

































Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
11 A P P E N D I X A This appendix provides a complete technical description of the test, along with experimental results from validation testing. Introduction and Generalized Procedure This section of Appendix A introduces the test and pro- vides a concise, generalized protocol for it. For specifics and details of the test as developed and practiced for the HM-14 project, refer to subsequent sections of this report appendix. Purpose of the Test The test procedure given here is intended for use in assess- ing the relative hazards of substances that emit flammable or toxic gases on contact with water by measuring the rate at which the substance produces gas when combined with water. Apparatus A block diagram for an apparatus recommended for this test is shown in Figure A-1. Alternative apparatus may be used. However, regardless of the apparatus used, it should meet the performance criteria outlined here. The apparatus should 1. Be gas tight and capable of safely withstanding internal pressures of at least 50 kPa gauge. 2. Allow for the safe combination of water with a water- reactive substance; provisions for this must include, but are not limited to, pressure relief of the apparatus at a pressure above 50 kPa gauge yet safely below a pressure at which the vessel might rupture. There should also be appropriate personal protective equipment for laboratory personnel and an appropriate laboratory workspace to house the apparatus including fume hoods, proper hazard communication procedures for laboratory personnel, and supervision and operation by qualified personnel. 3. Accommodate addition of the substance to water as well as, when required, the reverse order of addition. 4. Be capable of use with both solid and liquid substances. 5. Include accurate and precise monitoring of pressure as a function of time during testing, preferably using elec- tronic data logging at intervals as short as 2 seconds, with a pressure resolution greater than 0.1 kPa. 6. Accommodate calibration of the response of pressure to the volume of gas added to or produced within the apparatus to provide for conversion from observed pressure increases with the apparatus to volume of (as measured at ambient conditions of temperature and pressure) gas added to or produced within the apparatus (this may, for instance, be accomplished via the addition of known aliquots of gas at ambient pressure). 7. Allow, when the reactivity of the test substance warrants, for testing to occur under an inert atmosphere. Generalized Procedure The test method can be applied to solid and liquid sub- stances. In case a pyrophoric or air sensitive substance is tested, the test should be conducted under an inert atmosphere. When solids are being evaluated, the substance should be inspected for any particles of less than 500 µm diameter. If that powder constitutes more than 1% (mass) of the total, or if the substance is friable, then the whole of the sample should be ground to a powder before testing. Charge the apparatus with water. Water should be ISO 3696 (1987) grade 2 or better; in cases where it is judged likely that saltwater (3.5% USP grade NaCl in ISO 3696 (1987) grade 2 or better water) will result in a greater rate of gas production, then saltwater should be used. The mass of water should be measured, and the total volume of water should not occupy more than ~ 2.5% of the internal volume of the apparatus. Full Technical Test Description
12 For instance, 10.0 g (10.0 ml) could be used in an apparatus with internal volume of 400 ml. Note that if the flammable or toxic gas produced on contact with water is known to have appreciable solubility in water, the amount of water should be reduced to ~0.5% of the internal volume of the apparatus. If the substance under test is a solid, add it to the appara- tus, but in a way that does not yet put it in contact with the liquid water in the apparatus. If the substance under test is a liquid, proceed directly to the next paragraph. Use an amount of test substance chosen so that complete reaction with water, via the reaction expected according to established chemical knowledge or else established in separate testing, would cre- ate an amount of gas with a volume of ~ 1â3 of the internal volume of the apparatus. Close the apparatus and check, or, if not previously mea- sured, measure the apparatus P/V calibration (see item [6] under the apparatus specification). Equilibrate the apparatus pressure with ambient pressure. Combine the test substance with the water in the appara- tus. In the case of liquid substances, this can be accomplished by adding the test substance directly to the apparatus in a manner that brings it into immediate contact with the water, with good mixing, while maintaining the gas-tight integrity of the apparatus. For a solid substance, operate the appara- tus so that the test substance that was inserted as described above is rapidly mixed with the water while maintaining the gas-tight integrity of the apparatus. In the case of liquid sub- stances, use an amount of test substance chosen so that com- plete reaction with water, according to the reaction expected according to established chemical knowledge, or else estab- lished in separate testing, would create an amount of gas with a volume of ~ 1â3 of the internal volume of the apparatus. Monitor the pressure (and, therefore, volume of gas pro- duced) within the apparatus as a function of time (see item [2] under the apparatus specification). Continue monitoring until a steady state is observed. If the change in pressure is too low for accurate measure- ment, repeat the test using a carefully increased amount of substance; exercise care to not exceed the apparatus capac- ity. If necessary, continue to repeat the test using carefully increased amounts of substance, until a readily and accurately measurable response is observed. An ideal result would be an increase in pressure of from 10 to 25 kPa. Once a satisfactory response is achieved, conduct 4 additional replicate runs to obtain a total of 5 measurements. For each of the 5 measurements made, find the period of time during the reaction that shows the greatest rate of pressure increase (gas production). This may be as short as the inter- val between consecutive data points (2 seconds) or a few data points. In this case, convert the observed change in pressure to a net change in volume; this divided by the elapsed time consti- tutes the raw gas production rate (volume/time; e.g., liters/min or liters/hour). Alternatively, it may be a longer duration over which a nearly linear increase in pressure with time occurs. In this case, convert the rate of pressure increase represented by the slope of a line fitted to the data in that period of time to a rate of gas production, which then constitutes the raw gas production rate (volume/time; e.g., liters/min or liters/hour). Normalize the raw gas production rate for each measure- ment to the amount of substance used, to obtain a specific gas evolution rate (volume/(timeî°mass); e.g., liters/kg-min or liters/kg-hour). Combine the 5 specific gas evolution rate measurements to obtain an average of the observed specific gas evolution rates, and the sample standard deviation. These form the nominal specific gas evolution rate and precision estimate. Particularly in the case of liquid test substances, consider whether the observed gas production is due to reaction with water or to evaporation of the material or substance under test. This should be assessed by a qualified chemist, taking into con- sideration the observed yield of gas vs. the yield expected (low yields of gas may reflect evaporation) and the magnitude of the change in pressure (small changes, comparable to the vapor pressure of the test substance, may reflect evaporation). If the observed gas production may credibly be due simply to evapo- ration of the test substance, then the measurement should be repeated using a reversed order of addition. For all substances, the possibility that gas evolution has been masked (fully or to some extent) by absorption of gas by excess water in the appa- ratus should also be considered. If this credibly may be occur- ring, then the measurement should be repeated using a reversed order of addition. If a low amount of gas is formed relative to the quantity expected, absorption by water may be an issue. For the reversed order of addition, an amount of water chosen such that complete reaction via the reaction expected according to established chemical knowledge or else estab- lished in separate testing, would create an amount of gas with Figure A-1. Block diagram of the recommended apparatus.
13 a volume of ~ 1â3 of the internal volume of the apparatus should be added to an excess of the substance under test. The amount of substance under test should not occupy more than 2.5% of the internal volume of the apparatus, and often an amount in the range of 0.5% to 1% of the internal volume of the appa- ratus will be sufficient. When normalizing the raw gas produc- tion rate to a specific production rate, use an amount of test substance in the denominator that corresponds to the amount of test substance predicted by chemical knowledge to be con- sumed in a complete reaction with the amount of water used. Generally, a comparison of the specific gas evolution rates and overall gas yields from the two orders of addition will make it clear which observation best represents gas production from the reaction between the test substance and water. For instance: 1. If the addition of the substance to water yields an increase in pressure similar to what would be expected solely from the vapor pressure of the substance and the amount of gas produced is low in comparison to what is expected, while the addition of water to the substance produces an amount of gas close to what is expected with a pressure increase greater than the vapor pressure of water, then the latter result should be used. 2. If the addition of the substance to water yields an increase in pressure in excess of what would be expected solely from the vapor pressure of the substance, the amount of gas produced is similar to what is expected, and the specific rate of gas production is greater than for the addition of water to sub- stance, then the former result should form the basis of clas- sification in Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria, 5th revised edition. 3. For intermediate cases, the judgment of a qualified, inde- pendent chemist should be used to determine which result best represents the rate of gas production from reaction with water. Precautions Important Safety Point This test is intended to measure the evolution of gas when reac- tive materials are combined with water. Some of these materials may react violently with water, and many may need to be han- dled under dry, inert atmosphere prior to their careful reaction with water in order to preserve their integrity and to preclude the possibility of hazardous reactions. Furthermore, some of these materials may (by definition) produce toxic gases when combined with water. Important Safety Point These materials should be han- dled by trained, qualified per- sonnel with experience in handling water reactive and/or air-sensitive materials using appropriate laboratory facilities and proper personal protective equipment (PPE). Laboratory facilities should include properly designed and operating fume hoods in addition to other facilities and equipment that may be required. Typical PPE will include flame-retardant labo- ratory coats (preferably using intrinsically flame-retardant materials, such as Nomex® fabric), safety glasses, face shields, chemically resistant gloves, and other equipment as needed. There are several texts and other resources on the topics of laboratory safety and the handing of air-sensitive materials that should be consulted prior to work.1 Important Safety Point This test should be carried out under the supervision of a quali- fied, experienced chemist who is thoroughly familiar with the materials being handled, their reactivity, and water and air sensitive materials in general. Finally, this report appendix should be read in its entirety prior to attempting any testing. Example Test Apparatus A block diagram of an experimental apparatus meeting the requirements set forth in the generalized proposed test proce- dure above is shown in Figure A-2. The reaction flask is a heavy walled glass reactor with a total internal volume of ~ 400â450 cc. A typical vendor dia- gram of such a flask is shown in Figure A-3. 1 Information provided here comprises suggestions; personnel on hand, carrying out the experiments, are responsible for taking all needed pre- cautions. Resources on air-free and air-sensitive techniques include: (a) CRC Handbook of Laboratory Safety, 5th Ed. Furr, A. K. (2000). (b) Handbook of Chemical Health and Safety, Alaimo, R. J. Ed. (2001). (c) Identifying and Evaluating Hazards in Research Laboratories, American Chemical Society (2013); available online (as of 2013.09.30) at: http://cen.acs.org/content/dam/cen/static/pdfs/ ACSHazardAnalysis20130904.pdf. (d) University of California online Laboratory Safety Fundamentals training program, available (as of 2013.09.30) at: http://info. ucanr.org/safety/lab/story.html. (e) The Manipulation of Air-Sensitive Compounds, 2nd Edition; Duward F. Shriver; M. A. Drezdzon (1986). (f) Synthesis of Organometallic Compounds: A Practical Guide Komiya, S. (1997). (g) Sigma-Aldrich Technical Bulletin AL-134 Handling Air-Sensitive Reagents. Available online (as of 2013.09.30) at http://www.sig maaldrich.com/etc/medialib/docs/Aldrich/Bulletin/al_techbull_ al134.Par.0001.File.tmp/al_techbull_al134.pdf . (h) Guidelines for Handling Air-Sensitive Compounds; Gill, GB; Whiting, DA, Aldrichimica Acta, 1986, 19(2), 31â41. Available online (as of 2013.09.30) at: http://wolfweb.unr.edu/~wchalifoux/ PDFs/handlingairsensitivereagents.pdf. (i) Safe Laboratory Practices: Working with Air-Sensitive or Highly Reactive Compounds, Stanford University, 2/13/09, rev 10/15/10â OHS Report#:09-016a, available online (as of 2013.09.30) at: http://www.stanford.edu/dept/EHS/prod/documents/09-016.pdf. (j) Aluminum AlkylsâSafe Handling, Heck, WB; Johnson, RL Ind. Eng. Chem., 1962, 54 (12), pp. 35â38, DOI: 10.1021/ie50636a007.
