1
Introduction1
The volume and complexity of information about individual patients is greatly increasing with use of electronic records and personal devices. Potential effects on medical product development in the context of this wealth of real-world data2 could be numerous and varied, ranging from the ability to determine both large-scale and patient-specific effects of treatments to the ability to assess how therapeutics affect patients’ lives through measurement of lifestyle changes. However, mechanisms to facilitate efficient use of real-world data to meet the decision-making needs of myriad stakeholders have not been established. Traditional efficacy clinical trials are designed to test novel medical treatments in ideal, controlled circumstances. Clinical practice is much more diverse, and efficacy in practice (i.e., effectiveness) is affected by patient adherence, co-morbidities, concomitant
___________________
1 The planning committee’s role was limited to planning the workshop, and the Proceedings of a Workshop was prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants, and have not been endorsed or verified by the National Academies of Sciences, Engineering, and Medicine, and they should not be construed as reflecting any group consensus.
2 Real-world data are “data collected from sources outside of traditional clinical trials. These sources may include large simple trials, or pragmatic clinical trials, prospective observational or registry studies, retrospective database studies, case reports, administrative and health care claims, electronic health records, data obtained as part of a public health investigation or routine public health surveillance, and registries (e.g., device, procedural, or disease registries). The data [are] typically derived from electronic systems used in health care delivery, data contained within medical devices, and/or in tracking patient experience during care, including in home-use settings” (FDA, 2016, p. 4).
treatments, and other factors. Real-world evidence, which the U.S. Food and Drug Administration (FDA) has characterized as health care information aggregated from sources outside traditional clinical research settings (Sherman et al., 2016), has been touted as a way to generate a more complete understanding of treatment usage, effectiveness, and value.
In the current drug development paradigm, however, real-world evidence has primarily been applied in early discovery and in the postmarket phase for safety surveillance and comparative effectiveness evaluations (see Figure 1-1). Although this has led to many valuable insights, the larger promise of real-world evidence has not yet been fulfilled. On October 19, 2016, the Forum on Drug Discovery, Development, and Translation (the Forum) of the National Academies of Sciences, Engineering, and Medicine (the National Academies) held a workshop to facilitate dialogue among
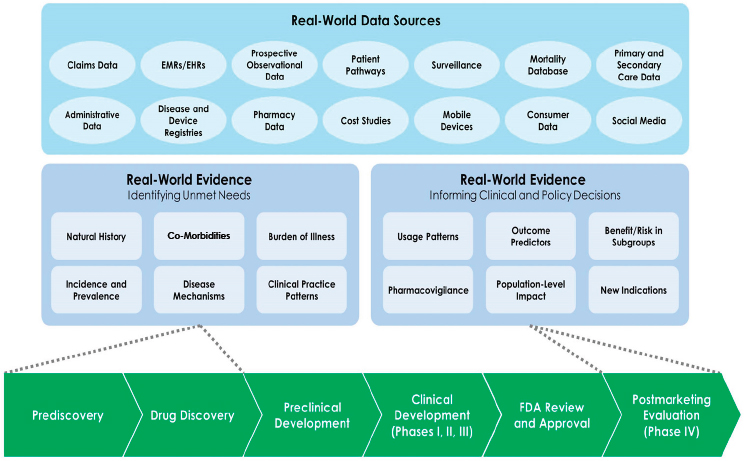
NOTES: Real-world evidence could inform all phases of treatment discovery and development, although thus far has been more commonly used to inform early development decisions and postmarketing safety surveillance or comparative effectiveness studies. By contrast, clinical development and review have tended to use more idealized and tightly controlled data sources for efficacy trials. EHR = electronic health record; EMR = electronic medical record.
SOURCE: Galson and Simon, 2016.
stakeholders about the opportunities and challenges for incorporating real-world evidence into all stages of the process for the generation and evaluation of therapeutics (see Box 1-1 for the full Statement of Task). This workshop builds on previous workshops sponsored by the Forum that in
recent years have focused on clinical trials, data sharing, and regulatory science.3
The potential applications of real-world evidence are numerous (see Box 1-2), and there are many remaining challenges surrounding its generation, accessibility and distribution, and use. To focus the discussons, presenters were asked not to delve into detailed technical aspects such as statistical methodologies, but instead, to share their perspectives on unmet stakeholder needs and opportunities to generate new kinds of evidence that meet those needs.
___________________
3 Publications from previous National Academies workshops with particular relevance to the topic of real-world evidence include
- Large Simple Trials and Knowledge Generation in a Learning Health System: Workshop Summary (IOM, 2013b), which focused on opportunities to advance a learning health system and improve the efficiency of drug development by integrating research at the point of care through large simple trials;
- Sharing Clinical Research Data: Workshop Summary (IOM, 2013c), which examined the benefits, barriers, and strategies to enhancing the sharing of clinical research data; and
- Advancing the Discipline of Regulatory Science for Medical Product Development: An Update on Progress and a Forward-Looking Agenda: Workshop Summary (NASEM, 2016), which touched on the integration and use of “big data” (e.g., data from electronic health records, registries, social media) in clinical research and regulatory decision making.
ORGANIZATION OF THE PROCEEDINGS OF A WORKSHOP
This Proceedings of a Workshop was prepared by the rapporteurs as a factual summary of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual workshop participants and have not been endorsed or verified by the Forum or the National Academies, and they should not be construed as reflecting any group consensus. The workshop was webcast live and online participants were able to contribute to the discussions through the hashtag #RealWorldEvidence. The slide presentations and videos are archived on the Forum website.4
The proceedings is organized as follows:
- Chapter 2 introduces the topic of real-world evidence in greater detail by describing some priorities for improving evidence generation to support decision making on approval and use of therapeutics as proposed by several diverse stakeholders, including regulators, patients, health care providers, payers, and industry.
- Chapter 3 characterizes some sources of real-world data and what can be learned from them.
-
Chapter 4 summarizes discussion of four case studies that highlight how real-world evidence has been incorporated into medical product development and evaluation processes, and the opportunities and challenges to build from these successful use cases. The four case studies were as follows:
- Salford Lung Study
- Transcatheter Valve Therapy (TVT) Registry
- Sentinel Initiative
- Observational Health Data Sciences and Informatics
- Chapter 5 summarizes some practical strategies for expanding the incorporation of real-world evidence into the generation and evaluation of therapeutics, including potential key next steps.
___________________
4 For more information, see https://www.nationalacademies.org/hmd/Activities/Research/DrugForum/2016-OCT-19.aspx (accessed November 16, 2016).
This page intentionally left blank.






