Summary
The organ donation and transplantation system strives to honor the gift of donated organs by fully using those organs to save and improve the quality of the lives of their recipients. Organ transplantation has become the optimal treatment for many end-stage organ-specific diseases. However, there are not enough donated organs to meet the demand. Some donated organs may not be recovered, some recovered organs may not be transplanted, and some transplanted organs may not function adequately. Yet, almost all transplantation research to date has focused on transplant recipients and on ways to improve transplantation processes and post-transplant health outcomes rather than on how to enhance the quality and increase the quantity of organs that can be recovered from deceased donors and then successfully transplanted. Organ donor intervention research can test and assess interventions (e.g., medications, devices, and donor management protocols) to maintain or improve organ quality prior to, during, and following transplantation. The intervention is administered either while the organ is still in the deceased donor or after it is recovered from the donor but before it is transplanted into a recipient.
Organ donor intervention research presents new challenges to the organ donation and transplantation community because of ethical questions about who should be considered a human subject in a research study, whose permission and oversight are needed, and how to ensure that such research does not threaten the equitable distribution of a scarce and valuable resource. Therefore, organ donor intervention research requires extensive oversight and careful planning to ensure that the integrity of the donation and transplantation process is maintained and that fully
using the gift of the donated organ has the highest priority in all phases of research.
THE DEMAND FOR ORGAN TRANSPLANTATION
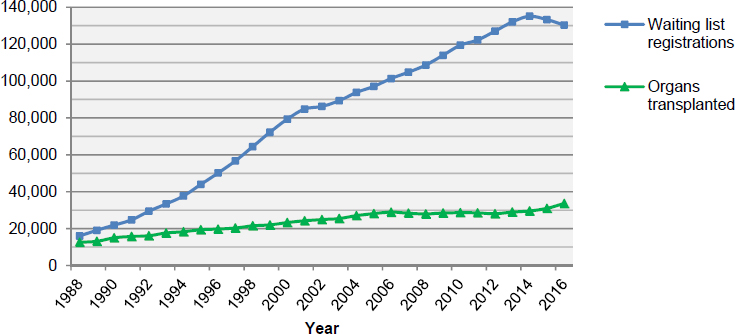
The number of organs transplanted has increased in recent decades. In 2016, approximately 82 percent of organs transplanted in the United States were from deceased donors—27,630 organs were transplanted from 9,971 deceased individuals, while an additional 5,980 organs were transplanted from living donors. In comparison, 10,794 organs were transplanted from deceased donors in 1988, and an additional 1,829 organs were transplanted from living donors. The outcomes for transplant recipients, including graft survival, have also improved. However, the growth in the number of patients awaiting organ transplantation has outpaced the growth in the number of organs being transplanted (see Figure S-1). As of July 13, 2017, there were 117,154 transplant candidates awaiting an organ.
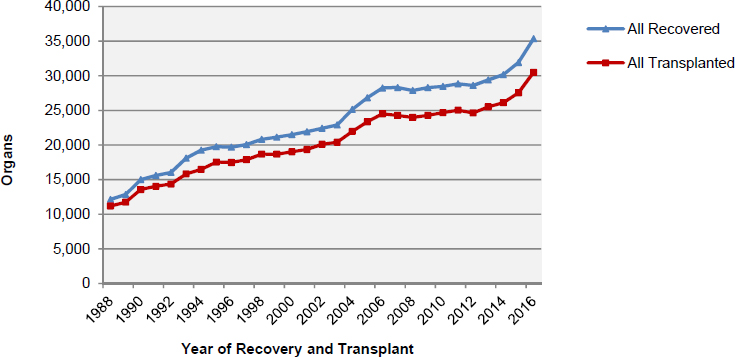
The supply of organs available for transplantation is affected by several factors including the number of potential organ donors and the public’s willingness to donate organs. Additionally, every year a number of of donor organs are not transplanted (see Figure S-2). For example, donated organs may not be transplanted because of the condition of the donated organ or because it is not possible to allocate an organ within the timeframe during which the organ is viable for transplantation. A donated organ may be determined to be unsuitable for transplantation based on a variety of factors such as the health of the deceased donor, the cause of death, or functional or anatomic abnormalities found in a potential donor or donor organ.