14 Assembled, the apparatus appears as shown in Figures A-4, A-5, and A-6. The pictured apparatus, along with a selection of gas- tight and disposable syringes, a laboratory balance, a nitrogen purged glove-bag (or, alternatively, a glove-box), a properly installed and functioning fume hood, access to reagents and distilled or deionized water, and an appropriate selection of PPE are all that is required for this test. Detailed Procedure Overview The flow chart shown in Figure A-7 provides an overview of the procedure. Subsequent text and flow charts provide expla- nation and additional information. âWaterâ refers to distilled or de-ionized (DI) water, unless otherwise noted (e.g., in tests that intentionally use saltwater containing 3.5% NaCl by weight). The first issue to consider is whether the identity of gases that might be evolved is known. Generally, this should be the case. Triethylaluminum, for instance (UN 3394; Organo- metallic substance, liquid, pyrophoric, water-reactive; Divi- sion 4.2), is known to produce ethane (or, ethylene, if excess heating occurs) gas on contact with water. Substances like this that produce gases, such as hydrogen or hydrocarbons, with limited solubility in water can usually be tested using Approach (A) (see Figure A-7), with excess water present and without undue concern for absorption of gas into the water influencing the result. Still, care should be taken to either limit the volume of water present to a modest amount (i.e., < 10 g, or < 2.5% of apparatus volume) or to account for its possible impact on the P/V calibration (by conducting the P/V calibration with water present, see below); this limitation is reflected in the proposed next text for Test N.5. TiCl4 (UN 1838), as another example, is known to produce HCl (g) on contact with water; likewise, anhydrous AlCl3 (UN 1726) can produce HCl (g) in contact with water. HCl (g) Figure A-2. Block diagram of the recommended apparatus. Figure A-3. Vendor drawing of reaction vessel.
15 Figure A-4. Complete reaction apparatus with analog gauge and timer for manual data acquisition (far left), with pressure transducer (center left) as configured for use with liquids, with solids addition tube in place (center right) for use with solids, and with solids addition tube after addition of solids (far right). Figure A-5. Detail of solids addition tube assembly empty (top) and after charging (bottom) with a water reactive (but not air sensitive) solid. Figure A-6. Detail of liquid addition/septum closure fitting with septum installed (not visible). Note that this is a friction fit closure of the vessel.
16 is very soluble in water and, as a result, the presence of excess water in the apparatus can mask the formation of gas. For this reason, extra care is needed in work with this type of material. Fortunately, experience shows that in many cases these materials can still be tested using Approach (A), adding the water-reactive materials to water. In this case, however, the amount of water should be kept to a practical minimum. In some cases, however, work with Approach (A) may make it apparent that it may be desirable to use Approach (B) (see Figure A-7), in which limiting amounts of water are added to the water-reactive materials under test. This approach is discussed further, below. In all cases, in addition to knowing the identity and prop- erties of gases that may form, it will also be necessary to have at least some idea of the expected amount of gas likely to form. If a low amount of gas is formed relative to the quantity expected, absorption by water may be an issue. With that information in mind, it is possible to turn to the specifics of the testing by either approach, in the sections that follow. Approach (A) Approach (A) can be used when absorption of evolved gas by water either will not be an issue or is a manageable issue (as demonstrated by the test). When absorption of evolved gas by the water present becomes a problem, Approach (B) should be used. Note that, particularly when the test material or substance is relatively volatile, Approach (A) may appear to be measuring gas evolution when, in fact, the gas being mea- sured is simply vapor from evaporation of the test material or substance. Personnel conducting the test should be alert to this possibility; if this is the case, Approach (B) should be used. Specifics of Approach (A) will vary slightly, depending upon whether a solid or a liquid material/substance is being tested (see Figure A-8). As noted in Figure A-8, assemble the clean and dry appara- tus components (as shown in Figures A-2 and A-4) using either a septum closure or a solids addition tube in the center open- ing of the flask and either a threaded plug or threaded septum adapter in one of the threaded ports (depending upon the type of material under test). When using the solids addition tube, calibration should first be conducted with the empty tube in place, after which the tube should be charged with the required amount of water-reactive material/water-reactive substance. Note that in Figure A-8, within the right-hand branch, where the apparatus is charged with both water and water-reactive material/water-reactive substance, they are kept separate; the water-reactive material/water-reactive substance is kept within the solids addition tube, and the water is charged to Figure A-7. Flow chart for testing.
17 the reaction flask. Further, note that in the case of air sensitive and/or highly reactive materials, the solids addition tube may need to be charged under an inert atmosphere. This can be done in a glove box, if available (and necessary), or in a simple nitrogen purged glove bag, with the choice being made by a competent chemist familiar with the materials in question and the safe handling of air-sensitive materials. The weight of the charged solids addition apparatus should be recorded prior to installing it into the apparatus. At the end of the reaction, the empty apparatus will be re-weighed to determine the actual amount combined with water in the test. The P/V calibration is an important step. This is easily done by using a volume-calibrated syringe2 to add known volumes of gas under laboratory conditions. The gas should be chosen to match that in use for the experiment (i.e., air or an inert gas such as nitrogen). On commissioning, each flask and associated fittings, assembled as for use, should be characterized by sequential addition of 4 aliquots of 50 cc of gas at ambient conditions. This is readily accomplished with a syringe and via the septum fittings. Figures A-9 and A-10 show a typical calibration exercise. Note that the result of 4.27 cc/kPa suggests a total inter- nal volume for the apparatus (with that particular flask and configuration) of ~ 430 cc, as an internal pressure of 101 kPa gauge (1 atm gauge) would indicate that a volume of gas equal to the internal volume of the apparatus had been added. As indicated in Figure A-8, a check of the P/V calibration should be included with each test run. This can be done with the addition of just two aliquots of gas (as illustrated in Figure A-10). Important Safety Point At this point, it is worth noting that the apparatus shown in Fig- ures A-2 and A-4 is intended to contain gas as it is produced and therefore will become pressurized (as shown, for instance, in Figure A-9) during testing. For this reason, estimating the maximum amount of gas that can be produced and scaling of the reaction so that it is within the capacity of the apparatus are part of the protocol (see below). Figure A-9 illustrates that pressures of at least 40â50 kPa gauge can be tolerated by the apparatus, corresponding to produced volumes of gas of ~ 200 cc. Pres- sure relief for the apparatus is provided by the friction fit of the septum closure (see Figure A-6) or the friction fit of the solids addition tube (see Figure A-5). If the pressure exceeds ~ 50 kPa, this friction fit closure will yield, venting the apparatus. Experi- ence shows that the closure will vent in the 40â50 kPa gauge pressure range and at somewhat lower pressures if left stand- ing for long periods or not firmly installed. Venting is not vio- lent and occurs with a soft (but definite) âpopâ as the closure is expelled. The closure is expelled with enough force to reach the hood ceiling, but the rebound is gentle and is comparable to dropping the closure from a height of a few feet. Users com- missioning new apparatus should verify both tolerance of the planned pressures and successful relief performance. Although the research teamâs experience has been good, and the few occasions where venting occurred posed no prob- lems, a portable blast shield is employed for initial testing of a new material, until its behavior is well established. Dur- ing all testing, and particularly during initial screening, all appropriate PPE (such as, but not limited to, lab coats, face shields, and gloves) is employed. 2 It is easy to calibrate or verify the volume graduations on a syringe by using DI water, a NIST (or equivalent) traceably calibrated balance, and a NIST (or equivalent) traceably calibrated thermometer, along with stan- dard reference values for the density of water. In our hands, we found that the existing graduations on commercial syringes were accurate to ± 1%. Figure A-8. Flow chart for Approach (A), adding solid or liquid water reactive material/substance to water. Note that, for amounts of water in excess of 10 g, water should be added prior to the P/V calibration check, so that the calibration reflects the free gas-volume in the apparatus, with water present.
18 Figure A-9. P/V calibration run. Note that the small drop in pressure on addition of the 2nd and subsequent aliquots is believed to be due to equilibration of the (slightly) pressurized vessel with the ambient pressure volume in the syringe. Figure A-10. Example of a P/V calibration curve for an apparatus as shown in Figure A-4 (center left). The open circles (4 total, slope of fitted line îµ 4.444 cc/kPa) represent the series of 4 additions of 50.0 cc of nitrogen gas to an empty apparatus, from the initial commissioning of this apparatus. The crosses (2 total, slope of 4.435 cc/kPa) reflect measurements made immediately prior to a test run, at a later date.
19 Important Safety Point Particular care to choose and use appropriate equipment and PPE should be taken during handling and dispensing of the water- reactive materials or water-reactive substances. The most reac- tive materials will require the greatest care and precautions (see Note 1, p.13). Important Safety Point The amount of water-reactive material/water-reactive substance to use in testing is determined by trial, starting with an amount of water-reactive material/water-reactive substance known (or estimated with confidence by a qualified chemist) to produce at most an amount of gas within the capacity of the apparatus (i.e., ⤠1â3 of the internal volume, ⤠140 cc in the case of the 430 cc apparatus used in Figures A-8 and A-9). See Figure A-10.3 Once the apparatus is calibrated and/or closed, water can be added, either via the septum closure or through the unused threaded opening. After that, the vessel should be vented to equilibrate the internal and external pressure. Water is nor- mally added via syringe, all at once, and the amount delivered is determined by weighing and difference. For liquid water reactive materials/substances, the substance is added from a pre-weighed syringe all at once via the septum closure, and the amount added is determined by weighing and difference. For solids, the solids addition tube is simply tipped up and the material added all at once. Again, the actual amount of water-reactive material/water-reactive substance delivered is determined by weighing before and after addition and using the difference as the mass. For both solids and liquids, trials are conducted iteratively (see Figure A-11), starting with amounts known to be within the capacity of the apparatus and increas- ing until convenient, measurable, and reproducible results are obtained. Pressure and temperature are monitored and recorded as the reaction occurs. The course of trials and replicate runs should proceed as shown in Figure A-11.3 Calculate expected volumes at 21.1 °C and 101.56 kPa. Figure A-11. Flow chart illustrating the process of conducting iterative runs to establish the amounts needed to obtain satisfactory results.
