord with government recommendations for higher inputs for Bt cotton. Forster et al. (2013) commented that the differences might have been more modest in their experiment than in surveys because the insect-pest problems in non-GE cotton were managed better in their experiment than in typical farms.
Kathage and Qaim (2012) surveyed cotton farmers in the Indian states of Maharashtra, Karnataka, Andhra Pradesh, and Tamil Nadu in 2002, 2004, 2006, and 2008. Controlling for all other factors, they found Bt cotton that controlled cotton bollworm (Helicoverpa armigera) had a yield advantage of 51 kilograms/hectare, a 24-percent increase over non-GE cotton yields during the period of the study. Their analysis led them to conclude that per-hectare yield benefits probably increased from 2002–2004 to 2006–2008. They hypothesized that the growth in yield advantage could be attributed to the increase in the availability of varieties with Bt starting in 2006 and the introduction of new Bt traits to the market around the same time.
A meta-analysis of 19 studies conducted in India with data from 2002 to 2008 reported that Bt cotton had a 33-percent yield advantage per hectare over non-Bt cotton (Witjaksono et al., 2014).11Stone (2011) found an average yield increase of 18 percent from 2003, when no Bt cotton was planted by the farmers sampled in four villages, to 2007, when farmers in the same villages planted Bt cotton almost exclusively. However, he noted that yields in Andhra Pradesh, the state where the villages were, did not so much increase as return to the peak that was achieved in 1994. Romeu-Dalmau et al. (2015) also raised the issue of whether the type of cotton grown could play a part in yield outcomes. They compared Bt cotton G. hirsutum L. with non-Bt cotton G. arboretum, a variety commonly grown in India before a U.S. variety of G. hirsutum was introduced in the 1980s. The authors interviewed 36 farmers who operated less than 5 hectares of land. Under rain-fed conditions in Maharashtra, India, yields for Bt G. hirsutum were not greater.
In a survey of cotton farmers in Punjab, Pakistan—248 of whom grew Bt cotton and 104 non-Bt cotton—Abedullah et al. (2015) reported a yield advantage of 26 percent for farmers of Bt cotton. The study was conducted from December 2010 to February 2011, the first cotton-growing season after Pakistan approved commercial planting of Bt cotton. As part of the study, they examined farmer assets. Their findings were consistent with Stone’s (2011) point that early adopters have more assets. Bt adopters were different in several ways: they had more education, more land, and more access to credit. They also were more likely to own a tractor and to have been aware of Bt cotton before nonadopters.
__________________
11 Data collected by Kathage and Qaim (2012) and Stone (2011) were included in this meta-analysis.
A meta-analysis of 17 studies conducted in China with data from 1999 to 2005 reported that Bt cotton had an 18.4-percent yield advantage (480 kilograms/hectare) over non-Bt cotton (Witjaksono et al., 2014). Surveys of 500 farmers conducted by the Center for Chinese Agricultural Policy in two cotton-growing regions in 2004, 2006, and 2007 reported that mean yields of Bt cotton were at least 500 kilograms/hectare greater than non-Bt cotton yields (Pray et al., 2011). In 2006, however, only 14 farmers reported growing non-Bt cotton, and the number had shrunk to four in 2007, so the reported differences were not robust. Qiao (2015) looked at the yield effect of Bt cotton throughout China since its introduction and found that it had a positive effect on yield that was stable from the adoption of Bt cotton in 1997 through the end of the study in 2012.
Cotton is a major cash crop for Burkina Faso. Although its production is much smaller than that of the world’s largest producers (China and India), Burkina Faso was the 10th-largest producer of cotton in 2013. Bt cotton was introduced there commercially in 2008. In an experiment conducted before commercialization on two sites in 2003, 2004, and 2005, Héma et al. (2009) compared a U.S.-developed Bt cotton containing the endotoxins Cry1Ac and Cry2Ab with three other treatments: a non-Bt local variety with standard insecticide applications, a non-Bt local variety without insecticide application, and a U.S. non-Bt variety without insecticide application. The Bt toxins in the GE variety targeted cotton bollworm and cotton leafroller (Syllepte derogate). Yields varied in space and time. At one site, the Bt variety had greater yields of seed cotton than the other tests in 2003. In 2004, there was no difference among the four varieties. The authors posited that differences were lacking in that year because insect-pest pressure was low. In 2005, yields from the Bt and insecticide-treated local varieties were statistically equivalent and yielded significantly more than the other two varieties. At the other site, the yields of the Bt variety and treated local variety were equivalent and were greater than the yields of other two varieties in all 3 years.
A survey of 160 rural households in 10 villages in the three cotton-growing regions of Burkina Faso was conducted in 2009, when roughly 30 percent of cotton hectares were planted with Bt cotton. Vitale et al. (2010) reported that there was an average yield advantage of 18.2 percent for Bt cotton over non-Bt cotton in all three regions. There was statistical interaction between the yield advantage and the specific region; the greatest advantage was 36.6 percent and the least was 14.3 percent. The authors hypothesized that the range in yield effect was due to differences in insect-pest populations among the regions. By 2012, Bt varieties were planted on 51 percent of cotton hectares in Burkina Faso (James, 2012).
Fernandez-Cornejo et al. (2014) reviewed three experiments and six surveys of Bt cotton production in the United States published in 1997–
2007. Greater yield of Bt than non-Bt varieties was reported in two of three experiments and in all surveys. The authors offered a caveat about survey results because “Bt use is not random. Surveyed farmers are not randomly assigned to a treatment group (adopters) and a control group (nonadopters). Consequently, adopters and nonadopters may be systematically different from one another (for example, in terms of management ability).”
Luttrell and Jackson (2012) compiled data on U.S. cotton crop loss to insects in 2000–2007. The estimated average of the percentage of crop loss to all insects (targets and nontargets of Bt) was lower for Bt cotton than for non-GE cotton (4.13 percent versus 6.46 percent), but no difference in yield between Bt and non-GE cotton was identified. Kerns et al. (2015) evaluated yields of one non-Bt variety and four Bt varieties of cotton in field plot tests in Arkansas, Louisiana, Mississippi, and Tennessee in 2014. When all varieties were sprayed for caterpillars, the Bt varieties still had a yield advantage of 9–52 percent, depending on location.
Bt Eggplant
As of 2015, Bt eggplant (Solanum melongena) was grown commercially only in Bangladesh. It was engineered for resistance to fruit and shoot borer (Leucinodes orbonalis) and first commercialized in spring 2014, when 20 farmers in four regions planted one of the four Bt varieties of eggplant (locally known as brinjal) on a total of 2 hectares (Choudhary et al., 2014). Krishna and Qaim (2008) summarized data provided to them from research-managed field trials conducted by MAHYCO, a seed company, but none of the data was published. In several Indian states during the mid-2000s, they found yield of uninfected fruit to be 117 percent greater in Bt eggplant hybrids than in insecticide-treated isogenic non-Bt hybrids. When the Bt hybrids were compared by the company with popular open-pollinated varieties of eggplant, the yield benefit grew to 179 percent. Krishna and Qaim predicted that under field conditions the yield advantage of Bt eggplant hybrids over non-Bt hybrids would be 40 percent and over open-pollinated varieties 60 percent. Results of large-scale field trials conducted by the Indian Institute of Vegetable Research during 2007–2008 and 2008–2009 were similar. Seven Bt eggplant hybrids were planted in eight locations alongside non-Bt hybrids. When Bt hybrids were compared with the non-Bt varieties into which the Bt trait had been introgressed, the yield of the Bt hybrids was 37.3 percent more than that of the non-Bt hybrids, but no statistics were presented. The yield increased to 54.9 percent when the comparison was with other popular hybrids, but again no statistics were presented (Kumar et al., 2010). The yield gains in both studies were due to the reduced damage from fruit and
shoot borer. Andow (2010) argued that losses in non-Bt eggplant for subsistence farmers were not as high as other studies had estimated because these farmers have outlets for selling or consuming damaged fruit, whereas large-scale commercial farmers do not.
Bt Poplar Trees
Poplar trees with Bt have been planted in China since field testing began in 1994, but they were not approved for commercialization until 2005. Populus nigra has been genetically engineered with Bt toxins targeted at poplar looper (Apochima cinerarius) and clouded drab moth (Orthosia incerta Hufnagel) (Hu et al., 2001). Although poplar can be grown for fuel, fiber, and forest products, poplar plantations in China have been used primarily to provide environmental protection and afforestation in northern China (Hu et al., 2001; Sedjo, 2005). Therefore, yield effects have not been an outcome of interest in the study of Bt poplar in China.
Field trials elsewhere have found some effect on yield of Bt traits in poplar in which Bt genes were inserted into specific clonal lines. In a screening trial of four paired clonal lines of poplar (one Populus deltoides × Populus nigra hybrid and three Populus trichocarpa × Populus deltoides crosses) in the Pacific Northwest of the United States, plant growth in three of the clonal lines with Bt gene insertion (expressing Cry3Aa) was not substantially different from that in their paired non-Bt line (Klocko et al., 2014). The average volume growth of one of the Bt Populus trichocarpa × Populus deltoides crosses was greater than its control based on measurements in year-1 and year-2. After the screening trial, the Populus deltoides × Populus nigra hybrid was used in a large-scale trial. From season 1 to season 2, net volume growth in Bt trees was an average of 8 percent greater than that in the controls (Klocko et al., 2014). Hjältén et al. (2012) compared aspen (Populus tremula × Populus tremuloides) clones expressing Bt toxins with isogenic non-Bt clones. The trees were planted in pots in a greenhouse. The authors found that the Bt trees were shorter than the non-Bt clones in the absence of the targeted insect, brassy willow-leaf beetle (Phratora vitellinae). However, the Bt trees were taller when beetle populations were great enough to cause substantial defoliation. Thus, there is evidence that GE insect resistance addresses yield-reducing factors in trees.
FINDING: Although results are variable, Bt traits available in commercial crops from introduction in 1996 to 2015 have in many locations contributed to a statistically significant reduction in the gap between actual yield and potential yield when targeted insect pests caused substantial damage to non-GE varieties and synthetic chemicals did not provide practical control.
FINDING: In areas of the United States where adoption of Bt maize or Bt cotton is high, there is statistical evidence that insect-pest populations are reduced regionally, and the reductions benefit both adopters and nonadopters of Bt crops.
FINDING: In surveys of farmers’ fields, differences in yield between Bt and non- Bt varieties may be due to differences between the farmers who do and who do not plant the Bt varieties. These differences could inflate the apparent yield advantage of the Bt v arieties if Bt -adopting farmers on the average have other production advantages over non- Bt –adopting farmers.
FINDING: In experimental plots, the difference in yield between Bt and non- Bt varieties is sometimes demonstrated to be due to decreased insect damage to the Bt variety, but in cases in which comparisons are not between true isolines, differences may be due to other characteristics of the Bt varieties or to a combination of crop variety and decreased insect-pest damage. These differences could confound the estimation of the apparent yield advantage of the Bt varieties.
RECOMMENDATION: In future experimental and survey studies that compare crop varieties with IR traits with those without the traits, it is important to assess how much of the difference in yield is due to decreased insect damage and how much may be due to other factors.
Changes in Insecticide Use Due to Insect-Resistant Crops
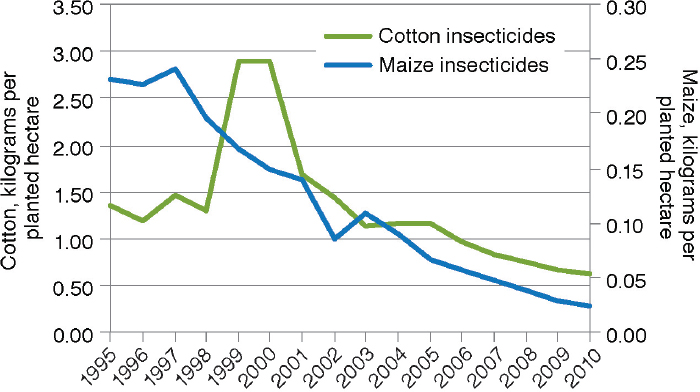
There have been numerous studies of changes in insecticide use on large-scale and small-scale farms as a result of the adoption of crops that produce Bt toxins. There is no question of whether GE crops have changed the amounts of insecticides used by adopting farmers. The debate is over the magnitude and direction of the changes. The meta-analysis by Klümper and Qaim (2014), for example, documented a 39-percent reduction of insecticide quantity from the adoption of Bt cotton and maize (n=108). The 2010 National Research Council report on impacts of GE crops in the United States reviewed data from USDA on insecticide use in cotton and maize from 1996 through 2007 and found a clear pattern of decline in both crops in pounds of active insecticidal ingredient (a.i.) applied per acre (NRC, 2010a).12Fernandez-Cornejo et al. (2014) extended the assessment
__________________
12 For example, pounds of a.i. applied per acre dropped from 0.23 in 1996 to 0.05 in 2007 for maize and from 1.6 in 1996 to 0.7 in 2007 for cotton.
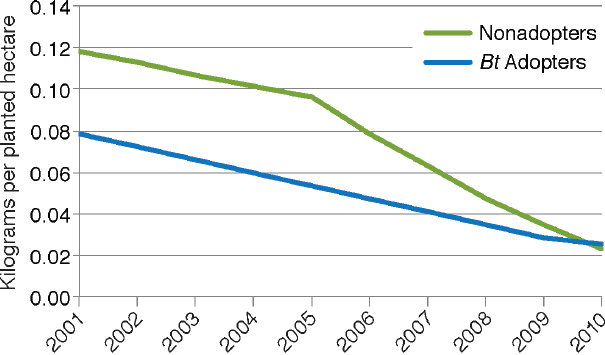
of USDA data through 2010 as illustrated in Figure 4-4. They also found that the reduction was apparent for both adopters and nonadopters of Bt maize (Figure 4-5). The decrease for nonadopters could be due to the regional decline in European corn borer populations (see Box 4-2).
A survey of farmers in the Philippines (Sanglestsawai et al., 2014) found that the amount of insecticide used on Bt maize was one-third and one-fourth of the amount used on non-Bt maize in the two growing seasons analyzed (2003–2004 and 2007–2008).
The committee did not find studies on the effects of Bt maize on insecticide use on small farm situations, presumably because insecticides are not typically used on the non-GE maize on these farms.
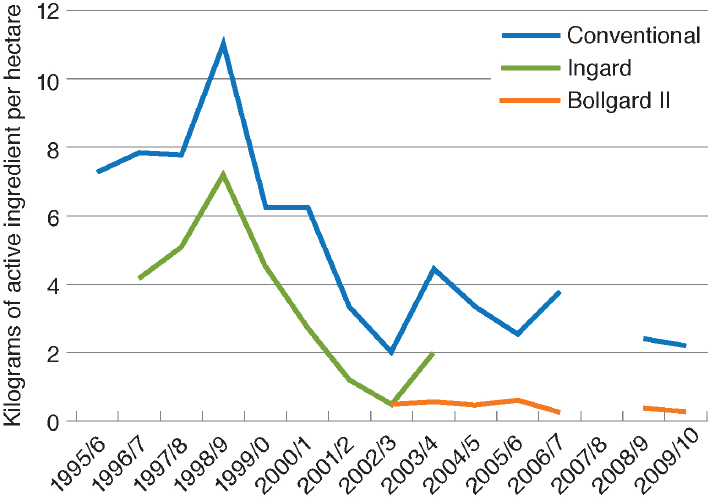
In Australia, the adoption of Bt cotton was slower than in the United States because, in efforts to slow the evolution of insect pests resistant to Bt, the Australians limited farms to planting 30 percent of their area in Bt cotton until 2003, when the single Bt toxin variety INGARD® was replaced with a two-toxin variety Bollgard® II. As can be seen in Figure 4-6, there has been a dramatic decline in insecticide use in Australia both in Bt cotton and in non-Bt cotton (Wilson et al., 2013).
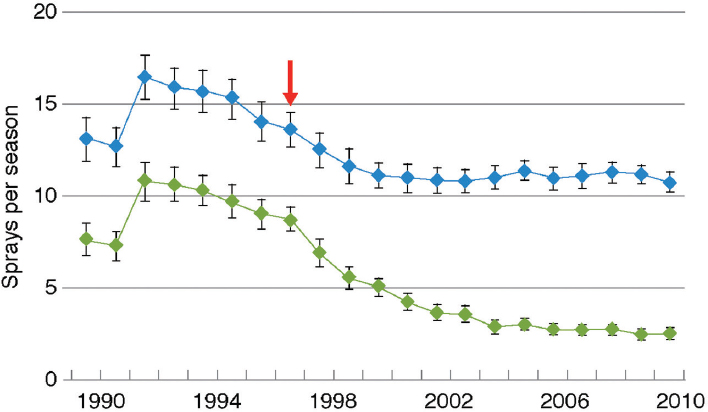
Adoption of Bt cotton in China was rapid: The percentage of farmland planted to Bt cotton rose to more than 95 percent by 2011 (Lu et al., 2012). The increase in use of Bt cotton resulted in reduced density of the target pest, Helicoverpa armigera, and to a decrease in overall use of insecticide on cotton (Figure 4-7).
SOURCE: Fernandez-Cornejo et al. (2014).
SOURCE: Fernandez-Cornejo et al. (2014).
SOURCE: Wilson et al. (2013).
NOTE: No data were collected in 2007–2008 because the cotton area was small owing to drought.
SOURCE: Lu et al. (2012).
NOTE: Blue dots are total insecticide; green dots are insecticide spray aimed at cotton bollworms. Red arrow indicates the year when Bt cotton was first commercialized.
The changes in insecticide applications resulting from the adoption of Bt cotton in India have been the subject of numerous studies, beginning around 2000 when availability of Bt seeds became widespread. Qaim and Zilberman (2003) analyzed data from field trials in 2001 and found that the amount of insecticide applied by Bt adopters was 69 percent less than by nonadopters (1.74 and 5.56 kilograms/hectare, respectively). Those results were extended by Sadashivappa and Qaim (2009), who found that average insecticide application on Bt cotton was 41 percent of that on non-Bt cotton, and by Kouser and Qaim (2011), who documented a 64-percent difference. Kouser and Qaim also showed that aggregate insecticide application declined for nonadopters of Bt cotton during the same period (they did not delineate pesticide categories—insecticides versus other pesticides). Similar results were reported by Stone (2011); the number of insecticide sprays applied by cotton growers in the Warangal District of Andhra Pradesh, India, fell by a statistically significant amount—more than half (54.7 percent)—from 2003 to 2007, with the largest reductions in areas with the greatest insecticide use.
Shankar et al. (2008) studied the relationship between insecticide use and Bt cotton in South Africa and found that farms using Bt cotton applied insecticide at 1.6 liters/hectare, and those with non-Bt cotton applied 2.4 liters/hectare.
Even though overall use of insecticides on maize and cotton in the United States has decreased, since 2003 there has been a substantial increase in treatment of maize, cotton, and soybean seed with neonicotinoid insecticides (Thelin and Stone, 2013). The committee received comments from the public that suggested that the increase could have been due to or associated with the increase in use of Bt crops. Douglas and Tooker (2015) provided a detailed assessment of U.S. data on the increase in use of neonicotinoids from the 1990s until 2011. It is clear that the increase was as dramatic in soybean as in maize. As of 2015, commercial soybean in the United States had not been engineered to produce Bt toxins, so the increase in neonicotinoid use in this crop clearly was not associated with the use of Bt varieties. Increases in use of neonicotinoids have also been seen in vegetables and fruits that are not genetically engineered. In the case of maize, the rates of use of neonicotinoids on seeds are too low to affect rootworms, and the Bt toxin in maize roots seems to affect only rootworms, so Bt and neonicotinoid insecticides act mostly as complementary pest-management tools (Petzold-Maxwell et al., 2013; Douglas and Tooker, 2015). However, another study suggested that a seed treatment could affect rootworm survival and might interact with Bt maize (Frank et al., 2015), so the potential for synergy between the two kinds of compounds in causing rootworm mortality should be further investigated. That overall insecticide use in maize and cotton has decreased even with the increase in use of neonicotinoid seed treatments is due in part to the fact that only about 0.001 kilogram of active ingredient of neonicotinoid is used per hectare13 and data on kilograms of seed-treatment chemicals do not seem to be reported in insecticide surveys conducted by USDA–NASS (Douglas and Tooker, 2015). One commonality between the use of Bt crops and the use of neonicotinoids is that the farmer’s decision to use either of them must be made before the beginning of the season, so use is prophylactic. Furthermore, farmer choice is sometimes constrained because most maize and cotton seed that is available is likely to produce at least one Bt toxin and be treated with a neonicotinoid insecticide.
