1
Introduction
The U.S. Social Security Administration (SSA) administers the Social Security Disability Insurance program (Title II of the Social Security Act) and the Supplemental Security Income (SSI) program (Title XVI of the Social Security Act). Title II pays disability benefits to people who are “insured” under the Act. The benefits are paid out of the Social Security trust fund, which is funded by the Social Security tax on individuals’ earnings. The Act also provides benefits to certain disabled dependents of insured individuals. Title XVI provides SSI payments to adults and children (under age 18) who are disabled and have limited income and resources.
When SSA evaluates disability claims based on a physical or mental impairment, it requires sufficient evidence to (1) establish the presence of a medically determinable physical or mental impairment(s), (2) assess the degree of functional limitation the impairment(s) imposes, and (3) project the probable duration of the impairment(s). Once SSA establishes the presence of a severe medically determinable physical or mental impairment(s), it determines whether the impairment(s) meets or medically equals (i.e., is equivalent in severity to) the criteria in the Listing of Impairments (the Listings), which are lists of medical conditions that qualify a candidate for disability benefits regardless of the applicant’s age, education, or work experience. The Listings are organized into 14 body systems for adults and 15 body systems for children. Each of the Listings includes impairments that, for adults, SSA considers severe enough to prevent any gainful activity and, for children, SSA considers severe enough to cause marked and severe functional limitations. If an individual does not meet Listings-level criteria, they can still qualify for disability further along in the sequential evaluation
process based on “residual functional capacity,” or functional limitations resulting from their medical impairments.
Special senses-related disorders that affect the ability to hear are evaluated under Listing 2.00 for adults and Listing 102.00 for children. SSA organizes the evaluation of hearing loss into two broad categories: hearing loss not treated with cochlear implantation and hearing loss treated with cochlear implantation.
The focus of this report is adults and children with cochlear implantation. In a Federal Register notice (Vol. 75, No. 105) published on June 2, 2010, SSA described in its rules and regulations the type of audiometric testing that is needed for those with cochlear implantation to be considered disabled until age 5 or for 1 year after implantation, whichever is later:
After that period, we [SSA] need word recognition testing performed with any age-appropriate version of the Hearing in Noise Test (HINT) or the Hearing in Noise Test for Children (HINT-C) to determine whether your impairment meets 102.11B. This testing must be conducted in quiet in a sound field. Your implant must be functioning properly and adjusted to your normal settings. The sentences should be presented at 60 dB HL (Hearing Level) and without any visual cues.
The current Listings that address hearing loss treated with cochlear implantation (2.11 and 102.11) contain criteria that evaluate hearing ability through a word recognition score determined using the Hearing in Noise Test (HINT) performed in a quiet sound field. To be used in SSA’s program, HINT testing must be performed on a person with properly functioning cochlear implants set at normal settings, with no visual testing cues, in a quiet sound field, and at 60 dB HL (decibel hearing level).1
Unlike the Listing for hearing loss in individuals with cochlear implants, the Listing for hearing loss not treated with cochlear implantation (2.10 and 102.10) does not specify a test (i.e., the HINT). Instead, it requires a “word recognition score of 40 percent or less in the better ear determined using a standardized list of phonetically balanced monosyllabic words.”
SSA seeks to generalize the listing criteria found in 2.11B and 102.11B (of Subpart P of Part 404, Listing of Impairments), the Listings for hearing loss treated with cochlear implantation, so that they can be evaluated with the results from hearing tests other than the HINT while maintaining similar levels of validity, specificity, sensitivity, and reliability.
___________________
1 dB HL = decibels in hearing level, the decibel measure displayed on an audiometer, normalized so that 0 dB HL = average normal for all frequencies.
STATEMENT OF TASK
SSA requested that a consensus committee of the National Academies of Sciences, Engineering, and Medicine identify and recommend generalized testing procedures and criteria for evaluating the level of functional hearing ability needed to make a disability determination in adults and children after cochlear implantation. The committee will produce a report detailing and supporting their findings, conclusions, and recommendations based on published evidence (to the extent possible) and professional judgment (where published evidence is lacking). The committee will:
- Identify and describe the salient test characteristics of the HINT, which is currently used to determine the functional hearing ability in adults or children with hearing loss treated with cochlear implantation, and provide recommendations as to how to generalize those characteristics into criteria that can be applied to other validated hearing tests for persons with cochlear implants.