20 Approach (B) Approach (B) (see Figure A-7) is generally only used with liquid water reactive materials/substances, though solids can be tested if necessary. It should be used when experience with Approach (A) shows that the amount of water present must be severely limited to avoid absorption of the gas produced in a reaction with water by excess water that remains in the reaction vessel. Approach (B) should also be employed when there is evidence that the gas observed in Approach (A) has more to do with evaporation of the test material or substance than its reaction with water. The overall approach is similar to that in Approach (A), but the order of addition is reversed. Also, the water-reactive material/water-reactive substance is present in excess, and water is the limiting reagent. A suitable amount of water- reactive material/water-reactive substance is charged to the apparatus for testing (2â10 g is typical); for liquids, enough must be used to permit evaporation to saturate the headspace while leaving some liquid within the reaction vessel. Also note that for substances with a significant vapor pressure, it may be necessary to (carefully and momentarily) vent the pressure to allow some of the initially present atmosphere to escape so that the equilibrium pressure is not too far above ambient. The amount of water is chosen to be such that results are within the capacity of the apparatus. Except for consider- ations of whether the reaction stoichiometry will be 1, 2, or (occasionally) 3 moles of gas per mole of water, the amounts here can be determined independently of the water-reactive material/water-reactive substance under test. In a 1:1 reac- tion, 100 mg of water yields 133 cc gas, and in a 1:2 reaction, 50 mg yields the same amount.4 Validation Test Results The examples included in this section illustrate the appli- cation of Approaches (A) and (B) within the scope of the new proposed test for N.5 and provide guidance (by way of example) on implementation of the new proposed test N.5. Example 1âDimethyldichlorosilane (UN 1162) Dimethyldichlorosilane ([CH3]2SiCl2, Aldrich, > 98.5%, UN 1162) was subjected to testing. This is an example of a liquid material likely to be a water-reactive material. CH SiCl l H O l Si CH O 2 HCl g 1 3 2 2 2 3 2 n ( )( )( ) ( ) ( ) ( ) ( ) + â â â + Equation (1) indicates that the potential for gas evolution from (CH3)2SiCl2 is ~ 378 l/kg(CH3)2SiCl2 after complete reaction at normal ambient conditions. This suggests that (CH3)2SiCl2 is a liquid water reactive material that produces a water sol- uble gas. The process shown in Figure A-7 suggests that this material should be tested according to Approach (A), using the minimum required water. Given the gas evolution poten- tial for (CH3)2SiCl2 and considering the process shown in Fig- ure A-11, preliminary experiments should be limited to ~ 0.3 g ([CH3]2SiCl2 maximum expected gas production, ~ 112 cc). A reaction apparatus as shown in Figure A-4, center left, configured for liquid addition was assembled, the P/V cali- bration checked (as shown in Figure A-10, data indicated with dash-dot pattern), and a trial run with 0.3230 g (CH3)2SiCl2 and 2.0585 g water. The results are shown in Figure A-12. Experience (see other validation testing results) shows that the results of Figure A-12 may be due to evaporation of the (CH3)2SiCl2 rather than gas production and/or that any gas being produced is being absorbed by the water. Indica- tors of this include the following: (a) the observed pressure increase when the (CH3)2SiCl2 was added (~ 1.3 kPa) cor- responds to only a small amount of gas evolution (~ 6 cc), and (b) it is a much smaller increase in pressure than would be expected even from just the vapor pressure of (CH3)2SiCl2 (b.p. 70 °C). The behavior shown in Figure A-12 was observed in additional experiments, with 0.3â0.5 g (CH3)2SiCl2 and 1â2 g water. While a rate could be calculated from the small increase in pressure (e.g., 300â700 l/kg-min observed over 4 tests), it seemed clear that absorption of gas by the water present in the reaction was occurring on a timescale similar to, or faster than, gas evolution (if it was occurring). Because this indicated that there were issues related to the solubility of the evolved gas (HCl) in water (see Figure A-7), tests with Approach (B), adding water to (CH3)2SiCl2, were conducted. Figure A-13 shows the results. Figure A-13 confirmed that the expected increase in pres- sure from evaporation of (CH3)2SiCl2 into the headspace would be larger than that observed on addition of (CH3)2SiCl2 in Figure A-12; further, very nearly the theoretical amount of gas evolution (i.e., 90% of theory) was observed when water was added. This supports the contention that gas evolution in Figure A-12 was occurring, but was masked by the absorp- tion of the gas by the water present. Fitting a line to the early, steepest part of the gas evolution curve in Figure A-13 (after correction for the small observed changes in temperature), yielded an initial raw gas evolution rate of 9472 kPa/day. Of the 2.15 g (CH3)2SiCl2 present, complete reaction would con- sume ~ 0.72 g, according to Eq. (1). Normalizing the raw gas evolution rate to that amount yields a specific gas evolution rate of 42 l/kg-min. Additional experiments were conducted, adding water to (CH3)2SiCl2, producing the results shown in Table A-1.4 Calculated for 21.1 °C and 101.56 kPa.
21 Figure A-12. Pressure vs. time for the pre-run apparatus calibration, water addition, and reaction of 0.3230 g (CH3)2SiCl2 with 2.0585 g water. The open circles show the observed pressure, and crosses show the observed temperature, vs. time as the reaction proceeds. Calibration occurred during the 1st 3 minutes of the experiment, with water addition shortly after that. The reaction was vented after water vapor pressure equilibration and the (CH3)2SiCl2 added after ~ 7 minutes. The âbumpâ at ~ 7:30 corresponds to the addition of (CH3)2SiCl2. Figure A-13. Pressure vs. time for the apparatus calibration, (CH3)2SiCl2 addition, and reaction of 2.1492 g (CH3)2SiCl2 with 0.0762 g water. (CH3)2SiCl2 addition occurred prior to 7:00, and the reactor was vented several times as the vapor pressure of (CH3)2SiCl2 equilibrated in the reactor headspace. Water addition was at ~ 25:00. The net increase in pressure (total) after water addition was ~ 40 kPa, corresponding to ~ 184 cc (~ 90% of theory). Mass DMDCS Mass Water Calibration Rate Rate Net gas observed Water/ DMDCS g g cc/kPa kPa/Day l/kg-min (% theory) mole/mole 2.1492 0.0762 4.61 9472 42 97 0.3 2.1710 0.0866 4.47 10203 39 89 0.4 2.1776 0.1357 4.31 11370 27 67* 0.6 1.3428 0.0728 4.72 9672 46 92 0.5 1.3282 0.1013 4.59 9612 32 77* 0.7 1.3933 0.1357 4.42 10208 25 34* 0.9 0.7291 0.0563 4.75 6295 39 73 0.7 *Reaction stopped early, as the pressure increase approached the relief pressure of the apparatus (or, in one case, vented). Table A-1. Summary of (CH3)2SiCl2 results.
22 The average of these results is 36 l/kg-min, with a relative standard deviation of 22% for the sample. Normally it would not be necessary to explore the impact of changes in the ratio of water to test material or substance (though it may often be useful to do this), but in this case that was also explored. In this case, it appears that the specific gas production rate does depend on the water/(CH3)2SiCl2, falling as that ratio increases (though, the raw rate increases). These effects are easily rationalized; as the water increases, the raw rate increases from the greater available water, but the specific rate falls because the amount of (CH3)2SiCl2 required for reaction (i.e., the denominator in the specific rate calcula- tion) also increases. If the raw rate increases, proportionally, less than the increase in the amount of water used, then the specific rate will fall. Example 2âSodium Amide (UN 1390) Sodium amide (NaNH2, Aldrich, 95%, UN 1390) was sub- jected to testing. NaNH2 is known5 to react violently with water to release ammonia. NaNH s H O l NaOH aq NH g 22 2 3 ( )( )( ) ( ) ( )+ â + Equation (2) indicates that the potential for gas evolution from NaNH2 is ~ 620 l/kgNaNH2 after complete reaction at nor- mal ambient conditions. This suggests that NaNH2 is a solid water reactive material that produces a water soluble gas. As the process shown in Figure A-7 suggests, this material should be tested according to Approach (A), using the minimum required water. Preliminary experiments should be limited to ~ 0.2 g NaNH2 (maximum expected gas production, ~ 125 cc). A reaction apparatus as shown in Figure A-4, center-right and far right, configured for solid addition was assembled, the P/V calibration checked (as in Figure A-13, data indicated with circles), and a trial run conducted with 0.1996 g NaNH2 (final weight determined by difference, after completion of the reaction) and 2.0747 g water. In this case (in contrast to Example 1), rapid initial evolu- tion of a substantial amount of gas (obviously in excess of any vapor pressure from NaNH2 solid) was observed (Fig- ure A-14). While some gas was subsequently absorbed, the initial evolution of gas appears to be substantially faster than the rate at which the gas is absorbed. As shown in Table A-2 and Figure A-15, similar rates and behavior were observed for a series of experiments with this material, so Approach (A) was judged to be suitable. Water was always present in excess (Approach [A]), and, while the water/NaNH2 ratio varied somewhat in testing (because of modest changes in the masses used from run to run), there is no obvious correlation of observed rate with the water/NaNH2 ratio. Consequently, all 5 results can be pooled, yielding a result of 9600 l/kg-min with a sample relative stan- dard deviation of 20%. Example 3âSodium Borohydride (UN 1426) Sodium borohydride (SBH, Sigma-Aldrich, ⥠98%, UN 1426) was subjected to testing. In principle, Sodium borohydride can Figure A-14. Pressure vs. time for the reaction of 0.1996 g NaNH2 with 2.0747 g water. Reaction is complete within 6 seconds, after which some absorption of the ammonia by the water present in the reaction occurs. The rate, based on gas formed after 2 seconds, is 12,000 l/kgNaNH2-min. As sometimes happens with reactions of (reactive) solids with water, the reaction is somewhat irregular. In this case, unlike reactions where HCl (g) forms, gas is reabsorbed, but not all of it, as the solubility of NH3 (g) in basic solution is lower than that of HCl (g) in neutral or basic solutions. 5 Cheremisinoff, N. P. Handbook of Industrial Toxicology and Hazardous Materials, CRC Press, 1999, p. 366.
23 react with water to form 4 moles of hydrogen, and it is already classified as a Division 4.3 WRS, with PG 1 assigned: NaBH s 4H O l NaB OH 4H g 34 2 4 2 ( )( ) ( ) ( ) ( )+ â + Since the hydrogen produced has low solubility in water, the left side of Figure A-7 applies, and Approach (A) was used. Equation (3) indicates that Sodium borohydride would release ~2550 l/kgSBH of hydrogen gas after complete reac- tion at normal ambient conditions. This suggests that initial experiments should use ~ 0.050 g of Sodium borohydride (expected yield, ~125 cc). An initial reaction with 0.0302 g Sodium borohydride and 2.0448 g water showed a very mild reaction, with ~ 20â25 cc of gas formed over 90 minutes (~ 33% yield of hydrogen), so the reaction was scaled up. Reaction between 0.4541 g Sodium borohydride and 3.9748 g water is shown in Figure A-16. Subsequently, the reaction was further scaled up to ~ 1 g Sodium borohydride /8 g water and ~ 2 g Sodium borohydride / 16 g water. These results are summarized in Table A-3 and Figure A-17. Here, as in Example 2 and despite careful work, significant standard deviations remain in the experimental results. The ori- gin of this is unclear, but presumably it is due to an underlying variability in the reactionâpossibly because of a strong depen- dence on subtle aspects of the experiment (e.g., details of mix- ing of solid and water). The results were obtained by the same worker, using the same set of apparatus, in the same laboratory, with multiple tests run in a single day. Also, the P/V response of the apparatus was calibrated prior to each run by the addition Table A-2. Summary of NaNH2 results. Mass NaNH2 Mass Water âP (Fastest) âV Rate Water/NaNH2 G g kPa cc l/kg-min mole/mole 0.2929 2.129 16.97 82.3 8430 15.7 0.1996 2.0747 17.13 79.8 11998 22.5 0.223 2.1240 14.70 65.7 8840 20.6 0.2141 2.0513 16.78 80.2 11239 20.7 0.1954 2.1305 10.42 48.0 7375 23.6 Average rate = 9600(2000), 20% relative standard deviation (RSD). Figure A-15. Results from runs of the reaction of NaNH2 with water at room temperature.