Although there is an overall reduction in the amount of synthetic insecticides used as a result of planting of Bt crops, Benbrook (2012) pointed out that a hectare of maize planted with a variety that has multiple Bt traits can produce 4.19 kilograms of Bt toxin per hectare. Data in the section of this chapter on environmental effects of GE crops indicates that Bt toxins are not having adverse environmental effects compared to non-Bt crop varieties. Bt toxins are proteins that are insect-specific, and they are rapidly destroyed by microbial action when the remains of GE crops decompose.
__________________
13 Managing Insect Pests in Organically Certified Field Corn. North Carolina State University Department of Entomology. Available at http://www.ces.ncsu.edu/plymouth/ent/neonicotinoidseedcoat.html. Accessed April 5, 2016.
Andow’s (2010) critique of Bt eggplant disagreed with the projection that Bt eggplant would reduce insecticide use for small-scale farmers. He hypothesized that the Bt variety would not be used by smallholders and therefore there would not be a decrease in insecticide use. At the time the committee was writing its report, Bt eggplant has been adopted by almost 150 farmers in Bangladesh. Its effect on insecticide use remained to be seen.
FINDING: In all cases examined, use of Bt crop varieties reduced application of synthetic insecticides in those fields. In some cases, the use of Bt crop varieties has also been associated with reduced use of insecticides in fields with non- Bt varieties of the crop and other crops.
Changes in Secondary Insect Pests Due to Bt Crops
The control of targeted species by Bt toxins sometimes provides an opportunity for populations of “secondary” insect species to increase. The secondary insect-pest populations increase because they are not susceptible to or have reduced susceptibility to the specific Bt trait in the crop. The insects would have been controlled by broad-spectrum insecticides that were used before the introduction of the Bt crop.
Bt cotton and maize are the most widely grown IR crops. The particular Bt proteins and their specific targets vary. Some are specific to some beetle species, others to the caterpillars of some moth species. One of the best examples of a secondary pest outbreak is in Bt cotton in China. In a 10-year study conducted from 1997 (when Bt cotton was introduced) through 2008, populations of a mirid bug (Heteroptera: Miridae), which is not affected by the Bt toxin in the cotton, steadily increased (Lu et al., 2010). The authors concluded that the increase was due to the mirid bugs’ preference for cotton, and they were controlled with insecticide applications before the introduction of Bt cotton. Furthermore, mirid bug populations increased in other host crops, and these increases correlated with the extent of Bt cotton planting in cotton-growing regions in China. Over the 10 years of the study, there was increased damage to cotton and the other host crops, and the number of insecticide applications for mirid bug control also increased even though overall insecticide use declined. A summary assessment of the effects of secondary pests on Bt cotton in China (Qiao, 2015) concluded that the effects were minor in comparison with the decreases in major insect pests and insecticide use.
In the Southeast of the United States, decreased insecticide use in Bt cotton has been associated with an increase in cotton yield loss due to the stink bugs Nezara viridula and Euschistus servus (Zeilinger et al., 2011); in the Midwest of the United States, the western bean cutworm (Striacosta albicosta) became a pest after introduction of Bt maize. Indirect evidence
indicates that the western bean cutworm became more common because it was not as affected by Bt toxins as the major caterpillar pests of maize, so it had an open ecological niche when the major insect pests were removed (Dorhout and Rice, 2010).
Although some secondary insect pests have increased in abundance as Bt crops have replaced broad-spectrum insecticides, Naranjo et al. (2008:163, 167), in a review of studies in and outside the United States on the effects of secondary insect pests, concluded that a “relatively large number of pest species that are not susceptible to the Bt toxins expressed in transgenic cottons affect cotton production worldwide. In general, most of these species exhibit the same pest status and continue to be managed identically in Bt and [non-Bt] cotton systems.” Catarino et al. (2015) reviewed some other cases in which indirect evidence suggests an increase in secondary insect pests in Bt cotton and Bt maize. They concluded that the secondary insect pests “may not be serious enough to undermine the use of the technology, but do require further exploration so that practical and economically viable advice can be given to farmers and so that regulators are aware of potential issues and risks during a crop’s approval phase.”
Resistance Evolution and Resistance Management in Bt Crops
The evolution of target insects with resistance to Bt toxins has resulted in substantial economic losses for farmers of Bt crops. The committee heard from members of the public, researchers, and farmers that such resistance is an indication that genetic-engineering technology is not sustainable, and it reviewed evidence of the problem.
In 1996, the U.S. Environmental Protection Agency (EPA) Pesticide Program Dialogue Committee proposed that, with respect to Bacillus thuringensis and other environment-friendly formulations, “development of resistance would cause the potential loss of a pesticide that was in the ‘public good’ ” (EPA, 1997). Although the EPA committee used the term public good, it was not clear about how to assess the term quantitatively and requested public comments (EPA, 1997). The comments submitted to EPA varied from supportive of the approach to strongly negative. In 2001, EPA clarified that it “considers protection of insect (pest) susceptibility of Bt to be in the ‘public good’” because it “determined that development of resistant insects would constitute an adverse environmental effect” (EPA, 2001). The EPA statements reinforced the agency’s early actions that required that applicants for registration of Bt crops develop and implement approaches for deploying the crops in ways that would delay evolution of resistance. External EPA science advisory panels endorsed the appropriate use of resistance-management strategies (EPA, 1998, 2002, 2011, 2014b).
Reports by the National Research Council in 2000 and 2010 described the scientific basis of resistance-management strategies for situations in which the pesticidal substance is produced by a plant (NRC, 2000, 2010a). Of the diverse potential strategies (Gould, 1998), the one most favored by EPA and industry is referred to as the high dose/refuge strategy. Only a short summary is provided here because details of this approach have been discussed in previous National Research Council reports. The high dose/refuge approach assumes that most alleles of genes that can confer high levels of resistance to a toxin must be homozygous (both gene copies have the resistance allele) to be able to overcome a high titer of the toxin and that such alleles are rare in an insect-pest population before use of the toxin. Furthermore, the approach requires that there be a “refuge” where insects lacking resistance can survive and preserve susceptibility alleles in the population. The refuge could be a planting of the crop itself that does not produce the toxin or of another crop or wild plant species that the insect pest feeds on but that does not contain the toxin.
The initial EPA mandates that crops have a high dose of toxin relative to insect-pest tolerances was fulfilled by Bt crops targeting some insect pests—for example, Colorado potato beetle (Leptinotarsa decemlineata), pink bollworm, and tobacco budworm (Heliothis virescens)—but not others—for example, cotton bollworm, fall armyworm, and western corn rootworm. For cases in which a high dose was lacking, theory clearly indicated that a much larger refuge was required to delay resistance (EPA, 2002). There is now empirical evidence that resistance has occurred less often when a high dose has been used, and there are no reported cases of resistance when a high dose and an appropriate refuge have been used together. Huang et al. (2011) pointed out that as of 2009 the three cases of field failures due to resistance were cases in which a Bt variety with a high dose for the target insect was not available or was not deployed. Tabashnik et al. (2013) found that in six of nine cases in which Bt plants met the high-dose standard there was either no decrease in target-insect susceptibility or fewer than 1 percent of individuals were resistant; however, in the 10 cases in which there was not a high dose, more than 1 percent of individuals were resistant and sometimes the toxin lost efficacy.
One problem with the industry resistance-management plans accepted by EPA is the lack of compliance with the mandated refuges by farmers (Goldberger et al., 2005; CSPI, 2009; Reisig, 2014). When refuges are planted, they are sometimes sprayed more than needed and this decreases the utility of the refuge. Other countries have also legislated refuge plans, but few have enforced them (for example, Kruger et al., 2012). Australia is an exception: There was strict maintenance of refuges for Bt cotton (Wilson et al., 2013).
The 2010 National Research Council report on the impacts of GE
crops in the United States (NRC, 2010a) documented a few cases of resistance (defined as a genetically based change in susceptibility to a toxin) but only one in which insect-pest damage in the field increased substantially. Since then, in the United States, there have been more cases of resistance defined broadly (Tabashnik et al., 2013) and one more case of field losses in the United States due to resistance of western corn rootworm (Gassmann et al., 2014; Wangila et al., 2015). Damaging levels of resistance have also evolved in pests in other countries, for example, pink bollworm in India (Bagla, 2010; Kranthi, 2015; Kasabe, 2016), African maize stem borer (Busseola fusca) in South Africa (Kruger et al., 2011), and fall armyworm in Brazil (Farias et al., 2014). In all of these cases there was lack of a high dose relative to the pest’s tolerance of the Bt toxin, a lack of a refuge for the pest, or both.
The case of pink bollworm resistance to Bt cotton is instructive. The first commercial cotton hybrids with one Bt toxin (Cry1Ac) were released in India in 2002. By 2005 in central and southern India about 93 percent of the cotton contained the Bt gene, and in 2008 a survey indicated 99-per-cent adoption, which meant that refuges were not planted (Kathage and Qaim, 2012). In 2009, Monsanto researchers confirmed field failures due to resistance in pink bollworm (Mohan et al., 2016). Cotton hybrids with two Bt toxins (Cry1Ac and Cry2Ab) were commercialized and replaced most single-toxin hybrids. By 2015, pink bollworm had evolved resistance to the dual-toxin cotton in the state of Gujarat and some parts of the states of Andhra Pradesh, Telangana, and Maharashtra and that caused estimated losses of 7–8 percent (Kranthi, 2015; Kasabe, 2016). Fortunately, the other cotton bollworm species (Helicoverpa armigera) has not evolved high enough levels of resistance to cause excess damage to the Bt cotton variety.
In addition to developing varieties with multiple Bt genes aimed at a single target insect pest, companies have also stacked Bt genes aimed at different pests. For example, Monsanto’s SmartStax® maize variety has two Bt genes targeted at the European corn borer and other Lepidoptera and two other Bt genes aimed at the western corn rootworm. Those stacked varieties can make resistance-management approaches complicated. For example, there are two general approaches for planting a refuge: having non-Bt seeds planted in fields next to the Bt crop or having Bt and non-Bt seeds mixed in the bags of maize seed. For the European corn borer, the best approach for refuge design is having a particular percentage of fields (or blocks of rows) planted in non-Bt seed to serve as refuges. For European corn borer, seed mixtures could be problematic because the insect larvae could move between Bt and non-Bt plants in the seed mixture and receive an intermediate dose of the toxin (Mallet and Porter, 1992; Gould, 1998). For corn earworm, a problem sometimes attributed to the use of seed mixtures is that the non-Bt plants can be pollinated by the Bt maize
in the mixture. For corn rootworm, a seed mixture or a block-to-block mixture should have similar effects. The result of that is that one-half or more of the kernels in the ears have Bt toxin, so when the corn earworm feeds on the ears of the “refuge” maize, it is exposed to Bt toxin, negating the utility of the refuge (Yang et al., 2014). It is important to note that with corn earworm, the Bt toxin levels do not result in a high dose, so the small refuges are never expected to be effective unless the resistance trait is genetically recessive (Gould, 1998; Brévault et al., 2015). It is assumed that for western corn rootworm a seed mixture of Bt and non-Bt seed is reasonable because the soil-dwelling larvae do not typically move between plants. In that case, the varieties available in 2015 did not produce a high dose, so the utility of the small current refuge would be limited with or without movement of larvae unless, again, the resistance trait was recessive. In an article (Andow et al., 2016), a group of 10 entomologists and economists who work on maize production concluded that “farmers should be encouraged to move away from a mentality of ‘what trait do I use’ to a multifaceted pest management approach. This integrated approach should start as soon as a new technology is commercialized, so that it can be more effectively stewarded by reducing the rate of resistance evolution, especially for traits with less than a high-dose.” The committee agrees that this would be an appropriate approach but that implementation would require carefully constructed, long-term incentives for farmers; farmers currently have little choice but to look for the next trait to come along. A publication by Badran et al. (2016) demonstrated a new technology that might be able to more quickly generate new Bt toxins and thus provide that next hoped-for new trait; however, at the time the committee was writing its report, it was only a proof of concept.
As described in Box 4-2, Bt crops have caused the European corn borer population to decline to the point where they are well below economic thresholds, so it often is not economically favorable for farmers to grow maize with the Bt toxins that are aimed primarily at the corn borers (Hutchison et al., 2010; Bohnenblust et al., 2014). The field-to-field (or blocks of rows) planting of Bt and non-Bt maize appears to have mitigated resistance evolution in the European corn borer, but a seed mix may compromise the refuge (NRC, 2010a; Carrière et al., 2016). This situation is suboptimal because, even though there are fewer corn borers and little damage, the same percentage of corn borers are being exposed to the Bt toxins no matter what their density, and unless total numbers of the pests in a region are below a million, it is the percentage exposure and not the number exposed that is expected to have the greatest effect on the rate at which resistance genes increase in frequency. For European corn borer, even in Wisconsin where, only about 3 percent of all maize plants are infested, the population of these insects is estimated at over 3 billion. It is not now
possible to purchase maize with Bt toxins aimed at western corn rootworm but without those aimed at European corn borer.
As trait stacking becomes more common and involves both insect pests and pathogens, providing optimal combinations of traits and refuges will become more important. It is difficult for seed providers to maintain inventories of multiple varieties that provide farmers with the ability to match traits with their specific needs, but that is an issue that should be addressed in order to slow the evolution of resistance.
As noted above, many countries do not enforce refuge guidelines. Another problem for developing countries is that the Bt toxins incorporated into crops as of 2015 have been designed mostly for insect pests of the United States. The major insect pests in developing counties are often different from those in the United States, and the Bt toxins in the crops might be only marginally useful for pests in those countries and more likely to cause evolution of resistance. The cases of the African maize stem borer (Kruger et al., 2011) and some armyworm species in Brazil (Bernardi et al., 2014) are examples of how Bt toxins developed for U.S. insect pests have suboptimal effects on pests in developing countries and resulted in evolution of resistance.
FINDING: The high dose/refuge strategy for delaying evolution of resistance to Bt toxins appears to have been successful, but deployment of crops with intermediate levels of Bt toxins and small refuges has sometimes resulted in the evolution of resistance in insect pests that erodes the benefits of the Bt crops.
FINDING: The widespread deployment of crops with Bt toxins has decreased some insect-pest populations to the point where it is economically realistic to increase plantings of crop varieties without a Bt toxin that targets these pests. Planting varieties without Bt under those circumstances would delay evolution of resistance further.
RECOMMENDATION: Given the theoretical and empirical evidence supporting the use of the high dose/refuge strategy to delay the evolution of resistance, development of crop varieties without a high dose of one or more toxins should be discouraged and planting of appropriate refuges should be incentivized.
RECOMMENDATION: Seed producers should be encouraged to provide farmers with high-yielding crop varieties that only have the pest resistance traits that are economically and evolutionarily appropriate for their region and farming situation.
EFFECTS RELATED TO THE USE OF HERBICIDE-RESISTANT CROPS
The committee looked at the effects of GE herbicide resistance on crop yield, herbicide use, weed species distribution, and the evolution of resistance to the GE trait in targeted weed species. As in the section on Bt crops, it relied in part on previous reviews but went beyond that in examining specific studies in order to provide the reader with the strengths and weaknesses of studies used to support specific claims about HR crops.
Yield Effects of Genetically Engineered Herbicide Resistance
As of 2015, GE herbicide resistance had been incorporated into soybean, maize, cotton, canola, sugar beet (Beta vulgaris), and alfalfa (Medicago sativa). With the exception of alfalfa, for which GE varieties are resistant only to glyphosate, varieties of those crops with GE resistance to other herbicides in addition to glyphosate had been developed (see Table 3-1), but not all were commercially available. In the first 20 years of GE crop production, glyphosate resistance was the predominant GE herbicide-resistant trait used by farmers.
Herbicide-Resistant Soybean
Areal et al. (2013) found no difference in yield between HR soybean and non-GE soybean on the basis of a meta-analysis of data collected in the United States, Canada, Argentina, and Romania in 1996–2003. Fernandez-Cornejo et al. (2014) found mixed results in their summary of studies on HR soybean in the United States published in 1995–2004. Three studies reported an increase in yield, one reported a small increase, one reported a small decrease, and four reported no difference.
In a field experiment in Brazil in the three crops seasons of 2007–2010, Bärwald Bohm et al. (2014) found that glyphosate-resistant soybean treated twice with glyphosate, 28 and 56 days after planting, yielded the same as the same glyphosate-resistant variety that was treated only once or that was hand-weeded instead of being sprayed with glyphosate. These yields also did not differ from those on a plot of non-HR isogenic soybean that was hand-weeded.
Another experiment in Brazil examined yield of glyphosate-resistant soybean at six locations in the growing seasons of 2003–2004, 2004–2005, and 2005–2006 (Hungria et al., 2014). Glyphosate-resistant soybean treated with glyphosate was compared with four other scenarios: the same HR variety treated with other herbicides typically used with non-HR soybean, the non-HR parent line of the HR soybean treated with other
herbicides typically used with non-HR soybean, the HR soybean with hand-weeding, and the non-HR parent line of the HR soybean with hand-weeding. No difference in yield was found between the plots with HR soybean (treated with glyphosate, treated with other herbicides, and hand-weeded) and those with non-HR soybean in five of the six locations.14 When the plots with HR soybean treated with glyphosate were compared with the plots with the HR soybean treated with other herbicides, yields for the HR soybean treated with glyphosate in four of the locations were greater. When HR soybean treated with glyphosate was compared with the non-HR parent line of the HR soybean treated with other herbicides, the yields for the HR soybean were greater in three of the locations.
In field experiments in Iowa conducted in 2007 and 2008, Owen et al. (2010) found that HR varieties (three resistant to glyphosate and three resistant to the herbicide glufosinate) had greater yields than three non-HR varieties. The result was the same when none of the varieties was treated with post-emergence herbicides or when the glyphosate-resistant varieties were treated with glyphosate, the glufosinate-resistant varieties were treated with glufosinate, and the non-HR varieties were treated with post-emergence herbicides. No differences in yield were observed among the HR varieties over the 2 years or in the experiments’ three sites. In a different experiment in Iowa in 2010 there were no differences in the mean yield between three populations of glyphosate-resistant soybean and three non-HR counterpart populations planted at four locations, with one exception: at one location, one of the glyphosate-resistant populations had a mean yield 1.6 percent greater than its counterpart (De Vries and Fehr, 2011).
Field experiments in two locations in Missouri during the summers of 2009 and 2010 compared different combinations of pre-emergence and post-emergence herbicide programs in non-GE soybean, glyphosate-resistant soybean, and glufosinate-resistant soybean (Rosenbaum et al., 2013). Averaged among locations and treatments, glufosinate-resistant soybean had the greatest yields (2,688 kilograms/hectare), followed by glyphosate-resistant soybean (2,550 kilograms/hectare) and non-GE soybean (2,013 kilograms/hectare). In control plots, to which no herbicides were applied, yields were similar in all three varieties; this indicated that glufosinate and glyphosate herbicide programs with GE soybean provided better control of competing weeds than did herbicide programs with non-GE soybean.