- Describe the characteristics of hearing tests, administered in the sound field, either binaurally or monaurally, in either quiet or noise, that are in use for those with cochlear implants, and describe to the degree possible:
- The availability of the selected tests with respect to the instruments themselves, trained administrators of the tests, and insurance coverage or costs incurred with testing;
- The patient burden of undergoing these tests;
- Whether testing procedures or parameters, or the appropriateness of the test itself, vary based on the age of the person being tested;
- Whether the test outcomes are expected to vary based on demographic or other patient characteristic factors, including repeated testing with the same instrument; and
- The validity, specificity, sensitivity, reliability, and generalizability of the tests.
- Among the hearing tests described in task 2, identify those with characteristics most similar to the HINT, determine which tests, performed in the sound field, either binaurally or monaurally, in either quiet or noise, produce measurements most closely analogous to the word recognition score of the HINT (given HINT testing parameters of properly functioning cochlear implants set at normal settings, with no visual testing cues, in a quiet sound field, at 60 dB HL), and describe to the degree possible:
-
- What differences exist between the identified tests and the HINT in terms of the specific elements of hearing ability they measure;
- The committee’s recommendations as to how scores from the identified tests can be compared or converted to equivalent scores on the HINT; and
- The committee’s recommendations for the scores on hearing tests that correspond to a level of functional hearing ability that causes marked and severe functional limitation in a child or that prevents an adult from doing any gainful activity, regardless of his or her age, education, or work experience, and whether those scores can be expressed in a form comparable between hearing tests such as percentile or standard deviation from the norm.
- Examine the special considerations inherent in evaluating hearing ability in persons with single-sided deafness or asymmetric hearing loss receiving a cochlear implant and describe:
- Any special considerations in the testing and treatment of persons with bilateral but unequal hearing loss;
- Whether there is a correlation between the presence and degree of hearing loss in the less-affected ear and the recovery time or treatment for individuals with single-sided deafness or asymmetric hearing loss receiving a cochlear implant in their more-affected ear;
- Whether there is a level of hearing ability in the less-affected ear which would render cochlear implantation in the more-affected ear immaterial with respect to meeting the severity of hearing loss in the Listings (i.e., would not prevent an adult from engaging in any gainful activity nor a child from having “marked” limitations in two domains of functioning or an “extreme” limitation in one domain2);
- Whether the tests identified in task 3 remain appropriate for testing hearing ability in persons with single-sided deafness or asymmetric hearing loss receiving a cochlear implant and why, and if there are any differences in how the tests should be administered or interpreted; and
- Whether the equivalent scores identified in task 3 remain accurate proxies for the HINT word recognition scores when assessing persons with single-sided deafness or asymmetric hearing loss receiving a cochlear implant.
___________________
2 See 20 Code of Federal Regulations 416.926a and DI (disability insurance) 25225.030, DI 25225.035, DI 25225.040, DI 25225.045, DI 25225.050, and DI 25225.055.
APPROACH TO THE TASK
The National Academies assembled a committee of experts to address the task. Members with diverse backgrounds and expertise were appointed to focus on the different aspects of the task. Specifically, the members have expertise in audiology, otolaryngology, cochlear implantation in both children and adults, hearing loss testing, biostatistics, and epidemiology.
The committee met four times. It sponsored one open meeting, which enabled SSA representatives and the committee members to interact directly and to discuss the committee’s charge. In its discussion with SSA, the committee interpreted its charge to provide SSA with a recommendation for tests that would be accessible and feasible for widespread use by audiology clinics. Furthermore, that guiding principle led the committee to search for tests that would align with standard clinical practice.
In support of the committee’s discussions and deliberations, the committee instructed the staff to conduct targeted literature searches and to gather information from relevant texts, scientific journals and professional societies, and federal sources.
The review began with a search of online databases for U.S. and international English-language literature from 2010 through 2020. This search covered PubMed and Scopus as well as SSA and the National Academies Press websites. The search terms used included the names of the hearing tests the committee members identified intersected with key words from each of the questions in the Statement of Task. Staff initially reviewed more than 1,132 titles and abstracts, which the committee members carefully reviewed for relevance to the committee’s task. Committee members and project staff identified additional literature as needed throughout the course of the study to supplement the initial search, using systematic reviews when available.