STUDY PROCESS AND TASK
This report focuses on the ethical, legal, regulatory, policy, and organizational issues relevant to the conduct of research in the United States involving deceased organ donors.1 This type of research is challenging to conduct under current policies and regulatory mechanisms concerning biomedical research. For these reasons, a group of organizations came forward to sponsor a study by the National Academies of Sciences, Engineering, and Medicine (the National Academies) on deceased organ donor intervention research: American Association for the Study of Liver Diseases, American Society of Transplant Surgeons, American Society of Transplantation, Association of Organ Procurement Organizations, Gift of Life Donor Program, Health Resources & Services Administration, Laura and John Arnold Foundation, National Institutes of Health (National Heart, Lung, and Blood Institute; National Institute of Allergy and Infectious Diseases; National Institute of Diabetes and Digestive and Kidney Diseases), National Kidney Foundation, OneLegacy Foundation, and The Transplantation Society. To address the statement of task (see Box S-1), the National Academies appointed a 12-member committee with expertise in organ transplant surgery, organ procurement, pediatrics, decision science, law, ethics, clinical trial research, and organ donation public awareness and education efforts.
___________________
1 Living donation is not included in the statement of task and thus not discussed in this report.
After examining the complexities and challenges surrounding organ donor intervention research, the committee identified six goals to guide its work (see Box S-2).
This report provides recommendations for how to conduct organ donor intervention research in a manner that maintains high ethical standards,
that ensures dignity and respect for deceased organ donors and their families, that provides transparency and information for transplant candidates who might receive a research organ, and that supports and sustains the public’s trust in the process of organ donation and transplantation.
THE ORGAN DONATION AND TRANSPLANTATION PROCESS
In the United States, organ donation and transplantation are accomplished through a cooperative, interdependent network of multidisciplinary, multi-institutional services. Oversight of this highly regulated process is coordinated by the federally mandated Organ Procurement and Transplantation Network (OPTN), which is operated by the United Network for Organ Sharing (UNOS). OPTN sets national policies that apply to all organizations involved in organ donation and transplantation, including organ procurement organizations (OPOs), donor hospitals, and transplantation programs and centers. Currently, 58 OPOs procure and distribute organs to 254 transplant hospitals. Different programs within these hospitals are responsible for the transplantation of specific organs and the follow-up care for organ transplant recipients. An extensive national computerized network is used to match donated organs with potential recipients. The network operates in units referred to as donation services areas (DSAs). Each DSA encompasses one OPO, the donor hospitals contracted to work with the OPO, and the assigned transplant hospitals, transplant programs, and histocompatibility labs that serve the area. DSAs are geographically and culturally diverse and work within the nation’s 11 transplant regions.
Individuals who need an organ transplant are placed on a waiting list for the type(s) of organ they need. When an organ becomes available and a potential recipient is identified, the potential recipient’s transplant team is notified and provided with details about the organ. If the organ is determined to be acceptable, the team contacts the potential recipient to determine his or her current state of health and interest in proceeding with the transplantation. In order to maintain the organ in optimal condition, the decision of whether to accept an organ offer needs to be made quickly—usually within 1 hour of the recipient team receiving the offer and accessing the deceased donor’s information—and the transplantation surgery proceeds as soon as possible after the organ is accepted and received.
THE POTENTIAL OF ORGAN DONOR RESEARCH
In the time between the declaration of the donor’s death and the procurement of the organs, the authorized OPO and donor hospital implement donor management protocols (e.g., administering medications and maintaining the donor’s body at a particular temperature). These protocols are designed to maintain the organs in the best possible condition by minimizing the organ stress, damage, and dysfunction until the organs are recovered.
If research followed by transplantation (organ donor intervention research) has been authorized, the research intervention would be administered to a deceased donor prior to organ recovery or to the target organ after the organ has been recovered but before transplantation. When the research intervention is administered prior to organ recovery and the intent is to have an effect on a specific organ (i.e., the target organ), the intervention could affect other organs from the same donor that may also be removed and transplanted after the intervention (i.e., non-target organs). As a result, many transplant recipients across multiple transplant centers could become human subjects in a single organ donor intervention research study. Organ donor intervention research therefore involves three different parties as potential participants in the research—organ donors, target organ recipients, and non-target organ recipients—with each deserving specific considerations, all of which are needed to ensure that a respectful, fair, and trustworthy donation and transplantation system is in place in the United States.
Deceased organ donor intervention research offers an opportunity to gain the knowledge needed to maximize the benefits of the gifts of donated organs. The committee’s work focused on identifying next steps in overcoming challenges so that such research can be conducted in the pursuit of improving the quality and increasing the quantity of organs available for transplantation.