Soybean with GE resistance to the imidazolinone class of herbicides was first approved for commercial production in 2010 in Brazil. In 2007–2008, Hungria and colleagues tested GE imidazolinone-resistant soybean against a non-HR isoline. HR soybean treated with an imidazolinone herbicide
__________________
14 The sixth location experienced drought, and yield data were collected only for one growing season.
was compared with HR soybean treated with other post-emergence herbicides and with the non-HR isoline treated with other post-emergence herbicides. No differences in yield were observed among the three treatments or over time (Hungria et al., 2015).
Gurian-Sherman (2009) also reported little or no effect on yield in a review of studies of HR soybean conducted in 1999–2006 in the United States. He raised the issue of yield drag, which was also discussed in the 2010 National Research Council report on GE crops, and yield lag (NRC, 2010a).15 Gurian-Sherman and the National Research Council report looked at the same studies from the early 2000s16 and found evidence of yield drag and yield lag. However, more recent studies, such as those reviewed above, demonstrate that yield drag and yield lag appear to have been overcome in HR soybean because the yields of HR soybean are the same as or more than the yields of non-HR soybean. As with some of the results described for Bt crops, Owen et al. (2010) hypothesized that the lower yield observed in a non-GE soybean (not treated with post-emergence herbicides) than in glyphosate-resistant soybean and glufosinate-resistant soybean (also not treated after emergence with their counterpart herbicides) in a 2007–2008 experiment could be due to yield lag in the genetic potential of the non-GE variety.
Herbicide-Resistant Maize
Thelen and Penner (2007) compared the yields of glyphosate-resistant maize treated with glyphosate and glyphosate-resistant maize treated with other herbicides. Three field sites in different counties in Michigan were monitored for 5 years (2002–2006). At two of the sites, there was no difference in the 5-year average yield between fields treated with glyphosate and fields treated with other herbicides. At the third site, the glyphosate-treated
__________________
15 “Yield lag is a reduction in yield resulting from the development time of cultivars with novel traits (in this case, glyphosate resistance and Bt). Because of the delay between the beginning of the development of a cultivar with a novel trait and its commercialization, the germplasm that is used has lower yield potential than the newer germplasm used in cultivars and hybrids developed in the interim. Consequently, the cultivars with novel traits have a tendency to initially yield lower than new elite cultivars without the novel traits. Over time, the yield lag usually disappears.
“Yield drag is a reduction in yield potential owing to the insertion or positional effect of a gene (along with cluster genes or promoters). This has been a common occurrence throughout the history of plant breeding when inserting different traits (e.g., quality, pest resistance, and quality characteristics). Frequently, the yield drag is eliminated over time as further cultivar development with the trait occurs.” (NRC, 2010a:142)
16 The National Research Council report (2010a) reviewed the same two reports reviewed in Gurian-Sherman (2009) and several others.
maize, averaged over 5 years, had a yield advantage over glyphosate-resistant maize treated with other herbicides.
Field studies in Illinois in 1999 and 2000 compared four kinds of maize hybrids suited to the growing region (Nolte and Young, 2002). One hybrid was non-GE, one was not genetically engineered but was resistant to the imidazolinone class of herbicides, one was genetically engineered with resistance to glyphosate, and one was genetically engineered with resistance to glufosinate. There was no difference in yield among the non-GE hybrid, the non-GE imidazolinone-resistant hybrid, and the GE glufosinate-resistant hybrid. In the first year, yield for the GE glyphosate-resistant hybrid was lower; in the second year, its yield was greater than that of the other hybrids. The authors hypothesized that the glyphosate-resistant hybrid was more sensitive to temperature and moisture stress and that its yield responded more to stressful growing conditions in the first year and more to ideal growing conditions in the second year.
Almost all the data on yield effects of HR maize come from North America. However, Gonzales et al. (2009) collected data from six provinces in the Philippines and found that three provinces reported a yield advantage (but with no statistical information) from HR maize when compared with average yield of non-GE hybrids in the wet season of 2007–2008. Three other provinces reported a yield disadvantage. In the dry season of 2007–2008, five provinces reported a yield advantage; the average yield in two other provinces was nearly equivalent between HR and non-GE maize. Two years later, in the wet season of 2010, Afidchao et al. (2014) reported that HR maize yielded the same as non-GE maize in the Isabela province of the Philippines.
Herbicide-Resistant Cotton
Most cotton varieties produced since 2005 in the United States have HR and IR traits. India, China, and Pakistan—the other large producers of cotton—grow Bt varieties. The wide adoption of Bt-HR varieties and Bt-only varieties means that little recent research has been devoted to comparing the yields of HR and non-HR cotton.
Herbicide-Resistant Canola
GE canola with resistance to glyphosate or glufosinate is in commercial production. In a study conducted over 3 years in Great Britain, GE glufosinate-resistant canola (oilseed rape) was found to be less invasive and persistent than the non-GE comparators (Crawley et al., 1993). Stringam et al. (2003) reviewed the introduction of GE varieties in Canada, using data from variety trials that compared GE and non-GE varieties and yield
estimates from producers. The data did include statistical comparisons. They found yield increases of as much as 39 percent for GE varieties, but most differences were smaller. Harker et al. (2000) reported that yields were greater when the GE glyphosate-resistant or glufosinate-resistant varieties were treated with glyphosate or glufosinate, respectively, compared with treatment with standard herbicides used in canola. Those yield increases were as much as 38 percent greater, which they attributed to improved weed control in some circumstances but also to higher potential yield in the germplasm of the HR varieties. In field studies conducted throughout Canada under different environmental conditions, yield of the GE and non-GE varieties was similar (Clayton et al., 2004). Beckie et al. (2011) reported that the rapid adoption of GE canola is due to better weed control and to greater yields and economic returns.
Herbicide-Resistant Sugar Beet
Kniss et al. (2004) compared yield of two non-GE and two GE glyphosate-resistant sugar beet varieties in studies conducted in Nebraska in 2001 and 2002 (before HR sugar beets were commercially sold). Although not reported to be isolines, the varieties were paired for evaluation on the basis of a high degree of shared genetic backgrounds. The non-GE varieties were treated with herbicides that would typically be used for weed control, but glyphosate was the only herbicide applied to the resistant varieties. In one case, the glyphosate-resistant variety (Beta 4546RR) produced greater sucrose content than the nonresistant variety (Beta 4546). The authors concluded that the difference was due to reduced herbicide injury and better weed control. In the other case, even though there were less crop injury and increased weed control, the GE sugar beet variety (HM 1640RR) did not have greater sucrose content than its nonresistant HM variety counterpart. The authors proposed that the difference in response was due to the Beta varieties’ greater genetic similarity than that of the HM varieties. Sucrose production is not controlled by a single gene, so the difference between the two sets of varieties would account for differences in sucrose concentration rather than in the resistance trait. The resistant Beta variety produced a greater yield and gross sucrose production than the nonresistant Beta variety in all but one treatment. The yield results of the HM varieties were less definitive, with few differences in yield or gross sucrose production. The authors concluded that planting a glyphosate-resistant variety versus a non–glyphosate-resistant variety will not necessarily return greater profits but that it is important to choose a variety that is high-yielding and locally adapted.
Kniss (2010) compared yield of non-GE and glyphosate-resistant sugar beet on a field scale in Wyoming in 2007, the first year of commercial
production. The fields were chosen on the basis of the criteria that paired fields with glyphosate-resistant sugar beets or non-GE sugar beets were managed by the same grower and had similar slopes, soil types, irrigation, and production histories. The growers controlled management decisions on both fields. Tillage was lower in the glyphosate-resistant sugar beet than in the non-GE sugar beet fields. Sugar content was similar in the resistant and nonresistant sugar beets, but yield was greater in the glyphosate-resistant sugar beet fields, so the gross sucrose production was greater. The study was conducted in only one year because in 2008 farmer adoption of the glyphosate-resistant varieties was so great that non-GE sugar beet fields were not available to repeat the study.
Other studies have compared the yield of glyphosate-resistant varieties after applications of glyphosate versus commonly used herbicides in the United States (Guza et al., 2002; Wilson et al., 2002; Armstrong and Sprague, 2010) and in Germany and Poland (Nichterlein et al., 2013). In the studies conducted in the United States, there was some variation among sites in weed control and yield. In Idaho, weed control and yield were comparable in plants treated with glyphosate and with the herbicides typically used in sugar beet production (Guza et al., 2002). In Nebraska, weed control was similar between the glyphosate treatments and the treatments with commonly used herbicides, but sucrose yield was reduced with the treatments with commonly used herbicides (Wilson et al., 2002). In Michigan, glyphosate provided better weed control than commonly used herbicides, but yield in kilograms of sugar was similar (Armstrong and Sprague, 2010). In the studies conducted in Germany and Poland of glyphosate-resistant sugar beet treated with glyphosate versus treatment with herbicides typically used in sugar beet production, yields of the former were greater only in some trials (Nichterlein et al., 2013). However, fewer herbicide applications were required, and there was a reduction in kilograms of active ingredients applied. Therefore, the authors concluded that growing glyphosate-resistant sugar beet would lead to a reduction in herbicide use.
Wilson et al. (2002) and Kemp et al. (2009) included glufosinate-resistant varieties in their studies. Weed control and yields were similar for all treatments. At the writing of this report, no glufosinate-resistant varieties were in commercial production.
Herbicide-Resistant Alfalfa
Glyphosate-resistant alfalfa has not been grown for as many years as the other resistant crops, so fewer studies of it have been published. In addition, alfalfa is a perennial crop, so evaluation of its field performance will require more years than for that of the annual crops discussed previously. Data on the yield of glyphosate-resistant alfalfa in peer-reviewed publica-
tions are sparse. One study compared yield, weed biomass, and forage quality of glyphosate-resistant alfalfa and non-GE alfalfa (Sheaffer et al., 2007). In the year of seeding and the following year, the two systems were similar in yield and forage quality.
FINDING: HR crops contribute to greater yield where weed control is improved because of the specific herbicides that can be used in conjunction with the HR crop.
Changes in Herbicide Use Due to Herbicide-Resistant Crops
Findings on the effect of glyphosate-resistant crops on the amount of herbicide applied per hectare of crop have been diverse. There is doubt about the utility of simply measuring the amount of herbicide without reference to the environmental and health effects per kilogram of each herbicide used. The committee first presents the reviews of data on amount of herbicide used and then examines the relevance of these data.
The assessment by Klümper and Qaim (2014) concluded that the amount of herbicide applied to HR soybean, maize, and cotton compared to their non-GE counterparts was essentially unchanged from non-HR counterparts (-0.6 percent; n=13). Barfoot and Brookes (2014) concluded that, on an overall global level for the period between 1996 and 2012, the volume of herbicide active ingredients applied decreased by 0.2 percent (soybean) to 16.7 percent (canola) and that only application to sugar beet increased (25.6 percent). Qaim and Traxler (2005) found that herbicide application rates for HR soybean in Argentina doubled during 1996–2001. Here the growers substituted glyphosate for herbicides in higher toxicity classes (Nelson and Bullock, 2003; Cerdeira and Duke, 2006).
In the United States, Fernandez-Cornejo et al. (2014) found that herbicide use on maize in terms of a.i. had decreased from about 2.9 kilograms/hectare in the early years of HR maize adoption to less than 2.2 kilograms/hectare in 2002. Herbicide use increased slightly from 2002 to 2010 (Figure 4-8). For soybean and cotton, there were initial decreases in a.i. per hectare, but amounts in 2008 (soybean) and 2010 (cotton) were greater than amounts in 1995 (Fernandez-Cornejo et al., 2014). USDA data were 1.6 kilograms/hectare for soybean in 2012 (USDA–NASS, 2013) and 2.1 kilograms/hectare for maize in 2014 (USDA–NASS, 2015). Statistical significance was not reported.
Benbrook (2012) also assessed USDA data and concluded (without a statistical analysis) that herbicide application rates in kilograms of a.i. per hectare per year were greater in 2006–2010 than in 1996 for soybean and cotton (soybean, 1.3 kilograms/hectare in 1996 and 1.6 kilograms/hectare in 2006; cotton, 2.1 kilograms/hectare in 1996 and 3.0 kilograms/hectare in
SOURCE: Fernandez-Cornejo et al. (2014).
2010) but less for maize (3.0 kilograms/hectare in 1996 and 2.5 kilograms/hectare in 2010). Benbrook pointed out that data on kilograms per hectare may be misleading because some herbicides are effective at about 1.0 kilograms/hectare and others at less than 0.1 kilograms/hectare. An overall decrease or increase in kilograms per hectare could simply reflect a change in use of high-efficacy and low-efficacy herbicides and not necessarily reflect increased desirability from a human-health or environmental perspective. To address that problem, some researchers, including Barfoot and Brookes (2014), use an environmental impact quotient (EIQ) for evaluating herbicides and insecticides (Kovach et al., 1992). However, EIQ has not been found to be a substantially better indicator of environmental impact than kilograms per hectare (Kniss and Coburn, 2015). Kniss and Coburn (2015) argued convincingly that neither kilograms per hectare nor EIQ is a useful metric and that only careful case-by-case evaluation of the environmental and health impacts of each herbicide will yield useful assessments. They recommended use of the EPA risk-quotient approach. Mamy et al. (2010) provided a comparative environmental risk assessment of glyphosate and other herbicides. Nelson and Bullock (2003) presented an approach for measuring a proxy for human toxicity risk per application of an herbicide.
Assessing the relative effects of diverse herbicides can be challenging, but it is clear that simply determining if the total kilograms/hectare of herbicide used per year has gone up or down is not useful for assessing human or environmental risks.
FINDING: The use of HR crops sometimes initially correlated with decreases in total amount of herbicide applied per hectare of crop per year, but the decreases have not generally been sustained. However, such simple determination of whether total kilograms of herbicide used per hectare per year has gone up or down is not useful for assessing changes in human or environmental risks.
RECOMMENDATION: Researchers should be discouraged from publishing data that simply compares total kilograms of herbicide used per hectare per year because such data can mislead readers.
Changes in Weed Species Densities Due to Herbicide-Resistant Crops
Once the HR crops are introduced and used continuously, the repeated applications of a single herbicide with no other weed-management techniques leads to increases in weed species that are not sensitive to the herbicide or that respond to other changes in production practices. Glyphosate controls many monocot and dicot weeds, but it does not control all weed species equally. Some of the weed species that are tolerant to glyphosate have become more problematic in glyphosate-resistant crops. In the United States, a survey of 12 weed scientists in 11 states in 2006 indicated that several weed species were increasing in glyphosate-resistant crops (Culpepper, 2006); they included morning glories (Ipomoea spp.), dayflowers (Commelina spp.), and sedges (Cyperus spp.). One weed species that decreased in density within crop fields was common milkweed (Asclepias syriaca) (Hartzler, 2010). Other shifts in weed species abundance were occurring in U.S. crop production areas, but they were attributed to time of application of the herbicide rather than to overall weed sensitivity to the herbicide. Owen (2008) and Johnson et al. (2009) reviewed the literature and found some strong shifts to weeds that are hard to manage, such as giant ragweed (Ambrosia trifida), horseweed (Conyza canadensis), common and narrowleaf lambsquarters (Chenopodium album), morning glories, and shattercane (Sorghum bicolor ssp. X. drummondii). Some of the changes in weed species at that time could also have been associated with the increase in use of no-till and reduced-till crop production practices.
Now that crops with stacked herbicide-resistant traits such as 2,4-D and glyphosate are being commercialized, it is possible that different weeds will increase or decrease, but such changes may not be problematic for pro-
duction agriculture unless previously minor weed species pose severe problems. Furthermore, as discussed below (see section “Herbicide-Resistant Crops and Weed Biodiversity”), the increased use of glyphosate does not appear to have affected the general diversity of weeds in cropping systems (Gulden et al., 2010; Schwartz et al., 2015).
FINDING: Both for insect pests and weeds, there is evidence that some species have increased in abundance as IR and HR crops have become widely planted. However, in only a few cases have the increases posed an agronomic problem.
Resistance Evolution and Resistance Management for Herbicide-Resistant Crops
When glyphosate-resistant crops were commercialized in the United States, neither USDA nor EPA required resistance-management plans to delay the evolution of glyphosate-resistant weeds. The repeated use of glyphosate on glyphosate-resistant crops, which were adopted rapidly and widely, quickly led to selection for weeds with evolved resistance to glyphosate (Box 4-3). In 2000, marestail (Conyza canadensis L.) was the first glyphosate-resistant weed to be confirmed in an HR cropping system (VanGessel, 2001). The resistant marestail was selected in glyphosate-resistant soybean within 3 years of the use of glyphosate alone for weed management. Glyphosate was used to suppress weeds in crops for many years before the advent of GE crops, typically by spraying before a crop plant emerged from the ground or after harvest. It is still used in crops without GE herbicide resistance. However, at least 16 of the 35 reported glyphosate-resistant weed species identified (Heap, 2016) evolved in fields where HR crops were grown. Glyphosate-resistant weeds have been identified in Argentina, Australia, Bolivia, Brazil, Canada, Chile, China, Colombia, Costa Rica, the Czech Republic, Greece, France, Indonesia, Israel, Italy, Japan, Malaysia, Mexico, New Zealand, Paraguay, Poland, Portugal, South Africa, Spain, Switzerland, Taiwan, the United States, and Venezuela (Heap, 2016). The National Research Council held a workshop to assess the resistance problem and potential solutions (NRC, 2012). A study conducted by the USDA Economic Research Service (Livingston et al., 2015) estimated the reduction in total returns in maize and soybean in the United States due to the cost of glyphosate-resistant weeds at $165/hectare and $56/hectare, respectively. Livingston and colleagues (2015:i) concluded that “managing glyphosate resistance is more cost effective than ignoring it, and after about 2 years, the cumulative impact of the returns received is higher when managing instead of ignoring resistance.” The committee could not find cost estimates for other countries. However, Binimelis
et al. (2009) quoted sources in Argentina to the effect that controlling glyphosate-resistant Johnsongrass (Sorghum halepense) increased soybean production cost by 19 percent and doubled herbicide costs.
Powles (2008) pointed out that in many regions in the world glyphosate resistance has not yet evolved and that some widely planted crops, such as wheat and rice, do not yet have commercially available glyphosate-resistant varieties. Powles made a strong case for learning from the problems in maize and soybean that continuous use of glyphosate will not be beneficial in the long term.
There is disagreement in the weed-science research community about the benefit of stacking multiple HR traits and spraying multiple herbicides for resistance management (Wright et al., 2010; Egan et al., 2011; Mortensen et al., 2012). Evolutionary theory suggests that combinations of herbicides in tank mixes are expected to substantially delay resistance compared to use of a single herbicide only when weeds that are resistant to one herbicide are still killed by the second herbicide in the tank mix (Tabashnik, 1989; Gould, 1995; Neve et al., 2014). The stacked HR traits that had been or were being commercialized when the committee wrote its report will provide resistance to various combinations of glyphosate, glufosinate, 2,4-D, and dicamba. Those herbicides have different sites of action, so crops with stacked HR traits could reduce specific selection pressure from glyphosate. However, that would not be the case for all weed species because some weeds are susceptible to only one herbicide in mixed herbicide applications. For example, glyphosate has activity on both monocots and dicots whereas 2,4-D and dicamba control only dicots; therefore, monocots exposed to a tank mix of glyphosate and 2,4-D or dicamba are functionally being controlled only with glyphosate.
Evans et al. (2016:74) analyzed resistance to glyphosate in the major weed common waterhemp (Amaranthus tuberculatus) on 105 farms in Illinois and determined that combined spraying of herbicides that have different sites of action to that weed (that is, tank mixes) reduced the likelihood of evolution of glyphosate-resistant waterhemp on a farm. From their large-scale study, the authors concluded that “although measures such as herbicide mixing may delay the occurrence of [resistance in weeds to glyphosate] or other HR weed traits, they are unlikely to prevent [it].”