COCHLEAR IMPLANTS
Cochlear implants are small electronic devices that help provide a sense of sound to profoundly deaf or severely hard-of-hearing individuals. They function differently from hearing aids, as implants do not amplify sounds to improve normal hearing; instead they give a person a representation of sounds in the environment, which in turn helps with understanding speech (NIDCD, 2017). Cochlear implants are surgically implanted and work by replacing the function of the damaged cochlea (inner ear) and stimulating the auditory nerve directly. The most recently published data state that in 2012 approximately 58,000 adults and 38,000 children had cochlear implants in the United States (NIDCD, 2017). Due to expansion of indications and implantation in the past decade, these numbers are
now a significant under-estimate. The American Cochlear Implant Alliance estimates that there were a total of 217,000 cochlear implant users in the United States in 2019. That estimate was based on a 9 percent annual growth rate from 2012 (ACIA, 2021).
Adults who lose some or most of their hearing as they age frequently benefit from cochlear implants, as they learn to associate the signals from the implant with sounds they remember, including speech, without requiring visual cues such as those provided by lipreading or sign language. For infants and young children without additional disabilities, cochlear implants placed early afford a child the ability to communicate via listening and spoken language at levels comparable to those of their same-age peers with typical hearing (Mayo Clinic, 2020; Niparko et al., 2010).
In early clinical trials, to qualify for cochlear implants, adults were required to score 0 percent on open-set measures of sentence recognition3 (Waltzman and Shapiro, 1999), and children were required to demonstrate bilateral profound sensorineural hearing loss, as the outcomes with cochlear implants were not known. As the safety and efficacy of cochlear implants became known, the criteria to receive a cochlear implant changed. Currently, three different manufacturers produce cochlear implants that have received U.S. Food and Drug Administration (FDA) approval in the United States. The approved indications for those devices vary depending on the timing of the approval and the stated goals of the clinical trial used to obtain FDA approval. Traditionally, most devices based candidacy on a sentence score for adults and on a word score for children. In 2013 and 2014 FDA approved two devices that used acoustic and electric hearing for rehabilitation (the Nucleus Hybrid Implant System and the MED-EL electric-acoustic stimulation, respectively). For each device candidacy was based on more lenient audiometric test results and on an aided word recognition score and not on a sentence score. In 2017 FDA approved the clinical trial to evaluate the Nucleus CI532 cochlear implant in adults (NCT03007472). In that trial, adults were considered to be candidates for this device if they obtained a test score of 40 percent correct or less in the ear to be implanted and 50 percent correct or less in the contralateral ear on a recorded monosyllabic word test presented at 60 dB sound pressure level (SPL), with A weighting (NLM, 2020). In 2019 FDA approved the MED-EL system for use in children (5 years of age and older) and adults with single-sided deafness and asymmetric hearing loss. Both approvals include indications that base candidacy for cochlear implantation on a word score for both children and adults, and represent a trend toward the
___________________
3 Open-set tasks are designed without limitations on the possible responses, such as an essay question on an exam or a speech recognition task for which the listener must provide a response without prompts.
use of monosyllabic word measures with both children and adults in the field of cochlear implants.
Cochlear implants work by bypassing the damaged areas of the ear and directly stimulating the auditory nerve. Signals generated by the implant are transmitted by way of the auditory nerve to the brain, which interprets the signals as sound (NIDCD, 2017). The implant consists of an external part that is located behind the ear and an additional part that is surgically placed under the skin (see Figure 1-1). An implant consists of the following parts:
- A microphone, which picks up sounds from the environment.
- A speech processor, which selects and arranges sounds picked up by the microphone.
- A transmitter and receiver/stimulator, which receives signals from the speech processor and converts them into electric impulses.
- An electrode array, which collects the impulses from the stimulator and sends them to different regions of the auditory nerve.