24 Mass SBH Mass Water Calibration Rate Rate Water/ SBH g g cc/kPa kPa/Day l/kg-hr mole/mole 0.4541 3.9748 4.65 173.7 74.1 18.4 0.9145 8.0338 4.70 498.7 106.8 18.4 0.9873 7.9741 4.70 601.4 119.3 17.0 1.8549 15.9241 4.73 673.4 71.5 18.0 2.0567 16.4623 4.85 1387 136.3 16.8 1.8373 16.6066 4.73 1234 132.4 19.0 2.0461 16.3986 4.53 1587 146.4 16.8 2.1084 16.3060 4.84 1524 145.8 16.2 1.9692 16.2761 4.70 1556 154.7 17.4 Average rate = 120(30), 25% RSD. Table A-3. Results from tests with Sodium borohydride. Rates were based on the steepest slope portion of the observed curves; reactions were typically monitored for 1.5 hours or more. Figure A-16. Results from a reaction between 0.4541 g Sodium borohydride and 3.9748 g water showing the smooth reaction, which achieved a near steady state reaction rate, producing a pressure increase of ~ 173.7 kPa/day in the reaction vessel. Conversion to volume of gas and normalization to the amount of Sodium borohydride used, yielded a specific gas production rate of 74 l/kgSBH-h. of known volumes of gas, as called for in the protocol. It may be that the nature of these reactions intrinsically limits precision. It is tempting to divide the results shown in Figure A-17 and Table A-3 into the two groups apparent in Figure A-17 and segregate them accordingly. However, a close look at the results shows that while most of the higher results came from the tests with ~2 g sodium borohydride, one of those experi- ments also yielded the lowest observed rate. Consequently, the case for segregation cannot be made, and all the results were pooled to yield a result of 120 l/kg-hr with a sample relative standard deviation of 25%. Example 4âAcetyl Chloride (UN 1717) Acetyl chloride (CH3COCl, Fluka, â¥99%, UN 1717) was subjected to testing. Acetyl chloride is well known to react vigorously with water; complete hydrolysis proceeds accord- ing to Equation (4). CH COCl l H O l CH CO H s HCl g 43 2 3 2 ( )( ) ( ) ( ) ( )+ â + This stoichiometry indicates that the potential for gas evolution from acetyl chloride is ~ 310 l/kgAcCl, and that it is a water reactive material that produces a water soluble gas.
25 This would suggest that ~ 0.4 g of acetyl chloride would yield ~ 125 cc of gas. Because this is a water-reactive material likely to produce a gas soluble in water, the right-hand portion of Figure A-7 applies, and a limited amount of water (e.g., 2.0 g) should be used. Note that with 0.4 g acetyl chloride, 2.0 g water provides ~ 20 moles of water for each mole of acetyl chloride. A reaction apparatus as shown in Figure A-4, center-left, configured for liquid addition was assembled, the P/V calibra- tion checked (as in Figure A-10, data indicated with dash-dot pattern), and a trial run conducted with 0.4384 g acetyl chloride and 2.0104 g water. As shown in Figure A-18, the rate observed for the first 6 secondsâthe fastest period of reactionâwas ~ 470 l/kg-min. However, the maximum transient yield of gas was only ~ 25 cc, corresponding to ~ 20% of theory. Replicate runs gave the results shown in Table A-4 and Fig- ure A-19. For acetyl chloride, a rapid initial production of gas was observed even though it was ultimately absorbed by the Figure A-17. Results for the 9 replicate runs for the reaction of sodium borohydride with water at room temperature, showing the observed specific rate of gas evolution as a function of water/ sodium borohydride ratio. The scatter in the x-direction (water/ sodium borohydride ratio) reflects the fact that targeted weights are not precisely achieved. This shows that the observed rate is not strongly dependent on water/ sodium borohydride ratio in this range, so all results can be pooled. Figure A-18. Pressure vs. time for reaction of 0.4384 g acetyl chloride is combined with 2.0104 g water. The fastest reaction is observed over the first 6 seconds after the materials are combined and corresponds to a rate of 468 l/kg-min. The maximum pressure observed corresponds to 25 cc of gas evolution or ~ 19% of the theoretical yield.
26 water. In the case of AlCl3, and TiCl4 (see Examples 5 and 9), the initial gas evolution was very rapid, reaction was obvious, the amount of gas transiently observed was a substantial frac- tion of the expected amount, and the pressure observed was obviously in excess of that likely to be due simply to the vapor pressure of the material under test. In this case, it is less clear whether the observed initial surge of pressure was due to gas production or vapor pres- sure of the acetyl chloride. Consequently, a series of experi- ments were conducted using Approach (B) with ~ 0.050 g of water (chosen because of the theoretical possibility of form- ing two moles of HCl per mole of water, see prior discussion of Approach [B]). This yielded the behavior shown in Fig- ure A-20, which is much more like the behavior expected for this reaction according to chemical principles, and it yields an amount of gas in line with expectations (See Table A-5). Replicates of the test yielded the results shown in Table A-5, with rates measured in the steepest part of the âSâ curve and (as in Example 1) normalized to the amount of acetyl chlo- ride required to react with the water added. The pooled result here appears to be 665 l/kg-min with a relative standard deviation of 9%. Example 4 (Repeat with Saltwater)âAcetyl Chloride (UN 1717) Acetyl chloride was selected for testing with saltwater (3.5% w/w NaCl in DI water) as well as DI water (preced- ing section). This work used Approach (B) and yielded the behavior shown in Figure A-21. Table A-4. Results from tests with acetyl chloride rates are based on the fastest period of gas evolution (e.g., the first several seconds, the actual duration of the fastest gas evolution varied from run to run). Average rate = 620(180), 30% RSD. Mass AcCl Mass Water âP (Fastest) âV Duration Rate Water/AcCl g g kPa cc m l/kg-min mole/mole 0.4384 2.0104 4.45 20.5 0.10 468 20.0 0.8765 2.0406 10.53 49.3 0.07 843 10.1 0.8595 2.0420 14.20 63.6 0.13 555 10.4 0.8621 2.0604 5.22 22.6 0.03 787 10.4 0.8649 2.1218 5.67 26.0 0.07 451 10.7 Figure A-19. Results from trial and replicate runs for the reaction of acetyl chloride with water at room temperature. This shows that the observed rate is not strongly dependent on water/acetyl chloride ratio in this range, so all results can be pooled.
27 Figure A-20. Pressure observed as a function of time when 0.0535 g water is added to 2.2596 g acetyl chloride. Table A-5. Results from tests with acetyl chloride rates are based on the fastest period of gas evolution (e.g., the first several seconds, the actual duration of the fastest gas evolution varied from run to run). Mass AcCl Mass Water âV (Fastest) V (total) V (% of theory) Duration Rate Water/AcCl g g cc cc m l/kg-min mole/mole 2.2172 0.0561 29.5 76.9 103% 0.17 724 0.110 2.2596 0.0535 25.1 67.3 94% 0.17 646 0.103 2.2133 0.0544 19.7 71.3 98% 0.13 624 0.107 2.2532 0.0585 31.3 79.3 101% 0.17 736 0.113 2.2578 0.0578 15.0 77.3 100% 0.10 596 0.112 Average rate = 665(60), 9% RSD. Figure A-21. Pressure vs. time for reaction of 0.0572 g saltwater with 2.2272 g acetyl chloride.
28 Qualitatively, it is clear that Figure A-21 shows the same behavior apparent in Figure A-20. Quantitatively, results from 5 replicate runs with saltwater showed an average specific rate of gas production of 790 l/kg- min with a relative standard deviation of 12%. This is slightly higher than the results with DI water (average of 665 l/kg- min with a relative standard deviation of 9%), but given the sample standard deviations, this difference cannot be consid- ered statistically significant. Example 5âAnhydrous Aluminum Chloride (UN 1726) Aluminum chloride (AlCl3, Sigma-Aldrich, 98%, UN 1726) was tested. This is an example of a solid material that poten- tially could be a water-reactive material (if gas is evolved on contact with water). An interesting nuance for AlCl3 is that it is not obvious in advance whether it will produce gas, and if so, whether that could be measured in the presence of water. AlCl3 is known to react vigorously with water, ultimately forming a hydrated salt:6 AlCl s excess H O l Al H O 3Cl aq 53 2 2 6 3[ ] ( )( ) ( ) ( ) ( )+ â ++ â However, it is also reported to fume on contact with water and release at least some HCl (g).7 In principle (and by ana- logy to materials like SiCl4), this could be due to a reaction such as Equation (6): AlCl s 6H O l Al OH H O 3HCl g 63 2 3 2 3 ( )( ) ( ) ( ) ( ) ( )+ â + If a transient reaction as in Equation (6) occurs, then AlCl3 might need to be classified as a water-reactive material in that it might emit a toxic gas, HCl (g), on contact with water. Should this (potentially transient) formation of 3 moles of HCl (g) per mole of AlCl3 occur, the potential gas production from AlCl3 is ~ 540 l/kgAlCl3. This would suggest that ~ 0.20 g AlCl3 might yield as much as 110 cc HCl (g). As that amount is within the capacity of the reaction apparatus (see Figure A-11), that was chosen as the starting amount. Because AlCl3 may produce a gas soluble in water, the right-hand portion of Figure A-7 applies, and a limited amount of water (e.g., 2.0 g) used. Note that with 0.20 g AlCl3, 2.0 g water still provides ~75 moles of water for each mole of AlCl3, or > 12 times the amount required for Equation (6) above. A reaction apparatus (as shown in Figure A-4, center-right and far-right) configured for solids addition was assembled, the P/V calibration checked (as in Figure A-10, data indicated with dash-dot pattern), and charged with 0.2143 g AlCl3 and 2.1085 g water. The AlCl3 was added to the water all at once, and the data shown in Figure A-22 obtained. As was the case for Example 2, AlCl3 has a negligible vapor pressure at room temperature, so the observed increase in pressure must be due to gas evolution. Since this was well within the apparatus capacity, testing was scaled up to ~ 0.4 g AlCl3 with 2.0 g water and replicates run. A typical result is shown in Figure A-23, all results are tabulated in Table A-6, and the specific rate results are graphed in Figure A-24. The preceding results suggest that Equation (5) is indeed occurring, but that it occurs by the rapid, stepwise reactions of equations (7)8 and (8). AlCl s 6H O l Al OH H O 3HCl g 73 2 3 2 3 ( )( ) ( ) ( ) ( ) ( )+ â + Al OH H O 3HCl g Al H O 3Cl aq 8 3 2 3 2 6 3[ ]( ) ( )( ) ( ) ( ) ( ) + â + + â This testing suggests that AlCl3 reacts very rapidly with water to produce HCl (g) with an average observed rate of ~ 7500 l/kgALCl3-min and an observed relative standard devia- tion of ~ 20%. While some of this HCl (g) might escape in an open environment, the gas is quickly absorbed by the water present in this closed apparatus, recombining with the inter- mediate aluminum product. Example 6âPhosphoryl Chloride (UN 1810) Phosphoryl chloride (POCl3, Fluka, â¥99%, UN 1810) was subjected to testing. Phosphoryl chloride is well known9 to react vigorously with water; complete hydrolysis proceeds (in principle) according to Equation (9). POCl l 3H O l H PO l 3HCl g 93 2 3 4 ( )( ) ( ) ( ) ( )+ â + Equation (9) indicates that the potential for gas evolution from POCl3 is ~ 470 l/kgPOCL3. This suggests that POCl3 is a water reactive material that produces a water soluble gas. This would suggest that ~ 0.3 g of POCl3 would yield ~ 140 cc of gas. Because this is a water-reactive material likely to produce a gas soluble in water, the right-hand portion of Figure A-7 applies, and a limited amount of water (e.g., 2.0 g) should be used. Note that with 0.3 g phosphoryl chloride, 2.0 g water provides > 50 moles of water for each mole of phosphoryl chloride. 6 Vincoli, J. W., Risk Management for Hazardous Chemicals, CRC Press, 1996, p. 75. 7 Many AlCl3 MSDS note the potential for AlCl3 to fume, producing HCl (g) in contact with water or humid air. 8 Equation (7) is identical to Equation (6), and reproduced here simply for convenience. 9 See, for instance, Inorganic Chemistry, Cotton, FA; Wilkinson, G; Murillo, CA; Bochmann, M, 1999, John Wiley & Sons, Inc., p. 404.