There is uncertainty regarding the best approaches for using single and multiple herbicides to delay resistance evolution in weeds. Spraying mixtures of herbicides could be useful, but theoretical and empirical evidence for the utility of this approach is weak. More research at the farm level and in experimental plots and biochemical, genomic, and population genetic research are needed to decrease the uncertainty and develop better resistance-management approaches. It is generally recognized that the less often a control measure is used, the longer it takes for resistance to evolve,
and a number of integrated weed-management approaches could decrease the need for heavy reliance on herbicides, but they are not widely practiced in large-scale cropping systems in the United States (Wiggins et al., 2015). The use of judicious tillage, a key component of integrated weed management (Mortensen et al., 2012), can be highly effective in suppressing herbicide-resistant weeds in some cropping systems (Kirkegaard et al., 2014). Tillage once every 5 years within no-till and reduced-till cropping systems can be done without detrimental effects on grain yield or soil properties (Wortmann et al., 2010; Giller et al., 2015). Other aspects of integrated weed management, such as crop rotation and use of cover crops, which in some areas of the United States are promoted with economic incentives to reduce nitrate leaching (Mortensen et al., 2012), fit well with approaches to conservation tillage. In general, integrated weed management requires a detailed understanding of the weed-community ecology in a specific area. Without availability of knowledgeable extension agents to assist farmers in implementing diverse approaches to suppress weed populations, it will be difficult for farmers to move away from intensive use of herbicides.
EPA’s 2014 document on the registration of the herbicide Enlist DuoTM, which contains a mixture of glyphosate and 2,4-D and is targeted at GE crops that are resistant to both, includes a requirement for the registering company to develop a resistance-management plan (EPA, 2014a). On the basis of the committee’s review of the theoretical and empirical literature, there is no scientific consensus on the best practices for delaying resistance simply through use of mixtures of herbicides. There is an obvious need for weed management that includes approaches other than continuous use of herbicides.
FINDING: Weed resistance to glyphosate is a problem and could be delayed by the use of resistance-management tactics especially in cropping systems and regions where weeds have not yet been exposed to continuous glyphosate applications.
RECOMMENDATION: To delay evolution of resistance to herbicides in places where GE crops with multiple HR traits are grown, integrated weed-management approaches beyond simply spraying mixtures of herbicides are needed. This will require effective extension programs and incentives for farmers.
RECOMMENDATION: Although multiple strategies can be used to delay weed resistance, there is insufficient empirical evidence to determine which strategy is expected to be most effective in a given cropping system. Therefore, research at the laboratory and farm level should be funded to improve resistance-management strategies.
YIELD EFFECTS OF GENETICALLY ENGINEERED HERBICIDE AND INSECT RESISTANCE
As of 2015, GE varieties of soybean, maize, and cotton with both HR and IR traits were available in some countries. Most varieties had only one HR trait, which was most often glyphosate resistance. Many varieties contained more than one Bt toxin to target different insect pests.
Bt-HR soybean was planted for commercial production in Brazil, Argentina, Paraguay, and Uruguay starting in 2013 (Unglesbee, 2014). In two environmental studies, Beltramin da Fonseca et al. (2013) found that number of pods per plant and yield for Bt-HR soybean were greater than those of non–Bt-HR soybean.
Nolan and Santos (2012) found that maize with GE traits of Bt targeting European corn borer and herbicide resistance had a yield advantage of 501 kilograms/hectare over a non-GE variety. The advantage for herbicide resistance with Bt targeting corn rootworm was even greater (921 kilograms/hectare). All three traits combined provided a yield advantage of 927 kilograms/hectare. In 2010, Afidchao et al. (2014) reported that Bt-HR maize yielded the same as non-GE maize in the Isabela province of the Philippines.
Bauer et al. (2006) compared two Bt-HR cotton varieties to their non-GE parents in field experiments planted on three dates in spring 2000 and 2001 in South Carolina. Lint yield was not different between the transgenic lines and the parent lines regardless of planting date.
ENVIRONMENTAL EFFECTS OF GENETICALLY ENGINEERED CROPS
Diverse views have been expressed about the possibility that GE crops have adverse environmental effects. They include declines in natural enemies of insect pests, in honey bees (Apis spp.), and in monarch butterflies (Danaus plexippus) and in plant and insect biodiversity in general. At a landscape level, there is concern that GE crops contaminate other crops and wild relatives through gene flow. There is also concern that GE crops have resulted in more use of monoculture over space and time because, with protection from insect pests and availability of more effective herbicides, it becomes more profitable to grow a single crop that has the highest economic return, even if that means ignoring crop-rotation practices. It has also been suggested that GE crops are causing more fertilizer and herbicide runoff into waterways. In this section, the committee examines the evidence regarding those concerns.
Effects of Genetically Engineered Crops on Biodiversity on Farms
With regards to biodiversity on the farm, the committee looked for changes in the abundance and diversity of insects and weeds in GE cropping systems and changes in the diversity of types of crops planted and the genetic diversity in each crop species.
Bt Crops and Arthropod Biodiversity
The National Research Council report on the impact of GE crops on farm sustainability in the United States noted that generalist predators tended to be unchanged or were more abundant where Bt crops replaced non-Bt crops, especially when the non-Bt crops were sprayed with synthetic insecticides (NRC, 2010a). However, there were no data for assessing whether that translated into more effective biological control on a farm scale. More recently, Lu et al. (2012) reported a widespread and large increase in generalist predators (ladybirds, lacewings, and spiders) in China in association with the adoption of Bt cotton. That increase in generalist predators spilled over on to non-Bt crops (maize, peanut, and soybean) and resulted in enhanced biological control of aphid pests. It is important to note that the reported effect arises because of a contrast between heavy insecticide use (pyrethroids and organophosphates) in nonorganic, non-Bt cotton and substantially reduced insecticide use in Bt cotton. The committee could find no other similar studies since publication of those results.
It is expected that when an insect-pest population declines dramatically because of Bt crops, as in the case of European corn borer in the United States, there will be an accompanying decline in any host-specific parasitoid or pathogen of the pest, and the parasitoid or pathogen could even become locally extinct (Lundgren et al., 2009). Under such conditions, if the pest later evolves resistance to the Bt toxin, it could increase in density because it would lack natural control. The committee was unable to find any quantitative studies that tested for reductions in pest-specific natural enemies.
Beyond examining natural enemies of crop insect pests, the National Research Council report on GE crop impacts examined effects of Bt crops on general arthropod biodiversity on farms (NRC, 2010a). In comparisons between Bt varieties of maize and cotton and nonorganic, non-Bt varieties with typical insecticide use, the report concluded that Bt crops can promote biodiversity. However, if the comparison is with absence of insecticide application, biodiversity was similar or lower with Bt crops. The report’s conclusions were based on meta-analyses, in which the results of a large number of laboratory and field studies were synthesized, and the weight of evidence depended on sample sizes, differences in means, and variability in the data (Marvier et al., 2007; Wolfenbarger et al., 2008). Later field studies
broadened the crops and species under consideration and arrived at similar conclusions (Lu et al., 2014; Neher et al., 2014). Hannula et al. (2014) reviewed the literature on potential effects of Bt crops on soil fungi. They found a high degree of variation among studies and concluded that more careful research approaches should be used to examine the crops case by case. As more is learned about the root microbiome, the feasibility of such studies will increase. There remains a need for continued meta-analyses and development of databases to aid in assessment of the effects of Bt crops on overall biodiversity. One such effort was under way for maize when the committee wrote its report (Romeis et al., 2014).
There is special concern about the effects of Bt maize pollen and nectar on honey bees because of their critical role in pollinating other crops. Duan et al. (2008) conducted a meta-analysis of 25 studies of Bt toxin effects on honey bee larvae and adults. They concluded that there was no evidence of any adverse effect on the honey bee but that “additional studies in the field may be warranted if stressors such as heat, pesticides, pathogens, and so on are suspected to alter the susceptibility of honey bees to Cry protein toxicity.” There is almost no Bt protein in nectar and little in pollen, so exposure of the honey bees is low. When honey bees were exposed to a dose that was about 50 times the dose expected from foraging on Bt maize varieties, there was no mortality, but there was some effect on learning by adults (Ramirez-Romero et al., 2008). The committee did not find any studies of the interaction of Bt pollen and exposure to neonicotinoid insecticides. In a review of honey bee toxicology, Johnson (2015) concluded that evidence from many studies indicates that Bt pollen and nectar are not harmful to honey bees. A 2013 National Research Council report raised concerns about the potential for synergistic interactions between toxins (NRC, 2013). The committee did not find studies that tested for synergy between Bt toxin in pollen and honey bee exposure to other toxins and stresses.
Herbicide-Resistant Crops and Weed Biodiversity
For HR crops, there is concern that the efficiency of post-emergence treatment with glyphosate is so high that it decreases weed abundance and diversity. That reduction in turn could affect vertebrate and invertebrate diversity (Lundgren et al., 2009). There have been shifts in the predominant weeds found in maize and soybean due to use of glyphosate-resistant varieties, as discussed above, but Owen (2008) and Johnson et al. (2009) found that the effects on weed biodiversity have been much less than initially expected and more complex. When weeds were controlled by a single application of glyphosate to HR maize and soybean, there was typically greater weed diversity and abundance than when other herbicides were applied to the non-GE counterparts. However, in HR sugar beet treated
with glyphosate, weed abundance was much lower than in non-GE sugar beet. In canola weed density was greater in the glyphosate-treated HR crop system than in the non-GE crop system early in the season but lower at another time of the season, while in maize weed density was always higher in the GE crop system (Heard et al., 2003).
Young et al. (2013) and Schwartz et al. (2015) reported results of a detailed U.S. study of weed seedbanks and aboveground weeds on 156 farm field sites in six states in the Southeast and the Midwest. The studies examined several cropping systems: a single continuous HR crop, a rotation of two HR crops, and a rotation of an HR crop with a non-HR crop. They found that the diversity of the weed community in farmers’ fields of maize, cotton, and soybean was strongly affected by geographical location and by the previous year’s crop. The cropping system had effects on specific weeds, but the overall diversity of weeds was affected much more strongly by location than by the cropping system. Schwartz et al. (2015:437) concluded that “diversification of the weed community, both in the weed seedbank and aboveground, is reflective of geographic region, cropping system being implemented and crop rotation, but not frequency of the use [of] the [glyphosate-resistant] crop trait.” Schwartz et al. emphasized that how the HR trait is integrated with other weed control strategies will determine the local weed composition.
Effects of Genetically Engineered Traits on Crop Diversity on Farms
Maintaining a diversity of crop species on farms and a diversity of varieties of each crop on a farm is generally considered to provide a buffer against outbreaks of insect pests and pathogens and insurance against year-to-year environmental fluctuations that could be especially damaging to one crop or variety (Hajjar et al., 2008; Davis et al., 2012; Mijatović et al., 2013). The committee received comments indicating concern that the adoption of GE crops was resulting in reduction of diversity in crops and varieties. It also heard from presenters that GE crops were crucial enablers for implementing specialized crop rotations.
Effect of Genetically Engineered Traits on Diversity of Crop Species. In a survey of U.S. counties from 1978 to 2012, Aguilar et al. (2015) found that the diversity of crop species had decreased by about 20 percent from 1987 to 2012. The decline was especially noticeable in the Midwest. It is difficult to attribute any of the change to the advent of GE crops inasmuch as no change in the trend since 1996 would generally fit the pattern of increased use of GE crops. Furthermore, commodity prices, costs of such inputs as seed and fertilizer, subsidies and societal priorities, water availability, and climatic conditions influence farmers’ choices about what to plant (NRC,
2010b). U.S. federal and state policies and their associated incentives have a powerful influence, as evidenced by the majority of U.S. farmland’s being managed in compliance with federally mandated Farm Bill guidelines in order to attract commodity payments or other subsidies (NRC, 2010b). Some subsidy programs and policies—such as the Energy Independence and Security Act of 2007 (110 P.L. 140), which establishes targets for use of renewable fuels, including biofuels made from maize and soybean—encourage planting of increased areas of commodity crops with concomitant decreases in crop diversity (Heinemann et al., 2014).
At the individual farm level in the United States, there is little evidence of a substantial shift toward continuous cropping (3 or more consecutive years of a single crop) of maize, soybean, and wheat since the introduction of GE maize and soybean (Wallander, 2013; Figure 4-9). In the Midwest, however, there is a pattern somewhat different from that in the rest of the United States: a doubling in frequency (from about 3.5 percent to about 7 percent) of continuous planting of maize for 4 consecutive years (Plourde et al., 2013). That pattern probably reflects maize prices.
Successful management of very large areas of these crops without rotation may be facilitated by GE varieties with HR or IR traits because these traits give farmers the flexibility to reduce tillage, reduce pesticide use, reduce reliance on crop rotation for weed or insect control, and re-
SOURCE: From Wallander (2013).
duce the use of herbicides with long residual times that can harm certain rotational crops. The committee heard from a USDA research entomologist (Lundgren, 2015) that the adoption of Bt maize made it easier for farmers to shift to maize monocropping (for example, when maize prices were high). Several recent studies that used U.S. Census of Agriculture Data, Cropland Data Layer (CDL), and digitized aerial photographs (especially those of smaller land areas than that of the Corn Belt) show that locations with high adoption rates of GE varieties had increased use of a short crop rotation of only maize and soybean. Fausti et al. (2012) showed that adoption of GE maize and soybean in South Dakota was faster than in any other state, with maize area planted with Bt or stacked GE hybrids increasing from 37 percent in 2000 to 71 percent in 2009. Over the same period, the proportion of cropland area planted to maize and soybean roughly doubled, from less than 25 percent to about 50 percent of planted hectares. Another possible cause of the shift is increased irrigation development, but increasing prices of both maize and soybean (especially in 2007–2009) are likely drivers and underscore the difficulties in attributing changes in cropping patterns to genetic-engineering technology.
The committee heard a presentation from an invited farmer (Hill, 2015), who indicated that some farmers rely on GE varieties of row crops to control weeds and enable crop rotations that include non-GE vegetables and other non-GE crops in which weed control is otherwise prohibitively expensive or difficult. For those farmers, GE crops are enabling the maintenance of more diverse cropping systems.
Effect of Genetically Engineered Traits on Genetic Diversity Within Crop Species. There is no doubt that genetic diversity within major crop species planted globally has declined over the last century. Gepts (2006:2281) points out that “in Mexico, only 20% of the maize types recorded in 1930 can now be found. Only 10% of the 10000 wheat varieties grown in China in 1949 remain in use.”
Although the number of varieties has declined, a meta-analysis of 44 journal articles examining trends from the 1920s through the 1990s in molecular-level (DNA marker) diversity among modern crop varieties of eight crops—including maize, soybean, and wheat—found no general loss of diversity over all crops but some increases and decreases in specific crops (van de Wouw et al., 2010). An invited presenter cautioned that widespread planting of varieties containing the same one or few successful GE trait insertion events that are backcrossed into many breeding lines could decrease genetic diversity and render a crop vulnerable to any pathogen or stress that may evolve to thrive on varieties containing flanking sequences of the single insertion event (Goodman, 2014). For example, a single insertion event of the Bt toxin Cry1Ac in cotton is found throughout the world,
often based on five or fewer backcrosses (Dowd-Uribe and Schnurr, 2016). The committee could find no evidence of a GE crop that resulted in lower genetic diversity and unexpected pathogen or stress problems, but there is evidence that, in breeding sorghum (Sorghum bicolor) for non-GE resistance to greenbug (Schizaphis graminum), there was a decline in overall genetic diversity of planted sorghum (Smith et al., 2010). That development points to the need for global monitoring of genetic diversity in crops. As made clear from the studies reviewed by van de Wouw et al. (2010) and later studies of genetic variation in crop varieties (for example, Smith et al., 2010; Choudhary et al., 2013), tools for careful monitoring of loss in genetic diversity are available if researchers can gain access to patented GE varieties of crops to conduct genetic analyses.
FINDING: Planting of Bt varieties of crops tends to result in higher insect biodiversity than planting of similar varieties without the Bt trait that are treated with synthetic insecticides.
FINDING: In the United States, farmers’ fields with glyphosate-resistant GE crops sprayed with glyphosate have similar or more weed biodiversity than fields with non-GE crop varieties.
FINDING: Since 1987, there has been a decrease in diversity of crops grown in the United States—particularly in the Midwest—and a decrease in frequency of rotation of crops. Studies could not be found that tested for a cause–effect relationship between GE crops and this pattern. Changes in commodity prices might also be responsible for this pattern.
FINDING: Although the number of available crop varieties declined in the 20th century, there is evidence that genetic diversity among major crop varieties has not declined in the late 20th and early 21st centuries since the introduction and widespread adoption of GE crops in some countries.
Effects of Genetically Engineered Crops at the Landscape and Ecosystem Levels
The discussion in the section above was confined to potential effects of GE crops on biodiversity on farms themselves. However, the committee also sought evidence of effects of GE crops on loss of the biodiversity found in natural environments, on population loss in species that move between farms and natural environments, and on the potential effects of genes from GE crops on adjoining unmanaged plant communities and on farms with-
out GE crops. Finally, the committee assessed evidence that GE crops have resulted in greater adoption of no-till and reduced-till cropping systems that can have beneficial effects on farms and beyond.
Genetically Engineered Crops and the Expansion of Agriculture into Unmanaged Environments
On the basis of the data presented on the effects of GE crops on biodiversity on farms, there is evidence of some changes in the specific weeds in fields due to herbicides used in association with GE crop varieties, although the overall plant biodiversity does not seem to change substantially (Young et al., 2013; Schwartz et al., 2015). However, the expansion of row crops into previously unmanaged environments is well known to cause a loss of plant and animal biodiversity (Tilman et al., 2001). If GE crops enable expansion of row crops into unmanaged areas, they are likely to affect landscape biodiversity.
Wright and Wimberly (2013) documented a net loss of grasslands of 530,000 hectares in the United States from 2006 to 2010. The land was converted to row crops from a number of environmentally sensitive land forms, including wetlands, highly erodible land, and land in the Conservation Reserve Program (a federal government program that pays farmers to take environmentally sensitive land out of production). Lark et al. (2015) reported similar changes from 2008 to 2012; their sampling indicated that about 0.42 million hectares (or about 14 percent) of the total recent conversion came from land sources that had not been cultivated for more than four decades. Although there is no analysis of whether adoption of GE crops played some part in fueling the conversion of natural lands to maize and other crops, the conversion appears mostly to be a response to both increased demand for liquid biofuels and rapidly increasing crop prices rather than to adoption of genetic-engineering technology, which was already widespread before the largest conversions of unmanaged lands.
Since the commercialization of glyphosate-resistant crops, there has been an expansion of soybean area in Argentina (Grau et al., 2005; Gasparri et al., 2013) and Brazil (Morton et al., 2006; Vera-Diaz et al., 2009; Lapola et al., 2010). The committee searched for information on whether any of the expansion was augmented or hastened by the use of GE soybean. Kaimowitz and Smith (2001) and Grau et al. (2005) argued that improvement in soybean varieties, including the glyphosate-resistance trait, enhanced expansion of soybean area, but they presented no evidence that the glyphosate-resistant trait itself was involved. It is generally possible for HR traits to enhance expansion of crops on previously diverse unmanaged lands, but the committee could not find any compelling evidence that such expansion has occurred.