The internal part of the implant is placed under the skin behind the ear during outpatient surgery. A thin wire and small electrodes lead to the cochlea (part of the inner ear). The wire sends signals to the vestibulocochlear
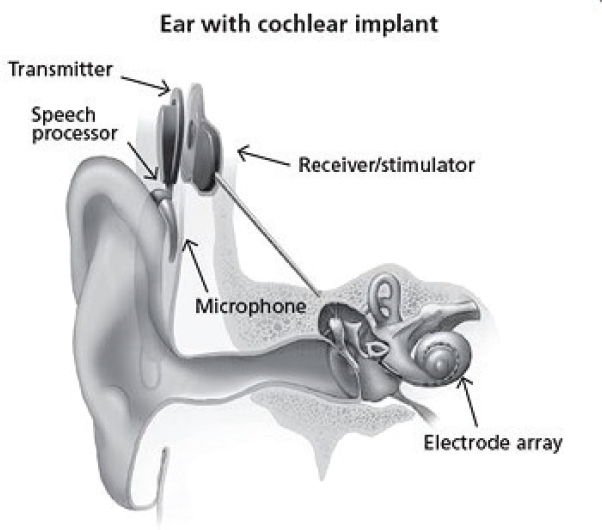
SOURCE: NIDCD, 2017.
nerve, which sends sound information to the brain to produce a hearing sensation. Although normal hearing is not restored, with appropriate therapy and practice the improved hearing experience can provide increased awareness of sounds in the environment as well as better communication through easier lipreading and listening (NIDCD, 2017).
Two recent literature reviews of post-implant performance were conducted by Boisvert et al. (2020) and by Buchman et al. (2020). Boisvert et al. (2020) reviewed 201 articles and reported that the average word recognition ability of implant recipients improved from 8.2 percent pre-implant to 53.9 percent post-implant and that 82 percent of post-lingually deafened adults and 53.4 percent of pre-lingually deafened adults demonstrated improvements of 15 percentage points or more. Buchman et al. (2020) reviewed 74 articles and identified 20 evidence-based consensus statements about cochlear implants, including
Cochlear implants significantly improve speech recognition in both quiet and moderate noise in adults with severe, profound, or moderate sloping to profound bilateral sensorineural hearing loss; these gains in speech recognition are likely to remain stable over time. (p. 947)
From 2000 to early 2020 cochlear implants were FDA-approved for use in children beginning at 12 months of age; in March 2020, Cochlear Ltd. (Sydney, Australia) received FDA approval to expand the labeled indications from 12 months to 9 months of age (FDA, 2020). For young children who are deaf or severely hard-of-hearing, using a cochlear implant exposes them to sounds during an optimal period in which to develop speech and language skills (NIDCD, 2017).
According to the National Institutes of Health, research has shown that children who receive a cochlear implant followed by intensive therapy from about 12–18 months of age are better able to hear and comprehend sound and music and to use spoken language than children who receive implants when they are older. Studies have also shown that many eligible children who receive a cochlear implant by 12–18 months of age develop language skills at a rate comparable to children with normal hearing and that many succeed in mainstream classrooms (FDA, 2018).
There is a growing body of literature demonstrating that children who receive cochlear implants before 12 months of age significantly outperform children implanted between 12 and 18 months on measures of language development (Bergeson et al., 2010; Dettman et al., 2016; Houston and Miyamoto, 2010; Houston et al., 2012a,b; Tobey et al., 2013), speech perception (Holman et al., 2013; Tajudeen et al., 2010), vocabulary (Hayes et al., 2009; Houston and Miyamoto, 2010; Tomblin et al., 2005), and speech intelligibility (i.e., how well others are able to understand one’s
speech) (Habib et al., 2010). The likely reason is that the typically developing infant undergoes significant auditory and language development in the first year of life—all of which may be missed or delayed for infants with severe-to-profound hearing loss. For example, by 6 months of age infants are linking sound patterns with meanings, including events (“bye-bye”), persons (“mommy”), and familiar objects (“nose”) (e.g., Bergelson and Swingley, 2012; Tincoff and Jusczyk, 1999, 2012). Word segmentation—the process by which a listener extracts meaningful units, such as individual words, from connected discourse—develops rapidly between 7.5 and 10.5 months (e.g., Jusczyk, 2002). By 8 months of age, infants exhibit long-term memory for newly acquired words, which is an important prerequisite for auditory-based language learning (Houston and Jusczyk, 2003; Jusczyk and Hohne, 1997).