Figure A-22. Pressure vs. time for reaction of 0.2143 g AlCl3 is with 2.1085 g water. Pressures reported are corrected via the ideal gas law for small temperature changes during the course of the reaction to yield an isothermal-equivalent result. Transient gas formation is observed (within the first 2 second interval, data points are separated by 2 seconds), but the gas formed is reabsorbed within 20â30 seconds. Applying the P/V calibration for this apparatus, the transient gas formation amounted to ~ 55 cc of gas (~47% of theory). This corresponds to a (transient) gas evolution rate of ~7700 l/kgAlCl3-min. Figure A-23. Pressure vs. time for the reaction of 0.3298 g AlCl3 with 2.1961 g water. The water was added at around 6 minutes, equilibration of reactor pressure with ambient pressure allowed at around 13 minutes. When AlCl3 is combined with the water, transient gas formation is observed, though in this case the gas formed is essentially completely reabsorbed within 20â30 seconds. Applying the P/V calibration for this apparatus, 99 cc of (~ 55% of theory) transient gas formation is observed. Given the short duration of the evolution, this corresponds to a gas evolution rate of ~ 7600 l/kg-min. Mass AlCl3 Mass Water âP (Fastest) âV Rate Water/AlCl3 g g kPa cc l/kg-min mole/mole 0.2143 2.1085 11.38 55.0 7695 72.8 0.5001 2.0452 31.93 157.4 9443 30.3 0.3184 2.1221 12.97 60.6 5707 49.3 0.3250 2.0760 24.05 107.7 9946 47.3 0.3928 2.1961 22.06 99.5 7599 41.4 0.3582 2.1036 14.66 71.0 5943 43.5 0.3442 2.1060 15.79 73.6 6413 45.3 Average rate = 7500(1700), 23% RSD. Table A-6. Results from 7 runs of AlCl3 reaction with water (AlCl3 added to water).
30 A reaction apparatus as shown in Figure A-4, center- left, configured for liquid addition was assembled, the P/V calibration checked (as in Figure A-10, data indicated with dash-dot pattern), and a trial run with 0.3485 g phosphoryl chloride and 2.077 g water. For this trial run, fairly slow and modest reaction was observed. In this case, a steady rate was observed, and the slope of the pressure vs. time curve was used to extract a reaction rate. (See Figure A-25.) Because this reaction rate and yield was modest, increasing amounts of POCl3 were used (see Table A-7). Interestingly, as the water/POCl3 ratio fell (i.e., the amount of POCl3 used increased, while keeping the water constant), the character of the reaction changed. In all cases, there was an induction period, after which a more rapid reaction was observed (see Figure A-26). This more rapid reaction proceeded over a suf- ficiently short period of time that a simple slope based on the change in pressure and elapsed time could be used to find the maximum raw and specific gas production rate. However, the observed rate was a strong function of the water/POCl3 ratio (see Table A-7 and Figure A-27). Also, at the higher POCl3 charges (lower water/POCl3 ratios), not all the gas was reabsorbed. These results clearly make it difficult to assign a definite rate to this reaction. 10 See, for instance, Inorganic Chemistry, Cotton, FA; Wilkinson, G; Murillo, CA; Bochmann, M, 1999, John Wiley & Sons, Inc., p. 271. Because of these results, a series of experiments using Approach (B) were also conducted. Interestingly, (see Fig- ures A-28 and A-29) these also showed a strong dependence on water/POCl3 ratio. In this case, it would appear that the most that can be said is the following: ⢠POCl3 reacts, when added to water, with a definite induc- tion period. While the materials can be mixed without immediate reaction, a rapid reaction may occur just a few moments after mixing. ⢠When the reaction occurs, the rate is highly dependent on the amounts of water and POCl3 involved. At water/POCl3 mole ratios below ~15 and above ~ 5, reaction can be rapid and produce gas at rates at least as high as 3000 l/kg-min. Example 7âSilicon Tetrachloride (UN 1818) Silicon tetrachloride (SiCl4, Aldrich, â¥99%, UN 1818) was subjected to testing. SiCl4 is well known10 to react vigorously Figure A-24. Results from one trial run (point at > 70 water/AlCl3) and 6 replicate runs for the reaction of AlCl3 with water at room temperature, showing the observed rate of gas evolution, normalized to the amount of AlCl3 used, as a function of the water/ AlCl3 ratio. The scatter in the x-direction (water/AlCl3 ratio) reflects the fact that targeted weights are not precisely achieved. This shows that the observed rate is not strongly dependent on the water/AlCl3 ratio in this range, so all results can be pooled.
31 Figure A-25. Pressure vs. time for the reaction of 0.3485 g phosphoryl chloride with 2.077 g water. In this reaction, a modest, sustained raw gas production rate was observed, of ~ 4300 kPa/day. This corresponds to a specific gas evolution rate of ~ 38 l/kg-min. The maximum pressure observed corresponds to ~ 11 cc of gas evolution or ~ 7% of the theoretical yield. Mass POCl3 Mass Water âP (Fastest) âV Duration Rate Rate Water/ POCl3 g g cc cc m kPa/day) l/kg-min mole/mole 0.3485 2.0767 4317.00 38 51.4 0.6945 2.0399 3.59 15.2 0.07 328 25.3 1.3100 2.0171 56.53 260.6 0.07 2984 13.3 1.0300 2.1082 32.22 142.3 0.07 2072 17.6 0.8449 2.038 10.57 44.7 0.07 794 20.8 Table A-7. Results from tests with phosphoryl chloride. Rates are based on either a sustained reaction or the fastest period of gas evolution. Figure A-26. Pressure vs. time when 1.31 g POCl3 is combined with 2.0171 g water. In this reaction, though not obvious in this trace, a definite induction period was observed, after which a very rapid reaction was observed. For the 4 fastest seconds, the rate was ~ 2900 l/kg-min. The maximum pressure observed corresponds to ~ 261 cc of gas evolution, or ~ 42% of the theoretical yield. Note that, in this case, not all of the gas was reabsorbed by the reaction media; the starting water/POCl3 ratio was ~13.
32 Figure A-27. Results from runs of the reaction of POCl3 with water at room temperature as the water/POCl3 ratio varies. This shows that the observed rate is strongly dependent on the water/POCl3 ratio in this range. Figure A-28. Specific gas production rates, normalized to POCl3 required for reaction, from the reaction of POCl3 with water via Approach (B). As in Figure A-24, there is a strong dependence on water/POCl3 ratio.
33 with water; complete hydrolysis proceeds (in principle) according to Equation (10). SiCl l 2H O l SiO s 4HCl g 104 2 2 ( )( ) ( ) ( ) ( )+ â + This stoichiometry indicates that the potential for gas evo- lution from SiCl4 is 570 l/kgSiCl4, and that SiCl4 is a water reac- tive material that produces a water soluble gas. This would suggest that ~ 0.25 g of SiCl4 would yield ~ 140 cc of gas. Because this is a water-reactive material likely to produce a gas soluble in water, the right-hand portion of Figure A-7 applies, and a limited amount of water (e.g., 2.0 g) should be used for Approach (A). Note that with 0.25 g SiCl4, 2.0 g water provides ~ 75 moles of water for each mole of SiCl4, or nearly 20 times the amount required for Equation (10). A reaction apparatus configured for liquid addition was assembled as shown in Figure A-4, center-left, the P/V cali- bration checked (as in Figure A-10, data indicated with dash- dot pattern), and a trial run with 0.2462 g SiCl4 and 1.9868 g water. This showed very rapid immediate reaction, but a fairly modest yield of gas, so the amount of SiCl4 was increased to ~ 0.6 g and replicate runs conducted. Note that the behavior of SiCl4 (i.e., in Figure A-30) is intermediate between that of (CH3)2SiCl2 (Figure A-12) and TiCl4 (Figure A-37). It shows a greater pressure increase than (CH3)2SiCl2, though it is still only a low fraction of the expected amount (e.g., ~ 12% of theory at max gas production in Figure A-30). In part, the increase relative to (CH3)2SiCl2 could be due to its lower boiling point (57. 6 vs. 70 °C). It shows substantially slower reaction than TiCl4 (Figure A-38), which also shows a larger fraction of theoretical gas evolution (~ 40%). The observations noted above make it difficult to be cer- tain that the gas evolution observed in Figure A-30, resulting in the measurements of Table A-8 and Figure A-31 are due to gas evolution from, or evaporation of, SiCl4. This makes SiCl4 a candidate for testing via Approach (B). However, in this case, attempts to add water to SiCl4 in a con- trolled fashion (i.e., ~ 0.50 g water to ~ 2.0 g SiCl4) failed because the water was immediately encased by reaction products. Fur- ther, though reaction had obviously occurred, there was no strong evidence of a measurable amount of gas evolution. This leaves no choice but to use the Approach (A) result of a specific rate of gas evolution of 1020 l/kg-hr with a relative sample standard deviation of 20%. Example 7 (Repeat with Saltwater)âSilicon Tetrachloride (UN 1818) Silicon tetrachloride was also selected for testing with salt- water (3.5% w/w NaCl in DI water) as well as DI water (pre- ceding section). For the reasons noted in the final paragraph of the preceding section, this work used Approach (A). Figure A-29. This figure combines the results shown in Figures A-27 and A-28 for the reaction of POCl3 with water at room temperature, comparing Approach (A) (right portion) with Approach (B) (left portion). This suggests that the rate of reaction between POCl3 and water depends strongly on the water/POCl3 ratio and peaks at or above 3000 l/kg-min.
34 Figure A-30. Pressure vs. time for the reaction of 0.6126 g SiCl4 with 2.0757 g water. The fastest reaction is observed over the first 4 seconds after the materials are combined and corresponds to a rate of 650 l/kgSiCl4-min. The maximum pressure observed corresponds to 41 cc of gas evolution, or ~ 11% of the theoretical yield. Mass SiCl4 Mass Water âP (Fastest) âV Rate Water/ SiCl4 g g kPa cc l/kg-min mole/mole 0.2462 1.9868 3.82 17.8 1085 76.1 0.603 2.106 8.99 41.2 1025 32.9 0.6000 2.0598 9.81 46.2 1154 32.4 0.6110 2.0694 10.56 48.1 1181 31.9 0.6126 2.0757 6.15 26.6 651* 32.0 Average rate = 1020(210), 20% RSD. Table A-8. Results from tests with SiCl4. Rates are based on the fastest period of gas evolution, (i.e., the first 4 seconds). Figure A-31. Results from trial and replicate runs for the reaction of SiCl4 with water at room temperature. This shows that the observed rate is not strongly dependent on the water/SiCl4 ratio in this range, so all results can be pooled.