ETHICAL PRINCIPLES
The committee considered its task under the assumptions that organ transplantation is a good that is worth pursuing and expanding and that it is thus important to increase the number of and improve the quality of organs for transplantation in order to save lives and improve recipients’ quality of life. A close analysis of the relevant ethical principles indicates several conditions under which organ donor intervention research can be both ethically justified and ethically conducted. These principles include
- Respect for persons: respect for persons’ autonomous choices
- Beneficence (utility): balance of probable benefits against probable harms
- Fairness: equitable distribution of benefits, risks, costs, and burdens
- Validity: generation of evidence that is sufficiently reliable to guide decision making
- Trustworthiness: confidence in and reliance on others to act competently and in accord with ethical principles and legal and regulatory standards
Much depends on developing a clear understanding of this research, what is required for it to succeed, and how various options in its pursuit might fulfill these ethical principles.
LEGAL, REGULATORY, AND POLICY FRAMEWORKS
Ethical principles are often embodied and embedded in laws, regulations, and policies. A good example is the Uniform Anatomical Gift Act (UAGA), some version of which guides the transfer of organs from deceased persons in each state, the District of Columbia, and Puerto Rico. Another good example is the Federal Policy for the Protection of Human Subjects (also termed the Common Rule), which is the core federal policy that governs federally funded and much privately funded research involving human subjects in the United States. Trust in the U.S. donation and transplantation system, including confidence and reliance that the organizations and health professionals involved will fully and fairly communicate the facts and the nuances of the complex donation and transplantation processes, is essential. However, questions remain as to what the transplantation community and society as a whole should do to maintain that trust, as opposed to what merely must be done to be in legal and regulatory compliance.
Improving Transparency and Public Trust in the Process of Authorizing Organ Donation
The United States’ organ donation system operates under an “opt-in” model in which the individual while alive or the next of kin or surrogate after the individual’s death must explicitly choose to donate organs. The Common Rule defines its regulations as covering living individuals and so does not apply to deceased organ donor intervention research itself. However, authorization for organ donation, including for research purposes, is still required under state laws based on the UAGA. The challenge is that the state laws vary regarding authorization for research followed by transplantation and there is no standard practice for recording an individual’s preferences for donating organs for the purpose of research followed by transplantation. Additionally, there are no requirements for what information about organ donation options, including research, should be provided to individuals who are contemplating registering to be an organ donor.
Messaging and communication strategies regarding organ donor intervention research need to be developed and thoroughly tested to meet the health literacy needs across the general public. It will be important to identify the potential benefits of organ transplantation with organs that have been subject to organ donor intervention research in awareness and educational programs about organ donation. This is particularly important for populations who may be suspicious of research because of a long history of biomedical research abuses. Racial and ethnic minorities tend to have lower rates of organ donation and to be less willing to participate in research. Social and economic marginalization, as well as distrust in medical research that has its roots in historical abuses, have likely made members of minority groups less likely to participate willingly in organ donation and research.
Recipients of Research Organs: Improving Consent and Ensuring Protections
The committee examined the effective and ethical implementation of the laws that ensure human research subject protections with particular attention to the following questions:
- Are recipients of research organs human research subjects?
- What are the issues regarding informed consent?
- How can the informed consent processes use risk stratification?
- How can consent be most effectively obtained given the time-sensitive nature of these decisions?
- What are the issues for post-transplant follow-up?
These questions raise a number of ethical and regulatory issues and the committee’s conclusions are summarized in Figure S-3.
Careful consideration is needed for how to most effectively inform transplant candidates about organ donor intervention research. The window of time during which an organ is viable for transplantation and during which many steps in the transplantation process must take place is limited. Additionally, transplant candidates receive a wealth of information from the time of intake through the time of discussing a transplant organ offer and need adequate time to fully learn about organ donor intervention research and make a determination about whether they would consider receiving a research organ.
In order to find a balance between the laws and regulations and the need to ensure that organs do not become unusable because of an excessive lapse of time, the committee proposes a two-stage process for obtaining consent from transplant candidates who could receive a research organ. In the first stage, information on organ donor intervention research is provided and the transplant candidate is asked to decide whether they would consider receiving a research organ. This first stage would be part of the clinical consent process that begins at the time of patient intake and continues through wait listing. The second stage would occur when an organ is being offered to the transplant candidate and would follow research informed consent processes as determined by the single institutional review board (IRB) for organ donor intervention research. The committee considered other options that would require revisions to the Common Rule, but concluded that the proposed two-stage process should stay within current human research subjects protection regulations and that this process offers the best opportunities to
- fully inform transplant candidates about organ donor intervention research at a time when they can consider the risks, benefits, and alternatives in depth;
- provide a thorough informed consent process for participation in research; and
- allow the process to be conducted as expeditiously as possible by only doing the more in-depth informed consent processes with those candidates who have expressed an interest in receiving a research organ.