Babies are exposed to multisensory stimuli shaping their auditory and language development with consistent auditory exposure to speech, music, and environmental sounds. Those auditory experiences are particularly influential during an infant’s waking hours; however, infants with severe-to-profound hearing loss experience auditory deprivation prior to initial hearing aid fitting and typically wear their hearing aids for less than 4 hours per day in the first year of life (Walker et al., 2015). Consequently, infants with severe-to-profound sensorineural hearing loss, for whom auditory access audibility is limited, are deprived of critical auditory-based, language-learning opportunities prior to cochlear implantation (Levine et al., 2016).
INTRODUCTION TO THE HEARING IN NOISE TEST
The HINT, first published in 1994, is the test that SSA currently uses to determine functional hearing ability in adults or children with hearing loss who have been treated with cochlear implantation. The HINT measures sentence recognition and is standardized to be administered with background noise, although SSA uses the HINT sentences in a quiet sound field. The HINT corpus is composed of 250 sentences, which are categorized into 25 lists. The sentences for the HINT were adapted from 336 Bamford-Kowal-Bench (BKB) sentences written in British English (Bench et al., 1979) to American English sentences of equivalent content and length (Starkey Research, 2020). During the test the subject uses both ears (binaural hearing) and is required to repeat sentences in a quiet environment and with competing noise presented from different directions (California Ear Institute, 2020).
The volume of each sentence is adjusted based on listener response. Following each correct response, the volume is decreased, which increases the level of difficulty for the next sentence on the list. Conversely, the volume of the sentence is increased after each incorrect response, which reduces
the difficulty for hearing each subsequent sentence. However, the level of background noise is held constant.
The HINT, as noted, was developed in 1994 to be adaptively measured (i.e., the signal-to-noise ratio was adjusted between trials according to whether the response was correct) in order to minimize floor and ceiling effects4 (Nilsson et al., 1994). However, improvements in cochlear implant technology have resulted in individuals with cochlear implants scoring consistently near the ceiling on the HINT sentence test. Specifically, unilateral cochlear implant recipients with post-lingual onset of deafness are routinely achieving 60 percent open-set monosyllabic word recognition, on average, on the HINT (Buchman et al., 2020; Holden et al., 2013). Indeed, an increasingly higher proportion of adult and pediatric implant recipients demonstrate at or near ceiling-level performance for tests of sentence recognition in quiet (Dunn et al., 2020; Gifford et al., 2018).
ORGANIZATION OF THE REPORT
Chapter 2 is a primer on assessing speech perception, familiarizing the reader with terms, concepts, and considerations for speech recognition test presentation level setup. It defines numerous terms and introduces speech and word tests and highlights the distinctions between them. It also provides the background material necessary to understand items 1, 2, and 3c in the Statement of Task. Chapter 3 addresses the Statement of Task’s item 1, which asks the committee to:
Identify and describe the salient test characteristics of the HINT, which is currently used to determine the functional hearing ability in adults or children with hearing loss treated with cochlear implantation, and provide recommendations as to how to generalize those characteristics into criteria that can be applied to other validated hearing tests for persons with cochlear implants.
Chapter 4 describes the Statement of Task’s item 2, that is,
Describe the characteristics of hearing tests, administered in the sound field, either binaurally or monaurally, in either quiet or noise, that are in use for those with cochlear implants, and describe to the degree possible, their availability, patient burden, the appropriateness of the test and whether test outcomes might vary based on demographic or other
___________________
4 The ceiling effect is observed when an independent variable no longer has an effect on a dependent variable or when the level above which variance in an independent variable is no longer measurable.
patient characteristic factors, and the validity, specificity, sensitivity, reliability, and generalizability of the tests.
Chapter 5 addresses the Statement of Task’s item 4, “examine special considerations in evaluating hearing ability in persons with single sided deafness or asymmetric hearing loss receiving a cochlear implant,” and Chapter 6 addresses item 3:
Among the hearing tests described in task 2, identify those with characteristics most similar to the HINT, determine which tests, performed in the sound field, either binaurally or monaurally, in either quiet or noise, produce measurements most closely analogous to the word recognition score of the HINT.
A list of acronyms and abbreviations can be found at the beginning of the report.