Genetically Engineered Crops, Milkweed, and Monarch Butterflies
Some concerns over the effect of GE crops on landscape biodiversity focus on communities of thousands of species, but one species has drawn more attention than any other in North America. Worries about effects of Bt maize on monarch butterflies began with the publication of laboratory experiments that demonstrated substantial effects of Bt maize pollen on growth and survival of monarch larvae (Losey et al., 1999). Because the monarch travels long distances and feeds in agricultural and nonagricultural areas, concern about the potential for death due to Bt was reasonable. Controversy over the validity of the research by Losey et al. (1999) and other research that did and did not find adverse effects on the monarch butterfly finally resulted in detailed, coordinated studies funded by U.S. and Canadian government agencies, universities, and industry. Results of these studies were peer-reviewed and published as six articles in the Proceedings of the National Academy of Sciences of the United States of America (Hellmich et al., 2001; Oberhauser et al., 2001; Pleasants et al., 2001; Sears et al., 2001; Stanley-Horn et al., 2001; Zangerl et al., 2001). The 2002 National Research Council report Environmental Effects of Transgenic Plants provided a detailed discussion of these studies (NRC, 2002:71–75) and concluded that one transgenic event in maize, Bt176, posed a risk to monarchs because of high levels of Bt toxin in the pollen, but that the vast majority of the Bt maize that was being grown in the United States did not pose such a risk. Bt176 was later removed from the market, thereby eliminating risk posed by that variety to monarch butterflies or other pollinators. The 2002 National Research Council report saw this portfolio of coordinated studies conducted with transparency and open access to data and supported by diverse funders as a model for dealing with controversial GE crop issues and suggested that “present public research programs, such as in Biotechnology Risk Assessment and Risk Management, will need to be expanded substantially”; the report specifically pointed to the USDA Biotechnology Risk Assessment Research Grants Program in this regard (NRC, 2002:197–198). The committee agrees with the recommendation of the 2002 committee because it has become clear that when studies are or are perceived to be controlled by the developers of the technology the legitimacy of the work is often questioned.
In addition to the potential for a direct effect of Bt maize on monarch butterfly populations, it is possible that HR crops indirectly affected monarch populations if they resulted in reducing the abundance of milkweed plants, which are the sole food source for monarch caterpillars. Hartzler (2010) documented a 90-percent decline in the area within Iowa agricultural fields occupied by milkweed from 1999 through 2009 that was due primarily to the use of glyphosate. Pleasants and Oberhauser (2013) used
those data and other data on abundance of milkweed in noncrop areas of Iowa to estimate the overall decline in milkweed. They estimated that milkweed abundance declined by 58 percent from 1999 to 2010; but on the basis of data showing more eggs laid on milkweed plants within crops, there was an estimated 81-percent decline in potential production of monarchs in Iowa. Data at that level of detail are not available for other areas of the monarch range. (Of course, decline in milkweed is likely to have been beneficial to some farmers but the specific impacts of milkweed on maize and soybean profitability are not available.)
There are data that demonstrated a decline in the density of monarchs at overwintering sites in Mexico. The average total hectares occupied by dense aggregations of adults during the winters of 1995–2002 was about 9.3, but the average for 2003–2011 was 5.5 with a general trend of decline (Brower et al., 2012). The decline has continued to below 0.7 hectares in 2014, but it was expected to increase in 2015 to 3–4 hectares (Yucatan Times, 2015).
The cause–effect relationship between lower abundance of milkweed in the United States and decreasing overwintering populations is uncertain. If lower abundance of milkweed is limiting the monarch populations, there is expected to be an indication of it in their population dynamics beyond winter habitats in Mexico. A series of articles published in 2015 examined data from researchers and citizen scientists collected in 1995–2014 on dynamics of monarch populations as they moved north in spring and began moving south in fall (Badgett and Davis, 2015; Crewe and McCracken, 2015; Howard and Davis, 2015, Nail et al., 2015; Ries et al., 2015; Steffy, 2015; Stenoien et al., 2015). There was year-to-year variation in the population sizes but little evidence of decline of the monarchs during that period. A general conclusion from the work was that “while the overwintering population (and early spring migration) appears to be shrinking in size, these early monarchs appear to be compensating with a high reproductive output, which allows the subsequent generations of monarchs to fully recolonize their breeding range in eastern North America” (Howard and Davis, 2015:669). The researchers recommended more detailed studies to understand what causes the fall decline. That recommendation was echoed in a paper by Inamine et al. (2016) that also could find no evidence that lower abundance of milkweed resulted in monarch decline. The authors hypothesized that such factors as low nectar abundance and habitat fragmentation could be affecting survival during fall migration.
Pleasants et al. (2016) critiqued the conclusion of no evidence of a decline drawn by Howard and Davis (2015), and Pleasants et al. have been rebutted in turn by Dyer and Forister (2016). Without detailed data, it is difficult to exclude the possibility that declines in the overwintering populations were caused by extreme weather events or parasites and pathogens.
Resolving this debate will require modeling and direct experimental assessment of the extent to which milkweed abundance affects monarch population size. A long-term study providing a complete life-cycle analysis of the monarch butterfly is called for.
The National Research Council reports, Genetically Modified Pest-Protected Plants: Science and Regulation (NRC, 2000) and Environmental Effects of Transgenic Plants (NRC, 2002) and Marvier et al. (2007) called for spatially explicit national-scale databases on GE crop plants, associated farming practices, and environmental data so that many of the questions surrounding sustainability and genetic-engineering technology could be answered. At the time of its review in 2015, the committee found such databases to be inadequate. That limits the ability to assess effects on abundance of monarchs and many other species.
Dispersal of Genes from Genetically Engineered Crops to Wild Species
Gene flow is the change in gene frequency in a population due to the introduction of a gene or genes through gametes, individuals, or groups of individuals from other populations (Slatkin, 1987). Seed, pollen, and spread by vegetative growth are considered in evaluating gene flow from GE crops to populations of wild relatives. The magnitude of gene flow via pollen depends on many factors, including pollination biology, inheritance of the trait, size of pollen source and sink, and pollen viability over time and distance. Gene flow in the field between compatible plants can occur when they are close enough for pollen to reach a receptive stigma, the plants have synchronous flowering, and there are no reproductive barriers.
Gene flow from GE crops via pollen to other sexually compatible species has long fueled the debate over the introduction of GE crops (for example, Snow and Palma, 1997). Many early concerns were based on the assumption that gene flow would increase the weediness of related species (Wolfenbarger and Phifer, 2000). However, GE crops approved as of 2015, especially in North America, have few sexually compatible weed species or naturalized plant species with which they could hybridize, so the focus has changed to emphasis on gene flow from GE to non-GE crops. The introduction of a GE crop with more sexually compatible wild species could have outcomes different from those observed so far. Release of a GE crop in the crop’s center of origin also has raised concerns about the preservation of genetic resources (Kinchy, 2012). If gene flow from a GE crop to a GE crop relative resulted in expression of a Bt toxin and that species was now protected from herbivore pests, it could outcompete other closely related species and decrease biodiversity. There is no evidence of such an occurrence.
Movement of a herbicide-resistant transgene to a related species has not been reported to increase competitiveness or weediness in the absence of
the herbicide. However, the selection pressure from the use of a herbicide will allow populations to expand as susceptible plants are removed. Populations of HR alfalfa, canola, and creeping bentgrass (Agrostis stolonifera) produce feral populations that survive outside cultivation, increase with selection pressure from herbicides, and continue to be a transgene pollen source (Knispel et al., 2008; Zapiola et al., 2008; Schafer et al., 2011; Bagavathiannan et al., 2012; Greene et al., 2015).
GE glyphosate-resistant feral alfalfa was found in seed production areas of California, Idaho, and Washington in 2011 and 2012. Twenty-seven percent of 404 sites where feral alfalfa plants were collected had GE plants (Greene et al., 2015). The authors did not determine if the feral populations were from seed or pollen dispersal. Although there are no wild or native species in the United States with which alfalfa can hybridize, feral populations will increase if glyphosate is the only herbicide used on roadsides and noncrop areas for vegetation management.
There are many reports of GE canola establishing outside cultivation (Pessel et al., 2001; Aono et al., 2006; Knispel and McLachlan, 2010; Schafer et al., 2011). Canola will cross with multiple related species (Warwick et al., 2003). Warwick et al. (2008) identified hybrids between GE herbicide-resistant Brassica napus (canola) and a weedy population of B. rapa. Multiple generations of crosses were identified in the population, which indicated that the transgene had persisted and was being transmitted over generations. Only one advanced backcross hybrid was found in the population, which showed that transgene introgression is rare in this system. The hybrids were reported to have reduced fitness, including reduced pollen viability, but the transgene persisted over a 6-year period. During that period, herbicide selection pressure did not occur. The results of this study indicate that transgenes may persist but competitiveness would not increase unless the herbicide is applied. Warwick et al. (2008:1393) concluded that “at present, there are no compelling data to suggest that the presence of an HR transgene in a wild or weedy relative is inherently risky.”
When the committee wrote its report, populations of glyphosate-resistant creeping bentgrass were still present in Oregon 13 years after seed-production fields were removed, despite yearly removal of GE plants (Mallory-Smith, personal observation). Populations of the glyphosate-resistant creeping bentgrass were identified in 2010 in Malheur County, Oregon, where no permit was issued for its production (Mallory-Smith, personal observation). Glyphosate-resistant bentgrass was selected because of the use of glyphosate for weed management on irrigation canals. It had spread over hundreds of kilometers of canals by the time it was identified and mitigation efforts were initiated. Creeping bentgrass hybridizes with wild and naturalized compatible relatives. Hybrids between the GE creeping bentgrass and wild and naturalized compatible species were identified
outside cultivation (Reichman et al., 2006; Zapiola and Mallory-Smith, 2012). Hybridization, further introgression, and selection pressure from glyphosate use on roadsides and waterways make it likely that the trait will remain in the environment.
There have been no field reports of increased competiveness from gene flow from Bt crops to related wild species. In one research study, transfer of the Bt trait from GE sunflowers to wild sunflowers reduced insect feeding injury on the wild sunflowers and increased their fecundity (Snow et al., 2003). In another research study, a Bt transgene was transferred from Brassica napus to wild B. juncea, and the progeny were backcrossed to produce a second generation of backcross offspring (Liu et al., 2015). In research plots, the Bt plants produced more biomass in pure stands with or without insect pressure than did the susceptible plants. In mixed stands, however, the susceptible plants produced more seeds when insects were not present than when insects were present. As the proportion of Bt plants increased with insect feeding pressure, biomass and seed production increased, indicating that the presence of the Bt plants may have provided a level of protection for the susceptible plants. In both cases, it is possible that gene flow would provide an advantage to wild populations over time. However, it should be noted that these are research studies with plants that have not been in commercial use.
FINDING: Although gene flow has occurred, no examples have demonstrated an adverse environmental effect of gene flow from a GE crop to a wild, related plant species.
Herbicide-Resistant Crops, Reduced Tillage, and Ecosystem Processes
No-till and reduced-till agricultural practices are known for decreasing wind and water erosion of soil (Montgomery, 2007). There are also claims that no-till and reduced-till agriculture often leads to enhanced soil carbon sequestration and reduces greenhouse-gas emissions (Barfoot and Brookes, 2014). However, many reports that claimed increases in soil carbon suffered methodological flaws: they failed to account for increases in soil bulk density and the lack of soil mixing under no-till (Ellert and Bettany, 1995; Wendt and Hauser, 2013). Other authors concluded that no-till has only a weak effect, if any, on greenhouse-gas emissions even when no-till is combined with mulch retention (Baker et al., 2007; Giller et al., 2009; Powlson et al., 2014). From an environmental perspective, the decrease in soil erosion alone is important.
The adoption of no-till and reduced-till methods began in the 1980s, and the rate of adoption increased because of a combination of factors: the advent of inexpensive and effective herbicides, development of new
machines to facilitate direct planting, and, in the United States, a new soil-conservation policy under the Food Security Act of 1985. Those factors favored the use of conservation tillage, in which soil cover of at least 30 percent is maintained as crop residues or other mulch to reduce erosion. Thus, the greatest expansion of no-till and conservation tillage and the concomitant reductions in soil erosion actually predate the release of the first HR varieties of maize and soybean in 1996 (NRC, 2010a).
The National Research Council report on the impacts of GE crops in the United States (NRC, 2010a) reviewed several studies that indicate that farmers who adopted HR crops were more likely to practice conservation tillage and vice versa. During the period 1997–2002, there was an increase in HR crops and conservation tillage (including no-till), but the direction of causation was not clear (Fernandez-Cornejo et al., 2012). In 1997, some 60 percent of the land planted with HR soybean was under no-till or conservation tillage compared with 40 percent of the land planted with non-GE soybean (Fernandez-Cornejo and McBride, 2002).
Adoption of HR varieties may have resulted in farmer decisions to use conservation tillage, or farmers who were using conservation tillage may have adopted HR crops more readily. The work of Mensah (2007) established a “two-way causal relationship”: both causal relationships were occurring at the same time. Fernandez-Cornejo et al. (2012) used state-level data from primary soybean-producing states to explore the causal relationships further and changes in herbicide use. Unlike previous researchers, they found that “HR soybean adoption has a positive and highly significant (P < 0.0001) impact on the adoption of conservation tillage” in the United States (Fernandez-Cornejo et al., 2012:236–237). They quantified it as an elasticity and found that a 1-percent increase in area of HR soybean resulted in a 0.21-percent increase in conservation tillage. A meta-analysis by Carpenter (2011) stated that from 1996 to 2008, adoption of conservation tillage increased from 51 to 63 percent of planted soybean hectares. Fernandez-Cornejo et al. (2014) also concluded that adopters of HR crops in the United States practice conservation tillage and no-till more than growers of non-GE varieties. That is especially evident with the adoption of HR soybean, although the conclusion also holds for cotton and maize. Those conclusions are based on aggregate trends and do not allow one to determine that the introduction of GE herbicide resistance is causing the adoption of no-till or that the increase in no-till is accompanied by adoption of GE herbicide resistance.
Globally, the effects of HR crop adoption on conservation tillage are less clear because the research has been sparse. The introduction of glyphosate-resistant soybean is cited as a contributing factor in the rapid increase of no-till in Argentina, where adoption of no-till increased from about one-third of soybean area in 1996 to over 80 percent in 2008 (Trigo
et al., 2009). Other factors also contributed to the expansion of no-till in Argentina, such as favorable macroeconomic policies, continued promotion efforts, and reduction in herbicide cost. Substantial growth in no-till production also occurred in Canada; from 1996 to 2005, the no-till canola area increased from 0.8 million hectares to 2.6 million hectares, about half the total canola area (Qaim and Traxler, 2005).
FINDING: Both GE crops and the percentage of cropping area farmed with no-till and reduced-till practices have increased over the last two decades. However, cause and effect are difficult to determine.
CONCLUSIONS
There have been strong claims made about the purported benefits and adverse effects of GE crops. The committee found little evidence to connect GE crops and their associated technologies with adverse agronomic or environmental problems. For example, the use of Bt crops or HR crops did not result in substantially reduced on-farm biodiversity, and sometimes their use resulted in increased biodiversity. In terms of benefits, the evidence was mixed. Bt crops have increased yields when insect-pest pressure was high, but there was little evidence that the introduction of GE crops were resulting in a more rapid yearly increases in on-farm crop yields in the United States than had been seen prior to the use of GE crops. Use of Bt crops is clearly associated with a decrease in the number of insecticide applications, but with HR crops the evidence is equivocal. Importantly, most studies only report the number of kilograms of pesticide used, but this metric does not necessarily predict environmental or health effects.
The quantitative contribution of GE crop traits themselves to yield in experimental plots was sometimes difficult to determine because the GE and non-GE varieties could differ in other yield-associated traits. In surveys on yield and insecticide and herbicide use in farmer fields, the different adoption rates of GE crops by farmers who had different land quality and financial resources confounded some results. There is a need for improved survey and experimental approaches that disentangle the effects of the GE trait itself from other factors that affect yield.
The evolution of resistance to Bt toxin in insect pests was found to be associated with the use of varieties without a high dose of Bt toxin or the absence of refuges. Evolved herbicide resistance in weeds was associated with the overuse of a single herbicide. If GE crops are to be used sustainably, regulations and incentives must be provided to farmers so that more integrated and sustainable pest management approaches become economically feasible.
Overall, the committee found no evidence of cause-and-effect relationships between GE crops and environmental problems However, the
complex nature of assessing long-term environmental changes often made it difficult to reach definitive conclusions. That is illustrated by the case of the decline in monarch butterfly populations. Detailed studies of monarch dynamics carried out as of 2015 did not demonstrate an adverse effect related to the increased glyphosate use, but there was still no consensus among researchers that the effects of glyphosate on milkweed has not caused decreased monarch populations.
The committee offers a number of recommendations regarding where investment of public resources in conducting careful experiments and analyses might enable society to make more rigorous assessments of the potential benefits and problems associated with GE crops that would be seen as more legitimate by concerned members of the public than experiments funded by the developers of the technology.
REFERENCES
Abedullah, S. Kouser, and M. Qaim. 2015. Bt cotton, pesticide use and environmental efficiency in Pakistan. Journal of Agricultural Economics 66:66–86.
Adamczyk, J.J., and D. Hubbard. 2006. Changes in populations of Heliothis virescens (F.) (Lepidoptera: Noctuidae) and Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in the Mississippi Delta from 1986 to 2005 as indicated by adult male pheromone traps. Journal of Cotton Science 10:155–160.
Afidchao, M.M., C.J.M. Musters, A. Wossink, O.F. Balderama, and G.R. de Snoo. 2014. Analysing the farm level economic impact of GM corn in the Philippines. NJAS–Wageningen Journal of Life Sciences 70–71:113–121.
Aguilar, J., G.G. Gramig, J.R. Hendrickson, D.W. Archer, F. Forcella, and M.A. Liebig. 2015. Crop species diversity changes in the United States: 1978–2012. PLoS ONE 10:e0136580.
Allen, K.C., and H.N. Pitre. 2006. Influence of transgenic corn expressing insecticidal proteins of Bacillus thuringiensis Berliner on natural populations of corn earworm (Lepidoptera: Noctuidae) and southwestern corn borer (Lepidoptera: Crambidae). Journal of Entomological Science 41:221–231.
Andow, D.A. 2010. Bt Brinjal: The Scope and Adequacy of the GEAC Environmental Risk Assessment. Available at http://www.researchgate.net/publication/228549051_Bt_Brinjal_The_scope_and_adequacy_of_the_GEAC_environmental_risk_assessment. Accessed October 23, 2015.
Andow, D.A., S.G. Pueppke, A.W. Schaafsma, A.J. Gassmann, T.W. Sappington, L.J. Meinke, P.D. Mitchell, T.M. Hurley, R.L. Hellmich, and R.P. Porter. 2016. Early detection and mitigation of resistance to Bt maize by western corn rootworm (Coleoptera: Chrysomelidae). Journal of Economic Entomology 109:1–12.
Aono, M., S. Wakiyama, M. Nagatsu, N. Nakajima, M. Tamaoki, A. Kubo, and H. Saji. 2006. Detection of feral transgenic oilseed rape with multiple-herbicide resistance in Japan. Environmental Biosafety Research 5:77–87.
Areal, F.J., L. Riesgo, and E. Rodríguez-Cerezo. 2013. Economic and agronomic impact of commercialized GM crops: A meta-analysis. Journal of Agricultural Science 151:7–33.
Armstrong, J.J.Q., and C.L. Sprague. 2010. Weed management in wide- and narrow-row glyphosate-resistant sugarbeet. Weed Technology 24:523–528.
Badgett, G., and A.K. Davis. 2015. Population trends of monarchs at a northern monitoring site: Analyses of 19 years of fall migration counts at Peninsula Point, MI. Annals of the Entomological Society of America 108:700–706.
Badran, A.H., V.M. Guzov, Q. Huai, M.M. Kemp, P. Vishwanath, W. Kain, A.M. Nance, A. Evdokimov, F. Moshiri, K.H. Turner, P. Wang, T. Malvar, and D.R. Liu. 2016. Continuous evolution of Bacillus thuringiensis toxins overcomes insect resistance. Nature 533:58–63.
Bagavathiannan, M.V., G.S. Begg, R.H. Gulden, and R.C. Van Acker. 2012. Modelling of the dynamics of feral alfalfa populations and its management implications. PLoS ONE 7:e39440.
Bagla, P. 2010. Hardy cotton-munching pests are latest blow to GM crops. Science 327:1439.
Baker, J.M., T.E. Ochsner, R.T. Venterea, and T.J. Griffis. 2007. Tillage and soil carbon sequestration—What do we really know? Agriculture, Ecosystems & Environment 118:1–5.