35 Interestingly, the behavior of SiCl4 with saltwater was (unlike acetyl chloride) qualitatively different than its behav- ior with DI water, as Figure A-32 illustrates. It may be that, in this case, it is possible to differentiate between the initial rapid increase in pressure due to evapora- tion of SiCl4 and a slower, sustained increase in pressure due to HCl (g) release, which either is produced more rapidly, or dissolves less rapidly, with saltwater. Replicate runs showed the slower, sustained gas evolution to be occurring at an average specific gas production rate of 14 l/kg-min with a relative standard deviation of 40%, while the initial pressure surge corresponded to 300 l/kg-min with a relative standard deviation of 40%. The latter is substan- tially slower than the 1020 l/kg-min observed with DI water. Example 8âThionyl Chloride (UN 1836) Thionyl chloride (SOCl2, Sigma-Aldrich, >99%, UN 1836) was subjected to testing. It is known to react with water to yield HCl (g) and SO2(g): SOCl l 2H O l SO g 2HCl g 112 2 2 ( ) ( )( ) ( ) ( )+ â + This stoichiometry indicates that the potential for gas evolu- tion from SOCl2 is (3 moles/mole) ~ 607 l/kgSOCl2, and that it is a water reactive material that produces water soluble gases. This would suggest that ~ 0.25 g of SOCl2 would yield ~ 150 cc of gas. Because this is a water-reactive material likely to pro- duce gases soluble in water, the right-hand portion of Figure A-7 applies, and a limited amount of water (e.g., 2.0 g) should be used. Note that with 0.25 g SOCl2, 2.0 g water provides ~ 50 moles of water for each mole of SOCl2, or > 25 times the amount required for Equation (11). A reaction apparatus configured for liquid addition was assembled as shown in Figure A-4, center-left, the P/V cali- bration checked (as in Figure A-10, data indicated with dash- dot pattern), and the apparatus charged with 2.0263 g water. For the screening test, 0.2495 g SOCl2 was added to the water all at once, with results shown in Figure A-33. Replicate testing with Approach (A) yielded the results in Table A-9 and Figure A-34. In this experiment, it is difficult to be certain that the pres- sure increase is due to gas evolution or simply evaporation of some of the SOCl2 (b.p. 74.6 °C). Because of the uncertainty, Approach (B) was checked. Figure A-35 shows the behavior observed with Approach (B). The results shown in Table A-10 strongly suggest that the actual rate should be measured with Approach (B). From these results using Approach (B), it seems clear that the specific rate of gas production for SOCl2 in contact with water is 370 l/kg-min, with a relative standard deviation of 30%. Example 8 (Repeat with Saltwater) Thionyl chloride was also selected for testing with saltwater (3.5% w/w NaCl in DI water) as well as DI water (preceding section). For the reasons noted in the preceding section, this work used Approach (B). From these results (Figure A-36 and Table A-11) using Approach (B) with brine, the specific rate of gas production for SOCl2 in contact with water is 210 l/kg-min with a rela- tive standard deviation of 33%. This is a barely statistically significant difference (p = 0.02 in in Studentâs t-test) from the result with water (370(110) l/kg-min, 30% RSD). Figure A-32. Pressure vs. time for the reaction of 0.7036 g SiCl4 with 2.1236 g saltwater. As with DI water, the fastest increase in pressure is observed over the first 4 seconds after the materials are combined and corresponds to a rate of 280 l/kgSiCl4-min. However, that pressure increase is very modest (~ 3 kPa, corresponding to only ~ 13 cc of gas), and it is followed by a sustained period of pressure increase (gas production). That pressure increase (~ 10 kPa) production corresponds to ~ 43 cc of gas production at a rate of 17 l/kg-min.
36 Figure A-33. Pressure vs. time for the reaction of 0.2495 g SOCl2 with 2.0263 g water (observed pressure and temperature shown). Fairly rapid (within ~15 seconds) gas formation is observed. Observed gas formation amounted to ~ 37 cc of gas (~25% of theory). This corresponds to a (transient) gas evolution rate of ~1400 l/kgSOCl2-min. Figure A-34. Results from trial and replicate runs for the reaction of SOCl2 with water at room temperature. This shows that the observed rate is not strongly dependent on the water/SOCl2 ratio in this range, so all results can be pooled. Mass SOCl2 Mass Water âP (Fastest) âV Rate Water/SOCl2 g g kPa cc l/kg-min mole/mole 0.2495 2.0263 4.95 22.7 1366 53.6 0.5022 2.0491 13.09 57.6 1720 26.9 0.5142 2.0619 11.64 49.3 1438 26.5 0.5337 2.0582 8.91 41.1 1154 25.5 0.4849 2.063 7.98 35.3 1091 28.1 Average rate = 1350(250), 20% RSD. Table A-9. Results from tests with SOCl2. Rates are based on the initial (4 seconds, maximum rate) gas evolution rate.
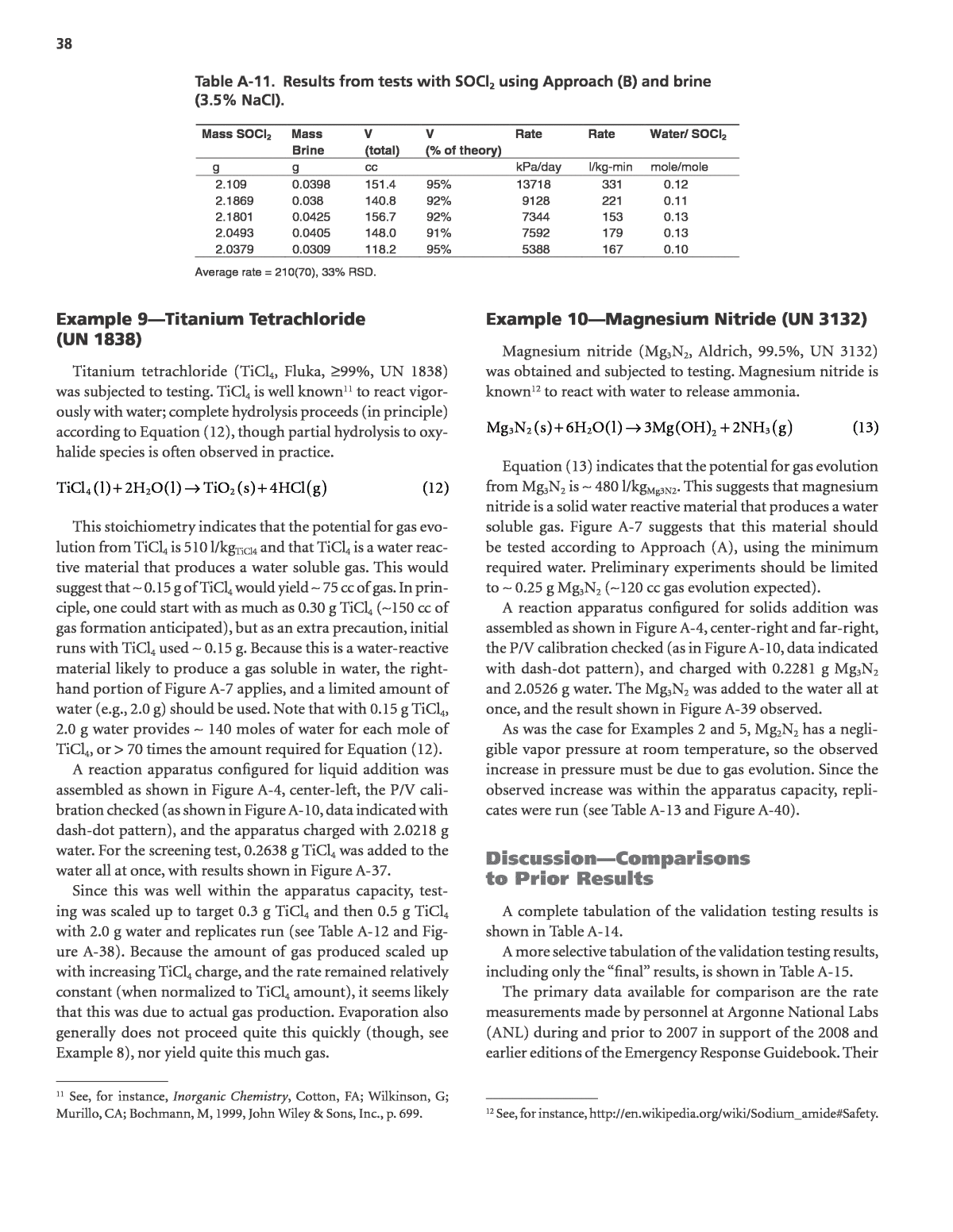
37 Figure A-35. Pressure vs. time for reaction of 0.0416 g water with 2.2269 g SOCl2. Smooth reaction with gas formation is observed. Observed gas formation here amounted to ~ 160 cc of gas, which was 96% of theory. The line fitted to the steepest part of the curve yielded a specific gas evolution rate, based on SOCl2 required for complete reaction, of 500 l/kg-min. Table A-10. Results from tests with SOCl2 using Approach (B). Mass SOCl2 Mass Water V (total) V (% of theory) Rate Rate Water/ SOCl2 g g cc kPa/day l/kg-min mole/mole 2.0583 0.0307 122.1 99% 9584 306 0.10 2.2269 0.0416 159.7 96% 22584 502 0.12 2.3782 0.0322 125.8 97% 14089 400 0.09 2.3617 0.0319 126.9 99% 16087 493 0.09 1.1189 0.029 128.5 111% 6746 224 0.17 1.1663 0.0271 119.8 110% 9086 301 0.15 Average rate = 370(110), 30% RSD. Figure A-36. Pressure vs. time for reaction of 0.0398 g brine (3.5% NaCl) with 2.109 g SOCl2. Smooth reaction with gas formation is observed. Observed gas formation here amounted to ~ 150 cc of gas, which was 95% of theory. The line fitted to the steepest part of the curve yielded a specific gas evolution rate, based on SOCl2 required for complete reaction, of 330 l/kg-min.
38 Example 9âTitanium Tetrachloride (UN 1838) Titanium tetrachloride (TiCl4, Fluka, â¥99%, UN 1838) was subjected to testing. TiCl4 is well known11 to react vigor- ously with water; complete hydrolysis proceeds (in principle) according to Equation (12), though partial hydrolysis to oxy- halide species is often observed in practice. TiCl l 2H O l TiO s 4HCl g 124 2 2 ( )( ) ( ) ( ) ( )+ â + This stoichiometry indicates that the potential for gas evo- lution from TiCl4 is 510 l/kgTiCl4 and that TiCl4 is a water reac- tive material that produces a water soluble gas. This would suggest that ~ 0.15 g of TiCl4 would yield ~ 75 cc of gas. In prin- ciple, one could start with as much as 0.30 g TiCl4 (~150 cc of gas formation anticipated), but as an extra precaution, initial runs with TiCl4 used ~ 0.15 g. Because this is a water-reactive material likely to produce a gas soluble in water, the right- hand portion of Figure A-7 applies, and a limited amount of water (e.g., 2.0 g) should be used. Note that with 0.15 g TiCl4, 2.0 g water provides ~ 140 moles of water for each mole of TiCl4, or > 70 times the amount required for Equation (12). A reaction apparatus configured for liquid addition was assembled as shown in Figure A-4, center-left, the P/V cali- bration checked (as shown in Figure A-10, data indicated with dash-dot pattern), and the apparatus charged with 2.0218 g water. For the screening test, 0.2638 g TiCl4 was added to the water all at once, with results shown in Figure A-37. Since this was well within the apparatus capacity, test- ing was scaled up to target 0.3 g TiCl4 and then 0.5 g TiCl4 with 2.0 g water and replicates run (see Table A-12 and Fig- ure A-38). Because the amount of gas produced scaled up with increasing TiCl4 charge, and the rate remained relatively constant (when normalized to TiCl4 amount), it seems likely that this was due to actual gas production. Evaporation also generally does not proceed quite this quickly (though, see Example 8), nor yield quite this much gas. Example 10âMagnesium Nitride (UN 3132) Magnesium nitride (Mg3N2, Aldrich, 99.5%, UN 3132) was obtained and subjected to testing. Magnesium nitride is known12 to react with water to release ammonia. Mg N s 6H O l 3Mg OH 2NH g 133 2 2 2 3 ( )( ) ( ) ( ) ( )+ â + Equation (13) indicates that the potential for gas evolution from Mg3N2 is ~ 480 l/kgMg3N2. This suggests that magnesium nitride is a solid water reactive material that produces a water soluble gas. Figure A-7 suggests that this material should be tested according to Approach (A), using the minimum required water. Preliminary experiments should be limited to ~ 0.25 g Mg3N2 (~120 cc gas evolution expected). A reaction apparatus configured for solids addition was assembled as shown in Figure A-4, center-right and far-right, the P/V calibration checked (as in Figure A-10, data indicated with dash-dot pattern), and charged with 0.2281 g Mg3N2 and 2.0526 g water. The Mg3N2 was added to the water all at once, and the result shown in Figure A-39 observed. As was the case for Examples 2 and 5, Mg2N2 has a negli- gible vapor pressure at room temperature, so the observed increase in pressure must be due to gas evolution. Since the observed increase was within the apparatus capacity, repli- cates were run (see Table A-13 and Figure A-40). DiscussionâComparisons to Prior Results A complete tabulation of the validation testing results is shown in Table A-14. A more selective tabulation of the validation testing results, including only the âfinalâ results, is shown in Table A-15. The primary data available for comparison are the rate measurements made by personnel at Argonne National Labs (ANL) during and prior to 2007 in support of the 2008 and earlier editions of the Emergency Response Guidebook. Their Table A-11. Results from tests with SOCl2 using Approach (B) and brine (3.5% NaCl). Mass SOCl2 Mass Brine V (total) V (% of theory) Rate Rate Water/ SOCl2 g g cc kPa/day l/kg-min mole/mole 2.109 0.0398 151.4 95% 13718 331 0.12 2.1869 0.038 140.8 92% 9128 221 0.11 2.1801 0.0425 156.7 92% 7344 153 0.13 2.0493 0.0405 148.0 91% 7592 179 0.13 2.0379 0.0309 118.2 95% 5388 167 0.10 Average rate = 210(70), 33% RSD. 11 See, for instance, Inorganic Chemistry, Cotton, FA; Wilkinson, G; Murillo, CA; Bochmann, M, 1999, John Wiley & Sons, Inc., p. 699. 12 See, for instance, http://en.wikipedia.org/wiki/Sodium_amide#Safety.