REFERENCES
ACIA (American Cochlear Implant Alliance). 2021. Cochlear implants: What is a cochlear implant? https://www.acialliance.org/page/CochlearImplant (accessed January 15, 2021).
Bench, J., A. Kowal, and J. Bamford. 1979. The BKB (Bamford-Kowal-Bench) sentence lists for partially-hearing children. British Journal of Audiology 13(3):108–112.
Bergelson, E., and D. Swingley. 2012. At 6–9 months, human infants know the meanings of many common nouns. Proceedings of the National Academy of Sciences 109(9):3253–3258.
Bergeson, T. R., D. M. Houston, and R. T. Miyamoto. 2010. Effects of congenital hearing loss and cochlear implantation on audiovisual speech perception in infants and children. Restorative Neurology and Neuroscience 28(2):157–165.
Boisvert, I., M. Reis, A. Au, R. Cowan, and R. C. Dowell. 2020. Cochlear implantation outcomes in adults: A scoping review. PLOS ONE 15(5):e0232421.
Buchman, C. A., R. H. Gifford, D. S. Haynes, T. Lenarz, G. O’Donoghue, O. Adunka, A. Biever, R. J. Briggs, M. L. Carlson, P. Dai, C. L. Driscoll, H. W. Francis, B. J. Gantz, R. K. Gurgel, M. R. Hansen, M. Holcomb, E. Karltorp, M. Kirtane, J. Larky, E. A. M. Mylanus, J. Thomas Roland, Jr., S. R. Saeed, H. Skarzynski, P. H. Skarzynski, M. Syms, H. Teagle, P. H. van de Heyning, C. Vincent, H. Wu, T. Yamasoba, and T. Zwolan. 2020. Unilateral cochlear implants for severe, profound, or moderate sloping to profound bilateral sensorineural hearing loss: A systematic review and consensus statements. JAMA Otolaryngology—Head & Neck Surgery 146(10):942–953.
California Ear Institute. 2020. Hearing in Noise Test (HINT). https://www.californiaearinstitute.com/audiology-services-hint-bay-area-ca.php (accessed October 17, 2020).
Dettman, S. J., R. C. Dowell, D. Choo, W. Arnott, Y. Abrahams, A. Davis, D. Dornan, J. Leigh, G. Constantinescu, R. Cowan, and R. J. Briggs. 2016. Long-term communication outcomes for children receiving cochlear implants younger than 12 months: A multicenter study. Otology and Neurotology 37(2):e82–e95.
Dunn, C. C., J. Oleson, A. Parkinson, M. R. Hansen, and B. J. Gantz. 2020. Nucleus Hybrid S12: Multicenter clinical trial results. Laryngoscope 130(10):E548–E558.
FDA (U.S. Food and Drug Administration). 2018. What is a cochlear implant? https://www.fda.gov/medical-devices/cochlear-implants/what-cochlear-implant (accessed October 17, 2020).
FDA. 2020. “Off-label” and investigational use of marketed drugs, biologics, and medical devices. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/label-and-investigational-use-marketed-drugs-biologics-and-medical-devices (accessed September 24, 2020).
Gifford, R. H., L. Loiselle, S. Natale, S. W. Sheffield, L. W. Sunderhaus, M. S. Dietrich, and M. F. Dorman. 2018. Speech understanding in noise for adults with cochlear implants: Effects of hearing configuration, source location certainty, and head movement. Journal of Speech, Language, and Hearing Research 61(5):1306–1321.
Habib, M. G., S. B. Waltzman, B. Tajudeen, and M. A. Svirsky. 2010. Speech production intelligibility of early implanted pediatric cochlear implant users. International Journal of Pediatric Otorhinolaryngology 74(8):855–859.
Hayes, H., A. E. Geers, R. Treiman, and J. S. Moog. 2009. Receptive vocabulary development in deaf children with cochlear implants: Achievement in an intensive auditory–oral educational setting. Ear and Hearing 30(1):128–135.
Holden, L. K., C. Brenner, R. M. Reeder, and J. B. Firszt. 2013. Postlingual adult performance in noise with HiRes 120 and ClearVoice Low, Medium, and High. Cochlear Implants International 14(5):276–286.
Holman, M. A., M. L. Carlson, C. L. W. Driscoll, K. J. Grim, R. S. Petersson, D. P. Sladen, and R. P. Flick. 2013. Cochlear implantation in children 12 months of age and younger. Otology and Neurotology 34(2):251–258.