GOAL 1: Improve transparency and public trust in the organ donation process for research followed by transplantation.
RECOMMENDATION 1: The Organ Procurement and Transplantation Network, organ procurement organizations (OPOs), the Health
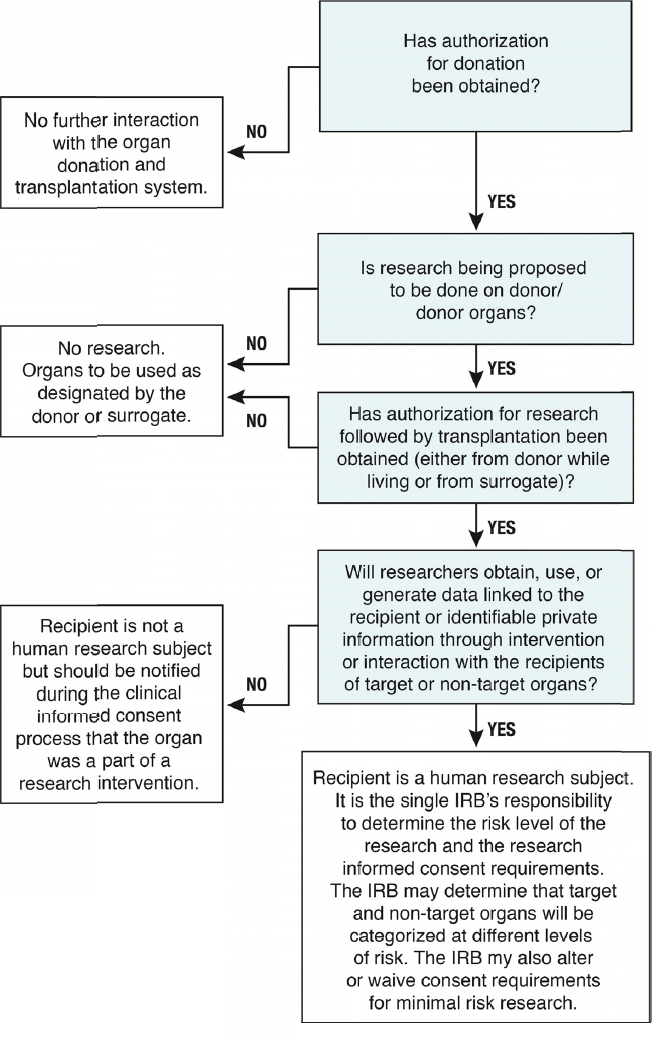
NOTE: IRB = institutional review board.
Resources & Services Administration, advocacy organizations, and professional associations involved in educating the general public and obtaining individual and surrogate authorization should explore, develop, and test communication strategies and materials that explain organ donor intervention research and should implement and disseminate those resources for which effective messaging has been identified. Information resources to be developed include
- Template language to be used by all U.S. organ donor registries (e.g., departments of motor vehicles [DMVs], national registry) to ensure consistency across registries in the language used to obtain authorization for organ donation. This language should explain organ donation options in language that takes into account the wide range of degrees of health literacy among the public.
- Templates for DMVs, OPOs, and other entities that advocate for organ/tissue donation to use for communicating a consistent set of facts about organ donor intervention research across websites and other dissemination methods.
- Standardized talking points for communicating with donor surrogates and families about organ donor intervention research. These should include, at a minimum, information about donation, transplantation, and research in language that takes into account the wide range of degrees of health literacy among the public.
GOAL 2: Improve the coordination and sharing of information about donor preferences.
RECOMMENDATION 2: All active donor registries in the United States should coordinate in order to ensure a single, unified secure national donor registry that is easily accessible to organ procurement organizations. All donor registry information collected by departments of motor vehicles should automatically feed into this single national registry. Model state legislation should be developed to facilitate this merger.
GOAL 3: Clarify legal guidance on organ donation for the purpose of research followed by transplantation (organ donor intervention research).
RECOMMENDATION 3: The National Conference of Commissioners on Uniform State Laws should explore revisions to the Uniform Anatomical Gift Act (UAGA) that would clarify the authorization of organ donation for the purpose of research followed by transplanta-
tion. The following possible clarifications to the UAGA should be considered:
- When a decedent has stated a general intent to make an anatomical gift, without further specification, research followed by transplantation is permitted.