Figure A-37. Pressure vs. time for reaction of 0.1638 g TiCl4 with 2.0218 g water. As in other examples, pressures are corrected for small temperature changes during the course of the reaction. Rapid (within 2 seconds) transient gas formation is observed (at around 21:40), but the gas formed is reabsorbed within ~ 2 minutes. The transient gas formation amounted to ~ 35 cc of gas (~40% of theory). This corresponds to a (transient) gas evolution rate of ~6400 l/kgTiCl4-m. Table A-12. Results from tests with TiCl4. Rates are based on the maximum amount of gas observed (uniformly observed in 2 seconds). Mass TiCl4 Mass Water âP (Fastest) âV Rate Water/ TiCl4 g g kPa cc l/kg-min mole/mole 0.1638 2.0218 7.57 34.7 6364 130.0 0.3463 2.1076 10.36 48.4 4191 64.1 0.5420 2.0725 23.34 104.6 5788 40.3 0.5720 2.0725 21.01 94.8 4970 38.1 0.5783 2.1515 24.52 118.7 6157 39.2 Average rate = 5500(900), 16% RSD. Figure A-38. Results from trial and replicate runs for the reaction of TiCl4 with water at room temperature. This shows that the observed rate is not strongly dependent on the water/TiCl4 ratio in this range, so all results can be pooled.
40 Figure A-39. Pressure vs. time for the reaction of 0.2281 g Mg3N2 with 2.0526 g water. Pressures reported are corrected via the ideal gas law for small temperature changes during the course of the reaction, to yield an isothermal-equivalent result. Transient gas formation is observed within the first 4-second interval; data points are separated by 2 seconds. Applying the P/V calibration for this apparatus, the transient gas formation amounted to ~ 104 cc of gas (~96% of theory). This corresponds to a (transient) gas evolution rate of ~6850 l/kgMg3N2-min. Figure A-40. Results from trial and replicate runs for the reaction of Mg3N2 with water at room temperature. This shows that the observed rate is not strongly dependent on the water/Mg3N2 ratio in this range, so all results can be pooled. Mass Mg3N2 Mass Water âP (Fastest) âV Rate Water/ Mg3N2 g g kPa cc l/kg-min mole/mole 0.2400 2.0043 19.5 86.6 5406 46.8 0.2281 2.0526 22.8 104.0 6848 50.4 0.2114 2.0510 30.5 138.3 9816 54.4 0.2330 2.0689 30.6 139.6 8986 49.8 0.2206 2.1200 28.2 125.9 8579 53.8 Average rate = 7900(1800), 23% RSD. Table A-13. Results from tests with Mg3N2. Rates are based on the maximum amount of gas observed (uniformly observed in 4 seconds).
41 Table A-14. Complete validation testing results. Material Approach Result (l/kg-min) Std. Dev. (l/kg-min) RSD UN Number (CH3)2SiCl2 B 36 8 22% 1162 NaNH2 A 9600 2000 20% 1390 NaBH4 A 2 (120 l/kg-h) 0.5 (30 l/kg-h) 25% 1426 CH3COCl A 620 180 30% 1717 CH3COCl B 665 60 9% 1717 CH3COCl B (saltwater) 790 95 12% 1717 AlCl3 A 7500 1700 23% 1726 POCl3 A, B 1-3000 (& up) n/a n/a 1810 SiCl4 A 1020 210 20% 1818 SiCl4 A (saltwater initial) 300 120 40% 1818 SiCl4 A (saltwater steady) 14 6 40% 1818 SOCl2 A (prob. evaporation) 1350 250 20% 1836 SOCl2 B 370 110 30% 1836 SOCl2 B (saltwater) 210 70 34% 1836 TiCl4 A 5500 900 16% 1838 Mg3N2 A 7900 1800 23% 3132 Approach: A = Substance added to water, B = water added to substance (Salt) indicates a test that used saltwater (3.5% NaCl in DI water) Table A-15. Selected final validation testing results. Material Approach Result (l/kg-min) Std. Dev. (l/kg-min) RSD UN Number (CH3)2SiCl2 B 36 8 22% 1162 NaNH2 A 9600 2000 20% 1390 NaBH4 A 2 (120 l/kg-h) 0.5 (30 l/kg-h) 25% 1426 CH3COCl B 665 60 9% 1717 AlCl3 A 7500 1700 23% 1726 POCl3 A, B 1-3000 (& up) n/a n/a 1810 SiCl4 A 1020 210 20% 1818 SOCl2 B 370 110 30% 1836 TiCl4 A 5500 900 16% 1838 Mg3N2 A 7900 1800 23% 3132 Approach: A = Substance added to water, B = water added to substance latest report was made in 2009.13 Workers at ANL used an apparatus where small quantities of water-reactive materials/ water-reactive substances could be combined either with stoichiometric amounts of water (ANL Method A) or 5:1 molar excess over the stoichiometric ratio of water: water- reactive material/water-reactive substance (ANL Method B). In the 2007 ANL work, gas evolution was measured by the displacement of a lubricated syringe, and the evolution of gas vs. time fitted with a first order rate equation to arrive at parameters that described the reaction. These parameters were l, a first order rate constant, and b, a ratio of the amount of gas evolution observed (Gasobs) to that expected (Gastheory). These, in principle,14 can be used to model the progress of reaction (Gasobs(t)) via the following equations: Let j(t) â« extent of reaction (i.e., from 0.000 to 1.000, where 0.000 = no reaction and 1.000 = complete reaction), where t â« time since the start of reaction. Then: t 1 exp , and 14t( )( )( ) ( )Ï = â âλ 13 See the report Development of the Table of Initial Isolation and Pro- tective Action Distances for the 2008 Emergency Response Guidebook prepared by D. F. Brown, H. M. Hartmann, W. A. Freeman, and W. D. Haney. ANL/DIS-09-2. Currently available online at http://phmsa.dot. gov/staticfiles/PHMSA/DownloadableFiles/Files/Argonne_Report.pdf. 14 i.e., to the extent that the parameters are accurate and that the reac- tion proceeds in a first order fashion, based on water-reactive materials/ water-reactive substances.
42 Gas t Gas t Gas 1 exp 15obs theory theory t( )( )( ) ( ) ( )= Î²Ï = β â âλ In making comparisons, it should be recalled that the rates shown in Tables A-14 and A-15 are observed initial rates of gas production, while the results from Argonne are parameters for an assumed (possibly, though not necessarily, correct) rate law. Equation (15) can be used to calculate the amount of gas expected to be formed at some time (t) and, from that, initial rates estimated for comparison. Because of the vary- ing nature and time frame for the reactions, it is difficult to directly compare the parameters and their predictions to the results here in a general sense. Instead, it is best to consider the comparisons on a case-by-case basis. Dimethyldichlorsilane ([CH3]2SiCl2, UN 1162) In the present work, Approach (B) (addition of water to water-reactive materials, from the present protocol) was used to test (CH3)2SiCl2. Reactions were run with a mole ratio of between 0.3:1 and 1.5:1 (water:[CH3]2SiCl2) and yielded an initial rate of 36 (8)15 l/kg-min. Behavior was as shown in Figure A-13 (see Example 1). The 2007 ANL work found behavior as shown in Fig- ure A-41.16 Qualitatively, the behavior apparent in Figure A-13 is com- parable to that in Figure A-41. The ANL workers reported values for l and b of 0.93 min-1 and 0.25, respectively, for Method B, which used a 5:1 molar excess over the stoichiometric ratio of water: water-reactive material/water-reactive substance. When comparing predictions from Equation (15) to results here, gas production needs to be compared for a similar point (i.e., the start) in the reaction and for a similar duration. Many of the tests here showed rapid initial rates and the determina- tion of rates on a time frame of seconds. Given that, it seems reasonable to calculate the overall rate of gas evolution from the start of the 1st order reaction for a duration that matches the observations here. For dimethyldichlorosilane, given the values for l and b, Equation (15) predicts a rate of gas generation of 82 l/kg-min over the first 6 seconds. This is actually fairly good (i.e., < one order of magnitude in difference) agreement for kinetic data like these, acquired with different apparatus and conditions. Acetyl Chloride (UN 1717) In the present work, Approach (B) was found to be correct for acetyl chloride, and reactions were run with a mole ratio of between ~0.1:1.0 (water: acetyl chloride). These yielded an initial rate of 665 (60) l/kg-min (note that Approach (A) actually yielded very similar results, of ~620 (180) l/kg-min). Behavior with Approach (A) was as shown in Figure A-20 (see Example 4). The 2007 ANL work found behavior as shown in Fig- ure A-42.17 These results have a reasonable amount of similarity to the present results. The ANL workers reported values for l and b of 6.38 min-1 and 0.70, respectively, for both Method A and B results.18 Since observed rates in this work were based on observations over the first 4â8 seconds of gas evolution, it seems appropriate to compare these predictions of gas production over the first 6 seconds of a 1st order reaction with these values for l and b. This has been done, and it yields a predicted net rate of gas evolution for 6 seconds of ~ 1000 l/kg-min with an apparent extent of reaction of 33%. This is actually fairly good agree- ment with the results reported here, of ~660 (60) l/kg-min. Considering the different apparatus, methods of measuring rates, the intrinsically fast nature of the reaction, the rapid reabsorption of HCl(g), and the differing water: acetyl chlo- ride ratios, this is an acceptable level of agreement. Aluminum Chloride (UN 1726) In the present work, reactions were run with mole ratios of ~ 30:1 to ~75:1 (water:AlCl3) and showed results that did not depend strongly upon the water:AlCl3 ratio (see Figure A-24, in 15 Parenthetical value is a sample standard deviation. 16 As a work of the U.S. government, ANL/DIS-09-2 is in the public domain. Figure A-41 is reproduced from p. D-17 of that report. Figure A-41. Results from ANL for (CH3)2SiCl2. 17 As a work of the U.S. government, ANL/DIS-09-2 is in the public domain. Figure A-42 is reproduced from p. D-34 of that report. 18 See Table C.1 of ANL/DIS-09-2 (footnote 13).