Barfoot, P., and G. Brookes. 2014. Key global environmental impacts of genetically modified (GM) crop use 1996-2012. GM Crops and Food: Biotechnology in Agricultural and the Food Chain 5:149–160.
Bärwald Bohm, G.M., C.V. Rombaldi, M.I. Genovese, D. Castilhos, B.J. Rodrigues Alves, and M.Gouvêa Rumjanek. 2014. Glyphosate effects on yield, nitrogen fixation, and seed quality in glyphosate-resistant soybean. Crop Science 54:1737–1743.
Bauer, P.J., D.D. McAlister, III, and J.R. Frederick. 2006. A comparison of Bollgard/glyphosate tolerant cotton cultivars to their conventional parents for open end yarn processing performance. Journal of Cotton Science 10:168–174.
Baute, T.S., M.K. Sears, and A.W. Schaafsma. 2002. Use of transgenic Bacillus thuringiensis Berliner corn hybrids to determine the direct economic impact of the European corn borer (Lepidoptera: Crambidae) on field corn in eastern Canada. Journal of Economic Entomology 95:57–64.
Beckie, H.J., K.N. Harker, A. Legere, M.J. Morrison, G. Seguin-Swartz, and K.C. Falk. 2011. GM canola: The Canadian experience. Farm Policy Journal 8:43-49.
Beltramin da Fonseca, P.R., M.G. Fernandes, W. Justiniano, L.H. Cavada, and J.A. Neta da Silva. 2013. Leaf chlorophyll content and agronomic performance of Bt and non-Bt soybean. Journal of Agricultural Science 5:117–125.
Benbrook, C.M. 2012. Impacts of genetically engineered crops on pesticide use in the U.S.—the first sixteen years. Environmental Sciences Europe 24:24.
Bernardi, O., R.J. Sorgatto, A.D. Barbosa, F.A. Domingues, P.M. Dourado, R.A. Carvalho, S. Martinelli, G.P. Head, and C. Omoto. 2014. Low susceptibility of Spodoptera cosmioides, Spodoptera eridania and Spodoptera frugiperda (Lepidoptera: Noctuidae) to genetically-modified soybean expressing Cry1Ac protein. Crop Protection 58:33–40.
Binimelis, R., W. Pengue, and I. Monterroso. 2009. “Transgenic treadmill”: Responses to the emergence and spread of glyphosate-resistant johnsongrass in Argentina. Geoforum 40:623–633.
Bohnenblust, E.W., J.A. Breining, J.A. Shaffer, S.J. Fleischer, G.W. Roth, and J.F. Tooker. 2014. Current European corn borer, Ostrinia nubilalis, injury levels in the northeastern United States and the value of Bt field corn. Pest Management Science 70:1711–1719.
Bowen, K.L., K.L. Flanders, A.K. Hagan, and B. Ortiz. 2014. Insect damage, aflatoxin content, and yield of Bt corn in Alabama. Journal of Economic Entomology 107:1818–1827.
Brévault, T., B.E. Tabashnik, and Y. Carrière. 2015. A seed mixture increases dominance of resistance to Bt cotton in Helicoverpa zea. Scientific Reports 5:9807.
Brower, L.P., O.R. Taylor, E.H. Williams, D.A. Slayback, R.R. Zubieta, and M.I. Ramírez. 2012. Decline of monarch butterflies overwintering in Mexico: Is the migratory phenomenon at risk? Insect Conservation and Diversity 5:95–100.
Buntin, G.D., R.D. Lee, D.L. Wilson, and R.M. McPherson. 2001. Evaluation of YieldGard transgenic resistance for control of fall armyworm and corn earworm (Lepidoptera: Noctuidae) on corn. Florida Entomologist 84:37–42.
Buntin, G.D., J.N. All, R.D. Lee, and D.L. Wilson. 2004. Plant-incorporated Bacillus thuringiensis resistance for control of fall armyworm and corn earworm (Lepidoptera: Noctuidae) in corn. Journal of Economic Entomology 97:1603–1611.
Carpenter, J.E. 2011. Impact of GM crops on biodiversity. GM Crops 2:7–23.
Carrière, Y., C. Ellers-Kirk, M. Sisterson, L. Antilla, M. Whitlow, T.J. Dennehy, and B.E. Tabashnik. 2003. Long-term regional suppression of pink bollworm by Bacillus thuringiensis cotton. Proceedings of the National Academy of Sciences of the United States of America 100:1519–1524.
Carrière, Y., J.A. Fabrick, and B.E. Tabashnik. 2016. Can pyramids and seed mixtures delay resistance to Bt crops? Trends in Biotechnology 34:291–302.
Catarino, R., G. Ceddia, F.J. Areal, and J. Park. 2015. The impact of secondary pests on Bacillus thuringiensis (Bt) crops. Plant Biotechnology Journal 13:601–612.
Cerdeira, A.L., and S.O. Duke. 2006. The current status and environmental impacts of glyphosate-resistant crops. Journal of Environment Quality 35:1633–1658.
Chang, J., D.E. Clay, S.A. Hansen, S.A. Clay, and T.E. Schumacher. 2014. Water stress impacts on transgenic drought-tolerant corn in the northern Great Plains. Agronomy Journal 106:125–130.
Choudhary, G., N. Ranjitkumar, M. Surapaneni, D.A. Deborah, A. Vipparla, G. Anuradha, E.A. Siddiq, and L.R. Vemireddy. 2013. Molecular genetic diversity of major Indian rice cultivars over decadal periods. PLoS ONE 8:e66197.
Choudhary, B., K.M. Nasiruddin, and K. Gaur. 2014. The Status of Commercialized Bt Brinjal in Bangladesh. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
Clayton, G.W., K.N. Harker, J.T. O’Donovan, R.E. Blackshaw, L.M. Dosdall, F.C. Stevenson, and T. Ferguson. 2004. Fall and spring seeding date effects on herbicide-tolerant canola (Brassica napus L.) cultivars. Canadian Journal of Plant Science 84:419–430.
Cotter, J. 2014. GE Crops—Necessary? Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, September 16, Washington, DC.
Crawley, M.J., R.S. Hails, M. Rees, D. Kohn, and J. Buxton. 1993. Ecology of transgenic oilseed rape in natural habitats. Letters to Nature 363:620–623.
Crewe, T.L., and J.D. McCracken. 2015. Long-term trends in the number of monarch butterflies (Lepidoptera: Nymphalidae) counted on fall migration at Long Point, Ontario, Canada (1995–2014). Annals of the Entomological Society of America 108:707–717.
Crost, B., and B. Shankar. 2008. Bt-cotton and production risk: Panel data estimates. International Journal of Biotechnology 10:123–131.
CSPI (Center for Science in the Public Interest). 2009. Complacency on the Farm. Washington, DC: CSPI.
Culpepper, A.S. 2006. Glyphosate-induced weed shifts. Weed Technology 20:277–281.
Culpepper, A.S., T.L. Grey, W.K. Vencill, J.M. Kichler, T.M. Webster, S.M. Brown, A.C. York, J.W. Davis, and W.W. Hanna. 2006. Glyphosate-resistant palmer amaranth (Amaranthus palmeri) confirmed in Georgia. Weed Science 54:620–626.
Davis, A.S., J.D. Hill, C.A. Chase, A.M. Johanns, and M. Liebman. 2012. Increasing cropping system diversity balances productivity, profitability and environmental health. PLoS ONE 7:e47149.
De Vries, B.D., and W.R. Fehr. 2011. Impact of the MON89788 event for glyphosate tolerance on agronomic and seed traits of soybean. Crop Science 51:1023–1027.
Dever, J. 2015. Conventional Breeding at Public Institutions. Webinar presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, January 27.
Dillehay, B.L., G.W. Roth, D.D. Calvin, R.J. Karatochvil, G.A. Kuldau, and J.A. Hyde. 2004. Performance of Bt corn hybrids, their near isolines, and leading corn hybrids in Pennsylvania and Maryland. Agronomy Journal 96:818–824.
Dorhout, D.L., and M.E. Rice. 2010. Intraguild competition and enhanced survival of western bean cutworm (Lepidoptera: Noctuidae) on transgenic Cry1Ab (MON810) Bacillus thuringiensis corn. Journal of Economic Entomology 103:54–62.
Douglas, M., and J.F. Tooker. 2015. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environmental Sciences & Technology 49:5088−5097.
Dowd-Uribe, B., and M.A. Schnurr. 2016. Burkina Faso’s reversal on genetically modified cotton and the implications for Africa. African Affairs 115:161–172.
Duan, J.J., M. Marvier, J. Huesing, G. Dively, and Z.Y. Huang. 2008. A meta-analysis of effects of Bt crops on honey bees (Hymenoptera: Apidae). PLoS ONE 3:e1415.
Duke, S.O. 2015. Perspectives on transgenic, herbicide-resistant crops in the USA almost 20 years after introduction. Pest Management Science 71:652–657.
Duvick, D.N. 2005. Genetic progress in yield of United States maize (Zea mays L.). Maydica 50:193–202.
Dyer, L.A., and M.L. Forister. 2016. Wherefore and whither the modeler: Understanding the population dynamics of monarchs will require integrative and quantitative techniques. Annals of the Entomological Society of America sav160.
Egan, J.F., B.D. Maxwell, D.A. Mortensen, M.R. Ryan, and R.G. Smith. 2011. 2,4-Dichloro-phenoxyacetic acid (2,4-D)–resistant crops and the potential for evolution of 2,4-D–resistant weeds. Proceedings of the National Academy of Sciences of the United States of America 108:E37.
Ellert, B.H., and J.R. Bettany. 1995. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Canadian Journal of Soil Science 75:529–538.
EPA (U.S. Environmental Protection Agency). 1997. Plant pesticides resistance management; Notice of meeting. Federal Register 62:19115–19117.
EPA (U.S. Environmental Protection Agency). 1998. Memorandum: Transmittal of the Final Report of the FIFRA Scientific Advisory Panel Subpanel on Bacillus thuringiensis (Bt) Plant-Pesticides and Resistance Management, Meeting held on February 9 and 10, 1998. Available at http://archive.epa.gov/scipoly/sap/meetings/web/pdf/finalfeb.pdf. Accessed November 22, 2015.
EPA (U.S. Environmental Protection Agency). 2001. Biopesticides Registration Action Document—Bacillus thuringiensis Plant-Incorporated Protectants. Available at http://www3.epa.gov/pesticides/chem_search/reg_actions/pip/bt_brad.htm. Accessed November 22, 2015.
EPA (U.S. Environmental Protection Agency). 2002. Memorandum: Transmittal of Meeting Minutes of the FIFRA Scientific Advisory Panel Meeting Held August 27-29, 2002. Available at http://archive.epa.gov/scipoly/sap/meetings/web/pdf/august2002final.pdf. Accessed November 22, 2015.
EPA (U.S. Environmental Protection Agency). 2011. Memorandum: Transmittal of Meeting Minutes of the FIFRA Scientific Advisory Panel Meeting Held December 8-9, 2010 to Address Scientific Issues Associated with Insect Resistance Management for SmartStax™ Refuge-in-the-Bag, a Plant-Incorporated Protectant (PIP) Corn Seed Blend. Available at http://archive.epa.gov/scipoly/sap/meetings/web/pdf/120810minutes.pdf. Accessed November 22, 2015.
EPA (U.S. Environmental Protection Agency). 2014a. Final Registration of Enlist Duo™ Herbicide. Available at http://www2.epa.gov/sites/production/files/2014-10/documents/final_registration_-_enlist_duo.pdf. Accessed November 24, 2015.
EPA (U.S. Environmental Protection Agency). 2014b. SAP Minutes No. 2014-01: A Set of Scientific Issues Being Considered by the Environmental Protection Agency Regarding Scientific Uncertainties Associated with Corn Rootworm Resistance Monitoring for Bt Corn Plant Incorporated Protectants (PIPs). Available at http://www2.epa.gov/sites/production/files/2015-06/documents/120413minutes.pdf. Accessed November 22, 2015.
Evans, J.A., P.J. Tranel, A.G Hager, B. Schutte, C. Wu, L.A. Chatham, and A.S. Davis. 2016. Managing the evolution of herbicide resistance. Pest Management Science 72:74–80.
Farias, J.R., D.A. Andow, R.J. Horikoshi, R.J. Sorgatto, P. Fresia, A.C. Santos, and C. Omoto. 2014. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Protection 64:150–158.
Fausti, S.W., T.M. McDonald, J.G. Lundgren, J. Li, A.R. Keating, and M. Catangui. 2012. Insecticide use and crop selection in regions with high GM adoption rates. Renewable Agriculture and Food Systems 27:295–304.
Fernandez-Cornejo, J., and W.D. McBride. 2002. Adoption of Bioengineered Crops. Agricultural Economic Report No. 810. Washington, DC: U.S. Department of Agriculture–Economic Research Service.
Fernandez-Cornejo, J., C. Hallahan, R. Nehring, S. Wechsler, and A. Grube. 2012. Conservation tillage, herbicide use, and genetically engineered crops in the United States: The case of soybeans. AgBioForum 15:231–241.
Fernandez-Cornejo, J., S.J. Wechsler, M. Livingston, and L. Mitchell. 2014. Genetically Engineered Crops in the United States. Washington, DC: U.S. Department of Agriculture–Economic Research Service.
Ferreira, S.A., K.Y. Pitz, R. Manshardt, F. Zee, M. Fitch, and D. Gonsalves. 2002. Virus coat protein transgenic papaya provides practical control of Papaya ringspot virus in Hawaii. Plant Disease 86:101–105.
Finger, R., N. El Benni, T. Kaphengst, C. Evans, S. Herbert, B. Lehmann, S. Morse, and N. Stupak. 2011. A meta analysis on farm-level costs and benefits of GM crops. Sustainability 3:743–762.
Forster, D., C. Andres, R. Verma, C. Zundel, M.M. Messmer, and P. Mäder. 2013. Yield and economic performance of organic and conventional cotton-based farming systems—results from a field trial in India. PLoS ONE 8:e81039.
Frank, D.L., R. Kurtz, N.A. Tinsley, A.J. Gassmann, L.J. Meinke, D. Moellenbeck, M.E. Gray, L.W. Bledsoe, C.H. Krupke, R.E. Estes, and P. Weber. 2015. Effect of seed blends and soil-insecticide on western and northern corn rootworm emergence from mCry3A+ eCry3.1Ab Bt maize. Journal of Economic Entomology 108:1260–1270.
Fuchs, M. and D. Gonsalves. 2008. Safety of virus-resistant transgenic plants two decades after their introduction: Lessons from realistic field risk assessment studies. Annual Review of Phytopathology 45:173–202.
FuturaGene. 2015. FuturaGene’s eucalyptus is approved for commercial use in Brazil. Available at http://www.futuragene.com/FuturaGene-eucalyptus-approved-for-commercial-use.pdf. Accessed September 23, 2015.
Gasparri, N.I., H.R. Grau, and J. Angonese. 2013. Linkages between soybean and neotropical deforestation: Coupling and transient decoupling dynamics in a multi-decadal analysis. Global Environmental Change 23:1605–1614.
Gassmann, A.J., J.L. Petzold-Maxwell, E.H. Clifton, M.W. Dunbar, A.M. Hoffmann, D.A. Ingber, and R.S. Keweshan. 2014. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proceedings of the National Academy of Sciences of the United States of America 111:5141–5146.
Gepts, P. 2006. Plant genetic resources conservation and utilization: The accomplishments and future of a societal insurance policy. Crop Science 46:2278–2292.
Giller, K.E., E. Witter, M. Corbeels, and P. Tittonell. 2009. Conservation agriculture and smallholder farming in Africa: The heretics view. Field Crops Research 114:23–34.
Giller, K.E., J.A. Andersson, M. Corbeels, J. Kirkegaard, D. Mortensen, O. Erenstein, and B. Vanlauwe. 2015. Beyond conservation agriculture. Frontiers in Plant Science 6:870.
Goldberger, J., J. Merrill, and T. Hurley. 2005. Bt corn farmer compliance with insect resistance management requirements in Minnesota and Wisconsin. AgBioForum 8:151–160.
Gonzales, L.A., E.Q. Javier, D.A. Ramirez, F.A Cariño, and A.R. Baria. 2009. Modern Biotechnology and Agriculture: A History of the Commercialization of Biotech Maize in the Philippines. Los Baños, Philippines: STRIVE Foundation.
Goodman, M. 2014. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, September 15, Washington, DC.
Gould, F. 1995. Comparisons between resistance management strategies for insects and weeds. Weed Technology 9:830–839.
Gould, F. 1998. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annual Review of Entomology 43:701–726.
Grau, H.R., N.I. Gasparri, and T.M. Aide. 2005. Agriculture expansion and deforestation in seasonally dry forests of north-west Argentina. Environmental Conservation 32:140–148.
Greene, S.L., S.R. Kesoju, R.C. Martin, and M. Kramer. 2015. Occurrence of transgenic feral alfalfa (Medicago sativa subsp. sativa L) in alfalfa seed production areas in the United States. PLoS ONE 10:e0143296.
Gulden, R.H., P.H. Sikkema, A.S. Hamill, F. Tardif, and C.J. Swanton. 2010. Glyphosate-resistant cropping systems in Ontario: Multivariate and nominal trait-based weed community structure. Weed Science 58:278–288.
Gurian-Sherman, D. 2009. Failure to Yield: Evaluating the Performance of Genetically Engineered Crops. Cambridge, MA: UCS Publications.
Gurian-Sherman, D. 2014. Remarks to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, September 16, Washington, DC.
Guza, C.J., C.V. Ransom, and C. Mallory-Smith. 2002. Weed control in glyphosate-resistant sugarbeet (Beta vulgaris L.). Journal of Sugar Beet Research 39:109–123.
Haegele, J.W., and F.E. Below. 2013. Transgenic corn rootworm protection increases grain yield and nitrogen use of maize. Crop Science 53:585–594.
Hajjar, R., D.I. Jarvis, and B. Gemmill-Herren. 2008. The utility of crop genetic diversity in maintaining ecosystem services. Agriculture, Ecosystems & Environment 123:261–270.
Hannula, S.E., W. de Boer, and J.A. van Veen. 2014. Do genetic modificiations in crops affect soil fungi? A review. Biology and Fertility of Soils 50:433–446.
Harker, K.N., R.E. Blackshaw, K.J. Kirkland, D.A. Derksen, and D. Wall. 2000. Herbicide-tolerant canola: Weed control and yield comparisons in western Canada. Canadian Journal of Plant Science 80:647–654.
Hartzler, R.G. 2010. Reduction in common milkweed (Asclepias syriaca) occurrence in Iowa cropland from 1999 to 2009. Crop Protection 29:1542–1544.
HASS (Hawaii Agricultural Statistic Service). 1993. Statistics of Hawaiian Agriculture 1992. Honolulu: Hawaii Department of Agriculture.
HASS (Hawaii Agricultural Statistic Service). 2000. Statistics of Hawaiian Agriculture 1998. Honolulu: Hawaii Department of Agriculture.
Heap, I. 2016. Weeds resistant to EPSP synthase inhibitors (G/9). The international survey of herbicide resistant weeds. Available at http://weedscience.org/summary/moa.aspx?MOAID=12. Accessed March 23, 2016.
Heard, M.S., C. Hawes, G.T. Champion, S.J. Clark, L.G. Firbank, A.J. Haughton, A.M. Parish, J.N. Perry, P. Rothery, R.J. Scott, M.P. Skellern, G.R. Squire, and M.O. Hill. 2003. Weeds in fields with contrasting conventional and genetically modified herbicide-tolerant crops: I. Effects on abundance and diversity. Philosophical Transactions of the Royal Society B 358:1819–1832.
Heinemann, J.A., M. Massaro, D.S. Coray, S.Z. Agapito-Tenfen, and J.D. Wen. 2014. Sustainability and innovation in staple crop production in the US Midwest. International Journal of Agricultural Sustainability 12:71–88.