- Organ procurement organizations should be explicitly empowered to seek from a donor’s surrogate the expansion of the authorization for an existing gift for any purpose to be used for research followed by transplantation.
The committee also considered two options for resolving the ambiguities in the UAGA and state laws, but sensitive to trust and transparency felt this issue requires more public consultation. Therefore, the committee recommends that the Organ Procurement and Transplantation Network and transplant community should engage in public consultation and determine whether to amend the UAGA and state laws to
- Specify that when the decedent has authorized transplantation this denotes that the gift is authorized for research followed by transplantation, or
- Specify research followed by transplantation as an additional purpose of donation that would be added to the list of choices for the donor.
GOAL 4: Promote informed consent for transplant recipients’ participation in organ donor intervention research in a manner that is compatible with the logistical complexities of organ transplantation.
RECOMMENDATION 4: Transplant centers and organ procurement organizations, in collaboration with the Organ Procurement and Transplantation Network/United Network for Organ Sharing, professional associations, and patient advocacy organizations should develop and implement a protocol for notifying and educating potential organ transplant recipients about the possibility of being offered an organ that has been exposed to a research intervention and seeking informed consent if they agree to be part of the research study. Specifically,
- At intake and at regular intervals thereafter, all potential recipients should be provided with information about organ donor intervention research and asked whether, at the time of organ offer, they would potentially consider accepting an organ (target organ or non-target organ) that was part of a research study. As a result of
- time constraints at the time of the organ offer for transplantation, only potential recipients who have previously agreed to consider research organs should be approached with the option to accept an available research organ.
- At the time of being offered an organ for transplantation, each transplant candidate who will potentially receive an organ that is part of a research study—be it a target organ or a non-target organ—should be provided with information about the specific research protocol and should follow the single institutional review board’s approved informed consent process for participating in that specific research study (including possible alteration or waiver of informed consent) and accepting the particular research organ offered. Given the importance of minimizing delays, information about the research protocol should be imparted through a process that ensures equitable, effective, and efficient placement and transplantation.
RESEARCH APPROVAL, IMPLEMENTATION, AND OVERSIGHT
According to the committee’s analysis, one major reason for the lag in organ donor intervention research is the lack of central oversight that is needed to overcome the complexities of this geographically and clinically dispersed research. Without a central organization that can coordinate and facilitate cooperative research among a large number of institutions, this promising research is not likely to proceed at the volume, quality, and pace needed. Moreover, oversight and monitoring are needed to ensure adherence to the relevant ethical, legal, and regulatory policies and thus to promote public trust. Several of the unique challenges to conducting organ donor intervention research illustrate the rationale for a more centralized research system:
- Brevity of the timeframe: Time is extremely limited due to concerns about the viability of the organs. Finding the appropriate recipient(s) and making the most of the gift of an organ or organs involves making rapid decisions, which in turn requires clearly defined, well-vetted, and centralized processes and policies.
- Target and non-target organs: Because much of this research is conducted prior to the recovery of the organs from the deceased donor, the intervention may have the potential to affect not only the target organ but also the non-target organs.
- Numerous and geographically dispersed stakeholders: An organ donor managed by 1 of the 58 OPOs in the United States can provide up to eight solid organs, each of which could be transplanted by different transplant programs across the country and allocated via varied distribution schemes. Donor intervention research in-
- volving donors, donor families, OPO staff, transplant staff, and recipients (target and non-target organ) adds another layer of complexity to this already multifaceted and time-driven system.
- Fairness: Donated organs are a scarce and valued national resource. The critical donor organ shortage and the life-and-death nature of organ donation and transplantation require a fair and equitable system for organ allocation. Organ allocation is, for the most part, moving away from a local and regional model toward a national model in which organs can be sent across the country. An oversight system must ensure that research activities do not substantially alter the way in which organs are distributed.
- Consistency: Successful research requires consistency of performance across the multiple institutions and disparate geographic locations. Consistency will be best achieved through centralization of clinical oversight and IRB functions.
- Efficiency: For organ donor intervention research to flourish, mechanisms need to be established to coordinate and facilitate initiation and implementation of multi-center research investigations across a wide geographic area. By reducing the number of parallel and dispersed processes, a centralized oversight approach diminishes major administrative barriers.