43 Example 5). The rates observed were in the range of ~ 6000 to 10,000 l/kg-min, or 7500 (1700) l/kg-min. Typically, gas forma- tion was transient, with rates based on 2 seconds of gas produc- tion, and ~ 55% was a typical maximum yield (see Figure A-23). In the 2009 report, ANL workers reported values for l and b of 30 min-1 and 0.20, respectively,19 for both Method A (3 mmol water, 1 mmol AlCl3) and Method B (15 mmol water, 1 mmol AlCl3). Those results were based on experi- ments conducted during 1999 and reported in 2000.20 These values are reported, even though the comments on the reac- tion stated that âGaseous product did not appear.â21 While the 2009 report (see footnote 13) included graphs of gas production vs. time, the earlier work did not. Given the report of values for l and b, and the observation of no (net?) gaseous product, it seems likely that the gas formation that led to the parameter values was transientâbut this is not certain. Taking the reported values for l and b at face value, they predict gas evolution over the 1st 2 seconds (to be comparable to the observations here) at a rate of ~ 2000 l/kg-min (and ~ 15% apparent reaction, 60% actual extent of reaction)âthis is again within order of magnitude agreement with the pres- ent results. As above, considering the different apparatus and methods and the nature of the reaction, this is an acceptable level of agreement. Phosphoryl Chloride (UN 1810) In the present work, reactions were run with a mole ratio of between 0.2:1 and 50:1 (water:POCl3) and showed results that depended strongly upon the water/POCl3 ratio (see Fig- ure A-29). For most tests using Approach (A), reported in this work, reactions occurred over 4 seconds. In the 2007 ANL work, behavior for POCl3 was as shown in Figure A-43.22 The ANL workers reported values for l and b of 6.0 min-1 and 0.23, respectively, based on the Method A result (3 mmol water, 1 mmol POCl3). For Method B (15 mmol water, 1 mmol POCl3), parameters could not be fit, and much less gas was observed. ANL workers also note that in their 2003 work the Method B experiment showed a transient spike of gas, followed by absorption of the gas. Using the ANL values of for l and b, from their Method A, and finding the gas evolution rate over the first 4 seconds of reaction gives a result of ~ 500 l/kg-min. When compared with the results shown in Figure A-28 (see Example 6) for water:POCl3 mole ratios of ~ 1:1 (not quite the 3:1 of Method A, but close), the present results actually overlap with the pre- diction of 500 l/kg-min. However, with higher water/POCl3 ratios (e.g., 14â18 mol water/1 mole POCl3, see Table A-7) that are not too far from the ANL Method B water:POCl3 ratios (i.e., 15:1), the current test shows much faster gas evolution from POCl3 and water (e.g., 2000â3000 l/kg-min). Thus, on an equal conditions basis, the results from the present work agree with those of the ANL work; however, Figure A-42. Results from ANL for acetyl chloride. 19 See Table C.1 of ANL/DIS-09-2 (footnote 13). 20 See the report Development of the Table of Initial Isolation and Pro- tective Action Distances for the 2000 Emergency Response Guidebook prepared by D. F. Brown, A. J. Polcastro, W. E. Dunn, R. A. Carhart, M. Lazaro, W. A. Freeman, and M. Krumpolc. ANL/DIS-00-1. Currently available online at http://urbansurvivallibrary.com/uploads/Hazmat_ Protective_Action_Distances.pdf. 21 See p. 143 of ANL/DIS-00-1 (footnote 20). Figure A-43. Results from ANL for phosphoryl chloride. 22 As a work of the U.S. government, ANL/DIS-09-2 is in the public domain. Figure A-43 is reproduced from p. D-65 of that report.
44 the wider exploration of conditions here showed that higher rates are possible. Silicon Tetrachloride (UN 1818) In the present work, reactions were run with a mole ratio of (mainly) ~ 30:1 (water:SiCl4) and showed results that did not depend strongly upon the water/SiCl4 ratio (see Figure A-31). Most of the rates observed were in the range from 1,000 to 1,200 l/kg-min, though one result was ~ 600 l/kg-min; over- all, 1000 (215) l/kg-min. Typically, gas formation was tran- sient, and ~ 10% was the maximum yield (see Figure A-30). In the 2007 ANL work, behavior for SiCl4 was as shown in Figure A-44.23 The ANL workers reported values for l and b of 1.81 min-1 and 0.30, respectively, based on the Method B result (10 mmol water, 1 mmol SiCl4, which is 5 times the stoichiometric ratio), which had the conditions closest to those reported here. Using the ANL values of for l and b, from their Method B, and finding the gas evolution rate over the first 4 seconds of reac- tion gives a result of ~ 290 l/kg-min. When compared with the results in Table A-8 (see Example 7) for water:SiCl4 mole ratios of ~ 30:1 ( 600â1,200 l/kg-min), there is again rough (order of magnitude) agreement. As for other materials discussed so far, considering the different apparatus and methods and the nature of the reaction, this is an acceptable level of agreement Thionyl Chloride (UN 1836) In the present work, under Approach (A) (see Example 8), reactions were run with mole ratios ~ 25:1 to 50:1 (water:SOCl2) 23 As a work of the US government, ANL/DIS-09-2 is in the public domain. Figure A-44 is reproduced from p. D-69 of that report. and showed results that did not depend strongly upon water/ SOCl2 ratio (see Figure A-34). The rates observed were in the range of 1,000 to 1,800 l/kg-min; 1,350 (250) l/kg-min. Typi- cally, gas formation was rapid, occurring over ~20 seconds, and ~ 25% was a typical maximum yield (see Figure A-33). How- ever, work with Approach (B) suggested that in Approach (A) the observed gas production rate was biased by evaporation of thionyl chloride. Approach (B) showed results more clearly due to reaction, in the range of 200â500 l/kg-min, with water limited. The ANL workers reported values for l and b of 2.75 min-1 and 1.00, respectively, for both Method A (2 mmol water, 1 mmol SOCl2) and Method B (10 mmol water, 1 mmol SOCl2) That result is based on experiments conducted in prior exercises (1999) and reported in 2005.24 Method A achieved 100% of the theoretical yield of gas after 5 minutes, and Method B after 2 minutes. Using the reported values of l and b for Method B, as before, predicts an initial (6 seconds) rate of ~ 1,500 l/kg-min. The present work suggests that this rate may be biased by gas formation from evaporation of thionyl chloride and that the actual rate of gas production from reaction with water is somewhat lower. Titanium Tetrachloride (UN 1838) In the present work, reactions were run with mole ratios ~ 40:1 to 140:1 (water:TiCl4) and showed results that did not depend strongly upon water/TiCl4 ratio (see Figure A-38). The rates observed were in the range of 4,000 to 6,500 l/kg- min; 5,500 (900) l/kg-min. Typically, gas formation was tran- sient, occurring over 2 seconds, and ~ 40% was a typical maximum yield (see Figure A-37, in Example 9). The ANL workers reported values for l and b of 1.35 min-1 and 0.13, respectively, for the Method B result (10 mmol water, 1 mmol TiCl4) and 1.35 min-1 and 0.20 for the Method A result (2 mmol water, 1 mmol TiCl4). Those results were based on experiments conducted in prior exercises (1999, 2003), and reported in 2005.25 In that work, it was reported that for Method A the water was immediately covered with a crust and that for Method B there was a peak of gas produc- tion followed by absorption. Figure A-44. Results from ANL for silicon tetrachloride. 24 See the report Development of the Table of Initial Isolation and Protec- tive Action Distances for the 2004 Emergency Response Guidebook pre- pared by D. F. Brown, W. A. Freeman, R. A. Carhart, and M. Krumpolc. ANL/DIS-05-2. Currently available online at http://www.ipd.anl.gov/ anlpubs/2005/09/53554.pdf. 25 See the report Development of the Table of Initial Isolation and Protec- tive Action Distances for the 2004 Emergency Response Guidebook pre- pared by D. F. Brown, W. A. Freeman, R. A. Carhart, and M. Krumpolc. ANL/DIS-05-2. Currently available online at http://www.ipd.anl.gov/ anlpubs/2005/09/53554.pdf.
45 Those observations are in qualitative agreement with the present work and, using the reported values of l and b for Method B as before, predict ~ 90 l/kg-min as the rate of gas formation for the first 2 seconds of reaction. This represents poorer agreement between the ANL work and present work than for most of the other materials for which comparison is possible. Summary of Comparisons Overall, the comparison between the present work and the prior ANL work shows generally good agreement: ⢠For (CH3)2SiCl2 the present work suggests a rate of 36 (8) l/kg-min. ANL workers reported qualitatively similar results and suggested that an initial rate of gas generation would be ~ 80 l/kg-min. ⢠For acetyl chloride, the present work suggests a rate of 665 (60) l/kg-min. ANL workers reported qualitatively similar results and suggested that an initial rate of gas generation would be ~ 1000 l/kg-min. ⢠For AlCl3, the present work suggests a rate of 7,500 (1,700) l/kg-min. ANL workers appear to report qualitatively simi- lar results, suggesting that an initial rate of gas generation would be ~ 2000 l/kg-min. ⢠For POCl3, the present work suggests a rate that depends strongly on water/POCl3 ratio and which can be as high as 2,000â3,000 l/kg-min, or possibly more. The ANL work appears to share some of this character, with their Method A and B results differing. The parameters reported there suggest an initial rate of gas generation of ~ 500 l/kg-min, which actually is in agreement with observations under similar conditions in the present work, but which is also somewhat lower than the highest rate observed here. ⢠For SiCl4, the present work suggests a rate of ~1,000 (200) l/kg-min. ANL workers appear to report qualitatively simi- lar results, suggesting that an initial rate of gas generation would be ~ 300 l/kg-min. ⢠For SOCl2, the present work suggests a rate of ~ 370 (110) l/kg-min. ANL workers appear to report higher results, which this work suggests were influenced by gas from the evaporation of SOCl2. ⢠For TiCl4, the present work suggests a rate of 5,500 (900) l/kg-min. ANL workers appear to report qualitatively simi- lar results, but suggest a much lower initial rate of gas gener- ation of ~ 90 l/kg-min. Possibly, this was due to poor mixing in their work, where âcrustâ formation was reported. The generally good agreement between the results begs the question: âShould the ANL approach be considered as a test methodology?â The answer to that question is, in principle, âYes.â However, the HMCRP Project HM-14 team thought that the method outlined in this appendix had several advantages. These include the following: ⢠Easy acquisition of data with a commercial, off-the-shelf pressure/temperature transducer. ⢠Easy calibration of the P/V response via additions of known volumes of gas, without concern about the force needed to displace the syringe or âwhiplashâ in the syringe displacement. ⢠Ability with Approach (A) to use larger molar excesses of water than the 5:1 ratio used in the ANL work. ⢠Ability to vary the relative amounts of water and test material used. ⢠Ease of achieving a leak-tight apparatus by eliminating the presence of tubing.