Hellmich, R.L., B.D. Siegfried, M.K. Sears, D.E. Stanley-Horn, M.J. Daniels, H.R. Mattila, T. Spencer, K.G. Bidne, and L.C. Lewis. 2001. Monarch larvae sensitivity to Bacillus thuringiensis-purified proteins and pollen. Proceedings of the National Academy of Sciences of the United States of America 98:11925–11930.
Héma, O., H.N. Somé, O. Traoré, J. Greenplate, and M. Abdennadher. 2009. Efficacy of transgenic cotton plant containing Cry1Ac and Cry2Ab genes of Bacillus thuringiensis against Helicoverpa armigera and Syllepte derogata in cotton cultivation in Burkina Faso. Crop Protection 28:205–214.
Hill, J. 2015. Pest Management in Corn and Vegetable Production. Presentation at National Academies Workshop on Comparing the Environmental Effects of Pest Management Practices Across Cropping Systems, March 4, Washington, DC.
Hjältén, J., E.P. Axelsson, T.G. Whitman, C.J. LeRoy, R. Julkunen-Tiitto, A. Wennström, and G. Pilate. 2012. Increased resistance of Bt aspens to Phratora vitellinae (Coleoptera) leads to increased plant growth under experimental conditions. PLoS ONE 7:e30640.
Howard, E., and A.K. Davis. 2015. Investigating long-term changes in the spring migration of monarch butterflies (Lepidoptera: Nymphalidae) using 18 years of data from Journey North, a citizen science program. Annals of the Entomological Society of America 108:664–669.
Hu, J.J., Y.C. Tian, Y.F. Han, L. Li, and B.E. Zhang. 2001. Field evaluation of insect-resistant transgenic Populus nigra trees. Euphytica 121:123–127.
Huang, F., D.A. Andow, and L.L. Buschman. 2011. Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomologia Experimentalis et Applicata 140:1–16.
Hungria, M., I. Carvalho Mendes, A. Shigueyoshi Nakatani, F. Bueno dos Reis-Junior, J.Z. Morais, M.C. Neves de Oliveria, M. Ferreira Fernandes. 2014. Effects of the glyphosate-resistance gene and herbicides on soybean: Field trials monitoring biological nitrogen fixation and yield. Field Crops Research 158:43–54.
Hungria, M., A. Shigueyoshi Nakatani, R.A. Souza, F.B. Sei, L.M. de Oliveira Chueire, and C. Arrabal Arias. 2015. Impact of the ahas transgene for herbicides resistance on biological nitrogen fixation and yield of soybean. Transgenic Research 24:155–165.
Hutchison, W.D., E.C. Burkness, P.D. Mitchell, R.D. Moon, T.W. Leslie, S.J. Fleischer, M. Abrahamson, K.L. Hamilton, K.L. Steffey, M.E. Gray, R.L. Hellmich, L.V. Kaster, T.E. Hunt, R.J. Wright, K. Pecinovsky, T.L. Rabaey, B.R. Flood, and E.S. Raun. 2010. Area-wide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 330:222–225.
Inamine, H., S.P. Ellner, J.P. Springer, and A.A. Agrawal. 2016. Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos 125:1081–1091.
Inman, M.D., D.L. Jordan, A.C. York, K.M. Jennings, D.W. Monks, W.J. Everman, S.L. Bollman, J.T. Fowler, R.M. Cole, and J.K. Soteres. 2016. Long-term management of Palmer amaranth (Amaranthus palmeri) in dicamba-tolerant cotton. Weed Science 6:161–169.
James, C. 2006. Global Status of Commercialized Biotech/GM Crops: 2006. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
James, C. 2008. Global Status of Commercialized Biotech/GM Crops: 2008. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
James, C. 2010. Global Status of Commercialized Biotech/GM Crops: 2010. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
James, C. 2012. Global Status of Commercialized Biotech/GM Crops: 2012. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
James, C. 2015. Global Status of Commercialized Biotech/GM Crops: 2015. Ithaca, NY: International Service for the Acquisition of Agri-biotech Applications.
Johnson, R.M. 2015. Honey bee toxicology. Annual Review of Entomology 60:415–434.
Johnson, W.G., V.M. Davis, G.R. Kruger, and S.C. Weller. 2009. Influence of glyphosate-resistant cropping systems on weed species shifts and glyphosate-resistant weed populations. European Journal of Agronomy 31:162–172.
Kaimowitz, D., and J. Smith. 2001. Soybean technology and the loss of natural vegetation in Brazil and Bolivia. Pp. 195–211 in Agricultural Technologies and Tropical Deforestation, A. Angelsen and D. Kaimowitz, eds. Oxon, UK: CABI Publishing.
Kasabe, N. February 19, 2016. Efforts on to Protect Cotton Crop from Pink Bollworm During Coming Season. Online. The Financial Express. Available at http://www.financialexpress.com/article/markets/commodities/efforts-on-to-protect-cotton-crop-from-pink-bollwormduring-coming-season/213353/. Accessed April 5, 2016.
Kathage, J., and M. Qaim. 2012. Economic impacts and impact dynamics of Bt (Bacillus thuringiensis) cotton in India. Proceedings of the National Academy of Sciences of the United States of America 109:11652–11656.
Kemp, N.J., E.C. Taylor, and K.A. Renner. 2009. Weed management in glyphosate- and glufosinate-resistant sugar beet. Weed Technology 23:416–424.
Kerns, D., G. Lorenz, J. Gore, D. Cook, A. Catchot, G. Studebaker, S. Stewart, S. Brown, N. Seiter, and R. Viator. 2015. Effectiveness of Bt cotton towards bollworms and benefit of supplemental oversprays. Pp. 819–829 in Proceedings of the 2015 Beltwide Cotton Conferences, January 5–7, San Antonio, TX.
Kinchy, A. 2012. Seeds, Science, and Struggle: The Global Politics of Transgenic Crops. Cambridge: The MIT Press.
Kirkegaard J.A., M.K. Conyers, J.R. Hunt, C.A. Kirkby, M. Watt, and G.J. Rebetzke. 2014. Sense and nonsense in conservation agriculture: Principles, pragmatism and productivity in Australian mixed farming systems. Agriculture, Ecosystems & Environment 187:133–145.
Klocko, A.L., R. Meilan, R.R. James, V. Viswanath, C. Ma, P. Payne, L. Miller, J.S. Skinner, B. Oppert, G.A. Cardineau, and S.H. Strauss. 2014. Bt-Cry3Aa transgene expression reduces insect damage and improves growth in field-grown hybrid poplar. Canadian Journal of Forest Research 44:28–35.
Klümper, W. and M. Qaim. 2014. A meta-analysis of the impacts of genetically modified crops. PLoS ONE 9:e111629.
Knispel, A.L., and S.M. McLachlan. 2010. Landscape-scale distribution and persistence of genetically modified oilseed rape (Brassica napus) in Manitoba, Canada. Environmental Science and Pollution Research 17:13–25.
Knispel, A.L., S.M. McLachlan, R.C. Van Acker, and L.F. Friesen. 2008. Gene flow and multiple herbicide resistance in escaped canola populations. Weed Science 56:72–80.
Kniss, A.R. 2010. Comparison of conventional and glyphosate-resistant sugarbeet the year of commercial introduction in Wyoming. Journal of Sugar Beet Research 47:127–134.
Kniss A.R., and C.W. Coburn. 2015. Quantitative evaluation of the Environmental Impact Quotient (EIQ) for comparing herbicides. PLoS ONE 10:e0131200.
Kniss, A.R., R.G. Wilson, A.R. Martin, P.A. Burgener, and D.M. Feuz. 2004. Economic evaluation of glyphosate-resistant and conventional sugar beet. Weed Technology 18:388–396.
Kouser, S., and M. Qaim. 2011. Impact of Bt cotton on pesticide poisoning in smallholder agriculture: A panel data analysis. Ecological Economics 70:2105–2113.
Kovach, J., C. Petzoldt, J. Degni, and J. Tette. 1992. A method to measure the environmental impacts of pesticides. New York Food and Life Sciences Bulletin 139:1–8.
Kranthi, K.R. 2015. Pink Bollworm Strikes Bt-Cotton. Cotton Statistics & News. Mumbai: Cotton Association of India.
Krishna, V.V., and M. Qaim. 2008. Potential impacts of Bt eggplant on economic surplus and farmers’ health in India. Agricultural Economics 38:167–180.
Krishna, V.V., M. Qaim, and D. Zilberman. 2016. Transgenic crops, production risk, and agrobiodiversity. European Review of Agricultural Economics 43:137–164.
Kruger, M., J.B.J. Van Rensburg, and J. Van den Berg. 2011. Resistance to Bt maize in Busseola fusca (Lepidoptera: Noctuidae) from Vaalharts, South Africa. Environmental Entomology 40:477–483.
Kruger, M., J.B.J. Van Rensburg, and J. Van den Berg. 2012. Transgenic Bt maize: Farmers’ perceptions, refuge compliance and reports of stem borer resistance in South Africa. Journal of Applied Entomology 136:38–50.
Kumar, S., P.A.L. Prasanna, and S. Wankhade. 2010. Economic Benefits of Bt Brinjal—An Ex-ante Assessment. New Delhi: National Centre for Agricultural Economics and Policy Research.
Lapola, D.M., R. Schaldach, J. Alcamo, A. Bondeau, J. Koch, C. Koelking, and J.A. Priess. 2010. Indirect land-use changes can overcome carbon savings from biofuels in Brazil. Proceedings of the National Academy of Sciences of the United States of America 107:3388–3393.
Lark, T.J., J.M. Salmon, and H.K. Gibbs. 2015. Cropland expansion outpaces agricultural and biofuel policies in the United States. Environmental Research Letters 10:044003.
Leibman, M., J.J. Shryock, M.J. Clements, M.A. Hall, P.J. Loida, A.L. McClerren, Z.P. McKiness, J.R. Phillips, E.A. Rice, and S.B. Stark. 2014. Comparative analysis of maize (Zea mays) crop performance: Natural variation, incremental improvements and economic impacts. Plant Biotechnology Journal 12:941–950.
Liesner, L. 2015. 2014 Arizona pink bollworm eradication program update. Pp. 848–851 in Proceedings of the 2015 Beltwide Cotton Conferences, January 5–7, San Antonio, TX.
Liu, Y-B., H. Darmency, C.N. Stewart, Jr., W. Wei, Z-X. Tang, and K-P. Ma. 2015. The effect of Bt-transgene introgression on plant growth and reproduction in wild Brassica juncea. Transgenic Research 24:537–547.
Livingston, M., J. Fernandez-Cornejo, J. Unger, C. Osteen, D. Schimmelpfennig, T. Park, and D. Lambert. 2015. The Economics of Glyphosate Resistance Management in Corn and Soybean Production. Washington, DC: U.S. Department of Agriculture–Economic Research Service.
Lobell, D.B., K.G. Cassman, and C.B. Field. 2009. Crop yield gains: Their importance, magnitudes and causes. Annual Review of Environment and Resources 34:179–204.
Losey, J.E., L.S. Raynor, and M.E. Carter. 1999. Transgenic pollen harms Monarch larvae. Nature 399:214.
Lu, Y., K. Wu, Y. Jiang, B. Xia, P. Li, H. Feng, K.A.G. Wyckhuys, and Y. Guo. 2010. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328:1151–1154.
Lu, Y., K. Wu, Y. Jiang, Y. Guo, and N. Desneux. 2012. Wide-spread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487:362–365.
Lu, Z.B., J.C. Tian, N.S. Han, C. Hu, Y.F. Peng, D. Stanley, and G.Y. Ye. 2014. No direct effects of two transgenic Bt rice lines, T1C-19 and T2A-1, on the arthropod communities. Environmental Entomology 43:1453–1463.
Lundgren, J. 2015. Risks of GM Crops and Sustainable Pest Management Alternatives. Presentation at National Academies Workshop on Comparing the Environmental Effects of Pest Management Practices Across Cropping Systems, March 4, Washington, DC.
Lundgren, J.G., A.J. Gassmann, J. Bernal, J.J. Duan, and J. Ruberson. 2009. Ecological compatibility of GM crops and biological control. Crop Protection 28:1017–1030.
Luttrell, R.G., and R.E. Jackson. 2012 Helicoverpa zea and Bt cotton in the United States. GM Crops and Food: Biotechnology in Agriculture and the Food Chain 3:213–227.
Mallet, J., and P. Porter. 1992. Preventing insect adaptation to insect-resistant crops: Are seed mixtures or refugia the best strategy? Proceedings of the Royal Society London B: Biological Sciences 250:165–169.
Mamy, L., B. Gabrielle, and E. Barriuso. 2010. Comparative environmental impacts of glyphosate and conventional herbicides when used with glyphosate-tolerant and non-tolerant crops. Environmental Pollution 158:3172–3178.
Manshardt, R. 2012. The papaya in Hawai’i. HortScience 47:1399–1404.
Marvier, M., C. McCreedy, J. Regetz, and P. Kareiva. 2007. A meta-analysis of effects of Bt cotton and maize on nontarget invertebrates. Science 316:1475–1477.
Mensah, E.C. 2007. Economics of Technology Adoption: A Simple Approach. Saarbrücken, Germany: VDM Verlag.
Micinski, S., D.C. Blouin, W.F. Waltman, and C. Cookson. 2008. Abundance of Helicoverpa zea and Heliothis virescens in pheromone traps during the past twenty years in northwestern Louisiana. Southwestern Entomologist 33:139–149.
Mijatović, D., F. van Oudenhoven, P. Eyzaguirre, and T. Hodgkin. 2013. The role of agricultural biodiversity in strengthening resilience to climate change: Towards an analytical framework. International Journal of Agricultural Sustainability 11:95–107.
Mohan, K.S., K.C. Ravi, P.J. Suresh, D. Sumerford, and G.P. Head. 2016. Field resistance to the Bacillus thuringiensis protein Cry1Ac expressed in Bollgard® hybrid cotton in pink bollworm, Pectinophora gossypiella (Saunders), populations in India. Pest Management Science 72:738–746.
Montgomery, D.R. 2007. Soil erosion and agricultural sustainability. Proceedings of the National Academy of Sciences of the United States of America 104:13268–13272.
Mortensen, D.A., J.F. Egan, B.D. Maxwell, M.R. Ryan, and R.G. Smith. 2012. Navigating a critical juncture for sustainable weed management. BioScience 62:75–84.
Morton, D.C., R.S. DeFries, Y.E. Shimabukuro, L.O. Anderson, E. Arai, F.D. Espirito-Santo, R. Freitas, and J. Morisette. 2006. Cropland expansion changes deforestation dynamics in the southern Brazilian Amazon. Proceedings of the National Academy of Sciences of the United States of America 103:14637–14641.
Nail, K.R., C. Stenoien, and K.S. Oberhauser. 2015. Immature monarch survival: Effects of site characteristics, density, and time. Annals of the Entomological Society of America 108:680–690.
Naranjo, S.E., J.R. Ruberson, H.C. Sharma, L. Wilson, and K.M. Wu. 2008. The present and future role of insect-resistant genetically modified cotton in IPM. Pp. 159–194 in Integration of Insect-Resistant Genetically Modified Crops with IPM Systems, J. Romeis, A.M. Shelton, and G.G. Kennedy, eds. Berlin: Springer.
Neher, D.A., A.W.N. Muthumbi, and G.P. Dively. 2014. Impact of coleopteran-active Bt maize on non-target nematode communities in soil and decomposing corn roots. Soil Biology & Biochemistry 76:127–135.
Nelson, D.S., and G.C. Bullock. 2003. Simulating a relative environmental effect of glyphosate-resistant soybeans. Ecological Economics 45:189–202.
Neve, P., R. Busi, M. Renton, and M.M. Vila-Aiub. 2014. Expanding the eco-evolutionary context of herbicide resistance research. Pest Management Science 70:1385–1393.
Nichterlein, H., A. Matzk, L. Kordas, J. Kraus, and C. Stibbe. 2013. Yield of glyphosate-resistant sugar beets and efficiency of weed management systems with glyphosate and conventional herbicides under German and Polish crop production. Transgenic Research 22:725–736.
Nichols, R.L, J. Bond, A.S. Culpepper, D. Dodds, V. Nandula, C.L. Main, M.W. Marshall, T.C. Mueller, J.K. Norsworthy, A. Price, M. Patterson, R.C. Scott, K.L. Smith, L.E. Steckel, D. Stephenson, D. Wright, and A.C. York. 2009. Glyphosate-resistant Palmer amaranth (Amaranthus palmeri) spreads in the southern US. Resistant Pest Management Newsletter 18:8–10.
Nolan, E., and P. Santos. 2012. The contribution of genetic modification to changes in corn yield in the United States. American Journal of Agricultural Economics 94:1171–1188.
Nolte, S.A., and B.G. Young. 2002. Efficacy and economic return on investment for conventional and herbicide-resistant corn (Zea mays). Weed Technology 16: 371–378.
Novacek, M., S.C. Mason, T.D. Galusha, and M. Yaseen. 2014. Bt transgenes minimally influence grain yield and lodging across plant population. Maydica 59:90–95.
NRC (National Research Council). 2000. Genetically Modified Pest-Protected Plants: Science and Regulation. Washington, DC: National Academy Press.
NRC (National Research Council). 2002. Environmental Effects of Transgenic Plants: The Scope and Adequacy of Regulation. Washington, DC: National Academy Press.
NRC (National Research Council). 2010a. The Impact of Genetically Engineered Crops on Farm Sustainability in the United States. Washington, DC: National Academies Press.
NRC (National Research Council). 2010b. Toward Sustainable Agricultural Systems in the 21st Century. Washington, DC: National Academies Press.
NRC (National Research Council). 2012. National Summit on Strategies to Manage Herbicide-Resistant Weeds: Proceedings of a Symposium. Washington, DC: National Academies Press.
NRC (National Research Council). 2013. Assessing Risks to Endangered and Threatened Species from Pesticides. Washington, DC: National Academies Press.
Oberhauser, K.S., M.D. Prysby, H.R. Mattila, D.E. Stanley-Horn, M.K. Sears, G. Dively, E. Olson, J.M. Pleasant. W.-K.F. Lam, and R.L. Hellmich. 2001. Temporal and spatial overlap between monarch larvae and corn pollen. Proceedings of the National Academy of Sciences of the United States of America 98:11913–11918.
Owen, M.D.K. 2008. Weed species shifts in glyphosate-resistant crops. Pest Management Science 64:377–387.
Owen, M.D.K., P. Pedersen, J.L. De Bruin, J. Stuart, J. Lux, D. Franzenburg, and D. Grossnickle. 2010. Comparisons of genetically modified and non-genetically modified soybean cultivars and weed management systems. Crop Science 50:2597–2604.
Ozelame, O., and T. Andreatta. 2013. Evaluation of technical and economic performance: A comparative study between hybrid corn and Bt corn. Custos e Agronegócio Online 9:210–232.
Pessel, F.D., J. Lecomete, V. Emeriau, M. Krouti, A. Messean, and P.H. Gouyon. 2001. Persistence of oilseed rape (Brassica napus L.) outside of cultivated fields. Theoretical and Applied Genetics 102:841–846.
Petzold-Maxwell, J.L., L.J. Meinke, M.E. Gray, R.E. Estes, and A.J. Gassmann. 2013. Effect of Bt maize and soil insecticides on yield, injury, and rootworm survival: Implications for resistance management. Journal of Economic Entomology 106:1941–1951.
Pleasants, J.M., and K.S. Oberhauser. 2013. Milkweed loss in agricultural fields because of herbicide use: Effect on the monarch butterfly population. Insect Conservation and Diversity 6:135–144.
Pleasants, J.M., R.L. Hellmich, G.P. Dively, M.K. Sears, D.E. Stanley-Horn, H.R. Mattila, J.E. Foster, P. Clark, and G.D. Jones. 2001. Corn pollen deposition on milkweeds in and near cornfields. Proceedings of the National Academy of Sciences of the United States of America 98:11919–11924.