Therefore, the committee recommends the use of a centralized oversight framework that consists of three affiliated entities: (1) a centrally administered and standing Donor-Research Oversight Committee (D-ROC); (2) a single IRB for organ donor intervention research; and (3) study-specific data and safety monitoring boards (DSMBs).
The committee envisions the D-ROC as a centrally administered standing committee. As part of its charter, D-ROC should be empowered to work with stakeholders to prioritize, review, implement, and track research protocols as well as to develop and disseminate information about organ donor intervention research. Core responsibilities of D-ROC should include
- Reviewing and prioritizing donor intervention proposals
- Assessing and monitoring the impact of organ donor intervention research on organ allocation and distribution
- Coordinating and facilitating clinical and research informatics and promoting communications
- Promoting effective trial design
- Maintaining liaisons with key external groups
The standard model of local IRB oversight for multi-site studies, in which each research institution must review and approve the research
protocol, is poorly suited to the context in which organ donor intervention studies take place. Because this type of research will likely involve coordination across multiple OPOs, donor hospitals, and transplant centers, it would be necessary to obtain consent from recipients across many sites in a short period of time and to have all potential sites in agreement with the centralized processes.
A single IRB for organ donor intervention research could oversee human research protections and ensure that processes are carried out in accord with relevant regulatory and policy requirements and guidance, particularly the Common Rule. Also, a single IRB would offer the advantages of developing and maintaining core expertise in organ donor intervention research. The committee recognizes that the IRB function could be done by (1) creating an independent central IRB or (2) contracting with an existing IRB that has appropriate scientific, ethical and regulatory expertise. The single IRB may be a free-standing (central) IRB or part of an academic medical center willing to serve as the IRB of record for the multiple sites. The committee believes that D-ROC should have flexibility in determining how to best constitute or contract out the single IRB’s functions.
DSMBs are independent committees that oversee the conduct of clinical trials and serve several broad purposes. First, they review incoming data in order to assure that the risk–benefit ratio of an ongoing trial has not shifted. The DSMB would establish study-stopping criteria based on outcomes for target and non-target organ recipients. The DSMB could determine that the investigation has become unsafe for participants and thus should be terminated early. Second, DSMBs can advise on and evaluate protocol amendments. For example, DSMBs can advise on broadening eligibility criteria in order to access a wider population. Third, DSMBs can evaluate whether patients need to be informed of new developments in a trial. The DSMBs for organ donor intervention research could be organized around a single research study or a set of studies. The key will be for D-ROC to have the administrative capacity to establish DSMBs as they are needed.
GOAL 5: Establish centralized management and oversight of organ donor intervention research in order to ensure equitable, transparent, and high-quality research.
RECOMMENDATION 5: The Organ Procurement and Transplantation Network, in collaboration with the National Institutes of Health, the Health Resources & Services Administration, organ procurement organizations, donor hospitals, transplantation centers and programs, professional associations, patient advocacy organizations, community representatives, and other relevant organizations, should establish and sustain a standing Donor-Research Oversight Committee (D-ROC)
to guide, coordinate, evaluate, prioritize, and disseminate research on deceased organ donor interventions. D-ROC should include the administrative structure to establish independent data safety monitoring boards to ensure the scientific integrity of organ donor intervention research and assess its risks and benefits as studies progress. A single institutional review board should be established or contracted with to ensure human subject research protections for donor intervention research studies.
GOAL 6: Promote transparency regarding organ donor intervention research and enable the implementation, tracking, and analysis of organ donor intervention research to improve transplantation outcomes.
RECOMMENDATION 6: The Donor-Research Oversight Committee, in collaboration with the Organ Procurement and Transplantation Network, the National Institutes of Health, the Health Resources & Services Administration, professional associations, organ procurement organizations, patient advocacy organizations, and transplant centers and programs should create organ donor intervention research electronic tools to ensure that organ donor intervention studies are listed on a publicly available website, that clinicians have the information to provide to potential recipients, that researchers can conduct studies effectively, that research outcomes are tracked and monitored appropriately, and that research outcomes are widely available in aggregate. These tools could use or link to new or current relevant databases but should, at the minimum, provide the following functions:
- Access to real-time study information used to maintain study continuity and monitor key elements of active studies necessary for project management;
- Additional data fields in UNet and other relevant databases to allow for the designation of the organ as a research organ and to note other relevant information about the research protocol for clinical use and in the tracking of research outcomes;
- An online registry of pending, approved, active, closed, and discontinued organ donor intervention research studies; and
- Links to research outcome data, abstracts, and scientific publications.
