Houston, D. M., and P. W. Jusczyk. 2003. Infants’ long-term memory for the sound patterns of words and voices. Journal of Experimental Psychology: Human Perception and Performance 29(6):1143–1154.
Houston, D. M., and R. T. Miyamoto. 2010. Effects of early auditory experience on word learning and speech perception in deaf children with cochlear implants: Implications for sensitive periods of language development. Otology and Neurotology 31(8):1248–1253.
Houston, D. M., J. Beer, T. R. Bergeson, S. B. Chin, D. B. Pisoni, and R. T. Miyamoto. 2012a. The ear is connected to the brain: Some new directions in the study of children with cochlear implants at Indiana University. Journal of the American Academy of Audiology 23(6):446–463.
Houston, D. M., J. Stewart, A. Moberly, G. Hollich, and R. T. Miyamoto. 2012b. Word learning in deaf children with cochlear implants: Effects of early auditory experience. Developmental Science 15(3):448–461.
Jusczyk, P. W. 2002. How infants adapt speech-processing capacities to native-language structure. Current Directions in Psychological Science 11(1):15–18.
Jusczyk, P. W., and E. A. Hohne. 1997. Infants’ memory for spoken words. Science 277(5334): 1984–1986.
Levine, D., K. Strother-Garcia, R. M. Golinkoff, and K. Hirsh-Pasek. 2016. Language development in the first year of life: What deaf children might be missing before cochlear implantation. Otology and Neurotology 37(2):e56–e62.
Mayo Clinic. 2020. Cochlear implants. https://www.mayoclinic.org/tests-procedures/cochlear-implants/about/pac-20385021 (accessed August 20, 2020).
NIDCD (National Institute on Deafness and Other Communication Disorders). 2017. Cochlear implants. https://www.nidcd.nih.gov/health/cochlear-implants (accessed August 19, 2020).
Nilsson, M., S. D. Soli, and J. A. Sullivan. 1994. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. Journal of the Acoustical Society of America 95(2):1085–1099.
Niparko, J. K., E. A. Tobey, D. J. Thal, L. S. Eisenberg, N. Y. Wang, A. L. Quittner, and N. E. Fink. 2010. Spoken language development in children following cochlear implantation. JAMA 303(15):1498–1506.
NLM (National Library of Medicine). 2020. Clinical evaluation of the Cochlear Nucleus CI532 cochlear implant in adults (SME). https://www.clinicaltrials.gov/ct2/show/NCT03007472?term=NCT03007472&draw=2&rank=1 (accessed December 4, 2020).
Starkey Research. 2020. The Hearing in Noise Test (HINT). https://starkeypro.com/research/research-resources/hearing-in-noise-test.html (accessed October 17, 2020).
Tajudeen, B. A., S. B. Waltzman, D. Jethanamest, and M. A. Svirsky. 2010. Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Otology and Neurotology 31(8):1254–1260.
Tincoff, R., and P. W. Jusczyk. 1999. Some beginnings of word comprehension in 6-month-olds. Psychological Science 10(2):172–175.
Tincoff, R., and P. W. Jusczyk. 2012. Six-month-olds comprehend words that refer to parts of the body. Infancy 17(4):432–444.
Tobey, E. A., D. Thal, J. K. Niparko, L. S. Eisenberg, A. L. Quittner, and N. Y. Wang. 2013. Influence of implantation age on school-age language performance in pediatric cochlear implant users. International Journal of Audiology 52(4):219–229.
Tomblin, J. B., B. A. Barker, L. J. Spencer, X. Zhang, and B. J. Gantz. 2005. The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. Journal of Speech, Language, and Hearing Research 48(4):853–867.
Walker, E. A., R. W. McCreery, M. Spratford, J. J. Oleson, J. Van Buren, R. Bentler, P. Roush, and M. P. Moeller. 2015. Trends and predictors of longitudinal hearing aid use for children who are hard of hearing. Ear and Hearing 36:38S–47S.
Waltzman, S. B., and W. H. Shapiro. 1999. Cochlear implants in children. Trends in Amplification 4(4):143–162.