Pleasants, J.M., E.H. Williams, L.P. Brower, K.S. Oberhauser, and O.R Taylor. 2016. Conclusion of no decline in summer monarch population not supported. Annals of the Entomological Society of America 109:169–171.
Plourde, J.D., B.C. Pijanowski, and B.K. Pekin. 2013. Evidence for increased monoculture in the Central United States. Agriculture, Ecosystem & Environment 165:50–59.
Powles, S.B. 2008. Evolved glyphosate-resistant weeds around the world: Lessons to be learnt. Pest Management Science 64:360–365.
Powlson, D.S., C.M. Stirling, M.L. Jat, B.G. Gerard, C.A. Palm, P.A. Sanchez, and K.G. Cassman. 2014. Limited potential of no-till agriculture for climate change mitigation. Nature Climate Change 4:678–683.
Pray, C.E., L. Nagarajan, J.K. Huang, R.F. Hu, and B. Ramaswami. 2011. The impact of Bt cotton and the potential impact of biotechnology on other crops in China and India. Pp. 83–114 in Genetically Modified Food and Global Welfare, C.A. Carter, G.C. Moschini, and I. Sheldon, eds. Bingley, UK: Emerald Group Publishing.
Qaim, M., and G. Traxler. 2005. Roundup Ready soybeans in Argentina: Farm level and aggregate welfare effects. Agricultural Economics 32:73–86.
Qaim, M., and D. Zilberman. 2003. Yield effects of genetically modified crops in developing countries. Science 299:900–902.
Qiao, F. 2015. Fifteen years of Bt cotton in China: The economic impact and its dynamics. World Development 70:177–185.
Ramirez-Romero, R., N. Desneux, A. Decourtye, A. Chaffiol, and M.H. Pham-Delègue. 2008. Does Cry1Ab protein affect learning performances of the honey bee Apis mellifera L. (Hymenoptera, Apidae)? Ecotoxicology and Environmental Safety 70:327–333.
Reay-Jones, F.P.F., and P. Wiatrak. 2011. Evaluation of new transgenic corn hybrids producing multiple Bacillus thuringiensis toxins in South Carolina. Journal of Entomological Science 46:152–164.
Reay-Jones, F.P.F., and D.D. Reisig. 2014. Impact of corn earworm injury on yield of transgenic corn producing Bt toxin in the Carolinas. Journal of Economic Entomology 107:1101–1109.
Reay-Jones, F.P.F., P. Wiatrak, and J.K. Greene. 2009. Evaluating the performance of transgenic corn producing Bacillus thuringiensis toxins in South Carolina. Journal of Agricultural and Urban Entomology 26:77–86.
Reichman, J.R., L.S. Watrud, E.H. Lee, C.A. Burdick, M.A. Bollman, M.J. Storm, G.A. King, and C. Mallory-Smith. 2006. Establishment of transgenic herbicide-resistant creeping bentgrass (Agrostis stolonifera L.) in nonagronomic habitats. Molecular Ecology 15:4243–4255.
Reisig, D. 2014. North Carolina Grower Experience with Crops Expressing Bt. Webinar presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, October 1.
Rice, M.E. and K. Ostlie. 1997. European corn borer management in field corn: A survey of perceptions and practices in Iowa and Minnesota. Journal of Production Agriculture 10:628–634.
Ries, L., D.J. Taron, and E. Rendón-Salinas. 2015. The disconnect between summer and winter monarch trends for the eastern migratory population: Possible links to differing drivers. Annals of the Entomological Society of America 108:691–699.
Romeis, J., M. Meissle, F. Alvarez-Alfageme, F. Bigler, D.A. Bohan, Y. Devos, L.A. Malone, X. Pons, and S. Rauschen. 2014. Potential use of an arthropod database to support the non-target risk assessment and monitoring of transgenic plants. Transgenic Research 23:995–1013.
Romeu-Dalmau, C., M.B. Bonsall, K.J. Willis, and L. Dolan. 2015. Asiatic cotton can generate similar economic benefits to Bt cotton under rainfed conditions. Nature Plants 1:15072.
Rosenbaum, K.K., R.E. Massey, and K.W. Bradley. 2013. Comparison of weed control, yield, and net income in conventional, glyphosate-resistant, and glufosinate-resistant soybean. Crop Management 12.
Ruffo, M.L., L.F. Gentry, A.S. Henninger, J.R. Seebauer, and F.E. Below. 2015. Evaluating management factor contributions to reduce corn yield gaps. Agronomy Journal 107:495–505.
Sadashivappa, P., and M. Qaim. 2009. Bt cotton in India: Development of benefits and the role of government seed price interventions. AgBioForum 12:172–183.
Sanglestsawai, S., R.M. Rejesus, and J.M. Yorobe. 2014. Do lower yielding farmers benefit from Bt corn? Evidence from instrumental variable quantile regressions. Food Policy 44:285–296.
Schafer, M.G., A.A. Ross, J.P. Londo, C.A. Burdick, E.H. Lee, S.E. Travers, P.K. Van de Water, and C.L. Sagers. 2011. The establishment of genetically engineered canola populations in the US. PLoS ONE 6:e25736.
Schwartz, L.M., D.J. Gibson, K.L. Gage, J.L. Matthews, D.L. Jordan, M.D.K. Owen, D.R. Shaw, S.C. Weller, R.G. Wilson, and B.G. Young. 2015. Seedbank and field emergence of weeds in glyphosate-resistant cropping systems in the United States. Weed Science 63:425–439.
Sears, M.K., R.L. Hellmich, D.E. Stanley-Horn, K.S. Oberhauser, J.M. Pleasants, H.R. Mattila, B.D. Siegfried, and G.P. Dively. 2001. Impact of Bt corn pollen on monarch butterfly populations: A risk assessment. Proceedings of the National Academy of Sciences of the United States of America 98:11937–11942.
Sedjo, R.A. 2005. Will developing countries be the early adopters of genetically engineered forests? AgBioForum 8:205–212.
Shankar, B., R. Bennett, and S. Morse 2008. Production risk, pesticide use and GM crop technology in South Africa. Applied Economics 40:2489–2500.
Sheaffer, C.C., D.J. Undersander, and R.L. Becker. 2007. Comparing Roundup Ready and conventional systems of alfalfa establishment. Forage and Grazinglands 5.
Shi, G., J.-P. Chavas, and J. Lauer. 2013. Commercialized transgenic traits, maize productivity, and yield risk. Nature Biotechnology 31:111–114.
Sinclair, T.R. 1994. Limits to crop yield? Pp. 509–532 in Physiology and Determination of Crop Yield, K.J. Boote, J.M. Bennett, T.R. Sinclair, and G.M. Paulsen, eds. Madison, WI: ASA, CSSA, SSSA.
Slatkin, M. 1987. Gene flow and the geographic structure of natural-populations. Science 236:787–792.
Smith, S., V. Primomo, R. Monk, B. Nelson, E. Jones, and K. Porter. 2010. Genetic diversity of widely used U.S. sorghum hybrids 1980–2008. Crop Science 50:1664–1673.
Snow, A.A., and P.M. Palma. 1997. Commercialization of transgenic plants: Potential ecological risks. Bioscience 47:86–96.
Snow, A.A., D. Pilson, L.H. Rieseberg, M.J. Paulsen, N. Pleskac, M.R. Reagon, D.E. Wolf, and S.M. Selbo. 2003. A Bt transgene reduces herbivory and enhances fecundity in wild sunflowers. Ecological Applications 13:279–286.
Sosnoskie, L.M., and A.S. Culpepper. 2014. Glyphosate-resistant palmer amaranth (Amaranthus palmeri) increases herbicide use, tillage, and hand-weeding in Georgia cotton. Weed Science 62:393–402.
Stanley-Horn, D.E., G.P. Dively, R.L. Hellmich, H.R. Mattila, M.K. Sears, R. Rose, L.C.H. Jesse, J.E. Losey, J.J. Obrycki, and L. Lewis. 2001. Assessing the impact of Cry1Abexpressing corn pollen on monarch butterfly larvae in field studies. Proceedings of the National Academy of Sciences of the United States of America 98:11931–11936.
Steffy, G. 2015. Trends observed in fall migrant monarch butterflies (Lepidoptera: Nymphalidae) east of the Appalachian Mountains in an inland stopover in southern Pennsylvania over an eighteen year period. Annals of the Entomological Society of America 108:718–728.
Stenoien, C., K.R. Nail, and K.S. Oberhauser. 2015. Habitat productivity and temporal patterns of monarch butterfly egg densities in the eastern United States. Annals of the Entomological Society of America 108:670–679.
Stringam, G.R., V.L. Ripley, H.K. Love, and A. Mitchell. 2003. Transgenic herbicide tolerant canola—the Canadian experience. Crop Science 43:1590–1593.
Stone, G.D. 2011. Field versus farm in Warangal: Bt cotton, higher yields, and larger questions. World Development 39:387–398.
Tabashnik, B.E. 1989. Managing resistance with multiple pesticide tactics: Theory, evidence, and recommendations. Journal of Economic Entomology 82:1263–1269.
Tabashnik, B.E., T. Brevault, and Y. Carriere. 2013. Insect resistance in Bt crops: Lessons from the first billion acres. Nature Biotechnology 31:510–521.
Tan, S., R.R. Evans, M.L. Dahmer, B.K. Singh, and D.L. Shaner. 2005. Imidazolinone-tolerant crops: History, current status and future. Pest Management Science 61:246–257.
Thelen, K.D., and D. Penner. 2007. Yield environment affects glyphosate-resistant hybrid response to glyphosate. Crop Science 47:2098–2107.
Thelin, G.P., and W.W. Stone. 2013. Estimation of Annual Agricultural Pesticide Use for Counties of the Conterminous United States, 1992–2009. Reston, VA: U.S. Geological Survey.
Tilman, D., J. Fargione, B. Wolff, C. D’Antonio, A. Dobson, R. Howarth, D. Schindler, W.H. Schlesinger, D. Simberloff, and D. Swackhamer. 2001. Forecasting agriculturally driven global change. Science 292:281–284.
Trigo, E., E. Cap, V. Malach, and F. Villareal. 2009. The Case of Zero-Tillage Technology in Argentina. Washington, DC: International Food Policy Research Institute.
Unglesbee, E. March 5, 2014. Soybeans: Monsanto Assessing Fit of Bt Varieties in the U.S. Online. AgFax. Available at http://agfax.com/2014/03/05/soybeans-monsanto-assessingfit-bt-varieties-u-s-dtn/. Accessed December 21, 2015.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2011. Bayer CropScience LP; Determination of nonregulated status of cotton genetically engineered for insect resistance and herbicide tolerance. Federal Register 76:63278–63279.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2015a. Determination of nonregulated status for Dow AgroSciences DAS-8191Ø-7 cotton. Available at https://www.aphis.usda.gov/brs/aphisdocs/13_26201p_det.pdf. Accessed April 14, 2016.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2015b. Record of Decision; Monsanto petitions (10-188-01p and 12-185-01p) for determination of nonregulated status for dicamba-resistant soybean and cotton varieties. Available at https://www.aphis.usda.gov/brs/aphisdocs/dicamba_feis_rod.pdf. Accessed April 14, 2016.
USDA–NASS (U.S. Department of Agriculture–National Agricultural Statistics Service). 2009. Hawaii Papayas. Available at http://www.nass.usda.gov/Statistics_by_State/Hawaii/Publications/Archive/xpap0809.pdf. Accessed April 17, 2016.
USDA–NASS (U.S. Department of Agriculture–National Agricultural Statistics Service). 2013. NASS Highlights: 2012 Agricultural Chemical Use Survey–Soybean. May 2013. Available at http://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Chemical_Use/2012_Soybeans_Highlights/ChemUseHighlights-Soybeans-2012.pdf. Accessed November 22, 2015.
USDA–NASS (U.S. Department of Agriculture–National Agricultural Statistics Service). 2015. NASS Highlights: 2014 Agricultural Chemical Use Survey–Corn. May 2015. Available at http://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Chemical_Use/2014_Corn_Highlights/ChemUseHighlights_Corn_2014.pdf. Accessed November 22, 2015.
van de Wouw, M., T. van Hintum, C. Kik, R. van Treuren, and B. Visser. 2010. Genetic diversity in twentieth century crop cultivars: A meta analysis. Theoretical & Applied Genetics 120:1241–1252.
VanGessel, M.J. 2001. Glyphosate-resistant horseweed from Delaware. Weed Science 49:703–705.
van Ittersum, M.K., and R. Rabbinge. 1997. Concepts in production ecology for analysis and quantification of agricultural input–output combinations. Field Crops Research 52:197–208. van Ittersum, M.K., K.G. Cassman, P. Grassini, J. Wolf, P. Tittonell, and Z. Hochman. 2013. Yield gap analysis with local to global relevance—a review. Field Crops Research 143:4–17.
Vera-Diaz, M.C., R.K. Kaufmann, and D.C. Nepstad. 2009. The Environmental Impacts of Soybean Expansion and Infrastructure Development in Brazil’s Amazon Basin. Medford, MA: Tufts University.
Vitale, J., G. Vognan, M. Ouattarra, and O. Traoré. 2010. The commercial application of GMO crops in Africa: Burkina Faso’s decade of experience with Bt cotton. AgBioForum 13:320–332.
Wallander, S. 2013. While Crop Rotations Are Common, Cover Crops Remain Rare. USDA Economic Research Service. Amber Waves, March. Available at http://www.ers.usda.gov/amber-waves/2013/march/while-crop-rotations-are-common-cover-crops-remain-rare/. Accessed August 11, 2015.
Wangila, D.S., A.J. Gassmann, J.L. Petzold-Maxwell, B.W. French, and L.J. Meinke. 2015. Susceptibility of Nebraska western corn rootworm (Coleoptera: Chrysomelidae) populations to Bt corn events. Journal of Economic Entomology 108:742–751.
Ward, S.M., T.M. Webster, and L.E. Steckel. 2013. Palmer amaranth (Amaranthus palmeri): a review. Weed Technology 12:12–27.
Warwick, S.I., M.-J. Simard, A. Lègére, H.B. Beckie, L. Braun, B. Zhu, P. Mason, G. Séguin-Swartz, and C.N. Stewart, Jr. 2003. Hybridization between transgenic Brassica napus L. and its wild relatives: Brassica rapa L., Raphanus raphanistum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) O.E. Schulz. Theoretical and Applied Genetics 107:528–539.
Warwick, S.I., A. Lègére, M.-J. Simard, and T. James. 2008. Do escaped transgenes persist in nature? The case of an herbicide resistance transgene in a weedy Brassica rapa population. Molecular Ecology 17:1387–1395.
Webster, T.M., and H.D. Coble. 1997. Changes in the weed species composition of the southern United States: 1974 to 1995. Weed Technology 11:308–217.
Webster, T.M., and T.L. Nichols. 2012. Changes in the prevalence of weed species in the major agronomic crops of the southern United States: 1994/1995 to 2008/2009. Weed Science 60:145–157.
Wendt, J.W., and S. Hauser. 2013. An equivalent soil mass procedure for monitoring soil organic carbon in multiple soil layers. European Journal of Soil Science 64:58–65.
WI Department of Agriculture. 2014. Wisconsin Pest Bulletin 59, November 13. Available at https://datcpservices.wisconsin.gov/pb/pdf/11-13-14.pdf. Accessed March 24, 2016.
Wiggins, M.S., M.A. McClure, R.M. Hayes, and L.E. Steckel. 2015. Integrating cover crops and POST herbicides for glyphosate-resistant Palmer amaranth (Amaranthus palmeri) control in corn. Weed Technology 29:412–418.
Wilson, L., S. Downes, M. Khan, M. Whitehouse, G. Baker, P. Grundy, and S. Maas. 2013. IPM in the transgenic era: A review of the challenges from emerging pests in Australian cotton systems. Crop and Pasture Science 64:737–749.
Wilson, R.G., C.D. Yonts, and J.A. Smith. 2002. Influence of glyphosate and glufosinate on weed control and sugarbeet (Beta vulgaris) yield in herbicide-tolerant sugarbeet. Weed Technology 16:66–73.
Witjaksono, J., X. Wei, S. Mao, W. Gong, Y. Li, and Y. Yuan. 2014. Yield and economic performance of the use of GM cotton worldwide over time: A review and meta-analysis. China Agricultural Economic Review 6:616–643.
Wolfenbarger, L.L., and P.R. Phifer. 2000. The ecological risks and benefits of genetically engineered plants. Science 290:2088–2093.
Wolfenbarger, L.L., S. Naranjo, J. Lundgren, R. Bitzer, and L. Watrud. 2008. Bt crop effects on functional guilds of non-target arthropods: A meta-analysis. PLoS ONE 3:e2118.
Wortmann, C.S., R.A. Drijber, and T.G. Franti. 2010. One-time tillage of no-till crop land five years post-tillage. Agronomy Journal 102:1302–1307.
Wright, C.K., and M.C. Wimberly. 2013. Recent land use change in the Western Corn Belt threatens grasslands and wetlands. Proceedings of the National Academy of Sciences of the United States of America 110:4134–4139.
Wright, T.R., G. Shan, T.A. Walsh, J.M. Lira, C. Cui, P. Song, M. Zhuang, N.L. Arnold, G. Lin, K. Yau, S.M. Russell, R.M. Cicchillo, M.A. Peterson, D.M. Simpson, N. Zhou, J. Ponsamuel, and Z. Zhang. 2010. Robust crop resistance to broadleaf and grass herbicides provided by aryloxyalkanoate dioxygenase transgenes. Proceedings of the National Academy of Sciences of the United States of America 107:20240–20245.
Wu, K.-M., Y.-H. Lu, H.-Q. Feng, Y.-Y. Jiang, and J.-Z. Zhao. 2008. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 321:1676–1678.
Yang, F., D.L. Kerns, G.P. Head, B.R. Leonard, R. Levy, Y. Niu, and F. Huang. 2014. A challenge for the seed mixture refuge strategy in Bt maize: Impact of cross-pollination on an ear-feeding pest, corn earworm. PLoS ONE 9:e112962.
Young, B.G., D.J. Gibson, K.L. Gage, J.L. Matthews, D.L. Jordan, M.D.K. Owen, D.R. Shaw, S.C. Weller, and R.G. Wilson. 2013. Agricultural weeds in glyphosate-resistant cropping systems in the United States. Weed Science 61:85–97.
Yucatan Times. November 17, 2015. Monarch butterfly population expected to quadruple in Mexico. Online. Available at http://www.theyucatantimes.com/2015/11/monarchbutterfly-population-expected-to-quadruple-in-mexico/. Accessed November 24, 2015.
Zangerl, A.R., D. McKenna, C.L. Wraight, M. Carroll, P. Ficarello, R. Warner, and M.R. Berenbaum. 2001. Effects of exposure to event 176 Bacillus thuringiensis corn pollen on monarch and black swallowtail caterpillars under field conditions. Proceedings of the National Academy of Sciences of the United States of America 98:11908–11912.
Zapiola M.L., and C.A. Mallory-Smith. 2012. Crossing the divide: Gene flow produces inter-generic hybrid in feral transgenic creeping bentgrass population. Molecular Ecology 21:4672–4680.
Zapiola, M.L., C.K. Campbell, M.D. Butler, and C.A. Mallory-Smith. 2008. Escape and establishment of transgenic glyphosate-resistant creeping bentgrass (Agrostis stolonifera) in Oregon, USDA: A 4-year study. Journal of Applied Ecology 45:486–494.
Zeilinger, A.R., D.M. Olson, and D.A. Andow. 2011. Competition between stink bug and heliothine caterpillar pests on cotton at within-plant spatial scales. Entomologia Experimentalis et Applicata 141:59–70.










































































